Abstract
Transmission electron microscopy has had a profound impact on our knowledge and understanding of viruses and bacteria. The 1000-fold improvement in resolution provided by electron microscopy (EM) has allowed visualization of viruses, the existence of which had previously only been suspected as the causative agents of transmissible infectious disease. Viruses are grouped into families based on their morphology. Viruses from different families look different and these morphological variances are the basis for identification of viruses by EM.
Electron microscopy initially came to prominence in diagnostic microbiology in the late 1960s when it was used in the rapid diagnosis of smallpox, by differentiating, on a morphological basis, poxviruses from the less problematic herpesviruses in skin lesions. Subsequently, the technique was employed in the diagnosis of other viral infections, such as hepatitis B and parvovirus B19. Electron microscopy has led to the discovery of many new viruses, most notably the various viruses associated with gastroenteritis, for which it remained the principal diagnostic method until fairly recent times. Development of molecular techniques, which offer greater sensitivity and often the capacity to easily process large numbers of samples, has replaced EM in many areas of diagnostic virology. Hence the role of EM in clinical virology is evolving with less emphasis on diagnosis and more on research, although this is likely only to be undertaken in specialist centres. However, EM still offers tremendous advantages to the microbiologist, both in the speed of diagnosis and the potential for detecting, by a single test, any viral pathogen or even multiple pathogens present within a sample.
There is continuing use of EM for the investigation of new and emerging agents, such as SARS and human monkeypox virus. Furthermore, EM forms a vital part of the national emergency response programme of many countries and will provide a frontline diagnostic service in the event of a bioterrorism incident, particularly in the scenario of a deliberate release of smallpox virus.
In the field of bacteriology, EM is of little use diagnostically, although some bacterial pathogens can be identified in biopsy material processed for EM examination. Electron microscopy has been used, however, to elucidate the structure and function of many bacterial features, such as flagellae, fimbriae and spores and in the study of bacteriophages. The combined use of EM and gold-labelled antibodies provides a powerful tool for the ultrastructural localisation of bacterial and viral antigens.
Keywords: Electron microscopy, Viruses, Bacteria
1. Introduction
Prior to the development of the transmission electron microscope, cells and bacteria could be observed by light microscopy, but the only viruses that could be visualised were the poxviruses. Although poxviruses were the largest of the viruses known at that time (approximately 250 nm), no morphological detail could be seen under an optimally adjusted light microscope. The resolution limit of light microscopy of approximately 200 nm formed a barrier to visualising all other viruses and bacterial ultrastructure. The transmission electron microscope (TEM) or ‘supermicroscope’ (Gabor, 1945) was first described by Max Knoll and Ernst Ruska in 1932 (Knoll and Ruska, 1932). The successful development of the TEM, with its resolution limit of about one thousand times greater than the light microscope and the subsequent, albeit limited, availability of commercial electron microscopes post 1945, revolutionised many aspects of virology and microbiology. Ernst Ruska's brother Helmut, was one of the first workers to visualise viruses (Kruger et al., 2000). Key events in the development of electron microscopy have recently been reviewed by Haguenau and colleagues (Haguenau et al., 2003). To fully exploit the potential of the TEM, new methods for specimen preparation and new procedures for fixation, embedding, sectioning and staining were developed (Nermut et al., 1987, Harris, 1997). Since viruses and bacteria are very small, it was also necessary to develop thin films to coat EM specimen grids to support these organisms. Early imaging of viruses and bacteria was accomplished by depositing metal atoms onto the organisms to provide the necessary contrast for their visualisation: this was known as the ‘shadow casting’ technique. The introduction of negative contrast staining, using solutions of heavy metal salts, greatly simplified specimen preparation and revealed far greater detail in the ultrastructure of viruses and bacteria (Brenner and Horne, 1959). Increased commercial production of TEMs made the instruments available to many institutions, including universities and hospital laboratories and, together with the new specimen preparation methods, allowed investigation of a whole range of infectious diseases.
Use of transmission electron microscopy for the study of micro-organisms peaked during the period 1970–1980 when it contributed to the discovery of several new viruses. Biological and medical journals of that era feature significant numbers of papers describing ultrastructural investigations of disease pathogenesis involving the study of many viruses and bacteria and their interaction with a range of cells and tissues.
Development of other techniques, such as immunofluorescence, enzyme-linked immune absorbent assays (EIAs) and, in particular, the advances in molecular biology, such as the polymerase chain reaction (PCR), have all contributed to a gradual decline in the use of EM within the fields of diagnostic virology, microbiology and medicine. By the mid 1990s many laboratories and organisations were forced to rationalise their EM facilities as a result of the introduction of these new methods and financial constraints, which would not allow the automatic replacement of costly electron microscopes on a one to one basis. This rationalisation allowed some facilities to survive while others were forced to close down.
This review endeavours to outline the historical, current and future uses of EM within the fields of virology and bacteriology from a mainly UK perspective.
2. Methods
2.1. Support films on specimen grids
For examination in a TEM, suspensions of viruses, bacteria and other particulate specimens must be supported on a thin film of plastic, carbon, or a combination of the two applied to the surface of an electron microscope specimen grid. Plastic support films must be of suitable thickness in order to be durable yet not degrade the resolution of the image produced by the microscope. Polyvinyl formal (Formvar) is commonly used (1–4% in a solvent), but other compounds such as nitrocellulose (Collodion) are also suitable. A carbon coating applied to the plastic film can enhance its stability and prevent deformation and rupture due to thermal stress caused by the electron beam. Plastic films are easier to prepare and are more durable than carbon support films, but where ultimate-resolution imaging is required, carbon films alone are preferred.
2.2. Metal shadowing
Metal shadowing was the early method used to add contrast to viruses and bacteria to enhance their visualisation by TEM. Metals (gold, platinum, chromium, molybdenum, tungsten) or metal alloys (gold/palladium, platinum/palladium) are vacuum deposited at an angle of between 30 and 45 ° onto the specimens adhering to the support film. This procedure, carried out within a vacuum coating unit, requires careful monitoring of the thickness of the metal to avoid masking surface detail on bacteria and viruses (Fig. 1 ). The method is technically demanding and proved non-ideal for routine examination of clinical specimens. The introduction of the far simpler negative staining technique relegated metal shadowing to specialised applications only, for example the visualisation of nucleic acids (Fig. 2 ).
Fig. 1.

Metal shadowed Vaccinia virus—this was the original method of producing contrast in microorganisms before the advent of negative staining. Vaccinia is about 250×200 nm in size.
Fig. 2.
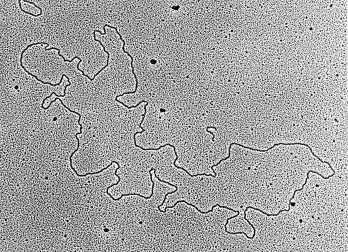
A preparation of plasmid DNA—low-angle metal shadowing is still used to visualise nucleic acids under the electron microscope.
2.3. Negative staining
The negative staining technique developed by Horne and colleagues was a major advance in diagnostic virology (Brenner and Horne, 1959, Horne and Wildy, 1963, Horne and Wildy, 1979). This technique uses the electron scattering power of heavy metal salts to provide contrast to viruses or bacteria so that surface detail may be seen. The convenience and simplicity of the method allows routine examination of many samples compared to the significantly more cumbersome metal shadowing technique. Phosphotungstic acid (PTA) is probably the most commonly used negative stain within diagnostic microbiology, but there are others that are used routinely (e.g. ammonium molybdate, uranyl acetate, sodium silicotungstate, methylamine tungstate). A solution of the stain is either mixed with a suspension of the virus or bacterial sample suspension before application to the grid or it may be applied to the grid after the sample has been adsorbed to the support film. The pH of the negative stain may have a significant effect on the detail seen and contrast produced in a specimen (Madeley, 1972). For instance, group B rotaviruses can be difficult to detect if the wrong stain is used (Nakata et al., 1987, Suzuki et al., 1987). Also, poxviruses show better ultrastructure with uranyl acetate staining, but this stain is not necessarily good for other viruses.
2.4. Resin embedding and thin sectioning
Resin embedding and thin sectioning enables the visualisation of virus replication within cells (Fig. 3 ) and the investigation of bacterial infection within cells and tissues. It is far more time consuming than the complementary technique of negative staining and is not normally used in routine diagnostic work with viruses and bacteria. However, some viruses, such as retroviruses, are difficult to see with negative staining and better results may be obtained from thin sections of infected cells.
Fig. 3.
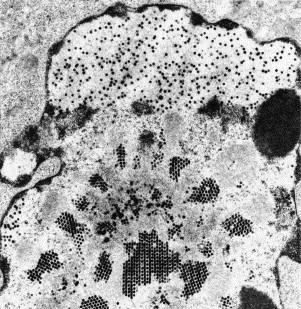
Thin section of a cultured cell containing replicating adenoviruses. Note crystalline arrays of virus assembling within the cell nucleus. Adenoviruses are about 80 nm in diameter.
Preservation (fixation) of the structural elements of infected cells or tissues in a life-like state is a critical initial stage of the technique. The fixative of choice is buffered glutaraldehyde, although others may be used (Sabatini et al., 1963). Normally, this primary fixation is followed by secondary fixation (post-fixation) in osmium tetroxide. Fixed specimens are dehydrated in ethanol and usually embedded in an epoxy resin, such as Araldite™ or Epon™, prior to thin sectioning. Ultrathin sections are cut from polymerised resin blocks and subsequently stained with heavy metal salts (usually uranyl acetate followed by lead citrate) to provide the contrast required.
