Abstract
The symptoms of rhinorrhea secondary to influenza and cold virus or seasonal and perennial allergic rhinitis are circadian rhythmic. Cough frequency and handkerchief use by persons suffering from virus-induced rhinorrhea are more prominent during the daytime, especially during the initial hours after awakening from nocturnal sleep. The elevation in sublingual temperature as well as the decrement in mental alertness associated with influenza in particular are more profound at this time. Sneezing, blocked nose, and runny nose secondary to allergic rhinitis are also greater in intensity during the morning in approximately 70% of sufferers. The day-night variation in symptom intensity amounts to approximately 20% of the 24-hour mean level. The treatment of these diseases and their symptoms has traditionally involved equal-interval, equal-dose (homeostatic) medication schedules. The effects of antihistamine and antiinflammatory medicines may be enhanced by timing them to the day-night temporal pattern in symptom manifestation and intensity to achieve an optimization of their beneficial effects with control of toxicity, that is, as a chronotherapy. (J ALLERGY CLIN IMMUNOL 1995;95:1084-96.)
Keywords: Circadian rhythms, colds, influenza, allergic rhinitis, rhinorrhea, chronotherapeutics
Biological functions are not constant over time as implied by the concept of homeostasis. Instead they undergo predictable and thus rhythmic variation over specific time domains—the 24 hours, month, and year, for example. Biological processes exhibit a rather precise time structure defined by both the totality of cycles of various period durations and the phase relationships (peak and trough times) of the rhythmic functions of the same period.1, 2 Chronobiology is the science concerned with the study of biological rhythms, their mechanisms, and the bioclocks that drive them. Chronobiological investigations demonstrate that the biological time structure is an endogenous, genetic trait.3, 4, 5 Rhythms are coordinated by specialized pacemaker clocks located in different tissues and organs of the body with the suprachiasmatic nuclei of the hypothalamus thought to be of greatest importance.6, 7, 8 Specific environmental time cues serve to set or reset endogenous biological clocks on a day-to-day basis.1, 2, 9, 10 In human beings the environmental light-dark cycle in conjunction with the daily schedule of activity and rest serve as primary time cues (synchronizers) of 24-hour rhythms.9, 10, 11
Circadian (circa = about; dies = a day) rhythms in particular are of critical importance in medicine. The findings of commonly performed diagnostic procedures, including blood pressure measurement, pulmonary function assessment, cutaneous allergy testing, and blood withdrawal for laboratory chemistry determinations, are strongly influenced by when they are done with regard to the circadian time structure.12, 13, 14, 15 Moreover, both the pharmacokinetics and dynamics of most classes of medications are dependent on circadian rhythmic phenomena of the gastrointestinal tract and other organs.16 Thus the kinetics and effects of medications can be very different when administered in the morning versus the evening.17, 18, 19 Finally, many diseases are affected by the circadian time structure, which leads to prominent 24-hour patterns in their manifestation and exacerbation. Asthma20 and ulcer21 disease tend to worsen or occur only at night; migraine headache,22 rheumatoid arthritis,23 exertional angina,24 myocardial infarction,25 and thrombotic stroke26 are most common or more severe during the initial hours of diurnal activity.1 Studies pertaining to the pathophysiology of these and other diseases are typically conducted during the daytime at the convenience of both the investigators and subjects. The findings of daytime-conducted studies, however, may not be representative, especially for those disorders that worsen or occur during the nighttime.
The chronobiology of asthma has been the subject of scientific investigation for more than 30 years. Most patients are prone to nocturnal exacerbations of dyspnea. Circadian rhythms in endocrine function, the autonomic nervous system, and airway inflammation are contributory.20 The signs and symptoms of infectious and allergic rhinitis are likely to be affected by these and other circadian functions and processes. The purpose of this article is to (1) review the evidence of circadian rhythms in the occurrence and severity of rhinorrhea and (2) examine circadian rhythm-based treatment strategies for improving the pharmacotherapy of viral and allergic rhinitis.
DAY-NIGHT PATTERNS IN COLD AND INFLUENZA SYMPTOMS
Temporal variation in the severity of cold and influenza symptoms has been little studied. Indeed, only two published reports could be located by a thorough review of the medical literature published during the past 20 years. The investigations of Smith et al.27 focused on the day-night pattern in symptom intensity of individuals challenged with cold or influenza viruses. Healthy volunteers without a history of allergic rhinitis, respiratory or other organic disease, and free of medication use served as subjects. Volunteers were quarantined for 3 days to ensure absence of infection. Thereafter, nasal drops containing either influenza B, corona virus or rhinoviruses RV2 and RV9, or saline as placebo were administered to different persons in a double-blind manner. After a 2- to 3-day incubation period while residing in a special study unit, subjects were assessed for signs and symptoms of infectious disease. Handkerchief use and sublingual temperature were assessed at specific times during the day and evening by subjects who had symptoms of the cold viruses. In addition, mental alertness was self-assessed four times daily by those who had symptoms of influenza B virus.
Colds
Ten volunteers developed moderate to mild cold symptoms after challenge with the rhinoviruses, and nine others developed primarily mild cold symptoms after provocation with the corona virus. Analysis of variance confirmed a statistically significant (p < 0.05) difference in the number of handkerchiefs used daily when subjects were symptomatic. Handkerchief use increased during the first 2 days of symptoms and gradually declined during the subsequent two. Handkerchief use was not random throughout the hours of daily activity (p < 0.05). It was most prominent in the morning after awakening, between 8:00 and 11:00 AM, and less so during the afternoon (Table I). On average, tissue use was more than twofold greater in the morning than in the afternoon or early evening during the last 2 days of symptoms. The cold virus had no effect on the circadian rhythm of sublingual temperature or mental alertness.
TABLE I.
Mean (±standard error) use of handkerchiefs by cold and influenza sufferers by clock time*
|
Clock times |
|||||||
|---|---|---|---|---|---|---|---|
| Type of virus | No. of subjects | Study days | 8-11 am | 11 am-2 pm | 2-5 pm | 5-8 pm | 8-11 pm |
| Cold | 19 | 1 and 2 | 4.6 ± 0.7 | 3.6 ± 0.9 | 3.7 ± 1.0 | 3.6 ± 0.9 | 4.0 ± 0.6 |
| Cold | 19 | 3 and 4 | 4.8 ± 1.1 | 2.8 ± 0.8 | 2.8 ± 1.0 | 1.8 ± 1.0 | 1.8 ± 0.6 |
| Influenza | 3 | All | 7.1 ± 2.8 | 1.9 ± 0.6 | 2.2 ± 1.2 | 1.9 ± 1.2 | 3.7 ± 2.4 |
Data from Smith A, et al. Chronobiol Intern 1988;5:441.
