Abstract
Objectives
To assess the value of PCT as a rapid and sensitive marker for diagnosis, prognosis, and therapy of lower respiratory tract bacterial infections necessitating antimicrobial treatment and comparing this marker with other markers of infections including C-reactive protein (CRP) and total white-blood cell counts (WBCs).
Patients and methods
Sixty Patients were enrolled in the study, they were subjected to complete history taking, physical examination, laboratory investigations including complete blood count, blood gases, blood chemistry, bacteriological culture for sputum and blood, serology for atypicals, and PCR for respiratory viruses, serum C-reactive protein (CRP) and PCT levels were measured. The patients were divided into two groups, group 1 included 26 patients who were culture negative for bacterial infection and group 2 included 34 patients who were culture positive. Group 2 patients were given antibiotic therapy according to the culture sensitivity.
Result
The results revealed that, there was no significant difference between group 1 and group 2 patients as regards age, sex, clinical manifestations, final diagnosis, white blood cell counts, blood gases, number of admitted patients, intensive care unit admission and length of hospital stay. A significant increase of PCT and CRP levels was detected in group 2 compared to group 1 at initial diagnosis. At cutoff value >0.5 ng/ml, PCT gave a sensitivity of 94.1%, specificity of 88.4%, positive predictive value (PPV) of 91.4%, negative predictive value (NPV) of 92% and diagnostic efficiency of 91.6% for diagnosis of respiratory tract bacterial infections. However, at a cutoff value >8 mg/L, CRP gave a sensitivity of 85.2%, specificity of 76.9%, PPV of 82.8%, NPV of 80% and diagnostic efficiency of 81.7%. After antibiotic therapy PCT and CRP levels dropped in group 2 patients as compared to their pre-treatment levels.
Conclusion
Serum PCT level could be used as a novel marker of lower respiratory tract bacterial infections for diagnosis, prognosis and follow up of therapy. This reduces side-effects of an unnecessary antibiotic use, lowers costs, and in the long-term, leads to diminishing drug resistance.
Keywords: Procalcitonin, Lower respiratory tract infection
Introduction
Acute respiratory infections (ARIs) comprise a large and heterogeneous group of infections, including bacterial infections, viral infections, and infections of other etiologies. Early initiation of adequate antibiotic therapy is the cornerstone in the treatment of bacterial ARIs and is associated with improved clinical outcomes [1]. However, overuse of antibiotics by over-prescription in outpatients and prolonged duration of antibiotic therapy in patients with bacterial ARIs in the hospital and intensive care setting is associated with increased resistance for common bacteria, high costs, and adverse drug reactions [2]. A novel approach to estimate the likelihood of bacterial infections and the severity of disease is the use of blood biomarkers mirroring the host response to infection, and the severity of infection. In recent years, procalcitonin (PCT) has emerged as a promising marker for the diagnosis of bacterial infections because higher levels are found in severe bacterial infections than in viral infections and nonspecific inflammatory diseases. Hence, PCT may be used to support clinical decision making for the initiation and discontinuation of antibiotic therapy [3].
Procalcitonin is a prohormone of the calcium homeostasis hormone calcitonin. In non-infectious conditions it is produced in the neuroendocrine medullary C-cells of the thyroid gland. In normal subjects circulating procalcitonin concentrations are low (<0.05 ng/mL), but bacterial infections selectively induce an increase in the concentration of procalcitonin (in parenchymal tissues) because both endotoxins (lipopolysaccharides) from the bacterial cell wall and host responses to infection stimulate the production of procalcitonin by cytokines, such as IL-1 b, tumor necrosis factor-α, and IL-6. The up-regulation of PCT correlates with the severity and extent of bacterial infections. This results in an accumulation of procalcitonin because, unlike neuroendocrine cells, parenchymal cells lack the ability to cleave procalcitonin into its mature form, calcitonin [4]. Conversely, interferon-γ, a cytokine released in response to viral infections, blocks the up-regulation of PCT, resulting in a higher specificity of PCT toward bacterial infections. Quantitatively, PCT may help distinguish severe bacterial infections from milder viral illnesses [5]. It is detectable within 2–4 h and peaks within 6–24 h (as opposed to CRP which begins to rise after 12–24 h and peaks at 48 h) [6]. PCT production is not impaired by neutropenia or other immunosuppressive states. PCT levels parallel the severity of the inflammatory insult or infections meaning those with more severe disease have higher levels [7]. Furthermore, procalcitonin has some utility as a prognostic indicator with higher serum concentrations related to the risk of mortality [8].
PCT has some advantages over other biomarkers in common clinical use such as C-reactive protein (CRP) and white blood cell count. These advantages include specificity for bacterial infection, the rapidity of its rise after an insult (6 h), the rapid decline with immune control on infection (half-life of 24 h), excellent correlation with severity of illness (higher levels in more severely ill), and the lack of impact of anti-inflammatory and immunosuppressive states on production [9]. PCT was used as a guide in the following situations:
-
•
Differentiation of bacterial verses viral respiratory tract infection.
-
•
Determination of the duration of antibiotic treatment in respiratory infections.
-
•
Diagnosis and monitoring of sepsis and septic shock.
