Abstract
Anatid herpesvirus 1 (AHV-1) CH virulent strain was first isolated from an infected duck and it was found that this virus strain could induce cytopathic effect (CPE) in duck embryo fibroblast (DEF). Following AHV-1 infection, DEF showed morphological changes such as cell rounding, improved refractivity and detachment from the culture surface. However, its pathological characteristics were not adequately known. Related studies were performed and the results showed that syncytium formation could be observed as the other type of CPE in AHV-1 infection. Hematoxylin-eosin staining and 4’, 6-diamidino-2-phenylindole (DAPI) staining of infected DEF were each used to visualize the shape and distribution of chromatin within nuclei and nuclear fragmentation was observed. Chromatin condensation and margination, as well as formation of apoptotic bodies were observed by transmission electron microscopy (TEM). DNA ladder formation was detected in AHV-1 infected cells and apoptosis of the infected DEF was also detected by flow cytometry analysis of Annexin V-FITC/PI staining method. Therefore, it was suggested that AHV-1 virulent strain can induce syncytium and apoptosis in DEF. Syncytium formation and apoptosis observed in this study may contribute to the elucidation of AHV-1 pathogenesis.
Keywords: Anatid herpesvirus 1, Duck embryo fibroblast, Syncytium, Apoptosis, Cytopathic effect
1. Introduction
Anatid herpesvirus 1 (AHV-1) is currently grouped in the family Herpesviridae (Kaleta, 1990) and AHV-1 infection (Attanasio et al., 1980) variously known as duck plague (DP) or duck virus enteritis (DVE) is one of the most widespread and devastating diseases of waterfowls in the family Anatidae and has severely affected the waterfowl industry because relatively high mortality could be observed and a wide host range including domestic and wild ducks, geese, swans and other birds mainly in the family Anatidae, order Anseriformes throughout the world are susceptible (Sandhu and Shawky, 2003).
A cell normally contains only one nucleus. However, a multinucleate cell (syncytium) formation by the joining together of two or more cells has been previously reported in certain types of infections caused by viruses, which include paramyxovirus (Shortridge et al., 1980, Horvath et al., 1992), retrovirus (Hildreth and Orentas, 1989), and herpesvirus (Nazerian and Purchase, 1970, Hamdy et al., 1974, Turner et al., 1998) and syncytia are one of the major cytopathic effects induced by these viruses. In this study, we investigated whether the AHV-1 can induce syncytium in infected DEF to provide more information on its pathogenesis study.
Apoptosis also named programmed cell death (PCD) is a physiologically important process that eliminates redundant, damaged, or infected cells and is defined by typical changes in cellular morphology and biochemical features, including DNA fragmentation, chromatin condensation, and cellular breakdown into apoptotic bodies (Kerr et al., 1972). Apoptosis as an energy-dependent process of cell suicide without provoking an inflammatory response (Vaux and Strasser, 1996) can be triggered by diverse intracellular and extracellular signals including virus infections. An increasing number of viruses or viral gene products have been reported to induce apoptosis (Groux et al., 1992, Ito et al., 1997, Ruggieri et al., 2007, Sanfilippo et al., 2004, Lovato et al., 2003, Sadzot-Delvaux et al., 1995, Aleman et al., 2001, Yuan et al., 2007) both in vitro and in vivo, which includes herpesvirus and is one of the cytolytic properties of viral infections and the phenotype of CPE in vitro.
During the recent years, AHV-1 gene related studies (Jia et al., 2009, Chang et al., 2009, Zhao et al., 2008) have been frequently noticed. However, study on AHV-1 induced apoptosis was not often observed. Since apoptosis plays an important role (Yuan et al., 2007) in the pathogenesis of AHV-1 infection in vivo, in this paper proper modifications were made based on the preliminary findings (Guo et al., 2007) and further steps were taken as well to investigate the AHV-1 induced apoptosis in vitro, which we believe will help clarify the pathological characteristics of AHV-1.
