Abstract
Severely dehydrated calves that are unable to suckle need intravenous fluids for effective resuscitation. Intravenous fluid therapy is also indicated for sick calves without obvious dehydration, such as calves with strong ion acidosis, ruminal acidosis (rumen drinkers), severe pneumonia, septicemia, or hypothermia. This article presents an updated overview of intravenous fluid therapy for calves, recent insights into the development of metabolic acidosis in young calves resulting from accumulation of D-lactate, a simplified algorithm for intravenous fluid therapy, and a procedure for ear vein catheterization under field conditions.
Keywords: Calves, Diarrhea, Dehydration, Acidosis, Intravenous fluid therapy
One of the most important factors in decreasing mortality associated with diarrhea in calves is the proper use of oral and intravenous (IV) fluid therapy. Sick calves without significant dehydration, as seen in other diseases (eg, persistent anorexia, septicemia, ruminal acidosis from ruminal drinking, severe pneumonia, and hypothermia) also may benefit from administration of IV fluids. The recommendations for type, amount, route, and rate of administration of solutions for IV fluid therapy in calves vary and often are too complicated or not suitable for use in field practice. This article presents a simplified protocol for administration of IV fluids and a simple technique for on-farm IV fluid therapy by ear vein catheterization in calves. Recent insights in the development of metabolic acidosis in calves and studies on IV fluid therapy are reviewed.
Diarrhea in neonatal calves remains the leading cause of morbidity and mortality in North America and Europe,1, 2 with no change in mortality rates between 1995 and 2001 in dairy heifer calves in the United States.1 Neonatal calf diarrhea is a complex disease that occurs predominantly during the first 4 weeks of life. Calves with diarrhea have an increased chance of fecal isolation of one or more viral (rotavirus, coronavirus), bacterial (Escherichia coli, Salmonella sp.), or protozoal (Cryptosporidium parvum, Eimeria sp.) pathogens than healthy control calves.3 Regardless of the origin, most calves with diarrhea have increased numbers of coliform bacteria in their small intestine (bacterial overgrowth), which contributes to morphologic damage of the intestinal mucosa and may result in increased susceptibility to bacteremia.4 Metabolic acidosis is a frequent consequence of gastrointestinal disease and is found in calves with dehydration and in clinically sick calves with minimal or no signs of dehydration, the so-called “acidosis-without-dehydration-syndrome.”5
The importance of bacterial overgrowth in the intestines of calves with diarrhea gained more attention when the role of D-lactate (the anion of D-lactic acid) in the development of metabolic acidosis was discovered. Production of D-lactic acid results from bacterial fermentation of carbohydrates in the gastrointestinal tract of milk-fed calves and is a common finding in sick calves with and without diarrhea. Studies have demonstrated that D-lactate is a major component of the metabolic acidosis generally present in calves with diarrhea, in calves with the “acidosis-without-dehydration-syndrome,” and in calves with ruminal acidosis from drinking milk into the rumen (ruminal drinking).6, 7, 8, 9, 10, 11, 12, 13, 14, 15 D-lactate also has been identified as the major component of metabolic acidosis in the floppy kid syndrome of young goat kids.16 Clinical signs of impaired central nervous system (CNS) function, including ataxia and coma in sick calves, have been historically attributed to metabolic acidosis; however, recent findings indicated that many of the clinical signs formerly attributed to acidosis are caused by an increase in D-lactate concentrations.17, 18 The pathophysiology of D-lactate accumulation is important for bovine practitioners to understand and is discussed in more detail in the next section.
Indications for intravenous fluid therapy
Dehydration and Electrolyte Imbalances
The pathophysiology of diarrhea includes increased intestinal secretion and decreased intestinal absorption of fluids along with increased passage of intestinal contents. Severe dehydration caused by fecal loss of fluids and electrolytes is a frequent complication of diarrhea and the primary indication for oral and intravenous fluid therapy. A higher total body water content (approximately 75% body weight) and a higher extracellular fluid volume content (approximately 45% body weight) in newborn calves make them more sensitive to fluid losses compared with adult cattle. The higher proportional water content does not serve as a water reservoir and has no protective effect against dehydration.19 Fecal fluid loss in calves with severe watery diarrhea can reach 13% to 18% of body weight per day and is probably underestimated in most cases.20 The highest fecal fluid loss reported was in a calf that lost 21% of its body weight over 24 hours, although ad libitum oral fluids were provided.20 The kidneys compensate for increased fluid loss in diarrhea by decreasing urine production; however, if losses exceed fluid intake, dehydration follows.21
Fecal losses of water occur in combination with losses of electrolytes, primarily sodium and potassium. Serum electrolyte concentrations are also affected by a reduced dietary (milk) intake and can be masked by hemoconcentration.22 The decrease in extracellular fluid volume decreases plasma volume and venous return, which results in extracellular dehydration and total body loss of water and electrolytes. Fluid and electrolyte losses in the early stages of calf diarrhea are primarily of secretory origin and—to a much lesser extent—of osmotic origin.23
Blood electrolyte concentrations can vary considerably in calves with diarrhea because most calves generally have been treated with oral fluids before presentation.20, 24, 25, 26 Mixing errors of oral electrolyte solutions often occur, and many calves do not have access to fresh water.26 In dehydrated calves with diarrhea, decreased serum concentrations of glucose, sodium, potassium, and chloride have been reported.27 Hyponatremia is generally present as a result of increased fecal loss (secretory diarrhea);22, 27, 28, 29 however, hypernatremia also may be present. This is particularly true in calves that have been treated or overtreated with oral electrolyte solutions and do not have access to fresh water26 or calves to which an electrolyte solution with excessive sodium concentration was fed (> 140 mEq/L). Total body potassium concentrations decrease in diarrhea; however, hyperkalemia is often present in calves with diarrhea with severe acidosis.22, 27 Hyperkalemia is thought to result from translocation of potassium from the intracellular to the extracellular compartment.19 An impaired renal excretion of potassium also may play a role in this not-fully-understood mechanism of hyperkalemia involving hyponatremia, hypo-osmolality, acidemia, and cellular hypoxia.5 Calcium concentrations are often low, and magnesium concentrations vary in calves with diarrhea.22, 30 Electrolyte imbalances (sodium, total calcium, magnesium) have been found to be present even 10 days after completion of therapy in calves that were successfully treated for diarrhea.30
Diarrhea in young calves causes a hypo-osmotic extracellular dehydration with decreased extracellular fluid volume (plasma and interstitial) and a small increase in intracellular fluid volume.31, 32, 33 In calves with chronic diarrhea or shortly before death, hyperosmotic dehydration may be present.21, 34 Electrolyte disturbances and type of dehydration (iso-, hypo-, or hyperosmotic) vary among individual cases and cannot be predicted from clinical findings without laboratory analysis. Scouring calves may be in poor body condition when they have not been fed milk for several days and were treated only with oral fluids.20, 35 Hypothermia is also a logical consequence in the pathophysiology of dehydration, and a decreased rectal temperature is frequently observed in calves with diarrhea.36, 37, 38
The traditional recommendation for IV fluid therapy is predominantly based on the degree of dehydration. A decrease of more than 8% body weight is believed to require IV fluid administration rather than only oral fluids for successful rehydration. Under field conditions, the indications for IV fluid therapy in calves may be extended to nondehydrated sick calves with gastrointestinal or other diseases.39, 40, 41 Calves with less severe dehydration may benefit from IV fluids if they show signs of severe depression or coma, are recumbent or severely depressed, or do not have a suckle reflex. In the authors' opinion, the indication for IV fluid therapy should be extended to some calves that may be less than 8% dehydrated. Regardless of the degree of clinical dehydration, when calves show signs of severe CNS depression or weakness, are comatose, are unable to stand, do not suckle for more than 24 hours, or have a rectal temperature of less than 100°F (38 °C) (in newborn calves), IV fluid therapy is indicated. The authors recommend IV fluid therapy for other diseases or syndromes in sick calves without diarrhea, including diseases that cause a decrease in oral fluid intake because of abdominal pain or an inability to suckle, which can be seen with severe respiratory distress.
Metabolic Acidosis and D-Lactate
Development of strong ion (metabolic) acidosis is common in calves with diarrhea and other gastrointestinal diseases. Our understanding of the pathophysiology of this acidosis has increased tremendously during the past decade, primarily because of discoveries by researchers in Europe and Canada on the significance of D-lactate in calves with gastrointestinal disorders. The importance of D-lactate in the pathophysiology of gastrointestinal diseases in calves has been elucidated in several clinical and experimental studies. Two thorough review articles were published recently on this subject;8, 11 however, a brief discussion is still warranted here. We have known for years that adult ruminants with acute rumen acidosis develop D-lactic acidosis after grain overfeeding.42 Only recently, however, were increased D-lactate concentrations identified to be responsible for most of the systemic acidemia that occurs in calves with the “acidosis-without-dehydration syndrome”12, 14 and for a major portion of the acidemia seen in calves with diarrhea.6, 7, 9, 10, 13, 43 In calves with ruminal acidosis after drinking or drenching of whole milk into the rumen, acidemia may follow after absorption of sufficient amounts of D-lactate from the gastrointestinal tract.15, 44
D- and L-lactate are end products of organic acids normally produced in the gastrointestinal tract by bacterial metabolism of carbohydrates without any deleterious consequences to the animal.45 In adult cattle with grain overload, fermentation of larger amounts of carbohydrates induces an increased concentration of organic acids that leads to a decrease in intraruminal pH. This decrease in pH favors the overgrowth of bacteria (mainly Lactobacillus spp.) that are able to produce D- and L-lactate in high quantities.8 In calves with diarrhea, the increased production of D- and probably L-lactate is thought to result from villous atrophy, with subsequent malabsorption and fermentation of nutrients by intestinal bacteria.8, 11 Both the L- and D-isomers of lactic acid can be absorbed from the gastrointestinal tract; however, the hepatic metabolism and renal excretion of D-lactate are significantly slower in ruminants compared with L-lactate. With increased production and absorption of L- and D-lactate, the metabolic acidosis that develops is primarily caused by increases in D-lactate concentrations.8
Development of metabolic acidosis in calves with diarrhea has long been attributed to (1) loss of bicarbonate ions (HCO3 -) in the feces, (2) decreased renal excretion of hydrogen ions (H+) associated with dehydration and decreased renal blood flow, and (3) the presence of unidentified organic acids in plasma.46, 47, 48, 49, 50 In the late 1980s, Naylor51 found that metabolic acidosis is based at least in part on the presence of increased L-lactate concentrations in the serum of calves with diarrhea younger than 8 days. Compared with the recent findings of elevated D-lactate concentrations in the blood, however, the small increases in L-lactate concentrations seen in earlier studies do not explain the high anion-gap (AG = ([Na+] + [K+]) - ([Cl-] + [HCO3 -])) often found in calves with diarrhea.6, 13, 48, 52 An increase in the anion gap is the result of an increased concentration of strong anions in blood, mainly from organic acids (eg, lactic acid). Experimental induction of severe dehydration (at least 14% body weight) produced only a mild L-lactic acidosis.53 The discovery of D-lactate ended speculation of which unidentified anion or anions might be most responsible for the increase in the anion gap. It is clear that increased D-lactate concentrations explain most of the acidemia and elevated anion gaps present in calves with diarrhea and in clinically sick calves without diarrhea and dehydration (“acidosis without dehydration syndrome”).
