Abstract
The association of Mycoplasma cynos with canine infectious respiratory disease is increasingly being recognised. This study describes the strain typing of 14 M. cynos isolates cultured from trachea and bronchoalveolar lavage samples of six dogs with respiratory disease, from two separate kennels in the United Kingdom. The genetic similarity of the isolates was investigated using pulsed-field gel electrophoresis (PFGE) and random amplified polymorphic DNA (RAPD). Most of the isolates from four dogs housed at a re-homing kennel were genetically similar and some isolates from different dogs were indistinguishable by both PFGE and RAPD. These isolates were cultured from dogs with non-overlapping stays in the kennel, which may indicate maintenance of some strains within kennels. A small number of isolates showed much greater genetic heterogeneity and were genetically distinct from the main group of M. cynos strains. There was also a high degree of similarity of the M. cynos type strain (isolated from a dog with respiratory disease in Denmark in 1971) to at least one of the United Kingdom isolates using PFGE analysis, which may suggest possible conservation of pathogenic strains of M. cynos.
Keywords: Mycoplasma cynos, Canine infectious respiratory disease (CIRD), Kennel cough, PFGE, RAPD
1. Introduction
Canine infectious respiratory disease (CIRD or kennel cough) is a multifactorial disease complex and the agents traditionally associated with this disease are Bordetella bronchiseptica, canine parainfluenza virus (CPIV), canine adenovirus (CAV), and canine herpesvirus (CHV). Recently, a novel canine respiratory coronavirus (CRCoV; Erles et al., 2003) and Streptococcus equi subsp. zooepidemicus (Chalker et al., 2003a) have also been found to be associated with the disease.
Within this microbial complex, Mycoplasma spp. are found to be ubiquitous in the upper respiratory tract of dogs and are thought to be normal flora (Rosendal, 1982, Randolph et al., 1993). However, mycoplasmas have also been the sole bacterial isolate in a number of clinical cases of canine respiratory disease, but unfortunately these isolates were not speciated and viral causes of CIRD were not investigated (Kirchner et al., 1990, Jameson et al., 1995, Chandler and Lappin, 2002). The involvement of M. cynos in CIRD has been noted for some time (Rosendal, 1972, Rosendal, 1978, Rosendal, 1982). Evidence for this has been mounting recently, as Chalker et al. (2004) found that M. cynos was the only mycoplasma significantly associated with canine respiratory disease. In addition, dogs entering a re-homing kennel that developed an antibody response to M. cynos were more likely to suffer respiratory disease (Rycroft et al., 2007). M. cynos has been isolated from dogs with pneumonia (Rosendal, 1972, Rosendal, 1978, Chvala et al., 2007) and was particularly abundant in the most necrotic areas of the lung (Chvala et al., 2007). Furthermore, M. cynos was the only detected agent in a case of severe bronchopneumonia in a litter of young puppies which resulted in the deaths of some puppies, but which was resolved in the surviving littermates after the administration of appropriate antibiotics (Zeugswetter et al., 2007).
Recently molecular epidemiological studies of isolates of the Mycoplasma species M. bovis (Kusiluka et al., 2000, McAuliffe et al., 2004), M. ovipneumoniae (Parham et al., 2006) M. gallisepticum and M. synoviae (Feberwee et al., 2005) have been conducted using the genetic typing techniques amplified fragment length polymorphism (AFLP), random amplified polymorphic DNA (RAPD) and pulsed-field gel electrophoresis (PFGE). This is the first genetic typing study performed on M. cynos.
2. Materials and methods
2.1. Mycoplasma cynos isolates
M. cynos isolates cultured from respiratory samples from dogs with moderate to severe respiratory disease were identified from an earlier large study. Isolation and identification of these isolates has been previously described (Chalker et al., 2004). Briefly, bronchoalveolar lavage (BAL) and trachea samples were obtained from euthanized dogs from a re-homing centre with a history of endemic CIRD (population A). Alternately, BAL samples were taken from dogs with persistent coughs at a training centre (population B). Dogs were graded for respiratory signs prior to sampling or euthanasia. M. cynos was cultured on Mycoplasma media (Mycoplasma Experience) and identified by PCR specific for the 16S/23S rRNA intergenic spacer region. Cultures of the single-cloned M. cynos isolates were stored frozen at −70 °C.
The type strain M. cynos H381 NCTC10142 was obtained from the National Collection of Type Cultures (NCTC), Collindale, London.