-
•
Prognostic usefulness in classifying the patients as low risk or high risk for bacterial infection and/or sepsis and prediction of mortality.
-
•
Monitoring the response to antibacterial therapy.
-
•
Differentiating bacterial verses viral meningitis.
-
•
Diagnosis of bacterial infection in neutropenic patients [10].
Decisions regarding antimicrobial therapy should NOT be based solely on procalcitonin serum concentrations
Procalcitonin should be placed into the clinical context of each patient scenario considering the site of possible infection, the likelihood of bacterial infection, and the severity of illness [11].
Most studies on sepsis have evaluated using PCT to initiate or discontinue antibiotics so, the decision to initiate therapy in the ICU should be driven by the severity of illness and clinical assessment of the likelihood of infection and PCT was used as a guide to assist when to initiate or stop antibiotics. PCT levels that are not declining or are increasing are strong prognostic indicators of lack of control on the infection by the host immune system and/or antibiotics. It has been recommended that further diagnostic procedures/imaging or broader spectrum antibiotics should be initiated based upon this rising value [12].
A recent study used PCT values of >1 μg/L as an “alert” to suggest broadening of therapy and further imaging. This high value can also give prognosis of an increased length of stay in the ICU and on the ventilator. In these situations, it is recommended to do careful reassessment of the patient for other sites/sources of infection or evidence of resistant pathogens and decisions regarding further interventions to be made based upon this evaluation [13].
Aim of the work
The aim of this review, was focusing on respiratory infections to give a potential usefulness on PCT as a guide in diagnosing bacterial infections, differentiating bacterial from viral diseases, prognosticating the severity of a patient’s condition, and guiding clinical decisions about when to initiate antibiotic therapy and when it can be safely discontinued.
Patients and methods
A total number of 60 patients (28 males, 32 females) were included in the study from December 2011 to January 2013; they were randomly selected from outpatient clinic and inpatient departments of Al Sabah Hospitals in Kuwait.
Inclusion criteria
Any patient presenting with fever, cough, expectoration and/or dyspnea and suspected to have lower respiratory tract infection as the main diagnosis including pneumonia, acute exacerbation of asthma, COPD and acute bronchitis was included.
The patients were subjected to the following
-
(1)
Detailed history and physical examination.
-
(2)
Chest radiography was done to all patients.
-
(3)
Sputum and blood were collected for bacterial culture by using specific media for each organism. Microorganisms were recorded if they were detected in sputum, blood cultures, or both.
-
(4)
Fresh heparinized arterial blood samples were collected for blood gases’ analysis, on room air (on AVL-autoanlyzer-Roche).
-
(5)
Serology for atypicals including Chlamydia pneumonia IgA/IgM/IgG, Coxiella bruntii IgM/IgG, legionella pneumonia IgM/IgG, Mycoplasma pneumonia IgA/IgM/IgG, using (Enzyme Linked Immunosorbent Assay) ELISA which is an immunoassay, suited to the determination of antibodies in the field of infectious serology. The reaction is based on the specific interaction of antibodies with their corresponding antigen. Therefore, the test strips of the SERION ELISA classic micro-titer plate are coated with specific antigens of the pathogen of interest. If antibodies in the patient́s serum sample are present, they bind to the fixed antigen. A secondary antibody, which has been conjugated with the enzyme alkaline phosphatase, detects the immune complex. The colorless substrate p-nitrophenylphosphate is then converted into the colored product p-nitro phenol. The signal intensity of the reaction product is proportional to the concentration of the analyte in the sample and is measured photometrically.
-
(6)
Urine test for Legionella antigen.
-
(7)
Sputum or nasopharyngeal swab for PCR to detect respiratory viruses included, Influenza A/B IgA/IgM/IgG, Para-influenza virus 1, 2, 3 IgA/IgG, Herpes simplex virus 1/2 IgA/IgM/IgG, Cytomegalovirus IgM/IgG, Epstein – Barr virus IgG/IgM,Herpes Simplex Virus 1/2 IgM/IgG. Pathogen detection by the polymerase chain reaction (PCR) is based on the amplification of specific regions of pathogen genome. In real time PCR the amplified product is detected via florescent dyes. These are linked to oligonucleotide probes that bind specifically to the amplified product. Monitoring the fluorescence intensities during the PCR run (i.e. real-time) allows the detection and quantification of the accumulating product without having to re-open the reaction tubes after the PCR run. The ARTUS SARS RG RT-PCR kit was used to detect the novel coronal virus. The artus SARS RG RT-PCR Kit is based on the amplification and simultaneous detection of a specific region of the SARS corona virus genome using real-time PCR. The kit provides high levels of specificity, sensitivity, and reproducibility. Each artus SARS RG RT-PCR Kit provides 4 quantitation standards. The use of standards enables accurate quantitation of viral load. In addition, the kit contains a second heterologous amplification system to identify possible PCR inhibition. This is detected as an internal control (IC) in a different fluorescence channel from the analytical PCR. The detection limit of the analytical SARS corona virus RT-PCR is not reduced.
-
(8)
Venous blood samples were collected from patients in 2 tubes, 2 ml of blood was delivered into EDTA tube to perform complete blood picture on the automatic cell counter.
