Abstract
Objectives
To determine the involvement of human metapneumovirus (HMPV) in childhood community-acquired alveolar pneumonia (CAAP) and compare the demographic, clinical, and laboratory features of HMPV-associated CAAP and CAAP associated with other respiratory viruses.
Study design
Nasopharyngeal wash specimens obtained prospectively over a 4-year period from children age < 5 years evaluated in the emergency department with radiologically diagnosed CAAP and from healthy controls were tested for HMPV by reverse-transcriptase polymerase chain reaction and for respiratory syncytial virus (RSV), adenovirus, influenza and parainfluenza viruses by direct immunofluorescence and culture.
Results
HMPV was detected in 108 of 1296 patients (8.3%) versus RSV in 23.1%, adenovirus in 3.4%, influenza A virus in 2.9%, and parainfluenza viruse in 2.9%. During the period of peak activity (November to May), HMPV was detected in 95 of 1017 patients (9.3%) and in 3 of 136 controls (2.2%) (P = .005). The patients with HMPV were older than those with RSV (P < .001) with a more common history of acute otitis media requiring tympanocentesis (P = .032), wheezing (P = .001) and gastrointestinal symptoms (P < .001) and a lower hospitalization rate (P = .005).
Conclusions
The high detection rate suggests an important role for HMPV in childhood CAAP. Our findings identify demographic and clinical features of HMPV-positive CAAP and its age-related impact on hospital admissions.
Keywords: CAAP, Community-acquired alveolar pneumonia; CRP, C-reactive protein; DFA, Direct immunofluorescence assay; ED, Emergency Department; HMPV, Human metapneumovirus; HMPVco, HMPV copathogen; LRTI, Lower respiratory tract infection; PCR, Polymerase chain reaction; RSV, Respiratory syncytial virus; RT-PCR, Reverse-transcriptase polymerase chain reaction; WBC, White blood cell
Human metapneumovirus (HMPV), discovered in the Netherlands in 2001, has been increasingly recognized as a major cause of lower respiratory tract infections (LRTIs) in young children worldwide.1, 2, 3, 4 This virus is detected in 5%-16% of children with an LRTI in the community, emergency room, and hospital settings, occurring in seasonal peaks with variable year-to-year activity.1, 2, 3, 4, 5, 6 The clinical features of HMPV infection closely resemble those of respiratory syncytial virus (RSV) infection.1, 2, 3, 4, 5, 6 Although the association between HMPV and bronchiolitis has been well documented, the extent of involvement of HMPV in pneumonia has not been assessed directly.
Alveolar pneumonia is often considered a bacterial infection, with pneumococcus the leading pathogen in all age groups.7, 8 But using the pneumococcal vaccine as an epidemiologic probe, Madhi et al9 recently demonstrated that the 9-valent pneumococcal conjugate vaccine substantially prevented viral-associated pneumonia. A subsequent analysis specifically revealed a 58% overall reduction in hospitalization for HMPV-associated clinical pneumonia in vaccinated children,10 suggesting an important involvement of HMPV as a copathogen in pneumonia. These findings provide indirect evidence that childhood pneumonia is commonly a result of Streptococcus pneumoniae and respiratory virus coinfection and suggest the frequent involvement of HMPV in such events. In a preliminary study comparing the clinical presentation of HMPV, RSV, and influenza A infection in young children hospitalized for a wide spectrum of LRTIs over a 1-year period, we found that HMPV infection was more often associated with a diagnosis of pneumonia compared with RSV and influenza A.11
The objectives of the present study were to determine the involvement of HMPV in radiologically diagnosed CAAP (often considered to have a bacterial etiology), and to compare the demographic, clinical, and laboratory features of children with HMPV-associated CAAP and children with CAAP associated with other respiratory viruses.
Methods
This 4-year prospective study was conducted from November 2001 through October 2005, a period before the introduction of the 7-valent pneumococcal conjugate vaccine in Israel. All children age < 5 years who were residents of southern Israel, evaluated in the pediatric emergency department (ED) of the Soroka University Medical Center (whether subsequently discharged to home or hospitalized), and met the World Health Organization's radiologic criteria for alveolar pneumonia12 were eligible for enrollment in the study.