The first two reports of D-lactic acidosis from France found a mean D-lactate concentration between 10 and 13 mmol/L in the plasma of clinically sick Charolais calves without signs of dehydration or diarrhea and a mean D-lactate concentration of 5 mmol/L in calves with simple diarrhea. This finding was compared with healthy control calves that had D-lactate concentrations between 1 and 2 mmol/L.12, 14 The mean L-lactate concentrations seen in acidotic calves (1–2.5 mmol/L) were much lower than the reported D-lactate levels. Subsequent studies from Canada and Germany showed that hyper-D-lactatemia is often present in calves with more severe diarrhea and dehydration. A blood D-lactate concentration above 3 mmol/L was found in 55% of 300 calves with neonatal diarrhea.9 Median D-lactate concentration was 4.1 mmol/L and ranged from 0 to 17.8 mmol/L. The correlation between D-lactate concentration and base deficit was statistically significant but not linear, and the degree to which D-lactate contributed to metabolic acidosis varied from calf to calf (Fig. 1 ). Calves with a severe metabolic acidosis (base deficit > 25 mmol/L) always had increased D-lactate concentrations, whereas calves with a less severe acidosis (base deficit between 10 and 25 mmol/L) had D-lactate concentrations that varied from 0 to 17.8 mmol/L.9, 10
Fig. 1.
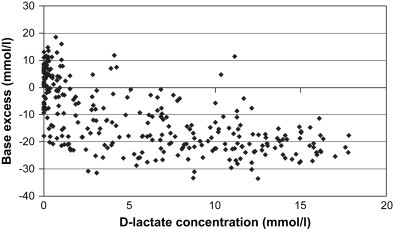
Blood base excess and serum D-lactate concentrations in 300 calves with neonatal diarrhea. (From Lorenz I. Influence of D-lactate on metabolic acidosis and on prognosis in neonatal calves with diarrhoea. J Vet Med A 2004;51:425–8; with permission.)
The concentration of D-lactate and L-lactate accounted for 64% of the elevated anion gap in one study,13 and significant correlations between D-lactate concentration and the anion gap were found in subsequent studies.6, 9 Ewaschuk and colleagues7 measured D- and L-lactate in rumen, blood, fecal, and urine samples in calves with diarrhea and in healthy control calves. Despite a markedly higher L-lactate concentration in rumen and fecal samples of diarrheic calves as compared with control calves, the L-lactate concentrations were not high enough to induce a systemic acidosis. The calves with diarrhea in that study had significantly higher values for D-lactate in all four samples (rumen, feces, blood, urine) than healthy control calves.7 The mean values for D-lactate were highest in the feces (25.4 mmol/L), followed by urine (19.2 mmol/L), rumen (17 mmol/L), and serum (13.9 mmol/L). The authors concluded that D-lactate production and absorption in the colon were more likely to have contributed to the systemic acidosis than other possible sites of absorption (rumen). Accumulated D-lactate may be effectively eliminated by the kidneys in nondehydrated calves.54 The role of IV fluid therapy to restore or maintain hydration status in sick calves is also important to speed elimination of D-lactate; however, kinetic studies of D-lactate elimination in calves are not currently available.
D-Lactate in Ruminal Drinker Calves
The role of D- and L-lactate in the pathophysiology of ruminal acidosis in adult cattle with grain overload was identified more than 40 years ago.42 Briefly, overfeeding with highly fermentable concentrates results in excessive bacterial fermentation of carbohydrates by the anaerobic bacteria of the rumen. Grain overload leads to production of short chain fatty acids and large amounts of D- and L-lactate.8 Because the ruminant liver is not efficient in metabolizing D-lactate, it begins to accumulate in the blood and systemic acidemia often follows. The same absorptive mechanisms for D-lactate in ruminating cattle may be responsible for systemic accumulation of D-lactate in preruminating calves with persistent ruminal acidosis from spilling milk into the rumen (ruminal drinking).15 Experimentally induced rumen acidosis in calves after repeated intraruminal force feeding of whole milk resulted in D-lactic acidosis. Calves developed hyper-D-lactatemia with concentrations between 6.8 and 11.1 mmol/L, generally in conjunction with metabolic acidosis, dehydration, severe depression, and lack of appetite or reduced suckle reflex.15 Calves that suffered from spontaneous diarrhea and concurrent ruminal acidosis (rumen pH < 6.0) had a higher mean D-lactate concentration (6.6 ± 5.2 mmol/L) in blood as compared with calves with a normal rumen pH (5.3 ± 5.4 mmol/L).9 The author stated that her study could not clarify whether the ruminal acidosis was a cause or a consequence of systemic D-lactic acidosis.
In summary, recent research has demonstrated that D-lactate is responsible for a major portion of the acidemia that generally accompanies diarrhea in calves suckling their dam or fed whole milk. D-lactic acidosis occurs after sufficient amounts of D-lactate have been absorbed from the gastrointestinal tract (primarily colon) and neutralization of the accumulated D-lactate by buffer mechanisms in the body becomes inadequate. Reports of D-lactate concentrations in calves with diarrhea that are being fed milk-replacer diets with or without antimicrobials are unavailable, so it is unknown whether these calves develop D-lactic acidosis to the same degree as calves on whole milk diets.
Metabolic Acidosis in Newly Born Calves With Asphyxia
A further indication for IV fluid therapy in calves is mixed respiratory and metabolic acidosis resulting from birth asphyxia.55 In calves that suffer from severely depressed uteroplacental gas exchange, the partial pressure of oxygen (pO2) decreases, which indicates hypoxia, and the partial pressure of CO2 (pCO2) increases, which indicates hypercapnia. The resulting mixed respiratory and metabolic acidosis is associated with increased mortality rates when venous blood pH falls below 7.2.56, 57 These calves benefit from IV fluid therapy with sodium bicarbonate solutions to increase blood pH.
Bacteremia, Sepsis, and Endotoxemia
Calves with neonatal diarrhea often have increased numbers of coliform bacteria in their small intestine.4 This bacterial overgrowth is associated with altered intestinal function, morphologic damage, and increased susceptibility to bacteremia. Studies have documented that bacteremia is a frequent complication of diarrhea in calves.58, 59 Fluid therapy is important for maintaining fluid and electrolyte balance in these calves; however, specific therapy of bacteremia focuses on administration of antibiotics (see the article by Constable found elsewhere in this issue.)
Assessing the need for intravenous fluid therapy
Food animal practitioners generally decide if IV fluid therapy is necessary in sick calves based on clinical examination rather than on laboratory values. Important clinical parameters to guide decision making on fluid therapy are obtained from the evaluation of hydration status and CNS function. Degree of enophthalmus is the best predictor of dehydration in calves, followed by skin elasticity determined on the neck and thorax.60 More detailed information on the clinical assessment of hydration status in calves is presented in the article by Smith on oral fluid therapy found elsewhere in this issue. In clinically sick calves, it is important to evaluate hydration status along with other clinical signs, including the ability of the calf to suckle, severity of CNS depression, and whether the calf can stand (degree of weakness). These factors in combination are used to determine whether IV fluid therapy is indicated.
Laboratory Tests to Aid the Assessment of Strong Ion Acidosis
Blood-gas and acid-base status can be determined in practice with a portable blood gas analyzer, such as the I-Stat unit (Heska Corporation, Fribourg, Switzerland). These laboratory analyzers are expensive, however, and are not used in most practices. Other less expensive methods for determination of acid-base status use a portable pH meter (Cardy Twin pH meter, Spectrum Technologies, Inc., Plainfield, IL)61 or the Harleco-System to determine total carbon dioxide concentration.24, 51 To the authors' knowledge, these methods are not widely used in bovine practice. A more detailed presentation of tools for laboratory assessment of acid-base status in bovine field practice is discussed elsewhere.62
The concentration of stereospecific D-lactate is determined by high-performance liquid chromatography or via enzymatic measurements.63 D-lactate has been determined in experimental studies and in teaching hospitals. Portable lactate analyzers for use in practice do exist, but they measure only L-lactate.62 Determination of D-lactate is not performed in most diagnostic laboratories, and simple assays for use in practice are not available.
Clinical Assessment of Metabolic Acidosis
Assessing and diagnosing metabolic acidosis on the basis of clinical signs are common practice. The predictive accuracy of the degree of metabolic acidosis on the basis of clinical signs has varied among studies. The clinical signs of neurologic depression (weakness, ataxia, and decreased menace, suckle, and panniculus reflex) correlated highly with the severity of metabolic acidosis in calves without dehydration.52 Also in calves with diarrhea, signs of CNS depression, ability to stand, and suckling force all correlated well with metabolic acidosis.36, 51, 64, 65 The degree of enophthalmos and peripheral skin temperature are important and obvious signs that determine whether IV fluid therapy is indicated; however, they do not correlate with the degree of metabolic acidosis.10, 24, 28, 36, 51, 52
An important discovery was that metabolic acidosis in calves with diarrhea varies during the first weeks of life. Naylor discovered that metabolic acidosis is less severe during the first week of life than in calves with diarrhea older than 8 days.36, 51 The base deficit in calves with diarrhea that were older than 1 week was almost twice as high as in calves that presented with diarrhea during the first week of life. Subsequent studies confirmed that calves with diarrhea older than 1 week of age usually exhibit a higher base deficit.24, 25, 50, 66, 67 On the basis of his findings, Naylor68 developed a chart for predicting the severity of metabolic acidosis based on body position, strength of suckle reflex, and age of the calf, with corresponding values for base deficit and bicarbonate requirements for the treatment of metabolic acidosis in calves with diarrhea younger or older than 8 days. This protocol became a popular approach to guide diagnosis and treatment of acidosis in calves with diarrhea and is presented in common veterinary medical textbooks. An even more simplified approach to fluid therapy in the field is presented later in this article.
Another study estimated the base deficit from suckling force or ability to stand without dividing calves into age groups.69 Data from 65 calves with diarrhea showed that when suckle reflex was strong, weak, or absent, the calves had a mean base deficit of 4.2 mEq/L, 11.4 mEq/L, or 21.5 mEq/L, respectively. The calves standing strongly, weakly, or unable to stand had a mean base deficit of 5.2 mEq/L, 7.8 mEq/L, and 19.1 mEq/L, respectively.69 Newer studies focusing on the clinical signs associated with D-lactic acidosis have reported only minor differences for the base deficit between calves standing securely compared with calves with diarrhea with a wobbly posture or not able to stand.10, 43 Severe metabolic acidosis from accumulation of D-lactate is likely present in depressed, wobbly, or recumbent calves with no or minimal diarrhea and dehydration.10, 12, 14, 24, 43, 52, 70
Clinical Assessment of D-Lactic Acidosis
The recent discovery that D-lactate is responsible for most of the acidemia present in calves with diarrhea with and without dehydration was accompanied by the discovery that D-lactate is also responsible for most of the CNS depression that was formerly been attributed to metabolic acidosis.10, 17, 18, 43 Practitioners have commonly used depression scores to predict the degree of metabolic acidosis in calves with diarrhea; however, a recent study questioned if the degree of acidosis can be predicted based on the severity of clinical signs, because administration of hydrochloric acid induced severe hyperchloremic metabolic acidosis but no abnormal clinical behavior.71 Other studies suggested that currently, D-lactate is the most important factor responsible for the clinical signs of weakness and CNS depression in calves with diarrhea.