-
(9)
The remaining amounts of blood were coagulated then centrifuged at 4000 rpm for 10 min, one part of the separated serum was used to assay blood chemistry (on Hitachi 911-Roche) and CRP concentration (on Axyem, Bayer-Germany), a value of CRP > 8 mg/L was considered abnormally elevated [14].
-
(10)
The second part of the serum was stored in aliquots at −70 °C for measurements of PCT until time of assay.
Serum PCT measurement was done using a luminescence immunoassay (LIA) for specific measurement of PCT in serum by using Elecyes 1020. In LIA, PCT covalently bound to paramagnetic particles for limited binding sites on acridine ester-labeled antibody. An inverse relationship exists between the concentrations of labeled antibody bound to the antigen PCT in the patient sample. This triggers the chemiluminescent reaction that results in the emission of photons of light. The photo multiplier tube detects the photon of light emitted and converts them into electrical pulses. The instrument counts these electrical pulses, reads the results off the master curve defined for the assay. The results were calculated from the standard curve in ng/ml. The detection limit of the assay was 0.1 ng/mL [15].
Patients were divided into two groups
Group 1: included 26 patients (12 males and 14 females), they were bacteriologically culture negative.
Group 2: included 34 patients (16 males and 18 females), they were bacteriologically culture positive, and were given antibiotic therapy according to the antibiotic sensitivity test.
Interpretation of procalcitonin levels
Normal: <0.1 ng/mL (infants >72 h – adults).
Suspected lower respiratory tract infection
-
•
0.1–0.25 ng/mL – Low likelihood for bacterial infection; antibiotics discouraged.
-
•
>0.25 ng/mL – Increased likelihood for bacterial infection; antibiotics encouraged [16].
Suspected sepsis: Strongly consider initiating antibiotics in all unstable patients.
-
•
0.1–0.5 ng/mL – Low likelihood for sepsis.
-
•
>0.5 ng/mL – Increased likelihood for sepsis.
-
•
>2.0 ng/mL – High risk of sepsis.
-
•
>10 ng/mL – Septic shock [17].
Higher PCT levels have been associated with a worse prognosis [5]. If antibiotics are administered, repeat procalcitonin testing every 2–3 days to consider early antibiotic cessation as shown in algorithm1 and 2 (Fig. 1 ).
Figure 1.
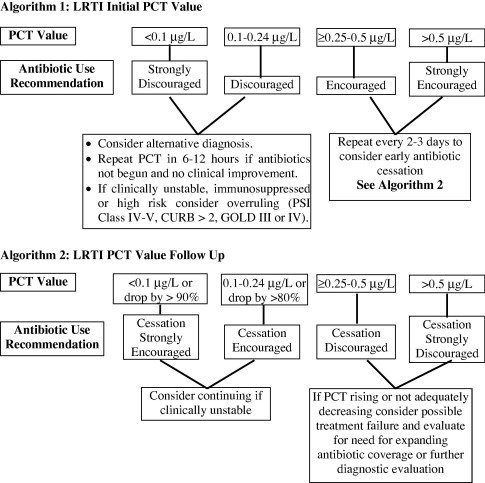
The use of PCT in the initiation and cessation of antibiotics [21].
Limitations of procalcitonin: False positive and false negative results can occur and the clinical context should guide interpretation of PCT results.
A false positive result, situations where the PCT elevations may be due to a non-bacterial causes [18]
-
•
Newborns (<48–72 h; after 72 interpret levels as usual).
-
•
Massive stress (severe trauma, surgery, cardiac shock, burns).
-
•
Treatment with agents which stimulate cytokines (anti-lymphocyte globulins, alemtuzumab, IL-2, granulocyte transfusion).
-
•
Malaria and some fungal infections.
-
•
Prolonged, severe cardiogenic shock or organ perfusion abnormalities.
-
•
Some forms of vasculitis and acute graft vs. host disease.
-
•
Paraneoplastic syndromes due to medullary thyroid and small cell lung cancer.
False negative results
-
(1)
PCT levels may not rise with localized infections as parapneumonic effusion, loculated infection (empyema), (osteomyelitis, localized abscess, etc.) [19].
-
(2)
If the sample is taken for PCT measurement early in the course of infection [20].
-
(3)
If the patient is taking steroids as they can inhibit the pathway for PCT production. This is important in patients with chronic lung conditions such as COPD, asthma or pulmonary fibrosis [19].
Result
The patients characteristics revealed that, no significant difference was detected between group 1 (culture negative group) and group 2 patients (culture positive group) as regards age, sex, symptoms, signs, body temperature and final diagnosis (Table 1 ).
Table 1.
