Abstract
Eukaryotic ribosomal frameshift signals generally contain two elements, a heptanucleotide slippery sequence (XXXYYYN) and an RNA secondary structure, often an RNA pseudoknot, located downstream. Frameshifting takes place at the slippery sequence by simultaneous slippage of two ribosome-bound tRNAs. All of the tRNAs that are predicted to decode frameshift sites in the ribosomal A-site (XXXYYYN) possess a hypermodified base in the anticodon-loop and it is conceivable that these modifications play a role in the frameshift process. To test this, we expressed slippery sequence variants of the coronavirus IBV frameshift signal in strains of Escherichia coli unable to modify fully either tRNALys or tRNAAsn. At the slippery sequences UUUAAAC and UUUAAAU (underlined codon decoded by tRNAAsn, anticodon 5′ QUU 3′), frameshifting was very inefficient (2 to 3%) and in strains deficient in the biosynthesis of Q base, was increased (AAU) or decreased (AAC) only two-fold. In E. coli, therefore, hypomodification of tRNAAsn had little effect on frameshifting. The situation with the efficient slippery sequences UUUAAAA (15%) and UUUAAAG (40%) (underlined codon decoded by tRNALys, anticodon 5′ mnm5s2UUU 3′) was more complex, since the wobble base of tRNALys is modified at two positions. Of four available mutants, only trmE (s2UUU) had a marked influence on frameshifting, increasing the efficiency of the process at the slippery sequence UUUAAAA. No effect on frameshifting was seen in trmC1 (cmnm5s2UUU) or trmC2 (nm5s2UUU) strains and only a very small reduction (at UUUAAAG) was observed in an asuE (mnm5UUU) strain. The slipperiness of tRNALys, therefore, cannot be ascribed to a single modification site on the base. However, the data support a role for the amino group of the mnm5 substitution in shaping the anticodon structure. Whether these conclusions can be extended to eukaryotic translation systems is uncertain. Although E. coli ribosomes changed frame at the IBV signal (UUUAAAG) with an efficiency similar to that measured in reticulocyte lysates (40%), there were important qualitative differences. Frameshifting of prokaryotic ribosomes was pseudoknot-independent (although secondary structure dependent) and appeared to require slippage of only a single tRNA.
Keywords: ribosomal frameshifting, tRNA anticodon modification, RNA pseudoknot, lysyl-tRNA, Q base
Abbreviations: RSV, Rous sarcoma virus; ORF, open reading frame; Q, queuosine; Y, wyebutoxine; HIV, human immunodeficiency virus; HTLV, human T-cell leukaemia virus; BLV, bovine leukaemia virus; IBV, infectious bronchitis virus; RRL, rabbit reticulocyte lysate; IPTG, isopropyl-β, d-thiogalactopyranoside; TGT, tRNA guanine transglycosylase; MMTV, mouse mammary tumour virus; pfu, plaque-forming units
Introduction
Several viruses use an efficient −1 ribosomal frameshifting mechanism to control expression of their replicases. Frameshifts of this class were first described as the process by which the gag-pol polyprotein of the retrovirus Rous sarcoma virus (RSV) is expressed from the overlapping gag and pol open reading frames (ORFs: Jacks & Varmus, 1985). Related frameshift signals have since been documented in an increasing number of systems, including several other retroviruses, a number of eukaryotic positive-strand RNA viruses, a double-stranded RNA virus of yeast, some plant RNA viruses and certain bacteriophage (reviewed by Brierley, 1995). The phenomenon is not restricted to viruses; frameshift signals of the “retrovirus type” have been described in a number of Escherichia coli insertion elements (reviewed by Chandler and Fayet 1993, Farabaugh 1996) and in a conventional cellular gene, the dna X gene of E. coli Blinkowa and Walker 1990, Flower and McHenry 1990, Tsuchihashi and Kornberg 1990, Tsuchihashi 1991. The mRNA signals that specify frameshifting appear to be composed of two essential elements; a heptanucleotide “slippery” sequence, where the ribosome changes reading frame, and a region of RNA secondary structure, often in the form of an RNA pseudoknot, located a few nucleotides downstream Jacks et al 1988a, Brierley et al 1989, ten Dam et al 1990. The molecular mechanism of the frameshift process is only poorly understood, but work from several groups supports a model (Jacks et al., 1988a) in which the elongating ribosome pauses upon encountering the region of mRNA secondary structure, facilitating realignment of the slippery sequence-decoding tRNAs in the −1 frame. The heptanucleotide stretch that forms the slippery sequence contains two homopolymeric triplets and conforms, in the vast majority of cases, to the motif XXXYYYN. Frameshifting at this sequence is thought to occur by simultaneous slippage of two ribosome-bound tRNAs, presumably peptidyl and aminoacyl tRNAs, which are translocated from the zero (X XXY YYN) to the −1 phase (XXX YYY: Jacks et al., 1988a). Following the slip, the tRNAs remain base-paired to the mRNA in at least two out of three anticodon positions. There is considerable experimental support for this model, particularly from site-directed mutagenesis studies Jacks et al 1988a, Dinman et al 1991, Dinman and Wickner 1992, Brierley et al 1992, sequencing of trans-frame proteins Hizi et al 1987, Jacks et al 1988a, Jacks et al 1988b, Weiss et al 1989, Nam et al 1993 and nucleotide sequence comparisons Jacks et al 1988a, ten Dam et al 1990. The protein sequencing studies indicate that the frameshift occurs at the second codon of the tandem slippery pair, i.e. at that codon decoded in the ribosomal aminoacyl (A) site (XXXYYYN). The importance of the A-site tRNA in frameshifting was also apparent from the mutagenesis studies; point mutations affecting the A-site tRNA were generally more inhibitory than those affecting the P-site tRNA.
A key question that has remained unanswered is whether frameshifting at the slippery sequence is mediated by canonical tRNAs, or requires the participation of special “shifty” tRNAs, more prone to frameshift than their “normal” counterparts (Jacks et al., 1988a). At naturally occurring frameshift sites, of the codons that are decoded in the ribosomal A-site prior to tRNA slippage (XXXYYYN), only five are represented in eukaryotes, AAC, AAU, UUA, UUC and UUU, and two in prokaryotes, AAA and AAG (Farabaugh, 1996). These codons are decoded by tRNAs with a highly modified base in the anticodon loop (see Hatfield et al., 1992 and references therein). In tRNAAsn(AAC, AAU), the wobble base is queuosine (Q), in tRNAPhe (UUC, UUU), wyebutoxine (Y) is present just 3′ of the anticodon, in tRNALys (AAA, AAG), the wobble base is 5-methylaminomethyl-2-thiouridine (mnm5s2U) (prokaryotes) and in tRNALeu(UUA), 2-methyl-5-formylcytidine is present at the wobble position (Debarros et al., 1996). Hatfield et al. (1992)have suggested that hypomodified variants of these tRNAs may exist that function as specific “shifty” tRNAs, since such variants will have a considerably less bulky anticodon and be more free to move around at the decoding site. Indirect support for this hypothesis comes from an examination of the modification status of the anticodons of the aminoacyl-tRNAs that are required for translation at and around the frameshift sites of human immunodeficiency virus type 1 (HIV-1), human T-cell leukaemia virus type I (HTLV-1) and bovine leukaemia virus (BLV: Hatfield et al., 1989). It was found that in HIV-1 infected cells, most of the tRNAPhe lacked Y and in HTLV-1 and BLV infected cells, most of the tRNAAsn lacked Q. However, research from other groups has suggested an alternative hypothesis, in which frameshifting is mediated by standard cellular tRNAs Tsuchihashi 1991, Tsuchihashi and Brown 1992, Brierley et al 1992. These authors propose that frameshifting at a particular site depends, amongst other parameters, upon the strength of the interaction between the slippery sequence codons and the tRNAs decoding it and that if this interaction is relatively weak, then slippage is more likely to occur. The strength of the interaction between mRNA and tRNA is likely to be influenced considerably by the kind of base-pair that forms between the 3′ base of the codon and the 5′ base of the anticodon (position 34) at the wobble position. Modification of the anticodon wobble base of frameshift site-decoding tRNAs may well influence this interaction and hence the level of frameshifting observed. A prediction of the hypothesis is that the anticodon modifications present in tRNAs that decode highly efficient slippery sequences reduce recognition of the corresponding codons.
Here, we have attempted to test these hypotheses by measuring directly the influence of tRNA anticodon modification on frameshifting. Our approach was to determine the efficiency of the frameshift signal of the coronavirus infectious bronchitis virus (IBV; Brierley et al 1987, Brierley et al 1989) in mutant strains of Escherichia coli unable to modify fully either tRNALys or tRNAAsn. The IBV frameshift signal, which is present at the overlap of the 1a and 1b ORFs of the virus genomic RNA, is a well-characterised eukaryotic system Brierley et al 1991, Brierley et al 1992 that comprises the slippery sequence UUUAAAC and a downstream RNA pseudoknot. We began by determining the components of the IBV signal required for efficient frameshifting in E. coli and then proceeded with the investigation of the role of tRNA anticodon modification in frameshifting. Four slippery sequence variants of the IBV site (UUUAAAX, where X was A, C, G or U) were tested, focusing specifically on the A-site decoding tRNAs. The results obtained indicate that in E. coli there is little influence of the tRNA modification status on frameshifting. Hypomodification of tRNAAsn had only a slight effect on frameshifting and of the tRNALys mutants tested, only trmE (anticodon s2UUU) had a marked influence, increasing the efficiency of the process at the slippery sequence UUUAAAA.
