Abstract
Background
Whether or not short-term exposure to particulate matter <2.5 μm in diameter (PM2.5) increases the risk of psychiatric emergency diseases is unclear.
Methods
The study was performed in a metropolis from January 2015 to December 2016. The exposure was PM2.5, and the confounders were weather (temperature and humidity) and other pollutants (PM10, SO2, CO, O3, and NO2). The outcomes were emergency department (ED) visits with psychiatric disease codes (F00-F99 in ICD10 codes). General additive models were used for the statistical analysis to calculate the adjusted relative risks (ARRs) and 95% confidence intervals (95% CIs) for the daily number of ED visits with a lag of 1 to 3 days following a 10 μg/m3 increase in PM2.5.
Results
During the study period, a total of 67,561 ED visits for psychiatric diseases were identified and tested for association with PM2.5. Daily ED visits for all psychiatric diseases were not associated with PM2.5 in the model that was not adjusted for other pollutants. The ARR (95% CI) in the model adjusted for SO2 was 1.011 (1.002–1.021) by 10 μg/m3 of PM2.5 on Lag 1 for all psychiatric diseases (F00-F99). The ARR (95% CI) in the model adjusted for O3 was 1.015 (1.003–1.029) by 10 μg/m3 of PM2.5 on Lag 1 for F40-F49 (Neurotic, stress-related and somatoform disorders).
Conclusion
An increase in PM2.5 showed a significant association with an increase in ED visits for all psychiatric diseases (F00-F99) and for neurotic, stress-related and somatoform disorders (F40-F49) on lag day 1.
Keywords: Particular matter, Emergency department, Psychiatry disease
1. Introduction
Psychiatric diseases account for approximately 6.2% of the global disease burden measured by disability adjusted life years (DALYs) and affected >400 million people globally in 2015 [1]. Approximately 4.3 million people visited emergency department for emergency psychiatric diseases in the USA, which accounted for 21 visits per 1000 adults in 2000 [2]. The risk factors must be identified to prevent acute exacerbation of psychiatric diseases.
PM2.5, which is the abbreviation for particulate matter 2.5 μm or less in diameter, is one of the most important potential environmental hazards for health conditions and mortality. Ambient PM2.5 exposure has been found to be a leading cause of death and disability worldwide [3]. A 10 μg/m3 increase in PM2.5 corresponds to a 0.68% increase in cardiovascular mortality [4]. Short-term exposure to PM2.5 induces respiratory hospital admissions [5,6], increases total cerebrovascular disease mortality [7], and even aggravates already diagnosed Parkinson's disease [8].
Although studies have investigated the relationship between emergency department visits for depression and ambient PM2.5 exposure [[9], [10], [11], [12]], studies on other emergency psychiatric diseases and PM2.5 are rare. Air pollutants may cause activation of inflammatory processes, the immune system, oxidative stress, and alterations in cerebral neurotransmitter concentrations [[13], [14], [15]], which can lead to mental or behavioral alterations. Therefore, an increase in PM2.5 may exacerbate emergency psychiatric diseases. The purpose of this study is to determine the association between ambient PM2.5 exposure and visits to the emergency department (ED) for emergency psychiatric diseases.
2. Methods
2.1. Study design and setting
This study is a cross-sectional observational study to evaluate the association between the number of patients visiting the ED for psychiatric diseases and the PM2.5 concentration in Seoul, Korea.
According to the National Statistical Office, the population of Seoul is 10 million people, which represents one-fifth of the Korean population. Seoul is one of the most highly populated cities in the world, with a population density of 16,492/km2 reported in 2017. Seoul has an area of 605 km2 and consists of 25 health and administrative districts. According to the Korea Meteorological Administration, the average annual temperature of Seoul is 12.5 °C (54.5 °F), but the temperature varies greatly depending on the season, with an average temperature in August of 25.7 °C (78.3 °F) and in January of −2.4 °C (27.7 °F). Seoul has a temperate climate with four distinct seasons. The annual average PM2.5 concentrations in Seoul were 23 μg/m3 and 26 μg/m3 in 2015 and 2016, respectively, according to the National Institute of Environmental Research.
According to statistics from the Ministry of Health and Welfare, there are 51 emergency departments in Seoul, which the national government has classified into three groups. These departments include 4 level 1 departments with a mission to provide a full range of emergency care services and disaster medical services that are staffed by emergency physicians 24 h per day and 7 days per week, 27 level 2 emergency departments where emergency physicians are on standby 24 h per day 365 days per year to provide general comprehensive emergency care services, and 20 level 3 emergency departments staffed by general physicians to provide general emergency care services. A total of 610 emergency medicine specialists, 217 residents, and 1290 nurses worked at the emergency departments in Seoul in 2016. A total of 1.8 million citizens visit the emergency departments annually in Seoul, including more than thirty thousand visits for psychiatric emergency diseases per year.
2.2. Data sources
We used four data sources for this study. The first data set for the Seoul population was collected from the National Statistical Office. The data set includes the population size, age distribution, gender distribution, and residence on a nationwide scale for policy making every year using family registry documents.
The second set of data on emergency department visits from the 51 hospitals in Seoul was obtained from the National Emergency Department Information System (NEDIS) of the National Emergency Medical Center (NEMC). The NEMC is the central headquarters of the emergency care system for quality assurance, evaluation, and emergency care program grants. The NEMC has collected NEDIS data since 2004 from 16 level 1 EDs; the NEMC collected data from 408 of the 413 emergency departments in the country in 2016 and from 403 emergency departments out of 420 in 2015. All 51 emergency departments in Seoul were included in both years. The NEDIS data include sex, age, when the event occurred, the patient's main symptom, the diagnosis, which emergency department the patient visited, and the address of the patient. The data are verified by the automatic system; if modification or reexamination is necessary, each emergency department revises the data [16].
The third set of meteorological data, including the daily mean temperature, relative humidity, and air pressure, was obtained from the Korean Meteorological Administration. The administration was established in 1949 and has collected meteorological data since that time. In Seoul, 35 automatic weather systems (AWSs) measure weather information every minute to inform citizens of weather situations and collect data for research. [17]
The fourth data source provided air pollutant monitoring data obtained from the Korean National Institute of Environmental Research. This data source provides the average concentrations of air pollutants, including PM2.5, PM10, carbon oxide (CO), nitric dioxide (NO2), sulfur dioxide (SO2), and ozone (O3), every hour using monitoring stations. The institute collects data from 40 monitoring stations in Seoul to provide information to citizens and researchers as a pollution alarm. [18]
2.3. Study population
The population aged 15 years and older in 2015 and 2016 was included in this study. We excluded children (<15 years old) and individuals who were not registered as residents at the district office, such as temporary visiting foreigners and travelers.