2.5. Detection and identification
Viruses are identified by their characteristic morphological features. For instance, poxviruses (Fig. 4 ) look quite different from herpes viruses (Fig. 5 ). If virus particles are present in large numbers, they may be seen by direct examination of negatively stained clinical specimens without any concentration. However, for detection of small particles like viruses, it is generally considered that there must be a minimum of 106–108 virus particles/ml present in a sample to allow detection and it is therefore often necessary to concentrate the particles by such methods as differential centrifugation or ammonium sulphate precipitation (Caul et al., 1978). For viral diagnosis, specimen grids need to be scanned at a suitable magnification (e.g. about 40,000× for skin lesion samples).
Fig. 4.
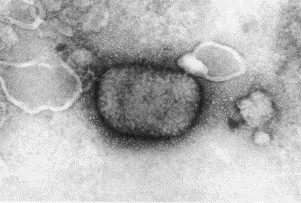
Monkeypox virus negatively stained with PTA—this is a large brick-shaped virus covered in ill-defined surface filaments. Monkeypox is an orthopox virus that normally infects certain rodents, but sometimes may infect humans. Monkeypox is about 250×200 nm in diameter.
Fig. 5.
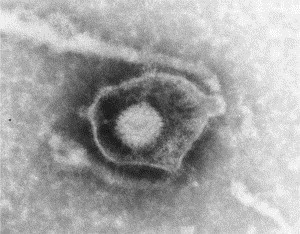
Preparation of a herpesvirus from a skin lesion negatively stained with PTA. The virion is surrounded by a limiting lipid bi-layer (‘fried egg’ appearance). This envelope is structurally and functionally an integral part of the virion. The virion appears to have a hexagonal outline (icosahedral morphology) and is covered with 152 tubular capsomeres. The members of this group of viruses all look identical, but each member of the group produces different symptoms. The internal virion of all herpes group viruses is about 100 nm in diameter and the complete virus with envelope is 150–180 nm.
2.6. Immune electron microscopy
Detection sensitivity may be increased and virus identification achieved using the technique of immune electron microscopy, which employs specific antiserum to bind to a viral antigen of interest. There are several immune electron microscopy (IEM) methods: immune clumping, solid phase immune electron microscopy (SPIEM) and immuno-gold labelling. The techniques have been particularly useful for detecting viruses causing hepatitis (Almeida et al., 1969, Feinstone et al., 1973, Balayan et al., 1983) and viruses associated with gastroenteritis. Both immune clumping and SPIEM have routine uses in virology, but immunogold labelling is mainly used in research investigations.
2.7. Immune clumping
Some of the smaller viruses lack distinctive morphological features and are thus difficult to discern from the background debris present in clinical specimens. In the immune clumping method, a virus specific detector antibody is mixed with the specimen suspension. Target virus, if present, will be aggregated by the antibody into clumps, which are much easier to detect than individual virus particles (Fig. 6 ). Examples of viruses detected by this method include hepatitis B virus (Bayer et al., 1968, Almeida et al., 1969) and parvovirus B19 (Cossart et al., 1975). Enteroviruses can also be typed using immune clumping with type-specific antisera (Lee et al., 1996).
Fig. 6.
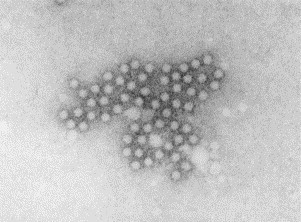
Parvovirus B19 particles negatively stained with PTA. These small round featureless viruses have been aggregated into a clump by specific antibody. Parvoviruses are 21–26 nm in diameter.
2.8. Solid phase immune electron microscopy (SPIEM)
The solid phase immune electron microscopy (SPIEM) method provides increased sensitivity over immune clumping, allowing detection of virus when only small numbers of particles are present in a clinical sample. SPIEM was originally developed for the detection of plant viruses (Derrick, 1973) and was subsequently adopted by electron microscopists working with a variety of human viruses. In SPIEM, a specimen grid with support film is coated with specific antibody. This antibody-coated grid is then floated on a drop of the virus sample under investigation. Virus particles are attracted onto the grid surface and these are spread individually over the grid rather than being aggregated into clumps. A major use of SPIEM has been for the detection of viruses associated with gastroenteritis, particularly noroviruses, which are often present in small numbers making detection by EM difficult (Lewis et al., 1988) and for enteric adenoviruses (Wood and Bailey, 1987).
2.9. Immuno-gold labelling
Immuno-gold labelling utilises specific antibodies tagged with small particles of colloidal gold as an electron dense marker. The technique may be used to localise antigens on whole viruses or bacteria or, for example, viral or bacterial antigens in thin sections of infected cells or tissues (Williams et al., 1987). Under the TEM, the location of the gold particles indicates the site of the antigen against which the antibody has been raised.
For detection of antigens in cells or tissues, a mild fixation is usually required in order to preserve sample ultrastructure yet retain antigenicity. This constraint normally precludes the use of osmium tetroxide. Dilute solutions of glutaraldehyde, formaldehyde or paraformaldehyde are commonly used fixatives for immunolabelling. Post-embedding immuno-labelling may be performed on thin sections prepared from organisms, infected cells or tissues which have been embedded in a hydrophilic resin (e.g. LR White or Lowicryl), which is compatible with antigen/antibody binding. Pre-embedding immuno-labelling of cells, permeabilised to allow access of the labelled antibody to the antigen, may also be may carried out. Should chemical fixation destroy the antigen of interest, cryofixation techniques are available (Harris, 1997). These procedures involve rapid freezing of the sample, followed by cryo-sectioning at a temperature of about minus 120 °C, using a cryo-ultramicrotome. Cryosections are collected on support films on specimen grids, thawed, incubated with labelled antibody and stained before TEM examination. Cryo techniques are highly specialised and normally undertaken for research rather than diagnostic purposes.
3. Development of the use of electron microscopy within virology and bacteriology
3.1. Bacteriology
The TEM has some limited applications in the field of diagnostic bacteriology. It can be used to identify structures and to locate antigens both on and within bacterial cells. It can also be useful in identifying some bacteria in biopsy samples. For example, spirochaete bacteria can be identified in intestinal biopsy samples, where, by light microscopy, they may be confused with the microvilli normally found on the surface of intestinal epithelial cells. Other bacteria such as, Mycobacterium avium intracellulare-complex organisms or Whipple's disease bacteria (Tropheryma whippelii, Fig. 7 ) may sometimes be found in intestinal biopsies. Bacteria with a spiral body forms, such as Helicobacter pylori (Fig. 8 ) and Helicobacter heilmani (synonym for Gastrospirillum hominis) can easily be identified in gastric biopsies by EM since the two organisms are morphologically distinct. (McNulty et al., 1989). In addition, the Q-fever bacterium, Coxiella burnetti, can be identified in native or prosthetic heart valves by electron microscopy (Isalska et al., 1996). However, such use of electron microscopy is not routine.
Fig. 7.
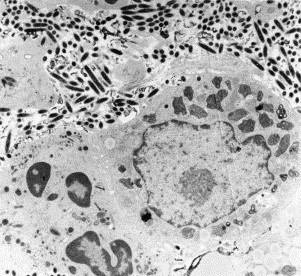
Thin section from a small intestinal biopsy showing Whipples disease bacteria (Tropheryma whippelii) in the lamina propria of an infected patient. Bacteria replicate extracellularly, but are phagocytosed by macrophages and after digestion are reduced to intracellular myelin figures.
Fig. 8.
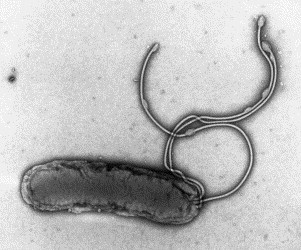
A negatively stained (PTA) preparation of Helicobacter pylori showing a terminal bunch of sheathed flagellar filaments. The flagellar sheath is thought to be acid resistant, an important adaptation to life in the gastric environment. H. pylori is 2–6 μm in length and 0.5 μm wide.
In research applications, many aspects of bacterial ultrastructure, such as motility, adhesion and plasmid transfer (via surface pili and fimbriae) and sporulation (in spore forming species), have been investigated and characterised by electron microscopy. Motility is an important attribute of a great many bacterial species and the best-understood form of this is swimming. Flagella are the structures responsible for this type of translational motility, but these appendages cannot normally be resolved under the light microscope. Under the TEM, bacterial flagella show a completely different structure from the flagella and cilia seen in higher organisms. The external part of the bacterial flagellum, which is normally visible, appears to be a structurally simple helical filament. However, at the base of the filament, a hook structure with complex basal architecture is embedded in the bacterial cell wall (Murray, 1978). The organisation of this basal complex varies depending on whether the bacterium is Gram positive or Gram negative, these two groupings having different cell wall structures (DePamphilis and Adler, 1971).
There are viruses, the bacteriophages, that exclusively infect bacteria (Fig. 9 ). These were amongst the first viruses imaged by electron microscopy (Gabor, 1945). Bacteriophages show a variety of morphologies (Kay, 1978), but usually consist of an icosahedral head and a long contractile tail. They can be used in ‘phage typing schemes’ to identify specific bacterial types and occasionally electron microscopy is used to characterise the bacteriophage involved (Sails et al., 1998).
Fig. 9.
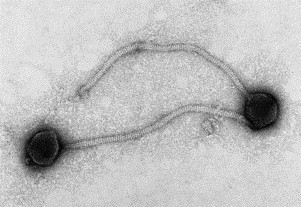
A negatively stained (PTA) preparation of a bacteriophage showing its icosahedral head and contractile tail. The phage head is about 100 nm in diameter.
