To the Editor:
Persistent hepatitis E virus (HEV) infection is recognised in immunocompromised patients, particularly solid organ transplant (SOT) recipients.1 The majority of cases are caused by genotype 3 (G3) HEV.1 Ribavirin monotherapy is considered first-line when the reduction of immunosuppression is contraindicated or unsuccessful.2 Treatment failure and relapse is recognised with limited alternative treatment options.3 Current guidelines suggest re-treatment with a prolonged course of ribavirin or, in cases of intolerance or non-response, pegylated interferon (PEG-IFN) (if not contraindicated).[2], [4] Sofosbuvir has been shown to inhibit G3 HEV in vitro, but to date no clinical cases treated with sofosbuvir have led to HEV clearance.3 Newer therapies are being investigated, but none have reached clinical use. We describe a case of persistent HEV infection demonstrating clinical phenotypic resistance to conventional treatments and the outcome of convalescent plasma (CP) therapy.
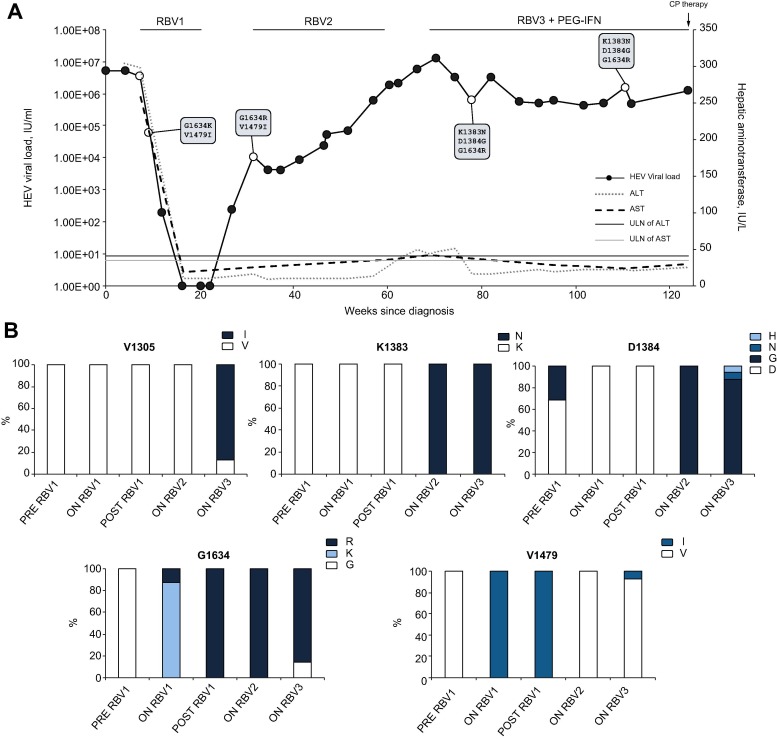
The patient was a 78-year-old male with persistent HEV infection on a background of underlying gastric marginal lymphoma on mucosa associated lymphoid tissue with omental involvement. He had been treated with chemotherapy, including RCVP [rituximab, cyclophosphamide, vincristine, prednisolone], R-CHOP [rituximab, cyclophosphamide, hydroxydaunomycin, vincristine, prednisolone] and radiotherapy 5 years previously and cured. As a result of this treatment he had chronic autoimmune neutropenia, which was supported with granulocyte colony stimulation factor. He was diagnosed with HEV viraemia (HEV G3c, 5.6 × 106 IU/ml, anti-HEV IgG 25.6 World Health Organization (WHO) units/ml) in March 2016, although he was first noted to have raised liver enzymes in November 2015. He was treated in early 2016 with a 12-week course of ribavirin (400 mg twice daily; estimated glomerular filtration rate 82 ml/min/1.73 m2; body weight 62 kg). HEV RNA was not detected in blood at weeks 8 and 12 of treatment, or in stool at the end of treatment. He relapsed 6 weeks after completing treatment. He received a second course of ribavirin 400 mg twice daily for 6 months, but HEV RNA levels remained high (6.0 × 105 IU/ml) so ribavirin was discontinued as he was experiencing side effects. On cessation of ribavirin he had a significant rise in liver enzyme levels. He was next treated with PEG-IFN and ribavirin (PEGASYS 180 µg weekly and ribavirin 400 mg twice daily) for 9 months but with no significant fall in HEV RNA. Illumina whole genome sequencing (HEV target-enrichment protocol, supplementary methods[5], [6] revealed the development of multiple mutations (K1383N, D1384G, and G1634R) in the RNA-dependent RNA-polymerase region previously described in patients failing ribavirin therapy.3 A further previously undescribed mutation, V1305I, was also detected during the third treatment course. Treatment and results are summarised in Fig. 1 .