*By analysis of variance, time-of-day differences in handkerchief use were statistically significant (p < 0.05) for symptomatic days 1 and 2 and 3 and 4 by cold and by influenza sufferers
Kuhn et al.28 assessed the circadian pattern of cough in 65 diurnally active university students suffering from acute respiratory illness, presumably the common cold. Participants had symptoms for no longer than 48 hour when studied. Cough frequency was determined by an audiorecording method. The total number of coughs was reported at 6-hour intervals during a 24-hour control baseline period and thereafter during treatment with guaifenesin (400 mg) or placebo every 6 hours for an additional 36 hours. Cough frequency was highest during the hours of activity and lowest overnight during the baseline period as well as during placebo and guaifenesin treatment (Fig. 1).
FIG. 1.

Circadian rhythm of cough frequency in groups of cold sufferers studied for 24 hours before and 36 hours during treatment with placebo or guaifenesin (400 mg) syrup four times daily at 6-hour intervals. Cough frequency is greater in the morning and afternoon than overnight. Sloping line depicts average rate of decline in cough frequency over 60 hours around which 24-hour variability is manifested. (Redrawn from Kuhn JJ, Hendley JO, Adams KF, et al. Antitussive effect of guaifensein in young adults with natural colds. Chest 1982;82:713-8.)
Influenza
Smith et al.27 studied nine volunteers, all women, who developed significant illness after challenge with influenza B virus. Data on handkerchief use, although restricted to three participants, showed prominent temporal variability (p < 0.05). Tissue use was greatest in the morning between 8:00 and 11:00 AM and lowest during the afternoon, with a moderate increase late in the evening (Table I). The number of tissues used in the morning was about threefold greater than in the afternoon or early evening. Moreover, the 24-hour mean sublingual temperature on symptomatic days was significantly increased over that of prechallenge baseline days (p < 0.05). The morning (8:00 AM) temperature was most affected by the viral infection (Table II). Alertness ratings during the symptomatic days were significantly reduced in comparison with the prechallenge baseline level (p < 0.05). Again, the effect was greatest at 8:00 AM. At this time alertness was reduced by 42% from the baseline value.
TABLE II.
Mean (±standard error) sublingual temperature and alertness self-ratings of influenza sufferers by clock time during baseline and symptomatic days*
|
Clock time |
||||||
|---|---|---|---|---|---|---|
| Study variable | No. of subjects | Experimental condition | 8 am | Noon | 5 pm | 10 pm |
| Temperature | 9 | Baseline | 36.27 ± 0.08 | 36.63 ± 0.09 | 36.55 ± 0.11 | 36.80 ± 0.10 |
| Temperature | 9 | Symptomatic | 37.26 ± 0.23 | 37.07 ± 0.21 | 37.12 ± 0.24 | 37.18 ± 0.22 |
| Alertness | 9 | Baseline | 40.80 ± 3.7 | 58.10 ± 6.0 | 60.30 ± 6.0 | 49.40 ± 5.5 |
| Alertness | 9 | Symptomatic | 23.60 ± 4.0 | 52.40 ± 6.0 | 51.40 ± 5.5 | 38.70 ± 5.5 |
Data from Smith A, et al. Chronobiol Intern 1988;5:441.
*Temperature in units of °C; alertness in arbitrary units of a visual analog scale (the greater the score the greater the self-rated alertness). Difference in 24-hour mean of temperature and alertness between baseline and symptomatic days significant by analysis of variance (p < 0.05). Significant interaction between time of day and experimental condition is indicative of a shift in maximal temperature from the evening during baseline condition to the morning during illness.
DAY-NIGHT PATTERN IN ALLERGIC RHINITIS
Patients who suffer from seasonal and perennial allergic rhinitis often complain of disturbed sleep at night as well as troublesome symptoms in the morning on awakening. Although patients and allergists are familiar with the evolution of symptoms during the 24 hours, relatively few medical publications have addressed their circadian patterns. Trousseau29 in 1865 was the first to write about the prominent morning manifestation and intensity of nasal symptoms. His observations on the clock-time patterning of symptoms led him to conclude that allergic rhinitis is a form of asthma that affects the nose (“L'asthme du nez”).
The quantitative study of the temporal variation in allergic rhinitis commenced during the 1970s. Nicholson and Bogie30 in Great Britain were apparently the first to assess the diurnal (daytime) variation in the commencement of “hay fever” symptoms. They used random location sampling to survey 2042 individuals. By questionnaire, the investigators identified so-called “hay fever” sufferers and their most troublesome symptoms (sneezing, wheezing, red itchy eyes, or stuffy nose). Specific questions solicited information about the time of day (before breakfast, in the morning, around lunch time, during the afternoon, in the evening, while in bed at night, or do not know) the most troublesome symptom began or was worst? Unfortunately, no information was ascertained about the use and scheduling of allergy or other medications.
A total of 246 persons were identified as “hay fever” sufferers. The most frequently reported main symptom was sneezing (82%), with “stuffy nose” (56%) and “red itchy eyes” (46%) next in prominence. Commencement of each of these symptoms was most frequent before breakfast and during the morning and least so during the middle of the day and late in the afternoon (Fig. 2). Wheeze and cough were experienced by 20% of the respondents. These symptoms, which are more indicative of asthma than rhinitis, occurred mostly while in bed at night and in the morning. Approximately 75% of the sample indicated that their most troublesome symptom was worse either in the evening while in bed at night or in the morning at the start of the activity period.
FIG. 2.

Day-night distribution of onset of "hay fever" symptoms by 246 presumably diurnally active British sufferers. Data obtained by survey questionnaire show that nasal and eye symptoms commence most commonly in the morning; this is not the case for cough and wheeze. (from Nicholson PA, Bogie W. Curr Med Res Opin 1973;1:395-401)
In 1982 Binder et al.31 reported on the day-night distribution of symptoms in 512 Finnish persons with a history of perennial allergic rhinitis and 462 others suffering from seasonal rhinitis. More than 90% of each group had experienced nasal allergy for at least a year; more than 50% had a 5-year or longer history of disease. No data were reported about the type and schedule of medication used for nasal allergy by the participants. Recall information pertaining to the dominating symptoms of nasal allergy and the time during the day when they were severest was obtained by questionnaire.
As in the Nicholson and Bogie study,30 sneezing was the most common major complaint of patients with both seasonal rhinitis (55%) and perennial (42%) rhinitis. Nasal stuffiness and drainage were the next most frequent main symptoms; they were experienced by 16% to 26% of participants. About 10% experienced dyspnea as the main symptom. Again, the morning was the most difficult time of the day for the sufferers regardless of the study group. Most patients with seasonal rhinitis (56%) and perennial (66%) rhinitis reported that their most severe symptom occurred in the morning; relatively few recalled their most severe symptom occurring during the evening or night (Fig. 3).