2. Materials and methods
2.1. Cell, virus and infection of DEF with AHV-1
Trypsinization of 9–11-day-old duck embryonated eggs was performed according to standard practice. The dispersed cells were dispensed into cell culture flasks and flat-bottomed six-well tissue culture plastic plates (Corning, Inc., New York, USA) with flying cover slips. Growth medium was Eagle's minimal essential medium that contained 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. Maintenance medium had 3% fetal bovine serum added. Cells were maintained at 37 °C with 5% CO2.
AHV-1 CH virulent strain, first isolated from an infected duck (negative of duck hepatitis B virus infection) with prominent lesions specific of AHV-1 infection and subsequently identified through specific methods, was from the Avian Disease Research Center of Sichuan Agricultural University (Yaan, Sichuan, China). AHV-1 stock was prepared through plaque assay and the titer of infectious virus was determined by the medium tissue culture infective dose (TCID50) method using DEF culture.
Replication of AHV-1 in DEF was performed as follows. Cells were washed twice with sterile phosphate-buffered solution (PBS), and then virus was inoculated onto monolayers about 90% confluency at time of infection. Virus adsorption was complete in 1 h at 37 °C and after that the inoculum was replaced by maintain medium and the DEF was incubated at 37 °C and in 5% CO2. The infected cell cultures were examined regularly under a TE2000U inverted microscope (Nikon, Inc., Kanagawa, Japan) for observation of CPE and images were recorded. The predetermined time points were usually at about 36 h postinfection.
2.2. Cytological analysis
AHV-1 infected and mock-infected DEF were collected at the predetermined time points after infection, washed in PBS, fixed in 4% paraformaldehyde in PBS (pH 7.4) for 60 min, stained with hematoxylin, and examined microscopically for observation of syncytium formation. The specimen collected as listed above were also stained with hematoxylin and eosin, and examined microscopically for observation of chromatin fragmentation.
2.3. Fluorescent microscopic examination of nuclear staining with DAPI
Detection of alterations in nuclear chromatin was conducted according to a slightly modified procedure previously described (Ruggieri et al., 2007). AHV-1 infected and mock-infected DEF were collected at the predetermined time points after infection, fixed in 4% paraformaldehyde for 60 min, permeabilized with 0.2% Triton-X 100 in PBS for 5 min, stained with 4’, 6-diamidino-2-phenylindole (DAPI) fluorochrome and analyzed under Nikon 80i microscope (Nikon, Inc., Kanagawa, Japan).
2.4. Transmission electron microscopy
Transmission electron microscopy of the AHV-1 infected and mock-infected cells were performed according to a slightly modified procedure previously described (Guo et al., 2006). Samples were collected at the predetermined time points after infection, fixed in 2.5% glutaraldehyde at 4 °C for 2 h. The cells were scraped from the flasks and centrifuged at 3000 rpm/min for 5 min. Then the supernatant was discarded and the pellets were mixed with 2% low melting-temperature agarose at 37 °C, and centrifuged at 6000 rpm/min for 10 min. Samples were post-fixed in 1.0% osmium tetroxide. After a stepwise dehydration in acetone, samples were embedded in epoxy resin 618 and polymerized at 80 °C for 72 h. Then, 50 nm ultra-thin sections were prepared with an LKB ultratome (LKB Instruments, Inc., Rockville, MD), collected on grids, and stained with uranyl acetate and lead citrate for subsequent examination with the Hitachi H-600-A2 transmission electron microscope at an accelerating voltage of 75 kV. Images were recorded on Kodak electron microscope film.
2.5. Analysis of low molecular weight DNA
Internucleosomal degradation of DNA, with appearance of DNA ladders due to activation of nuclear endonucleases is considered a hallmark in most cells undergoing apoptosis. To further ascertain that the AHV-1 virulent strain could induce apoptosis in DEF, DNA in AHV-1 infected DEF was extracted for DNA fragmentation analysis according to a slightly modified procedure previously described (Kim et al., 2002). Briefly, AHV-1infected and mock-infected cells that had grown in cell culture flasks were collected at the predetermined time and washed twice with PBS, then the cell monolayers were scraped by hand-held scraper, collected and resuspended in 600 μl ice-cold lysis buffer followed by a 20 min incubation on ice. High molecular weight DNA and cell debris were removed by centrifugation at 14,000 × g for 20 min at 4 °C. The supernatant fluid was treated with RNase A at 65 °C for 1 h, followed by proteinase K treatment at 50 °C for 1 h. DNA was extracted twice with phenol:chloroform, precipitated at −20 °C for 2 h in double volumes of ethanol and then centrifuged at 14,000 r/min for 10 min. The pellets were resuspended in 50 μl TE buffer and after electrophoresis, the 1.5% agarose gel was stained with ethidium bromide, visualized with a UV light transilluminator, and photographed.
