Abstract
In this study, the immunogenicity of chimeric 987P fimbriae on a Salmonella vaccine strain was improved by optimizing fimbrial expression. The constitutive tetA promoter and the in vivo activated nirB and pagC promoters were evaluated for their use to express two epitopes of the transmissible gastroenteritis virus (TGEV) spike protein carried by fimbriae which were displayed on a Salmonella vaccine strain. Constructs with the pagC promoter were shown to drive increased expression of chimeric 987P fimbriae in macrophages as well as in Mg2+-poor media, mimicking a major environmental signal found in Salmonella-containing endocytic vacuoles of macrophages. Mice immunized orally with a Salmonella vaccine strain which expressed chimeric fimbriae from the pagC promoter elicited significantly higher mucosal and systemic immune responses to both the 987P fimbriae and the TGEV epitopes than mice immunized with the same strain hosting a tetA or nirB promoter-driven expression plasmid. Moreover, only the Salmonella vaccine strains harboring a plasmid with the pagC promoter, with or without an additional tetA promoter in tandem, elicited neutralizing antibodies to TGEV. This indicated that the pagC promoter can be used successfully to improve epitope-display by chimeric fimbriae on Salmonella vaccine strains for the induction of a desired immune response.
Keywords: Fimbriae, TGEV, Salmonella typhimurium, Vaccine, pagC
1. Introduction
Transmissible gastroenteritis virus (TGEV) is a member of the Coronaviridae family in the Nidovirales order [1]. TGEV replicates both in the villous epithelial cells of the small intestine and in the lung cells of newborn piglets, resulting in a mortality of nearly 100% [2]. Protection of the newborn animals from TGEV infection requires the induction of secretory immunoglobulin A (IgA) in milk, especially in colostrum [2]. Typically, IgA-producing plasma cells in the mammary gland of pregnant sows originate from the gut-associated lymphoid tissues (GALT) where they were activated by different antigenic components. Thus, antigen-specific colostral antibodies can be induced by oral vaccines with enteric tropism [3], [4].
Several viral proteins are important for inducing immune responses to coronavirus: the spike protein (S), the nucleocapsid protein (N) and the membrane protein (M) [3], [5], [6]. Study of the induction of protective immunity to TGEV has focused on the spike protein (S), because it is the major inducer of TGEV neutralizing antibodies [7], [8] and mediates binding of TGEV to its cellular receptor aminopeptitase N [9]. The relevant epitopes for neutralization were mapped to the N terminal domain of S protein, and four major antigenic sites (A to D) were identified independently by two groups [7], [10]. Sites C and A (using the nomenclature of Delmas et al.) are involved in the induction of neutralization antibodies [7] and stretches of continuous residues in each site were shown to be recognized by neutralizing monoclonal antibodies [7], [10].
Various approaches have been used to develop TGEV vaccines. The current commercial vaccines include inactivated or live attenuated TGEV vaccines [11]. However, only oral vaccines containing live replicating organisms have been highly effective in inducing mucosal immune responses [2], [12]. New developments focused on the production and delivery of the S protein. S glycoprotein antigen produced in baculovirus [13], [14], or adenovirus [15] and Salmonella vectors expressing full-length or truncated S protein [16], [17] were investigated. Moreover, both purified chimeric CS31 and 987P fimbriae carrying TGEV C and/or A epitopes [18], [19] and Salmonella vaccine strains expressing chimeric 987P fimbriae carrying the TGEV C and A epitopes have been developed and shown to be immunogenic [20].
Salmonella spp. are facultative intracellular pathogens [21]. After oral administration, Salmonella penetrate through M cells into Peyer's patches, where most of them are observed in underlying macrophages [22], [23] or dendritic cells, as shown recently [24], [25]. These antigen presenting cells disseminate with their bacterial cargo to mesenteric lymph nodes, spleen and liver. Thus, attenuated Salmonella strains constitute attractive vehicles for the delivery of protective antigens to both the mucosal and systemic immune systems [26].
An effective vaccine against TGEV is still in demand, as TGEV infections continue to cause major economic losses to the swine industry. In an attempt to develop a live-attenuated and multivalent vaccine against diarrhea in pigs, we have constructed and studied a number of recombinant Salmonella strains which express chimeric 987P fimbriae carrying TGEV C and A epitopes [20]. In this paper, we demonstrate that immune responses to the 987P carrier as well as to the TGEV epitopes were enhanced by using the in vivo-inducible pagC promoter for fimbrial display on a Salmonella vector.
2. Materials and methods
2.1. Mice
Six-week-old female Balb/cByJ mice were obtained from the Jackson Laboratory and housed in filter-top cages in an air-conditioned animal facility. Water and food were provided ad libitum. Mice were adapted for 1 week after arrival before being used for immunization.
2.2. Bacterial strains, media and reagents
E. coli and Salmonella typhimurium strains used in this study are listed in Table 1 . Strains χ6212 and χ4550 were grown in LB medium with 50 μg/ml dl-α∋-diaminopimelic acid (DAP, Sigma, St Louis, MO). Medium components were purchased from Difco (Detroit, MI). Restriction and modification enzymes were from New England Biolabs, Inc. (Beverly, MA). Unless specified, reagents were purchased from Sigma.
Table 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Reference |
|---|---|---|
| E. coli | ||
| χ6212 | ΔasdA1 derivative of DH5α | [37] |
| S. typhimurium | ||
| χ4550 | gyrA1816 ΔasdA1 Δ(zhf-4::Tn10) Δcrp-1 Δcya-1 | [55] |
| Plasmid | ||
| pCS154 | pCS150 asd (Fas+) | [20] |
| pCS155 | pCS154 with the nirB promoter upstream fasA (Fas+) | [20] |
| pCS165 | pCS154 with the pagC promoter upstream fasA (Fas+) | This study |
| pCS173 | pCS165 with deletion of tetA promoter | This study |
| pCS176 | pCS155 with deletion of tetA promoter | This study |
2.3. Plasmid constructs
Standard procedures were used to construct the following plasmids. A DNA fragment comprising the pagC promoter was cloned from S. typhimurium χ4550 by PCR (upper primer 5′CGGGATCCGTTAACCACTCTTAATAATAAT, lower primer 5′CGGGATCCCGTGACGCTCCATCCGCAATAC) and was added to the Bam HI site just upstream of fasA in pCS154. This newly generated plasmid, pCS165, carries both the tetA and pagC promoters. Plasmid pCS173, which contains only the pagC promoter, was created by removing the smallest Hind III fragment containing the tetA promoter from pCS165. pCS176, which carries the nirB promoter alone, was constructed by removing the Hind III fragment of pCS155 containing the tetA promoter.