2.2. Bacterial and viral screening
Bacteriological screening of the samples has been previously described (Chalker et al., 2003a, Chalker et al., 2003b). Briefly, BAL and trachea samples were inoculated onto MacConkey agar and two blood agar plates (incubated aerobically and anaerobically) and incubated at 37 °C. Gram positive, catalase negative, beta-haemolytic colonies were identified as streptococci and sero-grouped into Lancefield Groups, then identified to the species level with API 20STREP (Biomerieux). Oxidase positive colonies with typical B. bronchiseptica growth characteristics were identified as such with API 20NE.
Virus screening of the samples has been previously described (Erles et al., 2004). Briefly, RNA and DNA were extracted from the respiratory tissue samples and PCR and reverse transcription-PCR were used to detect CPIV, canine herpesvirus (CHV), CAV, canine distemper virus (CDV), and CRCoV. In addition, RT-PCR for canine influenza virus (CIV) was carried out using primers AMP227F and AMP622R directed to the M gene (Ellis and Zambon, 2001). Equine influenza virus (H3N8) served as a positive control.
2.3. Pulsed-field gel electrophoresis
Aliquots (20 ml) of stationary phase M. cynos culture (maximum absorbance A 600 of approximately 0.3) were used for PFGE analysis. Cells were harvested by centrifugation (3500 × g for 20 min at 4 °C), washed three times with PBS buffer with 10% (w/v) glucose and resuspended in 300 ml cold PBS/glucose buffer. Agarose plugs were made from a 1:1 mixture of 2% low-melting-point agarose (Biorad) and the cell suspension. Plugs were incubated in lysis buffer (10 mM Tris–HCl, 1 mM EDTA, 1% lauroyl sarcosine, 1 mg/ml proteinase K) for 48 h at 56 °C. Plugs were washed four times with Tris–EDTA buffer for 30 min at 4 °C. Slices (2 mm) were cut aseptically from plugs and equilibrated in restriction buffer (Promega) for 1 h. Subsequently, restriction digestion was performed by using 30 U of SmaI (Promega) for 16 h according to the manufacturer’s instructions. The fragments were resolved on 1% pulsed field certified agarose (Biorad) gels using a CHEF-DRIII system (Biorad) at 6 V/cm, with a running time of 20 h at 14 °C; included angle of 120°; initial pulse time of 4 s; final pulse time of 40 s. Gels were stained with ethidium bromide (0.5 mg/ml) for 15 min, destained in distilled water for 1 h and photographed under UV light. A lambda ladder PFGE marker (Sigma) was used for fragment size determination. The Bionumerics package (Applied Maths) was used for gel analysis and dendrograms were produced using the Jaccard Coefficient and unweighted pair group method using arithmetic averages (UPGMA) cluster analysis.
2.4. RAPD
The single primer Hum4 5′-ACGGTACACT-3′ (Hotzel et al., 1998) was used for the generation of RAPD profiles. Amplification was performed in a 50-μl total reaction volume containing 100 ng of DNA sample, 10 mM Tris–HCl (pH 9.0), 1.5 mM MgCl2, 50 mM KCl, 0.1% Triton X-100, 0.2 mM each deoxynucleoside triphosphate, and 0.5 U of TaqGold (PerkinElmer). Cycling conditions included an initial denaturation step at 94 °C for 5 min, followed by 40 cycles of 94 °C for 15 s, 37 °C for 60 s and 72 °C for 90 s. The last cycle included a final elongation at 72 °C for 7 min. PCR products were resolved by electrophoresis on 10 cm 2% agarose gels at 60 mA for 1.5 h, stained with ethidium bromide and visualized under UV illumination. The Bionumerics package (Applied Maths) was used for gel analysis and dendrograms were produced using the Jaccard Coefficient and unweighted pair group method using arithmetic averages (UPGMA) cluster analysis.
3. Results
3.1. Dogs
Six dogs with moderate to severe respiratory disease from which M. cynos was isolated were identified from an earlier large study (Chalker et al., 2004). Four dogs were housed at a re-homing centre with a history of endemic CIRD (population A) and two dogs at a training centre (population B). All six dogs had respiratory disease with symptoms of either bronchopneumonia (respiratory score 5) or cough and nasal discharge (score 3). Trachea and/or BAL samples were taken from the dogs within 4 weeks of the first symptoms of CIRD. The dogs were 1–3 years old and of various breeds. The group consisted of entire and neutered males and females (Table 1 ).
Table 1.
