Abstract
The 30th International Conference on Antiviral Research (ICAR) was held in Atlanta, GA, USA from May 18 to 21, 2017. This report provides an account of award lectures, invited keynote addresses and oral presentations during the meeting. The 2017 Gertrude Elion Memorial Lecture Award by Michael Sofia highlighted one of the most important accomplishments in recent drug discovery in antiviral research, the identification of the hepatitis C virus direct-acting antiviral sofosbuvir and new alternatives to combat hepatitis B virus (HBV) infection. The Antonín Holý Lecture Award by David Chu on medicinal chemistry provided an overview of early developments of nucleoside analogs for the treatment of HIV and varicella zoster virus infection and how this knowledge serves to develop new drugs targeting HBV. Priscilla Yang gave the first ISAR Women in Science lecture. She reported on pharmacological validation of new antiviral targets for dengue, Zika and other flaviviruses. The William Prusoff Young Investigator Lecture Award by Maaike Everts described the Alabama Drug Discovery Alliance and the Antiviral Drug Discovery and Development Consortium, and how they are helping to accelerate the development of new antivirals. The 30th ICAR was a success in promoting new discoveries in antiviral drug development and research. The 31st ICAR will be held in Porto, Portugal, June 11–15, 2018.
Highlights
-
•
The 30th ICAR was held in Atlanta, Georgia, USA from May 18–21, 2017.
-
•
This article summarizes presentations by ISAR award recipients, principal invited lectures and keynote addresses.
-
•
Mini-symposium topics included antiviral immunity and emerging viral infections.
-
•
Other sessions and topics included hepatitis and retroviruses, respiratory viruses, DNA viruses and medicinal chemistry.
-
•
The 31st ICAR will be held in Porto, Portugal, June 11–15, 2018.
1. Introduction
The International Society for Antiviral Research (ISAR) sponsors an annual international meeting, the International Conference on Antiviral Research (ICAR). The 30th ICAR was held in Atlanta, Georgia, USA, from May 21–25, 2017. As in previous years (Vere Hodge, 2014, Vere Hodge, 2015, Vere Hodge, 2017), the meeting provided an interdisciplinary forum at which investigators involved in basic, translational, and clinical research worldwide met to review recent developments in all areas of antiviral research, drug and vaccine development. This is critical, because the successful discovery and development of new therapies requires close cooperation among scientists from diverse scientific disciplines. The overarching goal of the conference is to drive the discovery of new antiviral therapies by fostering collaboration among scientists from the fields of basic virology, medicinal chemistry, pharmacology, animal models of disease and toxicology in academia and the pharmaceutical industry.
This year, ICAR presented two symposia highlighting potential opportunities for both researchers and established collaborative work groups. The Antiviral Immunity Symposium discussed strategies to utilize the immune response of the host in conjunction with antiviral drugs to control chronic infections, such as hepatitis B virus (HBV) and human immunodeficiency virus (HIV) infections. New small molecules targeting viral entry for flavivirus or influenza virus infections were also discussed. The Emerging Infections Symposium discussed viral infections of global concern and the potential for antiviral drugs to treat infections and mitigate the effects of emerging epidemics, including hemorrhagic fever viruses, rodent-borne viruses and emerging coronavirus pandemic disease. The Respiratory Virus sessions described common respiratory viral diseases such as respiratory syncytial virus, human coronaviruses, and enteroviruses that cause significant morbidity and mortality. Other sessions included hepatitis viruses and retroviruses, emerging viruses, DNA and respiratory viruses and medicinal chemistry. The complete 30th ICAR program is available at http://c.ymcdn.com/sites/www.isar-icar.com/resource/resmgr/docs/ICAR_2017program_FINAL.pdf.
In this report, eight volunteer rapporteurs provide their summaries of principal scientific presentations at the 30th ICAR, hoping to effectively convey the speakers' goals and the results and conclusions of their talks. Because this article cannot cover all sessions, oral and poster presentations and other events, it should not be taken as a comprehensive account of the meeting. However, it does provide an overview of the Award lectures, invited keynote talks, and other presentations from the perspective of experts in antiviral research.
2. The ISAR Awards
2.1. Gertrude Elion Memorial Lecture Award: Michael J. Sofia, Arbutus Biopharma, Doylestown, PA
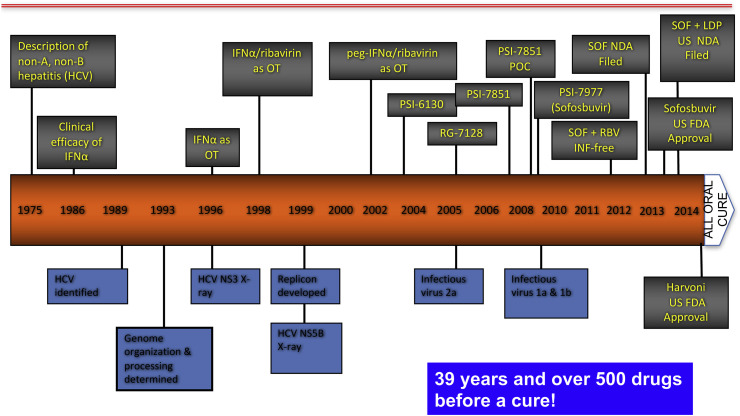
Michael (Mike) Sofia began his lecture by noting that experts once considered hepatitis B and C to pose a similar challenge to antiviral therapy, and did not predict that an effective cure for hepatitis C would be developed until the mid-2020s. The earlier than expected achievement of that goal (Fig. 1 ) can be attributed, in part, to the recognition by Pharmasset scientists some 15 years ago that the compound PSI-6130 (2′-deoxy-2′-fluoro-2′-C-methylcytidine) had several promising features: it was active against the NS5B polymerase of HCV genotypes 1–4, with a high barrier to resistance; had a good safety profile; and showed additive to synergistic activity in vitro in combination with ribavirin or interferon (Clark et al., 2005).
Fig. 1.
The long road to a cure of chronic hepatitis C.
The Pharmasset team then worked to overcome the limitations of PSI-6130, including relatively low uptake from the gastrointestinal tract, slow initial phosphorylation within cells and production of an inactive uridine metabolite. To improve uptake, they evaluated prodrugs, including the molecule RG7128, which proved to be well tolerated and performed well in combination with ribavirin or peg-IFN (Le Pogam et al., 2010). RG7128 showed additive potency when combined with a novel protease inhibitor (Gane et al., 2010). Although RG7128 continued to advance toward clinical use, it required large doses for efficacy, and it was clear that something better would be needed.
At that point, Mike's team decided to exploit the inherent enzymatic activity of hepatocytes, by creating a monophosphate prodrug that would undergo a series of cleavages to “un-mask” the phosphate group. The new compound proved to be active in genotype-1 patients, but was limited by the presence of active and inactive stereoisomers; final success was not achieved until a novel synthetic method produced the molecule now known as sofosbuvir (Ross et al., 2011). The true watershed moment came with the ELECTRON study in genotype 2 or 3 patients, in which a 12-week course of sofosbuvir plus ribavirin produced a 100% cure rate. The rest is history!
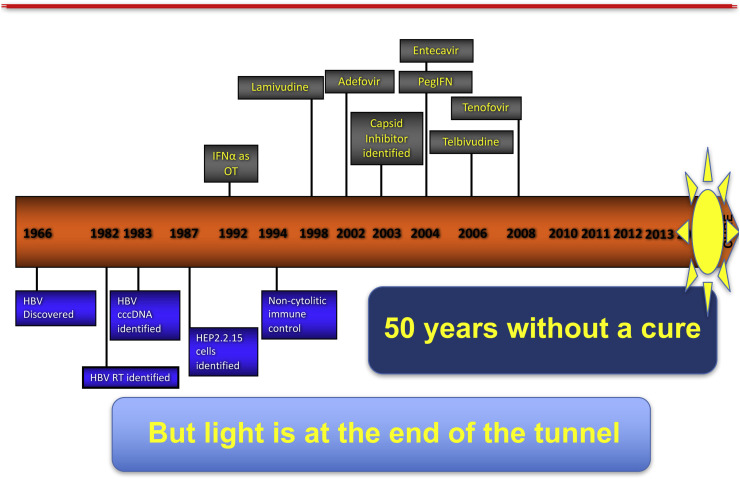
Mike then turned his attention to chronic hepatitis B, which is currently managed through long-term administration of nucleoside/tide analogs and peg-IFN, which suppresses viral replication and slows disease progression, but rarely produces a cure. Two factors are responsible for the limited efficacy of current treatment: the state of immune tolerance induced by massive, continuous production of subviral particles, and persistence of the cccDNA HBV genome in the nucleus of infected cells. This double barrier has discouraged research efforts: except for the new alafenamide prodrug of tenofovir, no new anti-HBV drug has been introduced in the past 10 years (Fig. 2 ).
Fig. 2.
A cure yet to be realized: chronic hepatitis B.
The remainder of his lecture described unpublished research by his team at Arbutus. Recognizing that curative therapy of hepatitis B must both inhibit viral replication and restore immune function, and will likely require a combination of drugs with different mechanisms of action, they are taking two different approaches. The first focuses on inhibiting assembly of the viral capsid, which is required for encapsidation of pregnomic RNA and the subsequent synthesis of cccDNA. The lead Arbutus compound AB-423 appears to act by binding to a site in the interface between capsid dimers that is highly conserved among viral genotypes. Testing in HBV-infected mice with fully humanized livers showed that ABV-423 significantly lowered viral DNA levels, and had an additive effect with entecavir.
The second approach aims to block all aspects of HBV replication simultaneously, through the delivery into hepatocytes of three different RNAi triggers targeting all four viral RNA transcripts. Arbutus researchers hope that, by abruptly halting antigen production, this approach will “reawaken” the immune system. The lead product, ARB-1467, targets conserved RNA sequences, and is expected to be effective against any viral genotype, regardless of treatment status and in combination with any other therapies. A second-generation RNAi product, ARB-1740, has shown greater potency and more prolonged activity in mice. As expected, triple combination therapy with entecavir or peg-IFN + AB-423 + AB-1740 is highly effective, producing the greatest delay in viral recovery and greatest suppression of serum HBsAg. Suppression of HBsAg production correlated with a gain in IFN-α expression, auguring the recovery of host immune responses.
ARB-423 has not yet been tested in humans, but a Phase II study in patients on chronic nucleoside analog therapy found that the addition of ARB-1467 decreased circulating HBsAg in both HBeAg-positive and -negative patients. These novel approaches offer the prospect of more effective suppression of HBV replication, but can they produce a cure? The answer should be only a few years away.
