Abstract
Purpose
To assess the response of rat urinary bladder regenerated by the homologous bladder acellular matrix graft (BAMG) to in vitro electrical and pharmacologic stimuli.
Materials and Methods
In Sprague-Dawley rats, partial cystectomy (>50%) was performed, followed by BAMG augmentation cystoplasty. After 4 months, organ bath studies of tissue strips in 10 were used to compare the contractility of the BAMG regenerates and the corresponding host detrusor smooth muscle.
Results
The BAMG regenerates exhibited contractile activity to electrical field stimulation and a qualitatively identical pattern of response to muscarinic, purinergic, alpha- and beta-adrenergic drug administration and nitric oxide. At 4 months after surgery, the maximum forces of contraction of the BAMG regenerates to carbachol stimulation amounted to close to 80% of the host bladder response. With electrical field stimulation, they equaled 44% and 62% of the host bladder response after 2.5 and 4 months, respectively. Histological and immunohistochemical studies confirmed the presence of receptors for neurotransmitters that these functional in vitro studies implied.
Conclusions
The present study provides further evidence that augmentation cystoplasty with the BAMG leads to functional regeneration of the rat bladder detrusor smooth muscle.
Key Words: bladder; transplantation, homologous; graft function, in vitro
To find a means of bladder augmentation that would avoid the complications encountered with the use of bowel segments, 1, 2 a great variety of synthetic and naturally derived biomaterials have been tested. Unfortunately, most of these graft materials have resulted in stone formation, collapse, infection, rejection, or extrusion and migration of the graft without adequate reconstruction of a functional bladder. 3, 4
Collagen-based and biodegradable materials, however, have been shown to have the best potential for regenerative and functional capacities. 5, 6, 7, 8 As such, the homologous bladder acellular matrix graft (BAMG) has recently been used successfully for augmentation cystoplasty in the rat model, leading to morphologic [9] and functional regeneration of detrusor smooth muscle. The bladders thus regenerated exhibited enhanced low-pressure reservoir function and the preservation of normal micturition in vivo.
To elucidate the mechanisms of functional innervation in the BAMG-regenerated rat bladder, in vitro electrical and pharmacologic stimulation techniques were used to study contractility and to characterize the expression of receptors for neurotransmitters.
MATERIALS AND METHODS
Preparation of the bladder acellular matrix graft.
The homologous BAMGs were freshly prepared from Sprague-Dawley rat bladders as previously described, [9] according to a method adapted from Meezan et al. [10] In brief, bladders from Sprague Dawley rats obtained from our institution's tissue-sharing program were excised and placed in a 35-mm. Petri dish containing 50 ml. of 10 mM phosphate-buffered saline (PBS, pH 7.0) and 0.1% sodium axide. The bladders were inverted and the mucosa was scraped off with a pair of glass slides. The remaining lamina propria and detrusor muscle were treated with 50 ml. of 10 mM PBS-0.1% sodium azide and stirred for 5 to 6 hours for partial cell lysis. Bladders were then washed with 40 ml. of PBS before treatment with 50 ml. of 1 M. sodium chloride containing 2000 Kunitz units DNase (Sigma; St. Louis, MO) and stirred for 6 to 8 hours. With this, cell lysis was complete and all the intracellular components were released. The samples were then treated with 50 ml. of 4% sodium desoxycholate containing 0.1% sodium azide and stirred for 5 to 6 hours to solubilize the lipid bilayer cell membranes and intracellular membrane lipids. This was repeated once. The resultant BAMG was washed three times with 50 ml. PBS and stored in 10% neomycin sulfate at 4C until grafted.
Surgical technique.
Male (n = 16) and female (n = 15) Sprague Dawley rats (age 2.9 +/− 0.19 months; weight 284.5 +/− 14.7 grams) were anesthetized with pentobarbital, 40 mg./kg. intraperitoneally. Through a midline lower abdominal incision, the bladder was exposed and partial cystectomy (>50%) was performed. The full-bladder-size BAMG (approximately 9 × 6 mm. undistended) was grafted to the remaining host bladder with a continuous polyfilament-coated 8 - 0 absorbable suture for both the anterior and posterior wall. To identify the matrix borders, 4 non-absorbable monofilament 7 - 0 button sutures were placed-one each at the anterior, posterior, left and right sides, 3 to 4 mm. apart. Suture integrity was tested by instillation of saline through a urethral tube in the female rats and by puncturing the posterior host bladder wall in the male. When satisfactory closure was obtained, the abdominal wall and the skin were closed in two layers. No drainage was used and no drugs were administered. All animals were housed under standard laboratory conditions.
Two animals were sacrificed at 2.5 months and two more at 8.5 months (urethane anesthesia [1.1 gm./kg. intraperitoneally] followed by bilateral thoracotomy). All remaining rats were sacrificed 4 months postoperatively. Of these, 6 were saved for histological and immunohistochemical evaluation only. The remaining bladders were subjected to immediate tissue bath studies. The earlier (2.5-month) and later (8.5-month) time points, although not central to the present in vitro study, were chosen to confirm observations in our previous investigation [9] in which characteristic histological changes were apparent at 2.5 months and neural regeneration continued beyond 4 months.
Tissue bath experiments: Tissue preparation.
The excised bladders were placed in a Petri dish with chilled Krebs solution and carefully dissected free from sparse adherent connective tissue. Full-thickness longitudinal bladder strips of uniform size (approximately 7 mm. long and 2 mm. wide) were obtained from both the regenerates and the host bladder wall. With the former, great care was taken to ensure that only regenerated bladder from within the area delineated by the marking sutures was used. The strips were mounted in the tissue bath to a glass tissue support hook on one side and an isometric force displacement transducer (Radnoti Glass Technology, Monrovia CA) on the other by means of two spring wire clips connected with 4 - 0 braided silk. All preparations were sufficiently durable to withstand the grip of the wire clips during repeated or continuous contractions. Peak contraction values were related to the weight of each individual muscle strip.
