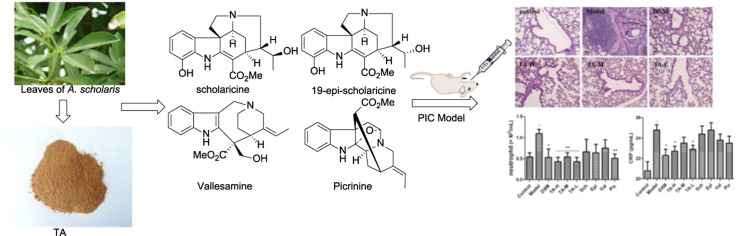
Abstract
Ethnopharmacological relevance
Leaf of Alstonia scholaris (L.) R. Br. (Apocynaceae), a wide used ethic-medicine in many Asia and Africa counties, has also been recorded as the common traditional Chinese medicine for treatment of illnesses in respiratory system by Dai people.
Aim of the study
To provide experimental data of clinical adaption of total indole alkaloids (TA) from leaf of A. scholaris for treating post-infectious cough in phase II clinical trial.
Materials and methods
To model post-infectious cough, all animals except control group were instilled intra-tracheal with lipopolysaccharide (LPS) (80 μg/50 µL/mouse), followed by subsequent exposure to cigarette smoke (CS) for 30 min per day for a total of 30 days. Mice were orally given TA at dose of 10, 25, 50 mg/kg, and four main alkaloids (Sch: scholaricine, Epi: 19-epischolaricine, Val: vallesamine, Pic: picrinine) once daily. Cellular infiltration was assessed in the broncho-alveolar lavage fluid (BALF). Expression of interleukin-6 (IL-6) and C-reactive protein (CRP) in the serum was determined, the superoxide dismutase (SOD) activity as well as malondialdehyde (MDA) content in the serum and homogenate were examined. Finally, histopathological examination in the lungs was assessed by H. E. staining.
Results
After administration of TA and four major alkaloids respectively, the symptoms of cough in mice were obviously attenuated. Total white blood cells (WBC) and neutrophils (NEU) amounts in BALF were reduced obviously and the pathological damage of lung was also attenuated. There was also significant reduction in IL-6, CRP, MDA and a marked improvement in SOD.
Conclusions
The efficacy of indole alkaloids against post-infectious cough (PIC) was shown in the down-regulation of inflammatory cells, cytokines, and the balance of antioxidants. What's more, the pharmacological effects of TA were better than single indole alkaloid, which might be related to the synergic effect of four major alkaloids.
Abbreviations: TA, total alkaloids; PIC, post-infectious cough; Sch, scholaricine; Epi, 19-epischolaricine; Val, vallesamine; Pic, picrinine; DXM, dexamethasone; BALF, broncho-alveolar lavage fluid; IL-6, Interleukin-6; CRP, C-reactive protein; MDA, malondialdehyde; SOD, superoxide dismutase; ELISA, enzyme-linked immunosorbent assay
Keywords: Leaf of Alstonia scholaris, Indole alkaloids, Scholaricine, 19-Epischolaricine, Vallesamine, Picrinine, Post-infectious cough
Graphical abstract
1. Introduction
Coughing is the classic symptoms of a cold, which has been divided into three types on the basis of its duration: acute cough (< 3 weeks), sub-acute cough (3–8 weeks), and chronic cough (> 8 weeks) (Irwin et al., 2006). Post-infectious cough (PIC) belongs to a type of sub-acute cough, and the percentage of it is 40% − 50%. Among the causes that can induce cough, the most common is represented by the infection of upper respiratory track, generally of viral origin. The most frequently implicated are picornavirus (rhinovirus and enterovirus) and coronavirus, followed by adenovirus, metapneumovirus, parainfluenza and influenza viruses, respiratory syncytial virus, and bocavirus (Dicpinigaitis et al., 2009, Footitt and Johnston, 2009, Jones and Stewart, 2002, Pappas et al., 2008, Regamey et al., 2008). Cough is the most common and troublesome symptom during airway infections, which is a major cause of significant morbidity an indeed mortality, and may last for several weeks or even months in some patients (Jones and Stewart, 2002), that bring the patient to the doctor (Footitt and Johnston, 2009).
The process of virus's replication in the airways includes the following parts: virus attachment to epithelial cells, cell membrane fusion and release of viral nucleocapsid and proteins into the cell cytoplasm (Jackson and Johnston, 2010). Some mediators, come from airway epithelial cells, activate the immune response by promoting the recruitment and activation of leukocytes (neutrophils, mast cells, monocytes, lymphocytes and cells NK) which provide a more effective response to different infections. Furthermore these cells release cytokines, chemokines, proteases, and oxygen free radicals that aggravate the injury of the airway structures caused by the virus (Biagioli et al., 1998, Jackson and Johnston, 2010, Rossi and Colin, 2015).
The pathogenesis of the PIC is as follows: extensive disruption of epithelial integrity, widespread airway inflammation, bronchial hyper responsiveness, and cough hypersensitivity (Betz et al., 2001, Cho et al., 2003, Morice, 2010, Zimmerman et al., 2000). Studies showed that the incidence of coughing after infection has an increased trend in recent years, which raised to the range from 25% to 50% during outbreaks of atypical pathogens’ infections (Ryan et al., 2012).
Although, a little of the complex pathogenesis of cough has been revealed by humans until now, but it at best increased the treatment options available (Morice et al., 2015). Many patients use over-the-counter drugs, for instance, cough suppressants and expectorants/ mucolytics, which often lack a clinically proven efficacy or have some activity, but at the expense of unpleasant or intolerable side effects. Two classes of cough suppressants are available: those with central action (codeine, dextromethorphan, and cloperastine) and those acting peripherally (levodropropizine). The only expectorant capable of inhibiting the cough reflex is guaifenesin. Meanwhile common side effects from these drugs are nausea, dry mouth, insomnia, dizziness, constipation, anorexia, fatigue, sleepiness and so on. A large part of income was spent on the treatment of cough globally (Morice, 2008). However, satisfactory results of all the medicine were not achieved due to the symptomatic treatment, side effects, and relapse after treatment. Additionally, montelukast, medicaments of a gleam for treating respiratory tract infection, was unresponsive to treatment for post-infectious cough (Wang et al., 2014). Therefore, an unmet need exists for the development of an effective therapeutic approach to prevent PIC.
