Graphical abstract
Keywords: Phosphonates, Isoxazolidines, Naphthalimides, Antiviral, Cytostatic
Abstract
A novel series of 5-arylcarbamoyl- and 5-arylmethyl-2-methylisoxazolidin-3-yl-3-phosphonates have been synthesized via cycloaddition of N-methyl-C-(diethoxyphosphoryl)nitrone with N-substituted naphthalimide acrylamides and N-allylnaphthalimides. All cis- and trans-isoxazolidine phosphonates obtained herein were assessed for antiviral activity against a broad range of DNA and RNA viruses. Isoxazolidines trans-9d and trans-9f exhibited the highest activity (EC50 = 8.9 μM) toward cytomegalovirus. Compounds cis- and trans-9d as well as cis- and trans-9f were found potent against HSV and Vaccinia viruses (EC50 in the 45–58 μM range), whereas isoxazolidines 10a and 10d suppressed replication of Coxsackie B4 and Punta Toro viruses (EC50 in the 45–73 μM range). Antiproliferative evaluation of all obtained isoxazolidines revealed the promising activity of cis-9b, cis-9d, trans-9d, cis-9e, trans-9e, cis-9f and trans-9f toward tested cancer cell lines with IC50 in the 1.1–19 μM range.
1. Introduction
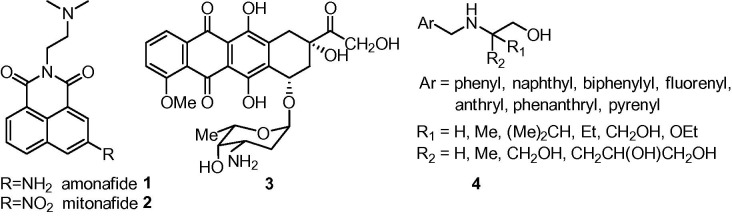
Despite significant achievements in cancer chemotherapy a search for more selective compounds with capability to better differentiate malignant tumors from normal cells and able to minimize side effects of current drugs continues. Taking into account their mechanisms of action, therapeutic agents belong to different pharmacological classes. Among them, those which interact with DNA, such as DNA alkylating agents, intercalators or groove binders, are of special interest.1, 2, 3 Intercalators constitute a well explored class of compounds because their mode of action is the most predictable and relies on inhibition of replication process by reversible binding to DNA.4, 5 The planar aromatic fragments of intercalators insert into the DNA double helix, thereby distorting the DNA backbone conformation and poison the DNA topoisomerases I or II.6 Several factors are responsible for stabilization a drug–DNA complex, namely stacking π-bond interactions, van der Waals forces and eventually hydrogen bonding between the aromatic fragment of a drug moiety and the purine/pyrimidine bases present in DNA strains. Intercalators can be classified according to their aromatic/heteroaromatic subunit or electrostatic potential they posses.2, 3 Among them, naphthalimide derivatives have been found promising as anticancer agents since they are able to intercalate into DNA and some of them, including amonafide 1 7, 8 and mitonafide 2,9 have reached clinical trials for treatment of solid tumors, however most of them showed poor therapeutic indices.
On the other hand, various functional groups present in the structure of intercalators may also contribute to the binding of a drug to DNA. The amino group of a sugar residue in doxorubicin 3 forms an ionic bond with the phosphate function of the DNA which results in additional and very efficient stabilization of locking the drug into an active site.10 There are also known groups of intercalators which activities are related to the specific shape of molecule rather than their aromatic ring system. Studies on intercalating properties of 2-[(arylmethyl)amino]-2-methyl-1,3-propanodiols (AMAPs) 4 proved that the amino side chain is responsible for DNA binding due to electrostatic interactions and enhanced activity which was observed for derivatives with 2-amino-1,3-propanediol moiety (Fig. 1 ).11, 12
Figure 1.
Structures of known intercalators.
Compounds 5 (Fig. 2 ) have been designed as new isoxazolidine-containing intercalators equipped with planar polycyclic aromatic frameworks at C3 and their cytotoxic and apoptotic properties have already been described.13, 14 Recently, we succeeded in the preparation of compounds 6 having 1- and 2-naphthyl substituents at C5 of an isoxazolidine ring and they proved cytotoxic against HeLa and K562 cell lines with IC50 values 50 and 90 μM, respectively.15 Moreover, isoxazolidines 7 containing at C5 a carbamoyl linker which separates the isoxazolidine ring and substituted phenyls suppressed divisions of three cancer cell lines at concentrations ranging from 228 to 102 μM.16
Figure 2.
Biologically active isoxazolidines.
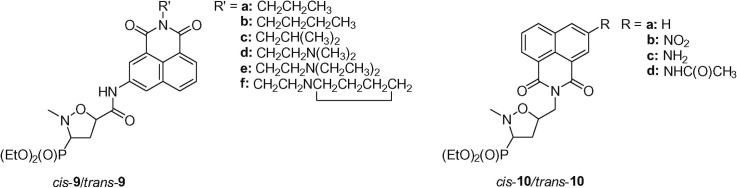
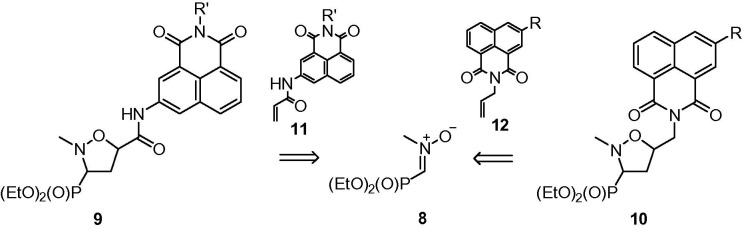
Herein, a new series of 5-arylcarbamoyl- and 5-arylmethylisoxazolidines substituted at C3 with a dialkoxyphosphoryl functionality 9 and 10 have been designed as a continuation of an ongoing project directed towards the construction of compounds with antineoplastic activity based on application of the phosphoryl nitrone 8 (Scheme 1 ).
Scheme 1.
Retrosynthesis of (isoxazolidinyl)phosphonates 9 and 10.
An idea behind designing compounds 9 and 10 is based on the combination of two biologically active moieties/pharmacophores, namely naphthalimide and (3-diethoxyphosphoryl)isoxazolidine units, with intention to obtain their active hybrids or conjugated drugs.17 Recently, similar approach was successfully applied by several research groups and resulted in synthesizing various series of amonafide-containing chimeras,18 including naphthalimides conjugated with carbazole,19 benzodiazepine,20, 21, 22 benzoic acid,23 aliphatic diamine chain24 as well as peptide nucleic acid.25 Our approach to the synthesis of phosphonylated intercalators 9 and 10 relies on the assumption that the designed molecules contain both aromatic systems able to intercalate into DNA and the diethoxyphosphoryl function at C3 of the isoxazolidine ring which could be further phosphorylated but also contains a non-hydrolysable C–P bond.26
2. Results and discussion
2.1. Chemistry
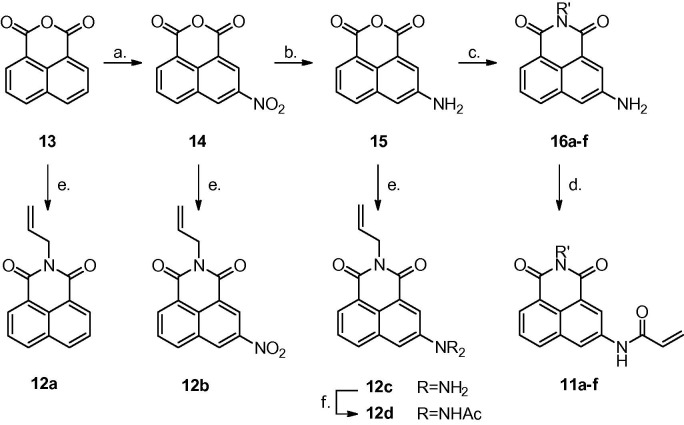
To the best of our knowledge all acrylamides 11 as well as allylated naphthalimides 12, except for 12a and 12b have not been described in the literature. For the purpose of this study, N-substituted acrylamides 11 were synthesized starting from commercially available naphthalic anhydride 13 via multistep procedures including nitration27 followed by a standard reduction of the nitro group,28 transformation of the aminoanhydride 15 into naphthalimides 16a–f by treatment with alkyl amines or ethylenediamines29 and finally preparation of acrylamides 11a–f from 16a–f and acryloyl chloride in the presence of triethylamine (Scheme 2 ). The series of dipolarophiles 12a–c were obtained in the reaction of naphthalic anhydrides 13, 14 and 15 with allylamine.29 Furthermore, naphthalimide 12c was converted into its acetyl derivative 12d.30
Scheme 2.
Reaction conditions: (a) H2SO4, HNO3; (b) SnCl2, HCl; (c) R′NH2, EtOH, see Table 1; (d) acryloyl chloride, NEt3, see Table 1; (e) allylamine, EtOH; (f) Ac2O.
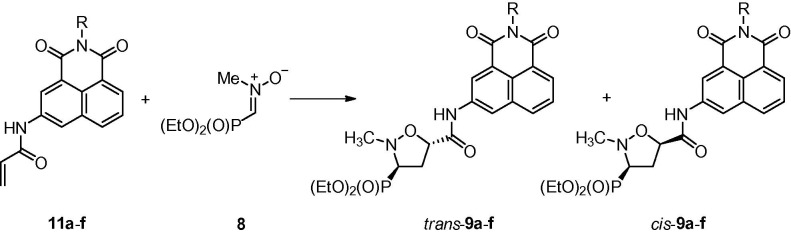
1,3-Dipolar cycloadditions of a nitrone 8 31 with naphthalimides 11a–f were performed in toluene or a toluene–chloroform mixture at 70 °C and led to the formation of diastereoisomeric mixtures of (3-diethoxyphosphoryl)isoxazolidines trans-9a–f and cis-9a–f (Scheme 3 ; Table 1 ). In all cases moderate trans/cis diastereoselectivities were observed with trans-isomers 9a–f predominating (de 56–72%). Purification of the crude mixtures of cycloadducts on silica gel columns resulted in separation of all major diastereoisomers trans-9a–f as well as all minor isomers cis-9a–f.
Scheme 3.
Reaction conditions: toluene or toluene–chloroform, 70 °C.
Table 1.
Isoxazolidines trans-9 and cis-9 obtained according to Scheme 3
| Entry | Acrylamide 11 | Ratio of trans-9/cis-9 | Yield (%) |
|---|---|---|---|
| R | |||
| A | 81:19 | trans-9a (63)a + cis-9a (11)a + trans-9a and cis-9a (11)b | |
| B | 84:16 | trans-9b (71)a + cis-9b (14)a + trans-9b and cis-9b (2)b | |
| C | 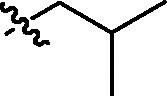 |
79:21 | trans-9c (67)a + cis-9c (19)a + trans-9c and cis-9c (5)b |
| D |  |
79:21 | trans-9d (33)a + cis-9d (8)a + trans-9d and cis-9d (52)b |
| E | 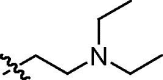 |
86:14 | trans-9e (32)a + cis-9e (11)a + trans-9e and cis-9e (54)b |
| F | 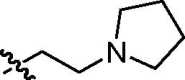 |
78:22 | trans-9f (31)a + cis-9f (8)a + trans-9f and cis-9f (50)b |
Yield of pure isomer.
Yield of pure mixture of cis- and trans-isomers.
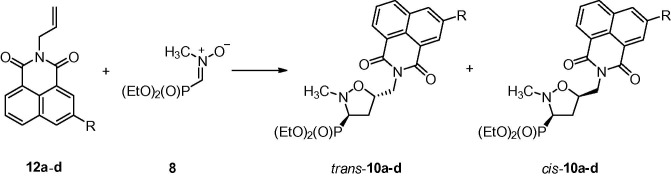
Reactions of a nitrone 8 with N-allylated naphthalimides 12a–d were carried out in toluene or toluene–chloroform solutions at 70 °C and gave mixtures of isomeric isoxazolidines trans-10a–d and cis-10a–d with good trans/cis diastereoselectivities (de 72–82%). Crude mixtures of cycloadducts were subjected to purification on silica gel columns and subsequent crystallization. However, attempts at separating diastereoisomers trans-10a–d and cis-10a–d failed leading to mixtures enriched in isomers trans-10 (Scheme 4 ; Table 2 ).
Scheme 4.
Reaction conditions: toluene or toluene–chloroform, 70 °C.
Table 2.
Isoxazolidines trans-10 and cis-10 obtained according to Scheme 4
| Entry | Imide 9 | Ratio of trans-10/cis-10 | Yielda (%) |
|---|---|---|---|
| R | |||
| A | H | 91:9 | trans-10a and cis-10a (76%; trans-10a/cis-10a = 93:7) |
| B | NO2 | 91:9 | trans-10b and cis-10b (67%; trans-10b/cis-10b = 94:6) |
| C | NH2 | 86:14 | trans-10c and cis-10c (32%; trans-10c/cis-10c = 93:7) |
| D | NHC(O)CH3 | 88:12 | trans-10d and cis-10d (73%; trans-10d/cis-10d = 93:7) |
Yield of pure mixture of cis- and trans-isomers.
The relative configurations of the isoxazolidines trans-9a–f and cis-9a–f were determined taking advantage of our previous studies on stereochemistry of cycloaddition of N-methyl-C-diethoxyphosphorylnitrone 8 with (hetero)arylacrylamides16 since similar 1H NMR spectral patterns for the respective series of isoxazolidines trans-9a–f and cis-9a–f were observed as compared to the previously described trans- and cis-5-(hetero)arylcarbamoyl-2-methylisoxazolidin-3-yl-3-phosphonates.16, 32 Briefly, for the major trans-isomers 9a–f the 3 E conformation (Fig. 3 ) of an isoxazolidine ring was established taking advantage of the diagnostic values of vicinal coupling constants [J CCCP = 7.7–8.9 Hz, J H3–H4α = 8.1–8.3 Hz, J H3–H4β = 8.5–8.9 Hz, J H4α–P = 9.4–10.3 Hz, J H4β–P = 15.7–16.1 Hz, J H4α–H5 = 5.5–5.8 Hz and J H4β–H5 = 8.7–8.9 Hz] extracted from the respective 1H and 13C NMR spectra. In this conformation the diethoxyphosphoryl group resides in the equatorial position of the isoxazolidine ring while carbamoyl substituents are located pseudoequatorially. Similarly, relative configurations of isoxazolidines trans-10a–d and cis-10a–d were established based on very close stereochemical outcomes found for the reaction between a nitrone 8 and N-allylated naphthalimides 12a–d as compared with already elaborated analogous reactions of a nitrone 8 and N-allylated nucleobases.33
Figure 3.