3.2. Virology
Viruses come in a whole range of sizes and shapes, but fall into three morphological groups characterised by (1) helical symmetry, (2) cubic or icosahedral symmetry, and (3) other or complex symmetry (Horne and Wildy, 1961, Horne, 1974, Nermut, 1987, Madeley and Field, 1988). Different shapes of viruses occur within each symmetry group. Viruses are taxonomically grouped in families and genera. Members of a family or genus have a similar morphological appearance and it is usually possible to identify which family or genus a virus belongs to on this basis, but not necessarily the specific virus type. For instance, the virus causing chicken pox, Herpes varicella, is identical in appearance to the herpes virus causing cold sores, Herpes simplex.
The smallest intact human viruses are about 18–26 nm in diameter (parvoviruses), the largest about 250 nm (poxviruses). There are also filamentous viruses, such as Marburg and Ebola, which cause haemorrhagic fevers with high mortality rates. These have a diameter of about 80 nm but show great variability in length, occasionally exceeding 20 μm. Smaller viruses are known, but these mainly infect bacteria (bacteriophages). The morphology of viruses is usually consistent within one group. For example all adenoviruses have icosahedral symmetry and are the same shape and size. Some viruses, such as the paramyxoviruses, which cause measles, mumps and other respiratory infections are pleomorphic (Fig. 10 ), but possess characteristic internal components (nucleocapsids with helical symmetry) of a specific diameter and structure. Paramyxoviruses contain a long, thin helical nucleocapsid 18–22 nm in diameter, with a ‘herringbone’ appearance, which is a diagnostic feature. It is the complex envelope of these viruses that gives rise to their variable size and shape and shows characteristic surface projections, which enable identification of their virus group as orthomyxovirus (e.g. influenza virus) or paramyxovirus. Observed morphology is a good predictor of other virus properties (Madeley, 1997) and these can be important in the investigation of new viruses.
Fig. 10.
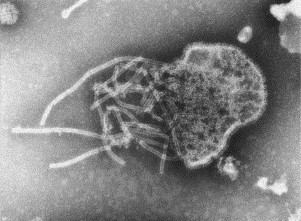
Paramyxovirus negatively stained with PTA. The nucleic acid (RNA) is protected by proteins (nucleocapsid), which give the filament a ‘herring bone’ appearance. This nucleocapsid is contained inside a membrane envelope that features various surface proteins and appears as a fringed envelope. In this micrograph, the virus membrane has ruptured and the nucleocapsid is spilling out. The nucleocapsid is 15–20 nm wide.
3.3. Rapid diagnosis of smallpox
Smallpox infection is a very serious human disease with high rates of morbidity and mortality. Following an intensive worldwide vaccination programme, the World Health Assembly announced in 1980 that smallpox had been eradicated (Henderson, 2002)—the first and so far the only human virus infection that has been successfully eradicated. Variola, the virus causing of smallpox, was endemic in some parts of the world, but outbreaks in large centres of population are known to have occurred during many periods of recorded human history up until the mid 20th century. Smallpox infection is fatal in up to 30% of infected individuals. Although classically seen as a skin infection, it is the release of virus from lesions in the respiratory tract and subsequent airborne transmission via the respiratory route that initiated outbreaks. Poxviruses are large viruses, the two major groups of which are orthopoxviruses (Fig. 4) and parapoxviruses (Fig. 11 ). Smallpox (Variola) is caused by an orthopoxvirus. Clinically, the skin lesions produced in chicken pox may easily be confused with those of smallpox. Vesicle fluid is very rich in virus particles and direct EM examination of vesicle fluid or scrapings of skin lesion leads to rapid identification of the virus group involved. Identification of a herpes virus (Fig. 5) rather than an orthopox virus negated the need to activate the public health measures (mass vaccination, quarantine, contact tracing) that would be required in the case of smallpox.
Fig. 11.
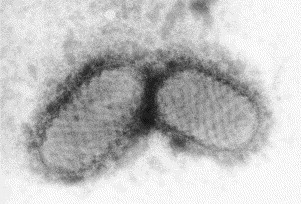
Two parapoxviruses negatively stained with PTA. This is a large ‘sausage-shaped’ virus with a distinctive surface filament that appears to wrap around the virion. The virus illustrated is a sheep pathogen (orf virus) that can sometimes infect the skin of humans. Orf virus is about 250×150 nm in size.
Although smallpox and some other poxviruses and herpesviruses were investigated by electron microscopy in the late 1940s (Nagler and Rake, 1948), it was not until the 1960s and 1970s that a number of electron microscopes were bought primarily by virology departments, for the diagnostic investigation of skin lesions (Long et al., 1970). In the UK, these microscopes were located in regional virus laboratories, Public Health Laboratories and some University Microbiology Departments, particularly in locations where imported cases of smallpox might possibly occur (Madeley, 1997). However, following the global eradication of smallpox, most laboratory stocks of the virus were destroyed and now only two centres officially hold smallpox virus, CDC, Atlanta, USA and Novosibirsk, Russian Federation (Henderson and Fenner, 2001). Although EM is no longer required for the diagnosis of smallpox, it continues to be used for detecting other poxviruses, herpesviruses and papilloma viruses in skin lesions.
3.4. Hepatitis viruses
Several viruses can infect the liver causing acute inflammation that may result in the patient becoming jaundiced. Some viral causes of hepatitis were originally investigated using electron microscopy.
3.5. Hepatitis B virus (Australia antigen)
In 1965, Blumberg and colleagues found an antibody in some haemophiliac patients, which reacted with an antigen in a serum from an Australian Aborigine and because of this, it became known as Australia antigen (Blumberg et al., 1965). Subsequently this antigen was found in about 20% of patients with viral hepatitis and is now known as hepatitis B virus surface antigen (HbsAg). For laboratory diagnosis of hepatitis B, the patient serum under investigation was mixed with a high titre immune serum and examined by EM (see Section 2.7). Under the electron microscope three types of particle may be identified (Fig. 12 ): small approximately 20 nm spheres, tubules 20 nm in diameter and up to 100 nm long and complex particles about 42 nm in diameter known as Dane particles. The Dane particle is the intact hepatitis B virus, whereas the spheres and tubules are excess viral protein (Dane et al., 1970). In the 1960s and 1970s, liver biopsies were sometimes performed on jaundiced patients and liver cells examined for the presence of Dane particles or core particles (internal components of Dane particles) migrating from the liver nuclei into the cytoplasm (Almeida et al., 1971). The immune electron microscopy method of detecting Australia antigen remained in use until about the mid 1980s when alternative methods of detection, such as radioimmuno assay, and later EIA became available.
Fig. 12.
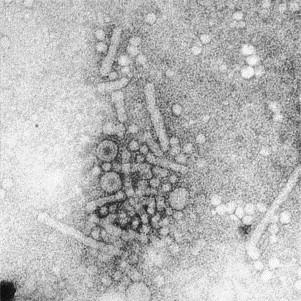
Negatively stained (PTA) spheres and tubules of hepatitis B surface antigen and Dane particles (infective virus) from the blood of an infected individual. The Dane particle is 40–45 nm in diameter and the surface antigen 18–22 nm wide.
3.6. Hepatitis A and hepatitis E
Unlike hepatitis B, which is a bloodborne infection, hepatitis A and hepatitis E are enterically transmitted infections. They may be transmitted directly between persons but may also be transmitted via contaminated food and water. Electron microscopy played a key role in the discovery of both of these viruses. Hepatitis A virus is a small icosahedral particle that has been classified with the Picornaviridae family. It was first identified by immune electron microscopy (IEM) using faecal specimens (virus) and sera (antibody) from volunteers who had been infected with hepatitis A (Feinstone et al., 1973). Hepatitis E virus was also discovered using IEM (Balayan et al., 1983). Morphologically this virus resembles the caliciviruses, but currently remains unclassified (Berke and Matson, 2000).
3.7. Parvovirus B19 in blood serum
The parvovirus group consists of small viruses, some of which cause disease in humans. Some human parvovirus infections were discovered by electron microscopy. For example, parvovirus B19 virus (Fig. 6) was discovered while looking for hepatitis B virus in serum by immune electron microscopy (Cossart et al., 1975). B19 is the cause of a common childhood rash disease, erythema infectiosum, colloquially known as ‘slapped cheek syndrome’ because of the bright red cheeks induced by the rash. We now know that this virus not only infects infants, but infection during pregnancy can seriously damage the developing foetus leading to hydrops fetalis and in some cases to spontaneous abortion. B19 infection is also a problem in individuals with chronic haemolytic disease (e.g. sickle cell anaemia), as infection can result in a significant and dangerous depression of haemoglobin levels (aplastic crisis). Development of other diagnostic techniques, particularly PCR, has resulted in EM now being rarely used for routine diagnosis of this virus, although it is occasionally used in reference laboratories where there are conflicting or equivocal results by other methods. Accurate diagnosis is important in pregnant women and those with haemolytic disorders as it can influence the clinical management of the patient.
3.8. Viral gastroenteritis
Gastroenteritis (diarrhoea and/or vomiting) is a common illness. There are many causative organisms, but most involve either bacterial infection (such as Camplylobacter or Salmonella) or viral infection. Infantile diarrhoea is a major cause of mortality in the developing world and even though relatively few infants die of gastroenteritis in the developed world, viral gastroenteritis is a leading cause of admissions to hospital of young children. In the 1950s and 1960s it was assumed that viruses were associated with some diarrhoeal illness, but it was only with the availability of electron microscopes in the 1970s that these viral causes of gastroenteritis were elucidated.
