Graphical abstract
Lonicera japonica has been used for thousands of years in China. Now, more than 140 chemical compounds have been isolated; and studies show Lonicera japonica possesses wide pharmacological actions. Meanwhile, it could be used as healthy food, ornamental groundcover, etc.
Abbreviations: ACV, acyclovir; AIV, avian influenza virus; ALT, alanine transarninase; AST, aspartate amino transferase; CAT, catalase; Cd, cadmium; CGN, carrageenan; COX, cycloxygenase; ConA, concanavalin A; CPE, cytopathologic effect; DAD, diode-array detection; DPPH, 1,1-diphenyl-2-picrylhydrazyl; ELSD, evaporative light scattering detectors; EtOAc, ethyl acetate; ERK, extracellular signal-regulated kinase; GC–MS, gas chromatography–mass spectrometry; GSH, glutathione; HDL-C, high density lipoprotein cholesterin; HIV-1, human immunodeficiency virus-1; HPLC, high performance liquid chromatography; HSV, Herpes simplex virus; HUVEC, Human Umbilical Vein Endothelial Cells; IC50, 50% inhibition concentration; JNK, Jun nuclear kinase; LED, Least Effective Dose; Lonicera japonica, Lonicera japonica Thunb.; LPS, lipopolysaccharide; MAPK, mitogen activated protein kinase; MDA, malondialdehyde; MEC, minimum effective concentration; MeOH, methyl alcohol; MIC, minimum inhibitory concentration; MPO, myeloperoxidase; MTT, methyl thiazolyl tetrazolium; MUFA, monounsaturated fatty acid; NDV, newcastle disease virus; NO, nitric oxide; PAPR2, proteinase-activated receptor 2; PDT, photodynamic therapy; PMNs, polymorphonuclear leukocytes; PUFA, polyunsaturated fatty acid; ROS, reactive oxygen species; RSV, respiratory syncytial virus; SARS coronavirus, severe acute respiratory syndromes coronavirus; SFA, saturated fatty acid; SI, selectivity index; SOD, superoxide dismutase; TCM, traditional Chinese medicine; TEAC, Trolox equivalent antioxidant capacity; TI, therapeutic index; TLC, thin layer chromatography; TNF-α, tumor necrosis factor-α; TOF-MS, time-of-flight mass spectrometry
Keywords: Lonicera japonica, Ethnopharmacology, Chlorogenic acid, Anti-inflammatory, Antiviral activity
Abstract
Ethnopharmacological relevance
Lonicera japonica Thunb. (Caprifoliaceae), a widely used traditional Chinese medicine, was known as Jin Yin Hua (Chinese:  ), Ren Dong and Japanese honeysuckle. It was taken to treat the exopathogenic wind-heat, epidemic febrile diseases, sores, carbuncles and some infectious diseases. At the same time, Lonicera japonica could be used as healthy food, cosmetics, ornamental groundcover, and so on.
), Ren Dong and Japanese honeysuckle. It was taken to treat the exopathogenic wind-heat, epidemic febrile diseases, sores, carbuncles and some infectious diseases. At the same time, Lonicera japonica could be used as healthy food, cosmetics, ornamental groundcover, and so on.
Aim of the review
The present paper reviewed the ethnopharmacology, the biological activities, toxicology and phytochemistry of Lonicera japonica.
Materials and methods
Information on Lonicera japonica was gathered via the Internet (using Google Scholar, Baidu Scholar, Elsevier, ACS, Medline Plus, CNKI and Web of Science) and libraries. Additionally, information also was obtained from some local books and brilliant scholars on ethnopharmacology.
Results
More than 140 chemical compounds have been isolated, and the main compositions are essential oils, organic acids and flavones, etc. Lonicera japonica and its active principles possess wide pharmacological actions, such as anti-inflammatory, antibacterial, antiviral, antioxidative and hepatoprotective activities.
Conclusions
As an important traditional Chinese medicine, further studies on Lonicera japonica can lead to the development of new drugs and therapeutics for various diseases, and how to utilize it better should be paid more attentions.
1. Introduction
Lonicera japonica Thunb. (Caprifoliaceae), also known as Japanese honeysuckle, Jin Yin Hua or Ren Dong, is native in the East Asian (He et al., 2010). Now as an ornamental groundcover, Lonicera japonica commonly planted in many areas for sprawling habit, numerous sweetly fragrant white flowers, attractive evergreen foliage, and become naturalized in Argentina, Brazil, Mexico, Australia, New Zealand and United States. Due to Lonicera japonica has escaped from cultivation in several places, becoming a major nuisance, and is restricted in parts of North America and New Zealand (Starr et al., 2003). But in China, 1500 years ago, Lonicera japonica has been planted largely in Fengqiu county of Henan province, and the flowers of Lonicera japonica have been used as the local and traditional medicine in clinical practice for the treatment of exopathogenic wind-heat, epidemic febrile diseases, sores, carbuncles, furuncles and some infection diseases. Scine 1995, Lonicera japonica has been listed in the Pharmacopoeia of the People's Republic of China and more than 500 prescriptions containing Lonicera japonica have been used to treat various diseases in China (http://www.zysj.com.cn). The modern pharmacological studies showed that Lonicera japonica and its active principles possessed wide pharmacological actions, such as antibacterial, anti-inflammatory, antiviral, antiendotoxin, blood fat reducing, antipyretic and other activities (Wang, 2008c). Most of these actions matched to those traditional uses seriously. At the same time, it was also used as food, healthy beverage in the world (Wang, 2010). Along with Lonicera japonica being used and cultivated in more and more countries, the chemical compounds have been extensively studied. Essential oils, organic acids, flavones, saponins, iridoids and inorganic elements as the main compositions were isolated and identified. Among of them, essential oils and chlorogenic acid have been proved with some good pharmacological effects, and were though as the active compounds of Lonicera japonica. In current Chinese Pharmacopoeia (Committee for the Pharmacopoeia of PR China, 2010), chlorogenic acid (1) has been officially used as the indicator compound to characterize the quality of this herb.
In this review, the advances in ethnopharmacology, phytochemistry, biological and pharmacological activities, and toxicology of Lonicera japonica are displayed, and the increasing data supports the utilization and the exploitation for new drug.
2. Botany and ethnopharmacology
2.1. Botany
According to the description of Wagner et al. (1999), Lonicera japonica is sprawling and twining lianas; young stems pubescent; leaves ovate, elliptic, oblong or broadly lanceolate, blades 3–8 cm long, 1–3.5 cm wide, pubescent, becoming glabrate above, entire or young lower leaves sometimes lobed; flowers 2 in axillary cymes, bracts 1–2 cm long, bracteoles suborbicular, ca. 1 mm long; corolla white, turning yellowish or tinged pink, 2-lipped, 2–3 cm long; berries bluish black, globose, 6–7 mm in diameter. The flowering duration of individual plant is usually 5–8 days, but the flowering period is from May to September in the field, can be divided into six stages, i.e. the juvenile bud stage, the third green stage, the second white stage, the complete white stage, the silver flowering stage and the gold flowering stage. Lonicera japonica often grows in hillside scrub, rocks pile and roadside, and the highest altitude is 1500 m. Due to its beautiful flowers and strong roots, Lonicera japonica was cultivated for people to watch, conserve water and soil in world. In traditional Chinese medicine, due to the outline form of sprawling and twining lianas, and the different flower colors, the dried flowers or flower buds of Lonicera japonica was named as Jin Yin Hua and Ren Dong in TCM. Both the chemical contents and compositions of Lonicera japonica flowers vary in a flowering-dependent characteristic with the collection time (Fig. 1 ) (Wang et al., 2009).
Fig. 1.
The flower and habitat of Lonicera japonica.
2.2. Ethnopharmacology
With a wide spectrum of biological and pharmacological properties, Lonicera japonica played a very important role in TCM. 3000 years ago, our ancestors have adopted it to cure some illnesses. Due to the effects of curing fever and swelling of body, ‘Ming Yi Bie Lu’ and ‘Shen Nong Ben Cao Jin’ has listed it as ‘top grade’ (Tao, 1986, Gu et al., 2007). Then, ‘Ben Cao Gang Mu’, the famous classical book of Chinese materia medica, has recorded that it could be applied to clear away the heat-evil, treat the swellings and dysentery, protect body and prolong life (Li, 1979, Wang, 2010). In addition, more than ten classical medicine books in China also have recorded this plant, and it has been used as the main composition in some famous prescription to treat various diseases (Table 1 ).