Results
Sequence requirements for IBV frameshifting in E. coli
The efficiency of frameshift signals of the IBV type in the eukaryotic rabbit reticulocyte lysate (RRL) in vitro translation system has been shown to be influenced by the nature of the slippery sequence, the integrity of the downstream RNA structure and the precise spacing between the two elements Brierley et al 1991, Brierley et al 1992. We began by confirming that these requirements were maintained inE. coli. Complementary DNAs (cDNAs) containing variants of the IBV signal were subcloned (see Materials and Methods) into the E. coli expression vector pET3xc (Studier et al., 1990) to create the pMM series of plasmids (see Figure 1). pET3xc contains the first 783 bp of coding sequence of the bacteriophage T7 gene 10, flanked by a T7 promoter and transcription termination signal. In E. coli BL21 cells, which contain an IPTG-inducible T7 RNA polymerase, a 261 amino acid residue portion of gene 10 is expressed from pET3xc as an abundant, Triton-insoluble product that is relatively easy to purify (see Materials and Methods). In the pMM plasmids, the IBV cDNAs were cloned in frame with and downstream of the gene 10 sequence of pET3xc at a unique BamHI site. In BL21 cells, these constructs were predicted to express a 33 kDa non-frameshifted product corresponding to ribosomes that terminated at the IBV 1a stop codon and a 50 kDa frameshift product from ribosomes which frameshifted prior to encountering the stop codon and continued to translate the 1b ORF in the −1 frame. The constructs tested are shown in Figure 2, the expressions in Figure 3 and the results are summarised in Table 2. For the pET3xc plasmid, which does not contain an IBV insertion, only two major species were present, the N-terminal portion of gene 10, with an apparent molecular mass of about 34 kDa and lysozyme, carried over from the purification procedure (see Materials and Methods). For the pMM expressions, three species were seen; lysozyme, a 36 kDa species corresponding to the non-frameshifted product and for most constructs, a 50 kDa frameshift product. The identity of the 36 kDa and 50 kDa proteins was confirmed by demonstrating that both proteins reacted in Western blots with a monoclonal antibody directed against the N terminus of gene 10 (AMS Biotechnology Ltd, Europe; data not shown). We also confirmed that the 36 kDa and 50 kDa proteins were restricted to the insoluble fraction of E. coli cell extracts; no soluble non-frameshifted or frameshifted protein was detected (data not shown).
Figure 1.
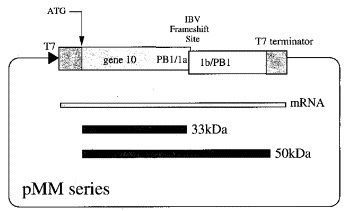
The basic plasmids used in this study, the pMM series, were prepared by subcloning 585 bp NheI-Eco RI fragments containing the IBV frameshift region from plasmids pFS7, pFS8 or mutant derivatives Brierley et al 1989, Brierley et al 1991 into BamHI-cleaved, plasmid pET3xc (Studier et al., 1990). Both fragment(s) and vector were end-filled using the Klenow fragment of DNA polymerase I prior to ligation with T4 DNA ligase. The resulting plasmids contain the IBV ORF 1a/1b frameshift signal (sequence information from base-pairs 12,286 to 12,511; Boursnell et al., 1987) flanked by portions of the influenza A/PR8/34 PB1 gene (sequence information from base-pairs 1140 to 1167 (5′) and 1167 to 1500 (3′); Young et al., 1983) located downstream of, and in frame with, the first 783 bp of coding sequence of the bacteriophage T7 gene 10. The ensemble is under the control of the bacteriophage T7 promoter and a T7 transcription termination signal is present at the end of the coding sequences. In the relevant T7-expressing bacteria (see Materials and Methods), the constructs are predicted to express a 33 kDa non-frameshifted product, corresponding to ribosomes that terminate at the IBV 1a stop codon and a 50 kDa frameshift product from ribosomes that frameshift prior to encountering the stop codon and continue to translate the 1b ORF in the −1 frame.
Figure 2.

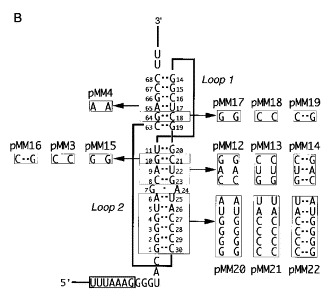
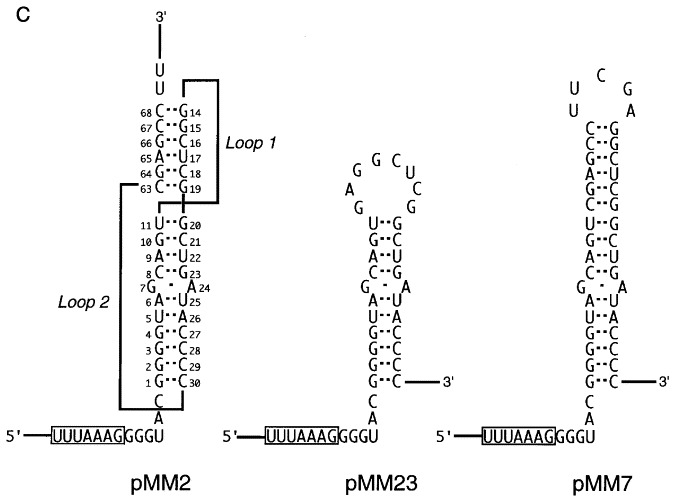
Frameshift constructs tested in E. coli. A, Slippery sequence variants. In each construct, the slippery sequence is boxed. Nucleotides that differ from the wild-type sequence (pMM2) are indicated in bold. In pMM10 and pMM11, the distance between the slippery sequence and RNA pseudoknot was decreased (GGG deleted) or increased (UAC inserted) by 3 nt, respectively. In pMM6 and pMM5, the pseudoknot was deleted (as in construct pFS7.6; Brierley et al., 1989). B, Pseudoknot variants. Complementary and compensatory changes were created within the pseudoknot region. In this representation of the pseudoknot, the stems are arranged vertically and the loops are shown as thick lines. For each base-pair(s) studied, the two complementary changes (no base-pairing) and the compensatory change (base-pairing restored) are boxed and labelled with a mutant number. C. Stem-loop constructs tested. Two constructs were tested that formed a stem-loop structure rather than a pseudoknot. Plasmid pMM23 forms only stem 1 due to a deletion that removed the downstream pseudoknot-forming region (as in construct pFS7.6; Brierley et al., 1989). Plasmid pMM7 is a stem-loop construct in which the stem nucleotides are of the same length and nucleotide composition as the stacked stems of the pseudoknot in pMM2 (as in construct pFS8.26; Brierley et al., 1991).
Figure 3.
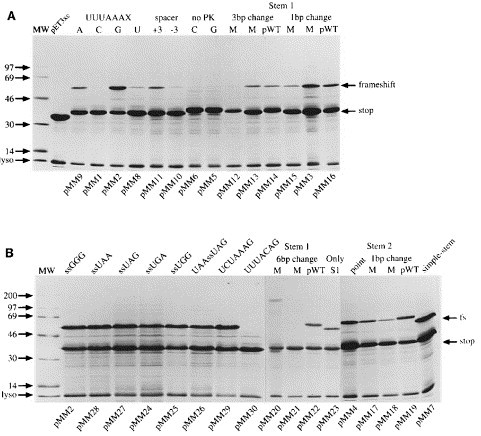
A and B, Frameshift assays in E. coli. Purified proteins expressed from the relevant frameshift plasmids were separated on SDS 15% polyacrylamide gels and detected by staining with Coomassie brilliant blue R as described in Materials and Methods. The non-frameshifted (stop) and frameshifted (frameshift or fs) products are indicated by arrows. Lysozyme (lyso) carried over from the purification procedure is indicated by an arrow. The relevant mutant number is indicated at the bottom of each track. A number of abbreviations are employed: MW, high molecular mass protein size standards (Amersham); UUUAAAX, variant slippery sequence where X is A, C, G or U as indicated above the relevant track; spacer, the slippery sequence-pseudoknot spacing distance was increased (+3 nt) or decreased (−3 nt) as indicated; no PK, pseudoknot deletion mutants with slippery sequence UUUAAAC (C) or UUUAAAG (G); stem 1 or stem 2 complementary (M) and compensatory (pWT) mutations were analysed in blocks of six (6 bp change), three (3 bp change) or single base-pairs (1 bp change); ssGGG, slippery sequence (UUUAAAG) and downstream codon are indicated; UAAssUAG, slippery sequence flanked by termination codons; point, single point mutation in stem 2 of construct pMM4; only S1, construct can form only stem 1; simple-stem, construct forming long stem-loop structure as detailed in Figure 2C.