Nasopharyngeal wash specimens were obtained daily from all children during specific hours (Sunday to Thursday between 8:00 a.m. and 12:00 p.m., except holidays). Thus, the nasopharyngeal wash often was obtained from hospitalized children the morning after hospitalization, but some of the outpatients were discharged from the ED before viral testing could be offered. Children encountered ≥ 48 hours after admission to the hospital were excluded, because their viral infection was considered a potential hospital-acquired infection.
Because we had only scan information on the prevalence of HMPV in healthy children, we included healthy controls as well. These were children age < 5 years admitted to the Soroka University Medical Center for elective surgery from whom specimens were obtained within 48 hours after admission. Control children were admitted all year-round, but only controls enrolled during the peak HMPV period were tested for HMPV (from November through May each year). Thus, the controls were not age-matched, but they were year- and season-matched. Accordingly, the results were adjusted for age and ethnic group in the analysis. Nasopharyngeal wash specimens obtained from both cases and controls whose legal guardians provided signed informed consent were analyzed. Detailed demographic, clinical, and laboratory data were collected from the medical files, and missing information was obtained by interviewing the parents or the child's primary care physician. Variables studied included age; sex; ethnic origin (Jewish or Bedouin children); number of siblings; history of wheezing or diagnosis of asthma, pneumonia, or otitis media; antibiotic treatment during the 3 months before enrollment; presence of clinical signs and symptoms; and maximal temperature, respiratory rate, oxygen saturation, white blood cell (WBC) and absolute neutrophil counts, and C-reactive protein (CRP) concentrations. Hospitalization, duration of hospitalization, and mortality also were recorded. The study design was approved by the institutional and national Ethics Committees and was performed in accordance with the Human Experimentation Guidelines of the Israeli Ministry of Health.
Definition of Alveolar Pneumonia
Chest radiographs, obtained within 24 hours of admission, were processed and archived by digital scanning (Hipax 3.27.1 X-ray image processing software; Ateinhart Medizinsysteme, Vorstetten, Germany). All radiographs were read in a blinded manner; the readers were not aware of the clinical and laboratory findings at the time of the reading. All radiographs were read prospectively and independently by 2 pediatric infectious disease specialists (R.D. and D.G) and a pediatric radiologist (J.B.-Z.). Radiographic findings were classified as alveolar pneumonia, nonalveolar pneumonia, or no pneumonia.12 Alveolar pneumonia was defined as a dense opacity appearing as a fluffy consolidation of a portion, total lobe, or the entire lung, often containing air on bronchography and sometimes associated with pleural effusion.12 The presence of radiologically diagnosed CAAP was confirmed on agreement of at least one of the study pediatric infectious disease specialists and the study pediatric radiologist.
Virus Detection
Nasopharyngeal wash specimens, obtained using 2 to 5 mL of 0.9% saline solution with a mucus extractor kit (Maersk Medical A/S, Lynge, Denmark), were sent to the Clinical Virology Laboratory within 6 hours. The specimens were diluted with 4 mL of RPMI culture medium (Kibbutz Beit Haemek, Israel). Three mL of sample was studied for the presence of RSV, influenza A and B viruses, parainfluenza viruses, and adenovirus by direct immunofluorescence assay (DFA), using commercial monoclonal antibodies (Chemicon, Temecula, California), and by tissue culture, as described previously.11 A second DFA was performed after 10 days of culture incubation. Thus, the final virologic results for all viruses (except HMPV) consisted of a combination of DFA applied on the initial sample, cytopathic effect as observed in tissue culture, and DFA applied to tissue culture cells. This combination enhanced our detection by ∼10% compared with DFA testing of the sample alone (data not shown). Residual aliquots were stored at -80°C until analysis for the presence of HMPV by reverse-transcription polymerase chain reaction (RT-PCR) at the Clinical Virology Laboratory of the Hadassah University Medical Center in Jerusalem. For HMPV detection, RNA was extracted from 140 μL of sample using the QIAamp Viral RNA Minikit (Qiagen, Hilden, Germany). Then 5 μL of the 60 μL of purified RNA solution was tested in a one-step real-time RT-PCR reaction, using conserved nucleoprotein gene primers and probe as described previously.13
Statistical Analysis
Data were recorded using the Access Microsoft Office software (Microsoft, Redmond, Washington), and statistical analysis was performed using SPSS version 14.0 (SPSS Inc., Chicago, Illinois). Univariate analysis was initially used to compare independent risk factors for HMPV-associated CAAP and those for CAAP associated with other respiratory viruses. The univariate analyses used the t-test or one-way ANOVA for quantitative dependent variables and the χ2 test or Fisher exact test for categorical dependent variables, as appropriate. Age is a common confounding variable and here was found to be a significant factor in virus distribution as well as in most potential risk factors tested, along with medical history–related and clinical variables. Thus, multivariate analyses were performed for all tested potential risk factors, controlling for age (< 12 months and ≥ 12 months). A P value < .05 was considered significant.