2.4. Macrophages
Both the murine macrophage-like cell line J774A.1 and murine peritoneal macrophages were used in this study. J774A.1 (ATCC TIB-67; kind gift of Dr Howard Goldfine) was maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 1.0 mM sodium pyruvate and 10% fetal bovine serum (DMEM-complete) at 37°C under an atmosphere of 5% CO2. Murine peritoneal macrophages were harvested as previously described [27]. Briefly, mice were euthanized by cervical dislocation, and 6–8 ml of Hanks balanced saline buffer (HBSS) was injected into the peritoneal cavity of each mouse. The abdomen of each mouse was massaged vigorously, and peritoneal macrophages were withdrawn using a syringe. Cells were pelleted by centrifugation (250×g, 10 min) and suspended in DMEM-complete. The cell suspensions were distributed in 24-well plates, and incubated at 37°C for 2 h to allow the macrophage to adhere to the plastic. Nonadherent cells were washed away with HBSS. These enriched populations of macrophages were then cultured in DMEM-complete at 37°C under an atmosphere of 5% CO2.
2.5. Peptides and fimbriae
The TGEV C and A peptides of the spike protein, corresponding to amino acid residues 379–388 and 521–531, respectively, were both synthesized with a cysteine added to their carboxy termini (SFFSYGEIPC and MKRSGYGQPIAC) at the Protein Chemistry Laboratory of the University of Pennsylvania School of Medicine. Fimbriae expressed on the bacterial surface were prepared by heat extraction, as described previously [28].
2.6. Seroagglutination and antibodies
Slide seroagglutination tests were performed with preadsorbed rabbit anti-987P antiserum [29], with an anti-TGEV-C epitope antiserum [19] and with an anti-TGEV A epitope antiserum [20].
2.7. SDS-PAGE and Western blotting
Bacterial pellets, isolated fimbriae or macrophage lysates were resuspended in sample buffer, boiled for 5 min, and the proteins were separated by SDS-PAGE. Western blots were probed with rabbit anti-987P fimbriae antibodies, using horseradish peroxidase (HRP)-conjugated secondary antibodies and enhanced chemiluminescence (ECL) for detection [20]. Relative amounts of chimeric FasA protein of the different constructs were evaluated by densitometry, using the NIH Image software (Division of Computer Research and Technology, National Institutes of Health, Bethesda, MD).
2.8. Phagocytosis assay
To determine the expression of chimeric fimbriae in macrophages, the bacteria were opsonized in 10% fresh mouse serum in phosphate-buffered saline (PBS) for 20 min and added to cell cultures at a multiplicity of infection of 10, as described elsewhere [30]. Internalization were allowed to proceed for 1 h. Nonphagocytosed bacteria were washed away with PBS. The infected macrophages were cultured in DMEM-complete containing 50 μg/ml gentamicin sulfate for 24 h. The macrophages were washed three times with PBS and lysed with 0.1% Triton X-100 in PBS to release the intracellular bacteria. The bacteria were then collected by centrifugation (4000×g, 5 min), solubilized in SDS-PAGE sample buffer and subjected to Western blotting.
2.9. Immunization and sampling
Mice were immunized and samples were collected and processed essentially as described previously [20], [31], [32]. For each immunization, a single colony of Salmonella was grown in L broth without any antibiotics at 37°C on a rotary shaker at 150 rev./min overnight. The bacterial cells were gently washed once and resuspended in sterile phosphate-buffered saline (PBS; 10 mM Na2HPO4, 1.8 mM KH2PO4, 2.7 mM KCl, 137 mM NaCl, pH 7.2) at the concentration of 1–5×1011 CFU/ml. Viable counts were performed on all inocula. Before immunization, the mice were deprived of food and water for 4 h. The mice were intubated with feeding needles for intragastrical delivery of 200 μl of bacterial suspensions and fasted for an additional 30 min. The mice were immunized twice at day 0 and 30. For each immunized group of mice, pooled fecal pellets were collected on a weekly basis. Approximately 500 mg feces were added to tubes containing 2 ml of a protease inhibitor solution (PBS with 0.5% BSA and a cocktail of protease inhibitors, Complete™, Boehringer Mannheim, Ger, using the manufacturer's recommended concentration). The fecal pellets were soaked in ice for 15 min and the tubes were agitated vigorously for 5 min twice on a vortex at maximum speed. The suspensions were centrifuged at 13 000×g for 15 min and the supernatants were stored at −20°C. To collect serum samples and intestinal secretions, mice were anesthetized with Metofane (methoxyflurane, Mallinckrodt Veterinary Inc., Mundelein, IL) and exsanguinated. Blood was collected by heart puncture. Whole small intestines, from duodenum to the ileo-cecal junction, were excised and luminal contents were carefully collected with the help of 3 ml of protease inhibitor solution introduced into intestinal lumens. Recovered intestinal contents were vortexed vigorously for 5 min. After centrifugation at 13 000×g for 15 min at 4°C, supernatants were collected and stored at −20°C.
2.10. ELISA
Individual mouse sera and intestinal secretions, and group-pooled fecal pellet extract were tested for immunoglobulin A (IgA) and IgG antibodies against 987P fimbriae, TGEV C or A peptides by enzyme-linked immunosorbent assay (ELISA) essentially as described [20], [29]. Briefly, 96-well ELISA plates (Immulon 4, Dynatech Laboratories, Inc., Chantilly, VA) were coated with isolated wild type 987P fimbriae (0.2 μg in 100 μl 0.1 M carbonate buffer, pH 9.6, per well) overnight at 4°C. TGEV C or A peptide(1.0 μg in 100 μl 0.1 M carbonate buffer, pH 9.6, per well) were coated to the plates using a microwave oven as described [33] and further overnight coating at 4°C. The plates were blocked with 0.5% BSA in PBS at 37°C for 2 h, washed four times with PBS and incubated with serial dilutions of body fluid samples in PBS–0.1% BSA–0.05% Tween-20 for 2 h at 37°C. After the second washing step, plates were incubated with HRP-conjugated anti-mouse IgG, IgG1, IgG2a or IgA antibodies at 37°C for 1 h. Following the last washing step, bound antibodies were detected by using o-phenylenediamine as the chromogenic reagent and reading the absorbance at 450 nm.
