Abstract
Local delivery of small interfering RNA (siRNA) to the lungs constitutes a promising new area in drug delivery. The present study evaluated parameters of importance for spray drying of siRNA-loaded poly(D,L-lactide-co-glycolide) (PLGA) nanoparticles (NPs) into nanocomposite microparticles intended for inhalation. The spray drying process was optimised using a statistical design of experiment and by evaluating powder characteristics upon systematic variation of the formulation parameters. Concentration, carbohydrate excipient (trehalose, lactose and mannitol) and the ratio of NP to excipient were varied to monitor the effects on moisture content, particle morphology, particle size and powder yield. The identified optimum conditions were applied for spray drying of siRNA-loaded nanocomposite microparticles, resulting in a product with a low water content (0.78% w/w) and an aerodynamic particle diameter considered suitable for inhalation. The use of mannitol in the formulation allowed a significantly lower moisture content than trehalose and lactose. The inclusion of 50% (w/w) or higher amounts of NPs resulted in a marked change in the surface morphology of the spray-dried particles. Importantly, the integrity and biological activity of the siRNA were preserved during the spray drying process. In conclusion, the present results show that spray drying is a suitable technique for producing nanocomposite microparticles comprising siRNA-containing PLGA NPs for potential use in inhalation therapy.
Keywords: Spray drying, Inhalation, PLGA nanoparticles, siRNA, Drug delivery
Graphical abstract
Nanocomposite microparticles, produced by spray drying, containing siRNA-loaded PLGA nanoparticles and mannitol, providing the possibility for local treatment with siRNA in the airways via inhalation.
1. Introduction
The concept of RNA interference (RNAi), mediated by small interfering RNA (siRNA), provides an opportunity for a whole new class of drugs directed towards disease-associated genes. However, before RNAi can be used to its full potential for treatment of a variety of diseases, challenges regarding the delivery of siRNA need to be overcome [1]. A promising approach to overcoming delivery challenges related to stability and cellular targeting of siRNA is local delivery of siRNA directly onto the diseased tissue using a delivery system capable of protecting the siRNA from degradation [1], [2]. Poly(D,L-lactide-co-glycolide) (PLGA) nanoparticles (NPs) have been proposed for delivering macromolecules, such as siRNA, to the cytosol of cells in vitro [3], [4]. The NPs have been shown to be taken up by endocytosis and can escape the endosomes to a certain degree before degradation [4] and subsequently release the drug, although the mechanism of endosomal escape is unclear at present. Once present in the cytosol, the PLGA is suggested to be degraded slowly, sustaining the release of encapsulated siRNA, a property which might be very useful for therapeutic purposes [3].
In this perspective, the airways are interesting sites for local delivery of siRNA. The lungs are exposed to a variety of pollutants on a daily basis and are therefore also susceptible to a wide range of diseases. Examples of airway-located diseases, which would be treatable with siRNA, are cancer and infections like Severe Acute Respiratory Syndrome (SARS) and influenza [2], [5]. Diseases may affect different parts of the airways, which branch out approximately 17 times before ending up in the alveoli [6]. However, many infections are localized in the lower airways [7], [8], so efficient delivery systems must be developed to treat airway diseases. Even though the total alveoli surface area of 100–150 m2 in adults provides a large surface for drug deposition [9], the branching, mucociliary clearance and breathing are mechanisms that prevent particles from entering the lower airways. To overcome these barriers and achieve particle deposition in the lower airways, the size distribution and aerodynamic behaviour of the particles intended for inhalation are of great importance. The aerodynamic diameter can be used to predict the behaviour of a given particle in an airstream and thereby predict the site of impact with airway epithelium. Larger particles with an aerodynamic diameter above 5 µm will most likely impact with the tissue of the mouth, throat or upper lung mucosa and be eliminated from there, whereas particles smaller than 1 µm will often suffer from being exhaled and thus not be deposited in the alveoli. When formulating drugs for pulmonary delivery, another issue to be considered is the clearance mechanism for particles deposited in the deeper areas of the lungs. The clearance in the deeper areas of the lungs is mostly provided by pulmonary macrophages, which are mobile phagocytic cells monitoring the inner surface of the alveoli by ingesting and degrading pathogens and particles. Even though micro-sized particles will be processed by the pulmonary macrophages, NPs with a diameter smaller than approximately 260 nm might escape macrophage clearance [10], [11].
To overcome the macrophage clearance and still benefit from a particle size suitable for deep lung deposition and sustained release, the present study employs nanocomposite microparticles consisting of co-spray-dried PLGA NPs and a carbohydrate excipient. The methodology of spray drying enables formation of particles of a size suitable for inhalation comprising both NPs and an excipient [12], [13], [14]. Furthermore, nanocomposite microparticles composed of PLGA NPs and sugars, such as trehalose and lactose, have been shown to decompose in the lung lining fluid, releasing the NPs from the formulation [12], [13], [14]. The spray drying process is widely applied for production of inhalable particles, and allows for a large degree of variation of the final product. Spray drying has previously been used for a number of applications, including the preparation of dry powder protein particles intended for inhalation [15], [16], as well as dry powder containing cationic PLGA nanoparticles, the latter to prevent aggregation and loss of transfection potential when subsequently complexed to pDNA [17].
During spray drying, a feed solution, suspension or emulsion of the compound(s) is dispersed into fine droplets, which are then dried by a hot air-stream. Several parameters in the drying process itself, as well as the composition of the feed formulation, greatly influence the characteristics of the dry end product [18], [19]. Carbohydrate excipients function as bulking agents that enable production of stable particles of a size suitable for inhalation and allow a rapid release of the NPs in the lung lining fluid upon inhalation. Also, the excipients are added to the formulation to provide some degree of protection of the NPs and the encapsulated drug (in this case siRNA) during the process of spray drying, in particular against shear forces and increased temperatures. Excipients like mannitol, trehalose and lactose are commonly used in spray drying with satisfying end products [13], [20]. These carbohydrates are dissolvable in water [21] and possess good safety profiles and are thus good choices for the production of the nanocomposite microparticles, which should decompose quickly in the lung lining fluid.
The objective of the current study was to identify optimal formulation variables and process parameters for spray drying of siRNA-loaded PLGA NPs. The success criterion was obtaining a product suitable for inhalation without compromising the integrity and biological activity of the active compound in the production. To the authors' knowledge, this is the first time that spray drying of a delivery vehicle containing siRNA intended for pulmonary delivery has been performed.
