Abstract
Monoclonal antibodies (mAbs) are increasingly being considered as agents to fight severe viral diseases. So far, they have essentially been selected and used on the basis of their virus-neutralizing activity and/or cell-killing activity to blunt viral propagation via direct mechanisms. There is, however, accumulating evidence that they can also induce long-lasting protective antiviral immunity by recruiting the endogenous immune system of infected individuals during the period of immunotherapy. Exploiting this property may revolutionize antiviral mAb-based immunotherapies, with benefits for both patients and healthcare systems.
Keywords: monoclonal antibodies, immunotherapy, antiviral therapy, vaccine-like effects, immune complexes, Fc receptors
Trends
Antiviral monoclonal antibodies (mAbs) are promising, high-added-value biotherapeutics. During recent years, the number of antiviral mAbs developed against both acute and chronic viruses has grown exponentially, some of them being currently tested in clinical trials.
Antiviral mAbs can be used to blunt viral propagation through direct effects. They can also engage the host's immune system, leading to the induction of long-lasting protective vaccine-like effects.
The assessment of mechanisms at play in the induction of vaccine-like effects by antiviral mAbs will help in improving antiviral treatments.
Exploiting this effect will translate into therapeutic benefit for patients. The benefit will also help healthcare systems through the reduction of treatment costs.
The Increasing Promise of Antiviral mAbs
Although mAbs currently constitute the main class of biotherapeutics, the possibility of using them to treat viral infections has received limited attention until recently. This contrasts with cancer and inflammatory diseases against which they are now widely utilized 1, 2, 3. This is also paradoxical if one considers that the first commercial mAb was an anti-respiratory syncytial virus (RSV) antibody used to fight a pediatric respiratory illness. This situation, however, is reversing. Indeed, a flurry of human, or humanized, mAbs against H5N1 influenza virus, human immunodeficiency virus (HIV), herpes simplex virus (HSV), cytomegalovirus (CMV), hepatitis C virus (HCV), Ebola virus, Marburg virus, severe acute respiratory syndrome (SARS) virus, dengue virus, rabies virus, Hendra virus, Nipah virus, yellow fever virus, and West Nile virus have been described in the past few years 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17. Some of these mAbs are currently being tested in the clinic and include second-generation mAbs with improved neutralizing activity and/or novel target specificities 6, 7, 18, 19, 20, 21, 22. This is especially true for HIV, which has dominated most of the reports dedicated to broadly neutralizing mAbs during the past 2 years. Finally, antiviral mAbs have already demonstrated partial efficacy when administered after HIV, HCV, or Ebola virus established infections 11, 23, 24, fueling the idea that they represent promising, high-added-value therapeutic agents.
Direct Mechanisms of Action of Antiviral mAbs
mAb therapy is a form of passive immunotherapy (see Glossary) that is classically intended to blunt viral infections via direct and rapid targeting of the infectious agent rather than via the triggering of a long-term immune response against it. This therapeutic approach contrasts with vaccine approaches that aim to stimulate the endogenous immune response of the host, in order to provide sustained protective immunity.
mAbs can diminish viral dissemination by direct action involving both their antigen-binding activity and the effector functions borne by their Fc fragment. So far, most antiviral mAbs with therapeutic potential have initially (and logically) been selected for their ability to neutralize virions via the recognition of viral surface antigens essential for receptor binding and/or entry into host cells. Furthermore, direct recognition has also been shown to inhibit cell–cell transmission of virions in certain settings.
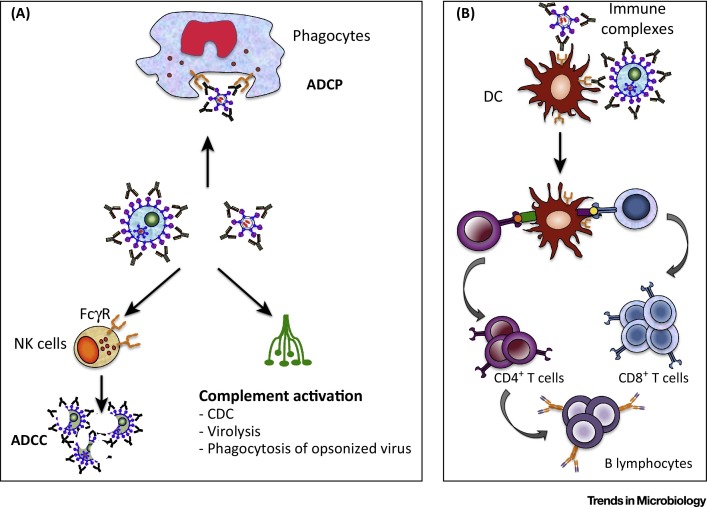
Most antiviral mAbs studied to date are immunoglobulin Gs (IgGs), that is, antibodies efficiently recognized by both the complement and the Fcγ receptors (FcγRs) borne by many cells of the immune system. In addition to complement-mediated inactivation of viral particles, and/or their phagocytosis, this also permits complement-dependent cytotoxicity (CDC), antibody-dependent cellular phagocytosis (ADCP), and antibody-dependent cell-mediated cytotoxicity (ADCC) to eliminate infected cells displaying viral antigens at their surface (Figure 1A). This, for instance, is the case of mAbs targeting the envelope glycoprotein (Env) of HIV (and other lenti-/retroviruses) that is exposed at the surface of virus-producing cells. Finally, Fc–FcγR interactions can also directly impact viral propagation via a mechanism called antibody-dependent, cell-mediated virus inhibition (ADCVI) (see 19, 25, 26, 27 for more information on direct Fc-mediated antiviral effects). Thus, during immunotherapies, mAbs can impact viral propagation via a variety of direct mechanisms, possibly varying according to the virus, the viral antigen recognized and the antibody itself. However, there is now accumulating evidence that antiviral mAbs, upon interaction with different components of the immune system, can also operate via indirect mechanisms, that is, engagement of the host immune response, with effects lasting well beyond the treatment itself. Thus, similarly to vaccine approaches, mAb treatment could lead to the stimulation of the endogenous humoral and cellular immune responses in such a manner as to provide protective immunity (‘vaccine-like effects’).
Figure 1.
Fc Fragment(Fc)-Mediated Activities of Antiviral Monoclonal Antibodies (mAbs). (A) Upon administration, an antiviral mAb can opsononize virus, as well as infected cells if the viral antigen is also expressed on their surface. This can lead to virus elimination by complement activation and/or phagocytosis mediated by cells of the innate immune system. Infected cells can also be eliminated by complement-dependent cytotoxicity (CDC) as well as by antibody-dependent cell-mediated cytotoxicity (ADCC) and/or antibody-dependent cellular phagocytosis (ADCP) mediated by FcγR-bearing effector cells. (B) Immune complexes (IC), constituted by mAb-coated virions or infected cells, can be recognized by FcγRs expressed on antigen-presenting cells such as dendritic cells (DC). Such IC recognition leads to enhanced antigen uptake and presentation, allowing induction of stronger humoral and cellular antiviral immune responses. Abbreviation: NK cells, natural killer cells.
Reasons to Suspect Indirect Long-Lasting Effects of Antiviral mAbs on Infected, Immunotreated Individuals’ Immunity
During the immunotherapy period, antiviral mAbs form immune complexes (ICs) with virions and, in some cases, infected cells. Such ICs can then be recognized by antigen-presenting cells (APCs) such as dendritic cells (DCs), which are central for any adaptive immune responses (Figure 1B). Surprisingly, until recently, the scientific and medical communities have largely failed to take into consideration that this could impact the endogenous immunity (i.e., the enhancement of antibody responses as well as cytotoxic T-cell responses) of infected, immunotherapy-treated patients. This is all the more puzzling as positive immunomodulatory (vaccine-like) effects, which strengthen the patients’ immune defenses against viral infections, would translate into obvious benefits, not only for the patients, but also for healthcare systems that would benefit from both reduced cost and duration of treatment.
