Abstract
Several investigations have shown that pentoxifylline possesses broad-spectrum antiviral activity against a range of RNA and DNA viruses. However, its ability to inhibit Japanese encephalitis virus (JEV) replication has not yet been studied. The present study was designed to investigate the antiviral activity of pentoxifylline against JEV in vitro and in vivo. The activity of pentoxifylline against JEV was evaluated in vitro using cytopathic effect inhibition and plaque reduction assays. Pentoxifylline was able to inhibit JEV replication in a dose-dependent manner at a 50% inhibitory concentration (IC50) of 50.3 μg/mL (0.00018 μM) and a therapeutic index (TI) of 10. Experiments to study the mechanism of antiviral action of pentoxifylline using in vitro translation of viral mRNA suggested that the drug did not interfere either with early or late protein synthesis but most likely exerted its action on virus assembly and/or release. Furthermore, the in vivo study showed that pentoxifylline at a concentration of 100 mg/kg and 200 mg/kg body weight was able to protect completely mice challenged with 50 × 50% lethal dose (LD50) of JEV.
Keywords: Antiviral agents, Japanese encephalitis virus, Pentoxifylline, Ribavirin
1. Introduction
Flaviviruses are important human pathogens causing a variety of diseases ranging from mild febrile illness to severe encephalitis and haemorrhagic fever. Among them, Japanese encephalitis virus (JEV) is a neuropathogenic virus commonly infecting children and is associated with acute encephalitis [1]. The disease burden of Japanese encephalitis (JE) is an increasing public health problem with an average of 50 000 cases per year in Asia [2], [3]. Despite this, the prospects for therapy of flavivirus infections are not encouraging, which has led to the unavailability of a specific and efficient antiviral agent against JEV [4]. This has rekindled the search for a drug that can inhibit JEV replication.
Pentoxifylline is a methylxanthine derivative and has been used for treating human vascular diseases [5]. Despite being a cardiovascular drug, pentoxifylline also demonstrated high antiviral activity against herpes simplex virus, vaccinia virus, rotavirus and tick-borne encephalitis virus, suggesting that this drug also has broad-spectrum virus inhibitory properties [6]. Furthermore, pentoxifylline also showed inhibition of human immunodeficiency virus (HIV) expression in acutely and chronically infected cells in vitro and in human peripheral blood mononuclear cells [7], [8]. However, the ability of pentoxifylline to inhibit JEV replication has not yet been studied. Therefore, the present study was designed to evaluate and specifically to understand the role of pentoxifylline as a potential therapeutic agent against JEV infection.
2. Materials and methods
2.1. Compounds
The compounds studied were an injectable form of pentoxifylline (20 mg/mL) (Trental™; Aventis, Paris, France) and ribavirin (200 mg) (Neaman, New York, NY).
2.2. Viruses and cells
A standard strain of JEV (P20778) was obtained from the National Institute of Virology, Pune, India. The Aedes albopictus (C6/36) mosquito cell line and porcine stable kidney (PS) cells were obtained from the National Centre for Cell Sciences, Pune, India, and maintained in minimum essential medium (MEM) with 10% foetal calf serum (FCS).
2.3. Animals
Random-bred Swiss albino mice (4 weeks old) were obtained from the Central Animal Research Facility, National Institute of Mental Health and Neurosciences (NIMHANS), Bangalore, India.
2.4. Cytotoxicity of pentoxifylline and ribavirin
The cytotoxicity of pentoxifylline and ribavirin was evaluated using a trypan blue exclusion assay [9], [10]. Briefly, PS cells grown to semiconfluence were exposed to five different concentrations of the compounds for 4 days and viable cells were counted using 2.5% trypan blue dye and a haemocytometer [10]. The concentration of compound that reduced cell growth by 50% was estimated as the 50% cytotoxic concentration (CC50). The effect of the compounds on cellular proliferation was also studied as described previously [10]. Ribavirin, a known inhibitor of flavivirus replication, was used in this study as a standard for comparing the results in all the experiments.
