Abstract
Because of its similarity to ageing in impaired immune efficiency 48 h after surgical procedures on young partially hepatectomised mice, partial hepatectomy/liver regeneration (pHx) provides a good model for the study of inflammation in ageing. In old age, high metallothionein (I+II) (MT) sequesters a substantial number of intracellular zinc ions consequently leading to low zinc ion bioavailability for an adequate immune response. Corticosterone and IL-6 affect MTmRNA induction in inflammation and after pHx against oxidative damage. The aim of this study was to investigate the role played by MT in conferring immune plasticity in ageing and in very old age using the pHx model. 48 h after their partial hepatectomy, the crude zinc balance was negative in young, old and very old mice coupled with increased MT, corticosterone, sIL-6R and IL-6. Concomitantly, Natural Killer (NK) cell activity and IL-2 production decreased. Complete restoration of the nutritional–endocrine–immune parameters occurred 15 days from the surgical procedures in young and very old mice, but not in old or transgenic mice overexpressing MT. A significant positive or inverse correlation among nutritional–endocrine–immune parameters exists in young and very old mice, but not in old mice during liver regeneration. Since MT also affects c-myc, the gene expression of c-myc declines from 48 h to days 7 and 15 after pHx in young and very old mice, but remains constantly high in old pHx mice for the same days. This circumstance leads to the appearance of tumours in the long run in old pHx mice and survival times that are shorter than old sham controls. Because complete remodelling also occurs in IL-6 and in sIL-6R in very old mice during liver regeneration, the pre-existing inflammation is not detrimental in very old age. As such, very old mice are still responsive to large inflammation, such as pHx, thanks to correct MT homeostasis. Correct MT homeostasis, via c-myc, is therefore pivotal in both suitable liver regeneration and in conferring immune plasticity with subsequent successful ageing. High MT plays an extremely harmful role in ageing: on one hand it lowers zinc ion bioavailability levels required for immune efficiency and on the other hand it increases c-myc expression. The combination of immune depression and enhanced c-myc, via high MT, may trigger the appearance of age-related degenerative diseases.
Keywords: IL-6, sIL-6R, NK cell activity, IL-2, Cancer, Successful ageing
1. Introduction
Healthy centenarians differ from “normal” aged individuals because of their optimal metabolic compensation and immune response as well as their ability to efficiently counter the alteration of the oxidative status typical of ageing (Franceschi et al., 1995). Though the molecular basis underpinning this exception has yet to be fully elucidated. A special asset of zinc-bound metallothionein (Zn–MT) (I+II) is known to play a central role both in zinc-related cell homeostasis during oxidative stress, inflammation and in immune response (Mocchegiani et al., 2000). Partial hepatectomy/liver regeneration (pHx) is a good model for the study of acute and constant inflammation in ageing because of its similarity to ageing and inflammation in impaired thymic endocrine activity and peripheral immune efficiency [Natural Killer (NK) cell activity and IL-2 production] (Mocchegiani et al., 1997) as well as in enhanced corticosterone (Shimada et al., 1996) and pro-inflammatory cytokines (IL-6 and TNF-alpha) (Kelley-Loughnane et al., 2002) in young pHx mice 48 h after partial hepatectomy. These altered immune and hormonal parameters are also a characteristic of inflammation and ageing (Mocchegiani et al., 2000). Therefore, the model of partial hepatectomy is useful for the study of inflammation in ageing, other than liver regeneration.
Many growth factors are involved in liver regeneration after pHx. IL-6 and corticosterone are co-mitogens during liver regeneration for hepatocytes crossing from the G0 to the G1 phase (Hoffman et al., 1994, Michalopoulos and DeFrances, 1997). However, without the presence of soluble IL-6 receptor (sIL-6R), IL-6 alone cannot provide the degree of stimulus necessary to promote hepatocyte proliferation (Maione et al., 1998). Moreover, IL-6 increases during inflammation for a prompt immune response (Buunsgaard et al., 2001), and some proto-oncogenes, such as c-myc, are involved from the first early phases in liver regeneration after pHx (Moser et al., 2001). In this context, Zn–MT plays a key role for four reasons: first, the MT gene expression is induced by corticosterone and IL-6 in inflammation (Andrews, 2000) and during liver regeneration (Tohyama et al., 1993) against oxidative damage; second MT affects the c-myc gene expression (Tohyama et al., 1993); third, in order to accomplish these tasks, MT sequesters intracellular zinc ions (Kagi and Shaffer, 1988), which are pivotal for immune efficiency including NK cell activity and IL-2 production (Mocchegiani et al., 1998); fourth, Zn–MT does not release zinc under constant stress, such as in ageing (Mocchegiani et al., 2002a), causing low free zinc ion bioavailability which immune efficiency and antioxidant activity depend on (Mocchegiani et al., 2000). Thus, the continuous sequestering of intracellular zinc by MT under conditions of constant stress may be harmful and offset the beneficial effects arising from transient stress, as can occur in young-adult mice (Kelly et al., 1996). Zn–MT, zinc ion bioavailability and immune performances undergo remodelling during liver regeneration in young but not in old pHx mice, suggesting that Zn–MT may be involved in affecting immune plasticity during inflammation (Mocchegiani et al., 1998). Such a remodelling is also due to a correct liver regeneration, via c-myc (Hoffman et al., 1994). No c-myc data exist in old pHx mice. The aim of this study was to investigate, through the pHx model, the role played by Zn–MT (I+II) in liver regeneration and in conferring immune plasticity, which is indispensable to successful ageing (Mocchegiani et al., 2002b). Young, old, very old and transgenic mice overexpressing MT (MT-I*) were used. We included the latter group of mice as their zinc ion bioavailability and immune response, both under normal conditions and the constant stress of being deprived of light for 10 days, are similar to that of old mice (Mocchegiani et al., 2002a).