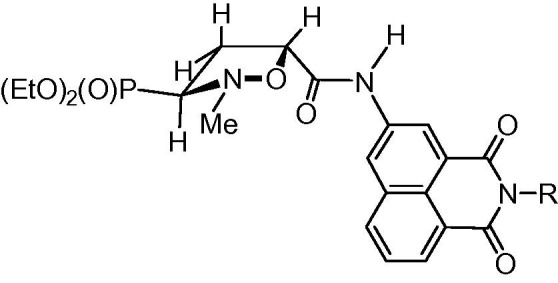
The 3E conformation of an isoxazolidine ring in trans-9a–f.
2.2. Antiviral and cytostatic evaluation
2.2.1. Antiviral activity
Pure 5-arylcarbamoyl-2-methylisoxazolidin-3-yl-3-phosphonates trans-9a–f and cis-9a–f and inseparable mixtures of 5-arylmethyl-2-methylisoxazolidin-3-yl-3-phosphonates trans-10a–d/cis-10a–d were evaluated for inhibitory activity against a wide variety of DNA and RNA viruses, using the following cell-based assays: (a) human embryonic lung (HEL) cells: herpes simplex virus-1 (KOS), herpes simplex virus-2 (G), thymidine kinase deficient (acyclovir resistant) herpes simplex virus-1 (TK− KOS ACVr), vaccinia virus, vesicular stomatitis virus and, adenovirus-2, cytomegalovirus (AD-169 strain and Davis strain), varicella-zoster virus (TK+ VZV strain and TK− VZV strain); (b) HeLa cell cultures: vesicular stomatitis virus, Coxsackie virus B4 and respiratory syncytial virus; (c) Vero cell cultures: para-influenza-3 virus, reovirus-1, Sindbis virus, Coxsackie virus B4, Punta Toro virus; (d) MDCK cell cultures: influenza A virus (H1N1 and H3N2 subtypes) and influenza B virus and (e) CrFK cell cultures: feline herpes virus (FHV) and feline corona virus (FIPV). Ganciclovir, cidofovir, acyclovir, brivudin, (S)-9-(2,3-dihydroxypropyl)adenine [(S)-DHPA], oseltamivir carboxylate, amantadine, rimantadine, ribavirin, dextran sulfate (molecular weight 5000, DS-5000), Hippeastrum hybrid agglutinin (HHA) and Urtica dioica agglutinin (UDA) were used as the reference compounds. The antiviral activity was expressed as the EC50: the compound concentration required to reduce virus plaque formation (VZV) by 50% or to reduce virus-induced cytopathogenicity by 50% (other viruses).
Some compounds of the series of isoxazolidines cis-9/trans-9 were found active against human cytomegalovirus with activities below 60 μM (Table 3 ). Among them, trans-9d (EC50 = 8.9 μM) and trans-9f (EC50 = 8.9–20 μM) exhibited the highest activity with EC50 values comparable to those found for the reference compound ganciclovir which is also an approved drug for HMCV, and approximately an order of magnitude lower than that assayed for cidofovir used as the second reference. However, the new-generation drug letermovir showed EC50 = 0.0046 ± 0.0019 μM against HCMV in AD169 strain and recently reached phase IIb of clinical trials.34
Table 3.
Cytotoxicity and antiviral activity against cytomegalovirus in HEL cell cultures
| Compound | R | Antiviral activity EC50a (μM) |
Cytotoxicity (μM) | |
|---|---|---|---|---|
| AD-169 strain | Davis strain | Cell morphology MCCb | ||
| cis-9a |  |
|||
| (exp. 1) | 45 | 45 | >100 | |
| (exp. 2) | 45 | 45 | >100 | |
| trans-9a | ||||
| (exp. 1) | >20 | 55 | >100 | |
| (exp. 2) | 49 | 45 | >100 | |
| cis-9b | ||||
| (exp. 1) | >20 | >20 | 100 | |
| (exp. 2) | >20 | >20 | 100 | |
| trans-9b | ||||
| (exp. 1) | >20 | >20 | 100 | |
| (exp. 2) | >20 | >20 | 100 | |
| cis-9c |  |
|||
| (exp. 1) | 45 | 45 | >100 | |
| (exp. 2) | 45 | 45 | >100 | |
| trans-9c | ||||
| (exp. 1) | >100 | >100 | >100 | |
| (exp. 2) | >100 | >100 | >100 | |
| cis-9d |  |
|||
| (exp. 1) | 45 | 41 | >100 | |
| (exp. 2) | 37 | 37 | >100 | |
| trans-9d | ||||
| (exp. 1) | 8.9 | 8.9 | 100 | |
| (exp. 2) | 8.9 | 8.9 | 100 | |
| cis-9e | 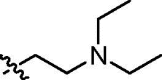 |
|||
| (exp. 1) | 100 | 100 | >100 | |
| (exp. 2) | 100 | >100 | >100 | |
| trans-9e | ||||
| (exp. 1) | 55 | 49 | >100 | |
| (exp. 2) | 55 | 63 | >100 | |
| cis-9f | 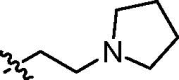 |
|||
| (exp. 1) | 45 | 45 | >100 | |
| (exp. 2) | 45 | 45 | >100 | |
| trans-9f | ||||
| (exp. 1) | 8.9 | 10.9 | 100 | |
| (exp. 2) | 10.9 | 20 | 100 | |
| Cidofovir | ||||
| (exp. 1) | 0.92 | 1.02 | >350 | |
| (exp. 2) | 0.92 | 1.14 | >350 | |
| Ganciclovir | ||||
| (exp. 1) | 7.1 | 7.1 | >350 | |
| (exp. 2) | 6.3 | 7.9 | >350 | |
Effective concentration required to reduce virus plaque formation by 50%. Virus input was 100 plaque forming units (PFU).
Minimum cytotoxic concentration that causes a microscopically detectable alternation of cell morphology.
Among the series of isoxazolidines cis-9/trans-9, majority of the derivatives was able to inhibit replication of TK+ and TK− VZV strains with EC50 values in the range of 20–45 μM (Table 4 ). The potency against TK+ VZV strain of the most active trans-9d (EC50 = 14–15 μM) was found an order or three orders of magnitude lower than that of the reference acyclovir (also the approved drug) or brivudin, respectively. However, activities of isoxazolidines studied (except trans-9c) against the TK− VZV strain compare favorably (EC50 = 20–45 μM) with that of the reference acyclovir (EC50 = 33–44 μM) being an order of magnitude less active than the second reference brivudin (EC50 = 1.0–4.9 μM).
Table 4.
Cytotoxicity and antiviral activity against varicella-zoster virus (VZV) in HEL cell cultures
| Compound | R | Antiviral activity EC50a (μM) |
Cytotoxicity (μM) | |
|---|---|---|---|---|
| TK+ VZV strain | TK− VZV strain | Cell morphology MCCb | ||
| cis-9a |  |
|||
| (exp. 1) | 20 | 38 | >100 | |
| (exp. 2) | 32 | 45 | >100 | |
| trans-9a | ||||
| (exp. 1) | 32 | 40 | >100 | |
| (exp. 2) | 28 | 41 | >100 | |
| cis-9b | ||||
| (exp. 1) | 20 | 38 | >100 | |
| (exp. 2) | 32 | 45 | >100 | |
| trans-9b | ||||
| (exp. 1) | 32 | 40 | >100 | |
| (exp. 2) | 28 | 41 | >100 | |
| cis-9c | 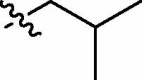 |
|||
| (exp. 1) | 45 | 45 | >100 | |
| (exp. 2) | 45 | 45 | >100 | |
| trans-9c | ||||
| (exp. 1) | >100 | >100 | >100 | |
| (exp. 2) | >100 | >100 | >100 | |
| cis-9d |  |
|||
| (exp. 1) | >20 | 20 | 100 | |
| (exp. 2) | 11 | 20 | 100 | |
| trans-9d | ||||
| (exp. 1) | 14 | 26 | >100 | |
| (exp. 2) | 15 | 34 | >100 | |
| cis-9e | 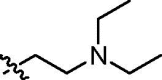 |
|||
| (exp. 1) | >20 | 36 | >100 | |
| (exp. 2) | 41 | 44 | >100 | |
| trans-9e | ||||
| (exp. 1) | 30 | 32 | 100 | |
| (exp. 2) | >20 | >20 | 100 | |
| cis-9f | 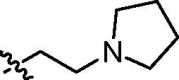 |
|||
| (exp. 1) | >20 | >20 | 100 | |
| (exp. 2) | >20 | 79 | >100 | |
| trans-9f | ||||
| (exp. 1) | >20 | >20 | 100 | |
| (exp. 2) | >20 | >20 | 100 | |
| Acyclovir | ||||
| (exp. 1) | 3.6 | 44 | >440 | |
| (exp. 2) | 1.5 | 33 | >440 | |
| Brivudin | ||||
| (exp. 1) | 0.042 | 1.0 | >300 | |
| (exp. 2) | 0.029 | 4.9 | >300 | |
Effective concentration required to reduce virus plaque formation by 50%. Virus input was 100 plaque forming units (PFU).
Minimum cytotoxic concentration that causes a microscopically detectable alternation of cell morphology.
On the other hand, none of the compounds from the series trans-10/cis-10 was found active either towards HCMV or against VZV.
Although the isoxazolidine derivatives equipped with N,N-dimethylaminoethyl (trans-9d and cis-9d) and 2-(pirolidyn-1-yl)ethyl (trans-9f) functions were found slightly active against three strains of herpes simplex virus with EC50 values ranging from 45 to 100 μM (Table 5 ) their potency seems marginal when compared with that of the four reference compounds (brivudin, cidofovir, ganciclovir and the FDA-approved acyclovir). However, activities of trans-9d, cis-9d, trans-9f and cis-9f against vaccinia virus (EC50 = 45–100 μM) are of considerable value since the reference acyclovir and ganciclovir were inactive while the two other reference compounds brivudin and cidofovir showed EC50 values of 17–22 and 22 μM (Table 5), respectively. Moreover, these compounds were not cytotoxic toward HEL cells (in which the viruses were replicated) at concentrations up to 100 μM or displayed a minimum cytotoxic concentration (MCC) of 100 μM.
Table 5.
Cytotoxicity and antiviral activity in HEL cell cultures
| Compound | R | MCCa (μM) | EC50b (μM) |
|||
|---|---|---|---|---|---|---|
| HSV-1 (KOS) | HSV-1 (TK− KOS ACVr) | HSV-2 (G) | Vaccinia virus | |||
| trans-9d | 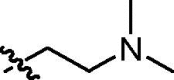 |
|||||
| (exp. 1) | ⩾100 | 45 | 45 | 45 | 45 | |
| (exp. 2) | ⩾100 | 45 | 45 | >100 | >100 | |
| cis-9d | ||||||
| (exp. 1) | >100 | 50 | 50 | 50 | 45 | |
| (exp. 2) | >100 | 58 | 58 | 58 | >100 | |
| trans-9f | 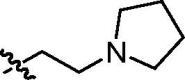 |
|||||
| (exp. 1) | ⩾100 | 45 | 45 | 45 | 45 | |
| (exp. 2) | ⩾100 | 45 | 45 | >100 | >100 | |
| cis-9f | ||||||
| (exp. 1) | >100 | >100 | >100 | 58 | 50 | |
| (exp. 2) | >100 | >100 | >100 | 100 | 58 | |
| Brivudin | ||||||
| (exp. 1) | >250 | 0.01 | 2.0 | 146 | 17 | |
| (exp. 2) | >250 | 0.05 | 10 | 250 | 22 | |
| Cidofovir | ||||||
| (exp. 1) | >250 | 1.0 | 1.2 | 1.0 | 22 | |
| (exp. 2) | >250 | 2.0 | 2.0 | 1.5 | 22 | |
| Acyclovir | ||||||
| (exp. 1) | >250 | 0.2 | 2.0 | 0.08 | >250 | |
| (exp. 2) | >250 | 0.2 | 2.0 | 0.2 | >250 | |
| Ganciclovir | ||||||
| (exp. 1) | >100 | 0.03 | 0.8 | 0.03 | >100 | |
| (exp. 2) | >100 | 0.02 | 0.5 | 0.03 | >100 | |
Minimum cytotoxic concentration that causes a microscopically detectable alternation of cell morphology.
Effective concentration that is required to reduce virus-induced cytopathogenicity by 50%.
In the second series of isoxazolidine phosphonates 10, trans-10a/cis-10a and trans-10d/cis-10d inhibited the replication of Coxsackie virus B4 at concentrations 45–73 μM which was significantly better than ribavirin (Table 6 ). On the other hand, only trans-10d/cis-10d showed a weak activity against Punta Toro virus (EC50 = 50–100 μM) which again was higher than that of ribavirin (Table 6). These compounds did not alter morphology of Vero cells where the antiviral assays were performed. Since no drugs for Coxackie and Punta Toro viruses have been approved so far, the urgency of search for active compounds is justified.
Table 6.
Cytotoxicity and antiviral activity in Vero cell cultures
| Compound | R | MCCa (μM) | EC50b (μM) |
|
|---|---|---|---|---|
| Coxsackie virus B4 | Punta Toro virus | |||
| trans-10a/cis-10a (93:7) | ||||
| (exp. 1) | H | >100 | 45 | >100 |
| (exp. 2) | >100 | 73 | >100 | |
| trans-10d/cis-10d(93:7) | ||||
| (exp. 1) | NHC(O)CH3 | >100 | 45 | 50 |
| (exp. 2) | >100 | 58 | 100 | |
| DS-10.000 (μg/ml) | ||||
| (exp. 1) | >100 | >100 | 45 | |
| (exp. 2) | >100 | >100 | 100 | |
| Ribavirin | ||||
| (exp. 1) | >250 | >250 | 112 | |
| (exp. 2) | >250 | >250 | 250 | |
Minimum cytotoxic concentration that causes a microscopically detectable alternation of cell morphology.
