Abstract
Trypanosoma cruzi is a flagellated protozoan belonging to the Trypanosomatidae family, the etiologic agent of Chagas disease. Currently, there is neither a licensed vaccine nor effective treatment, characterizing an unmet clinical need. The IgY refers to the egg yolk immunoglobulin (Y = yolk) and its production and use are subjects of many studies due to the diversity of its diagnostic and therapeutic applications. Several researchers have shown that the use of specific IgY may prevent and/or control infectious and parasitic diseases. Based on these evidences, the aim of this study was to immunize chickens with trypomastigotes of T. cruzi in order to produce highly effective and pure antibodies (IgY), as well as extract, characterize, quantify, and verify cytotoxic effects of IgY anti-T. cruzi. After the induction of IgY production by chickens, the eggs were collected and the IgY was extracted by method of precipitation of polyethylene glycol 6000. The IgY anti-T. cruzi characterization was performed using polyacrylamide gel electrophoresis (SDS-PAGE), western-blot and enzyme-linked immunosorbent assay (ELISA). Moreover, the cytotoxic or proliferative effects of IgY anti-T. cruzi was verified by MTT assay. The concentration of IgY in yolk was 8.41 ± 1.47 mg/mL. The characterization of IgY reveled bands of stained peptides with molecular weight between 75 and 50 kDa and 37 and 25 kDa. In the ELISA test was observed that there was antigen-antibody reaction throughout the sample period. The concentrations of 1, 5 and 10 mg/mL of IgY anti-T. cruzi presented no cytotoxic of proliferative effects in mononuclear and VERO cells in vitro. The results indicated that T. cruzi is able to generate a high production of specific immunoglobulins in chickens, it did not cause damage to the cell membrane and no proliferative effect.
Keywords: Chagas disease, Avian immunoglobulins, Egg yolk antibodies, Cytotoxic
1. Introduction
Chagas disease is a neglected tropical disease caused by Trypanosoma cruzi, an obligatory intracellular parasite widely spread in Latin America (Basso and Marini, 2015). This disease constitutes a serious worldwide public health issue (Coura and Dias, 2009), that causes a chronic parasitic condition characterized by a life-long infection of the host (Martin et al., 2015). Clinically, this disease may have an acute phase that lasts for 1 to 4 months characterized by patent parasitemia, and a chronic phase with a life-long phase including a subpatent parasitemia and tissue parasitism (Dias, 1997, Rassi et al., 2010). The diagnosis of T. cruzi infection during the acute phase is carried out by the direct detection of circulating parasites. However, during the chronic phase, the diagnosis is performed by conventional serologic assays (WHO, 2002, Otani et al., 2009, Carlier and Torrico, 2003).
Chemical drugs, such as benzimidazole and nifurtimox, are still used as the method of choice in controlling this disease (Urbina and Docampo, 2003). However, the treatment with benzimidazole has many side effects, prolonged treatment time, and low and variable efficacy in the chronic phase of this infection, which is the most prevalent form of the disease (Urbina and Docampo, 2003, Viotti et al., 2009). Thus, the search for more effective medications with less adverse effects is one of the main focuses of many studies (Coura, 2007). In this context, the search for alternative models for the treatment and diagnosis of T. cruzi is essential, mainly because it is a disease considered neglected. Specific antibodies for immunotherapy against this parasite might be a therapeutic alternative in controlling and/or prevention infections. Immunoglobulin Y (IgY) are polyclonal antibodies obtained from egg yolk (yolk = y) of immunized chickens. Such molecules have high specificity for binding and inactivating foreign substances, such as antigenic molecules that are able to invade the body (Karlsson et al., 2004). For this reason, IgY has been used for diagnosis and therapy (Reilly et al., 1997).
The IgY technology allows to obtain antibodies with high affinity and avidity (Karlsson et al., 2004). In addition, IgY shows numerous advantages when compared to mammalian antibodies, such as: similar functions of the mammalian IgG and IgE, no cross reaction with mammalian IgG, do not attack red blood cells, do not activate the complement system and the coagulation cascade. Furthermore, chickens are more able to produce antibodies than rabbits, goats, horses or rodents (Sampaio et al., 2014b, Chacana et al., 2004, Contreras et al., 2005).
Therefore, the aim of this study was to synthetize, extract, characterize and quantify antibodies IgY anti-T. cruzi extracted from eggs yolk of chickens immunized with trypomastigotes of T. cruzi. Moreover, effects of IgY anti-T. cruzi in vitro against mononuclear and VERO cells were performed, in order to evaluate possible collateral effects.
2. Materials and methods
All procedures in this study were approved by the Animal Welfare Committee of Ethics in Animal Experimentation of the Universidade Federal de Santa Maria (UFSM), under protocol number 5699170915.
2.1. IgY production, extraction and purification
2.1.1. The antigen
Trypomastigotes forms of T. cruzi (strain Y) was used as antigen to immunize the chickens, according the method described by Sampaio et al. (2014b), with some adaptations for T. cruzi, as follows below: cryopreserved trypomastigotes were inoculated into mice for reactivation and multiplication of strain, and blood samples were collected at the peak of parasitemia. In order to separate the parasites, blood was diluted in DMEM medium (1:1 v/v), followed by centrifugation (400 g for 10 min). The supernatant, rich of trypomastigotes, was collected and the amount of antigen was determined, aliquoted, and cryopreserved until use.