2.5. Screening for inhibition of virus-induced cytopathic effect (CPE)
The antiviral activity of pentoxifylline and ribavirin was initially determined using a CPE inhibition assay as described previously [10]. Briefly, a PS cell monolayer was infected with 1 multiplicity of infection (MoI) of virus and incubated for 2 h at 37 °C. At the end of the incubation period, the monolayer was rinsed with sterile phosphate-buffered saline (PBS) and then doubling dilutions of ribavirin and pentoxifylline (beginning with the CC50) were added and incubated for 3 days. The experiment was terminated when the virus control showed maximum CPE. The presence or absence of CPE was recorded microscopically every day and the plates were stained using crystal violet and compared with the virus control and drug control. All the experiments were run in triplicate to ensure reproducibility.
2.5.1. Confirmation of antiviral activity by the plaque reduction assay
The antiviral activity of pentoxifylline noted in the screening experiments was confirmed by the plaque reduction assay as described previously [10]. Briefly, PS cells grown to a confluent monolayer were infected with 1 MoI of JEV and adsorbed for 2 h at 37 °C. At the end of adsorption the monolayer was rinsed and 100 μL of MEM containing varying concentrations of pentoxifylline (500, 250, 125, 62.5 and 31.25 μg/mL) or ribavirin (50, 25, 12.5, 6.25 and 3.12 μg/mL) was added. The monolayer was then overlaid with maintenance medium containing 0.2% molten agarose (Sigma–Aldrich, St. Louis, MO). Appropriate controls were included in each run of the assay. Incubation was carried out at 37 °C for 3 days. At the end of the incubation period, monolayers were fixed and stained using 1% crystal violet and plaques were counted using a hand lens. All experiments were run in triplicate. Percentage inhibition of plaques was determined using the following formula:
The antiviral activity was expressed as 50% inhibitory concentration (IC50), which is the concentration of compound required to inhibit viral plaques by 50% compared with the virus control. The therapeutic potential and the specificity of action of the compounds were calculated as the therapeutic index (TI), which is the ratio of CC50 to IC50.
2.6. Determining the mechanism of action of pentoxifylline in relation to JEV replication
To understand the possible mechanism of action of pentoxifylline in relation to the replicative cycle of JEV, various in vitro experiments detailed below were carried out.
2.6.1. Determining the kinetics of JEV replication in PS cells
A 24-well plate containing sterile coverslips in each well was seeded with 4 × 104 cells/well and incubated to attain confluence. The monolayer was then infected with JEV (MoI = 1) for 1 h at 37 °C. Following incubation, the monolayer was rinsed and replenished with medium containing 1% FCS. This time point was considered as 0 h post infection. Subsequently at 2, 4, 6, 8, 10, 12, 14, 16 and 24 h post infection, the medium was harvested to determine the amount of extracellular virus released into the supernatant. At each time point, the coverslip containing cells was also removed, fixed in chilled acetone and stained by immunofluorescence assay (IFA) using a monoclonal antibody against the envelope protein of JEV to detect the cell-bound antigen [11].
2.6.2. Understanding the kinetics of antiviral activity of pentoxifylline
A 24-well plate was seeded with PS cells and incubated at 37 °C overnight. JEV was added to this monolayer and incubated for 2 h at 37 °C. At the end of virus adsorption, the monolayer was rinsed using sterile PBS and replenished with MEM containing 1% FCS. This time point was considered as 0 h post infection. Starting from the 0 h time point, 0.0017 μM pentoxifylline was added at 0, 2, 4, 6, 8, 10, 12, 14, 16 and 24 h post infection and incubated at 37 °C. The supernatant fluid was harvested from the respective wells at 48 h post infection. The fluid was divided into two parts. One part was used to determine the virus yield (50% tissue culture infective dose (TCID50)/mL) and the second part was used to detect the presence of soluble JEV antigen by antigen capture enzyme-linked immunosorbent assay (ELISA) as described elsewhere [12]. To detect cell-bound antigen, the coverslip cultures were fixed in chilled acetone for 30 min at 4 °C and stained using monoclonal antibody to JEV (clone F2C2) and anti-mouse IgG–fluorescein isothiocyanate (FITC) conjugate by indirect IFA.