Several objective reasons might explain this situation. First, most antiviral mAbs (if not all) were initially selected on the basis of their blunting effects on viral propagation. Second, models of viral infections in immunocompetent animals, allowing in-depth analyses of endogenous immune responses in the context of passive mAb-based immunotherapies, are scarce. This is especially true for relevant models of chronic infections by viruses such as HIV or HCV, against which most antiviral mAbs available to date were raised. Indeed, anti-HIV (and to a lesser extent anti-HCV) mAb activity was assessed mainly in immunocompromized, humanized mice reconstituting only partially the human immune system 28, 29, 30, 31, 32 and/or nonhuman primates (NHPs). Although NHPs are extremely useful for assessing the protective effects of anti-HIV mAbs, their use in the study of immunity is limited due to both technical and cost reasons. In contrast, mouse infection systems offer numerous immunologic tools and permit cost-effective experimentation on large cohorts. Third, most often antiviral mAbs have been generated in species different from those used in the in vivo experiments and therefore Fc-associated effector functions were not, or not fully, preserved. Finally, ethical, technical, and economic reasons, together with the low number of ongoing clinical trials, still limit this type of investigation in humans.
Despite this apparently unfavorable context, however, evidence gained in various animal models has recently demonstrated that vaccine-like effects can be induced by short mAb therapies. These experiments are reviewed below before potential improvements of the approach are discussed.
Lessons From the FrCasE Retrovirus Mouse Model
FrCasE retrovirus is a murine leukemia virus (MLV). FrCasE infection of newborn mice is reminiscent of perinatal infection by HIV, in that viral expansion occurs in an organism with developing immunity. It provides one of the rare models of chronic viral infection, in an immunocompetent animal, for which neutralizing mAbs of the same species (mouse) are available [33]. It was the first experimental system to permit the unambiguous demonstration of the possibility of inducing vaccine-like effects by mAb-based immunotherapy 34, 35, 36, 37.
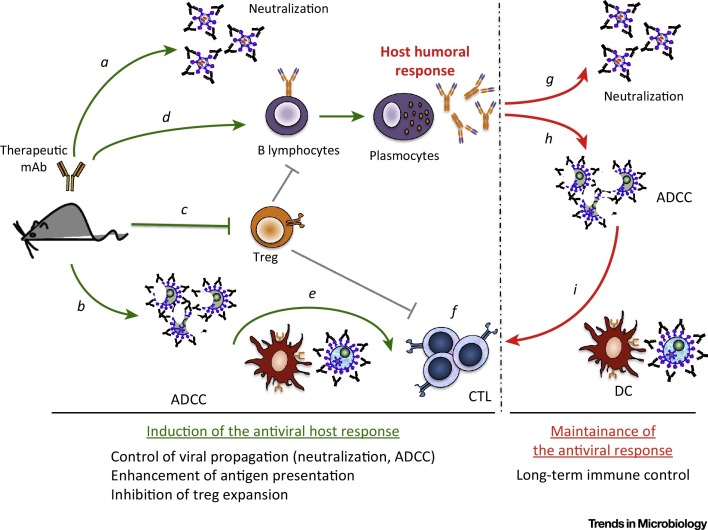
When inoculated into 8-day-old pups, FrCasE induces a fatal erythroleukemia (or neurodegeneration if inoculated earlier) associated with weak non-protective humoral and cellular antiviral immune responses. However, short treatments (a few days) with a neutralizing IgG2a (equivalent to IgG1 in humans) mAb, recognizing viral Env, shortly after infection allow infected mice to survive in good health and with a normal lifespan. Survival is associated with decreased viral propagation and, importantly, is strictly dependent on the development of a life-long protective endogenous antiviral immune response. This immunity is of the Th1 type with a humoral arm directed against the virus and a cytotoxic T-cell arm directed against infected/leukemic cells, both of them displaying strong memory responses in challenge experiments. Detailed analyses indicate that the induction of protective immunity is not the mere result of the reduction of the viral load during the immunotherapy but the consequence of a genuine immunomodulatory action of the therapeutic mAb involving various FcγR-dependent mechanisms 36, 37. In particular, the mAb induces Env-expressing infected cell lysis through a natural killer (NK) cell-dependent ADCC mechanism. This most likely provides an inflammatory environment favoring the induction of the antiviral immune response. Moreover, ICs formed with infected cells are more efficiently captured by DCs than infected cells alone, leading to stronger functional DC activation and elicitation of a potent antiviral cytotoxic T lymphocyte (CTL) response (Figure 2 , Key Figure). Importantly, the endogenous antiviral antibodies generated by the immunotherapy allow containment of viral propagation and contribute to the maintenance of a T-cell memory response once the therapeutic antibody has been eliminated from treated mice 36, 37. A third FcγR-dependent effect described is the inhibition of the expansion of regulatory T cells (Treg), which is essential for the induction of protective immunity [38] (Figure 2). Such a finding is, in fact, not surprising as Treg expansion is associated with all types of chronic viral infections and leads to dampening of the antiviral immune response, promoting chronicity and subsequent pathologic manifestations.
Figure 2.
Key Figure: Induction of Life-Long Protective Immunity Against FrCasE by Neutralizing Antiviral Monoclonal Antibodies (mAbs)
The administration, to FrCasE-infected mice, of a neutralizing antiviral IgG2a mAb directed to the viral Env glycoprotein limits viral propagation through neutralization of virus (a) and elimination of infected cells (b). At the same time, the immune complexes (ICs) formed with the virus, and probably more importantly with infected cells expressing Env at their surface, prevent the expansion of regulatory T cells (Treg cells) (c), which is necessary for the development of protective humoral (d) and cellular (e, f) responses by the host. In particular, the ICs formed between the therapeutic mAbs and infected cells activate dendritic cells (DCs), leading to an enhanced virus-specific CD8 T-cell response (f). Once the therapeutic antibody has been eliminated from treated mice, both arms of the adaptive immune system contribute to viral propagation control, as the endogenous antiviral antibodies, induced by the immunotherapy, control viral propagation (g, h) and contribute to the maintenance of a T-cell memory response. This requires the formation of ICs engaging residual infected cells and activation of DCs (i). Abbreviation: ADCC, antibody-dependent cell-mediated cytotoxicity.
Thus, the FrCasE model has provided the proof of concept that short mAb-based antiviral therapies can induce life-long protective immunity permitting an infected organism to survive in good health. Yet, a number of issues are still pending. In particular, it will be crucial to identify all of the cell types and molecular effectors involved in this process as well as to elucidate the mechanisms whereby long-term immunity is maintained in order to apply it to the treatment of human disease.
Can mAbs Directed to Human Viruses Also Generate Vaccine-like Effects?
Antiviral immune responses induced, or strengthened, by antiviral mAb-based immunotherapies have also been observed in various chronic and acute life-threatening human viruses (see summary in Table 1 ). This is most notable in preclinical NHP models of HIV infection using second-generation, broadly neutralizing antibodies (bnAbs). Such observations are all the more important now that these mAbs are being tested in clinical trials.
Table 1.