Characteristics of both patient groups.
| Variables | Group 1 (N = 26) | Group 2 (N = 34) | t-Test | P value |
|---|---|---|---|---|
| Age (mean ± SD) | 53.2 ± 9.3 | 48.2 ± 6.4 | 0.83 | >0.05 |
| Men/women | 12/14 | 16/18 | >0.05 | |
| Body temperature (°C)(mean ± SD) | 38.9 ± 1·2 | 39.1 ± 1·1 | 0.25 | >0.05 |
| Symptoms | No. (%) | No. (%) | χ2 | |
| Cough | 24 (92.3%) | 30 (88.2%) | 1.31 | >0.05 |
| Sputum (yellow or green) | 12 (46.2)% | 16 (47.1%) | 0.07 | >0.05 |
| Dyspnea | 16 (61.5%) | 23 (67.6%) | 1.92 | >0.05 |
| Signs: | ||||
| Rales | 13 (50%) | 18 (52.9%) | 0.67 | >0.05 |
| Wheezing | 11 (42.3%) | 15 (44.1%) | 0.32 | >0.05 |
| Chest radiograph infiltrate | 9 (34.6%) | 13 (38.2%) | 1.04 | >0.05 |
| Final diagnosis | No. (%) | No. (%) | χ2 | |
| Acute bronchitis | 8 (30.7%) | 13 (38.2%) | 2.01 | >0.05 |
| Acute exacerbation of asthma | 2 (7.7%) | 0 (0.0%) | 2.89 | >0.05 |
| Pneumonia | 10 (38.4%) | 11 (32.4%) | 1.06 | >0.05 |
| Acute exacerbation of COPD | 6 (23.1%) | 10 (29.4%) | 0.72 | >0.05 |
Table 2 shows a significant increase in CRP levels and a highly significant increase in PCT levels in group 2 as compared to group 1 at initial diagnosis. No significant difference as regards white blood cell counts or blood gases was detected. Also no significant difference was detected as regards number of admitted patients, intensive care unit admission or admitted days between group 1 and group 2 patients as shown in Table 3 .
Table 2.
Some laboratory variables in patients with lower respiratory tract infection.
| Variable | At Initial diagnosis |
|||
|---|---|---|---|---|
| Group1(n = 26) Mean ± SD | Group 2 (n = 34) Mean ± SD | Test value t p | ||
| White blood cell (×106/L) | 19.9 ± 4.9 | 22.6 ± 7.8 | 0.39 | >0.05 |
| CRP (mg/L) | 15.9 ± 6.8 | 44.8 ± 26.2 | 0.36 | <0.05⁎ |
| Procalcitonin (ng/mL) | 1.2 ± 0.9 | 4.8 ± 3.9 | 8.05 | <0.001⁎⁎ |
| Blood gases | ||||
| Pa O2 (mm Hg) | 86.9 ± 8.5 | 83.7 ± 9.4 | 0.61 | >0.05 |
| Pa CO2 (mm Hg) | 41.7 ± 8.8 | 42.8 ± 9.0 | 0.73 | >0.05 |
| pH | 7.3 ± 0.01 | 7.4 ± 0.02 | 0.08 | >0.05 |
P value is significant at < 0.05.
P value highly significant at < 0.001.
Table 3.
Hospital data of patients with lower respiratory tract infection.
| Variable | Group 1 (n = 26) | Group 2 (n = 34) | χ2 | P value |
|---|---|---|---|---|
| Admitted patients | 15 (57.7%) | 14 (41.2%) | 0.08 | >0.05 |
| Intensive care unit admission | 1 (3.8%) | 1 (2.9%) | 0.96 | >0.05 |
| Length of hospital stay | Mean ± SD 8.4 ± 3.1 | Mean ± SD 10.5 ± 5.1 | P value >0.05 |
Table 4 shows the number and the percentage of bacterial organisms that had been isolated from group 2 patients. In 11 patients with pneumonia, the Streptococcus pneumoniae, Morexella catarrhalis and Pseudomonas spp. were isolated. These bacteria were isolated from sputum of 5 cases (45.4%), from blood of 4 cases (36.4%) and from both of 2 cases (18.1%). In 10 patients with COPD, S. pneumoniae, Hemophilus influenza, Staphylococcus aureus and P. spp. were isolated. These bacteria were isolated from sputum of 4 cases (40%), from blood of 3 cases (30%) and from both of 3 cases (30%) while, S. pneumoiae, S. aureus, Pseudomonas spp. and M. catarrhalis were isolated from the 13 patients with bronchitis. These bacteria were isolated from sputum of 8 cases (61.5%), from blood of 4 cases (30.7%) and from both of one case (7.6%). Both blood and sputum cultures were negative in all cases that had acute exacerbation of asthma.
Table 4.
Organisms isolated in group 2 patients (positive culture) before treatment.
| Variables | Sputum (+ve) only | Blood culture (+ve) only | Both (+ve) |
|---|---|---|---|
| Pneumonia (n = 11) No.% | 5 (45.4%) | 4 (36.4%) | 2 (18.1%) |
| S. pneumoniae | 3 (27.3%) | 3 (27.3%) | 1 (9.1%) |
| M. catarrhalis | 1 (9.1%) | – | 1 (9.1%) |
| Pseudomonas spp | 1 (9.1%) | 1(9.1%) | – |
| COPD (n = 10) No.% | 4 (40%) | 3 (30%) | 3 (30%) |
| S. pneumoniae | 2 (20%) | 2 (20%) | 2 (20%) |
| H. influenzae | 1 (10%) | – | – |
| S. aureus | 1(10%) | 1 (10%) | 1(10%) |
| Pseudomonas spp. | - | 1(10%) | – |
| Bronchitis (n = 13) No.% | 8 (61.5%) | 4 (30.7%) | 1 (7.6%) |
| S. pneumoniae | 3 (23%) | 2(15.4%) | 1(7.6%) |
| S. aureus | 2 (15.4%) | 1(7.6%) | – |
| Pseudomonas spp. | 2(15.4%) | 1(7.6%) | – |
| M. catarrhalis | 1(7.6%) | – | – |
Table 5 demonstrates the value of PCT and CRP in diagnosis of the lower respiratory tract bacterial infection, at a cutoff value >0.5 ng/ml, PCT gave a sensitivity of 94.1%, specificity of 88.4%, positive predictive value (PPV) of 91.4%, negative predictive value (NPV) of 92% and diagnostic efficiency of 91.6%. While, at a cutoff value >8 mg/L, CRP gave a sensitivity of 85.2%, specificity of 76.9%, PPV of 82.8%, NPV of 80% and diagnostic efficiency of 81.7%.
