Abstract
Objective
This study investigated whether outpatient visits of acute upper respiratory infections for children aged less than 15 years are associated with temperature, air pollutants and circulating respiratory viruses in Taipei, Taiwan, from 2003 to 2007.
Methods
Outpatient records for acute upper respiratory infections (ICD9 CM codes: 460, 462, 463,464, 465.9 and 487) in a randomly selected sample (n=39,766 children in 2005) was used to estimate the cumulative relative risks (RR) associated with average temperature lasting for 8 days (lag 0–7 days), air pollutants (NO2, O3 and PM2.5) lasting for 6 days (lag 0–5 days), and virus-specific positive isolation rate lasting for 11 days (lag 0–10 days) using distributed lag non-linear models after controlling for relative humidity, wind speed, day of week, holiday effects and long-term trend.
Results
Average temperature of 33 °C was associated with the lowest risk for outpatient visits of acute upper respiratory infections. Relative to 33 °C, cumulative 8-day RR was highest at 15 °C of ambient average temperature [RR=1.94; 95% confidence interval (CI): 1.78, 2.11]. With the first quartile as reference, cumulative 6-day RRs were 1.25 (95% CI: 1.21, 1.29) for NO2, 1.04 (95% CI: 1.01, 1.06) for O3, and 1.00 (95% CI: 0.98, 1.03) for PM2.5 at the 95th percentile. Per-standard deviation (SD) increase of virus-specific isolation rate for influenza type A (SD=13.2%), type B (SD=8.76%), and adenoviruses (SD=5.25%) revealed statistical significance for overall 11-day RRs of 1.02 (95% CI: 1.01, 1.03), 1.05 (95% CI: 1.03, 1.06) and 1.04 (95% CI: 1.03, 1.05), respectively.
Conclusions
Current study suggested a positive association between outpatient visits for acute upper respiratory infections and ambient environment factors, including average temperature, air pollutants, and circulating respiratory viruses.
Keywords: Children, Upper respiratory infection, Air pollution, Respiratory viruses, Temperature
Highlights
► Outpatient visits of acute upper respiratory infections highly associated with lower average temperature. ► Daily concentrations of NO2 and ozone caused greater risks than particulate on acute upper respiratory infections. ► Isolation rates of influenza type A/B & adenoviruses were significantly allied with high acute upper respiratory infection.
1. Introduction
Acute upper respiratory infections are fairly common, for which children usually experience 3–8 episodes of it per year and 10–15% of the infected kids need to take at least 12 medical services for this particular type of infections annually (Denny, 1995). Typical symptoms of this group of diseases include sore throat, cough, fever, malaise and myalgia that may last for days (Simoes et al., 2006, West, 2002, WHO, 1995). They are rarely fatal but cause significant morbidity and economic burden (Gonzales et al., 2001). These diseases result in increasing frequency for suspending classes for school children (Davis, 1976).
More than 200 types of virus are reported associated with upper respiratory infections, including rhinovirus, coronaviruses, influenza viruses, parainfluenza viruses, adenoviruses and respiratory syncytial virus, human metapneumovirus, bocavirus, and polyomavirus (Brodzinski and Ruddy, 2009, Dasaraju and Liu, 1996, Denny, 1995, Treanor and Lee, 2010, West, 2002). Additionally, some specific bacteria, such as group A streptococci, were isolated from the patients with these diseases which could complicate their infections even further (Chi et al., 2003, Denny, 1995, Gonzales et al., 2001, Treanor and Lee, 2010).
Occurrence of acute upper respiratory infections is usually more common in seasons with lower temperature (Falagas et al., 2008, Liao et al., 2009, Makinen et al., 2009). Moreover, air pollutants, including NO2, O3, and particulate matter (PM), were also reported correlated with increased morbidity of respiratory infections (Burnett et al., 2001, Hwang and Chan, 2002, Lin et al., 2005, Lin et al., 2008, Peel et al., 2005, Schwartz et al., 1991, Wong et al., 2009, Wong et al., 2006). Even though the evidence of positive association between ambient environment and elevation of acute upper respiratory infections were revealed in literature, respiratory infections were believed to be more linked to the circulation of respiratory biological agents (Denny, 1995, Simoes et al., 2006, Treanor and Lee, 2010). Although some evidence about risk estimates of environmental risk factors showed various day lags resulting from displacement effect (harvesting effect) (Zanobetti et al., 2000), the association and interaction between acute upper respiratory infections and environmental and biological factors remains unclear. Moreover, latencies (the lag effects) of circulating respiratory viruses, air pollutants, and temperature led to more difficulties in clarifying the associations.
Therefore, current time series study aimed to clarify the acute to short-term effects of circulating respiratory viruses, daily temperature, and air pollutants on the outpatient visits of acute upper respiratory infections using chronological reimbursement claims of Taiwan national health insurance data for population aged less than 15 years old in Taipei, Taiwan.
2. Methods
2.1. Study setting
Data involved in present study included health insurance claims from the Taiwan National Health Research Institute, daily meteorological records from the Central Weather Bureau, daily air pollution monitoring records from the Taiwan Environmental Protection Administration, and daily virological surveillance data from the Taiwan Centers for Disease Control.
Over 96 percent of the 23-million population in Taiwan had been covered in the National Health Insurance program since 2000 (Bureau of National Health Insurance, 2001). Meanwhile, Taiwan National Health Research Institute also established a cohort with electronic reimbursement claim records consisting of a national representative random sample of one million people from the whole residents in Taiwan (Taiwan National Health Insurance Research Database, 2011). Individual identification number of citizenship was utilized for linking to the National Health Insurance electronic database for further analyses. Gender, date of birth, health care received by the subjects, dates of outpatient visits, inpatient admissions and discharges, and names of medical caregivers providing these services for this representative sample were thus retrieved.
Study subjects were population aged less than 15 years residing in Taipei Metropolitan, including Taipei City and New Taipei City (n=39,766 in 2005). Diagnoses were coded according to the 9th revision of International Classification of Diseases with clinical modification (ICD-9 CM). Records of outpatient visit for acute upper respiratory infections, which included common cold (ICD-9 CM code: 460), acute pharyngitis (code 462), acute tonsillitis (code 463), acute laryngitis (code 464), acute upper respiratory infection (code 465.9), and influenza (code 487), during the period of 2003–2007 were retrieved.