2.4. Main exposure and variables
We used the daily average concentration of the hourly PM2.5 for each district as the main exposure. The daily average concentration was provided by the data source. The variables included the daily mean temperature, relative humidity, air pressure, age, gender, day of the week, season, and patient addresses. The Middle East respiratory syndrome (MERS) outbreak occurred over a two-month period in 2015 when several EDs were closed, and the number of ED visits decreased. Therefore, the MERS outbreak period was added as a variable. The PM2.5 concentration was measured using the beta-ray absorption method, the PM10 concentration was measured using beta-ray absorption in μg/m3, CO was measured with the non-dispersive infrared method, NO2 was measured using chemiluminescence, SO2 with measured with ultraviolet fluorescence, and O3 was measured using ultraviolet photometry.
2.5. Outcome measurements
The outcome was the daily number of people who visited the emergency departments in Seoul from 2015 to 2016 with a diagnostic code for psychiatric diseases (International Classification of Disease-1oth version, Mental and Behavioral Disease, F00–F99, including F00-F09 (Organic, including symptomatic, mental disorders), F10-F19 (Mental and behavioral disorders due to psychoactive substance use), F20-F29 (Schizophrenia, schizotypal and delusional disorders), F30-F39 (Mood [affective] disorders), F40-F48 (Neurotic, stress-related and somatoform disorders), F50-F59 (Behavioral syndromes associated with physiological disturbances and physical factors), F60-F69 (Disorders of adult personality and behavior), F70-F79 (Mental retardation), F80-F89 (Disorders of psychological development), F90-F98 (Behavioral and emotional disorders with onset usually occurring in childhood and adolescence), and F99-F99 (Unspecified mental disorder)). The diagnostic codes for ED care were inputted by the emergency physicians when the patients were discharged to home, transferred to another hospital, or admitted to a ward. In addition to the ED diagnosis, the duty physicians inputted the diagnostic codes into the electronic medical records when the patients were discharged after admission. A maximum of 20 diagnostics codes can be uploaded to the NEDIS server. The NEDIS diagnostic codes are used for the National Emergency Department Evaluation Program, which is performed annually by the Ministry of Health and Welfare as a source of diagnostic codes, and are also used for the reimbursement program of the National Health Insurance Corporation. Therefore, most hospitals rigorously manage the diagnostic codes to ensure reliability and validity.
2.6. Statistical analysis
Demographic information was collected, including information for patients with emergency psychiatric diseases (age, gender, season, month, day of the week, disease category, MERS period, and district), weather (daily temperature, humidity, and pressure), and pollutants (PM10, CO, NO2, SO2, and O3). We determined the correlations between the pollutants using the R package version 3.2.3 (R Foundation for Statistical Computing, Vienna, Austria).
A time-series analysis using a generalized additive model (GAM) was used to calculate adjusted relative risks (ARRs) with 95% confidence intervals (95% CIs), because most epidemiological air pollution studies (cited in the second paragraph of the article) used this approach. The model was controlled for daily mean temperature, relative humidity, and mean air pressure, which were treated as continuous variables, as well as for the day of the week. The basic model is as follows: Log [E(Y)] = β0 + S1(X1) + S2(X2) + ⋯ + Sp(Xp) + β(PM2.5), where Y is the daily number of emergency department visits for psychiatric diseases, X1, …, Xp are covariates, S i() is a nonparametric smoother, and PM2.5 is the mean value for the corresponding day. A total of 4 degrees of freedom were selected to smooth the time trends. We examined the concentrations of the individual lag days 1 through 3 (lag 1 represents the PM2.5 level one day prior to the emergency room visit, lag 2 is the previous day, and so on). We tested the association between PM2.5 and all emergency psychiatric diseases and subgroups of emergency psychiatric diseases (F20 to F50) to calculate the ARRs (95% CIs) using the GAM model described above. All statistical analyses for the GAM data processing were performed using the SAS software, version 9.4 (SAS institute Inc., Cary, NC, USA).
3. Results
3.1. Basic characteristics
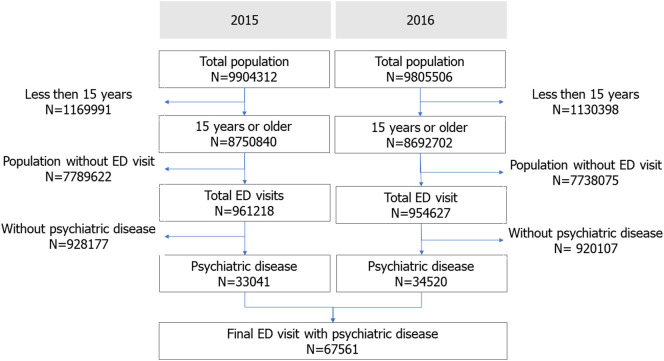
The study population over 15 years of age in Seoul included 8,734,321 citizens in 2015 and 8,675,108 citizens in 2016. In total, 67,561 patients with psychiatric emergencies (33,041 in 2015 and 34,520 in 2016) were analyzed (Fig. 1 ).
Fig. 1.
Study population.
ED, emergency department.
Table 1 shows the study population and the numbers of ED visits. A total of 67,561 emergency department visits were identified in Seoul from January 2015 to December 2016 (731 days). Emergency department visits for ICD codes F10-F19 (Mental and behavioral disorders due to psychoactive substance use) represented the largest subgroup, because alcohol abuse was included. Generally, the total ED visits were higher in women than in men, although this trend was not observed in some subgroups. More visits occurred in the summer than in the winter.
Table 1.