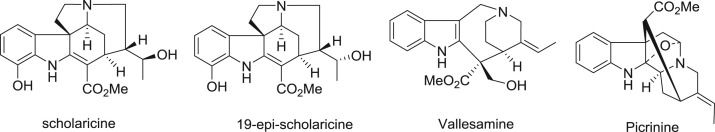
Alstonia scholaris is distributed in deciduous, evergreen forests and even in plains widely over the tropical regions of Africa and Asia (Li et al., 1995). Its leaves have long been used in “dai” ethno-pharmacy for the treatment of whooping cough (post infectious cough), chronic bronchitis, asthma, and other respiratory tract infections in Yunnan Province, PR China (Compiling Group of Yunnan Traditional Chinese Medicine, 1977). Phytochemical constituents of different parts of the plant were investigated intensively by our group (Cai et al., 2010, Cai et al., 2008a, Cai et al., 2008b, Cai et al., 2007, Chen et al., 2016, Du et al., 2007a, Du et al., 2007b, Feng et al., 2009, Feng et al., 2008, Liu et al., 2015, Pan et al., 2016, Qin et al., 2015a, Qin et al., 2015b, Xu et al., 2009, Yang et al., 2015a, Yang et al., 2015b, Yang et al., 2014a, Yang et al., 2014b, Zhang et al., 2014, Zhou et al., 2005). Meanwhile, the chemical profiling and metabolites of alkaloidal extract of A. scholaris were reported (Cao et al., 2015), in which scholaricine, 19-epischolaricine, vallesamine, and picrinine were the major indole alkaloids of its leaf ( Fig. 1.), and their pharmacokinetic behavior in rats was also investigated (Zhao et al., 2017). Moreover, A. scholaris extracts and alkaloids have shown antitussive, anti-asthmatic, expectorant (Shang et al., 2010a), analgesic, anti-inflammatory (Shang et al., 2010b), anti-airway inflammation (Zhao et al., 2016), and airways anti-allergic effect (Zhao et al., 2017) in vivo. The alkaloids also triggered β2 adrenergic receptor (β2AR) activation (Hou et al., 2012a) and inhibited the nuclear factor-κB (NF-κB) (Hou et al., 2012b) bioactivities in vitro. Based on the pre-clinical studies, the total indole alkaloids from A. scholaris (TA) has been registered as investigational new botanical drug (No. 2011L01436) and was approved for phase I/II clinical trials by China Food and Drug Administration (CFDA). A phase-I Single-center, randomized, double-blind, and placebo-controlled clinical trial has been completed, and the results support further phase-II clinical trials. The traditional use and our pharmacological evaluation assumed that the alkaloids from the leaf of A. scholaris might be used in treatment of respiratory diseases related to airway sub-acute inflammation. In this paper, we present the effect of alkaloids on the model of post-infectious cough.
Fig. 1.
Four major alkaloids from leaf of A. scholaris.
2. Materials and methods
2.1. Plant material
Leaves of A. scholaris were collected in June 2013 in Pu’er city of Yunnan Province, People's Republic of China, and identified by Dr. Xiao-Dong Luo, Kunming Institute of Botany, Chinese Academy of Sciences. A voucher specimen (Luo20130601) has been deposited in State Key Laboratory of Phytochemistry and Plant Resources in West China, Chinese Academy of Sciences.
2.2. Alkaloids preparation
The dried and powdered leaves of A. scholaris (1 kg) were extracted with 90% EtOH under reflux conditions (3 h × 4) and the solvent was evaporated in vacuo to get the ethanolic extract. The ethanolic extract was dissolved in 0.3% aqueous HCl solution and filtered; the residue was recognized as non-alkaloid fraction. Then the acidic solution, adjusted to pH 9–10 with 10% aqueous ammonia, was extracted with EtOAc to give TA fraction (10 g). Picrinine (10%), vallesamine (6%), sholaricine (6%), and 19-epischolaricine (2%) were isolated (Fig. 1) and kept in refrigerator in a previous phytochemical investigation from TA.
2.3. Chemicals
Lipopolysaccharide (LPS) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Hong mei brand cigarettes were purchased from Hongta Tobacco Group Co., Ltd. ELISA reagent sets for IL-6 and CRP were purchased from R&D Systems (Minneapolis, MN, USA). Malondialdehyde (MDA) and superoxide dismutase (SOD) determination kits were purchased from Jiancheng Bioengineering Institute of Nanjing (Jiangsu, China). All the other chemicals and solvents were of highest purity grade available.
2.4. Animals
Special pathogen-free male ICR mice, weighing approximately 22–25 g, were purchased from Kunming Medical University (licence number SYXK 2011–0004). All the animals were housed at room temperature (20–25 °C) and constant humidity (40–70%) under a 12 h light-dark cycle in SPF grade laboratory. Food and water were supplied ad libitum. Animals were acclimated for one week before treatment. Animal study was performed according to the international rules considering animal experiments and the internationally accepted ethical principles for laboratory animal use and care.
2.5. Experimental design
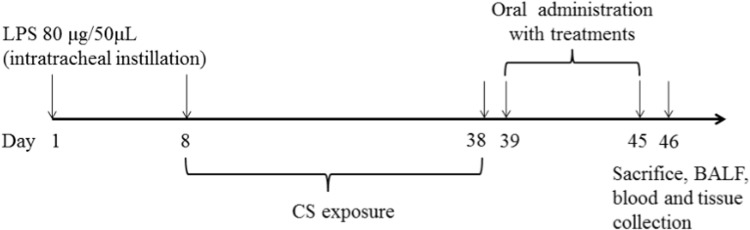
The experiment was performed according to previously described method with some modifications (Zhu and Li, 2014). To model post-infectious cough, mice were anesthetized by intraperitoneal injection of sodium pentobarbital. The trachea was intubated with a cannula (30 mm length and outer diameter 1.5 mm). Mice were placed in a supine position with the head elevated. With use of a micropipette and an intravenous catheter inserted through the intubated cannula, 50 µL of sterile saline containing 80 µg lipopolysaccharide and corresponding vehicle with 50 µL saline were instilled. On 8th day, all mice except control were placed in a smoke chamber and challenged with exposure to smoke of 5 cigarettes for 30 min/day for a total of 30 days. On 39th day after intra-tracheal instillation, the mice were randomly divided into 10 groups and treated as follows: (1) Control group (did not receive any intervention); (2) Model group: .5% carboxymethylcellulose intragastrically (i.g.), 20 mL/kg; (3) DXM: dexamethasone (i.g.), 1 mg/kg,; (4) TA-H: total indole alkaloids (i.g.), 50 mg/kg; (5) TA-M: total indole alkaloids (i.g.), 25 mg/kg; (6) TA-L: total indole alkaloids (i.g.), 10 mg/kg; (7) Sch: scholaricine (i.g.), 3 mg/kg; (8) Epi: 19-epischolaricine (i.g.), 1 mg/kg; (9) Val: vallesamine (i.g.), 3 mg/kg; (10) Pic: picrinine (i.g.), 5 mg/kg. Each group contained ten mice. A schematic diagram of the treatment schedule is shown in Fig. 2.
Fig. 2.
Experimental protocol for the establishment of LPS and CS induced PIC model and the treatment schedule.
2.6. Serum collection
On 46th day, animals were euthanized with an intraperitoneal injection of sodium pentobarbital (50 mg/kg) after overnight food deprivation. Then blood samples were collected via the orbital vein of mice. The serum samples were obtained by centrifuging blood samples at 4 °C, 4000 ×g for 15 min, and serum samples were prepared and stored at − 80 °C for the analysis of IL-6, CRP (Neumann et al., 2013), SOD, and MDA (Lemos et al., 2014). Operation was performed according to the manufacturer's specifications.
2.7. Bronchoalveolar lavage fluid(BALF)and cell enumeration
The right lungs were ligated and the left lungs were lavaged with 1.5 mL of autoclaved PBS for 3 times. The BALF was immediately centrifuged at 4 °C, 300 g for 10 min. The cell pellets were re-suspended in PBS, and the total cell number was counted using blood counting instrument (Wang et al., 2010, Zheng et al., 2009).
