Abstract
Objective
To focus on the potential beneficial effects of the pleiotropic effects of dipeptidyl peptidase-4 inhibitors (DPP4is) on attenuating progression of diabetic kidney disease in reducing the long-term effect of the acute kidney injury (AKI) to chronic kidney disease (CKD) transition.
Patients and Methods
Data from the National Health Insurance Research Database from January 1, 1999, to July 31, 2011, were analyzed, and patients with diabetes weaning from dialysis-requiring AKI were identified. Cox proportional hazards models and inverse-weighted estimates of the probability of treatment were used to adjust for treatment selection bias. The outcomes were incident end-stage renal disease (ESRD) and mortality, major adverse cardiovascular events, and hospitalized heart failure.
Results
Of a total of 6165 patients with diabetes weaning from dialysis-requiring AKI identified, 5635 (91.4%) patients were DPP4i nonusers and 530 (8.6%) patients were DPP4i users. Compared with DPP4i nonusers, DPP4i users had a lower risk of ESRD (hazard ratio, 0.81; 95% CI, 0.70-0.94; P=.04) and all-cause mortality (hazard ratio, 0.28; 95% CI, 0.23-0.34; P<.001) after adjustments for CKD, advanced CKD, and angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker use. In contrast, the risk of major adverse cardiovascular events and hospitalized heart failure did not differ significantly between groups.
Conclusion
Dipeptidyl peptidase-4 inhibitor users had a lower risk of ESRD and mortality than did nonusers among patients with diabetes after weaning from dialysis-requiring AKI. Therefore, a prospective study of AKI to CKD transitions after episodes of AKI is needed to optimally target DPP4i interventions.
Abbreviations and Acronyms: AKI, acute kidney injury; AKI-D, dialysis-requiring acute kidney injury; CKD, chronic kidney disease; DM, diabetes mellitus; DPP4, dipeptidyl peptidase-4; DPP4i, dipeptidyl peptidase-4 inhibitior; ESRD, end-stage renal disease; hHF, hospitalized heart failure; HR, hazard ratio; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; IPTW, inverse probability of treatment weighting; KIM-1, kidney injury molecule-1; MACE, major adverse cardiovascular event; MI, myocardial infarction; MPR, medication possession ratio; NHI, National Health Insurance; NHIRD, National Health Insurance Research Database
Diabetes mellitus (DM) is known to worsen outcomes of cardiovascular and renal diseases. The DM milieu potentially increases the risk of acute kidney injury (AKI) in addition to long-term mortality and morbidity by increasing the ischemia sensitivity of the kidney.1 Acute kidney injury is known to increase the risk of chronic kidney disease (CKD) and end-stage renal disease (ESRD),2 especially in diabetes, and is becoming an increasing burden on health care resources.3 Recently, the American Society of Nephrology’s Acute Kidney Injury Advisory Group has highlighted the transition of care as a potential opportunity to reduce the long-term effect of AKI.4 However, there is paucity of data on which interventions can reduce morbidity and mortality in AKI and acute kidney disease survivors.
Dipeptidyl peptidase-4 (DPP4) inhibition is a new treatment approach for DM.5 Dipeptidyl peptidase-4 inhibitors (DPP4is) protect the kidney via their anti-inflammatory activity at an early stage of diabetic nephropathy.6 Beyond lowering glucose levels, DPP4 inhibition ameliorates kidney fibrosis through its antioxidant properties and protein-protein interactions.7 Nonetheless, information is limited on the clinical outcomes of patients with diabetes who develop AKI after receiving DPP4is. However, the outcome from an episode of AKI cannot simply be regarded as the binary administration for long term renal replacement therapy or recovery. Thus, our study aimed to examine the effect of DPP4is on outcomes after weaning from dialysis-requiring AKI (AKI-D), the most severe form of AKI and focused on the risk of ESRD, mortality, and cardiovascular outcomes.
Patients and Methods
Data Source
The National Health Insurance (NHI) program offers comprehensive medical care coverage to more than 99% of the country’s population of 23 million inhabitants. Taiwan’s National Health Research Institutes released the National Health Insurance Research Database (NHIRD) for research purposes, with data encrypted to protect privacy. This database contains all information on outpatient consultations, hospitalizations, procedures, and prescriptions recorded within the NHI system. The NHI data are reliable because the NHI Administration routinely audits claims data to prevent fraud in the NHI program.8 Disease diagnoses registered in the NHIRD are classified according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). The baseline comorbidities were compiled from at least 3 outpatient visits or 1 inpatient claim within the 1 year before the index hospitalization for first dialysis. This rule was constructed on the basis of a relatively strict criterion and was well validated with good predictive power.8, 9, 10, 11, 12, 13
As all personal information is de-identified in the NHIRD, informed consent was waived and this study was exempt from a full ethical review by the institutional review board of the National Taiwan University Hospital (institutional review board number 201212021RINC).
Study Cohort
We identified adult patients with DM according to ICD-9-CM codes 580.x, 581.x, and 584.x at hospital discharge. Dialysis-requiring AKI were identified using International Classification of Diseases, Ninth Revision codes for AKI (584.3, 634.3, 635.3, 636.3, 637.3, 638.3, 639.3, 669.3, or 958.5) along with procedure codes for short term dialysis, and the procedure code was cofinanced by the NHI with high accuracy.9 Furthermore, we used a selection period of 90 days to define ESRD because all patients receiving dialysis for more than 90 days in Taiwan can apply to the NHI for catastrophic illness registration cards.14
Figure 1 depicts the algorithm used for patient selection. The study participants were selected from all citizens with AKI-D covered by Taiwan’s NHI from January 1, 1999, to July 31, 2011. The definition of DM in this cohort was based on the following criteria: having visited at least 3 outpatient clinics or having at least 1 admission with a diagnosis of DM (ICD-9-CM code 250.x). The diagnostic accuracy of DM has previously been validated with good predicting power.8, 15 We used a 1-year cutoff period immediately before the index hospitalization to identify preadmission AKI and dialysis. Patients with preadmission AKI or ESRD and those who had undergone a kidney transplant were excluded.
Figure 1.
Flow diagram for selecting study patients. Patients hospitalized between January 1, 1999, and July 31, 2011, were screened using inclusion and exclusion criteria. A total of 6165 patients were identified for the final analysis. AKI = acute kidney injury; DPP4i = dipeptidyl peptidase-4 inhibitor; NHIRD = National Health Insurance Research Database.
Exposure to Medication
Dipeptidyl peptidase-4 inhibitors have been available in Taiwan since March 2009; thus, we included all patients diagnosed with type 2 DM between March 1, 2009, and June 30, 2011. After inclusion, all patients were followed up until December 31, 2011, allowing at least a half-year follow-up to estimate the risk of outcome events. Dipeptidyl peptidase-4 inhibitor users were identified and enrolled after hospital discharge after the first weaning from AKI-D. The index date was the date of the first DPP4i prescription. Participants in the control cohort (patients without DPP4i use) were assigned the same index dates as the corresponding patients in the DPP4i cohort. To investigate patients’ medication adherence and its effect on mortality, we calculated each patient’s medication possession ratio (MPR)16 for DPP4is and selected the patient group with an MPR not less than 70% as a target group to contrast with DPP4i nonusers. Patients who used DPP4is at 1 year were enrolled, and the mean MPR of DPP4is was 82.8%.