Tissue bath and recording and stimulating equipment.
The 30-ml. double-chambered Quiet Bath (Radnoti Glass Technology) was used. Its working chamber was connected to a second chamber with 95% oxygen and 5% CO2 infusion. The gas flow induced circulation of Krebs solution, which was warmed to 37C by an external heating circuit (circulating pump Thermomix BU, Braun Instruments, Burlingame CA). Forces lower than 1 mNewton (0.1 gm.) without bubble artifacts could be measured in the working chamber. The transducer signals were fed into a thermal array recorder (Gould TA 4000, Gould Inc., Valley View OH). For tissue stimulation, vertical L-shaped, custom-made platinum iridium electrodes (15 mm. long, 0.18 mm. diameter) separated by 10 mm. were used with a Grass S44 stimulator (Grass Instrument Co., Quincy MA). The tissue was mounted parallel to the electrodes at a preload of 25 mNewton (2.5 gm.), resulting in a resting tension of about 8 mNewton (0.8 gm.) at the end of a 60-minute equilibration period. A custom-made current-distribution box supplied supramaximal current of 0.14 amperes (A) at 15 V, which was sequentially delivered to two of eight chambers.
Electrical field stimulation.
Based on preliminary length-tension, frequency-response and current-response studies of normal rat detrusor smooth muscle, we used the following supramaximal stimulation parameters: bipolar, monophasic balance-charged rectangular pulses; 1-msec pulse duration; 1 to 80 pulses per second (pps) frequency; 10-second stimulation trains; 2-minute intervals between stimulations; 0.14 A amplitude at 15 V.
Solutions.
Krebs (mmol.): NaCl 118.1, KCl 4.6, MgSO4 1.2, KH2 PO (4) 1.2, NaHCO3 25.0, CaCl2 2.5, glucose 11.0. For “high-potassium” Krebs buffer solution (KCl 60 mM.), NaCl was replaced with equimolar amounts of KCl. All solutions were made fresh from stock solutions.
Drugs.
Pharmacologic stimulation was used to evaluate the regeneration of specific neural components. alpha, beta-Methylene adenosine-5′-triphosphate dilithium salt (alpha, beta-ATP; Sigma Chemical Co., St. Louis MO; M 6517), atropine sulfate (Sigma A 0257), carbachol (Sigma C 4382), (+/−)-isoproterenol (Sigma I 5627), sodium nitroprusside (Sigma S 0501), norepinephrine (Sigma A 7256) and tetrodotoxin (Sigma T 5651) were diluted in saline. Propranolol (SoloPak Lab, IL) and valethamate bromide (Murel [R], Ayerst Lab, NY) were available as ready-mix solutions at concentrations of 1 and 10 mg./ml., respectively. The amounts and volumes added to the bath from stock solutions were as follows: alpha, beta-methylene ATP 1 × 10−7 M and 1 × 10−6 M, 1 ml. each [the latter delivered twice]; atropine 1 × 10 (−6) M, 1 ml.; carbachol 10−9 to 10−3 M, 1 ml. each; isoproterenol 1 × 10−4 M, 1 ml.; nitroprusside 1 × 10−4 M, 1 ml.; norepinephrine 5.6 × 10−4 M, 1 ml.; propranolol 3 × 10−6 M, 0.9 ml.; tetrodotoxin 0.4 × 10−6 M, 1 ml.; valethamate bromide 2.6 × 10−5 M, 1 ml.
To minimize artifacts related to tissue exhaustion or drug interference, each muscle strip was subjected to only one series of electrical field stimulations followed by administration of only one of the agonists/antagonists. To avoid pharmacologic interference with the reagents, bladders used in the tissue bath experiments were not used for histological or immunohistochemical evaluation.
Staining.
Some of the reserved specimens were fixed in 10% buffered formalin for at least 24 hours. After dehydration in graded ethanol solutions, the specimens were embedded in paraffin, sectioned (5 micro m.) and stained with trichrome for collagen and smooth muscle, hematoxylin and eosin (H&E) for nuclei, alpha-actin for smooth muscle, and the non-specific neuronal protein gene product (PGP 9.5) for nerves. PGP 9.5 represents a major protein component of the neural cytoplasm and therefore labels more nerve fibers than other general nerve markers. [11] Enzyme histochemistry for acetyl-cholinesterase (AChE was performed after pre-treating sections with 10 (−5) M tetraisoproply pyrophosphoramide (Sigma) for 30 minutes to block the non-specific cholinesterase staining. The remainder of the AChE evaluation was carried out according to the method of El-Badawi and Schenk. [12] Tissue for adrenergic staining was snap-frozen in liquid nitrogen after embedding in OCT. It was then cut in 10-micro m. sections, mounted on Superfrost [TM] slides and air-dried. Adrenergic staining was conducted with the modified glyoxylic acid monoamine histofluorescent technique described by de la Torre and Surgeon. [13] For NADPH-diaphorase staining, slides were incubated for 45 minutes at room temperature in a buffer containing (per milliliter) 1 mg. NADPH, 0.1 mg. nitroblue tetrazolium, and 0.2% triton X-100. The reaction was terminated by washing with buffer, and the sections were coverslipped with a 9:1 mixture of glycerol/phosphate buffer (pH 8.6). Areas staining for NADPH-diaphorase were seen as a dark blue precipitate against a pale blue, nonstained background. [14]
Statistics.
Student's t test was used to compare the responses between control and BAMG-regenerated bladder strips; p values< 0.05 were considered statistically significant. All data are presented as mean +/− S.E.M.
RESULTS
Mortality.