FIG. 3.

Day-night pattern in occurrence of most severe symptom of presumbably diurnally active Finnish patients suffering from seasonal and perennial rhinitis. Data obtained by anamnestic recall demonstrate that the most difficult time of day is the morning for most persons. (from Binder E, et al. Allergy 1982;37:389-96. © 1982 by Munksgaard international Publishers Ltd., Copenhagan, Danmark.)
Investigations by Reinberg et al.32 in 1988 were concerned with quantifying the nature and magnitude of the day-night variation in the symptom intensity of allergic rhinitis. The epidemiologic study involved 17 centers throughout France. The diagnosis of allergic rhinitis was based on conventional clinical criteria: positive skin reactions to antigens, total and differential white cell count, immunoglobulin E concentration, and medical history. A total of 765 persons participated. Each self-assessed the severity of their symptoms using visual analog scales at least four times daily—on awakening in the morning, before lunch and dinner, and before retiring to bed for nighttime sleep. Participants were to forego the use of all medications starting 2 days before and until completion of the 7-day span of data collection.
The times of self-assessments by the 765 participants were distributed almost equally between the hours of 6:00 AM and midnight. Graphs of the 3-hour averages of symptom intensity scores show the prominent day-night variation in the severity of sneezing as well as blocked and runny nose (Fig. 4). All the symptoms were worse in the morning at the start of the activity period. However, a second, less prominent evening peak in symptom intensity was also apparent. The magnitude of the day-night variation in the other surveyed symptoms—eye irritation, itchy nose, cough, and dyspnea—was trivial. This pattern was comparable in men and women, smokers, and nonsmokers, and in patients with recent and long-term history of the disease.
FIG. 4.
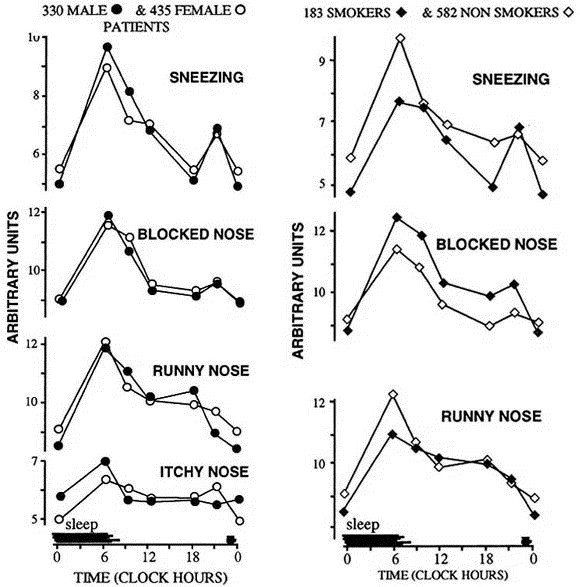
Day-night variation in occurrence and intensity of allergic rhinitis in 765 diurnally active French study participants. Data were obtained by self-assessment of symptom intensity four times daily with visual analog scales. Morning prominent and evening secondary peaks in nasal symptoms are evident in both men and women (left) and smokers and nonsmokers. (From Reinberg A, et al. J ALLERGY CLIN IMMUNOL 1988;81:51-62
An objective assessment of the circadian rhythmicity in each symptom was achieved by cosinor analysis.33 The time series data were approximated by a 24-hour in-period cosine curve by the method of least squares to statistically validate circadian rhythmicity by the zero-amplitude test. If rhythm detection was documented, it was described by the following parameters: the mesor, M (midline estimating statistic of rhythm, a rhythm-adjusted 24-hour mean); amplitude, A (one-half the peak-to-trough variation due to rhythmicity), and acrophase, ø (peak time referenced to local midnight of the approximating cosine curve).
By cosinor analysis statistically significant circadian rhythms were validated for the symptoms of sneezing plus blocked and runny nose (Table III). The acrophase of the rhythms was comparable and ranged between 5:00 AM (blocked nose) to 6:45 AM (runny nose). The double amplitude, 2A, representative of the entire 24-hour peak-to-trough variation in symptom intensity, was appreciable. The double amplitude for the rhythms in sneezing and blocked nose amounted to 23% M (the rhythm-adjusted 24-hour average of all the ratings). For runny nose it was slightly less, around 18% M. The symptoms of itchy nose, eye irritation, cough, and dyspnea were not circadian rhythmic by cosinor analysis.
TABLE III.
Circadian change in symptom intensity of 765 patients allergic rhinitis*
| Amplitude, A |
Acrophase, ø |
|||
|---|---|---|---|---|
| Type of symptom | Rhythm detection, p | Mesor mean ± SEM | (95% confidence limits) | |
| Sneezing | < 0.001 | 7.46 ± 0.13 | 0.87 (0.21 - .43) | 06:00 (02:54-09:06) |
| Blocked nose | < 0.001 | 10.05 ± 0.14 | 1.13 (0.51-1.75) | 05:00 (03:24-06:36) |
| Runny nose | < 0.001 | 10.27 ± 0.14 | 0.93 (0.28-1.48) | 06:42 (03:48-09:36) |
Data from Reinberg A, et al. J ALLERGY CLIN IMMUNOL 1988;81:51-62.
*Mesor (rhythm-adjusted mean), amplitude (one-half peak-trough variation), and acrophase (peak time) of approximated 24-hour cosine curve. M and A in arbitrary units from analog scales; acrophase expressed as hours and minutes referenced to local midnight
CIRCADIAN RHYTHMS IN RHINORRHEA: TREATMENT IMPLICATIONS
The pharmacokinetics and dynamics of many medications are circadian rhythm dependent. The science of chronopharmacology is concerned with the influences of circadian and other rhythms of the gastrointestinal tract, liver, kidney, and other organs on the absorption, distribution, elimination, and effects of medications.17, 18, 19 Administration–time-dependent differences in the pharmacokinetics of a second-generation H1-receptor antagonist, mequitazine, were demonstrated by Reinberg et al.34 In addition, the duration and the time to peak effect after administration of a first-generation antihistamine, cyproheptadine, and three other commonly prescribed H1-receptor antagonists, mequitazine, clemastine, and terfenadine (Fig. 5), were shown to vary according to administration time with reference to circadian rhythms.35, 36
FIG. 5.