The first descriptions of D-lactate suggested that it was the major cause of metabolic acidosis in nondehydrated calves without significant diarrhea, a syndrome that is characterized by CNS depression, ataxia, recumbency, and coma.12, 14 A D-lactate concentration up to 2 mmol/L is considered normal;44 however, values consistently associated with abnormal clinical signs have not been established. Markedly elevated D-lactate concentrations in calves with diarrhea are found when calves show a wobbly posture or cannot stand, exhibit a tired, listless, or comatose behavior, and have a delayed, incomplete, or completely absent palpebral reflex.10, 11, 43 The mean D-lactate concentration (between 10 and 11 mmol/L) was approximately four times higher in calves with diarrhea showing these abnormal signs compared with calves with normal posture (secure standing), alert behavior, or a prompt and complete palpebral reflex.10 Mean D-lactate concentration was 11.0 ± 3.6 mmol/L in calves with diarrhea that were unable to rise or had a wobbly posture; calves that were able to stand without difficulty had a mean D-lactate of 2.4 ± 2.1 mmol/L.10 Only minor differences were noted for the corresponding base deficit values (18.9 ± 3.9 mEq/L in wobbly or recumbent calves and 16.1 ± 3.5 mEq/L in calves standing securely).
A staggering and drunken appearance also has been described and is often found in calves with diarrhea and ruminal drinking in the authors' experience. D-lactic acidosis in experimentally induced ruminal acidosis was characterized by depression, reduced or absent suckle reflex, dehydration, and recumbency.15 The degree of dehydration does not correlate well with the degree of acidosis10, 64, 72 or D-lactate concentration.9 A negative correlation between D-lactate concentration in blood and dehydration was described in one study, however.9 Higher D-lactate concentrations were present in calves with a normal or slightly sunken position of the eyeballs than in calves with clearly sunken eyeballs.10 The author speculated that this finding may be explained by the preselection of severely sick calves admitted to a teaching hospital.
Increased D-lactate concentrations in blood and cerebrospinal fluid (CSF) are associated with signs of dysfunction of the CNS because D-lactic acid has been identified as a neurotoxic agent.18 Two studies clearly demonstrated that impaired neurologic function in healthy calves can be reproduced by administration of hypertonic sodium-D-lactate or isotonic DL-lactic acid.17, 18 Calves given D-lactate showed a delayed palpebral reflex, appeared tired and closed their eyes, and had a staggering (ataxic) gait, and some lay down with one foreleg extended backward parallel to the body (a sign seen in some weak calves requiring IV fluid therapy in the authors' experience).17 The suckle reflex was not depressed in a study that experimentally induced a short-term (2 hours) D-lactic acidosis;17 nor was it depressed after a short-term (2 hours) hyperchloremic metabolic acidosis after the administration of hydrochloric acid to healthy calves.71 The infusion of isotonic DL-lactic acid, L-lactic acid, and hydrochloric acid over 6 hours in another study did result in depression of the suckle reflex, however.18 The weak suckle reflex was more strongly correlated with CSF bicarbonate concentration, base excess values, and blood pH than it was with CSF D-lactate concentrations.18 It seems that increases in D-lactate alone do not impair the suckle reflex and that a decrease in CSF pH is needed to reduce a calf's suckling ability.17, 18, 71 Researchers do not completely understand how the decrease in suckling behavior develops in calves with diarrhea.
In summary, the age of a calf needs to be taken into consideration when assessing the severity of metabolic acidosis and determining bicarbonate requirements of calves with diarrhea.51 Calves with diarrhea and dehydration during their first week of life are less acidotic than older calves and require less sodium bicarbonate to correct their acidemia. Calves that are unable to stand or have a weak or absent suckle reflex have a more severe acidosis and require intravenous sodium bicarbonate to correct their acidemia. D-lactic acidosis may be present in sick calves with or without diarrhea and dehydration that are recumbent or wobbly, tired, listless or comatose, and have a delayed, incomplete, or absent palpebral reflex. If the suckle reflex is absent or weak or the calf chews irregularly instead of suckling normally, D-lactic acidosis may be the underlying disease state. Clinical assessment for weakness and ability to stand is performed by careful manipulation to help calves stand up (not performed in comatose calves), for duration of anorexia by obtaining history and determining suckle reflex, and for hypothermia by palpation of extremities and recording rectal temperature.
Indications for intravenous fluid therapy
The major indications for IV fluid therapy in neonatal calves are (1) dehydration, (2) severe depression, weakness, or inability to stand, (3) anorexia for more than 24 hours, and (4) hypothermia (temperature < 100°F [38.0 °C]) in newborn calves. An estimated dehydration of more than 8% of the calf's body weight is the most widely accepted indication for IV fluid administration,73 although experimental evidence supporting this as the most appropriate intervention point is currently unavailable. One study demonstrated that calves dehydrated more than 8% required at least 24 hours to be adequately rehydrated with orally administered electrolyte solutions.74 Calves that are recumbent, severely depressed, or comatose and calves without a suckle reflex also need IV fluid therapy. Calves with rapidly progressing dehydration and consistent profuse watery diarrhea should be treated intravenously rather than rehydrated by ororuminal intubation. If treatment with oral fluids is not successful and only a weak suckle reflex is present, initial IV restoration of fluid and electrolyte deficits is preferred. Collapsed dehydrated calves in severe hypovolemic shock are not able to rapidly resorb sufficient amounts of oral or subcutaneously administered fluids and should receive IV rehydration.74 Resuscitation by IV fluid administration restores oxygen delivery and removes the metabolic products of poorly perfused tissues.60
IV fluids are also recommended in sick calves that show signs of CNS depression and other underlying diseases.39, 40, 41 Severely depressed calves with suspected acidemia (most likely D-lactic acidosis) but without clinical signs of dehydration need IV alkalinizing fluids to restore a normal acid-base status.14, 52, 70 Severely depressed calves with acidemia need IV alkalinizing fluids to help to decrease D-lactate concentrations.5, 14 The authors also recommend IV fluid therapy for other diseases or syndromes in sick calves without diarrhea but with a decrease in oral fluid intake from pain or inability to suckle caused by severe respiratory distress. Finally, IV fluid therapy is needed for resuscitation of newly born calves that suffer from asphyxia and mixed respiratory-metabolic acidosis.56, 57
Goals of intravenous fluid therapy
In a previous edition of this article, the goals of IV fluid therapy in calves were defined as (1) correcting extracellular dehydration and restoring circulating blood volume, (2) correcting metabolic acidosis (increase blood pH >7.20), (3) correcting mental depression and restoring the suckle reflex, (4) correcting electrolyte abnormalities, (5) correcting the energy deficit, and (6) facilitating repair of damaged intestinal surface.73 Because of the importance of D-lactate in sick calves with gastrointestinal disease, another goal of fluid therapy in calves is to decrease the concentration of D-lactate. This goal was defined by Naylor and colleagues5 after D-lactate was identified as an important factor of metabolic acidosis that acts as a neurotoxic agent. The reduction of D-lactate helps to correct depression and restore the suckle reflex. Current research efforts concentrate on the development and evaluation of efficient strategies to decrease D-lactate concentrations.43, 54, 75 The administration of IV fluids may speed renal elimination of D-lactate and removal from body compartments such as the brain and CSF.54
Administration of IV sodium bicarbonate in calves with D-lactic acidosis had no significant effect on blood D-lactate concentrations 4 hours after starting buffer therapy and did not correct the base deficit in 53% of calves, despite correct calculation of bicarbonate requirements.43 After 24 hours, the mean D-lactate concentration decreased from 10 mmol/L before therapy to 5.4 mmol/L with administration of sodium bicarbonate followed by isotonic saline. Whether IV fluid therapy alone is sufficient to reduce the D-lactate levels found in acidotic calves with diarrhea is questionable. Administration of oral electrolyte solutions with a high strong ion difference for at least 2 days may help hasten the renal excretion of D-lactate and avoid relapses. A Canadian study reported significant reductions of D-lactate concentrations within 24 hours after starting a treatment protocol with a combination of IV fluids, oral electrolytes, and antibiotics.75 This study examined the effects of orally administered Lactobacillus rhamnosus GG, a probiotic bacteria that does not produce D-lactate. Unfortunately, the probiotic had no effect on D-lactate levels in serum and feces. The exact treatment plan was not presented in this study; however, combining IV buffer therapy and oral electrolytes with antibiotics seems logical for the treating D-lactic acidosis in calves with diarrhea and preventing relapses by controlling bacterial D-lactate production in the gastrointestinal tract with antimicrobials. A study from France suggested milk withdrawal for a short period combined with administration of antibiotics for the treatment of D-lactic acidosis in addition to administration of sodium bicarbonate.14 Further studies are desperately needed to evaluate the efficacy of various treatment protocols in calves with D-lactic acidosis.
Correcting the energy deficit by adding dextrose seems like a logical indication for IV fluid therapy in calves with diarrhea, but it should be used cautiously. A recent study found that the addition of 100 or 400 g of dextrose to 10 L of isotonic saline and sodium bicarbonate solution for the treatment of calves with diarrhea was associated with a decrease in voluntary milk intake as compared with calves that did not receive dextrose.76 Another deleterious effect of dextrose administration in adult cattle was a decrease in serum phosphorus concentration.77 A dextrose-enriched solution seems to be beneficial in newborn calves for the treatment of severe hypothermia during the first 24 hours of life.
Solutions for intravenous administration
Food animal practitioners need only a few crystalloid solutions for effective IV fluid therapy in calves, including isotonic and hypertonic saline (NaCl), isotonic and hypertonic sodium bicarbonate (NaHCO3), acetated or lactated Ringeŕs solution, and concentrated solutions of dextrose. These solutions are commercially available in most countries and come in plastic bags or bottles that are convenient to use and easily attachable in the environment of single-housed or tied (tethered) calves. If solutions are not commercially available or too expensive for routine use in calves, preparation of homemade, nonsterile solutions with clean tap water has been recommended78 and is presented elsewhere.79 This approach is questioned if approved products for use in food animals are available at reasonable costs.
Types of Solutions
Solutions for IV application are classified as crystalloid or colloid and—according to their osmolarity—as hypotonic, isotonic, or hypertonic.80 Balanced crystalloid solutions have a composition similar to extracellular fluid (ie, lactated Ringer's solution), whereas unbalanced solutions differ from extracellular fluid (ie, 0.9% NaCl). The solutes of crystalloid solutions form a true solution, can be crystallized, and are distributed throughout the extracellular fluid space into all body fluid compartments. Compounds of crystalloid solutions are electrolytes, mainly based on sodium and chloride, and organic compounds like dextrose or lactate. Sodium is the backbone of the extracellular fluid, and solutions based on sodium are always indicated in hypovolemia.80 To increase or maintain the extracellular volume, isotonic solutions (approximately 300 mOsm/L) must contain a sodium concentration of at least 140 mEq/L. Solutions that contain less sodium do not resuscitate dehydrated calves as effectively as isotonic saline that has a sodium concentration of 154 mEq/L.81 Sodium-containing solutions should not be used in calves with severe hypoalbuminemia because they decrease plasma albumin concentration and oncotic pressure, which forces fluid movement into the interstitial space and exacerbates tissue edema.80 Calves known to have hypoalbuminemia before the start of IV fluid therapy benefit from the administration of colloid solutions or blood transfusion.
