Abstract
Plausible representatives of plasmacytoid dendritic cells (pDCs) in pigs have been characterized as being CD4hiCD172lo. Due to their paucity in blood, we utilized novel fluorescent-activated cell sorting procedures to isolate them from PBMC. The resultant subset was greater than 98% homogeneous in regards to the selected phenotype and contained the preponderance of individuals secreting IFN-α after exposure to a known stimulant, transmissible gastroenteritis virus (TGEV). In addition to being a potent source of IFN-α, other properties of these porcine CD4hiCD172lo cells including their morphological transition from a plasma cell-like shape during quiescence to one resembling a dendritic cell (DC) after activation by TGEV and their relatively strong constitutive expression of interferon regulatory factor-7 (IRF-7) conformed to the expectations of genuine pDCs. While a substantial IFN-α response was also elicited from the porcine pDCs by pseudorabies virus (PrV), swine influenza virus (SIV), and TLR7 and 9 agonists, there was an agent-dependent induction of varying amounts of IL-2, IL-6, IL-8, IL-12, IFN-γ, and TNF-α. Notably, porcine reproductive and respiratory syndrome virus (PRRSV) failed to provoke the pDCs to secrete any of the measured cytokines except IL-2. Moreover, whereas pDCs exposed to TGEV or the TLR9 agonist rapidly increased IRF-7 production and morphed into DCs with enhanced CD80/86 expression, similar alterations were not observed during incubation with PRRSV. This atypical response of pDCs to PRRSV may contribute to its pathogenesis, which unlike that associated with PrV, SIV or TGEV includes persistent infection and limited development of protective immunity.
Keywords: FACS, Immunity, Innate immunity, Interferon-α, Plasmacytoid Dendritic cell, PRRS, Vaccine
1. Introduction
Despite their relatively low frequency (0.2–0.8%) in the general PBMC population, plasmacytoid dendritic cells (pDCs) represent an important component of the innate immune system (Liu, 2005, Fitzgerald-Bocarsly et al., 2008). Within a few hours after encountering a pathogen, individual cells can release type I IFN in the range of 3–10 pg—a quantity that is 100–1000-fold more than the amount produced by any other type of blood cell (Siegal et al., 1999). This prodigious response is partly due to their rather unique expression of toll-like receptors (TLR) 7 and 9 that interact with single-stranded RNA molecules with a high percentage of guanosine or uridine residues or single-stranded DNA strands having unmethylated CpG motifs, respectively (Gilliet et al., 2008). Foreign entities such as viruses are endocytosed into pDC endosomes where engagement of ligands in their genomes with the appropriate TLR triggers a MyD88-dependent pathway leading to ubiquitination and phosphorylation of interferon regulatory factor (IRF)-7. The modified IRF-7 then translocates to the nucleus where it is involved in autocrine regulation of gene expression and transcription of the IFN genes (Kawai et al., 2004, Honda et al., 2005). Although other cytokines such as TNF-α and IL-6 are also produced by stimulated pDCs (Liu, 2005), their rapid secretion of copious amounts of type I IFNs such as IFN-α (Cella et al., 1999, Siegal et al., 1999) helps establish an antiviral state (Cervantes-Barragan et al., 2007). Moreover, activated pDCs influence the development of adaptive immunity by inducing the differentiation of B cells (Jego et al., 2003, Poeck et al., 2004) and effector T cells and also by promoting the generation of regulatory T cells (Cella et al., 2000, McKenna et al., 2005, Ito et al., 2007).
Phenotypically, human pDCs have been characterized as being CD4+ but lacking lineage markers common to B (CD19, B cell receptor), myeloid (CD11b, CD11c, CD13, CD14, CD33), NK (CD16, CD56), and T (CD3, γδ T cell receptor) cells (Perussia et al., 1985, Cella et al., 1999, Liu, 2005). Quiescent pDCs constitutively express IRF-7 and the immediate availability of this factor is essential for their ability to promptly react to the presence of viruses (Coccia et al., 2004, Dai et al., 2004). Upon activation, pDCs undergo a morphological transition from their hallmark plasmacytoid shape into a form associated with mature dendritic cells (DCs) (Grouard et al., 1997, Soumelis and Liu, 2006). Moreover, their expression of MHC class II proteins as well as the co-stimulatory molecules, CD80 and CD86, is increased (Perussia et al., 1985, Svensson et al., 1996, Cella et al., 2000, Bauer et al., 2001).
Originally, the term pDC was reserved for plasmacytoid T cells/monocytes that represented a unique subset of DC precursors and exhibited a plasmacytoid morphology. At that time, this designated population had not yet been compared to blood cells that produced large amounts of IFN-α after exposure to a virus and were called interferon-producing cells or natural interferon-producing cells (NIPCs) (Liu, 2005, Fitzgerald-Bocarsly et al., 2008). Later, when it was demonstrated that human pDCs and NIPCs were identical (Cella et al., 1999, Siegal et al., 1999), pDCs became the preferred designation. However, in the case of swine, the equivalence of these two populations has not been formally established.
Porcine NIPCs were initially described as non-adherent, non-T, non-B cells that expressed CD4 and MHC class II surface markers and secreted IFN-α after incubation with the porcine coronavirus, transmissible gastroenteritis virus (TGEV) (Charley and Lavenant, 1990). Their low frequency in the blood (maximum of 40–110 cells per 105 PBMC) was shown to vary and be influenced by the pig's age, genetics, and physiological status (Nowacki et al., 1993). Moreover, they were found to migrate into the lymphoid tissue of virus-infected animals (Riffault et al., 1997, Riffault et al., 2001). TGEV-responsive NIPCs were later defined as being in the CD1− CD4+CD14−CD172lo subset of PBMC based on magnetic separation or flow cytometric analyses of mAb-treated blood mononuclear cells (Summerfield et al., 2003). A similar phenotype was also noted for those PBMC activated by certain oligodeoxynucleotides (ODN) containing unmethylated CpG motifs to produce IFN-α (Domeika et al., 2004, Guzylack-Piriou et al., 2004). In addition to TGEV and some ODNs, porcine NIPCs can be stimulated by classic swine fever virus (Balmelli et al., 2005), foot and mouth disease virus (FMDV) complexed with FMDV-specific Igs (Guzylack-Piriou et al., 2006), pseudorabies virus (PrV) (Domeika et al., 2004), and swine influenza virus (SIV) (Summerfield and McCullough, 2009).
Porcine NIPCs were originally enriched from PBMC by first removing plastic-adherent cells (mainly monocytes) and then collecting the low-density fraction of the remaining population after centrifugation in a metrizamide gradient (Nowacki and Charley, 1993). This protocol was later improved by using anti-CD3, anti-CD8 and anti-CD45RA mAbs for depletion of B, NK and T cells prior to separation of CD14+ cells from the CD14− NIPCs with a MACS system (Summerfield et al., 2003) or by just utilizing mAbs recognizing CD172 and either CD4 or CD14 in conjunction with magnetic sorting (Guzylack-Piriou et al., 2004). However, greater consistency, ease of preparation, and a decreased potential for procedural-generated artifacts should be obtainable by fluorescent-activated cell sorting (FACS) purification of NIPCs. In this regard, as described here, we have successfully used this approach, first with a traditional device in combination with prior depletion of B and T cells, and later by just two rounds of sorting with a newer, more sophisticated instrument. In either case, the selected CD4hiCD172lo subset could be purified to approximately 98% homogeneity with retention of the ability to express IFN-α after exposure to TGEV. They were defined as being pDCs based on their plasmacytoid morphology, greatest constitutive expression of IRF-7 among phenotypically distinct PBMC subsets, and unique responsiveness to virus stimulation characterized by profuse secretion of IFN-α and physical conversion into a DC appearance with an accompanying enhanced synthesis of CD80/86. Moreover, depending on the type of virus or TLR agonist used, a combination of IL-2, IL-6, IL-8, IL-12, IFN-γ, and TNF-α were released by the cells. In contrast, the purified pDCs appeared to be nearly unresponsive to the arterivirus, porcine reproductive and respiratory syndrome virus (PRRSV), in that virus-exposed cells did not mature into DCs, increase IRF-7 expression or synthesize IFN-α but did secrete IL-2.
2. Materials and methods
2.1. Preparation of porcine PBMC
Fresh heparinized venous blood was obtained from cross-bred Yorkshire × Landrace pigs housed at the University of Illinois Veterinary Medicine Research Farm. Samples were diluted with HBSS (Mediatec, Herndon, VA, USA) and subjected to density centrifugation through Ficoll-Hypaque 1077 (Sigma, St. Louis, MO, USA) gradients. The separated PBMC were washed twice with HBSS and then suspended in RPMI complete medium: RPMI 1640 medium with l-glutamine (Mediatech) supplemented with 5% FBS (GIBCO®, Invitrogen, Grand Island, NY, USA), 100 U/ml penicillin, 0.1 mg/ml streptomycin, 1 mM sodium pyruvate, 1× nonessential amino acids (Mediatech) 100 U/ml gentamicin and 250 mM 2-ME (Sigma).
2.2. Isolation of porcine CD4hiCD172lo cells
Purification of the CD4hiCD172lo subset of the porcine PBMC population was accomplished by either of two methods. In the first procedure, PBMC were incubated with anti-porcine CD2 (MSA4), CD3 (3Bb-238E6) and CD21 (BBb11C9) mAbs (Haverson et al., 2001) in PBS supplemented with 1.0% BSA (PBS–BSA) for 30 min at 4 °C, washed twice with PBS–BSA, and then incubated with PBS–BSA containing goat anti-mouse IgG microbeads (Miltenyi Biotec, Auburn, CA, USA) for 30 min at 4 °C. After one wash with PBS–BSA, the PBMC were passed through a MACS column (Miltenyi Biotec). Unbound, lineage-negative (CD2−CD3−CD21−) cells were incubated at 4 °C in PBS–BSA containing anti-swine CD4 mAb 74-12-4 (Haverson et al., 2001) conjugated to Alexa Fluor 488 and biotinylated, anti-swine CD172 mAb 74-22-15 (Southern Biotech, Birmingham, AL, USA). After 30 min, the cells were washed twice with PBS–BSA, incubated in the presence of streptavidin (SA) conjugated to PE (Southern Biotech), CyChrome (eBiosciences, San Diego, CA, USA) or Alexa Fluor 647 (Molecular Probes®, Invitrogen) for 30 min at 4 °C, and again washed twice in PBS–BSA buffer. Then, the CD4hiCD172lo subset was electrostatically separated from the remaining PBMC (primarily CD4−CD172hi cells) by using a MoFlo Sorter (Dako Cytomation, Ft. Collins, CO, USA).
Alternatively, porcine CD4hiCD172lo cells were isolated directly from non-fractionated PBMC populations after this heterogeneous group was stained only for the presence of CD4 and CD172 markers as described above. In this case, a novel, high-speed cell sorting device, Reflection™ (iCyt, Champaign, IL, USA), was configured to perform an enrichment and subsequent purification step of the targeted (electronically gated) CD4hiCD172lo cells.