Table 2.
| Construct | Feature | Efficiency (%) | |
|---|---|---|---|
| A.Slip-site variants | pMM9 | UUUAAAA | 15 |
| pMM1 | UUUAAAC | 2 | |
| pMM2 | UUUAAAG | 40 | |
| pMM8 | UUUAAAU | 3 | |
| pMM29 | UCUAAAG | 40 | |
| pMM30 | UUUACAG | 1 | |
| B.Flanking codons | pMM28 | UUAUUUAAAGUAA | 40 |
| pMM27 | UUAUUUAAAGUAG | 32 | |
| pMM24 | UUAUUUAAAGUGA | 29 | |
| pMM25 | UUAUUUAAAGUGG | 41 | |
| pMM26 | UAAUUUAAAGUAG | 33 | |
| C.Spacing variants | pMM11 | Spacer +3 nucleotides | 8 |
| pMM10 | Spacer −3 nucleotides | 2 | |
| D.PK mutations | pMM6 | Pseudoknot deletion | 1 |
| pMM5 | Pseudoknot deletion | 1 | |
| pMM23 | Stem 2 deleted | 22 | |
| pMM7 | Long stem-loop | 32 | |
| pMM12 | Stem 1 3 bp change | 8 | |
| pMM13 | Stem 1 3 bp change | 10 | |
| pMM14 | Stem I pseudo-wt | 12 | |
| pMM15 | Stem 1 1 bp change | 10 | |
| pMM3 | Stem 1 1bp change | 19 | |
| pMM16 | Stem 1 pseudo-wt | 20 | |
| pMM20 | Stem 1 6 bp change | 2 | |
| pMM21 | Stem 1 6 bp change | 2 | |
| pMM22 | Stem 1 pseudo-wt | 38 | |
| pMM4 | Stem 2 point mutation | 15 | |
| pMM17 | Stem 2 1 bp change | 15 | |
| pMM18 | Stem 2 1 bp change | 9 | |
| pMM19 | Stem 2 pseudo-wt | 37 | |
| E.Role of tRNA anticodon modification | |||
| Slip sequence tested | Host/modification | ||
| UUUAAAC | SJ1512/wt Q+ | 2 | |
| SJ1515/tgt1 Q− | 1 | ||
| K12Δtgt Q− | 1 | ||
| UUUAAAU | SJ1512/wt Q+ | 3 | |
| SJ1515/tgt1 Q− | 6 | ||
| K12Δtgt Q− | 5 | ||
| UUUAAAA | TG1/wt | 15 | |
| TH48/wt | 15 | ||
| TH49/trmC1 | 16 | ||
| TH69/trmC2 | 15 | ||
| DEV1/wt | 15 | ||
| DEV16/trmE | 32 | ||
| TH79/wt | 15 | ||
| TH78/trmE | 32 | ||
| TH160/wt | 15 | ||
| TH159/asuE | 15 | ||
| UUUAAAG | TG1/wt | 40 | |
| TH48/wt | 41 | ||
| TH49/trmC1 | 40 | ||
| TH69/trmC2 | 40 | ||
| DEV1/wt | 40 | ||
| DEV16/trmE | 48 | ||
| TH79/wt | 40 | ||
| TH78/trmE | 49 | ||
| TH160/wt | 40 | ||
| TH159/asuE | 33 |
Each value quoted represents the average of three to five independent measurements, which varied by less than 5%; i.e. a measurement of 40% frameshift efficiency was between 38% and 42%. The abbreviations used are: PK, pseudoknot; wt, wild-type.
Slippery sequence requirements
The generality of the simultaneous slippage model of frameshifting for sites expressed in E. coli is not fully established. It was important, therefore, to determine whether frameshifting at the IBV slippery sequence in E. coli deviated from the conventional simultaneous slippage mechanism ascertained in RRL. We created a series of mutations at or around the IBV slippery sequence and tested for frameshifting by expression in E. coli BL21 (see Figure 3A). The wild-type IBV slippery sequence, UUUAAAC(pMM1), decoded by tRNAAsn (anticodon 5′ QUU 3′) stimulated only low-levels of frameshifting (2%), as did UUUAAAU (pMM8, 3%). In E. coli, therefore, tRNAAsn is considerably less slippery than it is in eukaryotic cells or the RRL (Brierley et al., 1989). In contrast, tRNALys (anticodon 5′ U8UU 3′; where U8 is mnm5s2U), which reads UUUAAAA(pMM9) and UUUAAAG(pMM2), was highly slippery; frameshifting at these sites occurring with great efficiency (15% and 40%, respectively). The slipperiness of E. coli tRNALys has been documented and will be discussed in detail later. In E. coli the hierarchy of frameshifting for the seventh nucleotide of the slippery sequence (UUUAAAN) was N = G > A ⪢ U = C. This is almost the reverse of the situation seen in RRL, where the hierarchy for N is C > A = U ⪢ G (Brierley et al., 1992), and is consistent with earlier studies of frameshifting in E. coli Weiss et al 1989, Tsuchihashi and Brown 1992. The introduction of different termination codons (UGA, pMM24; UAG, pMM27; UAA, pMM28) or an alternative sense codon (UGG, pMM25) immediately downstream of the slippery sequence had little effect on frameshifting, although a discernible reduction was seen with the UAG (32%) and UGA (29%) terminators. By flanking the slippery sequence with termination codons (pMM26), one immediately downstream (UGA) in the zero phase and a second immediately upstream (UAA) in the −1 phase, we were able to confirm that frameshifting takes place within the UUUAAAG stretch (Figure 3B). As with pMM27, we observed a slight reduction in frameshift efficiency with this construct, which had UAG as the downstream termination codon (see Discussion). We also introduced mutations within the slippery sequence. Unsurprisingly, the sequence UUUACAG (pMM30) was non-functional, since his mutation would prevent slippage of the A-site decoding tRNA. However a P-site mutation, UCUAAAG (pMM29) was fully competent in frameshifting, suggesting that in the case of the IBV signal in E. coli, the process does not involve simultaneous slippage of two tRNAs, but rather −1 slippage of a single tRNALys (from AAG to AAA).
RNA secondary structure requirements
Efficient frameshifting at the IBV signal, at least in the RRL, depends upon the RNA pseudoknot structure, which cannot be replaced functionally by a stable stem-loop structure of the same predicted size and nucleotide composition as the stacked stems of the pseudoknot (Brierley et al., 1991). We investigated whether the RNA secondary structure requirements for frameshifting in E. coli were conserved by measuring the frameshift efficiency of a series of pseudoknot mutants (see Figure 2, Figure 3). Complete removal of the pseudoknot dramatically reduced frameshifting, irrespective of whether the slippery sequence was UUUAAAC (pMM6) or UUUAAAG (pMM5). The pattern of frameshifting observed for mutations within the pseudoknot closely paralleled that seen in the RRL, in that destabilization of either stem of the pseudoknot reduced frameshifting efficiency (pMM3, 12, 13, 15, 20, 21 in stem 1; pMM4, 17, 18 in stem 2) and compensatory mutations predicted to restore the structure in general increased frameshifting (pMM16, 22 in stem 1; pMM19 in stem 2). Of the compensatory mutants in stem 1, two of the three analysed had frameshift efficiencies considerably below that seen for the wild-type structure (pMM14, 12% and pMM16, 20%). This is a phenomenon that has been observed in a number of studies of eukaryotic frameshifting (see ten Dam et al., 1995) and may reflect a functional requirement for a particular pseudoknot conformation that is imprecisely reproduced in some of the compensatory mutants. The efficiencies of frameshifting measured for the stem 2 point mutations (pMM4, 15%; pMM17, 15% and pMM18, 9%) were considerably greater than that seen previously in RRL, where frameshifting in these mutants is reduced to about 5% (Brierley et al., 1991). This supports the idea that efficient frameshifting in E. coli can be mediated by simple hairpin loop stimulators. Indeed, in construct pMM23, which contains the potential for formation of only stem 1, frameshifting occurred efficiently (22%). Moreover, when the pseudoknot of pMM2 was replaced by a large stem-loop structure of the same predicted size and nucleotide composition as the stacked stems of the pseudoknot (pMM7), frameshifting occurred at a high level in E. coli cells (32%), in contrast to the situation in RRL, where such a structure promotes only low levels (1 to 2%) of frameshifting. This observation highlights an important difference between prokaryotic and eukaryotic ribosomes in terms of their response to RNA stimulators associated with frameshift sites. A similarity that is maintained, however, is the necessity for precise spacing between the stimulatory structure and the slippery sequence. As in the RRL (Brierley et al., 1989), increasing (pMM11) or decreasing (pMM10) the spacing distance by three nucleotides greatly reduced frameshifting at the IBV site in E. coli.