Effective concentration that is required to reduce virus-induced cytopathogenicity by 50%.
None of the isoxazolidines cis-9/trans-9 and trans-10 and cis-10 phosphonates showed activity against the other tested viruses, including adenovirus-2, vesicular stomatitis virus, respiratory syncytial virus, para-influenza-3 virus, reovirus-1, Sindbis virus, influenza A and B viruses, feline herpes virus, and feline corona virus.
2.2.2. Cytostatic activity
The 50% cytostatic inhibitory concentration (IC50) causing a 50% decrease in cell proliferation was determined against murine leukemia L1210, human lymphocyte CEM and human cervix carcinoma HeLa cells. The synthesized compounds showed differences in their antiproliferative activity with IC50 ranging from 1.1 to 180 μM (Table 7 ). Among isoxazolidines having a carbamoyl linker, structure–activity relationship studies indicated that the presence of a tertiary nitrogen atom in a side chain significantly increased cytostatic activity of the tested compounds. Phosphonates trans-9a–c and cis-9a–c having straight aliphatic chains were considerably less cytostatic (IC50 = 20–85 μM) than those containing tertiary amino functions, namely trans-9d–f and cis-9d–f. Moreover, compounds trans-9a and cis-9c decreased viability of the MDCK cell line at concentrations of 46.2 and 51.8 μM (CC50), respectively. Furthermore, both trans- and cis-isoxazolidines 9d, 9e and 9f having aminoalkyl substituents appeared to be the most cytostatic towards L1210, CEM and HeLa cell lines with IC50’s below 23 μM. Among them, trans-9d and cis-9d, which can be considered as amonafide analogues, as well as trans-9f and trans-9f proved highly cytostatic toward murine leukemia L1210 and human cervix carcinoma HeLa cells (IC50 = 1.1–3.3 μM) and their potency was only an order of magnitude lower than that of a reference 5-fluorouracil. Besides their high cytostatic activity, compounds trans-9d, cis-9d, trans-9f and cis-9f were found to reduce viability of MDCK and CrFK cells at concentrations in the range of 95.2–62.2 μM (CC50). In addition, phosphonates trans-9a, trans-9b, cis-9b, cis-9c, trans-9e and cis-9e exhibited high cytostatic activity against human lymphocyte cells (CEM), comparable to that of 5-fluorouracil, while trans-9d and trans-9f were even five-fold more active than 5-fluorouracil. Furthermore, although no correlation between configuration of isoxazolidines trans-9a–c/cis-9a–c and trans-9e/cis-9e and their biological activity was observed, trans-configured isoxazolidines trans-9d and trans-9f were found two-fold more cytostatic than the corresponding cis-isomers cis-9d and cis-9f.
Table 7.
Inhibitory effect of the tested compounds against the proliferation of murine leukemia (L1210), human T-lymphocyte (CEM) and human cervix carcinoma cells (HeLa)
| Compound | R | IC50a (μM) |
CC50b (μM) |
|||
|---|---|---|---|---|---|---|
| L1210 | CEM | HeLa | MDCK | CrFK | ||
| cis-9a | 76 ± 16 | 47 ± 18 | 70 ± 41 | >100 | >100 | |
| trans-9a | 41 ± 7 | 28 ± 4 | 74 ± 11 | 46.2 | >100 | |
| cis-9b | 28 ± 7 | 19 ± 1 | 25 ± 3 | >100 | >100 | |
| trans-9b | 85 ± 54 | 25 ± 7 | 78 ± 3 | >100 | >100 | |
| cis-9c | 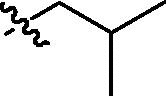 |
73 ± 27 | 26 ± 3 | 49 ± 21 | 51.8 | >100 |
| trans-9c | 72 ± 52 | 48 ± 10 | 56 ± 27 | >100 | >100 | |
| cis-9d | 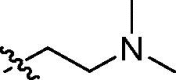 |
2.9 ± 0.2 | 6.0 ± 1.1 | 3.3 ± 0.3 | >100 | 62.2 |
| trans-9d | 1.5 ± 0.3 | 3.4 ± 0.4 | 1.8 ± 0.9 | 75.2 | 95.2 | |
| cis-9e | 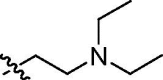 |
16 ± 4 | 23 ± 0 | 7.3 ± 2.4 | >100 | >100 |
| trans-9e | 11 ± 0 | 21 ± 1 | 13 ± 5 | >100 | >100 | |
| cis-9f | 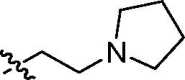 |
4.4 ± 0.1 | 12 ± 1 | 3.0 ± 0.6 | >100 | 89.1 |
| trans-9f | 1.7 ± 0.4 | 3.9 ± 0.0 | 1.1 ± 0.0 | 87.3 | >100 | |
| trans-10a/cis-10a (93:7) | H | 180 ± 47 | 111 ± 14 | 77 ± 20 | >100 | >100 |
| trans-10b/cis-10b (94:6) | NO2 | 34 ± 6 | 51 ± 6 | 20 ± 3 | >100 | >100 |
| trans-10c/cis-10c (93:7) | NH2 | 95 ± 42 | 99 ± 2 | 101 ± 58 | >100 | >100 |
| trans-10d/cis-10d (93:7) | NHC(O)CH3 | 136 ± 14 | 125 ± 4 | 111 ± 3 | >100 | >100 |
| 5-Fluorouracil | 0.33 ± 0.17 | 18 ± 5 | 0.54 ± 0.12 | — | — | |
50% inhibitory concentration or compound concentration required to inhibit tumor cell proliferation by 50%.
50% cytotoxic concentration, as determined by measuring the cell viability with the colorimetric formazan-based MTS assay.
The second series of 5-arylmethyl-2-methylisoxazolidin-3-yl-3-phosphonates trans-10/cis-10 also showed considerable cytostatic activity against all three tested cancer cell lines (IC50 from 20 to 180 μM). Isoxazolidines trans-10b/cis-10b substituted with the nitro group at C5 of a naphthalimide unit emerged as the most cytostatic with the highest potency toward HeLa cell line (IC50 = 20 μM). None of the evaluated compounds trans-10/cis-10 decreased viability of MDCK and CrFK cells at concentration up to 100 μM.
3. Conclusions
Two new series of 5-arylcarbamoyl-2-methylisoxazolidin-3-yl-3-phosphonates trans-9 and cis-9 and 5-arylmethyl-2-methylisoxazolidin-3-yl-3-phosphonates trans-10 and cis-10 have been obtained from N-methyl-C-(diethoxyphosphoryl)nitrone 8 and the respective N-substituted naphthalimide acrylamides or N-allylated naphthalimides via the 1,3-dipolar cycloaddition.
All synthesized isoxazolidine phosphonates trans-9 and cis-9 as well as the respective mixtures of trans-10/cis-10 were evaluated against a variety of DNA and RNA viruses. Several of these derivatives showed some activity against varicella-zoster virus and cytomegalovirus. Among all tested compounds, isoxazolidines trans-9d and trans-9f exhibited the highest activity (EC50 = 8.9 μM) toward cytomegalovirus, comparable to the activity of ganciclovir, the approved drug which was used as the reference compound. The isoxazolidines 9 (except trans-9c) appeared active against the TK− VZV strain and the potency of compound cis-9d (EC50 = 20 μM) compares favorably with that of the reference acyclovir (EC50 = 33–44 μM) which is also the approved drug.
Some of the tested compounds were also endowed with the antiviral activity against HSV and Vaccinia (cis- and trans-9d, cis- and trans-9f, EC50 in the 45–58 μM range), Coxsackie B4 and Punta Toro (10a and 10d, EC50 in the 45–73 μM range) viruses and although for the Coxsackie virus B4 compounds 10a and 10d are even more active than ribavirin used as the reference compound their potency is not large enough to deserve further studies.
Cytostatic activity of trans-9, cis-9 and trans-10/cis-10 was evaluated on L1210, CEM and HeLa cell lines and for the most cytostatic compounds (cis-9b, cis-9d, trans-9d, cis-9e, trans-9e, cis-9f and trans-9f), IC50 values were found in the 1.1–12 μM range. Generally, compounds with a carbamoyl linker trans-9/cis-9 were more active than trans-10/cis-10 having a methylene bridge.
4. Experimental section
1H NMR spectra were taken in CDCl3 on the following spectrometers: Varian Mercury-300 and Bruker Avance III (600 MHz) with TMS as internal standard. 13C NMR spectra were recorded for CDCl3 solution on the Varian Mercur-300 machine at 75.5 MHz, while for DMSO solution on Bruker Avance III at 151.0 MHz. 31P NMR spectra were performed in CDCl3 solution on the Varian Mercury-300 at 121.5 MHz or on Bruker Avance III at 243.0 MHz. IR spectra were measured on an Infinity MI-60 FT-IR spectrometer. Melting points were determined on Boetius apparatus and are uncorrected. Elemental analyses were performed by the Microanalytical Laboratory of this Faculty on Perkin–Elmer PE 2400 CHNS analyzer. The following adsorbents were used: column chromatography, Merck silica gel 60 (70–230 mesh); analytical TLC, Merck TLC plastic sheets silica gel 60 F254.
4.1. General procedure for the preparation of 1,8-naphthalimides 16a–f and 12a–c
A suspension of 1,8-naphthalic anhydride 13, 14 or 15 (1.00 mmol), appropriate aliphatic amine, substituted ethylenediamine or allylamine (2.00 mmol) in ethanol (10 mL) was heated under reflux for 3 h. After evaporation of a solvent under reduced pressure the crude product was purified on a silica gel column with chloroform/methanol mixtures (100:1, 50:1 v/v) to give the corresponding 1,8-naphthalimides 16a–f and 12a–c.
4.1.1. N-Propyl-3-amino-1,8-naphthalimide (16a)
Yield: 74%; yellow amorphous solid (crystallized from chloroform/hexane) mp 202–203 °C; IR (KBr, cm−1) ν max: 3473, 3368, 1688, 1646, 1580, 1341, 1308, 1220, 778, 744; 1H NMR (300 MHz, CDCl3) δ: 8.31 (dd, 1H, J = 7.3, 1.1 Hz), 8.02 (d, 1H, J = 2.3 Hz), 7.92 (dd, 1H, J = 8.3, 1.1 Hz), 7.60 (dd, 1H, J = 8.3, 7.3 Hz), 7.30 (d, 1H, J = 2.3 Hz), 4.18 (br s, 2H, NH 2), 4.13 (d, 2H, J = 7.5 Hz, CH 2CH2CH3), 1.81–1.69 (m, 2H, CH2CH 2CH3), 1.01 (d, 3H, J = 7.5 Hz, CH2CH2CH 3); 13C NMR (75.5 MHz, CDCl3) δ: 164.51 (s, C(O)), 164.28 (s, C(O)), 145.33, 133.50, 131.71, 127.54, 127.30, 123.79, 122.67, 122.62, 122.11, 114.05, 42.18 (s, CH2CH2CH3), 21.71 (s, CH2 CH2CH3), 11.85 (s, CH2CH2 CH3). Anal. Calcd for C15H14N2O2: C, 70.85; H, 5.55; N, 11.02; found: C, 70.61; H, 5.70; N, 10.77.
4.1.2. N-Isobutyl-3-amino-1,8-naphthalimide (16c)
Yield: 77%; yellow amorphous solid (crystallized from chloroform/hexane) mp 185–186 °C; IR (KBr, cm−1) ν max: 3452, 3357, 1692, 1657, 1627, 1581, 1447, 1343, 1297, 1222, 1072, 783; 1H NMR (300 MHz, CDCl3) δ: 8.33 (dd, 1H, J = 7.3, 1.0 Hz), 8.03 (d, 1H, J = 2.4 Hz), 7.93 (dd, 1H, J = 8.3, 1.0 Hz), 7.60 (dd, 1H, J = 8.3, 7.3 Hz), 7.31 (d, 1H, J = 2.4 Hz), 4.15 (br s, 2H, NH 2), 4.03 (d, 2H, J = 7.3 Hz, CH 2CH(CH3)2), 2.28–2.18 (m, 1H, CH2CH(CH3)2), 0.98 (d, 6H, J = 6.7 Hz, CH2CH(CH 3)2); 13C NMR (75.5 MHz, CDCl3) δ: 164.79 (s, C(O)), 164.57 (s, C(O)), 145.33, 133.50, 131.67, 127.61, 127.31, 123.75, 122.72, 122.59, 122.21, 114.05, 47.40 (s, CH2CH(CH3)2), 27.69 (s, CH2 CH(CH3)2), 20.62 (s, CH2CH(CH3)2). Anal. Calcd for C16H16N2O2: C, 71.62; H, 6.01; N, 10.44; found: C, 71.42; H, 5.84; N, 10.19.
4.1.3. N-(2-Propenyl)-3-amino-1,8-naphtalimide (12c)
Yield: 92%; orange amorphous solid mp 221–222 °C; IR (KBr, cm−1) ν max: 3455, 3364, 1688, 1644, 1615, 1578, 1448, 1379, 1330, 1237, 1180, 777, 744; 1H NMR (300 MHz, CDCl3) δ: 8.32 (dd, 1H, J = 7.3, 1.0 Hz), 8.03 (d, 1H, J = 2.4 Hz), 7.93 (dd, 1H, J = 8.1, 1.0 Hz), 7.60 (dd, 1H, J = 8.1, 7.3 Hz), 7.30 (d, 1H, J = 2.4 Hz), 5.99 (ddt, 1H, J = 17.0, 10.3, 5.7 Hz, CH2CH CH2), 5.31 (dq, 1H, J = 17.0, 1.3 Hz, CH2CH CH 2), 5.20 (dq, 1H, J = 10.3, 1.3 Hz, CH2CH CH 2), 4.79 (dt, 2H, J = 5.7, 1.3 Hz, CH 2CH CH2), 4.17 (br s, 2H, NH 2); 13C NMR (151.0 MHz, DMSO) δ: 163.92 (s, C(O)), 163.75 (s, C(O)), 148.35, 134.05, 133.46, 132.03, 127.39, 125.96, 122.93, 122.26, 122.13, 121.11, 116.65, 112.35, 42.08 (s, CH2CH CH2). Anal. Calcd for C15H12N2O2: C, 71.42; H, 4.79; N, 11.10; found: C, 71.14; H, 4.88; N, 10.96.