2. Materials and methods
2.1. Materials
Enhanced green fluorescent protein (EGFP) and luciferace (FLuc) Dicer substrate asymmetric duplex siRNAs were provided by Integrated DNA Technologies BVBA (Leuven, Belgium) as dried, annealed, purified and desalted duplexes. Sequences were as follows: EGFP, sense 5′-pACCCUGAAGUUCAUCUGCACCACcg-3′, antisense 5´-CGGUGGUGCAGAUGAACUUCAGGGUCA-3´, FLuc sense 5′-pGGUUCCUGGAACAAUUGCUUUUAca-3′, antisense 5´-UGUAAAAGCAAUUGUUCCAGGAACCAG-3´ [22]. Lower case letters are 2´-deoxyribonucleotides and underlined capital letters are 2´-O-methylribonucleotides. PLGA (lactide:glycolid molar ratio of 75:25, M w: 20 kDa) was purchased from Wako Pure Chemical Industries, Ltd (Osaka, Japan). Polyvinylalcohol (PVA) 403 with an 80.0% degree of hydrolysis was provided by Kuraray (Osaka, Japan). All other general chemicals were obtained commercially at analytical grade.
2.2. Preparation of PLGA NPs
Two different techniques were employed to prepare siRNA-loaded and non-loaded PLGA NPs. The use of the two different methods was required since it was not possible, in the small-scale lab available, to produce larger amounts of non-loaded NPs required for process optimization by the double emulsion solvent evaporation (DESE) method. However, NPs produced by the two methods had similar characteristics and are thus considered to be comparable (results not shown).
The DESE method was used for production of siRNA-loaded NPs. In brief, 250 µL of an 8 µM siRNA solution in TE-buffer (10 mM Tris–HCl, 1 mM EDTA, pH 7.5) was added to 500 µL of a 60 mg/mL solution of PLGA in dichloromethane, and the mixture was sonicated for 90 s to obtain an emulsion. A volume of 2 mL 2% (w/v) PVA in diethylpyrocarbonate (DEPC)-treated water was added to the emulsion, and a second sonication of 1 min was performed, giving a double emulsion. The double emulsion was subsequently diluted with 4 mL of 2% (w/v) PVA in DEPC-treated water and left for 3 h under stirring to evaporate the dichloromethane. For isolation of NPs, the dispersion was centrifuged for 12 min at 4 °C and 18,000 ×g. The supernatant was discarded, and the NP pellet was redispersed in DEPC-treated water. Centrifugation and redispersion of the NPs were repeated three times.
The “modified spontaneous emulsification solvent diffusion” (modified-SESD) method was used for the production of non-loaded PLGA NPs, employed in the process optimization [23]. A mixture consisting of 7.5 mL acetone and 5 mL methanol was used to dissolve 500 mg of PLGA. The polymer solution was then added dropwise to 50 mL 4% (w/v) aqueous PVA solution at a rate of approximately 2 mL/min. The PVA solution was continuously stirred by a Eurostar basic propeller mixer (IKA Labortechnik, Germany) at 400 rpm concomitant with addition of the polymer solution. NPs were isolated as described above but with centrifugation at 41,000 ×g for 12 min.
2.3. Spray drying
The nanocomposite microparticles were prepared by spray drying of a PLGA NP suspension in different aqueous carbohydrate solutions using a Büchi B-290 spray dryer (Büchi Labortechnik AG, Postfach, Switzerland). The spray dryer was a co-current model, equipped with a nozzle atomizer using nitrogen as the atomizing gas. The nozzle orifice diameter was 0.7 mm. Dry particles were separated from the airstream by centrifugal forces using a high-performance cyclone (Büchi Labortechnik AG, Postfach, Switzerland). The applied process parameters (Table 1 ) were chosen on the basis of pilot experiments providing a dry powder which could be reconstituted into a NP suspension without any marked change in the NP size distribution (data not shown).
Table 1.
Spray drying process parameters.
| Parameter | Level |
|---|---|
| Feed rate | 0.3 mL/min |
| Atomizing air flow | 473 L/h |
| Aspirator capacity | 70% |
| Inlet temperature | 45 °C |
| Approximate outlet temperaturea | 30 °C |
Outlet temperature measured between drying chamber and cyclone.
2.4. Experimental design and analysis
The formulation parameters evaluated by design of experiment were i) type of carbohydrate excipient, ii) ratio of NPs to excipient and iii) total concentration of carbohydrate excipient and NP in the feed solution (Table 2 ). A factorial 2 × 2 × 3 experimental design with 6 centre points was set up using the software SAS JMP® (SAS Institute Inc., Cary, NC, USA).The complete design is shown in Table 3 . Analysis of the results was carried out in SAS JMP® using the standard least squares approach.
Table 2.
Formulation parameters included in the experimental design.
| Low level | Centre level | High level | |
|---|---|---|---|
| Concentration (mg/mL)a | 10 | 20 | 30 |
| Ratio (w/w)b | 0.2 | 0.5 | 0.8 |
| Excipient | Mannitol | Lactose | Trehalose |
Total amount of carbohydrate excipient and NP in the feed solution.
Relative amount of NPs (by weight) compared to the total amount of dry substance.
Table 3.
Responses for optimization samples.