2.11. Dot blot assay
Nitrocellulose strips were spotted with TGEV C and TGEV A peptides, the strips were incubated with gut washes, and the blots were developed with HRP-conjugated with goat anti-mouse IgG or IgA antibodies and visualized by ECL, as described previously [20].
2.12. Virus seroneutralization test
TGEV neutralization was determined using a limiting dilution microassay with 96-well plates [34]. Briefly, 50 μl of serial twofold dilutions of antisera were mixed with an equal volume of virus suspension containing 100 TCID50 of TGEV Purdue-115 strain (kind gift from Dr Linda J. Saif). After incubation at 37°C for 1 h, 4×104 trypsinized swine testis (ST) cells (ATCC CRL-1746) in 100 μl DMEM supplemented with 10% fetal bovine serum and 0.1 mM non-essential amino acids were added to the antiserum–virus mixtures. Neutralization titers were determined 48 h later and calculated as the mean of the highest dilution that neutralized 100% of the cytopathic effect in duplicate experiments. Positive and negative reference sera were included in each experiment.
2.13. Statistical analysis
Groups of log-transformed data were compared by using the unpaired Student's t-test [35]. Probability (P) values of less than 0.05 indicated that the groups were significantly different.
3. Results
3.1. Construction of the in vivo inducible expression plasmids
To stabilize the antigen-expressing multicopy-number plasmids in live Salmonella vectors in vivo, we used the asd balanced lethal system [36], [37]. In plasmid pCS154, expression of the chimeric 987P fimbriae is driven by the promoter for the tetracycline resistance gene tetA [20]. Seroagglutination determined a high and constant level of 987P fimbriation of S. typhimurium χ4550/pCS154 in the absence or presence of subminimal inhibitory concentrations of tetracycline (data not shown), suggesting that pCS154 mediated-fimbrial expression was constitutive and not regulated by a TetR repressor [38]. The immunogenicity of chimeric 987P fimbriae expressed by Salmonella crp cya vaccine strains was shown previously to be improved by constructing and using pCS155 which contains a synthetic DNA fragment encoding the nirB promoter inserted between the tetA promoter and the fasA gene of pCS154 [20]. In this study, a similar strategy was used to construct plasmids containing the pagC promoter. A 294-bp fragment of DNA encompassing the pagC promoter of S. typhimurium χ4550 was cloned by standard PCR. The amplified fragment was inserted between the tetA promoter and the fasA gene of pCS154, generating pCS165. Consequently, both pCS155 and pCS165 have two promoters driving the expression of chimeric 987P fimbriae. To construct expression plasmids carrying only the in vivo-inducible promoter nirB or pagC, the tetA promoter-containing Hind III fragments of pCS155 and pCS165 were deleted, resulting in pCS176 and pCS173, respectively.
3.2. Environmental regulation of chimeric 987P fimbriae on Salmonella
Whether each plasmid construct contained the sequences necessary to drive gene expression after induction by the appropriate environmental signal was determined in vitro. The expression of chimeric 987P fimbriae was studied in S. typhimurium χ4550 carrying plasmids pCS165, pCS173 or pCS176 using different growth conditions. The amounts of fimbriae isolated from the same numbers of bacteria were compared on SDS-polyacrylamide gels. To test activation of the nirB promoter in χ4550/pCS176, the bacteria were grown statically in a capped-flask to reduce the oxygen level in the culture [39]. The aerated culture expressed a low level of fimbriae which increased threefold when the bacteria were cultured statically in a capped-flask (Fig. 1 , lanes 8–9). The pagC promoter in pCS165 or pCS173 were repressed by supplementing the bacterial culture medium with MgCl2. As the concentration of MgCl2 increased, the level of fimbrial expression decreased (Fig. 1, lanes 2–7). In LB media supplemented with 10 mM MgCl2, χ4550/pCS173 merely expressed fimbriae, while χ4550/pCS165 still expressed relatively high levels of fimbriae. This result was expected, since pCS165 can also drive fimbrial expression from its tetA promoter (Fig. 1). LB by itself contains approximately 0.23 mM Mg2+ (Difco, typical analysis sheets), thus, fimbrial expression might be even higher with bacteria grown under the Mg2+-limiting conditions (1 μM) shown to best signal the induction of PhoP-activated gene transcription [40]. Taken together, the data confirmed that fimbriae expression from the constructs with the pagC promoter are regulated by Mg2+ concentrations.
Fig. 1.
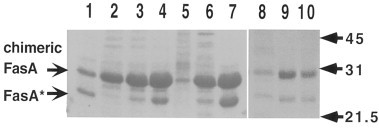
SDS-PAGE of isolated chimeric 987P fimbriae from S. typhimurium χ4550 with various plasmids grown under different conditions. χ4550/pCS154 grown aerated in LB (lanes 1 and 10); χ4550/pCS165 grown aerated in LB (lane 4) supplemented with 1 mM (lane 3) or 10 mM MgCl2 (lane 2); χ4550/pCS173 grown aerated in LB (lane 7) supplemented with 1 mM (lane 6) or 10 mM MgCl2 (lane 5); χ4550/pCS176 grown aerated (lane 8) or statically in capped-flasks (lane 9) in LB. The bands corresponding to the chimeric fimbrial subunit FasA and to its cleavage product (FasA*), as observed previously [20], are indicated on the left, and protein molecular weights (kDa) are indicated on the right.
3.3. Expression of chimeric fimbriae by Salmonella in macrophages
To verify that the pagC promoter in plasmids pCS165 and pCS173 can be induced in macrophages, the expression of chimeric fimbriae by Salmonella constructs in both J774A.1 macrophage-like cell line and fresh peritoneal macrophages were analyzed by Western blotting. S. typhimurium χ4550 expressing the fimbriae from the pagC promoter by harboring pCS165 or pCS173, produced high levels of chimeric fimbriae in both the J774A.1 cells (Fig. 2 A, lanes 2 and 3) and the peritoneal macrophages (Fig. 2B, lanes 2 and 3). In contrast, S. typhimurium χ4550 expressing the fimbriae from the tetA promoter of pCS154 (Fig. 2, lane 1) or from the nirB promoter of pCS176 (Fig. 2, lane 4) showed distinctively lower levels of chimeric fimbriae in both kinds of macrophages.
Fig. 2.