Taiwan Centers for Disease Control launched a nationwide virus surveillance network in 1999 (Taiwan Centers for Disease Control, 2011) and contracted with 8 regional clinical virology laboratories of medical centers to real-time monitor the circulating viruses country wide. Community sentinel physicians (from sentinel physician-based surveillance system) across the nation collected nasal and/ or throat specimens daily from patients with one or more symptoms of upper and lower respiratory tract infections, including cough, sore throat, tonsillitis, pharyngitis, pneumonia, and bronchiolotis. These collected specimens were delivered to regional contracted laboratories to examine the viral types, including influenza type A, influenza type B, parainfluenza viruses, adenoviruses, and respiratory syncytial virus, using viral isolation, immunofluorescent assay by type-specific monoclonal antibodies (Dako, Cambridgeshire, United Kingdom), and reverse-transcriptase polymerase chain reaction for subtype identification (Taiwan Centers for Disease Control, 2007, Shih et al., 2005). The Kun-Yan Central Laboratory of the Taiwan Centers for Disease Control was responsible for the final confirmation of cases based on laboratory results and reported symptoms. This study used laboratory-based virological surveillance data from 2003 to 2007 in Taipei (identification results from clinical virology laboratories of Tri-Service General Hospital, National Taiwan University Hospital, and Chang Gung Memorial Hospital in Linkou) that contained information for scrambled patient identification, gender, birthday, residential area, dates of disease onset and admission, results of viral isolation and names of medical caregivers providing services. Daily virus-specific isolation rates were calculated using the number of positive identified respiratory infections specimens divided by the total specimens from regional reference virology laboratories.
Taiwan Environmental Protection Administration established the Taiwan Air Quality Monitoring Network in 1993, consisting of 74 stationary monitoring stations nationwide in 2008 (Taiwan Environmental Protection Administration, 2011). Concentrations of ambient air pollutants–particulate matter less than 2.5 μm in aerodynamic diameter (PM2.5) by beta-ray absorption measurement, nitrogen dioxide (NO2) by chemiluminescene measurement, and ozone (O3) by ultraviolet absorption measurement were recorded hourly. This study utilized daily average levels of NO2 and O3 monitored at 13 general ambient stations and daily measurements of PM2.5 (10 days missing data among 1826 days from 2003 to 2007) obtained from Hsinchuang supersite station in Taipei metropolitan, 2003–2007. Locations of monitoring stations were shown in Fig. 1.
Fig. 1.
Locations for general ambient stations and weather station in Taipei metropolitan.
Central Weather Bureau provides 24-h weather records (average temperature, maximum/ minimum temperature, relative humidity, and barometric pressure) from 25 real-time weather monitoring stations in Taiwan (Central Weather Bureau, 2011). In our analyses, we used daily weather measurements from Taipei weather station that is most representative to the ambient temperature exposures of local population (Fig. 1).
2.2. Statistical analysis
This study applied a distributed lag non-linear model (DLNM) with Poisson distribution (Gasparrini et al., 2010) to assess the effects of daily average temperatures, concentrations for NO2, O3 and PM2.5, and rates of positive isolated viruses to the risk of outpatient visits for acute upper respiratory infections. To model the non-linear and delayed effects for temperature and air pollutants, lag-stratified natural cubic spline (NS) DLNM models were adopted for analysis. Relative risks of temperature and air pollutants were estimated by cross-basis function in DLNM models. The cross-basis function contains the dimensions of variables and lag days. This study placed the knots of variables at equally spaced quantiles of the predictor, and the knots of lag at equally spaced values on the log scale of lags.
Sensitivity analyses were conducted to evaluate the covariates and their degrees of freedom (df) in the model. We compared the risk estimates and model fittings for various combinations of relative humidity (NS, df set from 4 to 6), wind speed (NS, df set from 4 to 6), long-term time trend (NS, df set from 4 to 12), cross-basis of air pollutants (NS, df set from 4 to 5 for concentrations), and cross-basis of average temperature (NS, df set from 4 to 6 for temperature). The lag structure between temperature and outpatient visits of acute upper respiratory infections was assessed for lags 0–5 days, 0–7 days, 0–10 days, 0–20 days, and 0–30 days. The lag structure between air pollutants and outpatient visits of acute upper respiratory infections was assessed using lags 0–2 days, 0–5 days, and 0–10 days. Lag stratification of temperature and air pollutants were both set at 3 df. This study used Akaike’s information criterion (AIC) for model selection (Akaike, 1973). Lower AIC value indicates a better model.
Considering both model fitting and acute to short-term effects of ambient environment, daily average temperature was set at NS with 6 df (approximately 4 °C for 1 df), and cumulative 8-day (from current day to maximum lag of 7 days) relative risks (RR) and 95% confidence intervals (CI) for outpatient visits associated with specific temperatures at 5th, 50th and 95th percentiles were estimated by comparing with the temperature of lowest outpatient visits for acute upper respiratory infections. Air pollutants, NO2, O3 and PM2.5, were set at NS with 5 df. Six-day cumulative effects (lag 0–5 days) were estimated by comparing concentrations of air pollutants at 75th and 95th percentiles with concentrations at 25th (Q1) percentile (Andersen et al., 2011, Cheng et al., 2009).
Meanwhile, this study assumed linear relationship above the threshold (i.e., zero isolation for respiratory viruses was set as baseline) between daily virus-specific isolation rate and acute upper respiratory infections, and the lag effects were assessed from lag 0 to maximum lag 10 days (set at NS function with 3 df) after considering the latent period and infectious period for respiratory viruses (Lessler et al., 2009). Relative risks and their 95% confidence intervals for acute upper respiratory infections associated with one standard deviation (SD) increase of daily virus-specific isolation rates were reported in this study.
Natural cubic spline with 6 df was applied in daily measurements of relative humidity (RH) and wind speed (WS). Long-term trend (NS, 12 df per year), holiday effects, and effect from day of the week were also adjusted in the models. Moreover, year was treated as an extra categorical variable in models to control for annual variation in health outcomes (e.g., the sudden drop of outpatient visits due to epidemic of severe acute respiratory syndrome in 2003). The model for expected outpatient visits of acute upper respiratory infections (Y) at day (t) was:
where is the expected outpatient visits on day t. A linear relationship was assumed between outpatient visits and daily virus-specific isolation rate (, i=1–5 for five main categories of respiratory virus) with zero thresholds and effects were accumulated for 10-day lag maximum; represents the NS for measurements of air pollutants (j=1–3 for PM2.5, O3, and NO2, set at df=5) on day t. Effects were accumulated for 6 days (lag 0–lag 5 days) under 3 df lag stratification; Six degrees of freedom was set for average temperature on day t (), and the effect was accumulated for 8 days (lag 0–7 days) under 3 df lag stratification. The smoother term of time (“time” in the model) was set to 12 df per year. Cumulative relative risks were estimated from exponential of cumulative risk estimate () over the lag days (K) (Peng and Dominici, 2008):
Data manipulation and all the statistical analyses were performed using SAS version 9.1 (SAS Institute Inc., Cary, NC, USA) and statistical environment R 2.15 (dlnm package).