2. Material and methods
2.1. Animals
We used ten 2–3-month-old (young age) Balb/c inbred male mice, ten 20-month-old mice (old age) and ten 28–30-month-old (very old age) mice. The mice were housed in non-galvanised plastic cages (five to six mice per cage) and fed with standard pellet food (Nossan, Italy) and tap water ad libitum. Under our housing conditions, the life-span was of 30 months (Mocchegiani et al., 1998). Since there was an approximate 50% survival rate of 20 months, mice of this age were considered “old” (Mocchegiani et al., 1998). Ten young transgenic male mice over-expressing MT (MT-I*) (Jackson Lab., Bar Harbor, ME, USA) were also used. C57BL/6J mice were used as controls of MT-I* mice. The animals were maintained on a 12-h light:12-h dark cycle from 07:00 to 19:00 h at a constant temperature (20±1 °C) and a constant level of humidity (50±5%). Even though intrinsic genetic variability is almost absent in inbred Balb/c mice, environmental factors, such as stress, caused by conventional in-house breeding condition nevertheless produced genetic variability (Wesselkamper et al., 2000). Indeed because environmental factor-induced variability is also fundamental in man (Houlston and Tomlinson, 1998), the data yielded by our very old inbred mice may be comparable and reflect successful ageing in human.
2.2. Partial hepatectomy (pHx)
Young, old and very old Balb/c mice were partially hepatectomised (pHx) under ether anaesthesia by aseptic extirpation of the median lobe. To avoid diurnal variability, all operations were performed between 08:00 and 09:00 h (Mocchegiani et al., 1997). The animals were killed at 48 h and on day 15 following their hepatectomy using ether (ten young, ten old and ten very old animals for each time interval considered). Ten young sham, ten old sham, and ten very old sham mice were used as controls at time 0. Heparinised blood samples were collected by cardiac puncture for cytokines and corticosterone determinations in the plasma. The spleen was removed and teased for testing NK cell cytotoxicity. The liver was removed and frozen in liquid nitrogen for MTmRNA determination. Since no differences were found between sham-operated and control mice (Mocchegiani et al., 1997), sham-control mice (as time 0) were herein used. Moreover, no differences existed between inbreed Balb/c and C57BL/6J mice in nutritional–immune–endocrine response (Mocchegiani et al., 2002a).
2.3. Survival and histological analysis
For survival analysis (Kaplan–Meier), 30 mice (20 months old) were partially hepatecomised. 30 old sham mice of the same age were used as controls. The mice were censored every 3 days. Because the maximum life span of mice in our breeding conditions is of 30 months, 20 months are considered as “old age” and 30 mice are sufficient for survival analysis from this age (Mocchegiani et al., 1998). Another two groups of 20 old pHx and 20 old sham mice were used for histological analysis in order to determine physio/pathological conditions which are in turn necessary to reflect survival analysis and causes of death. For this purpose, all the mice belonging to the latter two groups were killed 1 month after their partial hepatectomy (i.e. at 21 months of age) as this is a sufficient time in which to show definitive hepatocytes differentiation and regeneration after a partial hepatectomy (Gordon et al., 2000). Immediately after euthanasia necropsies were performed and tissue specimens, from organs of the splanchnic cavities (thorax, abdomen), were collected and fixed in a phosphate formalin buffer pH 7.4. Tissues were paraffin wax embedded (56–58 °C) and microtome sections 5 μm thick were cut for routine histological staining.
Before surgical procedures, the health status of the mice was monitored with bacteriological analysis at bronchoalveolar and gastrointestinal levels using standard laboratory methods. Serological analysis (Sendai virus, mouse hepatitis virus, mycoplasma pulmonis and corona virus) (ELISA Kits) following FELASA guidelines (FELASA, 1994), was carried out (INRCA Veterinary Service).
2.4. Nutritional zinc assessment
For acclimatisation purposes, the animals were housed in non-galvanised metabolic cages (Techniplast, Italy) for a period of 7 days prior to their partial hepatectomy. The crude zinc balance was performed in ten operated mice (young, old and very old) for each of the time interval considered (48 h and 15 days) from surgical procedures. Faecal weight was determined in humid faeces. Zinc present in food, water, urine and faeces was measured for each animal every day. Crude zinc balance is the difference between zinc uptake and zinc loss and indirectly represents the amount of zinc in the body (Turnlund et al., 1986).
2.5. Zinc determination
Zinc determination in urine was performed in AAS on 24 h urine collected in non-galvanised metabolic cages and zinc content in faeces, food and water was carried out by AAS using methods extensively described elsewhere (Mocchegiani et al., 1997).
2.6. NK cell assay
NK splenocytes activity, as described elsewhere (Mocchegiani et al., 1997), was measured using YAC-1 lymphoma cell line as a target. 1×105 ml−1 target cells and 2×106 ml−1 liver effector lymphocytes were used. 100 μCi of 51Cr was used as a marker for NK lysis. The data were expressed in Lytic Unit 20/107 cells.
2.7. IL-2, IL-6, sIL-6R and corticosterone plasma determination
Plasma interleukin IL-2 and IL-6 levels were measured using ELISA Kits (Endogen, USA). The data were expressed in pg ml−1. The sensitivity of the kit was ≤7 pg ml−1 for IL-6 and ≤3 pg ml−1 for IL-2. Plasma (diluted 1:40) sIL-6R was tested using sIL-6R Quantikine ELISA kit (R&D Systems, Minneapolis, USA). The data were expressed in ng ml−1. The sensitivity of the kit was ≤140 pg ml−1. Plasma corticosterone levels were determined by RIA rat-corticosterone-3H kit (ICN, USA). The data were expressed in ng ml−1. The percentage of cross-reaction with other steroids was <0.01. The sensitivity of the kit was of 0.05 ng ml−1.
2.8. RT-PCR analysis
Total RNA was extracted from frozen liver using Tri-Reagent according to the manufacture's instructions (Sigma, USA). 3 μg of total RNA was reverse transcribed using a reaction mixture containing 50 mM Tris–HCl (pH 8.3), 75 mM KCl, 3 mM MgCl2, 10 mM DTT, 150 ng oligo dT, 20 units of RNAse inhibitor, 0.5 mM deoxynucleotide triphosphates and 200 units M-MLV reverse transcriptase (Sigma). PCRs were performed using sense and antisense primers as follows: MT-I: 5′-ATGGACCCCAACTGCTCCTGCTCCACC-3′, 5′-GGGTGGAACTGTATAGGAAGACGCTGG-3′, c-myc: 5′-AAGCTGGTCTCGGAGAAGCTG-3′, 5′-GGTTTGCCTCTTCTCCACAGA-3′, β-actin: 5′-GGACTCCTATGTGGGTGACGAGG-3′, 5′-GGGAGAGCATAGCCCTCGTAGAT-3′. Conditions for amplification were as follows: for MT-I each cycle consisted of 94 °C, 0.30 min; 50 °C, 0.30 min; 72 °C, 0.30 min for 30 cycles; for c-myc each cycle consisted of 94 °C, 1 min; 54 °C, 1 min; 72 °C, 1 min for 35 cycles; for β-actin each cycle consisted of 94 °C, 1 min; 61 °C, 1 min; 72 °C, 1 min for 30 cycles. The products of the RT-PCR reactions were size-fractionated by 2% agars gel electrophoresis and visualised by staining with ethidium bromide. The data are also expressed as a relative unit determined by normalisation of the density of MT-1 or c-myc band to that of β-actin band, as suggested by Okuda et al. (1995).