2.8. Lung homogenate
Lung tissue supernatants were prepared as described previously (Tsai et al., 2014). After BALF collection, 50 mg lungs were homogenized in 0.45 mL of ice-cold PBS, the assay mixture was cooled on ice, followed by centrifugation at 1000 g for 10 min at 4 °C to obtain the supernatant, and stored at − 80 °C until required for SOD and MDA analysis.
2.9. Lung histopathology
The right lung tissues were embedded in paraffin, cut into sections of 5 µm thickness, and stained with hematoxylin and eosin (H. E.) to evaluate the general morphology, as reported previously (Ichiki et al., 2005). The degrees of peribronchial and perivascular inflammation were scored in a double-blind screen with two independent blind investigators using a subjective scale of 0–5 (0, normal lung; 1, structural lesions of bronchial mucosa and alveolar wall were mild; 2, focal lesions of the bronchial mucosa and alveolar wall structures; 3, lesions of bronchial mucosa and alveolar wall were patchy; 4, lesions of bronchial mucosa and alveolar wall were less than 1/2 lobectomy; 5, lesions of bronchial mucosa and alveolar wall were more than 1/2 lobectomy) as described elsewhere (El-Agamy, 2011, Li et al., 2011, Ma et al., 2012).
2.10. Statistical analyses
Results are presented as mean ± SEM (n = 10). Comparisons between 2 groups were made by using a 2-tailed Mann-Whitney test. Multiple comparisons were made by using 1-way ANOVA with the Turkey post-test or Kruskal Wallis analysis with the Dunn post-test, in which nonparametric analyses were appropriate. A P value of less than 0.05 was considered as significant for all the analyses.
3. Results
3.1. Observation on general symptom
In contrast to control, the mice model developed cough, dyspnea, bristling, huddling, reducing diet, reduction, and weight loss. During administration period, the symptoms of the treatment groups alleviated without dose-response dependence compared to the model group.
3.2. Effects of indole alkaloids on inflammatory cell count in BALF
Lungs were lavaged at the end of experiment and differential cells in BALF were counted to investigate the effect of TA on inflammatory cells influx. As shown in Table 1, number of WBC (p < 0.05 vs. control group) and neutrophils (p < 0.05 vs. control group) in BALF increased in mice model, they were 3.8 ± 0.8 and 1.1 ± 0.08, respectively, while the number of WBC in TA-L group and Epi group were 1.1 ± 0.3 and 1.6 ± 0.5 (both p < 0.05 vs. model group), respectively. The number of neutrophils in all the three TA groups as well as Pic group was 0.43 ± 0.08, 0.54 ± 0.08, 0.43 ± 0.08, and 0.51 ± 0.09 (all p < 0.01 vs. model group), respectively.
Table 1.
The production of total leukocytes in BALF after treatment.
| Groups | WBC (× 105/mL) | NEU (× 105/mL) |
|---|---|---|
| Control | 1.1 ± 0.5 | 0.54 ± 0.12 |
| Model | 3.8 ± 0.8△ | 1.10 ± 0.08△ |
| DXM | 1.1 ± 0.2* | 0.53 ± 0.16* |
| TA-H | 1.5 ± 0.4 | 0.43 ± 0.08** |
| TA-M | 1.6 ± 0.4 | 0.54 ± 0.08** |
| TA-L | 1.1 ± 0.3* | 0.43 ± 0.08** |
| Sch | 2.5 ± 1.1 | 0.66 ± 0.26 |
| Epi | 1.6 ± 0.5* | 0.64 ± 0.17 |
| Val | 1.8 ± 0.5 | 0.75 ± 0.16 |
| Pic | 2.1 ± 0.6 | 0.51 ± 0.09** |
Note: Data are expressed as mean ± SEM. Statistical differences are represented as △p < 0.05 vs. control, *p < 0.05, **p < 0.01 vs. model (n = 10 mice per group). WBC, white blood (cell) count. NEU, neutrophils. All groups were intra-gastrically administrated.
Control: challenged with saline.
Model: established by intratracheal instillation of lipopolysaccharide (LPS) and passive smoking.
DXM: dexamethasone, 1 mg/kg.
TA-H: administrated total alkaloids, 50 mg/kg.
TA-M: administrated total alkaloids, 25 mg/kg.
TA-L: administrated total alkaloids, 10 mg/kg.
Sch, scholaricine, 3 mg/kg.
Epi, 19-epischolaricine, 1 mg/kg.
Val, vallesamine, 3 mg/kg.
Pic, picrinine, 5 mg/kg.
3.3. Effect of indole alkaloids on IL-6 and CRP production in serum
IL-6 and CRP production in serum were analyzed by ELISA. As shown in Table 2, LPS instillation and CS significantly increased the levels of pro-inflammatory cytokines IL-6 and CRP in the serum as compared to model animals (both p < 0.05 vs. control group), these were 26.5 ± 1.1 and 24.8 ± 0.5, respectively. However, TA-L and Sch groups significantly reduced the level of IL-6-22.7 ± 0.6 and 22.2 ± 0.9, respectively, (both p < 0.05 vs. model group). The content of CRP was markedly decreased to 22.7 ± 0.5 and 22.9 ± 0.4 in TA-H and TA-L groups, respectively. DXM group showed similar effects on the above two parameters (all p < 0.05 vs. model group). The remaining treatment group had the trend of decrease, but there was no significant difference (p > 0.05 vs. model group).
Table 2.
The production of IL-6 and CRP in serum after treatment.
| Groups | IL-6 (pg/mL) | CRP (pg/mL) |
|---|---|---|
| Control | 20.8 ± 0.7 | 20.8 ± 0.9 |
| Model | 26.5 ± 1.1△ | 24.8 ± 0.5△ |
| DXM | 22.0 ± 0.6* | 22.3 ± 0.6* |
| TA-H | 23.2 ± 1.3 | 22.7 ± 0.5* |
| TA-M | 24.1 ± 0.8 | 23.5 ± 0.6 |
| TA-L | 22.7 ± 0.6* | 22.9 ± 0.4* |
| Sch | 22.2 ± 0.9* | 24.4 ± 0.8 |
| Epi | 25.2 ± 1.2 | 24.8 ± 0.7 |
| Val | 25 ± 1.1 | 23.8 ± 0.6 |
| Pic | 23.9 ± 1.2 | 23.5 ± 0.7 |
Note: Data are expressed as mean ± SEM. Statistical differences are represented as △p < 0.05 vs. control, *p < 0.05, **p < 0.01 vs. model (n = 10 mice per group). IL-6, interleukin-6. CRP, C-reactive protein. All groups were intra-gastrically administrated.
3.4. Effect of indole alkaloids on SOD and MDA activities in serum and lung
Oxidative stress plays an important role in the development of PIC. SOD and MDA activities in the serum and lung homogenate were determined to evaluate the effect of indole alkaloids on oxidative stress. As shown, model group resulted in marked decline of SOD activities in serum and homogenate ( Table 3) compared with the control group (both p < 0.05) which decreased to 91.5% and 83.0%, respectively. Encouragingly, TA-M and TA-L groups significantly promoted the percentage of SOD activities, respectively, to 114.3% and 110% in serum (both p < 0.05 vs. control group), and to 120.3% (p < 0.01 vs. control group) and 118.3% (p < 0.05 vs. control group) in lung homogenate.