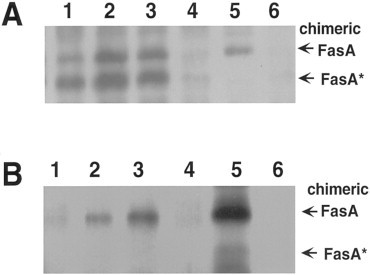
Western blot of chimeric fimbriae expressed by various derivatives of S. typhimurium χ4550 in J774A.1 macrophage-like cells (A) and peritoneal macrophages (B). Strain χ4550 containing the following plasmids were studied: pCS154 (lane 1); pCS165 (lane 2); pCS173 (lane 3); pCS176 (lane 4). In vitro grown χ4550/pCS154 were used as positive control (lane 5) and macrophages lysates were used as negative control (lane 6). The bands corresponding to the chimeric fimbrial subunit FasA and to its cleavage product (FasA*), as observed previously [20], are indicated on the right.
3.4. Systemic humoral immune responses against chimeric fimbriae
The immunogenicity of the chimeric 987P fimbriae expressed by S. typhimurium χ4550 hosting different plasmids was evaluated after oral immunization of Balb/c mice. 987P-specific serum IgG was detected in all mice immunized with S. typhimurium χ4550 vaccine strains regardless of which expression plasmid they harbored (Fig. 3 A). However, the antibody titers were significantly higher in the mice immunized with χ4550/pCS165 or χ4550/pCS173 than those in mice immunized with χ4550/pCS154 or χ4550/pCS176 (Fig. 3A). No other comparison in antibody titers identified significant differences. Thus, the data indicated that use of the plasmids with the pagC promoter significantly enhanced the immunogenicity of the chimeric 987P fimbriae. To better characterize the type of T helper cell response, the serum anti-987P fimbriae IgG1 and IgG2a subclasses of the different mice groups were determined. In mice immunized with strains expressing the fimbriae from the pagC promoter (with or without the tetA promoter), the anti-987P fimbriae IgG response was dominated by the IgG2a subclass (Fig. 3B). In contrast, mice immunized with strains expressing the fimbriae from the tetA or nirB promoter alone had a significantly lower IgG2a response. Statistically significant higher levels of IgG2a were detected (P<0.05) in the mice immunized with strains expressing the fimbriae from the pagC promoter, whereas statistically significant higher levels of IgG1 were detected (P<0.05) in the mice immunized with strains expressing the fimbriae from the tetA promoter alone. This suggested that mice from the former immunization groups developed a better type 1 helper T-cell (Th1) response to the 987P fimbriae than mice from the latter group.
Fig. 3.
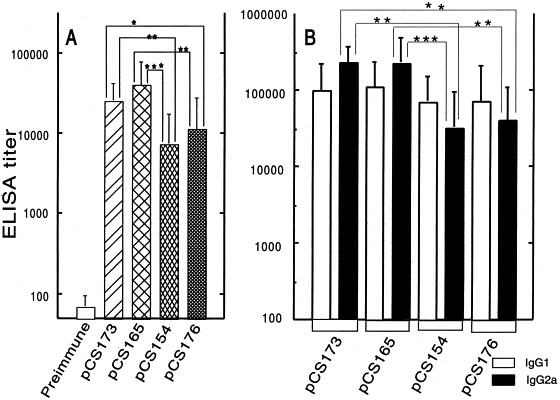
987P fimbriae-specific serum IgG titers (A), and IgG1- (white bars) and IgG2a- (solid bars) subclass titers (B). Balb/c mice were immunized orally with two doses (30-day interval) of S. typhimurium χ4550 containing pCS165, pCS173, pCS154 or pCS176. Sera collected before immunization were used as negative controls. The sera were measured individually and error bars represent the S.D. of the values for 10 mice. IgG titers elicited by S. typhimurium χ4550/pCS173 or χ4550/pCS165 were significantly higher than the titers induced by S. typhimurium χ4550/pCS154 or χ4550/pCS176 (*P<0.05; **P<0.01; ***P<0.001).
3.5. Intestinal humoral immune responses against chimeric fimbriae
The local gut anti-987P fimbriae IgA and IgG responses were measured by analyzing gut washes of immunized mice. Similar to the systemic humoral response described above, the mucosal anti-987P IgA (Fig. 4 A) and IgG (Fig. 4B) titers were much higher in the mice immunized with strain χ4550/pCS165 or χ4550/pCS173 than in the mice immunized with strain χ4550/pCS154 or χ4550/pCS176. There were no significant differences between the χ4550/pCS165- and χ4550/pCS173-immunized groups or between the χ4550/pCS154- and χ4550/pCS176-immunized mice. All the mucosal IgA titers correlated significantly with the serum IgG titers (correlation coefficient [r]=0.66; P<0.001). The kinetics of the mucosal antibody response were evaluated by analyzing extracts of fecal pellets. As expected, the responses were quite variable. After the first vaccination, the mice immunized with strain χ4550/pCS165 or χ4550/pCS173 developed higher mucosal IgA (Fig. 5 A) and IgG (Fig. 5B) responses for 2–3 weeks. After 3 weeks, mucosal antibody titers in most groups declined; however, both the mucosal IgA and IgG titers increased dramatically after the mice were boosted orally at 4 weeks. In all immunized groups, mucosal antibody titers reached a peak at 6 weeks and did not decrease significantly for at least two more weeks when the mice were sacrificed.
Fig. 4.
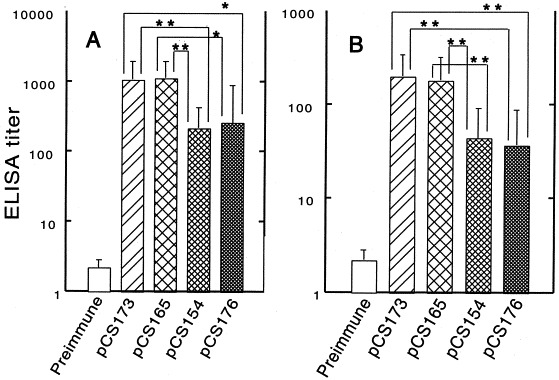
987P fimbriae-specific mucosal IgA (A) and IgG (B) titers. Balb/c mice were immunized orally with two doses (30-day interval) of S. typhimurium χ4550 containing pCS165, pCS173, pCS154 or pCS176. Gut washes were collected before immunization, to be used as negative controls, and at the end of the immunization experiments. The samples were measured individually and error bars represent the S.D. of the values for 10 mice. Mucosal IgA and IgG titers elicited by S. typhimurium χ4550/pCS173 or χ4550/pCS165 were significantly higher than the titers induced by S. typhimurium χ4550/pCS154 or χ4550/pCS176 (*P<0.05; **P<0.01).