2.2. Antonín Holý Memorial Lecture Award: Chung (David) K. Chu, University of Georgia
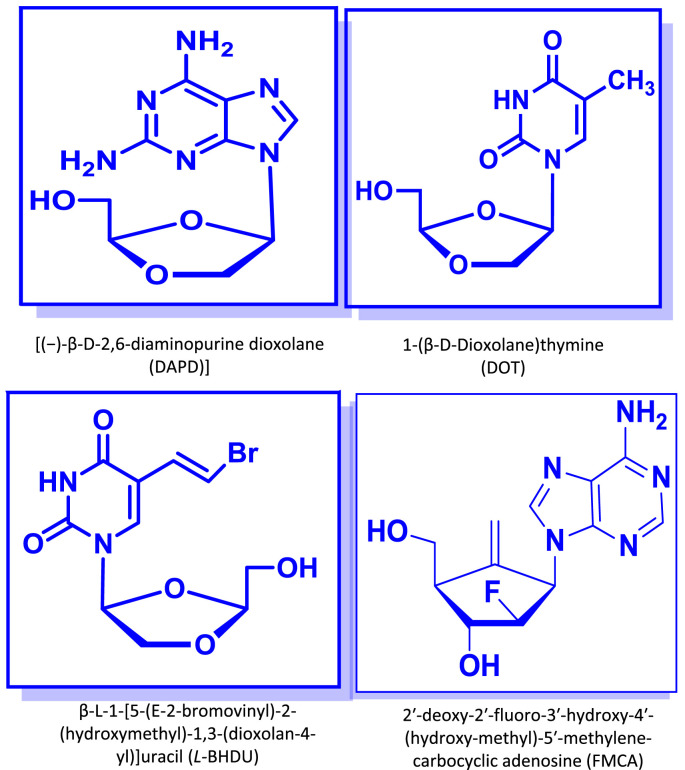
In the first part of his lecture, David Chu reviewed the synthesis and anti-HIV activity of diaminopurine dioxolanes (DAPD/amdoxovir) and dioxolane thymines (DOT) (Fig. 3 ), two classes of nucleoside analogs with potent in vitro and in vivo activity. During chemical synthesis and in vitro evaluation of dioxolane nucleosides as potential anti-HIV agents, DAPD (dioxolane-diaminopurine) was discovered as an interesting anti-HIV agent, which inhibited drug-resistant HIV mutants against AZT, 3TC (lamivudine) and non-nucleoside reverse transcriptase inhibitors (NNRTI) in vitro (Chen et al., 1996, Furman et al., 2001). It underwent Phase IIa clinical trials, which demonstrated efficacy in patients with drug-resistant HIV. DAPD (2,6-diamaino purine analog) (Fig. 3) is a biochemical pro-drug of a guanine analog (DXG), which is converted to a guanine analog by adenosine deaminase in vitro and in vivo. DAPD is more soluble in water than that of the guanine analog (DXG), which provides better physico-chemical properties as a drug.
Fig. 3.
Chemical structures of some of the compounds discussed during Dr. David Chu's lecture.
In order to understand the molecular mechanism of DAPD (or DXG triphosphate), and how and why the triphosphate is still effective against drug-resistant HIV, molecular modeling studies were conducted, from which it was found that the oxygen atom of the dioxolane-moiety promotes the binding of DAPD to the HIV-RT. Another dioxolane nucleoside that was discovered to be an interesting anti-HIV agent was DOT (dioxolane-thymine) (Fig. 3). DOT was effective against drug-resistant HIV mutants (K65R, L74V, M184V, T215Y, etc.) (Chu et al., 2005).
David then reviewed the history of a potential new therapy against varicella zoster virus (VZV), the cause of chickenpox in children and zoster (shingles) in older adults. In the USA and Europe, approved drugs include acyclovir and its derivatives and foscarnet. Brivudine (BVDU) (Fig. 3) is licensed in some European countries, but not in the USA, because the compound blocks the enzymatic degradation of the cancer drug 5-FU, and has caused severe toxicity and death in patients receiving both medications.
In an effort to identify an alternative to BVDU (a D-nucleoside) that would not block 5-FU catabolism, David and his colleagues synthesized and tested a series of L-analogs of BVDU in VZV-infected primary human foreskin fibroblasts and in skin organ culture (Choi et al., 2000, De et al., 2014). They identified β-L-1-[5-(E-2-bromovinyl)-2-(hydroxymethyl)-1,3-(dioxolan-4-yl)] uracil (L-BHDU) as an especially promising compound, with minimal cytotoxicity and 50% effective concentration (EC50) values against VZV significantly lower than those of acyclovir or foscarnet. L-BHDU was then further evaluated by Jennifer Moffat and colleagues in a model of VZV infection of human skin xenografts in SCID mice, and found to be more potent than acyclovir or valacyclovir. Importantly, HPLC analysis showed that, while BVDU prevented the breakdown of 5-FU, L-BHDU did not interfere with its catabolism, suggesting that the drug would be safe in cancer patients on 5-FU therapy. This is the first time that an L-nucleoside has been shown to be effective for herpes virus therapy.
Continuing his review of L-nucleosides as antiviral agents, David shifted the topic to hepatitis B. He first discussed the history of clevudine (2‘-fluoro-5-methyl-β-l-arabinofuranosyluracil, L-FMAU, Levovir®), which was shown 20 years ago to have good activity against HBV, and to be more potent than its D-analog (Ma et al., 1996). In work by Tennant, Korba and colleagues, clevudine was found to be highly effective in a woodchuck hepatitis model, rapidly inducing profound suppression of viremia that persisted up to 12 weeks after cessation of therapy (Peek et al., 2001). It was also highly effective in a Phase II/III clinical study, inducing a 4- to 5-log drop in viremia in HBeAg+ and – patients. Clevudine is now in use in South Korea and several other countries, but its Phase III development in the USA by Pharmasset was discontinued.
David concluded his lecture by reviewing the development of HBV inhibitors and a novel carbocyclic adenosine analog against HBV, 2′-deoxy-2′-fluoro-3′-hydroxy-4′-(hydroxymethyl)-5′-methylene-carbocyclic adenosine (FMCA) (Fig. 3), which is particularly useful because it is active against viruses that are resistant to both lamivudine and entecavir (Rawal et al., 2013). It has been synthesized as a phosphoramidate prodrug (FMCAP), which showed good efficacy in a hydrodynamic mouse model of hepatitis B. Its in vivo evaluation is ongoing.
2.3. The Women in Science Award presentation: Priscilla Yang, Harvard Medical School, Boston, MA
Priscilla Yang's research group focuses on pharmacological validation of new antiviral targets, with dengue (DENV), Zika (ZIKV) and other flaviviruses as principal targets. Efforts to block viral fusion and entry have traditionally been based on antibodies to the envelope (E) glycoprotein, but she reasoned that E could also be a target for small molecules, because the fusion process is catalytic: low endosomal pH triggers structural changes in E that lower the energy barrier for membrane fusion. However, in contrast to most small-molecule targets, E doesn't have a classical active site in which a covalent bond is made or broken, and it was also not clear where candidate drugs might bind. A possible ligand-binding pocket had been observed during initial efforts to determine the structure of E, when the protein was co-crystallized in the presence of the detergent beta-octoglucoside, but it was unclear whether this pocket was a bonafide antiviral target (Modis et al., 2003, Modis et al., 2004).
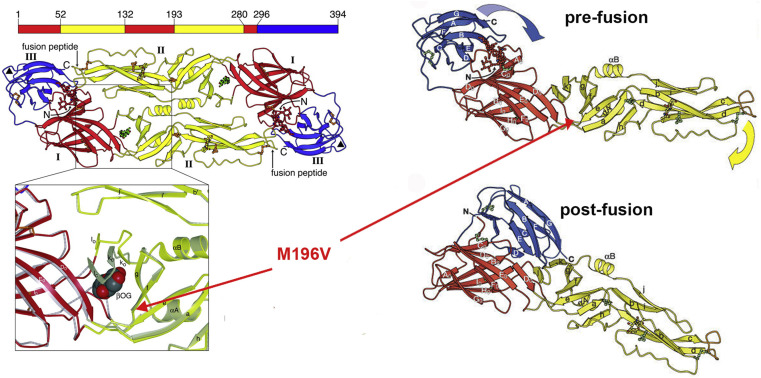
To answer that question, Priscilla's group began by using a cell-based phenotypic screen of DENV-infected cells to identify active compounds, detecting E expression by immunofluorescence and automated microscopy, followed by time-of-addition experiments to discriminate between inhibitors of virus entry and those blocking later steps in replication (Chu and Yang, 2007, Clark et al., 2016). Screening identified three series of small molecules -- 2,4-diaminopyrimidines, 4,6-disubstituted pyrimidines, and cyanohydrazones -- that interfere with entry. By linking the compounds to biotin or fluorophores, her group has shown that the inhibitors interact with the soluble pre-fusion E dimer, but not the post-fusion trimer (Clark et al., 2016) (Fig. 4 ), and that they block pH-triggered, E-mediated fusion of virions with liposomes (unpublished). However, they have not yet succeeded in obtaining high-resolution structures of any compound bound to recombinant prefusion E.
Fig. 4.
Structure of the dengue virus envelope glycoprotein, showing location of a ligand-binding pocket. Left: the pre-fusion dimer ribbon structure, with zoom showing detergent bound in the pocket. Right: monomers of the pre-fusion and post-fusion forms of E. Adapted from (Modis et al., 2003, Modis et al., 2004).
To identify the binding site, Priscilla's team performed serial virus passage in the presence of each compound, and found a resistance mutation, M196V, located at the base of a ligand-binding pocket between E domains I and II. Attempts to engineer resistance by introducing other mutations were unsuccessful, suggesting that modifying the binding site has a fitness cost. After examining the sequence diversity of the analogous pocket present in multiple DENV isolates, the team engineered six different substitutions into recombinant E proteins and measured Kd values for representative compounds from all three inhibitor series. All of the compounds were affected by at least 2 mutations in the pocket, but with different footprints; however, the footprints of inhibitors in the same class were more similar to each other than to the other two classes. Comparison of amino-acid sequences among different flavivirus species revealed that residues deep in the pocket are broadly conserved. When tested against Zika, West Nile and Japanese encephalitis viruses, the cyanohydrazone compounds had broad-spectrum activity, while the two pyrimidine series were active only against DENV.