| Run no. | Formulation parameters |
Responses |
|||||
|---|---|---|---|---|---|---|---|
| Excipient | Ratioa | Concentration (mg/mL) | Yield (%) | Moisture content (%) (n = 1) | MMAD (µm) (n = 3) | Morphology | |
| 1 | Trehalose | 0.2 | 10 | 47.8 | 5.6 | 2.93 ± 0.05 | Smooth |
| 2 | Trehalose | 0.5 | 20 | 39.2 | 4.7 | 2.81 ± 0.24 | Raisin |
| 3 | Lactose | 0.8 | 30 | 45.5 | 2.1 | 3.07 ± 0.03 | Raisin |
| 4 | Lactose | 0.8 | 10 | 19.5 | 1.5 | 2.25 ± 0.08 | Raisin |
| 5 | Mannitol | 0.5 | 20 | 48.9 | 0.5 | 3.25 ± 0.06 | Raisin |
| 6 | Trehalose | 0.8 | 10 | 19.3 | 2.2 | 2.76 ± 0.15 | Raisin |
| 7 | Mannitol | 0.2 | 10 | 34.6 | 0.5 | 3.41 ± 0.02 | Smooth |
| 8 | Trehalose | 0.5 | 20 | 39.4 | 4.0 | 4.21 ± 0.05 | Raisin |
| 9 | Mannitol | 0.5 | 20 | 43.9 | 0.4 | 3.07 ± 0.10 | Raisin |
| 10 | Lactose | 0.5 | 20 | 33.0 | 3.7 | 2.73 ± 0.27 | Raisin |
| 11 | Lactose | 0.2 | 10 | 41.8 | 7.0 | 2.92 ± 0.05 | Smooth |
| 12 | Lactose | 0.5 | 20 | 36.7 | 5.0 | 3.55 ± 0.13 | Raisin |
| 13 | Mannitol | 0.8 | 10 | 31.2 | 0.5 | 2.54 ± 0.45 | Raisin |
| 14 | Trehalose | 0.8 | 30 | 46.1 | 2.2 | 3.90 ± 0.06 | Raisin |
| 15 | Mannitol | 0.2 | 30 | 56.4 | 0.5 | 4.84 ± 0.12 | Smooth |
| 16 | Mannitol | 0.8 | 30 | 42.4 | 0.4 | 3.28 ± 0.10 | Raisin |
| 17 | Lactose | 0.2 | 30 | 44.9 | 4.8 | 5.36 ± 0.56 | Smooth |
| 18 | Trehalose | 0.2 | 30 | 51.3 | 5.1 | 5.62 ± 0.10 | Smooth |
Relative amount of NPs ( by weight) compared to the total amount of dry substance.
2.5. Characterization of nanocomposite microparticles
2.5.1. Yield
The dry powder yield was determined as the difference in weight of the sample vial before and after product collection. The weight difference was compared to the total dry mass used for the specific sample, and the yield in % was calculated. The moisture content in the dry powder was not considered when calculating the yield.
2.5.2. Powder moisture content
The water content of the powder was examined by thermogravimetric analysis, using a TGA 7 (Perkin Elmer, Waltham, Massachusetts, USA) with nitrogen purging. Samples were heated to 110 °C at a constant rate of 10 °C/min. The temperature was kept at 110 °C until a stable weight was obtained. The weight loss in percent due to water evaporation was subsequently calculated and defined as the moisture content.
2.5.3. Particle morphology
The surface morphology was examined using a scanning electron microscope (SEM) of the type JSM-5200 Scanning microscope (JEOL Ltd., Tokyo, Japan). A powder sample was sprinkled onto a SEM stub covered with double side carbon tape and subsequently sputter coated with gold prior to examination.
2.5.4. Aerodynamic particle size
The aerodynamic particle size was determined using an Aerodynamic Particle Sizer Spectrometer 3321 equipped with a Small-Scale Powder Disperser (TSI incorporated, Shoreview, MN, USA) used to generate the aerosol. The instrument employed a time of flight principle, measuring the velocity of the individual particles where the aerodynamic diameter was defined as the diameter of a spherical particle with the same velocity and behaviour as the analyzed particle. The resulting particle size distribution accounted for the density, was number based and was afterwards converted to a mass based particle size distribution by the software provided with the instrument. A few mg of spray dried powder was placed on the turntable inside the Small-Scale Powder Disperser and introduced into the Aerodynamic Particle Sizer by a Venturi aspirator. The flow rate and the rotation rate of the Small-Scale Powder Disperser were controlled to yield a particle concentration in the range of 0.3–3.0 mg/m3 and analysis was carried out continuously for 20 s. It was assumed that the shear forces present in the Small-Scale Powder Disperser were sufficient to deagglomerate the spray dried particles, which might not be the case for pharmaceutical devices used for inhalation therapy. For powders dispersed with metered dose inhalers the aerodynamic particle size obtained with the Aerodynamic Particle Sizer Spectrometer 3321 correlated well with the aerodynamic particle size obtained with cascade impactors [24], [25]. The formulation in the present study was spray dried and the dry powder was assumed to be chemically homogeneous making the chemical analysis of the cascade impactors less important. In the following text, the aerodynamic particle size is given as the mass median aerodynamic diameter (MMAD).
2.5.5. Content of PVA
The content of PVA in the NPs was examined by UV-spectroscopy, employing the method described by Barman et al. [26]. In brief, 5 mL of 1 N NaOH was added to a sample of 10 mg NPs (10 mg of PLGA as reference sample) and left while rotating until no solid was visible. 0.9 mL 37% (v/v) HCl was added. To 1 mL of sample solution, 0.9 mL of 3.7% (w/v) boric acid was added to form the borate salt, and subsequently 0.1 mL KI/I2 stock solution (3.32 g of KI and 2.54 g of I2 in 200 mL of deionized water) was added. The OD620 nm was measured on a Cary® 100 Bio UV–visible spectrophotometer (Varian Inc., Palo Alto, CA, USA) and the value for the reference sample was subtracted. The concentration was determined from a standard curve in the linear range of 0.01 mg–1 mg PVA.
2.5.6. Redispersibility
To evaluate the ability of the nanocomposite microparticles to decompose into PLGA NPs, 1 mL of water was added to approximately 1 mg of powder sample. After mixing, the sample was centrifuged for 12 min at 4 °C and 18,000 ×g and washed three times with water. The NPs were redispersed in water, and the size was determined by dynamic light scattering using the photon correlation spectroscopy (PCS) technique. The measurements were performed on diluted samples (n = 3) at 25 °C using a Malvern NanoZS (Malvern Instruments, Worcestershire, UK) equipped with a 633 nm laser and 173° detection optics. Malvern DTS v.5.10 software (Malvern Instruments, Worcestershire, UK) was used for data acquisition and analysis. A polystyrene size standard (220 ± 6 nm, Duke Scientific Corp., Duke, NC) was used to verify the performance of the instrument.
2.6. Stability and biological activity of siRNA
The integrity of the siRNA extracted from the spray-dried NPs was evaluated by gel electrophoresis to investigate the effects of the spray drying process on siRNA loaded into NPs. The biological activity was determined in transfection assays using the human epithelial non-small cell lung cancer cell line H1299 stably transfected with EGFP (a gift from Prof. Jørgen Kjems, University of Aarhus, Denmark).