Attempts to grow viruses thought to be associated with diarrhoeal symptoms in cell culture systems were common in the 1950s and 1960s. Some viruses could be grown from some stool samples, but it was subsequently shown that these were not the causative agents of viral gastroenteritis. Convincing evidence of an association between cultivable viruses and diarrhoea was rare. However, in the early 1970s, EM was used to examine diarrhoeal samples from patients with no bacterial cause for their symptoms and a number of new, non-cultivable viruses were discovered. Viruses were found to be common causes of infantile non-bacterial gastroenteritis and were responsible for many outbreaks of gastroenteritis in all age groups. The first of these viruses was Norwalk virus, which was derived from a community outbreak in Norwalk, OH, USA (Kapikian and Wyatt, 1972, Kapikian, 2000). Using IEM, Kapikian and colleagues were able to demonstrate the presence of virus in volunteers who developed enteric symptoms after ingesting stool filtrates from samples from this outbreak. Under the electron microscope, the virus was characterised by its round shape, small size of 30–35 nm and its ‘fuzzy’ or ragged edge (Fig. 13 ). Similar viruses with the same morphology were discovered elsewhere and were given interim names such as ‘Norwalk-like viruses’ or ‘small round structured viruses’ (SRSVs) to reflect their appearance in the electron microscope (Caul and Appleton, 1982). The interim names for this group of viruses have recently been reviewed and they are now officially known as ‘noroviruses’ (Mayo, 2002). Noroviruses are regarded as the commonest cause of non-bacterial gastroenteritis in developed countries. They are highly infectious, readily transmissible from person to person and are also the most frequent and important cause of foodborne viral infections (Appleton, 2000). Until fairly recently, EM was the main method of norovirus detection, but has been largely superseded by EIA and PCR methods.
Fig. 13.
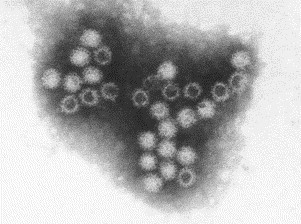
Norovirus negatively stained with PTA. This small virus is from the stool specimen of a patient with ‘winter vomiting disease’. Note the fuzzy or ragged-edged morphology of this virus and the presence of some empty virions. Norovirus is 30–35 nm in diameter.
In 1973 in Australia, Ruth Bishop and her team discovered a second virus associated with enteric symptoms in infants (Bishop et al., 1973). Using thin sections of resin embedded duodenal biopsy samples taken from infants with acute diarrhoea, she and her colleagues described a virus in the cytoplasm of duodenal enterocytes. Elsewhere, examination of stool samples from similar patients by negative staining also demonstrated the presence of this new virus (Flewett et al., 1973), which because of its' wheel-like ultrastructure (Fig. 14 ) was named ‘Rotavirus’. Rotaviruses are now considered to be the most important enteric viral infection in infancy and are associated with an estimated 600,000 deaths per year, particularly in the developing world (Parashar et al., 2003). In addition to human infection, rotaviruses also cause serious diarrhoeal disease in some animal species, such as cattle and sheep, where they have an economically important impact on farming.
Fig. 14.
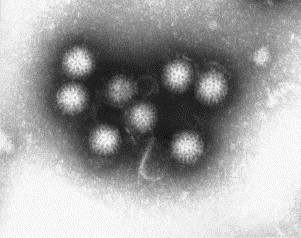
A negatively stained (PTA) preparation of rotaviruses from a diarrhoeal sample of a child. Note the spokes between the two shells of this virus, giving it a ‘wheel-like’ appearance. Rotaviruses are 70–75 nm in diameter.
Two new adenoviruses associated with enteric symptoms were discovered by EM in stool samples in 1975 by Flewett et al. (1975). Adenoviruses already comprised a large group of antigenically distinct viruses causing a wide range of symptoms from mild respiratory illness and sore throats to tumours. The two new adenoviruses, designated adenoviruses types 40 and 41 were specifically associated with gastroenteritis and formed a new group, the F adenoviruses (Gary et al., 1979). Unlike many other adenoviruses, these two new adenoviruses could not readily be cultured in the laboratory and hence became known as ‘fastidious adenoviruses’. All adenoviruses are morphologically identical. They are non-enveloped icosahedral viruses with a diameter of about 80 nm (Fig. 15 ).
Fig. 15.
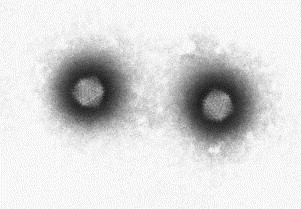
A negatively stained (PTA) preparation of two adenoviruses from a diarrhoeal sample of a child. Note the hexagonal outline (icosahedral shape) and spherical capsomeres making up the shell of this virion. Adenoviruses are around 80 nm in diameter.
Human astroviruses were discovered by EM of stool samples from infants with diarrhoea in 1975 (Appleton and Higgins, 1975, Madeley and Cosgrove, 1975). Astroviruses are morphologically distinct from other enteric viruses, being about 28 nm in diameter with a five or six-pointed surface star (Fig. 16 ). They often appear to be smooth-edged, but do not pack closely when observed in large groups. Surface projections were thought to be the reason that the viruses could not pack closely (Appleton and Higgins, 1975, Caul and Appleton, 1982) and the presence of these has been confirmed by Risco et al. (1995). Human astroviruses tend to cause a less severe diarrhoea in infants than rotavirus infection and eight astrovirus serotypes are known. Astroviruses have also been found in several other mammalian and avian species (Kurtz and Lee, 1987).
Fig. 16.
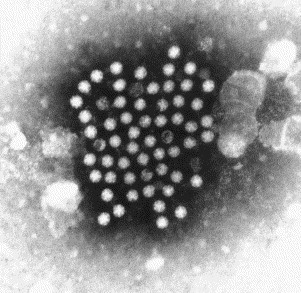
Negatively stained (PTA) preparation showing a group of astroviruses from a diarrhoeal sample from a childhood case of gastroenteritis. Note the surface star that is characteristic of this virus. Astroviruses are 27–28 nm in diameter.
Whilst looking at stool samples from infants with diarrhoea another virus related to the noroviruses but with distinctive morphology was found. This virus had the appearance of a calicivirus, being about 32–35 nm in diameter with surface hollows (Madeley and Cosgrove, 1976). These hollows (or cups) become filled with the negative stain, giving rise to a ‘star of David’ appearance in some orientations (Fig. 17 ). Enterically transmitted caliciviruses are now named sapoviruses (Kogasaka et al., 1981) and are most often associated with gastroenteritis in young children (whereas noroviruses infect all age groups). The two viruses have morphological similarities and both are classified in the Calicivirus family, although in separate genera.
Fig. 17.
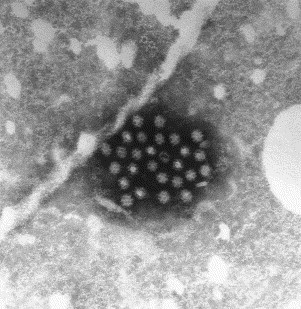
Negatively stained (PTA) preparation showing a group of sapoviruses from a diarrhoeal sample from a childhood case of gastroenteritis. Note surface cups that are characteristic of this virus and various orientations consistent with icosahedral symmetry. Sapoviruses are 33–35 nm in diameter.
Some electron microscopy units struggled to correctly identify the smaller and often taxonomically unclassified viruses found in faecal specimens, such as astroviruses and noroviruses. This led to an interim scheme for classification based on the morphological appearance of the various viruses in the electron microscope (Caul and Appleton, 1982). This ‘interim’ scheme remained useful for a number of years until advances in molecular techniques led to the development of alternative diagnostic tests and a more definitive classification for these viruses.
3.9. Investigation of outbreaks of viral gastroenteritis
The Public Health Laboratory Service (PHLS) within England and Wales, now superseded by the Health Protection Agency, originally sited many electron microscopes in laboratories near to significant concentrations of population to investigate gastroenteritis. However, by the late 1990s, EM within the PHLS was in decline due to the introduction of new methods of virus detection that were more sensitive than EM and could readily be used for dealing with large numbers of specimens, although initially these tests were only available in specialised laboratories. The reduction in EM facilities necessitated a rationalised virological EM specimen testing policy for the investigation of outbreaks of viral gastroenteritis (Curry et al., 1999). Large numbers of outbreaks with many specimens being generated from each outbreak, could not be handled by the remaining PHLS EM facilities and in order to give a good diagnostic service with limited facilities it was necessary for the number of samples examined from each outbreak to be restricted. It had been recognised that most community outbreaks of gastroenteritis were caused by noroviruses and hence if two or more samples were found to be norovirus positive, then the outbreak was declared to be a positive norovirus outbreak and no further samples were examined. This testing algorithm allowed EM units to continue to diagnose norovirus infections until widespread use of specific PCR tests for noroviruses were adopted in routine virus laboratories or specific norovirus EIAs became commercially available. Norovirus EIAs were introduced to the UK during the latter half of 2002. Norovirus EIA and PCR tests are in the process of or have now superseded EM as the front-line tests for the investigation of non-bacterial gastroenteritis outbreaks in routine virology laboratories.
3.10. Polyomaviruses
Two new human polyomaviruses, which are members of the papovavirus group, were discovered by electron microscopy. These viruses are called BK virus and JC virus. Both are about 45 nm in diameter and feature skewed symmetry of the proteins (capsomeres) making up the capsid of the virus. JC virus was originally discovered in thin sections of brain biopsies from patients with progressive multifocal leucoencephalopathy (Zu Rhein and Chou, 1965), whereas BK virus (Fig. 18 ) was found in negatively stained preparations of urine from organ transplant patients (Gardner et al., 1971). Infection is common and usually goes unnoticed, but virus may become reactivated and cause symptoms in immunosuppressed patients, such as organ transplant recipients. Immune EM was used for specific diagnosis of these two viruses until the development of molecular tests.
Fig. 18.