In an effort to better understand the chemical diversity that can engage the detergent-binding pocket, Priscilla's group developed an Alphascreen assay based on detecting the displacement of a biotinylated pyrimidine inhibitor bound to the target site. A screen of 20,000 compounds produced 8 leads for which the 50% inhibitory concentration (IC50) values correlated well with 90% effective concentration (EC90) values in cell culture and the Kd for recombinant E, indicating that the Alphascreen is a good tool for finding compounds that bind in the pocket and inhibit fusion. In collaboration with Steve Harrison and Luke Chao, they have used fluorophore-conjugated inhibitors and a single-particle fusion assay to examine the stoichiometry of inhibitor binding, and have found that approximately 12 molecules of the cyanohydrazone must bind to a VLP to inhibit fusion (each VLP has 120 copies of E) (Schmidt et al., 2012). Current efforts aim to determine if a greater number of pyrimidine molecules must bind to each viral particle to block fusion, and if the number correlates with the breadth of activity against different flaviviruses. They are also working to develop additional inhibitors to validate this antiviral strategy in vivo.
2.4. William Prusoff Young Investigator Award: Maaike Everts, University of Alabama (UAB) School of Medicine at Birmingham
Maaike Everts first described the Alabama Drug Discovery Alliance (ADDA), which was created in 2008 by Rich Whitley at the UAB and Jack Secrist at Southern Research (SR), when they recognized that their institutions possessed complementary scientific and management expertise, and that by working together they could cover most steps of discovering a new drug and moving it forward to the clinic (Everts et al., 2011). They also realized that the early phases of drug discovery, such as assay development and high-throughput screening (HTS), could best be funded by institutional investments, while later phases that require significant medicinal chemistry efforts should be supported by state and philanthropic dollars.
A key ingredient for the success of the ADDA has been regular meetings, in which each PI sits down with an interdisciplinary team to discuss progress and solve problems. Importantly, a licensing associate from the technology transfer office participates in the process from the beginning, long before any patent applications are filed. The ADDA now has about 20 projects in the pipeline, ranging from early assay development to Phase I clinical trials.
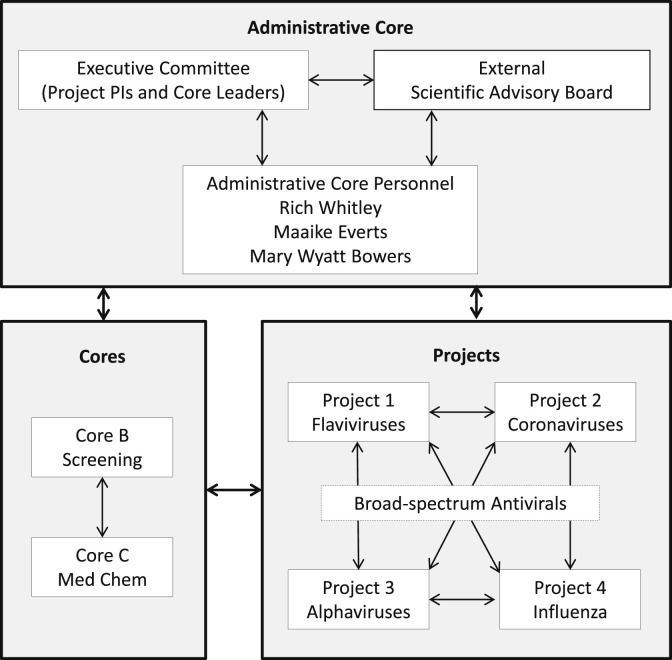
Based on the success of the ADDA, the UAB/SR team obtained a U19 grant from NIH in 2014 to establish the Antiviral Drug Discovery and Development Consortium (AD3C) (Fig. 5 ), which combines the expertise of prominent virologists in academic centers and the pharmaceutical industry with the HTS and medicinal chemistry resources of SR (Everts et al., 2016, Everts et al., 2017). The AD3C is currently focusing on countermeasure development against important pathogens in the flavivirus, alphavirus and coronavirus families and on influenza viruses. The Key factors for success factors have included a foundation of experience in basic virology, drug discovery and development; regular communication by phone and in face-to-face meetings; and an excellent advisory board to help with strategic decisions. Another major factor has been the sense of cohesion provided by Richard (Rich) Whitley, with his vast experience in virology and drug development, who serves as the nucleus for the consortium. The websites for the ADDA and AD3C are at: http://www.uab.edu/medicine/adda/ and https://www.uab.edu/medicine/ad3c/.
Fig. 5.
The structure of the Antiviral Drug Discovery and Development Consortium (AD3C) highlights the interconnections among the core groups and the projects, and the facilitating role of the administrative core (Core A) in moving projects forward through the pipeline.
3. Drug discovery and development 101
This year's session brought together individuals with expertise and experience relevant to creating a biotechnology company. Robert (Bob) Buckheit of Imquest Biosciences, Frederick, MD and Mike Sofia each presented their experience starting and leading successful companies from the perspectives of contract research and therapeutic discovery and development, respectively. Bob started with the introduction of the biotechnology industry, beginning with Genetech, and highlighted that the motivation to start such an enterprise is driven by the desire to do something good for society by translating academic discoveries to marketable products. Stressing the need for a solid business plan and honest assessment of the inherent risks, he emphasized the necessity of addressing an unmet need and assembling a high quality team.
Speaking about the challenges of starting a company focused on the discovery and development of therapeutics, Mike highlighted the challenges such as the problems of complex biology, long timelines, and low probability of success. He also stressed the many differences between a biotech company and “big pharma,” including the scope of resources, source of innovation, dependence on success of each individual program, decision making, risk threshold, and how a profit is made. According to Mike, the keys to success include addressing an unmet medical need that has a real market, leveraging innovation to address challenges and building a strong leadership team – echoing points raised by Bob Buckheit. Mike Sofia then walked the audience through the companies he has founded, including Transcell Technologies, Pharmasett and Oncore, the latter of which merged with Tekmira to create Arbutus.
The presentations by two successful CEOs were followed by lectures about intellectual property (IP) and steps in starting a biotech company. Rebecca Kaufman from King and Spaulding LLP, Atlanta, GA walked the audience through various aspects of intellectual property, including what a patent actually is, what is required to be granted a patent and why patent coverage should be pursued. She stressed the importance of an invention disclosure at the outset of the patent process.
In the final talk, Isaac R. Rodriguez-Chavez reviewed the top-10 steps in starting and funding a biotech company. He pointed out that while large pharma has been downsizing over recent years, the annual number of start-ups has grown and their success rate is 25–50%. While success stories abound, Isaac also highlighted some reasons for failure, including not understanding the value of the product or how to run a business. Circling back to messages by the first two speakers, he emphasized that the success of any start-up begins with a solid business plan. All in all, these experts provided conference attendees with an excellent overview of the requirements and risks of the start-up process.
4. Keynote addresses
4.1. The elusive rhinovirus C: Historical context and biological enigma. Ann Palmenberg, University of Wisconsin at Madison
Ann Palmenberg's lecture focused on the fast-developing story of the rhinovirus C (RV-C) species. Whereas types A and B have been known for many years, rhinovirus-C (RV-C) was discovered only in 2006, as part of SARS surveillance efforts. The RNA sequence revealed that this was a new type of rhinovirus, which had remained “hidden” because RV-C does not replicate in the standard rhinovirus cell culture assays – it uses another cellular receptor, cadherin-related protein 3 (CDHR3). The structural topography of RV-C differs from those of RV-A and RV-B, RV-C having spikes.
There are two forms of CDHR3 in the human population, Tyr529 and Cys529. In 2014, CDHR3 was reported to be an important susceptibility factor for asthma exacerbations, which are one of the most frequent causes of hospitalization during childhood (Bonnelykke et al., 2014). CDHR3 is highly expressed in airway epithelium. Children who are homozygous for Tyr529 have 5- to 10-fold higher risk of asthma exacerbations and hospitalization than those homozygous for Cys529.
The CDHR3 Tyr529 is the ancestral protein, found in many mammals and all nonhuman primates. Neanderthals are thought to have diverged from the modern human lineage about 300,000 years ago, and their DNA sequence shows that they retained the ancestral Tyr529 protein. Although Otzi, the 5000-year-old “Ice-man” found in the Alps, had the Tyr529 protein, the Cys529 allele was discovered in some early human specimens, indicating that this variant was spreading through the human population before the “out of Africa” event, around 50,000 years ago. Currently, the majority of human groups have both alleles encoding the Cys529 protein, ranging from 53% in Africa to 87% in Asia. Could it be that RV-C had evolved and exerted selective pressure on human evolution?
To answer this question, a phylogenetic tree was created from many unpassaged clinical isolates of rhinoviruses, types A, B and C. Preliminary analysis suggests an emergence of RV-C from RV-A about 6–10,000 years ago. The emergence of RV-C may explain why the Cys529 protein has become so dominant in the modern human population, but it does not account for the early appearance of this variant in ancient human DNA prior to the “out of Africa” migration.
4.2. Antivirals at the interface with public health: a case study of polio. Mark A. Pallansch, centers for disease control and prevention (CDC), Atlanta, GA
The Global Laboratory Network supporting the worldwide polio eradication initiative (GPEI) was started in 1988 with 4 partners, the World Health Organization, Rotary, CDC and UNICEF, with the aim of eradicating polio by 2000. Starting from a baseline of about 350,000 cases of polio per year in 125 countries, the burden of disease has been markedly reduced, and since 2000 there have been only a handful of cases each year. The oral vaccine has been effective in reducing the number of new cases, but a small reservoir of virus has been identified in immunocompromised subjects. During prolonged replication, the attenuating mutations are under negative selection pressure and there have been a few vaccine-associated paralytic polio cases, about 2 per 1,000,000 vaccinations, compared to 1 per 200 wild-type virus infections.
Various types of immune deficiency can lead to prolonged virus shedding (>6 months) but severe combined immunodeficiency (SCID) can result in chronic shedding (>5 years). Vaccine-derived polio virus in immunodeficient subjects has been given the label iVDPV. Since 1995, a registry has recorded all known iVDPV cases, initially mainly in well-resourced countries but increasingly now world-wide. Encouragingly, iVDPV shedders are now being identified at a greater rate, due to improved surveillance. In an iVDPV prevalence study, about 650 subjects have been enrolled.
Chronic shedding presents a hazard both to the individual (disease may progress to paralysis and death) and to the community (seeding new polio outbreaks). Therefore, the potential role for antiviral therapy is to be assessed. Two compounds, pocapavir (V-073, a capsid inhibitor) and V-7404 (3C protease inhibitor), have been identified as having the potential to be progressed quickly. As expected, the two drugs in combination cause a marked reduction of viral resistance frequency. However, it remains an open question whether the combination will have sufficiently high barrier to viral resistance in these immunodeficient subjects.