2.9. Statistical analysis
The differences between means were assessed using paired Student's t-test and one-way ANOVA test. χ 2-test was used in Table 2, Table 3. For survival analysis, the Kaplan–Meir test was used and the significance was Log-rank tested. Correlations were determined by linear regression analysis using the least square method. The differences between various regression lines were evaluated by analysis of covariance. Differences were considered to be significant when P<0.05.
Table 2.
Physio/pathological condition in old pHx mice after 1 month from pHx in comparison with old sham controls
| Physio/pathological condition | Frequency (%) | |
|---|---|---|
| Old pHx mice | Glomerulonephritis | 5 |
| Carcinoma | 15** | |
| Hyperplasia of BALT complex | 30* | |
| Carcinoma+glomerulonephritis | 0 | |
| Carcinoma+hyperplasia of BALT complex | 45* | |
| Glomerulonephritis+hyperplasia of BALT complex | 5 | |
| Glomerulonephritis+hyperplasia of BALT complex+carcinoma | 0 | |
| Health status | 0 | |
| Old sham mice | Glomerulonephritis | 5 |
| Carcinoma | 0 | |
| Hyperplasia of BALT complex | 45+ | |
| Carcinoma+glomerulonephritis | 0 | |
| Carcinoma+hyperplasia of BALT complex | 30++ | |
| Glomerulonephritis+hyperplasia of BALT complex | 0 | |
| Glomerulonephritis+hyperplasia of BALT complex+carcinoma | 0 | |
| Health status | 20° |
Carcinoma is referred to hepatoma+lung metastasis in old pHx mice. In old sham mice, hepatoma is not present, but exclusively lung carcinoma. *P<0.01 and **P<0.05 as compared with health status in old pHx mice and to the same pathology in old sham mice. +P<0.01 and ++P<0.05 as compared with health status in old sham mice. °P<0.05 as compared with old pHx mice. Glomerulonephritis is not significant when compared with health status in both groups of mice.
Table 3.
Physio/pathological condition in very old pHx mice after 1 month from pHx in comparison with very old sham controls
| Physio/pathological condition | Frequency (%) | |
|---|---|---|
| Very old pHx mice | Glomerulonephritis | 5 |
| Carcinoma | 0 | |
| Hyperplasia of BALT complex | 35** | |
| Carcinoma+glomerulonephritis | 0 | |
| Carcinoma+hyperplasia of BALT complex | 0 | |
| Glomerulonephritis+hyperplasia of BALT complex | 5 | |
| Glomerulonephritis+hyperplasia of BALT complex+carcinoma | 0 | |
| Health status | 50 | |
| Very old sham mice | Glomerulonephritis | 5 |
| Carcinoma | 0 | |
| Hyperplasia of BALT complex | 45 | |
| Carcinoma+glomerulonephritis | 0 | |
| Carcinoma+hyperplasia of BALT complex | 0 | |
| Glomerulonephritis+hyperplasia of BALT complex | 0 | |
| Glomerulonephritis+hyperplasia of BALT complex+carcinoma | 0 | |
| Health status | 50 |
**P<0.05 as compared with health status in very old pHx mice and to the same pathology in very old sham mice. Glomerulonephritis is present in similar percent in old sham and very old sham mice, whereas carcinoma is absent in very old sham mice and very old pHx mice in comparison with old sham mice and old pHx mice in Table 2 (P<0.001). Hyperplasia of BALT does not seem relevant between old sham mice and very old sham mice before and after 1 month from partial hepatectomy.
3. Results
3.1. Crude zinc balance, NK cell activity, IL-2, IL-6, sIL-6R and corticosterone plasma levels during liver regeneration in young, old and very old mice
Table 1 shows that the crude zinc balance was positive in sham young and sham very old mice, while it was negative in sham old mice. At 48 h after their partial hepatectomy, the crude zinc balance was negative in young, old and very old pHx mice with a more significant negativity in old pHx mice than in young and very old pHx mice (P<0.05). Positive values were newly observed at on day 15 following a partial hepatectomy in young and very old pHx mice but not in old pHx mice (Table 1). Young and very old pHx mice display significant reductions in NK cell activity and IL-2 production at 48 h after their partial hepatectomy as compared with respective sham controls (time 0) (P<0.01). Restoration occurred in the late period of compensatory liver growth (day 15) in young and very old pHx mice, but not in old pHx mice with no modifications as compared with respective controls (Table 1). IL-6, sIL-6R and corticosterone plasma levels increased in young and very old pHx mice at 48 h from partial hepatectomy as compared with respective sham controls (P<0.01), with restoration on day 15 of compensatory liver growth (Table 1). No significant modifications in IL-6, sIL-6R and corticosterone were observed in old pHx mice during liver regeneration (Table 1). It is noteworthy that IL-6 and sIL-6R are high in very old sham mice in comparison with young sham mice (P<0.01), but less high in comparison with old sham mice (Table 1). However, very old mice displayed the same trend in IL-6, sIL-6R and corticosterone of young mice during liver regeneration. Moreover, a small number (n=3) of old mice display IL-6 near to 16 pg ml−1: values similar to higher ones observed in very old mice (15.8 pg ml−1) at time 0. Therefore, relatively low levels of IL-6 may be considered a marker to reach successful ageing, as previously reported in very old humans (Bonafe et al., 2001).
Table 1.
