Abstract
In this study, aloe-emodin was identified as a potential interferon (IFN)-inducer by screening compounds from Chinese herbal medicine. Aloe-emodin showed low cytotoxicity to human HL-CZ promonocyte cells and TE-671 medulloblastoma cells and significantly activated interferon-stimulated response element (ISRE) and gamma-activated sequence (GAS)-driven cis-reporting systems. Moreover, aloe-emodin upregulated expression of IFN-stimulated genes such as dsRNA-activated protein kinase and 2′,5′-oligoisoadenylate synthase. Aloe-emodin resulted in significant activation of nitric oxide production. The antiviral activity of aloe-emodin against Japanese encephalitis virus (JEV) and enterovirus 71 (EV71) was evaluated using dose- and time-dependent plaque reduction assays in HL-CZ cells and TE-671 cells. The 50% inhibitory concentration (IC50) of aloe-emodin ranged from 0.50 μg/mL to 1.51 μg/mL for JEV and from 0.14 μg/mL to 0.52 μg/mL for EV71. Aloe-emodin showed clearly potent virus inhibitory abilities and achieved high therapeutic indices, in particular for HL-CZ cells. Therefore, the study demonstrated dose- and time-dependent actions of aloe-emodin on the inhibition of JEV and EV71 replication via IFN signalling responses.
Keywords: Aloe-emodin, Interferon signalling, Japanese encephalitis virus, Enterovirus 71
1. Introduction
Aloe-emodin (1,8-dihydroxy-3-hydroxymethyl-anthraquinone) is one of the natural anthraquinone derivatives from the root and rhizome of Rheum palmatum. Aloe-emodin has been reported to inhibit the replication of enveloped viruses including herpes simplex virus, influenza virus [1] and human cytomegalovirus [2]. In addition, other anthraquinone derivatives, including emodin, chrysophanic acid and hypericin, have demonstrated antiviral activities against hepatitis B virus [3] and poliovirus [4].
Type I and type II interferons (IFNs) are produced by leukocytes and fibroblasts in the non-specific host response to viral infection. IFNα and IFNβ show a therapeutic effect on viral infections, including coxsackievirus type A16 [5] and West Nile virus (WNV) [6]. IFNγ is involved in the activation of endogenous nitric oxide (NO) to inhibit viral replication in the macrophage and the central nervous system (CNS) [7]. Importantly, polyriboinosinic:polyribocytidylic acid (poly(I:C)), a potent IFN-inducer, has been demonstrated to improve the survival rate and to decrease tissue viral titres following enterovirus 71 (EV71) and WNV challenge in mice [6], [8]. Therefore, screening for IFN-inducers from the compounds of Chinese herbal medicine is an alternative approach to identify potent antiviral agents.
Japanese encephalitis virus (JEV) and EV71 are neurotropic viruses that spread particularly in the Asia–Pacific region [9], [10]. JEV and EV71 encephalitis are inflammatory diseases of the CNS, like poliomyelitis-like acute flaccid paralysis, aseptic meningitis and encephalitis. JEV and EV71 multiply in primary sites such as monocytes and myeloid and lymphoid cells and then travel from the periphery to the CNS [9], [10]. The antiviral approach against JEV and EV71 could be simultaneously set up in the same cell lines.
In this study, we aimed to identify antiviral herb compounds against JEV and EV71 in cell models such as the human TE-671 medulloblastoma cell line and HL-CZ promonocyte cell line. Aloe-emodin was identified from screening of IFN-inducers from Chinese herbal compounds showing IFN signalling induction in mammalian cells. Aloe-emodin revealed a dose-dependent effect on IFN expression and NO production as well as in vitro antiviral activities against JEV and EV71.