Eleven of the 31 rats died postoperatively; 8 of these (4 male/4 female) died within 48 hours of uremia consequent to urinary extravasation into the abdominal cavity. (The 4 male rats were found to have complete obstruction of the bladder neck and proximal urethra from a staghorn stone-like plug of coagula and defurfurated fibers of the BAMG.) Leakage occurred only at the site of anastomosis; a rupture of the graft itself was never seen. Two of the 11 (1 male/female) died after 6 and 10 days of infection (abscess, sepsis); 1 male rat died after 2 months from a Corona virus infection. Twenty surviving rats (10 male/10 female) were available for evaluation.
Bladder stone formation.
Within 4 months, bladder calculi (1 to 7 stones) occurred in 16 of 20 grafted rats (80%). They were composed of struvite (60 to 100%), apatite (40 to 100%), Newberyite (20 to 100%) and brushite (10 to 100%).
Electrical field stimulation.
The mean weight of the augmented bladders (n = 10) amounted to 222.7 +/− 14.8 mg. These bladders provided 19 BAMG-regenerated and 30 host bladder strips (see Table 1 ) weighing 29.5 +/− 4.0 mg. and 37.2 +/− 2.5 mg., respectively. Supramaximal electrical field stimulation at increasing frequencies showed contractile responses in all BAMG-regenerated strips that were qualitatively very similar to those of the host bladder smooth muscle strips (Figure 1 A). Peak contractions consistently occurred at a stimulation frequency of 80 pps. The maximum force of contraction of the BAMG regenerates amounted to 44% and 62% of host bladder wall tissue after 2.5 and 4 months, respectively (Figure 2 ). Tetrodotoxin (0.4 × 10−6 M) reduced the response to supramaximal electrical field stimulation by 39% in the regenerates and 64% in the host bladder strips. This effect was present at the first stimulation after 3 minutes and did not change with subsequent stimulations carried out at 6, 9 and 12 minutes after administration of the drug. There was also no significant change in resting tension between the stimulations.
Tension recordings in BAMG-regenerated and host bladder strips 4 months after surgery
| Strip Weight (mg.) | Tetrodotoxina (gm.) | Atropine Sulfateb (gm.) | Valethamate Bromideb (gm.) | Isoproterenolc (gm.) | Norepinephrinec (gm.) | Nitroprussided (gm.) | |
|---|---|---|---|---|---|---|---|
| BAMG- | 29.5 ± 4.0† | 1.86 ± 0.3 | 1.94 ± 0.58 | 0.76 ± 0.04 | 0.98 ± 0.351 | ||
| Regenerates | 1.13 ± 0.48 | 0.0 ± 0.04* | 0.0 ± 0.03* | 0.0 ± 0.01* | 0.0 ± 0.02* | 0.65 ± 0.1 | |
| (N = 19) | (N = 3) | (N = 3) | (N = 3) | (N = 2) | (N = 2) | (N = 4) | |
| Host | 37.2 ± 2.5 | 2.26 ± 0.43 | 2.42 ± 0.66 | 0.73 ± 0.04 | 2.03 ± 0.35 | ||
| Bladder | 0.81 ± 0.5* | 0.0 ± 0.03* | 0.0 ± 0.03* | 0 ± 0.04* | 0 ± 0.03* | 1.69 ± 0.3 | |
| (N = 30) | (N = 3) | (N = 5) | (N = 5) | (N = 2) | (N = 2) | (N = 8) | |
With supramaximal elecrical field stimulation before (upper line) and after administration of tetrodotoxin 0.4 × 10−6 M (lower line).
15 min. after carbachol stimulation 10−4 M (upper line) and after subsequent administration of atropine sulfate 10−6 M and valethamate bormide 2.6 × 10−5 M (lower line).
Before (upper line) and after (lower line) administration of isoproterenol 10−4 M and norepinephrine 5.6 × 10−4 M.
15 min. after potassium stimulation alone (upper line) and after subsequent administration of nitroprusside 10−4 M (lower line).
p<0.05 when compared with tension before administration of the drug.
p<0.05 when compared with host bladder strips.
Fig. 1.
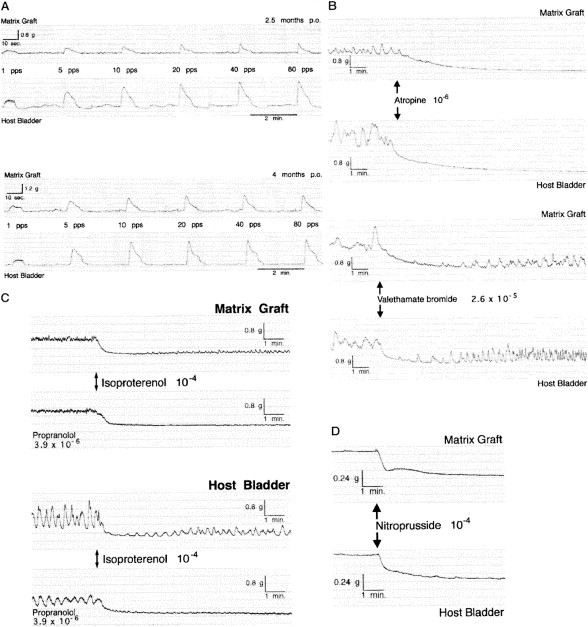
A, electrical field stimulation (0.14 A, 15 V, 1 to 80 pps) in BAMG regenerates and host bladder smooth muscle strips 2.5 and 4 months postoperatively shows increasing amplitudes of the contractile response in BAMG-regenerated strips, indicating gain in functional innervation and/or force of contraction over time. B, atropine (10−6 M) and valethamate bromide (2.6 × 10−5 M) completely abolish carbachol (10−4 M) response. Note that valethamate bromide-relaxed strips quickly resume enhanced spontaneous muscle activity. C, isoproterenol (10−4 M) lowers resting tension to zero in both BAMG regenerates and host bladder strips at 4 months after surgery. Pretreatment with propranolol (3 × 10−6 M) as specific beta1+2-adrenergic blocker does not inhibit isoproterenol-induced relaxation, but persistently prevents all strips from resuming spontaneous muscle activity. D, all potassium-contracted (60 mM) BAMG-regenerated and host bladder smooth muscle strips are relaxed to same extent by nitroprusside (10−4 M).
