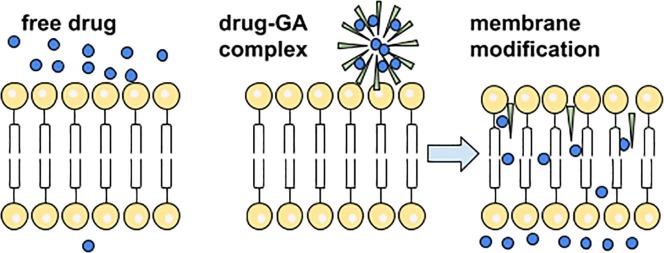
Graphical abstract
Keywords: Glycyrrhizic acid, Therapeutic activity, Self-association, Drug delivery, Membrane permeability, Lipid membrane, Complex formation
Highlights
-
•
Glycyrrhizic acid (GA), saponin of licorice shows wide range of biological activity.
-
•
Mechanism of GA activity on the cell and molecular level is rarely discussed.
-
•
GA activity could be caused by the cell membrane modification.
Abstract
Glycyrrhizic acid is the main active component of Licorice root which has been known in traditional Chinese and Japanese medicine since ancient times. In these cultures glycyrrhizic acid (GA) is one of the most frequently used drugs. However, only in 21-st century a novel unusual property of the GA to enhance the activity of other drugs has been discovered. The review describes briefly the experimental evidences of wide spectrum of own biological activities of glycyrrhizic acid as well as discusses the possible mechanisms of the ability of GA to enhance the activity of other drugs. We have shown that due to its amphiphilic nature GA is able to form self-associates in aqueous and non-aqueous media, as well as water soluble complexes with a wide range of lipophilic drugs. The main purpose of our review is to focus reader's attention on physicochemical studies of the molecular mechanisms of GA activity as a drug delivery system (DDS). In our opinion, the most intriguing feature of glycyrrhizic acid which might be the key factor in its therapeutic activity is the ability of GA to incorporate into the lipid bilayer and to increase the membrane fluidity and permeability. The ability of biomolecules and their aggregates to change the properties of cell membranes is of great significance, from both fundamental and practical points of view.
1. Introduction
Recent advances in nanotechnology and supramolecular chemistry significantly expand capabilities to address a wide range of medical problems. One of the most important scientific achievements is the use of nanoscale supramolecular aggregates to increase the solubility and stability of lipophilic drugs and to improve their targeted delivery to the source of the disease (Kumari et al., 2010, Patel and Chaudhari, 2012). It should be noted that the number of scientific papers devoted to the development of drug delivery systems (DDS), as well as the amount of funding for these works exceeded that for developing and researching new drugs. One of the purposes of such studies is the search for new non-toxic complexants that are able to protect guest molecules, reduce hydrophobicity, control their reactivity and improve the bioavailability and therapeutic activity of the drug molecule. The purpose of the present review is to introduce a novel unusual DDS – glycyrrhizic acid.
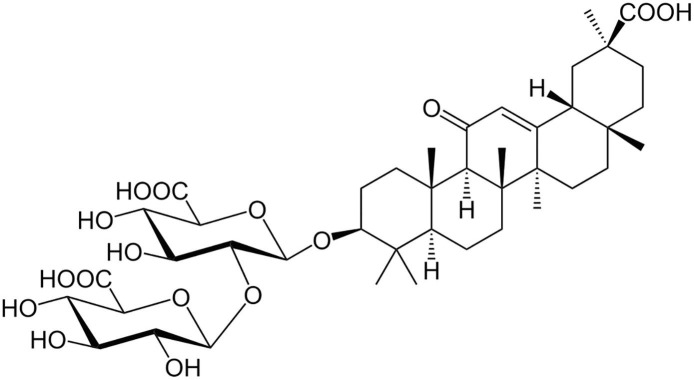
Glycyrrhizic acid (GA, or glycyrrhizin) is a saponin of licorice root. GA is an amphiphilic molecule: the hydrophilic part is represented by the glucuronic acid residues, and the hydrophobic part is the glycyrrhetic acid residue (Fig. 1 ). GA has very long history of use. It has been known since ancient times in ancient China, Egypt, Japan (Shibata, 2000). Licorice is widely used in traditional Chinese and Japanese medicine. For the past 50 years the biological and therapeutic activity of GA has been intensively investigated in Asia and Europe (Shibata, 2000, Ming and Yin, 2013, Su et al., 2017), and it was confirmed to be non-toxic (Ming and Yin, 2013).
Fig. 1.
The structure of Glycyrrhizic acid (GA).
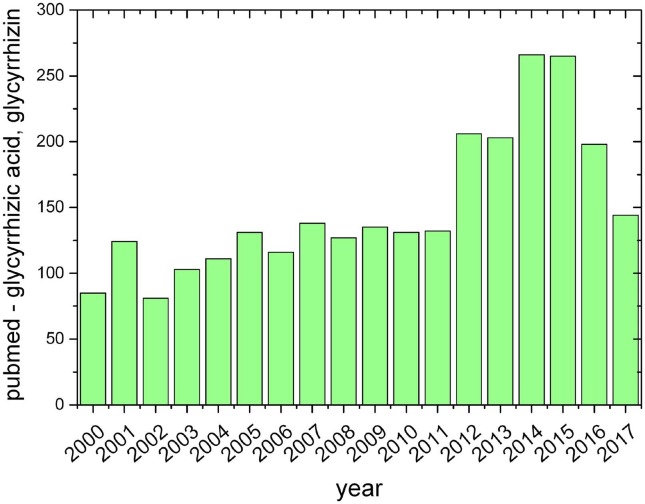
It should be noticed that GA is one of the most widely studied triterpene compounds. The number of references in PubMed since 2000 is more than 2500, and in 2015 and 2016 – more than 250 (Fig. 2 ). At the same time, the total number of publications devoted to the study of GA in Google Scholar is about 12,000, and the number of publications in 2015 is about 1000.
Fig. 2.
The total number of publications devoted to the study of glycyrrhizic acid since 2000 year.
It should be noticed that most of these works are devoted to own biological activity of GA (antiviral, anticancer etc.) and only a small part of them is devoted to possible applications of GA as drug carrier. And just a few studies contain attempts to understand and explain the mechanism of GA action as DDS. Only in last decades in addition to its own therapeutic activity, a novel unusual property of the GA has been discovered. In particular, the ability of GA to form inclusion complexes with a variety of drugs was demonstrated (Baltina, 2003, Apanasenko et al., 2015, Polyakov et al., 2008, Tolstikova et al., 2009, Polyakov and Leshina, 2011, Polyakov et al., 2013). These complexes show significant advantages over other known DDS. In addition, it was demonstrated the ability of GA to interact with cell membrane and to change membrane properties (Selyutina et al., 2016a, Selyutina et al., 2016b, Selyutina et al., 2014, Selyutina et al., 2017, Selyutina et al., 2015). Cell membrane forms the boundary between extracellular environment and intracellular space, provides cell integrity and implements barrier functions. Thus, understanding influence of drug delivery systems on functional properties of the cells (permeability, elasticity, lipid mobility and cell viability) is of particular interest. In this review we tried to collect and systematize the available data about GA both as biologically active molecule (in vivo and in vitro) and as drug carrier, in the hope of stimulating further discussions about the mechanism of GA action and about perspectives of GA application as a multifunctional drug carrier.
2. Antiviral activity of glycyrrhizic acid
There are a number of data on the antiviral activity of glycyrrhizin against various viruses. Thus, it was shown that GA at concentrations 0.04–4.8 mM inhibits the replication of the Epstein-Barr virus (human herpesvirus type 4 virus) in vitro (Lin, 2003), the GA selectivity index is 2 times higher than that of the antiviral drug Zidovudine, and 2 times lower than the popular drug Acyclovir. Also in this work it was established that the mechanism of GA action is not associated with direct inactivation of the virus. Presumably, the effect of GA occurs at the stage of virus penetration. It was shown that glycyrrhizic acid could inhibit the replication of coronavirus SARS in vitro (Hoever et al., 2005). Recent research of the antiviral activity of GA and its various derivatives were carried out on the Vero cell line obtained from the kidney epithelium taken from an African green monkey. It was found that at a concentration of 365 μM GA inhibits the cytopathic effect (destruction of cells) of the virus in 50% of infected cells. At the same time, the GA concentration at which the cell viability decreases by 50% is more than 24 mM.
It was shown that glycyrrhizin at a concentration 500 μg/ml in vitro inhibits cell destruction for three of the studied strains of the Japanese encephalitis virus: Nakayama, P-20778 and 821,564 XY48 after 96 h (Badam, 1997). A similar results were obtained for 1000 μg/ml of licorice extract and ammonium salt of GA. The minimum concentration for which inhibition was observed proved to be non-toxic for the pig's kidney (PS) cell lines and human cervical cancer (HeLa). The ability of GA to inhibit the replication of a number of other viruses is also established: vaccinia virus, herpes simplex virus type 1, Newcastle disease virus, poliovirus type 1, and vesicular stomatitis virus (Pompei et al., 1979, Pompei et al., 1980). These studies were performed in vitro on the human aneuploid cell line Hep2. It has been found that the addition of 8 mM GA to infected cell cultures inhibits both the growth of vaccinia viruses of the first type, Newcastle disease, and vesicular stomatitis, and their cytopathic effect. At the same time, the protection of cell cultures from viral exposure proved to be so effective that there are practically no differences between infected cells treated with GA and non-infected cells upon microscopic observation. Post-treatment (3 h after infection) by 8 mM GA also leads to suppression of virus growth and stops the progression of the cytopathic effect. The effect is also observed at GA concentrations of 4 mM and 2 mM, but is absent at a concentration of 1 mM. Along with this, an irreversible activation of the first herpes simplex virus is observed after treatment of infected cells with 8 mM glycyrrhizic acid in just 15 min. It should be noted that this effect is not observed for other viruses investigated in this work, even when processed up to 3 h. There is also no effect of GA on the first type of poliovirus.