Administration-time (7:00 AM versus 7:00PM) differences in terfenadine-and clemastine-induced inhibition of local cutaneous reaction to intradermally injected histamine (2 æg/0.1 ml). Double-blind protocol was used to test medication effects. Strength of antihistamine effect according to treatment time and dose is expressed as clock-time difference in cutaneous response to histamine during antihistamine versus placebo treatments. Antihistamine effect was longer in a dose-dependent manner when medications were ingested in the morning. Magnitude of peak effect was greater in a dose-dependent manner after evening treatment. (From Reinberg A, et al. Eur J Clin Pharmacol 1976;14:245-52, by permission of Elsevier Science Publishers.)
Traditionally, the treatment of human disease involves multiple or once-a-day equal-interval, equal-dose strategies to achieve the homeostatic goal of constancy of blood and tissue drug concentrations. We refer to these as homeostatic treatment strategies. However, the need for medication may not be constant throughout the 24 hours. Many chronic diseases, such as rheumatoid and osteoarthritis,23 ischemic heart disease,24, 25, 26 asthma,13, 20 and ulcer disease,21 for example, exhibit rather predictable variations in their occurrence or severity over a 24-hour period. The symptoms of infectious and allergic rhinitis also undergo predictable day-night variability; in most patients they are most intense in the morning upon arising from sleep and least troublesome in the afternoon. The demonstration of circadian patterns in the manifestation and intensity of human diseases, including rhinitis, suggests that the requirement for medication is not the same throughout the 24 hours. Indeed, the enhancement of the desired effects of medications or the control of their adverse reactions can be accomplished by administering them with reference to the biologic time structure of disease processes.1, 2, 18
The chronotherapy of antihistamines
The chronotherapy of allergic rhinitis with a second-generation H1-receptor antagonist (mequitazine, Pharmuka, France) was explored through a large multicenter study conducted by Reinberg et al. in France.36, 37 Different doses and regimens of mequitazine were administered in the morning and evening to comparable groups of diurnally active adult patients with allergic rhinitis. Participants conducted self-assessments of symptom intensity four times daily for 7 consecutive days to quantify the effect of the treatment regimen. Mequitazine was most effective in moderating the morning peak and overall 24-hour level of symptom intensity of allergic rhinitis when two thirds or all the 7.5 or 10 mg daily dose was ingested around dinner time. The medication was least effective when most of or all the daily dose was administered in the morning around breakfast time (Fig. 6). Evening administration (10 mg) was especially effective in patients who exhibited predominantly morning symptoms (Fig. 7). No sedative effect was observed. Moreover, one of the major side effects of the medication, dry mouth, was minimized or completely averted by the evening dosing schedules.36, 37 The chronotherapy of mequitazine for allergic rhinitis entails a daily treatment regimen in which all or most of the daily dose is administered in the evening.
FIG. 6.



Time-dependent effects of 7.5 mg of antihistamine mequitazine (Pharmaka, France) administrated in unequal morning (breakfast, B) and evening (dinner, D) doses for allergic rhinitis. When all or most of the daily dose of mequitazine was ingested in the evening, there was enhancement of drug effect. (From Reinberg A. Chronopharmacology of H1 - antihistamines. In: Lemmer BJ, ed. Chronopharmacology: cellular and biochemical interactions. New York: Marcel Dekker, 1989:115-35, by courtesy of Marcel Dekker.)
FIG. 7.

Differential effect of 10 mg of mequitazine as a once-daily evening versus morning strategy for treatment of allergic rhinitis in a group of 98 diurnally active adult patients with history of very prominent morning symptoms. Treatment effects were evaluated by self-assessments of symptoms with visual analog scales four times daily during a 7-day period. Evening in comparison with morning ingestion of antihistamine resulted in a statistically significant (p < 0.005) better control of symptoms. (From Reinberg A. Chronopharmacology of H1-antihistamines. In: Lemmer BJ, ed. Chronopharmacology: cellular and biochemical interactions. New York: Marcel Dekker, 1989:115-35, by permission of Marcel Dekker.)
Comparable study of other antihistaminic or decongestant medications is unknown. One company (Ferndale Laboratories) recommends that its decongestant-antihistamine product, Kronofed (60 mg pseudoephedrine and 4 mg chlorpheniramine) be taken in unequal doses, one third the daily dose in the morning and the remaining two thirds before bedtime. The rationale for this administration schedule appears be based on the fact that first-generation H1-antagonists exert a sedating effect; the greater evening dose helps induce sleep at night. However, the unequal dosing strategy might be more beneficial for the control of the disease than the equal-interval, equal-dose regimen, since it takes into account the circadian rhythmic aspects of allergic rhinitis.
Corticosteroids
Daily and alternate-day morning tablet corticotherapy with methylprednisolone was initially popularized during the 1960s; this constituted the first attempt to apply a chronotherapeutic strategy in clinical practice.38 In Europe another corticosteroid chronotherapy has been marketed.39, 40 It consists of giving two thirds the daily steroid dose at breakfast time (8:00 AM) and the remaining one third at 3:00 PM (about 8 hours into the activity span). The rationale for the chronotherapy of corticosteroids is the extensive amount of research demonstrating that the suppression of the hypothalamic-pituitary-adrenocortical axis is dependent on the circadian timing of synthetic corticosteroid hormones.41, 42, 43 Doses timed during the initial hours of the activity span, which correspond in time to the peak plasma cortisol level, are less adrenal suppressive than are those timed late in the evening or at night when the endogenous hormone level is low. Because supper and bedtime administration of tablet corticosteroids poses an elevated risk of hypothalamic-pituitary-adrenocortical suppression and perhaps other side effects, equal-interval, equal-dose (three to four-times daily) homeostatic treatment regimens are not recommended as a long-term treatment strategy.42, 43 In addition, morning and mid-day corticosteroid tablet administration is more effective in treating nocturnal asthma than is evening administration.32, 42
The use of tablet corticotherapy in the long-term management of rhinorrhea is a relatively uncommon practice except for the most severe cases. Topical corticosteroid aerosols are much more popular. Research on the chronotherapy of aerosol corticosteroids is just commencing.44, 45 Initial studies on asthmatic patients have been conducted recently by Pincus et al.45 In their studies once-daily afternoon (3:00 PM), high-dose aerosol corticotherapy was more effective on lung function than once-daily morning (8:00 AM) or evening (8:00 PM) timings. Exploration of the chronotherapy of topical corticosteroids for rhinorrhea is apparently yet to be initiated.
