Abstract
Respiratory virus infections in hematologic stem cell transplant recipients and patients with hematologic malignancies are increasingly recognized as a cause of significant morbidity and mortality. The often overlapping clinical presentation makes molecular diagnostic strategies imperative for rapid diagnosis and to inform understanding of the changing epidemiology of each of the respiratory viruses. Most respiratory virus infections are managed with supportive therapy, although there is effective antiviral therapy for influenza. The primary focus should remain on primary prevention infection control procedures and isolation precautions, avoidance of ill contacts, and vaccination for influenza.
Keywords: Respiratory virus infection, Hematopoietic stem cell transplant, RSV, Influenza, Parainfluenza, Human metapneumovirus, Rhinovirus, Coronavirus
Key points
-
•
The morbidity and associated complications of respiratory virus infections are greater in hematopoietic stem cell transplant recipients and patients with hematologic malignancy than in immunocompetent individuals, with severity of illness related to the degree of immunosuppression.
-
•
Molecular microbiologic testing is the gold standard for diagnosis, allowing differentiation of what are largely overlapping clinical syndromes.
-
•
Most of the respiratory viruses, apart from influenza and in some circumstances respiratory syncytial virus and adenovirus, are managed supportively.
-
•
Prevention is key and should focus on vaccination for influenza, avoidance of ill contacts, and compliance with principles of infection control.
Respiratory virus infections (RVIs) are increasingly recognized as a cause of significant morbidity and mortality in recipients of hematologic stem cell transplant (HCT) and patients with hematologic malignancy (HM).1, 2 With now widespread use of molecular diagnostics, the epidemiology and spectrum of clinical disease of these infections can be better characterized. Apart from influenza, the currently available antivirals are limited in efficacy and/or associated with potential for toxicity, thus emphasizing the importance of prevention strategies. This article provides a review of the epidemiology, clinical characteristics, management, and prevention of RVIs in HCT recipients and HM patients.
Epidemiology and transmission
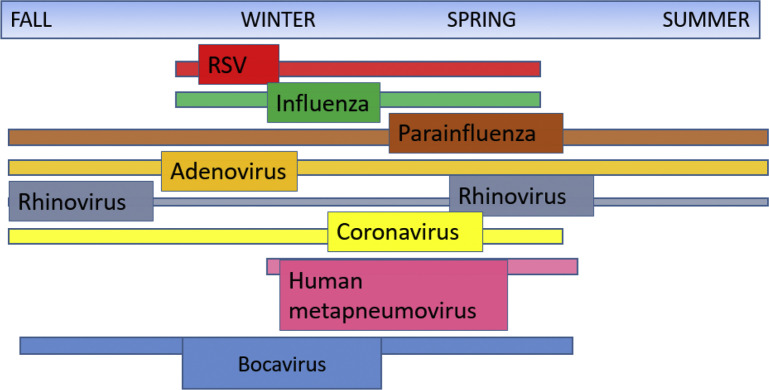
The reported incidence of RVIs (Table 1 ) is wide ranging, a consequence of variation in screening parameters, study population, and testing methodology. Seasonal trends and peaks in patients with HM and HCT recipients mirror those of the community RVI activity, with sometimes significant year-to-year variability in disease incidence and severity (Fig. 1 ). Older studies relied on less sensitive methods, such as viral culture and direct fluorescent antigen, likely underestimating the incidence of RVI.
Table 1.
Incidence of viral infections, rate of lower respiratory tract infection at diagnosis, and mortality rates for respiratory viral infections
| Respiratory Virus | Incidence (%) | Lower Respiratory Tract Infection at Diagnosis (%) | Mortality (%)c |
|---|---|---|---|
| Influenza A/B | 1.3–401, 3, 4, 5, 6, 7, 8, 9,a | 7–442, 3, 4, 5, 6, 7, 8, 10,a | 8–281, 2, 3, 5, 7, 8, 10,a |
| PIV | 3–274, 6, 11, 12,a | 7–502, 4, 5, 8, 13, 14, 15,a | 10–501, 2, 6, 8, 12, 14,a |
| RSV | 1–501, 3, 4, 6, 16, 17,a | 14–702, 3, 4, 5,a | 11–473, 5, 6, 8, 17, 18,a |
| HMPV | 2–111, 2, 3, 4, 19, 20,b | 5-412, 4, 8, 20, 21,b | 6–402, 19, 20, 21,b |
| Adenovirus | 1–301, 2, 4, 8, 22, 23, 24,a | 14–422, 3, 22, 23,a | 14–732, 8, 22, 23,a |
| Rhinovirus | 2–341, 2, 4, 8, 25,a | <5–272, 4, 8, 26,a | <5–412, 26, 27,a |
| CoV | 3–231, 2, 4, 25, 28,b | <52, 4, 8,b | <5–542, 28,b |
| Bocavirus | 1–32, 25, 29, 30,b | 02, 8, 25, 30,b | Not reported25, 29, 30,d |
Studies are a combination of PCR and traditional laboratory methods (eg, culture, direct fluorescent antibody, and enzyme immunoassay).
PCR-based studies.
Includes all-cause and attributable mortality with variable timeframe to death.
One mortality in a coinfected patient attributed to enterovirus/rhinovirus infection.
Fig. 1.
Northern hemisphere seasonality trends.
Although influenza and respiratory syncytial virus (RSV) typically have a defined seasonality, many of the other viruses can occur throughout the year and in overlapping time frames (see Fig. 1). This makes use of broad-range diagnostic strategies like multiplex polymerase chain reaction (PCR) critical, because it is often difficult to differentiate the virus type based on season or clinical presentation.
Respiratory viruses are transmitted by direct contact, fomite, or aerosolized droplet nuclei. Enveloped viruses, such as RSV, parainfluenza virus (PIV), coronavirus (CoV), rhinovirus, and influenza A/B, can remain viable on fomites from 2 hours to 72 hours.31 Transmission can occur through direct contact with infectious material and indirectly through fomites or inhalation of particles.
Clinical presentation
Clinical presentation is nonspecific and variable in severity, with the spectrum of illness ranging from pauci-symptomatic to respiratory failure. Clinical syndromes are categorized as upper respiratory tract infection (URTI), with rhinorrhea, nasal congestion, sinusitis, headache, otitis media, sore throat, malaise and/or fever, and lower respiratory tract infection (LRTI), with localized or diffuse pneumonia.9 Although RVI in the immunocompetent host typically is acute and self-limited, the presentation in HCT recipients in particular can be atypical, severe, and protracted. A majority present with fever and cough, but fever can be absent in approximately a third of the cases.7 Normal findings on chest auscultation with LRTI are present with greater frequency in the immunocompromised population.32
There is considerable overlap in radiographic findings for the RVIs and between RVIs and other infectious and noninfectious entities, on plain film and CT imaging of the chest. Radiographic abnormalities include diffuse bilateral ground-glass infiltrates, small multifocal nodules, bronchial vascular thickening, and/or airspace consolidation.33, 34, 35, 36 A majority of immunocompromised patients have radiographic studies performed and a little more than half have a radiographic abnormality at the time of diagnosis.32 Several studies in the radiology literature have proposed specific imaging features typifying particular RVIs. For example, a retrospective study of CT findings in HCT recipients found that those infected with RSV were more likely to have an airway-centric pattern of disease with tree-in-bud opacities and bronchial wall thickening with or without consolidation. Adenovirus appeared as multifocal consolidation or ground-glass opacities without inflammatory airway findings.37 Although these features may be suggestive, they are not highly specific.
Progression from URTI to LRTI occurs with variable frequency (see Table 1), dependent on RVI type and host factors, and is associated with an increased likelihood of a fatal outcome. The viruses most frequently associated with LRTI include influenza virus, PIV, RSV, and human metapneumovirus (HMPV). Risk factors associated with the development of LRTI include older age, lymphopenia, early post-transplant infection, myeloablative conditioning regimen, unmatched donor status, and conditions, such as graft-versus-host disease (GVHD), necessitating increased immunosuppression.2, 5 Variations in host immune status, through modulation of molecular and cellular response to infection, provide explanation for differences in clinical manifestation. For example, RSV elicits a strong neutrophilic response in the respiratory tract that corresponds with disease severity, whereas CD8+ T cells play a protective role in promoting viral clearance.38
Laboratory diagnosis
Given that the clinical presentations of the various RVIs are nonspecific, microbiologic testing is required, which in turn can inform patient-specific treatment decisions, isolation precautions, and epidemiologic investigation. Serologic testing has limited application given that reinfection is common over time and because humoral responses to viral infections are often not detectable or significantly impaired in the immunocompromised host. Reliance on viral culture is hampered by prolonged turnaround time as well as the abandonment of cell vial culture techniques by clinical laboratories, typically in favor of molecular techniques. That said, viral culture does allow for strain typing and is relevant to allow for characterization of point mutations associated with antiviral drug resistance. Antigen detection by enzyme-based immunoassays are available for some respiratory viruses but lack sufficient sensitivity, particularly for influenza.39, 40
In the current era, diagnosis of RVIs in stem cell transplant recipients and patients with HM is often reliant on molecular diagnostic strategies. In comparison with other methodologies, PCR offers more rapid turnaround time, higher sensitivity, and the ability to test for multiple viruses in a single test with multiplex platforms.39, 41, 42 The ability to detect multiple pathogens based on specific primers has revolutionized molecular diagnostics, allowing for panel-based testing driven by clinical syndrome.43 An important caveat of the high sensitivity of PCR is the detection of virus in the asymptomatic patient, which can represent subclinical infection, asymptomatic shedding, or a false-positive test result. PCR can be performed on various specimen types (eg, nasopharyngeal [NP] washes or swabs, oropharyngeal swab, and bronchoalveolar lavage [BAL] samples). The positive and negative predictive values of NP specimens were 86% and 94%, respectively, when compared with BAL specimens, in a study of paired NP and BAL PCR testing in HCT and HM patients with LRTI.44 Various studies have used quantitative viral load on respiratory and other specimens as a correlate of disease severity to predict outcomes and to gauge response to treatment.45, 46 That said, the practical clinical applications of quantitative PCR are limited at this time. One important exception to this is adenovirus infection, in which case viremia can predict risk for disseminated and end-organ disease as well as survival.47 Although not yet the realm of routine clinical diagnostics, next-generation sequencing techniques, such as whole-genome sequencing, hold potential for better informing RVI outbreak investigations (for example, in the health care setting) than traditional PCR-based diagnostic platforms.48
Complications and outcomes
Coinfection and alloimmune lung syndromes have been associated with RVIs in HCT recipients and have additional impact on morbidity and mortality, beyond the direct and attributable risk of respiratory tract infection. Coinfection with other viruses and superinfection with bacteria or fungi are well described in HCT recipients with RVI.3, 11, 13, 18, 49 RVI seems to be an important risk factor for the development of invasive aspergillosis in allogeneic HCT, although it is difficult to parse out the contribution of viral infection from the milieu of immunosuppression.50 Late airflow decline occurs in a significant proportion of HCT recipients with RVI in the first 100 days after transplant, particularly in those affected by RSV and PIV infection.51 There is a strong association between RVI after HCT and the development of what are termed alloimmune lung syndromes (eg, idiopathic pneumonia syndrome, bronchiolitis obliterans syndrome, and bronchiolitis obliterans organizing pneumonia), which in turn are associated with increased mortality.52 It is postulated that RVI in the early post-transplant period makes the lungs a target for alloimmunity.