2.6.1. Extraction and quantification of siRNA
siRNA was extracted from the NPs before and after spray drying by dissolving the PLGA matrix in chloroform. Briefly, an amount of powder corresponding to approximately 1 mg of NP was weighed. Samples consisting of nanocomposite microparticles were dissolved in 1 mL of water, the NPs were separated by centrifugation for 12 min at 4 °C and 18,000 ×g and the supernatant discarded. A volume of 200 µL CHCl3 and 500 µL TE-buffer was added, and the samples were rotated for 90 min. The two phases were separated by centrifugation for 20 min at 18,000 ×g and 4 °C, and the aqueous phase was collected.
The siRNA concentration in the extracts was determined using the RiboGreen® RNA quantitation reagent (Invitrogen A/S, Taastrup, Denmark). The concentration was determined using a standard curve ranging from 0 to 50 ng/mL, and samples were diluted to obtain values in this range. The fluorescence of the samples was measured using a FLUOstar OPTIMA fluorescence plate reader (BMG Labtech, Offenburg, Germany) at an excitation wavelength of 485 nm and an emission wavelength of 530 nm.
2.6.2. Integrity of siRNA
The integrity of extracted siRNA was evaluated on a 4–20% polyacrylamide gel containing TBE buffer (0.089 M Tris base, 0.089 M boric acid, and 2 mM sodium EDTA, pH 8.3). Electrophoresis was carried out in TBE buffer at a constant voltage of 100 V for 1 h. siRNA bands were visualized using an image station (Kodak Image Station 1000, Eastman Kodak Company, OR, US) after staining for 40 min with a 1:10,000 dilution of SYBR-Green II RNA gel stain (Invitrogen A/S, Taastrup, Denmark) in DEPC-treated water.
2.6.3. Biological activity of spray-dried siRNA
2.6.3.1. Cell culture
H1299 cells stably expressing EGFP were maintained in RPMI 1640 Medium (Fisher Scientific Biotech Line, Slangerup, Denmark) supplemented with 100 U/mL penicillin, 100 µg/mL streptomycin, 2 mM L-glutamine (all from Sigma-Aldrich, Brøndby, Denmark), 0.2 mg/mL Geneticin (Invitrogen A/S, Taastrup, Denmark) and 10% (v/v) fetal bovine serum (FBS) (PAA Laboratories GmbH, Pasching, Austria). Cells were grown in an atmosphere of 5% CO2/95% O2 at 37 °C, growth medium was replaced every 48 h, and the cells were sub-cultured approximately 1:5 twice a week. Prior to siRNA addition, H1299 cells were detached from culture flasks by incubating the cells for 5 min at 37 °C with trypsin/EDTA solution. The cells were seeded in 24-well tissue culture plates in 0.5 mL growth medium, at a density of 8 × 104 cells per well, and kept at normal culturing conditions for 24 h.
2.6.3.2. Transfections and flow cytometry
After 24 h (approximately 80% confluency), the cells were incubated with 300 µL of pre-mixed samples in fresh RPMI 1640 + 10% (v/v) FBS for 48 h at 37 °C. The samples consisted of either 2 nM siRNA extracted from NPs or MPs at a concentration of 1 mg/ml tested before or after spray drying and with or without the addition of lipofectamine 2000. In the case of the “extracted” control non-loaded samples, sample volumes were identical to siRNA-containing samples. Lipofectamine 2000 (Invitrogen A/S, Taastrup, Denmark) was added in amounts recommended by the manufacturer. After incubation, the cells were washed with 500 µL PBS, and 100 µL of TrypLETM Express (Invitrogen A/S, Taastrup, Denmark) was added. After incubation with TrypLETM Express for 5 min, 1 mL of growth medium was added, the cells were transferred to tubes and centrifuged for 10 min at 1076 ×g and 4 °C. The supernatant of each tube was removed, and the cells were redispersed in PBS containing 10% (v/v) FBS followed by a second centrifugation, after which the cells were redispersed in ice-cold PBS containing 10% (v/v) FBS (1 mL) and 1 µg/mL propidium iodide (PI) (Invitrogen A/S, Taastrup, Denmark) for staining of dead cells. The cells were analysed on a FACScan flow cytometer (Becton Dickinson, NJ, USA) using the CellQuest Software (Becton Dickinson). Dead cells were excluded based on the PI-staining.
2.7. Statistical analysis
Measurements were performed in triplicate, unless otherwise stated. Values are given as means ± SD. The statistical significance of the results obtained from cell transfections was determined using one-way analysis of variance (ANOVA) employing a confidence interval of 95%.
3. Results
The present study addresses the influence of different formulation and processing parameters on the characteristics of spray-dried nanocomposite microparticles. The results were evaluated to determine the optimal formulation for spray drying of PLGA NP-based nanocomposite particles. Further, siRNA-containing PLGA NPs were spray dried using the optimal formulation, and the siRNA extracted from the NPs was evaluated with respect to content, integrity and biological activity.
3.1. Nanocomposite microparticle optimization
In Table 3, the design of experiment is listed along with the responses. The samples were spray dried using non-loaded NPs for optimisation purposes.
The product yield after the spray drying process varied between 19.3 and 56.4%. The moisture content for all samples was relatively low, and the samples containing mannitol displayed considerably lower moisture content than the other samples. The majority of the samples showed a MMAD within the desired range of 1–5 µm, and only a few had a diameter slightly above 5 µm. All samples with a NP to excipient ratio of 0.2 appeared as particles with a smooth surface morphology, while samples having a ratio of 0.5 or 0.8 displayed corrugated and raisin-like surfaces (Fig. 1 ).
Fig. 1.
SEM images of samples containing co-spray-dried NPs and lactose (total dry substance concentration: 10 mg/mL). A: ratio 0.2. B: ratio 0.5.
The average diameters of the NPs before and after spray drying, as well as the polydispersity indexes, are shown in the supplementary data section. All samples could be redispersed completely into NPs with no marked change in the NP size, as compared to the size before spray drying.
Statistical evaluation was performed on data presented in Table 3. The analysed output values were moisture content, yield and MMAD. Fig. 2 shows the impact of the different formulation parameters on the measured responses.
Fig. 2.