The role of tRNA anticodon modification in frameshifting
To investigate the role of tRNA anticodon modification in the frameshift process, pairs of plasmids with variations in the last nucleotide of the slippery sequence (UUUAAAN) were expressed in E. coli strains deficient in tRNA modification (see Table 1). In these experiments, T7 RNA polymerase was provided by infecting cells with bacteriophage λ CE6 (see Materials and Methods), which contains the gene for T7 RNA polymerase under the control of the PL and PI promoters. Under these circumstances, we found that expression levels were generally lower than those seen upon IPTG-induction of BL21 cells and were influenced by the growth-rate of the cells (reflecting the efficiency of the λ CE6 infection). However, the signal to noise ratios (in terms of expressed proteins to cellular background) were sufficiently high that estimates of frameshifting efficiency were reliable and reproducible. We studied two anticodon modifications, the Q base of tRNAAsn and the mnm5s2U base of tRNALys (see Figure 4).
Table 1.
Bacterial strains used in this work
| Strain | Genotype | Reference or source |
|---|---|---|
| XA105 | araΔ (lac-proB) nalA rif thi metB argEam supG | Miller et al. (1977) |
| XA10B | araΔ (lac-proB) nalA argEamrif thi metB supB | Miller et al. (1977) |
| TH48 | As XA105 but fadL∷Tn10 | Hagervall & Björk (1984) |
| TH49 | AsTH48 but trmC2 | Hagervall & Björk (1984) |
| TH69 | As TH48 but trmC1 | Hagervall & Björk (1984) |
| DEV1 | thi1 rel1 spoT1 lacZ(UAG) | Elseviers et al. (1984) |
| DEV16 | As DEV 1 but valR, trmE | Elseviers et al. (1984) |
| TH78 | As TH79 but trmE | This work |
| TH79 | araΔ (lac-proB) nalA rif thi metB argEamvalRsupB | This work |
| TH159 | As TH160 but asuE107 | Hagervall & McCloskeya |
| TH160 | araΔ(lac-proB) nalA rif thi metB argEamsupB fadR∷Tn10 | Hagervall & McCloskeya |
| SJ1502 | araD139Δ(argF-lacU139) thi1 deoC relA1 rpsL150 | Reuter et al. (1991) |
| SJ1505 | As SJ1502 but tgt1 | Reuter et al. (1991) |
| K12Δtgt | Δ(tgt) | Kerstenb |
| TG1 | supE, thiΔ(lac-proAB) F′(traD36 proAB+lacIqlacZΔM15) hshΔ5 | Gibson et al. (1984) |
| BL21(DE3) | hsdS gal (λcIts857 ind1 Sam7 nin5 lacUV5-T7 gene 1) | Studier & Moffatt (1986) |
Unpublished results.
This strain was prepared in the laboratory of Professor Helga Kersten, Institüt für Biochemie, Universität Erlangen-Nürnberg, Fahrstrasse 17, D-8520 Erlangen, Germany.
Figure 4.
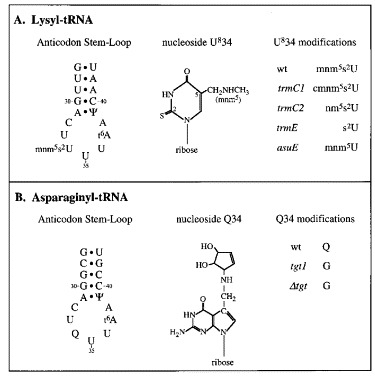
Anticodon modifications studied. The Figure shows the anticodon stem-loop nucleotides, the nucleoside modifications and the mutants tested for both lysyl and asparaginyl-tRNAs.
The role of Q
Plasmids pMM1 (UUUAAAC) and pMM8 (UUUAAAU) were expressed in a wild-type host (SJ1512) and in two strains deficient in the biosynthesis of Q due to a lack of functional tRNA guanine transglycosylase (TGT: SJ1515, K12 Δtgt). In these strains, Q is not incorporated into tRNA and tRNAAsn has the anticodon 5′ GUU 3′. As can be seen in Figure 5A, the absence of Q had only a modest effect on frameshifting, resulting in a twofold increase (UUUAAAU) or decrease (UUUAAAC) in frameshift efficiency (see Table 2). It is clear therefore that in E. coli, hypomodification of the anticodon of tRNAAsn does not lead to a dramatic increase or decrease in frameshifting.
Figure 5.
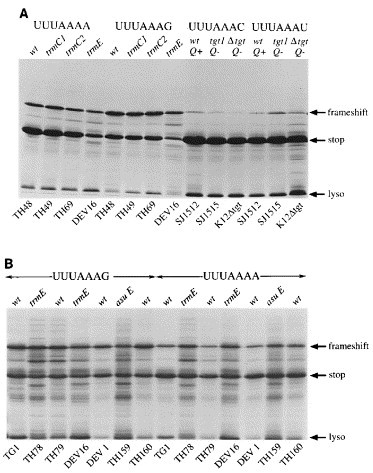
Frameshift assays in modification-deficient E. coli hosts. Frameshift plasmids were expressed in a variety of E. coli strains (see Table 1), the purified expression products separated on SDS 15% polyacrylamide gels and detected by staining with Coomassie brilliant blue R as described in Materials and Methods. The non-frameshifted (stop) and frameshifted (frameshift) products are indicated by arrows. Lysozyme carried over from the purification procedure is indicated by an arrow (lyso). The E. coli strain employed is indicated at the bottom of each track. The plasmids expressed were pMM1 (UUUAAAC), pMM2 (UUUAAAG), pMM8 (UUUAAAU) and pMM9 (UUUAAAA). The relevant genotype is indicated above each track (wt, wild-type with respect to the modification genotype); Q; Q status, either absent (−) or present (+). A, Bacteria cultured in LB medium; B, bacteria cultured in a defined minimal medium (see Materials and Methods), except asuE (LB medium).
The role of mnm5s2U
Unlike tRNAAsn, the anticodon wobble base of tRNALys is modified at two positions, at position 5, where hydrogen is replaced by a methylaminomethyl group and at position 2, where oxygen is replaced by sulphur. Although a number of E. coli mutants exist that are defective in the synthesis of mnm5s2U, a fully unmodified strain is not available. Plasmids pMM9 (UUUAAAA) and pMM2 (UUUAAAG) were expressed in wild-type hosts (TG1, TH48, DEV 1, TH79, TH160) and in the relevant defective strains listed in Table 1. Essentially, four mutants were examined; trmC1 (Hagervall & Bjork, 1984), which has the hypermodification 5-carboxymethylaminomethyl-2-thiouridine (cmnm5s2U; TH69); trmC2 (Hagervall & Bjork, 1984), containing the undermodified 5-aminomethyl-2-thiouridine (nm5s2U; TH49);trmE (Elseviers et al., 1984), which possesses only the 2-thiouridine substitution (s2U; TH78, DEV 16) and asuE107, which contains only the 5-methylaminomethyl substitution (mnm5U; TH159). In the trmC1, trmC2 and trmE strains, the presence of the sulphur atom was potentially problematic, since it can be replaced by selenium, which is thought to confer altered decoding properties (Wittwer & Ching, 1989). This was not a problem with the asuE strain; the level of mnm5Se2U has been measured in an asuE strain and was not detected (Kramer & Ames, 1988). Previous studies, however, have indicated that selenium is not detectably incorporated into tRNA when bacteria are cultured in minimal medium (Wittwer & Stadtman, 1986). Although these authors have suggested that this is also the case for LB medium, we decided to measure frameshifting in the trmC1, trmC2 and trmE strains during culture in a defined minimal medium prepared from chemicals of high purity in addition to assays in standard LB medium. The results of the analysis are shown in Figure 5 and are summarised in Table 2. The signal to noise ratio in the minimal medium assays was increased somewhat as a consequence of the slower growth-rate of the strains. The asuE strain (Figure 5B) grew very slowly in minimal medium and was only tested in LB medium. The frameshift efficiencies measured for the various mutants were not influenced by the growth medium. No effect on frameshifting was seen in the trmC1 or trmC2 strains when either the UUUAAAA or UUUAAAG-containing test plasmids were expressed. The most noticeable influence was in the trmE strain, where frameshifting was stimulated over twofold (from 15% to 32%) at the UUUAAAA site and also increased at the UUUAAAG site, although to a lesser extent (from 40% to 48%). These increases were seen in two independent trmE strains (TH78, DEV 16). Frameshifting in the asuE background was unaltered at the UUUAAAA site but was reduced a little at the UUUAAAG site (from 40% to 33%).