Demographic characteristics of emergency department patients with psychiatric diseases.
| Variables | Total |
2015 |
2016 |
||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| All | 67,561 | 100.0 | 30,472 | 100.0 | 32,997 | 100.0 | |
| Sex | Male | 30,193 | 44.7 | 14,481 | 47.5 | 15,712 | 47.6 |
| Female | 33,276 | 49.3 | 15,991 | 52.5 | 17,285 | 52.4 | |
| Age group, years | 15–29 | 9747 | 14.4 | 4624 | 15.2 | 5123 | 15.5 |
| 30–44 | 12,069 | 17.9 | 5743 | 18.8 | 6326 | 19.2 | |
| 45–59 | 16,756 | 24.8 | 8087 | 26.5 | 8669 | 26.3 | |
| 60–74 | 12,056 | 17.8 | 5899 | 19.4 | 6157 | 18.7 | |
| 75+ | 12,841 | 19.0 | 6119 | 20.1 | 6722 | 20.4 | |
| Season | Spring (March–May) | 16,973 | 25.1 | 7873 | 25.8 | 8378 | 25.4 |
| Summer (June–August) | 17,767 | 26.3 | 7670 | 25.2 | 8853 | 26.8 | |
| Autumn (September–November) | 16,867 | 25.0 | 7687 | 25.2 | 8285 | 25.1 | |
| Winter (December–February) | 15,954 | 23.6 | 7242 | 23.8 | 7481 | 22.7 | |
| Day of week | Sun | 12,011 | 17.8 | 5664 | 18.6 | 6347 | 19.2 |
| Mon | 8920 | 13.2 | 4383 | 14.4 | 4537 | 13.7 | |
| Tue | 8557 | 12.7 | 4168 | 13.7 | 4389 | 13.3 | |
| Wed | 8136 | 12.0 | 4048 | 13.3 | 4088 | 12.4 | |
| Thu | 8524 | 12.6 | 4145 | 13.6 | 4379 | 13.3 | |
| Fri | 8500 | 12.6 | 4032 | 13.2 | 4468 | 13.5 | |
| Sat | 8821 | 13.1 | 4032 | 13.2 | 4789 | 14.5 | |
| Disease category | F00-F09 | 13,092 | 19.4 | 6348 | 20.8 | 6744 | 20.4 |
| F10-F19 | 19,218 | 28.4 | 9050 | 29.7 | 10,168 | 30.8 | |
| F20-F29 | 4011 | 5.9 | 1889 | 6.2 | 2122 | 6.4 | |
| F30-F39 | 11,453 | 17.0 | 5608 | 18.4 | 5845 | 17.7 | |
| F40-F49 | 18,772 | 27.8 | 9105 | 29.9 | 9667 | 29.3 | |
| F50-F59 | 2164 | 3.2 | 978 | 3.2 | 1186 | 3.6 | |
| F60-F69 | 395 | 0.6 | 187 | 0.6 | 208 | 0.6 | |
| F70-F79 | 206 | 0.3 | 111 | 0.4 | 95 | 0.3 | |
| F80-F89 | 110 | 0.2 | 51 | 0.2 | 59 | 0.2 | |
| F90-F99 | 1504 | 2.2 | 702 | 2.3 | 802 | 2.4 | |
| MERS outbreak | No | 58,995 | 87.3 | 25,998 | 85.3 | 32,997 | 100.0 |
| Yes | 4474 | 6.6 | 4474 | 14.7 | 0 | 0.0 | |
| District | Gangnam | 3121 | 4.6 | 1506 | 4.9 | 1615 | 4.9 |
| Gangdong | 2549 | 3.8 | 1218 | 4.0 | 1331 | 4.0 | |
| Gangbuk | 2113 | 3.1 | 1068 | 3.5 | 1045 | 3.2 | |
| Gangseo | 2341 | 3.5 | 1158 | 3.8 | 1183 | 3.6 | |
| Gwanak | 3498 | 5.2 | 1718 | 5.6 | 1780 | 5.4 | |
| Gwangjin | 1671 | 2.5 | 726 | 2.4 | 945 | 2.9 | |
| Guro | 2415 | 3.6 | 1209 | 4.0 | 1206 | 3.7 | |
| Geumcheon | 1475 | 2.2 | 715 | 2.3 | 760 | 2.3 | |
| Nowon | 4021 | 6.0 | 1816 | 6.0 | 2205 | 6.7 | |
| Dobong | 2312 | 3.4 | 1071 | 3.5 | 1241 | 3.8 | |
| Dongdaemun | 4088 | 6.1 | 1844 | 6.1 | 2244 | 6.8 | |
| Dongjak | 2383 | 3.5 | 1060 | 3.5 | 1323 | 4.0 | |
| Mapo | 2143 | 3.2 | 1086 | 3.6 | 1057 | 3.2 | |
| Seodaemun | 2287 | 3.4 | 1158 | 3.8 | 1129 | 3.4 | |
| Seocho | 2151 | 3.2 | 1052 | 3.5 | 1099 | 3.3 | |
| Seongdong | 2023 | 3.0 | 944 | 3.1 | 1079 | 3.3 | |
| Seongbuk | 3303 | 4.9 | 1605 | 5.3 | 1698 | 5.1 | |
| Songpa | 2662 | 3.9 | 1271 | 4.2 | 1391 | 4.2 | |
| Yangcheon | 2052 | 3.0 | 1075 | 3.5 | 977 | 3.0 | |
| Yeongdeungpo | 2800 | 4.1 | 1327 | 4.4 | 1473 | 4.5 | |
| Yongsan | 1895 | 2.8 | 928 | 3.0 | 967 | 2.9 | |
| Eunpyeong | 2489 | 3.7 | 1274 | 4.2 | 1215 | 3.7 | |
| Jongro | 1786 | 2.6 | 927 | 3.0 | 859 | 2.6 | |
| Jung | 1543 | 2.3 | 788 | 2.6 | 755 | 2.3 | |
| Jungnang | 4348 | 6.4 | 1928 | 6.3 | 2420 | 7.3 | |
MERS, Middle East respiratory syndrome; F00-F99, Mental and Behavioral Disease, Foo-F99; F00-F09, Organic, including symptomatic, mental disorders; F10-F19, Mental and behavioral disorders due to psychoactive substance use; F20-F29, Schizophrenia, schizotypal and delusional disorders; F30-F39, Mood [affective] disorders; F40-F48, Neurotic, stress-related and somatoform disorders; F50-F59, Behavioral syndromes associated with physiological disturbances and physical factors; F60-F69, Disorders of adult personality and behavior; F70-F79, Mental retardation; F80-F89, Disorders of psychological development; F90-F98, Behavioral and emotional disorders with onset usually occurring in childhood and adolescence; F99-F99, Unspecified mental disorder.