Crude zinc balance and endocrine–immune parameters studied in young, old and very old mice in each time interval considered during compensatory liver growth from pHx
| Time from partial hepatectomy | Mice |
|||
|---|---|---|---|---|
| Young | Old | Very old | ||
| NK cell activity (L.U.20/107 cells) | t=0 (sham) | 25.2±3.2 | 8.5±2.0 | 12.5±2.0 |
| pHx 48 h | 14.7±5.1* | 7.8±1.3 | 6.5±1.4* | |
| pHx 15 days | 20.6±6.5 | 7.4±1.4 | 14.3±1.8 | |
| IL-2 (pg ml−1) | t=0 (sham) | 38.7±3.5 | 11.3±2.4 | 25.4±3.6 |
| pHx 48 h | 11±4.3* | 11.4±1.8 | 11.3±1.6* | |
| pHx 15 days | 36,8±4.5 | 11.3±1.7 | 26.4±3.4 | |
| Crude zinc balance (μg per day per mouse) | t=0 (sham) | +1.17±0.15 | −1.07±0.28 | +0.51±0.07 |
| pHx 48 h | −1.27±0.33 | −1.57±0.31** | -0.68±0.07 | |
| pHx 15 days | +1.25±0.24 | −0.97±0.18 | +0.37±0.04 | |
| Corticosterone (ng ml−1) | t=0 (sham) | 150±14 | 250±19 | 200±11 |
| pHx 48 h | 295±17* | 261±21 | 310±13* | |
| pHx 15 days | 143±12 | 249±23 | 199±14 | |
| IL-6 (pg ml−1) | t=0 (sham) | 7.8±3.9 | 25.8±9.8 | 12.6±3.2 |
| pHx 48 h | 26.7±6.6* | 26.4±7.6 | 26.4±9.4* | |
| pHx 15 days | 7.4±3.5 | 26.7±8.4 | 15.0±2.4 | |
| SIL-6R (ng ml−1) | t=0 (sham) | 19±4.6 | 45±9.1 | 25±5.1 |
| pHx 48 h | 45±8.3* | 47±8.4 | 47±15.8* | |
| pHx 15 days | 21±5.9 | 51±9.7 | 28±6.8 | |
*P<0.01 when compared with sham and 15 days from partial hepatectomy. **P<0.05 when compared with young and very old mice at 48 h.
3.2. MTmRNA during liver regeneration in young, old and very old mice
MTmRNA increases in old sham mice in comparison with young sham and very old sham mice (P<0.001) (Fig. 1 ). At 48 h from partial hepatectomy, MTmRNA increases in young and very old pHx mice in comparison with respective sham controls (P<0.001). No substantial variations occurs in old pHx mice at 48 h in comparison with respective controls (P>0.05) (Fig. 1). Complete MTmRNA downregulation is observed in young and very old mice in the late period of compensatory liver growth (day 15), but not in old mice (Fig. 1). No modifications in the pattern of MTmRNA are observed either during liver regeneration in transgenic MT-I* mice (Fig. 2 ) showing a close likeness to old pHx mice (Fig. 1). It is noteworthy that the differences in MT data among young, old and very old mice at time 0 obtained using RT-PCR reflect the values of liver MT protein using Ag+ saturation method (Mocchegiani et al., 2002b).
Fig. 1.
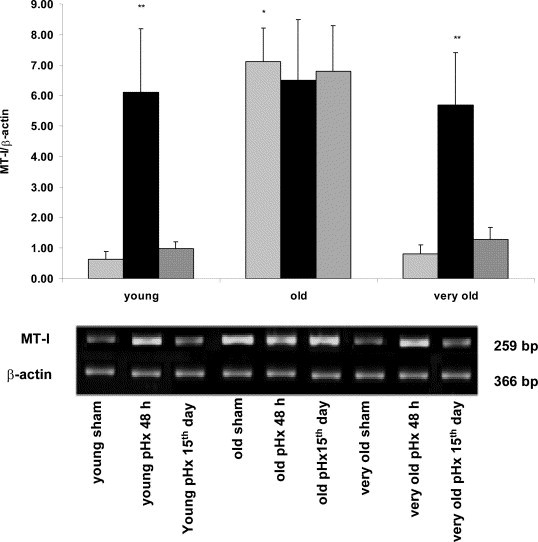
Liver MTmRNA (RT-PCR) concentrations in young, old and very old mice after partial hepatectomy/liver regeneration. [(▨) sham; (■)at 48 h; (▧) 15th day]. RT-PCR analysis of liver mRNA using specific primers for murine MT-I and β-actin under conditions described in Section 2 After mRNA isolation and cDNA synthesis, the amount of mRNA for MT-I was determined by semi-quantitative PCR. The densitometry analysis was also performed using Gel-Doc instrument (Bio-Rad, USA). The results are shown in the histograms and are expressed as MT-I/β-actin ratio. *P<0.001 when compared with young and very old mice (sham). **P<0.001 when compared with respective sham controls.
Fig. 2.
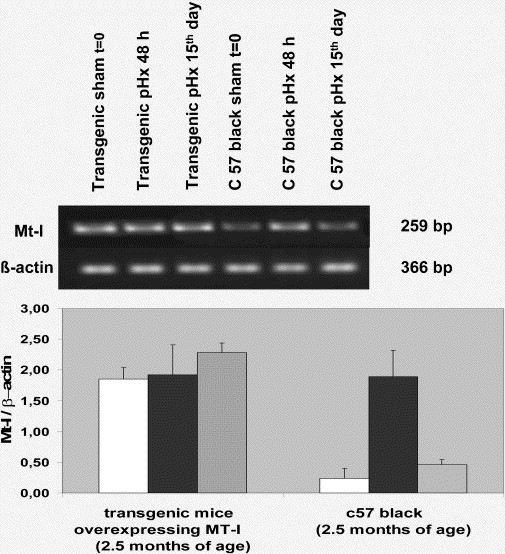
RT-PCR analysis of liver mRNA using specific primers for murine MT-I and β-actin under condition described in Section 2 in young transgenic mice overexpression MT and respective controls after partial hepatectomy/liver regeneration. [(□), sham; (■), at 48 h; (▧), 15th day]. After mRNA isolation and cDNA synthesis, the amount of mRNA for MT-I was determined by semi-quantitative PCR. The densitometry analysis was also performed using Gel-Doc instrument (Bio-Rad). The results are shown in the histograms and are expressed as MT-I/β-actin ratio. No differences exist in immune parameters between Balb/c and C5BL/6J mice used as controls of MT-I* mice (Mocchegiani et al., 2002a).