Table 1.
The traditional and clinical uses of Lonicera japonica in China.
| Preparation name | Main compositions | Traditional and clinical uses | References |
|---|---|---|---|
| Bao An Yan Shou Fang | Flos Lonicerae, Radix Glycyrrhizae | Curing some infection diseases | ‘Yi Fang Yi Jian’* |
| Bei Mu San | Flos Lonicerae, Bulbus Fritillariae Thunbergii | Curing mammary abscess | ‘Pu Ji Fang’ Vol. 325* |
| Fu Fang Jin Yin Hua Jiao Nang | Flos Lonicerae, Fructus Forsythiae, Radix Scutellariae | Clearing heat and detoxicating. Curing headache, fever, cough and toothache | ‘Chinese Medicine Dictionary’* |
| Gan Ju Tang | Flos Lonicerae, Radix Glycyrrhizae, Flos Chrysanthemi | Curing all furunculosis | ‘Chuan Mo You De Book’* |
| Hua Gan Xiao Du Tang | Flos Lonicerae, Fructus Gardeniae, Radix Glycyrrhizae, Radix Angelicae, Radix Angelicae Sinensis | Curing the pain of coastal regions | ‘Bian Zhen Lu’ Vol. 13* |
| Jia Wei Sheng Hua Tang | Flos Lonicerae, Fructus Forsythiae, Radix Glycyrrhizae, Olibanum, Myrrha and Radix Ginseng | Curing deficiency of both QI and blood after childbirth | ‘Jin Jian’ Vol. 48* |
| Jie Du Xian Cao | Fresh branches and leaves of Lonicera japonica | Curing late syphilis | ‘Shang Yi Da Quan’ Vol. 34* |
| Jin Pu Tang | Flos Lonicerae, Herba Taraxaci, Semen Benincasae, Radix Aucklandiae, Radix et Rhizoma Rhei | Clearing away heat evil, promoting diuresis and Qi to activate blood | ‘Zhu Ri Dong Fang’* |
| Jin Qi San | Flos Lonicerae, Radix Astragali, Radix Glycyrrhizae, Radix Rehmanniae, Radix Paeoniae Alba, Radix Angelicae Sinensis | Curing women’ acute pain and trug of hypogastrium | ‘Pu Ji Fang’ Vol. 335* |
| Jin Qiang Gao | Flos Lonicerae, Radix et Rhizoma Rhei, Herba Violae, Radix Arnebia, Radix Angelicae Sinensis, Eupolyphaga seu Steleophage, Cortex Phellodendri, Radix Glycyrrhizae, Radix Saposhnikoviae | Curing wound infection | ‘Zhong Yi Shang Ke Xue Jiang Yi’* |
| Jin Yin Hua San | Flos Lonicerae, Herb Schizonepetae, Fructus Cnidii, Radix seu Rhizoma Nardostachyos, Radix Angelicae, Semen Arecae, Natril Sulfas | Treating chancre sore | ‘Pu Ji Fang’, Vol. 301* |
| Jin Yin Hua Gao | Flos Lonicerae, Radix Glycyrrhizae, Herb Leonuri | Treating pregnancy carbuncle | ‘Chen Su An Fu Ke Bu Jie’, Vol. 3* |
| Jin Yin Hua Jiu (Wine) | Fresh leaf of Lonicera japonica | Curing superficial infection and furunculosis | ‘Gu Fang Hui Jin’* |
| Jin Yin Hua Tang Jiang | Vine of Lonicera japonica | Clearing heat and detoxicating. Curing fever, sore throat and so on | ‘Chinese Medicine Dictionary’* |
| Jin Yin Wine | Flos Lonicerae, Herba Taraxaci | Curing breast's bump | ‘Xian Nian Ji’ Vol. 3* |
| Ju Hua Jin Yin Hua Tang | Flos Lonicerae, Flos Chrysanthemi, Radix Platycodi, Radix Ophiopgonis, Radix Glycyrrhizae | Treating pharyngo-laryngitis chronica | ‘Dan Yan Fang’* |
| San Xing Tang | Flos Lonicerae, Herba Taraxaci, Radix Glycyrrhizae | Curing carbuncle in mouth | ‘Bian Zheng Lu’ Vol. 13* |
| Sheng Hua Tang | Flos Lonicerae, Radix Ginseng | Curing ulcer, the deficiency of Qi and blood | ‘Dong Tian Ao Zhi’ Vol. 14* |
| Shu Feng Qing Gan Tang | Flos Lonicerae, Radix Paeoniae Lactiflora, Herba Schizonepetae, Radix Saposhnikoviae, Rhizoma Chuanxiong, Herba Menthae Haplocalycis, Flos Chrysanthemi, Fructus Gardeniae, Radix Bupleuri, Fructus Forsythiae, Radix Glycyrrhizae, Radix Angelicae Sinensis | Clearing away heat evil, promoting diuresis and removing heat to brighten vision | ‘Yi Zong Jin Jian’ Vol. 65* |
| Wan Shan Wan | Flos Lonicerae, Radix Glycyrrhizae, Fructus Forsythiae, Spica Prunellae | Curing hemorrhoid | ‘Yan Fang Xin Bian’* |
| Xiao Du San | Flos Lonicerae, Fructus Forsythiae, Herba Schizonepetae, Radix Angelicae, Fructus Arctium, Radix Saposhnikoviae, Cortex Dictamni, Radix Paeoniae Lactiflora, Radix Glycyrrhizae, Fructus tribuli | Expelling wind and dispelling dampness, clearing away the heat-evil and expelling superficial evils | ‘Shang Yi Da Quan’* |
| Xiao Hua Tang | Flos Lonicerae, Radix Trichosanthis, Radix Angelicae Sinensis, Radix Glycyrrhizae, Gynura Bicolor, Medulla Tetrapanacis | Clearing away the heat-evil and expelling superficial evils, curing mammitis | ‘Dong Tian Ao Zhi’ Vol. 7* |
| Yin Hua Tang | Flos Lonicerae, Rhizoma Menispermi, Radix Trichosanthis, Bulbus Fritillariae Thunbergii, Radix Angelicae, Radix Saposhnikoviae, Radix Paeoniae Lactiflora, Olibanum, Myrrha, Radix Glycyrrhizae | Clearing away the heat-evil and expelling superficial evils, curing the phyma and body pain | ‘Gan Zu Wang Fang’* |
| Yin Hua Tea | Fresh Flos Lonicerae | Curing children’ parotitis and furunculosis sudariferus | ‘Chang Yong Zhong Cao Yao Shou Ce’* |
| Chinese Pharmacopoeia | |||
| Kang Gan Ke Li | Flos Lonicerae, Radix Paeoniae Lactiflora, Rhizoma Dryopteris Crassirhizomae | Curing headache, fever, cough and pharyngalgia | ‘Chinese Pharmacopoeia’** |
| Li Yan Jie Du Ke Li | Flos Lonicerae, Radix Isatis, Fructus Forsythiae, Herba Menthae Haplocalycis, Fructus Arctium, Fructus Crataegi, Radix Platycodi, Folium Isatidis, Bombyx Batryticatus, Radix Scrophulariae, Radix Scutellariae, Radix Rehmanniae, Radix Trichosanthis, Radix et Rhizoma Rhei, Bulbus Fritillariae Thunbergii, Radix Ophiopgonis | Curing anemopyretic tonsillitis, acute tonsillitis and anemopyretic laryngalgia | ‘Chinese Pharmacopoeia’** |
| Qin Guo Wan | Flos Lonicerae, Fructus Canarli, Radix Scutellariae, Rhizoma Menispermi, Radix Ophiopgonis, Radix Scrophulariae, Radix Paeoniae Alba, Radix Platycodi | Curing the swell of throat, celostomia, dry mouth and xeropulmonary cough | ‘Chinese Pharmacopoeia’** |
| Qin Re Jie Du Kou Fu Ye | Flos Lonicerae, Gypsum Fibrosum, Radix Scrophulariae, Radix Rehmanniae, Fructus Forsythiae, Fructus Gardeniae, Radix Scutellariae, Radix Gentianae, Radix Isatis, Rhizoma Anemarrhenae, Radix Ophiopgonis | Clearing away the heat-evil and expelling superficial evils | ‘Chinese Pharmacopoeia’** |
| Xiao Yin Pian | Flos Lonicerae, Radix Rehmanniae, Mudanpi, Radix Paeoniae Lactiflora, Radix Angelicae Sinensis, Radix Sophorae Flavescentis, Radix Scrophulariae, Fructus Arctium, Perioatracum Cicadae, Covtex Diatamni, Radix Saposhnikoviae, Folium Isatidis, Flos Carthami | Removing heat to cool blood, dispelling wind and arresting itching, curing pruritus | ‘Chinese Pharmacopoeia’** |