2. Materials and methods
2.1. Viruses and cells
JEV strain T1P1 [11] and an EV71 isolate from a throat culture were used in this study. BHK-21 cells were maintained in Modified Eagle's Medium (MEM) with 10% foetal bovine serum (FBS). TE-671 medulloblastoma cells were grown in MEM with 2 mM l-glutamine, 1 mM sodium pyruvate and 10% FBS. HL-CZ cells were incubated with RPMI 1640 medium containing 10% FBS.
2.2. Cytotoxicity test
Aloe-emodin, arecoline, catechin, shikonin, trans-cinnamic acid, hesperetin, scopoletin, daidzein and resveratrol were purchased from Sigma Chemical Co. (St Louis, MO). Cells were treated with various concentrations of each herb component for 48 h, followed by an MTT assay. Survival rates of cells were calculated as the ratio of the optical density at 570–630 nm (OD570–630) of treated cells to the OD570–630 of untreated cells. Quadruplicate wells were analysed for each concentration. The cytotoxic concentration showing 50% toxic effect (CC50) was determined using a computer program (provided by John Spouge, National Center for Biotechnology Information, National Institutes of Health).
2.3. Enzyme-linked immunosorbent assay (ELISA) for detection of IFNα expression
Following 48 h incubation of treated cells, IFNα was measured using an ELISA with anti-IFNα monoclonal antibody in quadruplicate wells. The ELISA product was developed using a chromogen solution containing ABTS (2,2′-azino-di-(3-ethylbenzthiazoline-6-sulfonate)) and hydrogen peroxide and then the absorbance at 405 nm (A405) was measured.
2.4. Transient transfections of cis-reporter plasmids for signalling pathway assay
Cells were transiently co-transfected with an internal control reporter pRluc-C1 plus the indicated cis-reporting plasmid pISRE-Luc, pGAS-Luc or pNFκB-Luc (Stratagene, La Jolla, CA). Cells were treated with each herbal compound at 1 μg/mL and incubated for 4 h and then firefly and Renilla luciferase enzymes were measured using the dual Luciferase Reporter Assay System (Promega, Madison, WI) and a TROPIX TR-717 luminometer (Applied Biosystems, Foster City, CA). The relative firefly luciferase activity was normalised by Renilla luciferase and compared with untreated cells.
2.5. Quantification of IFN-stimulating gene expression using real-time reverse transcription polymerase chain reaction (RT-PCR)
Quantification of mRNA was performed with TaqMan® real-time RT-PCR (Applied Biosystems) using 2.5 μL of reverse-transcribed cDNA. Oligonucleotide primers and TaqMan® probes for human protein kinase R (PKR) were as follows: forward primer, 5′-CAACC AGCGG TTGAC TTTTT-3′; reverse primer, 5′-ATCCA GGAAG GCAAA CTGAA-3′; and probe #50 (Universal ProbeLibrary probe). The forward primer for 2′,5′-oligoadenylate synthetase (OAS) was 5′-GATGT GGTTA GGTTT ATAGCTG-3′ and the reverse primer was 5′-TTGGG GGTTA GGTTT CTGCCTTT-3′. In addition, the forward primer for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was 5′-AGCCA CATCG CTCAG ACAC-3′ and the reverse primer was 5′-GCCCCA ATACG ACCAA ATCC-3′. The TaqMan® probe for GAPDH was probe #37. The measured amounts of PKR and OAS mRNA were normalised to the amount of GAPDH mRNA.
2.6. Measurement of NO production
Cells were loaded with DAF-2 DA for 3 h at 37 °C. After rinsing three times with serum-free MEM, cells were treated with each herbal compound. Fluorescence (excitation wavelength 480 nm, emission wavelength 530 nm) was measured using a BD FACSAria analyser (BD Biosciences, San José, CA).