2.1.2. Chicken immunization protocol
The chicken immunization protocol was as described by Sampaio et al. (2014b). Briefly, a solution containing trypomastigotes of T. cruzi was added to incomplete Freund's adjuvant (Difco®) at a 1:1 ratio, with a final volume of 1.0 mL. It was injected intramuscularly at five different sites of the pectoral muscle of two 25-week-old New Hampshire chickens. The interval between immunizations was set as 10 days (days 0, 10, 20, 30, 40, 50, 70 and 80), totaling eight inoculations, being 2.4 × 104 trypomastigotes for the first 4 inoculations and 7.2 × 103 trypomastigotes of T. cruzi for the other 4 inoculations. Chicken eggs were collected from the third week post-immunization and stored at 4 °C until they were processed. One chicken was not immunized and its eggs were used as controls.
2.1.3. Extraction of immunoglobulin IgY anti-T. cruzi
Extraction was performed by the method of precipitation of polyethylene glycol 6000 (PEG-6000) (Polson et al., 1980). Firstly, the yolk was gently separated and its fat was removed using PBS (1:2 ratio) and PEG 6000 at a final concentration of 3.5%. This mixture was held in a roller mix for 30 min and then centrifuged for 80 min at 4.000 rpm and 4 °C. The supernatant was removed, filtered in filter paper (28 μm), and the final volume measured. PEG 6000 was once again added (8.5%), and the solution kept in a roller mix for 10 min, and centrifuged for 30 min at 4000 rpm and 4 °C. In this stage the supernatant was discarded and the pellet was dissolved in 10 mL of PBS. A final step using PEG 6000 (12%) was performed as described previously. The final pellet was dissolved in 2.4 mL of PBS, and this solution was dialyzed overnight in saline solution (0.1%) at 4 °C. A final dialyzation was performed for three more hours, the extract was collected, placed in tubes, stored at − 20 °C, and lyophilized until further use.
2.1.4. IgY specific concentration
The specific concentration of IgY anti-T. cruzi was measured according to the Lambert-Beer's law using an extinction coefficient of 1.33 for IgY (Polson et al., 1980). The protein content of the samples was measured by the method of Bradford (1976), with Coomassie blue using bovine serum albumin as standard, and monitored by measuring the maximum absorbance of the solution at 595 nm.
2.2. Characterization of IgY
The IgY was characterize and evaluate regarding its specificity and sensibility to the antigen, as follow below:
2.2.1. The polyacrylamide gel electrophoresis (SDS-PAGE)
The IgY samples were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) at 12% (Bernardo, 2009). For this, 20 μL of the IgY samples collected from chickens on the week 3, 5, 7, 9, 11 and 13 post-immunization and a control sample of non-immunized chicken were mixed with 20 μL of the buffer (Laemmli Sample Buffer – BioRad®), boiled in a water bath at 95 °C for 5 min, and applied to the stacking gel wells in a final volume of 5 μL. The electrophoresis was submitted to a current of 70 V for 30 min and 120 V for 120 min. Finally, the gel was colored with Coomassie Blue R (Sigma-Aldrich®) for at least 1 h, and treated with a decolorizing solution (glacial acetic acid and methanol).
2.2.2. Western blot
Western blot was performed as described by Bernardo (2009). Initially, electrophoresis was performed under the same conditions as described above. Two gels were prepared: one for IgY samples (the same used in Section 2.2.1) and a second gel with samples of the antigen used to immunize chickens (trypomastigotes of T. cruzi) and samples of trypomastigotes of Trypanosoma evansi. After protein separation in polyacrylamide gels, they were electrophoretically transferred to a nitrocellulose membrane of 0.45 μm (BioRad) using a current of 140 V for 120 min. Finally, the membranes were removed carefully, and blocked overnight in a PBS-Tween solution with 5% skimmed milk powder. The membrane containing the IgY was washed three times with PBS-Tween, and incubated for 1 h with rabbit anti-chicken IgY peroxidase conjugate, diluted in PBS-Tween (1:2000) (Sigma-Aldrich®). The nitrocellulose membrane containing the antigens of T. evansi and T. cruzi was washed three times with PBS-Tween and incubated for one hour with the newly synthetized IgY anti-T. cruzi and the primary antibody control IgY. The membrane was washed again three times with PBS-Tween, incubated for one hour with rabbit anti-chicken antibody peroxidase conjugated. Membranes were washed three times with PBS-Tween, and finally incubated with a developing solution (DAB–diaminobenzidine, Tris HCl, Nickel sulfate 0.3%, and H2O2) for the visualization of any reactive band.