Inverse Probability of Treatment Weighting
To address confounding by observed covariates, we used inverse probability of treatment weighting (IPTW) methods, a form of propensity score–matching analysis. Weights were based on the results from a treatment selection model, which were estimated using logistic regression with receipt of DPP4i therapy as the dependent variable and baseline characteristics as independent variables. Variables used in the propensity score–matching analysis are included in the analysis if the P value was less than .10. Then each patient was weighted by the inverse probability of receiving the treatment that they actually received; the weight was calculated on the basis of the propensity score–matched value. Weights for DPP4i users were the inverse of the propensity score, and weights for DPP4i nonusers were the inverse of 1 – propensity score. The process of IPTW generates 2 new pseudo-cohorts. This process enabled us to preserve the sample size in the pseudo-cohorts close to the original cohorts, albeit not strictly equivalent.17, 18
After weighting, we assessed the balance of baseline characteristics among the treatment groups by using the chi-square test for categorical variables and the Student t test for continuous variables.19
Outcomes
The primary outcome in this study was all-cause mortality and ESRD after hospital discharge. Secondary outcomes were major adverse cardiovascular events (MACEs), defined as the incidence of coronary events that include nonfatal myocardial infarction (MI), coronary artery bypass graft, and coronary angiography. The International Classification of Diseases, Ninth Revision code for MI at hospitalization had high accuracy, as validated by previous studies.20 The records regarding coronary artery bypass graft and angiography were reliable because they were constructed on the basis of NHI procedure codes that were coupled to the NHI reimbursement system with routine auditing. We further defined patients with advanced CKD as those having a creatinine level of more than 6 mg/dL (to convert to mmol/L, multiply by 0.0259) with prescriptions for concomitant erythropoiesis-stimulating agents according to the reimbursement regulations of the NHI.9 Follow-up started on the first day of the use of DPP4is and ended on December 31, 2011, at the time of incident outcome of interest, or on the date of death, or on the last reimbursement record.
Statistical Analyses
The characteristics between nonusers and users were compared using the Student t test for age and the chi-square test for other variables. Incidence rates of outcomes of interest were compared between DPP4i users and nonusers using Poisson distributions. The treatment effects (hazard ratios [HRs] and 95% CIs) of the main outcomes of interest were modeled using Cox proportional hazards regression incorporated with the IPTW estimated using the propensity score–matching analysis. Importantly, the weighted Cox model, in addition to controlling CKD or advanced CKD and medical therapies, was estimated 1 year after discharge; angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker use was examined because these medications were prescribed after treatment assignment.
Sensitivity analyses were performed by estimating the overall HRs for users vs nonusers. To assess the potential heterogeneity of DPP4i treatment, the DPP4i effects on all-cause mortality, ESRD, MACEs, and hospitalized heart failure (hHF) were further analyzed using the post-IPTW cohort. We formally tested the first-order interactions using multivariable Cox proportional hazards models by entering interaction terms between DPP4i use and subgroup variables. Interactions between DPP4i use and clinically relevant variables, including other oral antidiabetic agents (ie, metformin, sulfonylurea, thiazolidinedione, insulin, meglitinide, and α-glucosidase inhibitors), were tested. Analyses were performed using SAS version 9.3 (SAS Institute Inc.). A P value less than .05 was considered statistically significant.
Results
Characteristics of the Study Population
A total of 173,045 participants with a diagnosis of AKI receiving hemodialysis between January 1, 1999, and July 31, 2011, were identified and recruited (Figure 1). Overall, a total of 6165 patients with type 2 DM who withdrew from dialysis were included in this analysis; and of these, 5635 patients were DPP4i nonusers and 530 patients were DPP4i users. The demographic and clinical characteristics of these cohorts before and after the IPTW were estimated are summarized in Table 1 . Dipeptidyl peptidase-4 inhibitor users were younger and had a lower proportion of chronic obstructive pulmonary disease and chronic liver disease. Furthermore, the user group was more likely to have received clopidogrel, statins, and other antidiabetic agents including thiazolidinediones, meglitinides, and α-glucosidase inhibitors.
Table 1.
Baseline Characteristics of the Study Population Before and After the Inverse Probability of Treatment Weighting Estimationab
| Characteristic | Before matching |
After matching |
||||
|---|---|---|---|---|---|---|
| DPP4i nonusers |
DPP4i users |
P value | DPP4i nonusers |
DPP4i users |
P value | |
| (n=5635) | (n=530) | (n=5635) | (n=530) | |||
| Age (y) | 68.93±11.38 | 65.74±11.5 | <.001 | 68.65±11.51 | 68.19±11.21 | .21 |
| Sex: male | 2678 (47.5) | 274 (51.7) | .07 | 2678 (47.9) | 274 (48.3) | .86 |
| Monthly income (New Taiwan $) | ||||||
| <19,100 | 3366 (59.7) | 306 (57.7) | .006 | 3366 (59.7) | 306 (57.2) | .26 |
| 19,100-41,999 | 2088 (37.1) | 193 (36.4) | 2088 (37) | 193 (38) | ||
| ≥42,000 | 181 (3.2) | 31 (5.9) | 181 (3.3) | 31 (4.8) | ||
| Hospital level | ||||||
| Urban | 2342 (41.6) | 218 (41.1) | .02 | 2342 (41.5) | 218 (40.9) | .28 |
| Suburban | 1288 (22.9) | 148 (27.9) | 1288 (23) | 148 (26.3) | ||
| Rural | 2005 (35.6) | 164 (30.9) | 2005 (35.5) | 164 (32.8) | ||
| Outpatient visits | ||||||
| <5 | 2129 (37.8) | 173 (32.6) | .002 | 2129 (37.2) | 173 (36.6) | .37 |
| 5-10 | 2989 (53) | 283 (53.4) | 2989 (53.3) | 283 (51.2) | ||
| 11-15 | 494 (8.8) | 70 (13.2) | 494 (9.1) | 70 (11.4) | ||
| >15 | 23 (0.4) | 4 (0.8) | 23 (0.5) | 4 (0.8) | ||
| Baseline comorbidities | ||||||
| Congestive heart failure | 1393 (24.7) | 119 (22.5) | .27 | 1393 (24.6) | 119 (22.6) | .33 |
| CKD | 1700 (30.2) | 140 (26.4) | .07 | 1700 (29.8) | 140 (28.7) | .65 |
| ACKD | 381 (6.8) | 46 (8.7) | .11 | 381 (6.8) | 46 (8.7) | .12 |
| COPD | 914 (16.2) | 46 (8.7) | <.001 | 914 (15.7) | 46 (12.5) | .06 |
| Dementia | 201 (3.6) | 8 (1.5) | .01 | 201 (3.5) | 8 (2.4) | .22 |
| Liver disease | 480 (8.5) | 21 (4) | <.001 | 480 (8.2) | 21 (6.5) | .20 |
| Peptic ulcer | 997 (17.7) | 78 (14.7) | .10 | 997 (17.4) | 78 (16) | .47 |
| PAD | 201 (3.6) | 7 (1.3) | .004 | 201 (3.4) | 7 (2.2) | .14 |
| Rheumatoid arthritis | 42 (0.8) | 1 (0.2) | .18 | 42 (0.7) | 1 (0) | .07 |
| Solid tumor | 267 (4.7) | 16 (3) | .08 | 267 (4.6) | 16 (4.4) | .81 |