Outcome of RVI in HCT recipients is often multifactorial in nature, related to both the direct respiratory impact of infection and the aforementioned complications that contribute to morbidity and mortality risk. Mortality rates vary among the different respiratory viruses and dependent on clinical presentation as well as underlying host factors. Risk factors for increased mortality include LRTI, mismatched allogeneic HCT, infection in the pre-engraftment phase (eg, health care–associated infection during the index transplant hospitalization), high-dose steroids at time of LRTI infection, oxygen requirement, and mechanical ventilation.4, 53, 54, 55 Mortality has been demonstrated to be particularly high for RVI outbreaks in the health care setting, likely a consequence of the high-risk nature of this patient segment and perhaps due to delays in diagnosis in this setting.54, 56, 57 Detection of RVI in the immediate pretransplant period is associated with both LRTI and with decreased survival at 100 days, with mortality greatest in symptomatic patients.54, 58
The 14-day overall mortality was noted to be 12% (6 of 49 with RVI) in a large contemporary multicenter prospective cohort study of allogeneic HCT recipients with diverse infections.59 Although this represents crude and not attributable mortality, short-term outcomes, as opposed to long-term outcomes, are more likely to be related to direct effects of viral infection. Attributable mortality for RVIs is highest in those with LRTI.2, 60 PIV, RSV, adenovirus, HMPV, and influenza are the viruses that most often present with LRTI, and in turn have the highest mortality rate (see Table 1). There have been attempts to develop severity scoring systems to predict risk of LRTI, progression of URTI to LRTI, and mortality after RVI in HCT recipients.61
Management
Management of RVI consists of supportive care and, when available and applicable, prompt initiation of antiviral therapy. Although a significant need, demonstration of clinical benefit in relevant host populations has been a challenge to new antiviral drug development. The use of adjuvant steroids is not advised, because high-dose steroids generally are associated with increased risk of progression to LRTI, prolonged viral shedding, and increased mortality.9, 21, 45, 53, 62, 63, 64 As such, consideration should be given to decreasing the steroid dose whenever feasible to less than 1 mg per/kg/d.
The identification of RVIs in the immediate pretransplant setting raises difficult management questions. Given the risk for increased complications and mortality, conditioning and transplant ideally should be delayed until the RVI is treated and/or there is clinical improvement.58 That said, the decision to delay transplant requires careful consideration, acknowledging both risk for relapse or progression of underlying disease and/or issues related to donor availability.
Prevention
Transmission of RVIs in the health care setting is well documented.9, 56, 57, 65, 66 Health care–associated outbreaks highlight the importance of monitoring patients and visitors for signs and/or symptoms of RVI. Symptomatic patients should be isolated and tested and ill visitors and health care workers excluded from contact with vulnerable populations. Strict compliance with infection control procedures, including isolation precautions and use of relevant personal protective equipment and hand hygiene, is critical to preventing spread of RVI. There are several guidelines available (eg, Centers for Disease Control and Prevention guidelines) that outline specific recommendations regarding isolation strategies.67 Patients with suspected or documented RVI should be placed on droplet and contact precautions.62 Specific isolation protocols for patients with RVI vary from institution to institution. Patient education on avoiding ill contacts and on hand hygiene and influenza vaccination for patients and their close contacts are important interventions to decrease risk for acquisition of RVI.
Prolonged shedding of RVI occurs not infrequently in this host population. Shedding for more than 12 weeks for greater than 10% of allogeneic recipients with either human rhinovirus (HRV) or human CoV (HCoV) infection was reported in 1 study.25 Prolonged asymptomatic shedding poses great challenges for infection prevention, with uncertainty regarding the degree of risk for transmission from asymptomatic shedders. Many centers opt to maintain isolation precautions until signs and symptoms have resolved and repeat respiratory virus testing is confirmed to be negative.
Virus specifics: outcomes and treatment
Influenza A/B
Influenza is a single-stranded enveloped RNA virus belonging to the Orthomyxoviridae family. Influenza A is divided into subtypes based on surface proteins, hemagglutinins, and neuraminidases, with antigenic drifts and shifts resulting from minor and major changes in hemagglutinins and neuraminidases, respectively. Influenza B is less prone to antigenic variation.68
The incidence of infection in HCT recipients and HM patients is driven by the activity of influenza in the community (see Table 1). The development of LRTI is associated with allogeneic HCT status, longer duration of symptoms prior to presentation, neutropenia, lymphopenia, and respiratory coinfection.3, 5, 61, 69 Risk factors associated with increased mortality include older age, LRTI, hypoxemia, mechanical ventilation, lymphopenia, neutropenia, and delayed antiviral therapy.45, 57, 69, 70 Disease severity and outcomes can vary based on the circulating strain, as exemplified by the 2009 H1N1 influenza pandemic, which resulted in greater illness severity and higher morality than seasonal influenza. LRTI occurred in 30% to 40% of patients, and the 30-day mortality rate for H1N1 among HCT recipients in 2009 was approximately 22%.10
Early recognition and treatment decreases the risk of progression to LRTI and improves survival.7, 9, 45 Antiviral treatment should be given as early as possible,7 regardless of the time from symptom onset to presentation in this patient population. Delayed administration has been shown to increase progression to LRTI and is associated with worse outcomes than early administration.45, 71 Neuraminidase inhibitors (NAIs) are the first-line agents for treatment of influenza A or influenza B. The currently available NAIs in the United States include oral oseltamivir, inhaled zanamivir, and intravenous peramivir. The standard dose of oseltamivir for treatment of influenza is 75 mg twice daily for 5 days. There are no data to support the use of high-dose oseltamivir rather than standard dose.71 The optimal treatment duration in the HCT and HM population is not clear, and prospective trials to address this important question are needed. A longer duration of treatment (eg, 10 days) can be considered given the prolonged shedding and the risk for reactivation after remission, particularly with occurrence of infection in the pre-engraftment period.72 The potential benefit of a longer course of antiviral therapy must be balanced against the concern and risk for emergence of resistance in this population. From the era of pandemic H1N1 in 2009, the preponderance of patients with emergence of oseltamivir-resistant virus (H275Y mutation, conferring cross-resistance to peramivir but not to zanamivir) were severely immunocompromised, mainly in HM patients and HCT recipients.73, 74, 75
There are several novel agents in development for treatment of influenza, although not much data is available in HCT recipients or HM patients. The NAI laninamivir octanoate is efficacious against oseltamivir-resistant strains and currently is used in Japan but is not yet Food and Drug Administration approved.76, 77, 78 S-033188, a selective inhibitor of influenza cap-dependent endonuclease, was recently Food and Drug Administration approved for uncomplicated influenza, including resistant strains, but has not yet been studied in complicated influenza or in this patient population.79 DAS181, a recombinant sialidase fusion protein with demonstrated activity against both influenza80 and PIV,81 has completed phase II study in immunocompetent adults with influenza.82 DAS181 seems a potential alternative for NAI-resistant influenza.83 Favipiravir, a viral polymerase inhibitor, is approved in Japan for use against NAI-resistant influenza. Data from a phase II clinical trials of favipiravir for uncomplicated influenza in healthy adults in the United States are forthcoming (https://clinicaltrials.gov/ct2/show/NCT02008344, https://clinicaltrials.gov/ct2/show/NCT02026349).
Adjunctive corticosteroids are generally not advised for the management of influenza. Data from a retrospective study of 143 HCT recipients with seasonal influenza on no, low-dose (<1 mg/kg/d), or high-dose (≥1 mg/kg/d) steroids, largely for management of GVHD, demonstrated no significant difference in development of LRTI, hypoxemia, need for mechanical ventilation, or death but a trend toward a lower risk of LRTI in the group on low-dose steroids.84 Although modest doses of steroids, if indicated for another cause, such as GVHD, may be continued after careful weighing of risks and benefits, steroids should not be started specifically for management of influenza infection.