3. Results
3.1. Characteristics of acute upper respiratory infections, atmospheric environment and circulating viruses
There were 666,653 outpatient visits for acute upper respiratory infections in total during 2003–2007 among the study population. In addition, a total of 12,190 biological specimens (72.3% was sampled from population aged less than 15 years) for virus surveillance were examined in the same period of time. The number of isolated specimens for influenza A, influenza B, parainfluenza viruses, adenoviruses and respiratory syncytial virus were 651, 499, 109, 739 and 43, respectively.
General features of average daily outpatient visits for acute upper respiratory infections, isolation rates for circulating viruses, and atmospheric environment during 2003–2007 in Taipei are described in Table 1. Daily isolation rates of circulating viruses were almost zero. Fig. 2 shows seasonal pattern in which outpatient visits of acute upper respiratory infections and isolation of influenza B were higher in cold months. Influenza A had the highest isolation rate in February, followed by a minor peak from June to August. Sudden drop of outpatient visits of acute upper respiratory infections happened in February because of Chinese New Year. Except the bimodal peaks of O3 (April–May and October–November), concentrations of PM2.5 and NO2 were found the highest in March–April.
Table 1.
Average daily measurements of outpatient visits for acute upper respiratory infections, temperature, air pollutants and circulating viruses from 2003 to 2007 in Taipei Metropolitan.
| Variables | Mean | Standard deviation | Minimum | Percentile |
Maximum | ||||
|---|---|---|---|---|---|---|---|---|---|
| 5th | 25th | 50th | 75th | 95th | |||||
| Atmospheric environment | |||||||||
| PM2.5 (μg/m3) | 30.1 | 15.8 | 1.37 | 9.82 | 18.6 | 26.9 | 38.8 | 60.6 | 109 |
| NO2 (ppb) | 23.4 | 7.78 | 3.54 | 11.8 | 18.4 | 22.5 | 27.4 | 38.6 | 55.2 |
| O3 (ppb) | 25.9 | 9.18 | 4.79 | 11.5 | 19.5 | 25.6 | 31.6 | 41.9 | 64.2 |
| Average Temperature (°C) | 23.5 | 5.34 | 8.30 | 14.1 | 19.3 | 24.0 | 28.0 | 30.8 | 33.0 |
| Relative humidity (%) | 75.6 | 8.83 | 51.0 | 61.0 | 69.0 | 75.0 | 82.0 | 90.0 | 98.0 |
| Wind speed (m/s) | 2.58 | 1.24 | 0 | 1.00 | 1.60 | 2.30 | 3.50 | 4.70 | 7.90 |
| Circulating viruses⁎ | |||||||||
| Specimen number | 6.89 | 5.05 | 1 | 1 | 3 | 6 | 9 | 16 | 54 |
| Influenza A, % | 5.56 | 13.2 | 0 | 0 | 0 | 0 | 0 | 28.6 | 100 |
| Influenza B, % | 2.87 | 8.76 | 0 | 0 | 0 | 0 | 0 | 22.2 | 100 |
| Parainfluenza viruses, % | 6.01 | 12.7 | 0 | 0 | 0 | 0 | 0.09 | 28.6 | 100 |
| Adenoviruses, % | 0.96 | 5.25 | 0 | 0 | 0 | 0 | 0 | 7.14 | 100 |
| Respiratory syncytial virus, % | 0.41 | 3.70 | 0 | 0 | 0 | 0 | 0 | 0 | 100 |
| Outpatient visits of AURIs† | 365 | 192 | 6 | 117 | 223 | 337 | 473 | 729 | 1,243 |
Number of virus-specific isolated specimens divided by the total specimens from the regional reference virology laboratories.
Study population was 39,766 in Taipei, 2005.
Fig. 2.
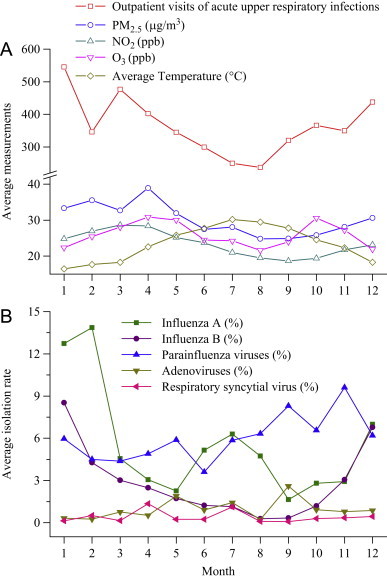
Patterns for outpatient visits of acute upper respiratory infections, ambient environment and virus-specific isolation rate by month in Taipei metropolitan, 2003–2007.
3.2. Cumulative relative risks
Fig. 3 shows the 8-day cumulative RR associated with average temperature, 6-day cumulative RRs associated with air pollutants (PM2.5, NO2 and O3), and 11-day cumulative RRs associated with virus-specific isolation rates for outpatient visits of acute upper respiratory infections using DLNM controlling for the daily RH, WS, holiday effects, day of a week, and smoothing long-term trend.
Fig. 3.
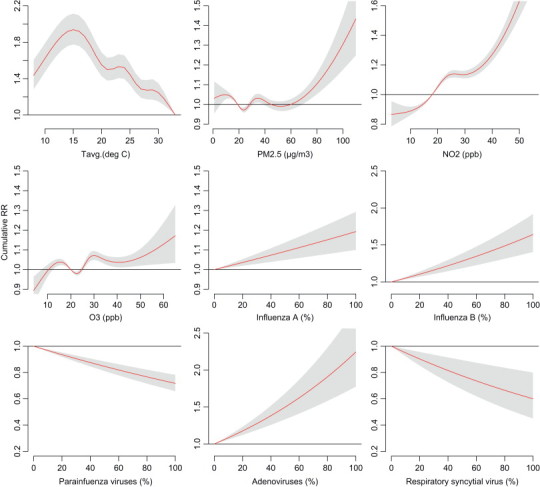
Cumulative 8-day (lag 0–7 days) relative risks (RR) for average temperature, cumulative 6-day (lag 0–5 days) RR for air pollutants (NO2, O3 and PM2.5), and cumulative 11-day (lag 0–10 days) RR for virus-specific positive isolation rate associated with outpatient visits of acute upper respiratory infections using DLNM model controlled effects of relative humidity, wind speed, long-term trend, day of the week, and holiday effects.