3.3. c-myc Gene expression during liver regeneration in young, old and very old mice
At time 0, the c-myc gene expression is already high in old sham controls in comparison with young and very old mice (P<0.01). It increases in all mice at 1 h after partial hepatectomy with progressive decrements at 24 and 48 h from surgical procedures and is significantly lacking on days 7 and 15 of liver regeneration exclusively in young and very old mice as compared with old mice (P<0.01) (Fig. 3 ). Indeed, c-myc is constantly high from 24 h to day 15 in old pHx mice with similar values of old sham controls (time 0) (Fig. 3). MT-I* mice display the same pattern of old mice in all time intervals considered after partial hepatectomy reinforcing the notion that MT over-expression is deleterious in constant inflammation with a likeness of MT-I* and old mice (Mocchegiani et al., 2002a). It is noteworthy that the differences in c-myc data among young, old and very old mice at time 0 obtained using RT-PCR reflect the values of c-myc quantification using immunocytochemical methods (Mocchegiani et al., unpublished observation).
Fig. 3.
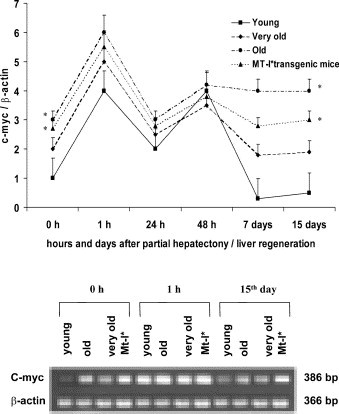
Liver c-myc mRNA (RT-PCR) concentrations in young, old, very old and MT-I* mice after partial hepatectomy/liver regeneration. RT-PCR analysis was performed using specific primers for mouse c-myc and β-actin under conditions described in Section 2. After mRNA isolation and cDNA synthesis, the amount of mRNA for c-myc was determined by semi-quantitative PCR. The densitometry analysis was also performed using Gel-Doc instrument (Bio-Rad). The results are expressed as c-myc/β-actin ratio. *P<0.01 as compared with young sham (time 0) and to young pHx mice (15th day), respectively.
3.4. Correlations during liver regeneration in young, old and very old mice
Significant inverse correlation exists in young and very old pHx mice, but not in old pHx mice, for the whole period of liver regeneration between MT and NK cell activity (r=−0.85, P<0.01; r=−0.68, P<0.05, respectively) and between MT and IL-2 (r=−0.78, P<0.01; r=−0.65, P<0.05, respectively). Significant positive correlation exists in young and very old pHx mice, but not in old pHx mice, for the whole period of liver regeneration between: MT and IL-6 or sIL-6R (r=0.76, P<0.01; r=0.61, P<0.05, respectively); MT and corticosterone (r=0.75, P<0.01, r=0.62, P<0.05, respectively); crude zinc balance and NK cell activity (r=0.75, P<0.01; r=0.67, P<0.05, respectively); crude zinc balance and IL-2 (r=0.73, P<0.01; r=0.60, P<0.05, respectively); c-myc and MT (r=0.85, P<0.01); c-myc and NK cell activity (r=0.77, P<0.01); c-myc and IL-2 (r=0.81, P<0.01).
3.5. Survival and physio/pathological analysis
Before survival and physio/pathological analysis, the health status of the chosen old mice was within the FELASA “conventional housing” normal rage (INRCA Veterinary Service). Old mice with no evident pathologies but underweight (≤20 g) were also discarded because of the presence of malnutrition (Mocchegiani et al., 1998) and the subsequent risk of cancer (Temple, 2002). Fig. 4 shows that the survival rate in old pHx mice is shorter (24 months) than in old sham controls (30 months) (P<0.01). The majority of deaths occurs at 21 months of age (i.e. after 1 month after partial hepatectomy). The cause of death is primarily due to cancer (hepatoma and lung metastasis) alone or associated with hyperplasia of bronchus associated lymphoid tissue (BALT) (90%) whereas the other 10% died for chronic glomerulonephritis. The histological analysis performed other groups of old pHx and sham old mice at 1 month from partial hepatectomy shows the presence of carcinoma alone or associated with hyperplasia of BALT complex (60%) in old pHx mice with respect to 30% in old sham controls (Table 2 ). Hyperplasia of BALT complex, on the whole, does not seem to constitute a factor in causing death (75% in both groups) (Table 2). Therefore, the differences in survival are due to cancer. Also, chronic glomerulonephritis does not seem to affect survival (5% in both groups) (Table 2). It is worthy to note that old sham mice (20%) show good health, which is not present in old pHx mice (0%), thus explaining the shorter survival time in old pHx mice with respect to old sham controls as well as the maximum life span of 30 months (Fig. 4). In view of this, the 20% of old sham mice showing good health could represent possible candidates for successful ageing, as has been reported for old humans (15–18%) (OCTO Study) (Wikby et al., 1998). Indeed, as reported in Table 3 , very old sham mice and very old pHx mice display no appearance of carcinoma both at time 0 and after 1 month from partial hepatectomy, respectively. Therefore, the absence of carcinoma is one of the requisites to reach successful ageing. Moreover, the health status is more present in very old sham mice (50%) than old sham mice (20%) (P<0.01). This means that a reduced inflammation in very old age (relatively low IL-6 at time 0, as shown in Table 1) is also one of the requisites for a good health status.
Fig. 4.
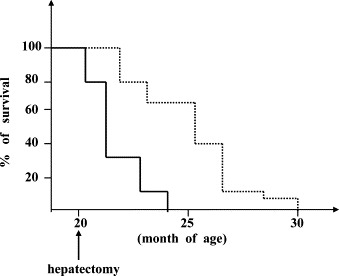
Percent of survival (Kaplan–Meier) in old pHx mice (direct line) and in old sham controls (hatched line) from the age of 20 months. The survival is reduced in old pHx mice in comparison with old sham controls (P<0.01, Log-rank test). High percent of death (90%) occurs at 21 months of age in old pHx mice due specially to the appearance of carcinoma (see Table 2).
4. Discussion
Compared with their respective sham controls, a negative crude zinc balance, reduced NK cell activity and decreased IL-2 production coupled with increased IL-6, sIL-6R and corticosterone and enhanced MTmRNA were recorded in young, old and very old mice 48 h after their partial hepatectomy. Restoration occurred at days 7 and 15 after the partial hepatectomy in young and very old mice, but not old mice.