Despite the abundance of studies of GA activity against various unbound DNA and RNA viruses, the mechanism of its antiviral effect remains unclear. It was shown that the effect of GA on herpes virus associated with Kaposi's sarcoma is related to inhibition of the synthesis of viral RNAs (Kang and Lieberman, 2011). The GA effect on the replication of various viruses was found in a number of works (Kimoto et al., 2005, Sekizawa et al., 2001, Baba and Shigeta, 1987). However, the fact that the GA antiviral activity is detected in relation to various weakly interconnected DNA and RNA viruses suggests that the GA effect is associated not only with the effect on the synthesis of RNA. It was established in (Lin, 2003) that when the GA is added 6 h after infection of the cells, no antiviral action is observed. At the same time with the addition of GA immediately after infection and washing of the GA after 5 h, the antiviral effect remains irreversible. Based on this, it is concluded that the GA selectively blocks the penetration of the virus into the cell, since this is a process that takes place approximately the first 5 h after infection. In the case of the virus of the reproductive-respiratory syndrome of pigs, it was shown that the GA action is related mainly to the stage of virus penetration and has little effect on the stages of absorption and release of the virus (Duan et al., 2015). In addition, the authors excluded the direct inhibitory effect of GA on viral particles. It has also been shown in a number of studies that GA and its derivatives prevent the penetration of a number of viruses through the plasma membrane (Lin, 2003, Hoever et al., 2005, Harada, 2005, Crance et al., 1994, Sui et al., 2010). In addition, it was found that the effect of GA leads to a decrease in the fluidity of the cell membranes (Harada, 2005, Wolkerstorfer et al., 2009). It was also established in (Matsumoto et al., 2013) that GA can prevent the spread of the virus by inhibiting the release of viral particles from the infected cell. Summarizing the results of these studies we can conclude that the key feature of GA responsible for its antiviral activity might be its membrane modifying ability. This question will be discussed below.
3. Anti-inflammatory activity
For a long time, glycyrrhizin is known as a compound with anti-inflammatory activity. A lot of studies were devoted to this issue. They consider the anti-inflammatory activity of GA mainly in terms of inhibiting the main mediators of inflammation, such as TNF-α, interleukins IL-1β and IL-6. It has been shown that glycyrrhizin inhibits its production in vitro. (Matsumoto et al., 2013) A number of studies associate the GA anti-inflammatory activity with its antioxidant activity. In particular, it has been shown that along with other components of licorice extract, glycyrrhizin inhibits the synthesis of NO and inflammatory cytokines in the model of lipopolysaccharide-stimulated microglial cells (Fu et al., 2014, Yu et al., 2015). Also GA, like a number of other terpenic acids, demonstrates antioxidant activity against H2O2 in the cells of human bronchial epithelium (Tsao and Yin, 2015). The protective mechanism of GA is caused by the fact that H2O2 reduces the expression of the regulator of apoptosis Bcl-2, while GA already reverses this effect at a concentration of 8 μM. Experiments show that GA does not decrease the viability of cells (Matsumoto et al., 2013, Tsao and Yin, 2015), while treatment with H2O2 decreases viability and increases DNA fragmentation in the tested cells (Tsao and Yin, 2015). Pre-treatment with glycyrrhizic acid in this case increases the viability of cells and reduces the fragmentation of DNA. In addition, glycyrrhizin reduces the production of caspase-3 and the oxidation and inflammation factors induced by H2O2 (Tsao and Yin, 2015, Akamatsu et al., 1991). It was demonstrated in (Tsao and Yin, 2015, Akamatsu et al., 1991) that GA significantly reduces the production of reactive oxygen species, in particular, O2 –, H2O2 and OH radicals, in vitro, by inhibiting neutrophil metabolism. In particular, this is also associated with the anti-inflammatory effect of GA. Another putative mechanism of GA anti-inflammatory activity is associated with inhibition of the synthesis of prostaglandin E2, which is one of the inflammation mediators (Ohuchi et al., 1981). In this study, macrophages were incubated for 20 h in a medium containing different GA concentrations. Significant inhibition of the synthesis of prostaglandin E2 by activated macrophages was observed at concentrations of 0.1 and 1 mg/ml. It was also established that GA in such concentrations is not toxic for macrophages.
One of the suggested mechanisms of GA action, as in the case of antiviral activity, is a change in the functional properties of the plasma membrane of cells treated with glycyrrhizin. In particular, antiviral action could be associated with degradation of lipid rafts, by GA extracting cholesterol, which makes it difficult to translate the TLR4 receptor into lipid rafts and inhibit the lipopolysaccharide-induced inflammatory response in RAW264.7 cells. TLR4, a membrane protein, belongs to the group of toll-like receptors involved in congenital immunity, is the main receptor of lipopolysaccharides in RAW264.7 cells, and therefore directly regulates the cellular response to bacterial lipopolysaccharides that are a marker of the infection (Matsumoto et al., 2013).
4. Hepatoprotective activity
A number of experimental data indicate the GA specificity to liver diseases. In particular, it has been established that glycyrrhizin demonstrates a hepatoprotective effect. Hepatoprotective effect against a number of toxic compounds is largely determined by the antioxidant activity of glycyrrhizic acid. Thus, it was shown that glycyrrhizin significantly reduces necrosis and apoptosis of liver cells of rats exposed to titanium dioxide nanoparticles (Orazizadehet al., 2014). Also in this study it was established that GA decreases lipid peroxidation and demonstrates antioxidant activity. It is suggested that a decrease in oxidative stress significantly causes a decrease in apoptosis and necrosis of hepatocytes induced by titanium dioxide. Hepatoprotective and antioxidant properties of glycyrrhizin are also manifested in relation to CCl4 (Huo et al., 2011, Yin et al., 2011, Liang et al., 2015), and a number of other cytotoxic compounds (Wan et al., 2009, Yin et al., 2008, Li et al., 2014, Wang et al., 2012, Hsiang et al., 2015). Some studies suggest a hepatoprotective function of GA including activity against liver cancer at the level of gene expression (Yin et al., 2011, Liang et al., 2015). In particular, it has been shown that GA regulates the expression of genes responsible for apoptosis and oxidative stress in human hepatoma cells (Wang et al., 2012).
GA anti-inflammatory and anti-apoptotic effect in the treatment of liver diseases is associated with suppression of tumor necrosis factor TNF-α and caspase-3, which causes GA hepatoprotective effect (El-Tahawy et al., 2011). Along with this, there are data indicating the manifestation of hepatoprotective function of GA by direct exposure to liver cells. In particular, Nakamura et al. induced the leakage of lactate dehydrogenase and glutamic oxaloacetic transaminase in a monolayer rat hepatocyte culture, treating these cells by CCl4. The leakage of this enzyme is caused by a change in the permeability of the membrane. The addition of glycyrrhizin caused a change in the release of the enzyme. A small changes were observed at glycyrrhizin concentration of 25 μg/ml, the maximum effect was observed at 200 μg/ml of glycyrrhizin. GA hepatoprotective effect could be connected with preventing a change in the permeability of the membranes of hepatocytes (Nakamura et al., 1985).
5. Anticancer activity
A number of studies have shown that glycyrrhizic acid demonstrates significant anticancer activity. It is able to induce apoptosis in tumor cells of various cancer types of cancer (Su et al., 2017, Hibasami et al., 2005, Thirugnanam et al., 2008). For example, daily intraperitoneal administration of 100 mg GA per kg of weight on mice with TF-1 tumor could reduce the growth of cancer cells and do not lead to body weight loss (Su et al., 2017). Drug combinations including GA were also studied. Viability of K562 cells (human chronic myeloid leukemia) was reduced by GA treatment at concentration higher than 2.0 mM. Combination of GA and anti-leukemic drug imatinib in the ratio 2 mM GA per 0.4 μM imatinib resulted in two fold increase of DNA fragmentation in K562 cell culture compared with caused by 2.0 mM GA or 0.4 μM imatinib alone (Hostetler et al., 2017).
Studies of GA influence on apoptosis in the other leukemia cells, WEHI-3 (mouse leukemia cells) shows the morphological changes of cells, which have dose-dependent character. DNA damage and fragmentation was observed in cells after 24 and 48 h after treatment. Treated cells also had higher yield of reactive oxygen species and apoptotic associated protein levels (Chueh et al., 2012).
It has been found that the addition of 10 μM glycyrrhetic (also known as glycyrrhitinic) acid (a metabolite of glycyrrhizic acid formed by its oral use, Fig. 3 ) to rat liver mitochondria leads to their loss of membrane potential, swelling of mitochondria, and the release of cytochrome C. This result indicates that glycyrrhetic acid is capable of initiating the opening of mitochondrial pores, and thus triggering a pro-apoptotic pathway (Salvi et al., 2003).
Fig. 3.