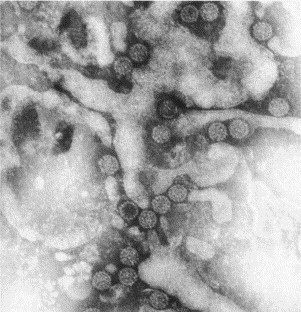
Negatively stained (PTA) preparation of polyomaviruses from a urine sample of a bone marrow transplant patient with symptoms of bloody urine. Polyomaviruses are 40–45 nm in diameter.
4. Current and future use of electron microscopy within virology
4.1. Skin lesion investigation and deliberate release of dangerous pathogens.
Electron microscopy still has an important role in the investigation of skin lesions as any viral pathogen involved can be detected and identified within 30 min of receipt of the sample. A skilled electron microscopist can distinguish herpesviruses (Fig. 5) from the various poxviruses (Fig. 4, Fig. 11) and papilloma viruses in skin scrapings or aspirated fluid from a lesion. Such investigation is important, particularly if the patient is immunocompromised or on a ward with other immunocompromised patients. Rapid availability of diagnostic results can positively influence patient management.
Electron microscopy will have a vital frontline diagnostic role in the event of biological agents being deliberately released into the community and it is, therefore, essential that expertise be maintained (Miller, 2003). The terrible events of September 11th 2001 and the unrelated deliberate release of anthrax spores in the USA at around the same time has alerted most countries to the serious consequences of deliberate release of other infectious agents such as smallpox. Diagnostic facilities must be in place to rapidly identify the causative agent present in atypical or suspicious skin lesions, be it smallpox or other viruses, so that unnecessary public panic can be avoided and appropriate control measures established. Electron microscopy has an important role in a rapid emergency diagnostic service and must be maintained as part of a balanced diagnostic response system, so that only orthopox samples are referred to specialist centres for definitive identification by molecular typing. Such a system requires a small network of well supported regionally based EM units with the necessary staff expertise in place to monitor viruses associated with skin lesions and to act as an early warning to the deliberate release of smallpox or other microbial agents. The risk of smallpox reappearing is small, but if a terrorist group were to obtain the virus, the possibility exists that it could be genetically modified to accentuate its pathogenic traits. A consequence of this would be that primers used in specific smallpox PCR tests might not amplify the nucleic acid, thereby rendering PCR as a means of diagnosis temporarily invalid. Such a modified virus would still be recognisable morphologically as a poxvirus under the electron microscope. This presents strong justification for the need to keep a network of microscopes and microscopists skilled in viral diagnosis, since EM is not dependant on detection of viral nucleic acid or expressed antigen.
Although smallpox is an exclusively human pathogen with no animal reservoir, other poxviruses can mimic its clinical symptoms. During 2003, monkeypox (a virus similar to smallpox) was detected by EM in humans in the USA (Fig. 4). Prior to this, it was thought that monkeypox only occurred in the tropical forest regions of Africa, where it was fatal in up to 10% of human cases. Some clinicians who had treated infected individuals in the USA outbreak had initially mistaken the monkeypox lesions for smallpox or chickenpox (Reed et al., 2004). Most infections appeared in owners of exotic pet rodents or workers in veterinary centres or pet shops. Other unrelated cases of unusual orthopoxvirus infections have recently been reported. A case of severe generalized cowpox infection has recently been seen in Finland and was diagnosed using EM, cell culture (virus isolation) and molecular techniques (Pelkonen et al., 2003). In the USA, a woman who was revaccinated against smallpox showed a variety of symptoms including lesions on the face, legs, trunk and upper extremities (Miller et al., 2003). The presence of orthopoxviruses in lesion fluid was demonstrated by electron microscopy. This was the first report of generalized vaccinia among civilians receiving vaccination against smallpox in the current heightened alert state and highlights the concerns associated with the re-introduction of widespread use of smallpox vaccine.
4.2. Investigation of viral gastroenteritis
The introduction of commercial EIAs for detection of rotaviruses, adenoviruses and noroviruses in many laboratories has greatly reduced the reliance on EM for investigation of both infantile gastroenteritis and outbreaks. Some virus laboratories that have retained EM still use it to examine EIA negative samples for other enteric viruses, for example, astroviruses and sapoviruses, and to investigate discrepant results from other tests. There is, however, great genetic diversity among norovirus strains, and strains may change from year to year. Noroviruses may not always be detectable by EIA or PCR assays in routine use, but may still be visualised by EM. If strains of noroviruses detected by EM are different from those included in the EIA or by the PCR primers, steps can be taken to modify or enhance these more specific diagnostic methods to take account of currently circulating strains.
Electron microscopy is still incorporated into surveillance programmes for gastroenteric illness and with the expected introduction of safe rotavirus vaccines, some virology EM units will be involved in monitoring emerging patterns of infantile gastroenteritis. First generation rotavirus vaccines had significant side effects, including intussusception (a form of intestinal obstruction), in a small number of cases (Murphy et al., 2001), but safer second-generation vaccines are in development. If widespread vaccination of infants is undertaken, EM will be used in specialist centres to monitor any possible changes in the pattern of infection by the different enteric viruses.
It is still the case that no pathogen is detected in 40% of incidents of non-bacterial gastroenteritis. There may be other viruses awaiting discovery and, if so, EM will have a role in this.
4.3. Investigation of immunocompromised patients
Electron microscopic investigation of samples from immunocompromised individuals remains an important use of EM. Immunocompromised patients with gastrointestinal symptoms should be investigated and because EM is a ‘catch all’ method any viral cause of symptoms can be elucidated on examination of negatively stained diarrhoeal samples providing the virus is present in sufficiently high numbers. In addition, investigation of skin lesions and bloody urine by EM can quickly reveal a possible viral cause of symptoms so that appropriate treatment can be instigated. Urine examination by EM can quickly reveal the presence of polyomavirus (BK) (Fig. 18) or adenoviruses (Gardner et al., 1971, Cotterill et al., 1992, Appleton, 2005). Typing of these viruses is now undertaken by other methods, usually PCR, but EM remains useful for obtaining a rapid diagnostic result.
Symptoms of viral infection can mimic organ rejection in transplant patients and hence rapid investigation of potential virus infection is vital. Treatment for organ rejection, by inducing further immunosuppression, would actually prolong virus replication. Herpesvirus infections, such as chicken pox, can be serious and even life threatening in immunocompromised patients, hence rapid diagnosis by EM can significantly aid patient management by indicating the need for the administration of antiviral drugs.
4.4. Investigation of new infectious agents
Electron microscopy showed its worth in the 2003 investigation of respiratory samples from patients with severe acute respiratory symptoms, particularly those in Hong Kong and Southern China or those travelling from that part of the world. Laboratory investigation, including EM, resulted in the discovery of the SARS agent (severe acute respiratory syndrome), a newly recognised viral infection of humans. The virus had the characteristic ultrastructural features of a coronavirus (Fig. 19 ). Such viruses are covered with club-shaped surface projections, resembling petals (Ksiazek et al., 2003, Falsey and Walsh, 2003). The value of EM was also demonstrated in Australia in 1995 during investigations of a respiratory infection that caused fatal disease in horses and humans. Investigations, including EM, showed that a new morbillivirus (Hendra virus, belonging to the family Paramyxoviridae) was the causative agent (Murray et al., 1995, Halpin et al., 2000). A related new virus, Nipah virus, has more recently been discovered, primarily in pig farmers, in Malaysia (Chua et al., 1999).
Fig. 19.
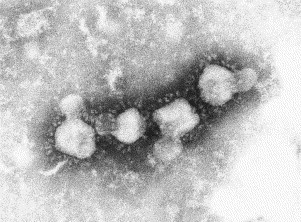
A negatively stained (PTA) preparation of the SARS virus showing the characteristic surface ‘petals’ of this newly recognised coronavirus. The SARS virus is about 100 nm in diameter.
4.5. Investigation of ‘exotic’ virus infections
The use of EM to investigate ‘exotic’ infections such as haemorrhagic fevers only takes place in specialist centres with the highest level of containment for infectious agents (Biosafety level 4). Viruses from a number of different families with different morphologies may cause haemorrhagic fever in humans. For example, the virus causing Lassa fever is an arenavirus while Crimean–Congo haemorrhagic fever is caused by a bunyavirus. Marburg and Ebola, the most dangerous of these viruses, are members of the filovirus family (Fig. 20 ). Some bunyaviruses, such as Rift Valley Fever virus and Hantavirus, cause severe and sometimes fatal encephalitis in humans. Other viruses causing severe encephalitis, such as West Nile Fever and Dengue, are flaviviruses. Humans are not the natural hosts for any of these zoonotic viruses and normally only become infected by exposure to the animal vector. Rodents are the natural hosts for arenaviruses, and bunyaviruses and flaviviruses are transmitted by arthropods, mainly blood-sucking ticks and mosquitoes. Despite much investigative work, the natural host of the filoviruses has yet to be identified. Most haemorrhagic fever viruses are associated with tropical regions, but recently interest has focused on West Nile Fever, which emerged in birds in New York in 1999. Human infections subsequently occurred throughout North America and there are now ongoing studies of bird flocks in several countries. The distinctive filamentous morphology of Filoviruses allows their detection by EM in acute phase patient serum. However, this technique is not routinely employed for the diagnosis of other haemorrhagic fever viruses, but is used in supporting research on the structure and pathogenesis of these agents and in the development of other diagnostic methods and therapeutic regimes. Most of these viruses have high mortality rates and must be inactivated before processing for EM. The fixatives used for this (such as buffered formaldehyde) must be carefully chosen so as not to mask or degrade the morphological features of interest.
Fig. 20.
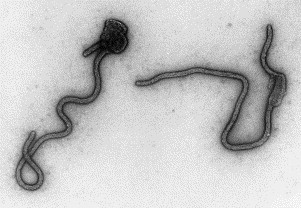
A negatively stained (PTA) preparation of Ebola virus, a cause of haemorrhagic fever. Ebola virus is about 80 nm wide, but very variable in length (600+nm).