Fig. 5.
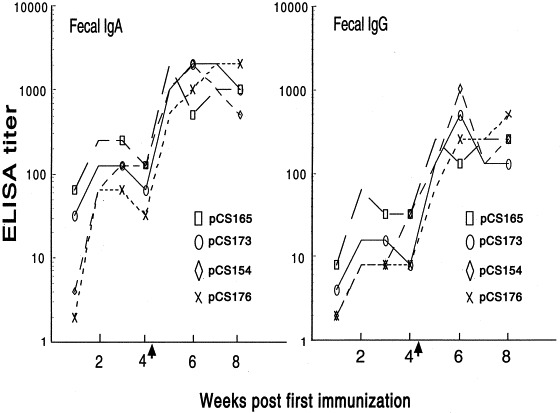
Kinetics of stool IgA (A) and IgG (B) titers. Balb/c mice were immunized orally with two doses (30-day interval) of S. typhimurium χ4550 containing pCS165, pCS173, pCS154 or pCS176. Arrows indicate booster immunization. Stool IgA and IgG were measured from pooled faecal pellets which were collected weekly.
3.6. Humoral immune responses against TGEV
The systemic humoral immune response to the TGEV C and A epitopes displayed by 987P fimbriae on the surface of S. typhimurium χ4550 vaccine strains were assessed by ELISA, dot blot assays and virus seroneutralization tests. Serum ELISA showed that strain χ4550/pCS165 and χ4550/pCS173 induced significantly higher anti-TGEV C peptide IgG titers than strains χ4550/pCS154 and χ4550/pCS176 (Fig. 6 ). All these anti-TGEV C peptide titers correlated significantly with the serum IgG titers against fimbriae (correlation coefficient [r]=0.68; P<0.001). No antibodies against the TGEV A peptide were detectable in the sera of immunized mice as determined by ELISA (data not shown). Most interestingly, 40%, namely eight out of 20 mice immunized with strain χ4550/pCS165 or χ4550/pCS173 developed serum TGEV-neutralizing antibodies, albeit at low titers (1:8 to 1:16). Moreover, these neutralization titers correlated significantly with the TGEV C peptide IgG ELISA titers (correlation coefficient [r]=0.93; P<0.001). In contrast, none of the mice immunized with strain χ4550/pCS154 or χ4550/pCS176 developed a detectable humoral antiviral immune response. Dot blot assays of gut washes showed that strain χ4550/pCS165 and χ4550/pCS173 induced anti-TGEV C peptide IgA in 10 out of 20 mice (50%), with titers reaching 10−2 (data not shown).
Fig. 6.
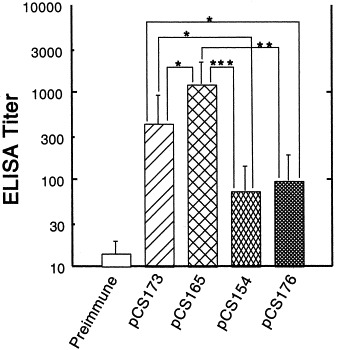
TGEV C epitope-specific serum IgG titers. Balb/c mice were immunized orally with two doses (30-day interval) of S. typhimurium χ4550 containing pCS165, pCS173, pCS154 or pCS176. Sera collected before immunization were used as negative controls. The sera were measured individually and error bars represent the S.D. of the values for 10 mice. IgG titers elicited by S. typhimurium χ4550/pCS165 were significantly higher than the titers induced by any of the three other strains, and IgG titers elicited by S. typhimurium χ4550/pCS173 were significantly higher than the titers induced by χ4550/pCS154 or χ4550/pCS176; (*P<0.05; **P<0.01; ***P<0.001).
4. Discussion
In this paper, we evaluated the use of combining the display of multimeric foreign epitopes on the surface of S. typhimurium with an in vivo-inducible promoter as a delivery system for vaccine development. The pagC promoter was particularly attractive, since it is activated in the environment of phagocytic vacuoles, after S. typhimurium is taken up by macrophages [41]. By genetically engineering asd +-stabilized plasmids expressing chimeric 987P fimbriae on S. typhimurium χ4550 cya crp asd under the control of the pagC promoter, the systemic and mucosal immune responses to the chimeric fimbriae was improved significantly, in comparison to a construct directing antigen expression from a constitutive tetA promoter or from the nirB promoter. Most importantly, and in addition to the finding that the pagC promoter was the most effective one for inducing anti-fimbriae antibodies, TGEV-neutralizing antibodies were induced only with S. typhimurium χ4550 constructs carrying this promoter for antigen expression. We had shown previously that chimeric fimbriae on S. typhimurium induced a better immune response when the fimbriae were expressed from a tandem promoter, where the in vivo-inducible nirB promoter was added to the constitutively-expressed tetA promoter (χ4550/pCS155) [20]. In this study, the new vaccine construct with the pagC promoter alone induces higher immune responses than the vaccine using the nirB promoter together with the tetA promoter [20]. Moreover, the immune response was improved by using a construct combining the pagC and tetA (χ4550/pCS165) promoters. Curiously, the consistent slight improvement in immune responses we observed with the pagC and tetA construct were statistically significant only for the TGEV C epitope, but not for the fimbrial antigen itself. Consistent with previous studies, the TGEV A epitope was not an effective immunogen for Balb/c mice [18], [20].
Among the various in vivo-inducible promoters, including the nirB, htrA, groE, osmC, katG, spv, dps and pagC promoters, which have been investigated to improve the immunogenicity of heterologous antigens expressed by Salmonella vectors in the last decade [31], [32], [42], [43], [44], the pagC promoter appears to be the most efficient one. This promoter is regulated through the PhoP/PhoQ two-component signal transduction system of Salmonella that senses environmental Mg2+ and Ca2+ concentrations [45], [46]. The pagC promoter is activated at relatively low concentrations of Mg2+, a condition which is found within Salmonella-containing vacuoles in macrophages [47], [48], [49]. As shown previously for single heterologous protein antigens [31], [45], it was demonstrated here that the pagC promoter can also drive 987P fimbrial expression in response to a low Mg2+ concentration, and thus activates 987P fimbriation in macrophages. In Salmonella, the pagC gene encodes an outer membrane protein which is essential for full bacterial virulence in Balb/c mice and is highly expressed in vivo. Thus, the observed induction of the neutralizing anti-TGEV immune response can most probably be attributed to the expression of large amounts of TGEV peptide antigen on the Salmonella and to the intracellular location of the bacteria in professional antigen-presenting cells.