Ipratropium bromide
An administration–time-dependent effect of aerosolized ipratropium bromide on pulmonary function was demonstrated by Gaultier et al.46 The effect of 80 μg of the drug on airway resistance and dynamic compliance of the lung was assessed in seven diurnally active healthy children. Assessments were conducted at four different times of day: 7:30 AM, 11:30 AM, 4:30 PM, and 10:30 PM, with the order of treatment timings randomized between subjects. Ipratropium bromide was most effective in reducing airway resistance when it was inhaled in the morning at 7:30 AM. The 80 μg dose had little impact on airway resistance when given at 10:30 PM; however, a dose of 200 μg was effective at this time. The effect of ipratropium bromide on dynamic lung compliance was also administration time dependent. It was greatest at 10:30 PM; at all other times of the day 80 μg of the drug had little or no influence on this pulmonary function measure. The results of this study are consistent with the hypothesis that vagal tone is circadian rhythmic and predominates at night.46
DISCUSSION
The investigation of circadian rhythms in disease requires the standardization and control of certain study variables. The staging of endogenous 24-hour rhythms is accomplished by one's daily activity (in light) and sleep (during darkness) routine. Night workers adhere to a different clock-hour schedule of sleep and activity than do day workers. Shiftworkers undergo readjustment of their circadian time structure with each rotation that entails alteration of the sleep-wake pattern. In the majority of studies on rhinorrhea, there was no standardization of the sleep-wake synchronizer schedule of circadian rhythms, nor was there mention of the subjects' sleep-wake routine. In addition, information on medication use was oftentimes lacking or incomplete. Even when it was known that patients received medication to manage allergic rhinitis, the dose and schedule of the medications were not always specified. In spite of these drawbacks in investigative methods, prominent day-night patterns in the symptoms of both viral and allergic rhinitis were detected. In the case of allergic rhinitis, the circadian variability was documented in studies conducted in different counties, in women and men, in the young and elderly, in smokers and nonsmokers, in newly diagnosed and chronic sufferers, and in patients with seasonal and perennial rhinitis.30, 31, 32, 47, 48, 49 In diurnally active individuals, symptoms most commonly started or were most severe during the morning after awakening from nighttime sleep.
The occurrence and severity of rhinorrhea, whether secondary to viral infection or allergen provocation, vary in a predictable-in-time pattern during the 24 hours.27 The study of viral rhinorrhea relied primarily on a single end point only: the number of handkerchiefs used throughout the hours of diurnal activity. Unfortunately, specific symptoms were not self-assessed repeatedly during the 24 hours; thus determination of the magnitude of the temporal variation could not be accomplished. Nonetheless, prominent day-night variation in the manifestation of the infectious disease was detected. In patients with mild to moderate symptoms of cold or influenza, handkerchief use was respectively one and one half to fourfold greater during the morning hours between 8:00 and 11:00 AM than during the afternoon. The effect of influenza on mental alertness and sublingual temperature was also time dependent. The decrement in alertness and increment in temperature were most pronounced in the morning. The frequency of coughing due to acute respiratory illness, most likely a cold virus, was greatest during the daytime and least overnight both before and with treatment by placebo or guaifenesin syrup.28
Most studies of the day-night variation in the symptom intensity of allergic rhinitis relied on patient recall. Questionnaires or diary cards were used to survey patients during clinical visits and home study or by mailings to randomly selected individuals.30, 33, 47, 48, 49 While this investigative method is useful for exploring the existence of temporal patterns in disease, it does not allow one to quantify the extent of variation in the symptom intensity experienced by patients. Reinberg et al.32 used a repeat-measures, self-assessment protocol to ascertain the occurrence and intensity of symptoms four times daily. They found that the magnitude of the 24-hour variation in the intensity of sneezing, nasal congestion, and nasal drainage was around 20% on average. However, because data collection was restricted to the hours of daytime activity only, the estimate of the extent of the variability in symptom intensity may be conservative. Patients oftentimes complain that their sleep is disturbed because of severe nasal congestion causing difficulty in breathing overnight. Thus the time when the nasal congestion of allergic and perhaps infectious rhinorrhea is most severe may be somewhat earlier than determined by surveys restricted to the daytime hours only. Close review of the published reports on allergic rhinitis reveal a second time, between supper and bedtime, when symptom intensity is elevated.31, 32, 49 The limited data published thus far on viral rhinorrhea reveal only a single morning time peak in symptoms during the 24 hours. The second peak of symptoms experienced by some sufferers of allergic rhinitis might represent a late-type reaction to antigens.50 Such reactions have also been reported for allergic asthma and dermatitis.51, 52
Why do the symptoms of these diseases vary to such an extent during the 24 hours? Although the mechanisms underlying the observed variation in rhinorrhea have yet to be elucidated, several explanations have been put forth. Some suggest that the increased morning use of handkerchiefs by cold and influenza sufferers represents the need to clear the upper airways of secretions that accumulate during nocturnal sleep. Others cite the role of the prone position during sleep as contributing to the worsening of nasal congestion overnight. The severity of morning symptoms of allergic rhinitis is commonly blamed on antigen exposure, particularly house dust mite, mold, and feathers, in the bedroom during nocturnal sleep. All such explanations are incomplete; they focus exclusively on temporal changes in the external environment without consideration of possible contributions of the circadian time structure.
The symptoms of rhinorrhea, regardless of origin, result from tissue injury caused by immunologic reactions and chemical mediator release associated with inflammation of the upper airways. The processes underlying the pathophysiology of inflammation are modulated by certain circadian rhythmic processes.53 The symptoms of other inflammatory diseases, such as asthma and rheumatoid arthritis, are worse during nocturnal sleep or in the morning on awakening. Laboratory rodent research reveals the rate and extent of the edematous response to phlogistic agents, the mucopolysaccharide carrageenan,54, 55 the antibiotic polymycin B, and croton oil,56 vary by up to threefold according to the circadian time of their administration. Time-dependent differences in the inflammatory response may be related to the circadian rhythm in cortisol.54 Experimentally induced edema is greatest when plasma cortisol level is lowest (during the rest span) and least when cortisol is highest (during the activity span); adrenalectomy results in loss of circadian rhythmicity in the edematous response. Rhythms in the bradykinin, prostaglandin E2, arachidonic acid, tissue blood flow, and the number and functional capacity of macrophages plus other inflammatory cells are thought to be involved.23, 57, 58, 59, 60, 61 Circadian rhythms in plasma cortisol and the autonomic nervous system plus day-night differences in airways inflammation, reactivity, and clearance may also play a role because they are known to contribute to the worsening of lung function and asthma overnight.13, 20
The traditional treatment of rhinorrhea consists of decongestant and H1-receptor antagonist medications. Topical nasal corticotherapy is used, particularly in the management of allergic rhinitis. Generally such medications are administered several times daily. Times of drug administration during the day and night are selected primarily to ensure patient compliance. Circadian dependencies and attributes of rhinorrhea, even if appreciated by the medical community, are unfortunately seldom taken into account in the scheduling of pharmacotherapy. Both the beneficial and side effect profile of medications may be circadian rhythm dependent.