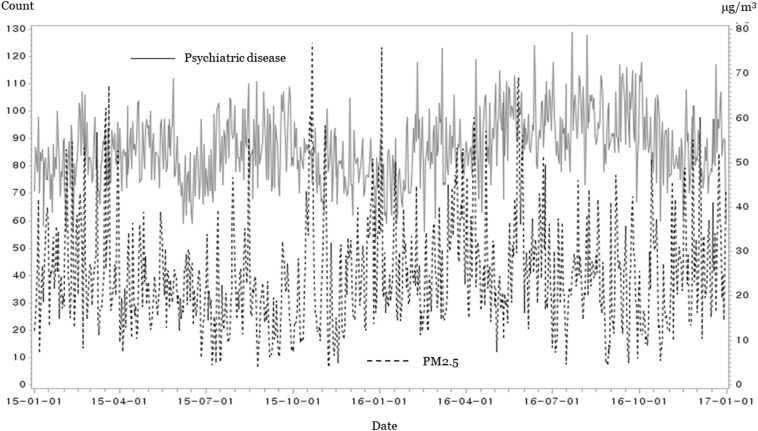
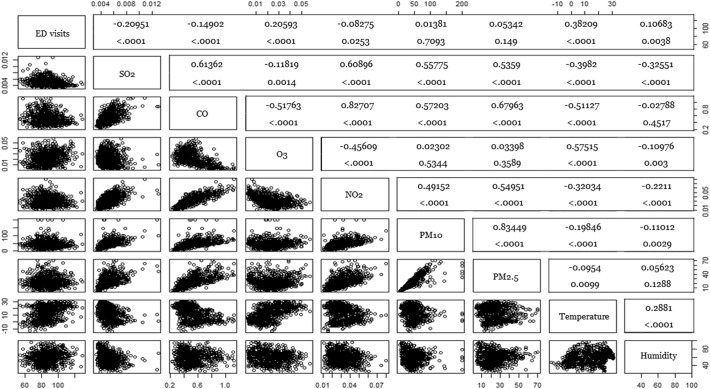
Table 2 summarizes the key variables. The mean ED (standard deviation) visits for psychiatric diseases and the mean PM2.5 level was 92 (12) and 24.8 (15.2) μg/m3. On 121 days corresponding to the 17th percentile, the daily mean PM2.5 was below 15 μg/m3, which was a level classified as “good” by the Korean Meteorological Administration. The daily mean PM2.5 was above 50 μg/m3, which was a “bad” level, on 11 days. Fig. 2 shows the number of ED visits for psychiatric diseases and the daily mean PM2.5 concentrations for each date. Fig. 3 shows correlations among the pollutants and ED visits. The ED visits for psychiatric disease were not significantly correlated with the daily PM2.5 concentrations (p = 0.149).
Table 2.
Daily statistics for weather factors and ambient pollutants.
| Variables | Mean | SD | Minimum | 25% | 50% | 75% | Maximum | IQR |
|---|---|---|---|---|---|---|---|---|
| ED visits for PD, N | 92 | 12 | 58 | 84 | 93 | 100 | 140 | 56 |
| Temperature, °C | 13.6 | 10.6 | −14.4 | 3.7 | 14.9 | 23.2 | 31.2 | 19.5 |
| Humidity, % | 59.5 | 14.4 | 36.6 | 48.4 | 60.1 | 69.5 | 97.3 | 21.1 |
| Pressure, hPa | 1016.4 | 8.5 | 1004.2 | 1009.3 | 1016.7 | 1023.1 | 1035.3 | 13.8 |
| PM2.5, μg/m3 | 24.8 | 15.2 | 0.0 | 14.0 | 22.0 | 32.0 | 168.0 | 18.0 |
| PM10, μg/m3 | 46.7 | 38.4 | 1.0 | 27.0 | 40.0 | 57.0 | 1160.0 | 30.0 |
| O3, ppm | 0.023 | 0.019 | 0.000 | 0.008 | 0.020 | 0.033 | 0.168 | 0.0 |
| NO2, ppm | 0.032 | 0.016 | 0.000 | 0.019 | 0.029 | 0.042 | 0.145 | 0.0 |
| CO, ppm | 0.523 | 0.242 | 0.000 | 0.400 | 0.500 | 0.600 | 3.400 | 0.2 |
| SO2, ppm | 0.005 | 0.002 | 0.000 | 0.004 | 0.005 | 0.006 | 0.030 | 0.0 |
SD, standard deviation; IQR, interquartile range; ED, emergency department; PD, psychiatric disease; PM2.5, particular mass <2.5 μm; PM10, particular mass <10 μm; O3, trioxide (ozone); NO2, nitric dioxide; CO, carbon oxide; SO2, sulfur dioxide.
Fig. 2.
Daily mean emergency department visits for psychiatric disease and the daily mean PM2.5 concentrations.
Fig. 3.
Correlation analysis between ED visits, pollutants, and weather.
ED, emergency department
PM2.5, particular mass <2.5 μm
PM10, particular mass <10 μm
O3, trioxide (ozone)
NO2, nitric dioxide
CO, carbon oxide
SO2, sulfur dioxide.
Table 3 shows the associations between pollutants (SO3, CO, O3, and NO2) and ED visits for psychiatric diseases in the GAM models. SO2 was significantly associated with decreased ED visits for all psychiatric diseases on lag day 1 (0.991, 0982–0.999). CO was also significantly associated with a decrease in ED visit due to F20-F29 (0.966, 0.944–0.989), whereas O3 was associated with increased ED visits (1.039, 1.001–1.079). Codes F40-F49 were significantly increased on lag day 1 by an increase in NO2 (1.015, 1.001–1.031).
Table 3.