2.3. Viruses and TLR agonists
Wild-type PRRSV strain 46448, obtained from NVSC, was expanded and titrated in MARC 145 cells (Meier et al., 2003) while the Becker strain of PrV was propagated in PK-15 cells. A H1N1 strain of SIV was provided by the Virology Section of the University of Illinois Veterinary Medicine Diagnostic Laboratory. TGEV (strain Purdue 115) was grown in swine testes cells. All viruses except PRRSV were used at doses that provoked maximum production of IFN-α by PBMC (PrV, TGEV, multiplicity of infection = 0.05; SIV, 50 hemagglutinating units). Due to the inability of PRRSV to elicit this type of response, exposure of PBMC or pDCs to PRRSV was performed at a multiplicity of infection of 0.1.
When required, incubation of the cells with either TLR7 agonist, imiquimod (InvivoGen, San Diego, CA) or a TLR9 agonist, a CpG-containing ODN type A (ODN D19; Qiagen, Valencia, CA) (Kamstrup et al., 2001), was conducted at final concentrations of 1 and 2 μg/ml, respectively.
2.4. Quantitation of IFN-α secretion by intact and fractionated porcine PBMC populations
Representatives of non-fractionated, porcine PBMC or its lineage positive (CD2+, CD3+, CD21+), CD4hiCD172lo, or monocyte (CD4−CD172hi) subsets derived during the depletion/sorting procedure were cultured at 2 × 105 cells/ml in RPMI complete medium in the presence/absence of TGEV for 16 h at 37 °C. The supernatant media were then separately assayed for the presence of IFN-α by using an ELISA. Briefly, individual wells of a Nunc Immulon II 96-well plate (Thermo Fisher Scientific, Inc., Rockford, IL, USA) that had been coated for 16 h at 4 °C with 50 μl of 5 μg/ml anti-pig IFN-α mAb F17 (PBL InterferonSource, Piscataway, NJ, USA) in 0.1 M carbonate buffer (pH 9.6) were washed 3 times with PBS containing 0.05% Tween 20 (PBS–T), and incubated with 200 μl milk blocking solution (BioFix, Owings Mills, MD, USA) for 1 h at 25 °C. After three washes with PBS–T, 50 μl culture supernatants and rIFN-α standards (PBL InterferonSource) diluted in RPMI complete medium were added to duplicate wells and placed for 1.5 h at 25 °C. After washing 5 times with PBS–T, each well was incubated with 50 μl of PBS–T containing 0.3 μg/ml biotin-labeled, anti-pig IFN-α mAb K9 (PBL InterferonSource) and 0.5% milk blocking solution at 25 °C for 1.5 h. After 5 washes with PBS–T, each well was incubated with 50 μl PBS–T containing 20 ng/ml SA conjugated to HRP (Biosource™, Invitrogen) for 20 min at 25 °C and then again washed 5 times with PBS–T. Color development was initiated at 25 °C with the addition of 100 μl TMB substrate (KPL, Gaithersburg, MD, USA) per well and terminated with 100 μl 1 M phosphoric acid. Optical densities were determined at 450 nm with a SPECTRAMAX Plus plate reader (Molecular Devices, Sunnyvale, CA). Results were averaged and the amounts of IFN-α were determined by comparison to a standard curve generated from the values obtained with the known quantities of IFN-α.
2.5. Detection of IFN-α secreting cells in intact and fractionated porcine PBMC populations
Individual, IFN-α secreting cells (SC) present in a non-fractionated, porcine PBMC population or its lineage positive (CD2+, CD3+, CD21+), CD4hiCD172lo, or monocyte (CD4−CD172hi) subsets were detected by means of an ELISPOT assay. For this purpose, 2 × 105 cells previously incubated in RPMI complete medium in the presence/absence of TGEV for 8 h at 37 °C were added to wells of an Immulon II 96-well plate that had already been coated with anti-pig IFN-α mAb F17 as described above for the IFN-α ELISA procedure and washed afterwards 3 times with sterile PBS. After 8 h at 37 °C, the cells were removed from the wells during three washes with PBS–T and then each well was sequentially incubated with PBS–T containing biotin-labeled, anti-pig IFN-α mAb K9 and PBS–T containing SA conjugated to HRP as described above for the IFN-α ELISA protocol. Finally, after being washed 5 times with PBS–T, each well was incubated with 100 μl TMB membrane substrate (KPL) for 30 min at 37 °C. The resultant blue spots on the well bottoms were enumerated and the values for duplicate samples averaged to provide frequencies of IFN-α SC per group.
2.6. Phenotypic analysis of porcine CD4hiCD172lo cells by three-color immunofluorescence flow cytometry
Staining of porcine PBMC consisted of various incubations in Flow PBS (PBS, 1.0% BSA, 0.01% sodium azide) containing the indicated mAbs or other components for 30 min at 4 °C with each step being terminated by one wash with non-supplemented Flow PBS. Initially, the PBMC were separately exposed to one of the following mAbs that recognize swine CD1a (76-7-4), CD2 (MSA4), CD3 (3Bb-238E6), CD8α (76-2-11), CD11a (BL1H8), CD11b (TMG 6-5), CD11c (S-HcL3), CD14 (biG 10/14), CD16 (G7), CD18 (MUC76a), CD21 (BBb11C9), CD29 (UCP1D2), CD44 (BAG40A), CD45RA (Mil 13), CD45RC (3a56), CD56 (MEM-188), CD163 (2A10/11), or MHC II DR (2E9/13) (Alvarez et al., 2000, Haverson et al., 2001) or just left untreated. Afterwards, the cells were sequentially incubated in Flow PBS containing: isotype-specific, goat anti-mouse Ig conjugated to PE (Southern Biotech), 2% normal mouse serum (Sigma) and 100 μg/ml mouse IgG (Zymed Laboratories, Invitrogen); anti-swine CD4 mAb conjugated to Alexa Fluor 488 and biotinylated, anti-swine CD172 mAb; and SA conjugated to Alexa Fluor 647. Multicolor immunoflourescence flow cytometric analysis of the stained cells was performed with a LSR II flow cytometer (BD Biosciences, San Jose, CA, USA). Data analyses, including the calculation of the robust coefficient of variation (RCV), and preparation of graphical representations were done with FlowJo software (Ashland, OR, USA).
2.7. Microscopic examination of purified porcine CD4hiCD172lo cells
For gross morphological examination, 105 purified porcine CD4hiCD172lo cells were cytospun onto glass slides, stained with Diff-Quick® (Dade Behring, Inc., Newark, DE, USA), and visualized by using light microscopy. For a more detailed assessment, the purified cells were submitted to the Center for Microscopic Imaging at the University of Illinois, Urbana, IL, where they were fixed, embedded, and examined with a Hitachi H600 transmission electron microscope.
2.8. Detection of intracellular IRF-7 in porcine PBMC and purified porcine pDCs
1 × 106 porcine PBMC were stained for CD4 and CD172 as described above. Afterwards, they were fixed in PBS containing 1% paraformaldehyde (Pierce Biotechnology, Thermo Fisher Scientific) for 20 min at 25 °C, washed with PBS, and permeabilized in PB buffer (PBS containing 0.1% Triton X-100 and 2% FBS) for 15 min at 25 °C. The cells were then incubated with PB containing rabbit anti-human IRF-7 polyclonal IgG or non-specific polyclonal IgG (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) in PB buffer for 30 min at 25 °C, washed twice with PB buffer, and incubated for 30 min at 25 °C in PB buffer containing polyclonal goat anti-rabbit IgG conjugated to Alexa Fluor 647 (Molecular Probes®). After another round of washing, the PBMC were again fixed in PBS containing 1% paraformaldehyde and their IRF-7 content analyzed by using a LSR II flow cytometer. Data analyses and preparation of graphical representations were done with FlowJo software.
3 × 105 enrichment-sorted porcine pDCs (see Fig. 5A, enrichment sort panel) were exposed to ODN D19, PRRSV, TGEV or just RPMI complete medium for 3 or 5 h. Afterwards, each sample was fixed, stained, and analyzed for the presence of intracellular IRF-7 as described above for similarly-treated PBMC. Fixed cells were also incubated with non-specific polyclonal IgG in lieu of anti-IRF-7 Ab for use as background controls.
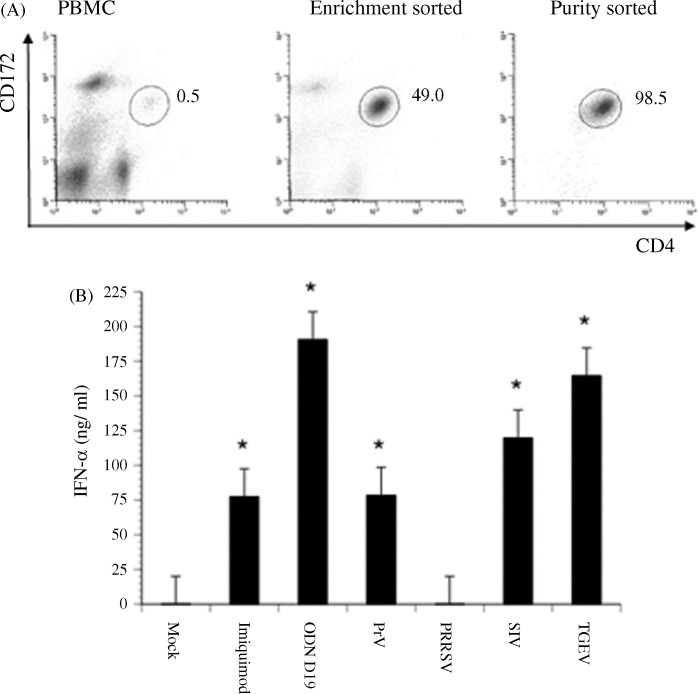
Fig. 5.
Porcine virus- or TLR agonist-mediated induction of IFN-α production by purified porcine pDCs. Two rounds of selection for the CD4hiCD172lo subset of porcine PBMC were performed with a Reflection™ high-speed cell sorter. (A) Histograms of non-fractionated porcine PBMC and of cells remaining after an enrichment or subsequent purity sort for a CD4hiCD172lo phenotype. The positions and frequencies of CD4hiCD172lo cells are shown throughout the isolation process. (B) Cumulative IFN-α response of purified CD4hiCD172lo cells (pDCs) to imiquimod, ODN D19, PrV, PRRSV, SIV, or TGEV. The quantities of IFN-α secreted by purity-sorted pDCs cultured alone (Mock) or with a porcine virus or TLR agonist for 24 h were determined by using an ELISA. Significant differences between the response of mock-treated and virus- or TLR agonist-stimulated pDCs are indicated by an (*p ≤ 0.05) or (**p ≤ 0.01). Bars represent the mean ± SEM of a representative experiment (n = 3).
2.9. Quantitation of cytokine secretion by purified porcine pDCs
Medium used to culture purified porcine pDCs at 2 × 105 cells/ml that had been left untreated or exposed to PrV, PRRSV, SIV, TGEV, imiquimod, or ODN D19 for 24 h was assayed for the presence of IFN-α by using the specific ELISA described above. For detection of IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, IFN-γ, and TNF-α, the Searchlight chemiluminescent multiplex assay was employed (Pierce Biotechnology, Thermo Fisher Scientific).