Table 3.
The production of SOD in serum and homogenate after treatment( mean ± SEM, U/mL).
| Groups | serum | homogenate |
|---|---|---|
| Control | 93.5 ± 1.7 | 61.3 ± 1.1 |
| Model | 85.5 ± 2.4△ | 53.4 ± 2.1△ |
| DXM | 86.9 ± 3.0 | 63.9 ± 1.8* |
| TA-H | 92.2 ± 2.7 | 54.0 ± 5.3 |
| TA-M | 97.7 ± 2.5* | 64.2 ± 1.5** |
| TA-L | 94.7 ± 2.2* | 63.1 ± 2.2* |
| Sch | 93.6 ± 3.9 | 58.4 ± 2.2 |
| Epi | 89.7 ± 6.9 | 54.2 ± 3.4 |
| Val | 91.7 ± 3.9 | 58.4 ± 1.8 |
| Pic | 91.2 ± 3.4 | 56.0 ± 4.0 |
Note: Data are expressed as mean ± SEM. Statistical differences are represented as △p < 0.05 vs. control, *p < 0.05, **p < 0.01 vs. model (n = 10 mice per group). SOD, superoxide dismutase. All groups were intra-gastrically administrated.
Meanwhile, the percentage of MDA content in serum and homogenate ( Table 4) was increased to 140.4% (p < 0.05 vs. control group) and 116.3% (p < 0.01 vs. control group) in model group, TA-L group also reduced MDA levels to 79.8% and 83.8% on the two samples (both p < 0.05 vs. model group). Val and Sch decreased the content of MDA significantly (p < 0.05/0.01 vs. model group), while Pic and Epi showed improving tendency (p > 0.05 vs. model group) in the two indices compared to the model group.
Table 4.
The production of MDA in serum and homogenate after treatment (nmol/mL).
| Groups | Serum | Homogenate |
|---|---|---|
| Control | 9.8 ± 0.9 | 6.4 ± 0.2 |
| Model | 13.7 ± 0.7△ | 7.4 ± 0.2△△ |
| DXM | 14.0 ± 1.2 | 6.2 ± 0.2** |
| TA-H | 11.9 ± 0.7 | 6.6 ± 0.4 |
| TA-M | 12.1 ± 2.0 | 6.2 ± 0.3 |
| TA-L | 11.0 ± 0.8* | 6.1 ± 0.4* |
| Sch | 12.2 ± 1.1 | 6.1 ± 0.4* |
| Epi | 11.6 ± 0.9 | 6.4 ± 0.5 |
| Val | 11.1 ± 0.4** | 7.1 ± 0.5 |
| Pic | 11.2 ± 1.4 | 6.2 ± 0.5 |
Note: Data are expressed as mean ± SEM. Statistical differences are represented as △p < 0.05, △△p < 0.01 vs. control, *p < 0.05, **p < 0.01 vs. model (n = 10 mice per group). MDA, malondialdehyde. All groups were intra-gastrically administrated.
3.5. Lung histopathology
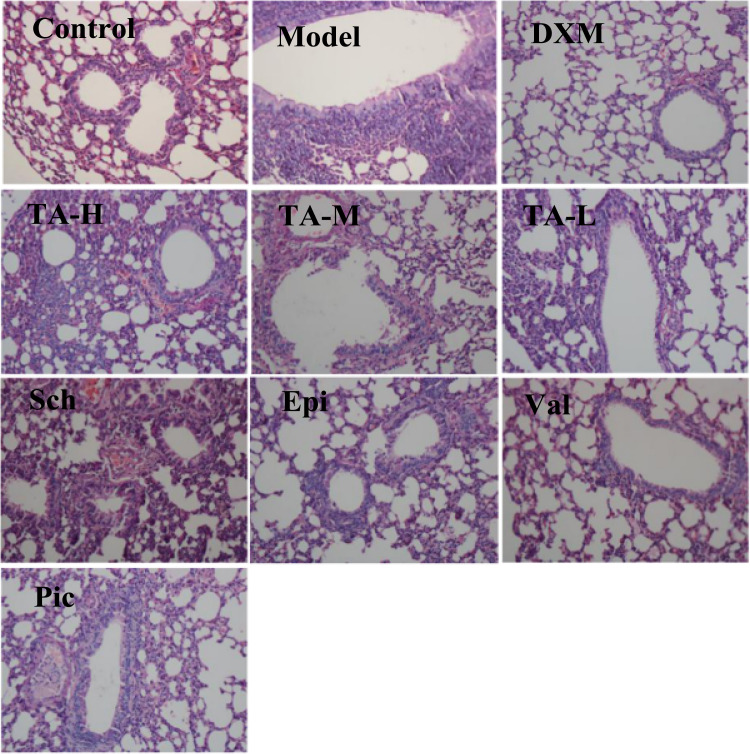
The production of inflammatory cells into the lungs of mice was also investigated by histopathological studies. LPS instillation and CS exposure provocated mice a marked infiltration of neutrophils around peribronchial and perivascular spaces compared to normal animals ( Fig. 3). Accordingly, the scores for total lung inflammation increased significantly ( Table 5). Besides, bronchia mucosal epithelium appeared swelling and shedding, and there was inflammatory cell infiltration exudate in the airway lumen. The infiltration of neutrophils in the bronchial mucosa was observed in control group. A few parts of infiltration of neutrophilic granulocytes were observed in pulmonary interstitial around the peribronchovascular. The results indicated that the infiltration of inflammatory cells reduced significantly in TA-treated mice compared to LPS and CS challenged mice, and four alkaloids (Sch, Epi, Val, and Pic) also prevented infiltration of inflammatory cells in varying degrees. DXM at 1 mg/kg treatment, used as the reference drug, improved the pulmonary histologic changes remarkably.
Fig. 3.
Representative microscopic sections of tissue demonstrating lung histology. The lung sections were stained with H&E and examined by light microscopy. Original magnification × 200.
Table 5.
Pathological changes after intratracheal LPS instillation and CS exposure.
| Groups | Dose (mg/kg) | Scores (means ± SEM) |
|---|---|---|
| Control | – | 0.9 ± 0.2 |
| Model | – | 3.0 ± 0.4△△ |
| DXM | 1.0 | 1.6 ± 0.3** |
| TA-H | 50.0 | 2.0 ± 0.2* |
| TA-M | 25.0 | 1.8 ± 0.3* |
| TA-L | 10.0 | 1.7 ± 0.3* |
| Sch | 3.0 | 2.0 ± 0.2* |
| Epi | 3.0 | 2.2 ± 0.2 |
| Val | 3.0 | 2.3 ± 0.4 |
| Pic | 5.0 | 2.0 ± 0.3* |
Note: Semi-quantitative analyses of inflammatory cell infiltrations in lung sections were performed as described in Materials and Methods. Lung tissues were fixed, sectioned at 5 µm, and stained with H&E for tissue neutrophils infiltration. Data are expressed as mean ± SEM. Statistical differences are represented as △△p < 0.01 vs. control, *p < 0.05, **p < 0.01 vs. model (n = 10 mice per group).