2.2.3. ELISA
The Enzyme Linked Immune Sorbent Assay (ELISA) was performed as described by Sampaio et al. (2014b). A 96-well ELISA plate was coated with T. cruzi antigen (1 μg/well) previously diluted in 0.05 M of carbonate buffer (pH 9.6). The plates were incubated overnight at 4 °C. After incubation, the plates were washed four times with saline buffer (SB - pH 7.2). Firstly, it was blocked with 100 μL of fetal bovine serum (FBS)-(Cripion®, SP, Brazil) (SB and FBS) per well, and incubated at 37 °C. After this, the plates were washed four times with SB-Tween (SB pH 7.4 with 0.05% Tween 20). IgY samples extracted at different weeks post-immunization (3, 5, 7, 9, 10, 11, 13) were diluted (1:1000, 1:2000, and 1:5000) in FBS and SB, distributed over the plates (100 μL/well), and incubated for 1 h at 37 °C. Then, plates were washed four times. Each sample tested was submitted to three repetitions per plate and two repetitions inter-plates. After incubation with IgY (primary antibody), the plates were washed four times with SBS-Tween and submitted to an incubation with a secondary antibody (rabbit anti-chicken IgY peroxidase conjugate, Sigma-Aldrich®) diluted at 1:3000 for 1 h at 37 °C. Finally, the plates were washed again, and 100 μL of the chromogenic substrate (orthophenylenediamine, OPD) was added. After 15 min, the reaction was blocked with 10 μL of H2SO4 and the reading was performed at 490 nm using a spectrophotometer.
2.3. Evaluation of IgY cytotoxicity
In order to evaluate whether IgY may cause a cytotoxic effects, we used the MTT assay in two cell lines: Peripheral blood mononuclear cells (non-adherent cells) and VERO cells (adherent cells).
2.3.1. Peripheral blood mononuclear cells (PBMC)
Samples of peripheral blood were supplied by the Clinical Analysis Laboratory of the Franciscan University Center. The project was approved by the Research Ethics Committee of the Franciscan University Center, CAAE (31211214.4.0000.5306). Peripheral blood samples were collected by venipuncture using a top Vacutainer® (BD Diagnostics, Plymouth, UK) and heparin tubes. These samples were drawn after 12 h of overnight fasting from three apparently healthy human volunteers (22–25 years old), nonsmokers that were not taking prescription drugs, with drinking habits of no more than two alcoholic beverages a week. Lymphocyte cells were separated by Histopaque-1077 (Sigma–Aldrich Co., St Louis, USA) under density gradient centrifugation using 4 mL of blood samples. After further centrifugation for 15 min at 2500g, cells were transferred to culture media containing 5 mL of RPMI 1640 supplemented with 10% of FBS, 1% penicillin and streptomycin. The cells were cultured in a 96-well microplate at an initial density of 2 × 105 for 24 and 72 h at 37 °C in a 5% humidified CO2 atmosphere (Wilms et al., 2005).
2.3.2. VERO cell culture
Green monkey kidney (VERO) cells (Adolpho Lutz Institute, São Paulo, Brazil) (20–30 passages) were cultured in 96-well plates (Corning, USA) with E-MEM supplemented with 10% (v/v) FBS (Gibco BRL), 1% (v/v) l-glutamine (200 mmol L− 1), 10 mg/mL ciprofloxacin (Baytril, Bayer), and 0.025 g/mL amphotericin B (Gibco BRL). The cells were maintained at 37 °C in a humidified incubator with a 5% CO2 atmosphere.
2.3.3. Cell viability
The cytotoxicity of IgY against lymphocytes (non-adherent) and VERO cells (adherent) was evaluated as described by Sagrillo et al. (2015), using MTT 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide colorimetric method based on the cleavage of the reagent by dehydrogenases in viable cells (Mosman, 1983). In microplates, cells were incubated at 37 °C in a humid atmosphere with 5% of CO2 for 24 and 72 h. At the end of incubation, 50 μL of MTT solution was added to each well and the cells were incubated for three more hours. The supernatant was removed and 200 μL of dimethyl sulfoxide (DMSO) was added to each well. The microplate was analyzed using an ELISA reader at a wavelength of 560 nm. As a control, cells were grown in a medium lacking the constituents. The experiment was performed in triplicate and treatments used as concentrations of 1, 5 and 10 mg/mL of IgY anti-T. cruzi. Finally, cell viability was determined and expressed as a percentage of the control value.
2.4. Statistical analyses
Normality and homoscedasticity were analyzed through the Shapiro-Wilk and Levene test, respectively. Significant differences between groups were analyzed and detected by two-way analyses of variance (ANOVA) for independent samples, followed by Tukey post-hoc test. The differences were considered to be statistically significant at p < 0.05.
3. Results
3.1. Concentration of IgY antibody
The production of IgY by immunized chickens was observed during the study period. The average concentration of IgY antibody purified between the 3rd and 13th week after immunization was 8.41 ± 1.47 mg/mL.