Fig. 2.
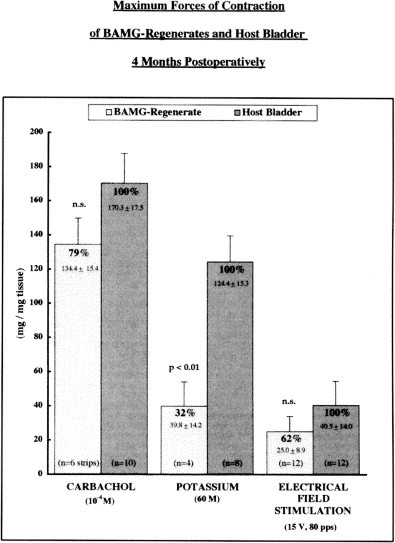
Maximum forces of contraction to carbachol, potassium and electrical field stimulation of BAMG regenerates and host bladder 4 months postoperatively.
Muscarinic responses:
Cumulative addition of carbachol (10−9 to 10−3 M) also elicited qualitatively identical contractions in the BAMG and host bladder strips. Peak contractions occurred at 10−4 M; again, they were smaller in the regenerates and amounted to 70% and 79% of host bladder after 2.5 and 4 months, respectively (Figure 2). Atropine (10−6 M) completely relaxed all carbachol-contracted strips, which then would not respond to electrical stimulation for at least 6 minutes. Administration of valethamate bromide (2.6 × 10−5 M), an atropine-like anticholinergic, papaverine-like musculotropic and ganglionoplegic drug, also completely reversed carbachol-induced contraction. In contrast to atropine's effect, however, valethamate bromide-relaxed strips did resume enhanced spontaneous muscle activity within 5 minutes after administration of the drug (Figure 1B).
Adrenergic responses:
The beta1+2. adrenergic agonist isoproterenol (10−4 M) relaxed all BAMG regenerates and host bladder strips by lowering their resting tension to zero. Incubation with the beta1+2 -adrenergic blocker propranolol (3 × 10−6 M) for 15 minutes before isoproterenol administration did not suppress relaxation, but it persistently prevented all strips from resuming spontaneous muscle activity (Figure 1C). The finding that propranolol was not able to inhibit isoproterenol-induced relaxation might indicate that isoproterenol given at the fairly high dose of 10−4 M was acting by a non-specific mechanism rather than by beta-receptor stimulation. The alpha1+2 -adrenergic agonist norepinephrine (5.6 × 10 (−4) M) also relaxed all BAMG regenerates and host bladder strips by zeroing their resting tension. Norepinephrine is not a pure alpha agonist, but can also be a potent beta agonist in the absence of a significant density of alpha-adrenergic receptors. The fact that norepinephrine relaxed the strips indicates that it was acting as a beta agonist in these studies.
Purinergic responses.
Acting as a purinergic receptor agonist, alpha,beta-methylene ATP (10−7 M and 10−6 M) caused a minute rise in smooth muscle tone (0.35 +/− 0.05 gm.) at the lower dose and a more pronounced contraction (0.55 +/− 0.05 gm.) lasting for 3 minutes at the higher dose. Thereafter, it showed almost complete desensitization to a second administration of the higher dose 11 minutes after the first. Again, the pattern and extent of the responses were identical in BAMG regenerates and host bladder strips (n = 2 each).
“High-potassium”
(60 mM) Krebs buffer solution evoked sustained contraction in both the BAMG-regenerated and host bladder smooth muscle strips. Once more, peak contractions were smaller in the regenerates, amounting to 32% of host bladder wall tissue at 4 months after grafting (Figure 2). Nitroprusside (1 × 10−4 M), as a non-adrenergic, non-cholinergic nitric oxide donor, relaxed the potassium-contracted BAMG regenerates and host bladder strips to the same extent (Figure 1D).
Histological and immunohistochemical examination.
On histological examination (see Figure 3 ), all BAMG regenerates showed a bladder wall structure qualitatively indistinguishable from the host bladder. The generally uniform urothelial lining was sometimes found to be hyperplastic in cases of extensive bladder stone formation. Differentiated muscularis mucosae and spatially oriented, well-developed detrusor smooth muscle were formed by alpha-actin-positive smooth muscle cells. In the 4-month specimen, the thickness of the muscle bundles seemed to decrease toward the center of the graft, whereas revascularization appeared to be equally pronounced at the edges and in the mid-area of the BAMG regenerates. The two 8.5-month specimens, however, seemed to be characterized by a more balanced dispersion of vessels and muscle fibers throughout the graft. The number of alpha-actin-positive cells appeared qualitatively stable and the density of blood vessels seemed to decrease in comparison with the 4-month specimen. The collagen content and density of small- and large-diameter blood vessels appeared qualitatively greater in the BAMG regenerates. The opposite seemed true for the amount of smooth muscle at 4 and 8.5 months after grafting. PGP-positive nerve fibers could be found predominantly next to muscle bundles and the luminal surface of the graft. Their size and number increased over time. These light-microscopic findings were consistent with previous studies from our lab that used digitized slides for a quantitative computer-assisted evaluation of graft differentiation over time. [9] Differential nerve staining revealed that cholinergic, adrenergic and NADPH diaphorase-positive fibers were present in the host bladder wall tissue as well as in the BAMG regenerates (Figure 4 ).
Fig. 3.