Results
A total of 3507 children with CAAP were seen at the Soroka University Medical Center between November 2001 and October 2005. Nasopharyngeal wash specimens were obtained from 1296 of these children (37%), of whom 997 (76.9%) were hospitalized. Of note, 137 of the children who were hospitalized for CAAP during the first year of the study also had been included in the analysis of a previous 1-year study that compared the features of HMPV, RSV, and influenza A virus in children hospitalized for a broad spectrum of LRTIs, including bronchiolitis, pneumonia, and exacerbation of asthma.11 In addition, nasopharyngeal wash specimens also were obtained from a total of 135 healthy controls enrolled between November and May in each of the study years.
The total number of patients in whom a virus was detected was 608 (46.9%). HMPV was identified in 108 children with CAAP (8.3%). HMPV was identified in 84 children (6.5%) as a single viral pathogen and in 24 children (1.8%) as a copathogen (HMPVco) with RSV (n = 10), adenovirus (n = 4), RSV and adenovirus (n = 3), RSV and influenza A virus (n = 1), cytomegalovirus (n = 3), influenza B virus (n = 2), or parainfluenza virus type 3 (n = 1).
Overall, HMPV was the second most common viral pathogen after RSV (identified in 23.1% of the children), followed by adenovirus (3.4%), influenza A (2.9%), and parainfluenza viruses (2.9%). Coinfection with more than one respiratory virus other than HMPV was found in an additional 3.2% of the children. A blood culture was obtained on enrollment in 3160 children (90.1%). An organism considered by the clinicians to be a true pathogen grew from 42 of these cultures (1.3%): S pneumoniae (n = 29), Hemophilus influenzae (nontypable, n = 3; type b, n = 2; type a, n = 1), Enterococcus spp (n = 2), Staphylococcus aureus (n = 2), Streptococcus pyogenes (n = 1), Brucella spp (n = 1), or Neisseria meningitidis group B (n = 1).
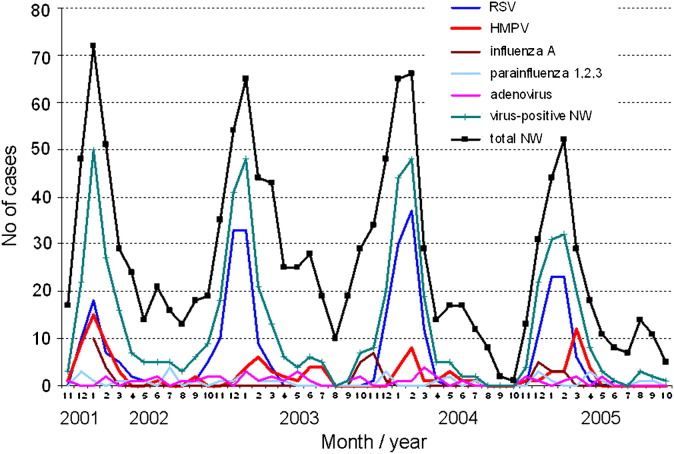
A clear seasonality of CAAP was observed, with 2 annual peaks: the major winter peak, usually from November through March, and a minor annual peak, usually from April through June. During the winter peaks (November through March), respiratory viruses were detected in the majority of patients with CAAP (550/1017 [54.1%]) (Figure 1 ). HMPV-associated CAAP overlapped with the winter seasonal peaks of RSV-associated CAAP, with further extension toward late winter and spring and occasionally early summer. Of the 108 cases of HMPV-associated CAAP, 95 (88%) occurred between November and May. During this peak period, HMPV was detected in 95 of 1017 patients with CAAP (9.3%) versus 3 of 136 healthy controls (2.2%) (P = .005). Some year-to-year variation in prevalence of HMPV-associated CAAP was detected during these months: 37/255 (14.5%), 17/291 (5.8%), 17/273 (6.2%), and 24/198 (12.1%) of all CAAP specimens were positive for HMPV in the first-, second-, third-, and fourth-year peaks, respectively.