| Shuang Huang Lian Shuan | Flos Lonicerae, Radix Scutellariae, Fructus Forsythiae | Curing upper respiratory tract infection and pneumonia | ‘Chinese Pharmacopoeia’** |
| Shuang Huang Lian Ke Li | Flos Lonicerae, Radix Scutellariae, Fructus Forsythiae | Dispelling the evil in the superficies with drugs of pungent taste and cool nature, and curing fever, cough and pharyngalgia | ‘Chinese Pharmacopoeia’** |
| Xiao Er Re Su Qin Kou Fu Ye | Flos Lonicerae, Radix Scutellariae, Radix Isatis, Radix Puerariae, Fructus Forsythiae, Radix Bupleuri, Radix et Rhizoma Rhei | Curing children headache, fever, nasal obstruction, cough and pharyngalgia | ‘Chinese Pharmacopoeia’** |
| Ying Huang Kou Fu Ye | Flos Lonicerae, Radix Scutellariae | Curing upper respiratory tract infection, acute tonsillitis and pharyngitis | ‘Chinese Pharmacopoeia’** |
| Zhi Zi Jin Hua Wan | Flos Lonicerae, Fructus Gardeniae, Rhizoma Coptidis, Radix Scutellariae, Cortex Phellodendri, Radix et Rhizoma Rhei, Rhizoma Anemarrhenae, Radix Pinelliae | Curing the swell of throat, constipation, conjunctival congestion, etc. | ‘Chinese Pharmacopoeia’** |
| Yin Qiao Jie Du Pian | Flos Lonicerae, Fructus Forsythiae, Herba Menthae Haplocalycis, Herba Schizonepetae, Fructus Arctium, Radix Platycodi, Folium lophatheri, Radix Glycyrrhizae | Curing pharwind-heat type common cold and headache, fever, cough | ‘Chinese Pharmacopoeia’** |
| Yin Qiao Tang | Flos Lonicerae, Fructus Forsythiae, Radix Scutellariae, Radix Bupleuri Chinensis, Herba Artemisiae, Fructus amomi, Almond, Semen Coicis, Radix Adenophorae, Rhizoma Phragmitis | Treating SARS in clinic | http://www.satcm.gov.cn |
| Healthy food | |||
| Feng Jiao Jin Yin Hua Qin Liang Tang | Flos Lonicerae, Propolius, Herba Menthae Haplocalycis | Moistening and cleaning throat | http://www.sda.gov.cn*** |
| Jin Yin Hua Qin Liang Tang | Flos Lonicerae, Fructus Canarli, Fructus momordicae, Semen Sterculiae Lychnophorae, Herba Menthae Haplocalycis | Moistening and cleaning throat | http://www.sda.gov.cn*** |
| Jin Yin Hua Li Yan Pian | Flos Lonicerae, Fructus momordicae, Rhizoma Imperatae, Herba Menthae Haplocalycis | Moistening and cleaning throat | http://www.sda.gov.cn*** |
| Jin Yin Hua Jiao Nang | Flos Lonicerae | Moistening and cleaning throat | http://www.sda.gov.cn*** |
| Jin Yin Hua Zhen Zhu Jiao Nang | Flos Lonicerae, Radix Salviae Miltiorrhizae, Herba Taraxaci, Mudanpi, Fructus Gardeniae, Radix et Rhizoma Rhei, Margarita | Curing acnes | http://www.sda.gov.cn*** |
| Jin Yin Hua Luo Han Guo Han Pian | Flos Lonicerae, Fructus momordicae, Flos Chrysanthemi, Semen Sterculiae Lychnophorae, Herba Menthae Haplocalycis | Moistening and cleaning throat | http://www.sda.gov.cn*** |
| Jin Yin Hua Pan Da Hai Chong Ji | Flos Lonicerae, Herba Taraxaci, Flos Chrysanthemi, Herb Houttuyniae, Fructus momordicae, Herba Menthae Haplocalycis, Radix Platycodi, Semen Sterculiae Lychnophorae | Moistening and cleaning throat | http://www.sda.gov.cn*** |
Cited from the Website: http://www.zysj.com.cn.
Cited from ‘Chinese Pharmacopoeia’.
Cited from the Website: http://www.sda.gov.cn.
Lonicera japonica has been planted and used as the local medicine in many places, especially in East Asian. In China, it is widely distributed in drainage areas of the Yellow River and Yangzi River, and largely cultivated in Longhui, Fengqiu, Pingyi and Fei counties of Hunan, Henan and Shandong provinces. According to the quality analyses, Lonicera japonica planted in Fengqiu county has the highest contents of chlorogenic acid with 4–6% (Wang, 2010). Since 1995, Lonicera japonica has been listed in the Pharmacopoeia of the People's Republic of China (Committee for the pharmacopoeia of PR China, 1995), and made to some preparations to treat chronic enteritis, pneumonia, acute tonsillitis, nephritis, acute mastitis, leptospirosis in clinic. Among of them, ‘Jin Yin Hua Jiu (Wine)’ has been used to clear away the heat-evil and expel superficial evils; ‘Jin Yin Hua Tang’ has been applied to clear heat and detoxicating, and so on (Table 1). Recently, Lonicera japonica also has been employed extensively to prevent and treat some serious viral diseases of human and veterinary, such as SARS coronavirus, H1N1 (Swine) flu virus, and being called the ‘bouvardin’ (Jiao, 2009).
Lonicera japonica was also employed as healthy beverage to improve body and prevent ills in China. In Qing dynasty, according to ‘Yan Shou Dan Fang’, it was used to moisturize the skin and rejuvenation (Chen, 2008). Modern pharmacological researches thought that these effects may be related to the active compositions volatile oils, chlorogenic acid and flavones. Myung et al. (2004) suggested that Lonicera japonica prepared by extraction of 70% methanol or 70% acetone followed by gamma irradiation treatment have a bright color, good tyrosinase inhibition, xanthine oxidase inhibition, and nitrite scavenging activities. It could use for the food or cosmetic industry as natural source of bioactive compound. And with other compositions, Lonicera japonica has been made healthy beverage through various technology, such as ‘Jin Yin Hua tea’, ‘Jin Yin Hua nutritive beverage’, ‘Jin Yin Hua acidophilous milk’, ‘Jin Yin Hua Wine’, ‘Jin Yin Hua oral liquid’ (He et al., 2010) (Table 1).
At the same time, Lonicera japonica has been made to the article of everyday uses and cosmetics, such as ‘Jin Yin Hua floral water’, ‘Jin Yin Hua facial mask’, especially it could be made to toothpaste which have the effects of preventing and treating the oral cavity's diseases (Jiao, 2009). Zhang (2008) investigated the antibacterial and antisepticize activities in cosmetics of the flower extracts of Lonicera japonica, the results showed that it had the marked antibacterial and antisepticize activities, and could be applied in cosmetics extensively. Shu et al. (2008) suggested that essential oils isolated from Lonicera japonica by supercritical extraction method would cover the smell from cigarettes and improve the quality. So Lonicera japonica would bring the social and economic values well.