2.6.3. Confirmation of the mechanism of action of pentoxifylline
To understand the mechanism of action of pentoxifylline, an in vitro translation experiment was carried out using commercially available Transcend™ Non-Radioactive Translation Detection System and Rabbit Reticulocyte Lysate (Promega, Madison, WI). A PS cell monolayer was adsorbed with JEV (MoI = 1) for 1 h. Following adsorption, monolayer was rinsed and 0.0017 μM pentoxifylline was added to one set of JEV-infected cells and incubated for 4 h. Pentoxifylline at the same concentration was added to a second set of monolayer cultures at 10 h. The plates were further incubated for 48 h at 37 °C. Appropriate virus and cell controls were included. At the end of incubation, the cells were treated with 750 μL of TRIzol reagent (Gibco, Rockville, MD) and viral RNA was extracted as per the manufacturer's instruction. The extracted RNA was subjected to real-time reverse transcriptase polymerase chain reaction (RT-PCR) using SYBR green I chemistry as described previously [13] with minor modifications [10] to ensure the presence of JEV RNA. The viral RNA obtained from JEV-infected cells, which encodes a 50-kDa protein, was subjected to in vitro translation using a commercial kit (Promega) as described previously [10]. After completion of the translation reaction, 1 μL of the product was subjected to sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE). The gel was electroblotted onto a polyvinylidene fluoride (PVDF) membrane. The membrane was reacted with specific monoclonal antibody to JEV and developed using diaminobenzidine and H2O2.
2.7. In vivo evaluation of pentoxifylline
Initially, non-toxic concentrations of pentoxifylline were determined by administering intraperitoneally 50, 100, 200 and 300 mg/kg body weight of the compound to different groups (n = 4 per group) of 4-week-old Swiss albino mice. All mice were observed for a period of 45 days for loss or gain in weight and other evidence of toxicity compared with the untreated normal mice. The therapeutic potential of pentoxifylline was then evaluated using a peripheral challenge model as described previously [10]. Briefly, 50 × 50% lethal dose (LD50) of JEV was injected intraperitoneally into four groups of 4-week-old mice (n = 4) and the blood–brain barrier (BBB) was breached 2 h later using 1% sterile starch. This was followed by intraperitoneal (i.p.) administration of pentoxifylline (50, 100 and 200 mg/kg body weight) twice daily into three groups of mice for 12 days. A fourth group of mice (n = 4) served as ‘no drug controls’. A fifth group of mice (n = 4) served as sham controls and received MEM intraperitoneally and starch intracerebrally. Mice were observed every day for 20 days post infection for the appearance of symptoms and death. At the end of the observation period the mice that survived the infection were sacrificed and their brains were harvested and subjected to JEV antigen detection by IFA, virus nucleic acid detection by real-time PCR and virus isolation as described previously [10].
3. Results
3.1. Antiviral screening of pentoxifylline by in vitro CPE inhibition assay
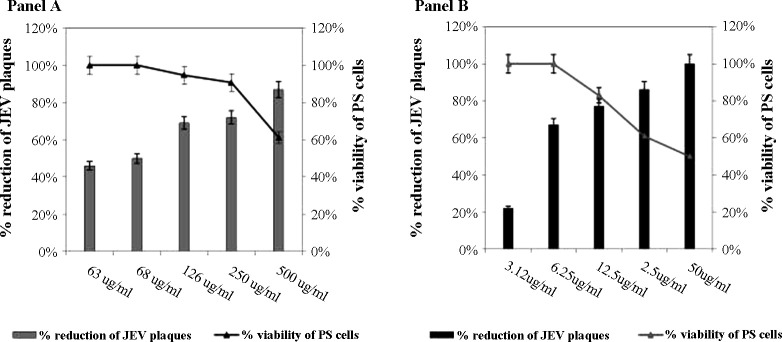
The CC50 was 500 μg/mL for pentoxifylline and 50 μg/mL for ribavirin. The antiviral activity of these two compounds was initially evaluated at a non-cytotoxic concentration (<CC50) against JEV using a CPE inhibition assay and subsequently evaluated by the plaque reduction assay. There was a dose-dependent reduction of viral plaques (Fig. 1 ) with an IC50 of 50.3 μg/mL (0.00018 μM) for pentoxifylline and 3.9 μg/mL (0.000016 μM) for ribavirin. The TI of ribavirin was 13 and the TI of pentoxifylline was 10, suggesting that both compounds are highly active against JEV.
Fig. 1.