2.6. Annexin V-FITC/PI stained fluorescence-activated cell sorter (FACS)
By staining cells with annexin V-FITC and PI, FACS was performed to distinguish and quantitatively determine the percentage of viable, apoptotic and necrotic cells after AHV-1 infection by using a PharmingenTM Annexin V-FITC apoptosis detection kit (BD Biosciences, San Diego, USA) under the manufacturer's instructions according to modified procedures previously described (Chen et al., 2008). AHV-1 infected and mock-infected DEF were each collected at the predetermined time points after trypsinization, and washed three times with cold PBS through centrifugation at 3000 r/min for 10 min. Then the cells were resuspended in 1× binding buffer at concentration of approximately 1.0 × 106 cells/ml, transferred into a 5-ml culture tube with a volume of 100 μl, and incubated with 5 μl FITC-conjugated annexin V and 5 μl of PI for 15 min at room temperature in a dark cabinet. After that, 1× binding buffer was added into each sample tube to bring the final volume to 500 μl. Then the samples were analyzed with FACSCalibur flow cytometer (BD corporation, San Jose, USA) and corresponding Cell Quest Research Software for Annexin V-FITC/PI stained FACS.
3. Results
3.1. Inverted microscopy observation
The AHV-1 infected and mock-infected cell cultures were examined regularly under inverted microscope for CPE observation and it was found that the AHV-1 infected DEF gradually developed the CPE including cell rounding and detachment at and after 24 h after inoculation. This consisted of scattered foci of plaque surrounded by rounded and refractile cells and the plaques were formed by cell detachment from the culture surface. On the cell monolayer, syncytia (Fig. 1 ) were observed as the other type of CPE in AHV-1 infection.
Fig. 1.
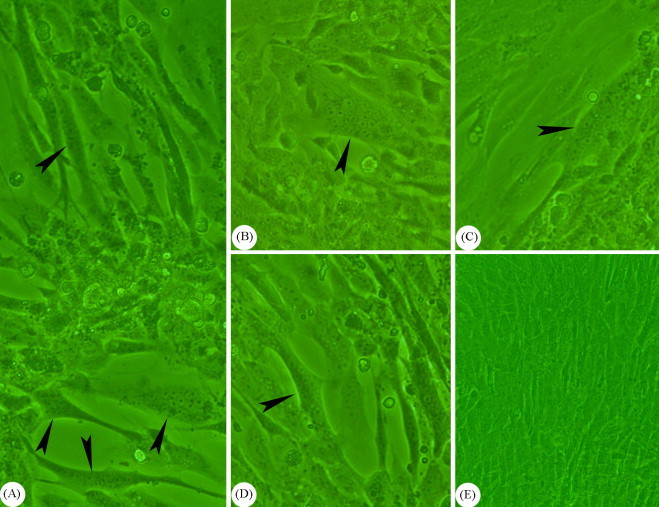
Inverted microscopic observation of the AHV-1 infected and mock-infected cell cultures. The cell monolayer was affected, resulting in syncytium formation (denoted by arrowheads in A, B, C, and D, 200×), which was observed as the other type of CPE in AHV-1 infection. No syncytium formation was observed in mock-infected cell cultures (E, 100×).
3.2. Cytological analysis
AHV-1 infected and mock-infected DEF were stained with hematoxylin solution and the microscopic examination demonstrated that AHV-1 could induce syncytium (Fig. 2 ), as a variant of the CPE in infected DEF. Staining with haematoxylin and eosin revealed chromatin fragmentation (Fig. 3 ) in AHV-1 infected DEF, which could serve as indication of apoptosis.
Fig. 2.