Figure 1.
Seasonal distribution of viruses associated with radiologically diagnosed CAAP over the 4 study years. NW, nasopharyngeal wash.
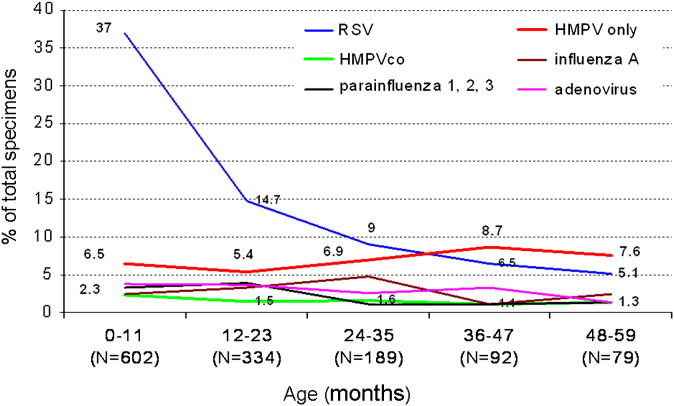
A detailed analysis of the proportion of virus detection by age revealed that in children under age 12 months (n = 602), RSV was significantly more common than HMPV (223 [37.0%] vs 39 [6.5%]; (P < .001) (Figure 2 ). The predominance of RSV was still significant during the second year of life: 49/334 (14.7%) versus 18/334 (5.4%) (P < .001); however, during the third to fifth year of life, the proportions of the 2 viruses were similar, with a decline in RSV detection. The number of children age 24 to 59 months who were admitted to the ED with HMPV-associated CAAP equaled that of those admitted with RSV-associated CAAP (n = 27). Of note, the proportion of virus-negative specimens increased from 222/602 (36.9%) in children under age 12 months to 466/694 (67.1%) in children age 12-59 months. The proportions of adenovirus, influenza A virus, and parainfluenza viruses remained relatively low in children with CAAP during the first 5 years of life.
Figure 2.
Proportion of virus detection by age in children with radiologically diagnosed CAAP, shown as the percentage of total nasopharyngeal wash specimens positive for each virus.
Children with HMPV-associated CAAP were significantly older than those with RSV-associated CAAP (Table I ). A history of acute otitis media requiring tympanocentesis was significantly more common in the children with HMPV-associated CAAP, even after adjustment for age (P = .032); a similar trend was found for a history of pneumonia (P = .097). Children with HMPVco were more likely to have had a history of wheezing or diagnosis of asthma in the last 3 months compared with those with HMPV as a single infection (P = .022). The groups did not differ significantly with regard to history of recent antibiotic treatment.
Table I.
Comparison of demographic characteristics and medical history of children with CAAP associated with HMPV, RSV, HMPVco, adenovirus, influenza A virus, and parainfluenza viruses
| Characteristic | HMPV (n = 84) | RSV (n = 299) | HMPVco (n = 24) | Adenovirus (n = 44) | Influenza A (n = 40) | Parainfluenza I-III (n = 37) | P value |
|---|---|---|---|---|---|---|---|
| Mean age, months | 19.1 ± 16.3 | 9.1 ± 10.1 | 14.7 ± 12.8 | 15.5 ± 12.6 | 18.3 ± 12.8 | 13.3 ± 11.6 | HMPV vs RSV, < .001 |
| Male sex | 52.4% | 50.5% | 58.3% | 61.4% | 55.0% | 37.8% | NS |
| Ethnicity, % Bedouins | 67.9% | 71.6% | 54.2% | 65.9% | 62.5% | 75.7% | NS |
| Number of siblings, mean ± SD | 4.5 ± 2.5 (n = 68) | 4.0 ± 2.5 (n = 229) | 4.0 ± 2.1 (n = 21) | 3.6 ± 2.1 (n = 42) | 3.7 ± 2.2 (n = 34) | 4.1 ± 2.7 (n = 34) | NS |
| History of asthma attack in the last 3 months | 14.1% (9/64) | 9.1% (19/209) | 40.0% (8/20) | 4.8% (2/42) | 15.2% (5/33) | 3.3% (1/30) | HMPVco vs HMPV, .022 |
| History of acute otitis media requiring tympanocentesis | 18.5% (12/65) | 7.6% (16/211) | 23.8% (5/21) | 15.0% (5/40) | 22.6% (7/31) | 18.5% (5/27) | HMPV vs RSV, .032∗ |
| History of pneumonia | 29.9% (20/67) | 13.5% (29/215) | 25.0% (5/20) | 26.2% (11/42) | 21.9% (7/32) | 23.3% (7/30) | HMPV vs RSV, .097∗ |
| History of antibiotic treatment during the last month | 38.0% (27/71) | 34.6% (74/214) | 55.0% (11/20) | 40.5% (17/42) | 57.1% (20/35) | 40.0% (12/30) | NS∗ |
NS, not significant.