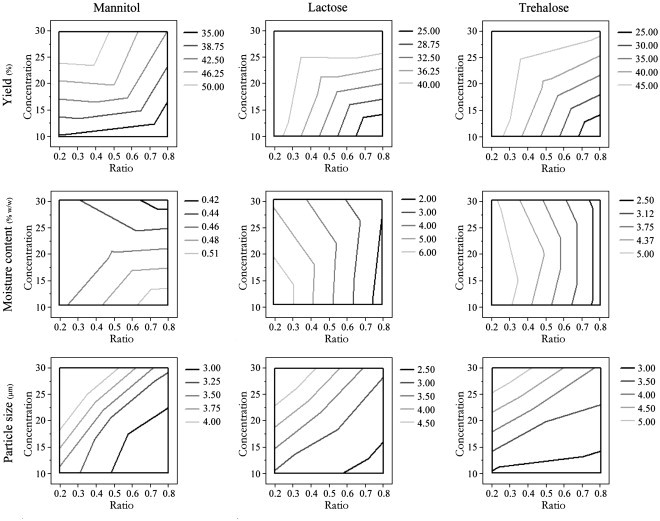
Contour plots showing the effect of parameters (yield, moisture content and particle size) on outcome, separated by excipient.
It is apparent from Fig. 2, that the products produced with lactose and trehalose possessed similar characteristics. Mannitol changed the effects exerted by the ratio and concentration somewhat as compared to the two other excipients. Table 4 shows the statistically significant parameters for each of the three evaluated responses. The scaled estimates provide an overview of the magnitude of the exerted effects found.
Table 4.
Statistical significance of formulation parameters.
| Moisture content |
Particle size |
Yield |
||||||
|---|---|---|---|---|---|---|---|---|
| P-value | Scaled estimate | P-value | Scaled estimate | P-value | Scaled estimate | |||
| Ratio | 0.0001 | − 1.21 | Ratio | 0.0023 | − 0.61 | Ratio | 0.0063 | − 6.06 |
| Mannitol | <0.0001 | − 2.36 | Concentration | 0.0005 | 0.77 | Concentration | 0.0016 | 7.71 |
| Lactose | 0.0003 | 1.20 | ||||||
| Trehalose | 0.0004 | 1.15 | ||||||
In brief, the moisture content was affected significantly by the type of excipient, and mannitol provided the lowest water content of the three examined excipients (P < 0.0001). The ratio of NPs to excipient also have a significant effect on the water content (P = 0.0001), since a high ratio resulted in a decrease in the water content. Factors showing a significant effect on the aerodynamic diameter of the nanocomposite microparticles were ratio (P = 0.0023) and concentration (P = 0.0005) of the sample. High ratios provided particles of slightly smaller MMAD, whereas high concentrations resulted in higher MMAD. The dry powder yield was significantly affected by the ratio of NPs to excipient (P = 0.0063), as increasing the ratio lead to a lower yield. Also, the yield was dependent on the concentration of total dry substance in the feed formulation (P = 0.0016), since a higher yield was obtained at increased concentrations. However, the effect of concentration on the yield could also be due to a reduced loss in the collection process, since larger amounts were used with increasing concentrations. To ensure that no bias was imposed on the result by the use of different batches of NPs, the effect of the batch of NPs on the different outcomes was examined. No significant difference was observed from the variation of the NP batches (data not shown).
The optimum formulation found to provide a low water content, a sufficient yield and a small particle size was with mannitol as the carbohydrate excipient, a total dry substance concentration of 30 mg/mL and a ratio of NPs to excipient of 0.2. Therefore, this formulation was used in the following experiments.
3.2. Characterisation of siRNA-containing nanocomposite microparticles
NPs containing siRNA were produced for spray drying into nanocomposite microparticles. The NPs had an average diameter of 262 ± 6.5 nm with a polydispersity index of 0.186 ± 0.019, and the encapsulation efficiency of the siRNA was 32 ± 5.5% corresponding to 381 ± 66 ng siRNA/mg nanoparticles with this particular production method. The resulting powder of nanocomposite microparticles had a MMAD of 4.99 ± 0.15 µm and a water content of 0.78 ± 0.067% (w/w, n = 2). The NPs were redispersible after spray drying into nanocomposite microparticles upon addition of DEPC-treated water (data not shown). The content of residual PVA in the siRNA-containing NPs was 6.5 ± 0.5% (w/w, n = 2), corresponding to a level of 1.3% w/w in the nanocomposite microparticles. This suggests that the PVA is relatively strongly bound to the NPs, which was also suggested in previous reports [23]. The amount of PVA present in the nanocomposite microparticles is relatively low, and should thus not present an obstacle for future inhalation therapy. The procedure of production of NPs and nanocomposite microparticles has been repeated several times with similar results.
The surface morphology of the siRNA-containing microparticles was examined by SEM. The microparticles displayed a relatively smooth surface morphology (Fig. 3 ), as expected from the previous investigations, since the employed ratio of NPs to carbohydrate was 0.2.
Fig. 3.
SEM image of siRNA-containing nanocomposite microparticles.
3.3. The siRNA is preserved during the spray drying process
The content of siRNA in the nanocomposite microparticles was extracted, and the total concentration was determined to be 232 ± 18 ng siRNA/mg microparticles, corresponding to 60% of the content in the NPs before spray drying. On investigation of the ratio of NPs to carbohydrate before and after spray drying, no difference was found, indicating that the loss of carbohydrate and NPs in the spray dryer was equal, and the calculations of the concentration of siRNA in the nanocomposite microparticles are valid. To ensure that the integrity of the siRNA was maintained during the spray drying process, non-denaturing PAGE was performed. The siRNA extracted from the nanocomposite microparticles appeared intact and of the same size as before the encapsulation and spray drying (Fig. 4 ).
Fig. 4.
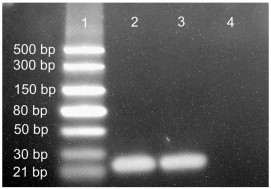
Lane 1, dsRNA marker; Lane 2, positive control; Lane 3, sample extracted from spray-dried particles; Well 4, sample extracted from spray-dried particles containing non-loaded NPs.
To prove that the siRNA in the nanocomposite microparticles maintained the biological activity, H1299 cells were transfected with siRNA extracted from the nanocomposite microparticles (Fig. 5A). The results showed that there was no significant difference between the activity of the siRNA extracted from nanocomposite microparticles and the positive control siRNA. No significant difference was observed between the blank and the negative control or the non-loaded nanocomposite microparticles (Fig. 5A). NPs containing siRNA were evaluated for their biological activity in the H1299 cell line. The evaluation of the gene silencing efficiency of the NP was done both before and after spray drying as well as with and without the addition of lipofectamine 2000 to the samples. Importantly, NPs mixed with lipofectamine 2000 provided 68% knockdown of EGFP expression in H1299 cells (Fig. 5B). None of the tested samples showed significantly higher cell death than the negative control, as determined from the PI staining (results not shown).