Fig. 1.
Clinical course of patient prior to convalescent plasma therapy. Clinical course of patient prior to CP therapy where (A) depicts viral load, liver enzyme values, antiviral treatment and in boxes the detection of mutations in our patient which have been reported in previous cases of treatment failure and (B) shows the relative proportion of amino acids detected at loci where mutations apparently became fixed in our patient (V1305I, K1383N, D1384G, G1634R) and previously described mutations (V1479I). The V1305I mutation has not previously been reported in association with treatment failure, however it was only detected at the final timepoint sampled during the third ribavirin course with PEG-IFN. The open circles represent samples submitted for Illumina whole genome sequencing. ALT, alanine aminotransferase; AST, aspartate aminotransferase; CP, convalescent plasma; IU, international units; PEG-IFN, pegylated interferon; RBV, ribavirin; ULN, upper limit of normal; VL, viral load.
In the absence of any other available treatment we attempted treatment with CP, whilst the patient continued therapy with PEG-IFN and ribavirin.
Four potential plasma donors were identified from blood donors who had had a recent primary HEV infection (<100 days) with development of high levels of detectable anti-HEV IgG (sample over cut-off of optical density values >15.0) and who had at least 1 HEV RNA negative plasma sample to demonstrate viral clearance. Archived samples were quantified for anti-HEV IgG by WHO units/ml and tested for the ability to neutralise HEV antigen (HEV-Ag) (Fig. S1). Anti-HEV IgG and HEV-Ag were detected using commercial assays (Fortress Diagnostics, Antrim, Northern Ireland, UK); HEV-Ag neutralisation was determined by a recently described method.7 HEV RNA was detected using an internally controlled and validated quantitative HEV PCR (limit of detection 22 IU/ml).8 Two donors were selected (donor C and D) whose plasma had greater than 10 WHO units/ml of anti-HEV IgG and highest HEV-Ag neutralising activity when tested at equivalent levels of anti-HEV IgG (between 2 and 5 WHO units/ml). Donor D had previously been infected with a G3c virus, but technical limitations prevented sequencing of the virus previously infecting donor C. Established apheresis protocols were followed;9 a convalescent HEV plasma team arranged plasma collections from the 2 selected donors. Plasma was collected from donor C over 9 weeks (3 × 280 ml; 16–25 weeks from initial viraemic sample) and from donor D over 4 weeks (5 × 280 ml; 31–35 weeks from initial viraemic sample). The donations were tested for blood borne viruses in line with national donor guidelines.10 The treatment was approved by the Newcastle upon Tyne Hospitals NHS Foundation Trust Clinical Governance Team and the Trust Medical Director. The patient provided written informed consent to the procedure and subsequent publication. The patient was admitted to the Liver unit, Freeman Hospital, Newcastle for observation and was given 3 transfusions of 560 ml CP 12 hours apart. Blood samples were taken according to the protocol (Table S1). At baseline his total white cell count was 7.13 × 109/L, (neutrophils 4.68 × 109/L; lymphocytes 1.1 × 109/L) and total serum IgG was 7.6 g/L, IgA 1.09 g/L and IgM 10.31 g/L with an IgM paraprotein.
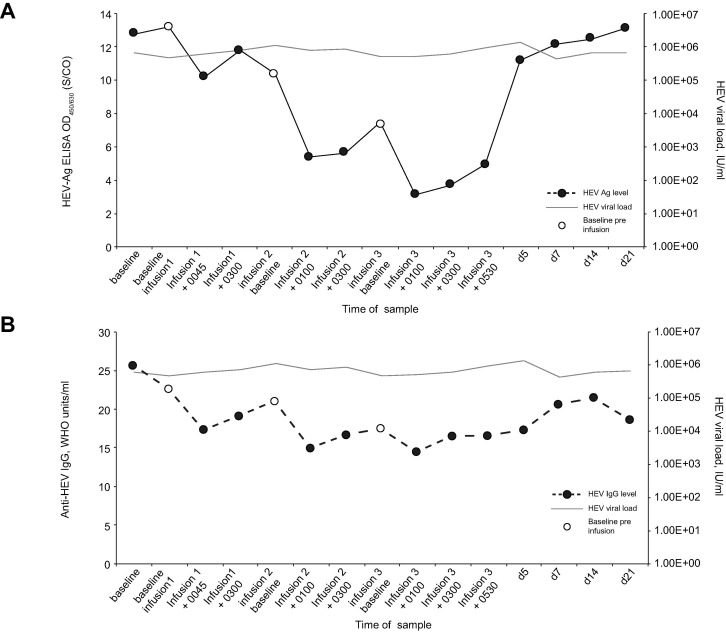
The patient reported no adverse effects during or after infusions. All observations (temperature, pulse rate, blood pressure, respiratory rate, oxygen saturations and level of consciousness) were normal during infusion and 2 hours post infusion. Blood tests showed no change in liver enzymes, blood counts or renal function during or after the infusion. Levels of plasma HEV RNA in the patient did not vary significantly after infusion or in the 21-day follow-up period (Fig. 2 ). Levels of detectable HEV-Ag showed a consistent pattern of falling at the 1-hour post-infusion timepoint, but this was not sustained at 3-hours post-infusion. Nevertheless, each baseline pre-infusion sample contained less detectable HEV-Ag than the previous baseline sample (Fig. 2A). HEV-Ag levels returned to baseline in the days following completion of infusions. Of particular note, the levels of detectable anti-HEV IgG showed a similar pattern to HEV-Ag levels, with a fall of 5.41, 6.09 and 3.00 WHO units/ml compared with the pre-infusion sample for infusions 1, 2 and 3 respectively (Fig. 2B).