Significant differences are highlighted in bold.
Adjusted for age.
Patients with HMPV-associated CAAP had a significantly higher rate of wheezing (P = .001) and gastrointestinal symptoms (P < .001 adjusted for age) than those with RSV-associated CAAP (Table II ). Gastrointestinal symptoms also were more common in those with HMPVco. There were no significant differences in respiratory rate, oxygen saturation, or duration of hospitalization in children with HMPV versus children with other respiratory viruses, or in children with HMPVco versus children with single HMPV detected.
Table II.
Comparison of clinical presentation and laboratory findings in children with HMPV, RSV, HMPVco, adenovirus, influenza A virus, and parainfluenza virus infection
| Characteristic | HMPV (n = 84) | RSV (n = 299) | HMPVco (n = 24) | Adenovirus (n = 44) | Influenza A (n = 40) | Parainfluenza I-III (n = 37) | P value |
|---|---|---|---|---|---|---|---|
| Upper respiratory infection | 64.8% (46/71) | 71.3% (186/261) | 77.3% (17/22) | 68.3% (28/41) | 78.4% (29/37) | 80.0% (28/35) | NS |
| Conjunctivitis | 10.4% (7/67) | 7.4% (16/215) | 8.7% (2/23) | 9.8% (4/41) | 8.8% (3/34) | 16.1% (5/31) | NS |
| Cough | 91.6% (76/83) | 95.2% (278/292) | 100% (24/24) | 88.6% (39/44) | 95.0% (38/40) | 94.4% (34/36) | NS |
| Acute otitis media | 49.2% (29/59) | 37.4% (79/211) | 57.9% (11/19) | 48.6% (18/37) | 43.3% (13/30) | 53.8% (14/26) | NS |
| Prolonged expirium | 33.3% (12/36) | 30.8% (64/208) | 38.5% (5/13) | 19.2% (5/26) | 38.9% (7/18) | 31.6% (6/19) | NS |
| Wheezing | 47.5% (19/40) | 23.2% (48/207) | 41.7% (5/12) | 19.2% (5/26) | 36.8% (7/19) | 31.6% (6/19) | HMPV vs RSV, .001∗; vs adenovirus, .026∗ |
| Gastrointestinal symptoms (overall) | 56.3% (40/71) | 31.2% (74/237) | 66.7% (16/24) | 58.1% (25/43) | 50.0% (18/36) | 48.5% (16/33) | HMPV vs RSV, < .001∗; HMPVco vs RSV, < .001 |
| Abdominal pain | 19.4% (12/62) | 7.3% (14/193) | 15.8% (3/19) | 10.0% (4/40) | 8.6% (3/35) | 3.6% (1/28) | HMPV vs RSV, .039∗ |
| Vomiting | 44.3% (31/70) | 25.5% (59/231) | 52.2% (12/23) | 48.8% (21/43) | 41.7% (15/36) | 45.5% (15/33) | HMPV vs RSV, .005∗; HMPVco vs RSV, .007 |
| Diarrhea | 11.8% (8/68) | 7.7% (17/221) | 22.7.% (5/22) | 14.3% (6/42) | 16.7% (6/36) | 3.2% (1/31) | HMPVco vs RSV, .036 |
| Mean respiratory rate, m/minute | 67.4 ± 105.4 (n = 82) | 74.9 ± 110.0 (n = 293) | 92.2 ± 193.9 (n = 24) | 120.4 ± 244.0 (n = 43) | 52.5 ± 16.2 (n = 39) | 109.1 ± 216.2 (n = 37) | NS∗ |
| Mean oxygen saturation, % | 92.1 ± 5.6 | 91.8 ± 4.5 | 91.8 ± 4.8 | 93.9 ± 3.7 | 92.1 ± 4.1 | 93.2 ± 4.2 | NS∗ |
| Percent hospitalized | 76.2% | 93.3% | 66.7% | 70.5% | 85.0% | 83.8% | HMPV vs RSV, .005∗ |
| Mean duration of hospitalization, days | 6.9 ± 5.7 | 7.3 ± 4.8 | 6.3 ± 4.5 | 5.8 ± 3.3 | 5.9 ± 3.5 | 6.4 ± 4.3 | NS∗ |
| Mean maximal temperature, °C | 38.9 ± 1.1 (n = 84) | 38.7 ± 1.1 (n = 295) | 39.2 ± 0.9 (n = 24) | 39.4 ± 0.9 (n = 43) | 39.1 ± 0.9 (n = 40) | 38.8 ± 1.3 (n = 36) | Adenovirus vs RSV, HMPV, and parainfluenza, < .05∗; vs influenza A .08∗ |
| Mean CRP concentration, mg/L | 39 ± 71 (n = 22) | 65 ± 96 (n = 62) | 27 ± 36 (n = 7) | 146 ± 150(n = 12) | 26 ± 25 (n = 12) | 66 ± 59 (n = 11) | Adenovirus vs HMPV, .021∗ |