4.1.4. N-(2-Propenyl-1,3-dioxo-2,3-dihydro-1H-benzo[de]isoquinolin-5-yl)acetamide (12d)
N-(2-Propenyl)-3-amino-1,8-naphthalimide 12c (1.00 mmol) was treated with acetic anhydride (3.00 mL, 31.55 mmol) and stirred for 5 h at room temperature. The reaction mixture was cooled to room temperature and cold water (10 mL) was added. Yellow precipitate was filtered off, washed several times with water and dried to afford naphthalimide 12d. Yield: 86%; yellow amorphous solid mp 253–254 °C; IR (KBr, cm−1) ν max: 3291, 3213, 1703, 1663, 1630, 1565, 1465, 1424, 1335, 1264, 1236, 1180, 880, 783, 745; 1H NMR (300 MHz, CDCl3) δ: 8.95 (s, 1H), 8.50 (d, 1H, J = 7.1 Hz), 8.27 (d, 1H, J = 2.2 Hz), 8.18 (d, 1H, J = 8.3 Hz), 7.73 (dd, 1H, J = 8.3, 7.1 Hz), 7.69 (br s, 1H, NH), 5.98 (ddt, 1H, J = 17.1, 10.5, 5.5 Hz, CH2CH CH2), 5.31 (d, 1H, J = 17.1 Hz, CH2CH CH 2), 5.21 (d, 1H, J = 10.5 Hz, CH2CH CH 2), 4.80 (d, 2H, J = 5.5 Hz, CH 2CH CH2), 2.31 (s, 3H, CH 3); 13C NMR (151.0 MHz, DMSO) δ: 169.50 (s, C(O)NH), 163.46 (s, C(O)), 163.24 (s, C(O)), 138.47, 134.11, 133.29, 132.49, 129.29, 127.86, 124.28, 124.15, 122.80, 122.03, 120.93, 116.83, 42.21 (s, CH2CH CH2), 24.56 (s, CH3C(O)). Anal. Calcd for C17H14N2O3: C, 69.38; H, 4.79; N, 9.52; found: C, 69.14; H, 4.88; N, 9.76.
4.2. General procedure for the preparation of acrylamides 11a–f
To a solution of an appropriate 3-amino-1,8-naphthalimide 16a–f (1.00 mmol) in dichloromethane (2 mL) triethylamine (1.10 mmol) was added. The mixture was cooled in an ice bath and acryloyl chloride (1.05 mmol) was added dropwise. The reaction mixture was stirred for 24 h at room temperature and extracted with water (3 × 3 mL). Subsequently, the inorganic layer was extracted with ethyl ether (3 × 5 mL). The combined organic layers were dried over anhydrous MgSO4 and filtered. After evaporation of solvents the residue was purified on a silica column with chloroform/methanol mixtures (100:1, 50:1 v/v) to afford the respective acrylamides 11a–f.
4.2.1. N-(2-Propyl-1,3-dioxo-2,3-dihydro-1H-benzo[de]isoquinolin-5-yl)propenamide (11a)
Yield: 57%; slightly yellowish amorphous solid (crystallized from chloroform/hexane) mp 223–224 °C; IR (KBr, cm−1) ν max: 3308, 2960, 1703, 1663, 1623, 1564, 1337, 1225, 786; 1H NMR (300 MHz, CDCl3) δ: 9.05 (s, 1H), 8.49 (dd, 1H, J = 7.3, 1.0 Hz), 8.30 (d, 1H, J = 2.2 Hz), 8.17 (dd, 1H, J = 8.3, 1.0 Hz), 7.87 (br s, 1H, NH), 7.72 (dd, 1H, J = 8.3, 7.3 Hz), 6.55 (dd, 1H, J = 16.8, 1.2 Hz, CH CH 2), 6.37 (dd, 1H, J = 16.8, 10.1 Hz, CH CH2), 5.89 (dd, 1H, J = 10.1, 1.2 Hz, CH CH 2), 4.13 (t, 2H, J = 7.6 Hz, CH 2CH2CH3), 1.79–1.69 (m, 2H, CH2CH 2CH3), 1.01 (t, 3H, J = 7.4 Hz, CH2CH2CH 3); 13C NMR (151.0 MHz, DMSO) δ: 164.24 (s, C(O)), 163.80 (s, C(O)), 163.58 (s, C(O)), 138.24, 134.12, 132.50, 131.97, 129.47, 128.22, 128.00, 124.54, 124.30, 123.12, 122.27, 121.48, 41.73 (s, CH2CH2CH3), 21.31 (s, CH2 CH2CH3), 18.82 (s, CH2CH2 CH3). Anal. Calcd for C18H16N2O3: C, 70.12; H, 5.23; N, 9.09; found: C, 70.37; H, 5.13; N, 8.98.
4.2.2. N-(2-Butyl-1,3-dioxo-2,3-dihydro-1H-benzo[de]isoquinolin-5-yl)propenamide (11b)
Yield: 70%; slightly yellowish amorphous solid (crystallized from chloroform/hexane) mp 235–236 °C; IR (KBr, cm−1) ν max: 3258, 2956, 1696, 1661, 1624, 1547, 1338, 1269, 1227, 1130, 786; 1H NMR (300 MHz, CDCl3) δ: 9.06 (d, 1H, J = 2.2 Hz), 8.49 (dd, 1H, J = 7.3, 1.0 Hz), 8.30 (d, 1H, J = 2.2 Hz), 8.17 (dd, 1H, J = 8.3, 1.0 Hz), 7.80 (br s, 1H, NH), 7.73 (dd, 1H, J = 8.3, 7.3 Hz), 6.55 (dd, 1H, J = 16.9, 1.2 Hz, CH CH 2), 6.36 (dd, 1H, J = 16.9, 10.1 Hz, CH CH2), 5.89 (dd, 1H, J = 10.1, 1.2 Hz, CH CH 2), 4.17 (t, 2H, J = 7.5 Hz, CH 2CH2CH2CH3), 1.76–1.66 (m, 2H, CH2CH 2CH2CH3), 1.51–1.41 (m, 2H, CH2CH2CH 2CH3), 0.97 (t, 3H, J = 7.1 Hz, CH2CH2CH2CH 3); 13C NMR (151.0 MHz, DMSO) δ: 164.24 (s, C(O)), 163.78 (s, C(O)), 163.55 (s, C(O)), 138.24, 134.13, 132.50, 131.97, 129.47, 128.23, 128.01, 124.54, 124.31, 123.15, 122.30, 121.50, 40.43 (s, NCH2CH2CH2CH3), 30.11 (s, CH2 CH2CH2CH3), 20.28 (s, CH2CH2 CH2CH3), 14.15 (s, CH2CH2CH2 CH3). Anal. Calcd for C19H18N2O3: C, 70.79; H, 5.63; N, 8.69; found: C, 70.44; H, 5.78; N, 8.43.
4.2.3. N-(2-Isobutyl-1,3-dioxo-2,3-dihydro-1H-benzo[de]isoquinolin-5-yl)propenamide (11c)
Yield: 76%; slightly yellowish amorphous solid (crystallized from chloroform/hexane) mp 213–214 °C; IR (KBr, cm−1) ν max: 3316, 2959, 1702, 1661, 1621, 1562, 1467, 1418, 1371, 1337, 1264, 1227, 888, 787; 1H NMR (300 MHz, CDCl3) δ: 9.06 (d, 1H, J = 1.8 Hz), 8.49 (dd, 1H, J = 7.2, 1.1 Hz), 8.31 (d, 1H, J = 1.8 Hz), 8.17 (dd, 1H, J = 8.1, 1.1 Hz), 7.89 (br s, 1H, NH), 7.72 (dd, 1H, J = 8.1, 7.2 Hz), 6.55 (dd, 1H, J = 16.9, 1.2 Hz, CH CH 2), 6.38 (dd, 1H, J = 16.9, 10.1 Hz, CH CH2), 5.89 (dd, 1H, J = 10.1, 1.2 Hz, CH CH 2), 4.04 (d, 2H, J = 7.3 Hz, CH 2CH(CH3)2), 2.29–2.16 (m, 1H, CH2CH(CH3)2), 0.97 (d, 6H, J = 6.5 Hz, CH2CH(CH 3)2); 13C NMR (75.5 MHz, CDCl3) δ: 164.93 (s, C(O)), 164.66 (s, C(O)), 164.47 (s, C(O)), 137.36, 134.02, 132.51, 130.84, 130.03, 128.37, 127.46, 124.94, 124.45, 122.79, 122.71, 122.07, 47.43 (s, CH2CH(CH3)2), 27.57 (s, CH2 CH(CH3)2), 20.42 (s, CH2CH(CH3)2). Anal. Calcd for C19H18N2O3: C, 70.79; H, 5.63; N, 8.69; found: C, 70.63; H, 5.84; N, 8.44.
4.2.4. N-(2-Dimethylamino)ethyl-1,3-dioxo-2,3-dihydro-1H-benzo[de]isoquinolin-5-yl)propenamide (11d)
Yield: 30%; yellowish amorphous solid (crystallized from chloroform/hexane) mp 208–210 °C; IR (KBr, cm−1) ν max: 3275, 3096, 2774, 1660, 1623, 1561, 1467, 1415, 1267, 1231, 1138, 786; 1H NMR (300 MHz, CDCl3) δ: 8.67 (br s, 1H, NH), 8.64 (s, 1H), 8.33 (dd, 1H, J = 7.3, 1.0 Hz), 7.99 (s, 1H), 7.90 (d, 1H, J = 8.2 Hz), 7.63 (dd, 1H, J = 8.2, 7.3 Hz), 6.52 (dd, 1H, J = 16.9, 1.6 Hz, CH CH 2), 6.38 (dd, 1H, J = 16.9, 9.9 Hz, CH CH2), 5.86 (dd, 1H, J = 9.9, 1.6 Hz, CH CH 2), 4.35 (t, 2H, J = 6.1 Hz, CH 2CH2N(CH3)2), 2.86 (t, 2H, J = 6.1 Hz, CH2CH 2N(CH3)2), 2.49 (s, 6H, CH2CH2N(CH 3)2); 13C NMR (75.5 MHz, CDCl3) δ: 164.37 (s, C(O)), 163.98 (s, C(O)), 163.35 (s, C(O)), 136.76, 133.62, 131.85, 130.95, 129.50, 128.43, 127.28, 124.17, 123.55, 122.33, 121.67, 121.67, 57.85 (s, CH2 CH2N(CH3)2), 46.05 (s, CH2CH2N(CH3)2), 37.89 (s, CH2CH2N(CH3)2). Anal. Calcd for C19H19N3O3: C, 67.64; H, 5.68; N, 12.46; found: C, 67.60; H, 5.43; N, 12.16.
4.2.5. N-(2-Diethylamino)ethyl-1,3-dioxo-2,3-dihydro-1H-benzo[de]isoquinolin-5-yl)propenamide (11e)
Yield: 64%; yellowish amorphous solid (crystallized from chloroform/hexane) mp 196–197 °C; IR (KBr, cm−1) ν max: 3278, 2969, 1700, 1662, 1624, 1542, 1466, 1341, 1263, 1227, 784; 1H NMR (300 MHz, CDCl3) δ: 8.96 (d, 1H, J = 2.1 Hz), 8.42 (dd, 1H, J = 7.2, 1.1 Hz), 8.41 (br s, 1H, NH), 8.30 (d, 1H, J = 2.1 Hz), 8.08 (dd, 1H, J = 8.2, 1.1 Hz), 7.67 (dd, 1H, J = 8.2, 7.2 Hz), 6.54 (dd, 1H, J = 16.9, 1.4 Hz, CH CH 2), 6.40 (dd, 1H, J = 16.9, 10.0 Hz, CH CH2), 5.86 (dd, 1H, J = 10.0, 1.4 Hz, CH CH 2), 4.27–4.22 (m, 2H, CH 2CH2N(CH2CH3)2), 2.78–2.73 (m, 2H, CH2CH 2N(CH2CH3)2), 2.64 (q, 4H, J = 7.1 Hz, CH2CH2N(CH 2CH3)2), 1.05 (t, 6H, J = 7.1 Hz, CH2CH2N(CH2CH 3)2); 13C NMR (75.5 MHz, CDCl3) δ: 164.49 (s, C(O)), 163.84 (s, C(O)), 163.77 (s, C(O)), 136.85, 133.81, 132.29, 130.83, 129.99, 128.90, 127.51, 124.84, 124.37, 122.88, 122.78, 122.00, 50.07 (s, CH2 CH2N(CH2CH3)2), 47.66 (s, CH2CH2N(CH2CH3)2), 38.36 (s, CH2CH2N(CH2CH3)2), 12.36 (s, CH2CH2N(CH2 CH3)2). Anal. Calcd for C21H23N3O3: C, 69.02; H, 6.34; N, 11.50; found: C, 68.88; H, 6.19; N, 11.34.