4. Discussion
The respiratory tract can be infected by a variety of bacteria, both gram positive and gram negative. The incidence of pneumonia is much higher for gram-negative infections than for gram-positive infections. Lipopolysaccharides (LPS), a large molecule consisting of a lipid and a polysaccharide, also known as lipoglycans and endotoxins, it was found in the outer membrane of Gram-negative bacteria and stimulate strong immune responses in animals, which may cause PIC with an inflammatory response (Kitamura et al., 2001, Saluk-Juszczak and Wachowicz, 2005). Several studies have shown that cigarette smoke has an impact on the immune function of the respiratory system, which induces the recruitment of inflammatory cells into the lungs and the release of pro-inflammatory cytokines, chemotactic factors, oxygen radicals and proteases (Joad et al., 2004, Karlsson et al., 1991).
In our present work, the content of pro-inflammatory cytokines (IL-6 and CRP) and lipid peroxidation products increased significantly. Besides, pathological change in the lung showed that many inflammatory cells were accumulated and infiltrated. A successful animal model of PIC was established and used by intra-tracheal instillation of LPS and CS exposure in mice, since the etiologies and mechanisms of PIC have been extensively investigated in number of experimental models.
Neutrophils are key blood cells, which play an important role in the pathogenesis of lung inflammation. A high number of neutrophils activate the reactive oxygen system and aggravate the development of respiratory diseases (Barnes, 2013, Cepkova and Matthay, 2006). Bronchoalveolar lavage fluid (BALF) has been extensively utilized for evaluating the alterations of lung microenvironment, including pulmonary epithelial integrity, cellular damage, and surface release/accumulation of cellular secretory products. As expected, the animals exposed to LPS and CS exhibited a significant increase of neutrophils in BALF, and was consistent with histological examination of the lung tissue in our study. Then, after the treatment of indole alkaloids, the number of total cells and neutrophils in BALF were inhibited, meanwhile lung's inflammatory cell infiltration alleviated.
Inflammation is controlled by various types of inflammatory mediators, including cytokines and chemokines which are associated with the recruitment of inflammatory cell and tissue destruction (Liaudet et al., 2002). Our research focused on the examination of interleukin (IL-6) and C-reactive protein (CRP). IL-6, an inflammatory and fibrogenic cytokine, is thought to play an important role in the development of lung disease (Jasiewicz et al., 2015). Recent studies demonstrated that IL-6 is marked increased in CS exposed animals (Li et al., 2009). CRP, an acute-phase protein, whose elevation accompanied by a remarkable pulmonary inflammation (Vos et al., 2009). Clinical trials reported that serum CRP concentrations of patients with COPD were higher than healthy controls (de Torres et al., 2006, Gan et al., 2004). In our investigation, mice intragastrically treated with indole alkaloids can effectively suppressed the elevated content of IL-6 and CRP produced by the exposure of LPS and CS in serum except the control group. The results suggest that the modulatory effect of pro-inflammatory cytokines may be one of the mechanisms of defending against infection.
It has been reported that the cigarette smoke contains many oxidants and free radicals, which causes the injury of alveolar epithelial cells and reduce anti-oxidizing abilities, and further leads to a large number of protein denaturation and apoptosis of surrounding tissues, and induces airway inflammation (William et al., 1983). SOD is an enzyme that exists in cells for removing oxyradicals, whose activity variation may represent the degree of tissue injury. Extracellular SOD might modulate neutrophil inflammation by reducing cytokine, released from macrophages, suggesting that extracellular SOD is an anti-inflammatory enzyme as well as a major anti-oxidant (Mates, 2000). Previous studies reported an increase of MDA concentration in the blood, BALF, and pulmonary tissues after the LPS administration (Shen et al., 2009), which was also observed in our experiment. In current study, we analyzed the anti-oxidant parameters (SOD and MDA) of serum and lung tissues, and found that the SOD activities were run-down significantly in all LPS and CS treated groups, but remarkably recovered in animals treated with alkaloids. Meanwhile, the promoted MDA in LPS and CS treated animals could be reduced.
Glucocorticoids with anti-inflammatory and anti-allergic activities are the most potent therapeutic agents, which are used to alleviate respiratory failure caused by neutrophilic granulocyte and alveolar macrophages in their metabolically activated states (Caramori and Adcock, 2003). Then, dexamethasone (DXM) was selected as a positive control of alkaloids to treat PIC. The results indicated that indole alkaloids from the leaf of Alstonia scholaris could well treat PIC in mice, which roughly equals to DXM in general (p > 0.05 vs. DXM group).
5. Conclusions
In conclusion, our study displayed that indole alkaloids could fight against PIC induced by LPS and CS exposure. However, the inhibition effect of TA was not appeared in a dosage dependent manner and superior to single alkaloids, simultaneously, four major alkaloids had different effects on different indicators, which assumed that the synergic effect between each component may compensate the drawback of single compound on PIC. And more importantly, this effect might be associated with the reduction of inflammatory infiltration and the improvement between oxidation and anti-oxidation.
Acknowledgements
The authors are grateful to the Ministry of Science and Technology of China (SQ2017YFC170594-07, 2014ZX09301307-003) for partial financial support, and to the analytical group of the Laboratory of Phytochemistry, Kunming Institute of Botany for spectral measurements.
Contributor Information
Xin-Hua Wang, Email: xinhuaw@gzhmu.edu.cn.
Xiao-Dong Luo, Email: xdluo@mail.kib.ac.cn.