Colloids are substances with a high molecular weight that are too large to pass through a semipermeable membrane. Colloid substances are restricted to the plasma compartment and provide sustained plasma volume expansion. Examples of colloid solutions are whole blood, blood substitutes, plasma, and high molecular glucose polymers such as dextran preparations (dextran-70) and hydroxyethyl starch preparations (hetastarch and pentastarch). The basics and the use of colloid solutions in cattle were discussed in the November 2003 issue of Veterinary Clinics of North America: Food Animal Practice.80
Alkalinizing Solutions
Because acidemia is common in calves with gastrointestinal disease requiring IV fluid therapy, these patients need administration of alkalinizing substances.82 Sodium bicarbonate is the alkalinizing agent of choice and is often recommended as a 1.3% isotonic solution (13 g NaHCO3/L).66, 73, 80, 83, 84, 85 Isotonic sodium bicarbonate has an effective strong ion difference of 155 mEq/L and is alkalinizing because it buffers hydrogen ions and increases the strong ion difference in blood. Many studies have documented that sodium bicarbonate is the most important buffer for the treatment of acidemia in calves with and without dehydration. Available hypertonic preparations of sodium bicarbonate include 4.2%, 5%, and 8.4% solutions with a theoretic osmolality of 1000 mOsm/L, 1190 mOsm/L, and 2000 mOsm/L, respectively. Hypertonic formulations of sodium bicarbonate are ideal for adding to larger quantities of isotonic saline to create a mildly hypertonic solution containing volume-expanding fluid and buffer.39, 66, 78 Sodium bicarbonate (NaHCO3) is available in 100-mL or 250-mL glass bottles as 4.2% or 8.4% hypertonic formulations, which provides for easy calculation of the volumes for buffer requirements because 1 mL of 8.4% sodium bicarbonate provides 1 mEq of buffer (HCO3 -). One or two (250 mL) bottles of 8.4% sodium bicarbonate can easily be added to a 5 L bag of isotonic saline with a large syringe and mixed on the farm just before administration to avoid potential contamination.39, 66 In North America, 500-mL bottles of 5% sodium bicarbonate are widely available and contain 0.6 mEq of HCO3 - per milliliter (298 mEq per bottle). Administration of undiluted 4.2% or 2.1% sodium bicarbonate has been recommended at a dosage of 500 mL to 1000 mL for resuscitation of comatose or severely acidotic calves before starting a volume-expanding or replacement solution.35
Administration of undiluted 8.4% hypertonic sodium bicarbonate solution should be used with caution, especially in dehydrated calves that are unable to suckle. Potential adverse effects of hypertonic sodium bicarbonate include hyperosmolality of extracellular fluid, hypokalemia, hypernatremia, hypocalcemia, and paradoxic intracellular and CSF acidosis. A recent study administered 8.4% hypertonic sodium bicarbonate (10 mL/kg over 8 minutes) or 5.85% hypertonic saline solution (5 mL/kg over 4 minutes) followed by oral electrolytes to dehydrated calves with diarrhea and severe acidosis.67 Administration of 8.4% hypertonic sodium bicarbonate resulted in greater cure rates as compared with hypertonic saline. Although no significant clinical side effects were observed, the authors warned against using hypertonic (high sodium) solutions in calves with hypernatremia.67 Hypertonic sodium bicarbonate should not be used in calves with diarrhea that have concurrent respiratory disease, because they may not be able to effectively exhale the excess CO2 generated in buffer reactions.
There are conflicting data on the ability of 8.4% hypertonic sodium bicarbonate solution to induce a paradoxic intracellular and CSF acidosis in normovolemic calves. The administration of 5 mL/kg of 8.4% NaHCO3 over 5 minutes to nondehydrated calves with an experimentally induced respiratory and metabolic acidosis did not result in a paradoxic acidosis of CSF in one study,55 whereas in another study a paradoxic CSF acidosis was reported in calves after treatment of an induced strong ion acidosis with sodium bicarbonate (formulation of solution not given).86 A 5% hypertonic sodium bicarbonate formulation has been used for the treatment of newborn calves with asphyxia accompanied by a mixed (respiratory and metabolic) acidosis.56, 57 The dose of sodium bicarbonate was determined on the basis of the base deficit and body weight. These two studies did not report significant side effects, and there was no increase in the partial pressure of carbon dioxide (pCO2) of blood to indicate the development of a paradoxic acidosis in blood.
Lactate and acetate are metabolizable bases and are included in popular polyionic solutions (lactated Ringer's and acetated Ringer's). Both substances produce an alkalinizing effect because they are metabolized predominantly to bicarbonate (HCO3 -); however, they do not alkalinize as rapidly as sodium bicarbonate.80 Sodium-L-lactate showed a delayed effect in increasing blood pH when compared with sodium bicarbonate.64 A theoretic disadvantage of commercially available lactated Ringer's solution is that the lactate is a racemic equimolar mixture of L-lactate and D-lactate, and it should be avoided in severely acidemic calves with a blood pH of less than 7.2 because D-lactate concentrations already may be increased.11, 14, 80 Acetated Ringer's solution is theoretically superior to lactated Ringer's solution because acetate is metabolized faster and alkalinization is more rapid. Acetate would not exacerbate D- and L-lactic acidosis.64 A disadvantage of commercially available acetated Ringer's solutions is that it contains gluconate, which is slowly metabolized by neonatal calves.87 Despite these concerns, lactated Ringer's solution is popular and still widely used by practitioners. In general, acetated or lactated Ringer's solution is preferred to correct a less severe acidemia (pH > 7.20 or base deficit < 10 mEq/L), and sodium bicarbonate should be used for the treatment of severe acidemia in sick calves.
Nonalkalinizing Solutions
Nonalkalinizing solutions are more frequently used in fluid therapy for adult cattle because they tend to get alkalemic instead of acidemic.82 Besides isotonic saline solution (0.9% NaCl), the classic balanced polyionic and isotonic crystalloid fluid for adult ruminants is Ringer's solution, which contains physiologic concentrations of sodium, potassium, calcium, and chloride. The addition of dextrose to IV fluid solutions is popular to provide energy during cold weather and counteract negative energy balance in calves with diarrhea. This practice should be questioned because of a recent study that showed that the addition of either 100 or 400 g of dextrose to 10 L of saline and sodium bicarbonate IV fluids (given over 24 hours) resulted in decreased milk intake as compared with calves that received the same fluids but without dextrose.76 In that study, adding dextrose to the fluids was also accompanied by an increase in the amount of sodium bicarbonate that was necessary to correct the base deficit.
Administration of crystalloid solutions that do not contain an alkalinizing agent have a strong ion difference of zero (ie, isotonic saline or Ringer's solution) and are acidifying because they decrease the normal strong ion difference in calves.80 Solutions without an alkalinizing agent induce a strong ion acidosis and should be used cautiously in calves with acidemia.74, 80 Several studies documented that isotonic saline or small amounts of hypertonic saline solutions do not significantly alter base deficits in healthy or acidemic calves.64, 67, 74, 87, 88
In summary, alkalinizing fluids are the appropriate choice for the IV rehydration of calves with diarrhea and dehydration (Fig. 2 ). Currently, isotonic sodium bicarbonate (1.3% = 13 g of NaHCO3/L = 155 mEq HCO3 −/L + 155 mEq Na+/L) at a dose of 1 to 4 L is the recommended solution for IV treatment of calves with diarrhea. Isotonic sodium bicarbonate rapidly corrects acidosis and dehydration and restores normal cellular function. When a calf's suckle reflex is re-established, further treatment can be given orally. Undiluted 8.4% hypertonic sodium bicarbonate solutions should be used with caution in severely dehydrated calves with diarrhea but are ideal for adding to larger quantities of isotonic saline. Correcting dehydration with rapid administration of small volumes of hypertonic saline solution (4–5 mL/kg body weight, 7.2% NaCl or 7.2% NaCl in 6% dextran-70) successfully resuscitates dehydrated calves but does not correct metabolic acidosis. Administration of hypertonic saline solutions should be accompanied by IV sodium bicarbonate in severely acidemic calves or by oral alkalinizing agents (acetate, propionate) in mildly to moderately acidemic calves.67 For additional information, a detailed review of solutions used for fluid and electrolyte therapy in ruminants was published in the November 2003 issue of Veterinary Clinics of North America: Food Animal Practice.80
Fig. 2.
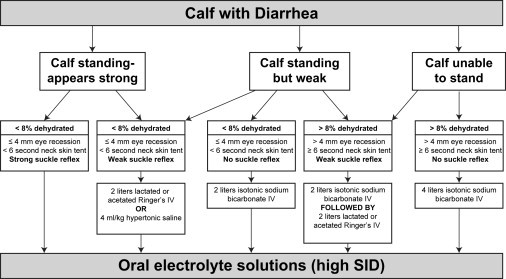
Algorithm for initial fluid therapy of dehydrated calves with diarrhea.
Auricular vein catheterization in calves
The focus of this section is on catheterization of the auricular vein in calves. Catheterization of the jugular vein is still widely used for fluid therapy in calves and adult cattle.35, 78 Other routes for administration of fluids in cattle have been presented but are not considered in this article. Schmid and Rüsse89 were the first to describe the technique of auricular vein catheterization in neonatal calves using a small flexible catheter, and the method quickly became popular among practitioners in Germany and other countries.90, 91, 92 Even in severely dehydrated calves, ear vein catheterization is possible, and a surgical cutdown of the jugular vein (which is often performed in severely dehydrated calves) can be avoided. Ear catheters allow the administration of adequate volumes of fluids by continuous drip infusion to calves.73, 91, 93, 94 Despite the lack of studies comparing the use of ear vein catheters with other approaches in calves, this technique is believed to result in fewer complications compared with jugular catheters.95 In pigs, long-term ear vein catheterization for 7 to 14 days was superior to jugular catheterization.96 In this study, jugular vein catheters occluded more often and caused thrombophlebitis, whereas ear catheters showed no reactions. A study that evaluated the use of auricular vein catheters in adult cattle stated that this technique is easy to perform, safe, and less expensive because fewer catheters need to be discarded.91
The ear vein approach initially should be practiced in calves with minimal or no dehydration before trying to catheterize severely dehydrated calves. Initially, it may be difficult to place a catheter into the ear of a severely dehydrated calf with a collapsed and small auricular vein. Blocking the vein with a tourniquet becomes essential for proper placement of the catheter. Small rubber bands are recommended and usually fit well around the base of the ear. To achieve better distention and visualization of the ear vein, gauze soaked with warm water can be applied to the ear for a short period of time to increase regional blood flow. The catheterization site needs to be shaved or clipped and prepared aseptically with iodine or alcohol before catheter insertion. The alcohol or iodine solution also acts as a lubricant and aids the advancement of the catheter into the ear. Lidocaine anesthesia of the venipuncture site is not necessary and is not recommended for ear vein catheterization. Subcutaneous administration of lidocaine creates a fluid bubble, which makes the vein difficult to visualize. Mild sedation with xylazine is occasionally necessary in vigorous calves that do not have any CNS depression; however, xylazine is not needed and should be avoided in severely dehydrated calves with hypovolemic shock because it further decreases blood pressure and causes the ear vein to collapse.
Current recommendations for ear vein catheterization in neonatal calves are to use a 22 gauge, 1-in (0.9 × 25 mm) over-the-needle catheter (ie, Vasocan Braunüle, B. Braun Melsungen AG, Melsungen, Germany) with a butterfly-shaped wing. Other suitable catheters for ear vein catheterization should incorporate a guide wire needle to facilitate easier placement. Older calves and adult cattle require larger gauge sizes for increased flow rates. A disadvantage of the over-the-needle catheters is that they may fray or splinter at the tip during insertion and then must be discarded. Advancing an already frayed catheter should be avoided because it damages the internal wall of the vein and increases the risk of thrombosis.97
The anatomy of the ear veins may differ slightly among calves. Normally there are one or two cranial, one medial, and one caudal ear vein that are large enough for catheterization. Identification of the ear vessels is important to avoid arterial puncture or placement of the catheter into the auricular artery. This artery is located between the cranial ear vein and the medial ear vein. It is usually more prominent than the ear veins and is visible even before applying a tourniquet. Identification of the auricular artery by palpating for a pulse is difficult; however, the artery feels harder than the veins and can be rolled under the skin. The author prefers the cranial ear vein for catheterization (Fig. 3A) followed by the medial vein. The cranial vein runs dorsally across the auricular pinna, which is flatter and more rigid than the caudal part of the ear. Catheterization should begin as far distally as possible to allow for repeated attempts more proximally if the first attempt is not successful. If the tip of the catheter lies too close to the base of the ear, the fluid flow rate may be slow.