4.2.6. N-(2-Pirolidyn-1-yl)ethyl-1,3-dioxo-2,3-dihydro-1H-benzo[de]isoquinolin-5-yl)propenamide (11f)
Yield: 43%; yellowish amorphous solid (crystallized from chloroform/hexane) mp 188–189 °C; IR (KBr, cm−1) ν max: 3254, 3096, 2966, 2786, 1698, 1661, 1562, 1467, 1418, 1349, 1268, 1232, 1131, 787; 1H NMR (300 MHz, CDCl3) δ: 8.77 (d, 1H, J = 1.9 Hz), 8.37 (dd, 1H, J = 7.1, 1.0 Hz), 8.37 (br s, 1H, NH), 8.10 (d, 1H, J = 1.9 Hz), 7.99 (dd, 1H, J = 8.3, 1.0 Hz), 7.66 (dd, 1H, J = 8.3, 7.1 Hz), 6.53 (dd, 1H, J = 16.9, 1.6 Hz, CH CH 2), 6.36 (dd, 1H, J = 16.9, 10.1 Hz, CH CH2), 5.87 (dd, 1H, J = 10.1, 1.6 Hz, CH CH 2), 4.37 (t, 2H, J = 6.6 Hz, CH 2CH2N(CH2CH2)2), 2.94 (t, 2H, J = 6.6 Hz, CH2CH 2N(CH2CH2)2), 2.80–2.71 (m, 4H, CH2CH2N(CH 2CH2)2), 1.86–1.79 (m, 4H, CH2CH2N(CH2CH 2)2); 13C NMR (75.5 MHz, CDCl3) δ: 164.25 (s, C(O)), 164.20 (s, C(O)), 163.60 (s, C(O)), 136.68, 133.83, 132.24, 130.87, 129.87, 128.84, 127.60, 124.70, 123.70, 122.95, 122.11, 121.95, 54.87 (s, CH2 CH2N(CH2CH2)2 and CH2CH2N(CH2CH2)2), 39.38 (s, CH2CH2N(CH2CH2)2), 23.91 (s, CH2CH2N(CH2 CH2)2). Anal. Calcd for C21H21N3O3: C, 69.41; H, 5.82; N, 11.56; found: C, 69.53; H, 5.74; N, 11.67.
4.3. General procedure for the preparation of isoxazolidines trans-9a–f and cis-9a–f
A mixture of the nitrone 8 (1.00 mmol), acrylamide 11a–f (1.00 mmol) and toluene or a toluene–chloroform mixture (2 mL, 1:1, v/v) was stirred at 70 °C for 24 h or until disappearance of the starting nitrone. After evaporation of solvents under reduced pressure the crude products were purified by silica gel chromatography with chloroform/methanol mixtures.
4.3.1. Diethyl cis-5-(N-propylnaphthalimide-3-ylcarbamoyl)-2-methylisoxazolidin-3-yl-3-phosphonate (cis-9a)
Yellow oil; IR (film, cm−1) ν max: 3317, 2968, 2934, 1696, 1661, 1542, 1430, 1339, 1234, 1052, 1025, 972; 1H NMR (300 MHz, CDCl3) δ: 9.31 (br s, 1H, NH), 8.94 (d, 1H, J = 2.1 Hz), 8.50 (dd, 1H, J = 7.3, 1.0 Hz), 8.41 (d, 1H, J = 2.1 Hz), 8.18 (dd, 1H, J = 8.1, 1.0 Hz), 7.73 (dd, 1H, J = 8.1, 7.3 Hz), 4.70 (dd, 1H, J = 8.7, 5.0 Hz, HC5), 4.24–4.05 (m, 6H, 2 × CH 2OP and CH 2CH2CH3), 3.20–2.98 (m, 2H, HC3 and H βC4), 3.02 (s, 3H, CH 3N), 2.94–2.81 (m, 1H, H αC4), 1.80–1.70 (m, 2H, CH2CH 2CH3), 1.30 (t, 3H, J = 7.0 Hz, CH 3CH2OP), 1.21 (t, 3H, J = 7.0 Hz, CH 3CH2OP), 1.02 (t, 3H, J = 7.4 Hz, CH2CH2CH 3); 13C NMR (151.0 MHz, CDCl3) δ: 170.81 (s, C(O)NH), 164.11 (s, C(O)), 163.81 (s, C(O)), 136.32, 133.63, 132.55, 129.90, 127.49, 125.22, 124.20, 123.54, 122.55, 121.89, 76.02 (d, J = 6.8 Hz, C5), 63.72 (d, J = 169.4 Hz, C3), 63.07 (d, J = 7.1 Hz, CH2OP), 62.02 (d, J = 6.8 Hz, CH2OP), 46.04 (d, J = 5.8 Hz, CH3N), 41.99 (s, CH2CH2CH3), 36.41 (s, C4), 21.39 (s, CH2 CH2CH3), 16.43 (d, J = 6.0 Hz, CH3CH2OP), 16.36 (d, J = 5.6 Hz, CH3CH2OP), 11.49 (s, CH2CH2 CH3); 31P NMR (121.5 MHz, CDCl3) δ: 21.31. Anal. Calcd for C24H30N3O7P: C, 57.25; H, 6.01; N, 8.35; found: C, 57.32; H, 5.92; N, 8.48.
4.3.2. Diethyl trans-5-(N-propylnaphtalimide-3-ylcarbamoyl)-2-methylisoxazolidin-3-yl-3-phosphonate (trans-9a)
Yellowish amorphous solid (crystallized from chloroform/hexane) mp 175–176 °C; IR (KBr, cm−1) ν max: 3241, 3067, 2967, 1697, 1664, 1567, 1339, 1266, 1214, 1049, 1018; 1H NMR (300 MHz, CDCl3) δ: 8.93 (d, 1H, J = 2.2 Hz), 8.57 (br s, 1H, NH), 8.51 (dd, 1H, J = 7.2, 1.1 Hz), 8.33 (d, 1H, J = 2.2 Hz), 8.18 (dd, 1H, J = 8.3, 1.1 Hz), 7.74 (dd, 1H, J = 8.3, 7.2 Hz), 4.69 (dd, 1H, J = 8.7, 5.8 Hz, HC5), 4.29–4.14 (m, 4H, 2 × CH 2OP), 4.15 (t, 2H, J = 7.5 Hz, CH 2CH2CH3), 3.19–3.06 (m, 1H, HC3), 3.08 (s, 3H, CH 3N), 3.07 (dddd, 1H, J = 16.1, 12.8, 8.7, 8.7 Hz, H βC4), 2.88 (dddd, 1H, J = 12.8, 9.9, 8.2, 5.8 Hz, H αC4), 1.80–1.72 (m, 2H, CH2CH 2CH3), 1.39 (t, 3H, J = 7.0 Hz, CH 3CH2OP), 1.37 (t, 3H, J = 7.0 Hz, CH 3CH2OP), 1.02 (t, 3H, J = 7.4 Hz, CH2CH2CH 3); 13C NMR (75.5 MHz, CDCl3) δ: 169.45 (s, C(O)NH), 163.72 (s, C(O)), 163.37 (s, C(O)), 135.71, 133.47, 132.18, 129.89, 127.46, 125.00, 124.00, 123.25, 122.23, 122.12, 76.58 (d, J = 8.6 Hz, C5), 63.57 (d, J = 164.3 Hz, C3), 63.41 (d, J = 6.3 Hz, CH2OP), 62.84 (d, J = 6.9 Hz, CH2OP), 46.84 (s, CH3N), 42.03 (s, CH2CH2CH3), 36.52 (s, C4), 21.48 (s, CH2 CH2CH3), 16.71 (d, J = 5.1 Hz, CH3CH2OP), 16.61 (d, J = 5.1 Hz, CH3CH2OP), 11.67 (s, CH2CH2 CH3); 31P NMR (121.5 MHz, CDCl3) δ: 20.52. Anal. Calcd for C24H30N3O7P: C, 57.25; H, 6.01; N, 8.35; found: C, 57.34; H, 5.92; N, 8.58.
4.3.3. Diethyl cis-5-(N-butylnaphthalimide-3-ylcarbamoyl)-2-methylisoxazolidin-3-yl-3-phosphonate (cis-9b)
Yellow oil; IR (film, cm−1) ν max: 2962, 2932, 1697, 1662, 1543, 1466, 1430, 1340, 1233, 1053, 1026, 753; 1H NMR (300 MHz, CDCl3) δ: 9.30 (br s, 1H, NH), 8.94 (d, 1H, J = 2.1 Hz), 8.50 (dd, 1H, J = 7.3, 1.2 Hz), 8.40 (d, 1H, J = 2.1 Hz), 8.17 (dd, 1H, J = 8.3, 1.2 Hz), 7.72 (dd, 1H, J = 8.3, 7.3 Hz), 4.71–4.67 (m, 1H, HC5), 4.24–4.04 (m, 4H, 2 × CH 2OP), 4.16 (t, 2H, J = 7.4 Hz, CH 2CH2CH2CH3), 3.14–3.05 (m, 2H, HC3 and H βC4), 3.01 (d, 3H, J = 0.6 Hz, CH 3N), 2.87–2.78 (m, 1H, H αC4), 1.77–1.67 (m, 2H, CH2CH 2CH2CH3), 1.51–1.39 (m, 2H, CH2CH2CH 2CH3), 1.30 (t, 3H, J = 7.1 Hz, CH 3CH2OP), 1.21 (t, 3H, J = 7.0 Hz, CH 3CH2OP), 0.98 (t, 3H, J = 7.3 Hz, CH2CH2CH2CH 3); 13C NMR (151.0 MHz, CDCl3) δ: 170.80 (s, C(O)NH), 164.09 (s, C(O)), 163.78 (s, C(O)), 136.31, 133.62, 132.53, 129.87, 127.48, 125.19, 124.18, 123.52, 122.54, 121.87, 76.01 (d, J = 6.8 Hz, C5), 63.70 (d, J = 169.6 Hz, C3), 63.07 (d, J = 6.3 Hz, CH2OP), 62.03 (d, J = 6.4 Hz, CH2OP), 46.03 (d, J = 5.8 Hz, CH3N), 40.28 (s, CH2CH2N(CH3)2), 36.39 (s, C4), 30.21 (s, CH2 CH2CH2CH3), 20.35 (s, CH2CH2 CH2CH3), 16.43 (d, J = 5.8 Hz, CH3CH2OP), 16.35 (d, J = 5.9 Hz, CH3CH2OP), 13.81 (s, CH2CH2CH2 CH3); 31P NMR (121.5 MHz, CDCl3) δ: 21.37. Anal. Calcd for C25H32N3O7P: C, 58.02; H, 6.23; N, 8.12; found: C, 58.30; H, 6.23; N, 8.00.
4.3.4. Diethyl trans-5-(N-butylnaphthalimide-3-ylcarbamoyl)-2-methylisoxazolidin-3-yl-3-phosphonate (trans-9b)
Yellowish amorphous solid (crystallized from chloroform/hexane) mp 157–158 °C; IR (KBr, cm−1) ν max: 3278, 2958, 1698, 1661, 1565, 1341, 1229, 1052, 1033, 970; 1H NMR (300 MHz, CDCl3) δ: 8.93 (d, 1H, J = 2.2 Hz), 8.57 (br s, 1H, NH), 8.51 (dd, 1H, J = 7.3, 1.2 Hz), 8.33 (d, 1H, J = 2.2 Hz), 8.18 (dd, 1H, J = 8.3, 1.2 Hz), 7.74 (dd, 1H, J = 8.3, 7.3 Hz), 4.69 (dd, 1H, J = 8.5, 5.6 Hz, HC5), 4.29–4.16 (m, 4H, 2 × CH 2OP), 4.18 (t, 2H, J = 7.4 Hz, CH 2CH2CH2CH3), 3.18–3.05 (m, 1H, HC3), 3.07 (s, 3H, CH 3N), 3.07 (dddd, 1H, J = 15.9, 12.7, 8.5, 8.5 Hz, H βC4), 2.88 (dddd, 1H, J = 12.7, 10.1, 8.1, 5.6 Hz, H αC4), 1.76–1.66 (m, 2H, CH2CH 2CH2CH3), 1.51–1.39 (m, 2H, CH2CH2CH 2CH3), 1.38 (t, 3H, J = 7.1 Hz, CH 3CH2OP), 1.37 (t, 3H, J = 7.1 Hz, CH 3CH2OP), 0.98 (t, 3H, J = 7.3 Hz, CH2CH2CH2CH 3); 13C NMR (75.5 MHz, CDCl3) δ: 169.47 (s, C(O)NH), 163.79 (s, C(O)), 163.45 (s, C(O)), 135.70, 133.55, 132.26, 129.98, 127.54, 125.10, 124.01, 123.36, 122.33, 122.17, 76.59 (d, J = 8.9 Hz, C5), 63.59 (d, J = 167.2 Hz, C3), 63.47 (d, J = 6.6 Hz, CH2OP), 62.87 (d, J = 6.9 Hz, CH2OP), 46.86 (s, CH3N), 40.37 (s, CH2CH2CH2CH3), 36.56 (s, C4), 30.31 (s, CH2 CH2CH2CH3), 20.51 (s, CH2CH2 CH2CH3), 16.74 (d, J = 5.1 Hz, CH3CH2OP), 16.64 (d, J = 5.1 Hz, CH3CH2OP), 14.02 (s, CH2CH2CH2 CH3); 31P NMR (121.5 MHz, CDCl3) δ: 20.55. Anal. Calcd for C25H32N3O7P: C, 58.02; H, 6.23; N, 8.12; found: C, 58.13; H, 6.20; N, 7.92.
4.3.5. Diethyl cis-5-(N-isobutylnaphthalimide-3-ylcarbamoyl)-2-methylisoxazolidin-3-yl-3-phosphonate (cis-9c)
Yellow oil; IR (film, cm−1) ν max: 2963, 1701, 1662, 1543, 1337, 1234, 1054, 1027, 755; 1H NMR (300 MHz, CDCl3) δ: 9.30 (br s, 1H, NH), 8.95 (d, 1H, J = 2.2 Hz), 8.50 (dd, 1H, J = 7.3, 1.1 Hz), 8.40 (d, 1H, J = 2.2 Hz), 8.18 (dd, 1H, J = 8.3, 1.1 Hz), 7.73 (dd, 1H, J = 8.3, 7.3 Hz), 4.69 (dd, 1H, J = 8.3, 4.6 Hz, HC5), 4.24–4.02 (m, 4H, 2 × CH 2OP), 4.05 (d, 2H, J = 7.3 Hz, CH 2CH(CH3)2), 3.16–3.01 (m, 2H, HC3 and H βC4), 3.01 (d, 3H, J = 1.0 Hz, CH 3N), 2.93–2.83 (m, 1H, H αC4), 2.28–2.19 (m, 1H, CH2CH(CH3)2), 1.30 (t, 3H, J = 7.0 Hz, CH 3CH2OP), 1.21 (t, 3H, J = 7.1 Hz, CH 3CH2OP), 0.99 (d, 6H, J = 6.7 Hz, CH2CH(CH 3)2); 13C NMR (151.0 MHz, CDCl3) δ: 170.80 (s, C(O)NH), 164.37 (s, C(O)), 164.08 (s, C(O)), 136.33, 133.59, 132.52, 129.95, 127.48, 125.23, 124.29, 123.46, 122.48, 121.86, 76.01 (d, J = 6.8 Hz, C5), 63.70 (d, J = 166.5 Hz, C3), 63.06 (d, J = 6.5 Hz, CH2OP), 63.02 (d, J = 6.8 Hz, CH2OP), 47.21 (s, CH2CH(CH3)2), 46.01 (d, J = 5.7 Hz, CH3N), 36.39 (s, C4), 27.39 (s, CH2 CH(CH3)2), 20.28 (s, CH2CH(CH3)2), 16.42 (d, J = 5.6 Hz, CH3CH2OP), 16.34 (d, J = 5.6 Hz, CH3CH2OP); 31P NMR (121.5 MHz, CDCl3) δ: 21.36. Anal. Calcd for C25H32N3O7P: C, 58.02; H, 6.23; N, 8.12; found: C, 58.15; H, 6.25; N, 7.87.