Given the significant morbidity associated with influenza infection, oseltamivir chemoprophylaxis (75 mg daily for 10–14 days postexposure or for the duration of the exposure in an outbreak setting) should be strongly considered for high-risk patients in the context of a relevant exposure or an outbreak.71, 85 A caution regarding the broad use of prophylaxis is the report of development of oseltamivir-resistant influenza infection in an HM patient after oseltamivir (75 mg twice per day for 7 days) for prophylaxis after an exposure and then subsequent induction chemotherapy, with confirmed transmission of oseltamivir-resistant virus to 3 other patients on the unit.66
Although immune response to vaccination is suboptimal, particularly in HCT recipients and HM patients, vaccination is the cornerstone of prevention of influenza infection and its attendant complications. Receipt of influenza vaccine has been associated not only with a decreased risk for influenza infection in HCT recipients but also with a decrease in influenza severity and complications if infection occurs despite vaccination.7 In a 5-year prospective observational study, allogeneic HCT recipients who received trivalent inactivated influenza vaccination had a significantly lower prevalence of virologically confirmed influenza infection (36% of vaccinated compared with 51% of nonvaccinated recipients; odds ratio [OR] 0.39), a decreased risk for progression to LRTI (OR 0.12) and a lower likelihood of influenza-related hospitalization compared with the nonvaccinated state.29 All individuals greater than 6 months of age without contraindication, including HCT recipients and HM patients, should receive the influenza vaccination annually. Vaccination at less than 6 months post-transplant has been demonstrated to result in poor immune response,86 and 2 doses of the influenza vaccine does not confer superior immunity compared with 1 dose.87 As such, standard-dose inactivated influenza vaccination is strongly recommended for HCT recipients greater than or equal to 6 months post-transplant88 and as early as 3 months to 4 months after transplant in patients without GVHD or otherwise requiring immune suppression89 or during community outbreaks.90 Live attenuated influenza vaccine is not recommended for HCT recipients or other immunocompromised hosts or for their close contacts.90 Pretransplant vaccination (≥2 weeks prior to conditioning) is recommended largely as a strategy to provide early post-transplant protection, although acknowledging seroprotection declines rapidly in the post-transplant setting.91 Given the vulnerability of this population and the suboptimal response to vaccination in the setting of immune compromise, vaccination of close contacts and health care workers is a critical arm of prevention.90, 92 HM patients receiving intensive chemotherapy or who have received anti–B-cell antibodies (eg, rituximab) in the preceding 6 months are unlikely to respond to influenza vaccination.90
Strategies to improve immune response to influenza vaccination include use of higher antigen dose and adjuvanted vaccines. In a large study of nonimmunocompromised adults greater than or equal to 65 years of age, high-dose trivalent vaccine resulted in significantly higher seroprotection rates and better protection against laboratory-confirmed influenza infection than standard-dose vaccine.93 A phase I study comparing high-dose to standard-dose trivalent inactivated vaccine in adult HCT recipients demonstrated a higher rate of seroprotective titers in the high-dose group.94 Small studies of adjuvanted vaccines and multidose vaccination have shown some promise, with improved immunogenicity to certain influenza strains, decreased hospital admission, and reduced mortality.95, 96, 97
Parainfluenza
PIVs are negative-sense, single-stranded, enveloped RNA viruses in the Paramyxoviridae family. There are 4 distinct serotypes, which include serotypes 1 and 3 in the Respirovirus genus and serotypes 2 and 4 in the Rubulavirus genus.
Epidemiologic and clinical features for each of the PIV serotypes vary widely. Although PIV2 and PIV3 circulate yearly, PIV1 has a biennial pattern. The incidence of symptomatic PIV in adult and pediatric HCT recipients is 3% to 27% (see Table 1). The reported incidence of PIV infection in HCT recipients may be falsely low due a larger percentage of asymptomatic or subclinical infections compared with other RVIs.15, 54 PIV3 is the predominant serotype associated with infection in HM patients and HCT recipients.11, 14, 54 Multiple studies have demonstrated PIV3, in comparison to the other serotypes, to be associated with more severe infection, increased risk for progression to LRTI, and higher mortality.12, 54 PIV3 infection is associated with a 1.3-fold increase in mortality, after adjustment for age, CMV serostatus, donor type, and underlying disease.12
In a large systematic review of 28 studies of PIV infection in HM patients and HCT recipients, LRTI occurred in 36% and 39% of patients, respectively. Risk factors for LRTI included allogeneic HCT, early post-transplant infection, lymphopenia, neutropenia, and use of steroids during the URTI phase.14 A retrospective study of 540 HCT recipients spanning 2 decades found an association of progression from URTI to LRTI with PIV3 serotype infection, presence of monocytopenia, greater than 1 mg/kg steroids prior to diagnosis, and copathogen detection. No patients who lacked all of these risk factors progressed to LRTI, whereas the progression risk increased to greater than 30% if 3 or more risk factors were present.64 The need for mechanical ventilation in those with LRTI was 43% and survival from onset of mechanical ventilation was 23%. Although overall mortality with PIV LRTI is high after transplant, mortality decreases with time from transplant.11 PIV-associated mortality is widely variable but is reported to be as high as 50% in those with copathogens (see Table 1).12
There is currently no licensed antiviral agent for the treatment of PIV infection. Despite data supporting in vitro PIV activity, numerous retrospective studies have shown lack of benefit of aerosolized or systemic ribavirin, with or without intravenous immunoglobulin (IVIG), in improving outcomes from an established LRTI or in preventing progression from URTI to LRTI12, 14, 64 or in decreasing viral shedding.12 There are few reports of successful treatment of HCT recipients with serious PIV infection with the investigational agent DAS181,98, 99, 100 and data from a phase 2 study, including HCT recipients and HM patients, are forthcoming (https://clinicaltrials.gov/ct2/show/NCT01644877?cond=das181&rank=9).
Respiratory Syncytial Virus
RSV is a single-stranded, enveloped, negative-sense RNA virus within the Pneumoviridae family, as is HMPV. Two antigenic subtypes (A and B) circulate seasonally, either simultaneously or alternately.
RSV arguably is one of the respiratory virus infections associated with the highest disease severity and mortality risk in HCT recipients. Age, lymphopenia, neutropenia, myeloablative conditioning regimens, pre-engraftment infection or recent HCT, use of marrow or cord blood as graft source, mismatched or unrelated donor, GVHD, high-dose steroids, delayed diagnosis, and detection of viral RNA in serum all have been associated with RSV LRTI or RSV-associated mortality.2, 3, 5, 60, 101, 102, 103, 104 Attempts have been made to develop an immunodeficiency score index for RSV-infected HCT recipients to quantify the risk for LRTI and mortality105 and to assist in decision making for initiating antiviral therapy.
The treatment of RSV infection in HCT recipients remains an area of significant controversy. The absence of high-quality prospective data, the toxicity and cost of available therapies, and the heterogeneity of host factors all contribute to uncertainty regarding the best approach to RSV management.106 The preponderance of existing literature draws on single-center retrospective studies, with their inherent biases and limitations.
Ribavirin, a guanosine analog, is available in aerosolized, intravenous, and oral forms. Important toxicities include myelosuppression and, with the aerosolized form, bronchospasm.107 Aerosolized ribavirin is both extremely costly in light of its orphan drug status108 and complicated to administer given it is a potential teratogen, requiring use of personal protective equipment and a special air flow environment. Palivizumab, an RSV-specific recombinant monoclonal antibody, or polyclonal IVIG has been used in combination with ribavirin, although their contribution to RSV treatment is controversial and difficult to quantify60, 103, 104 and their cost substantial, particularly for palivizumab. RSV monotherapy with palivizumab is neither supported by the literature nor recommended.109 RSV-specific IVIG is no longer available.
Historically, numerous publications have demonstrated a benefit of aerosolized ribavirin (alone or with antibody-based therapy) for RSV, with a decrease rate of progression from URTI to LRTI and a decrease in mortality.60, 103, 104 These studies, however, are severely limited on the basis of their single-center retrospective study design. A randomized controlled multicenter trial of aerosolized ribavirin for RSV URTI in HCT recipients was discontinued because of slow accrual, with failure to meet statistical significance for the primary endpoint of progression to LRTI.46 A trend toward decreasing viral load over time was observed in the group assigned to ribavirin. Definitive prospective data regarding the use of aerosolized ribavirin for RSV infection in this population are lacking and unlikely to be forthcoming.
Systemic ribavirin, by oral or intravenous route and with or without antibody-based therapy, has been explored as a less toxic alternative to nebulized ribavirin for the treatment of RSV, with small case series demonstrating tolerability and suggesting potential efficacy.110, 111, 112 There are more recent retrospective data comparing oral ribavirin with aerosolized ribavirin, providing increasing evidence to support the use of oral ribavirin as a safe, cost-effective, and potentially efficacious alternative to aerosolized ribavirin for HCT recipients and other severely immunocompromised hosts with RSV infection.113, 114
RSV treatment decisions are complicated, taking into account host factors that inform the risk of progression to LRTI, disease severity at presentation, drug toxicity and side-effect profile, and cost. Although the available literature supports the use of ribavirin for high-risk patients with RSV infection, there are profound limitations in generalizing treatment algorithms from uncontrolled studies. To this end, a small case series described low morbidity (19% progression to LRTI) and no mortality in 32 pediatric HCT recipients with RSV infection who were managed without ribavirin and with maintenance IVIG with or without palivizumab.115
Several small molecule antiviral agents are in early phase development or under study for RSV infection.116 Membrane fusion and RNA synthesis are important drug targets. GS-5806 (presatovir), a novel oral fusion protein inhibitor, showed promise in an early phase clinical trial but failed to demonstrate a reduction in nasal RSV viral load or symptom duration in lung transplant recipients.117 Similarly, ALN-RSV01 (asvasiran), a small interfering RNA targeting RSV replication, has not progressed due to limited impact on viral parameters and lack of improvement in symptomatic patients.118, 119 ALS-8176 (lumicitabine), an RNA polymerase inhibitor, was associated with a reduction in viral load and clinical severity compared with placebo in a phase II RSV challenge study in healthy adults,120 although the role of this and other agents awaits clarification in target populations. There are a few investigational agents in phase II study in HCT recipients, namely ALX-0171, a nanobody fusion inhibitor (https://clinicaltrials.gov/ct2/show/NCT03418571 and https://clinicaltrials.gov/ct2/show/NCT03468829)1, 16 and PC786, a non-nucleoside RSV L protein polymerase inhibitor (https://clinicaltrials.gov/ct2/show/study/NCT03715023). No RSV vaccine is currently available, although clinical trials are ongoing.
Adenovirus
Adenoviruses are a family of nonenveloped, double-stranded DNA viruses with more than 60 described serotypes categorized into 7 subgroups or species (A through G). Certain serotypes are associated with particular clinical syndromes, reflecting virus-specific tissue tropism for cellular receptors. Adenoviruses are nonenveloped viruses and can survive for extended periods on environmental surfaces and are resistant to killing by quaternary ammonium compounds, disinfectants widely used in the health care setting. Transmission can occur via aerosolized droplets or fomites as with other RVIs as well as by fecal-oral spread or through exposure to infected tissue or blood products.121 Infection in HCT recipients and other severely immunocompromised hosts can be a consequence of new acquisition or reactivation of endogenous infection.