These findings, yielded by the partial hepatectomy/liver regeneration (pHx) model, suggest that zinc-bound MT homeostasis is pivotal in conferring immune plasticity, which is indispensable to successful ageing (Mocchegiani et al., 2002b). The presence of a significant inverse or positive correlation between MTmRNA and nutritional or endocrine or immune parameters exclusively in young and very old pHx mice is consistent with this assumption. There is absolutely no doubt that MT increases after a partial hepatectomy (Tohyama et al., 1993) because MT induction is related to inflammation, which is a common event after partial hepatectomy (Fausto, 2000). The problem, however, is that enhanced MT sequesters many of the intracellular zinc ions with no subsequent zinc release during constant inflammation, including ageing (Mocchegiani et al., 2002a). High zinc-bound MT (Zn–MT) was always associated with a negative crude zinc balance in old mice at all the time intervals considered during liver regeneration whereas it is present in young and very old mice exclusively 48 h after their partial hepatectomy. Inflammation by high IL-6 provokes consistent zinc loss (Wapnir, 2000), but, at the same time, IL-6 affects MTmRNA induction (Andrews, 2000). Thus, the inflammation, on one hand, induces zinc loss from urine and faeces; while on the other hand, it induces MTmRNA induction. As a result, the crude zinc balance is negative, the zinc ion bioavailability is low and MT increases sequestering the remaining zinc ions, as reported in young mice during transient inflammation (Mocchegiani et al., 2002a). This phenomenon is marked in constant inflammation, such as in ageing. Indeed, the crude zinc balance is more negative (48 h) in old pHx mice than in others and strictly related to deeper inflammation (high constant IL-6, Table 1). Therefore, increments of Zn–MT and negative crude zinc balance are synergistic in ageing thus making the role of Zn–MT detrimental to immunity. The presence of thymic and immune dysfunctions in MT-I* mice (Mocchegiani et al., 2002a) with no changes in MTmRNA during liver regeneration (Fig. 2) suggest a similarity between MT-I* and old mice lending further support to the notion that MT overexpression is harmful for immune efficiency.
Conversely, when MTmRNA is low (at days 7 and 15 of liver regeneration in young and very old mice), the inflammation decreases (low IL-6, sIL-6R and corticosterone), the crude zinc balance regains positive values and the immune response is restored. Therefore, correct Zn-MT homeostasis is crucial in inducing good zinc ion bioavailability for immune efficiency and plasticity, which leads to successful ageing. Indeed, very old mice display the same remodelling as young mice in the nutritional–endocrine–immune response during liver regeneration. An intriguing point is related to the remodelling of sIL-6R as well during liver regeneration in young and very old mice, but not in old mice. It has been reported that the IL-6/sIL-6R complex is a primary stimulus to hepatocytes proliferation after pHx (Maione et al., 1998). But, at the same time, the constant high presence of this complex causes hepatocellular transformation with the appearance of liver tumours (Maione et al., 1998). Therefore, on the whole, such remodelling also correctly affects hepatocytes regeneration in young and very old mice. By contrast, carcinoma appears in old pHx mice in the long run coupled with shorter survival compared with that of the old sham controls (Fig. 4). Carcinogenesis may occur due to the high constant expression of c-myc proto-oncogene, which changes from being beneficial for liver regeneration during the first hours following the partial hepatectomy (Hoffman et al., 1994) to dangerous in the long run because of the possible abnormal proliferation of damaged liver cells as well. Indeed, the gene expression of c-myc is constantly high in old pHx, as well as in MT-I* mice, at days 7 and 15 following their partial hepatectomy in contrast to that of young and very old mice. Taking into account that MT induces c-myc gene expression (Tohyama et al., 1993), which in turn provokes proliferation in the presence of growth factors and apoptosis when such factors declines (Harrington et al., 1994), it is evident that liver regeneration (from 48 h to day 7) is correct in young and very old mice due to apoptosis indispensable in eliminating damaged liver cells and in blocking the compensatory liver growth, as normally occurs in young pHx rats (De Miglio et al., 1999, Moser et al., 2001). By contrast, the constant presence of growth factors (MT, IL-6/sIL-6R complex) in old and MT-I* pHx mice (from 48 h to days 7 and 15) may provoke continuos proliferation, via c-myc, of damaged liver cells too with the appearance of cancer in the long run, as is also shown by no health status in old pHx mice after 1 month following their partial hepatectomy in contrast to the old sham controls (Table 2). The presence of abnormal liver proliferation and tumours in IL-6/sIL-6R complex transgenic mice after a partial hepatectomy (Maione et al., 1998), the short survival of IL-6/sIL-6R complex transgenic mice (Maione et al., 1998), the short survival and the peripheral organs dysfuction in MT-transgenic mice (Quaife et al., 1999), which are also more susceptible to the lethal effect of cancer-provoking TNF injections (Waelput et al., 2001) and, finally, the correlation between high c-myc and depressed immune performances in old pHx mice (present study) and in pHx MT-I* mice (at 1 month after their partial hepatectomy) (E. Mocchegiani, unpublished results), are consistent with this interpretation. On the other hand, c-myc overexpression (Kawate et al., 1999), high MT (Ebadi and Swanson, 1988), persistent inflammation (high IL-6 and TNF-α) (Sharma and Anker, 2002), low zinc ion bioavailability and depressed immune functions (Mocchegiani et al., 1998) are common events in cancer. Therefore, a correct Zn–MT homeostasis is crucial in conferring both suitable liver regeneration and immune plasticity not only after partial hepatectomy, but also in obtaining healthy longevity. The correct Zn–MT homeostasis in lymphocytes taken from centenarians (Mocchegiani et al., 2002b) coupled with good immune performances (Franceschi et al., 1995) and (as indirect evidence) the presence of c-myc polymorphism in elderly cancer patients (Shih et al., 2002), support this assumption. In this context, the low gp130 and the reduced sIL-6R in centenarians despite of high IL-6 (Giuliani et al., 2001) is intriguing. Low gp130 also occurs in very old mice (Mocchegiani et al., 2002b). Consequently, inflammation is less detrimental in very old age as there is a loss of the target (i.e. gp130) of IL-6 or IL-6/sIL-6R complex, thereby explaining the similar patterns of nutritional–endocrine–immune parameters that are found in young and very old mice during liver regeneration. This means that very old mice are still responsive to severe inflammation, like pHx, just as young mice are, reflecting the major responsiveness to oxidative stress in human centenarians (Mecocci et al., 2000).