4.3.6. Diethyl trans-5-(N-isobutylnaphthalimide-3-ylcarbamoyl)-2-methylisoxazolidin-3-yl-3-phosphonate (trans-9c)
Yellowish amorphous solid (crystallized from chloroform/hexane) mp 165–166 °C; IR (KBr, cm−1) ν max: 3273, 3243, 2986, 2955, 1699, 1661, 1564, 1337, 1228, 1052, 1034, 973, 783; 1H NMR (300 MHz, CDCl3) δ: 8.93 (d, 1H, J = 2.2 Hz), 8.57 (br s, 1H, NH), 8.51 (dd, 1H, J = 7.3, 1.0 Hz), 8.33 (d, 1H, J = 2.2 Hz), 8.17 (dd, 1H, J = 8.3, 1.2 Hz), 7.74 (dd, 1H, J = 8.3, 7.3 Hz), 4.69 (dd, 1H, J = 8.7, 5.7 Hz, HC5), 4.29–4.18 (m, 4H, 2 × CH 2OP), 4.04 (d, 2H, J = 7.5 Hz, CH 2CH(CH3)2), 3.16–3.05 (m, 1H), 3.07 (s, 3H, CH 3N), 3.06 (dddd, 1H, J = 15.7, 12.8, 8.7, 8.7 Hz, H βC4), 2.87 (dddd, 1H, J = 12.8, 10.3, 8.1, 5.7 Hz, H αC4), 2.28–2.19 (m, 1H, CH2CH(CH3)2), 1.38 (t, 3H, J = 7.1 Hz, CH 3CH2OP), 1.37 (t, 3H, J = 7.1 Hz, CH 3CH2OP), 0.98 (d, 6H, J = 6.5 Hz, CH2CH(CH 3)2); 13C NMR (75.5 MHz, CDCl3) δ: 169.47 (s, C(O)NH), 164.21 (s, C(O)), 163.90 (s, C(O)), 135.69, 133.65, 132.43, 130.23, 127.68, 125.34, 124.13, 123.53, 122.46, 122.26, 76.53 (d, J = 8.9 Hz, C5), 63.68 (d, J = 166.9 Hz, C3), 63.54 (d, J = 6.6 Hz, CH2OP), 62.89 (d, J = 6.9 Hz, CH2OP), 47.38 (s, CH2CH(CH3)2), 46.93 (br s, CH3N), 36.72 (s, C4), 27.60 (s, CH2 CH(CH3)2), 20.52 (s, CH2CH(CH3)2), 16.78 (d, J = 5.1 Hz, CH3CH2OP), 16.71 (d, J = 5.4 Hz, CH3CH2OP); 31P NMR (121.5 MHz, CDCl3) δ: 20.53. Anal. Calcd for C25H32N3O7P: C, 58.02; H, 6.23; N, 8.12; found: C, 58.12; H, 5.99; N, 7.88.
4.3.7. Diethyl cis-5-[N-(2-dimethylamino)ethylnaphthalimide-3-ylcarbamoyl]-2-methylisoxazolidin-3-yl-3-phosphonate (cis-9d)
Yellow amorphous solid; mp 119–120 °C; IR (KBr, cm−1) ν max: 3274, 2980, 2776, 1695, 1662, 1544, 1465, 1430, 1341, 1237, 1026, 971, 751; 1H NMR (300 MHz, CDCl3) δ: 9.29 (br s, 1H, NH), 8.95 (d, 1H, J = 2.2 Hz), 8.50 (dd, 1H, J = 7.4, 1.2 Hz), 8.41 (d, 1H, J = 2.2 Hz), 8.18 (d, 1H, J = 8.3 Hz), 7.72 (dd, 1H, J = 8.3, 7.4 Hz), 4.69 (dd, 1H, J = 8.9, 4.8 Hz, HC5), 4.34 (t, 2H, J = 6.9 Hz, CH 2CH2N(CH3)2), 4.23–4.05 (m, 4H, 2 × CH 2OP), 3.18–3.00 (m, 2H, HC3 and H βC4), 3.01 (s, 3H, CH 3N), 2.87–2.78 (m, 1H, H αC4), 2.69 (t, 2H, J = 6.9 Hz, CH2CH 2N(CH3)2), 2.38 (s, 6H, CH2CH2N(CH 3)2), 1.30 (t, 3H, J = 7.0 Hz, CH 3CH2OP), 1.20 (t, 3H, J = 7.0 Hz, CH 3CH2OP); 13C NMR (151.0 MHz, CDCl3) δ: 170.83 (s, C(O)NH), 164.13 (s, C(O)), 163.83 (s, C(O)), 136.33, 133.73, 132.56, 129.98, 127.49, 125.26, 124.25, 123.45, 122.46, 121.93, 76.00 (d, J = 6.8 Hz, C5), 63.71 (d, J = 169.5 Hz, C3), 63.07 (d, J = 6.7 Hz, CH2OP), 63.02 (d, J = 7.0 Hz, CH2OP), 56.95 (s, CH2 CH2N(CH3)2), 46.03 (d, J = 5.9 Hz, CH3N), 45.67 (s, CH2CH2N(CH3)2), 38.15 (s, CH2CH2N(CH3)2), 36.41 (s, C4), 16.43 (d, J = 5.6 Hz, CH3CH2OP), 16.36 (d, J = 5.9 Hz, CH3CH2OP); 31P NMR (121.5 MHz, CDCl3) δ: 21.32. Anal. Calcd for C25H33N4O7P: C, 56.39; H, 6.25; N, 10.52; found: C, 56.20; H, 6.49; N, 10.39.
4.3.8. Diethyl trans-5-[N-(2-dimethylamino)ethylnaphthalimide-3-ylcarbamoyl]-2-methylisoxazolidin-3-yl-3-phosphonate (trans-9d)
Yellowish oil; IR (film, cm−1) ν max: 3248, 3092, 2943, 2776, 1700, 1662, 1562, 1465, 1430, 1340, 1236, 1052, 1025, 970, 751; 1H NMR (300 MHz, CDCl3) δ: 8.91 (d, 1H, J = 2.1 Hz), 8.61 (br s, 1H, NH), 8.50 (d, 1H, J = 7.1 Hz), 8.32 (d, 1H, J = 2.1 Hz), 8.16 (d, 1H, J = 8.5 Hz), 7.73 (dd, 1H, J = 8.5, 7.1 Hz), 4.69 (dd, 1H, J = 8.7, 5.8 Hz, HC5), 4.33 (t, 2H, J = 6.8 Hz, CH 2CH2N(CH3)2), 4.29–4.16 (m, 4H, 2 × CH 2OP), 3.18–3.05 (m, 1H, HC3), 3.07 (s, 3H, CH 3N), 3.06 (dddd, 1H, J = 16.0, 12.8, 8.7, 8.7 Hz, H βC4), 2.88 (dddd, 1H, J = 12.8, 9.6, 8.2, 5.8 Hz, H αC4), 2.66 (t, 2H, J = 6.8 Hz, CH2CH 2N(CH3)2), 2.36 (s, 6H, CH2CH2N(CH 3)2), 1.38 (t, 3H, J = 7.1 Hz, CH 3CH2OP), 1.37 (t, 3H, J = 7.0 Hz, CH 3CH2OP); 13C NMR (75.5 MHz, CDCl3) δ: 169.42 (s, C(O)NH), 163.92 (s, C(O)), 163.53 (s, C(O)), 135.71, 133.69, 132.30, 130.08, 127.58, 125.13, 123.93, 123.31, 122.28, 122.09, 76.57 (d, J = 8.9 Hz, C5), 63.65 (d, J = 170.9 Hz, C3), 63.50 (d, J = 6.6 Hz, CH2OP), 62.86 (d, J = 6.9 Hz, CH2OP), 57.21 (s, CH2 CH2N(CH3)2), 46.95 (s, CH3N), 45.90 (s, CH2CH2N(CH3)2), 38.33 (s, CH2CH2N(CH3)2), 36.57 (s, C4), 16.79 (d, J = 5.1 Hz, CH3CH2OP), 16.68 (d, J = 5.2 Hz, CH3CH2OP); 31P NMR (121.5 MHz, CDCl3) δ: 20.56. Anal. Calcd for C25H33N4O7P: C, 56.39; H, 6.25; N, 10.52; found: C, 56.18; H, 6.48; N, 10.45.
4.3.9. Diethyl cis-5-[N-(2-diethylamino)ethylnaphthalimide-3-ylcarbamoyl]-2-methylisoxazolidin-3-yl-3-phosphonate (cis-9e)
Yellowish oil; IR (film, cm−1) ν max: 3301, 2973, 2932, 1696, 1662, 1544, 1466, 1430, 1342, 1235, 1026, 972, 785; 1H NMR (300 MHz, CDCl3) δ: 9.29 (br s, 1H, NH), 8.95 (d, 1H, J = 2.2 Hz), 8.49 (dd, 1H, J = 7.3, 1.2 Hz), 8.40 (d, 1H, J = 2.2 Hz), 8.18 (d, 1H, J = 8.3 Hz), 7.72 (dd, 1H, J = 8.3, 7.3 Hz), 4.69 (dd, 1H, J = 8.7, 4.7 Hz, HC5), 4.31 (t, 2H, J = 7.1 Hz, CH 2CH2N(CH2CH3)2), 4.23–4.04 (m, 4H, 2 × CH 2OP), 3.20–2.98 (m, 2H, HC3 and H βC4), 3.01 (s, 3H, CH 3N), 2.98–2.80 (m, 1H, H αC4), 2.83 (t, 2H, J = 7.1 Hz, CH2CH 2N(CH2CH3)2), 2.69 (q, 4H, J = 7.0 Hz, CH2CH2N(CH 2CH3)2), 1.29 (t, 3H, J = 7.0 Hz, CH 3CH2OP), 1.20 (t, 3H, J = 7.0 Hz, CH 3CH2OP), 1.11 (t, 6H, J = 7.0 Hz, CH2CH2N(CH2CH 3)2); 13C NMR (151.0 MHz, CDCl3) δ: 170.86 (s, C(O)NH), 164.08 (s, C(O)), 163.78 (s, C(O)), 136.34, 133.77, 132.60, 129.95, 127.53, 125.23, 124.20, 123.44, 122.45, 121.98, 76.01 (d, J = 6.8 Hz, C5), 63.71 (d, J = 169.4 Hz, C3), 63.08 (d, J = 6.7 Hz, CH2OP), 63.01 (d, J = 6.8 Hz, CH2OP), 49.69 (s, CH2 CH2N(CH2CH3)2), 47.67 (s, CH2CH2N(CH2CH3)2), 46.04 (d, J = 5.8 Hz, CH3N), 37.85 (s, CH2CH2N(CH2CH3)2), 36.45 (s, C4), 16.43 (d, J = 5.6 Hz, CH3CH2OP), 16.36 (d, J = 6.0 Hz, CH3CH2OP), 12.06 (s, CH2CH2N(CH2 CH3)2); 31P NMR (121.5 MHz, CDCl3) δ: 21.31. Anal. Calcd for C27H37N4O7P: C, 57.85; H, 6.65; N, 9.99; found: C, 57.67; H, 6.62; N, 9.85.
4.3.10. Diethyl trans-5-[N-(2-diethylamino)ethylnaphthalimide-3-ylcarbamoyl]-2-methylisoxazolidin-3-yl-3-phosphonate (trans-9e)
Yellowish oil; IR (film, cm−1) ν max: 3249, 2974, 2933, 1698, 1662, 1563, 1466, 1430, 1341, 1235, 1054, 1027, 972, 755; 1H NMR (600 MHz, CDCl3) δ: 8.93 (d, 1H, J = 2.2 Hz), 8.64 (br s, 1H, NH), 8.51 (dd, 1H, J = 7.3, 0.8 Hz), 8.35 (d, 1H, J = 2.2 Hz), 8.18 (d, 1H, J = 8.0 Hz), 7.74 (dd, 1H, J = 8.0, 7.3 Hz), 4.71 (dd, 1H, J = 8.8, 5.6 Hz, HC5), 4.30 (t, 2H, J = 7.5 Hz, CH 2CH2N(CH2CH3)2), 4.28–4.19 (m, 4H, 2 × CH 2OP), 3.18–3.11 (m, 1H, HC3), 3.09 (s, 3H, CH 3N), 3.08 (dddd, 1H, J = 16.0, 12.7, 8.8, 8.8 Hz, H βC4), 2.89 (dddd, 1H, J = 12.7, 9.5, 8.3, 5.6 Hz, H αC4), 2.80 (t, 2H, J = 7.5 Hz, CH2CH 2N(CH2CH3)2), 2.68 (q, 4H, J = 7.1 Hz, CH2CH2N(CH 2CH3)2), 1.40 (t, 3H, J = 7.0 Hz, CH 3CH2OP), 1.38 (t, 3H, J = 7.0 Hz, CH 3CH2OP), 1.10 (t, 6H, J = 7.1 Hz, CH2CH2N(CH2CH 3)2); 13C NMR (75.5 MHz, CDCl3) δ: 169.46 (s, C(O)NH), 163.81 (s, C(O)), 163.47 (s, C(O)), 135.71, 133.68, 132.34, 130.04, 127.59, 125.16, 124.01, 123.35, 122.32, 122.23, 76.57 (d, J = 7.7 Hz, C5), 63.64 (d, J = 167.8 Hz, C3), 63.48 (d, J = 6.6 Hz, CH2OP), 62.86 (d, J = 6.9 Hz, CH2OP), 49.87 (s, CH2 CH2N(CH2CH3)2), 47.72 (s, CH2CH2N(CH2CH3)2), 46.96 (s, CH3N), 38.16 (s, CH2CH2N(CH2CH3)2), 36.63 (s, C4), 16.76 (d, J = 4.9 Hz, CH3CH2OP), 16.65 (d, J = 5.4 Hz, CH3CH2OP), 12.36 (s, CH2CH2N(CH2 CH3)2); 31P NMR (243.0 MHz, CDCl3) δ: 20.17. Anal. Calcd for C27H37N4O7P: C, 57.85; H, 6.65; N, 9.99; found: C, 57.87; H, 6.71; N, 9.91.