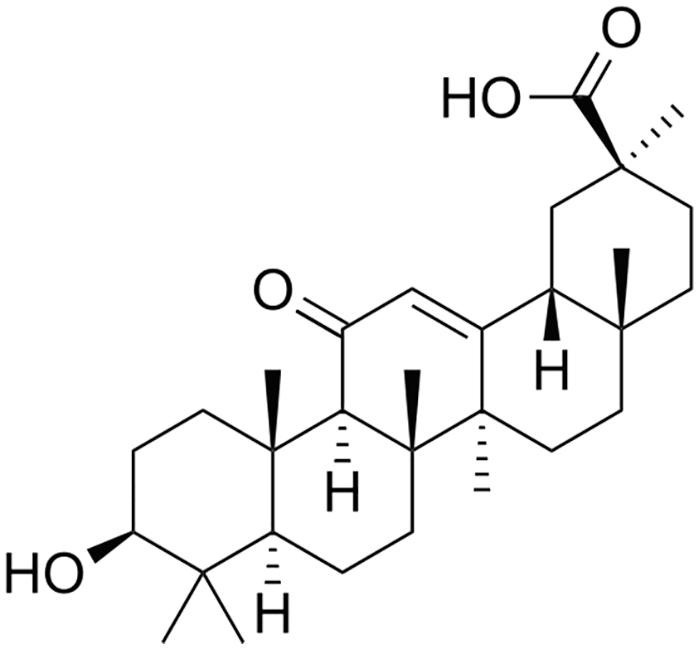
The structure of glycyrrhetic acid.
Further studies have shown that this effect is the result of the combined effect of glycyrrhetic acid and Ca2+, which can generate hydrogen peroxide by interacting with the mitochondrial respiratory chain. It oxidizes certain thiol groups and endogenous pyridine nucleotides, which leads to the opening of the mitochondrial pore (Fiore et al., 2004). This mechanism is also manifested when SiHa cells derived from cervical cancer material is treated with glycyrrhetic acid (Lee et al., 2008). This mechanism is of interest, since the formation of the mitochondrial pore is involved in various forms of cell damage, as well as apoptosis. The opening of mitochondrial pores causes the depolarization of the transmembrane potential, the release of Ca2+ and cytochrome C, and the loss of cancer cell viability (Mignotte and Vayssière, 1998).
6. Complex formation with drugs
One of the important problems of medical chemistry at the moment is the low bioavailability most of the used medicinal compounds. Low bioavailability is mainly determined by low solubility and low permeability or both. The solubility of drugs intended for oral administration is especially important. A possible way to solve this problem is the use of delivery systems in the form of complexes with highly soluble compounds. In many cases, this allows to significantly reduce the effective dose while preserving the therapeutic effect. The lipophilic drug molecule could pass through the lipid layer due to passive transport (against the concentration gradient), but for this it is necessary to achieve a sufficiently large concentration of the drug compound in the extracellular environment. However ∼ 30% of the released drug compounds are water insoluble. Taking into account that near 85% of the most popular drugs are taken orally, we could say that this trend the most promising.
A number of physicochemical studies show that the complexation of hydrophobic drugs with glycyrrhizic acid is capable of increasing the solubility of the drug by a factor of tens of times compared to the starting compound.
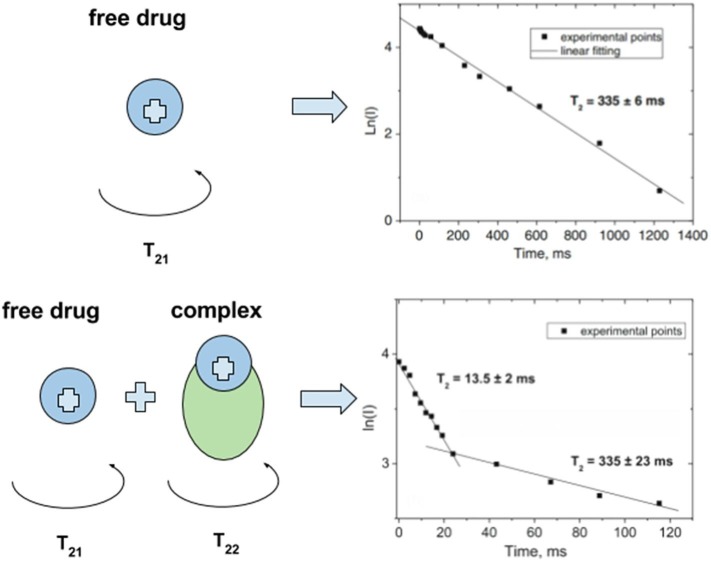
Various physicochemical methods have been applied to get the evidence of complexes formation between GA self-associates and drug molecules in solid state as well as in aqueous and non-aqueous solutions. Such complexes were investigated by Fourier-transform infrared spectroscopy, differential scanning calorimetry, X-ray diffraction and scanning electron microscopy techniques (Lekar et al., 2011a, Yang et al., 2015, Zu et al., 2013, Kong et al., 2017). The physicochemical properties and behavior of GA complexes in solution were characterized by NMR, mass spectroscopy, electrochemical and optical techniques (Baltina, 2003, Apanasenko et al., 2015, Konkina et al., 2015, Polyakov et al., 2005). In particular, changes of the mobility of a drug compound in the presence of GA were studied by 1H NMR spectroscopy to prove a formation of the inclusion complex (Apanasenko et al., 2015, Polyakov et al., 2008). The times of spin–lattice and spin–spin nuclear relaxation (T1 and T2, respectively) are very sensitive to changes in the mobility of molecules (Deese and Dartz, 1982, Bocian and Chan, 1978). Formation of inclusion complex results in decrease of the diffusion mobility of molecules and leads to decrease in the observed relaxation time of corresponding protons. The T2 is inversely proportional to the rotational correlation time and, as a result, depends on the rotational mobility of a molecule. Thus, T2 data could indicate is molecule in free of bound state. In the case of rapid exchange between the bound state and solution, the NMR signal decay in relaxation experiment is mono exponential, in the case of a slow exchange (in comparison with the relaxation time), the kinetics of the decay becomes bi-exponential:
The pre-exponential factors in this case correspond to the fraction of molecules with different rotational mobility (free and bound) (Fig. 4 ).
Fig. 4.
Influence of complex formation on the spin–spin relaxation times of molecules. Adopted from (Gluschenko, 2011).
Complexation of GA with different drugs was studied by mass spectroscopy, in particular, glycyrrhizin complexes with chloramphenicol antibiotic were studied by electrospray ionization mass spectrometry (Vetrova et al., 2015). The evidences of the complex formation were obtained by detecting changes in absorbance and infrared spectrum of chloramphenicol using ultraviolet/visible and infrared spectroscopy. In this study the complex formation of GA and chloramphenicol with the ratio 1:1 and 2:1 were registered in the negative ion mode. It was also established that GA derivatives forms complexes with guanine, adenine (Lekar et al., 2011b) and L-phenylalanine (Lekar et al., 2011a).
Different animal studies have shown that complexes of nootropic and tranquilizing drug phenibut with glycyrrhizin show an effect analogous to the effect of the drug itself in a dose reduced by 16 times (Tolstikova et al., 2009). In addition, these complexes increase the memory ability of animals and reduce such phenibut side effects as drowsiness and allergic reactions. At the same time, toxicity decreases by 1.7 times, and the amplitude of the therapeutic index increases by 17 times (Tolstikova et al., 2009). Similar action was detected for complexation of glycyrrhizin with other drugs, for example, nifedipine complexes with GA manifested antihypertensive therapeutic effect in a ten-fold reduced dose of nifedipine (Tolstikova et al., 2009, Polyakov and Leshina, 2011). The anti-inflammatory activity of GA and salbutamol was tested using tumor necrosis factor-alpha-induced NF-kappaB transcriptional activation reporter assay, I-kappaB Western blotting and interleukin-8 ELISA. [68]. It was established that GA at concentration 0.3 μM increased levels of mRNA of beta(2)-AR in vivo and the accumulation of cAMP in vitro. These results show that GA and salbutamol demonstrate synergistic anti-asthmatic effects. Complexes of GA with atorvastatin and simvastatin also demonstrated their higher effectiveness in comparison with pure drugs (Kong et al., 2017, Stakhneva et al., 2013). The amount of 3-hydroxy- 3-methyl-glutaryl-CoA reductase protein in rats decreased by 13 and 25% after 24 h of treatment with GA complex with atorvastatin and simvastatin, respectively. Activity of this enzyme decreased by 46% in rats treated with GA complex with atorvastatin (Stakhneva et al., 2013).
Optical spectroscopy was used to study the protective properties of complexes in solution. It is known that many drug compounds are characterized by a low stability in solutions. Some of them are photosensitive, susceptible to oxidation by free radicals formed in the body in vivo, etc. The complexation with glycyrrhizic acid in some cases can significantly improve the stability of the drug substance and protect it from undesirable effects. Thus the effect of various complexants including GA on the oxidative resistance of carotenoid molecules (lutein and zeaxanthin) was investigated (Apanasenko et al., 2015, Polyakov et al., 2013). Zeaxanthin and lutein have an important protective role in the eyes retina of humans and other mammals. The lack of these carotenoids can lead to eyes damage by the short-wavelength visible light and reactive oxygen species. It eventually leads to age-related macular degeneration and results in irreversible blindness. Zeaxanthin and lutein are not produced in the human body and should enter the body with food. But due to low solubility in water and instability of these molecules their practical use of its consumption is very limited. Preparation of supramolecular complexes of these carotenoids with GA, its disodium salt and natural polysaccharide arabinogalactan could minimize mentioned drawbacks and opens prospects for the use of such complexes in food industry and for the drug production. The complex formation of zeaxanthin and lutein was investigated by NMR relaxation, surface plasmon resonance, and optical absorption technique (Apanasenko et al., 2015, Polyakov et al., 2013). The authors have shown that complexes with polysaccharides and GA increase the solubility of carotenoids by more than 1000 times. Also, oxidation resistance of carotenoids in the reaction with ozone, peroxyl radicals and metal ions has been investigated. It was found that resistance to oxidation of lutein and zeaxanthin significantly increases in the complexes with glycyrrhizic acid and arabinogalactan (Fig. 5 ).