3.2. Gel electrophoresis SDS–PAGE
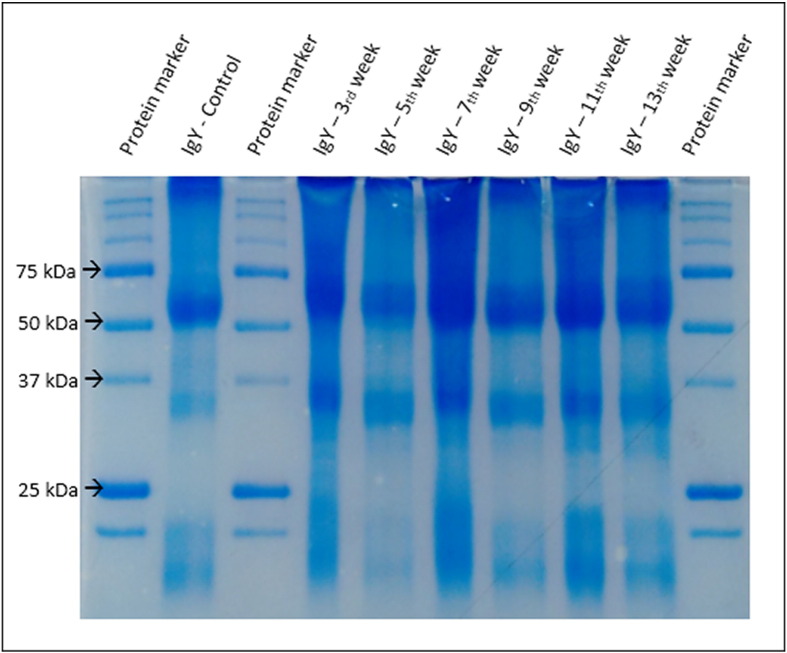
The polyacrylamide gel showed bands of stained peptides with molecular weight between 75 and 50 kDa (IgY heavy chain), 37 and 25 kDa (IgY light chain) (Fig. 1 ). These findings were obtained for all samples, which demonstrates that the extraction protocol was effective in separation the IgY.
Fig. 1.
IgY electrophoresis in polyacrylamide gel (10%) under reducing conditions, stained with Coomassie Blue R250 reagent (Bio-Rad) from samples of three immunized chickens. Protein marker - bands (250, 150, 100, 75, 50, 37, 25 and 20 kDa). The arrows indicates the intervals of the bands being, 75 and 50 kDa refers to heavy chain and 37 and 25 kDa refers to light chain of the IgY produced at 3, 5, 7, 9, 11 and 13 week post-immunization.
3.3. Western blot assay
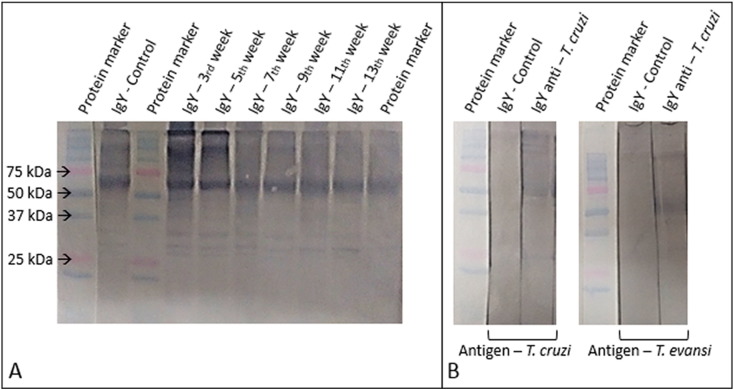
The results from the first Western blot, where IgY control samples and IgY from immunized chickens after 3, 5, 7, 9, 11 and 13 weeks of immunization can be seen in Fig. 2 A. This figure shows bands with molecular weight between 75 and 50 kDa and other bands between 37 and 25 kDa, corresponding to the heavy and light chains of IgY. Demonstrating that the bands found on the electrophoresis gel having the same molecular weight refer to IgY.
Fig. 2.
A) Immunoblot showing the characterization of IgY after the addition of the secondary antibody (rabbit anti-chicken IgY peroxidase conjugate, Sigma-Aldrich®). Protein marker - bands (250, 150, 100, 75, 50, 37, 25 and 20 kDa). The arrows indicate the bands (75, 50, 37 and 25 kDa) to demonstrate the range of IgY heavy and light chain bands of IgY produced at 3, 5, 7, 9, 11 and 13 week post-immunization and Control IgY. B) Immunoblot showing the specific recognition of IgY - anti-T. cruzi and IgY – Control, to the protein antigens from T. cruzi and T. evansi samples after addition of the peroxidase-conjugated secondary antibody.
The second membrane electrophoretically transferred (Fig. 2B) with antigen samples shows that IgY newly synthetized anti-T. cruzi reacted with proteins of both species of Trypanosoma, T. cruzi and T. evansi, which indicates a cross-reactivity. The immunoglobulin produced was able to recognize in T. cruzi several proteins, in this some homologous proteins in T. evansi were also recognized, demonstrating that IgY anti - T. cruzi can provide cross-reaction to other trypanosomatids and cannot be used in tests diagnoses specific. Also, results for IgY control were negative, indicating that the IgY produced has specificity for the target antigen.
3.4. ELISA assay
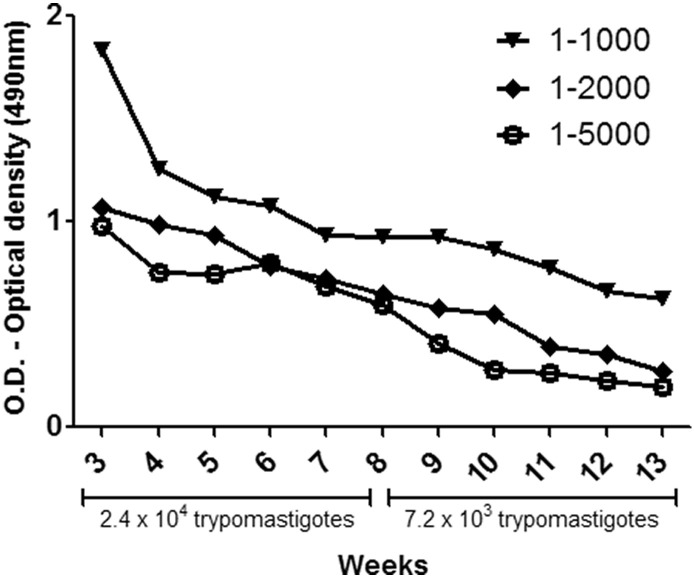
Positive results were obtained for all dilutions tested. There was an antigen-antibody reaction with an optical density (OD) between 0.196 ± 0.005 and 1.83 ± 0.002 (Fig. 3 ). It was also observed a decrease in the antigen-antibody reaction after the week 8, which refers to the reduction of trypomastigotes and the concentration of the antigen used to immunize chickens, thus, indicating a reduction of specific IgY anti-T. cruzi production.