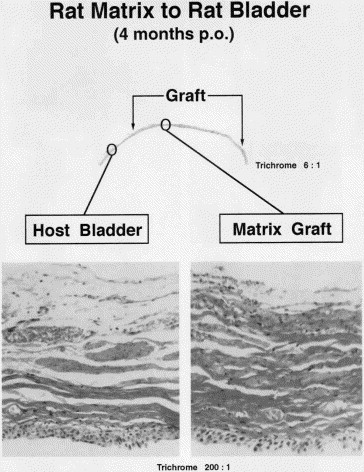
Trichrome section demonstrating the histological features of BAMG regenerate 4 months after grafting. All three layers of normal bladder wall are present, although collagen content (green) is greater and number and size of smooth muscle bundles (black arrows) is smaller than in corresponding host bladder. Pronounced neovascularization can be found even in midportion of graft.
Fig. 4.
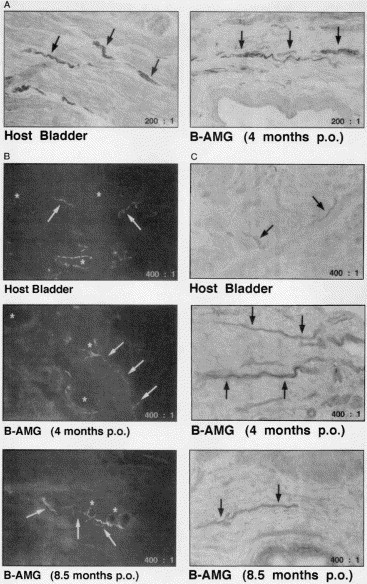
Differential nerve staining shows cholinergic (A), adrenergic (B) and NADPH diaphorase-positive (C) nerve fibers (arrows) in the BAMG-regenerated and host detrusor at 4 and 8.5 months after grafting. Note blood vessels walls (stars) exhibiting a characteristic circular arrangement of adrenergic nerve fibers.
DISCUSSION
Bladder reconstruction plays an essential role in the treatment of voiding disorders characterized by low bladder capacity or high intravesical pressures or both. [15] The ideal material as defined by Gleeson and Griffith [3] should be biocompatible and mechanically reliable, resist extraluminal infection, deter or tolerate intraluminal infection, and be easy to implant surgically. It should preserve renal function, provide adequate urinary storage at low pressure, and allow volitional, complete evacuation of urine per urethram. To achieve this goal, autoaugmentation techniques and a great variety of synthetic and naturally derived biomaterials have been used.
Synthetic materials have been unsuccessful because of foreign-body reactions, resulting in stone formation, collapse, infection, rejection, or extrusion and migration of the graft. 16, 17, 18, 19 They have been used primarily as temporary implants to allow bladder regeneration to occur, 20, 21 but the majority of studies confirm regeneration of transitional cell epithelial lining on the inner surface of the graft without adequate reconstruction of a functional detrusor muscle. Natural materials for bladder reconstruction have mostly retracted with time, 22, 23 and the alloplastic total bladder prosthesis is still at an investigational stage in animals.
Autoaugmentation by enterocystoplasty with either small bowel or colon has well-documented urodynamic benefits. [24] Because of complications, including metabolic acidosis, [2] rupture, [25] mucus production, chronic bacteriuria, stone formation, [26] and the potential for osteoporosis and malignancy, [27] the search for other suitable materials continues. Gastrocystoplasty circumvents some of these problems, but peptic ulcers and perforations, the hematuria/dysuria syndrome, and metabolic alkalosis negate some of its potential advantages over intestinal segments. [28] Recently the technique for enterocystoplasty lined with urothelium has been shown to increase bladder capacity while taking advantage of the inert properties of an intact urothelial lining. 29, 30 Gastrointestinal segments in general have proved to enhance bladder capacity and compliance, thus protecting the upper tract and renal function. Unfortunately, they are unable to support normal micturition, which often necessitates clean intermittent catheterization or other supportive measures to ensure complete bladder evacuation.
To overcome this functional shortcoming, natural and/or biodegradable materials serving as a scaffold for the ingrowth of host bladder wall components have been tried with encouraging results. 5, 31, 32, 33 The bladder wall tissue thus regenerated has shown the potential to provide functional augmentation without compromising voiding ability.
Previous histological studies demonstrated that the homologous bladder acellular matrix graft (BAMG) promotes the ingrowth of urothelium, smooth muscle, blood vessels and nerves in the rat. [9] In vivo studies found that bladders thus regenerated showed enhanced low-pressure reservoir function and the preservation of normal micturition. The present in vitro contractility studies were designed to characterize the function of the BAMG-regenerated rat bladder further, proposing that its functional innervation would be similar to that of the normal rat bladder.
Bladder stone formation, which occurred in 80% of the grafted animals, is a common finding after lower urinary tract surgery rat in the rat. 7, 9, 34, 35 It may potentially affect the in vitro contractility through chronic inflammation or by causing bladder hypertrophy and/or dilation through outlet obstruction. [36] To ensure the same starting point for our organ bath experiments, we therefore compared the BAMG-regenerated smooth muscle strips to strips from the same host bladder wall rather than to bladder strips from untreated, age-matched control rats. Although all strips were cut the same size, the weight of the regenerated and host bladder strips varied considerably, possibly because of differences in muscularization and collagen content and/or bladder hypertrophy. All peak contraction values were therefore related to the weight of each individual muscle strip (Figure 2).