In conclusion, pHx is a good model as it reveals the presence of a lesser degree of inflammation in very old age. This fact is due to a correct MT homeostasis, which in turn leads to suitable liver regeneration via c-myc conferring also immune plasticity even in very old age with subsequent successful ageing. Hence, in ageing high MT plays an extremely harmful role: on one hand it lowers zinc ion bioavailability levels required for immune efficiency; on the other hand increases c-myc expression. The combination of immune depression and enhanced c-myc, via high MT, may thus allow the appearance of age-related degenerative diseases. In order to further confirm this assumption, our lab is presently performing studies in MT-I* and MT null mice as well as in elderly infected and cancer patients.
Acknowledgements
Supported by INRCA, Italian Health Ministry (R.F.no. 99/107 to E.M.) and CEE (ImAginE no. QLK6-CT-1999-02031). We thank Professor Paul Bowerbank for revising the manuscript.
References
- Andrews G.K. Regulation of metallothionein gene expression by oxidative stress and metal ions. Biochem. Pharmacol. 2000;59:95–104. doi: 10.1016/s0006-2952(99)00301-9. [DOI] [PubMed] [Google Scholar]
- Bonafe M., Olivieri F., Cavallone L., Giovagnetti S., Marchegiani F., Cardelli M., Pieri C., Marra M., Antonicelli R., Lisa R., Rizzo M.R., Paolisso G., Monti D., Franceschi C. A gender-dependent genetic predisposition to produce high levels of IL-6 is detrimental for longevity. Eur. J. Immunol. 2001;31:2357–2361. doi: 10.1002/1521-4141(200108)31:8<2357::aid-immu2357>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Buunsgaard H., Pedersen M., Pedersen B.K. Aging and proinflammmatory cytokines. Curr. Opin. Hematol. 2001;8:131–136. doi: 10.1097/00062752-200105000-00001. [DOI] [PubMed] [Google Scholar]
- De Miglio M.R., Simile M.M., Muroni M.R., Pusceddu S., Calvisi D., Carru A., Seddaiu M.A., Daino L., Deiana L., Pascale R.M., Feo F. Correlation of c-myc overexpression and amplification with progression of preneoplastic liver lesions to malignancy in the poorly susceptible Wistar rat strain. Mol. Carcinog. 1999;25:21–29. [PubMed] [Google Scholar]
- Ebadi E., Swanson S. Zinc and metallothioneins in cancer. In: Ebadi M., editor. Nutrition, Growth and Cancer. Alan R. Liss; New York: 1988. pp. 161–175. [Google Scholar]
- Fausto N. Liver regeneration. J. Hepatol. 2000;32:19–31. doi: 10.1016/s0168-8278(00)80412-2. [DOI] [PubMed] [Google Scholar]
- FELASA (Working Group on Animal Health) Recommendations for the health monitoring of mouse, rat, hamster, guinea pigs and rabbit breeding colonies. Lab. Anim. 1994;28:1–30. doi: 10.1258/002367794781065933. [DOI] [PubMed] [Google Scholar]
- Franceschi C., Monti D., Sansoni C., Cossarizza A. The immunology of exceptional individuals: the lesson of centenarians. Immunol. Today. 1995;16:12–16. doi: 10.1016/0167-5699(95)80064-6. [DOI] [PubMed] [Google Scholar]
- Giuliani N., Sansoni P., Girasole G., Vescovini R., Passeri G., Passeri M., Pedrazzoni M. Serum interleukin-6, soluble interleukin-6 receptor and soluble gp130 exhibit different patterns of age- and menopause-related changes. Exp. Gerontol. 2001;36:547–557. doi: 10.1016/s0531-5565(00)00220-5. [DOI] [PubMed] [Google Scholar]
- Gordon G.J., Coleman W.B., Grisham J.W. Temporal analysis of hepatocyte differentiation by small hepatocyte-like progenitor cells during liver regeneration in retrorsine-exposed rats. Am. J. Pathol. 2000;157:771–786. doi: 10.1016/S0002-9440(10)64591-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington E.A., Bennett M.R., Fanidi A., Evan G.I. c-Myc-induced apoptosis in fibroblasts is inhibited by specific cytokines. EMBO J. 1994;13:3286–3295. doi: 10.1002/j.1460-2075.1994.tb06630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman A.L., Hugo R.R., Ljubimova J.U., Sher L., Podesta L.G., Demetriou A.A., Makowka L. Hepatic regeneration: current concepts and clinical implications. Semin. Liver Dis. 1994;14:190–210. doi: 10.1055/s-2007-1007311. [DOI] [PubMed] [Google Scholar]
- Houlston R.S., Tomlinson I.P. Modifier genes in humans: strategies for identification. Eur. J. Hum. Genet. 1998;6:80–88. doi: 10.1038/sj.ejhg.5200156. [DOI] [PubMed] [Google Scholar]
- Kagi J.H.R., Shaffer A. Biochemistry of metallothionein. Biochemistry. 1988;27:8509–8515. doi: 10.1021/bi00423a001. [DOI] [PubMed] [Google Scholar]
- Kawate S., Fukusato T., Ohwada S., Watanuki A., Morishita Y. Amplification of c-myc in hepatocellular carcinoma: correlation with clinic pathologic features, proliferative activity and p53 overexpression. Oncology. 1999;57:157–163. doi: 10.1159/000012024. [DOI] [PubMed] [Google Scholar]
- Kelley-Loughnane N., Sabla G.E., Ley-Ebert C., Aronow B.J., Bezerra J.A. Independent and overlapping transcriptional activation during liver development and regeneration in mice. Hepatology. 2002;35:525–534. doi: 10.1053/jhep.2002.31351. [DOI] [PubMed] [Google Scholar]
- Kelly E.J., Quaife C.F., Froelik G.J., Palmiter R.D. Metallothionein I and II against zinc deficiency and zinc toxicity. J. Nutr. 1996;126:1782–1790. doi: 10.1093/jn/126.7.1782. [DOI] [PubMed] [Google Scholar]