Fig. 3.
Optical density values obtained in the ELISA using purified IgY from egg yolk of immunized chickens. The samples tested were IgY extracted at different weeks after immunization (3, 4, 5, 6, 7, 8, 9, 10, 11, 12 and 13) at different concentrations (1:1000, 1:2000 and 1:5000). The arrow indicates the time of the last immunization.
3.5. MTT assay
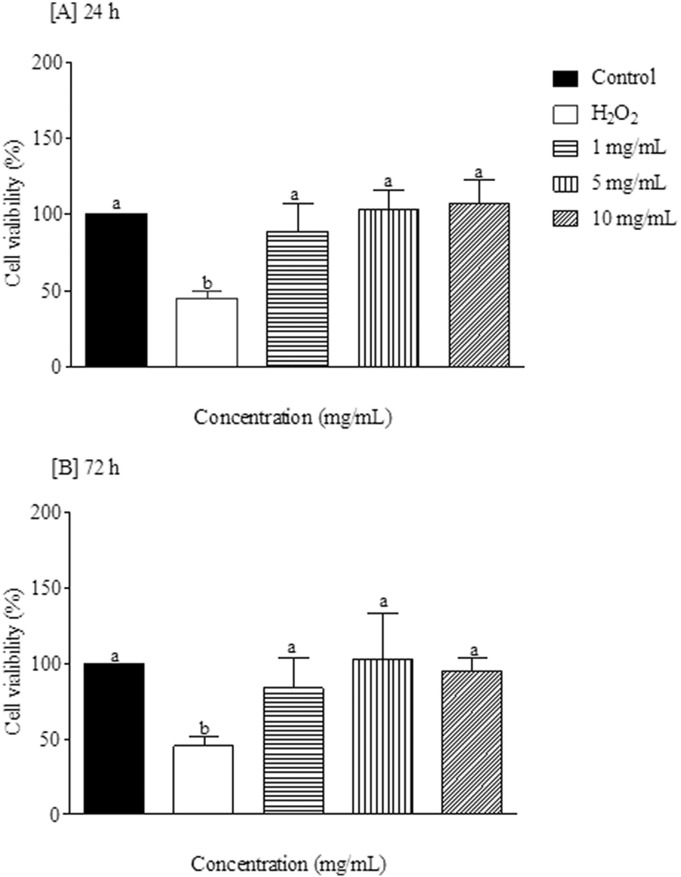
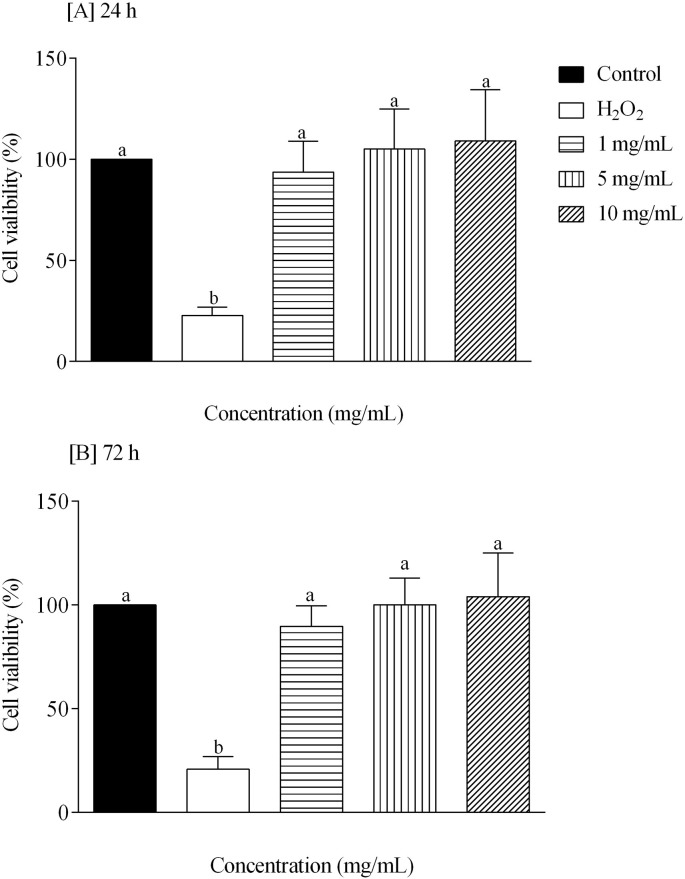
In order to determine whether IgY led to increased cellular toxicity, the MTT assay for cell viability was used. As shown in Fig. 4, Fig. 5 , IgY did not cause cytotoxicity effects at concentrations of 1, 5 and 10 mg/mL after 24 and 72 h post-incubation in PBMC and VERO cell cultures (p > 0.05).