Table 5.
Sensitivity, Specificity, PPV and NPV of serum procalcitonin and C- reactive protein for the diagnosis of respiratory tract infection.
| PCT (ng/ml) | CRP (mg/L) | |
|---|---|---|
| Cutoff value | >0.5 | >8 |
| Sensitivity | 94.1% | 85.2% |
| Specificity | 88.4% | 76.9% |
| PPV | 91.4% | 82.8% |
| NPV | 92% | 80% |
| Diagnostic efficiency | 91.6% | 81.7% |
PCT: procalcitonin CRP: C-reactive protein PPV: positive predictive value NPV: negative predictive value.
Table 6 shows a highly significant decrease in PCT and CRP levels in group 2 patients after antibiotic therapy. Also the white blood cell counts were significantly decreased as compared to pretreatment levels.
Table 6.
Comparison between laboratory tests before and after therapy in group 2 patients.
| Variable | Group 2 patients after antibiotic therapy |
|||
|---|---|---|---|---|
| Before treatment Mean ± SD | After treatment Mean ± SD | Test value t p | ||
| White blood cell counts (×106/L) | 22.6 ± 8.8 | 11.8 ± 6.9 | 3.59 | <0.05 |
| C-reactive protein (mg/L) | 49.8 ± 28.2 | 9.5 ± 4.3 | 4.36 | <0.001 |
| Procalcitonin (ng/mL) | 4.8 ± 3.9 | 0.8 ± 0.65 | 6.57 | <0.001 |
Statistical analysis
A statistical analysis of data was done using SPSS computer program version 12. A comparison between two parametric quantitative variables was done using student’s (t) test. Chi square (χ2) was used to compare between qualitative variables. P value < 0.05 was considered statistically significant and that >0.05 was considered insignificant. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and diagnostic efficiency were calculated.
Discussion
Identifying a true “gold standard” for the diagnosis of respiratory infections is often problematic. The use of blood and sputum cultures has significant limitations because of the duration of time required to obtain positive cultures and issues of colonization and contamination in addition to, the inability to grow certain bacteria in standard cultures [22].
In this study we evaluated the value of PCT as a rapid and sensitive marker for diagnosis of bacterial lower respiratory tract infections needing antimicrobial treatment and its value in the prognosis and follow up of therapy. We also assessed the value of procalcitonin (PCT) compared with other markers of infections including C-reactive protein (CRP) and total white-blood cell counts (WBCs) in predicting bacterial lower respiratory tract infections. The current study revealed that, there was no significant difference between bacterial culture negative group and culture positive group as regards age, sex, clinical manifestations, blood gases, number of admitted patients, intensive care unit admission or admitted days between both groups of patients. This may hinder the diagnosis and proper therapeutic interference.
Our data regarding the clinical diagnosis showed no statistical significant difference between culture positive and culture negative groups. This can be explained by the presence of different etiological factors causing respiratory tract infections. The culture used in this study was to detect bacterial infections only, so in culture negative cases, the infection may be due to other causes including viruses.
COPD and bronchial asthma exacerbation have been associated with a number of etiological factors which may be infectious or non infectious. Viral infections are important triggers for their exacerbations in up to 30% in the former and a higher percentage in the latter [23]. Controversy exists regarding the need of antimicrobial agents in acute exacerbation of COPD. Our findings are in agreement with those of Sethi and Murphy who found that, only a quarter of patients with acute exacerbations of COPD are estimated to benefit from the addition of antibiotics to treatment [24].
Our results demonstrate that both PCT and CRP have a superior discriminatory power to total WBCs in detecting bacterial lower respiratory tract infection. Total WBCs were elevated in both groups with no statistically significant difference.
CRP is an acute phase protein which has a plasma half-life that is constant under almost all conditions. CRP has been widely used clinically as a diagnostic tool for infection identification [25].
In the current study CRP was a good marker of bacterial lower respiratory tract infection. Its level was significantly increased in group 2 (culture positive patients) more than in group 1 (culture negative patients). Both white blood cell count and CRP are considered to be non-specific inflammatory parameters with changing reliability, denoting that, both may be not enough to diagnose the bacterial infection [26]. In our study after antibiotic therapy, PCT level was dropped in group 2 patients compared to their pre-treatment level.