- Maione D., DiCarlo E., Li W., Musiani P., Modesti A., Peters M., Rose-John S., Della Rocca C., Tripodi M., Lazzaro A., Taub R., Savino R., Ciliberto C. Coexpression of IL-6 and soluble IL-6R causes nodular regenerative hyperplasia and adenomas of the liver. EMBO J. 1998;17:5588–5597. doi: 10.1093/emboj/17.19.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecocci P., Polidori M.C., Troiano L., Cherubini A., Cecchetti R., Pini G., Straatman M., Monti D., Stahl W., Sies H., Franceschi C., Senin U. Plasma antioxidants and longevity: a study on healthy centenarians. Free Radic. Biol. Med. 2000;28:1243–1248. doi: 10.1016/s0891-5849(00)00246-x. [DOI] [PubMed] [Google Scholar]
- Michalopoulos G.K., DeFrances M.C. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- Mocchegiani E., Verbanac D., Santarelli L., Tibaldi A., Muzzioli M., Radosevic-Stasic B., Milin C. Zinc and metallothioneins on cellular immune effectiveness during liver regeneration in young and old mice. Life Sci. 1997;61:1125–1145. doi: 10.1016/s0024-3205(97)00646-2. [DOI] [PubMed] [Google Scholar]
- Mocchegiani E., Muzzioli M., Cipriano C., Giacconi R. Zinc, T-cell pathways, ageing: role of metallothioneins. Mech. Ageing Dev. 1998;106:183–204. doi: 10.1016/s0047-6374(98)00115-8. [DOI] [PubMed] [Google Scholar]
- Mocchegiani E., Muzzioli M., Giacconi R. Zinc and immunoresistance to infections in ageing: new biological tools. Trends Pharmacol. Sci. 2000;21:205–208. doi: 10.1016/s0165-6147(00)01476-0. [DOI] [PubMed] [Google Scholar]
- Mocchegiani E., Giacconi R., Cipriano C., Gasparini N., Orlando F., Stecconi R., Muzzioli M., Isani G., Carpenè E. Metallothionein (I+II) and thyroid-thymus axis efficiency in old mice: role of corticosterone and zinc supply. Mech. Ageing Dev. 2002;123:675–694. doi: 10.1016/S0047-6374(01)00414-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocchegiani E., Giacconi R., Cipriano C., Muzzioli M., Gasparini N., Moresi R., Stecconi R., Suzuki H., Cavalieri E., Mariani E. MtmRNA gene expression, via IL-6 and glucocorticoids, as potential genetic marker of immunosenescence: lessons from very old mice and humans. Exp. Gerontol. 2002;37:349–357. doi: 10.1016/s0531-5565(01)00202-9. [DOI] [PubMed] [Google Scholar]
- Moser M.J., Gong Y., Zhang M.N., Johnston J., Lipschitz J., Minuk G.Y. Immediate-early protooncogene expression and liver function following various extents of partial hepatectomy in the rat. Dig. Dis. Sci. 2001;46:907–914. doi: 10.1023/a:1010791915733. [DOI] [PubMed] [Google Scholar]
- Okuda Y., Nakatsuji Y., Fujimura H., Esumi H., Ogura T., Yanagihara T., Sakoda S. Expression of the inducible isoform of nitric oxide synthase in the central nervous system of mice correlates with the severity of actively induced experimental allergic encephalomyelitis. J. Neuroimmunol. 1995;62:103–112. doi: 10.1016/0165-5728(95)00114-h. [DOI] [PubMed] [Google Scholar]
- Quaife C.J., Kelly E.J., Masters B.A., Brinster R.L., Palmiter R.D. Ectopic expression of metallothionein-III causes pancreatic acinar cell necrosis in transgenic mice. Toxicol. Appl. Pharmacol. 1999;148:148–157. doi: 10.1006/taap.1997.8321. [DOI] [PubMed] [Google Scholar]
- Sharma R., Anker S.D. Cytokines, apoptosis and cachexia: the potential for TNF antagonism. Int. J. Cardiol. 2002;85:161–171. doi: 10.1016/s0167-5273(02)00244-9. [DOI] [PubMed] [Google Scholar]
- Shih C.M., Kuo Y.Y., Wang Y.C., Jian S.L., Hsu Y.T., Wu H.Y., Guo M.W., Wang Y.C. Association of L-myc polymorphism with lung cancer susceptibility and prognosis in relation to age-selected controls and stratified cases. Lung Cancer. 2002;36:125–132. doi: 10.1016/s0169-5002(01)00467-6. [DOI] [PubMed] [Google Scholar]
- Shimada M., Saitoh A., Kano T., Takenaka K., Sugimachi K. The effect of a perioperative steroid pulse on surgical stress in hepatic resection. Int. Surg. 1996;81:49–51. [PubMed] [Google Scholar]
- Temple N.J. Nutrition and diseases: challenges of research design. Nutrition. 2002;18:343–347. doi: 10.1016/s0899-9007(01)00759-6. [DOI] [PubMed] [Google Scholar]
- Tohyama C., Suzuki J.S., Hemelraad J., Nishimura N., Nishimura H. Induction of metallothionein and its localization in the nucleus of rat hepatocytes after partial hepatectomy. Hepatology. 1993;18:1193–1201. [PubMed] [Google Scholar]
- Turnlund J.R., Durkin N., Costa F., Margen S. Stable isotope studies of zinc absorption and retention in young and elderly men. J. Nutr. 1986;116:1239–1247. doi: 10.1093/jn/116.7.1239. [DOI] [PubMed] [Google Scholar]
- Waelput W., Broekaert D., Vandekerckhove J., Brouckaert P., Tavernier J., Libert C.A. Mediator role for metallothionein in tumor necrosis factor-induced lethal shock. J. Exp. Med. 2001;194:1617–1624. doi: 10.1084/jem.194.11.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wapnir R.A. Zinc deficiency, malnutrition and the gastrointestinal tract. J. Nutr. 2000;130(5S Suppl):1388S–1392S. doi: 10.1093/jn/130.5.1388S. [DOI] [PubMed] [Google Scholar]
- Wesselkamper S.C., Prows D.R., Biswas P., Willeke K., Bingham E., Leikauf G.D. Genetic susceptibility to irritant-induced acute lung injury in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2000;279:L575–582. doi: 10.1152/ajplung.2000.279.3.L575. [DOI] [PubMed] [Google Scholar]
- Wikby A., Maxson P., Olsson J., Johansson B., Ferguson F.G. Changes in CD8 and CD4 lymphocyte subsets, T cell proliferation responses and non-survival in the very old: the Swedish longitudinal OCTO-immune study. Mech. Ageing Dev. 1998;102:187–198. doi: 10.1016/s0047-6374(97)00151-6. [DOI] [PubMed] [Google Scholar]