It has been shown recently that fimbriae expressed by Salmonella vaccine strains stimulate a mixed T helper cell response [20], [50], [51]. Interestingly, a comparison of the anti-987P IgG1/IgG2a ratios of the animals immunized with strains χ4550/pCS165 or χ4550/pCS173 with the ratios of the animals immunized with strains χ4550/pCS154 or χ4550/pCS176 suggests that the former groups developed a biased Th1 response, while the latter groups developed a predominant Th2 response [50], [51], [52], [53]. Moreover, stronger mucosal antibody responses were developed in the former groups. Taken together, the data suggest that the pagC promoter is more appropriate than the nirB or tetA promoter for expressing antigens when a polarized Th1 response or a strong mucosal response is required for immunity, although more in-depth studies, including cytokine profiling, will be required for confirmation.
Compared to other expression methods for heterologous antigen display on Salmonella vaccines, the 987P fimbrial display system has several advantages that favor its use. First, fimbriae are polymeric proteins which allow inserted foreign epitopes to be presented hundreds of times along fimbrial threads, which themselves are numbered in the hundreds on the bacterial surface. Second, because enteroadhesion of 987P fimbriae is essentially mediated by its minor subunit FasG [28], [54], the major subunit FasA can be genetically modified as a carrier molecule without affecting the enteroadhesive property of the fimbriae [19]. Having demonstrated previously that the FasA subunit can be used as a carrier of protective epitopes of TGEV [19] and that Salmonella vaccine strains can be genetically manipulated to express the chimeric fimbriae [20], we combined these approaches with the beneficial aspects of in vivo expression by the pagC promoter as a novel strategy for designing better vaccines. The developed system was found to be efficient, since anti-TGEV neutralizing antibodies were detected for the first time after oral administration of a Salmonella vector delivering a TGEV immunogen. Moreover, the observed high titers of mucosal and systemic anti-987P antibodies, as well as the high titers of anti-Salmonella antibodies (data not shown), demonstrated the benefits of our combined approach to develop a multivalent vaccine targeting simultaneously major enteropathogens. It will be of interest to test whether the system can be developed further to display protective epitopes derived from other swine pathogens, such as rotavirus and foot-and-mouth disease virus. Most importantly, future studies will have to be undertaken in the pig to confirm our findings in the relevant host.
Acknowledgements
We are grateful to Dr Roy Curtiss, III for providing bacterial strains, Dr Linda Saif for providing the TGEV Purdue-115 strain, Dr Howard Goldfine for providing J774A.1 cells, and Dr Ronald Harty for critically reading the manuscript. This research was supported by USDA grant (#980-2623). H. Chen was partially supported by a Fellowship from the Nanjing Agricultural University and Ministry of Agriculture, China.
References
- 1.Cavanagh D. Nidovirales: a new order comprising Coronaviridae and Arteriviridae. Arch. Virol. 1997;142:629–633. [PubMed] [Google Scholar]
- 2.Saif L.J. Enteric viral infections of pigs and strategies for induction of mucosal immunity. Adv. Vet. Med. 1999;41:429–446. doi: 10.1016/S0065-3519(99)80033-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saif L.J., Jackwood D.J. Enteric virus vaccines: theoretical considerations, current status, and future approaches. In: Saif L.J., Theil K.W., editors. Viral Diarrhea of Man and Animals. CRC Press; Boca Raton, FL: 1990. pp. 313–329. [Google Scholar]
- 4.Butler J.E. Immunoglobulins and immunocytes in animal milks. In: Ogra P., Mestecky J., Lamm M., Strober W., Bienenstock J., McGhee J.R., editors. Mucosal Immunology. 2nd edn. Academic Press; New York: 1999. pp. 1531–1554. [Google Scholar]
- 5.Enjuanes L., Van der Zeijst B.A.M. Molecular basis of transmissible gastroenteritis virus epidemiology. In: Siddell S.G., editor. The Coronaviridae. Plenum; New Yoek: 1995. pp. 337–376. [Google Scholar]
- 6.Enjuanes L., Smerdou C., Castilla J. Development of protection against coronavirus induced diseases. A review. Adv. Exp. Med. Biol. 1995;380:197–211. doi: 10.1007/978-1-4615-1899-0_34. [DOI] [PubMed] [Google Scholar]
- 7.Delmas B., Rasschaert D., Godet M., Gelfi J., Laude H. Four major antigenic sites of the coronavirus transmissible gastroenteritis virus are located on the amino-terminal half of spike glycoprotein S. J. Gen. Virol. 1990;71:1313–1323. doi: 10.1099/0022-1317-71-6-1313. [DOI] [PubMed] [Google Scholar]
- 8.Delmas B., Gelfi J., Laude H. Antigenic structure of transmissible gastroenteritis virus. II. Domains of the peplomer glycoprotein. J. Gen. Virol. 1986;67:1405–1418. doi: 10.1099/0022-1317-67-7-1405. [DOI] [PubMed] [Google Scholar]
- 9.Delmas B., Gelfi J., L'Haridon R. Aminopeptidase N is a major receptor for the enteropathogenic coronavirus TGEV. Nature. 1992;357:417–420. doi: 10.1038/357417a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gebauer F., Posthumus W.P., Correa I. Residues involved in the antigenic sites of transmissible gastroenteritis coronavirus S glycoprotein. Virology. 1991;183:225–238. doi: 10.1016/0042-6822(91)90135-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pensaert M., Van Reeth K. Vaccines for swine. In: Pastoret P.-P., Blancou J., Vannier P., Verschueren C., editors. Veterinary Vaccinology. Elsevier; New York: 1997. pp. 372–394. [Google Scholar]