2.10. Detection of CD80/86 expression by purified porcine pDCs
4 × 105 enrichment-sorted porcine pDCs (see Fig. 5A, enrichment sort panel) were immediately processed as described below or first exposed to ODN D19, PRRSV, TGEV or just RPMI complete medium for 24 h. Afterwards, each sample was washed twice with Flow PBS and divided so that approximately one-fourth of each one could be cytospun onto glass slides and stained with Diff-Quick®. The remaining cells were incubated for 30 min at 4 °C in Flow PBS containing anti-CD80/86, biotinylated hCTLA-4muIg (Ancell, Bayport, MN), washed twice in Flow PBS, and incubated for 30 min at 25 °C in Flow PBS containing SA conjugated to CyChrome. After being washed twice more with Flow PBS, the cells were analyzed with a EPICS XL flow cytometer (Beckman Coulter, Inc., Fullerton, CA, USA). FlowJo software was used to prepare the graphical representations and for data analysis.
2.11. Statistic analyses
An unpaired t-test was performed to determine if significant differences existed between the quantities of each cytokine secreted by untreated pDCs as compared to ones incubated with a virus or TLR agonist.
3. Results
3.1. Purification of porcine CD4hiCD172lo cells
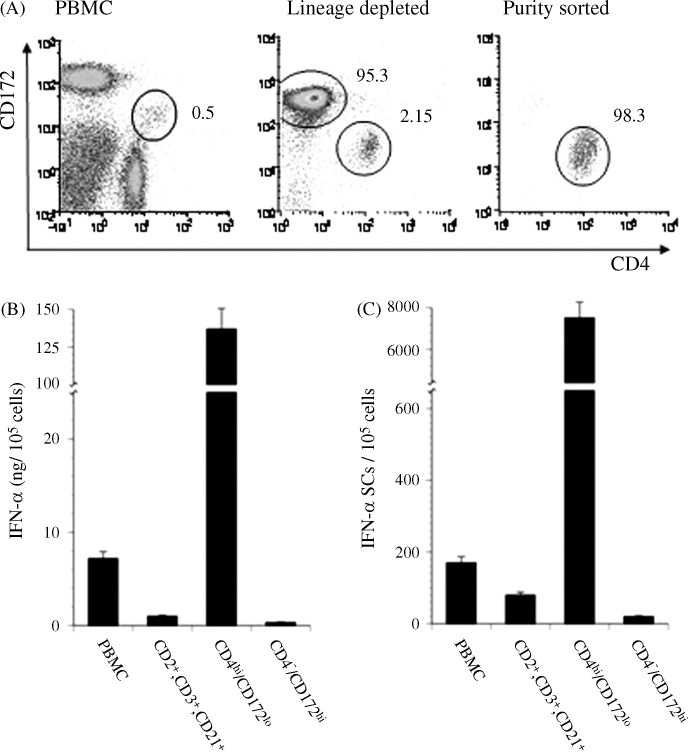
Previous characterizations of the predominant IFN-α producing members of the porcine PBMC population indicated that they expressed CD4 (Charley and Lavenant, 1990) and low levels of CD172, a member of the signal regulatory protein family (Summerfield et al., 2003). Likewise, in preliminary studies we had also associated the combination of these two cell-surface markers with the IFN-α responsiveness of some lymphocytes to viruses and accordingly intended to exploit this phenotype for developing a purification scheme. In this regard, when mAb-treated PBMC were subjected to two-color immunofluorescent analysis (Fig. 1A, PBMC panel), a low frequency (approximately 0.5%), CD4hiCD172lo subset was not only revealed but also appeared to be sufficiently, spatially distinct so as to be amendable to isolation by using a FACS procedure. However, at this time due to the limited efficiency of the available instrument, an initial step whereby cells expressing B and T cell lineage markers were depleted from freshly isolated PBMC samples was instituted. This procedure routinely enabled an approximately four-fold enrichment of CD4hiCD172lo cells (Fig. 1A, Lineage depleted panel) with the remainder being mostly CD172+ cells, which are monocytes. Afterwards, a single electronic sorting regularly resulted in the selection of a relatively homogeneous grouping (approximately 98% purity) of CD4hiCD172lo cells (Fig. 1A, purity-sorted panel). Overall, a nearly 200-fold enrichment of this minor PBMC component was achieved by using this process.
Fig. 1.
TGEV-mediated induction of IFN-α production by phenotypically distinct porcine PBMC. After depletion of lineage positive (CD2+, CD3+, and/or CD21+) cells by using MACS, the remaining porcine PBMC were separated into CD4hiCD172lo or predominately CD4−CD172hi subsets by using a MoFlo® cell sorter. (A) Histograms of non-fractionated, lineage depleted, and CD4hiCD172lo purity-sorted PBMC. The positions and frequencies of CD4hiCD172lo cells are shown throughout the purification process while those parameters for the CD4−CD172hi cells are only indicated in the Lineage depleted panel. (B and C) Cumulative and individual IFN-α responses of non-fractionated, lineage positive, CD4−CD172hi, and CD4hiCD172lo PBMC to TGEV. An intact PBMC sample and the designated subsets were separately cultured with TGEV for 16 h and then the quantities of secreted IFN-α and frequencies of IFN-α SC were determined by using an ELISA (B) and ELISPOT assay (C), respectively. Bars represent the mean ± SEM of a representative experiment (n = 3).
In agreement with other studies using either TGEV (Summerfield et al., 2003) or CpG-ODN (Domeika et al., 2004, Guzylack-Piriou et al., 2004) as stimulants, the preponderance of IFN-α production by activated porcine PBMC was found to be localized to the CD4hiCD172lo subset (approximately 137 ng/105 cells; Fig. 1B). In fact, only a very limited quantity of IFN-α was secreted by the fractions comprised primarily of CD172+ monocytes (<0.4 ng/105 cells) or cells of B or T lineage (3.2 ng/105 cells). Similarly, the combined number of TGEV-responsive cells in these two groups was approximately 80-fold lower than the frequency observed for the CD4hiCD172lo subset. Thus, this joint depletion/sorting method for selection of CD4hiCD172lo cells correlated with the purification of biologically active NIPCs.
3.2. Surface phenotyping of porcine CD4hiCD172lo cells
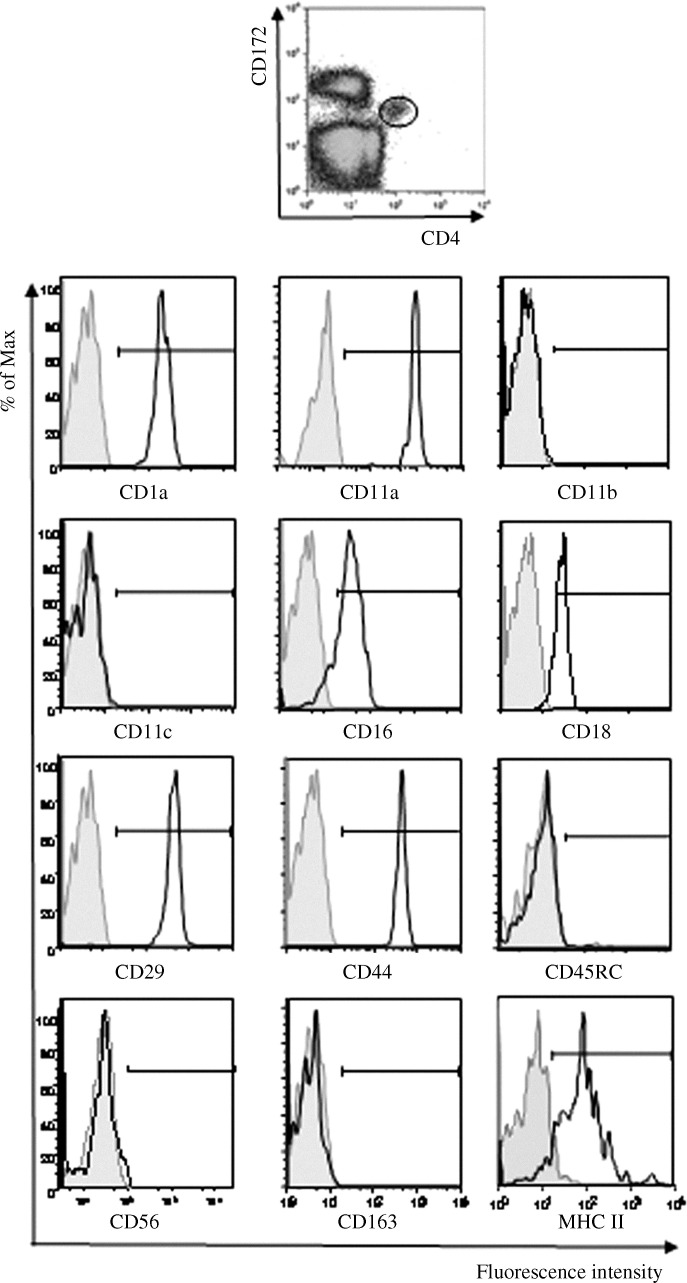
Human and mouse pDC/NIPCs do not express lineage-specific markers for B cells, T cells, NK cells or monocytes (Perussia et al., 1985, Liu, 2005). To determine if the CD4hiCD172lo subset of porcine PBMC acted in a similar manner and to thoroughly evaluate their surface phenotype, three-color immunofluorescence flow cytometric analyses of these cells stained for CD4, CD172, and a third variable protein were conducted (Fig. 2 ). Examination of the resultant histograms revealed a unimodal distribution of CD1a, CD11a, CD18, CD29, and CD44 among members of the CD4hiCD172lo subset and a fairly constant per cell amount of these proteins as evidenced by a low (<30) RCV in regards to the fluorescence intensity of the stained cells. A more variable level of expression of the CD16 was noted as reflected by larger variation in the fluorescence intensity (RCV = 48). Interestingly, while CD1 and CD16 were clearly present in this sampled population, their previous status was classified as being inconsistent (+/−) (Summerfield and McCullough, 2009). Overall, the relative level of expression of MHC class II molecules on individual CD4hiCD172lo cells was the most variable (RCV = 70). This phenomenon has been attributed to the CD4hiCD172lo cells being in different stages of maturation (Summerfield et al., 2003, Guzylack-Piriou et al., 2004). As previously reported (Nowacki and Charley, 1993, Summerfield et al., 2003, Domeika et al., 2004, Guzylack-Piriou et al., 2004), the myeloid cell marker CD14, the NK and T cell markers, CD2, CD3, CD8α, the B cell marker CD21 as well as the CD45 isoform CD45RA were not detected (data not shown). In addition, the myeloid cell markers CD11c, previously reported as being present (Summerfield et al., 2003), and CD11b, the CD45 isoform CD45RC, the NK cell adhesion molecule CD56, and the scavenger molecule CD163 seemed to be absent (Fig. 2). Thus, with the exception of CD16 that is found on the surface of macrophages, monocytes, NK cells, and neutrophils, the porcine CD4hiCD172lo subset does not express lymphoid or myeloid lineage cell markers.
Fig. 2.
Expression of surface markers by the CD4hiCD172lo subset of porcine PBMC. A portion of a porcine PBMC sample was stained for only CD4 and CD172 and used to prepare the top panel histogram in which the location of the CD4hiCD172lo subset is circled. Electronic gating on this demarcated region in similar histograms generated with other PBMC aliquots that were stained specifically for the indicated surface markers with the respective anti-CD mAb (clear areas) or non-specifically with a corresponding, anti-Ig isotype-matched mAb (shaded areas) prior to treatment with anti-CD4 and CD172 mAbs provided the resultant fluorescence distribution data shown in the lower graphs. Bars define the limits of fluorescent intensity representing a positive signal. Representative histograms (n = 3) are shown.