Fig. 5.
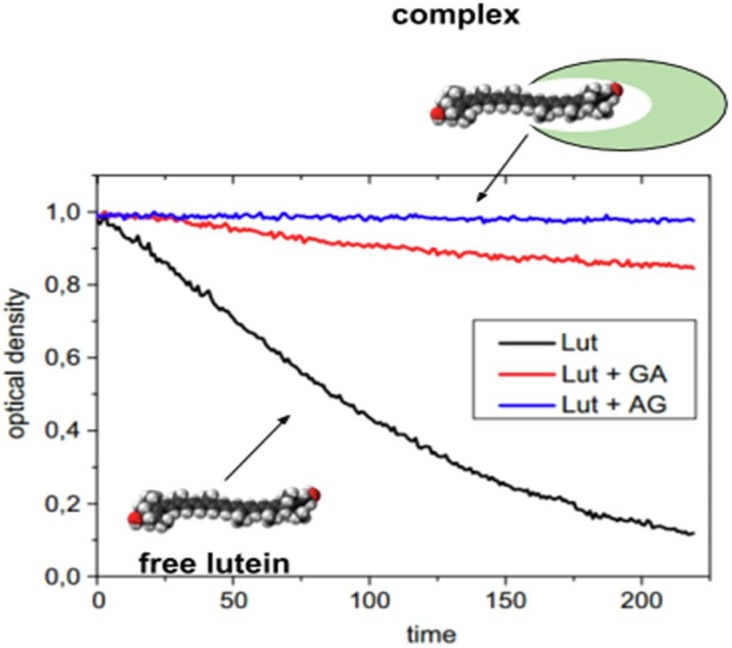
Kinetics of decay of lutein optical density in the absence and in the presence of GA (1 mM) and arabinogalactan (0.1 mM) in 75% water–ethanol solution during oxidation by ozone. Slow decay in the case of complex formation shows the oxidation stability of lutein. Adopted from Apanasenko et al. (2015).
The important feature of GA is its ability to reduce the cholesterol oxidation and to form water soluble complexes with cholesterol oxidation products (Gluschenko et al., 2011). This might be important factor for prevention of cholesterol plaques formation in blood vessels and for atherosclerosis development.
7. Self-association of GA and its use in drug delivery
The formation of inclusion complexes is facilitated by the formation of self-associates of GA molecules in solutions. As already discussed, glycyrrhizic acid is an amphiphilic molecule. The process of self-association of GA was studied by various methods. Thus, in (Kornievskaya et al., 2008) the formation of self-associates was proved by the NMR technique. It has been shown that micellization of glycyrrhizic acid occurs in water-methanol solutions with a low content of methanol (20%), whereas micelles do not form when the alcohol content is increased to 50%. The process of micellization and gelling of GA in a water-methanol mixture (20% methanol) was also studied by Petrova et al. (2016). It is shown that micelle formation occurs during hydrophobic interaction of the triterpene part of the glycyrrhizin. Studies of GA self-association by the molecular dynamics also demonstrated that the formation of aggregates occurs due to the hydrophobic interaction between terpenic residues (Zelikman et al., 2015). The study of GA micelles using small-angle scattering methods shows that GA forms rod-shaped micelles with a radius of 1.5 nm and a length of 21 nm, at a concentration of 5 mM in a solvent with pH = 5 (Matsuoka et al., 2015). Mass spectrometry study demonstrates that glycyrrhetic acid, an aglycone of glycyrrhizic acid, also forms self-associates (Lekar et al., 2016).
The ability of self-association in aqueous solutions and complexation with drugs makes GA a promising agent for drug delivery. Despite this, GA is usually used only as a targeting unit, in particular, for liver cells. There are currently very few studies devoted to the use of GA as an independent DDS.
An amorphous solid dispersion (SD) of curcumin (Cur) with disodium salt of glycyrrhizic acid (Na2GA) prepared by mechanical ball milling shows a marked improvement by factor about 20 in oral bioavailability in rats model (Zhang et al., 2018). Curcumin loaded micelles were self-formed when SD is dissolved in water. In vitro cytotoxic tests demonstrated higher cytotoxicity of Cur loaded GA micelles against glioblastoma U-87 MG cells than free Cur. Besides, an improvement of the membrane permeability of Cur was confirmed by parallel artificial membrane permeability assay (PAMPA). Employing solid dispersion of curcumin will allow the use of lower doses while maintaining efficacy and can help overcome some of the disadvantages of color, taste and smell.
Complexes of simvastatin with Na2GA also shows enhanced solubility and bioavailability (Kong et al., 2017). Simvastatin is an inhibitor of 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase and is widely used to control hyper-cholesterolemia and prevent cardiovascular diseases. Pharmacokinetic tests in vivo on laboratory animals showed an increase in drug bioavailability after its introduction as a complex with Na2GA.
The possibility of using glycyrrhizic acid micelles for the delivery of palitaxel, a cytotoxic anti-cancer drug characterized by low oral bioavailability (<2%), has been studied by Yang with coauthors (Yang et al., 2015). The GA micelles containing palitaxel obtained in this study have a size of about 250 nm, while the size of pure micelles is ∼ 80 nm, suggesting that the drug is located inside the micelles. In this case, transmission electron microscopy images show that all micelles are spherical. The solubility of the palitaxel as a component of aggregates with GA increases as a function of the GA concentration. The maximum observed increase was 200 times under GA concentration of 10 mM. As the result, paclitaxel-GA aggregates demonstrate an increase in oral bioavailability of approximately 6-fold compared to the initial compound in in vivo experiments, which is presumably due to increased absorption of the drug in the intestine.
The prospects of using GA micelles for transdermal delivery of podophyllotoxin (PT), a drug for the treatment of human papillomavirus type 6 and 11, were investigated in (Wang et al., 2016). These studies show that the solubility of podophyllotoxin in the water–ethanol mixture increases Caiapproximately five-fold with a 1:8 ratio of PT/GA in 25% ethanol. The sizes of pure micelles under these conditions were ∼6 nm, and the micelles containing the drug were ∼10 nm. Micelles containing PT have a longer release and penetration time than the PT solution, but a more stable concentration of PT in the skin for 12 h, and the final concentration of PT in the skin in the case of micelles was higher than in the case of solution.
The effect of GA and chitosan on the transdermal delivery of carvediol was also studied (Sapra et al., 2008). It has been found that the solubility of carvediol in solution containing 0.75% GA increases by a factor of 59 compared to the buffer solution. The permeability of carvediol through the skin in the presence of GA is also significantly higher than in a propylene glycol-ethanol mixture. The permeability of carvediol through the rat epidermis in a 0.75% GA solution increases 3-fold as compared to the buffer solution in vitro. Experiments also show that GA at concentrations higher than the critical concentration of micelle formation (∼1 mM) impairs the penetration of the drug into the epidermis. A presumptive mechanism for enhancing skin permeability is associated with modulation of the epidermal barrier state by action on the biochemical composition of the skin. The use of scanning electron microscopy and transmission electron microscopy also revealed that the use of GA leads to the formation of small pores in the stratum corneum.
A lot of studies are devoted to the use of GA and its metabolite glycyrrhetic acid as a targeting unit in liver therapy (Chen et al., 2016, Cai et al., 2016, Wu et al., 2017). As it was mentioned above, GA is effective hepatoprotective agent (see Section 4). The surface of hepatocytes is rich in glycyrrhetic acid receptors and this opens the prospect of using glycyrrhizic acids for targeted delivery. Wu et al. demonstrated the ability to use GA as a targeting agent for human serum albumin (HSA) nanoparticles loaded with resveratrol. Resveratrol is a natural compound which demonstrates anti-inflammatory, antimicrobial and possibly anticancer activity, but due to its low solubility its use is limited. It was shown that HSA nanoparticles conjugated with GA increase the solubility of resveratrol and besides that the drug release is persistent and slow. Fluorescence spectroscopy of FITC-labeled samples demonstrated that the uptake of HSA-GA nanoparticles loaded with resveratrol by HepG2 cells is much more effective than uptake of the nanoparticles without GA (Wu et al., 2017).
GA composition with anthelmintic drug praziquantel was studied in terms of bioavailability, solubility and permeability by various physicochemical techniques (Meteleva et al., 2019). The significant increase of praziquantel permeability through monolayer of Caco-2 cells in the composition with GA was observed by means of parallel artificial membrane permeability assay. In vivo studies on mice also have shown the increase of bioavailability by the factor of 3 under oral administration of composition (Meteleva et al., 2019).
Some studies indicate that not only the GA, but also glycyrrhetic acid (the metabolite of GA, Fig. 3) can be used for targeted drug delivery into liver cells (Chen et al., 2016, Chen et al., 2017, Cai et al., 2016, Singh et al., 2018). In particular, it was shown that glycyrrhetic acid could form supramolecular pro-gelator with curcumin, which demonstrated enhanced cellular uptake by HepG2 and better inhibition of cell growth cells in comparison with control naphthylacetic acid-curcumin hydrogel. So, such glycyrrhetic acid-curcumin hydrogel could be considered as a promising material for liver tumor chemotherapy (Chen et al., 2017). Fluorescence microscopic study of glycyrrhetic acid conjugated with coumarin-based fluoroprobe demonstrated that glycyrrhetic acid is selectively uptaken by liver cancer cells (HepG2 and Chang liver cancer cells). Also, a pro-drug epirubicin-glycyrrhetic acid (inactive form of epirubicin which can be activated by the rich esterase activity of the tumor cells microenvironment) demonstrated better selectivity to liver cancer cell, than free epirubicin (Singh et al., 2018). Cai and co-autores reviewed different drug delivery systems (liposomes, micelles, nanoparticles) modified with glycyrrhetinic acid. For all systems, the effect of glycyrretinic acid as a targeting agent for liver cells was observed (Cai et al., 2016).