In one study, Müller et al., evaluated PCT in a number of patients with community-acquired pneumonia (CAP) with typical pathogens, mostly Streptococcus pneumoniae. PCT was increased significantly in bacteremic patients compared with patients without an identified bacterial pathogen [27]. Another study focused on the potential of PCT to differentiate patients with a viral respiratory infection with or without bacterial super infection. This was investigated in patients with confirmed influenza A (H1N1) pneumonia in a critical care setting. PCT had differentiated the patients with bacterial super-infection from patients with viral pneumonia only [28]. Different studies have evaluated the prognostic potential of PCT in patients with respiratory infections, mainly in patients with CAP, the greatest benefit of PCT was found in patients classified as high risk by the pneumonia severity index score. Having a PCT < 0.1 ng/ml can exclude mortality in high-risk patients. Subsequent repeated measurements of PCT in this population demonstrated improved clinical outcomes with falling PCT levels [29]. In addition, the study found that PCT was more helpful in predicting serious adverse events, such as ICU admission or CAP-related complications [30].
Based on these studies, the prognostic usefulness of PCT in patients with respiratory infections may be summarized as follows [5]:
-
(1)
In low-risk patients with respiratory infections, PCT levels <0.25 ng/ml identify patients at lower risk of a bacterial cause and CAP and thus low mortality.
-
(2)
In low-risk patients with respiratory infections, PCT levels >0.25 ng/ml identify patients at higher risk of a bacterial cause and CAP and, perhaps, higher mortality.
-
(3)
In a high-risk population, PCT levels <0.1 ng/ml effectively decrease the likelihood of mortality from a bacterial cause, and other nonbacterial pathologies should be aggressively considered.
A number of protocols using PCT measurements can now be recommended (Fig 2 ) [5].
-
(1)
In patients with a low probability for a bacterial infection (e.g., patient with suspected non-pneumonic respiratory infection), a single PCT measurement and a cutoff of <0.25 ng/ml or certainly <0.1 ng/ml can exclude bacterial infection and there is no need to initiate an antibiotic therapy (Fig. 2A). Clinical follow-up with re-measurement of PCT within 6–24 h should be considered in all patients in whom antibiotics are withheld but who show clinical deterioration. If PCT is >0.25 ng/ml, and particularly >0.5 ng/ml, a bacterial infection becomes more likely and necessitates antibiotic therapy.
-
(2)
For patients who are clinically stable but presented with pneumonia, a PCT level >0.25 ng/ml strongly suggests that a bacterial infection and antibiotic therapy should be initiated, PCT should be reassessed every 2 days. Antibiotics may be discontinued if a patient shows clinical recovery and PCT decreases to <0.25 ng/ml (or by at least 80% to 90% from the peak level). Highly elevated PCT levels in this situation make bacteremic disease more likely and suggest that the infection may be more severe than expected based on clinical signs and symptoms. In patients suspected of having a pneumonia based on the presence of radiological infiltrates, a persistent (>24–48 h) PCT level of <0.1 ng/ml or even 0.1 ng/ml to <0.25 ng/ml argues against a typical bacterial infection, and physicians should consider the unusual pneumonias as pulmonary embolism, acute heart failure, bronchiolitis obliterans organizing pneumonia, Pneumocystis jiroveci pneumonia, and viral pneumonia particularly during flu season. If antibiotics are withheld initially, PCT should be rechecked after 6 to 24 h. If PCT levels are <0.25 ng/ml but bacterial infection is still highly suspected based on the clinical presentation or microbiologic results, antibiotic therapy should be considered. If PCT remains low during follow-up, early discontinuation of antibiotics should be considered, as well as an aggressive diagnostic workup for other causes.
-
(3)
For high-risk or patients in the ICU with severe respiratory infection (Fig 2B), empiric antibiotic therapy should be initiated. Still, an initial PCT level of <0.5 ng/ml argues against a typical bacterial infection, and other diagnoses should be considered, including viral causes, repeating the PCT level at 6–24 h in high-risk patients may be important so as not to miss the evolution of the inflammatory cascade, similar to the strategy used for serial troponins in monitoring cardiac ischemia. If PCT levels do not decrease or remaining high, treatment failure should be considered and reassessment of patients is recommended.
Figure 2.
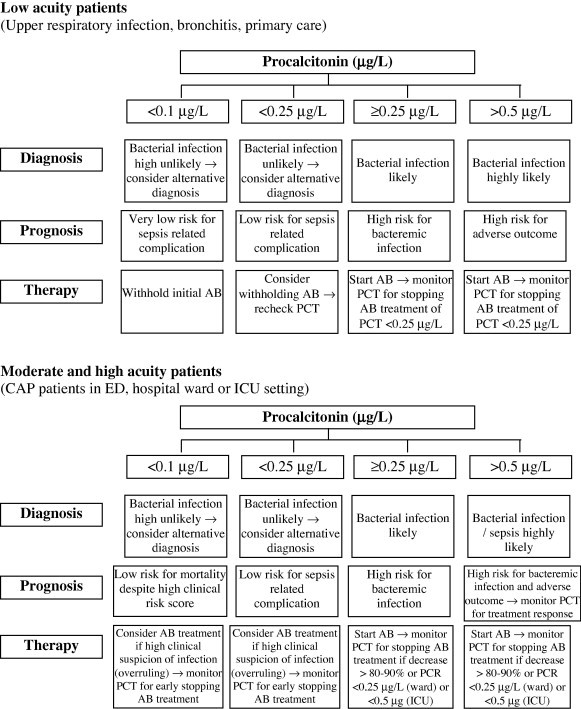
The usefulness of PCT in the diagnosis of bacterial infection, prognosis of severity and antibiotic therapy [5].