3.3. Morphological characterization of porcine CD4hiCD172lo cells
Human pDCs exhibit a distinct plasma cell-like morphology with an eccentric, kidney-shaped nucleus surrounded by a basophilic cytoplasm with a perinuclear pale Golgi zone (Siegal et al., 1999, Liu, 2005). To determine if CD4hiCD172lo cells displayed similar characteristics, freshly sorted representatives were treated with a histological stain and examined by using light microscopy. As seen in Fig. 3A, the dye-stained cells had a rounded to oval shape with an off-centered, reniform nucleus and a basophilic cytoplasm containing a prominent pale area that is adjacent to the nucleus and represents a negative image of the Golgi apparatus. Further examination of a CD4hiCD172lo cell with a transmission electron microscope revealed heterochromatin localized in the periphery of its nucleus and an extensive system of rough endoplasmic reticulum, a juxtanuclear Golgi apparatus and several mitochondria in its cytoplasm (Fig. 3C).
Fig. 3.
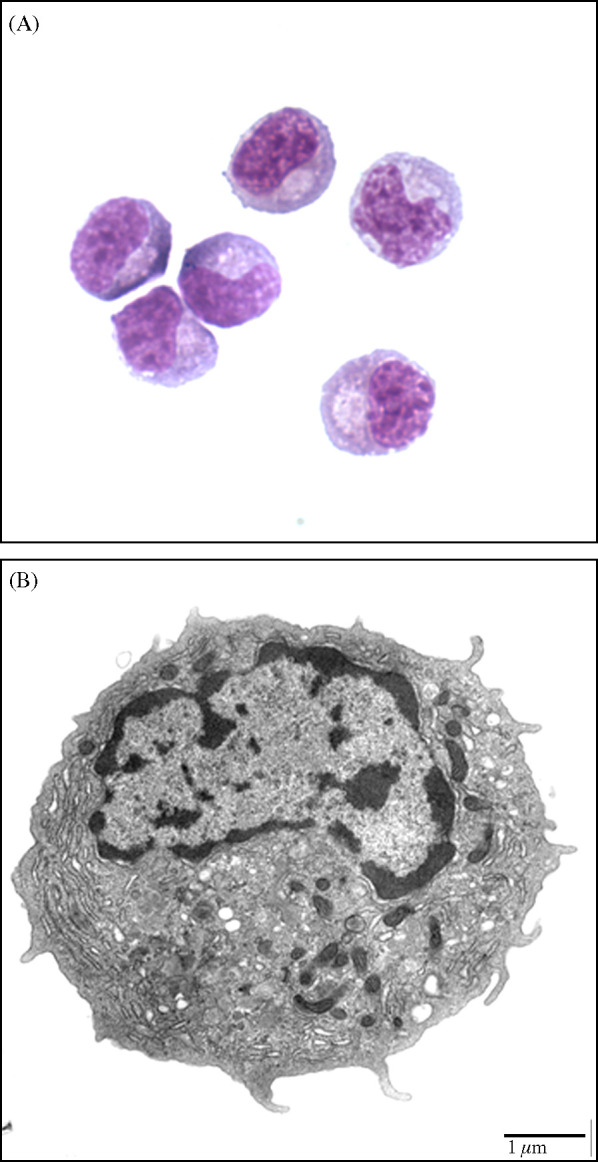
Morphology of CD4hiCD172lo cells purified from porcine PBMC by using the combined lineage depletion/phenotypic sorting procedure. (A) Light microscope (1000× magnification) image of Diff-Quick® stained cells. (B) Transmission electron microscopic image of a fixed cell.
3.4. Constitutive expression of IRF-7 by porcine CD4hiCD172lo cells
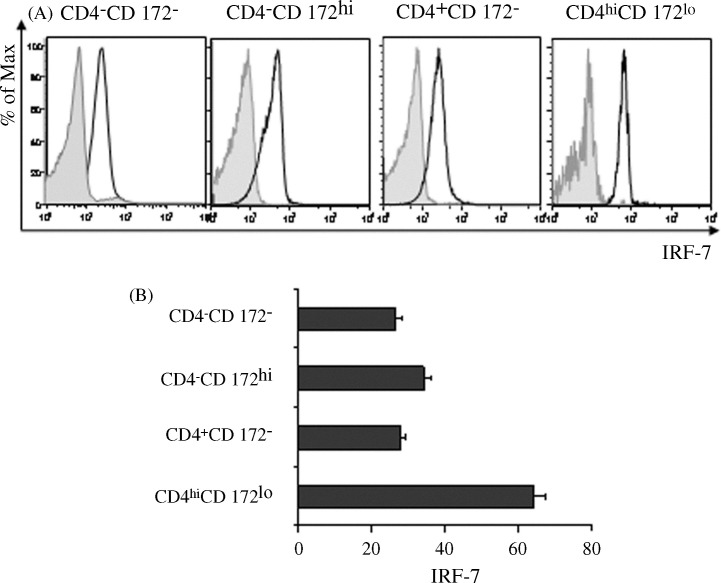
The pDC subset of human PBMC has been shown to constitutively express more IRF-7 than other leukocyte subpopulations such as monocytes and T cells (Izaguirre et al., 2003, Dai et al., 2004). This abundance is considered to be essential for promoting the rapid production of IFN-α by pDCs in response to interaction with a virus (Coccia et al., 2004, Dai et al., 2004). Consequently, porcine CD4hiCD172lo cells should be the prominent producer of IRF-7 among the pig's blood cells if they are pDCs. Indeed, a comparison of the IRF-7 distribution among various, gated porcine PBMC fractions previously stained for this transcription factor indicated that IRF-7 expression was greatest in the CD4hiCD172lo subset (Fig. 4A and B). Moreover, this subset was more homogeneous with respect to per cell amounts of IRF-7 as compared to that of the other examined groups, comprised of CD4−CD172− cells, monocytes (CD4−CD172hi) or CD4+ lymphocytes (CD4+CD172−). In this regard, while the RCV in relation to the individual fluorescence intensity of their stained members of these three groups were 45, 38, and 44, respectively, a lower value of 23 was determined for the CD4hiCD172lo subset. Therefore, based on their established status as the primary IFN-α producer, external and internal physical appearances as well as strong constitutive expression of IRF-7, the CD4hiCD172lo cells can be considered to be the porcine counterpart of human pDCs.
Fig. 4.
Constitutive expression of IRF-7 by porcine PBMC subsets. Stained porcine PBMC were virtually separated into CD4−CD172−, CD4−CD172hi, CD4+CD172−, and CD4hiCD172lo subsets in CD4 versus CD172 histograms (comparable to Fig. 1A, PBMC panel). (A) Fluorescent intensities of electronically gated PBMC subsets reacting with anti-IRF-7 polyclonal IgG (clear areas) or a non-specific counterpart (shaded areas). Prior to analysis, areas defining each respective subset in the two histograms were made spatially identical. (B) Mean fluorescence intensities (MFIs) of electronically gated, IRF-7 stained PBMC subsets. Vertical bars represent the mean ± SEM of a representative experiment (n = 3).
3.5. IFN-α and pro-inflammatory cytokine secretion by purified, porcine pDCs exposed to swine viruses and TLR7 and TLR9 agonists
Due to the advent of an innovative high-speed cell sorter, the original CD4hiCD172lo cell purification procedure was modified to eliminate the depletion step altogether and instead perform two rounds of positive phenotypic selection starting with an intact PBMC sample. With this improvement, a single, enrichment sort resulted in an increased frequency of porcine pDCs of nearly 200-fold, from approximately 0.5% to 49% (Fig. 5A). Moreover, the subsequent purity sort rendered a population that was >98% homogeneous and comparable to that achieved with the first protocol. Furthermore, a similar degree of cell viability (≥95%) was retained with either method (data not shown).
As with the CD4hiCD172lo cells isolated by using the former, more laborious exercise of combined depletion and sorting, the porcine pDCs prepared by the simplified, double-sorting method retained biological activity. They readily secreted IFN-α after exposure to TGEV and also to two other known virus stimulants, PrV (Domeika et al., 2004), and SIV (Summerfield and McCullough, 2009) (Fig. 5B). They also reacted to the TLR7 agonist, imiquimod (Hemmi et al., 2002), and to the TLR9 ligand, ODN D19 (Kamstrup et al., 2001). In contrast, detectable IFN-α production by the pDCs was not noted in the presence of PRRSV, even when the amount of this virus was increased 100-fold (data not shown). Moreover, there was also a lack of responsiveness by non-fractionated PBMC to the North American, Type II PRRSV strain used in this study (data not shown) as previously reported for a genetically distinct, European, Type I PRRSV isolate (Albina et al., 1998a).
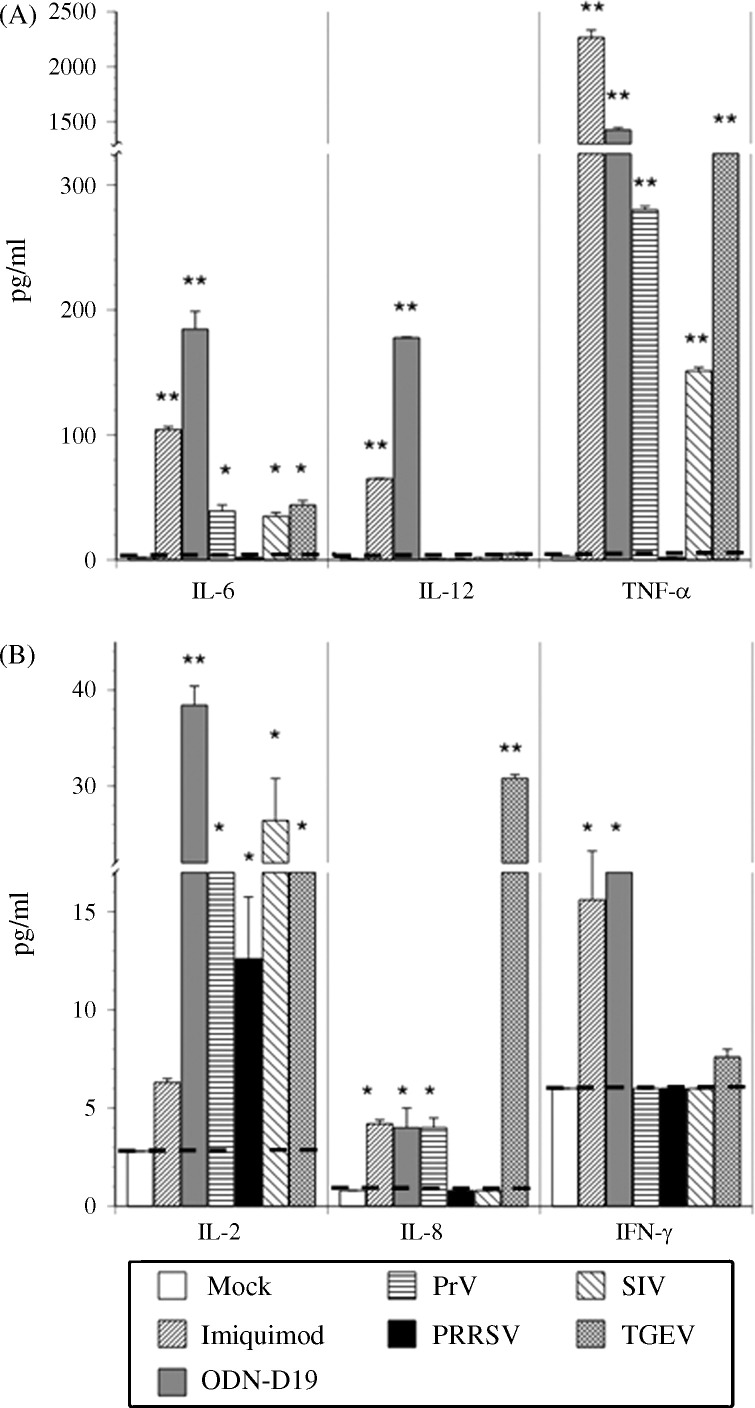
Remarkably, the secretion of other cytokines by the pDCs appeared to depend on the type of activating agent (Fig. 6 ). Of the six assayed proteins, IL-6 and TNF-α were the only two to be detected in significant amounts after exposure of the cells to any of the IFN-α inducing entities. Although IL-2 production was not elicited by imiquimod, all of the other agents, including PRRSV, were able to stimulate a low but detectable production of this cytokine. In contrast, both PrV and PRRSV failed to induce detectable IL-8 expression, while the other stimulants elicited a trace amount of this cytokine. Only the two TLR agonists provoked the secretion of a low (<200 pg/ml) amount of IL-12, as previously observed for porcine pDCs responding to similar types of ligands (Vincent et al., 2007), as well as a modest release of IFN-γ. IL-1, IL-4 and IL-10 were not detected in the medium of any of the treated pDCs (data not shown).