The spectrum of clinical disease in HCT recipients is broad, including URTI and/or LRTI, enterocolitis, hepatitis, nephritis, hemorrhagic cystitis, and meningoencephalitis.22, 121 Respiratory tract infection is caused most often by subgroups A, B, and C. The incidence is generally higher in the pediatric population.47, 122, 123 Disseminated disease occurs in up to 10% to 20% of patients and is associated with allogeneic HCT, receipt of a T-cell–depleted graft, infection in the early post-transplant period, presence of GVHD, use of systemic steroids, and lymphopenia.23, 24, 121, 124 The presence of viremia as well as the height of the viral load is a meaningful predictor of risk for invasive and disseminated disease as well as mortality.40, 47, 122, 125 Overall mortality for all syndromes can be high, especially so for patients with pneumonia or disseminated disease, particularly in recipients of T cell-depleted grafts.22, 40, 126
Monitoring with PCR and preemptive antiviral treatment is advocated by some experts and supported by several studies in high-risk HCT recipients, notably in the pediatric population.47, 126, 127 That said, other studies, including a prospective study in adult and pediatric non–T-cell–depleted recipients and a retrospective study in pediatric recipients of various graft types and conditioning regimens, have failed to demonstrate a benefit of monitoring and preemptive treatment.128, 129
Currently, there are no antivirals that have a formal indication for adenovirus infection. Cidofovir, a nucleotide analog that inhibits DNA polymerase, is active against all serotypes of adenovirus, with both in vitro and animal data demonstrating virologic and clinical activity, respectively.121 The human data supporting cidofovir for treatment of adenovirus infection in HCT recipients draw largely on retrospective studies demonstrating lower mortality rates in treated compared with untreated patients.122, 126 Pharmacologic disadvantages of cidofovir include active excretion of the unchanged drug in urine with resultant nephrotoxicity and low bioavailability, with limited concentration of the active phosphorylated compound in infected cells.126 Furthermore, toxicities of cidofovir are often dose limiting—mainly nephrotoxicity, myelosuppression, and ocular toxicity. Brincidofovir, an oral lipid ester of cidofovir with improved bioavailability and a better safety profile, is an investigational agent under study for the treatment of adenovirus infection in HCT recipients. Although uncontrolled series have suggested efficacy and tolerability of brincidofovir in treating adenovirus viremia and disease in HCT recipients,130, 131 a randomized placebo-controlled phase II trial failed to meet the primary endpoint of reduction in treatment failure and demonstrated a signal for diarrhea in the group assigned to brincidofovir.132 Although there are in vitro data to suggest that ribavirin has activity against adenovirus, in vivo data have not borne out clinical utility.22, 133 Clearance of viremia and survival are highly associated with lymphocyte reconstitution.134 With this in mind, adoptive T-cell transfer of adenovirus-specific donor T cells remains an area of active investigation.122 That said, despite well over a decade of work on adenovirus-specific cytotoxic T lymphocytes, this approach is far from routine for clinical application. In concert with consideration of antiviral treatment of serious adenovirus infection in HCT recipients, decrease in immune suppression as able also should be entertained.124
Human Metapneumovirus
HMPV, a large enveloped negative-sense RNA virus, is within the Pneumoviridae family, as is RSV. HMPV is a recently described viral infection, identified in 2001 with aid of molecular techniques.135
The reported incidence of HMPV has increased over time, in concert with improved molecular diagnostic capabilities. Knowledge of the disease spectrum continues to evolve, with increasing recognition of HMPV as a cause of RVI in the immunocompromised host population. A prospective study of URTI and LRTI in HM patients and HCT recipients with retrospective analysis for HMPV demonstrated an overall incidence of 9% with LRTI in 41% and death in 14% overall and in one-third of those with LRTI.20 A retrospective study spanning a decade identified 118 cases of HMPV in HCT recipients. LRTI occurred in 25% overall, and progression to LRTI was as high as 60% in those with steroid dose greater than or equal to 1 mg/kg and lymphopenia at time of presentation with URTI.21 A recent systematic review of the literature similarly found a low overall virus-associated mortality rate (6%), but substantially higher mortality (27%) for those with LRTI.2, 19
Although there are in vitro data136 and a few case series or case reports describing use of ribavirin with or without IVIG,137, 138 the preponderance of data, all retrospective in nature, fails to demonstrate a protective effect of ribavirin and/or IVIG in preventing HMPV LRTI and mortality.21, 139 An early but perhaps promising area of HMPV drug discovery research is the novel use of small interfering RNAs.140
Bocavirus
Human bocavirus (HBoV) is a small DNA virus within the Parvoviridae family. HBoV was first described in 2005 by molecular virus screening of NP aspirates from hospitalized children with LRTI.141 Although studies in immunocompetent children with LRTI have demonstrated an incidence as high as 19%, data from the HCT population indicate infrequent infection. A prospective single-center study from Spain spanning 30 months found HBoV in only 6 of 192 (3%) virologically documented RVI episodes in 79 consecutive allogeneic HCT recipients. Interestingly, 5 of the 6 had coinfection with another respiratory virus. Disease severity was not substantial, with only 1 case of LRTI, which was believed likely caused by enterovirus/rhinovirus and no deaths from respiratory failure in the 6 affected patients.28 Viral dissemination has been documented in transplant recipients, with detection of HBoV in blood and stool.142 Given the frequency of coinfection and the mild disease severity, many questions remain regarding the pathogenic potential of HBoV in HCT recipients and other immunocompromised hosts.143 Mode of transmission for HBoV is not well characterized. There is no specific antiviral treatment of HBoV infection.
Coronaviruses
CoVs are positive-sense, single-stranded, enveloped RNA viruses in the Coronaviridae family. CoVs are widespread among birds and animals. CoV in the alpha and beta genera have been associated with human infection: alpha-CoVs include HCoV-229E and HCoV-NL63, and beta-CoVs include HCoV-HKU1, HCoV-OC43, Middle East respiratory syndrome CoV, and severe acute respiratory syndrome CoV. HCoV-229E, HCoV-NL63, HCoV-HKU1, and HCoV-OC43 cocirculate during the nonsummer months.
A prospective surveillance study in HCT recipients detected HCoV in more than 10% of patients in the first year post-transplant, with approximately half of patients asymptomatic and with infrequent development of LRTI,25 suggesting infection is common but typically not severe. Not surprisingly, prospective and retrospective studies of symptomatic cohorts point to higher but variable morbidity and mortality.28, 30, 144 As with other RVIs, copathogen detection with HCoV is frequent,28, 30 making interpretation of attributable mortality complicated. That said, there are well-documented cases of severe and even fatal HCoV LRTI in HM patients and HCT recipients.145, 146 There is no specific antiviral treatment of CoV infection.
Rhinovirus
HRVs are small, single-stranded RNA viruses, members of the Picornaviridae family, genus Enterovirus. Rhinoviruses are classified into 3 species based on capsid features and sequencing: A, B, and C. Rhinoviruses circulate year-round, although with some seasonal clustering by species.27 In contemporary studies that use molecular diagnostic techniques, rhinovirus is the most frequently detected RVI in HM patients and HCT recipients.1, 2, 4, 8, 25, 27 It is important to recognize the limitation of the current diagnostic platforms in the inability to differentiate between rhinovirus and enterovirus.
Clinical presentation is highly variable. In the reported literature, most patients have URTI, although a sizable minority have LRTI. In a large contemporary study of symptomatic HM patients with HRV infection, approximately 30% presented with LRTI.27 Only 5% of those who presented at the URTI stage progressed to LRTI within 30 days, suggesting that symptomatic LRTI is uncommon. As with all RVIs and for HRV especially, it is likely that many studies overestimate the LRTI rate because they fail to account for the greater tendency of patients to seek medical care in the setting of more severe illness. Although some studies have found an association between HRV severity and LRTI and species type,147 this is not a consistent finding.27 Coinfection is common, particularly with LRTI, which makes interpretation of attributable morbidity and mortality challenging.26, 27, 148 In a large retrospective study of approximately 700 HCT recipients with HRV infection spanning more than 2 decades, the overall 90-day mortality in the 128 subjects with LRTI was 41% and was significantly associated with low monocyte count, oxygen requirement at diagnosis, and steroid dose greater than 1 mg/kg/d before diagnosis; survival was not affected by the presence of copathogens.26 As with other RVIs, early post-transplant infection is associated with greater disease severity.149 There is no specific antiviral treatment of HRV infection.
Footnotes
No disclosures.