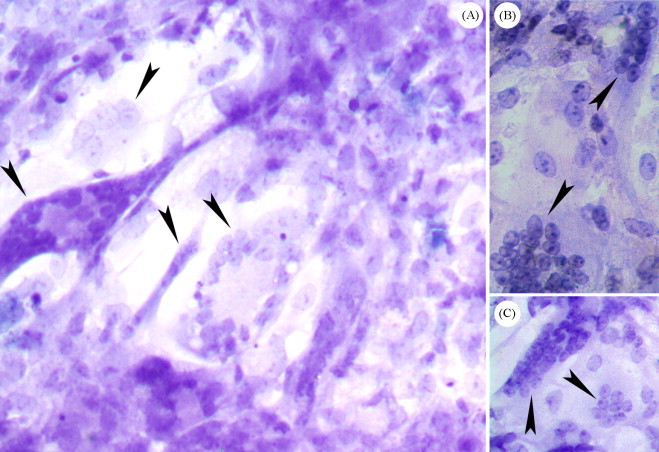
Cytological analysis of the AHV-1 infected DEF by hematoxylin staining. The microscopic examination demonstrated that AHV-1 can induce syncytium (denoted by arrowheads in A, B, 600×; and C, 400×), as a variant of the CPE in infected DEF.
Fig. 3.
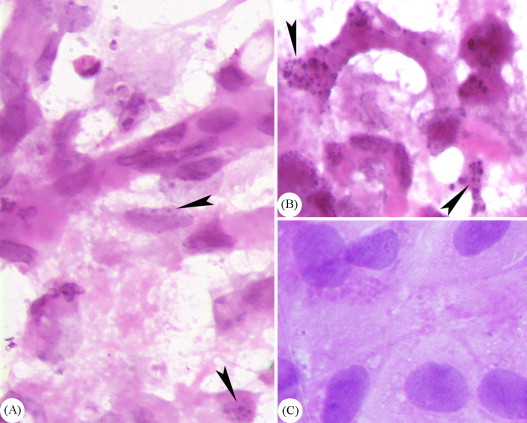
Cytological analysis of the AHV-1 infected DEF by haematoxylin and eosin staining. The microscopic examination demonstrated that AHV-1 can induce chromatin fragmentation (denoted by arrowheads in A, and B, 1000×) in AHV-1 infected DEF, which could serve as indication of apoptosis. No such change was observed in mock-infected cell cultures (C, 1000×).
3.3. Microscopic examination of nuclear staining with DAPI
Nuclei of AHV-1 infected and mock-infected DEF were stained with DAPI which binds to and stains the DNA in condensed chromatin. Fluorescence microscopic examination indicated that at the predetermined time points after infection, nuclei of infected cells appeared fragmented (Fig. 4A) and marginated (Fig. 4B). Typical apoptotic bodies, which are membrane bound portion of fragmented chromatin, could also be detected (Fig. 4C and D). In contrast, nuclei of control non-infected cells were uniformly stained without condensation (Fig. 4E). In addition, multinucleate cell (syncytium) formation could also be observed (Fig. 4F). These results suggested that apoptotic changes and syncytium formation could be induced in DEF by AHV-1 infection.
Fig. 4.
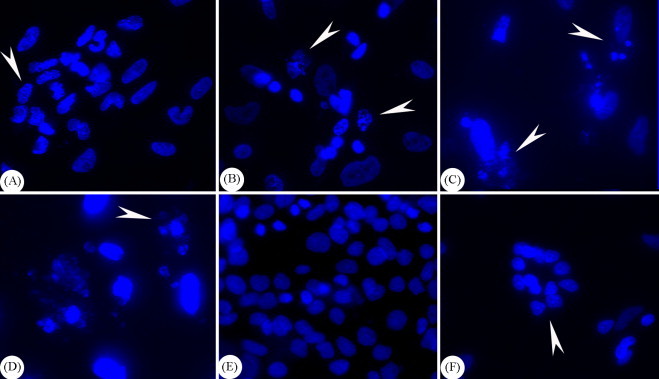
Microscopic examination of nuclear staining with DAPI. Fluorescence microscopic examination indicated that nuclei of infected cells appeared fragmented (denoted by arrowhead in A, 400×) and marginated (denoted by arrowheads in B, 600×). Typical apoptotic bodies could also be detected (denoted by arrowheads in C and D, 600×). Nuclei of mock-infected cells were uniformly stained without condensation (E, 400×). Multinucleate cell (syncytium) formation (denoted by arrowhead in F, 400×) could also be observed by this method.