Discussion
Signals for frameshifting in E. coli
The generality of the simultaneous slippage model of frameshifting for sites expressed in E. coli is not fully established. Weiss et al. (1989) have expressed a variant of the MMTV frameshift signal in E. coli and have shown that ribosomes respond to both of the tandem slippery codons of the MMTV frameshift signal as predicted by the simultaneous slippage model. At the frameshift signal of the E. coli dnaX gene, mutagenesis of the slippery sequence (A-AAA-AAG) has confirmed that both lysine codons are required for efficient frameshifting (Tsuchihashi & Brown, 1992). Similarly, frameshifting at the G-T ORF overlap required to produce a bacteriophage λ tail assembly protein occurs by a two tRNA slip (Levin et al., 1993). However, in the E. coli insertion element IS 1, frameshifting is known to occur by −1 slippage of a single lysyl tRNA at the sequence A-AAA (from the underlined codon onto the overlapping AAA codon), despite the fact that the A4 stretch is embedded within two potential and conventional slippery sequences (U-UUA-AAA-AAC; Sekine & Ohtsubo, 1992). An unexpected finding from our analysis of the slippery sequence requirements for IBV frameshifting in E. coli was the high efficiency of the UCUAAAG mutant (pMM29), since such a mutant has only low activity in RRL (Brierley et al., 1989). The simplest explanation for this observation is that frameshifting at the IBV site in E. coli occurs by slippage of a single tRNA at the second homopolymeric triplet of the slippery sequence (UUUAAAX) rather than by simultaneous slippage of two tRNAs. The P-site codon in the mutant, UCUAAAG, is probably decoded by a minor tRNALeu isoacceptor with anticodon 5′ UAG 3′ (Inokuchi & Yamao, 1995). If this tRNA were to slip into the −1 reading frame (in accordance with the simultaneous slippage model) it could form only a single G-U base-pair with the −1 frame codon. At present, therefore, we favour single-tRNA slippage. Whether tRNALys slips when in the P or A-site of the ribosome is not know. Recent evidence supports the idea that frameshifting at the HIV-1 slippery sequence (U-UUU-UUA) in E. coli occurs not by simultaneous slippage of P and A-site-bound tRNAs, but when these tRNAs are in the ribosomal E and P-sites (Horsfield et al., 1995). This hypothesis was proposed following the discovery that the presence of a termination codon immediately downstream of the U6A stretch reduced frameshifting some five- to tenfold in a manner that was independent of sequence context and could be modulated by prokaryotic release factor 2. These observations were consistent with the last six nucleotides of the slippery sequence occupying the E and P-sites and the termination codon the A-site prior to tRNA slippage. In the present study, we did detect a slight reduction in frameshift efficiency when the downstream terminators UAG and UGA were employed, raising the possibility that a fraction of the frameshift events monitored involve the E-site; but the data are most consistent with the slippery sequence occupying the standard P and A-sites. This conclusion is supported by the fact that, as is also seen in RRL Brierley et al 1989, Brierley et al 1992, the spacing distance between the slippery sequence and pseudoknot had to be maintained at six nucleotides for efficient frameshifting to occur. Protein sequencing studies and further mutagenesis experiments are in progress in an attempt to improve our understanding of the precise mechanism of tRNA slippage at the IBV site in E. coli.
The requirements for downstream RNA secondary structure were tested in constructs with the most efficient (UUUAAAG) slippery sequence, using a series of complementary and compensatory pseudoknot mutants. The response of prokaryotic ribosomes to these mutants was generally similar to that seen with the eukaryotic ribosomes of the RRL in vitro translation system (Brierley et al., 1991). However, an important difference was noted; when the IBV pseudoknot was replaced by a stable stem-loop structure of the same predicted size and nucleotide composition, frameshifting was maintained at a high level in E. coli cells, in contrast to the situation in RRL, where such a structure promotes only low levels of frameshifting. In naturally occurring frameshift sites in E. coli, the requirements for stimulatory RNA structures appear to be variable. At the G-T ORF overlap of bacteriophage λ, no stimulatory secondary structure is apparent (Levin et al., 1993). In contrast, the dnaX frameshift signal includes both a downstream stem-loop (Tsuchihashi & Kornberg, 1990) and an upstream stimulatory element formed between a Shine-Delgarno-like sequence (5′ AGGGaG 3′) located 10 nt upstream of the slippery sequence and the 3′-end of 16 S rRNA (3′ UCCUcC 5′: Larsen et al., 1994). Variation is also evident at the frameshift sites of bacterial insertion sequences (Chandler & Fayet, 1993). In IS3, a pseudoknot is required for efficient frameshifting (Sekine et al., 1994) and in IS150, stimulation is via a downstream stem-loop that may form a pseudoknot (Vögele et al., 1991). In contrast, although a number of potential RNA structures are predicted to form downstream of the slippery sequence of IS1 Sekine and Ohtsubo 1989, Escoubas et al 1991, they do not appear to play a role in frameshifting (Sekine & Ohtsubo, 1992). Whatever the case, a stimulatory structure is absolutely required for IBV frameshifting in E. coli; the UUUAAAG sequence alone was unable to stimulate detectable frameshifting. This is perhaps unsurprising, since in the expression constructs employed, no obvious Shine-Delgarno-like sequences are present upstream of the slippery sequence.
Influence of tRNA anticodon modification on frameshifting
Our investigation of the role of tRNA anticodon modification in frameshifting was prompted by the experiments reported by Hatfield et al 1989, Hatfield et al 1992, who proposed that hypomodification of the anticodons of those tRNAs implicated in decoding frameshift sites may promote efficient frameshifting. We began by expressing our frameshift reporter constructs in E. coli tgt mutants unable to biosynthesize Q. In these cells, however, we detected only a modest influence of hypomodified tRNAAsn, with frameshifting reduced by about twofold on the slippery sequence UUUAAAC and increased by a similar magnitude at the UUUAAAU site. So for tRNAAsn, at least in E. coli, anticodon hypomodification per se is insufficient to promote highly efficient frameshifting. The modest effects noted, however, are consistent with the view that the strength of the pre-slippage codon-anticodon interaction is important in frameshifting. The hypomodified variant of tRNAAsn with anticodon 5′ GUU 3′ would be expected to pair more strongly with the AAC codon than with AAU, and hence frameshifting should decrease at the AAC codon and increase at AAU, as was observed. The low levels of frameshifting seen in E. coli at slippery sequences decoded by tRNAAsn, with or without Q, is in contrast to the situation in higher eukaryotic cells, where tRNAAsn is highly slippery (Brierley et al., 1992), despite possessing an identical anticodon loop (Chen & Roe, 1978). The altered frameshift capacity may simply be a reflection of the eukaryotic translational environment, but a role for Q in eukaryotic frameshifting cannot be ruled out.
The situation with UUUAAAA and UUUAAAG, decoded by tRNALys, is more complex, since the wobble base of this tRNA (mnm5s2U) is modified at two positions (see Figure 4) and an E. coli strain expressing a fully unmodified tRNA is unavailable. In the present study, of the four available mutants with altered modification of the wobble base of tRNALys, only trmE (wobble base is 2-thiouridine) had a marked influence on frameshifting, increasing the efficiency of the process over twofold at UUUAAAA and to a lesser extent at UUUAAAG. The asuE mutant (wobble base mnm5U) showed a small reduction in frameshifting at UUUAAAG. The increased efficiency seen in the trmE strains is consistent with the idea that hypomodification of the anticodon stimulates frameshifting (Hatfield et al., 1992). However, this hypothesis cannot explain the intrinsic slipperiness of the hypermodified “wild-type” tRNALys. The biological role (or at least one of the roles) of the tRNALys modifications is thought to be in the regulation of the conformational flexibility or rigidity of the base to ensure the correct translation of codons during protein synthesis, particularly to prevent decoding of AAC and AAU. Proton NMR studies of the conformational characteristics of modified uridine bases (Yokoyama et al., 1985) indicate that the mnm5s2 modification introduces conformational rigidity such that the ribose exclusively adopts a C3′- endo form, which favours recognition of adenosine, allows weak recognition of guanosine and precludes binding to cytosine and uridine. In support of this, tRNALys has been shown to decode preferentially the AAA codon and has little affinity for AAG (Lustig et al., 1981). Tsuchihashi (1991) has rationalised the high-efficiency frameshift signal of the dnaX gene on the basis that the slippery sequence, AAAAAAG, is poorly recognised by tRNALys, which slips efficiently at the AAG codon due to a weak wobble base-pair. This concept is supported by the experiments reported by Tsuchihashi & Brown (1992), who were able to inhibit frameshifting at the dnaX site by expressing an additional tRNALys with anticodon 5′ CUU 3′. In these cells, frameshifting at the AAAAAAG slippery sequence is thought to have been prevented by the more stable recognition of the AAG codon by tRNALysCUU. We believe that the efficient frameshift observed at the IBV signal in E. coli (at UUUAAAG) is also a result of a restricted capacity of tRNALys to pair with AAG. The fact that the UUUAAAA slippery sequence is also highly permissive suggests that recognition of AAA by tRNALys is also unusual when compared, for example, with the decoding of AAC or AAU by tRNAAsn.