References
- 1.Paulsen G.C., Danziger-Isakov L. Respiratory viral infections in solid organ and hematopoietic stem cell transplantation. Clin Chest Med. 2017;38(4):707–726. doi: 10.1016/j.ccm.2017.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Renaud C., Campbell A.P. Changing epidemiology of respiratory viral infections in hematopoietic cell transplant recipients and solid organ transplant recipients. Curr Opin Infect Dis. 2011;24(4):333–343. doi: 10.1097/QCO.0b013e3283480440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martino R., Porras R.P., Rabella N., et al. Prospective study of the incidence, clinical features, and outcome of symptomatic upper and lower respiratory tract infections by respiratory viruses in adult recipients of hematopoietic stem cell transplants for hematologic malignancies. Biol Blood Marrow Transplant. 2005;11(10):781–796. doi: 10.1016/j.bbmt.2005.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D'Angelo C.R., Kocherginsky M., Pisano J., et al. Incidence and predictors of respiratory viral infections by multiplex PCR in allogeneic hematopoietic cell transplant recipients 50 years and older including geriatric assessment. Leuk Lymphoma. 2016;57(8):1807–1813. doi: 10.3109/10428194.2015.1113279. [DOI] [PubMed] [Google Scholar]
- 5.Ljungman P., Ward K.N., Crooks B.N., et al. Respiratory virus infections after stem cell transplantation: a prospective study from the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2001;28(5):479–484. doi: 10.1038/sj.bmt.1703139. [DOI] [PubMed] [Google Scholar]
- 6.Chemaly R.F., Ghosh S., Bodey G.P., et al. Respiratory viral infections in adults with hematologic malignancies and human stem cell transplantation recipients: a retrospective study at a major cancer center. Medicine (Baltimore) 2006;85(5):278–287. doi: 10.1097/01.md.0000232560.22098.4e. [DOI] [PubMed] [Google Scholar]
- 7.Kumar D., Ferreira V.H., Blumberg E., et al. A 5-year prospective multicenter evaluation of influenza infection in transplant recipients. Clin Infect Dis. 2018;67(9):1322–1329. doi: 10.1093/cid/ciy294. [DOI] [PubMed] [Google Scholar]
- 8.Shah D.P., Ghantoji S.S., Mulanovich V.E., et al. Management of respiratory viral infections in hematopoietic cell transplant recipients. Am J Blood Res. 2012;2(4):203–218. [PMC free article] [PubMed] [Google Scholar]
- 9.Chemaly R.F., Shah D.P., Boeckh M.J. Management of respiratory viral infections in hematopoietic cell transplant recipients and patients with hematologic malignancies. Clin Infect Dis. 2014;59(Suppl 5):S344–S351. doi: 10.1093/cid/ciu623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reid G., Huprikar S., Patel G., et al. A multicenter evaluation of pandemic influenza A/H1N1 in hematopoietic stem cell transplant recipients. Transpl Infect Dis. 2013;15(5):487–492. doi: 10.1111/tid.12116. [DOI] [PubMed] [Google Scholar]
- 11.Ustun C., Slaby J., Shanley R.M., et al. Human parainfluenza virus infection after hematopoietic stem cell transplantation: risk factors, management, mortality, and changes over time. Biol Blood Marrow Transplant. 2012;18(10):1580–1588. doi: 10.1016/j.bbmt.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nichols W.G., Corey L., Gooley T., et al. Parainfluenza virus infections after hematopoietic stem cell transplantation: risk factors, response to antiviral therapy, and effect on transplant outcome. Blood. 2001;98(3):573–578. doi: 10.1182/blood.v98.3.573. [DOI] [PubMed] [Google Scholar]
- 13.Nichols W.G., Gooley T., Boeckh M. Community-acquired respiratory syncytial virus and parainfluenza virus infections after hematopoietic stem cell transplantation: the Fred Hutchinson Cancer Research Center experience. Biol Blood Marrow Transplant. 2001;7(Suppl):11S–15S. doi: 10.1053/bbmt.2001.v7.pm11777098. [DOI] [PubMed] [Google Scholar]
- 14.Shah D.P., Shah P.K., Azzi J.M., et al. Parainfluenza virus infections in hematopoietic cell transplant recipients and hematologic malignancy patients: a systematic review. Cancer Lett. 2016;370(2):358–364. doi: 10.1016/j.canlet.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peck A.J., Englund J.A., Kuypers J., et al. Respiratory virus infection among hematopoietic cell transplant recipients: evidence for asymptomatic parainfluenza virus infection. Blood. 2007;110(5):1681–1688. doi: 10.1182/blood-2006-12-060343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ljungman P. Respiratory virus infections in stem cell transplant patients: the European experience. Biol Blood Marrow Transplant. 2001;7(Suppl):5S–7S. doi: 10.1053/bbmt.2001.v7.pm11777102. [DOI] [PubMed] [Google Scholar]
- 17.Khanna N., Widmer A.F., Decker M., et al. Respiratory syncytial virus infection in patients with hematological diseases: single-center study and review of the literature. Clin Infect Dis. 2008;46(3):402–412. doi: 10.1086/525263. [DOI] [PubMed] [Google Scholar]
- 18.Campbell A.P., Chien J.W., Kuypers J., et al. Respiratory virus pneumonia after hematopoietic cell transplantation (HCT): associations between viral load in bronchoalveolar lavage samples, viral RNA detection in serum samples, and clinical outcomes of HCT. J Infect Dis. 2010;201(9):1404–1413. doi: 10.1086/651662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shah D.P., Shah P.K., Azzi J.M., et al. Human metapneumovirus infections in hematopoietic cell transplant recipients and hematologic malignancy patients: a systematic review. Cancer Lett. 2016;379(1):100–106. doi: 10.1016/j.canlet.2016.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams J.V., Martino R., Rabella N., et al. A prospective study comparing human metapneumovirus with other respiratory viruses in adults with hematologic malignancies and respiratory tract infections. J Infect Dis. 2005;192(6):1061–1065. doi: 10.1086/432732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seo S., Gooley T.A., Kuypers J.M., et al. Human metapneumovirus infections following hematopoietic cell transplantation: factors associated with disease progression. Clin Infect Dis. 2016;63(2):178–185. doi: 10.1093/cid/ciw284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.La Rosa A.M., Champlin R.E., Mirza N., et al. Adenovirus infections in adult recipients of blood and marrow transplants. Clin Infect Dis. 2001;32(6):871–876. doi: 10.1086/319352. [DOI] [PubMed] [Google Scholar]
- 23.Yilmaz M., Chemaly R.F., Han X.Y., et al. Adenoviral infections in adult allogeneic hematopoietic SCT recipients: a single center experience. Bone Marrow Transplant. 2013;48(9):1218–1223. doi: 10.1038/bmt.2013.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramsay I.D., Attwood C., Irish D., et al. Disseminated adenovirus infection after allogeneic stem cell transplant and the potential role of brincidofovir - case series and 10 year experience of management in an adult transplant cohort. J Clin Virol. 2017;96:73–79. doi: 10.1016/j.jcv.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 25.Milano F., Campbell A.P., Guthrie K.A., et al. Human rhinovirus and coronavirus detection among allogeneic hematopoietic stem cell transplantation recipients. Blood. 2010;115(10):2088–2094. doi: 10.1182/blood-2009-09-244152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seo S., Waghmare A., Scott E.M., et al. Human rhinovirus detection in the lower respiratory tract of hematopoietic cell transplant recipients: association with mortality. Haematologica. 2017;102(6):1120–1130. doi: 10.3324/haematol.2016.153767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacobs S.E., Lamson D.M., Soave R., et al. Clinical and molecular epidemiology of human rhinovirus infections in patients with hematologic malignancy. J Clin Virol. 2015;71:51–58. doi: 10.1016/j.jcv.2015.07.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinana J.L., Madrid S., Perez A., et al. Epidemiologic and clinical characteristics of coronavirus and bocavirus respiratory infections after allogeneic stem cell transplantation: a prospective single-center study. Biol Blood Marrow Transplant. 2018;24(3):563–570. doi: 10.1016/j.bbmt.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pinana J.L., Perez A., Montoro J., et al. Clinical effectiveness of influenza vaccination after allogeneic hematopoietic stem cell transplantation: a cross-sectional prospective observational study. Clin Infect Dis. 2018 doi: 10.1093/cid/ciy792. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogimi C., Waghmare A.A., Kuypers J.M., et al. Clinical significance of human coronavirus in bronchoalveolar lavage samples from hematopoietic cell transplant recipients and patients with hematologic malignancies. Clin Infect Dis. 2017;64(11):1532–1539. doi: 10.1093/cid/cix160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boone S.A., Gerba C.P. Significance of fomites in the spread of respiratory and enteric viral disease. Appl Environ Microbiol. 2007;73(6):1687–1696. doi: 10.1128/AEM.02051-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Memoli M.J., Athota R., Reed S., et al. The natural history of influenza infection in the severely immunocompromised vs nonimmunocompromised hosts. Clin Infect Dis. 2014;58(2):214–224. doi: 10.1093/cid/cit725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franquet T., Rodriguez S., Martino R., et al. Thin-section CT findings in hematopoietic stem cell transplantation recipients with respiratory virus pneumonia. AJR Am J Roentgenol. 2006;187(4):1085–1090. doi: 10.2214/AJR.05.0439. [DOI] [PubMed] [Google Scholar]
- 34.Minnema B.J., Husain S., Mazzulli T., et al. Clinical characteristics and outcome associated with pandemic (2009) H1N1 influenza infection in patients with hematologic malignancies: a retrospective cohort study. Leuk Lymphoma. 2013;54(6):1250–1255. doi: 10.3109/10428194.2012.740558. [DOI] [PubMed] [Google Scholar]
- 35.Kanne J.P., Godwin J.D., Franquet T., et al. Viral pneumonia after hematopoietic stem cell transplantation: high-resolution CT findings. J Thorac Imaging. 2007;22(3):292–299. doi: 10.1097/RTI.0b013e31805467f4. [DOI] [PubMed] [Google Scholar]
- 36.Shiley K.T., Van Deerlin V.M., Miller W.T., Jr. Chest CT features of community-acquired respiratory viral infections in adult inpatients with lower respiratory tract infections. J Thorac Imaging. 2010;25(1):68–75. doi: 10.1097/RTI.0b013e3181b0ba8b. [DOI] [PubMed] [Google Scholar]