3.4. Transmission electron microscopy
Transmission electron microscopic examination of the AHV-1 infected and mock-infected cells indicated that chromatin condensation and margination (Fig. 5A) were observed. In addition, nuclei of infected cells appeared intensively stained and fragmented (Fig. 5B), blebbing of the cytoplasm was also observed (Fig. 5A). Typical apoptotic bodies, that are membrane bound portion of fragmented chromatin, could also be detected (Fig. 5C). In contrast, nuclei of control non-infected cells were uniformly stained without condensation (Fig. 5D).
Fig. 5.
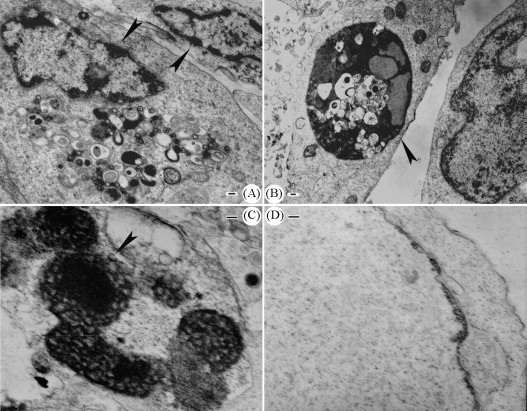
Transmission electron microscopic examination of the AHV-1 infected and mock-infected cells. Nuclei of infected cells appeared condensed and marginated (denoted by arrowhead in A), intensively stained and fragmented (denoted by arrowhead in B), blebbing of the cytoplasm was also observed (A). Typical apoptotic bodies could also be detected (denoted by arrowhead in C). Nuclei of mock-infected cells were uniformly stained without condensation (D). The bars represent 100 nm.
3.5. Analysis of low molecular weight DNA
DNA in the AHV-1 infected and mock-infected DEF was each extracted for DNA fragmentation analysis and as expected, the results revealed that oligonucleosomal DNA ladders was detected (Fig. 6 ) in AHV-1 infected cells, whereas in control cells DNA ladders was not detected. This result clearly indicates that AHV-1 infection induces DNA ladder formation, which is typical of apoptotic cells.
Fig. 6.
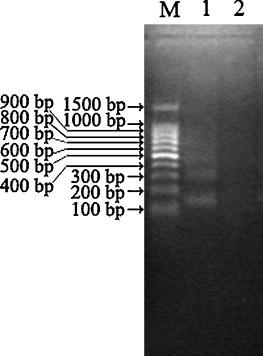
Analysis of low molecular weight DNA. Oligonucleosomal DNA ladders were detected in AHV-1 infected cells (Lane 1), whereas in mock-infected cells no DNA ladder was detected (Lane 2). Lane M: DNA molecular weight marker.
3.6. Annexin V-FITC/PI stained fluorescence-activated cell sorter (FACS)
Alterations of the plasma membrane, with translocation of phosphatidylserine from inner side of the plasma membrane to external surface are hallmark of apoptosis. The annexin V-FITC/PI stained fluorescence-activated cell sorter (FACS) analysis of the AHV-1 infected and mock-infected cells indicated that at the predetermined time points after infection, the percentage of apoptotic cells increased from 1.16% (Fig. 7B) in the mock-infected control culture to 6.94% (Fig. 7A) in the AHV-1 infected group. This result clearly indicates that AHV-1 infection induces membrane alterations characteristic of apoptotic cells in DEF culture.
Fig. 7.
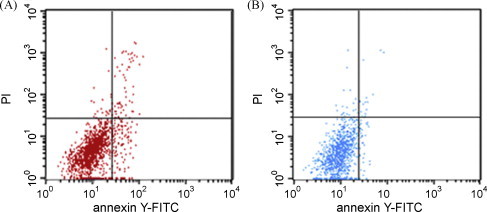
The annexin V-FITC/PI stained fluorescence-activated cell sorter (FACS) analysis of the AHV-1 infected and mock-infected cells. The percentage of apoptotic cells increased from 1.16% (B) in the mock-infected control culture to 6.94% (A) in the AHV-1 infected group.