References
- Barnes P.J. Corticosteroid resistance in patients with asthma and chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2013;131:636–645. doi: 10.1016/j.jaci.2012.12.1564. [DOI] [PubMed] [Google Scholar]
- Betz R., Kohlhaufl M., Kassner G., Mullinger B., Maier K., Brand P., Weber N., Haussinger K., Heyder J., Frankenberger M. Increased sputum IL-8 and IL-5 in asymptomatic nonspecific airway hyperresponsiveness. Lung. 2001;179:119–133. doi: 10.1007/s004080000055. [DOI] [PubMed] [Google Scholar]
- Biagioli M.C., Kaul P., Singh I., Turner R.B. The role of oxidative stress in rhinovirus induced elaboration of IL-8 by respiratory epithelial cells. Free Radic. Biol. Med. 1998;26:454–462. doi: 10.1016/s0891-5849(98)00233-0. [DOI] [PubMed] [Google Scholar]
- Cai X.H., Du Z.Z., Luo X.D. Unique monoterpenoid indole alkaloids from Alstonia scholaris. Org. Lett. 2007;9:1817–1820. doi: 10.1021/ol0705301. [DOI] [PubMed] [Google Scholar]
- Cai X.H., Liu Y.P., Feng T., Luo X.D. Picrinine-type alkaloids from the leaves of Alstonia scholaris. Chin. J. Nat. Med. 2008;6:20–22. [Google Scholar]
- Cai X.H., Tan Q.G., Liu Y.P., Feng T., Du Z.Z., Li W.Q., Luo X.D. A cage-monoterpene indole alkaloid from Alstonia scholaris. Org. Lett. 2008;10:577–580. doi: 10.1021/ol702682h. [DOI] [PubMed] [Google Scholar]
- Cai X.H., Shang J.H., Feng T., Luo X.D. Novel alkaloids from Alstonia scholaris. Z. Naturforsch. B J. Chem. Sci. 2010;65:1164–1168. [Google Scholar]
- Cao J., Shen H.M., Wang Q., Qian Y., Guo H.C., Li K., Qiao X., Guo D.A., Luo X.D., Ye M. Characterization of chemical constituents and rats metabolites of an alkaloidal extract of Alstonia scholaris leaves by liquid chromatography coupled with mass spectrometry. J. Chromatogr. B. 2015;1026:43–45. doi: 10.1016/j.jchromb.2015.07.044. [DOI] [PubMed] [Google Scholar]
- Caramori G., Adcock I. Pharmacology of airway inflammation in asthma and COPD. Pulm. Pharmacol. Ther. 2003;16:247–277. doi: 10.1016/S1094-5539(03)00070-1. [DOI] [PubMed] [Google Scholar]
- Cepkova M., Matthay M.A. Pharmacotherapy of acute lung injury and the acute respiratory distress syndrome. Eur. J. Intensive Care Med. 2006;21:119–143. doi: 10.1177/0885066606287045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.Y., Yang J., Yang X.W., Khan A., Liu L., Wang B., Zhao Y.L., Liu Y.P., Ding Z.T., Luo X.D. Alstorisine A, a nor-monoterpenoid indole alkaloid from cecidogenous leaves of Alstonia scholaris. Tetrahedron Lett. 2016;57:1754–1757. [Google Scholar]
- Cho Y.S., Park S.Y., Lee C.K., Lee E.Y., Shin J.H., Yoo B., Moon H.B. Enhanced cough response to hyperpnea with cold air challenge in chronic cough patients showing increased cough sensitivity to inhaled capsaicin. Allergy. 2003;58:486–491. doi: 10.1034/j.1398-9995.2003.00183.x. [DOI] [PubMed] [Google Scholar]
- Compiling Group of Yunnan Traditional Chinese Medicine . YunnanPeople's Press; Kunming: 1977. Yunnan Traditional Chinese Medicinal Plant. [Google Scholar]
- Dicpinigaitis P.V., Colice G., Goolsby M.J., Rogg G.I., Spector S.L., Winther B. Acute cough: a diagnostic and therapeutic challenge. Cough. 2009;5 doi: 10.1186/1745-9974-5-11. (11-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du G.S., Cai X.H., Shang J.H., Luo X.D. Non-alkaline constituents from leaf of Alstonia scholaris. Chin. J. Nat. Med. 2007;5:259–262. [Google Scholar]
- Du G.S., Shang J.H., Cai X.H., Luo X.D. Antitussive constituents from roots of Alstonia scholaris (Apocynaceae) Acta Bot. Yunnanica. 2007;29 (366-365) [Google Scholar]
- El-Agamy D.S. Nilotinib ameliorates lipopolysaccharide-induced acute lung injury in rats. Toxicol. Appl. Pharmacol. 2011;253:153–160. doi: 10.1016/j.taap.2011.03.023. [DOI] [PubMed] [Google Scholar]
- Feng T., Cai X.H., Du Z.Z., Luo X.D. Iridoids from the bark of Alstonia scholaris. Helv. Chim. Acta. 2008;91:2247–2251. [Google Scholar]
- Feng T., Cai X.H., Zhao P.J., Du Z.Z., Li W.Q., Luo X.D. Monoterpenoid indole alkaloids from the bark of Alstonia scholaris. Planta Med. 2009;75:1537–1541. doi: 10.1055/s-0029-1185900. [DOI] [PubMed] [Google Scholar]
- Footitt J., Johnston S.L. Cough and viruses in airways disease: mechanisms. Pulm. Pharmacol. Ther. 2009;22:108–113. doi: 10.1016/j.pupt.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan W.Q., Man S.F.P., Senthilselvan A., Sin D.D. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax. 2004;59:574–580. doi: 10.1136/thx.2003.019588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y.Y., Cao X.L., Dong L.Y., Wang L.Q., Cheng B.F., Shi Q., Luo X.D., Bai G. Bioactivity-based liquid chromatography-coupled electrospray ionization tandem ion trap/time of flight mass spectrometry for β 2 AR agonist identification in alkaloidal extract of Alstonia scholaris. J. Chromatogr. A. 2012;1227:203–209. doi: 10.1016/j.chroma.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Hou Y.Y., Cao X.L., Wang L.Q., Cheng B.F., Dong L.Y., Luo X.D., Bai G., Gao W.Y. Microfractionation bioactivity-based ultra performance liquid chromatography/quadrupole time-of-flight mass spectrometry for the identification of nuclear factor-κB inhibitors and β 2 adrenergic receptor agonists in an alkaloidal extract of the folk herb Alstonia scholaris. J. Chromatogr. B. 2012;908:98–104. doi: 10.1016/j.jchromb.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Ichiki H., Hoshino T., Kinoshita T., Imaoka H., Kato S., Inoue H., Nakamura H., Yodoi J., Young H.A., Aizawa H. Thioredoxin suppresses airway hyperresponsiveness and airway inflammation in asthma. Biochem. Biophys. Res. Commun. 2005;334:1141–1148. doi: 10.1016/j.bbrc.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Irwin R.S., Baumann H.M., Boulet L.P., Braman S.S. Diagnosis and management of cough: ACCP evidence-based clinical practice guidelines. Chest. 2006;129:138–146. doi: 10.1378/chest.129.1_suppl.25S. [DOI] [PubMed] [Google Scholar]