2.7. Dose- and time-dependent plaque reduction assays
The dose-dependent plaque reduction assay was performed using 10-fold dilutions of aloe-emodin in triplicate. Untreated and treated cells were infected at a multiplicity of infection of 1 with JEV or EV71. After 48 h of incubation at 37 °C, the cultured supernatant was harvested for determination of viral plaques using BHK-21 cell monolayers. The 50% inhibitory concentration (IC50) was the concentration required for 50% inhibition of the indicated virus plaques. The time-dependent plaque reduction assay was carried out in treated cells with 1 μg/mL aloe-emodin and in untreated cells. At 12, 24 and 48 h after inoculation, the cultured supernatant from treated and untreated cells was collected for plaque determination.
3. Results
3.1. IFNα expression induced by herbal compounds
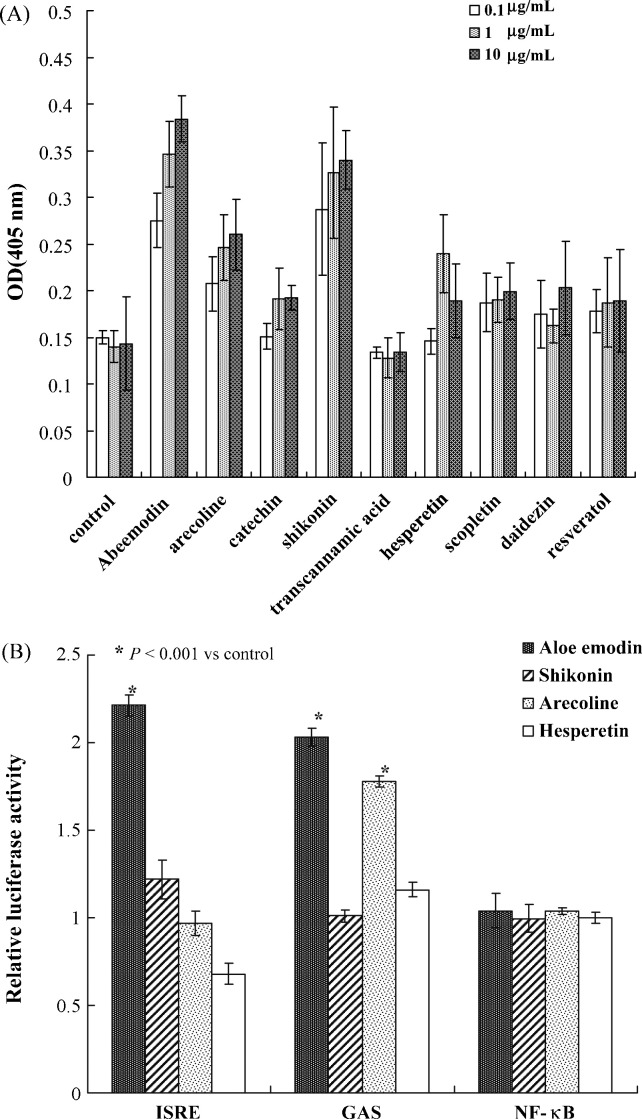
Initially, the cytotoxicity of nine herbal compounds, including aloe-emodin, arecoline, catechin, shikonin, trans-cinnamic acid, hesperetin, scopoletin, daidzein and resveratrol, was evaluated using MTT assays (Table 1 ). CC50 values of these nine components, except hesperetin, were >1000 μg/mL for both human cell lines, e.g. 2632 μg/mL for aloe-emodin for TE-671 cells and 2881 μg/mL for HL-CZ cells (Table 1). Subsequently, the level of IFNα expression in TE-671 cells was detected after 48 h of incubation with each herb compound using an ELISA assay (Fig. 1A). A dose-dependent increase in the level of IFNα expression was found in the cells treated with aloe-emodin, shikonin and arecoline. In particular, aloe-emodin showed a 2.5-fold increase in IFNα expression.
Table 1.