Antiviral Immune Responses Induced by Antiviral Monoclonal Antibodies (mAbs) Against Life-Threatening Human Virusesa
| Animal Model | Virus | Therapeutic Antibody | Administration | Clinical Outcome | Cellular Response | Humoral Response | Refs |
|---|---|---|---|---|---|---|---|
| Rhesus monkeys (Macaca mulatta) |
SIV smE660 (i.v.) |
SIVIG | i.v. (1 and 14 days post-infection) |
Delayed disease, increased survival rate; control of viremia (5-year follow-up) |
No difference in CD4+ T-cell counts, 30% animals develop an antiviral CTL response |
Acceleration of de novo nAb production | [39] |
| Rhesus monkeys (Macaca mulatta) |
SIV mac239 (intramuscular) |
SIVIG | i.v. (7 days post-infection) |
Reduction of viral loads | Polyfunctional Gag-specific T-cell response | Transient nAb response | 40, 41 |
| Pigtail macaques (Macaca nemestrina) |
SHIVSF162P3 (oral) |
SHIVIG + IgG1b12 | s.c. (24 h before challenge) |
Transient reduction in plasma viremia (6 months) |
No difference in CD4+ T-cell counts | Rapid appearance of nAb | [43] |
| Rhesus monkeys (Macaca mulatta) |
SHIVSF162P3 (oral) |
SHIVIG | s.c. (24 h before challenge) |
Protection from disease | No difference in total Gag-specific CD8+ T-cell response | Preservation of B cells: de novo nAb production (ADCVI) |
[44] |
| Rhesus monkeys (Macaca mulatta) |
SHIVSF162P3 (Intrarectal) |
PGT121+3BNC117+b12; PGT121+3BNC117; PGT121 |
i.v. (9 months post-infection) |
Decline in plasma viremia | Improved functionality of Gag-specific T-cell response | de novo nAb to SHIV-SF162P3 | [23] |
| Rhesus monkeys (Macaca mulatta) |
SHIV-1157ipEL-p (Intrarectal) |
HGN194 | i.v. (1 day before infection and 7 days post-infection) |
Aviremia, protection not reported |
Gag-specific T-cell response | N.D. | [46] |
| BALB/c mice | RSV r19F (Intranasal) |
131-2G mAb | i.p. (2 days before infection) |
Decreased virus replication | Increased Thf counts; decreased IL4, increased IFN-γ+ T cells; high counts of virus-specific CD8+ T cells |
Enhanced humoral response | [47] |
| African Green monkeys (Chlorocebus aethiops) |
HeV (Intratracheally) |
m102.4 | i.v. (10 h, 24 h or 72 h post-infection and 48 h later) |
Reduced viral loads | N.D. | Anti-HeV humoral response | [50] |
| African Green monkeys (Chlorocebus aethiops) |
NiV (Intratracheally) |
m102.4 | i.v. (24 h, 72 h, 60 h post-infection and 48 h later) |
Protection | N.D. | Antiviral humoral response | [51] |
Abbreviations: ADCVI, antibody-dependent cell-mediated viral inhibition; CTL, cytotoxic T lymphocyte; IL-4, interleukin-4; IFN-γ: interferon-γ; i.p., intraperitoneal; i.v., intravenous; nAbs, neutralizing antibodies; N.D., not determined; RSV, respiratory syncytial virus; SIVIG/SHIV, highly neutralizing polyclonal immune globulin prepared from hyperimmune SIV/SHIV-infected animals; s.c., subcutaneous; Tfh, follicular helper T cells.
Simian Immunodeficiency Virus (SIV)
Infection of macaques by different strains of SIV is widely used in NHP models of HIV infection, as well as in immunotherapy experiments. Several reports now indicate that short treatments of SIV-infected macaques with neutralizing antibodies can lead to enhanced antiviral immunity 39, 40. However, these experiments were performed with SIV-specific, highly neutralizing, polyclonal immune globulins (SIVIG) prepared from SIV-infected animals and not mAbs. In a first study, the SIVIG treatment could both significantly delay disease onset and increase the survival rate of SIVsmE660-infected Macaca mulatta macaques, with the long-term survivors showing accelerated de novo production of anti-SIV-neutralizing antibodies [39]. In another set of experiments, rhesus macaques treated with SIVIG shortly after SIVmac239 infection resulted in transiently detectable neutralizing-antibody responses followed by reduction in viral loads as compared to untreated macaques [41]. Interestingly, a virus-specific polyfunctional CD4+ T-cell response was also induced during the acute phase of infection and maintained elevated during the following chronic phase together with a CD8+ T-cell antiviral response measured by the appearance of CTLs specific to capsid (Gag). Intriguingly, no neutralizing antibodies could be measured during the latter phase (i.e., after SIVIG had disappeared) [40], underlining a striking difference with the M. mulata macaque and FrCasE models for reasons that will have to be elucidated. Moreover, DCs stimulated in vitro with SIVIG-preincubated SIV were shown to activate virus-specific CD4+ T lymphocytes in an Fc-dependent manner, suggesting that enhanced T-cell priming during the immunotherapy period is dependent on antibody-mediated virion uptake by these APCs [41]. Such a conclusion is consistent with a former observation that MHC class I-restricted cross-presentation of SIV capsid protein (Gag) is enhanced by anti-Gag antibodies to generate stronger Gag-specific CD8+ T-cell responses in SIV-infected macaques [42].
Thus, taken together, these experiments suggest that the administration of neutralizing antibodies can strengthen the antiviral immunity of lentivirus-infected organisms. Some of them also point to a crucial role for viral ICs in the observed effects.
Simian HIV
SHIV (simian HIV) is a chimeric virus in which HIV Env substitutes for that of SIV. Its use is pertinent to the assessment of the effects of HIV-neutralizing mAbs in NHP. Interestingly, 1-month-old pigtail macaques treated with the human HIV-neutralizing mAb b12 (one of the first to be generated) combined with polyclonal SHIVIG before oral challenge with the SF162P3 SHIV strain, developed an accelerated neutralizing antibody response and showed reduced plasma viremia for at least 6 months [43]. However, in this infection model, despite very high persistent viremia, there was no evidence of pathogenesis in the 6-month period of the study precluding the assessment of viral disease protection by the mAb-induced B-cell response. In contrast, such a study was performed using oral infection of 1-month-old rhesus macaques with the same virus, which leads to disease manifestation within 12 weeks. In this model, passive immunotherapy with polyclonal SHIVIG, 24 h prior to infection, reduced viral propagation, accelerated de novo neutralizing antibody production (also translating into ADCVI), and was associated with protection from disease [44]. These data are consistent with the concept of protective immunomodulatory effects induced by passive immunotherapies with neutralizing antibodies, at least when administered at the time of infection. These effects, however, also seem possible upon mAb treatment at a much later time. For example, the administration of either a cocktail of HIV-1-specific mAbs (PGT121, 3BNC117, and b12), or of the PGT121 mAb alone, 9 months after SHIV-SF162P3 infection of rhesus macaques resulted in the rapid decline of plasma viremia to undetectable levels correlating with a slight increase in neutralizing antibody titers. Interestingly, this humoral response was complemented by a T-cell response characterized by a significant improvement of the functionality of Gag-specific CD8+ and CD4+ T lymphocytes (manifested by a decreased expression of the exhaustion marker PD-1) despite there being no change in their numbers. Moreover, if virus rebounded in the majority of animals when serum mAb had disappeared, a subset of animals maintained long-term virological control in the absence of further mAb infusions [23]. Overall, these data demonstrate a strong therapeutic effect of potent neutralizing HIV-1-specific mAb in SHIV-infected rhesus monkeys as well as a positive impact on their immune response. These findings are potentially important, as very recently the 3BNC117 mAb has been shown to be safe and effective in reducing HIV-1 viremia in a phase 1 human clinical trial [45].