Antiviral activity of (A) pentoxifylline and (B) the standard antiviral agent ribavirin against Japanese encephalitis virus (JEV) evaluated using the plaque reduction assay. Dose-dependent reduction in JEV plaques obtained in porcine stable kidney (PS) cells with pentoxifylline (represented as bars), where the x-axis represents the various concentrations of the compound and the left-hand y-axis represents the percent reduction of JEV plaques. The viability of cells is represented as a line graph superimposed on the bar diagram, where the right-hand y-axis represents the percent viability of PS cells.
3.2. Kinetics of action of pentoxifylline in relation to the replication of JEV in vitro
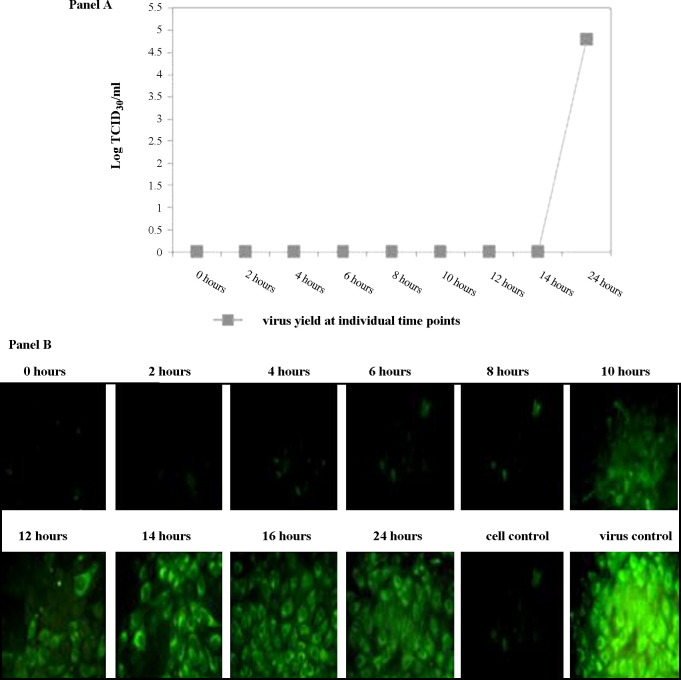
As a first step to understand the interactions of JEV with the compounds, experiments were designed to determine the kinetics of viral replication in vitro. It was noted that the earliest appearance of JEV antigen in infected PS cells was at 10 h post adsorption as detected by IFA (Fig. 2B). However, the first infectious progeny of virus was detected in the supernatant medium harvested at 14 h post adsorption (Fig. 2A). The cells stained at earlier time points (2, 4, 6 and 8 h) were not positive for viral antigen, and the supernatant harvested from them did not yield infectious virus. Based on these findings, it was concluded that a single replicative cycle of JEV in vitro in the PS cell line requires 14 h for completion.
Fig. 2.
Elucidation of a single replicative cycle of Japanese encephalitis virus in porcine stable kidney (PS) cells. PS cells were infected with a multiplicity of infection (MoI) of 1 of JEV at the 0 h time point. Virus yield in the supernatant and JEV antigen in the cells were examined at 2-h intervals up to 24 h post infection. (A) Yield of virus in the supernatant fluid. The x-axis represents the various time points at which virus yield was evaluated and the y-axis represents the virus yield (log 50% tissue culture infective dose (TCID50)/mL). (B) Detection of JEV-specific antigen using an immunofluorescence assay. It can be observed that the earliest appearance of cell-bound antigen was at 10 h post infection, whilst the earlier time points were negative for viral antigen (400×).