4. Discussion
Syncytia as one of the main cytopathic effects, can be formed when cells are infected with certain types of viruses including retrovirus, herpesvirus, paramyxoviruses and coronavirus. Infection of chick kidney cell monolayers with avian paramyxoviruses could lead to CPE consisting of small discrete syncytia (Shortridge et al., 1980) containing 2–6 nuclei which were formed by cell fusion and were visible from about 24 h onwards. In duckling kidney cell cultures infected by Muscovy duck reovirus, cytopathic changes in the form of large syncytia could be observed (Heffels-Redmann et al., 1992). When monolayers of primary hamster kidney cell cultures were infected with herpesvirus from turkeys, foci consisting of rounded refractile cells and syncytia were observed (Hamdy et al., 1974). In duck embryo fibroblast cultures infected by Marek's disease virus, well-developed, isolated microplaques were seen and these microplaques were composed of one or more syncytia (Nazerian and Purchase, 1970) in the center and many rounded and fusiform refractile cells in the periphery. All the polykarvocytes observed in this study were true multinucleate cells in which the cytoplasm was not divided by membranes partially separating the nuclei. From the cytological analysis and microscopic observation we can also know that the AHV-1 syncytia formation observed in this study was developed as result of “fusion of neighboring cells”. Polykaryocyte formation appears to be a general feature in herpesvirus-type infections (Nazerian et al., 1971) and since herpes simplex virus type 1 glycoproteins gB, gD, and gHgL are necessary and sufficient to mediate membrane fusion in a Cos cell transfection system (Turner et al., 1998), similar study could also be further undertaken to verify the related gene function of AHV-1.
Apoptosis represents an important antivirus defense mechanism of the host cell, and viruses have evolved strategies to regulate apoptosis in order to maximize the production of virus progeny and promote the spread of virus progeny to neighboring cells. Apoptosis induced by virulent AHV-1 in tissues of infected ducks has been investigated (Yuan et al., 2007) and in this study, by using DEF which is permissive to virulent AHV-1, we characterized apoptotic death of infected cells by morphologic and biochemical techniques.
Virus-induced apoptosis is a complex and important aspect of the pathogenesis of viral infections. In fact, in the case of virus-infected cells, the induction of cell death can either reduce viral spread in the host by early killing of infected cells, or facilitate viral progeny dissemination, through apoptotic bodies, which hide virus antigens and limit induction of inflammatory and immune responses (Koyama et al., 2000). Although it limits spreading of virus, apoptotic death of infected cells is still detrimental as it causes cell and tissue destruction.
AHV-1 could induce apoptosis in the lymphoid organs and apoptosis of lymphocytes may play an important role in the pathogenesis of duck viral enteritis (Yuan et al., 2007). Since AHV-1 infection is systemic and a wide range of cell and tissue types are susceptible (Sandhu and Shawky, 2003), the results of this study performed in vitro by using permissive duck embryo fibroblast would provide more valuable information on the pathological characteristics of AHV-1 which are still largely unknown, and along with the related in vivo study (Yuan et al., 2007) performed by using lymphoid organs, would contribute to the elucidation of AHV-1 pathogenesis by different routes.
In conclusion, this report showed that AHV-1 virulent strain could induce syncytium and apoptosis in duck embryo fibroblast and this finding may help to clarify the pathological characteristics of AHV-1.
Acknowledgements
We gratefully acknowledge the support of the National Natural Science Foundation of China (grant no. 30771598), the Cultivation Fund of the Key Scientific and Technical Innovation Project, department of Education of Sichuan Province (grant no. 07ZZ028), China Postdoctoral Science Foundation (grant no. 20060391027), New Century Excellent Talents program in University (grant no. NCET-06-0818), Scientific and Technological Innovation Major Project Funds in University (grant no. 706050), Program for Changjiang Scholars and Innovative Research Team in University (grant no. IRT0848), National Science and Technology Support Programs (grant no. 2007Z06-017), The earmarked fund for Modern Agro-industry Technology Research System (2009-2013) and Province Basic Research Program (grant no. 07JY029-016/07JY029-017/2008JO0003/2008JY0100/2008JY0102). The author wishes to thank our colleagues for their professional assistance and technical support to this study.