The notion that neutralizing mAb treatments can enhance antiviral immune responses is further supported by other experiments. For example, treating SHIV-1157pEL-p strain-infected rhesus macaques with the neutralizing mAb HGN194 led to both undetectable virus levels and the development of CD4+ and CD8+ Gag-specific responses, although disease progression was not assessed on the long term [46]. In addition, a single bnAb can control SHIV infection in immune-competent macaques but not in SHIV-infected humanized mice, suggesting that the macaque host immune response can contribute to viremia control and to immunotherapy success by preventing the resurgence of bnAb escape variants [30].
Thus, SHIV infection models strongly suggest that neutralizing anti-HIV antibody therapy may, at least to a certain extent, recruit the infected host immune system to generate an antiviral effect. An important issue is now to maximize this effect for efficient treatment of HIV-infected humans (see below).
Respiratory Syncytial Virus (RSV)
There is evidence that neutralizing mAb immunotherapies can affect endogenous antiviral immune response not only in chronic infections but also in acute infections. For example, the prophylactic treatment of RSV, an acute cytopathic virus, by a neutralizing mAb (131-2G) directed towards its G attachment protein (that also has strong immunosuppressive activity) can shift the adaptive immune response from the Th2- to the Th1-type [47]. Thus, 131-2G mAb-treated mice infected with the RSV r19F strain show an enhanced and sustained humoral response characterized by an increased ratio of anti-RSV IgG2a to IgG1, as compared with non-treated, infected mice. Moreover, the stimulation and skewing of the humoral response also correlate with an increase in follicular helper T cells (Tfh) and a higher frequency of CD8+ T cells directed to infected cells. It should be noted, however, that the effects on B-cell and T-cell responses are not Fc-dependent, as the 131-2G F(ab′) 2 fragment leads to the same shift of the adaptive immune response as the whole mAb 48, 49. These data suggest that neutralizing a virus via targeting one of its immunosuppressive proteins may represent an attractive approach to induce protective antiviral immunity.
Hendra and Nipah Henipaviruses
Hendra (HeV) and Nipah (NiV) are related acute and fatal henipaviruses against which therapeutic mAbs have also been developed. HeV challenge of African Green monkeys mirrors fatal human infection leading to death by day 8 post-infection. Upon rapid infusion of the human HeV-neutralizing mAb m102.4, infected monkeys survive after having transiently displayed neurologic symptoms [50]. Recovery from disease correlates with the mounting of a protective humoral immune response reducing the viral load. Interestingly, the 102.4 mAb cross-reacts with NiV and using it in post-exposure therapy of NiV-infected African green monkeys also leads to the development of an antiviral humoral response, which, most likely, both limits viral dissemination and contributes to protection from NiV disease [51]. Thus, the cross-reactive m102.4 mAb, which is currently being developed for human use as a Nipah- and Hendra-virus countermeasure [52], shows exceptional efficacy, not only because of its direct antiviral action, but probably also through its ability to affect endogenous immunity.
Improving Vaccine-like Effects of mAbs: Possible Future Directions
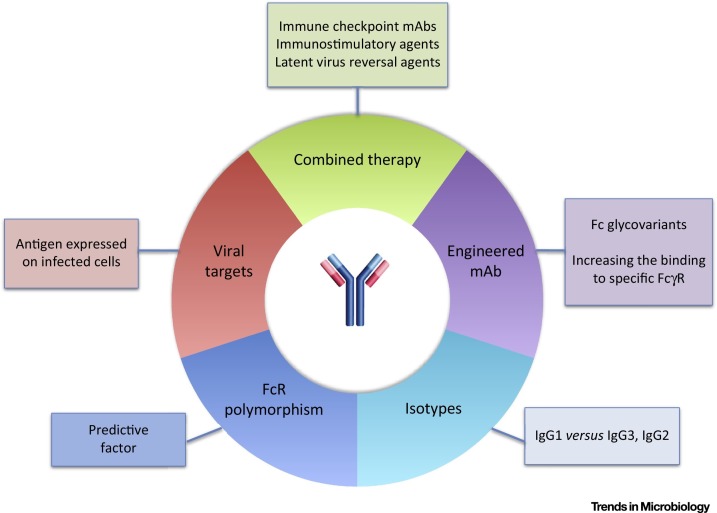
An important issue now is to translate the newly established concept of vaccine-like effects of antiviral mAbs into actual and efficient human medical applications. In-depth analysis of the underlying cellular and molecular mechanisms at play in the experimental settings already at hand (whether in rodents or in NHP) will certainly help towards this aim. However, three obvious lines of immunotherapy improvement can already be considered (Figure 3 ).
Figure 3.
Possible Improvements in Monoclonal Antibody(mAb)-Based Antiviral Immunotherapies. The identification of the molecular and cellular mechanisms responsible for the induction of vaccine-like effects by antiviral mAbs will be paramount to the improvement of future antiviral passive immunotherapies. They are currently the object of intense research. Furthermore, several possibilities pertaining to the conditions of mAb administration, the mAbs themselves, and their targets, can already be considered. These include combined therapies affecting viral propagation and/or enhancing immune responses; engineering mAbs to improve their effector functions, i.e., increasing their binding to Fcγ receptors (FcγRs) by affecting their glycosylation or their amino acid sequence; pertinent selection of their isotype; taking into account FcγR polymorphisms in patients to be treated; and selecting the most appropriate viral antigens.
Combined Therapies
There are reasons to suspect that combining mAb treatments with other therapies may strengthen or induce antiviral vaccine-like effects. A first approach might rely on inhibiting immunosuppressor mechanisms of infected, treated individuals, for example via depressing Tregs by treatments entailing either their depletion and/or loss of their activity (reviewed in [53]). This would be all the more pertinent because Treg expansion dampens antiviral immunity in a number of infections (see above). Targeting ‘immune checkpoints’ may represent another option. This could be achieved using the anti-PD-1 or anti-CTLA-4 mAbs that are currently used to treat cancer (reviewed in 54, 55. The fact that inhibiting the PD-1–PD-1L interaction enhances virus-specific cellular immune responses in different animal models of HCV [56], hepatitis B virus (HBV) 57, 58, HIV [59], SIV [60], and murine retrovirus [61] infections provides support for this idea, as well as the observations that PD-1 blockade stimulates anti-HBV cellular immunity in HBV-infected patients 62, 63 and is associated with the cure of a small subset of HCV-infected patients [64].
Combining antiviral mAbs with immunostimulatory agents can also be considered. Agonistic mAbs targeting T-cell costimulatory receptors such as CD40, OX40, GITR, and CD137 (reviewed in [65]) currently tested in the clinic may serve such a purpose, as the engagement of such costimulatory receptors is already known to enhance antiviral cellular immunity in different infection settings 66, 67, 68, 69. Alternatively, agonists of Toll-like receptors (TLRs) could be used, as, on one hand, activation of TLRs is essential for the development of many natural antiviral responses and, on the other hand, TLR7/8 and -9 agonists have already been shown to cooperate to enhance the anti-HIV-1 Env humoral response in rhesus macaques [70]. Further support for this idea comes from the observation that combining antitumor mAbs with different TLR agonists improves several cancer immunotherapies [71].