Fig. 2.
HEV markers throughout convalescent plasma therapy. Response of HEV-Ag levels (A) and anti-HEV IgG levels (B) taken from patient in receipt of CP therapy. The patient was given 3 infusions of CP (∼560 ml) 12 hours apart. Samples were diluted 1 log and 2 log in normal human plasma prior to testing for anti-HEV IgG and HEV-Ag respectively. CP, convalescent plasma; d, days; ELISA, enzyme-linked immunosorbent assay; hr, hours; IU, international units; S/CO, sample over cut-off of optical density values.
The rationale for CP as a therapeutic option was to provide higher levels of neutralising antibodies against HEV. Patients with persistent HEV infection appear to have a lower proportion of anti-HEV IgG capable of neutralising HEV-Ag than those in convalescence from acute HEV infection.11 Furthermore, studies have demonstrated a protective effect of CP in cynomolgus monkeys after an HEV challenge and a possible protective effect of replacement immunoglobulin against persistent HEV infection amongst antibody-deficient patients.[11], [12] In our case, treatment with HEV CP therapy was not effective in leading to a sustained reduction in HEV load. Understanding the reason for therapeutic failure is crucial to guide future research. CP has demonstrated mixed results for treating viral infections, with successful outcomes in the treatment of severe acute respiratory syndrome coronavirus and influenza A virus, but inconclusive results for Ebola virus disease.[13], [14] Since HEV has no latent stage nor does it integrate into host DNA there is biological plausibility that CP could be effective in treating HEV infection. However, to our knowledge CP therapy has not been used in an attempt to clear persistent HEV infection.
The mode of action of CP therapy is not well understood, but it is expected that neutralisation plays a significant role. HEV virions in plasma appear to circulate as quasi-enveloped particles which may protect them from neutralising antibodies and thus explain the failure of CP.15 However, passively transferred antibodies are effective in preventing liver disease after hepatitis A virus (HAV) infection which has similar quasi-enveloped virions.16 Access to important neutralisation epitopes may still be possible in endosomes after removal of the membrane, which is postulated for HAV.17
It is anticipated that higher titres of neutralising antibody will have greater therapeutic effect.18 Therefore, we identified potential high-titre apheresis donors amongst blood donors in convalescence from HEV infection by comparison of anti-HEV IgG titre and HEV-Ag neutralising capability. Despite this, our patient’s samples post infusion consistently had lower detectable anti-HEV IgG of between 3 and 6 WHO units/ml. This is intriguing and remains difficult to explain and we believe is not adequately explained purely by haemodilution from plasma volume, since pre-infusion levels of anti-HEV IgG in the plasma of our patient (25.6 WHO units/ml) were similar to or lower than the levels of detectable anti-HEV in donors C (21.6–22.7 WHO units/ml) or D (44.8–52.9 WHO units/ml). Nevertheless, there may simply have been insufficient antibodies in the plasma donations for therapeutic effect, especially given the fact that our patient was already seropositive with detectable anti-HEV IgG.