Inhibition of Japanese encephalitis virus (JEV) and enterovirus 71 (EV71) replication in TE-671 and HL-CZ cells treated with Chinese herbal compounds
| Virus/compound | Cell type | CC50 (μg/mL)a | IC50 (μg/mL)b | TIc |
|---|---|---|---|---|
| JEV | ||||
| Aloe-emodin | TE-671 | 2632 | 1.51 ± 0.05 | 1743 |
| Aloe-emodin | HL-CZ | 2881 | 0.50 ± 0.02 | 5762 |
| Shikonin | TE-671 | 1754 | 2.1 ± 0.5 | 835.2 |
| Shikonin | HL-CZ | >3000 | 1.2 ± 0.2 | >2500 |
| Arecoline | TE-671 | >3000 | 32.5 ± 0.4 | >92.3 |
| Arecoline | HL-CZ | >3000 | 10.5 ± 0.3 | >285.7 |
| Hesperetin | TE-671 | 574 | 3.6 ± 0.5 | 159 |
| Catechin | TE-671 | >3000 | N.D. | N.D. |
| Trans-cinnamic acid | TE-671 | >3000 | N.D. | N.D. |
| EV71 | ||||
| Aloe-emodin | TE-671 | 2632 | 0.14 ± 0.04 | 18 800 |
| Aloe-emodin | HL-CZ | 2881 | 0.52 ± 0.03 | 5540 |
CC50, 50% cytotoxic concentration; IC50, 50% inhibitory concentration; TI, therapeutic index; N.D., not detected.
Concentration (mean ± standard deviation) showing a 50% cytotoxic effect on the indicated cells, predicted using a computer program.
Concentration (mean ± standard deviation) required for 50% inhibition of the indicated virus plaques, predicted using a computer program.
Ratio of CC50 to IC50.
Fig. 1.
Induction of (A) interferon-alpha expression and (B) the in vivo signalling pathway by Chinese herbal compounds. The significance of the mean differences between groups was assessed by the χ2 test.
3.2. Signalling pathways induced by aloe-emodin
To examine induction of the IFN response, in vivo signalling pathways in HL-CZ cells treated with the herbal components were further detected using the dual-luciferase reporter assays (Fig. 1B). Cells were co-transfected with the firefly luciferase cis-reporter plasmid (pISRE-Luc, pGAS-Luc or pNFκB-Luc) and a Renilla luciferase reporter. After treatment for 4 h, the relative expression of firefly luciferase driven from the indicated cis-reporter plasmid was normalised by Renilla luciferase. The ratio of the firefly luciferase intensity revealed that aloe-emodin significantly increased the promoter activity of pISRE-Luc and pGAS-Luc by ca. 2.14- and 2.03-fold, respectively (Fig. 1B) (P < 0.001). Moreover, shikonin significantly increased the activity of an IFN-stimulated response element (ISRE)-containing promoter by ca. 1.2-fold, whilst arecoline activated the gamma-activated sequence (GAS)-containing promoter by 1.8-fold (P < 0.001). Aloe-emodin induced the activation of ISRE- and GAS-containing promoters associated with the activation of type I and II IFN responses.
3.3. Induction of IFN-stimulated gene expression by aloe-emodin
To detect the effect of each herb component on IFN-stimulated gene (ISG) expression, the mRNA levels of PKR and OAS in HL-CZ cells treated with 1 μg/mL aloe-emodin, shikonin, arecoline or hesperetin were quantified using real-time RT-PCR. After normalisation to GAPDH, aloe-emodin-treated cells showed an increase of 1294.4-fold of PKR mRNA and 8.6-fold of OAS mRNA compared with untreated HL-CZ cells, respectively. Moreover, aloe-emodin induced a more than two-fold increase in the level of the PKR mRNA compared with activation by shikonin, arecoline and hesperetin (P < 0.001). The result suggested that aloe-emodin more potently activated the expression of ISGs (PKR and OAS) than shikonin, arecoline and hesperetin.