Fig. 6.
Porcine virus- or TLR agonist-mediated induction of pro-inflammatory cytokine production by purified porcine pDCs. Purity-sorted porcine pDCs were cultured alone (Mock) or with imiquimod, ODN D19, PrV, PRRSV, SIV, or TGEV for 24 h and then the quantities of secreted IL-2, IL-6, IL-8, IL-12, TNF-α, and IFN-γ were determined by using the Searchlight chemiluminescent assay. Dotted lines indicate the level of detection (pg/ml) for each cytokine. Significant differences between this value and the quantity of respective cytokine released by virus- or TLR agonist-exposed pDCs are indicated by an (*p ≤ 0.05) or (**p ≤ 0.01). Bars represent the mean ± SEM of a representative experiment (n = 3).
3.6. Differential effect of swine viruses and a TLR9 agonist on IRF-7 expression by purified porcine pDCs
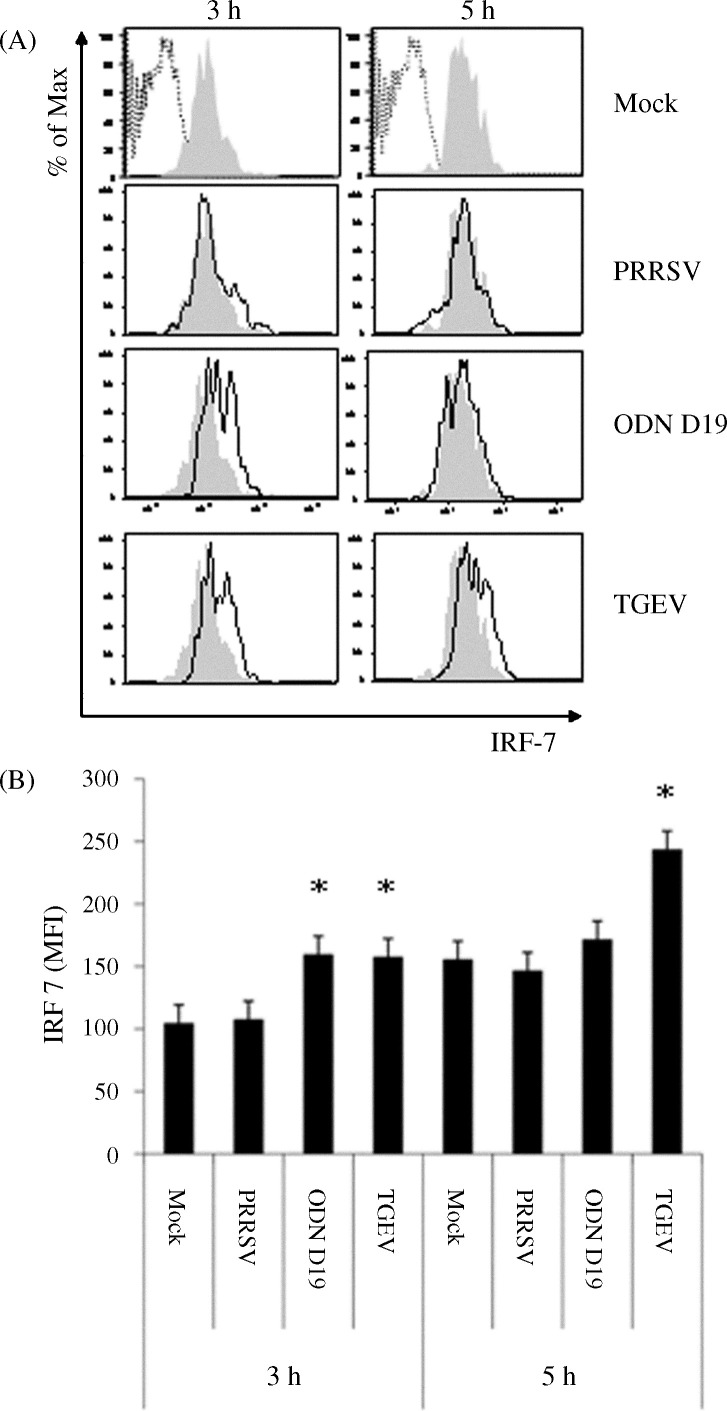
After a 6 or 9 h incubation period of human pDCs with external stimuli such as herpes simplex virus or IFN-α, the extent of IRF-7 expression was found to be greater than the already strong constitutive level (Izaguirre et al., 2003). To determine if a similar phenomenon occurred in activated porcine pDCs, the relative amounts of IRF-7 in DCs at 3 and 5 h post-exposure to ODN D19, PRRSV, and TGEV were compared to that in mock-treated cells. As seen in the histograms derived from the analysis of pDCs stained for the presence of intracellular IRF-7, an increase in fluorescent intensity was apparent at either both measurements or just at the earlier time for the TGEV- and ODN D19-treated cells, respectively (Fig. 7A). These visible changes were found to be statistically significant (p = 0.05) when the MFIs of these samples were compared to that of the respective, non-stimulated, temporal control (Fig. 7B). In contrast to the demonstrated positive impact of TGEV and ODN D19 on porcine pDC IRF-7 production, no alteration of this protein's quantity was noted in cells incubated with PRRSV.
Fig. 7.
Porcine virus- or TLR agonist-mediated impact on IRF-7 production by purified porcine pDCs. Purity-sorted porcine pDCs were cultured alone (Mock) or with ODN D19, PRRSV or TGEV for 3 or 5 h. (A) Histograms of IRF-7 stained pDCs previously incubated in the absence (shaded areas) or presence of a virus or TLR9 agonist (solid line, clear areas). The extent of binding of non-specific polyclonal IgG by mock-treated pDCs is indicated by the dotted line images. (B) MFIs of IRF-7 stained pDCs incubated in the absence (Fig. 8A, shaded areas) or presence of a virus or TLR9 agonist (Fig. 8A, solid line, clear areas). Significant differences between the response of mock-treated and virus- or TLR9 agonist-exposed pDCs are indicated by an (*p ≤ 0.05). Bars represent the mean ± SEM of a representative experiment (n = 3).
3.7. Differential effect of swine viruses and a TLR9 agonist on the maturation of purified porcine pDCs
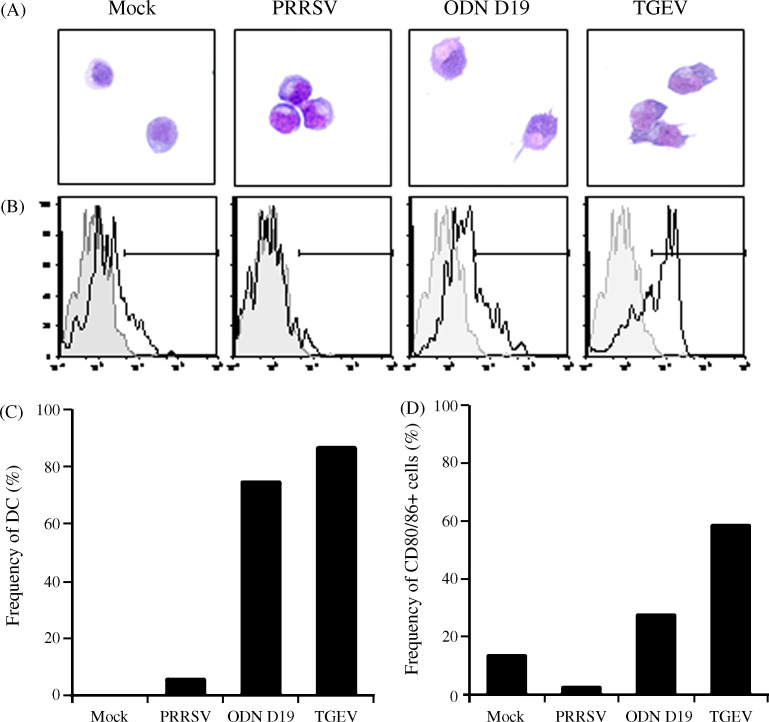
Activated human pDCs undergo a maturation process characterized by their morphological conversion into a DC appearance (Grouard et al., 1997, Siegal et al., 1999, Soumelis and Liu, 2006) and increased expression of co-stimulatory molecules such as CD80/86 (Svensson et al., 1996, Cella et al., 2000, Bauer et al., 2001). When porcine pDCs stimulated for 24 h were examined for these changes, the majority of the ODN D19- and TGEV-treated cells were found to be of a more irregular shape and to have developed dendritic processes (Fig. 8A and C). In contrast, a morphological alteration of the pDCs left untreated was not noted while only a minor percentage of those incubated with PRRSV had transitioned. Similarly, the frequency of CD80/86 cells was greater in the ODN D19-activated (approximately 2-fold) and TGEV-activated (approximately 5-fold) samples as compared to the mock-treated example (Fig. 8B and D). In this case, detectable expression of these two surface proteins by individual pDCs was reduced when PRRSV was present and the resultant value was somewhat intermediate between the approximately 13% and 1% determined for cultured (Fig. 8D) and freshly purified cells (data not shown), respectively. It should be noted that up-regulation of CD80/86 expression in pDCs just maintained in medium is not unique to porcine cells as their human counterparts act in a similar manner (Ghanekar et al., 1996).
Fig. 8.