Crucially we did not observe a significant fluctuation in the HEV RNA following any of the plasma infusions. However, we did observe a fall in HEV-Ag levels following each of the 3 infusions. Recent studies have identified 3 forms of this ORF2 antigen.19 It would be informative to understand whether all 3 forms of HEV-Ag fell post-infusion in our patient or whether the secreted form preferentially fell. We anticipate the fall in HEV-Ag levels we observed is due to binding and neutralisation of the secreted form of ORF2, since we would have expected the viral load to fall if virion-associated HEV-Ag had been bound and cleared from the circulation.20 Since the importance of host immunosuppression to the success of CP therapy is unknown we cannot be sure how important a functioning immune system is for treatment efficacy. Our patient had been heavily immunosuppressed previously and suffered from chronic neutropenia. Immune dysfunction may hinder the efficacy of CP therapy. The relative importance of B- and T-cell responses in clearing HEV infection is not understood, however T-cell responses are known to be important. HEV-specific T-cell responses detected in exposed healthy controls are absent in SOT recipients with persistent HEV infection and reducing immunosuppression targeting T-cells (e.g. tacrolimus) can lead to HEV clearance.21
We have described an unsuccessful treatment attempt of CP therapy in a case of treatment-refractory persistent HEV infection. New treatment approaches are required for those patients who fail conventional therapy.
Financial support
Additional virology including HEV-Ag neutralisation work and Illumina whole genome sequencing was funded by NHS Blood and Transplant.
Conflicts of interest
The authors declare no conflicts of interest.
Please refer to the accompanying ICMJE disclosure forms for further details.
Authors’ contributions
M Ankcorn – laboratory testing, co-ordination of testing, whole genome sequencing analysis, writing and review of manuscript. J Gallacher – clinical management of patient, writing and review of manuscript. S Ijaz – advised on clinical management and protocol of testing, revised manuscript. Y Taha – local virology liaison lead, data collection, review of manuscript. H Harvala – chair of NHSBT HEV Convalescent Plasma Group, review of manuscript. S MacLennan – co-ordination of apheresis plasma donations, review of manuscript. EC Thomson – whole genome sequencing and analysis, review of manuscript. C Davis – whole genome sequencing, review of manuscript. A da Silva Filipe – whole genome sequencing data acquisition and analysis, review of manuscript. K Smollett – whole genome sequencing, review of manuscript. M Niebel – whole genome sequencing analysis, review of manuscript. JB Singer – development of and advice using HEV GLUE platform, review of manuscript. MG Semple – developed treatment and monitoring protocol, revised manuscript. RS Tedder – advised on clinical management and protocol of testing, revised manuscript. S McPherson – clinical management of patient, co-ordination of CP administration, writing and review of manuscript.
Acknowledgements
The authors would to thank John Poh, Becky Haywood, Keerthana Jegatheesan, Justin Shute and Steve Dicks in the blood borne virus unit (BBVU), PHE Colindale team for diagnostic work and phylogenetics analysis. We are also grateful for and thank David Williams (PHE, London) and Sreenu Vattipally (CVR, Glasgow) for assistance with using programmes for analysis of short read viral sequences (Illumina). We would like to thank the NHSBT HEV Convalescent Plasma Group which was chaired by Dr Heli Harvala and included Dr Sheila MacLennan, Dr Rekha Anand, Alison Sivyer, Belinda Pelle and Susan Mahenny. Further thanks are extended to Kate Tettmar at the National Transfusion Microbiology Reference Laboratory (NTMRL), NHSBT, Colindale for helping co-ordinate transfer of samples from NTMRL to BBVU.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhep.2019.04.008.
Supplementary data
The following are the Supplementary data to this article:
References
- 1.Kamar N., Izopet J., Pavio N., Aggarwal R., Labrique A., Wedemeyer H. Hepatitis E virus infection. Nat Rev Dis Primers. 2017;3:17086. doi: 10.1038/nrdp.2017.86. [DOI] [PubMed] [Google Scholar]
- 2.McPherson S., Elsharkawy A.M., Ankcorn M., Ijaz S., Powell J., Rowe I. Summary of the British Transplantation Society UK guidelines for hepatitis E and solid organ transplantation. Transplantation. 2018;102:15–20. doi: 10.1097/TP.0000000000001908. [DOI] [PubMed] [Google Scholar]
- 3.Todt D., Meister T.L., Steinmann E. Hepatitis E virus treatment and ribavirin therapy: viral mechanisms of nonresponse. Curr Opin Virol. 2018;32:80–87. doi: 10.1016/j.coviro.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 4.European Association for the Study of the Liver (EASL) Clinical Practice Guidelines on hepatitis E virus infection. J Hepatol 2018;68:1256–1271. [DOI] [PubMed]