| Mean WBC count, cells/μL | 14.5 ± 6.9 (n = 83) | 14.6 ± 6.4 (n = 289) | 16.6 ± 6.4 (n = 24) | 20.6 ± 11.2 (n = 44) | 12.6 ± 5.4 (n = 40) | 14.6 ± 6.9 (n = 37) | Adenovirus vs RSV, HMPV, influenza A, and parainfluenza, < .05∗ |
| Mean neutrophil count, cells/μL | 8.9 ± 5.9 (n = 80) | 8.2 ± 5.2 (n = 285) | 10.3 ± 6.0 (n = 23) | 13.2 ± 8.4 (n = 44) | 7.4 ± 4.5 (n = 40) | 8.4 ± 4.5 (n = 37) | Adenovirus vs RSV, HMPV, influenza A, and parainfluenza, < .05∗ |
NS, not significant.
Significant differences are highlighted in bold.
Adjusted for age
Children with HMPV were less likely to be hospitalized than those with RSV (76.2% vs 93.3%; P = .005, adjusted for age). The maximal temperature on day of admission was usually high in all children with CAAP. Mean maximal temperature was higher for children with adenovirus infection compared with those with HMPV, RSV, or parainfluenza infection (Table II). Children with HMPV or HMPVco had intermediate values of CRP, WBC, and absolute neutrophil compared with children with other viruses. On the other hand, children with adenovirus infection and virus-negative children had higher values of CRP, WBC, and neutrophil counts.
Discussion
In this prospective study, HMPV was consistently detected each year in a substantial proportion of children with radiologically diagnosed CAAP often considered by clinicians to be of bacterial origin. It was detected in 8.3% of children with CAAP evaluated in the ED in southern Israel over a 4-year-period and in 9.3% of those evaluated during seasonal peaks occurring from November through May. The high rate of HMPV detection in children with CAAP versus the significantly lower rate in healthy controls suggests a pathogenic role of HMPV in CAAP.
The involvement of HMPV in pneumonia is supported by experimental models in animals and by studies of pneumococcal conjugate vaccine in humans. Animal studies have demonstrated the capability of the virus to replicate in the lower respiratory tract.14, 15 Specifically, long-term pulmonary inflammation has been recently described in HMPV-infected BALB/c mice.16 Furthermore, previous infection by HMPV has been shown to predispose mice to severe pneumococcal pneumonia.17 In humans, the prevalence of HMPV-associated pneumonia was lower in children receiving a pneumococcal conjugate vaccine compared with control children.9, 10 As expected in a population vaccinated for H influenzae type b, few children in our study had positive blood cultures, reflecting a limitation of the methods for diagnosis of bacterial pneumonia in general. Thus, the exact role of HMPV in bacterial coinfection and in the pathogenesis of pneumonia remains to be elucidated.
In this study, we defined pneumonia by positive chest radiography (appearance of alveolar densities, as per the World Health Organization's Pneumonia Vaccine Trial Investigators' Group12). But although radiologic findings are commonly accepted as the gold standard for defining pneumonia, there are no validated definitions of radiologic interpretation.18 The sensitivity and specificity of any morphological feature of chest radiographs in diagnosing bacterial or viral pneumonia is uncertain. Thus, the exact extent of HMPV association in all cases of pneumonia is unclear, and whether or not our study represents the true proportions of HMPV involvement in pneumonia manifestations other than CAAP is uncertain.