Porcine virus- or TLR agonist-mediated induction of the maturation of purified porcine pDCs. Purity-sorted porcine pDCs were cultured alone (Mock) or with ODN D19, PRRSV or TGEV for 24 h. (A) Light microscope (500× magnification) images of Diff-Quick® stained pDCs. (B) Histograms of CD80/86 stained pDCs that were either freshly purified (shaded areas) or previously incubated in the absence or presence of a virus or TLR9 agonist (solid line, clear areas). Horizontal bars define the limits of fluorescent intensity representing a positive signal. (C) Frequencies of pDCs exhibiting a DC morphology after incubation in the absence or presence of a virus or TLR9 agonist. Diff-Quick® stained pDCs having an irregular cell surface and dendrites were scored as having matured into DCs. (D) Frequencies of pDCs expressing CD80/86 after incubation in the absence or presence of a virus or TLR9 agonist. Values represent percentages of clear areas designated as being within the positive signal range in histograms of CD80/86 stained pDCs (B). A representative experiment (n = 3) is shown.
4. Discussion
We have ascertained that porcine CD4hiCD172lo leukocytes exhibited characteristics typical of pDCs in other mammalian species. For instance, our analysis of electronically gated pig CD4hiCD172lo cells revealed that, with the exception of CD16, their cell-surface phenotype was similar to that of human and murine pDCs (Liu, 2005) in that no B cell, monocyte, NK cell, or T cell lineage-specific markers were detected. Moreover, in comparison to three other gated PBMC subsets, members of this group were found to constitutively produce the greatest amounts of IRF-7 with the least degree of individual variation as previously observed for human (Izaguirre et al., 2003, Dai et al., 2004) and macaque (Chung et al., 2005) pDCs. Finally, by focusing on the FACS-purified porcine CD4hiCD172lo cells, we were able to demonstrate associated properties that were reminiscent of those ascribed to human pDCs (Grouard et al., 1997, Siegal et al., 1999, Cella et al., 2000, Liu, 2005, Fitzgerald-Bocarsly et al., 2008). These included: their prodigious ability to secrete IFN-α, their plasmacytoid appearance when quiescent, and their morphological conversion to DC-like shapes after exposure to viruses or TLR agonists with an accompanying transient or extended increase in IRF-7 and CD80/86 expression, respectively. Thus, based on these traits, we consider porcine CD4hiCD172lo cells to represent pDCs.
The detection of the αL integrin CD11a, the β1 integrin CD18, and the β2 integrin CD29 on the surface of porcine pDCs was not surprising since these types of molecules are involved in the recruitment of murine pDCs from blood into non-stimulated and inflamed lymph nodes via high endothelial venules (Yoneyama et al., 2004, Diacovo et al., 2005, Matsutani et al., 2007). In the pig, CD18 can interact with pDC-expressed CD11a to produce lymphocyte function-associated antigen (LFA)-1. The resultant heterodimer can bind to the intercellular adhesion molecule (ICAM)-1 expressed by activated endothelium at inflammatory sites (Dustin and Springer, 1988). Likewise, CD29 can form heterodimers, in this case with CD49d, to create the very late activation antigen (VLA)-4 that associates with CD44, also made by porcine pDCs, to enable leukocyte extravasation (Nandi et al., 2004) and trafficking of activated T cells to inflammatory regions (Steeber et al., 2005). In this regard, migration of TGEV-stimulated, pig NIPCs to lymphoid tissue (Riffault et al., 1997, Riffault et al., 2001, Pascale et al., 2008) and of non-activated cells to afferent lymph (Pascale et al., 2008) have been described.
As previously observed for human and macaque pDCs and porcine NIPCs, not every porcine pDC examined here produced IFN-α when cultured with a stimulant in vitro. For example, after exposure of human PBMC to the imidazoquinoline, resquimod, or to herpes simplex virus (HSV), only 31–36% (Gibson et al., 2002) or 25–60% (Feldman et al., 2001, Megjugorac et al., 2004), respectively, of the “gated” pDC cell subset contained detectable amounts of intracellular IFN-α. Although a wider variation in the frequencies of human and macaque pDCs responding to HSV was later reported, the maximum percentage did not exceed 80% or 82% for the two respective species (Chung et al., 2005). Similarly, 10–25% of porcine NIPCs (CD4+CD172lo PBMC subset) were identified as reacting to TGEV (Summerfield et al., 2003). Likewise, in the present study where an ELISPOT analysis was used in lieu of the previous multicolor immunofluorescence flow cytometry-based ones, 7–10% of the sorted porcine pDCs were found to have secreted IFN-α in response to TGEV stimulation. This lower frequency of responsive cells was probably not due to the isolation procedure negatively impacting functionality since concurrent exposure of the purified pDCs to TGEV and ODN D19 resulted in a cumulative increase in the number of IFN-α synthesizing cells (data not shown). Moreover, the majority (>70%) could still morph into DC after exposure to either TGEV or the TLR-9 agonist. Rather, this disparity may reflect differences in the spectrum of pDC function due to their relative proportions in the various stages of maturation (Duramad et al., 2003), the greater sensitivity of cytokine flow cytometry versus ELISPOT in detecting IFN-α production at the single cell level (Karlsson et al., 2003), and/or variance in the frequency of a pig's peripheral blood pDCs due to the animal's age and genetic factors (Nowacki et al., 1993).
When studying the biological and physical responses of PBMC subsets, relatively pure populations must be prepared so that features attributed to them do not reflect contributions by or interactions with other cell types. However, the traditional, magnetic bead-based procedures for isolation of porcine NIPCs may provide samples of insufficient homogeneity. In this regard, a FACS step was required to remove otherwise contaminating, myeloid dendritic cells (mDCs) from the human pDC subset before resolving their questionable, functional plasticity in regards to IFN-α and IL-12 production (Ito et al., 2006). Thus, our employment of novel sorting protocols for purifying members of the CD4hiCD172lo subset has circumvented a potentially similar problem regarding the characterization of activated and resting porcine pDCs.
With the exception of PRRSV, all other tested agents readily stimulated the purified porcine pDCs to produce IFN-α and TNF-α, two cytokines that promote the maturation of pDCs into DCs, and are typically made by human and murine pDCs (Liu, 2005, Fitzgerald-Bocarsly et al., 2008). Consequently, as observed, the majority of the ODN D19- and TGEV-treated cells underwent differentiation as indicated by morphological changes and the increased presence of cell-surface CD80/86 while the PRRSV-exposed cells remained relatively inert. Likewise, as with human pDCs, only the activated pDCs also secreted IL-6 which in conjunction with type I IFNs induces B cells to differentiate into Ab-secreting plasma cells (Jego et al., 2003, Poeck et al., 2004). Interestingly, the porcine pDCs universally responded to all of the viruses, including PRRSV, and both TLR agonists with secretion of IL-2. Although T cells primarily synthesize this cytokine, stimulated human pDCs can act in a similar manner and in doing so induce B, NK, and T cells to proliferate (Feau et al., 2005, Naranjo-Gomez et al., 2007). In contrast, synthesis of other cytokines appeared to be dependent on the type of eliciting agent. For instance, IL-8, a chemokine also produced by activated human pDCs and involved in the attraction of neutrophils to sites of inflammation (Penna et al., 2002, Bendriss-Vermare et al., 2005), was preferentially made by cells exposed to TGEV. Low quantities of IL-12 and IFN-γ were only secreted after induction by the TLR-7 and 9 ligands. IL-12 is also generated by human pDCs but only in very low amounts (Liu, 2005, Ito et al., 2006). Murine pDCs produce greater amounts of this cytokine (Asselin-Paturel et al., 2001) and an early developmental stage of these cells, the Ly6C-Ly49Q-subtype, secretes moderate amounts of IFN-γ (Vremec et al., 2007). Therefore, the observed cytokine response profiles of highly purified populations of porcine pDCs are consistent with those reported for other mammalian pDCs.
The inability of pDCs to produce IFN-α in response to PRRSV exposure could have ramifications that influence this virus's pathogenesis in the pig and extend to the current practice of immunizing with live virus. In this regard, it should be noted that both commercial vaccines and some swine farm-endemic viruses used for “controlled exposure” of naïve pigs have also been found to be incapable of eliciting IFN-α secretion by porcine pDCs (unpublished results). We have postulated (Meier et al., 2004, Royaee et al., 2004) that the absence of this innate immune response to infection or vaccination with PRRSV could be at least partly responsible for the belated production of specific virus-neutralizing antibodies (Lopez and Osorio, 2004) and the delayed onset and protracted development of a cell-mediated immune response of pigs against this virus (Meier et al., 2003). Accordingly, while type I cytokines are needed to incite the generation of virus-specific IFN-γ secreting T cells (Cella et al., 2000, Fitzgerald-Bocarsly et al., 2008), the quantity of this cytokine in the blood of PRRSV-infected pigs was conspicuously lower than that reported for animals receiving PrV or TGEV (Artursson et al., 1995, Albina et al., 1998b). Moreover, at the primary site of PRRSV replication, the lungs, IFN-α was either undetected or at a reduced amount in PRRSV-infected animals as compared to ones inoculated with porcine respiratory coronavirus, SIV, or TGEV (Albina et al., 1998b, VanReeth et al., 1999). Though at this location macrophages are the virus's primary target and as such would be considered to be a source of type I IFNs due to intracellular triggering by double-stranded, virus replicative intermediates, such cells exposed to PRRSV in vitro secreted undetectable or minor quantities of IFN-α (Albina et al., 1998a, Lee et al., 2004).
Although this is the first demonstration of the non-stimulatory nature of PRRSV towards porcine pDCs, this property is not unique to this pathogen. Other viruses have previously been shown to achieve the same outcome by not only suppressing the IFN-α response to themselves but also to other known stimulants. For instance, porcine circovirus type 2 can reduce porcine NIPC activity via interaction with its DNA genome (Vincent et al., 2007). A similar type of inhibition is directed to human pDCs by the V protein of measles virus (Pfaller and Conzelmann, 2008) and presumably the NS proteins of clinical isolates of respiratory syncytial virus (Schlender et al., 2005). Although a European strain of PRRSV did not exhibit such a repressive nature towards TGEV-activated NIPCs (Albina et al., 1998a), we have observed suppression of TGEV- and TLR9 agonist-induced production of IFN-α by purified porcine pDCs in the presence of either attenuated or virulent, North American PRRSV isolates (unpublished results). Therefore, like these other pathogens, PRRSV may render an afflicted host more susceptible to secondary infections due to blockage of the innate immune response provided by pDCs.
In closing, our data provide formal functional, morphological, and phenotypic evidence that the CD4hiCD172lo cell population found in swine represent the equivalent of pDCs in other species. Since the porcine pDCs were found to be nearly non-responsive to PRRSV but not to three other viruses or two TLR agonists, future studies will focus on understanding the mechanism(s) whereby PRRSV avoids inducing the typical activation of pDCs.
Acknowledgments
The authors would like to thank Mauricio Villamar for his excellent technical support and members of the University of Illinois Flow Cytometry Laboratory for their assistance with the initial FACS procedures. This study was supported by the National Research Initiative of the USDA Cooperative State Research, Education and Extension Service (CSREES) Coordinated Agricultural Project (CAP) number Q6706392373/2004-35605-14197, and by the National Research Initiative, Competitive Grants Program Project number 2004-35204-14954.