Fig. 3.
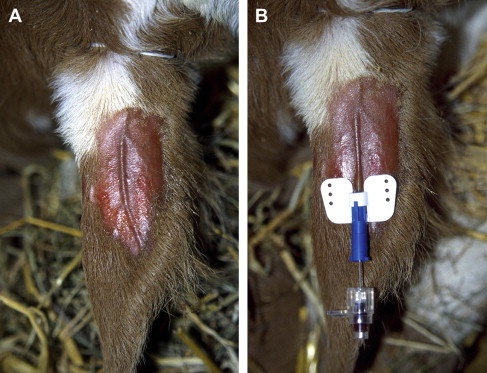
Placement of 22 gauge 1-inch (0.9 × 25 mm) catheter into cranial ear vein. (A) The vein is blocked with a rubber band at the base of the ear. The venipuncture site is shaved and sprayed with alcohol. (B) The catheter is advanced into the vein at least 0.5 in (1 cm) at once. The guide wire steel needle is then retracted slightly before the catheter is fully advanced into the ear vein. Confirm correct catheter position by checking for blood in the catheter hub and then withdraw guide wire needle completely.
Procedure for Auricular Vein Catheterization
- The following steps are used to proceed with auricular vein catheterization:
- 1.
-
2.Warm all fluids during cold weather.
-
3.Prepare four strips of tape 12 to 15 in (30–40 cm) long and attach them loose nearby.
-
4.Use right ear if right handed and left ear if left handed.
-
5.Place rubber band as a tourniquet around the base of the ear to distend veins (see Fig. 3A).
-
6.Spray the area for catheter placement, preferably the cranial third of the ear, vigorously with iodine and alcohol.
-
7.Shave dorsal pinna of the ear carefully with a single razor.
-
8.Spray the catheterization site again (or scrub with iodine solution and swab with alcohol) to achieve easier sliding of catheter.
-
9.Identify a suitable ear vein after allowing some time for the vessels to distend. A straight and 2-cm long vein is suitable (see Fig. 3A).
-
10.If the vein does not properly distend in severely dehydrated or hypothermic calves, apply a sponge or gauze soaked with warm water to increase blood flow to the ear.
-
11.Prepare the catheter by gently moving the needle of the catheter back and forth to allow easy retraction of the needle after being inserted. Hold ear straight with one hand and bend the ear a little.
-
12.Advance the tip of the catheter into the distended vein at least 0.5 in (1 cm) in one movement. Expect some head and/or ear shaking and be prepared to move with the calf.
-
13.Confirm that the catheter is in the vein by watching for blood flow into the hub of the catheter before slightly withdrawing the needle (Fig. 3B).
-
14.Advance the catheter completely into the vein and then remove the needle. Do not reinsert the needle into the catheter after withdrawing it because this may shear the catheter and potentially result in damage to the blood vessel.
-
15.Fix catheter with first tape strip to the ear and press tape firmly (Fig. 4A).
-
16.Remove tourniquet (rubber band) with razor or scissors.
-
17.Check for correct catheter placement by examining for blood flow out of the catheter hub or by flushing the catheter.
-
18.Hang the fluids, connect the fluid line (include an extension set if necessary), connect the fluid line, and open the fluids completely (again checking for correct catheter placement).
-
19.Tape the catheter with a loop of the fluid line (extension set) to the ear with a second strip of tape (Fig. 4B). If necessary, place a gauze roll of adequate size inside the ear to allow better and more forceful taping. Do not tape in front of the catheter tip.
-
20.Tape the fluid line to the base of the opposite ear with third strip of tape (Fig. 4C).
-
21.Avoid stress and manipulation in severely compromised calves. Usually calves with severe dehydration and acidosis are depressed or comatose. These cases require almost no assistance from another person to place an ear catheter. If calves are more active, however, it is the authors' preference to use the least amount of restraint possible and attempt placement of the catheter without assistance. If necessary, light sedation can be used.
Fig. 4.
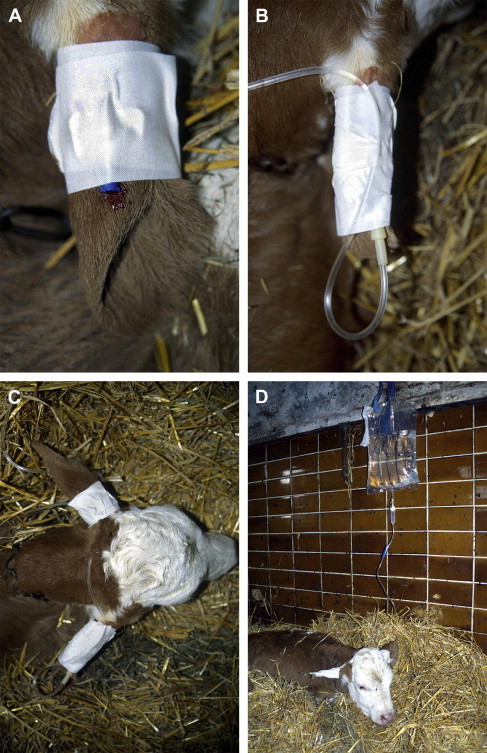
(A) Fixation of the ear vein catheter with one strip of adhesive tape after removing rubber band. Press adhesive tape firmly to the ear. (B) Fix the fluid line in a loop with second strip of adhesive tape. (C) Fix the fluid line to the opposite ear with a third strip of adhesive tape. (D) Administration of 5 L isotonic saline spiked with 250 mL 8.4% sodium bicarbonate by ear vein catheterization under field conditions.
Catheter-Related Complications
Over-the-needle catheters slide out easily when the animal moves or during manipulation of the catheter before securing it with tape.97 For secure fixation, the catheter can be sutured to the ear using an injection needle and monofilament suture material.73 Suturing the catheter to the ear is usually not necessary because most calves that require IV fluids are depressed or comatose and do not move vigorously. Attaching the catheter to the pinna with cyanoacrylate (tissue glue),92 using a gauze roll placed inside the ear, and taping the catheter with bandage material have been recommended for better catheter fixation.91 The risk of damaging catheters in ear veins attached with two strips of tape is minimal if the calf is confined. If the calf is able to move its head in and out of a gate or a hole in the hutch, however, the ear catheter may dislodge or the fluid line may become disconnected from the catheter. To avoid this complication, calves can be tethered during IV fluid therapy. The fluid line is not easily accessible for chewing or biting if the fluid bag is hanging above the calf.
Puncturing a vein damages the wall of the blood vessel and traumatizes the surrounding tissue. Bacterial contamination of the vessel often follows. Complications of IV catheterization include phlebitis and extravasation of fluids or blood with infiltration of surrounding tissue forming subcutaneous edema or hematoma.95, 97 The perivascular administration of fluids normally does not result in serious complications in calves if isotonic or mildly hypertonic solutions are administered. If the catheter is not correctly placed or is not advanced far enough into the vein, movement of the animal may cause displacement of the catheter. The signs of perivascular infusion include a slower infusion rate and swelling around the injection site. If the tip of the catheter is located close to the base of the ear, flow rates may be negatively affected.
Reports on catheter-related complications in cattle have focused primarily on problems associated with jugular vein catheterization. Thrombosis is the most frequent complication and results in thrombophlebitis, periphlebitis, and systemic infection.95, 97, 98, 99 Severe thrombophlebitis of the jugular veins in cattle is characterized by a painful and warm swelling around the jugular vein, increased rectal temperature, reduced feed intake, and abnormal behavior.99 Auricular vein catheterization is believed to result in fewer complications compared with jugular vein catheterization.91, 95 Severe infections have not been observed by the author after using the ear vein catheterization technique in hundreds of calves. Arterial puncture is a common complication of jugular catheterization, resulting in the formation of large hematomas. Puncture of the artery is also a serious complication of ear vein catheterization, although it can be avoided by identifying this vessel. If an ear artery is catheterized accidentally, the brighter color of the blood or a slow infusion rate is usually obvious. An ear vein catheter can be left in place for several days without significant complications. In the authors' experience, calves with ear catheters left in place for up to 7 days had only minor swelling with no evidence of thrombophlebitis. Most catheters can be removed by the producer after 2 days if the calf is able to suckle and maintain a normal hydration status. Changing ear catheters is not needed as often as compared with jugular catheters.91 Although it tends to be a common criticism of ear vein catheters, slow fluid flow rates are almost never a problem; however, ear catheters do tend to occlude more than jugular catheters.91
Administration of intravenous fluids
Many studies have presented various protocols for IV fluid therapy in calves; however, clinical research comparing the effectiveness of different protocols in dehydrated calves with diarrhea has not been done.80 In practice, fluid therapy has to be simple and cost effective and must be based on clinical signs that are easily assessed. Fig. 2 shows a simple flow chart with guidelines for resuscitation of dehydrated calves. The focus of this algorithm is on restoration of the suckle reflex to allow for further rehydration and maintenance fluid therapy to be given orally. European teaching hospitals and practitioners have recommended larger quantities (5–20 L) of volume-expanding and maintenance IV fluids for periods of 24 hours or longer.35, 78 To determine daily fluid requirements, estimated amounts for replacement, maintenance, and ongoing losses (for diarrhea) must be calculated. The quantity of replacement fluid in liters is calculated by multiplying the estimated dehydration in percentage with body weight in kilograms according to the following formula:
A maximum rate of 80 mL/kg/h for IV fluid administration has been used without inducing significant overhydration and hypertension.64 This rate is equivalent to a maximum fluid volume of 2.8 L/h for a 35-kg (77-lb) calf or 1 gallon (3.8 L) per hour for a 47-kg (104-lb) severely dehydrated calf. Higher flow rates are not recommended. Slower infusion rates of 30 to 50 mL/kg/h are often used to avoid overhydration and pulmonary edema. A recent study gave the first liter within 30 minutes and the subsequent dose of 3 L over the following 2.5 hours,100 which is in agreement with the slower rate of 30 to 40 mL/kg/h reported by Roussel.101 With a rate of 30 to 40 mL/kg/h, a 40-kg calf with 10% dehydration can be rehydrated within 3 to 4 hours. Daily maintenance fluid volumes of 80 to 100 mL/kg and ongoing losses of up to 7 L/d should be added to calculate the daily fluid requirements. If a calf can suckle after initial resuscitation, however, these fluid requirements can be given orally to reduce costs.