Effects of SO2, CO, O3, and NO2 on emergency department visits for psychiatric diseases.
| Exposure | All (F00-F99) |
F20-F29 |
F30-F39 |
F40-F49 |
F50-F59 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ARR | 95% CI | ARR | 95% CI | ARR | 95% CI | ARR | 95% CI | ARR | 95% CI | ||||||
| SO2, ppm | |||||||||||||||
| Lag 1 | 0.991 | 0.982 | 0.999 | 0.981 | 0.948 | 1.015 | 0.981 | 0.961 | 1.001 | 1.004 | 0.988 | 1.020 | 0.994 | 0.948 | 1.042 |
| Lag 2 | 1.000 | 0.992 | 1.009 | 1.003 | 0.970 | 1.038 | 1.000 | 0.980 | 1.020 | 1.002 | 0.986 | 1.017 | 1.000 | 0.952 | 1.051 |
| Lag 3 | 0.999 | 0.990 | 1.007 | 1.000 | 0.968 | 1.033 | 1.003 | 0.983 | 1.023 | 0.997 | 0.981 | 1.012 | 1.025 | 0.978 | 1.074 |
| CO, ppm | |||||||||||||||
| Lag 1 | 0.998 | 0.992 | 1.004 | 0.966 | 0.944 | 0.989 | 0.992 | 0.978 | 1.005 | 1.003 | 0.992 | 1.014 | 0.989 | 0.958 | 1.021 |
| Lag 2 | 1.000 | 0.994 | 1.006 | 0.991 | 0.968 | 1.015 | 1.008 | 0.994 | 1.022 | 0.999 | 0.988 | 1.010 | 1.003 | 0.973 | 1.035 |
| Lag 3 | 0.999 | 0.993 | 1.005 | 0.982 | 0.959 | 1.005 | 1.009 | 0.995 | 1.023 | 0.999 | 0.988 | 1.010 | 1.001 | 0.969 | 1.034 |
| O3, ppm | |||||||||||||||
| Lag 1 | 1.001 | 0.992 | 1.011 | 1.039 | 1.001 | 1.079 | 1.003 | 0.980 | 1.025 | 1.006 | 0.989 | 1.024 | 1.011 | 0.961 | 1.064 |
| Lag 2 | 0.998 | 0.988 | 1.007 | 1.037 | 0.999 | 1.077 | 1.006 | 0.984 | 1.029 | 1.005 | 0.988 | 1.022 | 0.992 | 0.943 | 1.043 |
| Lag 3 | 0.995 | 0.986 | 1.005 | 1.034 | 0.996 | 1.074 | 0.994 | 0.972 | 1.016 | 0.994 | 0.977 | 1.012 | 0.995 | 0.945 | 1.047 |
| NO2, ppm | |||||||||||||||
| Lag 1 | 1.000 | 0.992 | 1.008 | 0.996 | 0.965 | 1.028 | 0.993 | 0.975 | 1.012 | 1.015 | 1.001 | 1.031 | 0.990 | 0.949 | 1.032 |
| Lag 2 | 0.998 | 0.990 | 1.006 | 0.976 | 0.946 | 1.007 | 0.995 | 0.977 | 1.014 | 1.004 | 0.990 | 1.019 | 0.989 | 0.948 | 1.032 |
| Lag 3 | 0.998 | 0.990 | 1.006 | 0.991 | 0.961 | 1.022 | 1.010 | 0.991 | 1.029 | 0.994 | 0.980 | 1.009 | 0.992 | 0.951 | 1.034 |
Adjusted relative risks (ARRs) with 95 confidence intervals (95% CIs) were calculated using general additive models: Log [E(Y)] = β0+) + β(pollutants) S1(Temperature) + S2(humidity) + Sp(number + parameters (week, season, middle east respiratory syndrome period) (degree of freedom = 3). The beta coefficients of pollutants were calculated with relative risks with 95% confidence intervals. O3, trioxide (ozone) (ppm, pulse per minute); NO2, nitric dioxide (ppm, pulse per minute); CO, carbon oxide (ppm, pulse per minute); SO2, sulfur dioxide (ppm, pulse per minute); F00-F99, Mental and Behavioral Disease, F00-F99; F00-F09, Organic, including symptomatic, mental disorders; F10-F19, Mental and behavioral disorders due to psychoactive substance use; F20-F29, Schizophrenia, schizotypal and delusional disorders; F30-F39, Mood [affective] disorders; F40-F48, Neurotic, stress-related and somatoform disorders; F50-F59, Behavioral syndromes associated with physiological disturbances and physical factors; F60-F69, Disorders of adult personality and behavior; F70-F79, Mental retardation; F80-F89, Disorders of psychological development; F90-F98, Behavioral and emotional disorders with onset usually occurring in childhood and adolescence; F99-F99, Unspecified mental disorder.
3.2. Main analysis
Table 4 shows the associations between PM2.5 and ED visits for psychiatric diseases. ED visits for all psychiatric diseases were not associated with PM2.5 in the analyses that were not adjusted for the other pollutants. The ARR (95% CI) in the model adjusted for SO2 was 1.011 (1.002–1.021) for a 10 μg/m3 increment of PM2.5 on lag day 1 for all psychiatric diseases (F00-F99). The ARR (95% CI) in the model adjusted for O3 was 1.015 (1.003–1.029) for a 10 μg/m3 increment of PM2.5 on lag day 1 for F40-F49 (Neurotic, stress-related and somatoform disorders). The other models did not show any significant associations between PM2.5 and ED visits due to psychiatric diseases.
Table 4.
Effect of PM2.5 on emergency department visits for psychiatric diseases.