| SLE | 9 (0.2) | 0 (0) | .99 | 9 (0.2) | 0 (0) | .36 |
| Atrial fibrillation | 513 (9.1) | 28 (5.3) | .002 | 513 (8.8) | 28 (6.3) | .06 |
| Dyslipidemia | 1300 (23.1) | 205 (38.7) | <.001 | 1300 (24.5) | 205 (26.7) | .26 |
| Alzheimer disease | 13 (0.2) | 1 (0.2) | .99 | 13 (0.2) | 1 (0.2) | .82 |
| Parkinson disease | 135 (2.4) | 5 (0.9) | .03 | 135 (2.3) | 5 (1.2) | .09 |
| Hypertension medications | ||||||
| α-Blocker | 943 (16.7) | 74 (14) | .11 | 943 (16.7) | 74 (14.2) | .15 |
| β-Blocker | 2885 (51.2) | 306 (57.7) | .004 | 2885 (51.8) | 306 (51.9) | .97 |
| Calcium channel blocker | 4337 (77) | 404 (76.2) | .71 | 4337 (77) | 404 (77.4) | .87 |
| Diuretic | 4189 (74.3) | 375 (70.8) | .08 | 4189 (74.3) | 375 (71.1) | .11 |
| ACEi or ARB | 3824 (67.9) | 388 (73.2) | .01 | 3824 (68.2) | 388 (72.3) | .06 |
| Other medications | ||||||
| Aspirin | 875 (15.5) | 82 (15.5) | >.99 | 875 (15.6) | 82 (15.5) | .89 |
| Clopidogrel | 551 (9.8) | 89 (16.8) | <.001 | 551 (10.6) | 89 (12.3) | .26 |
| Ticlopidine | 307 (5.5) | 13 (2.5) | .002 | 307 (5.3) | 13 (3.6) | .11 |
| Warfarin | 199 (3.5) | 15 (2.8) | .46 | 199 (3.5) | 15 (4) | .60 |
| Proton-pump inhibitor | 809 (14.4) | 79 (14.9) | .75 | 809 (14.5) | 79 (15.6) | .48 |
| H2 blocker | 1106 (19.6) | 102 (19.3) | .86 | 1106 (19.5) | 102 (21) | .41 |
| Statin | 1608 (28.5) | 239 (45.1) | <.001 | 1608 (30) | 239 (33.2) | .14 |
| NSAID | 3109 (55.2) | 255 (48.1) | .002 | 3109 (54.6) | 255 (51.3) | .15 |
| Corticosteroid | 850 (15.1) | 56 (10.6) | .005 | 850 (14.9) | 56 (11.3) | .02 |
| SSRI | 159 (2.8) | 15 (2.8) | .99 | 159 (2.8) | 15 (3.4) | .39 |
| Nitrate | 96 (1.7) | 9 (1.7) | .99 | 96 (1.8) | 9 (2) | .82 |
| Antidiabetic agents | ||||||
| Metformin | 2552 (45.3) | 264 (49.8) | .05 | 2552 (45.3) | 264 (49.1) | .10 |
| Sulfonylurea | 3629 (64.4) | 353 (66.6) | .32 | 3629 (64.3) | 353 (65.9) | .44 |
| Thiazolidinedione | 508 (9) | 94 (17.7) | <.001 | 508 (9.2) | 94 (17) | <.001 |
| Insulin | 3219 (57.1) | 267 (50.4) | .003 | 3219 (57.1) | 267 (50.3) | .003 |
| Meglitinide | 870 (15.4) | 120 (22.6) | <.001 | 870 (15.6) | 120 (22.3) | <.001 |
| α-Glucosidase inhibitor | 647 (11.5) | 112 (21.1) | <.001 | 647 (11.7) | 112 (21) | <.001 |
ACEi = angiotensin-converting enzyme inhibitor; ACKD = advanced chronic kidney disease; ARB = angiotensin II receptor blocker; CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease; DPP4i = dipeptidyl peptidase-4 inhibitor; NSAID = nonsteroidal anti-inflammatory drug; PAD = peripheral artery disease; SLE = systemic lupus erythematosus; SSRI = selective serotonin reuptake inhibitor.
Data are presented as mean ± standardized difference or as No. (percentage).
Risk of ESRD, All-Cause Mortality, MACEs, and hHF
Compared with DPP4i nonusers, DPP4i users had a significantly lower risk of ESRD (HR, 0.81; 95% CI, 0.70-0.94; P=.04) and all-cause mortality (HR, 0.28; 95% CI, 0.23-0.34; P<.001) but DPP4is did not significantly affect the risk of an MACE (HR, 0.86; 95% CI, 0.71-1.04; P=.11) or hHF (HR, 1.17; 95% CI, 1.01-1.36; P=.13) (Table 2 ). The result was the same in a Cox model adjusted for the presence of CKD, advanced CKD, and angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker use.
Table 2.
Risk of End-Stage Renal Disease, Mortality, Major Adverse Cardiovascular Event, and Heart Failure Association With DPP4i Therapy Compare Nonusersa
| Outcome | DPP4is |
With vs without DPP4i therapyb |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Users |
Nonusers (reference) |
Unadjusted |
Inverse weighted |
Inverse weighted and adjustedc |
Inverse weighted and adjustedd |
|||||||||
| No. of events | Person-year | Incidence ratee (95% CI) | No. of events | Person-year | Incidence ratee (95% CI) | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| ESRD | 233 | 1449.9 | 160.7 (142.9-180.8) | 1968 | 12329.7 | 159.6 (153.3-166.2) | 1.04 (0.91-1.19) | .59 | 0.87 (0.75-1.00) | .06 | 0.85 (0.73-0.99) | .03 | 0.81 (0.70-0.94) | .04 |
| Mortality | 97 | 2113.4 | 45.9 (37.8-55.7) | 3652 | 18188.5 | 200.8 (195.0-206.7) | 0.23 (0.19-0.28) | <.001 | 0.26 (0.21-0.31) | <.001 | 0.26 (0.22-0.32) | <.001 | 0.28 (0.23-0.34) | <.001 |
| MACE | 138 | 1852.8 | 74.5 (63.4-87.4) | 1239 | 15964.5 | 77.6 (73.6-81.9) | 0.98 (0.82-1.17) | .80 | 0.87 (0.72-1.05) | .16 | 0.89 (0.73-1.07) | .21 | 0.86 (0.71-1.04) | .11 |
| hHFf | 207 | 1644.7 | 125.9 (110.8-143.0) | 1627 | 14433.9 | 112.7 (107.7-118.0) | 1.15 (0.99-1.33) | .06 | 1.18 (1.02-1.37) | .03 | 1.19 (1.02-1.38) | .02 | 1.17 (1.01-1.36) | .13 |
DPP4i = dipeptidyl peptidase-4 inhibitor; ESRD = end-stage renal disease; hHF = hospitalized heart failure; HR = hazard ratio; MACE = major adverse cardiovascular event.
Cox proportional hazards models used to compare DPP4i therapy with non-DPP4i.
After adjustment for age, sex, chronic kidney disease, and advanced chronic kidney disease.
After adjustment for age, sex, chronic kidney disease, advanced chronic kidney disease, and angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker use.
Per 103 person-years.
Hospitalization for a primary diagnosis of heart failure.
Interaction of DPP4is With Concomitant Use of Other Antidiabetic Agents
We further evaluated the effect of the interaction between DPP4i use and the concomitant use of other antidiabetic agents on outcomes. The effect of DPP4is on the risk of ESRD was consistent among patient subgroups when stratified by combination with other antidiabetic agents. However, DPP4is combined with insulin increased the risk of mortality (P=.004) and hHF (P<.001). In addition, DPP4is in combination with meglitinide increased the risk of mortality (P=.01) and α-glucosidase inhibitors increased the risk of hHF (P=.03) (Table 3 ).
Table 3.
Interaction of Medications of Interest With DPP4is to Predict Outcomes After Adding It to the Final Modela
| Medication | Interaction with DPP4is in the final model (adjustedb) |
|||||
|---|---|---|---|---|---|---|
| ESRD |
Mortality |
hHFc |
||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Metformin | 0.77 (0.57-1.03) | .08 | 0.74 (0.50-1.10) | .14 | 0.81 (0.61-1.07) | .13 |
| Sulfonylurea | 0.95 (0.71-1.27) | .73 | 0.71 (0.47-1.07) | .10 | 1.13 (0.83-1.53) | .45 |
| Thiazolidinedione | 0.77 (0.51-1.17) | .23 | 1.11 (0.65-1.91) | .69 | 1.10 (0.76-1.59) | .62 |
| Insulin | 1.14 (0.86-1.52) | .36 | 1.80 (1.20-2.69) | .004 | 1.82 (1.37-2.42) | <.001 |
| Meglitinide | 1.14 (0.83-1.55) | .43 | 1.77 (1.14-2.75) | .01 | 1.28 (0.93-1.76) | .14 |
| α-Glucosidase inhibitor | 0.79 (0.55-1.13) | .20 | 1.33 (0.81-2.16) | .26 | 1.44 (1.03-2.01) | .03 |
DPP4i = dipeptidyl peptidase-4 inhibitor; ESRD = end-stage renal disease; hHF = hospitalized heart failure; HR = hazard ratio.
After adjustment for age, sex, chronic kidney disease, advanced chronic kidney disease, and angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker use.
Hospitalization for a primary diagnosis of heart failure.