4.3. Impact of transmitted HIV phenotype on host-virus interactions and disease progression – implications for treatment and cure. Eric Hunter, Emory vaccine center, Atlanta, GA
HIV transmission studies in “serodiscordant” couples, in which one is HIV-positive and the other HIV-negative, allow examination of both the “donor” and “recipient” viruses. When recruiting couples into the study, counseling and supply of condoms reduces the expected transmission rate by more than two-thirds, but some transmissions do occur. In 75–80% of cases, the transmitted HIV is linked to the partner's virus.
HIV transmission involves a severe genetic bottleneck, especially when the male partner is newly infected, unless the male has genital lesions, in which case the bottleneck is less severe and comparable to that when the female partner is infected. HIV transmission involves both selection and chance. Of all the quasispecies present in the transmitting partner, there is a selection for virions that are closer to the consensus and have greater transmission fitness. The consequences of this selection have been followed in the newly infected partner for up to 8 years.
For years, the asymptomatic or set-point viral load (VL) has been known to correlate with disease progression. Despite selection during HIV transmission involves both selection and chance: individual virions differ in their replicative capacities (vRC) and in the epitopes which may or may not be detected by the new host's immune system. It has been shown that very early in infection, high-vRC viruses are linked to enhanced levels of inflammatory cytokines, driving T-cell dysfunction and increased viral burden in naïve CD4+ T cells and CD4+ memory T cells, which in turn is linked to a significantly faster decline of CD4 cells.
Prior immune selection history can render the transmitted virus either more or less visible to the new host's immune system. The hypothesis was that transmission of HIV Gag polymorphisms pre-adapted to the recipient's HLA alleles could reduce immune recognition of the transmitted founder virus (Mónaco et al., 2016). By chance, a proportion of the founder virus may already be adapted to escape the immune system. On the other hand, virions which retain the consensus epitopes will be more easily recognized by the immune system. Evidence to support this hypothesis was presented.
In conclusion, transmitted polymorphisms can impact HIV disease progression. With low vRC and original non-adapted epitopes, the disease will progress more slowly. High vRC and adapted epitopes will aid rapid disease progression. Alternatively, these functions can oppose each other, the balance defining the virulence.
4.4. Zika virus antiviral and vaccine development. Pei-Yong Shi, University of Texas Medical Branch, Galveston
Inactivated and subunit vaccines generally have a better safety profile, but live, attenuated vaccines may provide life-long immunity after a single dose. Pei-Yong has investigated two possible approaches to the development of live, attenuated vaccines against ZIKV: knocking out the viral methyltransferase active site, thus preventing the virus from acquiring a cap to protect the viral RNA, or deleting sections of the 3′ untranslated region (UTR). The latter was the focus of his ICAR presentation.
Perhaps surprisingly, a virus with a 10-nucleotide deletion (10-del ZIKV) seemed to be a more promising vaccine candidate than those with 20- or 30-nucleotide deletions. The A129 mouse model was used for initial evaluation, and safety and efficacy were then confirmed in rhesus macaques. The 10-del ZIKV vaccine was well tolerated, and a single dose gave protective immunity within 14 days post-vaccination in both mice and nonhuman primates. The vaccine prevented in utero transmission of ZIKV in pregnant mice and prevented testis damage in male mice, possibly answering two major concerns from clinical studies.
5. Antiviral immunity symposium
5.1. Targeting HIV reservoirs for stimulation and elimination. Jeff Murry, Gilead Sciences, Foster City, CA
Jeff Murry reviewed potential therapeutic approaches to “curing” HIV infection, and the role of virus latency and antiviral drug resistance as major barriers to a cure. He defined three types of cure 1) drug-free remission, 2) functional cure, and 3) sterilizing cure. Infected T cells serve as a reservoir for viral persistence, and can be the source for clonal expansion of latently infected cells. One strategy for elimination of these latently infected cells is a “shock and kill” treatment regimen, using latency-reversing agents such as toll-like receptor (TLR)-agonists. Immune activation stimulated by TLR-agonists stimulates HIV expression. Surprisingly, vaccination increases plasma viremia in HIV-infected individuals by activating T cells, which then increase virus expression. Jeff described research on the use of the TLR7 agonist GS-9620, a pattern recognition receptor (PRR) for ssRNA. GS-9620 activates CD8+ cells more than CD4+ cells, and when used as a therapeutic in combination with the anti-HIV monoclonal antibody PGT-121 produces a synergistic effect, leading to increased killing of infected cells. He also described the use of combination therapy with a TLR7 agonist and a therapeutic vaccine, with the intent of combining a latency-reversing agent with an immune activator.
5.2. Catch me if you can – using HBV immunity for therapy, Ulrike Protzer, Technical University of Munich, Germany
Ulrike Protzer described her studies of the immune response to HBV infection and possible strategies for stimulating immunity for therapy. She stated that T-cell responses can cure HBV infection in animal models. An interesting observation was that acutely infected patients have increased T cell responses, in contrast to chronically infected patients, who have decreased responses. Her research efforts focus on understanding the interaction between HBV and the host, then translating that knowledge into novel therapeutic approaches. Her lab has also established two HBV-transgenic mouse lines as models for vertical transmission, and has developed a mouse model of self-limited HBV infections.
Ulrike described several approaches for therapy, including the antiviral effects of cytokines and other immune mediators, antigen targeting by therapeutic vaccination, and adoptive transfer of antibody and T cells to reconstitute HBV-specific immunity. Therapeutic immune responses required low HBV load and the reactive T cells to be recruited to the liver. She discussed several recruitment strategies, including intra-hepatic myeloid-cell aggregates for T-cell population expansion (iMATES), chimeric-antigen receptor (CAR) T cells recognizing HBV antigens in the MHCII context, and bi-specific antibodies. In the chimeric mouse models, and with the possible exception of CAR, T-cells were most effective when the viral load was first lowered to below 400 International Units. Cytotoxic T cells can clear cccDNA in culture, with or without direct contact with the infected cells. To eliminate cccDNA, the ideal T-cell target must have dual activity, including both cytolytic and non-cytolytic effects. APOBEC 3A and 3B are involved in cccDNA degradation, and are up-regulated in HBV-infected human liver cells, but DNAses are also required. In conclusion, Ulrike suggested that, because immune tolerance must be overcome to cure chronic infection, her lab is evaluating strategies of therapeutic vaccination to redirect T cells to HBV-infected cells by generating recombinant T-cell receptors.
5.3. Host factors as potential targets for influenza virus antivirals. Adolfo Garcia-Sastre, Mount Sinai School of Medicine, New York City, NY
Adolfo Garcia-Sastre described three approaches to identifying host factors that may serve as influenza virus inhibitors. The first involves the use of siRNA screening assays to identify and confirm host factors required by influenza virus for RNA replication. Several were identified as inhibitors of transcriptional activation. A second approach is based on high-throughput screening (HTS) assays, in which a luciferase gene has replaced the hemagglutinin (HA) to identify host factors and potential antiviral targets. The HTS assay requires growing the recombinant viruses in HA-expressing cells to phenotypically complement them, resulting in a single growth cycle in non-complementing cells. In this assay, inhibitors that do not select for virus resistance indicate potential host factors as targets. The third approach involves identification of targets for influenza virus mRNA splicing and export. Adolfo's lab has identified cellular binding proteins that mediate viral M-protein mRNA splicing. He also described a novel TLR3 agonist, Riboxxol®, which produces an immune response that is an intermediate phenotype between TLR2 agonists and Poly I:C.
6. Emerging viruses
6.1. From basic science to promising antivirals for hemorrhagic fever viruses. Christina Spiropoulou, CDC, Atlanta GA
In her lecture, Christina Spiropoulou gave an overview of her team's efforts to develop new treatments for acute, severe viral diseases, focusing on three main areas. She first showed how a basic understanding of viral biology and pathogenesis can lead to novel therapies, using the example of hantavirus pulmonary syndrome. The development of increased vascular permeability in hantavirus infections, leading to hypotension and shock, can be modeled in vitro through the infection of cultured endothelial cells. Her group has demonstrated that tyrosine kinase inhibitors can reduce or prevent the development of vascular leak, both in infected primary human microvascular endothelial cells and in a rodent model, suggesting they would be of benefit for human patients (Bird et al., 2016).
In the second part of her lecture, Christina reviewed the contribution of her Branch to the discovery of experimental antivirals during the West Africa Ebola epidemic, and discussed how it shifted their approach to drug discovery. During the outbreak, her team screened a range of licensed therapeutic agents with the goal of repurposing them for treating Ebola, and also tested a large number of potential new antivirals. In a particularly successful effort, her group collaborated with researchers at Gilead Sciences to initially characterize and eventually demonstrate the effectiveness of the novel antiviral compound GS-5734 against Ebola virus (Lo et al., 2017) and they have since gone on to test it against lethal Nipah virus infection in nonhuman primates, in collaboration with Emmie de Wit and Heinz Feldmann at the NIH Rocky Mountain Laboratories. She closed her talk by referencing the recent development by the VSPB of high-throughput screening assays for antivirals against hemorrhagic fever viruses and other BSL-4 agents, and invited future collaborations with researchers who would like to test novel compounds, with the ultimate objective of finding clinical antiviral candidates.
6.2. Treatment and prevention of emerging rodent-borne viral diseases. David Safronetz, Public Health Agency of Canada, Winnipeg
David Safronetz began his presentation by acknowledging that the majority of the work he was presenting was conducted during his tenure in the Laboratory of Virology at the Rocky Mountain Laboratories (NIAID, NIH) in Hamilton MT. This session focused on two separate rodent-borne viral pathogens and the unique nuances associated with the preclinical development and testing of medical countermeasures to treat or prevent infections in human populations.
The first topic was New World hantaviruses, focusing primarily on Andes and Sin Nombre viruses which cause hantavirus pulmonary syndrome (HPS) in humans. Although several studies have superb protections rates in prophylactic immunization studies, the sporadic nature of human infection combined with relatively low incidence rates in many geographic regions suggest that therapeutics are required to treat as opposed to prevent these infections in all but high risk populations. Therein lies the problem in that there is only a single small animal disease model which involves infection of Syrian hamsters with Andes virus, the most prevalent South American species of hantavirus, and while the model recapitulates the disease manifestations of HPS accurately its predictive value for therapeutic studies involving immunomodulatory compounds remains unknown. It is clear that both antiviral treatments including ribavirin and favipiravir as well as therapeutic antibody treatment can prevent lethal disease in humans, currently treatment onset must be established prior to or very early after viremia onset which is well before humans develop clinical illness. Combination therapies need to be explored as well as further development of animal models, perhaps using similar strategies as the recently described nonhuman primate model for HPS in order advances in treatment options be made for this rare but frequently fatal disease.