Average temperature of 33 °C associated with the lowest risk for outpatient visits of acute upper respiratory infections. Compared with 33 °C as reference, cumulative 8-day RR was the highest as ambient average temperature at 15 °C with an RR equals to 1.94 (95% CI: 1.78, 2.11). Concentration of NO2 was most associated with outpatient visits of acute upper respiratory infections among these three air pollutants. Compared with measurements of 25th percentile, cumulative 6-day RRs were 1.25 (95% CI: 1.21, 1.29) for NO2, 1.04 (95% CI: 1.01, 1.06) for O3, and 1.00 (95% CI: 0.98, 1.03) for PM2.5 at 95th percentile of measurements. Cumulative RR was significant for PM2.5 only when its concentration exceeded 70< μg/m3 in Taipei metropolitan.
The cumulative risk of outpatient visits of acute upper respiratory infections were statistically elevated by daily isolations of influenza A, influenza B and adenoviruses, cumulative 11-day RRs were 1.02 (95% CI: 1.01, 1.03), 1.05 (95% CI: 1.03, 1.06) and 1.04 (95% CI: 1.03, 1.05) per SD increase for isolation rates, respectively.
3.3. Lag effects of risk factors
Fig. 4 shows the lag effects of average temperature on outpatient visits of acute upper respiratory infections based on reference temperature of 33 °C. Relative risks were higher in lower temperatures and earlier lag days. Risk was the highest at 15 °C at lag 0 day (RR=1.22; 95% CI: 1.19, 1.26).
Fig. 4.
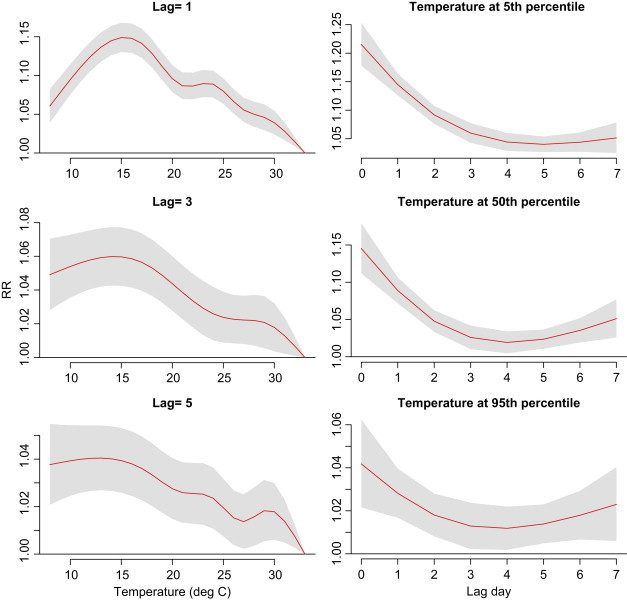
Lag associations between average temperature (baseline: 33 °C) and outpatient visits of acute upper respiratory infections. The DLNM model controlled effects of relative humidity, wind speed, long-term trend, day of the week, and holiday effects.
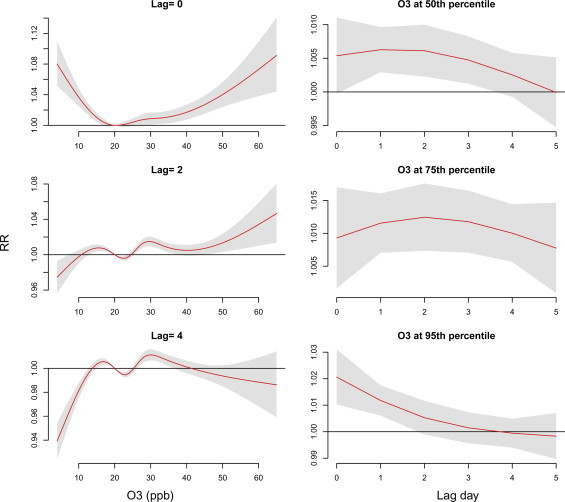
Lag effects varied with air pollutants—PM2.5, NO2 and O3 at measurements of 75th and 95th percentiles ( Fig. 5). Supplementary Figs. 1–3 also present the lag effects varied with the concentrations of these air pollutants. Risks of NO2 and O3 occurred earlier than PM2.5, RRs were 1.12 (95% CI: 1.10, 1.13) and 1.02 (95% CI: 1.01, 1.03), respectively, at lag 0 day as population exposed to measurements of 95th percentile. Outpatient visits of acute upper respiratory infections were negative associated with PM2.5 before lag 2 days. PM2.5 shows the highest relative risk at lag 4–5 days.
Fig. 5.
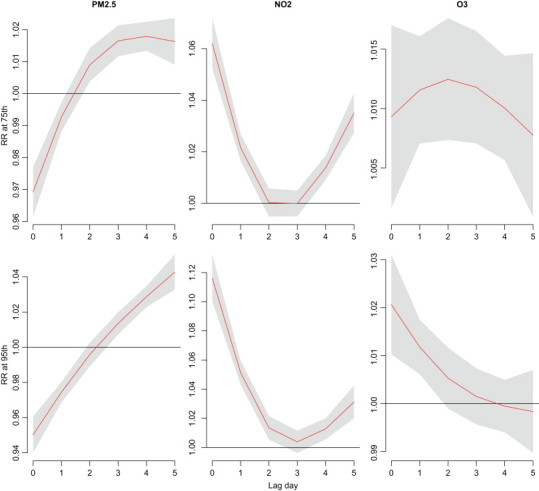
Lag associations between air pollutants at measurements of 75th and 95th percentiles (baseline: 25th percentile) and outpatient visits of acute upper respiratory infections using DLNM models controlling for relative humidity, wind speed, long-term trend, day of the week, and holiday effects.
Risks of acute upper respiratory infections were relevant to isolation rates of respiratory viruses, and the risks across 11-day lags varied with the type of respiratory viruses ( Fig. 6). Isolation of influenza B, parainfluenza viruses and adenoviruses associated with outpatient visits of acute upper respiratory infections in a V-shaped association within 11 lag days’ analysis. RRs were 1.01 (95% CI: 1.01, 1.02) for influenza B, 1.01 (95% CI: 1.00, 1.01) for parainfluenza viruses, and 1.01 (95% CI: 1.01, 1.01) for adenoviruses, as 1 SD increase for isolation rates at lag 0 day. Risks of isolation of influenza A were significant from lag 2–8 days and its RR was the highest at lag 4 days (RR=1.01, 95% CI: 1.01, 1.01, as 1SD increase for isolation rate).