Lastly, combining mAb therapy with latency reversal agents to flush out viruses from reservoirs might be used to stimulate anti-HIV immunity, as the release of viral antigens should favor the formation of ICs and, thereby, subsequent stimulation of APCs. Supporting the idea of possible cooperation between these two therapeutics, broadly neutralizing mAbs have already been shown capable of cooperating with several viral inducers to decrease the rebound of HIV-1 from its latent reservoirs in humanized mice [72]. Vaccine-like effects, however, could not be studied in this case.
Improving Fc-borne Effector Functions
Fc-borne effector functions are known to be essential for antiviral protection by various mAbs 27, 37, 73, 74, 75, 76, 77. This suggests that Fc engineering could represent an avenue to not only improve direct mAb antiviral efficacy but also to induce stronger vaccine-like effects. In particular, enhancing the affinity of mAbs for the FcγRs displayed by APCs could be achieved via the modification of the FcγRs’ peptide-binding motifs or via the production of mAbs with altered glycosylation (which can enhance binding to FcγR) 78, 79, 80, 81, 82. Indeed, Fc-engineered glycovariants were shown to enhance Fc-mediated reduction of viral replication and FcγR binding in different in vitro infection settings for Ebola virus and HIV 82, 83. Also supporting this idea, spontaneous control of HIV and improved antiviral activity correlated with a dramatic shift in the global antibody-glycosylation profile toward agalactosylated glycoforms [84]. Another interesting possibility of Fc engineering may aim at enhancing the binding to the neonatal Fc receptor (FcRn) expressed by various adult cell types [85]. Not only could this lead to increased mAb half-life in vivo [85] but also to stronger vaccine-like effects. Indeed, FcRn cross-linking on APCs by multivalent ICs has been shown to entail stronger humoral and cellular adaptive responses in certain infection settings 86, 87. In addition, an enhanced FcRn function of the broadly neutralizing mAb VRC01 improved protection against SHIV infection in macaques [88].
As not all IgG isotypes display equivalent effector functions, another possibility may reside in either antibody subclass selection or subclass switching via genetic engineering. Most human antiviral mAbs studied so far are IgG1s that display strong affinity for both FcγRs and complement. However, recent data suggest that other human IgG subtypes may exert stronger antiviral effects, at least under certain conditions. Thus, IgG2 antibodies to HIV Gag have been suggested to contribute to immune control of HIV infection by broadening and enhancing the function of IgG antibodies [89]. Moreover, increased vaccine efficacy and decreased risk of HIV-1 infection, in one of two clinical trials (RV144 and VAX003), was correlated with the development of highly functional anti-HIV IgG3 90, 91, 92.
Clearly, further work is still needed to establish how alteration of antibody effector function or isotype selection may be exploited to enhance or induce mAb-induced antiviral vaccine-like effects. This task will be complicated because different FcγRs are expressed at various levels by the different APCs and show functional polymorphism. Supporting this possibility, FcγRIIa polymorphism represents a genetic risk factor for latent Epstein–Barr virus (EBV) infection and expression of its oncogenic latency proteins [93]. Furthermore, FcγRIIa and FcγRIIIa polymorphisms are associated with variations in HIV disease progression [94], and progression of HIV infection has been associated with decreased expression of FcγRIIa [76]. In addition, FcγRIIc polymorphism has been shown to impact HIV-1 vaccine protection [95].
Infected Cells as Targets for Antiviral mAbs
Targeting viral antigens expressed on infected cells, rather than only on virions, might be rewarding in terms of induction of vaccine-like effects. This approach would mainly concern enveloped viruses, as they display their viral receptor-binding proteins at the surface of virus-producing cells. The fact that cellular ICs are crucial for inducing long-lasting immunity against FrCasE by neutralizing mAbs (see above) [36] substantiates this idea. In addition, HIV-infected cells are stronger inducers of innate immunity than cell-free virions [96], and HCV-infected hepatocytes have been reported to affect DC maturation and the subsequent activation of NK cells and T cells, whereas free virus could not [97], indicating that infected cells may be stronger inducers of immunity compared to virions. It is now important to establish whether the antigenic potential of such infected cells can be further enhanced upon opsonization with antiviral mAbs, as in the case of FrCasE.
Concluding Remarks
Over the past years, mounting evidence has revealed that antiviral mAbs may be used to recruit the endogenous immune systems of infected organisms to induce long-lasting vaccine-like effects. A major issue is now to translate this observation into human applications for the benefit of both patients and society (see Outstanding Questions). Much more research towards this aim is, however, still necessary. In particular, it will be essential to identify the molecular and cellular mechanisms whereby viral and cellular ICs formed during immunotherapy induce protective immunity and, then, determine the best means to exploit them therapeutically. It is also likely that improving the effector function of antiviral mAbs and/or combining them with other therapeutics will be necessary to achieve sterilizing immunity against infecting agents or, at least, their long-term containment.
Outstanding Questions.
What are the cellular types and molecular effectors involved in the induction of vaccine-like effects by antiviral monoclonal antibodies (mAbs)?
Which Fc-dependent effector functions is/are needed or involved in the induction of vaccine-like effects?
Can genetic engineering improve vaccine-like effects of antiviral mAbs?
Can combination therapies improve vaccine-like effects of antiviral mAbs?
When should mAb therapy be commenced?
Should mAb therapy be combined with other therapies?
What is the best approach to counteract immunosuppressive responses?
What is the best target for antiviral mAbs: virus and/or infected cells?
Can FcγR polymorphisms be used as a predictive factor for vaccine-like effects of antiviral mAbs?
Acknowledgments
This work was supported by grants from the Ligue Nationale contre le Cancer, the Fondation ARC, Sidaction and the Fondation pour la Recherche Médicale. M. Pelegrin, M. Naranjo-Gomez and M. Piechaczyk are part of the ‘MabImprove Labex’, a public grant overseen by the French National Research Agency (ANR) as part of the ‘Investissements d’Avenir’ program (reference: ANR-10-LABX -53-01) that also supported this work. We are grateful to Dr I. Robbins for critical reading of the manuscript.
Glossary
- Antibody-dependent cell-mediated cytotoxicity (ADCC)
a mechanism of cell-mediated immunity whereby effector cells of the immune system lyse a target cell that has been bound by specific antibodies. The Fc portions of the coating antibodies interact with Fc receptors that are expressed on immune effector cells (mainly natural killer cells or NKs), thereby initiating signalling cascades that result in the release of cytotoxic granules which induce the death of the antibody-coated cells.
- Antibody-dependent cellular phagocytosis (ADCP)
a mechanism of cell-mediated immunity whereby cells of the immune system phagocytose target cells that have been bound by specific antibodies. Similar to ADCC, the Fc portions of the coating antibodies interact with Fc receptors that are expressed on phagocytes (i.e., macrophages, granulocytes, and dendritic cells).
- Antibody-dependent cell-mediated virus inhibition (ADCVI)
a mechanism of antibody-mediated immunity wherey an infected target cell interacts with an effector cell expressing one or several FcγRs via a viral-specific antibody. ADCVI is a measure of the impact of antibody and FcγR-bearing effector cells on virus output from infected target cells and includes both lytic (i.e., ADCC) and non-lytic (i.e., production of chemokines) mechanisms leading to decreased viral spread.
- Complement-dependent cytotoxicity (CDC)
a mechanism of antibody-mediated immunity whereby antibody binding to the complement component C1q activates the classical complement cascade, leading to the formation of the membrane attack complex (the cytolytic end product of the complement cascade) and lysis of cells targeted by the antibody.
- Fab fragment
the region of an antibody that binds to antigens. It is composed of one constant and one variable domain of each of the heavy and light chains (VH and VL, respectively).