Details of dogs with respiratory disease from which M. cynos isolates were cultured, and bacteriology and virology screening results of trachea (T) and bronchoalveolar lavage (BAL) samples.
| Doga | Age (years) | Sexb | Breed | Respiratory scorec | Date sampled | Bacteriology | Virology |
|---|---|---|---|---|---|---|---|
| A-1 | 1 | MN | German shepherd | 3 | 3 June 1999 | B. bronchiseptica and M. spumans in BAL; M. cynos in BAL and T | Negative |
| A-2 | 2 | M | Staffordshire bull terrier | 3 | 3 June 1999 | B. bronchiseptica and Pasteurella spp. in BAL; M. cynos in BAL and T | CRCoV in T |
| A-3 | 2 | M | Dalmatian | 5 | 11 October 1999 | S. equi subsp. zooepidemicus in BAL; M. cynos and M. spumans in BAL and T | Negative |
| A-4 | 3 | FN | Mongrel | 5 | 24 February 2000 | S. equi subsp. zooepidemicus, Enterococcus spp., Ureaplasma spp. and Escherichia coli in BAL; M. canis and M. cynos in BAL and T | CHV in BAL |
| B-1 | 1 | MN | Labrador | 5 | 8 November 2000 | M. cynos in BALd | Negatived |
| B-2 | 1 | F | Labrador | 3 | 23 November 2000 | M. cynos in BALd | Negatived |
A: re-homing centre; B: training centre.
N: neutered.
3: cough and nasal discharge; 5: bronchopneumonia.
No trachea sample available.
3.2. Bacteriology and virology screening
M. cynos was cultured from the BAL of each dog and also the trachea where that sample was available (Table 1, Table 2 ).
Table 2.
Mycoplasma cynos isolate source and genetic typing groups.
| Dog | M. cynos isolate # | Source | PFGE group | RAPD group |
|---|---|---|---|---|
| A-1 | 185 | BAL | 1 | 1 |
| 210 | BAL | 1 | 1 | |
| 214 | BAL | 3 | 2 | |
| 253 | BAL | 1 | 1 | |
| 428 | T | 1 | 1 | |
| 429 | T | 1 | 1 | |
| A-2 | 190 | BAL | 1 | 1 |
| 417 | T | 1 | 1 | |
| A-3 | 191 | BAL | 1 | 1 |
| A-4 | 312 | BAL | 1 | 1 |
| 387 | T | 1 | 1 | |
| B-1 | 491 | BAL | 2 | 2 |
| B-2 | 492 | BAL | 2 | 2 |
| 510 | BAL | 3 | 1 | |
Testing of BAL samples from the two dogs from the training centre (B-1 and B-2) was negative for the viruses CRCoV, CHV, CPIV, CAV, CDV and CIV. In addition, these samples yielded no bacterial growth except that of M. cynos. In comparison, the four dogs from the re-homing kennel had other bacteria cultured from the respiratory samples (see Table 1).
3.3. PFGE analysis of M. cynos isolates
PFGE analysis of the M. cynos type strain and the 14 isolates from dogs with respiratory disease, resulted in six different PFGE profiles (Fig. 1 ). The PFGE profiles consisted of 3–5 DNA bands, which ranged in size between approximately 6 and 425 kb. The PFGE profiles of the isolates can be divided by similarity into three groups. Group 1 contains 10 isolates and it is a genetically homogeneous group with at least 78% similarity; isolates 185, 190, 191, 210, 253, 312, 387, 417, 428, and 429 all form this group. These are all of the isolates from the population A dogs except isolate 214 from dog A-1.
Fig. 1.
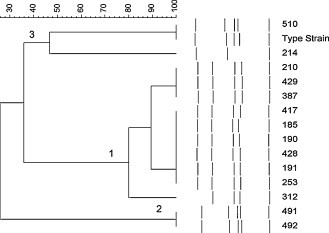
Similarity analysis of the SmaI PFGE profiles of the 14 M. cynos canine respiratory isolates from six dogs. Numbers 1–3 denote the groupings of similar profiles.
Group 2 contains 491 and 492 and these isolates are indistinguishable from each other but quite distinct to all the other isolates with only 28% similarity by cluster analysis. These isolates are from two different dogs from the training centre population (dogs B-1 and B-2).
The third group contains the type strain and isolates 510 and 214. The type strain and 510 are indistinguishable from each other, but 214 is distinctly different with only about 46% similarity to the other two. Isolate 510 was from dog B-2 while isolate 214 was from dog A-1.