Fig. 6.
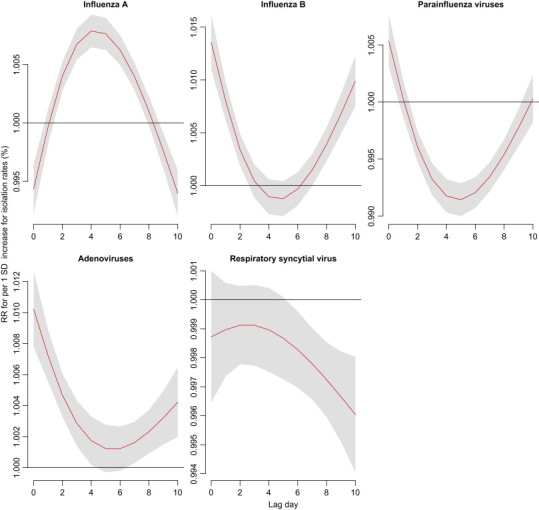
Lag associations between virus-specific isolation rate per SD increase and outpatient visits of acute upper respiratory infections using DLNM models controlling for relative humidity, wind speed, long-term trend, day of the week, and holiday effects.
4. Discussion
In present analyses, distributed lag non-linear models were performed to estimate the cumulative relative risks of average temperature, air pollutants, and circulating respiratory viruses on acute upper respiratory infections of a massive cohort aged less than 15 years old. Lower temperature, higher concentrations of NO2 and O3, and higher isolation rates of influenza A, influenza B and adenoviruses were particular related to elevated risks of acute upper respiratory infections outpatient visits in Taipei metropolitan.
Previous studies reported higher positive identification rate of circulating respiratory viruses in winter (Denny, 1995, Diaz et al., 2007). However, only influenza A and influenza B are shown to have seasonal variation in the present study (Fig. 1). The seasonal pattern is not statistically significant for parainfluenza viruses, adenoviruses and respiratory syncytial virus, which are commonly related with acute upper respiratory infections (Dasaraju and Liu, 1996). Variations of acute upper respiratory infections cannot be explained solely by circulating viruses revealed that the contributions from the risk factors other than circulating viruses should be further considered.
Present study revealed that daily outpatient visits for acute upper respiratory infections was significantly reversely related to average temperature, which was similar to what Falagas et al. (2008) reported. In literature, lower temperature and humidity were found correlated with respiratory tract infections (Liao et al., 2009, Makinen et al., 2009). Cold air could enhance the susceptibility of sensitive population by increasing vasoconstriction in upper airways and depressing the clearance mechanism of infections (Chu et al., 2006, Shephard and Shek, 1998). Additionally, transmission of suspected infectious bioaerosol (e.g., influenza viruses) has been reported negatively related with ambient temperature (Lowen et al., 2007). The present study detected significant correlations between average temperature and daily isolations for influenza A (correlations coefficient=−0.15, p<0.01), influenza B (correlations coefficient=−0.22, p<0.01), and adenoviruses (correlations coefficient=0.05, p<0.05). Future studies to address the effects of ambient environment and viral activity to upper respiratory infection are recommended. Besides, this study identified a negative association between average temperature and outpatient visits for temperatures ranged from 33 to 15 °C and a positive association between temperature lower than 15 °C (approximate 5th percentile of average temperature) and outpatient visits. A possible explanation is that people who have mild illness may prefer to stay in indoor environment and take actions in response to remit the symptoms themselves, rather than to seek medical help outside in cold temperature.
Experimental studies demonstrated that exposure to air pollutants synergistically caused viral or bacterial infections in airway epithelial cells (Chauhan and Johnston, 2003, Ciencewicki and Jaspers, 2007, Ciencewicki et al., 2008). Exposure to ozone may enhance sensitivity of inflammation (Ciencewicki et al., 2008) and thus increased the possibility of infections (Ciencewicki and Jaspers, 2007, Spannhake et al., 2002). But, ozone can also be protective because of its virucidal effect that relies on the exposure timing (before or after the respiratory infections) and duration (Ciencewicki and Jaspers, 2007). No matter the NO2 exposure is preceding or succeeding to respiratory viral infections, NO2 may decrease virus-specific immunity in mice and increase the inflammation of cells (Ciencewicki and Jaspers, 2007). The exposure of PM was suggested to decrease phagocytic ability and evoke infection in animal models, but the phenomenon was found specifically for respiratory syncytial virus only (Becker and Soukup, 1999, Kaan and Hegele, 2003, Lambert et al., 2003a, Lambert et al., 2003b).
Some epidemiological studies have identified the positive associations between PM10, NO2, O3 and CO and respiratory infections (Burnett et al., 2001, Hwang and Chan, 2002, Lin et al., 2005, Lin et al., 2008, Peel et al., 2005, Wong et al., 2006). Jaakkola et al. (1991) reported a 2-fold relative risk of acute upper respiratory infections in NO2-polluted city compared to less polluted cities for children aged less than 7 years old in Finland . NO2 and PM10 were also found strongly correlated with respiratory infections for children in Toronto (Lin et al., 2005). In Hong Kong, general practitioner visits for acute upper respiratory infections were reported associated with ambient NO2, O3, PM10 and PM2.5. Similar to our finding, Wong et al. (2006) also identified NO2 with the greatest effects following by O3 on acute upper respiratory infections . However, we identified the positive association between PM2.5 and acute upper respiratory infections when its concentration exceeded 70 μg/m3 in Taipei metropolitan. Numerous studies reported a positive association between PM and mortality/morbidity of cardiopulmonary diseases (Wilson et al., 2004). Relatively few studies evaluated both the effects of ambient environment and circulating respiratory viruses on acute respiratory infections. Wong et al. (2009) assessed the modification of influenza on acute respiratory infections and suggested that ambient influenza activity should be involved in the consideration of short-term effects of air pollutants. Therefore, more studies focusing on related issues are warranted in the future.
This study evaluated the risk pattern of PM10, PM2.5, daily hourly maximum O3, maximum 8-h moving average for O3, and daily 24-h average O3 relating to outpatient visits of acute upper respiratory infections. Based on model selection strategy of lower AIC value and relative risks estimated, the optimal model on prediction of acute upper respiratory infections was the one using 24-h average of PM2.5 and O3. Similar findings were reported in two previous papers (Schwartz and Neas, 2000, Wong et al., 2006), concluding that the magnitude of RR was higher for PM2.5 than PM10, in contrast to the study in Toronto (Lin et al., 2005).