Yokoyama et al. (1985) have suggested that both the 2 and 5-substituents contribute to the conformational rigidity of tRNALys, with the major input from the 2-thiocarbonyl group. The NMR studies by Agris and colleagues Sierzputowska-Gracz et al 1987, Agris et al 1992 indicate a role for only the 2-position thiolation. A second interaction, a hydrogen bond, has been proposed, which forms between the amino group of 5′-substitutents containing an aminomethyl moiety (mnm5 and cmnm5) and the 2′-OH residue of the adjacent, unmodified U33 base in the U-turn structure of the anticodon loop (Hillen et al 1978, Yokoyama and Nishimura 1995; and see Figure 4). Circular dichroism analysis of the tRNA suggests that the anticodon has an unusual conformation in which the wobble base is buried in the anticodon loop, possibly interacting via its 5′-substituent with the N6 threonylcarbamoyl (t6) modification of base A37 across the loop (Watanabe et al., 1993). Support for this idea comes from studies of a chemically synthesised short oligoribonucleotide comprising U33-mnm5s2U-U-U-t6A37 (cited by Agris, 1996). In this doubly modified pentamer, a unique interaction occurs between the two modifications that is thought to be between the amine group of mnm5 and the amino acid residue of t6A, by hydrogen or ionic bonding. Furthermore, model-building studies predict that the anticodon domain U-turn in tRNALys is at the mnm5s2U34 base rather than at the usual invariant residue U33.
The structural studies described above suggest that the intrinsic shiftyness of tRNALys arises from its inability to recognise efficiently lysine codons as a consequence of the anticodon modifications, which are required to prevent erroneous decoding of asparagine codons. In this light, we expected that gross alterations in the modification status (absence of thiolation in asuE, absence of the 5′-substituent in trmE) would result in a tRNA that would be less restricted and perhaps pair more readily with lysine codons. Following this logic, frameshifting would be reduced in these mutants, since the codon-anticodon interaction would be more stable. Clearly this was not the case, and at present we are unable to explain these observations satisfactorily. The most likely explanation is that possession of either of the two modifications is sufficient to reduce recognition of lysine codons and allow efficient frameshifting. The structural studies imply a role for the amino group of the 5′-substituent of tRNALys, most likely in inter-loop contact with t6A37. The increase in frameshifting seen with trmE, which does not possess the amino group, may well reflect the loss of such an interaction, resulting in altered recognition of the AAA codon (and to a lesser extent the AAG codon) by the mutant tRNA. It seems unlikely that the effect seen with trmE is related to how well the mutant tRNA is aminoacetylated; efficient aminoacetylation appears to be correlated with the presence of the s2 moiety in tRNAs of this class (Rogers et al., 1995). The lack of effect of the trmC1 and trmC2 mutations, which retain the amino group, may suggest that the conformation of tRNALys in these strains is not sufficiently different to influence codon recognition. In the case of trmC1 (cmnm5s2U; TH69) and trmC2 (nm5s2U; TH49), model-building studies (Lim, 1994) support the idea that the modified tRNAs would be expected to have similar decoding properties as the wild-type tRNA. Mutants defective in asuE, trmE and trmC all decrease the efficiency of the ochre suppressor tRNA supG, which is a derivative of tRNALys with anticodon 5′ mnm5s2UUA 3′ (reviewed by Björk, 1992). Whether this reflects a reduced affinity for the nonsense codon or another step in the suppression pathway is not clear, although the trmC1 mutation is known not to affect the binding properties of tRNALys to AAA or AAG programmed ribosomes (Elseviers et al., 1984).
In conclusion, our data are not wholly consistent with either of the hypotheses advanced to explain the role of tRNA anticodon modification in frameshifting. The observed effects of hypomodified tRNAAsn on frameshifting at UUUAAAU/C are most consistent with the idea that the strength of the codon-anticodon interaction determines frameshift efficiency rather than being mediated primarily by hypomodified tRNAs. However, the data obtained with variantly modified tRNAsLys are not readily explainable in terms of either model. Although the increased frameshifting seen in trmE strains is consistent with a role for hypomodified tRNAs, the inherent slipperiness of the wild-type hypermodified tRNALys argues strongly against this hypothesis. Nevertheless, the data obtained for the other tRNALys modification mutants are difficult to interpret solely in terms of the predicted strength of codon-anticodon recognition. All efficient −1 frameshift sites in E. coli employ tRNALys as the A-site decoding tRNA and in all likelihood, this tRNA has a very unusual anticodon structure. In attempting to compare the role of tRNA anticodon modification in frameshifting between prokaryotic and eukaryotic systems, we remain mindful that tRNALys may be a special case and that the role of modified bases in eukaryotic frameshifting needs to be tested directly. Such studies are underway.
Materials and methods
Site-specific mutagenesis
Site-directed mutagenesis was carried out by a procedure based on that of Kunkel (1985) as described (Brierley et al., 1989). Mutants were identified by dideoxy sequencing of single-stranded templates (Sanger et al., 1977).
Construction of plasmids
The starting plasmids pFS7, pFS8 or mutant derivatives Brierley et al 1989, Brierley et al 1991, which contain the IBV ORF 1a/1b frameshift signal flanked by portions of the influenza virus A/PR8/34 PB1 gene (Young et al., 1983), were subjected to site-directed mutagenesis. In most cases, this was to change the IBV slippery sequence from UUUAAAC to UUUAAAG. Following mutagenesis, 585 bp NheI-EcoRI fragments encompassing the frameshift region were subcloned from the mutated plasmids into BamHI-cleaved pET3xc, an E. coli expression vector (Studier et al., 1990). Both fragments and vector were end-filled using the Klenow fragment of DNA polymerase I prior to ligation with phage T4 DNA ligase. The resulting plasmids, which comprise the pMM series, are shown in Figure 1. The PB1:1a/1b:PB1 fragment is located downstream of, and in frame with, the first 783 bp of coding sequence of the bacteriophage T7 gene 10 and the ensemble is under the control of the bacteriophage T7 promoter.
Bacterial strains and culture conditions
Bacteria were grown at 37°C in rich medium (LB; Maniatis et al., 1982) or in minimal salt medium (M9; Maniatis et al., 1982) containing thiamine (5 mg/l), glucose (0.2%, w/v) and the required amino acids (50 mg/l). The M9 medium was prepared using reagents of high purity (Aldrich Chemical Company). The genotypes and origins of the E. coli strains used are given in Table 1. TH78 was constructed by transferring the trmE allele from DEV 16 into XA10B by phage P1 transduction, essentially according to Miller (1972). Transductants were selected for valine resistance and co-transduction of trmE monitored by screening for an Arg− phenotype. In this screen, the presence of trmE was observed as an antisuppresor activity of supB, which suppresses poorly the amber mutation in argE. A lack of mnm5s2U and the presence of s2U in tRNA from TH78 was verified by HPLC analysis according to Gehrke & Kuo (1990). TH79 is an isogenic trmE + strain. The isolation of the asuE107 mutation used in this study (TH159) will be described elsewhere. TH160 is an isogenic asuE+strain. Transfer RNA purified from TH159 was shown to contain mnm5U instead of mnm5s2U by combined liquid chromatography-mass spectrometry (LC/MS; unpublished results).
Frameshifting in E. coli BL21
The sequence requirements for IBV frameshifting in E. coli were investigated by expressing the pMM plasmid series in E. coli BL21/DE3/pLysS cells (Figure 1, Table 1), largely as described by Studier et al. (1990). These cells contain, under the control of the lac UV5 promoter, the gene for T7 RNA polymerase inserted within the int gene of the prophage DE3, a λ derivative. Expression of T7 RNA polymerase in BL21 cells can be induced by addition of isopropyl-β,d-thiogalactopyranoside (IPTG). Freshly transformed BL21 cells prepared by the method of Hanahan (1983)and containing plasmids of the pMM series were inoculated into 1.5 ml LB cultures containing ampicillin (50 μg/ml) and chloramphenicol (30 μg/ml). After three hours at 37°C, IPTG was added to 0.4 mM and incubation continued for a further three hours. Cells were pelleted, resuspended in 150 μl of lysis buffer (25 mM Tris (pH 8), 10 mM EDTA, 50 mM sucrose, 2 mg/ml lysozyme), placed on ice for 30 minutes and treated with DNase I (30 μg/ml) for a further 30 minutes at 37°C in the presence of 8 mM MgCl2 and 1 mM MnCl2. Detergent solution (300 μl of 20 mM Tris (pH 7.5), 2 mM EDTA, 0.2 M NaCl, 1% (w/v) deoxycholic acid, 1% (v/v) Nonidet P-40) was added and the insoluble material harvested by centrifugation at 8000 g for two minutes. The pellet was washed three times with 0.5% (v/v) Triton X-100, 1 mM EDTA and the Triton-insoluble material, which contained predominantly the pMM expression products, dissolved in 100 μl of sample buffer (Laemmli, 1970). Aliquots were analysed on SDS/15% (w/v) polyacrylamide gels according to standard procedures (Hames, 1991). Proteins were stained with Coomassie brilliant blue R (0.05%, w/v) in 10% (v/v) acetic acid, 50% (v/v) methanol, and destained in 10% acetic acid, 20% methanol. The relative abundance of non-frameshifted or frameshifted products was estimated (Adobe Photoshop and NIH Image software) by scanning densitometry and adjusted to take into account the differential size of the products. Scans were performed on gels whose proteins were stained to an intensity at the centre of the dynamic range of the scanner (Microtek IIXE Scanmaker). The frameshift efficiencies quoted in the text and summarised in Table 2 are the average of three to five independent measurements that varied by less than 5%, i.e. a measurement of 40% frameshift efficiency was between 38% and 42%.