The second topic was Lassa virus which causes Lassa fever, an acute hemorrhagic disease of humans. The relatively well defined endemic region across West Africa combined with predicted infection rates of several hundred thousand individuals annually suggest an immunization strategy would be beneficial in reduce the burden of this disease. However, the high genetic diversity associated with Lassa virus across the endemic regions raises the question of whether or not universal vaccine is achievable or if regional variants will be required. Many platforms have been evaluated though most only against a homotypic challenge most commonly with Lassa virus strain Josiah from Sierra Leone. Using a VSV-based Lassa vaccine similar to the recently deployed rVSV-EBOV vaccine, Dave's group has seen excellent results in challenge models against clade 4 and 5 viruses; however a lack of characterized models using Lassa clade 1, 2, and 3 isolates has slowed further laboratory investigations. Nevertheless, the VSV-Lassa vaccine is considered a frontline candidate for use in West Africa and it is receiving increased attention lately due to the rVSV-Ebola trails during recent West African outbreak.
Despite the recent success with the vaccine experiments, research into therapeutics to treat Lassa fever is also a priority given the viruses repeated introductions outside of West Africa, including into Europe and North America. While off-label use of ribavirin is currently considered the standard of care for humans, Dave's group has found that treatment of guinea pigs and NHPs with favipiravir demonstrates superior protection from lethal disease, and in the case of the guinea pigs model reverses signs of advanced disease even when treatment is initiated at 9 days post-infection, typically within 36–48 hours. Given that favipiravir is already licensed for treating influenza infections in humans in some countries, these results support further trials in treating Lassa fever in humans.
6.3. Flaviviral translation. Mariano Garcia-Blanco, University of Texas Medical Branch, Galveston
Mariano Garcia-Blanco and colleagues have conducted genome-scale RNAi screens to identify candidate host factors. They identified ribosomal proteins RPLP1 and RPLP2 (RPLP1/2) to be among the most crucial host factors required for DENV and YFV infection (Campos et al., 2017). RPLP1/2 are phosphoproteins that bind the ribosome through interaction with another ribosomal protein, RPLP0, to form a structure termed the ribosomal stalk. RPLP1/2 were validated as essential host factors for DENV, YFV, and ZIKV infection in human cell lines. RPLP1/2 knockdown strongly reduced early DENV protein accumulation, indicating a requirement for RPLP1/2 in viral translation.
Additionally, Mariano and colleagues interrogated a library of FDA-approved drugs for their ability to block infection of human HuH-7 cells by a newly isolated ZIKV strain (ZIKV MEX_I_7). More than 20 out of 774 tested compounds decreased ZIKV infection in their in vitro screening assay. Selected compounds were further validated for inhibition of ZIKV infection in human cervical, placental and neural stem cell lines, as well as primary human amnion cells. Established anti-flaviviral drugs (e.g. bortezomib and mycophenolic acid) and others that had no previously known anti-viral activity (e.g. daptomycin) were identified as inhibitors of ZIKV infection. This study identifies drugs that could be tested in clinical studies of ZIKV infection and provides a resource of small molecules to study ZIKV pathogenesis.
6.4. Broad-spectrum antivirals to prevent emerging coronavirus pandemic disease. Timothy Sheahan from the University of North Carolina at Chapel Hill.
Coronaviruses (CoV) have a proclivity to spread rapidly into new host species causing new disease. Severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) CoV successively emerged causing severe epidemic respiratory disease in immunologically naïve human populations throughout the globe. Broad-spectrum therapies capable of inhibiting CoV infections would address an immediate unmet medical need and could be invaluable in the treatment of emerging CoV infections. Timothy Sheahan and colleagues showed that a nucleotide prodrug GS-5734, currently in development to treat Ebola virus disease, can inhibit SARS-CoV and MERS-CoV replication in multiple in vitro systems including primary human airway epithelial cell cultures with submicromolar EC50 values (EC50 0.02–0.7 μM) with a good selectivity index (SI) in human and murine cell lines. GS-5734 was also effective against bat-CoVs, prepandemic bat-CoVs and circulating contemporary human CoV in primary human lung cells, thus demonstrating broad-spectrum anti-CoV activity. In a mouse model of SARS-CoV pathogenesis, prophylactic and early therapeutic administration of GS-5734 significantly reduced lung viral load and improved clinical signs of disease as well as respiratory functions in both adult and aged mice.
Sheahan and colleagues demonstrated that a small molecule inhibitor has potent in vitro and in vivo antiviral efficacy against multiple zoonotic, epidemic and contemporary human CoV. These data provide substantive evidence that GS-5734 may prove effective against endemic MERS-CoV in the Middle East, circulating human CoV, and emerging CoV of the future.
7. Emerging viruses
Maria Agostini from Vanderbilt University, Nashville, TN presented data on the antiviral activity of GS-441524 against CoV. GS-441524 is an analog of the adenosine C-nucleoside prodrug GS-5734. GS-441524 also showed broad anti-CoV activity, but was ∼3–19 less potent than GS-5734. The mutations conferring resistance to GS-441524 mapped to the finger domain of nsp12-RdRp, suggesting a similar mechanism of action as GS-5734. Overall, GS-5734 is broadly active against diverse CoVs and is an effective antiviral therapeutic against known and emerging CoVs.
Jinhong Chang from the Baruch S. Bloomberg Institute, Doylestown, PA presented data on the antiviral activity of the benzodiazepine compound BDAA against yellow fever virus (YFV). BDAA resistance mutations mapped to the YFV NS-4B protein, and BDAA specifically enhanced the host cellular cytokine response induced by YFV, but not other viruses. Time-of-addition experiments supported a proposed mechanism of action in which BDAA disrupts the integrity of the membrane-associated YFV RNA replication complex, suppressing viral replication, and also increases the exposure of viral RNA for an enhanced innate cytokine response.
Linlin Zhang from the Institute of Biochemistry, University of Lübeck, Germany investigated the antiviral activity of ZIKV protease inhibitors designed from the crystal structure of ZIKV NS2B-NS3pro in complex with a capsid dipeptide boronate inhibitor. ZIKV NS2B-NS3 protease is 40 fold more active compared to the proteases of the related flaviviruses WNV and DENV-2.
The development of animal models for emerging viruses is critical for antiviral development. Two talks from the Institute for Antiviral research (IAR), Utah State University discussed animal model development for emerging viruses. Justin Julander of the IAR presented data on the testing of live, attenuated ZIKV vaccine candidates CDX-ZKV-1 and CDX-ZKV-2 developed by Codagenix Inc. using a codon de-optimization approach. The two vaccine candidates were tested in the lethal AG129 Zika virus mouse model. These reverse-engineered synthetic, attenuated vaccine candidates showed dose-dependent protection, which correlated with neutralizing antibody levels present in the serum prior to virus challenge.
Bart Tarbet of the IAR discussed the development of both respiratory and neurological disease models for EV-D68 enterovirus infection in mice. After intranasal challenge, virus rapidly spreads from the lung to blood and to other tissues including the central nervous system. Respiratory disease signs included histological lesions, increased proinflammatory cytokines, and decreased lung function as measured by plethysmography. After intraperitoneal challenge, paralysis of the hind limbs was observed prior to mortality. Both respiratory and neurological signs could be prevented by administration of guanidine.
Kara Carter from Sanofi, Cambridge, MA discussed the development of recombinant human monoclonal antibodies targeting chikungunya virus (CHIKV). In two independent murine models of CHIKV disease, the recombinant antibodies produced dose-dependent reductions in viral titer in the joints, as well as in a nonhuman primate model of the disease. These candidates will continue to be developed for clinical therapeutic evaluation as well as prophylactic activity assessed in preclinical models.
Joana Rocha-Pereira from the Rega Institute for Medical Research in Leuven reported therapeutic activity of 7-deaza-2′-C- methyladenosine (7DMA) and 2′C-methyl cytidine (2CMC) against norovirus and rotavirus infections, both in vitro and in mouse models.
Nam-Joon Cho from the Nanyang Technological Institute, Singapore discussed the approach of using peptides to selectively destabilize lipid membranes as potential antiviral therapeutics. These peptides were active against multiple virus families and had a therapeutic index of 1000. Experiments in a model of dengue in humanized mice demonstrated reduced viral titers after treatment.
Victor De Filippis from Oregon Health and Science University in Portland, discussed the effect of the interferon (IFN)-activating small molecule AV-C in targeting viral infection (Zika, dengue and chikungunya). AV-C requires a TRIF-dependent signaling cascade that culminates in IRF-3 dependent expression and secretion of IFN-I to elicit antiviral responses. Additionally, human peripheral blood mononuclear cells treated with AV-C secreted pro-inflammatory cytokines that are linked with establishment of adaptive immune responses.
Julie Dyall from the NIH Integrated Research Facility, Frederick, MD discussed the antiviral activity of apilimod, a phosphatidylinositol-3-phosphate 5-kinase (PIKfyve) inhibitor, against EBOV and Marburg viruses. Apilimod inhibited EBOV and Marburg virus infection in Huh7, Vero E6 and primary human macrophage cells (EC50 10 nM). Mechanistic studies showed that apilimod blocks EBOV entry by blocking particle delivery into endolysosomes that contain Niemann-Pick C1, the intracellular receptor for EBOV.
Yasuteru Sakuari from the Texas Biomedical Research Institute, San Antonio discussed the discovery of multiple derivatives of the anti-malarial amodiaquine with anti-EBOV activity. In vitro screening revealed 14 compounds with potent activity and high selectivity indexes (>130).
Leen Delang from the Rega Institute in Leuven, discussed the role of mosquito vectors in the spread of drug-resistant viruses. She and her colleagues approached this question by looking at the replication and transmission abilities of wild-type CHIKV or resistant CHIKV variants (i.e. MADTP resistant CHIKV: mutation in nsp1 gene and T705 (favipiravir)-resistance CHIKV mutation in the RdRp gene). They found that the dissemination of favipiravir-resistant CHIKV within the mosquito and the spread of virus to the saliva was markedly decreased, compared to wild-type and MADTP-resistant viruses. Nevertheless, these viruses retained their drug-resistant phenotype in the mosquito.
8. Selected presentations
8.1. Hepatitis viruses
The 30th ICAR presentations summarized the great progress in hepatitis C therapy in the last 10 years, highlighted the few remaining challenges, and presented a number of approaches to move these successes to another hepatitis-causing virus, HBV. The differences between the replication cycles of HCV and HBV most relevant to antiviral therapy were highlighted. HCV is an RNA virus that replicates exclusively in the cytoplasm, without a DNA intermediate or any other means to establish persistent or latent infections, whereas the HBV replication cycle includes a closed-circular cDNA (cccDNA) nuclear-persistent form. The former can therefore be cured with any drug that efficiently inhibits genome replication or infection of new cells, whereas the latter also requires the elimination of cccDNA reservoirs. Nonetheless, the presenters were in general optimistic that lessons learned from HCV and HIV, together with the advances in antiviral therapy and immunology, will eventually lead to a definitive HBV cure. A number of presentations clearly reflected this consensus.