3.4. RAPD analysis of M. cynos isolates
When the same M. cynos isolates were subjected to analysis with RAPD with the primer Hum4, 12 different profiles were obtained (Fig. 2 ). The profiles consisted of 3–13 bands which ranged in size between approximately 240 and 2200 bp. Two broad groups of similar isolates were formed. The type strain and the 11 isolates 185, 190, 191, 210, 253, 312, 387, 417, 428, 429 and 510 had similar profiles and are considered to be a homogeneous group with more than 68% similarity (group 1). This group comprises all of the isolates from the population A dogs, except for isolate 214, but also includes 510 from dog B-2 and the type strain.
Fig. 2.
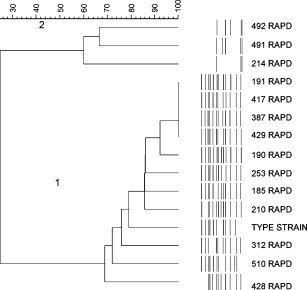
Similarity analysis of the profiles produced by RAPD with the primer Hum4. Numbers 1 and 2 denote the groupings of similar profiles.
Isolates 214, 491 and 492 formed a heterogeneous group about 60% similar to each other, but only about 26% similar to the group 1 isolates. These isolates are from dogs B-1, B-2 and isolate 214 from dog A-1.
The PFGE and RAPD grouping of isolates is summarised in Table 2.
4. Discussion
This is the first genetic typing study of M. cynos. The isolates from each kennel were found to be genetically similar. Indeed, isolates from dogs that had been housed in the same kennel 4 and 8 months apart were found to be indistinguishable using both genetic analysis methods (isolates 417 and 191 from dogs A-2 and A-3, and isolates 429 and 387 from dogs A-1 and A-4, respectively). The dogs had stayed at the kennels for between 8 and 16 days. This may suggest that there is maintenance of M. cynos strains within a kennel situation. M. cynos can be isolated from the upper respiratory tract of healthy dogs (Chalker et al., 2004) and it is probable that some strains are passed between subsequent dogs, resulting in the survival of these strains. In addition, environmental survival may aid the continued existence of some strains. Although the environmental survival of M. cynos is not known, the environmental survival of other mycoplasma species varies from a week to several months (Nagatomo et al., 2001) and M. cynos can be isolated from the air (Chalker et al., 2004). Recently it has been shown that biofilm formation is important for persistence of mycoplasmas and may aid environmental survival (McAuliffe et al., 2006), it seems feasible that M. cynos may be able to persist in the kennel environment as an adherent biofilm layer.
The M. cynos type strain was isolated from the lung of a dog with CIRD in Denmark in 1971 (Rosendal, 1973). This M. cynos type strain was indistinguishable by PFGE to isolate 510 from dog B-2 and was more than 68% similar by RAPD analysis to 11 of the M. cynos isolates from both kennels. The high degree of similarity of the type strain to these United Kingdom isolates from 1999 to 2000 suggests a low level of diversity of this organism in CIRD. However, this study also shows that some isolates have a relatively low level of similarity with each other (for example isolates 214, 491 and 492 appear to be dissimilar to the group 1 isolates). Indeed, this study suggests the potential for mixed M. cynos infections, as the same bronchoalveolar lavage sample from dog B-2 yielded M. cynos isolates 492 and 510, which are dissimilar strains. Similarly, the BAL sample from dog A-1 resulted in the culture of the 214 isolate which was dissimilar to the other isolates from this sample. A larger strain typing study of more isolates is required to consolidate these observations.
M. cynos was the only CIRD agent detected in two out of the six dogs (dogs B-1 and B-2). Similarly, recently Zeugswetter et al. (2007) described lethal bronchopneumonia in puppies where M. cynos was the only CIRD agent detected from the puppies. Mycoplasmas have been the sole bacterial isolate in a number of other cases of CIRD, but unfortunately these isolates were not speciated (Kirchner et al., 1990, Jameson et al., 1995, Chandler and Lappin, 2002). However, in the current study, both dogs were on a course of antibiotics preceding the sampling date (dog B-1 cephalosporin; B-2 erythromycin), which may have precluded the isolation of other bacterial agents. Likewise, in the case of Zeugswetter et al. (2007), the puppies had been treated with amoxicillin prior to isolation of M. cynos from the lung tissue.