3. Phytochemistry
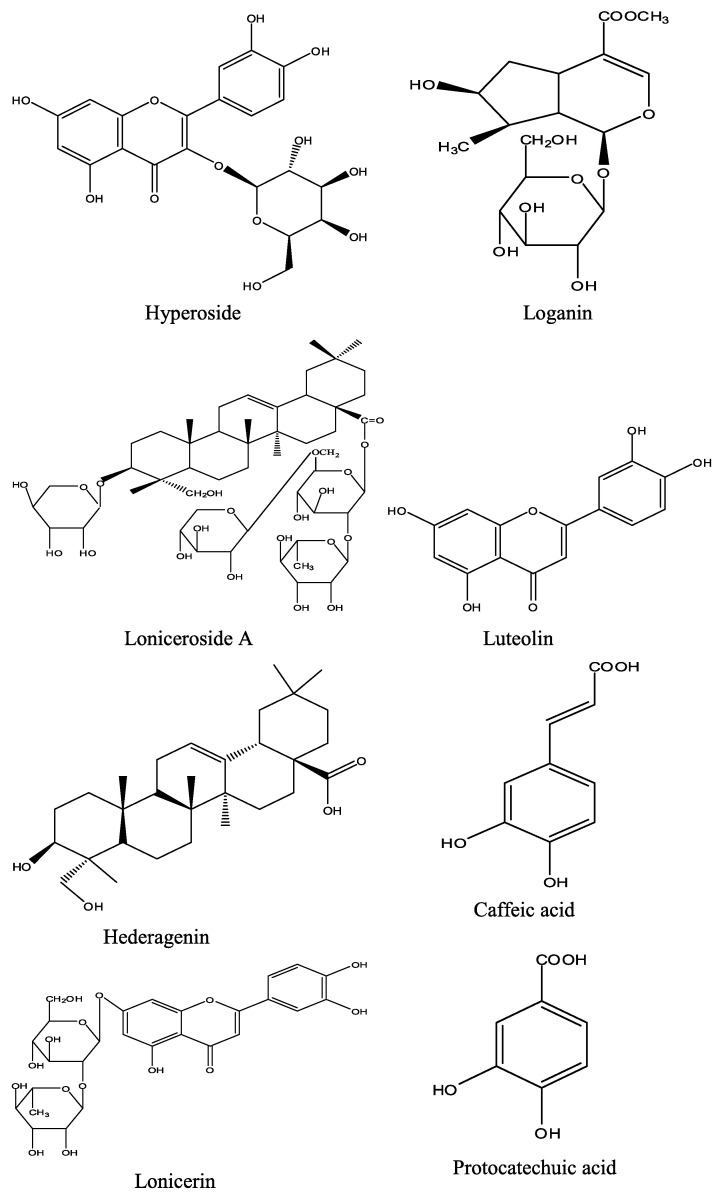
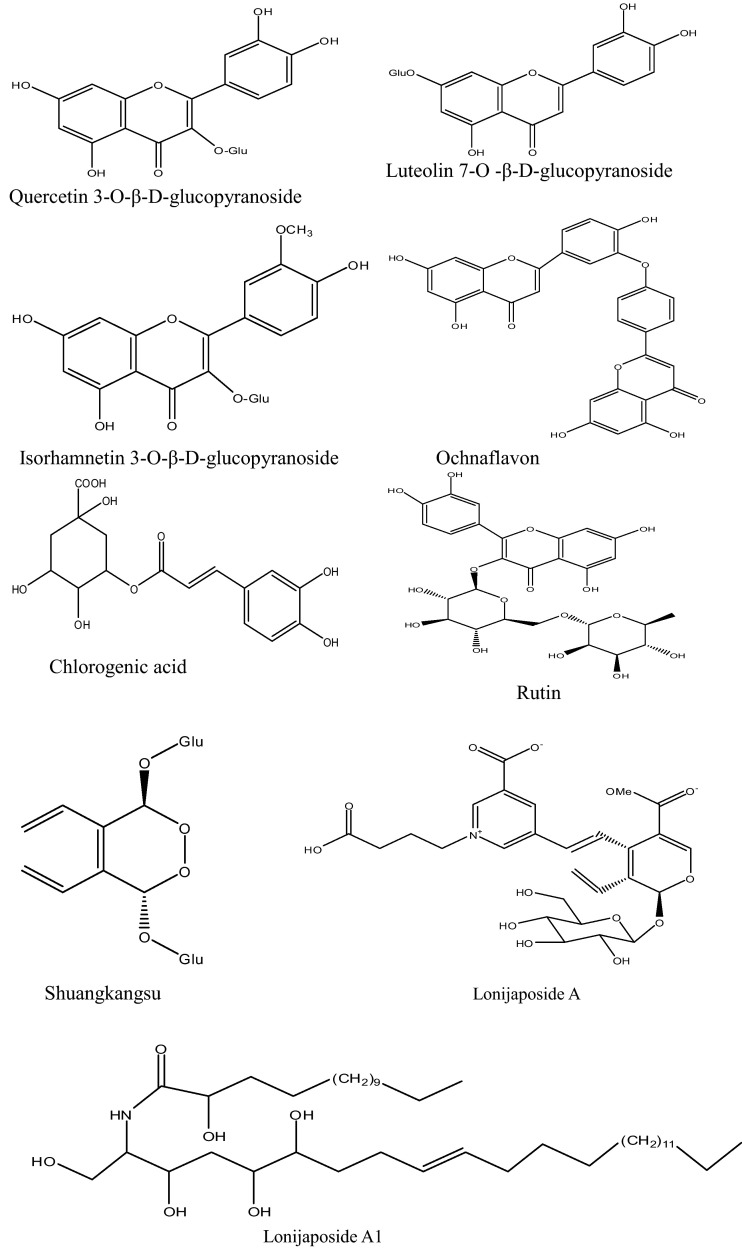
More than 140 compounds have been isolated and identified from Lonicera japonica so far. As one of the important chemical composition, essential oils were analyzed through GC–MS method, and linalool, hexadecanoic acid, octadecadienoic acid, ethyl palmitate and dihydrocarveol were the main compounds. At the same time, Lonicera japonica was abounds with flavones, organic acids, triterpenoid saponins, and iridoids (Table 2 and Fig. 2 ). Some of them displayed many bioactivities in vivo or in vitro (Table 3 ). And the different chemical compositions of Lonicera japonica will provide the foundation well of the different pharmacology activities.
Table 2.
The compounds isolated from Lonicera japonica (the structure of main compounds illustrated in Fig. 2).
| No. | Compounds | Resource | References |
|---|---|---|---|
| Organic acids | |||
| 1 | Chlorogenic acid | Whole plant | Yip et al. (2006) |
| 2 | Isochlorogenic acid | Whole plant | Yip et al. (2006) |
| 3 | Caffeic acid | Flowers | Choi et al. (2007) |
| 4 | Hexadecanoic acid | Whole plant | Huang et al. (1996) |
| 5 | Myristic acid | Whole plant | Huang et al. (1996) |
| 6 | 3,5-O-dicaffeoylquinic acid | Whole plant | Iwanhashi and Negoroy (1986) |
| 7 | 4,5-O-dicaffeoylquinic acid | Whole plant | Iwanhashi and Negoroy (1986) |
| 8 | 3,4-O-dicaffeoylquinic acid | Whole plant | Iwanhashi and Negoroy (1986) |
| 9 | 1,3-O-dicaffeoylquinic acid | Whole plant | Iwanhashi and Negoroy (1986) |
| 10 | 3-Ferulicoylquinic | Whole plant | Iwanhashi and Negoroy (1986) |
| 11 | 4-Ferulicoylquinic | Whole plant | Iwanhashi and Negoroy (1986) |
| 12 | 5-O-caffeoylquinic acid | Whole plant | Qi et al. (2009) |
| 13 | 4-O-caffeoylquinic acid | Whole plant | Qi et al. (2009) |
| 14 | Caffeoyl-CH2-O-quinic acid | Whole plant | Qi et al. (2009) |
| 15 | 1,5-O-dicaffeoylquinic acid | Whole plant | Qi et al. (2009) |
| 16 | 1,4-O-dicaffeoylquinic acid | Whole plant | Qi et al. (2009) |
| 17 | Methylated dicaffeoylquinic acid | Whole plant | Qi et al. (2009) |
| 18 | Oleanolic acid 28-α-O-l-rhamnopyranosyl-(1 → 2)-[β-d-xylopyranosyl(1 → 6)]-β-d-glu-copyranosyol ester | Flowers | Choi et al. (2007) |
| 19 | 3,5-O-dicaffeoylquinic acid methyl ester | Flower buds | Lee et al. (2010) |
| Peng et al. (2000) | |||
| 20 | Methyl chlorogenate | Flower buds | Lee et al. (2010) |
| 21 | 3-O-caffeoylquinic acid butyl ester | Flower buds | Ren et al. (2008) |
| 22 | 3-O-caffeoylquinic acid | Flower buds | Peng et al. (2000) |
| 23 | 3-caffeoylquinic acid methyl ester | Flower buds | Peng et al. (2000) |
| 24 | 3,5-dicaffeoylquinic acid buthyl ester | Flower buds | Peng et al. (2000) |
| 25 | Vanillic acid 4-O-β-d-(6-O-benzoylglucopyranoside) | Flower buds | Lee et al. (2010) |
| 26 | Protocatechuic acid | Flowers | Choi et al. (2007) |
| Yip et al. (2006) | |||
| 27 | Chlorogenic acid butyl ester | Flower buds | Wang (2008c) |
| 28 | Chlorogenin tetraacetate | Flower buds | Lou et al. (1996) |
| 29 | 5-Feruloylquinic acids | Aerial Parts | Shanghai Institute of Pharmaceutical Industry (1975) |
| 30 | Methyl 3,5-di-O-caffeoylquinic acid | Whole plant | Chang et al. (1995) |
| 31 | Methyl 3,4-di-O-caffeoylquinic acid | Whole plant | Chang et al. (1995) |
| 32 | Caffeic acid methyl ester | Whole plant | Ma et al. (2005) |
| Flavones | |||
| 33 | Chrysoeriol | Flowers | Choi et al. (2007) |
| 34 | Chrysoeirol-7-O-neohesperidoside | Aerial parts | Choi et al. (2007) |
| 35 | Luteolin | Flowers | Choi et al. (2007) |
| Leaves | Yip et al. (2006) | ||
| Kumar et al. (2005) | |||
| 36 | Chrysoeriol 7-O-β-d-glucopyranoside | Flowers | Choi et al. (2007) |
| 37 | Isorhamnetin 3-O-β-d-glucopyranoside | Flowers | Choi et al. (2007) |
| 38 | Isorhamnetin 3-O-β-d-rutinoside | Flower buds | Wang (2008c) |
| 39 | Kaempferol 3-O-β-d-glucopyranoside | Flowers | Choi et al. (2007) |
| 40 | Kaempferol 3-O-β-d-rutinoside | Flower buds | Wang (2008c) |
| 41 | Quercetin 3-O-β-d-glucopyranoside | Flowers | Choi et al. (2007) |
| Gao and Mu (1995) | |||
| 42 | Luteolin 7-O-α-d-glucoside | Flowers | Choi et al. (2007) |
| Gao and Mu (1995) | |||
| 43 | Luteolin-7-O-β-d-galactoside | Flowers | Choi et al. (2007) |
| Gao and Mu (1995) | |||
| 44 | Hyperoside | Aerial parts | Zhang et al. (2006) |
| 45 | Lonicerin | Whole plant | Lee et al., 1995a, Lee et al., 1995b |
| 46 | Hydnocarpin | Aerial parts | Son et al. (1992) |
| Gao and Mu (1995) | |||
| 47 | Quercetin | Aerial parts | Son et al. (1992) |
| 48 | Astragalin | Aerial parts | Son et al. (1992) |
| 49 | Isoquercitrin | Aerial parts | Son et al. (1992) |
| 50 | Rhoifolin | Aerial parts | Son et al. (1992) |
| 51 | Flavoyadorinin-B | Flower buds | Lee et al. (2010) |
| 52 | Rutin | Flower buds | Ren et al. (2008) |
| 53 | Tricin-7-O-β-d-glucoside | Flower buds | Ren et al. (2008) |
| 54 | Chrysin | Leaves | Kumar et al. (2005) |
| 55 | Eriodictyol | Aerial parts | Zhang et al. (2006) |
| 56 | Apigenin | Aerial parts | Zhang et al. (2006) |
| 57 | Corymbosin | Aerial parts | Huang et al. (1996) |
| 58 | 5-Hydroxy-3′,4′,7-trimethoxylflavone | Aerial parts | Huang et al. (1996) |
| 59 | Ochnaflavone | Whole plant | Son et al. (2006) |
| Son et al. (1992) | |||
| 60 | Ochnaflavone 4′-O-methylether | Aerial parts | Son et al. (1992) |
| 61 | 3′-O-methyl loniflavone [5,5″,7,7″-tetrahydroxy 3′-methoxy 4′,4′″-biflavonyl ether | Leaves | Kumar et al. (2005) |
| 62 | Loniflavone [5,5″,7,7″,3′-pentahydroxy 4′,4′″-biflavonyl ether | Leaves | Kumar et al. (2005) |
| Iridoids | |||
| 63 | Loganin | Whole plant | Lee et al. (1995a) |