Fig. 5.
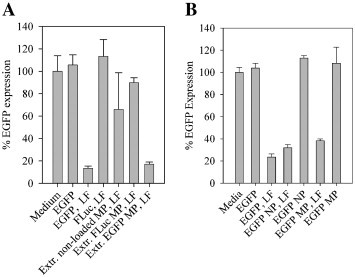
EGFP expression in H1299 cells after incubation for 48 h. A: Samples of extractions from nanocomposite microparticles (MP), lipofectamine 2000 (LF) was used as a transfection reagent for samples containing free siRNA. B: Samples of siRNA containing NPs not processed by spray drying and NPs redispersed from nanocomposite microparticles (MP) with and without the addition of lipofectamine 2000 (LF).
4. Discussion
The effect of different variables of the spray drying process on the outcome of the nanocomposite microparticles was investigated using non-loaded NPs. A statistical design of experiment approach was used in order to extract as much information as possible from the results. The nanocomposite microparticles were all shown to be of acceptable quality, proving that the chosen fixed parameters were suitable. However, nanocomposite microparticles containing mannitol showed a significantly lower water content than the other formulations. The low water content in the powder is highly desirable, since it will decrease interparticulate cohesion and thereby increase the respirability of the powder [15], [27]. A high water content might also have a negative effect on the stability of the product [15]. For these reasons, mannitol was chosen for all succeeding studies. The ratio of NPs to excipient was also shown to have an effect on the water content; increasing the ratio provided a lower moisture content. However, this effect was minor compared to the effect of using mannitol and was thus not taken into consideration. Since all samples containing mannitol had acceptable water content, no further attempts were made to reduce the water content.
The concentration and the ratio of NPs to excipient had a significant effect on the particle size. The concentration of total dry substance in the feed formulation had a positive effect on the particle size, as the particle size increased with a higher concentration. This effect has previously been described in literature [16], [28], [29], [30] and is thought to be caused by a larger amount of dry substance in each droplet after atomisation in the spray dryer. The ratio had a small negative effect on the particle size, as the particle size decreased when the amount of NPs in the feed formulation was increased. This could be correlated to the observation of corrugated particles appearing when higher amounts of NPs were used. The corrugated surface morphology changes the aerodynamic properties of the particles and thus also the MMAD.
The ratio and concentration in the feed formulation were also shown to have an effect on the yield of dry powder. Increased concentrations resulted in a higher yield, whereas a high ratio reduced the yield. An increase in yield at higher concentrations has previously been reported [16], [19] and corresponds well with the results. Since the cyclone used for product recovery relies on centrifugal forces for collection of the final product, larger particles will have a higher recovery rate. As it has already been shown that increased concentrations lead to larger MMAD, the observation that increasing concentrations increase the yield corresponds well with this. The finding that increasing the ratio provides lower yields also corresponds with this, since the increased ratio has been shown to decrease the MMAD. Besides providing the optimal formulation, the experimental setup for determination of the optimal factors proved that the fixed spray drying parameters were suitable for the formulation of nanocomposite microparticles with a size range applicable for inhalation, since almost all samples were of the appropriate size and with moderate water content. Thus, based on the results of the statistical evaluation a high concentration and a low ratio combined with mannitol as excipient were chosen for further studies.
As mentioned above, it has been reported previously that mannitol, when present in larger amounts, can have a negative effect on the respirable fraction of spray-dried particles, probably due to formation of particle aggregates [20]. This effect is not seen from the values measured in this study. Particles containing mannitol in this study have very low water content, and this may be the reason for the different results, since the particle aggregation will be significantly reduced. The analysis method in this study differs from the method used for measuring the respirable fraction in the study by Bosquillon et al. [20], where the methods of tap density and electrical zone sensing on spray-dried powders suspended in saline was combined to provide an aerodynamic diameter. The aerodynamic behaviour was investigated using an Andersen cascade impactor. The difference in analysis methods could also explain the difference in results, but it is very likely that the low moisture content seen in this study provides a significant reduction in particle aggregation leading to the different conclusions. The redispersibility of non-loaded NPs from the spray-dried powders were successfully demonstrated for the developed method of spray drying for each of the three different excipients, and it is highly likely that the results for siRNA-loaded NPs spray dried with mannitol will also apply for nanocomposite particles with the remaining two excipients.
The surface morphology of the produced nanocomposite microparticles was also investigated. There was a difference in surface corrugation when the content of NPs was varied. Some degree of surface corrugation has been shown to be beneficial for particles intended for inhalation, and it is suggested that the improved aerosolization properties of corrugated particles is not only due to the lower aerodynamic particle size but also to a reduction in interparticulate cohesion [31], [32]. Surface corrugation has also been reported to reduce the influence of the inhaler device on the aerosol generation [32]. Even a small degree of corrugation is sufficient to obtain the described effects [31]. Corrugated particles might also be more appropriate for dissolution in the lung fluid due to a larger surface area. This could be an advantage in the case of the nanocomposite microparticles since the rapid release of the NPs into the lung fluid is necessary to avoid macrophage clearance. The discovered possibility of modifications of the surface corrugation in the nanocomposite microparticles represents an interesting possibility for further optimisation of the system for delivery by inhalation. In addition, since the siRNA-containing NPs were produced by a modified version of the double emulsion solvent evaporation method, the size of the NPs can easily be modified to provide smaller particles by making small changes to the production method. Smaller NPs could possibly minimize loss by macrophage clearance of released NPs following inhalation [10], [11].