8. Permeability enhancement and membrane-modifying activity
In addition to solubility enhancement of lipophilic drugs in the complexes with GA, a number of studies indicate the drug permeability enhancement in the presence of GA. It provides broad prospects of applying of glycyrrhizic acid as a multifunctional DDS. It was also established that GA enhance absorption effects of the variety of drugs (Kong et al., 2017, Sapra et al., 2008, Radwant and Aboul-Enein, 2002, Chen et al., 2009, Chakotiya et al., 2016). In view of the fact that the movement of small drug molecules through gastrointestinal epithelium is regulated by the distension and constriction of the tight junctions, observed effects could be connected with the ability of glycyrrhizin to enhance the penetration of molecules into the cells. During transport, the drug molecule must overcome many barriers in the form of monolayered and multilayered membranes. Despite the cellular structures are not all the same, influence factors and the through ways of drugs are similar in different cells. A number of results indicates that GA may enhance the drug penetration into the cells by influencing the cell membrane properties (Selyutina et al., 2016a, Selyutina et al., 2016b, Selyutina et al., 2014, Selyutina et al., 2017, Selyutina et al., 2015, Meteleva et al., 2019). In particular, it was shown that GA could enhance the permeability of erythrocytes and K562 cells for formate ions (Selyutina et al., 2016b, Selyutina et al., 2014, Selyutina et al., 2017). It is suggested that permeability enhancement could be due to GA membrane-modifying activity. To confirm this hypothesis the interaction of glycyrrhizin with palmitoyl-oleoyl-phosphatidylcholine (POPC) liposomes, dioleyl-phosphatidylcholine (DOPC) and dipalmitoyl-phosphatidylcholine (DPPC) bilayers were studied by NMR and molecular dynamic (MD) techniques. NMR study showed that joint action of 0.5 mM cholesterol and 0.5 mM glycyrrhizin leads to the penetration of Pr ions through bilayer (Selyutina et al., 2016a). Also, in the presence of glycyrrhizin 2-fold acceleration of the transport of formate ions through red blood cell membrane compared with the untreated samples was observed (Selyutina et al., 2016b). It may be connected with GA penetration into the lipid bilayer (Selyutina et al., 2016a). GA in water could easily connect to the bilayer surface and then penetrated into that. It is preferably located in the “outer” half-layer and could freely exchange between the polar part of half-layer and its hydrophobic interior (Fig. 6 ). During the penetration it could carry a few water molecules inside the bilayer. It could explain observed acceleration of small molecules transport through the membrane (Selyutina et al., 2016a). Observed effects could be connected with GA self-association. For example, the maximum acceleration of the rate of formate-ions exchange through the erythrocyte membrane was noticed at a glycyrrhizin concentration near critical micelle-formation concentration (about 10−3 M) (Selyutina et al., 2016b).
Fig. 6.
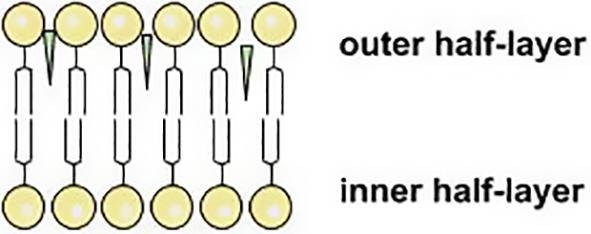
Schematic representation of GA incorporation into lipid bilayer.
Molecular dynamics modelling revealed that GA could penetrate in bilayers of different types of lipids. But only for the DPPC membrane, the most rigid one of the three types studied, it was found that GA could penetrate into the “inner” half-layer. As a result, it forms thinning which could lead to pore formation make the bilayer permeable for Pr ions (Fig. 7 ).
Fig. 7.
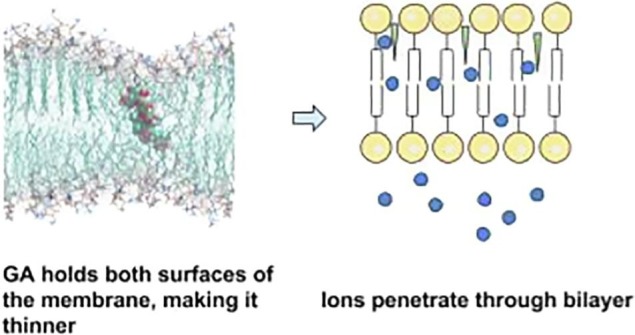
Possible mechanism of GA influence on membrane permeability. GA incorporates into lipid membrane and makes it thinner. Adopted from (Selyutina et al., 2016a).
Sapra et al. (2008) demonstrated the ability of GA to create small pores on the layer surface and intracellular lipid bilayer disordering in rat epidermis in vitro, that may be involved into the mechanism of the transdermal delivery of carvedilol. Also, observed pore formation and carrying of water molecules by GA may contribute into the passive transport through lipid membrane of molecules in supramolecular complex with GA. So, such DDS based on GA may not only increase the solubility of low-soluble drugs, but also enhance its penetration into the cells.
9. Safety concerns of GA
Glycyrrhizic acid as part of the licorice extract was approved by US Food and Drug Administration (FDA) as an existing food sweetener (Omar et al., 2012). Cosmetic Ingredient Review in 2007 reported that the intraperitoneal median lethal dose (LD50) for glycyrrhetinic acid (metabolite of GA, Fig. 3) in mice was 308 mg/kg and the oral LD50 was >610 mg/kg. The oral LD50 in rats was reported to be 610 mg/kg. Higher LD50 were reported for salts. Also, in a study of 39 volunteers there was no toxic effect observed under the oral administration of 2 mg/kg/day of GA for 8 weeks (Cosmetic Ingredient Review, 2007). In 1991, the European Union proposed a provisional figure of 100 mg/day as the upper limit for ingestion of GA and this limit was confirmed by the Scientific Committee on Food (Omar et al., 2012). European Food Safety Authority in 2015 concluded that glycyrrhizic acid ammoniated is safe at a concentration of 1 mg/kg complete feed for all species except chickens for fattening and laying hens (EFSA, 2015).
The main undesirable side-effects of licorice relate to its mineralocorticoid activity. Glycyrrhetic acid, an active metabolite of GA, inhibits the enzyme 11β-hydroxysteroid dehydrogenase which converts cortisol to inactive cortisone. This inhibition leads to disbalance in cortisol-cortisone conversion and activation of mineralocorticoid receptors by cortisol. It results in a state of apparent mineralocorticoid excess (AME) (Olukoga and Donaldson, 2000, Omar et al., 2012). AME includes hypokalaemic alkalosis, water and sodium retention with a tendency to hypertension, kaliuresis and suppression of the renin-angiotensin-aldosterone axis. It was shown that licorice-induced effect on the activity of 11β-hydroxysteroid dehydrogenase lasts for about two weeks following cessation of intake of the compound, during which period urinary glycyrrhetic acid concentration gradually falls (Olukoga and Donaldson, 2000).
10. Conclusions
Development of new safe and effective agents for drug delivery gains more and more popularity nowadays. Glycyrrhizin as the main active component of the licorice root which showed an extensive pharmacological activity is believed to have a great future. As it was discussed above, GA shows own pharmacological (antiviral, anti-inflammatory, anticancer, hepatoprotective, etc.) activity as well as enhances therapeutic effect of other drug molecules. One of the explanations of different types of GA activity, whether antiviral, anti-inflammatory, hepatoprotective or anti-cancer, is the GA membrane-modifying activity (Fig. 8 ). We have demonstrated several examples which illustrate the changes of membrane permeability, fluidity, pores formation and changes of trans-membrane potential by GA. These results are confirmed by the various physicochemical studies of the GA-membrane interaction.
Fig. 8.
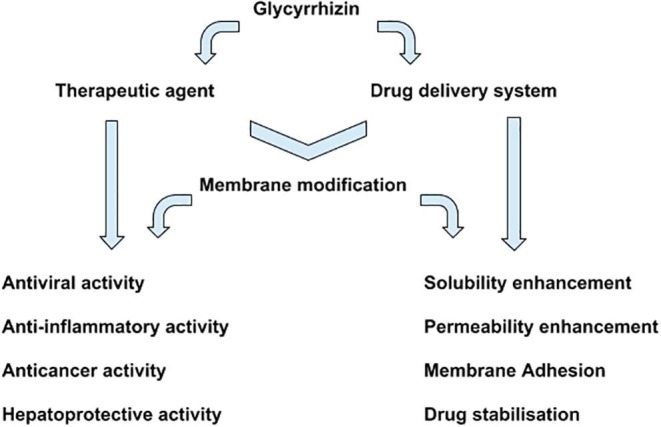
Possible ways of GA biological activity.
A lot of studies have also shown that drug solubility increases significantly in the supramolecular complex with GA. Solubility enhancement can facilitate the passive transport of drug molecules through cell membrane in supramolecular complex with GA. Permeability enhancement also could be caused by the GA incorporation into the cell membrane and pore formation in the lipid membrane under the action of GA. As a result, in addition to enhancement of the solubility and stability of drugs, glycyrrhizin could also affect their pharmacokinetic properties. In combination with its own biological activity such feature makes GA a perspective multifunctional drug delivery system.