Measurements of buffer needs are based on formulas for extracellular base excess (from blood gas analysis) or plasma total carbon dioxide concentration. Values calculated from blood gas analysis multiply base deficit with body weight and with a factor that considers the volume of distribution for bicarbonate ions in the body (0.5–0.6) according to the following formula:51, 101
Values above 1.0 for calculating bicarbonate requirements have been reported—a finding that was attributed to ongoing fecal and renal losses of bicarbonate, along with ongoing production of organic acids in the gastrointestinal tract.50 Another study recommended a distribution factor of 1.0 to consider the ongoing losses that accompany diarrhea.93 Values of 1.0 or more seem much too high to be used to correct existing deficits as supported by a report from Japan, which found a much lower distribution factor of bicarbonate (0.367) in dehydrated calves with diarrhea.84 More recent research showed that calves with acidemia from D-lactic acidosis need more sodium bicarbonate to buffer the metabolic acidosis and to decrease D-lactate concentration below 3 mmol/L.43
Practitioners must rely on clinical signs and the guidelines developed by Naylor based on standing ability, suckling force, and age of calves with diarrhea to predict if alkalinizing therapy is indicated and how much isotonic sodium bicarbonate should be administered. Because determining the severity of acidosis on the farm is difficult and costly, buffer administration is commonly done without any laboratory data. The clinical response of the calf to IV fluid therapy must be monitored. Urination within 30 to 60 minutes, improvement of mental and hydration status, and, most importantly, restoration of the suckle reflex are monitored as responses to treatment.78, 102 Recumbent calves should stand within a few hours of IV fluid therapy. If the suckle reflex does not return after IV buffer therapy, other diseases, such as septicemia, omphalitis, or pneumonia, should be ruled out.103
An easy but successful guideline for the treatment of severely dehydrated calves with diarrhea and acidosis is administration of isotonic sodium bicarbonate solution at approximately 10% body weight over a period of several hours.94 This protocol had a success rate of 91% without calculating buffer requirements, fluid rates, or maintenance requirements. Ninety calves with a mean base deficit of −19.0 ± 3.8 mEq/L (pH 7.06 ± 0.22) received approximately 10% of their body weight as isotonic sodium bicarbonate solution (1.26% = 12.6 g/L instead of the more widely used 1.3%). The first half was given rapidly over 2 to 3 hours, which equals an administration rate of approximately 20 mL/kg/h. After 3 hours, when approximately 5% body weight of isotonic sodium bicarbonate had been infused, 77% of the calves were able to suckle water again. Milk replacer was continuously fed in that study; however, oral electrolytes were not given. Additional replacement therapy with 2 to 4 L of Ringer's or isotonic sodium chloride was given to 16 calves.94 Another practical technique for a 40- to 50-kg, severely dehydrated and comatose calf is to give 2 L of isotonic sodium bicarbonate rapidly and then switch fluid to an isotonic mixture of sodium chloride and sodium bicarbonate.104
A Simplified Protocol for Farm Intravenous Fluid Therapy
A simplified protocol for IV fluid therapy by ear vein catheterization in calves used in the authors' practice is presented in this section. Calves are examined for dehydration by evaluating the eyeball position within the orbit, for weakness and ability to stand by careful manipulation (not performed in comatose calves), for duration of anorexia by obtaining history and determining suckle reflex, and for hypothermia by palpating extremities and oral cavity and obtaining rectal temperature.
The standard treatment protocol for IV fluid therapy consists of a 5-L bag of isotonic saline (0.9% NaCl) to which 250 mL of 8.4% hypertonic sodium bicarbonate (total of 250 mEq HCO3 -) is added (Fig. 5 ). This mixture creates a slightly hypertonic solution and is recommended for use in calves younger than 1 week. All solutions are commercially available, and isotonic saline solution is supplied in plastic bags that are easily attachable in the calf's environment (see Fig. 4D). Calves that present with relapses after administration of this standard protocol receive another treatment when they are able to suckle. Calves that fail to significantly improve in attitude, have a weak or absent suckle reflex, or show consistent weakness after one or two administrations of the standard (5-L) IV fluid solution are generally suspected of having a more severe acidosis. These calves receive fluids that contain a higher amount of sodium bicarbonate, especially when they are older than 1 week of age. Up to 750 mL of 8.4% sodium bicarbonate (750 mEq HCO3 -) can be added to the 5-L bag of isotonic saline. Calves with ongoing relapses of dehydration or depression after repeated administrations of this standard IV protocol, with or without a higher dose of sodium bicarbonate, receive a transfusion of whole blood (800–1000 mL) through the same fluid line. All IV fluid therapy is performed by ear vein catheterization using a 22-gauge IV catheter placed under aseptic conditions. In calves with dehydration or severe depression, a maximum flow rate is preferred, which allows administration of 1 to 2 L/h. In normally hydrated calves, slower flow rates are used (0.5–0.75 L/h). Milk may be withheld for one feeding if D-lactic acidosis is suspected; however, milk feeding normally is continued.
Fig. 5.
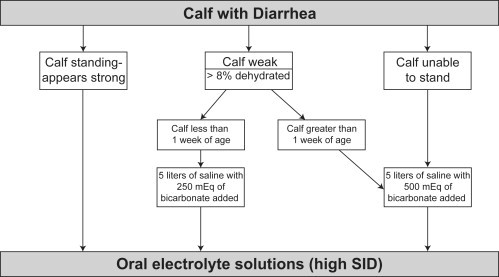
Simplified algorithm for fluid therapy of dehydrated calves. This approach requires that the practitioner carry only 5-L bags of 0.9% saline and 250-mL or 500-mL bottles of hypertonic sodium bicarbonate in practice. 8.4% sodium bicarbonate contains 1 mEq of bicarbonate per milliliter (so a 250-mL bottle equals 250 mEq of bicarbonate). 5% sodium bicarbonate contains 0.6 mEq of bicarbonate per milliliter (so a 500-mL bottle contains 298 mEq of bicarbonate).
Complications of intravenous fluid therapy
Technical Problems
Complications associated with IV catheterization were previously described. Problems in maintaining a continuous fluid line on farm for extended periods of time are primarily caused by lack of observation. Fluids may run out, which is usually followed by clotting of the catheter. Twisting or kinking of the fluid line is frequently observed if the lines are in place for several days and if the calf begins to recover and starts moving around in the stall or hutch. It is difficult to perform long-term (maintenance) IV fluid therapy without fluid line problems in an unobserved calf. When the calf begins moving freely in its stall, the infusion line can be coiled and is often occluded after bending. Complications with maintaining a permanent fluid line can be reduced by using coiled infusion sets, self-retractable dog leashes, or elastic bands92 to which the infusion line is taped. Because of the difficulty associated maintaining a fluid line for long-term fluid therapy, maintenance fluid therapy in calves is best performed orally.
Hypothermia
Hypothermia is often a significant pre-existing problem in newborn calves without a suckle reflex during the first 24 hours of life and in calves with diarrhea and dehydration.37 Particularly during periods of cold weather, administration of larger fluid volumes can exacerbate the hypothermia. IV therapy during cold weather conditions with large fluid volumes is almost impossible without creating or exacerbating hypothermia. Calves that receive IV fluids should be moved inside a building where external heating can be provided efficiently by heat lamps or some other type of heat source. The fluid line may be wrapped or looped around a heat lamp or passed through the metal cage of the heat lamp. In freezing weather conditions, however, it is almost impossible to avoid creating some degree of hypothermia in sick calves with or without dehydration that receive large volumes of IV fluids.
Overhydration
Overhydration may occur when fluids are administered too rapidly or with administration rates that increase intravascular pressure to an extent that pulmonary edema develops.105 Clinical signs of overhydration and pulmonary edema include nasal discharge, tachypnea, tachycardia, coughing, and wet lung sounds (crackles).95 If the central venous pressure exceeds 12 cm of water (measured at the level of the scapulohumeral joint in calves), the vascular pressure is too high and the infusion rate should be reduced or stopped temporarily.64 In a study that used a high flow rate (80 mL/kg/h), central venous pressure increased in almost one third of dehydrated calves but signs of pulmonary edema were not detected.64 In practice, it is not possible to determine an accurate body weight. An infusion rate slower than 80 mL/kg/h is preferred to avoid overhydration and development of pulmonary, interstitial, or cerebral edema. Anemia and hypoproteinemia also may follow overhydration, leading to hypoxia if the packed cell volume falls below 15%. Formation of interstitial edema occurs if total protein concentrations drop below 4 mg/dL.95 To the authors' knowledge, these complications have not been reported in the literature of calves and may be overlooked because of lack of observation on the farm. It seems unlikely that severe overhydration of calves would occur with flow rates that can be accomplished through a small (22-gauge) ear vein catheter, however.
Other Potential Adverse Effects of Intravenous Fluid Administration
The ideal composition of IV fluids for the treatment of calves with diarrhea and dehydration is unknown, and no studies have compared the effectiveness of different IV fluid therapy protocols on long-term survival of calves with diarrhea. Potential adverse effects of IV fluid administration on serum concentration of electrolytes, enzymatic activities, and voluntary intake of milk or oral electrolytes have not been investigated extensively. One study reported that the administration of isotonic sodium bicarbonate in calves with diarrhea was followed by a decrease in concentrations of potassium, magnesium, total calcium, and ionized calcium.22 The clinical significance of these findings is unknown, and the authors did not recommend any treatments other than the administration of sodium bicarbonate or calcium.22 In newborn calves with a mixed respiratory and metabolic acidosis (birth asphyxia), the administration of a hypertonic 5% sodium bicarbonate solution was followed by a decrease in total calcium concentration; however, concentrations of potassium, magnesium, and inorganic phosphorus were not affected 120 minutes after treatment.57 A study on the long-term effects of neonatal diarrhea and its treatment with IV sodium bicarbonate, glucose, and electrolyte solutions followed by oral electrolytes found significantly lower serum concentrations of sodium, magnesium, and total calcium up to 10 days after the last treatment was administered compared with healthy control calves.30 Base excess values of treated calves were significantly lower than those found in healthy control calves, and potassium concentrations were lower, although not statistically significant.
References
- 1.United States Department of Agriculture . National Animal Health Monitoring System; Fort Collins (CO): 2002. Part II: changes in the United States dairy industry, 1991–2002. #N388.0603. [Google Scholar]
- 2.Svensson C., Linder A., Olsson S.O. Mortality in Swedish dairy calves and replacement heifers. J Dairy Sci. 2006;89:4769–4777. doi: 10.3168/jds.S0022-0302(06)72526-7. [DOI] [PubMed] [Google Scholar]
- 3.Haschek B., Klein D., Benetka V. Detection of bovine torovirus in neonatal calf diarrhoea in lower Austria and Styria (Austria) J Vet Med B Infect Dis Vet Public Health. 2006;53:160–165. doi: 10.1111/j.1439-0450.2006.00936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Constable P.D. Antimicrobial use in the treatment of calf diarrhea. J Vet Intern Med. 2004;18:8–17. doi: 10.1111/j.1939-1676.2004.tb00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naylor JM, Zello GA, Abeysekara S. Advances in oral and intravenous fluid therapy of calves with gastrointestinal disease. In: Proceedings of the 24th World Buiatrics Congress. Nice, France; 2006. p. 139–50.
- 6.Ewaschuk J.B., Naylor J.M., Zello G.A. Anion gap correlates with serum D- and DL-lactate concentration in diarrheic neonatal calves. J Vet Intern Med. 2003;17:940–942. doi: 10.1111/j.1939-1676.2003.tb02539.x. [DOI] [PubMed] [Google Scholar]
- 7.Ewaschuk J.B., Naylor J.M., Palmer R. D-Lactate production and excretion in diarrheic calves. J Vet Intern Med. 2004;18:744–747. doi: 10.1892/0891-6640(2004)18<744:dpaeid>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 8.Ewaschuk J.B., Naylor J.M., Zello G.A. D-Lactate in human and ruminant metabolism. J Nutr. 2005;135:1619–1625. doi: 10.1093/jn/135.7.1619. [DOI] [PubMed] [Google Scholar]
- 9.Lorenz I. Investigations on the influence of serum D-lactate levels on clinical signs in calves with metabolic acidosis. Vet J. 2004;168:323–327. doi: 10.1016/j.tvjl.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 10.Lorenz I. Influence of D-lactate on metabolic acidosis and on prognosis in neonatal calves with diarrhoea. J Vet Med A Physiol Pathol Clin Med. 2004;51:425–428. doi: 10.1111/j.1439-0442.2004.00662.x. [DOI] [PubMed] [Google Scholar]
- 11.Lorenz I. D-Lactic acidosis in calves. Vet J, in press; corrected proof available online doi:10.1016/j.tvjl.2007.08.028.