- Jackson D.J., Johnston S.L. The role of viruses in acute exacerbations of asthma. J. Allergy Clin. Immunol. 2010;125:1178–1187. doi: 10.1016/j.jaci.2010.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasiewicz M., Knapp M., Waszkiewicz E., Ptaszynska-Kopczynska K., Szpakowicz A., Sobkowicz B., Musial W.J., Kaminski K.A. Enhanced IL-6 trans-signaling in pulmonary arterial hypertension and its potential role in disease-related systemic damage. Cytokine. 2015;76:187–192. doi: 10.1016/j.cyto.2015.06.018. [DOI] [PubMed] [Google Scholar]
- Joad J.P., Munch P.A., Bric J.M., Evans S.J., Pinkerton K.E., Chen C.Y., Bonham A.C. Passive smoke effects on cough and airways in young guinea pigs: role of brainstem substance P. Am. J. Respir. Crit. Care Med. 2004;169:499–504. doi: 10.1164/rccm.200308-1139OC. [DOI] [PubMed] [Google Scholar]
- Jones B.F., Stewart M.A. Duration of cough in acute upper respiratory tract infections. Aust. Fam. Physician. 2002;31:971–973. [PubMed] [Google Scholar]
- Karlsson J.A., Zackrisson C., Lundberg J.M. Hyperresponsiveness to tussive stimuli in cigarette smoke-exposed guinea-pigs_ a role for capsaicin-sensitive, calcitonin gene-related peptide-containing nerves. Acta Physiol. Scand. 1991;141:445–454. doi: 10.1111/j.1748-1716.1991.tb09105.x. [DOI] [PubMed] [Google Scholar]
- Kitamura Y., Hashimoto S., Mizuta N., Kobayashi A., Kooguchi K., Fujiwara I., Nakajima H. Fas/FasL-dependent apoptosis of alveolar cells after lipopolysaccharide-induced lung injury in mice. Am. J. Respir. Crit. Care Med. 2001;163:762–769. doi: 10.1164/ajrccm.163.3.2003065. [DOI] [PubMed] [Google Scholar]
- Lemos A.J.J.M., Peixoto C.A., Teixeira Á.A.C., Luna R.L.A., Rocha S.W.S., Santos H.M.P., Silva A.K.S., Nunes A.K.S., Wanderley-Teixeira V. Effect of the combination of metformin hydrochloride and melatonin on oxidative stress before and during pregnancy, and biochemical and histopathological analysis of the livers of rats after treatment for polycystic ovary syndrome. Toxicol. Appl. Pharmacol. 2014;280:159–168. doi: 10.1016/j.taap.2014.05.015. [DOI] [PubMed] [Google Scholar]
- Li P.T., Leeuwenberg A.J.M., Middleton D.J. Flora of China. In: Wu Z.Y., Raven P.H., editors. Apocynaceae. Beijing St. Louis Science Press. and Missouri Botanical Garden Press; Bei Jing: 1995. pp. 143–188. [Google Scholar]
- Li Y., Yao J.H., Hu X.W., Fan Z., Huang L., Jing H.R., Liu K.X., Tian X.F. Inhibition of Rho kinase by fasudil hydrochloride attenuates lung injury induced by intestinal ischemia and reperfusion. Life Sci. 2011;88:104–109. doi: 10.1016/j.lfs.2010.10.028. [DOI] [PubMed] [Google Scholar]
- Li Y.T., He B., Wang Y.Z. Exposure to cigarette smoke upregulates AP-1 activity and induces TNF-alpha overexpression in mouse lungs. Inhal. Toxicol. 2009;21:641–647. doi: 10.1080/08958370802322596. [DOI] [PubMed] [Google Scholar]
- Liaudet L., Pacher P., Mabley J.G., VIRÁG L.S., Soriano F.G., HASKÓ G.R., SZABÓ C. Activation of poly (ADP-Ribose) polymerase-1 is a central mechanism of lipopolysaccharide-induced acute lung inflammation. Am. J. Respir. Crit. Care Med. 2002;165:372–377. doi: 10.1164/ajrccm.165.3.2106050. [DOI] [PubMed] [Google Scholar]
- Liu L., Chen Y.Y., Qin X.J., Wang B., Jin Q., Liu Y.P., Luo X.D. Antibacterial monoterpenoid indole alkaloids from Alstonia scholaris cultivated in temperate zone. Fitoterapia. 2015;105:160–164. doi: 10.1016/j.fitote.2015.06.019. [DOI] [PubMed] [Google Scholar]
- Ma W.J., Bao M.J., Zhu J.P., Yao H.Y., Xie Y.C., Guan Y., Li F.F., Dong X.W., Zheng Y.M., Xie Q.M. Oral administration of allergen extracts from mugwort pollen desensitizes specific allergen-induced allergy in mice. Vaccine. 2012;30:1437–1444. doi: 10.1016/j.vaccine.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Mates J. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology. 2000;153:83–104. doi: 10.1016/s0300-483x(00)00306-1. [DOI] [PubMed] [Google Scholar]
- Morice A.H. Chronic cough epidemiology. Chron. Respir. Dis. 2008;5:43–47. doi: 10.1177/1479972307084252. [DOI] [PubMed] [Google Scholar]
- Morice A.H. The cough hypersensitivity syndrome: a novel paradigm for understanding cough. Lung. 2010;188(Suppl 1):S87–S90. doi: 10.1007/s00408-009-9185-z. [DOI] [PubMed] [Google Scholar]
- Morice A.H., Kantar A., Dicpinigaitis P.V., Birring S.S., McGarvey L.P., Chung K.F. Treating acute cough: wet versus dry - have we got the paradigm wrong? ERJ Open Res. 2015;1:1–2. doi: 10.1183/23120541.00055-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann D., Beermann S., Burhenne H., Glage S., Hartwig C., Seifert R. The dual H3/4R antagonist thioperamide does not fully mimic the effects of the ‘standard’H4R antagonist JNJ 7777120 in experimental murine asthma. Naunyn. Schmiede. Arch. Pharmacol. 2013;386:983–990. doi: 10.1007/s00210-013-0898-4. [DOI] [PubMed] [Google Scholar]
- Pan Z.Q., Qin X.J., Liu Y.P., Wu T., Luo X.D., Xia C.F. Alstoscholarisines H-J, indole alkaloids from Alstonia scholaris: structural evaluation and bioinspired synthesis of alstoscholarisine H. Org. Lett. 2016;18:654–657. doi: 10.1021/acs.orglett.5b03583. [DOI] [PubMed] [Google Scholar]
- Pappas D.E., Hendley O.J., Hayden F.G., Winther B. Symptom profile of common colds in school-aged children. Pediatr. Infect. Dis. J. 2008;27:8–11. doi: 10.1097/INF.0b013e31814847d9. [DOI] [PubMed] [Google Scholar]
- Qin X.J., Zhao Y.L., Lunga P.K., Yang X.W., Song C.W., Cheng G.G., Liu L., Chen Y.Y., Liu Y.P., Luo X.D. Indole alkaloids with antibacterial activity from aqueous fraction of Alstonia scholaris. Tetrahedron. 2015;71:4372–4378. [Google Scholar]
- Qin X.J., Zhao Y.L., Song C.W., Wang B., Chen Y.Y., Liu L., Li Q., Li D., Liu Y.P., Luo X.D. Monoterpenoid indole alkaloids from inadequately dried leaves of Alstonia scholaris. Nat. Prod. Bioprospect. 2015;5:185–193. doi: 10.1007/s13659-015-0066-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regamey N., Kaiser L., Roiha H.L., Deffernez C., Kuehni C.E., Latzin P., Aebi C., Frey U. Viral etiology of acute respiratory infections with cough in infancy: a community-based birth cohort study. Pediatr. Infect. Dis. J. 2008;27:100–105. doi: 10.1097/INF.0b013e31815922c8. [DOI] [PubMed] [Google Scholar]