Fig. 4.
Percentage of mononuclear cells (PBMC) viability after exposure to different concentrations of IgY anti-T. cruzi after 24 [A] and 72 h [B]. Different letters indicate statistical difference considering p < 0.05 using a Tukey post-hoc test.
Fig. 5.
Percentage of VERO cells viability after exposure to different concentrations of IgY anti-T. cruzi after 24 [A] and 72 h [B]. Different letters indicate statistical difference considering p < 0.05 using a Tukey post-hoc test.
4. Discussion
Identification of alternative treatments that are more efficient and less toxic against T. cruzi are necessary, since several resistance and toxic effects are reported using the conventional drugs (Viotti et al., 2009). Therefore, therapeutic immunization/immunotherapy using T. cruzi antigens can also be used to control the infection and reduce disease progression. Our results demonstrated that it is possible to produce IgY through the inoculation of trypomastigotes forms of T. cruzi in chickens from eggs yolks. The method of immunization recommended by Sampaio et al. (2014b) demonstrated to be an effective and easy method to be implemented for production of IgY anti-T. cruzi.
Our results showed the production of IgY after the inoculation of 104 and 103 trypomastigotes of T. cruzi, which produced an average of 8.41 mg/mL of IgY, whereas 107 trypomastigotes of T. evansi produced an average of 2.7 mg/mL (Sampaio et al., 2014b). This difference might be related to the immunization protocol, since we used only 10 days of intervals. Moreover, this difference may occur due a greater immune response mounted by the chickens opposite to the antigenic epitopes of T. cruzi antigenic epitopes that may be more complex than T. evansi. In addition, regarding the ELISA results, higher optical densities were observed up to 8 weeks, coinciding with the inoculation of a greater number of trypomastigotes (2.4 × 104 trypomastigotes/mL), which shows that the antigen concentration is closely related to the specific production of IgY. Furthermore, there was a major reduction of IgY activity, and this can be due to a reduction in the immune responses of the animals against the antigen, since after several inoculations this antigen cannot be seen as a challenge anymore.
Currently, there are several methods to isolate and purify functional active chicken IgY from egg yolk, even for large scale purification (Munhoz et al., 2014). These methods can be performed by salt precipitation (Deignan et al., 2000, Chacana et al., 2003), chromatographic techniques (Meulenaer and Huyghebaert, 2001), and ultrafiltration (Kim and Nakai, 1998). We choose to use the salt precipitation method using PEG–6000 as described by Polson et al. (1980) since it is an easy to perform. This technique is widely described in the literature as effective, high-yield antibody, besides having the advantage of possibility of handling at room temperature without risk of denaturation of the immunoglobulin (Akita and Nakai, 1993).
The specificity and characterization of anti-T. cruzi IgY were confirmed by polyacrylamide electrophoresis gel and Western blot. The molecular weight of 75 kDa (50 for the heavy chain and 25–37 for the light chain) found in our study was similar to that described by Contreras et al. (2005), where avian antibodies were produced by intradermal immunization of chickens with T. cruzi epimastigotes. However, the literature reports that the light chains of IgY can weight 18 kDa (Sun et al., 2001) to 21 kDa (Hatta et al., 1993), differing from our results. There are other molecular weight characterization of different IgY as the IgY anti-Staphylococcus aureus with light chain of 15–25 kDa and heavy chain of 55–70 kDa (Reddy et al., 2014), and IgY anti-Schistosoma japonicum with light and heavy chain between 25 and 68 kDa, respectively (Cai et al., 2012.), demonstrating that there is a certain variability in the molecular weight of IgY produced.
IgY technology has been used in many medical areas, especially for diagnosis purposes. Specific chicken antibodies have been successfully used against a wide variety of antigens (Munhoz et al., 2014). Various authors reported the efficiency, sensitivity, and specificity of diagnostic tests using IgY from various microorganisms such as Toxoplasma gondii, Schistosoma japonicum, Trichinella spiralis and coronavirus (Ferreira Júnior et al., 2012, Cai et al., 2012, Wang et al., 2012, Palaniyappan et al., 2012). The results of our experiment regarding IgY anti-T. cruzi indicate that this antibody could not be used in a diagnostic test, since it showed cross-reactivity with antigens of T. evansi. But on the other hand, this finding leaves the possibility that IgY could be used as immunotherapy against many trypanosomes.
The MTT assay is widely used to evaluate the cell viability, as well as the level of mitochondrial metabolic activity of viable cells, with high degree of accuracy (Mosman, 1983). In the present study, the IgY concentration of 10 mg/mL was able to maintain cell viability in all exposure times tested using PBMC and VERO cells, and it did not cause damage to DNA. Our results are similar to those found by Sampaio et al. (2014a), using IgY anti-T. evansi against PBMC cells on concentrations of 1, 5 and 10 mg/mL, other important blood protozoan belonging to the Trypanosomatidae family. Thus, we can conclude that all concentrations of IgY may be considered safe against these cells.