Subgroup Analyses of Outcomes of Interest
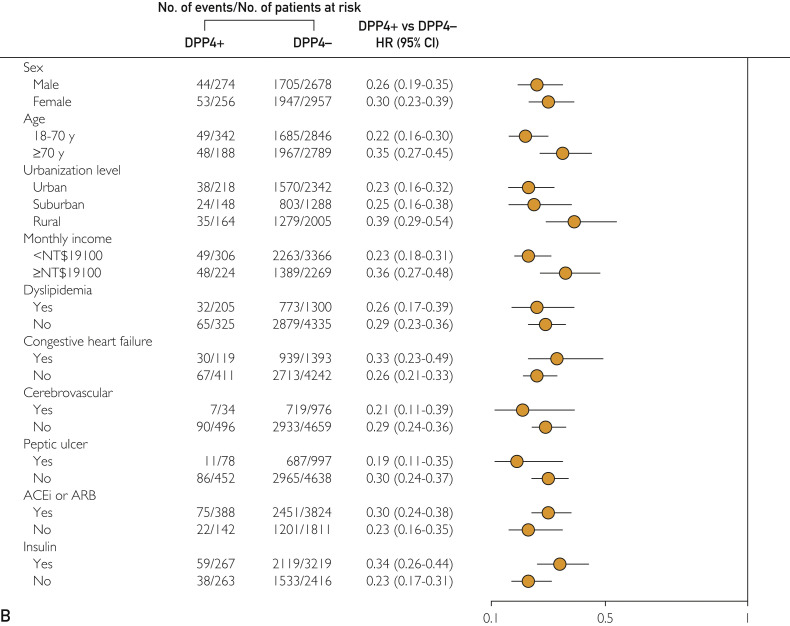
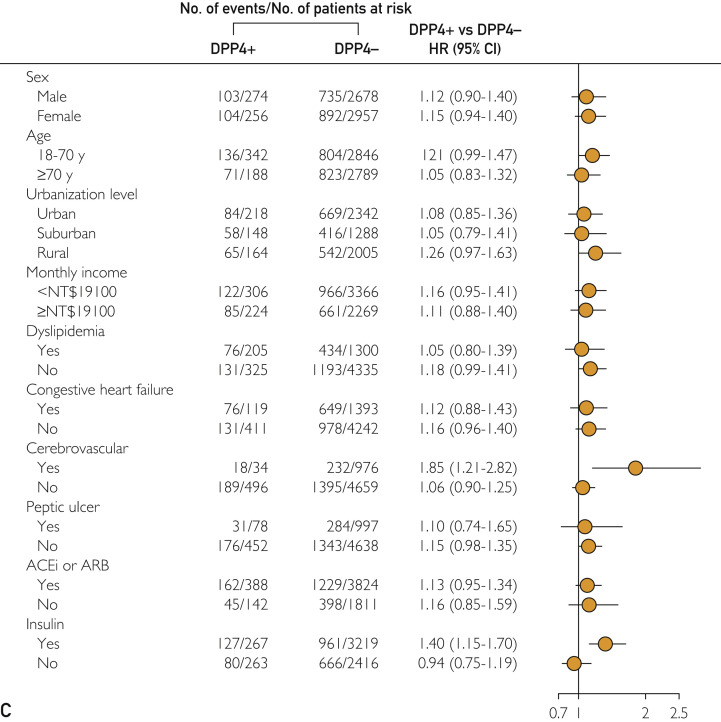
The results of subgroup analyses of different outcomes are presented in Figure 2 . Dipeptidyl peptidase-4 inhibitor use was consistently associated with a lower probability of long-term ESRD (Figure 2, A) and mortality risk (Figure 2, B) across various patient groups with respect to baseline comorbidities. For hHF, patients with previous cerebrovascular disease and insulin use had a higher risk of hHF (Figure 2, C).
Figure 2.
Adjusted HRs for the long-term risk of (A) incident end-stage renal disease, (B) all-cause mortality, and (C) hospitalized heart failure among DPP4i users and nonusers, and subgroup analysis with respect to premorbid risk and concomitant medications that was further adjusted for age, sex, chronic kidney disease, advanced chronic kidney disease, and ACEi or ARB use. ACEi = angiotensin-converting enzyme inhibitor; ARB = angiotensin II receptor blocker; DPP4i = dipeptidyl peptidase-4 inhibitor; HR = hazard ratio; NT$ = New Taiwan dollar.
Discussion
Our research is the first attempt to examine the effects of DPP4i use on renal outcomes in patients with diabetes weaning from AKI-D. Our results highlight that DPP4is decreased the risk of mortality and ESRD by 72% and 19%, respectively, during a mean follow-up of 3.3 years in the study cohort. Moreover, the risk of MACE and hHF was not significantly increased in DPP4i users.
Dipeptidyl Peptidase-4 Inhibitor Use Decreases Subsequent ESRD
Acute kidney injury episodes are associated with a cumulative risk of developing advanced CKD in patients with DM.21 Many studies have reported an associated increased risk of ESRD subsequent to AKI.22, 23 Despite survival and withdrawal from dialysis, AKI increases the incidence of de novo CKD, long-term dialysis, and death.24, 25 Hypoxia serves as a key player in AKI pathophysiology and is the final common pathway from CKD to ESRD.26 Acute kidney injury contributes to tubular atrophy, interstitial fibrosis, and peritubular capillary effacement, and thus maladaptive repair after AKI leads to accelerated kidney aging and CKD.27, 28 The pathological processes initiated during AKI (eg, hypoxia, cellular senescence, maladaptive repair, and inflammation) have been proposed to characterize AKI to CKD transitions, primarily via a self-perpetuating tubulo-interstitial fibrosis pathway.29 Renal repair is maladaptive because of tissue responses including inflammation, fibrosis from activation of interstitial myofibroblasts, and vascular rarefaction and often leads to persistent cell and tissue malfunction and eventually chronic fibrotic kidney disease.29, 30
Several studies have illustrated the pleiotropic effects of DPP4is in delaying renal function deterioration. Dipeptidyl peptidase-4 inhibitors may prevent inflammation and fibrosis of the heart and kidney by decreasing the oxidative stress response of heart and kidney tissues.31 Experimental studies using various diabetic models suggest that incretins protect the vascular endothelium from injury by binding to glucagon-like peptide 1 receptors, thereby ameliorating oxidative stress and the local inflammatory response, which reduces albuminuria and inhibits glomerular sclerosis.32 The renal effects of DPP4is might be explained indirectly by glucose-independent mechanisms such as an improvement in blood pressure control33 via down-regulation of the sodium/hydrogen antiporter 3 in the proximal tubule.34 Vildagliptin, a DPP4i, was reported to decrease apoptosis, as evidenced by a 2-fold decrease in B cell lymphoma-2–associated X protein/B cell lymphoma-2 messenger RNA expression and significantly decreased messenger RNA expression of the proinflammatory marker C-X-C motif chemokine 10 in the ischemia-reperfusion injury animal model.35 Macrophage phenotype switching from M1 to M2 subtype facilitates renal repair after AKI.36 In an animal study, sitagliptin-mediated inhibition of early atherosclerosis was due to M2 polarization during monocyte differentiation via stromal cell-derived factor 1/C-X-C chemokine receptor type 4 signaling,37, 38 which facilitated kidney repair. Renal recovery in AKI is compromised by perturbations of the cell cycle with arrest in the G2 phase and production of proinflammatory and profibrotic signals.29 It has been suggested that DPP4is may improve oxygen supply and mitigate the inflammatory response after ischemia-reperfusion injury by improving recovery of sublethally injured cells. Furthermore, in the immune system, DPP4 acts as a marker of T-cell activation in which it functions as a costimulatory molecule.39 Kidney injury molecule-1 (KIM-1) is expressed in activated CD4+ T cells as well as in injured renal tubular epithelial cells; thus, chronic KIM-1 expression may provide a mechanistic link between AKI and subsequent renal fibrosis.40 It is possible that DPP4is inactivate CD4+ T cells, which have been identified as the primary pathogenic T cell in experimental AKI,41 and may attenuate KIM-1 expression.