References
- Aleman N., Quiroga M.I., López-Peña M., Vázquez S., Guerrero F.H., Nieto J.M. Induction and inhibition of apoptosis by pseudorabies virus in the trigeminal ganglion during acute infection of swine. J. Virol. 2001;75:469–479. doi: 10.1128/JVI.75.1.469-479.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attanasio R., Olson R., Johnson J.C. Improvement in plaquing methods for the enumeration of Anatid herpesvirus (Duck plague virus) Intervirology. 1980;14:245–252. doi: 10.1159/000149193. [DOI] [PubMed] [Google Scholar]
- Chang H., Cheng A., Wang M., Guo Y., Xie W., Luo Q. Complete nucleotide sequence of the duck plague virus gE gene. Arch. Virol. 2009;154:163–165. doi: 10.1007/s00705-008-0284-6. [DOI] [PubMed] [Google Scholar]
- Chen S., Cheng A., Wang M., Peng X. Detection of apoptosis induced by new type gosling viral enteritis virus in vitro through fluorescein annexin V-FITC/PI double labeling. World J. Gastroenterol. 2008;14:2174–2178. doi: 10.3748/wjg.14.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groux H., Torpier G., Monte D., Mouton Y., Capron A., Ameisen J.C. Activation-induced death by apoptosis in CD4+ T cells from human immunodeficiency virus-infected asymptomatic individuals. J. Exp. Med. 1992;175:331–340. doi: 10.1084/jem.175.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y., Cheng A., Wang M., Zhou Y., Yuan G. Studies on morphogenesis of duck enteritis virus CH virulent isolate in infected duck embryo fibroblasts. Acta Vet. Zoo. Sin. 2006;37:274–280. [Google Scholar]
- Guo Y., Cheng A., Wang M., Jia R., Wen M., Zhou W., Chen X. Duck embryo fibroblast apoptosis induced by duck viral enteritis virus CH isolate. Chin. Poult. 2007;29:9–11. [Google Scholar]
- Hamdy F., Holt S.C., Sevoian M. Ultrastructure of hamster kidney cell culture infected with herpesvirus. Infect. Immun. 1974;10:270–276. doi: 10.1128/iai.10.1.270-276.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffels-Redmann U., Müller H., Kaleta E.F. Structural and biological characteristics of reoviruses isolated from Muscovy ducks (Cairina moschata) Avian Pathol. 1992;21:481–491. doi: 10.1080/03079459208418866. [DOI] [PubMed] [Google Scholar]
- Hildreth J.E., Orentas R.J. Involvement of a leukocyte adhesion receptor (LFA-1) in HIV-induced syncytium formation. Science. 1989;244:1075–1078. doi: 10.1126/science.2543075. [DOI] [PubMed] [Google Scholar]
- Horvath C.M., Paterson R.G., Shaughnessy M.A., Wood R., Lamb R.A. Biological activity of paramyxovirus fusion proteins: factors influencing formation of syncytia. J. Virol. 1992;66:4564–4569. doi: 10.1128/jvi.66.7.4564-4569.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M., Watanabe M., Kamiya H., Sakurai M. Herpes simplex virus type 1 induces apoptosis in peripheral blood T lymphocytes. J. Infec. Dis. 1997;175:1220–1224. doi: 10.1086/593672. [DOI] [PubMed] [Google Scholar]
- Jia R., Cheng A., Wang M., Xin H., Guo Y., Zhu D., Qi X., Zhao L., Ge H., Chen X. Analysis of synonymous codon usage in the UL24 gene of duck enteritis virus. Virus genes. 2009;38:96–103. doi: 10.1007/s11262-008-0295-0. [DOI] [PubMed] [Google Scholar]
- Kaleta E.F. Herpesviruses of birds. Avian Pathol. 1990;19:193–211. doi: 10.1080/03079459008418673. [DOI] [PubMed] [Google Scholar]
- Kerr J.F., Wyllie A.H., Currie A.R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T.S., Benfield D.A., Rowland R.R.R. Porcine reproductive and respiratory syndrome virus-induced cell death exhibits features consistent with a nontypical form of apoptosis. Virus Res. 2002;85:133–140. doi: 10.1016/s0168-1702(02)00029-1. [DOI] [PubMed] [Google Scholar]