- Rossi G.A., Colin A.A. Infantile respiratory syncytial virus and human rhinovirus infections: respective role in inception and persistence of wheezing. Eur. Respir. J. 2015;45:774–789. doi: 10.1183/09031936.00062714. [DOI] [PubMed] [Google Scholar]
- Ryan N.M., Vertigan A.E., Ferguson J., Wark P., Gibson P.G. Clinical and physiological features of postinfectious chronic cough associated with H1N1 infection. Respir. Med. 2012;106:138–144. doi: 10.1016/j.rmed.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Saluk-Juszczak J., Wachowicz B. The proinflammatory activity of lipopolysaccharide. Post. Biochem. 2005;51:280–287. [PubMed] [Google Scholar]
- Shang J.H., Cai X.H., Feng T., Zhao Y.L., Wang J.K., Zhang L.Y., Yan M., Luo X.D. Pharmacological evaluation of Alstonia scholaris: anti-inflammatory and analgesic effects. J. Ethnopharmacol. 2010;129:174–181. doi: 10.1016/j.jep.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Shang J.H., Cai X.H., Zhao Y.L., Feng T., Luo X.D. Pharmacological evaluation of Alstonia scholaris: anti-tussive, anti-asthmatic and expectorant activities. J. Ethnopharmacol. 2010;129:293–298. doi: 10.1016/j.jep.2010.03.029. [DOI] [PubMed] [Google Scholar]
- Shen W., Gan J., Xu S., Jiang G., Wu H. Penehyclidine hydrochloride attenuates LPS-induced acute lung injury involvement of NF-κB pathway. Pharmacol. Res. 2009;60:296–302. doi: 10.1016/j.phrs.2009.04.007. [DOI] [PubMed] [Google Scholar]
- de Torres J.P., Cordoba-Lanus E., Lopez-Aguilar C., Muros de Fuentes M., Montejo de Garcini A., Aguirre-Jaime A., Celli B.R., Casanova C. C-reactive protein levels and clinically important predictive outcomes in stable COPD patients. Eur. Respir. J. 2006;27:902–907. doi: 10.1183/09031936.06.00109605. [DOI] [PubMed] [Google Scholar]
- Tsai C.L., Lin Y.C., Wang H.M., Chou T.C. Baicalein, an active component of Scutellaria baicalensis, protects against lipopolysaccharide-induced acute lung injury in rats. J. Ethnopharmacol. 2014;153:197–206. doi: 10.1016/j.jep.2014.02.010. [DOI] [PubMed] [Google Scholar]
- Vos R., Vanaudenaerde B.M., De Vleeschauwer S.I., Willems-Widyastuti A., Dupont L.J., Van Raemdonck D.E., Verleden G.M. C-reactive protein in bronchoalveolar lavage fluid is associated with markers of airway inflammation after lung transplantation. Transpl. P. 2009;41:3409–3413. doi: 10.1016/j.transproceed.2009.09.029. [DOI] [PubMed] [Google Scholar]
- Wang K., Birring S.S., Taylor K., Fry N.K., Hay A.D., Moore M., Jin J., Perera R., Farmer A., Little P., Harrison T.G., Mant D., Harnden A. Montelukast for postinfectious cough in adults: a double-blind randomised placebo-controlled trial. Lancet Respir. Med. 2014;2:35–43. doi: 10.1016/S2213-2600(13)70245-5. [DOI] [PubMed] [Google Scholar]
- Wang Y.J., Jiang Y.L., Tang H.F., Zhao C.Z., Chen J.Q. Zl-n-91, a selective phosphodiesterase 4 inhibitor, suppresses inflammatory response in a COPD-like rat model. Int. Immunopharmacol. 2010;10:252–258. doi: 10.1016/j.intimp.2009.11.008. [DOI] [PubMed] [Google Scholar]
- William A.P., Donald G.P., Daniel F.C. Electron-spin resonance study of mainstream and sidestream cigarette smoke: nature of the free radicals in gas-phase smoke and in cigarette tar. Environ. Health Per. 1983;47:345–355. doi: 10.1289/ehp.8347345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Feng T., Cai X.H., Luo X.D. A new C13-norisoprenoid from leaves of Alstonia scholaris. Chin. J. Nat. Med. 2009;7:21–23. [Google Scholar]
- Yang X.W., Qin X.J., Zhao Y.L., Lunga P.K., Li X.N., Jiang S.Z., Cheng G.G., Liu Y.P., Luo X.D. Alstolactines A-C, novel monoterpenoid indole alkaloids from Alstonia scholaris. Tetrahedron Lett. 2014;55:4593–4596. [Google Scholar]
- Yang X.W., Yang C.P., Jiang L.P., Qin X.J., Liu Y.P., Shen Q.S., Chen Y.B., Luo X.D. Indole alkaloids with new skeleton activating neural stem cells. Org. Lett. 2014;16:5808–5811. doi: 10.1021/ol5029223. [DOI] [PubMed] [Google Scholar]
- Yang X.W., Luo X.D., Lunga P.K., Zhao Y.L., Qin X.J., Chen Y.Y., Liu L., Li X.N., Liu Y.P. Scholarisines H-O, novel indole alkaloid derivatives from long-term stored Alstonia scholaris. Tetrahedron. 2015;71:3694–3698. [Google Scholar]
- Yang X.W., Song C.W., Zhang Y., Khan A., Jiang L.P., Chen Y.B., Liu Y.P., Luo X.D. Alstoscholarisines F and G, two unusual monoterpenoid indole alkaloids from the leaves of Alstonia scholaris. Tetrahedron Lett. 2015;56:6715–6718. [Google Scholar]
- Zhang Z.Y., Luo X.D., Li S. Comparative genetic and chemical profiling performed on Alstonia scholaris in China and its implications to standardization of traditional Chinese medicine. J. Med. Plants Res. 2014;8:301–306. [Google Scholar]
- Zhao Y.L., Shang J.H., Pu S.B., Wang H.S., Wang B., Liu L., Liu Y.P., H.M S., Luo X.D. Effect of total alkaloids from Alstonia scholaris on airway inflammation in rats. J. Ethnopharmacol. 2016;178:258–265. doi: 10.1016/j.jep.2015.12.022. [DOI] [PubMed] [Google Scholar]
- Zhao Y.L., Cao J., Shang J.H., Liu Y.P., Khan A., Wang H.S., Qian Y., Liu L., Ye M., Luo X.D. Airways antiallergic effect and pharmacokinetics of alkaloids from Alstonia scholaris. Phytomedicine. 2017;27:63–72. doi: 10.1016/j.phymed.2017.02.002. [DOI] [PubMed] [Google Scholar]
- Zheng H., Liu Y., Huang T., Fang Z., Li G., He S. Development and characterization of a rat model of chronic obstructive pulmonary disease (COPD) induced by sidestream cigarette smoke. Toxicol. Lett. 2009;189:225–234. doi: 10.1016/j.toxlet.2009.06.850. [DOI] [PubMed] [Google Scholar]
- Zhou H., He H.P., Luo X.D., Wang Y.H., Yang X.W., Di Y.T., Hao X.J. Three new indole alkaloids from the leaves of Alstonia scholaris. Helv. Chim. Acta. 2005;88:2508–2512. [Google Scholar]
- Zhu J., Li F.X. Effects of Qufeng Xuanfei decoction in animal model of post-infectious cough. Cell Biochem. Biophys. 2014;70:881–885. doi: 10.1007/s12013-014-9994-4. [DOI] [PubMed] [Google Scholar]
- Zimmerman B., Silverman F.S., Tarlo S.M., Chapman K.R., Kubay J.M., Urch B. Induced sputum: comparison of postinfectious cough with allergic asthma in children. J. Allergy Clin. Immunol. 2000;105:495–499. doi: 10.1067/mai.2000.104933. [DOI] [PubMed] [Google Scholar]