Frameshifting in modification-deficient E. coli strains
The influence of tRNA anticodon modification on IBV frameshifting was probed by expressing pMM plasmids in modification-deficient E. coli strains (Table 1). Since the strains were non-lysogenic for DE3, expression of T7 RNA polymerase was achieved by infecting plasmid-bearing cells with λ CE6 (AMS Biotechnology UK Ltd). This phage contains the gene for T7 RNA polymerase under the control of the PL and PI promoters. Stocks of CE6 were prepared in E. coli LE392 by plate lysis (Maniatis et al., 1982), collected by centrifugation (100,000 g for two hours) and resuspended at 1012 pfu/ml. Modification-deficient strains harbouring pMM plasmids were grown until the absorbance at 600 nm was between 0.6 and 0.8, and infected with CE6 at 10 to 20 pfu/cell. After three hours, the cells were harvested and pMM expression products purified and analysed as above.
Acknowledgements
We thank Professor Glenn Björk for his support and advice, Professor Helga Kersten and members of her laboratory for the kind gift of E. coli strains and Dr Paul Digard for helpful comments on the manuscript. This work was supported by the Medical Research Council, UK, the Swedish Cancer Society (project no. 680 to Glenn R. Björk) and the Swedish Natural Science Research Council (project no. BBU2930-150 to Glenn R. Björk).
Footnotes
Edited by J. Karn
References
- Agris P.F. The importance of being modified: roles of modified nucleosides and Mg2+in RNA structure and function. Prog. Nucl. Acid Res. Mol. Biol. 1996;53:79–129. doi: 10.1016/s0079-6603(08)60143-9. [DOI] [PubMed] [Google Scholar]
- Agris P.F, Sierzputowska-Gracz H, Smith W, Malkiewicz A, Sochacka E, Nawrot B. Thiolation of uridine carbon-2 restricts the motional dynamics of the transfer RNA wobble position nucleoside. J. Am. Chem. Soc. 1992;114:2652–2656. [Google Scholar]
- Björk G.G. The role of modified nucleosides in tRNA interactions. In: Hatfield D.L, Lee B.J, Pirtle R.M, editors. Transfer RNA in Protein Synthesis. CRC Press; Boca Raton, FL: 1992. pp. 23–85. [Google Scholar]
- Blinkowa A.L, Walker J.R. Programmed ribosomal frameshifting generates the Escherichia coli DNA polymerase III γ subunit from within the τ subunit reading frame. Nucl. Acids Res. 1990;18:1725–1729. doi: 10.1093/nar/18.7.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boursnell M.E.G, Brown T.D.K, Foulds I.J, Green P.F, Tomley F.M, Binns M.M. Completion of the sequence of the genome of the coronavirus avian infectious bronchitis virus. J. Gen. Virol. 1987;68:57–77. doi: 10.1099/0022-1317-68-1-57. [DOI] [PubMed] [Google Scholar]
- Brierley I. Ribosomal frameshifting on viral RNAs. J. Gen. Virol. 1995;76:1885–1892. doi: 10.1099/0022-1317-76-8-1885. [DOI] [PubMed] [Google Scholar]
- Brierley I, Boursnell M.E.G, Binns M.M, Bilimoria B, Blok V.C, Brown T.D.K, Inglis S.C. An efficient ribosomal frame-shifting signal in the polymerase-encoding region of the coronavirus IBV. EMBO J. 1987;6:3779–3785. doi: 10.1002/j.1460-2075.1987.tb02713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley I, Digard P, Inglis S.C. Characterisation of an efficient coronavirus ribosomal frameshifting signal: requirement for an RNA pseudoknot. Cell. 1989;57:537–547. doi: 10.1016/0092-8674(89)90124-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley I, Rolley N.J, Jenner A.J, Inglis S.C. Mutational analysis of the RNA pseudoknot component of a coronavirus ribosomal frameshifting signal. J. Mol. Biol. 1991;220:889–902. doi: 10.1016/0022-2836(91)90361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley I, Jenner A.J, Inglis S.C. Mutational analysis of the “slippery sequence” component of a coronavirus ribosomal frameshifting signal. J. Mol. Biol. 1992;227:463–479. doi: 10.1016/0022-2836(92)90901-U. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler M, Fayet O. Translational frameshifting in the control of transposition in bacteria. Mol. Microbiol. 1993;7:497–503. doi: 10.1111/j.1365-2958.1993.tb01140.x. [DOI] [PubMed] [Google Scholar]
- Chen E.Y, Roe B.A. The nucleotide sequence of rat liver tRNAAsn. Biochem. Biophys. Res. Commun. 1978;82:235–246. doi: 10.1016/0006-291x(78)90601-0. [DOI] [PubMed] [Google Scholar]
- Debarros J.P.P, Keith G, Eladlouni G, Glasser C, Mack A.L, Dirheimer G, Desgres J. 2′- O -Methyl-5-formylcytidine (F(5)CM), a new modified nucleotide at the wobble position of 2 cytoplasmic tRNAs (Leu) (NAA) from bovine liver. Nucl. Acids Res. 1996;24:1489–1496. doi: 10.1093/nar/24.8.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinman J.D, Wickner R.B. Ribosomal frameshifting efficiency and gag/gag - pol ratio are critical for yeast M1 double-stranded RNA virus propagation. J. Virol. 1992;66:3669–3676. doi: 10.1128/jvi.66.6.3669-3676.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinman J.D, Icho T, Wickner R.B. A −1 ribosomal frameshift in a double-stranded RNA virus of yeast forms a gag-pol fusion protein. Proc. Natl Acad. Sci. USA. 1991;88:174–178. doi: 10.1073/pnas.88.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escoubas J, Prere M, Fayet O, Salvignol I, Galas D, Zerbib D, Chandler M. Translational control of transposition activity of the bacterial insertion sequence IS1. EMBO J. 1991;10:705–712. doi: 10.1002/j.1460-2075.1991.tb08000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elseviers D, Petrullo L.A, Gallagher P.J. Novel E. coli mutants deficient in the biosynthesis of 5-methylaminomethyl-2-thiouridine. Nucl. Acids Res. 1984;12:3521–3534. doi: 10.1093/nar/12.8.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farabaugh P.J. Programmed translational frameshifting. Microbiol. Rev. 1996;60:103–134. doi: 10.1128/mr.60.1.103-134.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flower A.M, McHenry C.S. The gamma subunit of DNA polymerase III holoenzyme of Escherichia coli is produced by ribosomal frameshifting. Proc. Natl Acad. Sci. USA. 1990;87:3713–3717. doi: 10.1073/pnas.87.10.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrke C.W, Kuo K.C. Ribonucleoside analysis by reversed-phase high performance liquid chromatography. In: Gherke C.W, Kuo K.C.T, editors. Chromatography and Modification of Nucleosides. Part A: Analytical Methods for Major Modified Nucleosides. Elsevier; Amsterdam: 1990. pp. 3–27. [Google Scholar]
- Gibson T, Stockwell P, Ginsburg M, Barrell B. Homology between 2 EBV early genes and HSV ribonucleotide reductase and K-38 genes. Nucl. Acids Res. 1984;12:5087–5099. doi: 10.1093/nar/12.12.5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagervall T.G, Björk G.R. Genetic mapping and cloning of the gene (trmC) responsible for the synthesis of tRNA (mnm5s2U)methyltransferase in Escherichia coli K12. Mol. Gen. Genet. 1984;196:201–207. doi: 10.1007/BF00328051. [DOI] [PubMed] [Google Scholar]
- Hames B.D. An introduction to polyacrylamide gel electrophoresis. In: Hames B.D, Rickwood D, editors. Gel Electrophoresis of Proteins: A Practical Approach. IRL Press; Oxford: 1991. pp. 1–19. [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hatfield D, Feng Y.-X, Lee B.J, Rein A, Levin J.G, Oroszlan S. Chromatographic analysis of the aminoacyl-tRNAs which are required for translation of codons at and around the ribosomal frameshift sites of HIV, HTLV-1 and BLV. Virology. 1989;173:736–742. doi: 10.1016/0042-6822(89)90589-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield D, Levin J.G, Rein A, Oroszlan S. Translational suppression in retroviral gene expression. Advan. Virus Res. 1992;41:193–239. doi: 10.1016/S0065-3527(08)60037-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillen W, Egert E, Lindner H.J, Gassen H.G. Restriction or amplification of wobble recognition. FEBS Letters. 1978;94:361–364. doi: 10.1016/0014-5793(78)80977-6. [DOI] [PubMed] [Google Scholar]
- Hizi A, Henderson L.E, Copeland T.D, Sowder R.C, Hixson C.V, Oroszlan S. Characterisation of mouse mammary tumor virus gag - pro gene products and the ribosomal frameshift site by protein sequencing. Proc. Natl Acad. Sci. USA. 1987;84:7041–7045. doi: 10.1073/pnas.84.20.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsfield J.A, Wilson D.N, Mannering S.A, Adamski F.M, Tate W.P. Prokaryotic ribosomes recode the HIV-1 gag-pol −1 frameshift sequence by an E/P site post-translocation simultaneous slippage mechanism. Nucl. Acids Res. 1995;23:1487–1494. doi: 10.1093/nar/23.9.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inokuchi H, Yamao F. Structure and expression of prokaryotic tRNA genes. In: Söll D, RajBhandary U.L, editors. tRNA: Structure, Biosynthesis and Function. ASM Press; Washington, DC: 1995. pp. 17–30. [Google Scholar]