Electrical field stimulation techniques were used to demonstrate contractility through activation of intrinsic nerves [37] within the BAMG-regenerated tissue and the host bladder wall, thus showing that PGP-positive nerve fibers had reinnervated the regenerated smooth muscle cells. Tetrodotoxin, which abolishes neurogenic action potentials, decreased electrical field stimulation-induced contractions, providing further evidence of the presence of neural components. [38]
Various agonists (carbachol, isoproterenol, norepinephrine, alpha, beta-methylene ATP) and antagonists (atropine, valethamate bromide, propranolol) were used to infer functionally the presence of muscarinic, adrenergic and purinergic receptors 39, 40, 41, 42, 43 in the regenerated and host bladder wall. They produced concentration-dependent tonic contraction and relaxation, respectively, in the BAMG regenerates and normal bladder strips. In addition, immunohistochemistry confirmed the existence of cholinergic and adrenergic nerve fibers (Figure 4), thereby exemplifying the regenerative potential of visceral nerves, as had been previously demonstrated in the rat for somatic nerves. 44, 45, 46 Muscarinic stimulation proved to be the strongest of the contractile stimuli studied, in conformity with our own and other in vitro studies of normal rat detrusor. 8, 36, 47, 48
Potassium was chosen as a non-adrenergic, non-cholinergic mechanism to induce contraction through smooth muscle cell depolarization by direct chemical changes of the membrane potential. Potassium-contracted BAMG-regenerated and host bladder strips were all susceptible to nitroprusside-induced relaxation, further evidencing a possible involvement of the nitric oxide pathway in non-adrenergic, non-cholinergic relaxation of rat detrusor smooth muscle. 49, 50 This finding was supported by immunohistochemical staining for NADPH-diaphorase, thus identifying neuronal nitric oxide-containing nerve fibers within the BAMG regenerates and host bladder tissue (Figure 4).
Because of its muscarinic, purinergic, alpha- and beta-adrenergic receptors, BAMG-regenerated detrusor proved capable of generating contractile and relaxatory responses through the neurotransmitter-based mechanisms of normal bladder. The patterns of response to all the aforesaid electrical and pharmacologic stimuli were qualitatively almost identical in the BAMG regenerates and host bladder strips, whereas the forces of contraction were quantitatively smaller in the regenerated bladder wall tissue (Figure 2), possibly resulting from the smaller muscle mass and higher collagen content in the BAMG regenerates. Previous studies from our laboratory have shown, however, that such a reduction in contractile force does not compromise normal voiding of BAMG-regenerated bladders in vivo.
CONCLUSION
Augmentation cystoplasty with the bladder acellular matrix graft in rats results in regeneration of the detrusor. Bladders thus regenerated exhibit functional and histological evidence of innervation that is similar to normal. This would allow them to work in coordination with the host bladder components to generate adequate intravesical pressure to produce sustained voiding. The results of the present in vitro study further demonstrate the important regenerative and functional capacities and clinical potential of the acellular matrix graft in bladder reconstruction.
Footnotes
Supported by Deutsche Forschungsgemeinschaft Grants Pi 272/1-2 and Da 409/1-1 and NIH Grant RO1 NS18029.
REFERENCES
- 1.Khoury J.M., Timmons S.L., Corbel L., Webster G.D. Complications of enterocystoplasty. Urology. 1992;40:9. doi: 10.1016/0090-4295(92)90428-y. [DOI] [PubMed] [Google Scholar]
- 2.McDougal W.S. Metabolic complications of urinary intestinal diversion. J. Urol. 1992;147:1199. doi: 10.1016/s0022-5347(17)37517-1. [DOI] [PubMed] [Google Scholar]
- 3.Gleason M.J., Griffith D. The use of alloplastic biomaterials in bladder substitution. J. Urol. 1992;148:1377. doi: 10.1016/s0022-5347(17)36916-1. [DOI] [PubMed] [Google Scholar]
- 4.Pust R. [Urinary bladder plastic enlargement with the use of autologous, homologous and heterologous skin transplants] Fortschr. Med. 1979;97:561. [PubMed] [Google Scholar]
- 5.Atala A., Freeman M.R., Vacanti J.P., Shepard J., Retik A.B. Implantation in vivo and retrieval of artificial structures consisting of rabbit and human urothelium and human bladder muscle. J. Urol. 1993;150:608. doi: 10.1016/s0022-5347(17)35561-1. [DOI] [PubMed] [Google Scholar]
- 6.Kropp B.P., Rippy M.K., Badylak S.F., Adams M.C., Keating M.A., Rink R.C., Thor K.B. Regenerative urinary bladder augmentation using small intestinal submucosa: urodynamic and histopathologic assessment in long-term canine bladder augmentations. J. Urol. 1996;155:2098. doi: 10.1016/s0022-5347(01)66117-2. [DOI] [PubMed] [Google Scholar]
- 7.Sutherland R.S., Baskin L.S, Hayward S.W., Cunha G.R. Regeneration of bladder urothelium, smooth muscle, blood vessels and nerves into an acellular tissue matrix. J. Urol. 1996;156:571. doi: 10.1097/00005392-199608001-00002. [DOI] [PubMed] [Google Scholar]