Although several small-scale clinical trials have found that a DPP4is significantly decreased the urine albumin-to-creatinine ratio,42, 43, 44 3 large-scale, randomized, double-blind studies have revealed that adding a DPP4i to routine care did not appear to increase the risk of renal failure.45, 46, 47 Clinical trials to date have not provided a clear consensus on the renal effect of these drugs in patients with type 2 DM. Enhanced follow-up of renal function of patients who have recovered from temporary dialysis may be warranted, and DPP4is may be candidate agents for preventing renal disease progression in patients with DM weaning from AKI-D.
Dipeptidyl Peptidase-4 Inhibitors Decreased Subsequent Mortality But Not MACE or hHF After AKI
Acute kidney injury requiring temporary dialysis increases the long-term risk of coronary events and all-cause mortality.9 The reduction in MI or cardiovascular events observed with short-term DPP4i treatment did not persist over the long term.48, 49 Dipeptidyl peptidase-4 inhibitors may have had a cardiovascular benefit in the low-risk patients enrolled and a neutral effect on those at high risk or with previous cardiovascular events participating in the cardiovascular outcome trial.45, 47, 50 Dipeptidyl peptidase-4 inhibitors were found to prevent the development of aortic and endothelial stiffness via decreased fibroblast growth factor 23, oxidative stress, and increased Klotho expression in mice.51 High levels of serum fibroblast growth factor 23 and Klotho deficiency were associated with an increased risk of coronary heart disease, heart failure, and cardiovascular mortality.52, 53 Dipeptidyl peptidase-4 inhibitors improved cardiac function and decreased the infarct size after MI through stromal cell-derived factor 1α/C-X-C chemokine receptor type 4/signal transducer and activator of transcription 3 signaling pathways in cardiomyocytes.53 Accordingly, a preliminary report establishes that both vildagliptin and sitagliptin treatments reduce intima media thickness, a surrogate marker for early atherosclerosis.54
In this study, we found that the use of DPP4is in patients with diabetes after weaning from AKI-D resulted in a lower risk of all-cause mortality. A recent meta-analysis reported that DPP4is decreased the risk of all-cause mortality in patients with CKD.55 Furthermore, Mogensen et al56 illustrated a lower mortality rate in the Danish population with diabetes taking incretin-based drugs. Recent observational data also confirm that DPP4is may improve cardiac and all-cause mortality in patients with DM with hHF57 and even in patients with preexisting heart failure.58
We further found that DPP4is may decreased the risk of MACE-related mortality and severe sepsis (Supplemental Table 1, available online at http://www.mayoclinicproceedings.org). The use of DPP4is could explain the decreased risk of mortality in our study. One study found that the use of sitagliptin in patients with type 2 diabetes after recent acute MI was not associated with an increased risk of adverse cardiovascular events,59 and another study noted that DPP4is improved long-term survival in patients with diabetes after first acute MI.60
Diabetes and infectious causes of death are linked to inflammation, and it has become increasingly appreciated that CKD is characterized by a state of chronic inflammation.61 There is a wealth of evidence indicating that disorders of both innate and adaptive immune systems contribute to an increased rate of infections in the course of CKD and diabetes.62
Dipeptidyl peptidase-4 inhibition may have pleiotropic effects, modulating the immune response by binding DPP4 receptors of immune cells63 or culprit pathogens, such as coronavirus64 and hepatitis C virus.65 The DPP4i sitagliptin reduced the lipopolysaccharide-induced inflammatory response, which was mediated by the nuclear factor κB signaling pathway. Although it is an animal study, it may hint that DPP4is may have a function in cardiac remodeling attributed to sepsis-induced inflammation.66
Although no concomitant effect on incident ESRD has been described, our study found that DPP4is combined with insulin or meglitinide therapy increased the risk of mortality in patients with diabetes after AKI. The concept is in line with the observation that meglitinide or other antidiabetic agent–induced hypoglycemia eventually contributed to cardiovascular events and all-cause mortality.67, 68 A recent study also reported that meglitinide combined with insulin will increase hypoglycemia in patients with advanced CKD.68 Accordingly, DPP4is will augment the long-term effects of insulin on subsequent heart failure in post–AKI-D care.
Strengths and Limitations
Our study has several strengths. First, this study is the first to report an association of DPP4i use with a lower risk of ESRD and all-cause mortality in patients with diabetes weaning from AKI-D. Our study population included only patients with DM who were hospitalized for AKI, and the result is consistent in patients with comorbidities such as congestive heart failure and cardiovascular disease and in those who had received angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers. Second, our data, derived from a current nationwide population-based cohort, enabled tracing of nearly all the AKI episodes associated with DPP4i use. All claims records of outpatient visits and hospital admissions were included, and diagnoses were included from both sources.
Our study has some limitations that should be acknowledged. First, given the impossibility of treatment randomization in this retrospective and observational study, the influence of potential confounding factors not evaluated herein may have biased the results. For example, laboratory data (eg, hemoglobin A1c level) were not available in the NHIRD claims data. However, we used surrogate indicators to adjust for patients’ baseline diabetes severity, such as the number of outpatient visits. We further added the frequency of hemoglobin A1c measurements to the final model and found similar results (Supplemental Result 1, available online at http://www.mayoclinicproceedings.org). High blood pressure is a well-known key for progression of kidney disease and death, but there were no blood pressure data in the NHIRD. We used joint modeling of multiple diseases to capture the effect of hypertension to investigate geographic variations in risk.69 Recent studies reported that congestive heart failure,70, 71 atrial fibrillation,72 and peripheral artery disease73 are highly related to hypertension and could be used as proxies for hypertension. Although we do not have any variable directly reflecting hypertension, we could assume that we have controlled the effect of hypertension because we have included the above-mentioned 3 diseases as proxies for hypertension and added them to the final model (Supplemental Result 2, available online at http://www.mayoclinicproceedings.org). To further avoid potential residual confounding due to inadequate adjustment for unevaluated biases, we used the IPTW model to balance every clinical characteristic between the 2 groups. In addition, we matched patients using the propensity score analysis with propensity score and reanalyzed mortality by using propensity score–adjusted logistic regression. Consistent with our IPTW analysis, the propensity score–matching analysis revealed that the use of DPP4is mitigates mortality in patients with diabetes after weaning from AKI-D (Supplemental Table 2, available online at http://www.mayoclinicproceedings.org).
Second, the implicit association of insulin use with more intensive glycemic control may have potentially confounded our results, although the results of the stratified analysis according to insulin use were consistent with those of the main analysis. Although there are no estimated glomerular filtration rate and CKD stage classification in the NHIRD, we have devised a way to differentiate the severity of CKD using the NHI data. We categorized patients with CKD with concomitant erythropoiesis-stimulating agents prescription as those with “advanced CKD.”9 More than 75% patients with advanced CKD are found to be anemic.74 Therefore, patients with such prescription are highly likely to have advanced CKD. We adjusted preadmission CKD, advanced CKD, postdischarge CKD, and advanced CKD, and found that in patients with advanced CKD, the use of DPP4is after discharge was independently associated with decreased mortality. Most importantly, these factors exhibited a severity-dependent risk to mortality. Finally, a future study will clarify the mechanisms underlying the differences in incident ESRD for different DPP4is. However, our results may only be generalizable to the population with DM, with an AKI episode covered by the universal health care insurance program.
Conclusion
Our findings suggest that the use of DPP4is in patients with diabetes weaning from AKI-D was associated with a decreased risk of ESRD and mortality. These findings extend those of previous studies on the safety of DPP4i use and may shed further light on the management of AKI to CKD transitional care and the potential renal effects, which may aid in treatment decisions in routine clinical practice.
Acknowledgments
We sincerely thank all staff of the Taiwan Clinical Trial Consortium (TCTC).