References
- Albina E., Carrat C., Charley B. Interferon-alpha response to swine arterivirus (PoAV), the porcine reproductive and respiratory syndrome virus. J. Interferon Cytokine Res. 1998;18:485–490. doi: 10.1089/jir.1998.18.485. [DOI] [PubMed] [Google Scholar]
- Albina E., Piriou L., Hutet E., Cariolet R., L’Hospitalier R. Immune responses in pigs infected with porcine reproductive and respiratory syndrome virus (PRRSV) Vet. Immunol. Immunopathol. 1998;61:49–66. doi: 10.1016/S0165-2427(97)00134-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez B., Domenech N., Alonso F., Sanchez C., Gomez del Moral M., Ezquerra A., Dominguez J. Molecular and functional characterization of porcine LFA-1 using monoclonal antibodies to CD11a and CD18. Xenotransplantation. 2000;7:258–266. doi: 10.1034/j.1399-3089.2000.00574.x. [DOI] [PubMed] [Google Scholar]
- Artursson K., Lindersson M., Varela N., Scheynius A., Alm G.V. Interferon-alpha production and tissue localization of interferon-alpha/beta producing cells after intradermal administration of Aujeszky's disease virus-infected cells in pigs. Scan. J. Immunol. 1995;41:121–129. doi: 10.1111/j.1365-3083.1995.tb03543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselin-Paturel C., Boonstra A., Dalod M., Durand I., Yessaad N., Dezutter-Dambuyant C., Vicari A., O’Garra A., Biron C., Briere F., Trinchieri G. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat. Immunol. 2001;2:1144–1150. doi: 10.1038/ni736. [DOI] [PubMed] [Google Scholar]
- Balmelli C., Vincent I.E., Rau H., Guzylack-Piriou L., McCullough K., Summerfield A. Fc gamma RII-dependent sensitisation of natural interferon-producing cells for viral infection and interferon-alpha responses. Eur. J. Immunol. 2005;35:2406–2415. doi: 10.1002/eji.200525998. [DOI] [PubMed] [Google Scholar]
- Bauer M., Redecke V., Ellwart J.W., Scherer B., Kremer J.P., Wagner H., Lipford G.B. Bacterial CpG-DNA triggers activation and maturation of human CD11c−CD123+ dendritic cells. J. Immunol. 2001;166:5000–5007. doi: 10.4049/jimmunol.166.8.5000. [DOI] [PubMed] [Google Scholar]
- Bendriss-Vermare N., Burg S., Kanzler H., Chaperot L., Duhen T., de Bouteiller O., D’agostini M., Bridon J.M., Durand I., Sederstrom J.M., Chen W., Plumas J., Jacob M.C., Liu Y.J., Garrone P., Trinchieri G., Caux C., Briere F. Virus overrides the propensity of human CD40L-activated plasmacytoid dendritic cells to produce Th2 mediators through synergistic induction of IFN-gamma and Th1 chemokine production. J. Leukoc. Biol. 2005;78:954–966. doi: 10.1189/jlb.0704383. [DOI] [PubMed] [Google Scholar]
- Cella M., Facchetti F., Lanzavecchia A., Colonna M. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent Th1 polarization. Nat. Immunol. 2000;1:305–310. doi: 10.1038/79747. [DOI] [PubMed] [Google Scholar]
- Cella M., Jarrossay D., Facchetti F., Alebardi O., Nakajima H., Lanzavecchia A., Colonna M. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat. Med. 1999;5:919–923. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- Cervantes-Barragan L., Zust R., Weber F., Spiegel M., Lang K.S., Akira S., Thiel V., Ludewig B. Control of coronavirus infection through plasmacytoid dendritic-cell-derived type I interferon. Blood. 2007;109:1131–1137. doi: 10.1182/blood-2006-05-023770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charley B., Lavenant L. Characterization of blood mononuclear cells producing IFN alpha following induction by coronavirus-infected cells (porcine transmissible gastroenteritis virus) Res. Immunol. 1990;141:141–151. doi: 10.1016/0923-2494(90)90133-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung E., Amrute S.B., Abel K., Gupta G., Wang Y., Miller C.J., Fitzgerald-Bocarsly P. Characterization of virus-responsive plasmacytoid dendritic cells in the rhesus macaque. Clin. Diagn. Lab. Immunol. 2005;12:426–435. doi: 10.1128/CDLI.12.3.426-435.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia E.M., Severa M., Giacomini E., Monneron D., Remoli M.E., Julkunen I., Cella M., Lande R., Uze G. Viral infection and Toll-like receptor agonists induce a differential expression of type I and lambda interferons in human plasmacytoid and monocyte-derived dendritic cells. Eur. J. Immunol. 2004;34:796–805. doi: 10.1002/eji.200324610. [DOI] [PubMed] [Google Scholar]
- Dai J., Megjugorac N.J., Amrute S.B., Fitzgerald-Bocarsly P. Regulation of IFN regulatory factor-7 and IFN-alpha production by enveloped virus and lipopolysaccharide in human plasmacytoid dendritic cells. J. Immunol. 2004;173:1535–1548. doi: 10.4049/jimmunol.173.3.1535. [DOI] [PubMed] [Google Scholar]
- Diacovo T.G., Blasius A.L., Mak T.W., Cella M., Colonna M. Adhesive mechanisms governing interferon-producing cell recruitment into lymph nodes. J. Exp. Med. 2005;202:687–696. doi: 10.1084/jem.20051035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domeika K., Magnusson M., Eloranta M.L., Fuxler L., Alm G.V., Fossum C. Characteristics of oligodeoxyribonucleotides that induce interferon (IFN)-alpha in the pig and the phenotype of the IFN-alpha producing cells. Vet. Immunol. Immunopathol. 2004;101:87–102. doi: 10.1016/j.vetimm.2004.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duramad O., Fearon K.L., Chan J.H., Kanzler H., Marshall J.D., Coffman R.L., Barrat F.J. IL-10 regulates plasmacytoid dendritic cell response to CpG-containing immunostimulatory sequences. Blood. 2003;102:4487–4492. doi: 10.1182/blood-2003-07-2465. [DOI] [PubMed] [Google Scholar]
- Dustin M.L., Springer T.A. Lymphocyte function-associated antigen-1 (LFA-1) interaction with intercellular adhesion molecule-1 (ICAM-1) is one of at least three mechanisms for lymphocyte adhesion to cultured endothelial cells. J. Cell Biol. 1988;107:321–331. doi: 10.1083/jcb.107.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feau S., Facchinetti V., Granucci F., Citterio S., Jarrossay D., Seresini S., Protti M.P., Lanzavecchia A., Ricciardi-Castagnoli P. Dendritic cell-derived IL-2 production is regulated by IL-15 in humans and in mice. Blood. 2005;105:697–702. doi: 10.1182/blood-2004-03-1059. [DOI] [PubMed] [Google Scholar]
- Feldman S., Stein D., Amrute S., Denny T., Garcia Z., Kloser P., Sun Y., Megjugorac N., Fitzgerald-Bocarsly P. Decreased interferon-α production in HIV-infected patients correlates with numerical and functional deficiencies in circulating type 2 dendritic cell precursors. Clin. Immunol. 2001;101:201–210. doi: 10.1006/clim.2001.5111. [DOI] [PubMed] [Google Scholar]
- Fitzgerald-Bocarsly P., Dai J., Singh S. Plasmacytoid dendritic cells and type I IFN: 50 years of convergent history. Cytokine Growth Factor Rev. 2008;19:3–19. doi: 10.1016/j.cytogfr.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanekar S., Zheng L., Logar A., Navratil J., Borowski L., Gupta P., Rinaldo C. Cytokine expression by human peripheral blood dendritic cells stimulated in vitro with HIV-1 and herpes simplex virus. J. Immunol. 1996;157:4028–4036. [PubMed] [Google Scholar]
- Gibson S.J., Lindh J.M., Riter T.R., Gleason R.M., Rogers L.M., Fuller A.E., Oesterich J.L., Gorden K.B., Qiu X., McKane S.W., Noelle R.J., Miller R.L., Kedl R.M., Fitzgerald-Bocarsly P., Tomai M.A., Vasilakos J.P. Plasmacytoid dendritic cells produce cytokines and mature in response to the TLR7 agonists, imiquimod and resiquimod. Cell. Immunol. 2002;218:74–86. doi: 10.1016/s0008-8749(02)00517-8. [DOI] [PubMed] [Google Scholar]
- Gilliet M., Cao W., Liu Y.J. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat. Rev. Immunol. 2008;8:594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- Grouard G., Rissoan M.C., Filgueira L., Durand I., Banchereau J., Liu Y.J. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J. Exp. Med. 1997;185:1101–1111. doi: 10.1084/jem.185.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzylack-Piriou L., Balmelli C., McCullough K.C., Summerfield A. Type-A CpG oligonucleotides activate exclusively porcine natural interferon-producing cells to secrete interferon-alpha, tumor necrosis factor-alpha and interleukin-12. Immunology. 2004;112:28–37. doi: 10.1111/j.1365-2567.2004.01856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzylack-Piriou L., Bergamin F., Gerber M., McCullough K.C., Summerfield A. Plasmacytoid dendritic cell activation by foot-and-mouth disease virus requires immune complexes. Eur. J. Immunol. 2006;36:1674–1683. doi: 10.1002/eji.200635866. [DOI] [PubMed] [Google Scholar]
- Haverson K., Saalmuller A., Alvarez B., Alonso F., Bailey M., Bianchi A.T., Boersma W.J., Chen Z., Davis W.C., Dominguez J., Engelhardt H., Ezquerra A., Grosmaire L.S., Hamilton M.J., Hollemweguer E., Huang C.A., Khanna K.V., Kuebart G., Lackovic G., Ledbetter J.A., Lee R., Llanes D., Lunney J.K., McCullough K.C., Molitor T., Nielsen J., Niewold T.A., Pescovitz M.D., de la Lastra J.M., Rehakova Z., Salmon H., Schnitzlein W.M., Seebach J., Simon A., Sinkora J., Sinkora M., Stokes C.R., Summerfield A., Sver L., Thacker E., Valpotic I., Yang H., Zuckermann F.A., Zwart R. Overview of the Third international workshop on swine leukocyte differentiation antigens. Vet. Immunol. Immunopathol. 2001;80:5–23. doi: 10.1016/s0165-2427(01)00290-2. [DOI] [PubMed] [Google Scholar]
- Hemmi H., Kaisho T., Takeuchi O., Sato S., Sanjo H., Hoshino K., Horiuchi T., Tomizawa H., Takeda K., Akira S. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat. Immunol. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- Honda K., Yanai H., Negishi H., Asagiri M., Sato M., Mizutani T., Shimada N., Ohba Y., Takaoka A., Yoshida N., Taniguchi T. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434:772–777. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- Ito T., Yang M., Wang Y.H., Lande R., Gregorio J., Perng O.A., Qin X.F., Liu Y.J., Gilliet M. Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J. Exp. Med. 2007;204:105–115. doi: 10.1084/jem.20061660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T., Kanzler H., Duramad O., Cao W., Liu Y. Specialization, kinetics, and repertoire of type 1 interferon responses by human plasmacytoid predendritic cells. Blood. 2006;107:2423–2431. doi: 10.1182/blood-2005-07-2709. [DOI] [PubMed] [Google Scholar]