Importantly, the data confirms that the siRNA is preserved during the spray drying process. The siRNA extracted from the microparticles was shown to be of the same size as before spray drying when analysed by PAGE. To ensure that the biological activity of the siRNA was preserved, two cell transfection studies were performed. The first study showed that the biological activity of the siRNA was retained after spray drying by evaluating the gene silencing effect of siRNA extracted from NPs combined with the commercial transfection reagent lipofectamine 2000 (Fig. 5A). The second study evaluated the silencing efficiency of the NPs before and after spray drying and with or without the addition of lipofectamine 2000 (Fig. 5B). The NPs displayed a good gene silencing efficiency in combination with lipofectamine 2000. However, there was no observable effect with the NPs without lipofectamine. Preliminary results indicate that the lack of silencing activity for the NPs alone might be due to a very slow siRNA release, which can be accelerated in the presence of lipofectamine 2000, resulting in the release of a dose sufficient for gene silencing within the 48 h of the in vitro experiment. No cellular toxicity was observed upon addition of lipofectamine 2000. In addition, the NPs seem to be capable of endosomal escape, which would therefore not be the cause of the lack of silencing in this short-term cell study. We are currently testing the effect of modified PLGA matrixes on the release profile and the gene knock-down efficiency of the siRNA-loaded NPs. Such modifications should ideally not compromise the favourable safety profile of the polymer, and still enable the preparation of nanocomposite microparticles with optimal characteristics for inhalation therapy.
Although there seems to be a small degree of degradation during the spray drying process, the remaining siRNA is intact and functional. At least two mechanisms are suggested to be responsible for this protection: 1) The PLGA matrix protects the siRNA during the spray drying process, and 2) the excipients enhance the in-process stability of the siRNA. The ratio of NPs to excipient was assessed after spray drying in order to eliminate an uneven loss of NPs and carbohydrate excipient as a cause of the decrease in siRNA content. The ratio was not affected by the spray drying, suggesting that the decrease in siRNA content must be due to degradation.
5. Conclusion
A method has been optimised for the spray drying of nanocomposite microparticles consisting of mannitol and PLGA NPs containing siRNA. The NPs used for spray drying were easily redispersed when the nanocomposite microparticles were dissolved in water. The siRNA encapsulated inside the NPs could be extracted and successfully used for gene knockdown in a cell transfection study using a commercial transfection agent. Also, the NPs showed a significant knockdown when mixed with lipofectamine 2000, probably due to a change of the release profile making the silencing measurable in a short term in vitro study. The ratio of NPs to excipient was found to have a significant effect on the surface morphology of the spray-dried nanocomposite microparticles. This discovery could provide the basis for further optimisation of the nanocomposite microparticles with respect to aerosolization properties. This study has proven for the first time that it is possible to obtain a spray-dried formulation containing siRNA with characteristics known to be optimal for inhalation therapy, without compromising the activity of the siRNA. Future work will address the preparation and in vivo test of this type of nanocomposite microparticles.
Acknowledgements
We are grateful to the Alfred Benzon Foundation (D.C.), the Danish Research Council for Technology and Production Sciences [Grant No. 26-02-0019 (D.M.K.J.)] and the Danish Agency for Science, Technology and Innovation for the financial support. We also acknowledge the Alfred Benzon Foundation and Drug Research Academy for co-funding the FLUOstar OPTIMA plate reader. Dorthe Kyed Ørbæk and Maria Læssøe Pedersen have been of great assistance in the practical work with the SEM and cell cultivation, respectively. In addition, this paper has been carried out with the financial support from the Commission of the European Communities, Priority 3 “Nanotechnologies and Nanosciences, Knowledge Based Multifunctional Materials, New Production Processes and Devices” of the Sixth Framework Programme for Research and Technological Development (Targeted Delivery of Nanomedicine: NMP4-CT-2006-026668). It does not necessarily reflect its views and in no way anticipates the Commission's future policy in this area.
Footnotes
Supplementary data associated with this article can be found in the online version, at doi:10.1016/j.jconrel.2009.10.010.
Appendix A. Supplementary data
References
- 1.Xie F.Y., Woodle M.C., Lu P.Y. Harnessing in vivo siRNA delivery for drug discovery and therapeutic development. Drug Discov. Today. 2006;11:67–73. doi: 10.1016/S1359-6446(05)03668-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Durcan N., Murphy C., Cryan S.A. Inhalable siRNA: potential as a therapeutic agent in the lungs. Mol. Pharm. 2008;5:559–566. doi: 10.1021/mp070048k. [DOI] [PubMed] [Google Scholar]
- 3.Yuan X., Li L., Rathinavelu A., Hao J., Narasimhan M., He M., Heitlage V., Tam L., Viqar S., Salehi M. SiRNA drug delivery by biodegradable polymeric nanoparticles. J. Nanosci. Nanotechnol. 2006;6:2821–2828. doi: 10.1166/jnn.2006.436. [DOI] [PubMed] [Google Scholar]
- 4.Panyam J., Zhou W.Z., Prabha S., Sahoo S.K., Labhasetwar V. Rapid endo-lysosomal escape of poly(DL-lactide-co-glycolide) nanoparticles: implications for drug and gene delivery. FASEB J. 2002;16:1217–1226. doi: 10.1096/fj.02-0088com. [DOI] [PubMed] [Google Scholar]
- 5.Thomas M., Lu J.J., Chen J., Klibanov A.M. Non-viral siRNA delivery to the lung. Adv. Drug Deliv. Rev. 2007;59:124–133. doi: 10.1016/j.addr.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patton J.S. Mechanisms of macromolecule absorption by the lungs. Adv. Drug Deliv. Rev. 1996;19:3–36. 19. [Google Scholar]