4.3.11. Diethyl cis-5-[N-(2-pirolidyn-1-yl)ethylnaphthalimide-3-ylcarbamoyl]-2-methylisoxazolidin-3-yl-3-phosphonate (cis-9f)
Yellowish oil; IR (film, cm−1) ν max: 3276, 2971, 2791, 1691, 1662, 1543, 1430, 1338, 1234, 1052, 971, 785, 750; 1H NMR (600 MHz, CDCl3) δ: 9.30 (br s, 1H, NH), 8.96 (d, 1H, J = 2.0 Hz), 8.52 (d, 1H, J = 7.2 Hz), 8.43 (d, 1H, J = 2.0 Hz), 8.19 (d, 1H, J = 8.1 Hz), 7.74 (dd, 1H, J = 8.1, 7.2 Hz), 4.71 (dd, 1H, J = 9.2, 5.0 Hz, HC5), 4.40 (t, 2H, J = 7.3 Hz, CH 2CH2N(CH2CH2)2), 4.24–4.08 (m, 4H, 2 × CH 2OP), 3.15–3.08 (m, 2H, HC3 and H βC4), 3.04 (s, 3H, CH 3N), 2.93–2.88 (m, 1H, H αC4), 2.88–2.83 (m, 2H, CH2CH 2N(CH2CH2)2), 2.71 (br s, 4H, CH2CH2N(CH 2CH2)2), 1.83 (br s, 4H, CH2CH2N(CH2CH 2)2, 1.32 (t, 3H, J = 7.0 Hz, CH 3CH2OP), 1.23 (t, 3H, J = 7.0 Hz, CH 3CH2OP); 13C NMR (151.0 MHz, CDCl3) δ: 170.84 (s, C(O)NH), 164.07 (s, C(O)), 163.75 (s, C(O)), 136.32, 133.72, 132.58, 129.95, 127.50, 125.26, 124.21, 123.50, 122.51, 121.92, 76.00 (d, J = 7.3 Hz, C5), 63.72 (d, J = 169.1 Hz, C3), 63.08 (d, J = 6.7 Hz, CH2OP), 62.99 (d, J = 6.8 Hz, CH2OP), 54.35 (s, CH2CH2N(CH2CH2)2), 53.63 (s, CH2 CH2N(CH2CH2)2), 46.04 (d, J = 5.7 Hz, CH3N), 39.21 (s, CH2CH2N(CH2CH3)2), 36.44 (s, C4), 23.64 (s, CH2CH2N(CH2 CH2)2, 16.43 (d, J = 5.7 Hz, CH3CH2OP), 16.36 (d, J = 5.6 Hz, CH3CH2OP); 31P NMR (243 MHz, CDCl3) δ: 20.86. Anal. Calcd for C27H35N4O7P: C, 58.06; H, 6.32; N, 10.03; found: C, 57.84; H, 6.39; N, 9.75.
4.3.12. Diethyl trans-5-[N-(2-pirolidyn-1-yl)ethylnaphthalimide-3-ylcarbamoyl]-2-methylisoxazolidin-3-yl-3-phosphonate (trans-9f)
Yellowish oil; IR (film, cm−1) ν max: 3271, 2970, 1698, 1661, 1563, 1465, 1430, 1337, 1233, 1027, 970, 784, 749; 1H NMR (600 MHz, CDCl3) δ: 8.94 (d, 1H, J = 2.1 Hz), 8.60 (br s, 1H, NH), 8.53 (dd, 1H, J = 7.3, 0.9 Hz), 8.36 (d, 1H, J = 2.1 Hz), 8.19 (d, 1H, J = 8.0 Hz), 7.76 (dd, 1H, J = 8.0, 7.3 Hz), 4.71 (dd, 1H, J = 8.9, 5.5 Hz, HC5), 4.39 (t, 2H, J = 7.3 Hz, CH 2CH2N(CH2CH2)2), 4.29–4.19 (m, 4H, 2 × CH 2OP), 3.17–3.09 (m, 1H, HC3), 3.10 (s, 3H, CH 3N), 3.08 (dddd, 1H, J = 16.1, 12.9, 8.9, 8.9 Hz, H βC4), 2.89 (dddd, 1H, J = 12.9, 9.4, 8.3, 5.5 Hz, H αC4), 2.85 (t, 2H, J = 7.3 Hz, CH2CH 2N(CH2CH2)2), 2.70 (br s, 4H, CH2CH2N(CH 2CH2)2), 1.82 (br s, 4H, CH2CH2N(CH2CH 2)2), 1.41 (t, 3H, J = 7.1 Hz, CH 3CH2OP), 1.39 (t, 3H, J = 7.1 Hz, CH 3CH2OP); 13C NMR (151.0 MHz, CDCl3) δ: 169.48 (s, C(O)NH), 163.88 (s, C(O)), 163.55 (s, C(O)), 135.68, 133.64, 132.40, 130.09, 127.59, 125.27, 123.97, 123.49, 122.43, 122.20, 76.38 (d, J = 8.9 Hz, C5), 63.67 (d, J = 170.4 Hz, C3), 63.35 (d, J = 6.5 Hz, CH2OP), 62.69 (d, J = 6.9 Hz, CH2OP), 54.34 (s, CH2CH2N(CH2CH2)2), 53.64 (s, CH2 CH2N(CH2CH2)2), 46.72 (s, CH3N), 39.27 (s, CH2CH2N(CH2CH3)2), 36.56 (s, C4), 23.61 (s, CH2CH2N(CH2 CH2)2, 16.52 (d, J = 5.9 Hz, CH3CH2OP), 16.45 (d, J = 5.7 Hz, CH3CH2OP); 31P NMR (243.0 MHz, CDCl3) δ: 20.13. Anal. Calcd for C27H35N4O7P: C, 58.06; H, 6.32; N, 10.03; found: C, 58.22; H, 6.28; N, 9.83.
4.4. General procedure for the preparation of isoxazolidines trans-10a–d and cis-10a–d
A mixture of the nitrone 8 (1.00 mmol), N-(2-propenyl)naphthalimide 12a–d (1.00 mmol) and toluene or a toluene–chloroform mixture (2 mL, 1:1, v/v) was stirred at 70 °C for 24 h or until disappearance of the starting nitrone. After evaporation of solvents under reduced pressure the crude products were purified by silica gel chromatography with chloroform/methanol mixtures.
4.4.1. Diethyl trans-5-(1,3-dioxo-2,3-dihydro-1H-benzo[de]izochinolin-2-ylmethyl)-2-methylisoxazolidin-3-yl-3-phosphonate (trans-10a)
White amorphous solid (crystallized from chloroform/hexane) mp 175–176 °C; IR (KBr, cm−1) ν max: 2981, 1702, 1662, 1591, 1328, 1234, 1056, 1035, 973; (signals of trans-10a were extracted from the spectra of a 93:7 mixture of trans-10a and cis-10a); 1H NMR (300 MHz, CDCl3) δ: 8.61 (dd, 2H, J = 7.2, 1.1 Hz), 8.23 (dd, 2H, J = 8.1, 1.1 Hz), 7.76 (dd, 2H, J = 8.1, 7.2 Hz), 4.56–4.44 (m, 2H, HC5 and CH 2N), 4.33–4.28 (m, 1H, CH 2N), 4.22–4.11 (m, 4H, 2 × CH 2OP), 3.09 (ddd, 1H, J = 9.5, 7.2, 2.3 Hz, HC3), 2.89 (d, 3H, J = 0.8 Hz, CH 3N), 2.65 (dddd, 1H, J = 18.0, 12.5, 7.2, 7.2 Hz, H βC4), 2.44 (dddd, 1H, J = 12.5, 11.6, 9.5, 6.9 Hz, H αC4), 1.33 (t, 3H, J = 7.0 Hz, CH 3CH2OP), 1.32 (t, 3H, J = 7.0 Hz, CH 3CH2OP); 13C NMR (75.5 MHz, CDCl3) δ: 164.16 (s, 2 × C(O)), 134.14, 131.60, 131.47, 128.21, 126.98, 122.50, 74.96 (d, J = 7.6 Hz, C5), 64.06 (d, J = 168.6 Hz, C3), 63.34 (d, J = 6.6 Hz, CH2OP), 62.53 (d, J = 6.9 Hz, CH2OP), 46.71 (br s, CH3N), 42.77 (s, CH2N), 36.55 (s, C4), 16.81 (d, J = 6.0 Hz, CH3CH2OP), 16.73 (d, J = 6.0 Hz, CH3CH2OP); 31P NMR (121.5 MHz, CDCl3) δ: 22.67. Anal. Calcd for C21H25N2O6P: C, 58.33; H, 5.83; N, 6.48; found: C, 58.28; H, 5.59; N, 6.61 (obtained on a 93:7 mixture of trans-10a and cis-10a).
4.4.2. Diethyl trans-5-(5-nitro-1,3-dioxo-2,3-dihydro-1H-benzo[de]izochinolin-2-ylmethyl)-2-methylisoxazolidin-3-yl-3-phosphonate (trans-10b)
Grey amorphous solid (crystallized from chloroform/hexane) mp 197–198 °C; IR (KBr, cm−1) ν max: 2985, 1715, 1672, 1600, 1541, 1343, 1326, 1233, 1033, 974, 799; (signals of trans-10b were extracted from the spectra of a 94:6 mixture of trans-10b and cis-10b); 1H NMR (300 MHz, CDCl3) δ: 9.32 (d, 1H, J = 2.2 Hz), 9.14 (d, 1H, J = 2.2 Hz), 8.79 (dd, 1H, J = 7.3, 1.2 Hz), 8.44 (dd, 1H, J = 8.2, 1.2 Hz), 7.95 (dd, 1H, J = 8.2, 7.3 Hz), 4.56 (dd, 1H, J = 12.5, 7.7 Hz, CH 2N), 4.47 (dddd, 1H, J = 7.7, 7.2, 7.2, 4.0 Hz, HC5), 4.30 (dd, 1H, J = 12.5, 4.0 Hz, CH 2N), 4.25–4.12 (m, 4H, 2 × CH 2OP), 3.09 (ddd, 1H, J = 9.6, 7.2, 2.4 Hz, HC3), 2.88 (d, 3H, J = 1.0 Hz, CH 3N), 2.69 (dddd, 1H, J = 18.1, 12.5, 7.2, 7.2 Hz, H αC4), 2.41 (dddd, 1H, J = 12.5, 11.9, 9.6, 7.2 Hz, H βC4), 1.34 (t, 3H, J = 7.1 Hz, CH 3CH2OP), 1.33 (t, 3H, J = 7.0 Hz, CH 3CH2OP); 13C NMR (75.5 MHz, CDCl3) δ: 163.07 (s, C(O)), 162.50 (s, C(O)), 146.43, 135.66, 134.61, 131.04, 130.24, 129.09, 129.02, 124.55, 124.44, 123.07, 74.53 (d, J = 7.6 Hz, C5), 63.94 (d, J = 169.3 Hz, C3), 63.13 (d, J = 6.5 Hz, CH2OP), 62.42 (d, J = 6.7 Hz, CH2OP), 46.46 (s, CH3N), 43.05 (s, CH2N), 36.34 (s, C4), 16.51 (d, J = 6.3 Hz, CH3CH2OP), 16.47 (d, J = 6.1 Hz, CH3CH2OP); 31P NMR (121.5 MHz, CDCl3) δ: 22.49. Anal. Calcd for C21H24N3O8P: C, 52.83; H, 5.07; N, 8.80; found: C, 52.73; H, 4.82; N, 8.52 (obtained on a 94:6 mixture of trans-10b and cis-10b).