- 37.Miller W.T., Jr., Mickus T.J., Barbosa E., Jr., et al. CT of viral lower respiratory tract infections in adults: comparison among viral organisms and between viral and bacterial infections. AJR Am J Roentgenol. 2011;197(5):1088–1095. doi: 10.2214/AJR.11.6501. [DOI] [PubMed] [Google Scholar]
- 38.Russell C.D., Unger S.A., Walton M., et al. The human immune response to respiratory syncytial virus infection. Clin Microbiol Rev. 2017;30(2):481–502. doi: 10.1128/CMR.00090-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuypers J., Campbell A.P., Cent A., et al. Comparison of conventional and molecular detection of respiratory viruses in hematopoietic cell transplant recipients. Transpl Infect Dis. 2009;11(4):298–303. doi: 10.1111/j.1399-3062.2009.00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ganzenmueller T., Buchholz S., Harste G., et al. High lethality of human adenovirus disease in adult allogeneic stem cell transplant recipients with high adenoviral blood load. J Clin Virol. 2011;52(1):55–59. doi: 10.1016/j.jcv.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 41.Zitterkopf N.L., Leekha S., Espy M.J., et al. Relevance of influenza a virus detection by PCR, shell vial assay, and tube cell culture to rapid reporting procedures. J Clin Microbiol. 2006;44(9):3366–3367. doi: 10.1128/JCM.00314-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu M., Qin X., Astion M.L., et al. Implementation of filmarray respiratory viral panel in a core laboratory improves testing turnaround time and patient care. Am J Clin Pathol. 2013;139(1):118–123. doi: 10.1309/AJCPH7X3NLYZPHBW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramanan P., Bryson A.L., Binnicker M.J., et al. Syndromic panel-based testing in clinical microbiology. Clin Microbiol Rev. 2018;31(1) doi: 10.1128/CMR.00024-17. [pii:e00024-17] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hakki M., Strasfeld L.M., Townes J.M. Predictive value of testing nasopharyngeal samples for respiratory viruses in the setting of lower respiratory tract disease. J Clin Microbiol. 2014;52(11):4020–4022. doi: 10.1128/JCM.01944-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choi S.M., Boudreault A.A., Xie H., et al. Differences in clinical outcomes after 2009 influenza A/H1N1 and seasonal influenza among hematopoietic cell transplant recipients. Blood. 2011;117(19):5050–5056. doi: 10.1182/blood-2010-11-319186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boeckh M., Englund J., Li Y., et al. Randomized controlled multicenter trial of aerosolized ribavirin for respiratory syncytial virus upper respiratory tract infection in hematopoietic cell transplant recipients. Clin Infect Dis. 2007;44(2):245–249. doi: 10.1086/509930. [DOI] [PubMed] [Google Scholar]
- 47.Lee Y.J., Chung D., Xiao K., et al. Adenovirus viremia and disease: comparison of T cell-depleted and conventional hematopoietic stem cell transplantation recipients from a single institution. Biol Blood Marrow Transplant. 2013;19(3):387–392. doi: 10.1016/j.bbmt.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Houlihan C.F., Frampton D., Ferns R.B., et al. Use of whole-genome sequencing in the investigation of a nosocomial influenza virus outbreak. J Infect Dis. 2018;218(9):1485–1489. doi: 10.1093/infdis/jiy335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hardak E., Avivi I., Berkun L., et al. Polymicrobial pulmonary infection in patients with hematological malignancies: prevalence, co-pathogens, course and outcome. Infection. 2016;44(4):491–497. doi: 10.1007/s15010-016-0873-3. [DOI] [PubMed] [Google Scholar]
- 50.Martino R., Pinana J.L., Parody R., et al. Lower respiratory tract respiratory virus infections increase the risk of invasive aspergillosis after a reduced-intensity allogeneic hematopoietic SCT. Bone Marrow Transplant. 2009;44(11):749–756. doi: 10.1038/bmt.2009.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Erard V., Chien J.W., Kim H.W., et al. Airflow decline after myeloablative allogeneic hematopoietic cell transplantation: the role of community respiratory viruses. J Infect Dis. 2006;193(12):1619–1625. doi: 10.1086/504268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Versluys A.B., Rossen J.W., van Ewijk B., et al. Strong association between respiratory viral infection early after hematopoietic stem cell transplantation and the development of life-threatening acute and chronic alloimmune lung syndromes. Biol Blood Marrow Transplant. 2010;16(6):782–791. doi: 10.1016/j.bbmt.2009.12.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Renaud C., Xie H., Seo S., et al. Mortality rates of human metapneumovirus and respiratory syncytial virus lower respiratory tract infections in hematopoietic cell transplantation recipients. Biol Blood Marrow Transplant. 2013;19(8):1220–1226. doi: 10.1016/j.bbmt.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kakiuchi S., Tsuji M., Nishimura H., et al. Human parainfluenza virus type 3 infections in patients with hematopoietic stem cell transplants: the mode of nosocomial infections and prognosis. Jpn J Infect Dis. 2018;71(2):109–115. doi: 10.7883/yoken.JJID.2017.424. [DOI] [PubMed] [Google Scholar]
- 55.Fisher B.T., Danziger-Isakov L., Sweet L.R., et al. A multicenter consortium to define the epidemiology and outcomes of inpatient respiratory viral infections in pediatric hematopoietic stem cell transplant recipients. J Pediatr Infect Dis Soc. 2017;7(4):275–282. doi: 10.1093/jpids/pix051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maziarz R.T., Sridharan P., Slater S., et al. Control of an outbreak of human parainfluenza virus 3 in hematopoietic stem cell transplant recipients. Biol Blood Marrow Transplant. 2010;16(2):192–198. doi: 10.1016/j.bbmt.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Espinosa-Aguilar L., Green J.S., Forrest G.N., et al. Novel H1N1 influenza in hematopoietic stem cell transplantation recipients: two centers' experiences. Biol Blood Marrow Transplant. 2011;17(4):566–573. doi: 10.1016/j.bbmt.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 58.Campbell A.P., Guthrie K.A., Englund J.A., et al. Clinical outcomes associated with respiratory virus detection before allogeneic hematopoietic stem cell transplant. Clin Infect Dis. 2015;61(2):192–202. doi: 10.1093/cid/civ272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schuster M.G., Cleveland A.A., Dubberke E.R., et al. Infections in Hematopoietic cell transplant recipients: results from the organ transplant infection project, a multicenter, prospective, cohort study. Open Forum Infect Dis. 2017;4(2):ofx050. doi: 10.1093/ofid/ofx050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shah J.N., Chemaly R.F. Management of RSV infections in adult recipients of hematopoietic stem cell transplantation. Blood. 2011;117(10):2755–2763. doi: 10.1182/blood-2010-08-263400. [DOI] [PubMed] [Google Scholar]
- 61.Kmeid J., Vanichanan J., Shah D.P., et al. Outcomes of influenza infections in hematopoietic cell transplant recipients: application of an immunodeficiency scoring index. Biol Blood Marrow Transplant. 2016;22(3):542–548. doi: 10.1016/j.bbmt.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Waghmare A., Englund J.A., Boeckh M. How I treat respiratory viral infections in the setting of intensive chemotherapy or hematopoietic cell transplantation. Blood. 2016;127(22):2682–2692. doi: 10.1182/blood-2016-01-634873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Damlaj M., Bartoo G., Cartin-Ceba R., et al. Corticosteroid use as adjunct therapy for respiratory syncytial virus infection in adult allogeneic stem cell transplant recipients. Transpl Infect Dis. 2016;18(2):216–226. doi: 10.1111/tid.12513. [DOI] [PubMed] [Google Scholar]
- 64.Seo S., Xie H., Leisenring W.M., et al. Risk factors for parainfluenza virus lower respiratory tract disease after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2018;25(1):163–171. doi: 10.1016/j.bbmt.2018.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hoellein A., Hecker J., Hoffmann D., et al. Serious outbreak of human metapneumovirus in patients with hematologic malignancies. Leuk Lymphoma. 2016;57(3):623–627. doi: 10.3109/10428194.2015.1067699. [DOI] [PubMed] [Google Scholar]
- 66.Chen L.F., Dailey N.J., Rao A.K., et al. Cluster of oseltamivir-resistant 2009 pandemic influenza A (H1N1) virus infections on a hospital ward among immunocompromised patients–North Carolina, 2009. J Infect Dis. 2011;203(6):838–846. doi: 10.1093/infdis/jiq124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Siegel J.D., Rhinehart E., Jackson M., et al. Health Care Infection Control Practices Advisory Committee 2007 guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007;35(10 Suppl 2):S65–S164. doi: 10.1016/j.ajic.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ariza-Heredia E.J., Chemaly R.F. In: Transplant infections. Fourth Edition. Ljungman P., Snydman D., Boeckh M., editors. Springer International Publishing; Cham (Switzerland): 2016. Influenza and parainfluenza infection in hematopoietic stem cell and solid organ transplant recipients; pp. 563–580. [Google Scholar]
- 69.Nichols W.G., Guthrie K.A., Corey L., et al. Influenza infections after hematopoietic stem cell transplantation: risk factors, mortality, and the effect of antiviral therapy. Clin Infect Dis. 2004;39(9):1300–1306. doi: 10.1086/425004. [DOI] [PubMed] [Google Scholar]
- 70.Ljungman P., de la Camara R., Perez-Bercoff L., et al. Outcome of pandemic H1N1 infections in hematopoietic stem cell transplant recipients. Haematologica. 2011;96(8):1231–1235. doi: 10.3324/haematol.2011.041913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Uyeki T.M., Bernstein H.H., Bradley J.S., et al. Clinical practice guidelines by the infectious diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenza. Clin Infect Dis. 2019;68(6):895–902. doi: 10.1093/cid/ciy874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Machado C.M., Boas L.S., Mendes A.V., et al. Use of Oseltamivir to control influenza complications after bone marrow transplantation. Bone Marrow Transplant. 2004;34(2):111–114. doi: 10.1038/sj.bmt.1704534. [DOI] [PubMed] [Google Scholar]