5. Conclusion
The results indicated that T. cruzi is able to generate a humoral immune response in chickens, resulting in a synthesis of high specific immunoglobulins against T. cruzi, that did not cause damage to the cell membrane, as well as no proliferative effect in mononuclear and VERO cells culture.
Contributor Information
Thirssa H. Grando, Email: thirssa.grando@smail.ufsm.br.
Silvia Gonzalez Monteiro, Email: sgmonteiro@uol.com.br.
References
- Akita E.M., Nakai S. Comparison of four purification methods for the production of immunoglobulin from eggs laid by hens immunized with an enterotoxigenic E. coli strain. J. Immunol. Methods. 1993;160:207–214. doi: 10.1016/0022-1759(93)90179-b. [DOI] [PubMed] [Google Scholar]
- Basso B., Marini V. Experimental Chagas disease in Balb/c mice previously vaccinated with T. rangeli. II. The innate immune response shows immunological memory: reality or fiction. Immuno. 2015;220:428–436. doi: 10.1016/j.imbio.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Bernardo A.R. Universidade Federal Rural do Rio de Janeiro; Seropédica-RJ: 2009. Tecnologia IgY: Produção de anticorpos aviários para Leishmania (Leishmania) amazonensis com o uso ético dos animais de experimentação. (60 pp. Dissertação (Mestrado em Ciências)) [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cai Y., Guo J., Chen S.H., Tian L.G., Steinmann P., Chen M.X., Li H., Ai L., Chen J.X. Chicken egg yolk antibodies (IgY) for detecting circulating antigens of Schistosoma japonicum. Parasitol. Int. 2012;61:385–390. doi: 10.1016/j.parint.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Carlier Y., Torrico F. Congenital infection with Trypanosoma cruzi: from mechanisms of transmission to strategies for diagnosis and control. Rev. Soc. Bras. Med. Trop. 2003;6:767–771. doi: 10.1590/s0037-86822003000600024. [DOI] [PubMed] [Google Scholar]
- Chacana P.A., Schade R., Terzolo H.R. A new bacterium suitable for egg yolk immunoglobulin (IgY) large-scale chromatographic purification. ALTEX. 2003;3:165. [Google Scholar]
- Chacana P.A., Terzolo H.R., Calzado E.G., Schade R. Tecnologia IgY e aplicaciones de los anticuerpos de yema de huevo de gallina. Rev. Med. Vet. 2004;85:179–189. [Google Scholar]
- Contreras V.T., Lima A.R., Navarro M.C., Arteaga R.Y., Graterol D., Cabello L., Farias M. Produción y purificación de anticuerpos (IgY) a partir de huevos de gallinas inmuizadas com epimastigotas de Trypanosoma cruzi. Salus online. 2005;9:33–44. [Google Scholar]
- Coura J.R. Chagas disease: what is known and what is needed - a background article. Mem. Inst. Oswaldo Cruz. 2007;102:113–122. doi: 10.1590/s0074-02762007000900018. [DOI] [PubMed] [Google Scholar]
- Coura J.R., Dias J.C.P. Epidemiology, control and surveillance of Chagas disease – 100 years after its discovery. Mem. Inst. Oswaldo Cruz. 2009;104:31–40. doi: 10.1590/s0074-02762009000900006. [DOI] [PubMed] [Google Scholar]
- Deignan T., Kelly J., Alwan A., O'Farrelly C. Comparative analysis of methods of purification of egg yolk immunoglobulin. Food Agric. Immunol. 2000;12:77–85. [Google Scholar]
- Dias J.C.P. Control de la transmisión de la enfermedad de Chagas en Brasil. Medicina. 1997;55:7–9. [Google Scholar]
- Ferreira Júnior A., Santiago F.M., Silva M.V., Ferreira F.B., Macêdo Júnior A.G., Mota C.M., Faria M.S., Silva Filho H.H., Silva D.A.O., Cunha-Júnior J.P., Mineo J.R., Mineo T.W.P. Production, characterization and applications for Toxoplasma gondii-specific polyclonal chicken egg yolk immunoglobulins. PLoS One. 2012;7:40391. doi: 10.1371/journal.pone.0040391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatta H., Tsuda K., Akachi S., Kim M., Yamamoto T. Productivity and some properties of egg yolk antibody (IgY) against human rotavirus compared of rabbit IgG. Biosci. Biotechnol. Biochem. 1993;57:450–454. doi: 10.1271/bbb.57.450. [DOI] [PubMed] [Google Scholar]
- Karlsson M., Larsson A., Kollberg H. Chicken IgY: utilizing the evolutionary advantage. Worlds Poult. Sci. J. 2004;60:341–348. [Google Scholar]
- Kim H., Nakai S. Simple separation of immunoglobulin from egg yolk by ultrafiltration. J. Food Sci. 1998;63:485–490. [Google Scholar]
- Martin D.L., Lowe K.R., McNeill T., Thiele E.A., Roellig D.M., Zajdowicz J., Hunter S.A., Brubaker S.A. Potential sexual transmission of Trypanosoma cruzi in mice. Acta Trop. 2015;149:15–18. doi: 10.1016/j.actatropica.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulenaer B., Huyghebaert A. Isolation and purification of chicken egg yolk immunoglobulins: a review. Food Agric. Immunol. 2001;13:275–288. [Google Scholar]