- Izaguirre A., Barnes B.J., Amrute S., Yeow W.S., Megjugorac N., Dai J., Feng D., Chung E., Pitha P.M., Fitzgerald-Bocarsly P. Comparative analysis of IRF and IFN-alpha expression in human plasmacytoid and monocyte-derived dendritic cells. J. Leukoc. Biol. 2003;74:1125–1138. doi: 10.1189/jlb.0603255. [DOI] [PubMed] [Google Scholar]
- Jego G., Palucka A.K., Blanck J.P., Chalouni C., Pascual V., Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19:225–234. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- Kamstrup S., Verthelyi D., Klinman D.M. Response of porcine peripheral blood mononuclear cells to CpG-containing oligodeoxynucleotides. Vet. Microbiol. 2001;78:353–362. doi: 10.1016/s0378-1135(00)00300-x. [DOI] [PubMed] [Google Scholar]
- Karlsson A.C., Martin J.N., Younger S.R., Bredt B.M., Epling L., Ronquillo R., Varma A., Deeks S.G., McCune J.M., Nixon D.F., Sinclair E. Comparison of the ELISPOT and cytokine flow cytometry assays for the enumeration of antigen-specific T cells. J. Immunol. Methods. 2003;283:141–153. doi: 10.1016/j.jim.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Kawai T., Sato S., Ishii K.J., Coban C., Hemmi H., Yamamoto M., Terai K., Matsuda M., Inoue J., Uematsu S., Takeuchi O., Akira S. Interferon-alpha induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat. Immunol. 2004;5:1061–1068. doi: 10.1038/ni1118. [DOI] [PubMed] [Google Scholar]
- Lee S.M., Schommer S.K., Kleiboeker S.B. Porcine reproductive and respiratory syndrome virus field isolates differ in in vitro interferon phenotypes. Vet. Immunol. Immunopathol. 2004;102:217–231. doi: 10.1016/j.vetimm.2004.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.J. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu. Rev. Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- Lopez O.J., Osorio F.A. Role of neutralizing antibodies in PRRSV protective immunity. Vet. Immunol. Immunopathol. 2004;102:155–163. doi: 10.1016/j.vetimm.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Matsutani T., Tanaka T., Tohya K., Otani K., Jang M.H., Umemoto E., Taniguchi K., Hayasaka H., Ueda K., Miyasaka M. Plasmacytoid dendritic cells employ multiple cell adhesion molecules sequentially to interact with high endothelial venule cells molecular basis of their trafficking to lymph nodes. Int. Immunol. 2007;19:1031–1037. doi: 10.1093/intimm/dxm088. [DOI] [PubMed] [Google Scholar]
- McKenna K., Beignon A.S., Bhardwaj N. Plasmacytoid dendritic cells: linking innate and adaptive immunity. J. Virol. 2005;79:17–27. doi: 10.1128/JVI.79.1.17-27.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megjugorac N.J., Young H.A., Amrute S.B., Olshalsky S.L., Fitzgerald-Bocarsly P. Virally stimulated plasmacytoid dendritic cells produce chemokines and induce migration of T and NK cells. J. Leukoc. Biol. 2004;75:504–514. doi: 10.1189/jlb.0603291. [DOI] [PubMed] [Google Scholar]
- Meier W.A., Galeota J., Osorio F.A., Husmann R.J., Schnitzlein W.M., Zuckermann F.A. Gradual development of the interferon-gamma response of swine to porcine reproductive and respiratory syndrome virus infection or vaccination. Virology. 2003;309:18–31. doi: 10.1016/s0042-6822(03)00009-6. [DOI] [PubMed] [Google Scholar]
- Meier W.A., Husmann R.J., Schnitzlein W.M., Osorio F.A., Lunney J.K., Zuckermann F.A. Cytokines and synthetic double-stranded RNA augment the T helper 1 immune response of swine to porcine reproductive and respiratory syndrome virus. Vet. Immunol. Immunopathol. 2004;102:299–314. doi: 10.1016/j.vetimm.2004.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi A., Estess P., Siegelman M. Bimolecular complex between rolling and firm adhesion receptors required for cell arrest; CD44 association with VLA-4 in T cell extravasation. Immunity. 2004;20:455–465. doi: 10.1016/s1074-7613(04)00077-9. [DOI] [PubMed] [Google Scholar]
- Naranjo-Gomez M., Oliva H., Climent N., Fernandez M.A., Ruiz-Riol M., Bofill M., Gatell J.M., Gallart T., Pujol-Borrell R., Borras F.E. Expression and function of the IL-2 receptor in activated human plasmacytoid dendritic cells. Eur. J. Immunol. 2007;37:1764–1772. doi: 10.1002/eji.200636980. [DOI] [PubMed] [Google Scholar]
- Nowacki W., Cederblad B., Renard C., La Bonnardiere C., Charley B. Age-related increase of porcine natural interferon alpha producing cell frequency and of interferon yield per cell. Vet. Immunol. Immunopathol. 1993;37:113–122. doi: 10.1016/0165-2427(93)90059-D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowacki W., Charley B. Enrichment of coronavirus-induced interferon-producing blood leukocytes increases the interferon yield per cell: a study with pig leukocytes. Res. Immunol. 1993;144:111–120. doi: 10.1016/0923-2494(93)80066-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascale F., Contreras V., Bonneau M., Courbet A., Chilmonczyk S., Bevilacqua C., Epardaud M., Niborski V., Riffault S., Balazuc A.M., Foulon E., Guzylack-Piriou L., Riteau B., Hope J., Bertho N., Charley B., Schwartz-Cornil I. Plasmacytoid dendritic cells migrate in afferent skin lymph. J. Immunol. 2008;180:5963–5972. doi: 10.4049/jimmunol.180.9.5963. [DOI] [PubMed] [Google Scholar]
- Penna G., Vulcano M., Roncari A., Facchetti F., Sozzani S., Adorini L. Cutting edge: differential chemokine production by myeloid and plasmacytoid dendritic cells. J. Immunol. 2002;169:6673–6676. doi: 10.4049/jimmunol.169.12.6673. [DOI] [PubMed] [Google Scholar]
- Perussia B., Fanning V., Trinchieri G. A leukocyte subset bearing HLA-DR antigens is responsible for in vitro alpha interferon production in response to viruses. Nat. Immun. Cell Growth Regul. 1985;4:120–137. [PubMed] [Google Scholar]
- Pfaller C.K., Conzelmann K.K. Measles virus V protein is a decoy substrate for IkappaB kinase alpha and prevents Toll-like receptors 7/9-mediated interferon induction. J. Virol. 2008;82:12365–12373. doi: 10.1128/JVI.01321-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeck H., Wagner M., Battiany J., Rothenfusser S., Wellisch D., Hornung V., Jahrsdorfer B., Giese T., Endres S., Hartmann G. Plasmacytoid dendritic cells, antigen, and CpG-C license human b cells for plasma cell differentiation and immunoglobulin production in the absence of T-cell help. Blood. 2004;103:3058–3064. doi: 10.1182/blood-2003-08-2972. [DOI] [PubMed] [Google Scholar]
- Riffault S., Carrat C., Besnardeau L., La Bonnardiere C., Charley B. In vivo induction of interferon-alpha in pig by non-infectious coronavirus: tissue localization and in situ phenotypic characterization of interferon-alpha-producing cells. J. Gen. Virol. 1997;78(Pt 10):2483–2487. doi: 10.1099/0022-1317-78-10-2483. [DOI] [PubMed] [Google Scholar]
- Riffault S., Carrat C., van Reeth K., Pensaert M., Charley B. Interferon-alpha-producing cells are localized in gut-associated lymphoid tissues in transmissible gastroenteritis virus (TGEV) infected piglets. Vet. Res. 2001;32:71–79. doi: 10.1051/vetres:2001111. [DOI] [PubMed] [Google Scholar]
- Royaee A.R., Husmann R.J., Dawson H.D., Calzada-Nova G., Schnitzlein W.M., Zuckermann F.A., Lunney J.K. Deciphering the involvement of innate immune factors in the development of the host response to PRRSV vaccination. Vet. Immunol. Immunopathol. 2004;102:199–216. doi: 10.1016/j.vetimm.2004.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlender J., Hornung V., Finke S., Gunthner-Biller M., Marozin S., Brotzka K., Moghim S., Endres S., Hatmann G., Conzelmann K.K. Inhibition of Toll-like receptor 7- and 9-mediated alpha/beta interferon production in human plasmacytoid dendritc cells by respiratory syncitial virus and measles virus. J. Virol. 2005;79:5507–5515. doi: 10.1128/JVI.79.9.5507-5515.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegal F.P., Kadowaki N., Shodell M., Fitzgerald-Bocarsly P.A., Shah K., Ho S., Antonenko S., Liu Y.J. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- Soumelis V., Liu Y.J. From plasmacytoid to dendritic cell: morphological and functional switches during plasmacytoid pre-dendritic cell differentiation. Eur. J. Immunol. 2006;36:2286–2292. doi: 10.1002/eji.200636026. [DOI] [PubMed] [Google Scholar]
- Steeber D.A., Venturi G.M., Tedder T.F. A new twist to the leukocyte adhesion cascade: intimate cooperation is key. Trends Immunol. 2005;26:9–12. doi: 10.1016/j.it.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Summerfield A., Guzylack-Piriou L., Schaub A., Carrasco C.P., Tache V., Charley B., McCullough K.C. Porcine peripheral blood dendritic cells and natural interferon-producing cells. Immunology. 2003;110:440–449. doi: 10.1111/j.1365-2567.2003.01755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfield A., McCullough K.C. The porcine dendritic cell family. Dev. Comp. Immunol. 2009;33:299–309. doi: 10.1016/j.dci.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson H., Johannisson A., Nikkila T., Alm G.V., Cederblad B. The cell surface phenotype of human natural interferon-alpha producing cells as determined by flow cytometry. Scand. J. Immunol. 1996;44:164–172. doi: 10.1046/j.1365-3083.1996.d01-289.x. [DOI] [PubMed] [Google Scholar]
- VanReeth K., Labarque G., Nauwynck H., Pensaert M. Differential production of proinflammatory cytokines in the pig lung during different respiratory virus infections: correlations with pathogenicity. Res. Vet. Sci. 1999;67:47–52. doi: 10.1053/rvsc.1998.0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent I.E., Balmelli C., Meehan B., Allan G., Summerfield A., McCullough K.C. Silencing of natural interferon producing cell activation by porcine circovirus type 2 DNA. Immunology. 2007;120:47–56. doi: 10.1111/j.1365-2567.2006.02476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vremec D., O’Keeffe M., Hochrein H., Fuchsberger M., Caminschi I., Lahoud M., Shortman K. Production of interferons by dendritic cells, plasmacytoid cells, natural killer cells, and interferon-producing killer dendritic cells. Blood. 2007;109:1165–1173. doi: 10.1182/blood-2006-05-015354. [DOI] [PubMed] [Google Scholar]
- Yoneyama H., Matsuno K., Zhang Y., Nishiwaki T., Kitabatake M., Ueha S., Narumi S., Morikawa S., Ezaki T., Lu B., Gerard C., Ishikawa S., Matsushima K. Evidence for recruitment of plasmacytoid dendritic cell precursors to inflamed lymph nodes through high endothelial venules. Int. Immunol. 2004;16:915–928. doi: 10.1093/intimm/dxh093. [DOI] [PubMed] [Google Scholar]