Conclusion
-
(1)
PCT is not a stand-alone test and will not replace clinical evaluations of patients. PCT needs to be interpreted in consideration of the clinical setting.
-
(2)
PCT mirrors the patient’s response to infection and thus, the extent and severity of infection.
-
(3)
PCT can rule out bacterial infection and provide information about patient recovery.
-
(4)
PCT proved as a good diagnostic, prognostic and follow up of therapy.
Conflict of Interest
None declared.
Footnotes
Peer review under responsibility of The Egyptian Society of Chest Diseases and Tuberculosis.
References
- 1.Philipp S., Matthias B., Mirjam C., Daiana S. Procalcitonin to guide initiation and duration of antibiotic treatment in acute respiratory infections: an individual patient data meta-analysis. Clin Infect Dis. 2012;55(5):651–662. doi: 10.1093/cid/cis464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawrence K.L., Kollef M.H. Antimicrobial stewardship in the intensive care unit: advances and obstacles. Am J Respir Crit Care Med. 2009;179:424–434. doi: 10.1164/rccm.200809-1394CP. [DOI] [PubMed] [Google Scholar]
- 3.Schuetz P., Albrich W., Christ-Crain M., Chastre J., Mueller B. Procalcitonin for guidance of antibiotic therapy. Expert Rev Anti Infect Ther. 2010;8:575–587. doi: 10.1586/eri.10.25. [DOI] [PubMed] [Google Scholar]
- 4.Linscheid P., Seboek D., Schaer D.J., Zulewski H., Keller U., Muller B. Expression and secretion of procalcitonin and calcitonin gene-related peptide by adherent monocytes and by macrophage-activated adipocytes. Crit Care Med. 2004;32(8):1715–1721. doi: 10.1097/01.ccm.0000134404.63292.71. [DOI] [PubMed] [Google Scholar]
- 5.Philipp S., Devendra N., Jeffrey L. Role of procalcitonin in managing adult patients with respiratory tract infections. Chest. 2012;141:1063–1073. doi: 10.1378/chest.11-2430. [DOI] [PubMed] [Google Scholar]
- 6.Kibe S., Adams K., Barlow G. Diagnostic and prognostic biomarkers of sepsis in critical care. J Antimicrob Chemother. 2011;66(2):33–40. doi: 10.1093/jac/dkq523. [DOI] [PubMed] [Google Scholar]
- 7.Christ C., Muller B. Biomarkers in respiratory tract infections: diagnostic guides to antibiotic prescription, prognostic markers and mediators. Eur Respir J. 2007;30:556–573. doi: 10.1183/09031936.00166106. [DOI] [PubMed] [Google Scholar]
- 8.Schuetz P., Albrich W., Mueller B. Procalcitonin for diagnosis of infection and guide to antibiotic decisions: past, present and future. BMC Med. 2011;9:107. doi: 10.1186/1741-7015-9-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rune A., Jens-Ulrik J. Procalcitonin-guided antibiotic treatment of respiratory tract infections in a primary care setting: are we there yet? Prim Care Respir J. 2011;20(4):360–367. doi: 10.4104/pcrj.2011.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christ-Crain M., Stolz D., Bingisser R., Müller C., Miedinger D., Huber P.R., Zimmerli W., Harbarth S., Tamm M., Müller B. Procalcitonin guidance of antibiotic therapy in community-acquired pneumonia. Am J Respir Crit Care Med. 2006;174:84–93. doi: 10.1164/rccm.200512-1922OC. [DOI] [PubMed] [Google Scholar]
- 11.Schuetz P., Christ-Crain M., Thomann R. Effect of procalcitonin based guidelines vs. standard guidelines on antibiotic use in lower respiratory tract infections. The ProHOSP randomized controlled trial. JAMA. 2009;302:1059–1066. doi: 10.1001/jama.2009.1297. [DOI] [PubMed] [Google Scholar]
- 12.Bouadma L., Luyt C.E., Tubach F., Cracco C., Alvarez A., Schwebel C., Schortgen F., Lasocki S., Veber B., Dehoux M., Bernard M., Pasquet B., Régnier B., Brun-Buisson C., Chastre J., Wolff M. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units: a multicenter randomized controlled trial. Lancet. 2010;375:463–474. doi: 10.1016/S0140-6736(09)61879-1. [DOI] [PubMed] [Google Scholar]
- 13.Daniel D., Frank D., Beat M., Werner C. Efficacy and safety of procalcitonin-guided antibiotic therapy in lower respiratory tract infections. Antibiotics. 2013;2:1–10. doi: 10.3390/antibiotics2010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schuetz P., Christ-Crain M., Albrich W., Zimmerli W., Mueller B. ProHOSP study group. guidance of antibiotic therapy with procalcitonin in lower respiratory tract infections: insights into the ProHOSP study. Virulence. 2010;1(2):88–92. doi: 10.4161/viru.1.2.10488. [DOI] [PubMed] [Google Scholar]