As described above, Mike Sofia reviewed the history of the development of effective direct acting antivirals (Li and De Clercq, 2017) for HCV therapies, then continued with new HBV capsid inhibitors, and Ulrike Protzer discussed the induction of curative immunity against chronic HBV by lowering the viral load and targeting immune cells. From these and all ensuing talks, a common theme emerged that approaches using antivirals to reduce viral load, in combination with vaccines or immune-modulators to then eliminate cccDNA reservoirs, will be the most likely to succeed at curing HBV. There was also a consensus that combination therapies will be essential for hepatitis B, as they have been for HIV and HCV infections.
Most of the presentations in the hepatitis virus session described potential novel antivirals, whereas several posters focused on a selection of approaches to induce stronger HBV immunity. John Tavis of Saint Louis University, Saint Louis, MO, proposed the RNaseH domain of the HBV polymerase as a target for antivirals that can be used in combination therapies. The RNaseH is a difficult enzyme to work with, as there are no in vitro systems in which to perform large scale screens. First-generation assays were under-sensitive, resulting in under-estimation of the number of inhibitors. John's group has focused on known HIV RNaseH inhibitors as the starting compounds, eventually identifying 77 inhibitors. The RNaseH from 13 HBV isolates (genotypes B, C, and D) were cloned. Their activities varied by approximately 10-fold, but all were sensitive to two of the inhibitors. Inhibition was additive with an experimental capsid inhibitor and synergistic with lamivudine. Two compounds inhibited viremia in Fah−/−/Rag2−/−/Il2rg−/− (FRG) chimeric mice by 0.4–1.3 logs, although they were limited by toxicity. Selection for resistance has not been observed so far. A poster by the same group further described RNaseH inhibitors derived from N-hydroxyisoquinoline-diones and related poly-oxygenated heterocycles. EC50s were in the submicromolar to micromolar range and 50% cytotoxic concentrations (CC50s) in the mid- to high-micromolar range. As expected, the inhibitors block synthesis of (+) strand DNA, resulting in accumulation of RNA:DNA heteroduplexes in vitro and in cells. These presentations highlighted the potential of RNaseH inhibitors for HBV therapy, while also noting challenges in identifying and developing these compounds, as in vitro assays still have some intrinsic limitations.
A talk by Shuo Wu of the Blumberg Institute and a poster presented by Masaaki Toyama of Kagoshima University, Japan, discussed two groups of capsid inhibitors, benzamide and pyrimidotriazinone derivatives, which were identified by cell-based or in silico screening of approximately 30,000 compounds or 170,000 structures, respectively. The benzamide-derived compounds identified by Wu's group were modeled to bind to a hydrophobic pocket in the capsid, which was shown to be targeted by other capsid inhibitors, and mutations in this pocket disrupted antiviral activity. The inhibitors are insensitive to capsid phosphorylation. They induced the formation of empty capsids and inhibited assembly by mixes of drug-sensitive and drug-resistant capsid molecules.
A poster presentation by Andrea Cuconati of Arbutus Biopharma described the combinatorial effects of capsid inhibitors or lipid-formulated siRNA nanoparticles, and pegylated interferon with the nucleoside/tide analogs entecavir, TDF and TAF. Two-way combinations were evaluated in stably-transfected HBV replicating cell lines and in HBV-infected primary human hepatocytes. All combinations tested for viral DNA and secreted HBV proteins were synergistic or additive, and no antagonistic effects were detected. Taken together, these presentations support the further development of capsid assembly inhibitors.
Cellular proteins were also examined as potential targets for HBV therapy. A presentation by David Durantel from INSERM, Lyon, France discussed inhibitors of Polo-like kinase 1 (PLK1), an enzyme which is activated during woodchuck hepatitis virus carcinogenesis, in hepatocellular carcinoma and by the HBV X antigen. PLK1 is involved in cell-cycle regulation and DNA repair.
Phosphorylation/dephosphorylation cycles of capsid are required for HBV encapsidation. PLK1 was induced by HBV, even in non-dividing primary human hepatocytes. siRNA and small molecule PLK1 inhibitors showed that it participates in HBV replication. Capsid assembly was inhibited by PLK1 inhibition, and PLK1 phosphorylates the capsid after priming phosphorylation by CDK2 or SRPK. The PLK inhibitor BI-2536 lowered viremia in FRG mice by 1.5 log at 10 mg/kg daily without any effect on sAg expression or obvious toxic effects. PLK inhibitors were proposed as components of combinatorial therapies.
Two other talks focused on modulation of antiviral cellular responses. A presentation by Emily Thi from Arbutus Biopharma discussed the effects of STING agonists on HBV infection. The model is that STING activation results in reactivation of immune responses, thus breaking immunotolerance. In support of this model, it was previously found that activation of STING in macrophages resulted in inhibition of HCV in cultured hepatoma cells, and that, like several other viruses, HBV inhibits STING. Two STING agonists, DMXAA and a chemically-modified 2′-3′-cGAMP, a physiological STING agonist, decreased viremia and liver HBV load in the adenovirus mouse model. Treatment with DMXAA (25mg/kg daily for 7 days) caused rapid reduction, and repeated treatments equaled or surpassed inhibition by IFN treatment, although inhibition was highly variable. As DMXAA and the chemically modified 2′-3′-cGAMP are structurally different, it is unlikely that the antiviral effects resulted from off-target effects. The half-lives of the molecules are vastly different, but this may not be important for STING activators. The effects of STING modulation on T-cell proliferation or on dendritic cells were not evaluated.
Another presentation by David Durantel focused on IFN and TLR agonists as immunomodulators to reactivate responses against HBV. A TLR-7 agonist, GS-9629 had shown good activity in woodchucks and chimpanzees, but was not active in humans, as only low doses could be used. Moreover, TLR-7 is not expressed in hepatocytes, and TLR-7 agonists can therefore only act indirectly. TLR-2 and -3, in contrast, are expressed in hepatocytes. Several TLR agonists were tested in HBV infected hepatocytes between days 7 and 14 after infection. cccDNA was reduced by only 50%, whereas viral antigens were fully inhibited, which indicates that some silencing mechanism is partly involved. Pam3CSK4 (TLR-1 and 2) and poly-IC (TLR-3) had the best antiviral effect, and no rebound within 10 days after stopping treatment.
A pure TLR3-agonist already discussed by Adolfo Garcia-Sastre, Riboxxxol®, which was tested in vivo, was equally active. Riboxxxol induces expression of genes in both NF-κB and IFN pathways. Treatment of infected hepatocytes with Riboxxxol or with conditioned medium from Riboxxxol-treated PBMCs resulted in similar antiviral effects, indicating that TLR-3 agonists act both directly on infected hepatocytes and indirectly, through activation of immune cells. The antiviral activity of the TLR-2 and -3 agonists was neutralized by IFN and IL6. Unfortunately, TLR-2 -3 agonists are more challenging to formulate than TLR7-agonists.
The 30th ICAR highlighted the great successes in hepatitis C therapy and the renewed interest in HBV as a target for antiviral intervention, with the ultimate goal of a functional cure. The general consensus was that combination therapies and immunomodulation will both be necessary to achieve this goal. New viral and cellular targets are being explored, including cellular protein kinases and viral RNaseH and capsid.
8.2. Respiratory viruses
Stephan Ludwig of the Institute of Virology, Westfälische Wilhelms-University Münster, Germany, reported that repurposing kinase inhibitors against influenza virus is a strategy to target cellular factors essential for virus replication, aiming for a broad antiviral activity spectrum and prevention of resistance. The Raf/MEK/ERK signaling cascade is required for influenza A virus (IAV) replication, triggering the nuclear export of newly assembled viral ribonucleoproteins (vRNPs). Inhibition of this cascade was previously shown to inhibit viral replication. Here, the first licensed MEK inhibitor for treatment of malignant melanoma, i.e. trametinib (GSK-1120212, Mekinist®) and CI-1040 (PD184352) being developed by Pfizer, were shown to efficiently block replication of different influenza subtypes, both in vitro and in vivo. These compounds enhanced mice survival and reduced lung viral titers even when treatment started 48 h after infection, providing a prolonged treatment window compared to oseltamivir (Haasbach et al., 2017). Confocal microscopy studies demonstrated that MEK inhibitors impaired nuclear export of vRNPs. Trametinib also limited hyper-expression of several cytokines. Atriva Therapeutics is currently developing antiviral MEK-inhibitors against influenza.
Sharon Tamir from Karyopharm Therapeutics, Newton, MA reported on the inhibition of another cellular target, nuclear export, which is required for export of important IAV cargos such as vRNPs. Verdinexor (KPT-335), an inhibitor of the nuclear export protein, exportin 1 (XPO1), is a broad-spectrum antiviral agent with efficacy against respiratory syncytial virus (RSV), HCV, HIV and Venezuelan equine encephalitis virus (VEEV). In 2015, a randomized, double-blind, placebo-controlled, dose-escalating Phase I clinical trial of verdinexor was conducted in healthy human volunteers in Australia to evaluate the safety and tolerability of verdinexor. The drug was generally safe and well tolerated. Verdinexor was evaluated in a lethal mouse model of IAV infection in BALB/c mice, in comparison with the neuraminidase inhibitor oseltamivir. Verdinexor showed robust antiviral activity and a long therapeutic window. When the initiation of treatment was delayed for up to 4 days post-challenge, the reduction in lung viral titers was equivalent to that seen when dosing was begun 1 day post-inoculation. Verdinexor also proved synergistic with oseltamivir.
Jennifer Pickens from Karyopharm Therapeutics reported that KPT-335 also modulated trafficking of the RSV matrix (M) protein, which is a critical mediator of RSV replication and viral egress. It is the only viral protein trafficked within the nucleus through the action of importin-b1 and XPO1. KPT-335 inhibited RSV replication in cell culture, therapeutically and prophylactically, resulted in mild nuclear accumulation of the RSV M protein, reduced XPO1 expression, increased p53 expression (without induction of the caspase 3/7 apoptotic pathway) and modulated immune factors, as evidenced by changes in pro-inflammatory cytokine expression.