- Mosman T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Munhoz L.S., Vargas G.D., Fischer G., Lima M., Esteves P.A., Hübner S.O. Avian IgY antibodies: characteristics and applications in immunodiagnostic. Cienc. Rural. 2014;44:153–160. [Google Scholar]
- Otani M.M., Vinelli E., Kirchhoff L.V., del Pozo A., Sands A., Vercauteren G., Sabino E.C. WHO comparative evaluation of serologic assays for Chagas disease. Transfusion (Paris) 2009;49:1076–1082. doi: 10.1111/j.1537-2995.2009.02107.x. [DOI] [PubMed] [Google Scholar]
- Palaniyappan A., Das D., Kammila S., Suresh M.R., Sunwoo H.H. Diagnostics of severe acute respiratory syndrome-associated coronavirus (SARS-CoV) nucleocapsid antigen using chicken immunoglobulin Y. Poult. Sci. 2012;91:636–642. doi: 10.3382/ps.2011-01916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polson A., von Wechmar M.B., van Regenmortel M.H. Isolation of viral IgY antibodies from yolks of immunized hens. Immunol. Commun. 1980;9:475–493. doi: 10.3109/08820138009066010. [DOI] [PubMed] [Google Scholar]
- Rassi A., Jr., Rassi A., Marin-neto J.A. Chagas disease. Lancet. 2010;375:1388–1402. doi: 10.1016/S0140-6736(10)60061-X. [DOI] [PubMed] [Google Scholar]
- Reddy P., Ramlal S., Sripathy M.H., Batra H.V. Development and evaluation of IgY Immuno Capture PCR ELISA for detection of Staphylococcus aureus enterotoxin A devoid of protein A interference. J. Immunol. Methods. 2014;408:114–122. doi: 10.1016/j.jim.2014.05.012. [DOI] [PubMed] [Google Scholar]
- Reilly M.R., Domingo R., Sandhu J. Oral delivery of antibodies: future pharmacokinetic trends. Clin. Pharmacokinet. 1997;32:313–323. doi: 10.2165/00003088-199732040-00004. [DOI] [PubMed] [Google Scholar]
- Sagrillo M.R., Garcia L.F.M., de Souza Filho O.C., Duarte M.M.M.F., Ribeiro E.E., Cadoná F.C., da Cruz I.B.M. Tucumã fruit extracts (Astrocaryum aculeatum Meyer) decrease cytotoxic effects of hydrogen peroxide on human lymphocytes. Food Chem. 2015;173:741–748. doi: 10.1016/j.foodchem.2014.10.067. [DOI] [PubMed] [Google Scholar]
- Sampaio L.C.L., Baldissera M.D., Sagrilo M.R., Heres T.S., Oliveira C.B., Stainki D.R., Monteiro S.G. In vitro cytotoxicity and genotoxicity of chicken egg yolk antibodies (igy) against Trypanosoma evansi in human lymphocytes. Int J Pharm Pharm Sci. 2014;6:167–170. [Google Scholar]
- Sampaio L.C.L., Baldissera M.D., Grando T.H., Gressler L., Capeleto D.M., de As M.F., de Jesus F.P., dos Santos A.G., Jr., Anciuti A.N., Colonetti K., Stainki D.R., Monteiro S.G. Production, purification and therapeutic potential of egg yolk antibodies for treating Trypanosoma evansi infection. Vet. Parasitol. 2014;204:96–103. doi: 10.1016/j.vetpar.2014.05.032. [DOI] [PubMed] [Google Scholar]
- Sun S., Mo W., Ji Y., Liu S. Preparation and mass spedtrometric study of egg yolk antibody (IgY) against rabies virus. Rapid Commun. Mass Spectrom. 2001;15:708–712. doi: 10.1002/rcm.271. [DOI] [PubMed] [Google Scholar]
- Urbina J.A., Docampo R. Specific chemotherapy of Chagas disease: controversies and advances. Trends Parasitol. 2003;19:495–501. doi: 10.1016/j.pt.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Viotti R., Vigliano C.A., Alvarez M.G., Lococo B.E., Petti M.A., Bertocchi G.L., Armenti A.H. The impact of socioeconomic conditions on chronic Chagas disease progression. Rev. Esp. Cardiol. 2009;62:1224–1232. doi: 10.1016/s1885-5857(09)73349-3. [DOI] [PubMed] [Google Scholar]
- Wang Z., Fu G.Y., Jing F.J., Jin J., Ren H.J., Jiang P., Cui J. Detection of Trichinella spiralis circulating antigens in serum of experimentally infected mice by an IgY-mAb sandwich ELISA. Foodborne Pathog. Dis. 2012;9:727–733. doi: 10.1089/fpd.2012.1157. [DOI] [PubMed] [Google Scholar]
- WHO . WHO; Genebra: 2002. World Health Organization. Control of Chagas Disease: Second Report of the WHO Expert Committee. (Technical Report Series, v. 905). [PubMed] [Google Scholar]
- Wilms L.C., Hollman P.C.H., Boots A.W., Kleinjans J.C.S. Protection by quercetin and quercetin-rich fruit juice against induction of oxidative DNA damage and formation of BPDE-DNA adducts in human lymphocytes. Mutat. Res. 2005;582:155–162. doi: 10.1016/j.mrgentox.2005.01.006. [DOI] [PubMed] [Google Scholar]