The chronotherapy of the local and systemic manifestations of cold and influenza viruses has not yet been investigated. Studies pertaining to the chronotherapy of allergic rhinitis are limited. The work of Reinberg et al. cited herein indicate that the efficiency of the second-generation antihistamine, mequitazine, can be enhanced by ingesting most of or the entire daily dose around dinner time.36, 37 This chronotherapeutic approach appears to be especially beneficial for those suffering primarily from morning symptoms of rhinorrhea. Other individualized, programmed in-time dosing regimens may be necessary to optimally manage patients who have different temporal patterns.
Topical corticosteroid aerosols are commonly prescribed to treat allergic rhinitis. Generally, this type of medication is used two to four times daily at equal intervals and equal doses to maintain tissue drug levels at near constant levels. Several published studies confirm the expectation that topical corticosteroids, in comparison with antihistamines alone, achieve a greater level of symptom relief. Moreover, the findings of two clinical trials involving topical corticosteroids imply that both the morning and afternoon peaks in symptom intensity or occurrence observed in some patients can be moderated by equal-interval, equal-dose treatment regimens.48, 49 The advantage of a chronotherapy for aerosol corticosteroids for allergic rhinitis has yet to be explored. Initial chronotherapeutic trials on asthma patients with corticosteroid aerosols show that the therapeutic efficiency varies with time of administration; high-dose inhalation corticotherapy timed to the middle of the patient's activity span (about 8 hours after awakening from nighttime sleep) results in a greater improvement in pulmonary function as measured by spirometry than does morning or evening once-a-day administration.45
Circadian rhythmicity in the effect of ipratropium bromide on rhinorrhea has yet to be explored. Vagal tone is stronger at night than during the day when sympathetic tone predominates.13, 20, 62, 63, 64 The findings of Gaultier et al.46 demonstrate the effect of the drug on airway resistance and dynamic compliance of the lung in healthy children is administration time dependent. Ipratropium bromide was effective in reducing lung airway resistance when inhaled in the morning in a dose of 80 μg but not in the evening. However, a larger dose of 200 μg was effective at this time, suggesting that the drug must be used in a higher dose at night when vagal tone is strongest to be optimally effective.
Circadian rhythms in the manifestation and severity of nasal symptoms secondary either to cold and influenza viruses or allergens must be taken into consideration to fully elucidate the underlying pathophysiology of the disease processes and to improve pharmacotherapy. This article has focused on the 24-hour pattern in rhinorrhea and its treatment; the complete management of rhinorrhea also takes into account annual and monthly (in premenopausal women) patterns.32, 36
Acknowledgements
We are grateful to Mr. Daniel Hermann, Ms. Dottsie Natt, Ms. Peggy Powell, and Mr. Verghese Thomas for their valuable assistance with the manuscript.
Footnotes
From the Hermann Chronobiology Center and Environmental Sciences, University of Texas-Houston School of Public Health,a Chronobiologie et Chronopharmacologie, Fondation Adolphe de Rothschild,b Paris, and Ecole de Pharmacie, Universite Laval, Cite Universitaire,c Quebec.
Reprint requests: Michael Smolensky, PhD, University of Texas-Houston School of Public Health, Rm. 442, Houston, TX 77025.
0091-6749/95 $3.00 + 0 1/0/63462
References
- 1.Reinberg A, Smolensky MH. Springer-Verlag; New York: 1983. Biological rhythms and medicine. [Google Scholar]
- 2.Touitou Y, Haus E, editors. Springer-Verlag; Berlin: 1992. (Biologic rhythms in clinical and laboratory medicine.). [Google Scholar]
- 3.Sehgal A, Man B, Price JL. New clock mutations in Drosophila. In: Hrushesky WJM, Langer R, Theeuwes F, editors. Vol. 618. 1991. pp. 1–10. (Temporal control of drug delivery. Ann NY Acad Sci). [Google Scholar]
- 4.Reinberg A, Touitou Y, Restoin A. The genetic background of circadian and ultradian rhythm patterns of 17-hydroxycorticosteroids: a cross-twin study. J Endocrinol. 1985;105:247–253. doi: 10.1677/joe.0.1050247. [DOI] [PubMed] [Google Scholar]
- 5.Ralph MR, Menaker M. A mutation of the circadian system in golden hamsters. Science. 1988;241:1225–1227. doi: 10.1126/science.3413487. [DOI] [PubMed] [Google Scholar]
- 6.Halberg F. Temporal coordination of physiologic function. Cold Spring Harbor Symp Quant Biol. 1960;25:289–310. doi: 10.1101/sqb.1960.025.01.031. [DOI] [PubMed] [Google Scholar]
- 7.Ralph MR, Foster RG, Davis FC, Menaker M. Transplantation suprachiasmatic nucleus determines circadian period. Science. 1990;247:975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- 8.Rietveld WJ. The suprachiasmatic nucleus and other pacemakers. In: Toutiou Y, Haus E, editors. Springer-Verlag; Berlin: 1992. pp. 55–64. (Biological rhythms in clinical and laboratory medicine.). [Google Scholar]
- 9.Wever R. Springer-Verlag; Heidelberg: 1979. The circadian system of man: results of experiments under temporal isolation. [Google Scholar]
- 10.Czeisler CA, Allan JS, Strogatz SH. Bright light resets the human circadian pacemaker independent of the timing of the sleep-wake cycle. Science. 1986;223:667–671. doi: 10.1126/science.3726555. [DOI] [PubMed] [Google Scholar]
- 11.Halberg F, Simpson H. Circadian acrophase of human 17-hydroxycorticosteroid excretion referenced to midsleep rather than midnight. Hum Biol. 1967;39:405–413. [PubMed] [Google Scholar]