Jia Meng, from Alios Biopharma, Inc., South San Francisco, CA reported the activity of ALS-8112/ALS-8176 against human metapneumovirus (hMPV), a virus first identified in the Netherlands in 2001 and the second leading cause of pediatric lower respiratory tract infections. ALS-8112 is the parent molecule of ALS8176 (lumicitabine), a nucleoside prodrug inhibitor of RSV replication currently under clinical evaluation. ALS-8112 demonstrated potent activity against various strains of hMPV (EC50 values in the 0.035–0.5 μM range). Its triphosphate form was recognized as a substrate by RSV and hMPV polymerases causing immediate chain termination and it did not inhibit polymerases from host or viruses unrelated to RSV and hMPV. These results support the further investigation of ALS-8176 for the treatment of hMPV infection in both pediatric and adult patients.
Erik Rhoden, from the CDC, Atlanta described the activity of antifungal azoles against enterovirus (EV) and parechovirus A3 (Par-A3). Par-A3 causes severe illness in young infants, including sepsis meningitis and encephalitis, highlighting the need for antiviral therapy. Two FDA-approved antifungal azoles, i.e. itraconazole (ITZ) and posaconazole (PSZ) as well as the PI4KIIIβ inhibitor enviroxime and the oxysterol-binding protein inhibitor 25-hydroxycholesterol (25HC) had broad-spectrum anti-EV A-D activity, consistent with a similar mechanism of action against EV. Only ITZ and PSZ proved active against Par-A3, pointing to a different mechanism of action. ITZ and PSZ target an early step of the Par-A3 life cycle, displaying antiviral effects during pretreatment, co-addition, cell-free virus-compound pre-incubation, inactivation and attachment. Ketoconazole, fluconazole and voriconazole had no anti-EV or anti-Par-A3 activity. Interestingly, ITZ and PSZ were specific inhibitors of Par-A3 as other parechovirus types were not inhibited. Further studies are required to identify potential receptors involved in Par-A3 infection.
8.3. DNA viruses
Herpesvirus DNA replication relies on several processes typically catalyzed by enzymes in the nucleotidyltransferase (NTS) superfamily. These enzymes play a role in nucleic acid metabolic events, including RNA and DNA digestion, DNA recombination, DNA integration, replication fork repair, DNA repair, and microRNA (miRNA) maturation and function. Viral NTS enzymes include the HBV RNase H, the HIV RNase H and HIV integrase. Several compounds selected for their ability to suppress HIV or HBV RNase H activity were previously reported to inhibit HSV replication in cell culture.
Lynda Morrison from Saint Louis University School of Medicine identified 15 natural tropolones that reduced HSV replication by 3–6 logs at 5 μM in cell culture. She also reported the anti-HSV activity of synthetic tropolones that were screened first by a colorimetric assay, then by a plaque-reduction assay, with the goals of determining a preliminary structure-activity relationship (SAR) and providing a starting point for future optimization studies. An intact α-hydroxytropolone (αHT) pharmacophore was advantageous for anti-HSV potency and additional αHT derivatives were therefore synthesized. The two most powerful inhibitors shared a common biphenyl side-chain, were capable of inhibiting HSV-1 and HSV-2 with an EC50 of 81–210 nM and a therapeutic index >1000. They also strongly inhibited acyclovir-resistant mutants. Resistance to NTS inhibitors evolved slowly, and αHT derivatives presented a higher barrier to selection for viral resistance than acyclovir. Synergy between acyclovir and one of the αHT derivatives was reported. Troponoid drugs are envisioned to be employed alone or in combination with existing anti-HSV drugs to suppress HSV replication in order to prevent viral shedding and to limit the development of drug resistance.
Because the selection for herpesvirus drug resistance is a well-recognized problem among diverse populations of patients with impaired immune systems, a translational research platform RegaVir (www.regavir.org) was set up in Belgium for typing drug resistance among patients who fail anti-herpesvirus therapy. Graciela Andrei from the Rega Institute, Leuven presented several human cytomegalovirus (HCMV) case studies, which highlighted:
-
•
the usefulness of rapid genotyping for the adjustment of antiviral therapy;
-
•
the emergence of multiple drug-resistance, due either to infection with a virus having a single mutation conferring resistance to the currently approved drugs ganciclovir, cidofovir and foscavir, or caused by co-infection with viruses with distinct genotypes;
-
•
the compartmentalization of drug-resistant HCMV;
-
•
the advantage of next-generation sequencing for detecting minor populations of drug-resistant viruses;
-
•
the emergence of resistance to investigational anti-HCMV drugs (e.g. maribavir); and
-
•
the urgent need for novel anti-HCMV agents to reduce morbidity and mortality caused by drug-resistant viruses and therapy of congenital infections.
In the search for anti-adenovirus (AdV) agents, Karoly Toth from the Saint Louis University School of Medicine presented the in vitro and in vivo activity of USC-187, an alkyl tyrosinamide-ester prodrug of the nucleotide analog HPMPA (considered the adenine counterpart of cidofovir). USC-187 proved active against multiple AdV serotypes in cell culture and was effective against AdV-C6 in hamsters immunosuppressed by treatment with cyclophosphamide. Oral administration of USC-187 completely prevented or significantly decreased mortality, viral titers and liver pathology when administered up to 4 days post-AdV-C6 intravenous challenge. In a respiratory AdV-C6 challenge model, pathologic changes were not only the result of viral replication. The drug (administered orally at 10 mg/ml bid starting 1 day before virus inoculation) diminished viral titers in the lung, but this did not result in decreased disease, and eventually the pathology was exacerbated by drug administration, as evaluated by loss of body weight, indicating that USC-087 was toxic at the concentration used. Fine-tuning of drug concentrations in the intranasal AdV-C6 challenge model is planned.
9. Medicinal chemistry
Steven De Jonghe from the Rega Institute, Leuven proposed cyclin G-associated kinase (GAK) as a target for HCV therapy GAK has emerged as a promising drug target for treatment of RNA virus infections. GAK is a host cell kinase and its depletion by siRNA was shown to inhibit two distinct steps of the HCV replication cycle, viral entry and infectious virus assembly. The hit-to-lead optimization process of isothiazolo[4,3-b]pyridines led to the development of the first selective GAK inhibitors, which were also active against dengue and Ebola virus.
T-705 (favipiravir), which is metabolized to its biologically active ribonucleoside triphosphate, has been extensively studied as a potent antiviral agent. However, the corresponding nucleoside and nucleotide derived from T-705 were found to be quite unstable and their synthesis problematic. Johanna Huchting from Hamburg University, Germany has developed a reliable protocol for the synthesis of the T-705-ribonucleoside and its 5′-monophosphate. She also presented the first mechanistic study of T-705-ribonucleoside degradation, using methods such as UV/Vis and NMR spectroscopy, HPLC and HR-ESI-MS. The data revealed significant lability of both the glycosidic bond and the heterocycle (6-fluoro-3-hydroxypyrazine-2-carboxamide) itself, even under very mild conditions. The degradation is proposed to begin with a nucleophilic displacement of the fluorine atom for the hydroxyl group, followed by destruction of the heterocyclic moiety and glycosidic bond cleavage. The decomposition of the nucleobase after the incorporation of the T-705-nucleoside triphosphate into the viral RNA can also lead to a mutagenic effect, representing one of the possible mechanisms of action of favipiravir.
Non-nucleoside reverse transcriptase inhibitors (NNRTIs) represent an extensively studied group of compounds for the treatment of HIV-1 infections. An advantage of NNRTIs, compared to nucleoside and nucleotide analogs, is that they do not require intracellular metabolization. Ondřej Baszczyňski from the Institute of Organic Chemistry and Biochemistry of the Czech Academy of Sciences in Prague, Czech Republic disclosed a novel type of NNRTI bearing a 5,6,7,8-tetrahydropteridine core. Quite complicated and low-yielding synthesis afforded a series of compounds, of which the best candidate exhibited single-digit nanomolar activity against wild-type and two highly prevalent HIV-1 mutants (K103N and Y181C), as well as improved solubility compared to the FDA-approved NNRTIs.
The presentation by Izzat Raheem from Merck, West Point, NY dealt with a new class of HIV integrase strand-transfer inhibitors (InSTIs), for which the lead compounds have revealed excellent antiviral activity, but limited oral absorption. A unique phosphate/carbonate acetal double-prodrug approach was therefore developed that achieved significantly elevated plasma exposure.
Lak Shin Jeong from Seoul National University, South Korea described the design and synthesis of novel fluorocarbocyclic nucleosides, several of which inhibited ZIKV and CHIKV in the sub-micromolar range (EC50 0.2–0.3 μM). The activity of the compounds appears to correlate with the direct inhibition of both S-adenosylhomocysteine hydrolase and viral RNA polymerases.
Radim Nencka from the Institute of Organic Chemistry and Biochemistry of the Czech Academy of Sciences in Prague emphasized the urgent need to develop potent inhibitors of emerging flaviviruses, especially those easily transmitted via mosquitoes and ticks (thus called arthropod-borne viruses or arboviruses). His team recently evaluated a series of nucleoside and nucleotide analogs against ZIKV, West Nile virus (WNV) and tick-borne encephalitis virus (TBEV). Triphosphate derivatives of selected nucleosides were prepared for the evaluation of their inhibitory activities on ZIKV RNA-dependent RNA polymerase, an assay the team successfully established for routine compound screening. The promising results may lead to the design of novel types of nucleoside analogs and hopefully to the development of potent antivirals against these emerging human pathogens.
Acknowledgements
We thank all the speakers of the 30th ICAR for their contribution to a successful meeting and all those who collaborated in the making of this report.