| Exposure | All (F00-F99) |
F20-F29 |
F30-F39 |
F40-F49 |
F50-F59 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ARR | 95% CI | ARR | 95% CI | ARR | 95% CI | ARR | 95% CI | ARR | 95% CI | ||||||
| PM2.5 | |||||||||||||||
| Lag 1 | 1.000 | 0.994 | 1.007 | 0.984 | 0.958 | 1.012 | 0.999 | 0.983 | 1.016 | 1.008 | 0.995 | 1.021 | 0.982 | 0.946 | 1.019 |
| Lag 2 | 1.002 | 0.995 | 1.008 | 1.000 | 0.973 | 1.027 | 1.010 | 0.994 | 1.026 | 1.002 | 0.990 | 1.015 | 0.985 | 0.949 | 1.023 |
| Lag 3 | 0.998 | 0.991 | 1.005 | 0.983 | 0.957 | 1.010 | 1.005 | 0.989 | 1.022 | 1.001 | 0.988 | 1.013 | 1.004 | 0.966 | 1.044 |
| PM2.5 + SO2 | |||||||||||||||
| Lag 1 | 1.011 | 1.002 | 1.021 | 0.991 | 0.956 | 1.028 | 1.018 | 0.996 | 1.039 | 1.010 | 0.993 | 1.026 | 0.975 | 0.928 | 1.025 |
| Lag 2 | 1.003 | 0.994 | 1.013 | 0.998 | 0.963 | 1.035 | 1.018 | 0.996 | 1.039 | 1.002 | 0.986 | 1.019 | 0.979 | 0.932 | 1.028 |
| Lag 3 | 0.998 | 0.989 | 1.007 | 0.982 | 0.947 | 1.018 | 1.008 | 0.987 | 1.030 | 1.002 | 0.985 | 1.019 | 0.974 | 0.925 | 1.025 |
| PM2.5 + CO | |||||||||||||||
| Lag 1 | 1.005 | 0.995 | 1.015 | 1.034 | 0.993 | 1.077 | 1.019 | 0.995 | 1.044 | 1.007 | 0.988 | 1.026 | 0.985 | 0.932 | 1.042 |
| Lag 2 | 1.004 | 0.994 | 1.014 | 1.021 | 0.980 | 1.063 | 1.005 | 0.982 | 1.030 | 1.005 | 0.986 | 1.024 | 0.962 | 0.909 | 1.017 |
| Lag 3 | 0.998 | 0.988 | 1.009 | 1.000 | 0.960 | 1.042 | 0.991 | 0.967 | 1.016 | 0.998 | 0.979 | 1.017 | 1.013 | 0.956 | 1.074 |
| PM2.5 + O3 | |||||||||||||||
| Lag 1 | 1.003 | 0.996 | 1.010 | 0.986 | 0.959 | 1.013 | 1.003 | 0.987 | 1.019 | 1.015 | 1.003 | 1.029 | 0.980 | 0.944 | 1.018 |
| Lag 2 | 1.001 | 0.994 | 1.008 | 0.999 | 0.972 | 1.027 | 1.009 | 0.993 | 1.025 | 1.003 | 0.990 | 1.016 | 0.980 | 0.944 | 1.018 |
| Lag 3 | 0.998 | 0.992 | 1.005 | 0.980 | 0.954 | 1.007 | 1.006 | 0.990 | 1.023 | 1.003 | 0.990 | 1.016 | 1.013 | 0.974 | 1.053 |
| PM2.5 + NO2 | |||||||||||||||
| Lag 1 | 1.002 | 0.994 | 1.010 | 0.997 | 0.964 | 1.030 | 1.004 | 0.985 | 1.024 | 1.009 | 0.994 | 1.025 | 0.984 | 0.941 | 1.030 |
| Lag 2 | 1.004 | 0.996 | 1.012 | 1.009 | 0.976 | 1.043 | 1.008 | 0.989 | 1.028 | 1.007 | 0.992 | 1.023 | 0.985 | 0.941 | 1.031 |
| Lag 3 | 0.999 | 0.991 | 1.007 | 0.988 | 0.956 | 1.021 | 1.001 | 0.982 | 1.021 | 1.000 | 0.985 | 1.016 | 1.015 | 0.968 | 1.063 |
| PM2.5 + PM10 | |||||||||||||||
| Lag 1 | 1.000 | 0.999 | 1.000 | 0.998 | 0.995 | 1.001 | 1.000 | 0.999 | 1.002 | 0.998 | 0.994 | 1.003 | 1.001 | 0.998 | 1.003 |
| Lag 2 | 1.000 | 0.999 | 1.001 | 1.000 | 0.997 | 1.003 | 1.001 | 0.999 | 1.003 | 1.000 | 0.996 | 1.004 | 1.001 | 0.999 | 1.003 |
| Lag 3 | 1.000 | 0.999 | 1.000 | 0.998 | 0.996 | 1.001 | 0.999 | 0.998 | 1.001 | 0.997 | 0.993 | 1.001 | 1.000 | 0.997 | 1.002 |
Adjusted relative risks (ARRs) with 95 confidence intervals (95% CIs) were calculated using general additive models: Log [E(Y)] = β0 + β(pollutants) + S1(pollutants) + S1(Temperature) + S2(humidity) + Sp(number) + β(pollutants) + parameters (week, season, middle east respiratory syndrome period) (degree of freedom = 3). The beta coefficients of PM2.5 were calculated with relative risks with 95% confidence intervals; PM2.5, particular mass <2.5 μm (μg/m3); PM10, particular mass <10 μm (μg/m3); O3, trioxide (ozone) (ppm, pulse per minute); NO2, nitric dioxide (ppm, pulse per minute); CO, carbon oxide (ppm, pulse per minute); SO2, sulfur dioxide (ppm, pulse per minute); F00-F99, Mental and Behavioral Disease; F00-F09, Organic, including symptomatic, mental disorders; F10-F19, Mental and behavioral disorders due to psychoactive substance use; F20-F29, Schizophrenia, schizotypal and delusional disorders; F30-F39, Mood [affective] disorders; F40-F48, Neurotic, stress-related and somatoform disorders; F50-F59, Behavioral syndromes associated with physiological disturbances and physical factors; F60-F69, Disorders of adult personality and behavior; F70-F79, Mental retardation; F80-F89, Disorders of psychological development; F90-F98, Behavioral and emotional disorders with onset usually occurring in childhood and adolescence; F99-F99, Unspecified mental disorder.
4. Discussion
We found a significant association between the PM2.5 concentration and emergency department visits for psychiatric diseases on lag 1 day in the adjusted models. ED visits due to all types of psychiatric diseases following an increase in PM2.5 were increased on lag day 1 after adjustment for SO2. ED visits with codes F40-F49 were also increased on lag day 1 by an increase in PM2.5 after adjustment for O3.
Our study did not show consistent effects due to increases in pollutants, as reported in previous studies. One possible reason is the relatively low PM2.5 levels in Seoul in this study setting compared with other studies. The mean (standard deviation) of PM2.5 was 24.8 (15.2), and only 31 days had PM2.5 concentrations >50 μg/m3.
Among the 40 air pollutant monitoring stations, 25 are located in each district of Seoul at community service centers or other public buildings, such as high schools or museums. Their average height from the ground is 14.6 m. Because these buildings and centers are located in residential areas and are highly accessible, measurements from these stations represent the air that the citizens breathe. A total of 15 stations are located at road sides, such as main intersections or public transport stations, where the floating population is very high. Because data from the roadside stations might show different trends than the data from the residential area stations and the roadside stations were not located in all districts, data from the roadside stations were excluded.