- 12.Smolensky MH, Tatar SE, Bergman SA. Circadian rhythmic aspects of human cardiovascular function: a review by chronobiologic statistical methods. Chronobiologia. 1976;3:337–371. [PubMed] [Google Scholar]
- 13.Smolensky MH, Barnes PJ, Reinberg A, McGovern JP., Chronobiology and asthma I: day-night differences in bronchial patency and dyspnea and circadian rhythm dependencies. J Asthma. 1986;23:321–341. doi: 10.3109/02770908609073179. [DOI] [PubMed] [Google Scholar]
- 14.McGovern JP, Smolensky MH, Reinberg A. Circadian and circamensual rhythmicity in cutaneous reactivity to histamine and allergenic extracts. In: McGovern JP, Smolensky MH, Reinberg A, editors. Charles C Thomas; Springfield, Ill: 1977. pp. 79–116. (Chronobiology in allergy and immunology.). [Google Scholar]
- 15.Haus E, Lakatua D, Swoyer J, Sackett-Lundeen L. Chronobiology in hematology and immunology. Am J Anat. 1993;168:467–517. doi: 10.1002/aja.1001680406. [DOI] [PubMed] [Google Scholar]
- 16.Vener KJ, Moore JG. Chronobiologic properties of the alimentary canal affecting xenobiotic absorption. Annu Rev Chronopharmacol. 1988;4:257–281. [Google Scholar]
- 17.Reinberg A, Smolensky MH. Circadian changes in drug disposition in man. Clin Pharmacokinet. 1982;7:401–420. doi: 10.2165/00003088-198207050-00002. [DOI] [PubMed] [Google Scholar]
- 18.Lemmer BJ, editor. Marcel Dekker; New York: 1989. (Chronopharmacology: cellular and biochemical interactions.). [Google Scholar]
- 19.Bruguerolle B. Chronopharmacology. In: Touitou Y, Haus E, editors. Springer-Verlag; Berlin: 1982. pp. 114–137. (Biological rhythms in clinical and laboratory practice.). [Google Scholar]
- 20.Martin RJ, editor. Futura Publishing Co; Mt. Kisco, N.Y: 1993. (Nocturnal asthma: mechanism and treatment.). [Google Scholar]
- 21.Moore JG, Smolensky MH. Biological rhythms in gastrointestinal function and processes: implication for the pathogenesis and treatment of peptic ulcer disease. In: Swabb EA, Szabo S, editors. Marcel Dekker; New York: 1991. pp. 55–85. (Ulcer disease: investigation and basis for treatment.). [Google Scholar]
- 22.Solomon GD. Circadian rhythms and migraine. Cleveland Clin J Med. 1992;59:326–329. doi: 10.3949/ccjm.59.3.326. [DOI] [PubMed] [Google Scholar]
- 23.Labrecque G. Inflammatory reaction and disease. In: Touitou Y, Haus E, editors. Springer-Verlag; Berlin: 1992. pp. 483–492. (Biological rhythms in clinical and laboratory medicine.). [Google Scholar]
- 24.Pepine CJ. Circadian variations in myocardial ischemia. JAMA. 1991;65:386–390. [PubMed] [Google Scholar]
- 25.Muller JE, Stone PH, Turin ZA. The MILIS study group: circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med. 1985;313:1315–1322. doi: 10.1056/NEJM198511213132103. [DOI] [PubMed] [Google Scholar]
- 26.Ridker PM, Willich SN, Muller JE, Hennekens CH. Aspirin, platelet aggregation, and the circadian variation in acute thrombotic events. Chronobiol Intern. 1991;8:327–335. doi: 10.3109/07420529109059169. [DOI] [PubMed] [Google Scholar]
- 27.Smith A, Tyrrell D, Coyle K. Diurnal variation in the symptoms of colds and influenza. Chronobiol Intern. 1988;5:411–416. doi: 10.3109/07420528809067786. [DOI] [PubMed] [Google Scholar]
- 28.Kuhn JJ, Hendley JO, Adams KF. Antitussive effect of guaifensein in young adults with natural colds. Chest. 1982;82:713–718. doi: 10.1378/chest.82.6.713. [DOI] [PubMed] [Google Scholar]
- 29.Trousseau A. Vol 2. Bailliere; Paris: 1865. p. 373. (Clinique medicale de L'Hotel dieu Paris). [Google Scholar]
- 30.Nicholson PA, Bogie W. Diurnal variation in the symptoms of hay fever: implications for pharmaceutical development. Curr Med Res Opin. 1973;1:395–401. doi: 10.1185/03007997309111700. [DOI] [PubMed] [Google Scholar]
- 31.Binder E, Holopainen E, Malmberg H, Salo O. Anamnestic data in allergic rhinitis. Allergy. 1982;37:389–396. doi: 10.1111/j.1398-9995.1982.tb02317.x. [DOI] [PubMed] [Google Scholar]
- 32.Reinberg A, Gervais P, Levi F. Circadian and circannual rhythms of allergic rhinitis: an epidemiologic study involving chronobiologic methods. J ALLERGY CLIN IMMUNOL. 1988;81:51–62. doi: 10.1016/0091-6749(88)90220-5. [DOI] [PubMed] [Google Scholar]
- 33.Nelson E, Tong YK, Jueng-Kuen L, Halberg F. Methods for cosinor rhythmometry. Chronobiologia. 1979;6:305–323. [PubMed] [Google Scholar]
- 34.Reinberg A, Levi F, Fourtillan JP. Antihistamine and other effects of 5 mg mequitazine vary between morning and evening acute administration. Annu Rev Chronopharmacol. 1984;1:57–60. [Google Scholar]
- 35.Reinberg A, Levi F, Guillet P. A chronopharmacologic study of antihistamines with special reference to terfenadine. Eur J Clin Pharmacol. 1976;14:245–252. doi: 10.1007/BF00560457. [DOI] [PubMed] [Google Scholar]
- 36.Reinberg A. Chronopharmacology of H1-antihistamines. In: Lemmer BJ, editor. Marcel Dekker; New York: 1989. pp. 115–135. (Chronopharmacology: cellular and biochemical interactions.). [Google Scholar]
- 37.Reinberg A, Gervais P, Ugolini C. A multicentric chronotherapeutic study of mequitazine in allergic rhinitis. Annu Rev Chronopharmacol. 1985;3:441–444. [Google Scholar]
- 38.Harter JG, Reddy WJ, Thorn GW. Studies on an intermittent corticosteroid dosage regimen. N Engl J Med. 1963;296:591–595. doi: 10.1056/NEJM196309192691201. [DOI] [PubMed] [Google Scholar]
- 39.Reinberg A, Gervais P, Chaussade M. Circadian changes in the effectiveness of corticosteroids in eight patients with allergic asthma. J ALLERGY CLIN IMMUNOL. 1983;71:425–433. doi: 10.1016/0091-6749(83)90073-8. [DOI] [PubMed] [Google Scholar]
- 40.Reinberg A, Guillet P, Gervais P. One-month chronocorticotherapy (Dutimelan 8-15 mite): control of the asthma condition without adrenal suppression and circadian alteration. Chronobiologia. 1977;4:295–312. [PubMed] [Google Scholar]