- 15.Snider R.H., Nylen E.S., Becker K.L. Procalcitonin and its component peptides in systemic inflammation: immunochemical characterization. J Investig Med. 1997;45:552–560. [PubMed] [Google Scholar]
- 16.Burkhardt O., Ewig S., Haagen U. Procalcitonin guidance and reduction of antibiotic use in acute respiratory tract infection. Eur Respir J. 2010;36(3):601–607. doi: 10.1183/09031936.00163309. [DOI] [PubMed] [Google Scholar]
- 17.Jensen J.U., Hein L., Lundgren B., Bestle M.H., Mohr T.T., Andersen M.H., Thornberg K.J., Løken J., Steensen M., Fox Z., Tousi H., Søe-Jensen P., Lauritsen A., Strange D., Petersen P.L., Reiter N., Hestad S., Thormar K., Fjeldborg P., Larsen K.M., Drenck N.E., Ostergaard C., Kjær J., Grarup J., Lundgren J.D. Procalcitonin-guided interventions against infections to increase early appropriate antibiotics and improve survival in the intensive care unit. Crit Care Med. 2011;39:2048–2258. doi: 10.1097/CCM.0b013e31821e8791. [DOI] [PubMed] [Google Scholar]
- 18.Becker K.L., Snider R., Nylen E.S. Procalcitonin assay in systemic inflammation, infection, and sepsis: clinical utility and limitations. Crit Care Med. 2008;36(3):941–952. doi: 10.1097/CCM.0B013E318165BABB. [DOI] [PubMed] [Google Scholar]
- 19.Kruger S., Ewig S., Kunde J., Hartmann O., Marre R., Suttorp N. Assessment of inflammatory markers in patients with community-acquired pneumonia—Influence of antimicrobial pre-treatment results from the german competence network capnetz. Clin Chim Acta. 2010;411:1929–1934. doi: 10.1016/j.cca.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Christ C., Muller B. Procalcitonin in bacterial infections-Hype, hope, more or less? Swiss Med Wkly. 2005;135:451–460. doi: 10.4414/smw.2005.11169. [DOI] [PubMed] [Google Scholar]
- 21.Philipp S., Victor C., Matthias B., Jeffrey L.G. Procalcitonin algorithms for antibiotic therapy decisions. Arch Intern Med. 2011;171(15):1322–1331. doi: 10.1001/archinternmed.2011.318. [DOI] [PubMed] [Google Scholar]
- 22.Mandell L., Wunderink R., Anzueto A. Infectious diseases society of America; American thoracic society. Infectious diseases society of America/American Thoracic society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44:27–72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenberg S.B., Allen M., Wilson J., Atmar R.L. Respiratory viral infections in adults with and without chronic obstructive pulmonary disease. J Respir Crit Care Med. 2000;162:167–173. doi: 10.1164/ajrccm.162.1.9911019. [DOI] [PubMed] [Google Scholar]
- 24.Sethi S., Murphy T.F. Bacterial infection in chronic obstructive pulmonary disease: a state-of-the-art review. Clin Microbiol Rev. 2005;14:336–363. doi: 10.1128/CMR.14.2.336-363.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matson A., Soni N., Sheldon J. C-reactive protein as a diagnostic test of sepsis in the critically ill. Anaesth Intensive Care. 1991;19:182–186. doi: 10.1177/0310057X9101900204. [DOI] [PubMed] [Google Scholar]
- 26.Lorrot, M; Moulin F; cost J; Ravilly S; guerin S, belon P, and Gendrel D, Procalcitonin on pediatric emergency: comparison with C-reactive, interleukin 6 and interferon alpha in differentiation between bacterial and viral infections, Press-med Jan, 29 (3) (2000) 128–134. [PubMed]
- 27.Müller F., Christ-Crain M., Bregenzer T. ProHOSP Study Group. Procalcitonin levels predict bacteremia in patients with community-acquired pneumonia: a prospective cohort trial. Chest. 2010;138(1):121–129. doi: 10.1378/chest.09-2920. [DOI] [PubMed] [Google Scholar]
- 28.Cuquemelle E., Soulis F., Villers D. A/H1N1 REVA-SRLF Study Group. Can procalcitonin help identify associated bacterial infection in patients with severe influenza pneumonia? A multicentre study. Intensive Care Med. 2011;37(5):796–800. doi: 10.1007/s00134-011-2189-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schuetz P., Suter-Widmer I., Chaudri A. Prognostic value of procalcitonin in community-acquired pneumonia. Eur Respir J. 2011;37(2):384–392. doi: 10.1183/09031936.00035610. [DOI] [PubMed] [Google Scholar]
- 30.Krüger S., Ewig S., Kunde J. Assessment of inflammatorymarkers in patients with community-acquired pneumonia–influence of antimicrobial pre-treatment: results from the German competence network CAPNETZ. Clin Chim Acta. 2010;411:1929–1934. doi: 10.1016/j.cca.2010.08.004. [DOI] [PubMed] [Google Scholar]