Latency effect of risk factors is of great importance in health risk assessment. Lin et al. (2008) indicated that ozone level was correlated with 2-day lag of hospital admission for respiratory diseases in childhood. Fusco et al. (2001) found acute respiratory infections significantly associated with the levels of NO2 at lag 0 day , which was consistent to our observations. We have tried to evaluate the lag effects for virus-specific isolation rate on outpatient visits of acute upper respiratory infections. This study found influenza B and adenoviruses associated with acute upper respiratory infections at lag 0–3 days and lag 7–10 days, and lag effects were significant from lag 2–8 days for influenza A. In this study, we observed clear lag effects varying with covariates and their levels throughout evaluating periods (Fig. 4, Fig. 5, Fig. 6). Therefore, using DLNM to calculate the cumulative relative risks of outpatient visits for acute upper respiratory infections to risk factors on a given day and lag days would be a more sensible method.
Significance of covariates in original analyses remained in sensitivity analyses, no matter how the df was changed. However, relative risk estimations for temperature and air pollutants were the highest when long-term trend (time) was set at 5–7 per year (other settings are the same with this study). RR for average temperature at measurement of 5th percentile was 2.25 (95% CI: 2.09, 2.40) when time was set at 6 per year and 1.27 (95% CI: 1.23, 1.31) for NO2 at measurement of 95th percentile when time was set at 7 per year. Relative risks for daily isolations of respiratory viruses were the highest when time was set at 5 per year for influenza B and 9 per year for adenoviruses (other settings are the same with this study). RRs for per SD increase of isolation rates were 1.11 (95% CI: 1.10, 1.12) when time was set at 5 per year for influenza B, and 1.06 (95% CI: 1.05, 1.07) when time was set at 9 per year for adenoviruses.
The major advantage of current study was the massive population-representative sample, in contrast to other countries without overall coverage of health insurance. However, limited information of other kinds of circulating virus is a flaw in this study. Based on emerging infectious diseases policy of Taiwan CDC, influenza A, influenza B, parainfluenza viruses, adenoviruses and respiratory syncytial virus are the major targets for investigations. Therefore, the effect of other respiratory viruses, including rhinoviruses, coronaviruses, human metapneumovirus, bocavirus, polyomavirus, and bacterial infections which have been reported in recent studies (Brodzinski and Ruddy, 2009, Dasaraju and Liu, 1996, Denny, 1995, Mackie, 2003, Treanor and Lee, 2010, Wang et al., 2006) was out of the scope of present study. In addition, the influence from government-funded seasonal influenza vaccination campaign starting in 1999 in Taiwan was not evaluated in the present study, because Taiwan CDC did not establish a complete database for advanced evaluation. Moreover, current study utilized data of a cohort with one million subjects which was started in 2000 that implied the lack of information for children aged younger than 2 years during the study periods (2003–2007). Therefore, we were unable to generate the study outcomes to younger kids.
With the evidence given by present study, we suggest that average temperature and concentrations of ambient NO2 in urban areas could cause fairly strong adverse effects on acute upper respiratory infections, rather than what incipiently known that they primarily come from circulating respiratory viruses. Further studies are in need to clarify the mechanisms about temperature, air pollutants, and virulence of respiratory viruses for various susceptible populations.
In conclusion, this study used distributed lag non-linear models to assess the cumulative relative risks of atmospheric environment and circulating respiratory viruses associated with outpatient visits for acute upper respiratory infections. In addition to elevated risks associated with lower average temperature, daily concentrations of NO2 also contributed to higher risks and followed by the isolation rate of influenza type B and adenoviruses.
Funding
This study was supported by National Science Council (NSC 96-EPA-Z-002–005-, NSC 96–3111-B-033–001 and NSC 99–2221-E-033–052) of Taiwan.
Acknowledgements
We appreciate the authorities of Taiwan National Health Research Institute, Taiwan Environmental Protection Administration, Taiwan centers for Disease control and Taiwan Central Weather Bureau for providing research data. Interpretations and conclusions herein do not represent those of these agencies.
Footnotes
Supplementary data associated with this article can be found in the online version at 10.1016/j.envres.2012.09.002.
Appendix A. Supplementary materials
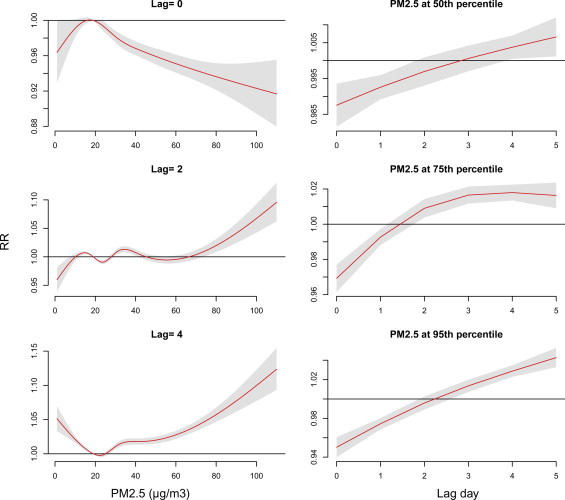
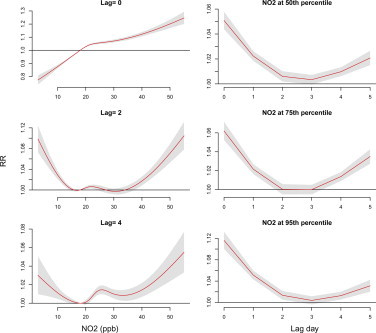
Supplementary material
References
- Akaike H. Information theory and an entension of the maximum likelihood principle. 2nd Int. Symp. on Inf. Theory. 1973:267–281. [Google Scholar]
- Andersen Z.J., Bonnelykke K., Hvidberg M., Jensen S.S., Ketzel M., Loft S. Long-term exposure to air pollution and asthma hospitalisations in older adults: a cohort study. Thorax. 2011;67:6–11. doi: 10.1136/thoraxjnl-2011-200711. [DOI] [PubMed] [Google Scholar]
- Becker S., Soukup J.M. Exposure to urban air particulates alters the macrophage-mediated inflammatory response to respiratory viral infection. J. Toxicol. Environ. Health A. 1999;57:445–457. doi: 10.1080/009841099157539. [DOI] [PubMed] [Google Scholar]
- Brodzinski H., Ruddy R.M. Review of new and newly discovered respiratory tract viruses in children. Pediatr. Emerg. Care. 2009;25:352–360. doi: 10.1097/PEC.0b013e3181a3497e. quiz 361-353. [DOI] [PubMed] [Google Scholar]
- Bureau of National Health Insurance . Bureau of National Health Insurance, Department of Health, Executive Yuan, Republic of China; Taipei: 2001. National Health Insurance Profile [in Chinese] [Google Scholar]
- Burnett R.T., Smith-Doiron M., Stieb D., Raizenne M.E., Brook J.R., Dales R.E. Association between ozone and hospitalization for acute respiratory diseases in children less than 2 years of age. Am. J. Epidemiol. 2001;153:444–452. doi: 10.1093/aje/153.5.444. [DOI] [PubMed] [Google Scholar]
- Central Weather Bureau, 2011. The Central Weather Bureau online information: 〈http://www.cwb.gov.tw/eng/index.htm〉. Accessed date: 2011/05/12.