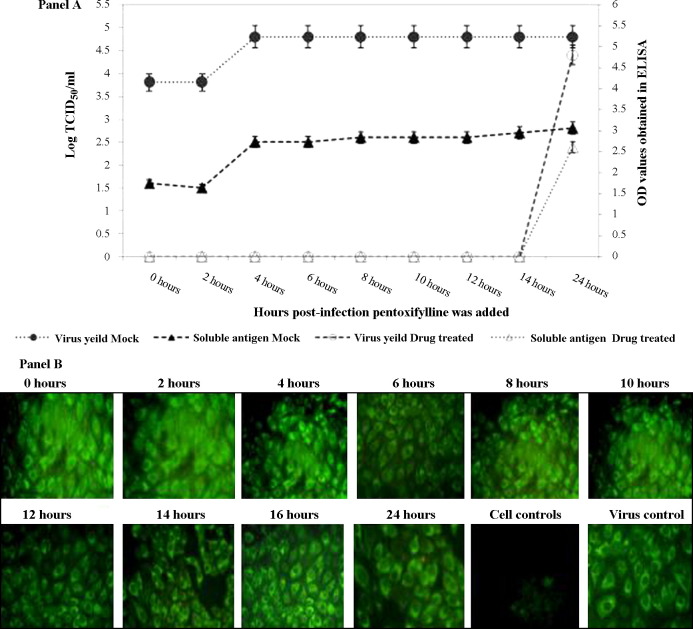
The antiviral activity of pentoxifylline was subsequently investigated in relation to the kinetics of JEV replication. A non-toxic concentration of pentoxifylline was added at various time points following entry of JEV into PS cells and the experiments were terminated following 48 h of incubation. Pentoxifylline at a concentration of 0.0017 μM was able to inhibit JEV replication completely when added to the infected monolayer up to 14 h post infection, as evidenced by the absence of virus yield and soluble antigen. However, beyond 14 h post infection pentoxifylline did not completely inhibit JEV replication (Fig. 3A). An intriguing observation was the presence of viral antigen expressed in the pentoxifylline-treated cells from 0 to 14 h, the time points that inhibited virus yield (Fig. 3B).
Fig. 3.
(A) Addition of pentoxifylline to virus-infected porcine stable kidney (PS) cells was staggered (see Section 2.6.2 for details). The x-axis represents the various time points at which pentoxifylline was added following adsorption of Japanese encephalitis virus onto PS cells. Note that there was no virus yield (represented as log 50% tissue culture infective dose (TCID50)/mL on the left-hand y-axis) in drug-treated cells (○) until 14 h post infection, after which virus yield steadily increased to attain levels similar to that obtained in untreated cells (●). The right-hand y-axis represents the optical density (OD) values obtained in the JEV antigen capture enzyme-linked immunosorbent assay (ELISA). Soluble JEV antigen was measured in the supernatant fluids obtained at 48 h after the experiment (see Section 2.6.2 for details) in both drug-treated (△) and untreated (▴) cells. Note the absence of soluble antigen in the drug-treated cells until 14 h post infection, after which it was detectable. (B) Detection of JEV-specific antigen using an immunofluorescence assay. It can be observed that JEV-infected monolayers treated with pentoxifylline were positive for viral antigen at all time points post infection (400×).
3.3. Confirmation of the mechanism of action of pentoxifylline
The presence of viral antigen noted in the pentoxifylline-treated cells from 0 to 14 h (Fig. 3B) prompted us to confirm whether translation of viral proteins remained unaffected. Therefore, viral RNA extracted from a series of time-point experiments was subjected to in vitro translation using a commercial kit. The viral RNA obtained from JEV-infected cells treated with pentoxifylline at two time points (4 and 10 h) showed the presence of a 50-kDa protein that reacted with JEV-specific monoclonal antibodies in a Western blot (Fig. 4 ).
Fig. 4.
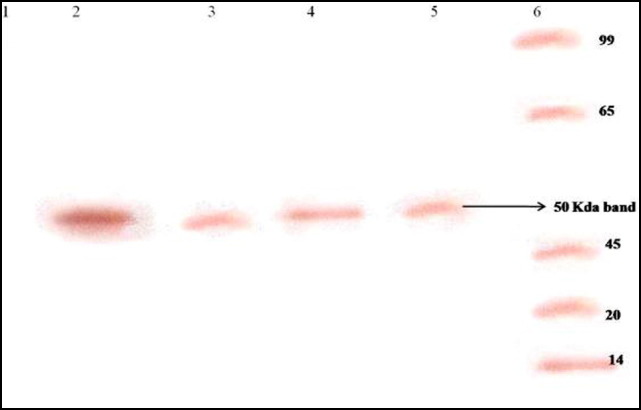
Western blot illustrating the effect of pentoxifylline on Japanese encephalitis virus translation using an in vitro translation kit. Lane 1, uninfected cell control; lanes 2 and 4, in vitro translation products of RNA obtained from JEV-infected porcine stable kidney (PS) cells (untreated) at 4 and 10 h post infection, respectively; lanes 3 and 5, in vitro translation products of RNA obtained from JEV-infected PS cells treated with pentoxifylline for 4 and 10 h, respectively. Note the presence of a 50-kDa JEV-specific protein in pentoxifylline-treated JEV-infected cells at 4 and 10 h. Lane 6, molecular weight markers.