3.4. Effect of aloe-emodin on NO production
Since IFNγ is involved in activation of endogenous NO to inhibit viral replication [7], the production of NO in herb compound-treated HL-CZ cells was assessed using the NO-specific fluorescent dye DAF-2 DA. Aloe-emodin and arecoline caused a greater DAF signal compared with the control and shikonin (P < 0.001), indicating that aloe-emodin and arecoline led to greater NO production. The results suggested that aloe-emodin could activate IFNγ-mediated NO production.
3.5. Antiviral activity against JEV and EV71
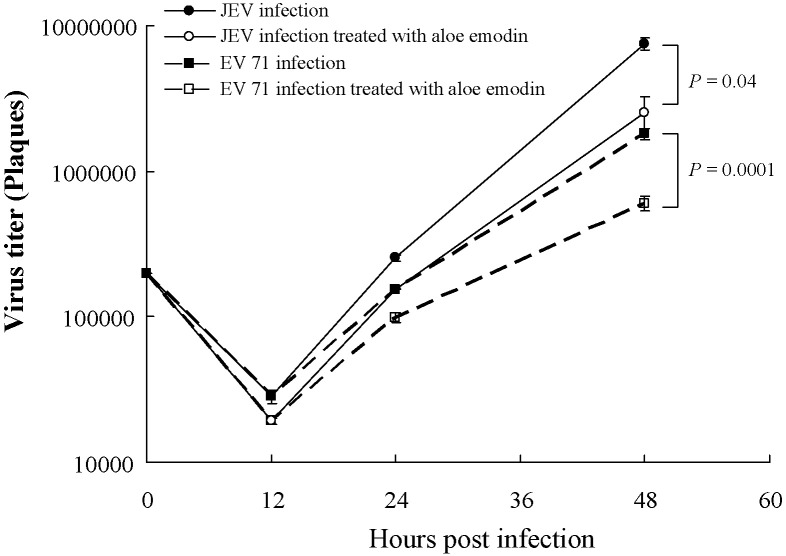
To test the antiviral activity, the dose- and time-dependent effects of herbal compounds against JEV and EV71 were determined using the plaque assay (Fig. 2 ; Table 1). Aloe-emodin, shikonin and arecoline showed dose-dependent inhibition of JEV replication in cell culture assay. IC50 values of aloe-emodin against JEV replication were 0.50 ± 0.02 μg/mL in HL-CZ cells and 1.51 ± 0.05 μg/mL in TE-671 cells. IC50 values of shikonin for inhibition of JEV replication were 1.2 ± 0.2 μg/mL in HL-CZ cells and 2.1 ± 0.5 μg/mL in TE-671 cells. In addition, IC50 values of arecoline were 10.5 ± 0.3 μg/mL in HL-CZ cells and 32.5 ± 0.4 μg/mL in TE-671 cells. On the other hand, IC50 values of aloe-emodin for inhibition of EV71 replication were 0.52 ± 0.03 μg/mL in HL-CZ cells and 0.14 ± 0.04 μg/mL in TE-671 cells. The therapeutic index (TI) of aloe-emodin for anti-JEV activity was >1500, whilst the TI for anti-EV71 activity was >5000. Moreover, aloe-emodin had higher TI values against JEV and EV71 than shikonin and arecoline. In the time-dependent assay, aloe-emodin significantly inhibited the early stages of JEV and EV71 replication in TE-671 and HL-CZ cells at a low concentration (1 μg/mL) (Fig. 2). The results demonstrated that aloe-emodin potently inhibited in vitro JEV and EV71 replication in a dose- and time-dependent manner.
Fig. 2.
Time-dependent inhibition of Japanese encephalitis virus (JEV) and enterovirus 71 (EV71) replication in HL-CZ cells.