- Chauhan A.J., Johnston S.L. Air pollution and infection in respiratory illness. Br. Med. Bull. 2003;68:95–112. doi: 10.1093/bmb/ldg022. [DOI] [PubMed] [Google Scholar]
- Cheng M.F., Tsai S.S., Chiu H.F., Sung F.C., Wu T.N., Yang C.Y. Air pollution and hospital admissions for pneumonia: are there potentially sensitive groups? Inhal. Toxicol. 2009;21:1092–1098. doi: 10.3109/08958370902744855. [DOI] [PubMed] [Google Scholar]
- Chi H., Chiu N.C., Li W.C., Huang F.Y. Etiology of acute pharyngitis in children: is antibiotic therapy needed? J. Microbiol. Immunol. Infect. 2003;36:26–30. [PubMed] [Google Scholar]
- Chu Y.H., Wu C.C., Wang H.W. Effect of cooling on electrical field stimulation and norepinephrine-induced contraction in isolated hypertrophic human nasal mucosa. Am. J. Rhinol. 2006;20:471–475. doi: 10.2500/ajr.2006.20.2942. [DOI] [PubMed] [Google Scholar]
- Ciencewicki J., Jaspers I. Air pollution and respiratory viral infection. Inhal. Toxicol. 2007;19:1135–1146. doi: 10.1080/08958370701665434. [DOI] [PubMed] [Google Scholar]
- Ciencewicki, J., Trivedi, S., Kleeberger, S.R., 2008. Oxidants and the pathogenesis of lung diseases. J. Allergy. Clin. Immunol. 122, 456–468; quiz 469-470 . [DOI] [PMC free article] [PubMed]
- Dasaraju P.V., Liu C. Infections of the respiratory systems. In: Baron S., editor. Medical Microbiology. University of Texas Medical Branch at Galveston; Galveston: 1996. pp. 1103–1115. [PubMed] [Google Scholar]
- Davis D.J. Measurements of the prevalence of viral infections. J. Infect. Dis. 1976;133(Suppl):A3–5. doi: 10.1093/infdis/133.supplement_2.a3. [DOI] [PubMed] [Google Scholar]
- Denny F.W., Jr. The clinical impact of human respiratory virus infections. Am. J. Respir. Crit. Care Med. 1995;152:S4–12. doi: 10.1164/ajrccm/152.4_Pt_2.S4. [DOI] [PubMed] [Google Scholar]
- Diaz A., Barria P., Niederman M., Restrepo M.I., Dreyse J., Fuentes G. Etiology of community-acquired pneumonia in hospitalized patients in chile: the increasing prevalence of respiratory viruses among classic pathogens. Chest. 2007;131:779–787. doi: 10.1378/chest.06-1800. [DOI] [PubMed] [Google Scholar]
- Falagas M.E., Theocharis G., Spanos A., Vlara L.A., Issaris E.A., Panos G. Effect of meteorological variables on the incidence of respiratory tract infections. Respir. Med. 2008;102:733–737. doi: 10.1016/j.rmed.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Fusco D., Forastiere F., Michelozzi P., Spadea T., Ostro B., Arca M. Air pollution and hospital admissions for respiratory conditions in Rome, Italy. Eur. Respir. J. 2001;17:1143–1150. doi: 10.1183/09031936.01.00005501. [DOI] [PubMed] [Google Scholar]
- Gasparrini A., Armstrong B., Kenward M.G. Distributed lag non-linear models. Stat. Med. 2010;29:2224–2234. doi: 10.1002/sim.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales R., Malone D.C., Maselli J.H., Sande M.A. Excessive antibiotic use for acute respiratory infections in the United States. Clin. Infect. Dis. 2001;33:757–762. doi: 10.1086/322627. [DOI] [PubMed] [Google Scholar]
- Hwang J.S., Chan C.C. Effects of air pollution on daily clinic visits for lower respiratory tract illness. Am. J. Epidemiol. 2002;155:1–10. doi: 10.1093/aje/155.1.1. [DOI] [PubMed] [Google Scholar]
- Jaakkola J.J., Paunio M., Virtanen M., Heinonen O.P. Low-level air pollution and upper respiratory infections in children. Am. J. Public Health. 1991;81:1060–1063. doi: 10.2105/ajph.81.8.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaan P.M., Hegele R.G. Interaction between respiratory syncytial virus and particulate matter in guinea pig alveolar macrophages. Am. J. Respir. Cell Mol. Biol. 2003;28:697–704. doi: 10.1165/rcmb.2002-0115OC. [DOI] [PubMed] [Google Scholar]
- Lambert A.L., Mangum J.B., DeLorme M.P., Everitt J.I. Ultrafine carbon black particles enhance respiratory syncytial virus-induced airway reactivity, pulmonary inflammation, and chemokine expression. Toxicol. Sci. 2003;72:339–346. doi: 10.1093/toxsci/kfg032. [DOI] [PubMed] [Google Scholar]
- Lambert A.L., Trasti F.S., Mangum J.B., Everitt J.I. Effect of preexposure to ultrafine carbon black on respiratory syncytial virus infection in mice. Toxicol. Sci. 2003;72:331–338. doi: 10.1093/toxsci/kfg031. [DOI] [PubMed] [Google Scholar]
- Lessler J., Reich N.G., Brookmeyer R., Perl T.M., Nelson K.E., Cummings D.A. Incubation periods of acute respiratory viral infections: a systematic review. Lancet Infect. Dis. 2009;9:291–300. doi: 10.1016/S1473-3099(09)70069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C.M., Chang S.Y., Chen S.C., Chio C.P. Influenza-associated morbidity in subtropical Taiwan. Int. J. Infect. Dis. 2009;13:589–599. doi: 10.1016/j.ijid.2008.09.019. [DOI] [PubMed] [Google Scholar]
- Lin M., Stieb D.M., Chen Y. Coarse particulate matter and hospitalization for respiratory infections in children younger than 15 years in Toronto: a case-crossover analysis. Pediatrics. 2005;116:e235–240. doi: 10.1542/peds.2004-2012. [DOI] [PubMed] [Google Scholar]