Despite the variety of available data on the complexation of GA with drugs, very little papers are devoted to the application of the GA as the independent drug delivery system. Most of the papers are devoted to its use as a modifying agent for DDS, increasing its specificity for liver cells. But taking into account the variety of drugs which could be included in supramolecular complexes with glycyrrhizin and observed increase of cell membrane permeability in the presence of GA, and also the GA affinity to liver cells, we can conclude that GA could be used as effective and relatively cheap targeted drug delivery system. In addition, glycyrrhizic acid can be considered as universal nonselective carrier. At this moment a number of in vivo studies have been performed which prove the therapeutic potential of GA as multifunctional DDS for drugs with low solubility and low permeability. Animal studies demonstrate significant enhancement of bioavailability and therapeutic activity of these drugs in the compositions with GA.
The development of novel multifunctional DDS that could influence on the properties of cell membranes is of huge importance, from both fundamental and practical points of view. Taking into account the diversity and importance of the functions of the biological membrane in the vital activity of the living cell, the study of changes in the properties of the lipid membrane by various drug and biologically active molecules is one of the most important tasks of biophysics.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The reported research was funded by Russian Foundation for Basic Research (grants № 15-29-05792, 17-43-540175, 18-33-00662) and by Russian Ministry of Science and Education (0304-2017-0009).
References
- Akamatsu H., Komura J., Asada Y., Niwa Y. Mechanism of anti-inflammatory action of glycyrrhizin: effect on neutrophil functions including reactive oxygen species generation. Planta Med. 1991;57:119–121. doi: 10.1055/s-2006-960045. [DOI] [PubMed] [Google Scholar]
- Apanasenko I.E., Selyutina O.Yu., Polyakov N.E., Suntsova L.P., Meteleva E.S., Dushkin A.V., Vachali P., Bernstein P.S. Solubilization and stabilization of macular carotenoids by water soluble oligosaccharides and polysaccharides. Arch. Biochem. Biophys. 2015;572:58–65. doi: 10.1016/j.abb.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba M., Shigeta S. Antiviral activity of glycyrrhizin against varicella-zoster virus in vitro. Antiviral Res. 1987;7:99–107. doi: 10.1016/0166-3542(87)90025-8. [DOI] [PubMed] [Google Scholar]
- Badam L. In vitro antiviral activity of indigenous glycyrrhizin, licorice and glycyrrhizic acid (Sigma) on Japanese encephalitis virus. J. Commun. Dis. 1997;29:91–99. [PubMed] [Google Scholar]
- Baltina L.A. Chemical modification of glycyrrhizic acid as a route to new bioactive compounds for medicine. Curr. Med. Chem. 2003;10(2):155–171. doi: 10.2174/0929867033368538. [DOI] [PubMed] [Google Scholar]
- Bocian F., Chan S.I. NMR studies of membrane structure and dynamics. Annu. Rev. Phys. Chem. 1978;29:307–335. [Google Scholar]
- Cai Y., Xu Y., Chan H.F., Fang X., He C., Chen M. Glycyrrhetinic acid mediated drug delivery carriers for hepatocellular carcinoma therapy. Mol. Pharm. 2016;13:699–709. doi: 10.1021/acs.molpharmaceut.5b00677. [DOI] [PubMed] [Google Scholar]
- Chakotiya A.S., Tanwar A., Narula A., Sharma R.K. Alternative to antibiotics against Pseudomonas aeruginosa: effects of Glycyrrhiza glabra on membrane permeability and inhibition of efflux activity and biofilm formation in Pseudomonas aeruginosa and its in vitro time-kill activity. Microb. Pathog. 2016;98:98–105. doi: 10.1016/j.micpath.2016.07.001. [DOI] [PubMed] [Google Scholar]
- Chen G., Li J., Cai Y., Zhan J., Gao J., Song M., Shi Y., Yang Z. A glycyrrhetinic acid-modified curcumin supramolecular hydrogel for liver tumor targeting therapy. Sci. Rep. 2017;7:44210. doi: 10.1038/srep44210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Ding H., Zhou J., Zhao X., Zhang J., Yang C., Li K., Qiao M., Hu H., Ding J., Zhao X. Novel glycyrrhetinic acid conjugated pH-sensitive liposomes for the delivery of doxorubicin and its antitumor activities. RSC Adv. 2016;6:17782–17791. [Google Scholar]
- Chen L., Yang J., Davey A.K., Chen Y.X., Wang J.P., Liu X.Q. Effects of diammonium glycyrrhizinate on the pharmacokinetics of aconitine in rats and the potential mechanism. Xenobiotica. 2009;39(12):955–963. doi: 10.3109/00498250903271997. [DOI] [PubMed] [Google Scholar]
- Chueh F.S., Hsiao Y.T., Chang S.J., Wu P.P., Yang J.S., Lin J.J., Chung J.G., Lai T.Y. Glycyrrhizic acid induces apoptosis in WEHI-3 mouse leukemia cells through the caspase- and mitochondria-dependent pathways. Oncol. Rep. 2012;28(6):2069–2076. doi: 10.3892/or.2012.2029. [DOI] [PubMed] [Google Scholar]
- Cosmetic Ingredient Review, Washington DC 20036, USA Final report on the safety assessment of Glycyrrhetinic Acid, Potassium Glycyrrhetinate, Disodium Succinoyl Glycyrrhetinate, Glyceryl Glycyrrhetinate, Glycyrrhetinyl Stearate, Stearyl Glycyrrhetinate, Glycyrrhizic Acid, Ammonium Glycyrrhizate, Dipotassium Glycyrrhizate, Disodium Glycyrrhizate, Trisodium Glycyrrhizate, Methyl Glycyrrhizate, and Potassium Glycyrrhizinate. Int. J. Toxicol. 2007;26(Suppl 2):79–112. doi: 10.1080/10915810701351228. [DOI] [PubMed] [Google Scholar]
- Crance J.M., Lévêque F., Biziagos E., van Cuyck-Gandré H., Jouan A., Deloince R. Studies on mechanism of action of glycyrrhizin against hepatitis A virus replication in vitro. Antiviral Res. 1994;23:63–76. doi: 10.1016/0166-3542(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Deese L.J., Dartz E.A. Proton NMR T1, T2, and T1r relaxation studies of native and reconstituted sarcoplasmic reticulum and phospholipid vesicles. Biophys. J. 1982;37:207–216. doi: 10.1016/S0006-3495(82)84670-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan E., Wang D., Fang L., Ma J., Luo J., Chen H., Li K., Xiao S. Suppression of porcine reproductive and respiratory syndrome virus proliferation by glycyrrhizin. Antiviral Res. 2015;120:122–125. doi: 10.1016/j.antiviral.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Food Safety Authority (EFSA) Scientific Opinion on the safety and efficacy of glycyrrhizic acid ammoniated (chemical group 30, miscellaneous substances) when used as a flavouring for all animal species. EFSA J. 2015;13(1):3971. [Google Scholar]
- El-Tahawy N.F., Ali A.H., Saied S.R., Wahab Z.A. Effect of glycyrrhizin on lipopolysaccharide/D-galactosamine-induced acute hepatitis in albino rats: a histological and immunohistochemical study. Egypt. J. Histol. 2011;34:518–527. [Google Scholar]
- Fiore C., Salvi M., Palermo M., Sinigaglia G., Armanini D., Toninello A. On the mechanism of mitochondrial permeability transition induction by glycyrrhetinic acid. Biochim. Biophys. Acta (BBA) – Bioenergetics. 2004;1658:195–201. doi: 10.1016/j.bbabio.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Fu Y., Zhou E., Wei Z., Song X., Liu Z., Wang T., Wang W., Zhang N., Liu G., Yang Z. Glycyrrhizin inhibits lipopolysaccharide-induced inflammatory response by reducing TLR4 recruitment into lipid rafts in RAW264.7 cells. BBA. 2014;1840:1755–1764. doi: 10.1016/j.bbagen.2014.01.024. [DOI] [PubMed] [Google Scholar]
- Gluschenko O.Yu., Polyakov N.E., Leshina T.V. NMR relaxation study of cholesterol binding with plant metabolites. Appl. Magn. Reson. 2011;41:283–294. [Google Scholar]
- Harada S. The broad anti-viral agent glycyrrhizin directly modulates the fluidity of plasma membrane and HIV-1 envelope. Biochem. J. 2005;392:191–199. doi: 10.1042/BJ20051069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibasami H., Iwase H., Yoshioka K., Takahashi H. Glycyrrhizin induces apoptosis in human stomach cancer KATO III and human promyelotic leukemia HL-60 cells. Int. J. Mol. Med. 2005;16:233–236. [PubMed] [Google Scholar]
- Hoever G., Baltina L., Michaelis M., Kondratenko R., Baltina L., Tolstikov G.A., Doerr H.W., Cinatl J., Jr. J. Med. Chem. 2005;48:1256–1259. doi: 10.1021/jm0493008. [DOI] [PubMed] [Google Scholar]