| 64 | Sweroside | Flower buds | Song et al. (2006) |
| Machida et al. (1995) | |||
| 65 | 7-O-ethyl sweroside | Flower buds | Song et al. (2006) |
| 66 | 7-Epi vogeloside | Flower buds | Song et al. (2006) |
| 67 | Secoxyloganin | Flower buds | Song et al. (2006) |
| 68 | Secoxyloganin 7-butyl ester | Flower buds | Song et al. (2006) |
| 69 | 7-Dimethyl-secologanoside | Flower buds | Song et al. (2006) |
| 70 | Centauroside | Flower buds | Song et al. (2006) |
| 71 | Secologanic acid | Flower buds | Qi et al. (2009) |
| 72 | Secologanin | Flower buds | Machida et al. (1995) |
| 73 | Secologanin dimethyl acetal | Flower buds | Machida et al. (1995) |
| 74 | Kingiside | Flower buds | Son et al. (1994) |
| 75 | Vogeloside | Flower buds | Kakuda et al. (2000) |
| 76 | Epi-vogeloside | Flower buds | Kakuda et al. (2000) |
| 77 | Dehydrornorronisi | Flower buds | Li et al. (2003) |
| 78 | Ketologanin | Flower buds | Song (2008) |
| 79 | 7α-Morroniside | Flower buds | Song (2008) |
| 80 | 7β-Morroniside | Flower buds | Song (2008) |
| 81 | Secologanoside | Flower buds | Song (2008) |
| 82 | Lonijaposide A | Flower buds | Song et al. (2008) |
| 83 | Lonijaposide B | Flower buds | Song et al. (2008) |
| 84 | Lonijaposide C | Flower buds | Song et al. (2008) |
| 85 | Lonijaposide D | Flower buds | Song (2008) |
| 86 | Lonijaposide E | Flower buds | Song (2008) |
| 87 | Lonijaposide F | Flower buds | Song (2008) |
| 88 | Lonijaposide G | Flower buds | Song (2008) |
| 89 | Lonijaposide H | Flower buds | Song (2008) |
| 90 | Lonijaposide I | Flower buds | Song (2008) |
| 91 | Lonijaposide J | Flower buds | Song (2008) |
| 92 | Lonijaposide K | Flower buds | Song (2008) |
| 93 | Lonijaposide L | Flower buds | Song (2008) |
| 94 | l-Phenylalaninosecologanin | Stems, leaves | Machida et al. (2002) |
| 95 | 7-O-(4-β-d-glucopyranosyloxy-3-methoxy-benzoyl) secologanolic acid | Stems, leaves | Machida et al. (2002) |
| 96 | 6′-O-(7α-hydroxyswerosyloxy) loganin | Stems, leaves | Machida et al. (2002) |
| 97 | (Z)-aldosecologanin | Stems, leaves | Machida et al. (2002) |
| 98 | (E)-aldosecologanin | Stems, leaves | Machida et al. (2002) |
| 99 | Loniceracetalide A | Flower buds | Kakuda et al. (2000) |
| 100 | Loniceracetalide B | Flower buds | Kakuda et al. (2000) |
| Saponins | |||
| 101 | 3-O-α-l-arabinopyranosyl-28-O-[β-d-glucopyranosyl(1 → 6)-β-d-glucopyranosyl] oleanolic acid | Aerial parts | Kawai et al. (1988) |
| 102 | 3-O-[α-l-rahmnopyranosyl(1 → 2)-α-l-arabinopyranosyl]-28-O-β-d-glucopyran-osyl hederagenin | Aerial parts | Kawai et al. (1988) |
| 103 | 3-O-[α-l-rahmnopyranosyl(1 → 2)-α-l-arabinopyranosyl]-28-O-[β-d-glucopyranosyl(1 → 6)-β-d-glucopyranosyl] oleanolic acid | Aerial parts | Kawai et al. (1988) |
| 104 | 3-O-[α-l-rahmnopyranosyl(1 → 2)-α-l-arabinopyranosyl]-28-O-[6-acetyl-β-d-glucopyranosyl(1 → 6)-β-d-glucopyranosyl] hederagenin | Aerial parts | Kawai et al. (1988) |
| 105 | 3-O-α-l-rhamnopyranosyl-(1 → 2)-α-l-arabinopyranosy hederagenin 28-O-β-d-xylpyranosyl(1 → 6)-β-d-glucopyranosyl ester | Flower buds | Lou et al. (1996) |
| 106 | 3-O-α-l-arabinopyranosy hederagenin 28-O-α-d-rhamnopyranosyl (1 → 2) [β-d-xyl pyranosyl(1 → 6)-β-d-glucopyranosyl ester | Flower buds | Lou et al. (1996) |
| 107 | 3-O-α-l-rhamnopyranosyl-(1 → 2)-α-l-arabinopyranosy hederagenin 28-O-α-d-rhamnopyranosyl(1 → 2)[β-d-xyl pyranosyl(1 → 6)-β-d-glucopyranosyl ester | Flower buds | Lou et al. (1996) |
| 108 | 3-O-β-d-glucopyranosyl-(1 → 4)-β-l-glucopyranosyl(1 → 3)-α-l-rhamno pyranosyl(1 → 2)-α-l-arabinopyranosy hederagenin28-O-β-d-glucopyranosyl-(1 → 6)-β-d-glucopyranosyl ester | Flower buds | Chen et al. (2000) |
| 109 | Hederagenin-3-O-α-l-rhamnopyranosyl(1 → 2)-α-l-arabinopyranoside | Flower buds | Chen et al. (2000) |
| 110 | 3-O-α-l-rhamnopyranosyl-(1 → 2)-α-l-arabinopyranosy hederagenin 28-O-β-d-glucopyranosyl(1 → 6)-β-d-glucopyranosyl ester | Flower buds | Chen et al. (2000) |
| 111 | 3-O-β-d-glucopyranosyl-(1 → 3)-α-l-rhamnopyranosyl(1 → 2)-α-l-arabino pyranosyl hederagenin 28-O-β-d-glucopy ranosyl-(1 → 6)-β-d-glucopyranosyl ester | Flower buds | Chen et al. (2000) |
| 112 | Loniceroside A | Whole plant | Son et al. (1994) |
| Lee et al. (1995a) | |||
| 113 | Loniceroside B | Whole plant | Son et al. (1994) |
| Lee et al. (1995a) | |||
| 114 | Loniceroside C | Aerial parts | Kwak et al. (2003) |
| 115 | Loniceroside D | Flower buds | Lin et al. (2008) |
| 116 | Loniceroside E | Flower buds | Lin et al. (2008) |
| 117 | Macranthoidin A | Flower buds | Ren et al. (2008) |
| 118 | Macranthoidin B | Flower buds | Ren et al. (2008) |
| 119 | Dipsacoside B | Flower buds | Ren et al. (2008) |
| 120 | Hederagenin-28-O-[β-d-glucopyranosyl-(1 → 6)-β-d-glucopyranosyl] ester | Flower buds | Ren et al. (2008) |
| 121 | Macranthoside B | Flower buds | Ren et al. (2008) |
| 122 | Macranthoside A | Flower buds | Ren et al. (2008) |
| 123 | 3-O-[α-l-rhamnopyranosyl-(1 → 2)-α-l-arabinopyranosyl] hederagenin | Flower buds | Ren et al. (2008) |
| 124 | Saponin 1 | Flower buds | Qi et al. (2009) |
| 125 | Saponin 4 | Flower buds | Qi et al. (2009) |
| 126 | Hederagenin 3-O-α-l-arabinopyranoside | Flowers | Choi et al. (2007) |
| 127 | Hederagenin | Whole plant | Lee et al. (1995a) |
| 128 | Oleanolic acid | Flower buds | Wang (2008c) |
| Others | |||
| 129 | Lonijaposide A1 | Flowers | Kumar et al. (2006) |
| 130 | Lonijaposide A2 | Flowers | Kumar et al. (2006) |
| 131 | Lonijaposide A3 | Flowers | Kumar et al. (2006) |
| 132 | Lonijaposide A4 | Flowers | Kumar et al. (2006) |
| 133 | Lonijaposide B1 | Flowers | Kumar et al. (2006) |
| 134 | Lonijaposide B2 | Flowers | Kumar et al. (2006) |