- 8.Vaught J.D., Kropp B.P., Sawyer B.D., Rippy M.K., Badylak S.F., Shannon H.E., Thor K.B. Detrusor regeneration in the rat using porcine small intestinal submucosal grafts: functional innervation and receptor expression. J. Urol. 1996;155:374. [PubMed] [Google Scholar]
- 9.Probst M., Dahiya R., Carrier S., Lipkin D., Tanagho E.A. Reproduction of functional smooth muscle tissue and partial bladder replacement. Br. J. Urol. 1997;79:505. doi: 10.1046/j.1464-410x.1997.00103.x. [DOI] [PubMed] [Google Scholar]
- 10.Meezan E., Hjelle J.T., Brendel K. A simple, versatile, nondisruptive method for the isolation of morphologically and chemically pure basement membranes from several tissues. Life Sci. 1975;17:1721. doi: 10.1016/0024-3205(75)90119-8. [DOI] [PubMed] [Google Scholar]
- 11.Gulbenkian S., Wharton J., Polak J.M. The visualization of cardiovascular innervation in the guinea pig using an antiserum to protein gene product 9.5 (PGP 9.5) J. Auton. Nerv. Syst. 1987;18:235. doi: 10.1016/0165-1838(87)90122-6. [DOI] [PubMed] [Google Scholar]
- 12.El-Badawi A., Schenk E.A. Histochemical methods for separate, consecutive and simultaneous demonstration of acetylcholinesterase and norepinephrine in cryostat sections. J. Histochem. Cytochem. 1967;15:580. doi: 10.1177/15.10.580. [DOI] [PubMed] [Google Scholar]
- 13.de la Torre J.C., Surgeon J.W. A methodological approach to rapid and sensitive monoamine histofluorescence using a modified glyoxylic acid technique: the SPG method. Histochemistry. 1976;49:81. doi: 10.1007/BF00495672. [DOI] [PubMed] [Google Scholar]
- 14.Sutherland R.S., Kogan B.A., Piechota H.J., Bredt D.S. Vesicourethral function in mice with genetic disruption of neuronal nitric oxide synthase. J. Urol. 1997;157:1109. [PubMed] [Google Scholar]
- 15.Mitchell M.E., Gonzales R., Cabral B.H., Bauer S.B., Gearhart J.P., Filmer R.B. Bladder augmentation problems in neurovesical dysfunction. Dialogues Ped. Urol. 1987;10:1. [Google Scholar]
- 16.Barrett D.M., Donovan M.G. Prosthetic bladder augmentation and replacement. Semin. Urol. 1984;2:167. [PubMed] [Google Scholar]
- 17.Bohne A.W., Urwiller K.L. Experience with urinary bladder regeneration. J. Urol. 1957;77:725. doi: 10.1016/S0022-5347(17)66624-2. [DOI] [PubMed] [Google Scholar]
- 18.Stanley T.H., Feminella J.G., Jr., Priestley J.B., Lattimer J.K. Subtotal cystectomy and prosthetic bladder replacement. J. Urol. 1972;107:783. doi: 10.1016/s0022-5347(17)61139-x. [DOI] [PubMed] [Google Scholar]
- 19.Swinney J., Tomlinson B.E., Walder D.N. Urinary tract substitution. Br. J. Urol. 1961;33:414. [Google Scholar]
- 20.Taguchi H., Ishizuka E., Saito K. Cystoplasty by regeneration of the bladder. J. Urol. 1977;108:752. doi: 10.1016/s0022-5347(17)58181-1. [DOI] [PubMed] [Google Scholar]
- 21.Tsuji I., Shiraishi Y., Kassai T., Kunishima K., Orikasa S., Abe N. Further experimental investigations on bladder reconstruction without using the intestine. J. Urol. 1967;97:1021. doi: 10.1016/S0022-5347(17)63168-9. [DOI] [PubMed] [Google Scholar]
- 22.Baret A.C., De Muth W.E., Murphy J.J., Muir W.W. Experimental repair of extrophy of the bladder without cystectomy by using a free fascial graft. Surg. Gynecol. Obstet. 1953;97:633. [PubMed] [Google Scholar]
- 23.Kelami A. Lyophilized human dura as a bladder wall substitute: experimental and clinical results. J. Urol. 1971;105:518. doi: 10.1016/s0022-5347(17)61563-5. [DOI] [PubMed] [Google Scholar]
- 24.Sidi A.A., Reinberg Y., Gonzalez R. Influence of intestinal segment and configuration on the outcome of augmentation enterocystoplasty. J. Urol. 1986;136:1201. doi: 10.1016/s0022-5347(17)45282-7. [DOI] [PubMed] [Google Scholar]
- 25.Bauer S.B., Hendren W.H., Kozakewich H., Maloney S., Colodny A.H., Mandell J, Retik A.B. Perforation of the augmented bladder. J. Urol. 1992;148:699. doi: 10.1016/s0022-5347(17)36698-3. [DOI] [PubMed] [Google Scholar]
- 26.Golomb J., Klutke C.G., Raz S. Complications of bladder substitution and continent urinary diversion. Urology. 1989;34:329. doi: 10.1016/0090-4295(89)90435-4. [DOI] [PubMed] [Google Scholar]
- 27.Filmer R.B., Spencer J.R. Malignancies in bladder augmentations and intestinal conduits. J. Urol. 1990;143:671. doi: 10.1016/s0022-5347(17)40055-3. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell M., Burns M. Augmentation cystoplasty with stomach. In: Webster G.D., Kirby B., King L.R., editors. Reconstructive Urology. Blackwell Scientific; Oxford: 1993. pp. 439–444. [Google Scholar]
- 29.Buson H., Manivel R., Long R.M.D., Gonzales R. Seromuscular colocystoplasty lined with urothelium: experimental study. Urology. 1994;44:743. doi: 10.1016/s0090-4295(94)80220-3. [DOI] [PubMed] [Google Scholar]
- 30.Gonzales R., Buson H., Reid C., Reinberg Y. Seromuscular colocystoplasty lined with urothelium. Experience with 16 patients. Urology. 1995;45:124. doi: 10.1016/s0090-4295(95)97364-8. [DOI] [PubMed] [Google Scholar]
- 31.Knapp P.M., Lingeman J.E., Siegel Y.I., Badylak S.F., Demeter R.J. Biocompatibility of small-intestinal submucosa in urinary tract as augmentation cystoplasty graft and injectable suspension. J. Endourol. 1994;8:125. doi: 10.1089/end.1994.8.125. [DOI] [PubMed] [Google Scholar]