- 12.Saif L.J. Mucosal immunity: an overview and studies of enteric and respiratory coronavirus infections in a swine model of enteric disease. Vet. Immunol. Immunopathol. 1996;54:163–169. doi: 10.1016/S0165-2427(96)05702-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shoup D.I., Jackwood D.J., Saif L.J. Active and passive immune response to transmissible gastroenteritis virus (TGEV) in swine inoculated with recombinant baculovirus-expressed TGEV spike glycoproteins vaccines. Am. J. Vet. Res. 1997;58:242–250. [PubMed] [Google Scholar]
- 14.Sestak K., Meister R.K., Hayes J.R. Active immunity and T-cell populations in pigs intraperitoneally inoculated with baculovirus-expressed transmissible gastroenteritis virus structural proteins. Vet. Immunol. Immunopathol. 1999;70:203–221. doi: 10.1016/S0165-2427(99)00074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torres J.M., Alonso C., Ortega A., Mittal S., Graham F., Enjuanes L. Tropism of human adenovirus type 5-based vectors in swine and their ability to protect against transmissible gastroenteritis coronavirus. J. Virol. 1996;70:3770–3780. doi: 10.1128/jvi.70.6.3770-3780.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smerdou C., Urniza A., Curtiss I.I.I.R., Enjuanes L. Characterization of transmissible gastroenteritis coronavirus S protein expression products in avirulent S. typhimurium Δcya Δcrp persistence, stability and immune response in swine. Vet. Microbiol. 1996;48:87–100. doi: 10.1016/0378-1135(95)00141-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smerdou C., Antón I.M., Plana J., Curtiss I.I.I.R., Enjuanes L. A continuous epitope from transmissible gastroenteritis virus S protein fused to E. coli heat-labile toxin B subunit expressed by attenuated Salmonella induces serum and secretory immunity. Virus Res. 1996;41:1–9. doi: 10.1016/0168-1702(95)01265-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Der Vartanian M., Girardeau J.-P., Martin C. An Escherichia coli CS31A fimbrillum chimera capable of inducing memory antibodies in outbred mice following booster immunization with the entero-pathogenic coronavirus gastroenteritis virus. Vaccine. 1997;15:111–120. doi: 10.1016/S0264-410X(96)00172-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rani D.B.R., Bayer M.E., Schifferli D.M. Polymeric display of immunogenic epitopes from herpes simplex virus and transmissible gastroenteritis virus surface proteins on an enteroadherent fimbria. Clin. Diagn. Lab. Immunol. 1999;6:30–40. doi: 10.1128/cdli.6.1.30-40.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen H., Schifferli D.M. Mucosal and systemic immune responses to chimeric fimbriae expressed by Salmonella vaccine strains. Infect. Immun. 2000;66:3129–3139. doi: 10.1128/iai.68.6.3129-3139.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finlay B.B., Falkow S. Salmonella as an intracellular parasite. Mol. Microbiol. 1989;3:1833–1841. doi: 10.1111/j.1365-2958.1989.tb00170.x. [DOI] [PubMed] [Google Scholar]
- 22.Jones B.D., Ghori N., Falkow S. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer's patches. J. Exp. Med. 1994;180:15–23. doi: 10.1084/jem.180.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones B.D., Falkow S. Salmonellosis: host immune responses and bacterial virulence determinants. Annu. Rev. Immunol. 1996;14:533–561. doi: 10.1146/annurev.immunol.14.1.533. [DOI] [PubMed] [Google Scholar]
- 24.Marriott I., Hammond T.G., Thomas E.K., Bost K.L. Salmonella efficiently enter and survive within cultured CD11c+ dendritic cells initiating cytokine expression. Eur. J. Immunol. 1999;29:1107–1115. doi: 10.1002/(SICI)1521-4141(199904)29:04<1107::AID-IMMU1107>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 25.Hopkins S.A., Niedergang F., Corthesy-Theulaz I.E., Kraehenbuhl J.-P. A recombinant Salmonella typhimurium vaccine strain is taken up and survives within murine Peyer's patch dendritic cells. Cell. Microbiol. 2000;2:59–68. doi: 10.1046/j.1462-5822.2000.00035.x. [DOI] [PubMed] [Google Scholar]
- 26.Sirard J.C., Niedergang F., Kraehenbuhl J.P. Live attenuated Salmonella: a paradigm of mucosal vaccines. Immunol. Rev. 1999;171:5–26. doi: 10.1111/j.1600-065x.1999.tb01340.x. [DOI] [PubMed] [Google Scholar]
- 27.Fortier A., Falk L.A. Isolation of murine macrophages. In: Coligen J.E., Kruisbeek A.M., Margulies D.H., Shevach E.M., Strober W., editors. Current Protocols in Immunology. Wiley; New York: 1994. pp. 14.1.1–14.1.6. [Google Scholar]
- 28.Khan A.S., Schifferli D.M. A minor 987P protein different from the structural fimbrial subunit is the adhesin. Infect. Immun. 1994;62:4233–4243. doi: 10.1128/iai.62.10.4233-4243.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schifferli D.M., Abraham S.N., Beachey E.H. Use of monoclonal antibodies to probe subunit- and polymer specific epitopes of 987P fimbriae of Escherichia coli. Infect. Immun. 1987;55:923–930. doi: 10.1128/iai.55.4.923-930.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rhen M., Riikonen P., Taira S. Transcriptional regulation of Salmonella enterica virulence plasmid genes in cultured macrophages. Mol. Microbiol. 1993;10:45–56. doi: 10.1111/j.1365-2958.1993.tb00902.x. [DOI] [PubMed] [Google Scholar]
- 31.Dunstan S.J., Simmons C.P., Strugnell R.A. Use of in vivo-regulated promoters to deliver antigens from attenuated Salmonella enterica var. Typhimurium. Infect. Immun. 1999;67:5133–5141. doi: 10.1128/iai.67.10.5133-5141.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberts M., Li J., Bacon A., Chatfield S. Oral vaccination against tetanus: comparison of the immunogenicities of Salmonella strains expressing fragment C from the nirB and htrA promoters [published erratum appears in Infect Immun 1999;67(1):468] Infect. Immun. 1998;66:3080–3087. doi: 10.1128/iai.66.7.3080-3087.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang L.Z., Gong Y.F., Fang Y., Zhang Y.S., Gu F.S. Use of microwaves in immunoenzyme techniques. Clin. Chem. 1993;39:2021. [PubMed] [Google Scholar]