A detailed demographic and clinical analysis identified medical history and clinical characteristics that were more common in children with HMPV-associated CAAP compared with those with CAAP associated with other viruses, despite a general overlap with RSV. As reported previously for children with LRTIs in general,4, 6, 11 children with HMPV-associated CAAP were significantly older than those with RSV-associated CAAP. Interestingly, a history of otitis media requiring tympanocentesis was significantly more common in children with HMPV than in those infected with RSV. A history of pneumonia appeared to be more common in children infected with HMPV, although the difference was not statistically significant (P = .097). These differences could not be attributed simply to older age, because they remained significant after adjustment for age.
On presentation, HMPV-associated CAAP was associated with a significantly higher rate of previous wheezing or asthma diagnosis and a higher rate of gastrointestinal symptoms compared with RSV-associated CAAP. The predominance of pulmonary obstructive findings is in accordance with the physiological airway hyperresponsiveness induced by HMPV in experimental infection.16 The mechanism of gastrointestinal symptomatology, also more commonly observed in patients with HMPVco, remains unknown. Importantly, children with HMPV were less likely to be hospitalized than children with RSV; however, there was no significant difference in any variable reflecting disease severity, such as respiratory rate, oxygen saturation, or duration of hospitalization. With regard to the extent of inflammatory response, including maximal temperature, CRP level, WBC count, and neutrophil count, children with HMPV did not have unique features compared with children with any other virus tested except adenovirus. It is noteworthy that adenovirus-related CAAP, like virus-negative CAAP, was associated with higher WBC and neutrophil counts compared with other respiratory viruses.
The relative proportions of HMPV-associated and RSV-associated CAAP varied with age. Whereas RSV accounted for the majority of virus-associated CAAP in children under age 12 months, declining after the first year of life, the proportion of HMPV infections gradually increased with age. In children age 2 to 4 years, the burden of ED evaluation for HMPV-associated CAAP equaled that of RSV-associated CAAP. The significant increase in the proportion of virus-negative specimens after the first year of life suggests an increasing role for other viral agents or pure bacterial etiologies in older children with CAAP. It should be noted that analysis of rhinovirus and coronavirus was not included in the present study. Interestingly, we have also shown that influenza virus, the leading viral etiology of CAAP in adults, played only a minor role in childhood CAAP, detected in only 2.9% of the patients.
The only virus assayed by PCR in the present study was HMPV; all of the other viruses were assayed by DFA and enhanced tissue culture. In our experience, PCR did not add more than an additional 10% to the sensitivity of respiratory virus testing over the enhanced DFA method (data not shown). Similar relative sensitivity has been demonstrated recently in a comparison of DFA and real-time PCR for the detection of HMPV.19 Thus, we doubt that the differences seen in this study between HMPV and the other viruses was due to detection bias.
Acknowledgments
We thank Niveen Saleh, BSc, for her assistance.
Footnotes
Supported by research grants from the Israeli Ministry of Science, MedImmune, Wyeth, and GlaxoSmithKline. The study sponsors had no involvement in study design, the collection, analysis, and interpretation of data, the writing of the report, or the decision to submit the manuscript for publication. No honorarium, grant, or other form of payment was given to anyone to produce the manuscript. The authors declare no conflicts of interest.