- 41.Ceresa F, Angeli A, Buccuzzi G, Molino G. Once-a-day neurally stimulated and basal ACTH secretion phases in man and their response to corticoid inhibition. J Clin Endocrinol. 1969;29:1074–1082. doi: 10.1210/jcem-29-8-1074. [DOI] [PubMed] [Google Scholar]
- 42.Reinberg A, Smolensky MH, D'Alonzo GE, McGovern JP. Chronobiology of asthma. III: timing corticotherapy to biological rhythms to optimize treatment goals. J Asthma. 1988;25:219–248. doi: 10.3109/02770908809071368. [DOI] [PubMed] [Google Scholar]
- 43.Reinberg AE. Chronopharmacology of corticosteroids and ACTH. In: Lemmer BJ, editor. Marcel Dekker; New York: 1989. pp. 137–167. (Chronopharmacology: cellular and biochemical interactions.). [Google Scholar]
- 44.Toogood JH, Baskerville JC, Jennings B. Influence of dosing frequency and schedule on the response of chronic asthmatics to the aerosol steroid, budesonide. J ALLERGY CLIN IMMUNOL. 1982;70:288–298. doi: 10.1016/0091-6749(82)90065-3. [DOI] [PubMed] [Google Scholar]
- 45.Pincus DJ, Szefler SJ, Ackerson LM, Martin RJ. Chronotherapy of asthma with inhaled steroids: the effect of dosage timing on drug efficacy. J ALLERGY CLIN IMMUNOL (Submitted for publication). [DOI] [PubMed]
- 46.Gaultier C, Reinberg A, Girard F. Circadian rhythms in lung resistance and dynamic lung compliance of healthy children: effect of two bronchodilators. Respir Physiol. 1977;31:169–182. doi: 10.1016/0034-5687(77)90100-1. [DOI] [PubMed] [Google Scholar]
- 47.Sibbald B, Rink E. Epidemiology of seasonal and perennial rhinitis: clinical presentation and medical history. Thorax. 1991;46:895–901. doi: 10.1136/thx.46.12.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wihl J-L, Nuchel Petersen B, Nuchel Petersen L. Effect of the nonsedative H1-receptor antagonist astemizole in perennial allergic rhinitis and nonallergic rhinitis. J ALLERGY CLIN IMMUNOL. 1985;75:720–727. doi: 10.1016/0091-6749(85)90100-9. [DOI] [PubMed] [Google Scholar]
- 49.Munch EP, Soborg M, Norreslet TT, Mygind N. A comparative study of dexchlorpheniramine maleate sustained release tablets and budesonide nasal spray in seasonal allergic rhinitis. Allergy. 1983;38:517–552. doi: 10.1111/j.1398-9995.1983.tb02361.x. [DOI] [PubMed] [Google Scholar]
- 50.Gosset P, Malaquin F, Deneste Y. Interleukin-6 and interleukin-1 alpha production is associated with antigen-induced late nasal response. J ALLERGY CLIN IMMUNOL. 1993;92:878–890. doi: 10.1016/0091-6749(93)90066-o. [DOI] [PubMed] [Google Scholar]
- 51.Wasserfallen JF, Leuenberger P, Pecound A. Effect of cetirizine, a new H1 antihistamine, on early and late allergic reactions in a bronchial provocation test with allergen. J ALLERGY CLIN IMMUNOL. 1993;91:1189–1197. doi: 10.1016/0091-6749(93)90322-7. [DOI] [PubMed] [Google Scholar]
- 52.Nish WA, Charlesworth MG, Davis TL. The effect of immunotherapy on the cutaneous late phase response to antigen. J ALLERGY CLIN IMMUNOL. 1994;93:484–493. doi: 10.1016/0091-6749(94)90358-1. [DOI] [PubMed] [Google Scholar]
- 53.Labrecque G, Reinberg AE. Chronopharmacology of nonsteroid anti-inflammatory drugs. In: Lemmer BJ, editor. Marcel Dekker; New York: 1989. pp. 545–579. (Chronopharmacology: cellular and biochemical interactions.). [Google Scholar]
- 54.Labrecque G, Doré F, Bélanger PM. Circadian variation of carrageneenan paw-edema in the rat. Life Sci. 1981;28:1337–1343. doi: 10.1016/0024-3205(81)90406-9. [DOI] [PubMed] [Google Scholar]
- 55.Loubaris N, Cros G, Serrrano JJ. Circadian and circannual variations of the carrageenan inflammatory effect in the rat. Life Sci. 1983:1349–1353. doi: 10.1016/0024-3205(83)90809-3. [DOI] [PubMed] [Google Scholar]
- 56.Soliman KFA, Soliman MR, Owasogo JO, Walker CA. Diurnal variation in the phylogistic response of rats to inflammatory agents. J Pharm Pharmacol. 1983;35:388–389. doi: 10.1111/j.2042-7158.1983.tb02964.x. [DOI] [PubMed] [Google Scholar]
- 57.Labrecque G, Doré F, Bélanger PM. Circadian variations in bradykinin-induced paw edema, in the activity of plasma bradykininase and kininogen in the rat. Proc Can Fed Biol Soc. 1981;24:803. [Google Scholar]
- 58.Doré F, Labrecque G, Bélanger PM, D'Auteuil C. Chronobiologic studies on the hypotensive effect of prostaglandin E2 and arachidonic acid in the rat. Chronobiol Intern. 1984;1:273–278. doi: 10.3109/07420528409063907. [DOI] [PubMed] [Google Scholar]
- 59.Labrecque G, Doré F, Bélanger PM, Carter V. Chronobiological study of plasma exudation in carrageenan-paw oedema in the rats. Agents Actions. 1984;14:719–722. doi: 10.1007/BF01978914. [DOI] [PubMed] [Google Scholar]
- 60.Bureau JP, Coupe M, Labrecque G, Vago P. Chronobiologie de l'inflammation. Pathologie et Biologie (Paris) 1987;35:942–950. [PubMed] [Google Scholar]
- 61.Young MRI, Matthews JP, Kanabrocki EL. Circadian rhythmometry of serum interleukin-2, interleukin-10, tumor necrosis factor-alpha, and granulocyte-macrophage-colony-stimulating factor in adult men. Chronobiol Intern. 1995;12:19–27. doi: 10.3109/07420529509064496. [DOI] [PubMed] [Google Scholar]
- 62.Varbanova A, Nikolov N, Doneshka P. Fluctuations in the vagal and sympathetic tone connected with the circadian cycle and the sleep-wake cycle. Agressologie. 1975;16:27–33. [PubMed] [Google Scholar]
- 63.Reinberg A, Gervais P, Morin M, Abulker C. Rythme circadien humain du seuil de la réponse bronchique á l'acétylcholine. CR Acad Sci (Paris) 1971;272:1879–1881. [PubMed] [Google Scholar]
- 64.Reinberg A, Ghata J, Halberg F. Rythmes circadiens du pouls, de la pression artérielle, des excrétions urinaires en 17-hydroxycorticostéroides, catécholamines et potassium chez l'homme adulte sain, actif et au repos. Ann Endocrinol. 1970;31:277–287. [PubMed] [Google Scholar]