- Hostetler B.J., Uchakina O.N., Ban H., McKallip R.J. Treatment of hematological malignancies with glycyrrhizic acid. Anticancer Res. 2017;37(3):997–1004. doi: 10.21873/anticanres.11409. [DOI] [PubMed] [Google Scholar]
- Hsiang C.Y., Lin L.J., Kao S.T., Lo H.Y., Chou S.T., Ho T.Y. Glycyrrhizin, silymarin, and ursodeoxycholic acid regulate a common hepatoprotective pathway in HepG2 cells. Phytomedicine. 2015;22:768–777. doi: 10.1016/j.phymed.2015.05.053. [DOI] [PubMed] [Google Scholar]
- Huo H.Z., Wang B., Liang Y.K., Bao Y.Y., Gu Y. Hepatoprotective and antioxidant effects of licorice extract against CCl4-induced oxidative damage in rats. Int. J. Mol. Sci. 2011;12:6529–6543. doi: 10.3390/ijms12106529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H., Lieberman P.M. Mechanism of glycyrrhizic acid inhibition of Kaposi’s sarcoma-associated herpesvirus: disruption of CTCF-cohesin-mediated RNA polymerase II pausing and sister chromatid cohesion. J. Virol. 2011;85:11159–11169. doi: 10.1128/JVI.00720-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimoto M., Kawai R., Mitsui T., Harada Y., Sato A., Yokoyama S., Hirao I. Site-specific incorporation of fluorescent probes into RNA by specific transcription using unnatural base pairs. Nucleic Acids Symp. Ser. 2005;49:287–288. doi: 10.1093/nass/49.1.287. [DOI] [PubMed] [Google Scholar]
- Kong R., Zhu X., Meteleva E.S., Chistyachenko Y.S., Suntsova L.P., Polyakov N.E., Khvostov M.V., Baev D.S., Tolstikova T.G., Yu J., Dushkin A.V., Su W. Enhanced solubility and bioavailability of simvastatin by mechanochemically obtained complexes. Int. J. Pharm. 2017;534(1–2):108–118. doi: 10.1016/j.ijpharm.2017.10.011. [DOI] [PubMed] [Google Scholar]
- Konkina I.G., Shitikova O.V., Lobov A.N., Murinov Yu.I., Bachurin S.O. Host—guest complexation in the glycyrrhizic acid—2,8dimethyl5[2 ´(6methylpyridin3yl)ethyl]2,3,4,5tetrahydro1Hpyrido[4,3b]indole system. Russ. Chem. Bull., Int. Ed. 2015;64(6):1385–1393. [Google Scholar]
- Kornievskaya V.S., Kruppa A.I., Leshina T.V. Investigation of the influence of supramolecular structures of glycyrrhizic acid on the reactivity of organic compounds by 1H NMR and CIDNP. Vestnik NSU. 2008;3:37–46. (in russian) [Google Scholar]
- Kumari A., Yadav S.K., Yadav S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B. 2010;75:1–18. doi: 10.1016/j.colsurfb.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Lee C.S., Kim Y.J., Lee M.S., Han E.S., Lee S.J. 8β-Glycyrrhetinic acid induces apoptotic cell death in SiHa cells and exhibits a synergistic effect against antibiotic anti-cancer drug toxicity. Life Sci. 2008;83:481–489. doi: 10.1016/j.lfs.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Lekar A.V., Vetrova E.V., Borisenko N.I., Yakovishin L.A., Grishkovets V.I., Borisenko S.N. Electrospray ionization mass spectrometry of mixtures of triterpene glycosides with L-phenylalanine. J. Appl. Spectrosc. 2011;78(4):501–505. [Google Scholar]
- Lekar A.V., Vetrova E.V., Borisenko N.I., Yakovishin L.A., Grishkovets V.I. Mass spectrometry of triterpene glycosides molecular complexation with purine bases of nucleic acids. Russ. J. Bioorg. Chem. 2011;37(5):609–613. doi: 10.1134/s1068162011050116. [DOI] [PubMed] [Google Scholar]
- Lekar A.V., Vetrova E.V., Borisenko N.I., Yakovishin L.A., Grishkovets V.I., Borisenko S.N. A mass spectrometry study of the self-association of glycyrrhetinic acid molecules. Russ. J. Bioorg. Chem. 2016;42:716–720. [Google Scholar]
- Li J.Y., Cao H.Y., Liu P., Cheng G.H., Sun M.Y. Glycyrrhizic acid in the treatment of liver diseases: literature review. Biomed Res. Int. 2014;2014 doi: 10.1155/2014/872139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang B., Guo X.L., Jin J., Ma Y.C., Feng Z.Q. Glycyrrhizic acid inhibits apoptosis and fibrosis in carbon-tetrachloride-induced rat liver injury. World J. Gastroenterol. 2015;21:5271–5280. doi: 10.3748/wjg.v21.i17.5271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J.C. Mechanism of action of glycyrrhizic acid in inhibition of Epstein-Barr virus replication in vitro. Antiviral Res. 2003;59:41–47. doi: 10.1016/s0166-3542(03)00030-5. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y., Matsuura T., Aoyagi H., Matsuda M., Hmwe S.S., Date T., Watanabe N., Watashi K., Suzuki R., Ichinose S., Wake K., Suzuki T., Miyamura T., Wakita T., Aizaki H. Antiviral activity of glycyrrhizin against hepatitis C virus in vitro. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0068992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka K., Miyajima R., Ishida I., Karasawa S., Yoshimura T. Aggregate formation of glycyrrhizic acid. Colloids Surf. A. 2015;500:112–117. [Google Scholar]
- Meteleva E.S., Chistyachenko Y.S., Suntsova L.P., Khvostov M.V., Polyakov N.E., Selyutina O.Yu., Tolstikova T.G., Frolova T.S., Mordvinov V.A., Dushkin A.V., Lyakhov N.Z. Disodium salt of glycyrrhizic acid – a novel supramolecular delivery system for anthelmintic drug praziquantel. J. Drug Delivery Sci. Technol. 2019 [Google Scholar]
- Mignotte B., Vayssière J.L. Mitochondria and apoptosis. Eur. J. Biochem. 1998;252:1–15. doi: 10.1046/j.1432-1327.1998.2520001.x. [DOI] [PubMed] [Google Scholar]
- Ming L.J., Yin A.C. Therapeutic effects of glycyrrhizic acid. Nat. Prod. Commun. 2013;8(3):415–418. [PubMed] [Google Scholar]
- Nakamura T., Fujii T., Ichihara A. Enzyme leakage due to change of membrane permeability of primary cultured rat hepatocytes treated with various hepatotoxins and its prevention by glycyrrhizin. Cell Biol. Toxicol. 1985;1:285–295. doi: 10.1007/BF00118193. [DOI] [PubMed] [Google Scholar]
- Ohuchi K., Kamada Y., Levine L., Tsurufuji S. Glycyrrhizin inhibits prostaglandin E2 production by activated peritoneal macrophages from rats. Prostaglandins Med. 1981;7:457–463. doi: 10.1016/0161-4630(81)90033-1. [DOI] [PubMed] [Google Scholar]
- Olukoga A., Donaldson D. Liquorice and its health implications. J. R. Soc. Promot. Health. 2000;120(2):83–89. doi: 10.1177/146642400012000203. [DOI] [PubMed] [Google Scholar]
- Omar H.R., Komarova I., El-Ghonemi M., Fathy A., Rashad R., Abdelmalak H.D., Yerramadha M.R., Ali Y., Helal E., Camporesi E.M. Licorice abuse: time to send a warning message, Therapeutic. Adv. Endocrinol. Metab. 2012;3(4):125–138. doi: 10.1177/2042018812454322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orazizadeh M., Fakhredini F., Mansouri E., Khorsandi L. Effect of glycyrrhizic acid on titanium dioxide nanoparticles-induced hepatotoxicity in rats. Chem. Biol. Interact. 2014;220:214–221. doi: 10.1016/j.cbi.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Patel D.P., Chaudhari B.G. Application of supramolecules in drug delivery. J. Curr. Pharm. Res. 2012;9:1–5. [Google Scholar]
- Petrova S.S., Schlotgauer A.A., Kruppa A.I., Leshina T.V. Self-association of glycyrrhizic acid. NMR Study. Zeitschrift für Physikalische Chemie. 2016;231:1–17. [Google Scholar]
- Polyakov N.E., Khan V.K., Taraban M.B., Leshina T.V. Complex of calcium receptor blocker nifedipine with glycyrrhizic acid. J. Phys. Chem. B. 2008;112:4435–4440. doi: 10.1021/jp076850j. [DOI] [PubMed] [Google Scholar]
- Polyakov N.E., Khan V.K., Taraban M.B., Leshina T.V., Salakhutdinov N.F., Tolstikov G.A. Complexation of lappaconitine with glycyrrhizic acid: stability and reactivity studies. J. Phys. Chem. B. 2005;109(51):24526–24530. doi: 10.1021/jp053434v. [DOI] [PubMed] [Google Scholar]
- Polyakov N.E., Leshina T.V. Glycyrrhizic acid as a novel drug delivery vector. Synergy of drug transport and efficacy. Open Conf. Proc. J. 2011;2:64–72. [Google Scholar]
- Polyakov N.E., Magyar A., Kispert L.D. Photochemical and optical properties of water-soluble xanthophyll antioxidants: aggregation vs complexation. J. Phys. Chem. B. 2013;117:10173–10182. doi: 10.1021/jp4062708. [DOI] [PubMed] [Google Scholar]
- Pompei R., Flore O., Marccialis M.A., Pani A., Loddo B. Glycyrrhizic acid inhibits virus growth and inactivates virus particles. Nature. 1979;281:689–690. doi: 10.1038/281689a0. [DOI] [PubMed] [Google Scholar]
- Pompei R., Pani A., Flore O., Marcialis M.A., Loddo B. Antiviral activity of glycyrrhizic acid. Experientia. 1980;36:304. doi: 10.1007/BF01952290. [DOI] [PubMed] [Google Scholar]