- F(ab′)2
two Fab fragments linked by a short fragment of the constant parts of the antibody (hinge region). It has the same affinity as the whole antibody and it is divalent.
- Fc-engineered glycovariants
engineered antibodies in which the glycosylation pattern has been modified to enhance its effector functions.
- Fc fragment
the fragment crystallizable region (Fc region) is the tail region of an antibody that interacts with cell-surface receptors called Fc receptors and some proteins of the complement system. This property allows antibodies to activate the immune system.
- Fc receptors (FcRs)
receptors for the Fc portion of immunoglobulins. They constitute a family of cell-surface molecules expressed on many cells of the immune system. There are several different types of FcR, which are classified based on the type of antibody that they recognize. For example, those that bind the most common class of antibody, IgGs, are called Fc-gamma receptors (FcγR).
- Immune checkpoints
a number of different inhibitory pathways of the immune system necessary to maintain self-tolerance and to modulate the duration and magnitude of immune responses in order to minimize collateral tissue damage. Many such immune checkpoints involve ligand–receptor interactions between T cells and antigen-presenting cells (i.e., PD1–PD1L/PD2L; CTLA4–CD86/CD80).
- Neonatal FcR (FcRn)
an Fc receptor that is structually related to MHC class I molecules and protects IgG from degradation, resulting in a long serum half-life. Additionally, FcRn mediates IgG transfer from a mother to her fetus, thereby providing passive immunity.
- Passive immunotherapy
the transfer to nonimmune patients of active antibodies (or other immune-system components) that are produced outside of the body (i.e., in the laboratory or isolated from immunized donors). This approach can help combat diseases for which no treatments or vaccines are available by providing short-term immunity.
- Regulatory T cells (Treg)
a subpopulation of T cells with immunosuppressive activities. These cells are observed in all cases of chronic viral infections and lead to dampened antiviral immune responses.
- Vaccine
a biological preparation that confers protective immunity against a specific disease. It usually employs an innocuous form of the disease agent (such as killed or weakened bacteria or viruses) to stimulate the body's immune system to recognize the agent as foreign and destroy it. It also confers immunological memory against such an agent.
References
- 1.Beck A. Strategies and challenges for the next generation of therapeutic antibodies. Nat. Rev. Immunol. 2010;10:345–352. doi: 10.1038/nri2747. [DOI] [PubMed] [Google Scholar]
- 2.Reichert J.M. Antibodies to watch in 2015. MAbs. 2015;7:1–8. doi: 10.4161/19420862.2015.988944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vacchelli E. Trial watch: monoclonal antibodies in cancer therapy. Oncoimmunology. 2013;2:e22789. doi: 10.4161/onci.22789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berry J.D., Gaudet R.G. Antibodies in infectious diseases: polyclonals, monoclonals and niche biotechnology. Nat. Biotechnol. 2011;28:489–501. doi: 10.1016/j.nbt.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bossart K.N. A neutralizing human monoclonal antibody protects against lethal disease in a new ferret model of acute nipah virus infection. PLoS Pathog. 2009;5:e1000642. doi: 10.1371/journal.ppat.1000642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Both L. Monoclonal antibodies for prophylactic and therapeutic use against viral infections. Vaccine. 2013;31:1553–1559. doi: 10.1016/j.vaccine.2013.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corti D., Lanzavecchia A. Broadly neutralizing antiviral antibodies. Annu. Rev. Immunol. 2013;31:705–742. doi: 10.1146/annurev-immunol-032712-095916. [DOI] [PubMed] [Google Scholar]
- 8.Krawczyk A. Overcoming drug-resistant herpes simplex virus (HSV) infection by a humanized antibody. Proc. Natl. Acad. Sci. U.S.A. 2013;110:6760–6765. doi: 10.1073/pnas.1220019110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marasco W.A., Sui J. The growth and potential of human antiviral monoclonal antibody therapeutics. Nat. Biotechnol. 2007;25:1421–1434. doi: 10.1038/nbt1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flego M. Clinical development of monoclonal antibody-based drugs in HIV and HCV diseases. BMC Med. 2013;11:4. doi: 10.1186/1741-7015-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qiu X. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature. 2014;514:47–53. doi: 10.1038/nature13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sautto G. Possible future monoclonal antibody (mAb)-based therapy against arbovirus infections. Biomed. Res. Int. 2013;2013:838491. doi: 10.1155/2013/838491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams K.L. Therapeutic efficacy of antibodies lacking Fcgamma receptor binding against lethal dengue virus infection is due to neutralizing potency and blocking of enhancing antibodies [corrected] PLoS Pathog. 2013;9:e1003157. doi: 10.1371/journal.ppat.1003157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dejnirattisai W. A new class of highly potent, broadly neutralizing antibodies isolated from viremic patients infected with dengue virus. Nat. Immunol. 2015;16:170–177. doi: 10.1038/ni.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flyak A.I. Mechanism of human antibody-mediated neutralization of Marburg virus. Cell. 2015;160:893–903. doi: 10.1016/j.cell.2015.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qin Y. Characterization of a large panel of rabbit monoclonal antibodies against HIV-1 gp120 and isolation of novel neutralizing antibodies against the V3 loop. PLoS ONE. 2015;10:e0128823. doi: 10.1371/journal.pone.0128823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan Y. A novel humanized antibody neutralizes H5N1 influenza virus via two different mechanisms. J. Virol. 2015;89:3712–3722. doi: 10.1128/JVI.03014-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen J. Immunology. Bound for glory. Science. 2013;341:1168–1171. doi: 10.1126/science.341.6151.1168. [DOI] [PubMed] [Google Scholar]
- 19.Euler Z., Alter G. Exploring the potential of monoclonal antibody therapeutics for HIV-1 eradication. AIDS Res. Hum. Retroviruses. 2014;31:13–24. doi: 10.1089/aid.2014.0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang J. Broad and potent HIV-1 neutralization by a human antibody that binds the gp41-gp120 interface. Nature. 2014;515:138–142. doi: 10.1038/nature13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klein F. Antibodies in HIV-1 vaccine development and therapy. Science. 2013;341:1199–1204. doi: 10.1126/science.1241144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwong P.D. Broadly neutralizing antibodies and the search for an HIV-1 vaccine: the end of the beginning. Nat. Rev. Immunol. 2013;13:693–701. doi: 10.1038/nri3516. [DOI] [PubMed] [Google Scholar]
- 23.Barouch D.H. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature. 2013;503:224–228. doi: 10.1038/nature12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Jong Y.P. Broadly neutralizing antibodies abrogate established hepatitis C virus infection. Sci. Transl. Med. 2014;6:254ra129. doi: 10.1126/scitranslmed.3009512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forthal D.N., Moog C. Fc receptor-mediated antiviral antibodies. Curr. Opin. HIV AIDS. 2009;4:388–393. doi: 10.1097/COH.0b013e32832f0a89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hessell A.J., Haigwood N.L. Neutralizing antibodies and control of HIV: moves and countermoves. Curr. HIV/AIDS Rep. 2012;9:64–72. doi: 10.1007/s11904-011-0105-5. [DOI] [PubMed] [Google Scholar]