- 73.Centers for Disease Control and Prevention (CDC) Oseltamivir-resistant novel influenza A (H1N1) virus infection in two immunosuppressed patients - Seattle, Washington, 2009. MMWR Morb Mortal Wkly Rep. 2009;58(32):893–896. [PubMed] [Google Scholar]
- 74.Tramontana A.R., George B., Hurt A.C., et al. Oseltamivir resistance in adult oncology and hematology patients infected with pandemic (H1N1) 2009 virus, Australia. Emerg Infect Dis. 2010;16(7):1068–1075. doi: 10.3201/eid1607.091691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Graitcer S.B., Gubareva L., Kamimoto L., et al. Characteristics of patients with oseltamivir-resistant pandemic (H1N1) 2009, United States. Emerg Infect Dis. 2011;17(2):255–257. doi: 10.3201/eid1702.101724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kashiwagi S., Watanabe A., Ikematsu H., et al. Long-acting neuraminidase inhibitor laninamivir octanoate as post-exposure prophylaxis for influenza. Clin Infect Dis. 2016;63(3):330–337. doi: 10.1093/cid/ciw255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Watanabe A., Chang S.C., Kim M.J., et al. Long-acting neuraminidase inhibitor laninamivir octanoate versus oseltamivir for treatment of influenza: a double-blind, randomized, noninferiority clinical trial. Clin Infect Dis. 2010;51(10):1167–1175. doi: 10.1086/656802. [DOI] [PubMed] [Google Scholar]
- 78.Watanabe A. A randomized double-blind controlled study of laninamivir compared with oseltamivir for the treatment of influenza in patients with chronic respiratory diseases. J Infect Chemother. 2013;19(1):89–97. doi: 10.1007/s10156-012-0460-1. [DOI] [PubMed] [Google Scholar]
- 79.Hayden F.G., Sugaya N., Hirotsu N., et al. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med. 2018;379(10):913–923. doi: 10.1056/NEJMoa1716197. [DOI] [PubMed] [Google Scholar]
- 80.Belser J.A., Lu X., Szretter K.J., et al. DAS181, a novel sialidase fusion protein, protects mice from lethal avian influenza H5N1 virus infection. J Infect Dis. 2007;196(10):1493–1499. doi: 10.1086/522609. [DOI] [PubMed] [Google Scholar]
- 81.Moscona A., Porotto M., Palmer S., et al. A recombinant sialidase fusion protein effectively inhibits human parainfluenza viral infection in vitro and in vivo. J Infect Dis. 2010;202(2):234–241. doi: 10.1086/653621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moss R.B., Hansen C., Sanders R.L., et al. A phase II study of DAS181, a novel host directed antiviral for the treatment of influenza infection. J Infect Dis. 2012;206(12):1844–1851. doi: 10.1093/infdis/jis622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Triana-Baltzer G.B., Sanders R.L., Hedlund M., et al. Phenotypic and genotypic characterization of influenza virus mutants selected with the sialidase fusion protein DAS181. J Antimicrob Chemother. 2011;66(1):15–28. doi: 10.1093/jac/dkq387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Boudreault A.A., Xie H., Leisenring W., et al. Impact of corticosteroid treatment and antiviral therapy on clinical outcomes in hematopoietic cell transplant patients infected with influenza virus. Biol Blood Marrow Transplant. 2011;17(7):979–986. doi: 10.1016/j.bbmt.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vu D., Peck A.J., Nichols W.G., et al. Safety and tolerability of oseltamivir prophylaxis in hematopoietic stem cell transplant recipients: a retrospective case-control study. Clin Infect Dis. 2007;45(2):187–193. doi: 10.1086/518985. [DOI] [PubMed] [Google Scholar]
- 86.Machado C.M., Cardoso M.R., da Rocha I.F., et al. The benefit of influenza vaccination after bone marrow transplantation. Bone Marrow Transplant. 2005;36(10):897–900. doi: 10.1038/sj.bmt.1705159. [DOI] [PubMed] [Google Scholar]
- 87.Karras N.A., Weeres M., Sessions W., et al. A randomized trial of one versus two doses of influenza vaccine after allogeneic transplantation. Biol Blood Marrow Transplant. 2013;19(1):109–116. doi: 10.1016/j.bbmt.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ljungman P., Cordonnier C., Einsele H., et al. Vaccination of hematopoietic cell transplant recipients. Bone Marrow Transplant. 2009;44(8):521–526. doi: 10.1038/bmt.2009.263. [DOI] [PubMed] [Google Scholar]
- 89.Avetisyan G., Aschan J., Hassan M., et al. Evaluation of immune responses to seasonal influenza vaccination in healthy volunteers and in patients after stem cell transplantation. Transplantation. 2008;86(2):257–263. doi: 10.1097/TP.0b013e3181772a75. [DOI] [PubMed] [Google Scholar]
- 90.Rubin L.G., Levin M.J., Ljungman P., et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58(3):e44–e100. doi: 10.1093/cid/cit684. [DOI] [PubMed] [Google Scholar]
- 91.Ambati A., Boas L.S., Ljungman P., et al. Evaluation of pretransplant influenza vaccination in hematopoietic SCT: a randomized prospective study. Bone Marrow Transplant. 2015;50(6):858–864. doi: 10.1038/bmt.2015.47. [DOI] [PubMed] [Google Scholar]
- 92.Frenzel E., Chemaly R.F., Ariza-Heredia E., et al. Association of increased influenza vaccination in health care workers with a reduction in nosocomial influenza infections in cancer patients. Am J Infect Control. 2016;44(9):1016–1021. doi: 10.1016/j.ajic.2016.03.024. [DOI] [PubMed] [Google Scholar]
- 93.DiazGranados C.A., Dunning A.J., Kimmel M., et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med. 2014;371(7):635–645. doi: 10.1056/NEJMoa1315727. [DOI] [PubMed] [Google Scholar]
- 94.Halasa N.B., Savani B.N., Asokan I., et al. Randomized double-blind study of the safety and immunogenicity of standard-dose trivalent inactivated influenza vaccine versus high-dose trivalent inactivated influenza vaccine in adult hematopoietic stem cell transplantation patients. Biol Blood Marrow Transplant. 2016;22(3):528–535. doi: 10.1016/j.bbmt.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 95.Ambati A., Einarsdottir S., Magalhaes I., et al. Immunogenicity of virosomal adjuvanted trivalent influenza vaccination in allogeneic stem cell transplant recipients. Transpl Infect Dis. 2015;17(3):371–379. doi: 10.1111/tid.12382. [DOI] [PubMed] [Google Scholar]
- 96.Natori Y., Humar A., Lipton J., et al. A pilot randomized trial of adjuvanted influenza vaccine in adult allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2017;52(7):1016–1021. doi: 10.1038/bmt.2017.24. [DOI] [PubMed] [Google Scholar]
- 97.de Lavallade H., Garland P., Sekine T., et al. Repeated vaccination is required to optimize seroprotection against H1N1 in the immunocompromised host. Haematologica. 2011;96(2):307–314. doi: 10.3324/haematol.2010.032664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chalkias S., Mackenzie M.R., Gay C., et al. DAS181 treatment of hematopoietic stem cell transplant patients with parainfluenza virus lung disease requiring mechanical ventilation. Transpl Infect Dis. 2014;16(1):141–144. doi: 10.1111/tid.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Waghmare A., Wagner T., Andrews R., et al. Successful treatment of parainfluenza virus respiratory tract infection with DAS181 in 4 immunocompromised children. J Pediatr Infect Dis Soc. 2015;4(2):114–118. doi: 10.1093/jpids/piu039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Salvatore M., Satlin M.J., Jacobs S.E., et al. DAS181 for treatment of parainfluenza virus infections in hematopoietic stem cell transplant recipients at a single center. Biol Blood Marrow Transplant. 2016;22(5):965–970. doi: 10.1016/j.bbmt.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 101.Seo S., Campbell A.P., Xie H., et al. Outcome of respiratory syncytial virus lower respiratory tract disease in hematopoietic cell transplant recipients receiving aerosolized ribavirin: significance of stem cell source and oxygen requirement. Biol Blood Marrow Transplant. 2013;19(4):589–596. doi: 10.1016/j.bbmt.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim Y.J., Guthrie K.A., Waghmare A., et al. Respiratory syncytial virus in hematopoietic cell transplant recipients: factors determining progression to lower respiratory tract disease. J Infect Dis. 2014;209(8):1195–1204. doi: 10.1093/infdis/jit832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shah D.P., Ghantoji S.S., Shah J.N., et al. Impact of aerosolized ribavirin on mortality in 280 allogeneic haematopoietic stem cell transplant recipients with respiratory syncytial virus infections. J Antimicrob Chemother. 2013;68(8):1872–1880. doi: 10.1093/jac/dkt111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Waghmare A., Campbell A.P., Xie H., et al. Respiratory syncytial virus lower respiratory disease in hematopoietic cell transplant recipients: viral RNA detection in blood, antiviral treatment, and clinical outcomes. Clin Infect Dis. 2013;57(12):1731–1741. doi: 10.1093/cid/cit639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shah D.P., Ghantoji S.S., Ariza-Heredia E.J., et al. Immunodeficiency scoring index to predict poor outcomes in hematopoietic cell transplant recipients with RSV infections. Blood. 2014;123(21):3263–3268. doi: 10.1182/blood-2013-12-541359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Griffiths C., Drews S.J., Marchant D.J. Respiratory syncytial virus: infection, detection, and new options for prevention and treatment. Clin Microbiol Rev. 2017;30(1):277–319. doi: 10.1128/CMR.00010-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Whimbey E., Champlin R.E., Englund J.A., et al. Combination therapy with aerosolized ribavirin and intravenous immunoglobulin for respiratory syncytial virus disease in adult bone marrow transplant recipients. Bone Marrow Transplant. 1995;16(3):393–399. [PubMed] [Google Scholar]
- 108.Chemaly R.F., Aitken S.L., Wolfe C.R., et al. Aerosolized ribavirin: the most expensive drug for pneumonia. Transpl Infect Dis. 2016;18(4):634–636. doi: 10.1111/tid.12551. [DOI] [PubMed] [Google Scholar]
- 109.de Fontbrune F.S., Robin M., Porcher R., et al. Palivizumab treatment of respiratory syncytial virus infection after allogeneic hematopoietic stem cell transplantation. Clin Infect Dis. 2007;45(8):1019–1024. doi: 10.1086/521912. [DOI] [PubMed] [Google Scholar]
- 110.Gueller S., Duenzinger U., Wolf T., et al. Successful systemic high-dose ribavirin treatment of respiratory syncytial virus-induced infections occurring pre-engraftment in allogeneic hematopoietic stem cell transplant recipients. Transpl Infect Dis. 2013;15(4):435–440. doi: 10.1111/tid.12092. [DOI] [PubMed] [Google Scholar]