- 34.Laude H., Chapsal J.M., Gelfi J., Labiau S., Grosclaude J. Antigenic structure of transmissible gastroenteritis virus. I. Properties of monoclonal antibodies directed against virion proteins. J. Gen. Virol. 1986;67:119–130. doi: 10.1099/0022-1317-67-1-119. [DOI] [PubMed] [Google Scholar]
- 35.Mittrücker H.W., Köhler A., Mak T.W., Kaufmann S.H. Critical role of CD28 in protective immunity against Salmonella typhimurium. J. Immunol. 1999;163:6769–6776. [PubMed] [Google Scholar]
- 36.Galán J.E., Nakayama K., Curtiss I.I.I.R. Cloning and characterization of the asd gene of Salmonella typhimurium: use in stable maintenance of recombinant plasmids in Salmonella vaccine. Gene. 1990;94:29–35. doi: 10.1016/0378-1119(90)90464-3. [DOI] [PubMed] [Google Scholar]
- 37.Nakayama K., Kelly S.M., Curtiss I.I.I.R. Construction of an Asd+ expression vector: stable maintenance and high expression of cloned genes in Salmonella vaccine strain. Bio/technology. 1988;6:693–697. [Google Scholar]
- 38.Unger B., Becker J., Hillen W. Nucleotide sequence of the gene, protein purification and characterization of the pSC101-encoded tetracycline resistance-gene-repressor. Gene. 1984;31:103–108. doi: 10.1016/0378-1119(84)90199-9. [DOI] [PubMed] [Google Scholar]
- 39.Oxer M.D., Bentley C.M., Doyle J.G., Peakman T.C., Charles I.G., Makoff A.J. High level heterologous expression in E. coli using the anaerobically-activated nirB promoter. Nucleic Acids Res. 1991;19:2889–2892. doi: 10.1093/nar/19.11.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soncini F.C., Véscovi G.E., Solomon F., Groisman E.A. Molecular basis of the magnesium deprivation response in Salmonella typhimurium: Identification of PhoP-regulated genes. J. Bacteriol. 1996;178:5092–5099. doi: 10.1128/jb.178.17.5092-5099.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller S.I. PhoP/PhoQ: macrophage-specific modulators of Salmonella virulence? Mol. Microbiol. 1991;5:2073–2078. doi: 10.1111/j.1365-2958.1991.tb02135.x. [DOI] [PubMed] [Google Scholar]
- 42.Everest P., Frankel G., Li J., Lund P., Chatfield S., Dougan G. Expression of LacZ from the htrA, nirB and groE promoters in a Salmonella vaccine strain: influence of growth in mammalian cells. FEMS Microbiol. Lett. 1995;126:97–102. doi: 10.1111/j.1574-6968.1995.tb07398.x. [DOI] [PubMed] [Google Scholar]
- 43.McSorley S.J., Xu D., Liew F.Y. Vaccine efficacy of Salmonella strains expressing glycoprotein 63 with different promoters. Infect. Immun. 1997;65:171–178. doi: 10.1128/iai.65.1.171-178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marshall D.G., Haque A., Fowler R. Use of the stationary phase inducible promoters, spv and dps, to drive heterologous antigen expression in Salmonella vaccine strains. Vaccine. 2000;18:1298–1306. doi: 10.1016/s0264-410x(99)00417-x. [DOI] [PubMed] [Google Scholar]
- 45.García Véscovi E., Soncini F.C., Groisman E.A. Mg++ as an extracellular signal: Environmental regulation of Salmonella virulence. Cell. 1996;84:165–174. doi: 10.1016/s0092-8674(00)81003-x. [DOI] [PubMed] [Google Scholar]
- 46.Vescovi E.G., Ayala Y.M., Di Cera E., Groisman E.A. Characterization of the bacterial sensor protein PhoQ. Evidence for distinct binding sites for Mg2+ and Ca2+ J. Biol. Chem. 1997;272:1440–1443. doi: 10.1074/jbc.272.3.1440. [DOI] [PubMed] [Google Scholar]
- 47.Alpuche-Aranda C.M., Swanson J.A., Loomis W.P., Miller S.I. Salmonella typhimurium activates virulence gene transcription within acidified macrophage phagosomes. Proc. Natl. Acad. Sci. USA. 1992;89:10079–10083. doi: 10.1073/pnas.89.21.10079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Groisman E.A. The ins and outs of virulence gene expression: Mg2+ as a regulatory signal. Bioessays. 1998;20:96–101. doi: 10.1002/(SICI)1521-1878(199801)20:1<96::AID-BIES13>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 49.Hohmann E.L., Oletta C.A., Loomis W.P., Miller S.I. Macrophage-inducible expression of a model antigen in Salmonella typhimurium enhances immunogenicity. Proc. Natl. Acad. Sci. USA. 1995;92:2904–2908. doi: 10.1073/pnas.92.7.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ascon M.A., Hone D.M., Walters N., Pascual D.W. Oral immunization with a Salmonella typhimurium vaccine vector expressing recombinant enterotoxigenic Escherichia coli K99 fimbriae elicits elevated antibody titers for protective immunity. Infect. Immun. 1998;66:5470–5476. doi: 10.1128/iai.66.11.5470-5476.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pascual D.W., Hone D.M., Hall S. Expression of recombinant enterotoxigenic Escherichia coli colonization factor antigen I by Salmonella typhimurium elicits a biphasic T helper cell response. Infect. Immun. 1999;67:6249–6256. doi: 10.1128/iai.67.12.6249-6256.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dunstan S.J., Simmons C.P., Strugnell R.A. Comparison of the abilities of different attenuated Salmonella typhimurium strains to elicit humoral immune responses against a heterologous antigen. Infect. Immun. 1998;66:732–740. doi: 10.1128/iai.66.2.732-740.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stevens T.L., Bossie A., Sanders V.M. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature. 1988;334:255–258. doi: 10.1038/334255a0. [DOI] [PubMed] [Google Scholar]
- 54.Choi B.-K., Schifferli D.M. Lysine residue 117 of the FasG adhesin of enterotoxigenic Escherichia coli is essential for binding of 987P fimbriae to sulfatide. Infect. Immun. 1999;67:5755–5761. doi: 10.1128/iai.67.11.5755-5761.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schödel F., Kelly S.M., Peterson D.L., Milich D.R., Curtiss R., 3rd Hybrid hepatitis B virus core-pre-S proteins synthesized in avirulent Salmonella typhimurium and Salmonella typhi for oral vaccination. Infect. Immun. 1994;62:1669–1676. doi: 10.1128/iai.62.5.1669-1676.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]