- Lin S., Bell E.M., Liu W., Walker R.J., Kim N.K., Hwang S.A. Ambient ozone concentration and hospital admissions due to childhood respiratory diseases in New York State, 1991–2001. Environ. Res. 2008;108:42–47. doi: 10.1016/j.envres.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Lowen A.C., Mubareka S., Steel J., Palese P. Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathog. 2007;3:1470–1476. doi: 10.1371/journal.ppat.0030151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie P.L. The classification of viruses infecting the respiratory tract. Paediatr. Respir. Rev. 2003;4:84–90. doi: 10.1016/S1526-0542(03)00031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinen T.M., Juvonen R., Jokelainen J., Harju T.H., Peitso A., Bloigu A. Cold temperature and low humidity are associated with increased occurrence of respiratory tract infections. Respir. Med. 2009;103:456–462. doi: 10.1016/j.rmed.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Peel J.L., Tolbert P.E., Klein M., Metzger K.B., Flanders W.D., Todd K. Ambient air pollution and respiratory emergency department visits. Epidemiology. 2005;16:164–174. doi: 10.1097/01.ede.0000152905.42113.db. [DOI] [PubMed] [Google Scholar]
- Peng R.D., Dominici F. Springer; 2008. Statistical Methods for Environmental Epidemiology with R-A Case Study in Air Pollution and Health. [Google Scholar]
- Schwartz J., Neas L.M. Fine particles are more strongly associated than coarse particles with acute respiratory health effects in schoolchildren. Epidemiology. 2000;11:6–10. doi: 10.1097/00001648-200001000-00004. [DOI] [PubMed] [Google Scholar]
- Schwartz J., Spix C., Wichmann H.E., Malin E. Air pollution and acute respiratory illness in five German communities. Environ. Res. 1991;56:1–14. doi: 10.1016/s0013-9351(05)80104-5. [DOI] [PubMed] [Google Scholar]
- Shephard R.J., Shek P.N. Cold exposure and immune function. Can. J. Physiol. Pharmacol. 1998;76:828–836. doi: 10.1139/cjpp-76-9-828. [DOI] [PubMed] [Google Scholar]
- Shih S.R., Chen G.W., Yang C.C., Yang W.Z., Liu D.P., Lin J.H. Laboratory-based surveillance and molecular epidemiology of influenza virus in Taiwan. J. Clin. Microbiol. 2005;43:1651–1661. doi: 10.1128/JCM.43.4.1651-1661.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoes E.A.F., Cherian T., Chow J., Shahid-Salles S.A., Laxminarayan R., John T.J. Oxford University Press; New York: 2006. Chapter 25: Acute Respiratory Infections in Children Disease Control Priorities in Developing Countries. pp. 483–498. [Google Scholar]
- Spannhake E.W., Reddy S.P., Jacoby D.B., Yu X.Y., Saatian B., Tian J. Synergism between rhinovirus infection and oxidant pollutant exposure enhances airway epithelial cell cytokine production. Environ. Health Perspect. 2002;110:665–670. doi: 10.1289/ehp.02110665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taiwan Centers for Disease Control. CDC Annual Report 2007. In: Centers for Disease Control, Department of Health, Executive Yuan. Taiwan Centers for Disease Control, Taipei, 2007. Available at: 〈http://www.cdc.gov.tw/uploads/files/5b2072e6-e3be-42b3-a019-0325cb4e807a.pdf〉. Accessed date: 2012/05/10.
- Taiwan Centers for Disease Control. Taiwan’s virus surveillance network. Available at: 〈http://www.cdc.gov.tw/english/info.aspx?treeid=00ed75d6c887bb27&nowtreeid=f0131176aa46d5db&tid=F664EBB290D1FB7F〉. Accessed date: 2012/05/10.
- Taiwan Environmental Protection Administration, 2011. Taiwan Air Quality Monitoring Network. Available at: 〈http://taqm.epa.gov.tw/taqm/en/PsiAreaHourly.aspx〉. Accessed date: 2011/05/12.
- Taiwan National Health Insurance Research Database. Available at: 〈http://w3.nhri.org.tw/nhird/en/Data_Subsets.html#S3〉. Accessed date: 2011/05/12.
- Treanor J., Lee F.E.-H. Viral infections. In: Mason R.J., editor. Murray & Nadel’s Textbook of Respiratory Medicine. PA: WB Saunders; Philadelphia: 2010. Chapter 31. [Google Scholar]
- Wang S.M., Liu C.C., Wang H.C., Su I.J., Wang J.R. Human metapneumovirus infection among children in Taiwan: a comparison of clinical manifestations with other virus-associated respiratory tract infections. Clin. Microbiol. Infect. 2006;12:1221–1224. doi: 10.1111/j.1469-0691.2006.01540.x. [DOI] [PubMed] [Google Scholar]
- West J.V. Acute upper airway infections. Br. Med. Bull. 2002;61:215–230. doi: 10.1093/bmb/61.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 1995. Management of Acute Respiratory Infections in Children: Practical Guidelines for Outpatient Care.
- Wilson A.M., Salloway J.C., Wake C.P., Kelly T. Air pollution and the demand for hospital services: a review. Environ. Int. 2004;30:1109–1118. doi: 10.1016/j.envint.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Wong C.M., Yang L., Thach T.Q., Chau P.Y., Chan K.P., Thomas G.N. Modification by influenza on health effects of air pollution in Hong Kong. Environ. Health Perspect. 2009;117:248–253. doi: 10.1289/ehp.11605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T.W., Tam W., Tak Sun Yu.I., Wun Y.T., Wong A.H., Wong C.M. Association between air pollution and general practitioner visits for respiratory diseases in Hong Kong. Thorax. 2006;61:585–591. doi: 10.1136/thx.2005.051730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanobetti A., Wand M.P., Schwartz J., Ryan L.M. Generalized additive distributed lag models: quantifying mortality displacement. Biostatistics. 2000;1:279–292. doi: 10.1093/biostatistics/1.3.279. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material