- 12.Navetat H., Biron P., Contrepois M. Paralysing gastroenteritis: disease or syndrome? Bull Acad Vét Fr. 1997;70:327–336. [Google Scholar]
- 13.Omole O.O., Nappert G., Naylor J.M. Both L- and D-lactate contribute to metabolic acidosis in diarrheic calves. J Nutr. 2001;131:2128–2131. doi: 10.1093/jn/131.8.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schelcher F, Marcillaud S, Braun JP, et al. Metabolic acidosis without dehydration and no or minimal diarrhoea in suckler calves is caused by hyper-D-lactatemia. In: Proceedings of the 20th World Buiatrics Congress. Sydney, Australia; 1998. p. 371–4.
- 15.Gentile A., Sconza S., Lorenz I. D-Lactic acidosis in calves as a consequence of experimentally induced ruminal acidosis. J Vet Med A Physiol Pathol Clin Med. 2004;51:64–70. doi: 10.1111/j.1439-0442.2004.00600.x. [DOI] [PubMed] [Google Scholar]
- 16.Bleul U., Schwantag S., Stocker H. Floppy kid syndrome caused by D-lactic acidosis in goat kids. J Vet Intern Med. 2006;20:1003–1008. doi: 10.1892/0891-6640(2006)20[1003:fkscbd]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 17.Lorenz I., Gentile A., Klee W. Investigations of D-lactate metabolism and the clinical signs of D-lactataemia in calves. Vet Rec. 2005;156:412–415. doi: 10.1136/vr.156.13.412. [DOI] [PubMed] [Google Scholar]
- 18.Abeysekara S., Naylor J.M., Wassef A.W.A. D-Lactic acid-induced neurotoxicity in a calf model. Am J Physiol. 2007;293:E558–E565. doi: 10.1152/ajpendo.00063.2007. [DOI] [PubMed] [Google Scholar]
- 19.Hartmann H., Finsterbusch L., Lesche R. Fluid balance of calves. II. Fluid volume in relation to age and the influence of diarrhoea. Arch Exp Vet Med. 1984;38:913–922. [PubMed] [Google Scholar]
- 20.Doll K., Weirather P., Küchle H.M. Calf diarrhoea as a herd problem: effects of husbandry and management and frequent errors in treatment. Prakt Tierarzt. 1995;76:995–1004. [Google Scholar]
- 21.Hartmann H., Reder S. Effect of dehydration on functional indicators of fluid metabolism in calves and the efficacy of rehydration with crystalline or colloidal saline infusions. Tierärztl Prax. 1995;23:342–350. [PubMed] [Google Scholar]
- 22.Grove-White D., Michell A.R. Iatrogenic hypocalcemia during parenteral fluid therapy of diarrhoeic calves. Vet Rec. 2001;149:203–207. doi: 10.1136/vr.149.7.203. [DOI] [PubMed] [Google Scholar]
- 23.Doll K. Studies on the secretory process and osmotic mechanism in the pathogenesis of neonatal diarrhoea in calves. In: Proceedings of the 18th World Buiatrics Congress. Bologna, Italy; 1994. p. 411–4.
- 24.Grove-White D.H., White D.G. Diagnosis and treatment of metabolic acidosis in calves: a field study. Vet Rec. 1993;133:499–501. doi: 10.1136/vr.133.20.499. [DOI] [PubMed] [Google Scholar]
- 25.Berchtold J. Untersuchungen zur Diagnose und Behandlung systemischer Azidosen bei Kälbern [thesis]. [Investigations on the diagnosis and treatment of systemic acidosis in calves]. Berlin: Free University of Berlin; 1998 [in German].
- 26.Abutarbush S.M., Petrie L. Treatment of hypernatremia in neonatal calves with diarrhea. Can Vet J. 2007;48:184–187. [PMC free article] [PubMed] [Google Scholar]
- 27.Maach L., Gründer H.D., Boujija A. Clinical and haematological investigations in newborn Holstein-Friesian calves with diarrhoea in Morocco. Dtsch Tierärztl Wochenschr. 1992;99:133–140. [PubMed] [Google Scholar]
- 28.Constable P.D., Walker P.G., Morin D.E. Clinical and laboratory assessment of hydration status of neonatal calves with diarrhea. J Am Vet Med Assoc. 1998;212:991–996. [PubMed] [Google Scholar]
- 29.Constable P.D., Stämpfli H.R., Navetat H. Use of a quantitative strong ion approach to determine the mechanism for acid-base abnormalities in sick calves with or without diarrhea. J Vet Intern Med. 2005;19:581–589. doi: 10.1892/0891-6640(2005)19[581:uoaqsi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 30.Bostedt H., Hermühlheim H., Bleul U. Studies on the convalescent phase of calves after neonatal diarrhoea. Prakt Tierarzt. 2000;81:301–312. [Google Scholar]
- 31.Fayet J.C. Plasma and fecal osmolality, water kinetics, and body fluid compartments in neonatal calves with diarrhoea. Br Vet J. 1971;127:37–44. doi: 10.1016/s0007-1935(17)37787-4. [DOI] [PubMed] [Google Scholar]
- 32.Phillips R.W., Lewis L.D., Knox K.L. Alterations in body water turnover and distribution in neonatal calves with acute diarrhea. Ann N Y Acad Sci. 1971;176:321–343. [Google Scholar]
- 33.Phillips R.W., Lewis L.D. Viral induced changes in intestinal transport and resultant body fluid alterations in neonatal calves. Ann Rech Vet. 1973;4:87–98. [Google Scholar]
- 34.Hartmann H., Meyer H., Steinbach G. Influence of diarrhoea on electrolyte content and osmolarity of the blood of calves. Monatsh Veterinarmed. 1983;38:292–296. [Google Scholar]
- 35.Rademacher G., Lorenz I., Klee W. Feeding and treatment of calves with neonatal diarrhoea. Tierärztl Umsch. 2002;57:177–189. [Google Scholar]
- 36.Naylor J.M. A retrospective study of the relationship between clinical signs and severity of acidosis in diarrheic calves. Can Vet J. 1989;30:577–580. [PMC free article] [PubMed] [Google Scholar]
- 37.Cambier C., Clerbaux T., Detry B. Effects of intravenous infusions of sodium bicarbonate on blood oxygen binding in calves with diarrhoea. Vet Rec. 2005;156:706–710. doi: 10.1136/vr.156.22.706. [DOI] [PubMed] [Google Scholar]
- 38.Gökce G., Gökce H.I., Erdogan H.M. Investigation of the coagulation profile in calves with neonatal diarrhea. Turk J Vet Anim Sci. 2006;30:223–227. [Google Scholar]
- 39.Berchtold J., Prechtl J. Orale und parenterale Flüssigkeitstherapie – Das Kalb als Intensivpatient in der Praxis. Nutztierpraxis Aktuell. 2002;2:12–15. [Google Scholar]
- 40.Berchtold J., Prechtl J. Technik der Ohrvenen-Infusion beim Kalb. Fachpraxis – Zeitschrift für die Tierarztpraxis. 2003;(27):5–8. [Google Scholar]
- 41.Prechtl J, Berchtold J, Brunner B. Simplified intravenous (IV) fluid therapy of calves in practice [abstract 538]. In: Proceedings of the 22nd World Buiatrics Congress. Hannover, Germany; 2002. p. 171–2.
- 42.Dunlop R.H., Hammond P.B. D-lactic acidosis of ruminants. Ann N Y Acad Sci. 1965;119:1109–1132. doi: 10.1111/j.1749-6632.1965.tb47466.x. [DOI] [PubMed] [Google Scholar]
- 43.Lorenz I., Vogt S. Investigations on the association of D-lactate blood concentration with the outcome of therapy of acidosis, and with posture and demeanor in young calves with diarrhoea. J Vet Med A. 2006;53:490–494. doi: 10.1111/j.1439-0442.2006.00863.x. [DOI] [PubMed] [Google Scholar]
- 44.Grude T. Laktat in Blut, Harn und Pansensaft von Kälbern, insbesondere bei “Pansentrinkern” [Lactate levels in blood, urine, and rumen liquor in calves, with special reference to “ruminal drinkers”] [thesis]. Munich: University of Munich; 1999 [in German].
- 45.Ewaschuk J.B., Zello G.A., Naylor J.M. Metabolic acidosis: separation methods and biological relevance of organic acids and lactic acid enantiomers. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;781:39–56. doi: 10.1016/s1570-0232(02)00500-7. [DOI] [PubMed] [Google Scholar]
- 46.Tennant B., Harrold D., Reina-Guerra M. Physiologic and metabolic factors in the pathogenesis of neonatal infections in calves. J Am Vet Med Assoc. 1972;161:993–1007. [PubMed] [Google Scholar]
- 47.Groutides C., Michell A.R. Changes in plasma composition in calves surviving or dying from diarrhea. Br Vet J. 1990;146:205–210. doi: 10.1016/s0007-1935(11)80003-5. [DOI] [PubMed] [Google Scholar]
- 48.Hartmann H., Berchtold J., Hofmann W. Pathophysiological aspects of acidosis in diarrhoeic calves. Tierärztl Umsch. 1997;52:568–574. [Google Scholar]
- 49.Stocker H., Lutz H., Kaufmann C. Acid-base disorders in milk-fed calves with chronic indigestion. Vet Rec. 1999;145:340–346. doi: 10.1136/vr.145.12.340. [DOI] [PubMed] [Google Scholar]
- 50.Grove-White D.H. Pathophysiology and treatment of metabolic acidosis in the diarrhoeic calf. Bovine Practitioner. 1997;31:56–60. [Google Scholar]
- 51.Naylor J.M. Severity and nature of acidosis in diarrheic calves over and under one week of age. Can Vet J. 1987;28:168–173. [PMC free article] [PubMed] [Google Scholar]
- 52.Kasari T.R., Naylor J.M. Further studies on the clinical features and clinicopathological findings of a syndrome of metabolic acidosis with minimal or no dehydratation in neonatal calves. Can J Vet Res. 1986;50:502–508. [PMC free article] [PubMed] [Google Scholar]
- 53.Walker P.G., Constable P.D., Morin D.E. A reliable, practical, and economical protocol for inducing diarrhea and severe dehydration in the neonatal calf. Can J Vet Res. 1998;62:205–213. [PMC free article] [PubMed] [Google Scholar]
- 54.Zello G.A., Janzen A., Abeysekara S. Urinary excretion of both D- and L-lactate using a calf-infusion model. FASEB J. 2008;22(1205):5. [abstract] [Google Scholar]
- 55.Berchtold J.F., Constable P.D., Smith G.W. Effects of intravenous hyperosmotic sodium bicarbonate on arterial and cerebrospinal fluid acid-base status and cardiovascular function in calves with experimentally induced respiratory and strong ion acidosis. J Vet Intern Med. 2005;19:240–251. doi: 10.1892/0891-6640(2005)19<240:eoihsb>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 56.Bleul U., Bachofner C., Stocker H. Comparison of sodium bicarbonate and carbicarb for the treatment of metabolic acidosis in newborn calves. Vet Rec. 2005;156:202–206. doi: 10.1136/vr.156.7.202. [DOI] [PubMed] [Google Scholar]
- 57.Bleul U.T., Schwantag S.C., Kähn W.K. Effects of hypertonic sodium bicarbonate solution on electrolyte concentrations and enzyme activities in newborn calves with respiratory and metabolic acidosis. Am J Vet Res. 2007;68:850–857. doi: 10.2460/ajvr.68.8.850. [DOI] [PubMed] [Google Scholar]
- 58.Fecteau G., Paré J., Van Metre D.C. Use of a clinical sepsis score for predicting bacteremia in neonatal dairy calves on a calf rearing farm. Can Vet J. 1997;38:101–104. [PMC free article] [PubMed] [Google Scholar]
- 59.Lofstedt J., Dohoo I.R., Duizer G. Model to predict septicemia in diarrheic calves. J Vet Intern Med. 1999;13:81–88. doi: 10.1111/j.1939-1676.1999.tb01134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Constable P.D., Walker P.G., Morin D.E. Use of peripheral temperature and core-temperature difference to predict cardiac output in dehydrated calves housed in a thermoneutral environment. Am J Vet Res. 1998;59:874–880. [PubMed] [Google Scholar]
- 61.Nappert G., Naylor J.M. A comparison of pH determination methods in food animal practice. Can Vet J. 2001;42:364–367. [PMC free article] [PubMed] [Google Scholar]
- 62.Rollin F. Tools for a prompt cowside diagnosis: what can be implemented by the bovine practitioner? In: Proceedings of the 24th World Buiatrics Congress. Nice, France; 2006. p. 89–99.