The National Taiwan University Hospital Study Group for Acute Renal Failure (NSARF) includes the following: Vin-Cent Wu, MD, PhD, Department of Internal Medicine, National Taiwan University Hospital, Taipei; Tai-Shuan Lai, MD, PhD, Department of Internal Medicine, National Taiwan University Hospital, Taipei; Yu-Feng Lin, MD, Department of Internal Medicine, National Taiwan University Hospital, Taipei; I-Jung Tsai, MD, PhD, Department of Pediatrics, National Taiwan University Children’s Hospital, Taipei; Chun-Fu Lai, MD, PhD, Department of Internal Medicine, National Taiwan University Hospital, Taipei; Tao-Min Huang, MD, Department of Internal Medicine, National Taiwan University Hospital, Taipei; Tzong-Shinn Chu, MD, PhD, Department of Internal Medicine, National Taiwan University Hospital, Taipei; Yung-Ming Chen, MD, Department of Internal Medicine, National Taiwan University Hospital, Taipei; Jian-Jhong Wang, MD, Department of Internal Medicine, Chi Mei Medical Center, Liouying, Tainan; Yu-Hsing Chang , MD, Department of Internal Medicine, National Taiwan University Hospital, Taipei; Cheng-Yi Chen, MD, Department of Internal Medicine, Mackay Memorial Hospital, Hsinchu; Chih-Chung Shiao, MD, Department of Internal Medicine, Saint Mary’s Hospital Luodong, Yilan; Wei-Jie Wang, MD, PhD, Department of Internal Medicine, Taoyuan General Hospital, Ministry of Health and Welfare, Taoyuan; Jui-Hsiang Lin, MD, Department of Internal Medicine, Taoyuan General Hospital, Ministry of Health and Welfare, Taoyuan; Che-Hsiung Wu, MD, Division of Nephrology, Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Taipei; Yu-Chang Yeh, MD, PhD, Department of Anesthesiology, National Taiwan University Hospital, Taipei; Chien-Heng Lai, RN, Department of Surgery, National Taiwan University Hospital, Taipei; Li-Jung Tseng, RN, Department of Surgery, National Taiwan University Hospital, Taipei; Chih-Jen Wu, MD, PhD, Department of Internal Medicine, Mackay Memorial Hospital, Taipei; and Kwan-Dun Wu, MD, PhD, Department of Internal Medicine, National Taiwan University Hospital, Taipei, Taiwan.
Drs Cheng-Yi Chen and Vin-Cent Wu equally contributed to the work.
Footnotes
Grant Support: This study was supported by Taiwan’s National Science Council (grant nos. 101-2314-B-002-085-MY3, 102-2314-B-002-140-MY2, and 104-2314-B-002-125-MY3) and National Taiwan University Hospital (grant nos. 106-FTN20, 106-P02, UN106-014, 106-S3582, 105-P05, VN105-04, 105-S3061, VN104-07, and 104-S2718). This work was also supported by the Ministry of Science and Technology (MOST) of the Republic of China (Taiwan) (grant no. MOST 106-2321-B-182-002). The study is partly based on data provided by the Bureau of National Health Insurance, Department of Health, Taiwan. The interpretation and conclusions contained in this article do not represent those of the Bureau of National Health Insurance, Department of Health; National Health Research Institutes; or National Taiwan University Hospital. Some of the authors are employed by 2 organizations financially supporting the study: National Taiwan University Hospital and National Health Research Institutes. The other funding organization, National Science Council, played no role in the design and conduct of the study; in the collection, management, analysis, and interpretation of the data; and in the preparation, review, and approval of the manuscript.
Potential Competing Interests: The authors report no competing interests.
Contributor Information
National Taiwan University Study Group on Acute Renal Failure:
Vin-Cent Wu, Tai-Shuan Lai, Yu-Feng Lin, I-Jung Tsai, Chun-Fu Lai, Tao-Min Huang, Tzong-Shinn Chu, Yung-Ming Chen, Jian-Jhong Wang, Yu-Hsing Chang, Cheng-Yi Chen, Chih-Chung Shiao, Wei-Jie Wang, Jui-Hsiang Lin, Che-Hsiung Wu, Yu-Chang Yeh, Chien-Heng Lai, Li-Jung Tseng, Chih-Jen Wu, and Kwan-Dun Wu
Supplemental Online Material
Supplemental material can be found online at: http://www.mayoclinicproceedings.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
References
- 1.Allison S.J. Acute kidney injury: mechanism of AKI sensitivity in diabetic nephropathy. Nat Rev Nephrol. 2014;10(9):484. doi: 10.1038/nrneph.2014.125. [DOI] [PubMed] [Google Scholar]
- 2.Ishani A., Xue J.L., Himmelfarb J. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol. 2009;20(1):223–228. doi: 10.1681/ASN.2007080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chawla L.S., Bellomo R., Bihorac A., Acute Disease Quality Initiative Workgroup 16 Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol. 2017;13(4):241–257. doi: 10.1038/nrneph.2017.2. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein S.L., Jaber B.L., Faubel S., Chawla L.S., Acute Kidney Injury Advisory Group of American Society of Nephrology AKI transition of care: a potential opportunity to detect and prevent CKD. Clin J Am Soc Nephrol. 2013;8(3):476–483. doi: 10.2215/CJN.12101112. [DOI] [PubMed] [Google Scholar]
- 5.Drucker D.J. Therapeutic potential of dipeptidyl peptidase IV inhibitors for the treatment of type 2 diabetes. Expert Opin Investig Drugs. 2003;12(1):87–100. doi: 10.1517/13543784.12.1.87. [DOI] [PubMed] [Google Scholar]
- 6.Kodera R., Shikata K., Takatsuka T. Dipeptidyl peptidase-4 inhibitor ameliorates early renal injury through its anti-inflammatory action in a rat model of type 1 diabetes. Biochem Biophys Res Commun. 2014;443(3):828–833. doi: 10.1016/j.bbrc.2013.12.049. [DOI] [PubMed] [Google Scholar]
- 7.Shi S., Koya D., Kanasaki K. Dipeptidyl peptidase-4 and kidney fibrosis in diabetes. Fibrogenesis Tissue Repair. 2016;9:1. doi: 10.1186/s13069-016-0038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng C.L., Kao Y.H., Lin S.J., Lee C.H., Lai M.L. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf. 2011;20(3):236–242. doi: 10.1002/pds.2087. [DOI] [PubMed] [Google Scholar]
- 9.Wu V.C., Wu C.H., Huang T.M., NSARF Group Long-term risk of coronary events after AKI. J Am Soc Nephrol. 2014;25(3):595–605. doi: 10.1681/ASN.2013060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang W.J., Chao C.T., Huang Y.C., National Taiwan University Study Group on Acute Renal Failure The impact of acute kidney injury with temporary dialysis on the risk of fracture. J Bone Miner Res. 2013;29(3):676–684. doi: 10.1002/jbmr.2061. [DOI] [PubMed] [Google Scholar]
- 11.Wu V.C., Hu Y.H., Wu C.H., TAIPAI Study Group Administrative data on diagnosis and mineralocorticoid receptor antagonist prescription identified patients with primary aldosteronism in Taiwan. J Clin Epidemiol. 2014;67(10):1139–1149. doi: 10.1016/j.jclinepi.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 12.Wu C.S., Lai M.S., Gau S.S., Wang S.C., Tsai H.J. Concordance between patient self-reports and claims data on clinical diagnoses, medication use, and health system utilization in Taiwan. PLoS One. 2014;9(12):e112257. doi: 10.1371/journal.pone.0112257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu Y.T., Wu S.C., Lee Y.C., Lai M.S., Tam S.C. Assessing measures of comorbidity using National Health Insurance Databases. Taiwan J Public Health. 2010;29(3):191–200. [Google Scholar]
- 14.Lin Y.F., Ko W.J., Chu T.S., NSARF Study Group The 90-day mortality and the subsequent renal recovery in critically ill surgical patients requiring acute renal replacement therapy. Am J Surg. 2009;198(3):325–332. doi: 10.1016/j.amjsurg.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 15.Lin C.C., Lai M.S., Syu C.Y., Chang S.C., Tseng F.Y. Accuracy of diabetes diagnosis in health insurance claims data in Taiwan. J Formos Med Assoc. 2005;104(3):157–163. [PubMed] [Google Scholar]