4. Discussion
In this study, aloe-emodin significantly induced the expression of IFNα, PKR and OAS, the activation of ISRE and GAS promoters, and the production of NO (Fig. 1). Aloe-emodin showed dose- and time-dependent inhibitory activities on the replication of JEV and EV71 in TE-671 and HL-CZ cells, but showed low cytotoxicity to TE-671 and HL-CZ cells (Fig. 2; Table 1). In addition, aloe-emodin was a potential antiviral agent, with an IC50 value of 0.5 μg/mL on the JEV replication in HL-CZ cells and 0.14 μg/mL on EV71 replication in TE-671 cells. The antiviral efficacy of aloe-emodin against JEV was similar to a previous report in that aloe-emodin was found to have IC50 values of 1.5–6.0 μg/mL for inactivating enveloped viruses including herpes simplex virus type 1 and type 2, varicella-zoster virus, pseudorabies virus and influenza virus [1]. However, our result that aloe-emodin significantly inhibited the non-enveloped virus EV71 was not consistent with the previous report showing no antiviral effect on the replication of rhinovirus [1]. Therefore, we suggest that the antiviral action of aloe-emodin could be not of cell-type and virus-type specificity.
The mode of action of antiviral activity by anthraquinones has been demonstrated through both virucidal and non-virucidal mechanisms [1], [2], [4]. In this study, aloe-emodin has been further demonstrated to activate ISREs, to induce ISG expression (including PKR and OAS) and to increase production of IFNα (Fig. 1). In addition, aloe-emodin induced activation of the IFNγ activation sequence using in vivo signalling pathway assays (Fig. 1B), associated with the generation of endogenous NO (data not shown). NO has been found to inhibit viral replication, including JEV [7] and SARS coronavirus [12]. Importantly, IFNγ has been demonstrated to act synergistically with IFNα and IFNβ to inhibit viral replication potently [7]. Our findings suggested that aloe-emodin might be a potent component for activation of type I and II IFN signalling against viral replication.
In this study, shikonin also effectively inhibited JEV replication in human medulloblastoma cells and promonocyte cells (Table 1). The anti-JEV mechanism of shikonin was not clearly found to be associated with activation of in vivo ISRE, GAS and NF-κB signalling pathways (Fig. 1B). However, shikonin (1 μg/mL) increased levels of PKR and OAS in HL-CZ cells by 1087- and 5-fold, respectively, compared with mock cells. Shikonin was hypothesised to be an effective inhibitor of protein–protein interactions with multiple targets in both the intracellular and extracellular compartments owing to several therapeutic applications [13]. Therefore, we suggest that the mechanism of anti-JEV activity of shikonin could involve multiple targets for anti-JEV activity. Arecoline, a major areca nut alkaloid, showed less anti-JEV efficacy than aloe-emodin and shikonin (Table 1). Arecoline has been demonstrated to activate the GAS signalling pathway (Fig. 1B) and to enhance NO production. However, several side effects of arecoline have been reported, such as immunosuppression, hepatotoxicity, depression of antioxidant status and attenuation of cytochrome P450 1A1 activation in liver cells [14].
Treatment for viral encephalitis is still unknown. Recently, a case of WNV meningoencephalitis was reported to be successfully treated with IFNα-2b [6]. In addition, poly(I:C), a potent IFN-inducer, has been demonstrated to improve the survival rate and to decrease tissue viral titres following EV71 and WNV challenge in mice [6], [8]. In this study, aloe-emodin has been demonstrated to be a potent IFN-inducer that significantly activates type I and II IFN responses and effectively inhibits the replication of JEV and EV71 in human neural and blood cell lines. Therefore, aloe-emodin could be useful for the development of antiviral agents.
Acknowledgments
The authors would like to thank Mr Kuan-Hsun Lin and the colleagues at the Clinical Virology Laboratory, Department of Laboratory Medicine, China Medical University Hospital, Taiwan.
Funding: National Science Council of R.O.C. (Taiwan) (NSC95-2745-B-039-003-URD); and China Medical University (CMU95-054).
Competing interests: None declared.
Ethical approved: Not required.