- 7.Kuiken T., Taubenberger J.K. Pathology of human influenza revisited. Vaccine. 2008;26(Suppl 4):D59–D66. doi: 10.1016/j.vaccine.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicholls J.M., Butany J., Poon L.L., Chan K.H., Beh S.L., Poutanen S., Peiris J.S., Wong M. Time course and cellular localization of SARS-CoV nucleoprotein and RNA in lungs from fatal cases of SARS. PLoS Med. 2006;3:e27. doi: 10.1371/journal.pmed.0030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brain J.D. Inhalation, deposition, and fate of insulin and other therapeutic proteins. Diabetes Technol. Ther. 2007;9(Suppl 1):S4–S15. doi: 10.1089/dia.2007.0228. [DOI] [PubMed] [Google Scholar]
- 10.Dailey L.A., Jekel N., Fink L., Gessler T., Schmehl T., Wittmar M., Kissel T., Seeger W. Investigation of the proinflammatory potential of biodegradable nanoparticle drug delivery systems in the lung. Toxicol. Appl. Pharmacol. 2006;215:100–108. doi: 10.1016/j.taap.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 11.Groneberg D.A., Witt C., Wagner U., Chung K.F., Fischer A. Fundamentals of pulmonary drug delivery. Respir. Med. 2003;97:382–387. doi: 10.1053/rmed.2002.1457. [DOI] [PubMed] [Google Scholar]
- 12.Tomoda K., Ohkoshi T., Nakajima T., Makino K. Preparation and properties of inhalable nanocomposite particles: effects of the size, weight ratio of the primary nanoparticles in nanocomposite particles and temperature at a spray-dryer inlet upon properties of nanocomposite particles. Colloids Surf. B. Biointerfaces. 2008;64:70–76. doi: 10.1016/j.colsurfb.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 13.Tomoda K., Ohkoshi T., Kawai Y., Nishiwaki M., Nakajima T., Makino K. Preparation and properties of inhalable nanocomposite particles: effects of the temperature at a spray-dryer inlet upon the properties of particles. Colloids Surf. B. Biointerfaces. 2008;61:138–144. doi: 10.1016/j.colsurfb.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 14.Makino K., Terada H., Nakajima T., Tomoda K. EP20070738833 20070316. Dec 24 2008. Nanocomposite particle. [Google Scholar]
- 15.You Y., Zhao M., Liu G., Tang X. Physical characteristics and aerosolization performance of insulin dry powders for inhalation prepared by a spray drying method. J. Pharm. Pharmacol. 2007;59:927–934. doi: 10.1211/jpp.59.7.0003. [DOI] [PubMed] [Google Scholar]
- 16.Maltesen M.J., Bjerregaard S., Hovgaard L., Havelund S., van de Weert M. Quality by design — spray drying of insulin intended for inhalation. Eur. J. Pharm. Biopharm. 2008;70:828–838. doi: 10.1016/j.ejpb.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 17.Takashima Y., Saito R., Nakajima A., Oda M., Kimura A., Kanazawa T., Okada H. Spray-drying preparation of microparticles containing cationic PLGA nanospheres as gene carriers for avoiding aggregation of nanospheres. Int. J. Pharm. 2007;343:262–269. doi: 10.1016/j.ijpharm.2007.05.042. [DOI] [PubMed] [Google Scholar]
- 18.Masters K. SprayDryConsult International; Charlottenlund: 2002. Spray drying in practice. [Google Scholar]
- 19.Seville P.C., Li H.Y., Learoyd T.P. Spray-dried powders for pulmonary drug delivery. Crit. Rev. Ther. Drug Carrier Syst. 2007;24:307–360. doi: 10.1615/critrevtherdrugcarriersyst.v24.i4.10. [DOI] [PubMed] [Google Scholar]
- 20.Bosquillon C., Lombry C., Preat V., Vanbever R. Influence of formulation excipients and physical characteristics of inhalation dry powders on their aerosolization performance. J. Control. Release. 2001;70:329–339. doi: 10.1016/s0168-3659(00)00362-x. [DOI] [PubMed] [Google Scholar]
- 21.O'Neil . The Merck index. 13. ed. Merck Research Laboratories, Whitehouse Station; 2001. [Google Scholar]
- 22.Rose S.D., Kim D.H., Amarzguioui M., Heidel J.D., Collingwood M.A., Davis M.E., Rossi J.J., Behlke M.A. Functional polarity is introduced by Dicer processing of short substrate RNAs. Nucleic Acids Res. 2005;33:4140–4156. doi: 10.1093/nar/gki732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murakami H., Kobayashi M., Takeuchi H., Kawashima Y. Preparation of poly(DL-lactide-co-glycolide) nanoparticles by modified spontaneous emulsification solvent diffusion method. Int. J. Pharm. 1999;187:143–152. doi: 10.1016/s0378-5173(99)00187-8. [DOI] [PubMed] [Google Scholar]
- 24.Rebits L.G., Bennett D.J., Bhagwat P.A., Morin A., Sievers R.E. Method for quantifying the sample collected by an Andersen Cascade Impactor using total organic carbon analysis. J. Aerosol. Sci. 2007;38:1197–1206. [Google Scholar]
- 25.Stein S.W., Myrdal P.B., Gabrio B.J., Obereit D., Beck T.J. Evaluation of a new Aerodynamic Particle Sizer Spectrometer for size distribution measurements of solution metered dose inhalers. J. Aerosol. Med. 2003;16:107–119. doi: 10.1089/089426803321919870. [DOI] [PubMed] [Google Scholar]
- 26.Barman S.P., Lunsford L., Chambers P., Hedley M.L. Two methods for quantifying DNA extracted from poly(lactide-co-glycolide) microspheres. J. Control. Release. 2000;69:337–344. doi: 10.1016/s0168-3659(00)00308-4. [DOI] [PubMed] [Google Scholar]
- 27.Chew N.Y., Chan H.K. The role of particle properties in pharmaceutical powder inhalation formulations. J. Aerosol. Med. 2002;15:325–330. doi: 10.1089/089426802760292672. [DOI] [PubMed] [Google Scholar]
- 28.Corrigan D.O., Corrigan O.I., Healy A.M. Physicochemical and in vitro deposition properties of salbutamol sulphate/ipratropium bromide and salbutamol sulphate/excipient spray dried mixtures for use in dry powder inhalers. Int. J. Pharm. 2006;322:22–30. doi: 10.1016/j.ijpharm.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 29.Elversson J., Millqvist-Fureby A., Alderborn G., Elofsson U. Droplet and particle size relationship and shell thickness of inhalable lactose particles during spray drying. J. Pharm. Sci. 2003;92:900–910. doi: 10.1002/jps.10352. [DOI] [PubMed] [Google Scholar]
- 30.Mosen K., Backstrom K., Thalberg K., Schaefer T., Kristensen H.G., Axelsson A. Particle formation and capture during spray drying of inhalable particles. Pharm. Dev. Technol. 2004;9:409–417. doi: 10.1081/pdt-200035795. [DOI] [PubMed] [Google Scholar]
- 31.Chew N.Y., Tang P., Chan H.K., Raper J.A. How much particle surface corrugation is sufficient to improve aerosol performance of powders? Pharm. Res. 2005;22:148–152. doi: 10.1007/s11095-004-9020-4. [DOI] [PubMed] [Google Scholar]
- 32.Chew N.Y., Chan H.K. Use of solid corrugated particles to enhance powder aerosol performance. Pharm. Res. 2001;18:1570–1577. doi: 10.1023/a:1013082531394. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