- 16.Kozma C.M., Dickson M., Phillips A.L., Meletiche D.M. Medication possession ratio: implications of using fixed and variable observation periods in assessing adherence with disease-modifying drugs in patients with multiple sclerosis. Patient Prefer Adherence. 2013;7:509–516. doi: 10.2147/PPA.S40736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Austin P.C. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med. 2014;33(7):1242–1258. doi: 10.1002/sim.5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu S., Ross C., Raebel M.A., Shetterly S., Blanchette C., Smith D. Use of stabilized inverse propensity scores as weights to directly estimate relative risk and its confidence intervals. Value Health. 2010;13(2):273–277. doi: 10.1111/j.1524-4733.2009.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Austin P.C., Mamdani M.M. A comparison of propensity score methods: a case-study estimating the effectiveness of post-AMI statin use. Stat Med. 2006;25(12):2084–2106. doi: 10.1002/sim.2328. [DOI] [PubMed] [Google Scholar]
- 20.Cheng C.L., Lee C.H., Chen P.S., Li Y.H., Lin S.J., Yang Y.H. Validation of acute myocardial infarction cases in the National Health Insurance Research Database in Taiwan. J Epidemiol. 2014;24(6):500–507. doi: 10.2188/jea.JE20140076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thakar C.V., Christianson A., Himmelfarb J., Leonard A.C. Acute kidney injury episodes and chronic kidney disease risk in diabetes mellitus. Clin J Am Soc Nephrol. 2011;6(11):2567–2572. doi: 10.2215/CJN.01120211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rimes-Stigare C., Frumento P., Bottai M. Evolution of chronic renal impairment and long-term mortality after de novo acute kidney injury in the critically ill: a Swedish multi-centre cohort study. Crit Care. 2015;19:221. doi: 10.1186/s13054-015-0920-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coca S.G. Outcomes and renal function trajectory after acute kidney injury: the narrow road to perdition. Kidney Int. 2017;92(2):288–291. doi: 10.1016/j.kint.2017.03.044. [DOI] [PubMed] [Google Scholar]
- 24.Lai C.F., Wu V.C., Huang T.M., National Taiwan University Hospital Study Group on Acute Renal Failure (NSARF) Kidney function decline after a non-dialysis-requiring acute kidney injury is associated with higher long-term mortality in critically ill survivors. Crit Care. 2012;16(4):R123. doi: 10.1186/cc11419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu V.C., Shiao C.C., Chang C.H. Long-term outcomes after dialysis-requiring acute kidney injury. Biomed Res Int. 2014;2014:365186. doi: 10.1155/2014/365186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nangaku M., Hirakawa Y., Mimura I., Inagi R., Tanaka T. Epigenetic changes in the acute kidney injury-to-chronic kidney disease transition. Nephron. 2017;137(4):256–259. doi: 10.1159/000476078. [DOI] [PubMed] [Google Scholar]
- 27.Hörbelt M., Lee S.Y., Mang H.E. Acute and chronic microvascular alterations in a mouse model of ischemic acute kidney injury. Am J Physiol Renal Physiol. 2007;293(3):F688–F695. doi: 10.1152/ajprenal.00452.2006. [DOI] [PubMed] [Google Scholar]
- 28.Zager R.A., Johnson A.C., Becker K. Acute unilateral ischemic renal injury induces progressive renal inflammation, lipid accumulation, histone modification, and “end-stage” kidney disease. Am J Physiol Renal Physiol. 2011;301(6):F1334–F1345. doi: 10.1152/ajprenal.00431.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Venkatachalam M.A., Weinberg J.M., Kriz W., Bidani A.K. Failed tubule recovery, AKI-CKD transition, and kidney disease progression. J Am Soc Nephrol. 2015;26(8):1765–1776. doi: 10.1681/ASN.2015010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forni L.G., Darmon M., Ostermann M. Renal recovery after acute kidney injury. Intensive Care Med. 2017;43(6):855–866. doi: 10.1007/s00134-017-4809-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alam M.A., Chowdhury M.R., Jain P., Sagor M.A., Reza H.M. DPP-4 inhibitor sitagliptin prevents inflammation and oxidative stress of heart and kidney in two kidney and one clip (2K1C) rats. Diabetol Metab Syndr. 2015;7:107. doi: 10.1186/s13098-015-0095-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hendarto H., Inoguchi T., Maeda Y. GLP-1 analog liraglutide protects against oxidative stress and albuminuria in streptozotocin-induced diabetic rats via protein kinase A-mediated inhibition of renal NAD(P)H oxidases. Metabolism. 2012;61(10):1422–1434. doi: 10.1016/j.metabol.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 33.von Websky K., Reichetzeder C., Hocher B. Physiology and pathophysiology of incretins in the kidney. Curr Opin Nephrol Hypertens. 2014;23(1):54–60. doi: 10.1097/01.mnh.0000437542.77175.a0. [DOI] [PubMed] [Google Scholar]
- 34.Girardi A.C., Fukuda L.E., Rossoni L.V., Malnic G., Reboųcas N.A. Dipeptidyl peptidase IV inhibition downregulates Na+-H+ exchanger NHE3 in rat renal proximal tubule. Am J Physiol Renal Physiol. 2008;294(2):F414–F422. doi: 10.1152/ajprenal.00174.2007. [DOI] [PubMed] [Google Scholar]
- 35.Glorie L.L., Verhulst A., Matheeussen V. DPP4 inhibition improves functional outcome after renal ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2012;303(5):F681–F688. doi: 10.1152/ajprenal.00075.2012. [DOI] [PubMed] [Google Scholar]
- 36.Lee S., Huen S., Nishio H. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol. 2011;22(2):317–326. doi: 10.1681/ASN.2009060615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brenner C., Franz W.M., Kühlenthal S. DPP-4 inhibition ameliorates atherosclerosis by priming monocytes into M2 macrophages. Int J Cardiol. 2015;199:163–169. doi: 10.1016/j.ijcard.2015.07.044. [DOI] [PubMed] [Google Scholar]
- 38.Brenner C., Kränkel N., Kühlenthal S. Short-term inhibition of DPP-4 enhances endothelial regeneration after acute arterial injury via enhanced recruitment of circulating progenitor cells. Int J Cardiol. 2014;177(1):266–275. doi: 10.1016/j.ijcard.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 39.Morimoto C., Schlossman S.F. The structure and function of CD26 in the T-cell immune response. Immunol Rev. 1998;161:55–70. doi: 10.1111/j.1600-065x.1998.tb01571.x. [DOI] [PubMed] [Google Scholar]
- 40.Humphreys B.D., Xu F., Sabbisetti V. Chronic epithelial kidney injury molecule-1 expression causes murine kidney fibrosis. J Clin Invest. 2013;123(9):4023–4035. doi: 10.1172/JCI45361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akcay A., Nguyen Q., He Z. IL-33 exacerbates acute kidney injury. J Am Soc Nephrol. 2011;22(11):2057–2067. doi: 10.1681/ASN.2010091011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tani S., Nagao K., Hirayama A. Association between urinary albumin excretion and low-density lipoprotein heterogeneity following treatment of type 2 diabetes patients with the dipeptidyl peptidase-4 inhibitor, vildagliptin: a pilot study. Am J Cardiovasc Drugs. 2013;13(6):443–450. doi: 10.1007/s40256-013-0043-2. [DOI] [PubMed] [Google Scholar]
- 43.Groop P.H., Cooper M.E., Perkovic V., Emser A., Woerle H.J., von Eynatten M. Linagliptin lowers albuminuria on top of recommended standard treatment in patients with type 2 diabetes and renal dysfunction. Diabetes Care. 2013;36(11):3460–3468. doi: 10.2337/dc13-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fujita H., Taniai H., Murayama H. DPP-4 inhibition with alogliptin on top of angiotensin II type 1 receptor blockade ameliorates albuminuria via up-regulation of SDF-1α in type 2 diabetic patients with incipient nephropathy. Endocr J. 2014;61(2):159–166. doi: 10.1507/endocrj.ej13-0305. [DOI] [PubMed] [Google Scholar]