References
- 1.van den Hoogen B.G., de Jong J.C., Groen J., Kuiken T., de Groot R., Fouchier R.A. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7:719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jartti T. van den Hoogen B, Garofalo RP, Osterhaus AD, Ruuskanen O. Metapneumovirus and acute wheezing in children. Lancet. 2002;360:1393–1394. doi: 10.1016/S0140-6736(02)11391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams J.V., Harris P.A., Tollefson S.J., Halburnt-Rush L.L., Pingsterhaus J.M., Edwards K.M. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N Engl J Med. 2004;350:443–450. doi: 10.1056/NEJMoa025472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamelin M.E., Abed Y., Boivin G. Human metapneumovirus: a new player among respiratory viruses. Clin Infect Dis. 2004;38:983–990. doi: 10.1086/382536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maggi F., Pifferi M., Vatteroni M., Fornai C., Tempestini E., Anzilotti S. Human metapneumovirus associated with respiratory tract infections in a 3-year study of nasal swabs from infants in Italy. J Clin Microbiol. 2003;41:2987–2991. doi: 10.1128/JCM.41.7.2987-2991.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kahn J.S. Epidemiology of human metapneumovirus. Clin Microbiol Rev. 2006;19:546–557. doi: 10.1128/CMR.00014-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Facklam R.R., Breiman R.F. Current trends in bacterial respiratory pathogens. Am J Med. 1992;91(Suppl 6A):38–118. doi: 10.1016/0002-9343(91)90301-d. [DOI] [PubMed] [Google Scholar]
- 8.Mulholland K. Strategies for the control of pneumococcal diseases. Vaccine. 1999;17(Suppl 1):S79–S84. doi: 10.1016/s0264-410x(99)00112-7. [DOI] [PubMed] [Google Scholar]
- 9.Madhi S.A., Klugman K.P. Vaccine Trialist Group. A role for Streptococcus pneumoniae in virus-associated pneumonia. Nat Med. 2004;10:811–813. doi: 10.1038/nm1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madhi S.A., Ludewick H., Kuwanda L., Niekerk N., Cutland C., Little T. Pneumococcal coinfection with human metapneumovirus. J Infect Dis. 2006;193:1236–1243. doi: 10.1086/503053. [DOI] [PubMed] [Google Scholar]
- 11.Wolf D.G., Greenberg D., Kalkstein D., Shemer-Avni Y., Givon-Lavi N., Saleh N. Comparison of human metapneumovirus, respiratory syncytial virus and influenza A virus lower respiratory tract infections in hospitalized young children. Pediatr Infect Dis J. 2006;25:320–324. doi: 10.1097/01.inf.0000207395.80657.cf. [DOI] [PubMed] [Google Scholar]
- 12.Pneumonia Vaccine Trial Investigators' Group . World Health Organization; Geneva: 2001. Standardization of interpretation of chest radiographs for the diagnosis of pneumonia in children. WHO/V&B/01.35. [Google Scholar]
- 13.Maertzdorf J., Wang C.K., Brown J.B., Quinto J.D., Chu M., de Graaf M. Real-time reverse-transcriptase PCR assay for detection of human metapneumoviruses from all known genetic lineages. J Clin Microbiol. 2004;42:981–986. doi: 10.1128/JCM.42.3.981-986.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacPhail M., Schickli J.H., Tang R.S., Kaur J., Robinson C., Fouchier R.A. Identification of small-animal and primate models for evaluation of vaccine candidates for human metapneumovirus (hMPV) and implications for hMPV vaccine design. J Gen Virol. 2004;85:1655–1663. doi: 10.1099/vir.0.79805-0. [DOI] [PubMed] [Google Scholar]
- 15.Kuiken T., van den Hoogen B.G., van Riel D.A., Laman J.D., van Amerongen G., Sprong L. Experimental human metapneumovirus infection of cynomolgus macaques (Macaca fascicularis) results in virus replication in ciliated epithelial cells and pneumocytes with associated lesions throughout the respiratory tract. Am J Pathol. 2004;164:1893–1900. doi: 10.1016/S0002-9440(10)63750-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamelin M.E., Prince G.A., Gomez A.M., Kenkead R., Boivin G. Human metapneumovirus infection induces long-term pulmonary inflammation associated with airway obstruction and hyperresponsiveness in mice. J Infect Dis. 2006;193:1634–1642. doi: 10.1086/504262. [DOI] [PubMed] [Google Scholar]
- 17.Kukavica-Ibrulj I., Hamelin M.E., Prince G.A., Gagnon C., Bergeron Y., Bergeron M.G. Infection with human metapneumovirus predisposes mice to severe pneumococcal pneumonia. J Virol. 2009;83:1341–1349. doi: 10.1128/JVI.01123-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Madhi S.A., Klugman K.P. World Health Organisation definition of “radiologically confirmed pneumonia” may underestimate the true public health value of conjugate pneumococcal vaccines. Vaccine. 2007;25:2413–2419. doi: 10.1016/j.vaccine.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 19.Landry M.L., Cohen S., Ferguson D. Prospective study of human metapneumovirus detection in clinical samples by use of light diagnostics direct immunofluorescence reagent and real-time PCR. J Clin Microbiol. 2008;46:1098–1100. doi: 10.1128/JCM.01926-07. [DOI] [PMC free article] [PubMed] [Google Scholar]