- Radwant M.A., Aboul-Enein H.Y. The effect of oral absorption enhancers on the in vivo performance of insulin-loaded poly(ethylcyanoacrylate) nanospheres in diabetic rats. J. Microencapsul. 2002;19(2):225–235. doi: 10.1080/02652040110081406. [DOI] [PubMed] [Google Scholar]
- Salvi M., Fiore C., Armanini D., Toninello A. Glycyrrhetinic acid-induced permeability transition in rat liver mitochondria. Biochem. Pharmacol. 2003;66:2375–2379. doi: 10.1016/j.bcp.2003.08.023. [DOI] [PubMed] [Google Scholar]
- Sapra B., Jain S., Tiwary A.K. Transdermal delivery of carvedilol containing glycyrrhizinand chitosan as permeation enhancers: biochemical. Biophys., Microsc. Pharmacodynamic Eval., Drug delivery. 2008;15:443–454. doi: 10.1080/10717540802327047. [DOI] [PubMed] [Google Scholar]
- Sekizawa T., Yanagi K., Itoyama Y. Glycyrrhizin increases survival of mice with herpes simplex encephalitis. Acta Virol. 2001;45:51–54. [PubMed] [Google Scholar]
- Selyutina O.Y., Polyakov N.E., Korneev D.V., Zaitsev B.N. Effect of glycyrrhizic acid on hemolysis of red blood cells and properties of cell membranes. Russ. Chem. Bull. 2014;63(5):1201–1204. [Google Scholar]
- Selyutina O.Y., Apanasenko I.E., Shilov A.G., Khalikov S.S., Polyakov N.E. Effect of natural polysaccharides and oligosaccharides on the permeability of cell membranes. Russ. Chem. Bull. 2017;66(1):129. [Google Scholar]
- Selyutina O.Y., Apanasenko I.E., Polyakov N.E. Membrane-modifying activity of glycyrrhizic acid. Russ. Chem. Bull. 2015;64(7):1555–1559. [Google Scholar]
- Selyutina O.Y., Apanasenko I.E., Kim A.V., Shelepova E.A., Khalikov S.S., Polyakov N.E. Spectroscopic and molecular dynamics characterization of glycyrrhizin membrane-modifying activity. Colloids Surf., B: Biointerfaces. 2016;147:459–466. doi: 10.1016/j.colsurfb.2016.08.037. [DOI] [PubMed] [Google Scholar]
- Selyutina O.Y., Polyakov N.E., Korneev D.V., Zaitsev B.N. Influence of glycyrrhizin on permeability and elasticity of cell membrane: perspectives for drugs delivery. Drug Delivery. 2016;23(3):858–865. doi: 10.3109/10717544.2014.919544. [DOI] [PubMed] [Google Scholar]
- Shibata S. A drug over the millennia: pharmacognosy, chemistry, and pharmacology of licorice. Yakugaku Zasshi-J. Pharm. Soc. Jpn., 2000. 2000;120(10):849–862. doi: 10.1248/yakushi1947.120.10_849. [DOI] [PubMed] [Google Scholar]
- Singh H., Kim S.J., Kang D.H., Kim H.-R., Sharma A., Kim W.Y., Kang C., Kim J.S. Glycyrrhetinic acid as a hepatocyte targeting unit for an anticancer drug delivery system with enhanced cell type selectivity. Chem. Commun. 2018;87:12353–12356. doi: 10.1039/c8cc05175e. [DOI] [PubMed] [Google Scholar]
- Stakhneva E.M., Vavilin V.A., Ragino Yu.I., Safronova O.G., Shintyapina A.B., Ivanova M.V. Effects of simvaglyzin and atorvaglyzin on the expression of 3-hydroxy-3-methyl-glutaryl-CoA reductase in rat liver. Bull. Exp. Biol. Med. 2013;156(1):63–65. doi: 10.1007/s10517-013-2278-y. [DOI] [PubMed] [Google Scholar]
- Su X., Wu L., Hu M., Dong W., Xu M., Zhang P. Glycyrrhizic acid: a promising carrier material for anticancer therapy. Biomed. Pharmacother. 2017;95:670–678. doi: 10.1016/j.biopha.2017.08.123. [DOI] [PubMed] [Google Scholar]
- Sui X., Yin J., Ren X. Antiviral effect of diammonium glycyrrhizinate and lithium chloride on cell infection by pseudorabies herpesvirus. Antiviral Res. 2010;85:346–353. doi: 10.1016/j.antiviral.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirugnanam S., Xu L., Ramaswamy K., Gnanasekar M. Glycyrrhizin induces apoptosis in prostate cancer cell lines DU-145 and LNCaP. Oncol. Rep. 2008;20:1387–1392. [PubMed] [Google Scholar]
- Tolstikova T.G., Khvostov M.V., Bryzgalov A.O. The complexes of drugs with carbohydrate-containing plant metabolites as pharmacologically promising agents. Mini-Rev. Med. Chem. 2009;9:1317–1328. doi: 10.2174/138955709789878123. [DOI] [PubMed] [Google Scholar]
- Tsao S.M., Yin M.C. Antioxidative and antiinflammatory activities of asiatic acid, glycyrrhizic acid, and oleanolic acid in human bronchial epithelial cells. J. Agric. Food Chem. 2015;63:3196–3204. doi: 10.1021/acs.jafc.5b00102. [DOI] [PubMed] [Google Scholar]
- Vetrova E.V., Lekar A.V., Filonova O.V., Borisenko S.N., Maksimenko E.V., Borisenko N.I. Study of molecular complexation of glycyrrhizic acid with chloramphenicol by electrospray ionization mass spectrometry. J. Nat. Sci. Biol. Med. 2015:S40–S43. doi: 10.4103/0976-9668.166070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan X.Y., Luo M., Li X.D., He P. Hepatoprotective and anti-hepatocarcinogenic effects of glycyrrhizin and matrine. Chem. Biol. Interact. 2009;181:15–19. doi: 10.1016/j.cbi.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhao B., Wang S., Liang Q., Cai Y., Yang F., Li G. Formulation and evaluation of novel glycyrrhizic acid micelles for transdermal delivery of podophyllotoxin. Drug Delivery. 2016;23:1623–1635. doi: 10.3109/10717544.2015.1135489. [DOI] [PubMed] [Google Scholar]
- Wang Y.G., Zhou J.M., Ma Z.C., Li H., Liang Q.D., Tan H.L., Xiao C.R., Zhang B.L., Gao Y. Pregnane X receptor mediated-transcription regulation of CYP3A by glycyrrhizin: a possible mechanism for its hepatoprotective property against lithocholic acid-induced injury. Chem. Biol. Interact. 2012;200:11–20. doi: 10.1016/j.cbi.2012.08.023. [DOI] [PubMed] [Google Scholar]
- Wolkerstorfer A., Kurz H., Bachhofner N., Szolar O.H. Glycyrrhizin inhibits influenza A virus uptake into the cell. Antiviral Res. 2009;83:171–178. doi: 10.1016/j.antiviral.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., Lian B., Deng Y., Feng Z., Zhong C., Wu W., Huang Y., Wang L., Zu C., Zhao X. Resveratrol-loaded glycyrrhizic acid-conjugated human serum albumin nanoparticles wrapping resveratrol nanoparticles: preparation, characterization, and targeting effect on liver tumors. J. Biomater. Appl. 2017;32(2):1–15. doi: 10.1177/0885328217713357. [DOI] [PubMed] [Google Scholar]
- Yang F.H., Zhang Q., Liang Q.Y., Wang S.Q., Zhao B.X., Wang Y.T., Cai Y., Li G.F. Bioavailability enhancement of paclitaxel via a novel oral drug delivery system: paclitaxel-loaded glycyrrhizic acid micelles. Molecules. 2015;20:4337–4356. doi: 10.3390/molecules20034337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin G., Cao L., Xu P., Jeney G., Nakao M., Lu C. Hepatoprotective and antioxidant effects of Glycyrrhiza glabra extract against carbon tetrachloride (CCl4)-induced hepatocyte damage in common carp (Cyprinus carpio) Fish Physiol. Biochem. 2011;37:209–216. doi: 10.1007/s10695-010-9436-1. [DOI] [PubMed] [Google Scholar]
- Yin J., Li D., Hu W., Meng Q. Effects of glycyrrhizic acid on cocklebur-induced hepatotoxicity in rat and human hepatocytes. Phytother. Res. 2008;22:395–400. doi: 10.1002/ptr.2336. [DOI] [PubMed] [Google Scholar]
- Yu J.Y., Ha J.Y., Kim K.M., Jung Y.S., Jung J.C., Oh S. Anti-Inflammatory activities of licorice extract and its active compounds, glycyrrhizic acid, liquiritin and liquiritigenin, in BV2 cells and mice liver. Molecules. 2015;20:13041–13054. doi: 10.3390/molecules200713041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Polyakov N.E., Chistyachenko Yu.S., Khvostov M.V., Frolova T.S., Tolstikova T.G., Dushkin A.V., Su W. Preparation of curcumin self-micelle solid dispersion with enhanced bioavail-ability and cytotoxic activity by mechanochemistry. Drug Delivery. 2018;25:198–209. doi: 10.1080/10717544.2017.1422298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelikman M.V., Kim A.V., Medvedev N.N., Selyutina O.Yu., Polyakov N.E. Structure of dimers of glycyrrhizic acid in water and their complexes with cholesterol: molecular dynamics simulation. J. Struct. Chem. 2015;56:67–76. [Google Scholar]
- Zu Y., Meng L., Zhao X., Ge Y., Yu X., Zhang Y., Deng Y. Preparation of 10-hydroxycamptothecin-loaded glycyrrhizic acid-conjugated bovine serum albumin nanoparticles for hepatocellular carcinoma-targeted drug delivery. Int. J. Nanomed. 2013;8:1022–1207. doi: 10.2147/IJN.S40493. [DOI] [PMC free article] [PubMed] [Google Scholar]