- Jacks T, Varmus H.E. Expression of the Rous sarcoma virus pol gene by ribosomal frameshifting. Science. 1985;230:1237–1242. doi: 10.1126/science.2416054. [DOI] [PubMed] [Google Scholar]
- Jacks T, Power M.D, Masiarz F.R, Luciw P.A, Barr P.J, Varmus H.E. Characterisation of ribosomal frameshifting in HIV-1 gag-pol expression. Nature. 1988;331:280–283. doi: 10.1038/331280a0. [DOI] [PubMed] [Google Scholar]
- Jacks T, Madhani H.D, Masiarz F.R, Varmus H.E. Signals for ribosomal frameshifting in the Rous sarcoma virus gag-pol region. Cell. 1988;55:447–458. doi: 10.1016/0092-8674(88)90031-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer G.F, Ames B.N. Isolation and characterisation of a selenium metabolism mutant of Salmonella typhimurium. J. Bacteriol. 1988;170:736–743. doi: 10.1128/jb.170.2.736-743.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T.A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc. Natl Acad. Sci. USA. 1985;82:488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U.K. Cleavage of structural proteins during the assembly of the bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larsen B, Wills N.M, Gesteland R.F, Atkins J.F. rRNA-mRNA base-pairing stimulates a programmed −1 ribosomal frameshift. J. Bacteriol. 1994;176:6842–6851. doi: 10.1128/jb.176.22.6842-6851.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M.E, Hendrix R.W, Casjens S.R. A programmed translational frameshift is required for the synthesis of a bacteriophage λ tail assembly protein. J. Mol. Biol. 1993;234:124–139. doi: 10.1006/jmbi.1993.1568. [DOI] [PubMed] [Google Scholar]
- Lim V.I. Analysis of action of wobble nucleoside modifications on codon-anticodon pairing within the ribosome. J. Mol. Biol. 1994;240:8–19. doi: 10.1006/jmbi.1994.1413. [DOI] [PubMed] [Google Scholar]
- Lustig F, Elias P, Axberg T, Samuelsson T, Tittawella I, Lagerkvist U. Codon reading and translational error. Reading of glutamine and lysine codons during protein synthesis in vitro. J. Biol. Chem. 1981;256:2635–2643. [PubMed] [Google Scholar]
- Maniatis T, Fritsch E.F, Sambrook J. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1982. [Google Scholar]
- Miller J. Experiments in Molecular Genetics. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1972. [Google Scholar]
- Miller J.H, Ganem D, Lu P, Schmitz A. Genetic studies of the lac repressor I. Correlation of mutational sites with specific amino acid residues: construction of a colinear gene-protein map. J. Mol. Biol. 1977;109:275–301. doi: 10.1016/s0022-2836(77)80034-x. [DOI] [PubMed] [Google Scholar]
- Nam S.H, Copeland T.D, Hatanaka M, Oroszlan S. Characterisation of ribosomal frameshifting for expression of pol gene products of human T-cell leukemia virus type I. J. Virol. 1993;67:196–203. doi: 10.1128/jvi.67.1.196-203.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter, K., Slany, R., Ullrich, F. & Kersten, H. (191). Structure and function of Escherichia coli genes involved in biosynthesis of the deazaguanine derivative queuine, a nutrient factor for eukaryotes. J. Bacteriol. 173, 2256–2264. [DOI] [PMC free article] [PubMed]
- Rogers K.C, Crescenzo A.T, Söll D. Aminoacetylation of transfer RNAs with 2-thiouridine derivatives in the wobble position of the anticodon. Biochimie. 1995;77:66–74. doi: 10.1016/0300-9084(96)88106-5. [DOI] [PubMed] [Google Scholar]
- Sanger F, Nicklen S, Coulson A.R. DNA sequencing with chain-terminating inhibitors. Proc. Natl Acad. Sci. USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine Y, Ohtsubo E. Frameshifting is required for production of the transposase encoded by insertion sequence 1. Proc. Natl Acad. Sci. USA. 1989;86:4609–4613. doi: 10.1073/pnas.86.12.4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine Y, Ohtsubo E. DNA sequences required for translational frameshifting in production of the transposase encoded by IS1. Mol. Gen. Genet. 1992;235:317–324. doi: 10.1007/BF00279377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine Y, Eisaka N, Ohtsubo E. Translational control in production of transposase and in transposition of insertion sequence IS3. J. Mol. Biol. 1994;235:1406–1420. doi: 10.1006/jmbi.1994.1097. [DOI] [PubMed] [Google Scholar]
- Sierzputowska-Gracz H, Sochacka E, Malkiewicz A, Kuo K, Gehrke C.W, Agris P.F. Chemistry and structure of modified uridines in the anticodon, wobble position of transfer RNA are determined by thiolation. J. Am. Chem. Soc. 1987;109:7171–7177. [Google Scholar]
- Studier F.W, Moffatt B.A. Use of bacteriophage T7 RNA polymerase to direct selective high level expression of cloned genes. J. Mol. Biol. 1986;189:113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Studier F.W, Rosenberg A.H, Dunn J.J, Dubendorff J.W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- ten Dam E.B, Pleij C.W.A, Bosch L. RNA pseudoknots: translational frameshifting and readthrough on viral RNAs. Virus Genes. 1990;4:121–136. doi: 10.1007/BF00678404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Dam E, Verlaan P, Pleij C. Analysis of the role of the pseudoknot component in the SRV-1 gag - pro ribosomal frameshift signal: loop lengths and stability of the stem regions. RNA. 1995;1:146–154. [PMC free article] [PubMed] [Google Scholar]
- Tsuchihashi Z. Translational frameshifting in the Escherichia coli dnaX gene in vitro. Nucl. Acids Res. 1991;19:2457–2462. doi: 10.1093/nar/19.9.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchihashi Z, Brown P.O. Sequence requirements for efficient translational frameshifting in the Escherichia coli dnaX gene and the role of an unstable interaction between tRNALys and an AAG lysine codon. Genes Dev. 1992;6:511–519. doi: 10.1101/gad.6.3.511. [DOI] [PubMed] [Google Scholar]
- Tsuchihashi Z, Kornberg A. Translational frameshifting generates the gamma subunit of DNA polymerase III holenzyme. Proc. Natl Acad. Sci. USA. 1990;87:2516–2520. doi: 10.1073/pnas.87.7.2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vögele K, Schwartz E, Welz C, Schiltz E, Rak B. High level ribosomal frameshifting directs the synthesis of IS150 gene products. Nucl. Acids Res. 1991;19:4377–4385. doi: 10.1093/nar/19.16.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Hayashi N, Oyama A, Nishikawa K, Ueda T, Miura K. Unusual anticodon loop structure found in E. coli lysine tRNA. Nucl. Acids Res. 1993;22:79–87. doi: 10.1093/nar/22.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R.B, Dunn D.M, Shuh M, Atkins J.F, Gesteland R.F. E. coli ribosomes re-phase on retroviral frameshift signals at rates ranging from 2 to 50 per cent. Nature New. Biol. 1989;1:159–169. [PubMed] [Google Scholar]
- Wittwer A.J, Ching W.-M. Selenium containing tRNAGlu and tRNALys from Escherichia coli: purification, codon specificity and translational specificity. Biofactors. 1989;2:27–34. [PubMed] [Google Scholar]
- Wittwer A.J, Stadtman T.C. Biosynthesis of 5-methylaminomethyl-2-selenouridine, a natural occurring nucleoside in E. coli tRNA. Arch. Biochem. Biophys. 1986;248:540–550. doi: 10.1016/0003-9861(86)90507-2. [DOI] [PubMed] [Google Scholar]
- Yokoyama S, Nishimura S. Modified nucleosides and codon recognition. In: Söll D, RajBhandary U.L, editors. tRNA: Structure, Biosynthesis and Function. ASM Press; Washington, DC: 1995. pp. 207–223. [Google Scholar]
- Yokoyama S, Watanabe T, Murao K, Ishikura H, Yamaizumi Z, Nishimura S, Miyazawa T. Molecular mechanism of codon recognition by tRNA species with modified uridine in the first position of the anticodon. Proc. Natl Acad. Sci. USA. 1985;82:4905–4909. doi: 10.1073/pnas.82.15.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J.F, Desselberger U, Graves P, Palese P, Shatzman A, Rosenberg M. Cloning and expression of influenza virus genes. In: Laver W.G, editor. The Origin of Pandemic Influenza Viruses. Elsevier Science; Amsterdam: 1983. pp. 129–138. [Google Scholar]