References
- 1.Sydiskis R.J., Owen D.G., Lohr J.L., Rosler K.H., Blomster R.N. Inactivation of enveloped viruses by anthraquinones extracted from plants. Antimicrob Agents Chemother. 1991;35:2463–2466. doi: 10.1128/aac.35.12.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnard D.L., Huffman J.H., Morris J.L., Wood S.G., Hughes B.G., Sidwell R.W. Evaluation of the antiviral activity of anthraquinones, anthrones and anthraquinone derivatives against human cytomegalovirus. Antiviral Res. 1992;17:63–77. doi: 10.1016/0166-3542(92)90091-i. [DOI] [PubMed] [Google Scholar]
- 3.Shuangsuo D., Zhengguo Z., Yunru C. Inhibition of the replication of hepatitis B virus in vitro by emodin. Med Sci Monit. 2006;12:BR302–BR306. [PubMed] [Google Scholar]
- 4.Semple S.J., Pyke S.M., Reynolds G.D., Flower R.L. In vitro antiviral activity of the anthraquinone chrysophanic acid against poliovirus. Antiviral Res. 2001;49:169–178. doi: 10.1016/s0166-3542(01)00125-5. [DOI] [PubMed] [Google Scholar]
- 5.Sasaki O., Karaki T., Imanishi J. Protective effect of interferon on infections with hand, foot, and mouth disease virus in newborn mice. J Infect Dis. 1986;153:498–502. doi: 10.1093/infdis/153.3.498. [DOI] [PubMed] [Google Scholar]
- 6.Lewis M., Amsden J.R. Successful treatment of West Nile virus infection after approximately 3 weeks into the disease course. Pharmacotherapy. 2007;27:455–458. doi: 10.1592/phco.27.3.455. [DOI] [PubMed] [Google Scholar]
- 7.Lin Y.L., Huang Y.L., Ma S.H. Inhibition of Japanese encephalitis virus infection by nitric oxide: antiviral effect of nitric oxide on RNA virus replication. J Virol. 1997;71:5227–5235. doi: 10.1128/jvi.71.7.5227-5235.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu M.L., Lee Y.P., Wang Y.F. Type I interferons protect mice against enterovirus 71 infection. J Gen Virol. 2005;86:3263–3269. doi: 10.1099/vir.0.81195-0. [DOI] [PubMed] [Google Scholar]
- 9.Burke D.S., Monath T.P. Flaviviridae. In: Knipe D.M., Howley P.M., editors. Fields virology. 4th ed. Lippincott-Williams and Wilkins; New York: 2001. pp. 1043–1125. [Google Scholar]
- 10.McMinn P.C. An overview of the evolution of enterovirus 71 and its clinical and public health significance. FEMS Microbiol Rev. 2002;26:91–107. doi: 10.1111/j.1574-6976.2002.tb00601.x. [DOI] [PubMed] [Google Scholar]
- 11.Lin C.W., Huang H.D., Shiu S.Y. Functional determinants of NS2B for activation of Japanese encephalitis virus NS3 protease. Virus Res. 2007;127:88–94. doi: 10.1016/j.virusres.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 12.Keyaerts E., Vijgen L., Chen L., Maes P., Hedenstierna G., Van Ranst M. Inhibition of SARS-coronavirus infection in vitro by S-nitroso-N-acetylpenicillamine, a nitric oxide donor compound. Int J Infect Dis. 2004;8:223–226. doi: 10.1016/j.ijid.2004.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen X., Yang L., Oppenheim J.J., Howard M.Z. Cellular pharmacological study of shikonin derivatives. Phytother Res. 2002;16:199–209. doi: 10.1002/ptr.1100. [DOI] [PubMed] [Google Scholar]
- 14.Chang E.E., Miao Z.F., Lee W.J. Arecoline inhibits the 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced cytochrome P450 1A1 activation in human hepatoma cells. J Hazard Mater. 2007;146:356–361. doi: 10.1016/j.jhazmat.2006.12.035. [DOI] [PubMed] [Google Scholar]