- 63.Lorenz I., Hartmann I., Gentile A. Determination of D-lactate in calf serum samples: an automated assay. Comp Clin Path. 2003;12:169–171. [Google Scholar]
- 64.Kasari T.R., Naylor J.M. Clinical evaluation of sodium bicarbonate, sodium L-lactate, and sodium acetate for the treatment of acidosis in diarrheic calves. J Am Vet Med Assoc. 1985;187:392–397. [PubMed] [Google Scholar]
- 65.Geishauser T., Thünker B. Metabolic acidosis in diarrhoeic neonatal calves: estimation using suckling reflex and standing ability. Prakt Tierarzt. 1997;78:600–605. [Google Scholar]
- 66.Grove-White D., Michell A.R. Comparison of the measurement of total carbon dioxide and strong ion difference for the evaluation of metabolic acidosis in diarrhoeic calves. Vet Rec. 2001;148:365–370. doi: 10.1136/vr.148.12.365. [DOI] [PubMed] [Google Scholar]
- 67.Koch A., Kaske M. Clinical efficacy of intravenous hypertonic saline solution or hypertonic bicarbonate solution in the treatment of inappetent calves with neonatal diarrhea. J Vet Intern Med. 2008;22:202–211. doi: 10.1111/j.1939-1676.2007.0029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Naylor J.M. Neonatal ruminant diarrhea. In: Smith B.P., editor. Large animal internal medicine. 4th edition. Elsevier; St. Louis: 2008. p. 356. [Google Scholar]
- 69.Geishauser T., Thünker B. Metabolic acidosis in diarrhoeic calves: treatment using isomolar sodium bicarbonate. Prakt Tierarzt. 1997;78:595–600. [Google Scholar]
- 70.Kasari T.R., Naylor J.M. Metabolic acidosis without clinical signs of dehydration in young calves. Can Vet J. 1984;25:394–399. [PMC free article] [PubMed] [Google Scholar]
- 71.Gentile A., Lorenz I., Sconza S. Experimentally induced systemic hyperchloremic acidosis in calves. J Vet Intern Med. 2008;22:190–195. doi: 10.1111/j.1939-1676.2008.0028.x. [DOI] [PubMed] [Google Scholar]
- 72.Wendel H., Sobotka R., Rademacher G. Studies on the correlation between blood acidosis and clinical signs in calves with neonatal diarrhoea. Tierärztl Umsch. 2001;56:351–356. [Google Scholar]
- 73.Berchtold J. Intravenous fluid therapy of calves. Vet Clin North Am Food Anim Pract. 1999;15:505–531. doi: 10.1016/S0749-0720(15)30161-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Constable P.D., Gohar H.M., Morin D.E. Use of hypertonic saline-dextran solution to resuscitate hypovolemic calves with diarrhea. Am J Vet Res. 1996;57:97–104. [PubMed] [Google Scholar]
- 75.Ewaschuk J.B., Zello G.A., Naylor J.M. Lactobacillus GG does not affect D-lactic acidosis in diarrheic calves in a clinical setting. J Vet Intern Med. 2006;20:614–619. doi: 10.1111/j.1939-1676.2006.tb02905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gareis A. Die Bedeutung von Glukosezusatz zur Infusionslösung bei Kälbern mit Neugeborenendiarrhoe [Significance of glucose additions to infusions in calves with neonatal diarrhea] [thesis]. Munich: University of Munich; 2003 [in German].
- 77.Grünberg W., Morin D.E., Drackley J.K. Effect of continuous intravenous administration of a 50% dextrose solution on phosphorus homeostasis in dairy cows. J Am Vet Med Assoc. 2006;229:413–420. doi: 10.2460/javma.229.3.413. [DOI] [PubMed] [Google Scholar]
- 78.Grove-White D. Practical intravenous fluid therapy in the diarrhoeic calf. In Pract. 2007;29:404–408. [Google Scholar]
- 79.Corke M.J. Economical preparation of fluids for intravenous use in cattle practice. Vet Rec. 1988;122:305–307. doi: 10.1136/vr.122.13.305. [DOI] [PubMed] [Google Scholar]
- 80.Constable P.D. Fluid and electrolyte therapy in ruminants. Vet Clin North Am Food Anim Pract. 2003;19:557–597. doi: 10.1016/s0749-0720(03)00054-9. [DOI] [PubMed] [Google Scholar]
- 81.Groutides C., Michell A.R. Intravenous solutions for fluid therapy in calf diarrhoea. Res Vet Sci. 1990;49:292–297. [PubMed] [Google Scholar]
- 82.Roussel A.R., Cohen N.D., Holland P.S. Alterations in acid-base balance and serum electrolyte concentrations in cattle: 632 cases (1984–1994) J Am Vet Med Assoc. 1998;212:1769–1775. [PubMed] [Google Scholar]
- 83.Iwabuchi S., Suzuki K., Abe I. Comparison of the effects of isotonic and hypertonic sodium bicarbonate solutions on acidemic calves experimentally induced by ammonium chloride administration. J Vet Med Sci. 2003;65:1369–1371. doi: 10.1292/jvms.65.1369. [DOI] [PubMed] [Google Scholar]
- 84.Suzuki K., Abe I., Iwabuchi S. Evaluation of isotonic sodium bicarbonate solution for alkalizing effects in conscious calves. J Vet Med Sci. 2002;64:699–703. doi: 10.1292/jvms.64.699. [DOI] [PubMed] [Google Scholar]
- 85.Suzuki K., Kato T., Tsunoda G. Effect of intravenous infusion of isotonic sodium bicarbonate solution on acidemic calves with diarrhea. J Vet Med Sci. 2002;64:1173–1175. doi: 10.1292/jvms.64.1173. [DOI] [PubMed] [Google Scholar]
- 86.Zello G.A., Abeysekara S., Lohmann K.L. Bicarbonate treatment of acidosis produces paradoxical acidosis in the brain. FASEB J. 2007;21:838.4. [abstract] [Google Scholar]
- 87.Naylor J.M., Forsyth G.W. The alkalinizing effects of metabolizable bases in the healthy calf. Can J Vet Res. 1986;50:509–516. [PMC free article] [PubMed] [Google Scholar]
- 88.Walker P.G., Constable P.D., Morin D.E. Comparison of hypertonic saline-dextran solution and lactated Ringer's solution for resuscitating severely dehydrated calves with diarrhea. J Am Vet Med Assoc. 1998;213:113–121. [PubMed] [Google Scholar]
- 89.Schmid G., Rüsse M. Technique for the continuous infusion into an ear vein of the newborn calf. Berl Münch Tierärztl Wochenschr. 1983;96:189–191. [PubMed] [Google Scholar]
- 90.Glawischnig E., Gerber N., Schlerka G. Continuous drip infusion for calves with severe acidosis. Tierärztl Umsch. 1990;45:562–569. [Google Scholar]
- 91.Roussel A.J., Taliofera L., Navarre C.B. Catheterization of the auricular vein in cattle: 68 cases (1991–1994) J Am Vet Med Assoc. 1996;208:905–907. [PubMed] [Google Scholar]
- 92.Garcia J.P. A practitioner's views on fluid therapy in calves. Vet Clin North Am Food Anim Pract. 1999;15:533–543. doi: 10.1016/s0749-0720(15)30162-6. [DOI] [PubMed] [Google Scholar]
- 93.Geishauser T. Intravenöse Dauertropfinfusion zur Durchfallbehandlung beim Kalb. Prakt Tierarzt. 1992;78:595–600. [Google Scholar]
- 94.Kümper H. Kälberdurchfall mit schwerer Allgemeinstörung: Therapiemöglichkeiten unter Praxisbedingungen. In: Proceedings (Large Animal) BpT-Kongress. Münster, Germany; 1997. p. 27–9.
- 95.Hartmann H. Technik, Überwachung und Komplikationen der Infusionstherapie. In: Hartmann H., Staufenbiel R., editors. Flüssigkeitstherapie bei Tieren. Gustav Fischer Verlag; Jena–Stuttgart (Germany): 1995. pp. 112–159. [Google Scholar]
- 96.van Leengoed L.A., de Vrey P., Verheijden J.H.M. Intravenous catheterization in pigs: an evaluation of two methods. Zentralbl Veterinarmed A. 1987;34:549–556. doi: 10.1111/j.1439-0442.1987.tb00327.x. [DOI] [PubMed] [Google Scholar]
- 97.Hansen B.D. Technical aspects of fluid therapy. In: DiBartola S.P., editor. Fluid, electrolyte, and acid-base disorders in small animal practice. 3rd edition. Saunders Elsevier; St. Louis (MO): 2006. pp. 344–376. [Google Scholar]
- 98.Pusterla N., Braun U. Prophylaxis of intravenous catheter-related thrombophlebitis in cattle. Vet Rec. 1996;139:287–289. doi: 10.1136/vr.139.12.287. [DOI] [PubMed] [Google Scholar]
- 99.Pusterla N., Braun U. Ultrasonographic evaluation of the jugular vein of cows with catheter-related thrombophlebitis. Vet Rec. 1995;137:431–434. doi: 10.1136/vr.137.17.431. [DOI] [PubMed] [Google Scholar]
- 100.Bouda J, Medina CM, Nunez OL, et al. Microbiology and clinicopathological findings in calves with acute diarrhea and suggested intravenous fluid therapy. In: Proceedings of the 20th World Buiatrics Congress. Sydney, Australia; 1998. p. 375–9.
- 101.Roussel A.J. Principles and mechanics of fluid therapy in calves. Compend Cont Educ Pract Vet. 1983;5:S332–S336. [Google Scholar]
- 102.Roussel A.J., Kasari T.R. Using fluid and electrolyte replacement therapy to help diarrheic calves. Vet Med. 1990;85:303–304. [Google Scholar]
- 103.Rebhun W.C. Infectious diseases of the gastrointestinal tract. In: Rebhun W.C., editor. Diseases of dairy cattle. Lippincott Williams & Wilkins; Philadelphia: 1995. pp. 161–162. [Google Scholar]
- 104.Radostits O.M. Treatment and control of neonatal diarrhea in calves. J Dairy Sci. 1975;58:464–470. doi: 10.3168/jds.S0022-0302(75)84589-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rollin F. Practical parenteral fluid therapy in the bovine species. Ann Méd Vét. 1997;141:89–111. [Google Scholar]