- 27.Su B., Moog C. Which antibody functions are important for an HIV vaccine? Front. Immunol. 2014;5:289. doi: 10.3389/fimmu.2014.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akkina R. New generation humanized mice for virus research: comparative aspects and future prospects. Virology. 2013;435:14–28. doi: 10.1016/j.virol.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brehm M.A. Generation of improved humanized mouse models for human infectious diseases. J. Immunol. Methods. 2014;410:3–17. doi: 10.1016/j.jim.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein F. Enhanced HIV-1 immunotherapy by commonly arising antibodies that target virus escape variants. J. Exp. Med. 2014;211:2361–2372. doi: 10.1084/jem.20141050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lux A., Nimmerjahn F. Of mice and men: the need for humanized mouse models to study human IgG activity in vivo. J. Clin. Immunol. 2012;33(Suppl. 1):S4–S8. doi: 10.1007/s10875-012-9782-0. [DOI] [PubMed] [Google Scholar]
- 32.Shultz L.D. Humanized mice for immune system investigation: progress, promise and challenges. Nat. Rev. Immunol. 2012;12:786–798. doi: 10.1038/nri3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Portis J.L. Neurodegenerative disease induced by the wild mouse ecotropic retrovirus is markedly accelerated by long terminal repeat and gag-pol sequences from nondefective Friend murine leukemia virus. J. Virol. 1990;64:1648–1656. doi: 10.1128/jvi.64.4.1648-1656.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gros L. Induction of long-term protective antiviral endogenous immune response by short neutralizing monoclonal antibody treatment. J. Virol. 2005;79:6272–6280. doi: 10.1128/JVI.79.10.6272-6280.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gros L. Endogenous cytotoxic T-cell response contributes to the long-term antiretroviral protection induced by a short period of antibody-based immunotherapy of neonatally infected mice. J. Virol. 2008;82:1339–1349. doi: 10.1128/JVI.01970-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michaud H.A. A crucial role for infected-cell/antibody immune complexes in the enhancement of endogenous antiviral immunity by short passive immunotherapy. PLoS Pathog. 2010;6:e1000948. doi: 10.1371/journal.ppat.1000948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nasser R. Long-lasting protective antiviral immunity induced by passive immunotherapies requires both neutralizing and effector functions of the administered monoclonal antibody. J. Virol. 2010;84:10169–10181. doi: 10.1128/JVI.00568-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nasser R. Control of regulatory T cells is necessary for vaccine-like effects of antiviral immunotherapy by monoclonal antibodies. Blood. 2013;121:1102–1111. doi: 10.1182/blood-2012-06-432153. [DOI] [PubMed] [Google Scholar]
- 39.Haigwood N.L. Passive immunotherapy in simian immunodeficiency virus-infected macaques accelerates the development of neutralizing antibodies. J. Virol. 2004;78:5983–5995. doi: 10.1128/JVI.78.11.5983-5995.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamamoto T. Polyfunctional CD4+ T-cell induction in neutralizing antibody-triggered control of simian immunodeficiency virus infection. J. Virol. 2009;83:5514–5524. doi: 10.1128/JVI.00145-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamamoto H. Post-infection immunodeficiency virus control by neutralizing antibodies. PLoS ONE. 2007;2:e540. doi: 10.1371/journal.pone.0000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Villinger F. Evidence for antibody-mediated enhancement of simian immunodeficiency virus (SIV) Gag antigen processing and cross presentation in SIV-infected rhesus macaques. J. Virol. 2003;77:10–24. doi: 10.1128/JVI.77.1.10-24.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ng C.T. Passive neutralizing antibody controls SHIV viremia and enhances B cell responses in infant macaques. Nat. Med. 2010;16:1117–1119. doi: 10.1038/nm.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jaworski J.P. Neutralizing polyclonal IgG present during acute infection prevents rapid disease onset in simian-human immunodeficiency virus SHIVSF162P3-infected infant rhesus macaques. J. Virol. 2013;87:10447–10459. doi: 10.1128/JVI.00049-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caskey M. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature. 2015 doi: 10.1038/nature14411. Published online April 8, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watkins J.D. An anti-HIV-1 V3 loop antibody fully protects cross-clade and elicits T-cell immunity in macaques mucosally challenged with an R5 clade C SHIV. PLoS ONE. 2011;6:e18207. doi: 10.1371/journal.pone.0018207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boyoglu-Barnum S. Prophylaxis with a respiratory syncytial virus (RSV) anti-G protein monoclonal antibody shifts the adaptive immune response to RSV rA2-line19F infection from Th2 to Th1 in BALB/c mice. J. Virol. 2014;88:10569–10583. doi: 10.1128/JVI.01503-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kauvar L.M. Therapeutic targeting of respiratory syncytial virus G-protein. Immunotherapy. 2010;2:655–661. doi: 10.2217/imt.10.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Swedan S. Multiple functional domains and complexes of the two nonstructural proteins of human respiratory syncytial virus contribute to interferon suppression and cellular location. J. Virol. 2011;85:10090–10100. doi: 10.1128/JVI.00413-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bossart K.N. A neutralizing human monoclonal antibody protects African Green monkeys from hendra virus challenge. Sci. Transl. Med. 2011;3:105ra103. doi: 10.1126/scitranslmed.3002901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Geisbert T.W. Therapeutic treatment of Nipah virus infection in nonhuman primates with a neutralizing human monoclonal antibody. Sci. Transl. Med. 2014;6:242ra282. doi: 10.1126/scitranslmed.3008929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Broder C.C. A treatment for and vaccine against the deadly Hendra and Nipah viruses. Antiviral. Res. 2013;100:8–13. doi: 10.1016/j.antiviral.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nishikawa H., Sakaguchi S. Regulatory T cells in cancer immunotherapy. Curr. Opin. Immunol. 2014;27:1–7. doi: 10.1016/j.coi.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 54.Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharma P., Allison J.P. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161:205–214. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fuller M.J. Immunotherapy of chronic hepatitis C virus infection with antibodies against programmed cell death-1 (PD-1) Proc. Natl. Acad. Sci. U.S.A. 2013;110:15001–15006. doi: 10.1073/pnas.1312772110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu J. Enhancing virus-specific immunity in vivo by combining therapeutic vaccination and PD-L1 blockade in chronic hepadnaviral infection. PLoS Pathog. 2014;10:e1003856. doi: 10.1371/journal.ppat.1003856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tzeng H.T. PD-1 blockage reverses immune dysfunction and hepatitis B viral persistence in a mouse animal model. PLoS ONE. 2012;7:e39179. doi: 10.1371/journal.pone.0039179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seung E. PD-1 blockade in chronically HIV-1-infected humanized mice suppresses viral loads. PLoS ONE. 2013;8:e77780. doi: 10.1371/journal.pone.0077780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Velu V. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458:206–210. doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dietze K.K. Combining regulatory T cell depletion and inhibitory receptor blockade improves reactivation of exhausted virus-specific CD8+ T cells and efficiently reduces chronic retroviral loads. PLoS Pathog. 2013;9:e1003798. doi: 10.1371/journal.ppat.1003798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bengsch B. Restoration of HBV-specific CD8+ T cell function by PD-1 blockade in inactive carrier patients is linked to T cell differentiation. J. Hepatol. 2014;61:1212–1219. doi: 10.1016/j.jhep.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 63.Sherman A.C. Augmentation of hepatitis B virus-specific cellular immunity with programmed death receptor-1/programmed death receptor-L1 blockade in hepatitis B virus and HIV/hepatitis B virus coinfected patients treated with adefovir. AIDS Res. Hum. Retroviruses. 