4.4.3. Diethyl trans-5-(5-amino-1,3-dioxo-2,3-dihydro-1H-benzo[de]izochinolin-2-ylmethyl)-2-methylisoxazolidin-3-yl-3-phosphonate (trans-10c)
Yellow amorphous solid (crystallized from chloroform/hexane) mp 126–127 °C; IR (KBr, cm−1) ν max: 3431, 3353, 1692, 1651, 1619, 1236, 1056, 1021, 969, 778; (signals of trans-10c were extracted from the spectra of a 93:7 mixture of trans-10c and cis-10c); 1H NMR (300 MHz, CDCl3) δ: 8.30 (dd, 1H, J = 7.3, 1.0 Hz), 8.00 (d, 1H, J = 2.3 Hz), 7.93 (dd, 1H, J = 8.2, 1.0 Hz), 7.59 (dd, 1H, J = 8.2, 7.3 Hz), 7.29 (d, 1H, J = 2.3 Hz), 4.53–4.42 (m, 2H, HC5 and CH 2N), 4.31–4.25 (m, 1H, CH 2N), 4.23–4.11 (m, 4H, 2 × CH 2OP), 3.09 (ddd, 1H, J = 9.6, 7.2, 2.2 Hz, HC3), 2.89 (d, 3H, J = 0.8 Hz, CH 3N), 2.64 (dddd, 1H, J = 18.2, 12.5, 7.2, 7.2 Hz, H αC4), 2.43 (dddd, 1H, J = 12.5, 11.8, 9.6, 7.2 Hz, H βC4), 1.33 (t, 3H, J = 7.1 Hz, CH 3CH2OP), 1.32 (t, 3H, J = 7.1 Hz, CH 3CH2OP); 13C NMR (75.5 MHz, CDCl3) δ: 164.41 (s, C(O)), 164.12 (s, C(O)), 145.70, 133.25, 131.78, 127.23, 126.91, 123.04, 122.85, 122.07, 121.87, 113.86, 75.17 (d, J = 7.4 Hz, C5), 63.92 (d, J = 170.9 Hz, C3), 63.26 (d, J = 6.5 Hz, CH2OP), 62.74 (d, J = 6.9 Hz, CH2OP), 46.76 (br s, CH3N), 42.64 (s, CH2N), 36.36 (s, C4), 16.77 (d, J = 5.7 Hz, CH3CH2OP), 16.70 (d, J = 5.7 Hz, CH3CH2OP); 31P NMR (121.5 MHz, CDCl3) δ: 22.73. Anal. Calcd for C21H26N3O6P: C, 56.37; H, 5.86; N, 9.39; found: C, 56.42; H, 5.88; N, 9.36 (obtained on a 93:7 mixture of trans-10c and cis-10c).
4.4.4. Diethyl trans-5-(5-acetamido-1,3-dioxo-2,3-dihydro-1H-benzo[de]izochinolin-2-ylmethyl)-2-methylisoxazolidin-3-yl-3-phosphonate (trans-10d)
Yellow amorphous solid (crystallized from chloroform/hexane) mp 166–168 °C; IR (KBr, cm−1) ν max: 3299, 2982, 1628, 1660, 1553, 1255, 1044, 973, 784; (signals of trans-10d were extracted from the spectra of a 93:7 mixture of trans-10d and cis-10d); 1H NMR (300 MHz, CDCl3) δ: 8.81 (d, 1H, J = 2.0 Hz), 8.41 (d, 1H, J = 7.1 Hz), 8.35 (br s, NH), 8.22 (d, 1H, J = 2.0 Hz), 8.07 (d, 1H, J = 8.1 Hz), 7.67 (dd, 1H, J = 8.1, 7.1 Hz), 4.55–4.46 (m, 2H, HC5 and CH 2N), 4.29–4.13 (m, 5H, CH 2N and 2 × CH 2OP), 3.17 (ddd, 1H, J = 9.5, 7.0, 2.3 Hz, HC3), 2.91 (s, 3H, CH 3N), 2.72 (dddd, 1H, J = 18.0, 12.7, 7.0, 7.0 Hz, H αC4), 2.45 (dddd, 1H, J = 12.7, 11.9, 9.5, 7.0 Hz, H βC4), 2.30 (s, 3H, CH 3C(O)), 1.34 (t, 3H, J = 7.1 Hz, CH 3CH2OP), 1.33 (t, 3H, J = 7.1 Hz, CH 3CH2OP); 13C NMR (75.5 MHz, CDCl3) δ: 169.74 (s, C(O)NH), 163.98 (s, C(O)), 163.47 (s, C(O)), 137.34, 133.80, 132.20, 129.62, 127.16, 124.42, 124.24, 122.37, 121.80, 121.66, 75.25 (d, J = 6.2 Hz, C5), 63.93 (d, J = 169.4 Hz, C3), 63.34 (d, J = 6.7 Hz, CH2OP), 63.03 (d, J = 7.1 Hz, CH2OP), 46.87 (br s, CH3N), 42.70 (s, CH2N), 36.27 (s, C4), 24.69 (s, CH3C(O)), 16.78 (d, J = 5.5 Hz, CH3CH2OP), 16.71 (d, J = 5.6 Hz, CH3CH2OP); 31P NMR (121.5 MHz, CDCl3) δ: 22.70. Anal. Calcd for C23H28N3O7P: C, 56.44; H, 5.77; N, 8.59; found: C, 56.53; H, 5.64; N, 8.67 (obtained on a 93:7 mixture of trans-10d and cis-10d).
4.5. Antiviral activity assays
The compounds were evaluated against different herpes viruses, including herpes simplex virus type 1 (HSV-1) strain KOS, thymidine kinase-deficient (TK−) HSV-1 KOS strain resistant to ACV (ACVr), herpes simplex virus type 2 (HSV-2) strain G, varicella-zoster virus (VZV) strains Oka and YS, TK− VZV strains 07-1 and YS-R, human cytomegalovirus (HCMV) strains AD-169 and Davis as well as feline herpes virus (FHV), the poxvirus vaccinia virus (Lederle strain), para-influenza-3 virus, reovirus-1, Sindbis virus, Coxsackie virus B4, Punta Toro virus, respiratory syncytial virus (RSV), feline coronovirus (FIPV) and influenza A virus subtypes H1N1 (A/PR/8), H3N2 (A/HK/7/87) and influenza B virus (B/HK/5/72) and human immune deficiency virus (5HVV-1 and HIV-2). The antiviral assays, other than HIV, were based on inhibition of virus-induced cytopathicity or plaque formation in human embryonic lung (HEL) fibroblasts, African green monkey kidney cells (Vero), human epithelial cervix carcinoma cells (HeLa), Crandell-Rees feline kidney cells (CRFK), or Madin Darby canine kidney cells (MDCK). Confluent cell cultures in microtiter 96-well plates were inoculated with 100 CCID50 of virus (1 CCID50 being the virus dose to infect 50% of the cell cultures) or with 20 plaque forming units (PFU) and the cell cultures were incubated in the presence of varying concentrations of the test compounds. Viral cytopathicity or plaque formation (VZV) was recorded as soon as it reached completion in the control virus-infected cell cultures that were not treated with the test compounds. Antiviral activity was expressed as the EC50 or compound concentration required to reduce virus-induced cytopathicity or viral plaque formation by 50%.
4.6. Cytotoxicity assays
Cytotoxicity measurements were based on the inhibition of cell growth. HEL cells were seeded at a rate of 5 × 103 cells/well into 96-well microtiter plates and allowed to adhere and proliferate for 24 h. Then, medium containing different concentrations of the test compounds was added. After 3 days of further incubation at 37 °C, the cell number was determined with a Coulter counter. The cytostatic concentration was calculated as the CC50, or the compound concentration required reducing cell proliferation by 50% relative to the number of cells in the untreated controls. CC50 values were estimated from graphic plots of the number of cells (percentage of control) as a function of the concentration of the test compounds. Alternatively, cytotoxicity of the test compounds was expressed as the minimum cytotoxic concentration (MCC) or the compound concentration that caused a microscopically detectable alteration of cell morphology.
Acknowledgments
The authors wish to express their gratitude to Mrs. Leentje Persoons, Mrs. Frieda De Meyer, Mrs. Lies Van den Heurck and Mrs. Lizette van Berckelaer for excellent technical assistance. The synthetic part of this work was supported by the Medical University of Łódź, Poland, internal funds (503/3-014-1/503-01 and 502-03-/3-014-01/502-34-020). The biological part of this work was supported by the KU Leuven, Belgium, (GOA 10/014).
References and notes
- 1.Hendry L.B., Mahesh V.B., Bransome E.D., Jr., Ewing D.E. Mutat. Res. 2007;623:53. doi: 10.1016/j.mrfmmm.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 2.Martínez R., Chacón-García L. Curr. Med. Chem. 2005;12:127. doi: 10.2174/0929867053363414. [DOI] [PubMed] [Google Scholar]
- 3.Braña M.F., Cacho M., Gradillas A., de Pascual-Teresa B., Ramos A. Curr. Pharm. Design. 2001;7:1745. doi: 10.2174/1381612013397113. [DOI] [PubMed] [Google Scholar]
- 4.Lerman L.S. J. Mol. Biol. 1961;3:18. doi: 10.1016/s0022-2836(61)80004-1. [DOI] [PubMed] [Google Scholar]
- 5.Hogan M., Dattagupta N., Crothers D.M. Biochemistry. 1979;18:280. doi: 10.1021/bi00569a007. [DOI] [PubMed] [Google Scholar]
- 6.Pommier Y. Chem. Rev. 2009;109:2894. doi: 10.1021/cr900097c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheithauer W., Dittrich C., Kornek G., Haider K., Linkesch W., Gisslinger H., Depisch D. Breast Cancer Res. Treat. 1991;20:63. doi: 10.1007/BF01833358. [DOI] [PubMed] [Google Scholar]
- 8.Costanza M.E., Berry D., Henderson I.C., Ratain M.J., Wu K., Shapiro C., Duggan D., Kalra B., Berkowitz I., Lyss A.P. Clin. Cancer Res. 1995;1:699. [PubMed] [Google Scholar]
- 9.Rosell R., Carles J., Abad A., Ribelles N., Barnadas A., Benavides A., Martin M. Invest. New Drugs. 1992;10:171. doi: 10.1007/BF00877242. [DOI] [PubMed] [Google Scholar]
- 10.Henry D.W. Cancer Treat. Rep. 1979;63:845. [PubMed] [Google Scholar]
- 11.Bair K.W., Andrews C.W., Tuttle R.L., Knick V.C., Cory M., McKee D.D. J. Med. Chem. 1991;34:1983. doi: 10.1021/jm00111a010. [DOI] [PubMed] [Google Scholar]
- 12.Bair K.W., Tuttle R.L., Knick V.C., Cory M., McKee D.D. J. Med. Chem. 1990;33:2385. doi: 10.1021/jm00171a012. [DOI] [PubMed] [Google Scholar]
- 13.Rescifina A., Chiacchio M.A., Corsaro A., De Clercq E., Iannazzo D., Mastino A., Piperno A., Romeo G., Romeo R., Valveri V. J. Med. Chem. 2006;49:709. doi: 10.1021/jm050772b. [DOI] [PubMed] [Google Scholar]
- 14.Rescifina A., Chiacchio U., Piperno A., Sordino S. New J. Chem. 2006;30:554. [Google Scholar]
- 15.Piotrowska D.G., Cieślak M., Królewska K., Wróblewski A.E. Arch. Pharm. Chem. Life Sci. 2011;11:301. doi: 10.1002/ardp.201000282. [DOI] [PubMed] [Google Scholar]
- 16.Kokosza K., Balzarini J., Piotrowska D.G. Bioorg. Med. Chem. 2013;21:1097. doi: 10.1016/j.bmc.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bansal Y., Silakari O. Eur. J. Med. Chem. 2014;76:31. doi: 10.1016/j.ejmech.2014.01.060. [DOI] [PubMed] [Google Scholar]
- 18.Lv M., Xu H. Curr. Med. Chem. 2009;16:4797. doi: 10.2174/092986709789909576. [DOI] [PubMed] [Google Scholar]
- 19.Rozovsky A., Regozin E., Oron-Herman M., Albeck A., Gellerman G. Eur. J. Org. Chem. 2015:1811. [Google Scholar]
- 20.Kamal A. Bioorg. Med. Chem. Lett. 2002;12:1933. doi: 10.1016/s0960-894x(02)00326-8. [DOI] [PubMed] [Google Scholar]
- 21.Kamal A., Ramu R., Tekumalla V. Bioorg. Med. Chem. 2008;16:7218. doi: 10.1016/j.bmc.2008.06.034. [DOI] [PubMed] [Google Scholar]
- 22.Rettig M., Kamal A., Ramu R., Mikolajczyk J., Weisz K. Bioorg. Med. Chem. 2009;17:919. doi: 10.1016/j.bmc.2008.11.033. [DOI] [PubMed] [Google Scholar]
- 23.Wu A., Mei P., Xu Y., Qian X. Chem. Biol. Drug Des. 2011;78:841. doi: 10.1111/j.1747-0285.2011.01232.x. [DOI] [PubMed] [Google Scholar]
- 24.Tian Z.-Y., Li J.-H., Li Q., Zang F.-L., Zhao Z.-H., Wang C.-J. Molecules. 2014;19:7646. doi: 10.3390/molecules19067646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikeda H., Nakamura Y., Saito I. Tetrahedron Lett. 2002;43:5525. [Google Scholar]
- 26.Hilderbrand R.L. CRC Press; Boca Raton: 1983. The Role of Phosphonates in Living Systems. [Google Scholar]
- 27.Ares J.J., Kador P.F., Miller D.D. J. Med. Chem. 1986;29:2384. doi: 10.1021/jm00161a040. [DOI] [PubMed] [Google Scholar]
- 28.Amegadzie, A. K.; Carey, M. E.; Domagala, J. M.; Huang, L.; Micentich, R. G.; Sanchez, J. P.; Singh, R.; Stier, M. A.; Vaisburg, A. US 6362181B1, 2002.
- 29.Li X., Lin Y., Yuan Y., Liu K., Qian X. Tetrahedron. 2011;67:2299. [Google Scholar]
- 30.Van Quaquebeke E., Mahieu T., Dumont P., Dewelle J., Ribaucour F., Simon G., Sauvage S., Gaussin J.-F., Tuti J., El Yazidi M., Van Vynckt F., Mijatovic T., Lefranc F., Darro F., Kiss R. J. Med. Chem. 2007;50:4122. doi: 10.1021/jm070315q. [DOI] [PubMed] [Google Scholar]
- 31.Piotrowska D.G. Tetrahedron Lett. 2006;47:5363. [Google Scholar]
- 32.Kokosza K., Balzarini J., Piotrowska D.G. Nucleosides, Nucleotides Nucleic Acids. 2014;33:552. doi: 10.1080/15257770.2014.909046. [DOI] [PubMed] [Google Scholar]
- 33.Łysakowska M., Balzarini J., Piotrowska D.G. Arch. Pharm. Chem. Life Sci. 2014;347:341. doi: 10.1002/ardp.201300382. [DOI] [PubMed] [Google Scholar]
- 34.Goldner T., Hempel C., Ruebsamen-Schaeff H., Zimmermann H., Lischka P. Antimicrob. Agents Chemother. 2015;58:610. doi: 10.1128/AAC.01794-13. [DOI] [PMC free article] [PubMed] [Google Scholar]