- 45.Scirica B.M., Bhatt D.L., Braunwald E., SAVOR-TIMI 53 Steering Committee and Investigators Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369(14):1317–1326. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 46.Cornel J.H., Bakris G.L., Stevens S.R., TECOS Study Group Effect of sitagliptin on kidney function and respective cardiovascular outcomes in type 2 diabetes: outcomes from TECOS. Diabetes Care. 2016;39(12):2304–2310. doi: 10.2337/dc16-1415. [DOI] [PubMed] [Google Scholar]
- 47.White W.B., Cannon C.P., Heller S.R., EXAMINE Investigators Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369(14):1327–1335. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- 48.Savarese G., Perrone-Filardi P., D’Amore C. Cardiovascular effects of dipeptidyl peptidase-4 inhibitors in diabetic patients: a meta-analysis. Int J Cardiol. 2015;181:239–244. doi: 10.1016/j.ijcard.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 49.Wu D., Li L., Liu C. Efficacy and safety of dipeptidyl peptidase-4 inhibitors and metformin as initial combination therapy and as monotherapy in patients with type 2 diabetes mellitus: a meta-analysis. Diabetes Obes Metab. 2014;16(1):30–37. doi: 10.1111/dom.12174. [DOI] [PubMed] [Google Scholar]
- 50.Green J.B., Bethel M.A., Armstrong P.W., TECOS Study Group Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373(3):232–242. doi: 10.1056/NEJMoa1501352. [published correction appears in N Engl J Med. 2015] [DOI] [PubMed] [Google Scholar]
- 51.Manrique C., Habibi J., Aroor A.R. Dipeptidyl peptidase-4 inhibition with linagliptin prevents western diet-induced vascular abnormalities in female mice. Cardiovasc Diabetol. 2016;15:94. doi: 10.1186/s12933-016-0414-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu M.C., Kuro-o M., Moe O.W. The emerging role of Klotho in clinical nephrology. Nephrol Dial Transplant. 2012;27(7):2650–2657. doi: 10.1093/ndt/gfs160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lutsey P.L., Alonso A., Selvin E. Fibroblast growth factor-23 and incident coronary heart disease, heart failure, and cardiovascular mortality: the Atherosclerosis Risk in Communities study. J Am Heart Assoc. 2014;3(3):e000936. doi: 10.1161/JAHA.114.000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barbieri M., Rizzo M.R., Marfella R. Decreased carotid atherosclerotic process by control of daily acute glucose fluctuations in diabetic patients treated by DPP-IV inhibitors. Atherosclerosis. 2013;227(2):349–354. doi: 10.1016/j.atherosclerosis.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 55.Monami M., Ahrén B., Dicembrini I., Mannucci E. Dipeptidyl peptidase-4 inhibitors and cardiovascular risk: a meta-analysis of randomized clinical trials. Diabetes Obes Metab. 2013;15(2):112–120. doi: 10.1111/dom.12000. [DOI] [PubMed] [Google Scholar]
- 56.Mogensen U.M., Andersson C., Fosbøl E.L. Cardiovascular safety of combination therapies with incretin-based drugs and metformin compared with a combination of metformin and sulphonylurea in type 2 diabetes mellitus—a retrospective nationwide study. Diabetes Obes Metab. 2014;16(10):1001–1008. doi: 10.1111/dom.12314. [DOI] [PubMed] [Google Scholar]
- 57.Sato A., Yoshihisa A., Kanno Y. Associations of dipeptidyl peptidase-4 inhibitors with mortality in hospitalized heart failure patients with diabetes mellitus. ESC Heart Fail. 2016;3(2):77–85. doi: 10.1002/ehf2.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ou S.M., Chen H.T., Kuo S.C., Chen T.J., Shih C.J., Chen Y.T. Dipeptidyl peptidase-4 inhibitors and cardiovascular risks in patients with pre-existing heart failure. Heart. 2017;103(6):414–420. doi: 10.1136/heartjnl-2016-309687. [DOI] [PubMed] [Google Scholar]
- 59.Wang S.H., Chen D.Y., Lin Y.S. Cardiovascular outcomes of sitagliptin in type 2 diabetic patients with acute myocardial infarction, a population-based cohort study in Taiwan. PLoS One. 2015;10(6):e0131122. doi: 10.1371/journal.pone.0131122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang M.T., Lin S.C., Tang P.L. The impact of DPP-4 inhibitors on long-term survival among diabetic patients after first acute myocardial infarction. Cardiovasc Diabetol. 2017;16(1):89. doi: 10.1186/s12933-017-0572-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kaysen G.A. The microinflammatory state in uremia: causes and potential consequences. J Am Soc Nephrol. 2001;12(7):1549–1557. doi: 10.1681/ASN.V1271549. [DOI] [PubMed] [Google Scholar]
- 62.Kato S., Chmielewski M., Honda H. Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol. 2008;3(5):1526–1533. doi: 10.2215/CJN.00950208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Poppel P.C., Gresnigt M.S., Smits P., Netea M.G., Tack C.J. The dipeptidyl peptidase-4 inhibitor vildagliptin does not affect ex vivo cytokine response and lymphocyte function in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2014;103(3):395–401. doi: 10.1016/j.diabres.2013.12.039. [DOI] [PubMed] [Google Scholar]
- 64.Raj V.S., Mou H., Smits S.L. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495(7440):251–254. doi: 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yanai H. Dipeptidyl peptidase-4 inhibitor sitagliptin significantly reduced hepatitis C virus replication in a diabetic patient with chronic hepatitis C virus infection. Hepatobiliary Pancreat Dis Int. 2014;13(5):556. doi: 10.1016/s1499-3872(14)60308-8. [DOI] [PubMed] [Google Scholar]
- 66.Lin C.H., Lin C.C. Sitagliptin attenuates in flammatory responses in lipopolysaccharide-stimulated cardiomyocytes via nuclear factor-κB pathway inhibition. Exp Ther Med. 2016;11(6):2609–2615. doi: 10.3892/etm.2016.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hsu P.F., Sung S.H., Cheng H.M. Association of clinical symptomatic hypoglycemia with cardiovascular events and total mortality in type 2 diabetes: a nationwide population-based study. Diabetes Care. 2013;36(4):894–900. doi: 10.2337/dc12-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu P.C., Wu V.C., Lin C.J., NRPB Kidney Consortium Meglitinides increase the risk of hypoglycemia in diabetic patients with advanced chronic kidney disease: a nationwide, population-based study. Oncotarget. 2017;8(44):78086–78095. doi: 10.18632/oncotarget.17475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Best N., Hansell A.L. Geographic variations in risk: adjusting for unmeasured confounders through joint modeling of multiple diseases. Epidemiology. 2009;20(3):400–410. doi: 10.1097/EDE.0b013e31819d90f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tadic M., Cuspidi C., Bombelli M., Grassi G. Right heart remodeling induced by arterial hypertension: could strain assessment be helpful? J Clin Hypertens (Greenwich) 2018;20(2):400–407. doi: 10.1111/jch.13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.He J., Ogden L.G., Bazzano L.A., Vupputuri S., Loria C., Whelton P.K. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med. 2001;161(7):996–1002. doi: 10.1001/archinte.161.7.996. [DOI] [PubMed] [Google Scholar]
- 72.Wachtell K. Atrial fibrillation is target organ damage caused by an impaired haemodynamic state. Heart. 2018;104(15):1234–1235. doi: 10.1136/heartjnl-2017-312778. [DOI] [PubMed] [Google Scholar]
- 73.Clement D.L., De Buyzere M.L., Duprez D.A. Hypertension in peripheral arterial disease. Curr Pharm Des. 2004;10(29):3615–3620. doi: 10.2174/1381612043382819. [DOI] [PubMed] [Google Scholar]
- 74.Astor B.C., Muntner P., Levin A., Eustace J.A., Coresh J. Association of kidney function with anemia: the Third National Health and Nutrition Examination Survey (1988-1994) Arch Intern Med. 2002;162(12):1401–1408. doi: 10.1001/archinte.162.12.1401. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.