2013;29:665–672. doi: 10.1089/aid.2012.0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gardiner D. A randomized, double-blind, placebo-controlled assessment of BMS-936558, a fully human monoclonal antibody to programmed death-1 (PD-1), in patients with chronic hepatitis C virus infection. PLoS ONE. 2013;8:e63818. doi: 10.1371/journal.pone.0063818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aranda F. Trial watch: immunostimulatory monoclonal antibodies in cancer therapy. Oncoimmunology. 2014;3:e27297. doi: 10.4161/onci.27297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goulding J. OX40:OX40L axis: emerging targets for improving poxvirus-based CD8(+) T-cell vaccines against respiratory viruses. Immunol. Rev. 2012;244:149–168. doi: 10.1111/j.1600-065X.2011.01062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee S. Targeting CD137 enhances vaccine-elicited anti-respiratory syncytial virus CD8+ T cell responses in aged mice. J. Immunol. 2014;192:293–299. doi: 10.4049/jimmunol.1300453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vezys V. 4-1BB signaling synergizes with programmed death ligand 1 blockade to augment CD8 T cell responses during chronic viral infection. J. Immunol. 2011;187:1634–1642. doi: 10.4049/jimmunol.1100077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao Y. Targeting 4-1BB (CD137) to enhance CD8 T cell responses with poxviruses and viral antigens. Front. Immunol. 2012;3:332. doi: 10.3389/fimmu.2012.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moody M.A. Toll-like receptor 7/8 (TLR7/8) and TLR9 agonists cooperate to enhance HIV-1 envelope antibody responses in rhesus macaques. J. Virol. 2014;88:3329–3339. doi: 10.1128/JVI.03309-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sommariva M. High efficacy of CpG-ODN, cetuximab and cisplatin combination for very advanced ovarian xenograft tumors. J. Transl. Med. 2013;11:25. doi: 10.1186/1479-5876-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Halper-Stromberg A. Broadly neutralizing antibodies and viral inducers decrease rebound from HIV-1 latent reservoirs in humanized mice. Cell. 2014;158:989–999. doi: 10.1016/j.cell.2014.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bournazos S. humanized mice to study FcgammaR function. Curr. Top. Microbiol. Immunol. 2014;382:237–248. doi: 10.1007/978-3-319-07911-0_11. [DOI] [PubMed] [Google Scholar]
- 74.Bournazos S. Broadly neutralizing anti-HIV-1 antibodies require Fc effector functions for in vivo activity. Cell. 2014;158:1243–1253. doi: 10.1016/j.cell.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.DiLillo D.J. Broadly neutralizing hemagglutinin stalk-specific antibodies require FcgammaR interactions for protection against influenza virus in vivo. Nat. Med. 2014;20:143–151. doi: 10.1038/nm.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dugast A.S. Decreased Fc receptor expression on innate immune cells is associated with impaired antibody-mediated cellular phagocytic activity in chronically HIV-1 infected individuals. Virology. 2011;415:160–167. doi: 10.1016/j.virol.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hessell A.J. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 78.Lazar G.A. Engineered antibody Fc variants with enhanced effector function. Proc. Natl. Acad. Sci. U.S.A. 2006;103:4005–4010. doi: 10.1073/pnas.0508123103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moldt B. A nonfucosylated variant of the anti-HIV-1 monoclonal antibody b12 has enhanced FcgammaRIIIa-mediated antiviral activity in vitro but does not improve protection against mucosal SHIV challenge in macaques. J. Virol. 2012;86:6189–6196. doi: 10.1128/JVI.00491-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Natsume A. Fucose removal from complex-type oligosaccharide enhances the antibody-dependent cellular cytotoxicity of single-gene-encoded bispecific antibody comprising of two single-chain antibodies linked to the antibody constant region. J. Biochem. 2006;140:359–368. doi: 10.1093/jb/mvj157. [DOI] [PubMed] [Google Scholar]
- 81.Niwa R. IgG subclass-independent improvement of antibody-dependent cellular cytotoxicity by fucose removal from Asn297-linked oligosaccharides. J. Immunol. Methods. 2005;306:151–160. doi: 10.1016/j.jim.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 82.Zeitlin L. Enhanced potency of a fucose-free monoclonal antibody being developed as an Ebola virus immunoprotectant. Proc. Natl. Acad. Sci. U.S.A. 2011;108:20690–20694. doi: 10.1073/pnas.1108360108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chung A.W. Identification of antibody glycosylation structures that predict monoclonal antibody Fc-effector function. AIDS. 2014;28:2523–2530. doi: 10.1097/QAD.0000000000000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ackerman M.E. Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity. J. Clin. Invest. 2013;123:2183–2192. doi: 10.1172/JCI65708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Giragossian C. Neonatal Fc receptor and its role in the absorption, distribution, metabolism and excretion of immunoglobulin G-based biotherapeutics. Curr. Drug Metab. 2013;14:764–790. doi: 10.2174/13892002113149990099. [DOI] [PubMed] [Google Scholar]
- 86.Baker K. The role of FcRn in antigen presentation. Front. Immunol. 2014;5:408. doi: 10.3389/fimmu.2014.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rath T. Regulation of immune responses by the neonatal fc receptor and its therapeutic implications. Front. Immunol. 2015;5:664. doi: 10.3389/fimmu.2014.00664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ko S.Y. Enhanced neonatal Fc receptor function improves protection against primate SHIV infection. Nature. 2014;514:642–645. doi: 10.1038/nature13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.French M.A. Isotype-switched immunoglobulin G antibodies to HIV Gag proteins may provide alternative or additional immune responses to ‘protective’ human leukocyte antigen-B alleles in HIV controllers. AIDS. 2013;27:519–528. doi: 10.1097/QAD.0b013e32835cb720. [DOI] [PubMed] [Google Scholar]
- 90.Chung A.W. Polyfunctional Fc-effector profiles mediated by IgG subclass selection distinguish RV144 and VAX003 vaccines. Sci. Transl. Med. 2014;6:228ra238. doi: 10.1126/scitranslmed.3007736. [DOI] [PubMed] [Google Scholar]
- 91.Yates N.L. Vaccine-induced Env V1-V2 IgG3 correlates with lower HIV-1 infection risk and declines soon after vaccination. Sci. Transl. Med. 2014;6:228ra239. doi: 10.1126/scitranslmed.3007730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zolla-Pazner S. Vaccine-induced IgG antibodies to V1V2 regions of multiple HIV-1 subtypes correlate with decreased risk of HIV-1 infection. PLoS ONE. 2014;9:e87572. doi: 10.1371/journal.pone.0087572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Diamantopoulos P.T. Correlation of Fc-gamma RIIA polymorphisms with latent Epstein-Barr virus infection and latent membrane protein 1 expression in patients with low grade B-cell lymphomas. Leuk. Lymphoma. 2013;54:2030–2034. doi: 10.3109/10428194.2012.762512. [DOI] [PubMed] [Google Scholar]
- 94.Cocklin S.L., Schmitz J.E. The role of Fc receptors in HIV infection and vaccine efficacy. Curr. Opin. HIV AIDS. 2014;9:257–262. doi: 10.1097/COH.0000000000000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li S.S. FCGR2C polymorphisms associate with HIV-1 vaccine protection in RV144 trial. J. Clin. Invest. 2014;124:3879–3890. doi: 10.1172/JCI75539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lepelley A. Innate sensing of HIV-infected cells. PLoS Pathog. 2011;7:e1001284. doi: 10.1371/journal.ppat.1001284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ebihara T. Hepatitis C virus-infected hepatocytes extrinsically modulate dendritic cell maturation to activate T cells and natural killer cells. Hepatology. 2008;48:48–58. doi: 10.1002/hep.22337. [DOI] [PubMed] [Google Scholar]