4.6. Investigation of recombinant proteins, antigens and viral capsids
Recombinant virus proteins and recombinant virus-like particles (virus capsids) can also be investigated by EM. The use of EM for such investigations is restricted to specialist centres, but shows the value of a combined molecular and EM approach to the production and characterisation of recombinant viral proteins (Cohen and Richmond, 1982, Clarke et al., 1998). Although not of direct clinical relevance, this use of EM can be important in research applications and particularly in the production of antigens for vaccine and assay development (Warnes et al., 1995).
4.7. Investigation of cytopathic effect (CPE) in cell culture
Incubation of patient samples in cultured cell lines to induce growth of virus is still a relatively common procedure in most virus laboratories, although routine use of this method is also in decline. Viruses often produce characteristic cytopathic effects in cell cultures, which may be observed by light microscopy to allow indirect identification of the viral agent involved. Atypical cytopathic effects or cytopathic effects induced by new agents sometimes require investigation by EM (Ksiazek et al., 2003). This is a minor use of EM in most laboratories, but may aid and speed up the identification of viral pathogens.
5. Discussion
The role of EM in bacteriology and virology has continually evolved since its introduction. As newer methods of virus detection have become available, so EM has been utilised in new and different areas. The great expansion of diagnostic electron microscopy that occurred in the1970s and 1980s, mainly for the detection and characterisation of the causative agents of viral gastroenteritis, has ended. EM is now applied to the investigation of a much wider range of viruses, but this is becoming confined to fewer specialist centres, which are usually associated with laboratories undertaking research activities as well as routine diagnostic microbiology. However, maintaining facilities and ensuring availability of experienced microscopists is now becoming a problem in many parts of the UK.
There is a very pertinent saying—‘a picture paints a thousand words’. In the field of EM, the production of ‘mere pictures’ has belittled the technique in some quarters in recent years and yet the information contained within micrographs combined with skilled interpretation by an experienced microscopist can lead investigators in new directions or give clues as to the identity of new organisms (Orenstein, 2000). The SARS virus, a new human viral pathogen, required the use of several investigative methods, including EM, to determine that it was a coronavirus.
Attitudes to EM have also become very polarised. Some workers regard EM as ‘cumbersome and time consuming’ (Visvesvara et al., 1994) or laborious (Fedorko and Hijazi, 1996), whereas others are very positive and enthusiastic about the usefulness of the technique (Madeley, 2003, Biel and Gelderblom, 1999, Biel and Madeley, 2001, Hazelton and Gelderblom, 2003). Certainly laboratory managers faced with limited budgets are, almost inevitably, going to opt for the new molecular technologies, which offer the promise of greater sensitivity and specificity, rather than invest in expensive new electron microscopes. Also, specimens for EM require individual examination by an experienced electron microscopist, a process that cannot be automated and results in a relatively limited throughput of specimens. Economic arguments can be misleading, however. Over the long-term, despite the high initial capital costs, EM comes out as a very cost effective technique. Unlike other modern diagnostic equipment, electron microscopes have a useful life expectancy of around 20 years. In addition, consumable costs are almost negligible compared to expensive molecular diagnostic reagents. Electron microscopy still has much to offer and if such narrow attitudes persist, there may be a need for a national strategy to be put in place in order to safeguard EM capability within virology in the UK. This may mean concentrating EM in a few specialist centres.
Electron microscopy still has a role as a diagnostic tool for the investigation of gastrointestinal virus infections. Indeed, it remains the only ‘catch-all’ method, capable of detecting multiple infections in one test. If EIAs and PCR testing predominate, then a battery of tests may be required to detect multiple infections and this may negate some of the perceived advantages of these new alternative diagnostic techniques. Despite this, EIAs and PCR methods are now perceived as more convenient, sensitive, relatively cheap and do not requiring highly skilled staff. However, most gastroenteritis viruses contain an RNA genome, susceptible to spontaneous mutation, which may alter the RNA or expressed proteins, making them undetectable by existing EIAs or PCR assays. It is, therefore, prudent that EM should remain a part of the diagnostic regime to enable genetically mutated enteric viruses to remain detectable on the basis of their characteristic morphology.
Electron microscopy also has a particular advantage in other viral diagnostic applications. The rapid provision of results when investigating skin lesions is currently of high priority to counter the threat posed by terrorist groups who potentially may be able to obtain stocks of pathogens, such as smallpox, with the intention to release such agents into largely non-immune population centres (widespread vaccination ceased with the worldwide eradication of smallpox). EM is currently a frontline diagnostic test in the emergency response plans of the UK and many other countries for such a scenario. It is vitally important that EM facilities involved in such emergency response participate in external quality control schemes to maintain and augment recognition skills for potential viral bioterrorism agents.
Conventional silver-based ‘wet’ photography is now in decline and EM is currently embracing digital image capture. Although expensive to retrofit to electron microscopes, digital EM cameras allow rapid acquisition of images and their instant sharing between networked facilities. This is particularly important in relation to bioterrorism, as images of viruses may be quickly scrutinised by geographically separated experts. Digital photography is opening up a new area of communication between microscopists. It is making it easier to provide images and to promote enthusiasm for EM to a wider audience both scientific and non-scientific.
Electron microscopy should have a continuing role in virological and bacteriological investigations, but this requires that its use must be encouraged and supported. In the biomedical field, this may mean that electron microscope facilities have to be centralised to ensure a sufficient volume of work is undertaken to keep the cost of investigations at an acceptable level. One way forward is for virologists who still require occasional access to EM to form partnerships with other users for whom EM is still seen as an essential part of the diagnostic and research process. An example of this approach is virology users linking with established EM units undertaking the investigation of, for example, renal disease where EM remains an important investigative technique (Pearson et al., 1994, Shore and Moss, 2002).
Although molecular techniques are having and will have an increasingly important role in virology and bacteriology, EM still has much to contribute. Electron microscopy should be seen as being capable of producing different, but equally relevant, information about infectious agents. Electron microscopy is complementary to other investigative techniques, not a rival to them. Using such a combined approach will enable us to elucidate many new aspects of both previously known and emerging organisms and the diseases they produce in humans and animals.
The future for electron microscopy within virology and microbiology lies in its contribution to research, in the epidemiology of enteric viruses and the rapid differential diagnosis of skin lesion viruses, where EM examination may rapidly exclude the presence of serious bioterrorism-related agents. Discrepant laboratory results can also be investigated and possible reagent contamination readily identified. In addition EM is being increasingly used in development and quality control of vaccines in specialist centres.
The role of electron microscopy within the diagnosis and study of infectious disease is at a critical period. In order to secure its' long-term future, a new generation of electron microscopists must be encouraged to acquire the required technical skills. Most importantly, the significant knowledge and experience required for meaningful image interpretation, must be handed on by the declining band of skilled microscopists while they are still in post. Institutions with a commitment to electron microscopy must ensure sensible programmes of succession planning are put in place to ensure the long-term survival of electron microscopy and its essential contribution to microbiology.
Acknowledgements
We would like to acknowledge the assistance of Dr Sandy Smith, Dr John Kennaugh and Mrs Collette Curry in writing this review and Mrs Christina Paddon, Mrs Hilary Cotterill and Miss Patricia Rowland for assistance with the illustrations.
References
- Almeida J.D., Zuckerman A.J., Taylor P.E., Waterson A.P. Immune electron microscopy of the Australia-SH (serum hepatitis) antigen. Microbios. 1969;1:117–123. [Google Scholar]
- Almeida J.D., Rubenstein D., Stott E.J. New antigen-antibody system in Australia-antigen-positive hepatitis. Lancet. 1971;II:1225–1227. doi: 10.1016/s0140-6736(71)90543-5. [DOI] [PubMed] [Google Scholar]
- Appleton H. Control of food-borne viruses. British Medical Bulletin. 2000;56:172–183. doi: 10.1258/0007142001902879. [DOI] [PubMed] [Google Scholar]
- Appleton H. Electron microscopy in virology. In: Crocker J., Burnett D., editors. The Science of Laboratory Diagnosis. second ed. Wiley; 2005. (Chapter 18) [Google Scholar]
- Appleton H., Higgins P.G. Viruses and gastroenteritis in infants. Lancet. 1975;I:1297. doi: 10.1016/s0140-6736(75)92581-7. [DOI] [PubMed] [Google Scholar]
- Balayan M.S., Andjaparidze A.G., Saninskaya S.S., Ketiladze E.S., Braginsky D.M., Savinov A.P., Poleschuk V.F. Evidence for a virus in non-A, non-B hepatitis transmitted by the faecal oral route. Intervirology. 1983;20:23–31. doi: 10.1159/000149370. [DOI] [PubMed] [Google Scholar]
- Bayer M.E., Blumberg B.S., Werner B. Particles associated with Australia antigen in the sera of patients with leukaemia, Down's syndrome and hepatitis. Nature. 1968;218:1057–1059. doi: 10.1038/2181057a0. [DOI] [PubMed] [Google Scholar]
- Berke T., Matson D.O. Reclassification of the Caliciviridae into distinct genera and exclusion of hepatitis E virus from the family on the basis of comparative phylogenetic analysis. Archives of Virology. 2000;145:1421–1436. doi: 10.1007/s007050070099. [DOI] [PubMed] [Google Scholar]
- Biel S.S., Gelderblom H.R. Diagnostic electron microscopy is still a timely and rewarding method. Journal of Clinical Virology. 1999;13:105–119. doi: 10.1016/S1386-6532(99)00027-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biel S.S., Madeley D. Diagnostic virology—the need for electron microscopy: a discussion paper. Journal of Clinical Virology. 2001;22:1–9. doi: 10.1016/s1386-6532(01)00151-2. [DOI] [PubMed] [Google Scholar]
- Bishop R.F., Davidson G.P., Holmes I.H., Ruck B.J. Virus particles in epithelial cells of duodenal mucosa from children with acute non-bacterial gastroenteritis. Lancet. 1973;ii:1281–1283. doi: 10.1016/s0140-6736(73)92867-5. [DOI] [PubMed] [Google Scholar]
- Blumberg B.S., Alter H.J., Visnich S. A ‘new’ antigen in leukaemia sera. Journal of the American Medical Association. 1965;191:541–546. doi: 10.1001/jama.1965.03080070025007. [DOI] [PubMed] [Google Scholar]
- Brenner S., Horne R.W. A negative staining method for high resolution electron microscopy of viruses. Biochemica et Biophysica Acta. 1959;34:103–110. doi: 10.1016/0006-3002(59)90237-9. [DOI] [PubMed] [Google Scholar]
- Caul E.O., Appleton H. The electron microscopical and physical characteristics of small round human faecal viruses: an interim scheme for classification. Journal of Medical Virology. 1982;9:257–265. doi: 10.1002/jmv.1890090403. [DOI] [PubMed] [Google Scholar]
- Caul E.O., Ashley C.R., Eggleston S. An improved method for the routine identification of faecal viruses using ammonium sulphate precipitation. FEMS Microbiology Letters. 1978;4:1–7. [Google Scholar]
- Chua K.B., Goh K.J., Wong K.T., Kamarulzaman A., Tan P.S., Ksiazek T.G., Zaki S.R., Paul G., Lam S.K., Tan C.T. Fatal encephalitis due to Nipah virus among pig-farmers in Malaysia. Lancet. 1999;354:1257–1259. doi: 10.1016/S0140-6736(99)04299-3. [DOI] [PubMed] [Google Scholar]
- Clarke, I.N., Lambden, P.R., Caul, E.O., 1998. Human enteric RNA viruses: caliciviruses and astroviruses. In: Topley and Wilson, nineth ed. Hodder Arnold.