- 32.Novick A.C., Straffon R.A., Koshino I., Banowsky L.H., Levin H., Kambic H. Experimental bladder substitution using a biodegradable graft of natural tissue. J. Biomed. Material Res. 1978;12:125. doi: 10.1002/jbm.820120202. [DOI] [PubMed] [Google Scholar]
- 33.Scott R., Mohammed R., Gorham S.D., French D.A., Monsour M.J., Shivas A., Hyland T. The evolution of a biodegradable membrane for use in urological surgery. A summary of 109 in vivo experiments. Br. J. Urol. 1988;62:26. doi: 10.1111/j.1464-410x.1988.tb04259.x. [DOI] [PubMed] [Google Scholar]
- 34.Liang D.S., Liang M.D. Bladder muscle regeneration. Invest. Urol. 1974;12:5. [PubMed] [Google Scholar]
- 35.Little J.S., Jr., Klee L.W., Hoover D.M., Rink R.C. Long-term histopathological changes observed in rats subjected to augmentation cystoplasty. J. Urol. 1994;152:720. doi: 10.1016/s0022-5347(17)32690-3. [DOI] [PubMed] [Google Scholar]
- 36.Saito M., Longhurst P.A., Tammela T.L., Wein A.J., Levin R.M. Effects of partial outlet obstruction of the rat urinary bladder on micturition characteristics, DNA synthesis and the contractile response to field stimulation and pharmacological agents. J. Urol. 1993;150:1045. doi: 10.1016/s0022-5347(17)35683-5. [DOI] [PubMed] [Google Scholar]
- 37.Malmgren A., Uvelius B., Andersson K.-E., Andersson P.O. Urinary bladder function in rats with hereditary diabetes insipidus; a cystometrical and in vitro evaluation. J. Urol. 1992;148:930. doi: 10.1016/s0022-5347(17)36780-0. [DOI] [PubMed] [Google Scholar]
- 38.Maggi C.A., Santicioli P., Meli A. Pharmacological evidence for the existence of two components in the twitch response to field stimulation of detrusor strips from the rat urinary bladder. J. Auton. Pharmacol. 1985;5:221. doi: 10.1111/j.1474-8673.1985.tb00123.x. [DOI] [PubMed] [Google Scholar]
- 39.Brading A.F., Williams J.H. Contractile responses of smooth muscle strips from rat and guinea-pig urinary bladder to transmural stimulation: effects of atropine and alpha, beta-methylene ATP. Br. J. Pharmacol. 1990;99:493. doi: 10.1111/j.1476-5381.1990.tb12956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burnstock C., Cocks T., Kasakov L., Hong H.K. Direct evidence for ATP release from non-adrenergic, non cholinergic (purinergic) nerves in the guinea-pig taenia coli and bladder. Eur. J. Pharmacol. 1978;49:145. doi: 10.1016/0014-2999(78)90070-5. [DOI] [PubMed] [Google Scholar]
- 41.Igawa Y., Mattiasson A., Andersson K.-E. Functional importance of cholinergic and purinergic neurotransmission for micturition contraction in the normal, unanaesthetized rat. Br. J. Pharmacol. 1993;109:473. doi: 10.1111/j.1476-5381.1993.tb13593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kasakov L., Burnstock G. The use of slowly degradable analog, alpha, beta-methylene ATP, to produce desensitization of the P2-purinoceptor: effect on non-adrenergic, non-cholinergic responses of the guinea-pig urinary bladder. Eur. J. Pharmacol. 1982;86:291. doi: 10.1016/0014-2999(82)90330-2. [DOI] [PubMed] [Google Scholar]
- 43.Vale J.A., Liu K., Whitfield H.N., Trott K.R. Post-irradiation bladder dysfunction: muscle strip findings. Urol. Res. 1994;22:51. doi: 10.1007/BF00431549. [DOI] [PubMed] [Google Scholar]
- 44.Danielsen N., Dahlin L.B., Lee Y.F., Lundborg G. Axonal growth in mesothelial chambers. The role of the distal nerve segment. Scand. J. Plast. Reconstr. Surg. 1983;17:119. doi: 10.3109/02844318309013106. [DOI] [PubMed] [Google Scholar]
- 45.Hagg T., Gulati A.K., Behzadian M.A., Vahlsing H.L., Varon S., Manthorpe M. Nerve growth factor promotes CNS cholinergic axonal regeneration into acellular peripheral nerve grafts. Exp. Neurol. 1991;112:79. doi: 10.1016/0014-4886(91)90116-t. [DOI] [PubMed] [Google Scholar]
- 46.Warburton A.L., Santer R.M. Sympathetic and sensory innervation of the urinary tract in young adult and aged rats: a semi-quantitative histochemical and immunohistochemical study. Histochem. J. 1994;26:127. doi: 10.1007/BF00157961. [DOI] [PubMed] [Google Scholar]
- 47.Chun A.L., Wallace L.J., Gerald M.C., Wein A.J., Levin R.M. Effects of age on urinary bladder function in the male rat. J. Urol. 1989;141:170. doi: 10.1016/s0022-5347(17)40634-3. [DOI] [PubMed] [Google Scholar]
- 48.Malmgren A., Ekblad E., Sundler F., Andersson K.-E., Andersson P.O. Muscarinic supersensitivity in the rat urinary bladder after capsaicin pretreatment. Acta Physiol. Scand. 1990;138:377. doi: 10.1111/j.1748-1716.1990.tb08860.x. [DOI] [PubMed] [Google Scholar]
- 49.Andersson K.-E., Persson K. The L-arginine/nitric oxide pathway and non-adrenergic, non-cholinergic relaxation of the lower urinary tract. Gen. Pharmacol. 1993;24:833. doi: 10.1016/0306-3623(93)90156-r. [DOI] [PubMed] [Google Scholar]
- 50.Persson K., Igawa Y., Mattiasson A., Andersson K.-E. Effects of inhibition of the L-arginine/nitric oxide pathway in the rat lower urinary tract in vivo and in vitro. Br. J. Pharmacol. 1992;107:178. doi: 10.1111/j.1476-5381.1992.tb14483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