M. cynos has also been previously implicated in canine respiratory disease along with other bacterial or viral pathogens (Rosendal, 1978, Chalker et al., 2004, Chvala et al., 2007). This was also found in the current study as other respiratory pathogens apart from M. cynos were detected in the four dogs from the re-homing kennel, for example B. bronchiseptica, S. equi subsp. zooepidemicus, CHV and CRCoV. Multi-pathogen respiratory disease is commonly reported and it has been suggested that the pathogens may interact synergistically to produce disease (Randolph et al., 1993).
Escherichia coli, which was detected in one dog in the present study, has been previously isolated from BAL from a puppy with CIRD and was thought to be a contaminant (Williams et al., 2006). This is likely to be the case in this study as Enterococcus spp. was co-isolated from the same sample. In addition, M. spumans was isolated from two dogs, one of which also had M. canis and Ureaplasma spp., however, these species were not found to be significantly associated with respiratory disease in dogs (Chalker et al., 2004).
In summary, the PFGE and RAPD genetic typing methods were in basic agreement and showed that many of the isolates were highly similar. Strain maintenance is suggested by strains which are indistinguishable by genetic typing, being isolated from dogs housed months apart within the same kennel. There was also a high degree of similarity of the M. cynos type strain (isolated from a dog with respiratory disease in Denmark in 1971) to at least one of these United Kingdom isolates, which suggests possible conservation of pathogenic strains of M. cynos.
Acknowledgements
We thank Dr. V. Chalker for the M. cynos isolates and The Royal Veterinary College Bacteriology Lab for bacteriological screening of dog respiratory samples. We thank Mitesh Patel for his assistance with the molecular typing.
References
- Chalker V.J., Brooks H.W., Brownlie J. The association of Streptococcus equi subsp. zooepidemicus with canine infectious respiratory disease. Vet. Microbiol. 2003;95:149–156. doi: 10.1016/S0378-1135(03)00155-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalker V.J., Toomey C., Opperman S., Brooks H.W., Ibuoye M.A., Brownlie J., Rycroft A.N. Respiratory disease in kennelled dogs: serological responses to Bordetella bronchiseptica lipopolysaccharide do not correlate with bacterial isolation or clinical respiratory symptoms. Clin. Diagn. Lab. Immunol. 2003;10:352–356. doi: 10.1128/CDLI.10.3.352-356.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalker V.J., Owen W.M., Paterson C., Barker E., Brooks H., Rycroft A.N., Brownlie J. Mycoplasmas associated with canine infectious respiratory disease. Microbiology. 2004;150:3491–3497. doi: 10.1099/mic.0.26848-0. [DOI] [PubMed] [Google Scholar]
- Chandler J.C., Lappin M.R. Mycoplasmal respiratory infections in small animals: 17 cases (1988–1999) J. Am. Anim. Hosp. Assoc. 2002;38:111–119. doi: 10.5326/0380111. [DOI] [PubMed] [Google Scholar]
- Chvala S., Benetka V., Mostl K., Zeugswetter F., Spergser J., Weissenbock H. Simultaneous canine distemper virus, canine adenovirus type 2, and Mycoplasma cynos infection in a dog with pneumonia. Vet. Pathol. 2007;44:508–512. doi: 10.1354/vp.44-4-508. [DOI] [PubMed] [Google Scholar]
- Ellis J.S., Zambon M.C. Combined PCR-heteroduplex mobility assay for detection and differentiation of influenza A viruses from different animal species. J. Clin. Microbiol. 2001;39:4097–4102. doi: 10.1128/JCM.39.11.4097-4102.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erles K., Toomey C., Brooks H.W., Brownlie J. Detection of a group 2 coronavirus in dogs with canine infectious respiratory disease. Virology. 2003;310:216–223. doi: 10.1016/S0042-6822(03)00160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erles K., Dubovi E.J., Brooks H.W., Brownlie J. Longitudinal study of viruses associated with canine infectious respiratory disease. J. Clin. Microbiol. 2004;42:4524–4529. doi: 10.1128/JCM.42.10.4524-4529.