| 135 | 5-Hydroxymethyl-2-furfural | Flowers | Choi et al. (2007) |
| 136 | 1-O-methyl-myo-inositol | Flower buds | Wang (2008c) |
| 137 | Nonacontane | Flower buds | Wang (2008c) |
| 138 | β-Sitosterol | Flower buds | Wang (2008c) |
| 139 | Sucrose | Flower buds | Wang (2008c) |
| 140 | Glucose | Flower buds | Wang (2008c) |
| 141 | Shuangkangsu | Flowers | Li (2008a) |
| 142 | (+)-N-(3-methybutyryl-β-d-glucopyranoyl)-nicotinate | Flower buds | Song (2008) |
| 143 | (+)-N-(3-methybut-2-enoyl-β-d-glucopyranoyl)-nicotinate | Flower buds | Song (2008) |
| 144 | 5′-O-methyladenosine | Flower buds | Song (2008) |
| 145 | Guanosine | Flower buds | Song (2008) |
| 146 | Adenosine | Flower buds | Song (2008) |
| 147 | Syringin | Flower buds | Song (2008) |
Fig. 2.
The chemical structure of main compounds from Lonicera japonica.
Table 3.
The activities of some compounds from Lonicera japonica.
| Compounds | Effects | In vivo | In vitro | Reference |
|---|---|---|---|---|
| Caffeic acid | Antioxidative activity | Showed marked antioxidant and scavenging activities with IC50 values of 5.72 μM for DPPH radicals, and 3.18 μM for ONOO− | Choi et al. (2007) | |
| Chlorogenic acid | Anti-tumor activity | With IC50 values of 55 μmol/L and corresponding cell (HepG2 cell) viabilities was 62% ± 4%. And the cytotoxicities of chlorogenic acid were partially eliminated by the antioxidant effect of N-acetyl-l-cysteine (NAC) | Yip et al. (2006) | |
| Antibacterial activity | Compared to the Gram-positive bacteria, the chlorogenic acid to Gram-negative bacteria's bacteriostasis activeness was stronger; the minimum inhibitory concentration of chlorogenic acid to shigella and salmonella was 0.125 mg/ml, almost the same to 0.1 mg/ml kanamycin | Xu (2008) | ||
| MIC was 0.025 g/ml, 0.025 g/ml, 0.1 g/ml and 0.8 g/ml against Escherichia coli, Sarcina luteus, Bacillus subtillis and Staphylococcus aureus | Wu (2005) | |||
| Antioxidative activity | The DPPH scavenging activity was 74% at the dose of 0.1 g/ml | Wu (2005) | ||
| Antiviral activity | At the doses of 0.05 mg/ml, 0.1 mg/ml, 0.4 mg/ml, 0.8 mg/ml and 0.8 mg/ml, it respectively inhibited respiratory syncytia virus, coxsackie B3 virus, adeno-associated 7 viruses, adeno-associated 3 viruses and Coxsackie B5 virus | Hu et al. (2001) | ||
| The 0% toxic dose, minimum effective concentration and therapeutic index against to human cytomegalovirus were 100 μg/ml, 1 μg/ml and 100, respectively | Chen et al. (2009) | |||
| Anti-inflammatory activity | It (5, 10, 15 mmol/L) decreased the expression of NF-kB P65 induced by LPS at 4 h (P < 0.05), and the concentration of NO at 6 h. At the same time, it would increase the decrease of activity of GSH-Px induced by LPS at 6 h (P < 0.05) | Huo et al. (2003) | ||
| Hypoglycemic activity | 1 mM inhibited about 40% of glucose-6-phosphatase activity (P < 0.05) in the microsomal fraction of hepatocytes. It promoted a significant reduction (P < 0.05) in the plasma glucose peak at 10 and 15 min during the oral glucose tolerance test, probably by attenuating intestinal glucose absorption. This suggested a possible role for it as a glycaemic index lowering agent and highlighting it as a compound of interest for reducing the risk of developing type 2 diabetes | Bassoli et al. (2008) | ||
| Dicaffeoylquinic acids | Antiviral activity | Results showed that 3,5-dicaffeoylquinic acid and two analogues were potent and selective inhibitors of HIV-1 IN in vitro. All of the dicaffeoylquinic acids were found to inhibit HIV-1 replication at concentrations ranging from 1 to 6 μM in T cell lines, whereas their toxic concentrations in the same cell lines were >120 μM. In addition, it inhibited HIV-1 IN in vitro at submicromolar concentrations. So the dicaffeoylquinic acids as a class are potent and selective inhibitors of HIV-1 IN and form important lead compounds for HIV drug discovery | Robinson et al. (1996) | |
| Hederagenin | Anti-inflammatory activity | 100 mg/kg showed anti-inflammatory activity in the same model with 42% and 23% inhibition rates (P < 0.001) | Lee et al. (1995a) | |
| Hyperoside | Antibacterial activity | It showed a excellent antibacterial effect on SA strains with a low MIC of 0.5–1 mg/ml, and the MIC of 2 mg/ml for strains of Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumonia were observed | Tang (2008) | |
| The antibacterial activity with synergistic effect | The FIC index indicated that the addition effects were found in 55%, 30%, 25% and 15% of MRSA strains (n = 20) when hyperoside combined with oxacillin, benzylpenicillin, gatifloxacin and levofloxacin, respectively. Results suggested that hyperoside could enhance the anti-MRSA efficiency of these β-lactams or quinolones. It combined with chlorogenic acid showed an obvious cumulate bactericidal action on Pseudomonas aeruginosa ATCC27853 with a FIC index of 0.75 | Tang (2008) | ||
| Hepatoprotective | 80 μg/mL exhibited the best protective effects for hepatocytes injured by CCl4, characterized as the levels of ALT, AST and MDA decreasing, elevation of GSH level and survival hepatocytes increasing with little damage in cell structure. The significant hepatoprotective effects for CCl4-attatcked rats were found in three dosages of hyperoside (10, 20 and 30 mg/kg) with the obvious improve biochemical indexes and liver histopathology examination. The results of animals received hyperoside of 30 mg/kg were almost similar to that of normal controls | Tang (2008) | ||
| Anti-tumor activity | 5, 10, 20, 25 μg/mL have inhibitory effect on Hep-2 cells when it was used as photosensitizer in PDT or as radiosensitizer in radiotherapy. And this indicate that hypericin may be a hopeful agent to treat laryngeal carcinoma | Sun (2002) | ||