3.4. In vivo evaluation of compounds against JEV using a mouse model
It was observed that i.p. administration of 50, 100 and 200 mg/kg body weight was non-toxic to mice, whilst 300 mg/kg body weight showed signs of toxicity (loss of weight and death) and was therefore found to be unsuitable for use in the study. The therapeutic potential of pentoxifylline was evaluated in mice using a peripheral challenge model. Three groups (n = 4 per group) of 4-week-old Swiss albino mice infected with 50× LD50 of JEV by the peripheral route were administered 50, 100 and 200 mg/kg body weight of pentoxifylline by the i.p. route twice daily. Mice that received 200 and 100 mg/kg body weight of pentoxifylline showed 100% protection, whilst mice that received 50 mg/kg body weight of pentoxifylline showed a 50% reduction in mortality. On the other hand, mice that did not receive the drug succumbed to encephalitis within 7 days.
Mice that survived the challenge post treatment were sacrificed and their brains were harvested and subjected to virus isolation, detection of viral antigen and viral RNA by RT-PCR. Infectious virus could not be isolated from brain tissues obtained from mice that survived the challenge. Similarly, viral antigen could not be demonstrated in the brain smears by immunofluorescent staining using monoclonal antibodies to JEV. However, viral nucleic acid was detected in the brain homogenate by RT-PCR, suggesting that viral RNA was present in the brain of animals that survived JEV infection following treatment with pentoxifylline.
4. Discussion
The use of agents that can interfere with viral replication and concomitantly suppress viral infection by modulating the immune system is one of the strategies that can be used to prevent viral infections. JEV infection generates a rapid inflammatory response following entry into the host, and elevated levels of tumour necrosis factor-alpha (TNFα) have been correlated with poor outcome in JE patients [14]. The pathophysiology of central nervous system inflammation in JEV may be attributed to TNFα, which ranges from promoting viral replication to evasion of host defences [15]. These observations raise the possibility that a compound capable of blocking TNFα could reduce the severity of JEV infection. Pentoxifylline is a known inhibitor of TNFα production [16]. Furthermore, recent studies have shown that pentoxifylline exhibits antiviral activity against severe acute respiratory syndrome (SARS) virus and HIV [7], [17]. This study was therefore designed to investigate the antiviral activity of pentoxifylline against JEV in vitro and in vivo.
In the present study, it was observed that pentoxifylline inhibited JEV replication post-entry into the cell. Our preliminary experiments indicated that this compound neither interfered with viral adsorption nor possessed any virucidal (inactivating) property (data not presented). The extent of antiviral activity was then confirmed using a plaque reduction assay. The results of the confirmatory plaque reduction assay suggested that pentoxifylline and ribavirin were able to inhibit JEV replication in a dose-dependent manner (Fig. 1). There was a significant reduction in JEV plaques by pentoxifylline at a concentration well below the CC50, with an IC50 of 50.3 μg/mL (0.00018 μM), whilst the IC50 of ribavirin was 3.9 μg/mL (0.000016 μM). This suggests that both the compounds possess significant inhibitory potential against JEV. It was further observed that both the compounds were highly active against JEV, with a TI of 10 and 13 for pentoxifylline and ribavirin, respectively.
There are no extensive in vitro studies reported to date on the mechanism of antiviral action of pentoxifylline. In the absence of such information, we asked two crucial questions pertaining to the antiviral activity of pentoxifylline. (i) What is the probable stage at which pentoxifylline inhibits virus replication? And (ii) how late can addition of pentoxifylline to infected cells be delayed to achieve complete inhibition of virus replication? To answer these questions, we used an experimental approach similar to that described by Baginiski et al. [18]. Pentoxifylline was added at 2-h intervals post infection until the completion of one replicative cycle of JEV. It appeared that pentoxifylline was able to inhibit JEV replication very late in the replication cycle. This assumption is based on several findings obtained in this study: (i) the presence of cell-bound antigen detected by IFA at all time points (Fig. 3A) suggests that protein translation is unaffected by the drug; (ii) the presence of a 50 kDa in vitro translation product (Fig. 4) obtained at both 4 h and 10 h post infection further affirmed that translation of viral proteins was unaffected by the drug; and (iii) neither infectious virus nor soluble viral antigen could be detected (Fig. 3B) in the supernatant of drug-treated cells until 14-h post infection. Taken together, these three observations suggest that pentoxifylline was indeed able to inhibit JEV replication at a stage beyond viral translation, probably interfering with assembly and/or release of the virion. Had pentoxifylline interfered with early or late protein translation, detection of JEV antigen by IFA in drug-treated cells would not have been possible (Fig. 3A).