Air pollutants are hypothesized to affect the mental status through activation of inflammatory processes, the alteration of immune system, influence on oxidative stress, and alterations in cerebral neurotransmitter concentrations. Because absorption and chemical reactions take time, using the daily mean concentrations of pollutants rather than other values, such as the maximum, is a reasonable approach. Although most studies use daily mean concentrations of air pollutants except for O3, one journal uses the standard deviations of air pollutants (PM10) as well as the daily mean concentrations [19]. Because air pollutants increase and decrease through chemical reactions, the air pollutant concentrations are expected to increase or decrease faster when the daily mean concentration is higher. Because the daily mean concentration and standard deviation were positively associated, as mentioned in one article [19], we only used the daily mean concentration. In most studies, researchers use daily maximal 8-hour averages [20,21] or the daily maximum values between 0900 and 1800 h in the case of O3 [22]. This approach is reasonable, because the main cause of O3 is ultraviolet waves generated by the sun. However, the pollution ozone can be produced by traffic exhaust which may be increased in the evening to night time in a metropolis. [23]
Nonetheless, in our data, the O3 concentrations were sometimes higher during the night than during the day. Because using the daily maximal 8-hour average of O3 would represent a time bias, we used the daily mean O3 concentration.
Three time-series analyses in China investigated the associations between air pollution and hospital admissions for psychiatric diseases. The study in Tianjin reported that an increase of 10 μg/m3 in the 2-day average concentrations of PM10, SO2, and NO2 corresponded to increases in daily hospital admissions of 0.15%, 0.49%, and 0.57%, respectively [24]. According to a study in Shanghai, increases in PM10, SO2, and CO increased hospital admissions for psychiatric diseases by 1.27%, 6.88%, and 0.16% per 10 μg/m3 increase, respectively [25]. The study in Beijing reported that PM10 showed a significant association with hospital admissions for psychiatric diseases [26]. Although the results of the three studies were different, PM10 was commonly associated with hospital admissions for psychiatric diseases. The types of pollutants that showed significant associations with emergency room visits varied by study, as did the extent to which air pollutants affected hospital visits. These inconsistent outcomes may be due to the different climatic environments of different cities. Unlike Korea, the reason for the significant results in China may be that the PM2.5 concentration in China is 203 times higher than the concentration. Although PM2.5 had a significant effect over a time span longer than 3 days, we only evaluated the association between PM2.5 and ED visits for a lag of 1 to 3 days. Previous studies have included longer lags for mortality outcomes due to pollutants. However, a maximum lag of three days was used in the GAM model in this study to test the abrupt association between exposure and outcomes.
Some studies reported significant associations of specific subtypes of psychiatric diseases with air pollutants. Both the study conducted in Seoul, Korea, and the study conducted in Canada reported that SO2, PM10, NO2, and CO were positively associated with emergency department visits for depression [11,12]. The study in Beijing also reported that an increase in the PM10 concentration of 10 μg/m3 corresponded to an increase in hospital admissions for schizophrenia of 1.74% [26]. In the study conducted in Seoul, Korea, the association was more significant when the patients were older and had background illnesses, such as cardiovascular diseases and diabetes mellitus.
4.1. Limitations
In the GAM models, we adjusted for weather factors, such as the daily mean temperature and humidity. Those weather factors were correlated with ED visits for psychiatric diseases (p < 0.001, and p = 0.0038, respectively). Although 35 AWSs in Seoul measure weather factors, not all 35 AWSs measure relative humidity and air pressure. Therefore, we used data from one of the central station named ‘Seoul’ located in Jongro district. The Korean Meteorological Administration also uses the measurement values from this ‘Seoul’ station as representative weather values for Seoul.
We measured the numbers of ED visits for psychiatric disease. The NEDIS database was started to collect basic, clinical, diagnostic, and prognostic information for all emergency department patients in 2004. The data are used for ED evaluation programs by the Ministry of Health and Welfare and for reimbursement of the national health insurance program. Although the diagnostic codes in usual claims data may be incorrect in number of patients, NEDIS is required to include the exact diagnostic codes for the annual national evaluation program for all EDs.
The study was performed in Seoul, which is located in East Asia with four distinguishable seasons. These study findings may not be generalizable to other cities and climates.
5. Conclusion
Based on a two-year observational study in a metropolis, an increase in PM2.5 shows significant associations with emergency department visits for all psychiatric diseases (F00-F99) and neurotic, stress-related and somatoform disorders (F40-F49) after a lag of one day. However, PM2.5 did not increase the risk of or exacerbate other psychiatric diseases, such as major depression (mood disorder) or psychosis (schizophrenia). Additional research could develop a public health alert system for specific air pollutants associated with psychiatric emergencies, which could be used to prevent psychiatric emergencies in vulnerable populations.
Funding acknowledgement
This study was supported and funded by the Korea Centers for Disease Control & Prevention (No. 2017P140200).
Ethic approval
The study was approved by the institutional review board of the study institution and informed consent was waivered. The all databases were opened to public to be used in researches and did not have any individual private information.
Consent for publication
This manuscript is an original article, all of the authors have agreed to the submission and the manuscript is not under consideration by any other journal.
Acknowledgement of funding source
This study was supported by the Korea Centers for Disease Control and Prevention (CDC). The study was funded by the Korea CDC (No. 2017P140200).
Footnotes
Authors' contribution: All authors contributed to this study; Kim SH analyzed the data and drafted the manuscript. Ro YS, Song KJ, and Kong SY contributed to data collection. Ko SY and Lee SY contributed to provide major comments for the study design and analysis. All authors contributed to revision of the manuscript. Shin SD designed the study and has responsible for the study.