| Isorhamnetin 3-O-β-d-glucopyranoside | Antioxidative activity | Showed marked antioxidant and scavenging activities with IC50 values of 11.76 μM for DPPH radicals, and 3.34 μM for ONOO− | Choi et al. (2007) | |
| Loganin | Anti-inflammatory activity | 100 mg/kg presented anti-inflammatory activity against mouse ear edema induced by croton-oil and arachidonic acid with 20% and 19% inhibition rates | Lee et al. (1995a) | |
| Loniceroside A | Anti-inflammatory activity | 100 mg/kg showed anti-inflammatory activity against mouse ear edema induced by croton-oil and arachidonic acid with 34% and 23% inhibition rates. Although far less potent than prednisolone (52% and 36%), it was comparable to aspirin at the dose of 100 mg/kg. 100 mg/kg/day also could reduce adjuvant-induced arthritis in rats (P < 0.05). The reference compound showed potent activity at a dose of 20 mg/kg/day (P < 0.01). At the same time, it (100 mg/kg, p.o.) against mouse ear edema provoked by croton oil with 30.2% inhibition rate |
Lee et al. (1995a) Kwak et al. (2003) |
|
| Loniceroside C | Anti-inflammatory activity | At the doses of 50, 100, 200 mg/kg (p.o.), it showed antiinflammatory activity against mouse ear edema provoked by croton oil with 15%, 31% and 28.7% inhibition rates, respectively | Kwak et al. (2003) | |
| Lonicerin | Anti-inflammatory activity | It presented anti-inflammatory activity against mouse ear edema induced by croton-oil with 39% (P < 0.001) | Lee et al. (1995a) | |
| Luteolin | Antioxidative activity | |||
| Anti-inflammatory activity | It effectively inhibited the lipopolysaccharide (LPS)-induced tumor necrosis factor-α, interleukin-6 and inducible nitric oxide production in vitro, protect against LPS-induced lethal toxicity by inhibiting pro-inflammatory molecule expression in vivo and reducing leukocyte infiltration in tissues | Park et al. (2005), Xagorari et al. (2001), Kotanidou et al. (2002) | ||
| Anti-tumor activity | The MTT assay showed that was 20% viability when HepG2 hepatocellular carcinoma cells were incubated at 100 μmol/L. IC50 values of 40 μmol/L and corresponding cell viabilities of 53% ± 5%. The cytotoxicities were partially eliminated by the antioxidant effect of N-acetyl-l-cysteine | Yip et al. (2006) | ||
| Anti-5-lipoxygenase activity | It presented the 5-lipoxygenase inhibitory activities with 97% inhibition at 20 μM. Nordihydroguaiaretic acid was used as a reference compound with 100% inhibition at 20 μM | Lee et al. (2010) | ||
| Luteolin 7-O-β-d-glucopy ranoside | Antioxidative activity | Showed marked antioxidant and scavenging activities with IC50 values of 9.97 μM for DPPH radicals, and 3.18 μM for ONOO− | Choi et al. (2007) | |
| Ochnaflavon | Anti-inflammatory activity | It inhibited cyclooxygenase-2 (COX-2) dependent phases of prostaglandin D2 (PGD2) generation in bone marrow-derived mast cells with IC50 values of 0.6 μM. Western blotting showed that the decrease in quantity of the PGD2 product was accompanied by a decrease in the COX-2 protein level. And this compound could consistently inhibit the production of leukotriene C4, with an IC50 value of 6.56 μM. So ochnaflavone has a dual cyclooxygenase-2/5-1ipoxygenase inhibitory activity. It also strongly inhibited degranulation reaction, with an IC50 value of 3.01 μM | Son et al. (2006) | |
| At 10 μM, ochnaflavone showed the suppressive activity against lymphocyte proliferation induced by Con A or LPS | Lee et al. (1995b) | |||
| Protocate chuic acid | Antioxidative activity | Showed marked antioxidant and scavenging activities with IC50 values of 7.21 μM for DPPH radicals, and 1.47 μM for ONOO− | Choi et al. (2007) | |
| Anti-tumor activity | It was capable of stimulating the c-Jun N-terminal kinase (JNK) and p38 subgroups of the mitogen-activated protein kinase (MAPK) family. It induced cell death was rescued by specific inhibitors for JNK and p38, with IC50 values of 60 μmol/L | Yip et al. (2006) | ||
| Quercetin 3-O-β-d-glu copyranoside | Antioxidative activity | Showed marked antioxidant and scavenging activities with IC50 values of 4.60 μM for DPPH radicals, and 1.76 μM for ONOO− | Choi et al. (2007) | |
| Rutin | Anti-apoptotic activity | Improved I/R-induced myocardial contractile function and reduced infarct size (32.0% ± 6.0%). Rutin administration also inhibited apoptosis in myocardial tissues in I/R rats by increasing Bcl-2/bax ratio and decreasing active caspase-3 expression. These results suggest that rutin reduced oxidative stress-mediated myocardial damage in vitro and in vivo model, which might be useful in treatment of myocardial infarction | Rutin decreased expression of both cleaved from of caspase-3 (P < 0.01, at 20 μM) and increased Bcl-2/Bax ratio in H9c2 cells. The protective effect of rutin was inhibited by PI3K inhibitor or ERK inhibitor. It increased phosphorylation of ERK and Akt in H9c2 cells. These anti-apoptotic effects of rutin were confirmed both by annexin-V and TUNEL assay | Jeong et al. (2009) |
| Shuangkangsu | Antiviral activity | It inhibited markedly influenza B virus and influenza A3 virus (P < 0.5). IC50 < 0.31 mg/embroy, therapeutic index (TI) > 32. And also could inhibit respiratory syncytial virus (RSV) (P < 0.005), IC50 = 0.9 mg/ml, TI = 6.2 | Li (2008a) |
3.1. Essential oils
As one of the important compositions, essential oils exist in the aerial parts of Lonicera japonica, flower (fresh and dry), leaves and vines. Due to the difference habitat, the harvest time, medicinal parts, extraction methods and the processing, the contents and components of essential oils are different.