Having established that pentoxifylline was able to inhibit JEV replication in vitro, we investigated its therapeutic potential in an in vivo mouse model. Indeed, this drug at concentrations of 100 mg/kg and 200 mg/kg body weight was able to protect mice completely against a lethal challenge (50× LD50) of JEV. Although viral RNA was demonstrated in the brains of all drug-treated mice that survived JEV infection, there were no classical signs of illness observed in any of the mice. This indicates that the virus crossed the BBB following i.p. injection but failed to replicate in the presence of the compounds since there was no viral antigen demonstrable in the brain smears of drug-treated mice. To ascertain that the viral RNA detected in the brain was not due to persistence of infection, virus isolation was carried out using a C6/36 monolayer. It was observed that all the mice that survived the infection failed to demonstrate replicating virus. This indicates the lack of infectious virus in the brains of mice that survived treatment with pentoxifylline. Notwithstanding these observations, it must be emphasised that pentoxifylline is a potent inhibitor of TNF [16]. Recently, it has been reported that JEV induces apoptosis in cell lines via the endoplasmic reticulum stress pathway [19]. The underlying mechanism of neuronal apoptosis in JEV has been attributed to the role of the tumour necrosis factor receptor (TNFR) superfamily [20]. Therefore, it is also possible that apart from exhibiting anti-JEV activity, pentoxifylline could have also modulated the TNF responses and protected mice against lethal challenge. However, further studies are warranted to investigate the immunomodulatory role of pentoxifylline in JEV infection.
In conclusion, this study has presented unambiguous evidence of anti-JEV activity of pentoxifylline, which necessitates the evaluation of this compound in controlled clinical trials.
Acknowledgments
The authors wish to acknowledge Dr. Suresh Chandra, Senior Veterinarian, CARF, NIMHANS, Bangalore, India, for supplying the animals used in this study.
Funding: No funding sources.
Competing interests: None declared.
Ethical approval: Approval was obtained from the Institutional Animal Ethics Committee (IAEC), NIMHANS, Bangalore, India (AEC/XVI/4/146/2004-2005). The care of animals was in accordance with institutional guidelines.
References
- 1.Chen C.J., Raung S.L., Kuo M.D., Wang Y.M. Suppression of Japanese encephalitis virus infection by non-steroidal anti-inflammatory drugs. J Gen Virol. 2002;83:1897–1905. doi: 10.1099/0022-1317-83-8-1897. [DOI] [PubMed] [Google Scholar]
- 2.Kalita J., Misra U.K. EEG in Japanese encephalitis: a clinico-radiological correlation. Electroencephalogr Clin Neurophysiol. 1998;106:238–243. doi: 10.1016/s0013-4694(97)00123-5. [DOI] [PubMed] [Google Scholar]
- 3.Misra U.K., Kalita J., Srivastava M. Prognosis of Japanese encephalitis: a multivariate analysis. J Neurol Sci. 1998;161:143–147. doi: 10.1016/s0022-510x(98)00265-2. [DOI] [PubMed] [Google Scholar]
- 4.Leyssen P., Erik D.C., Johan N. Perspectives for treatment of infections with Flaviviridae. Clin Microbiol Rev. 2000;13:67–82. doi: 10.1128/cmr.13.1.67-82.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huh P.D., Kotasek H.S., Jacob G.M., Vercellotti, Hammerschimidt D.E. Mechanism of action of pentoxifylline in peripheral vascular disease: inhibition of platelet and granulocyte responsiveness. Clin Res. 1985;33:866–878. [Google Scholar]
- 6.Amvros’eva T.V., Votiakov V.I., Andreeva O.T., Vladyko G.V., Nikolaeva S.N., Orlova S.V. New properties of trental as an inhibitor of viral activity with a wide range of activity [in Russian] Vopr Virusol. 1993;38:230–233. [PubMed] [Google Scholar]