References
- Bird B.H., Shrivastava-Ranjan P., Dodd K.A., Erickson B.R., Spiropoulou C.F. Effect of Vandetanib on Andes virus survival in the hamster model of Hantavirus pulmonary syndrome. Antivir. Res. 2016;132:66–69. doi: 10.1016/j.antiviral.2016.05.014. [DOI] [PubMed] [Google Scholar]
- Bonnelykke K., Sleiman P., Nielsen K., Kreiner-Moller E., Mercader J.M., Belgrave D., den Dekker H.T., Husby A., Sevelsted A., Faura-Tellez G., Mortensen L.J., Paternoster L., Flaaten R., Molgaard A., Smart D.E., Thomsen P.F., Rasmussen M.A., Bonas-Guarch S., Holst C., Nohr E.A., Yadav R., March M.E., Blicher T., Lackie P.M., Jaddoe V.W., Simpson A., Holloway J.W., Duijts L., Custovic A., Davies D.E., Torrents D., Gupta R., Hollegaard M.V., Hougaard D.M., Hakonarson H., Bisgaard H. A genome-wide association study identifies CDHR3 as a susceptibility locus for early childhood asthma with severe exacerbations. Nat. Genet. 2014;46:51–55. doi: 10.1038/ng.2830. [DOI] [PubMed] [Google Scholar]
- Campos R.K., Wong B., Xie X., Lu Y.-F., Shi P.-Y., Pompon J., Garcia-Blanco M.A., Bradrick S.S. RPLP1 and RPLP2 are essential flavivirus host factors that promote early viral protein accumulation. J. Virol. 2017;91 doi: 10.1128/JVI.01706-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Boudinot F.D., Chu C.K., McClure H.M., Schinazi R.F. Pharmacokinetics of (-)-beta-D-2-aminopurine dioxolane and (-)-beta-D-2-amino-6-chloropurine dioxolane and their antiviral metabolite (-)-beta-D-dioxolane guanine in rhesus monkeys. Antimicrob. Agents Chemother. 1996;40:2332–2336. doi: 10.1128/aac.40.10.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y., Li L., Grill S., Gullen E., Lee C.S., Gumina G., Tsujii E., Cheng Y.C., Chu C.K. Structure-activity relationships of (E)-5-(2-bromovinyl)uracil and related pyrimidine nucleosides as antiviral agents for herpes viruses. J. Med. Chem. 2000;43:2538–2546. doi: 10.1021/jm990543n. [DOI] [PubMed] [Google Scholar]
- Chu C.K., Yadav V., Chong Y.H., Schinazi R.F. Anti-HIV activity of (-)-(2R,4R)-1- (2-hydroxymethyl-1,3-dioxolan-4-yl)-thymine against drug-resistant HIV-1 mutants and studies of its molecular mechanism. J. Med. Chem. 2005;48:3949–3952. doi: 10.1021/jm050060l. [DOI] [PubMed] [Google Scholar]
- Chu J.J., Yang P.L. c-Src protein kinase inhibitors block assembly and maturation of dengue virus. Proc. Natl. Acad. Sci. U. S. A. 2007;104:3520–3525. doi: 10.1073/pnas.0611681104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M., Midituru C., Schmidt A., Zhu X., Pitts J., Wang J., Potisopon S., Zhang J., Wojciechowski A., Chu J.-H., Gray N., Yang P. GNF-2 inhibits dengue virus by targeting Abl kinases and the viral E protein. Cell Chem. Biol. 2005;23:443–452. doi: 10.1016/j.chembiol.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M.J., Miduturu C., Schmidt A.G., Zhu X., Pitts J.D., Wang J., Potisopon S., Zhang J., Wojciechowski A., Hann Chu J.J., Gray N.S., Yang P.L. GNF-2 inhibits dengue virus by targeting Abl kinases and the viral E protein. Cell Chem. Biol. 2016;23:443–452. doi: 10.1016/j.chembiol.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De C., Liu D., Zheng B., Singh U.S., Chavre S., White C., Arnold R.D., Hagen F.K., Chu C.K., Moffat J.F. beta-l-1-[5-(E-2-bromovinyl)-2-(hydroxymethyl)-1,3-(dioxolan-4-yl)] uracil (l-BHDU) prevents varicella-zoster virus replication in a SCID-Hu mouse model and does not interfere with 5-fluorouracil catabolism. Antivir. Res. 2014;110:10–19. doi: 10.1016/j.antiviral.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everts M., Cihlar T., Bostwick J.R., Whitley R.J. Accelerating drug development: antiviral therapies for emerging viruses as a model. Annu. Rev. Pharmacol. Toxicol. 2017;57:155–169. doi: 10.1146/annurev-pharmtox-010716-104533. [DOI] [PubMed] [Google Scholar]
- Everts M., Knight W.B., Harris D.R., Secrist J.A., 3rd, Whitley R.J. The Alabama Drug Discovery Alliance: a collaborative partnership to facilitate academic drug discovery. Pharm. Res. 2011;28:1454–1459. doi: 10.1007/s11095-011-0432-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everts M., Suto M.J., Painter G.R., Whitley R.J. Consortia's critical role in developing medical countermeasures for re-emerging viral infections: a USA perspective. Future Virol. 2016;11:187–195. doi: 10.2217/fvl-2015-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman P.A., Jeffrey J., Kiefer L.L., Feng J.Y., Anderson K.S., Borroto-Esoda K., Hill E., Copeland W.C., Chu C.K., Sommadossi J.P., Liberman I., Schinazi R.F., Painter G.R. Mechanism of action of 1-beta-D-2,6-diaminopurine dioxolane, a prodrug of the human immunodeficiency virus type 1 inhibitor 1-beta-D-dioxolane guanosine. Antimicrob. Agents Chemother. 2001;45:158–165. doi: 10.1128/AAC.45.1.158-165.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gane E.J., Roberts S.K., Stedman C.A., Angus P.W., Ritchie B., Elston R., Ipe D., Morcos P.N., Baher L., Najera I., Chu T., Lopatin U., Berrey M.M., Bradford W., Laughlin M., Shulman N.S., Smith P.F. Oral combination therapy with a nucleoside polymerase inhibitor (RG7128) and danoprevir for chronic hepatitis C genotype 1 infection (INFORM-1): a randomised, double-blind, placebo-controlled, dose-escalation trial. Lancet. 2010;376:1467–1475. doi: 10.1016/S0140-6736(10)61384-0. [DOI] [PubMed] [Google Scholar]
- Haasbach E., Müller C., Ehrhardt C., Schreiber A., Pleschka S., Ludwig S., Planz O. The MEK-inhibitor CI-1040 displays a broad anti-influenza virus activity in vitro and provides a prolonged treatment window compared to standard of care in vivo. Antivir. Res. 2017;142:178–184. doi: 10.1016/j.antiviral.2017.03.024. [DOI] [PubMed] [Google Scholar]
- Le Pogam S., Seshaadri A., Ewing A., Kang H., Kosaka A., Yan J.M., Berrey M., Symonds B., De La Rosa A., Cammack N., Najera I. RG7128 alone or in combination with pegylated interferon-alpha2a and ribavirin prevents hepatitis C virus (HCV) Replication and selection of resistant variants in HCV-infected patients. J. Infect. Dis. 2010;202:1510–1519. doi: 10.1086/656774. [DOI] [PubMed] [Google Scholar]
- Li G.D., De Clercq E. Current therapy for chronic hepatitis C: the role of direct-acting antivirals. Antivir. Res. 2017;142:83–122. doi: 10.1016/j.antiviral.2017.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo M.K., Jordan R., Arvey A., Sudhamsu J., Shrivastava-Ranjan P., Hotard A.L., Flint M., McMullan L.K., Siegel D., Clarke M.O., Mackman R.L., Hui H.C., Perron M., Ray A.S., Cihlar T., Nichol S.T., Spiropoulou C.F. GS-5734 and its parent nucleoside analog inhibit Filo-, Pneumo-, and Paramyxoviruses. Sci. Rep. 2017;7:43395. doi: 10.1038/srep43395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T., Pai S.B., Zhu Y.L., Lin J.S., Shanmuganathan K., Du J., Wang C., Kim H., Newton M.G., Cheng Y.C., Chu C.K. Structure–activity relationships of 1-(2-Deoxy-2-fluoro-beta-L-arabinofuranosyl)pyrimidine nucleosides as anti-hepatitis B virus agents. J. Med. Chem. 1996;39:2835–2843. doi: 10.1021/jm960098l. [DOI] [PubMed] [Google Scholar]
- Modis Y., Ogata S., Clements D., Harrison S.C. A ligand-binding pocket in the dengue virus envelope glycoprotein. Proc. Natl. Acad. Sci. U. S. A. 2003;100:6986–6991. doi: 10.1073/pnas.0832193100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modis Y., Ogata S., Clements D., Harrison S.C. Structure of the dengue virus envelope protein after membrane fusion. Nature. 2004;427:313–319. doi: 10.1038/nature02165. [DOI] [PubMed] [Google Scholar]
- Mónaco D.C., Dilernia D.A., Fiore-Gartland A., Yu T., Prince J.L., Dennis K.K., Qin K., Schaefer M., Claiborne D.T., Kilembe W., Tang J., Price M.A., Farmer P., Gilmour J., Bansal A., Allen S., Goepfert P., Hunter E. Balance between transmitted HLA preadapted and nonassociated polymorphisms is a major determinant of HIV-1 disease progression. J. Exp. Med. 2016;213:2049–2063. doi: 10.1084/jem.20151984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peek S.F., Cote P.J., Jacob J.R., Toshkov I.A., Hornbuckle W.E., Baldwin B.H., Wells F.V., Chu C.K., Gerin J.L., Tennant B.C., Korba B.E. Antiviral activity of clevudine [L-FMAU, (1-(2-fluoro-5-methyl-beta, L-arabinofuranosyl) uracil)] against woodchuck hepatitis virus replication and gene expression in chronically infected woodchucks (Marmota monax) Hepatology. 2001;33:254–266. doi: 10.1053/jhep.2001.20899. [DOI] [PubMed] [Google Scholar]
- Rawal R.K., Singh U.S., Chavre S.N., Wang J., Sugiyama M., Hung W., Govindarajan R., Korba B., Tanaka Y., Chu C.K. 2′-Fluoro-6′-methylene-carbocyclic adenosine phosphoramidate (FMCAP) prodrug: in vitro anti-HBV activity against the lamivudine-entecavir resistant triple mutant and its mechanism of action. Bioorg. Med. Chem. Lett. 2013;23:503–506. doi: 10.1016/j.bmcl.2012.11.027. [DOI] [PubMed] [Google Scholar]
- Ross B.S., Reddy P.G., Zhang H.R., Rachakonda S., Sofia M.J. Synthesis of diastereomerically pure nucleotide phosphoramidates. J. Org. Chem. 2011;76:8311–8319. doi: 10.1021/jo201492m. [DOI] [PubMed] [Google Scholar]
- Schmidt A.G., Lee K., Yang P.L., Harrison S.C. Small-molecule inhibitors of dengue-virus entry. PLoS Pathog. 2012;8:e1002627. doi: 10.1371/journal.ppat.1002627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vere Hodge R.A. Meeting report: 27th International conference on antiviral research, in Raleigh, NC, USA. Antivir. Res. 2014;111:143–153. doi: 10.1016/j.antiviral.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vere Hodge R.A. Meeting report: 28th international conference on antiviral research in Rome, Italy. Antivir. Res. 2015;123:172–187. doi: 10.1016/j.antiviral.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vere Hodge R.A. Meeting report: 29th international conference on antiviral research in La Jolla, CA, USA. Antivir. Res. 2017;137:23–40. doi: 10.1016/j.antiviral.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]