- Cohen B.J., Richmond J. Electron microscopy of hepatitis B core antigen synthesised in E. coli. Nature. 1982;296:677–678. doi: 10.1038/296677a0. [DOI] [PubMed] [Google Scholar]
- Cossart Y.E., Field A.M., Cant B., Widdows D. Parvovirus-like particles in human sera. Lancet. 1975;1:72–73. doi: 10.1016/s0140-6736(75)91074-0. [DOI] [PubMed] [Google Scholar]
- Cotterill H.A., Macaulay M.E., Wong V. Reactivation of polyomavirus in bone marrow transplant recipients. Journal of Clinical Pathology. 1992;45:445. doi: 10.1136/jcp.45.5.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry A., Bryden A., Morgan-Capner P., Fox A., Guiver M., Martin L., Mutton K., Wright P., Mannion P., Westwell A., Cheesbrough J., Ashton I., Blackley A. A rationalised virological electron microscope specimen testing policy. Journal of Clinical Pathology. 1999;52:471–474. doi: 10.1136/jcp.52.6.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dane D.S., Cameron C.H., Briggs M. Virus-like particles in serum of patients with Australia-antigen-associated hepatitis. Lancet. 1970;I:695–698. doi: 10.1016/s0140-6736(70)90926-8. [DOI] [PubMed] [Google Scholar]
- DePamphilis M.L., Adler J. Fine structure and isolation of the hook-basal body complex of flagella from Escherichia coli and Bacillus subtilis. Journal of Bacteriology. 1971;105:384–395. doi: 10.1128/jb.105.1.384-395.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrick K.S. Quantitative assay for plant viruses using serologically specific electron microscopy. Virology. 1973;56:652–653. doi: 10.1016/0042-6822(73)90068-8. [DOI] [PubMed] [Google Scholar]
- Falsey A.R., Walsh E.E. Novel coronavirus and severe acute respiratory syndrome. Lancet. 2003;361:1312–1313. doi: 10.1016/S0140-6736(03)13084-X. (April 19th) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorko D.P., Hijazi Y.M. Application of molecular techniques to the diagnosis of microsporidial infection. Emerging Infectious Diseases. 1996;2:183–191. doi: 10.3201/eid0203.960304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstone S.M., Kapikian A.Z., Purcell R.H. Hepatitis A: detection by immune electron microscopy of a virus-like antigen associated with acute illness. Science. 1973;182:1026–1028. doi: 10.1126/science.182.4116.1026. [DOI] [PubMed] [Google Scholar]
- Flewett T.H., Bryden A.S., Davies H.A. Virus particles in gastroenteritis. Lancet. 1973;ii:1497. doi: 10.1016/s0140-6736(73)92760-8. [DOI] [PubMed] [Google Scholar]
- Flewett T.H., Bryden A.S., Davies H.A., Morris C.A. Epidemic viral enteritis in a long-stay children's ward. Lancet. 1975;I:4–5. doi: 10.1016/s0140-6736(75)92370-3. [DOI] [PubMed] [Google Scholar]
- Gabor D. Electronic Engineering Technical Monographs. Hulton Press; 1945. The electron microscope. [Google Scholar]
- Gardner S.D., Field A.M., Coleman D.V., Hulme B. New human papovavirus (BK) isolated from urine after transplantation. Lancet. 1971;1:1253–1257. doi: 10.1016/s0140-6736(71)91776-4. [DOI] [PubMed] [Google Scholar]
- Gary G.W., Jr, Hierholzer J.C., Black R.E. Characteristics of noncultivable adenoviruses associated with diarrhea in infants: a new subgroup of human adenoviruses. Journal of Clinical Microbiology. 1979;10:96–103. doi: 10.1128/jcm.10.1.96-103.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haguenau F., Hawkes P.W., Hutchinson J.L., Satiat-Jeunemaître B., Simon G.T., Williams D.B. Key events in the history of electron microscopy. Microscopy and Microanalysis. 2003;9:96–138. doi: 10.1017/S1431927603030113. [DOI] [PubMed] [Google Scholar]
- Halpin K., Young P.L., Field H.E., Mackenzie J.S. Isolation of Hendra virus from pteropid bats: a natural reservoir of Hendra virus. Journal of General Virology. 2000;81:1927–1932. doi: 10.1099/0022-1317-81-8-1927. [DOI] [PubMed] [Google Scholar]
- Harris J.R. Microscopy Handbooks 35. Bios Scientific Publishers in association with the Royal Microscopical Society; 1997. Negative staining and cryoelectron microscopy. [Google Scholar]
- Hazelton P.R., Gelderblom H.R. Electron microscopy for rapid diagnosis of infectious agents in emergent situations. Emerging Infectious Diseases. 2003;9:294–303. doi: 10.3201/eid0903.020327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson D.A. Countering the posteradication threat of smallpox and polio. Clinical Infectious Diseases. 2002;34:79–84. doi: 10.1086/323897. [DOI] [PubMed] [Google Scholar]
- Henderson D.A., Fenner F. Recent events and observations pertaining to smallpox virus destruction in 2002. Clinical Infectious Diseases. 2001;33:1057–1059. doi: 10.1086/323808. [DOI] [PubMed] [Google Scholar]
- Horne R.W. Academic Press; New York: 1974. Virus Structure. pp. 1–52. [Google Scholar]
- Horne R.W., Wildy P. Symmetry in virus architecture. Virology. 1961;15:348–373. doi: 10.1016/0042-6822(61)90366-x. [DOI] [PubMed] [Google Scholar]
- Horne R.W., Wildy P. Virus structure revealed by negative staining. Advances in Virus Research. 1963;10:101–170. doi: 10.1016/s0065-3527(08)60698-3. [DOI] [PubMed] [Google Scholar]
- Horne R.W., Wildy P. An historical account of the development and applications of the negative staining technique to the electron microscopy of viruses. Journal of Microscopy. 1979;117:103–122. doi: 10.1111/j.1365-2818.1979.tb00234.x. [DOI] [PubMed] [Google Scholar]
- Isalska B.J., Curry A., Stanbridge T.N., Tweddle D., Caul E.O. Electron microscopy and serological features of a patient with Q fever prosthetic valve endocarditis. Journal of Clinical Pathology. 1996;49:679–681. doi: 10.1136/jcp.49.8.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapikian A.Z. The discovery of the 27-nm Norwalk virus: an historic perspective. Journal of Infectious Diseases. 2000;181(Suppl. 2):S295–S302. doi: 10.1086/315584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapikian A.Z., Wyatt R.G. Visualisation by immune electron microscopy of a 27-nm particle associated with infectious nonbacterial gastroenteritis. Journal of Virology. 1972;10:1075–1081. doi: 10.1128/jvi.10.5.1075-1081.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay D. Interactions between phage and bacteria. In: Norris J.R., Richmond M.H., editors. Essays in Microbiology. Wiley; New York: 1978. pp. 1–32. [Google Scholar]
- Knoll M., Ruska E. Das elektonenmikroscop. Zeitschrift fur Physik. 1932;78:318–329. [Google Scholar]
- Kogasaka R., Nakamura S., Chiba S. The 33- to 39-nm virus-like particles tentatively designated as Sapporo agent, associated with an outbreak of acute gastroenteritis. Journal of Medical Virology. 1981;8:187–193. doi: 10.1002/jmv.1890080305. [DOI] [PubMed] [Google Scholar]
- Kruger D.H., Schneck P., Gelderblom H.R. Helmut Ruska and the visualization of viruses. Lancet. 2000;355:1713–1717. doi: 10.1016/s0140-6736(00)02250-9. [DOI] [PubMed] [Google Scholar]
- Ksiazek T.G., Erdman D., Goldsmith C.S. A novel coronavirus associated with severe acute respiratory syndrome. New England Journal of Medicine. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- Kurtz, J.B., Lee, T.W., 1987. Astroviruses: human and animal. In: Novel Diarrhoea Viruses. CIBA Foundation Symposium 128. Wiley, New York. pp. 92–107. [DOI] [PubMed]
- Lee T.W., Megson B., Kurtz J.B. Enterovirus typing by immune electron microscopy. Journal of Medical Microbiology. 1996;44:151–153. doi: 10.1099/00222615-44-2-151. [DOI] [PubMed] [Google Scholar]