- 5.Thomson E., Ip C.L., Badhan A., Christiansen M.T., Adamson W., Ansari M.A. Comparison of next-generation sequencing technologies for comprehensive assessment of full-length hepatitis C viral genomes. J Clin Microbiol. 2016;54:2470–2484. doi: 10.1128/JCM.00330-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singer J.B., Thomson E.C., McLauchlan J., Hughes J., Gifford R.J. GLUE: a flexible software system for virus sequence data. BMC Bioinf. 2018;19:532. doi: 10.1186/s12859-018-2459-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ankcorn M.J., Ijaz S., Haywood B., Neuberger J., Elsharkawy A.M., Maggs J. Confirmation of specificity of reactivity in a solid phase ELISA for the detection of hepatitis E viral antigen improves utility of the assay. J Virol Methods. 2018;252:42–48. doi: 10.1016/j.jviromet.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Garson J.A., Ferns R.B., Grant P.R., Ijaz S., Nastouli E., Szypulska R. Minor groove binder modification of widely used TaqMan probe for hepatitis E virus reduces risk of false negative real-time PCR results. J Virol Methods. 2012;186:157–160. doi: 10.1016/j.jviromet.2012.07.027. [DOI] [PubMed] [Google Scholar]
- 9.van Griensven J., Edwards T., de Lamballerie X., Semple M.G., Gallian P., Baize S. Evaluation of convalescent plasma for Ebola virus disease in guinea. N Engl J Med. 2016;374:33–42. doi: 10.1056/NEJMoa1511812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joint United Kingdom (UK) Blood Transfusion and Tissue Transplantation Services Professional Advisory Committee. Guidelines for the Blood Transfusion Services in the UK. Available from: https://www.transfusionguidelines.org/red-book. 2013 [cited 2019 March 3].
- 11.Ankcorn M., Moreira F., Ijaz S., Symes A., Buckland M.S., Workman S. Absence of persistent Hepatitis E virus infection in antibody-deficient patients is associated with the transfer of antigen neutralising antibodies from immunoglobulin products. J Infect Dis. 2018 doi: 10.1093/infdis/jiy504. [DOI] [PubMed] [Google Scholar]
- 12.Tsarev S.A., Tsareva T.S., Emerson S.U., Govindarajan S., Shapiro M., Gerin J.L. Successful passive and active immunization of cynomolgus monkeys against hepatitis E. Proc Natl Acad Sci U S A. 1994;91:10198–10202. doi: 10.1073/pnas.91.21.10198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu G., Wong G., Su S., Bi Y., Plummer F., Gao G.F. Clinical evaluation of Ebola virus disease therapeutics. Trends Mol Med. 2017;23:820–830. doi: 10.1016/j.molmed.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mair-Jenkins J., Saavedra-Campos M., Baillie J.K., Cleary P., Khaw F.M., Lim W.S. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211:80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagashima S., Takahashi M., Kobayashi T., Tanggis, Nishizawa T., Nishiyama T. Characterization of the quasi-enveloped hepatitis E virus particles released by the cellular exosomal. Pathway. J Virol. 2017;91 doi: 10.1128/JVI.00822-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Victor J.C., Monto A.S., Surdina T.Y., Suleimenova S.Z., Vaughan G., Nainan O.V. Hepatitis A vaccine versus immune globulin for postexposure prophylaxis. N Engl J Med. 2007;357:1685–1694. doi: 10.1056/NEJMoa070546. [DOI] [PubMed] [Google Scholar]
- 17.Feng Z., Hensley L., McKnight K.L., Hu F., Madden V., Ping L. A pathogenic picornavirus acquires an envelope by hijacking cellular membranes. Nature. 2013;496:367–371. doi: 10.1038/nature12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown J.F., Dye J.M., Tozay S., Jeh-Mulbah G., Wohl D.A., Fischer W.A., 2nd Anti-Ebola virus antibody levels in convalescent plasma and viral load after plasma infusion in patients with Ebola virus disease. J Infect Dis. 2018 doi: 10.1093/infdis/jiy199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montpellier C., Wychowski C., Sayed I.M., Meunier J.C., Saliou J.M., Ankavay M. Hepatitis E virus lifecycle and identification of 3 forms of the ORF2 capsid protein. Gastroenterology. 2018;154 doi: 10.1053/j.gastro.2017.09.020. 211–223 e218. [DOI] [PubMed] [Google Scholar]
- 20.Yin X., Ying D., Lhomme S., Tang Z., Walker C.M., Xia N. Origin, antigenicity, and function of a secreted form of ORF2 in hepatitis E virus infection. Proc Natl Acad Sci U S A. 2018 doi: 10.1073/pnas.1721345115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suneetha P.V., Pischke S., Schlaphoff V., Grabowski J., Fytili P., Gronert A. Hepatitis E virus (HEV)-specific T-cell responses are associated with control of HEV infection. Hepatology. 2012;55:695–708. doi: 10.1002/hep.24738. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.