- 7.Fazely F., Dezube B.J., Ryan J., Pardee A.B., Ruprecht R.M. Pentoxifylline (Trental) decreases the replication of the human immunodeficiency virus type 1 in human peripheral blood mononuclear cells and in cultured T cells. Blood. 1991;77:1653–1656. [PubMed] [Google Scholar]
- 8.Fox L.M., Kinter A.L., Poli G., Fauci A.S. Pentoxifylline inhibits HIV replication in acutely infected primary cells and chronically infected U1 cells through multiple mechanisms. Program and Abstracts of the First National Conference on Human Retrovirus and Related Infections; December 12–16; Washington DC; 1993. p. 119. [Google Scholar]
- 9.Crance J.M., Scarmozzino N., Jouan A., Garin D. Interferon, ribavirin, 6-azauridine and glycyrrhizin: antiviral compounds active against pathogenic flavivirus. Antiviral Res. 2003;57:73–79. doi: 10.1016/s0166-3542(02)00185-7. [DOI] [PubMed] [Google Scholar]
- 10.Sebastian L., Desai A., Madhusudana S.N., Yogeeswari Y., Sriram D., Ravi V. N-methylisatin-β-thiosemicarbazone derivative (SCH 16) is an inhibitor of Japanese encephalitis virus infection in vitro and in vivo. Virol J. 2008;5:64. doi: 10.1186/1743-422X-5-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desai A., Ravi V., Guru S.C., Shankar S.K., Kaliaperumal V.G., Chandramuki A. Detection of autoantibodies to neural antigens in the CSF of Japanese encephalitis patients and co-relation of findings with the outcome. J Neurol Sci. 1994;122:109–116. doi: 10.1016/0022-510x(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 12.Whitby K., Pierson T.C., Geiss B., Lane K., Engle M., Zhou Y. Castanospermine, a potent inhibitor of dengue virus infection in vitro and in vivo. J Virol. 2005;79:8698–8706. doi: 10.1128/JVI.79.14.8698-8706.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shu P.Y., Chang S.F., Kuo Y.C., Yueh Y.Y., Chien L.J., Sue C.L. Development of group and serotype specific one-step Syber Green-I based real time reverse transcription PCR assay for dengue virus. J Clin Microbiol. 2003;41:2408–2416. doi: 10.1128/JCM.41.6.2408-2416.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ravi V., Parida S., Desai A., Chandramukhi A., Gourie-Devi M., Grau G.E. Correlation of tumor necrosis factor levels in the serum and cerebrospinal fluid with clinical outcome in Japanese encephalitis patients. J Med Virol. 1997;51:132–136. [PubMed] [Google Scholar]
- 15.Campbell I.L. Cytokines in viral disease. Curr Opin Immunol. 1991;3:486–491. doi: 10.1016/0952-7915(91)90008-o. [DOI] [PubMed] [Google Scholar]
- 16.Schade U.F. Pentoxifylline increases survival in murine endotoxin shock and decreases formation of tumor necrosis factor. Circ Shock. 1990;31:171–181. [PubMed] [Google Scholar]
- 17.Martin J.F.B., Jimenez J.L., Munoz-Fernandez M.A. Pentoxifylline and severe acute respiratory syndrome (SARS): a drug to be considered. Med Sci Monit. 2003;9:41–46. [PubMed] [Google Scholar]
- 18.Baginski S.G., Pevear D.C., Seipel M., Sun S.C.C., Benetatos C.A., Chunduru S.K. Mechanism of action of a pestivirus antiviral compound. Proc Natl Acad Sci USA. 2000;97:7981–7986. doi: 10.1073/pnas.140220397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hase T., Summers P.L., Dubois D.R. Ultrastructural changes of mouse brain neurons infected with Japanese encephalitis virus. Int J Exp Pathol. 1990;71:493–505. [PMC free article] [PubMed] [Google Scholar]
- 20.Chen G., Goeddel D.V. TNF-R1 signaling: a beautiful pathway. Science. 2002;296:1634–1635. doi: 10.1126/science.1071924. [DOI] [PubMed] [Google Scholar]