- 111.Marcelin J.R., Wilson J.W., Razonable R.R., Mayo Clinic Hematology/Oncology and Transplant Infectious Diseases Services Oral ribavirin therapy for respiratory syncytial virus infections in moderately to severely immunocompromised patients. Transpl Infect Dis. 2014;16(2):242–250. doi: 10.1111/tid.12194. [DOI] [PubMed] [Google Scholar]
- 112.Gorcea C.M., Tholouli E., Turner A., et al. Effective use of oral ribavirin for respiratory syncytial viral infections in allogeneic haematopoietic stem cell transplant recipients. J Hosp Infect. 2017;95(2):214–217. doi: 10.1016/j.jhin.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 113.Foolad F., Aitken S.L., Shigle T.L., et al. Oral versus aerosolized ribavirin for the treatment of respiratory syncytial virus infections in hematopoietic cell transplantation recipients. Clin Infect Dis. 2018 doi: 10.1093/cid/ciy760. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Trang T.P., Whalen M., Hilts-Horeczko A., et al. Comparative effectiveness of aerosolized versus oral ribavirin for the treatment of respiratory syncytial virus infections: a single-center retrospective cohort study and review of the literature. Transpl Infect Dis. 2018;20(2):e12844. doi: 10.1111/tid.12844. [DOI] [PubMed] [Google Scholar]
- 115.El-Bietar J., Nelson A., Wallace G., et al. RSV infection without ribavirin treatment in pediatric hematopoietic stem cell transplantation. Bone Marrow Transplant. 2016;51(10):1382–1384. doi: 10.1038/bmt.2016.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Brendish N.J., Clark T.W. Antiviral treatment of severe non-influenza respiratory virus infection. Curr Opin Infect Dis. 2017;30(6):573–578. doi: 10.1097/QCO.0000000000000410. [DOI] [PubMed] [Google Scholar]
- 117.Gottlieb J.T.F., Haddad T. A phase 2b randomized controlled trial of presatovir, an oral RSV fusion inhibitor, for the treatment of respiratory syncytial virus (RSV) in lung transplant (LT) recipients. J Heart Lung Transplant. 2018;37(4):S155. [Google Scholar]
- 118.DeVincenzo J., Lambkin-Williams R., Wilkinson T., et al. A randomized, double-blind, placebo-controlled study of an RNAi-based therapy directed against respiratory syncytial virus. Proc Natl Acad Sci U S A. 2010;107(19):8800–8805. doi: 10.1073/pnas.0912186107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gottlieb J., Zamora M.R., Hodges T., et al. ALN-RSV01 for prevention of bronchiolitis obliterans syndrome after respiratory syncytial virus infection in lung transplant recipients. J Heart Lung Transplant. 2016;35(2):213–221. doi: 10.1016/j.healun.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 120.DeVincenzo J.P., McClure M.W., Symons J.A., et al. Activity of oral ALS-008176 in a respiratory syncytial virus challenge study. N Engl J Med. 2015;373(21):2048–2058. doi: 10.1056/NEJMoa1413275. [DOI] [PubMed] [Google Scholar]
- 121.Ison M.G. Adenovirus infections in transplant recipients. Clin Infect Dis. 2006;43(3):331–339. doi: 10.1086/505498. [DOI] [PubMed] [Google Scholar]
- 122.Lee Y.J., Prockop S.E., Papanicolaou G.A. Approach to adenovirus infections in the setting of hematopoietic cell transplantation. Curr Opin Infect Dis. 2017;30(4):377–387. doi: 10.1097/QCO.0000000000000379. [DOI] [PubMed] [Google Scholar]
- 123.Bruno B., Gooley T., Hackman R.C., et al. Adenovirus infection in hematopoietic stem cell transplantation: effect of ganciclovir and impact on survival. Biol Blood Marrow Transplant. 2003;9(5):341–352. doi: 10.1016/s1083-8791(03)00102-2. [DOI] [PubMed] [Google Scholar]
- 124.Chakrabarti S., Mautner V., Osman H., et al. Adenovirus infections following allogeneic stem cell transplantation: incidence and outcome in relation to graft manipulation, immunosuppression, and immune recovery. Blood. 2002;100(5):1619–1627. doi: 10.1182/blood-2002-02-0377. [DOI] [PubMed] [Google Scholar]
- 125.Schilham M.W., Claas E.C., van Zaane W., et al. High levels of adenovirus DNA in serum correlate with fatal outcome of adenovirus infection in children after allogeneic stem-cell transplantation. Clin Infect Dis. 2002;35(5):526–532. doi: 10.1086/341770. [DOI] [PubMed] [Google Scholar]
- 126.Lindemans C.A., Leen A.M., Boelens J.J. How I treat adenovirus in hematopoietic stem cell transplant recipients. Blood. 2010;116(25):5476–5485. doi: 10.1182/blood-2010-04-259291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lion T., Kosulin K., Landlinger C., et al. Monitoring of adenovirus load in stool by real-time PCR permits early detection of impending invasive infection in patients after allogeneic stem cell transplantation. Leukemia. 2010;24(4):706–714. doi: 10.1038/leu.2010.4. [DOI] [PubMed] [Google Scholar]
- 128.Ohrmalm L., Lindblom A., Omar H., et al. Evaluation of a surveillance strategy for early detection of adenovirus by PCR of peripheral blood in hematopoietic SCT recipients: incidence and outcome. Bone Marrow Transplant. 2011;46(2):267–272. doi: 10.1038/bmt.2010.86. [DOI] [PubMed] [Google Scholar]
- 129.Walls T., Hawrami K., Ushiro-Lumb I., et al. Adenovirus infection after pediatric bone marrow transplantation: is treatment always necessary? Clin Infect Dis. 2005;40(9):1244–1249. doi: 10.1086/429235. [DOI] [PubMed] [Google Scholar]
- 130.Florescu D.F., Pergam S.A., Neely M.N., et al. Safety and efficacy of CMX001 as salvage therapy for severe adenovirus infections in immunocompromised patients. Biol Blood Marrow Transplant. 2012;18(5):731–738. doi: 10.1016/j.bbmt.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hiwarkar P., Amrolia P., Sivaprakasam P., et al. Brincidofovir is highly efficacious in controlling adenoviremia in pediatric recipients of hematopoietic cell transplant. Blood. 2017;129(14):2033–2037. doi: 10.1182/blood-2016-11-749721. [DOI] [PubMed] [Google Scholar]
- 132.Grimley M.S., Chemaly R.F., Englund J.A., et al. Brincidofovir for asymptomatic adenovirus viremia in pediatric and adult allogeneic hematopoietic cell transplant recipients: a randomized placebo-controlled phase II trial. Biol Blood Marrow Transplant. 2017;23(3):512–521. doi: 10.1016/j.bbmt.2016.12.621. [DOI] [PubMed] [Google Scholar]
- 133.Lankester A.C., Heemskerk B., Claas E.C., et al. Effect of ribavirin on the plasma viral DNA load in patients with disseminating adenovirus infection. Clin Infect Dis. 2004;38(11):1521–1525. doi: 10.1086/420817. [DOI] [PubMed] [Google Scholar]
- 134.Heemskerk B., Lankester A.C., van Vreeswijk T., et al. Immune reconstitution and clearance of human adenovirus viremia in pediatric stem-cell recipients. J Infect Dis. 2005;191(4):520–530. doi: 10.1086/427513. [DOI] [PubMed] [Google Scholar]
- 135.van den Hoogen B.G., de Jong J.C., Groen J., et al. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7(6):719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wyde P.R., Chetty S.N., Jewell A.M., et al. Comparison of the inhibition of human metapneumovirus and respiratory syncytial virus by ribavirin and immune serum globulin in vitro. Antiviral Res. 2003;60(1):51–59. doi: 10.1016/s0166-3542(03)00153-0. [DOI] [PubMed] [Google Scholar]
- 137.Shahda S., Carlos W.G., Kiel P.J., et al. The human metapneumovirus: a case series and review of the literature. Transpl Infect Dis. 2011;13(3):324–328. doi: 10.1111/j.1399-3062.2010.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hoppe B.P., de Jongh E., Griffioen-Keijzer A., et al. Human metapneumovirus in haematopoietic stem cell transplantation recipients: a case series and review of the diagnostic and therapeutic approach. Neth J Med. 2016;74(8):336–341. [PubMed] [Google Scholar]
- 139.El Chaer F., Shah D.P., Kmeid J., et al. Burden of human metapneumovirus infections in patients with cancer: Risk factors and outcomes. Cancer. 2017;123(12):2329–2337. doi: 10.1002/cncr.30599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nitschinsk K.M., Clarke D.T., Idris A., et al. RNAi targeting of human metapneumovirus P and N genes inhibits viral growth. Intervirology. 2018;61(3):149–154. doi: 10.1159/000491927. [DOI] [PubMed] [Google Scholar]
- 141.Allander T., Tammi M.T., Eriksson M., et al. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci U S A. 2005;102(36):12891–12896. doi: 10.1073/pnas.0504666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Schenk T., Strahm B., Kontny U., et al. Disseminated bocavirus infection after stem cell transplant. Emerg Infect Dis. 2007;13(9):1425–1427. doi: 10.3201/eid1309.070318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Schildgen O., Muller A., Allander T., et al. Human bocavirus: passenger or pathogen in acute respiratory tract infections? Clin Microbiol Rev. 2008;21(2):291–304. doi: 10.1128/CMR.00030-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hakki M., Rattray R.M., Press R.D. The clinical impact of coronavirus infection in patients with hematologic malignancies and hematopoietic stem cell transplant recipients. J Clin Virol. 2015;68:1–5. doi: 10.1016/j.jcv.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pene F., Merlat A., Vabret A., et al. Coronavirus 229E-related pneumonia in immunocompromised patients. Clin Infect Dis. 2003;37(7):929–932. doi: 10.1086/377612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Oosterhof L., Christensen C.B., Sengelov H. Fatal lower respiratory tract disease with human corona virus NL63 in an adult haematopoietic cell transplant recipient. Bone Marrow Transplant. 2010;45(6):1115–1116. doi: 10.1038/bmt.2009.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ferguson P.E., Gilroy N.M., Faux C.E., et al. Human rhinovirus C in adult haematopoietic stem cell transplant recipients with respiratory illness. J Clin Virol. 2013;56(3):255–259. doi: 10.1016/j.jcv.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ison M.G., Hayden F.G., Kaiser L., et al. Rhinovirus infections in hematopoietic stem cell transplant recipients with pneumonia. Clin Infect Dis. 2003;36(9):1139–1143. doi: 10.1086/374340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ghosh S., Champlin R., Couch R., et al. Rhinovirus infections in myelosuppressed adult blood and marrow transplant recipients. Clin Infect Dis. 1999;29(3):528–532. doi: 10.1086/598627. [DOI] [PubMed] [Google Scholar]