- Lewis D.C., Lightfoot N.F., Pether J.V.S. Solid-phase immune electron microscopy with human immunoglobulin M for serotyping of Norwalk-like viruses. Journal of Clinical Microbiology. 1988;26:938–942. doi: 10.1128/jcm.26.5.938-942.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long W.L., Noble J., Jr., Murphy F.A., Herrmann K.L., Lourie B. Experience with electron microscopy in the differential diagnosis of smallpox. Applied Microbiology. 1970;20:497–504. doi: 10.1128/am.20.3.497-504.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeley C.R. first ed. Churchill Livingstone; 1972. Virus Morphology. [Google Scholar]
- Madeley C.R. Origins of electron microscopy and viral diagnosis. Journal of Clinical Pathology. 1997;50:454–456. doi: 10.1136/jcp.50.6.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeley C.R. Diagnosing smallpox in possible bioterrorist attack. Lancet. 2003;January:97–98. doi: 10.1016/s0140-6736(03)12241-6. [DOI] [PubMed] [Google Scholar]
- Madeley C.R., Cosgrove B.P. 28 nm particles in faeces in infantile gastroenteritis. Lancet. 1975;II:451–452. doi: 10.1016/s0140-6736(75)90858-2. [DOI] [PubMed] [Google Scholar]
- Madeley C.R., Cosgrove B.P. Caliciviruses in man. Lancet. 1976;I:199–200. doi: 10.1016/s0140-6736(76)91309-x. [DOI] [PubMed] [Google Scholar]
- Madeley C.R., Field A.M. second ed. Churchill Livingstone; 1988. Virus Morphology. [Google Scholar]
- Mayo M.A. A summary of taxonomic changes recently approved by ICTV. Archives of Virology. 2002;147:1655–1656. doi: 10.1007/s007050200039. [DOI] [PubMed] [Google Scholar]
- McNulty C.A.M., Dent J.C., Curry A., Uff J.S., Ford G.A., Gear M.W.L., Wilkinson S.P. New spiral bacterium in gastric mucosa. Journal of Clinical Pathology. 1989;42:585–591. doi: 10.1136/jcp.42.6.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S.E. Bioterrorism and electron microscopic differentiation of poxviruses from herpesviruses: dos and don'ts. Ultrastructural Pathology. 2003;27:133–140. doi: 10.1080/01913120309932. [DOI] [PubMed] [Google Scholar]
- Miller J.R., Cirino N.M., Philbin E.F. Generalized vaccinia 2 days after smallpox revaccination. Emerging Infectious Diseases. 2003;9:1649–1650. doi: 10.3201/eid0912.030592. (letters) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy T.V., Gargiullo P.M., Massoudi M.S., Nelson D.B., Juman A.O., Okoro C.A., Zanardi L.R., Setia S., Fair E., LeBaron C.W., Wharton M., Livengood J.R. Intussusception among infants given an oral rotavirus vaccine. New England Journal of Medicine. 2001;344:564–572. doi: 10.1056/NEJM200102223440804. [DOI] [PubMed] [Google Scholar]
- Murray R.G.E. Form and function—1 bacteria. In: Norris J.R., Richmond M.H., editors. Essays in Microbiology. Wiley; New York: 1978. pp. 1–31. [Google Scholar]
- Murray K., Selleck P., Hooper P., Hyatt A., Gould A., Gleeson L., Westbury H., Hiley L., Selvey L., Rodwell B., Ketterer P. A morbillivirus that caused fatal disease in horses and humans. Science. 1995;268:94–97. doi: 10.1126/science.7701348. [DOI] [PubMed] [Google Scholar]
- Nagler F.P.O., Rake G. The use of electron microscope in diagnosis of variola, vaccinia, and varicella. Journal of Bacteriology. 1948;55:45–51. doi: 10.1128/jb.55.1.45-51.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata S., Petrie B.L., Calomeni E.P., Estes M.K. Electron microscopy procedure influences detection of rotaviruses. Journal of Clinical Microbiology. 1987;25:1902–1906. doi: 10.1128/jcm.25.10.1902-1906.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nermut M.V. General principles of virus architecture. In: Nermut M.V., Steven A.C., editors. Animal Virus Structure. vol. 3. Elsevier; Amsterdam: 1987. pp. 3–18. (Perspectives in Medical Virology). [Google Scholar]
- Nermut M.V., Hockley D.J., Gelderblom H. Electron microscopy: methods for studies of virus particles and virus infected cells. In: Nermut M.V., Steven A.C., editors. Animal Virus Structure. vol. 3. Elsevier; Amsterdam: 1987. pp. 21–33. (Perspectives in Medical Virology). [Google Scholar]
- Orenstein J.M. Isn't a picture still worth a thousand words? Ultrastructural Pathology. 2000;24:67–74. doi: 10.1080/01913120050118530. [DOI] [PubMed] [Google Scholar]
- Parashar U.D., Hummelman E.G., Bresee J.S., Miller M.A., Glass R.I. Global illness and deaths caused by rotavirus disease in children. Emerging Infectious Diseases. 2003;9:565–572. doi: 10.3201/eid0905.020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson J.M., McWilliam L.J., Coyne J.D., Curry A. Value of electron microscopy in diagnosis of renal disease. Journal of Clinical Pathology. 1994;47:126–128. doi: 10.1136/jcp.47.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkonen P.M., Tarvainen K., Hynninen A., Kallio E.R.K., Henttonen H., Palva A., Vaheri A., Vapalahti O. Cowpox with severe generalized eruption, Finland. Emerging Infectious Diseases. 2003;9:1458–1461. doi: 10.3201/eid0911.020814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed K.D., Melski J.W., Graham M.B., Regnery R.L., Sotir M.J., Wegner M.V., Kazmierczak J.J., Stratman E.J., Li Y., Fairley J.A., Swain G.R., Olson V.A., Sargent E.K., Kehl S.C., Frace M.A., Kline R., Foldy S.L., Davis J.P., Damon I.K. The detection of monkeypox in humans in the Western hemisphere. New England Journal of Medicine. 2004;350:342–350. doi: 10.1056/NEJMoa032299. [DOI] [PubMed] [Google Scholar]
- Risco C., Carrascosa J.L. Ultrastructure of human astrovirus serotype 2. Journal of General Virology. 1995;76:2075–2080. doi: 10.1099/0022-1317-76-8-2075. [DOI] [PubMed] [Google Scholar]
- Sabatini D.D., Bensch K., Barnett R.J. Cytochemistry and electron microscopy. The preservation of cellular structure and enzymatic activity by aldehyde fixation. Journal of Cell Biology. 1963;17:19–58. doi: 10.1083/jcb.17.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sails A.D., Wareing D.R.A., Bolton F.J., Fox A.J., Curry A. Characterisation of 16 Campylobacter jejuni and C. coli typing bacteriophages. Journal of Medical Microbiology. 1998;47:123–128. doi: 10.1099/00222615-47-2-123. [DOI] [PubMed] [Google Scholar]
- Shore I., Moss J. Electron microscopy in diagnostic renal pathology. Current Diagnostic Pathology. 2002;8:207–215. [Google Scholar]
- Suzuki H., Chen G.M., Hung T. Effects of two negative staining methods on the Chinese atypical rotavirus. Archives of Virology. 1987;94:305–308. doi: 10.1007/BF01310723. [DOI] [PubMed] [Google Scholar]
- Visvesvara G.S., Leitch G.J., Da Silva A.J., Croppo G.P., Moura H., Wallace S., Slemenda S.B., Schwartz D.A., Moss D., Bryan R.T., Pieniazek N.J. Polyclonal and monoclonal antibody and PCR-amplified small-subunit rRNA identification of a microsporidian, Encephalitozoon hellem, isolated from an AIDS patient with disseminated infection. Journal of Clinical Microbiology. 1994;32:2760–2768. doi: 10.1128/jcm.32.11.2760-2768.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnes A., Fooks A.R., Dowsett A.B., Wilkinson G.W.G., Stephenson J.R. Expression of the measles virus nucleoprotein gene in Escherichia coli and assembly of nucleocapsid-like structures. Gene. 1995;160:173–178. doi: 10.1016/0378-1119(95)00227-w. [DOI] [PubMed] [Google Scholar]
- Williams A., Baskerville A., Dowsett A.B., Conlan W. Immunocytochemical demonstration of the association between Legionella pneumophila, its tissue-destructive protease and pulmonary lesions in experimental Legionnaires' disease. Journal of Pathology. 1987;153:257–264. doi: 10.1002/path.1711530310. [DOI] [PubMed] [Google Scholar]
- Wood D.J., Bailey A.S. Detection of adenovirus types 40 and 41 in stool specimens by immune electron microscopy. Journal of Medical Virology. 1987;21:191–199. doi: 10.1002/jmv.1890210211. [DOI] [PubMed] [Google Scholar]
- Zu Rhein G.M., Chou S.-M. Particles resembling pavova viruses in human cerebral demyelinating disease. Science. 1965;148:1477–1479. doi: 10.1126/science.148.3676.1477. [DOI] [PubMed] [Google Scholar]