References
- 1.Organization WH . World Health Organization; 2015. Health in 2015: from MDGs, millennium development goals to SDGs, sustainable development goals. [Google Scholar]
- 2.Hazlett S.B., McCarthy M.L., Londner M.S., Onyike C.U. Epidemiology of adult psychiatric visits to US emergency departments. Acad Emerg Med. 2004;11:193–195. [PubMed] [Google Scholar]
- 3.Lim S.S., Vos T., Flaxman A.D., Danaei G., Shibuya K., Adair-Rohani H. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao L., Liang H.R., Chen F.Y., Chen Z., Guan W.J., Li J.H. Association between air pollution and cardiovascular mortality in China: a systematic review and meta-analysis. Oncotarget. 2017;8:66438–66448. doi: 10.18632/oncotarget.20090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vahedian M., Khanjani N., Mirzaee M., Koolivand A. Associations of short-term exposure to air pollution with respiratory hospital admissions in Arak. Iran J Environ Health Sci Eng. 2017;15:17. doi: 10.1186/s40201-017-0277-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yorifuji T., Suzuki E., Kashima S. Hourly differences in air pollution and risk of respiratory disease in the elderly: a time-stratified case-crossover study. Environ Health Glob Access Sci Source. 2014;13:67. doi: 10.1186/1476-069X-13-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y., Eliot M.N., Wellenius G.A. Short-term changes in ambient particulate matter and risk of stroke: a systematic review and meta-analysis. J Am Heart Assoc. 2014;3 doi: 10.1161/JAHA.114.000983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee H., Myung W., Kim D.K., Kim S.E., Kim C.T., Kim H. Short-term air pollution exposure aggravates Parkinson's disease in a population-based cohort. Sci Rep. 2017;7:44741. doi: 10.1038/srep44741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szyszkowicz M. Air pollution and emergency department visits for depression in Edmonton, Canada. Int J Occup Med Environ Health. 2007;20:241–245. doi: 10.2478/v10001-007-0024-2. [DOI] [PubMed] [Google Scholar]
- 10.Szyszkowicz M., Kousha T., Kingsbury M., Colman I. Air pollution and emergency department visits for depression: a multicity case-crossover study. Environ Health Insights. 2016;10:155–161. doi: 10.4137/EHI.S40493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szyszkowicz M., Rowe B.H., Colman I. Air pollution and daily emergency department visits for depression. Int J Occup Med Environ Health. 2009;22:355–362. doi: 10.2478/v10001-009-0031-6. [DOI] [PubMed] [Google Scholar]
- 12.Cho J., Choi Y.J., Suh M., Sohn J., Kim H., Cho S.-K. Air pollution as a risk factor for depressive episode in patients with cardiovascular disease, diabetes mellitus, or asthma. J Affect Disord. 2014;157:45–51. doi: 10.1016/j.jad.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Raison C.L., Capuron L., Miller A.H. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sirivelu M.P., Mohankumar S.M., Wagner J.G., Harkema J.R., Mohankumar P.S. Activation of the stress axis and neurochemical alterations in specific brain areas by concentrated ambient particle exposure with concomitant allergic airway disease. Environ Health Perspect. 2006;114:870–874. doi: 10.1289/ehp.8619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Berlo D., Albrecht C., Knaapen A.M., Cassee F.R., Gerlofs-Nijland M.E., Kooter I.M. Comparative evaluation of the effects of short-term inhalation exposure to diesel engine exhaust on rat lung and brain. Arch Toxicol. 2010;84:553–562. doi: 10.1007/s00204-010-0551-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeong J., Shin S.D., Kim H., Hong Y.C., Hwang S.S., Lee E.J. The effects of celebrity suicide on copycat suicide attempt: a multi-center observational study. Soc Psychiatry Psychiatr Epidemiol. 2012;47:957–965. doi: 10.1007/s00127-011-0403-7. [DOI] [PubMed] [Google Scholar]
- 17.Cho E.J., Shin S.D., Jeong S., Kwak Y.H., Suh G.J. The effect of atmosphere temperature on out-of-hospital cardiac arrest outcomes. Resuscitation. 2016;109:64–70. doi: 10.1016/j.resuscitation.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Kang S.H., Heo J., Oh I.Y., Kim J., Lim W.H., Cho Y. Ambient air pollution and out-of-hospital cardiac arrest. Int J Cardiol. 2016;203:1086–1092. doi: 10.1016/j.ijcard.2015.11.100. [DOI] [PubMed] [Google Scholar]
- 19.Kim H., Lee J.-T., Hong Y.-C., Yi S.-M., Kim Y. Evaluating the effect of daily PM10 variation on mortality. Inhal Toxicol. 2004;16:55–58. doi: 10.1080/08958370490443042. [DOI] [PubMed] [Google Scholar]
- 20.Kang S.-H., Heo J., Oh I.-Y., Kim J., Lim W.-H., Cho Y. Ambient air pollution and out-of-hospital cardiac arrest. Int J Cardiol. 2016;203:1086–1092. doi: 10.1016/j.ijcard.2015.11.100. [DOI] [PubMed] [Google Scholar]
- 21.Chen C., Liu C., Chen R., Wang W., Li W., Kan H. Ambient air pollution and daily hospital admissions for mental disorders in Shanghai, China. Sci Total Environ. 2018;613:324–330. doi: 10.1016/j.scitotenv.2017.09.098. [DOI] [PubMed] [Google Scholar]
- 22.Lim Y.-H., Kim H., Kim J.H., Bae S., Park H.Y., Hong Y.-C. Air pollution and symptoms of depression in elderly adults. Environ Health Perspect. 2012;120:1023. doi: 10.1289/ehp.1104100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith R.B., Fecht D., Gulliver J., Beevers S.D., Dajnak D., Blangiardo M. Impact of London's road traffic air and noise pollution on birth weight: retrospective population based cohort study. BMJ. 2017;359:j5299. doi: 10.1136/bmj.j5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tong L., Li K., Zhou Q. Season, sex and age as modifiers in the association of psychosis morbidity with air pollutants: a rising problem in a Chinese metropolis. Sci Total Environ. 2016;541:928–933. doi: 10.1016/j.scitotenv.2015.09.066. [DOI] [PubMed] [Google Scholar]
- 25.Chen C., Liu C., Chen R., Wang W., Li W., Kan H. Ambient air pollution and daily hospital admissions for mental disorders in Shanghai, China. Sci Total Environ. 2017;613–614:324–330. doi: 10.1016/j.scitotenv.2017.09.098. [DOI] [PubMed] [Google Scholar]
- 26.Gao Q., Xu Q., Guo X., Fan H., Zhu H. Particulate matter air pollution associated with hospital admissions for mental disorders: a time-series study in Beijing, China. Eur Psychiatry. 2017;44:68–75. doi: 10.1016/j.eurpsy.2017.02.492. [DOI] [PubMed] [Google Scholar]