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feberwee A., Dijkstra J.R., von Banniseht-Wysmuller T.E., Gielkens A.L., Wagenaar J.A. Genotyping of Mycoplasma gallisepticum and M. synoviae by amplified fragment length polymorphism (AFLP) analysis and digitalized random amplified polymorphic DNA (RAPD) analysis. Vet. Microbiol. 2005;111:125–131. doi: 10.1016/j.vetmic.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Hotzel H., Schneider B., Sachse K. Investigation of Mycoplasma bovis field isolates using PCR fingerprinting. In: Leori G., Santini F., Scanziani E., Frey J., editors. vol. 2. European Commission; Brussels: 1998. pp. 17–19. (Mycoplasma of Ruminants: Pathogenicity, Diagnostics, Epidemiology and Molecular Genetics). [Google Scholar]
- Jameson P.H., King L.A., Lappin M.R., Jones R.L. Comparison of clinical signs, diagnostic findings, organisms isolated, and clinical outcome in dogs with bacterial pneumonia: 93 cases (1986-1991) J. Am. Vet. Med. Assoc. 1995;206:206–209. [PubMed] [Google Scholar]
- Kirchner B.K., Port C.D., Magoc T.J., Sidor M.A., Ruben Z. Spontaneous bronchopneumonia in laboratory dogs infected with untyped Mycoplasma spp. Lab. Anim. Sci. 1990;40:625–628. [PubMed] [Google Scholar]
- Kusiluka L.J., Kokotovic B., Ojeniyi B., Friis N.F., Ahrens P. Genetic variations among Mycoplasma bovis strains isolated from Danish cattle. FEMS Microbiol. Lett. 2000;192:113–118. doi: 10.1111/j.1574-6968.2000.tb09368.x. [DOI] [PubMed] [Google Scholar]
- McAuliffe L., Kokotovic B., Ayling R.D., Nicholas R.A. Molecular epidemiological analysis of Mycoplasma bovis isolates from the United Kingdom shows two genetically distinct clusters. J. Clin. Microbiol. 2004;42:4556–4565. doi: 10.1128/JCM.42.10.4556-4565.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuliffe L., Ellis R.J., Miles K., Ayling R.D., Nicholas R.A. Biofilm formation by mycoplasma species and its role in environmental persistence and survival. Microbiology. 2006;152:913–922. doi: 10.1099/mic.0.28604-0. [DOI] [PubMed] [Google Scholar]
- Nagatomo H., Takegahara Y., Sonoda T., Yamaguchi A., Uemura R., Hagiwara S., Sueyoshi M. Comparative studies of the persistence of animal mycoplasmas under different environmental conditions. Vet. Microbiol. 2001;82:223–232. doi: 10.1016/s0378-1135(01)00385-6. [DOI] [PubMed] [Google Scholar]
- Parham K., Churchward C.P., McAuliffe L., Nicholas R.A., Ayling R.D. A high level of strain variation within the Mycoplasma ovipneumoniae population of the UK has implications for disease diagnosis and management. Vet. Microbiol. 2006;118:83–90. doi: 10.1016/j.vetmic.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Randolph J.F., Moise N.S., Scarlett J.M., Shin S.J., Blue J.T., Bookbinder P.R. Prevalence of mycoplasmal and ureaplasmal recovery from tracheobronchial lavages and prevalence of mycoplasmal recovery from pharyngeal swab specimens in dogs with or without pulmonary disease. Am. J. Vet. Res. 1993;54:387–391. [PubMed] [Google Scholar]
- Rosendal S. Mycoplasmas as a possible cause of enzootic pneumonia in dogs. Acta Vet. Scand. 1972;13:137–139. [PubMed] [Google Scholar]
- Rosendal S. Mycoplasma cynos, a new canine Mycoplasma species. Int. J. Syst. Bacteriol. 1973;23:49–54. [Google Scholar]
- Rosendal S. Canine mycoplasmas: pathogenicity of mycoplasmas associated with distemper pneumonia. J. Infect. Dis. 1978;138:203–210. doi: 10.1093/infdis/138.2.203. [DOI] [PubMed] [Google Scholar]
- Rosendal S. Canine mycoplasmas: their ecologic niche and role in disease. J. Am. Vet. Med. Assoc. 1982;180:1212–1214. [PubMed] [Google Scholar]
- Rycroft A.N., Tsounakou E., Chalker V. Serological evidence of Mycoplasma cynos infection in canine infectious respiratory disease. Vet. Microbiol. 2007;120:358–362. doi: 10.1016/j.vetmic.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M., Olver C., Thrall M.A. Transtracheal wash from a puppy with respiratory disease. Vet. Clin. Pathol. 2006;35:471–473. doi: 10.1111/j.1939-165x.2006.tb00168.x. [DOI] [PubMed] [Google Scholar]
- Zeugswetter F., Weissenbock H., Shibly S., Hassan J., Spergser J. Lethal bronchopneumonia caused by Mycoplasma cynos in a litter of golden retriever puppies. Vet. Rec. 2007;161:626–627. doi: 10.1136/vr.161.18.626. [DOI] [PubMed] [Google Scholar]