- Koyama A.H., Fukumori T., Fujita M., Irie H., Adachi A. Physiological significance of apoptosis in animal virus infection. Microbes Infect. 2000;2:1111–1117. doi: 10.1016/s1286-4579(00)01265-x. [DOI] [PubMed] [Google Scholar]
- Lovato L., Inman M., Henderson G., Doster A., Jones C. Infection of cattle with a bovine herpesvirus 1 strain that contains a mutation in the latency-related gene leads to increased apoptosis in trigeminal ganglia during the transition from acute infection to latency. J. Virol. 2003;77:4848–4857. doi: 10.1128/JVI.77.8.4848-4857.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazerian K., Purchase H.G. Combined fluorescent-antibody and electron microscopy study of Marek's disease virus-infected cell culture. J. Virol. 1970;5:79–90. doi: 10.1128/jvi.5.1.79-90.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazerian K., Lee L.F., Witter R.L., Burmester B.R. Ultrastructural studies of a herpesvirus of turkeys antigenically related to Marek's disease virus. Virology. 1971;43:442–452. doi: 10.1016/0042-6822(71)90316-3. [DOI] [PubMed] [Google Scholar]
- Ruggieri A., Trani L.D., Gatto I., Franco M., Vignolo E., Bedini B., Elia G., Buonavoglia C. Canine coronavirus induces apoptosis in cultured cells. Vet. Microbiol. 2007;121:64–72. doi: 10.1016/j.vetmic.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadzot-Delvaux C., Thonard P., Schoonbroodt S., Piette J., Rentier B. Varicella-zoster virus induces apoptosis in cell culture. J. Gen. Virol. 1995;76:2875–2879. doi: 10.1099/0022-1317-76-11-2875. [DOI] [PubMed] [Google Scholar]
- Sandhu T.S., Shawky S.A. Duck virus enteritis (Duck plague) In: Saif Y.M., Barnes H.J., Glisson J.R., editors. Diseases of Poultry. 11th ed. Iowa State Press; Ames: 2003. pp. 354–363. [Google Scholar]
- Sanfilippo C.M., Chirimuuta F.N.W., Blaho J.A. Herpes simplex virus type 1 immediate-early gene expression is required for the induction of apoptosis in human epithelial HEp-2 Cells. J. Virol. 2004;78:224–239. doi: 10.1128/JVI.78.1.224-239.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortridge K.F., Alexander D.J., Collins M.S. Isolation and properties of viruses from poultry in Hong Kong which represent a new (sixth) distinct group of avian paramyxoviruses. J. Gen. Virol. 1980;49:255–262. doi: 10.1099/0022-1317-49-2-255. [DOI] [PubMed] [Google Scholar]
- Turner A., Bruun B., Minson T., Browne H. Glycoproteins gB, gD, and gHgL of herpes simplex virus type 1 are necessary and sufficient to mediate membrane fusion in a Cos cell transfection system. J. Virol. 1998;72:873–875. doi: 10.1128/jvi.72.1.873-875.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaux D.L., Strasser A. The molecular biology of apoptosis. Proc. Natl. Acad. Sci. U.S.A. 1996;93:2239–2244. doi: 10.1073/pnas.93.6.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan G., Cheng A., Wang M., Han X., Zhou Y., Liu F. Preliminary study on duck enteritis virus-induced lymphocyte apoptosis in vivo. Avian Dis. 2007;51:546–549. doi: 10.1637/0005-2086(2007)51[546:PSODEV]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Zhao L., Cheng A., Wang M., Yuan G., Jia R., Zhou D., Qi X., Ge H., Sun T. Identification and characterization of duck enteritis virus dUTPase gene. Avian Dis. 2008;52:324–331. doi: 10.1637/8169-110607-ResNote.1. [DOI] [PubMed] [Google Scholar]