3.1.1. The different habitat
From the dry flower of Lonicera japonica in Henan province, 27 compounds were identified, mainly including aromadendrene, linalool and geraniol, and most of them belong to monoterpenes and sesquiterpenes. But from the dry flower of Lonicera japonica in Shandong province, more than 65 compounds were identified, the main compound was hexadecanoic acid, and the others were aldehydes, ketones, acids, esters and so on (Wang, 2010). In 2009, Lin (2009) found that the main compounds of essential oils in Fujian province were hexadecanoic acid, methyl linolenate, methylpalmita, etc. At the same time, Ikeda et al. has studied the volatile components of the concrete from flowers of Lonicera japonica with GC–MS in Japan. 150 compounds, made up of 36 hydrocarbons, 28 alcohols, 21 aldehydes, 12 ketones, 38 esters, 15 miscellaneous were identified. The important components that characterize the volatiles of honeysuckle flowers were identified as linalool, (Z)-jasmone, (Z)-jasmin lactone, methyl jasmonate, and methyl epi-jasmonate. In addition, changes of the volatile components emitted from the living flowers throughout the whole day were investigated by dynamic headspace analysis using GC and GC–MS, and the strongest odor was found to be emitted in the middle of the night (Ikeda et al., 1994). In 2010, Feng (2010) identified the content of chlorogenic acid from Lonicera japonica in different habitats by TLC. Results showed the content were 3.43%, 3.14%, 1.62% and 2.11% in Shanxi, Shandong, Hubei and Henan, respectively.
So the difference habitat would change the proportion of the chemical compounds. And the chemical compositions have strong relationship with the habitat of traditional Chinese medicine.
3.1.2. The different harvest time
Yang and Zhao (2007) have investigated the compositions of essential oils from Lonicera japonica between June and August. The results displayed that 32, 54 and 74 compounds were identified by GC–MS from flowers at June, July and August, respectively. The mainly increased compositions were low molecular number and low-boiling point compounds. But the compounds with high content were similar at each month, which were linalool (13.47%, 13.47%, 7.92%), dibutylphthalate (10.26%, 7.54%, 7.67%) and carvacrol (7.92%, 10.09% and 6.67%). Then, the highest levels of chlorogenic acid were found at the second white and complete white flowering stages. The results indicated that the best time to harvest Lonicera japonica flowers for essential oils was the silver flowering stage, and for chlorogenic acid was the second white or complete white flowering stage (Wang et al., 2009).
3.1.3. The different medicinal parts
Wu et al. (2009) has analyzed the components of essential oils in different parts of Lonicera japonica. 85 compounds were identified with GC–MS method, and only seven of which were mutual in the buds, leaves and stems, the main compounds were benzaidehyde, hexadecanoic acid, diethyl phthalate and hexadecanoyl, respectively. At the same time, the marked difference was showed in the main compositions of the buds, leaves and stems. The content of alkanes in stems was the highest, and in leaves was the lowest; the content of aldehydes in leaves was the highest, and in buds was the lowest; the content of the acids in buds was the highest, and in stems was not found. This difference of the chemical compositions implied the leaves and stems could not be used as the succedaneum of the buds in TCM.
In 2008, essential oils from the buds, silver flower and golden flower of Lonicera japonica have been studied with GC–MS method. 39, 48 and 39 compounds were identified respectively and only 10 of which were mutual. At the process of buds changing to golden flowers, the contents of alkanes, alcohols and ketones increased gradually, but the contents of acids and esters reduced gradually (Wang et al., 2008a, Wang et al., 2008b). At the same time, essential oils from the flowers and stems of Lonicera japonica have been studied. 36 constituents are isolated and identified in all, of which 28 from flowers and 26 from stems. 18 compounds are found simultaneously in both crude drugs and they account for 85.23%, 83.42%, respectively. And palmatic acid and linoleic acid are the highest principles (Li et al., 2002a). So, different medical parts should be selected for different diseases.
3.1.4. The different extraction methods
With the wide action and utilization of the essential oil from Lonicera japonica, different extraction methods have been employed to extract it. In 2009, Du et al. (2009) indicated that with the fresh flowers homogenate method, the main compounds were propylbenzene (12.40%), ethyl benzene (8.58%), benzoic aldehyde (8.04%), translinalool (4.72%) and isophytol (2.94%), but with the steam distillation method, the main components were cyclohexano (8.06%), cyclohexylisooxalic ester (3.45%), methylcyclohexane (12.35%) and n-hexadecanoic acid (12.56%). These suggested that different extraction methods would result in different contents and compositions of essential oils.
3.1.5. The different processing
In the fresh flowers, the content of linalool was more than 14%, and other oils compositions were low-boiling unsaturated terpenes. But in the dry flowers, the content of hexadecanoic acid was more than 26%, and linalool less than 0.4%. Obviously, the fragrant composites have been lost by heating and lighting in the drying process (Ji et al., 1990).
In a word, different habitat, harvest time, medicinal parts, extraction methods, and drying process would result in different chemical compositions and the different quality of Lonicera japonica flowers. From above studies, it can be suggested that the middle of China was the best habitat; the complete white and silver flower period were the preferable harvest time; the best medicinal part was flower, and leaver and stem could be used as supplement for some particular object; low temperature and no-lighting was in favor of the essential oil in the dry and extract processes.
3.2. Organic acids
Organic acids is another important compositions of Lonicera japonica, and it mainly contains chlorogenic acid (1), isochlorogenic acid (2), caffeic acid (3), hexadecanoic acid (4), etc. (Zhang et al., 2000). In 1996, hexadecanoic acid (4) and myristic acid (5) have been isolated from the chloroform extracts of Lonicera japonica (Huang et al., 1996), and tetraacetyl-phthalein chlorogenic acid was obtained from the aqueous extracts of Lonicera japonica (Lou et al., 1996). At the same time, six isomers of isochlorogenic acids have been identified, including 3,5-O-dicaffeoylquinic acid (6), 4,5-O-dicaffeoylquinic acid (7), 3,4-O-dicaffeoylquinic acid (8), 1,3-O-dicaffeoylquinic acid (9), 3-ferulicoylquinic (10) and 4-ferulicoylquinic (11) (Iwanhashi and Negoroy, 1986).
As a major bioactive component of the flowers, chlorogenic acid (1) has been received much attention. Studies showed the chlorogenic acid have stronger bacteriostasis activity to Gram-negative bacteria than Gram-positive bacteria. The minimum inhibitory concentration (MIC) of chlorogenic acid (1) to shigella and salmonella was 0.125 mg/ml, almost the same to 0.1 mg/ml kanamycin (Xu, 2008). And the MIC values against Escherichia coli, Sarcina luteus, Bacillus subtillis and Staphylococcus aureus were 0.025 g/ml, 0.025 g/ml, 0.1 g/ml and 0.8 g/ml (Wu, 2005). Meanwhile, at the doses of 0.05 mg/ml, 0.1 mg/ml, 0.4 mg/ml, 0.8 mg/ml and 0.8 mg/ml, chlorogenic acid (1) presented the significant antiviral activity to respiratory syncytia virus, coxsackie B3 virus, adeno-associated 7 viruses, adeno-associated 3 viruses and Coxsackie B5 virus respectively (Hu et al., 2001). The 0% toxic dose, minimum effective concentration and therapeutic index against to human cytomegalovirus were 100 μg/ml, 1 μg/ml and 100, respectively (Chen et al., 2009).
3.3. Flavones
Up to now, about 30 flavones have been isolated from Lonicera japonica. Gao and Mu (1995) isolated quercetin-3-O-β-d-glucoside (41), luteolin-7-O-α-d-glucoside (42), luteolin-7-O-β-d-galactoside (43), and hyperoside (44) from n-butanol extracts. Corymbosin (57) and 5-hydroxy-3′,4′,7-trimethoxylflavone (58) were isolated from the chloroform extracts (Huang et al., 1996). In 2005, Kumar et al. isolated two new biflavones, 3′-O-methyl loniflavone [5,5″,7,7″-tetrahydroxy 3′-methoxy 4′,4‴-biflavonyl ether] (57) and loniflavone [5,5″,7,7″,3′-pentahydroxy 4′,4‴-biflavonyl ether] (58), from the leaves of Lonicera japonica in India (Kumar et al., 2005). At the same time, colorimetric method was used to compare the contents of flavones from the different habitat. The results showed that the contents were more than 4.31% from Lonicera japonica in Rizhao (Shandong). And the contents were 1.83%, 2.02%, 2.47% and 0.65% in Pinyi (Shandong), Fenqiu (Henan), Xinmi (Henan) and Nanjin (Jiangsu), respectively. Xing et al. (2002) used the ultraviolet spectrophotometry to determine the contents of flavones in different medical parts. The results indicated that the contents in leaves were the highest, and another is flower, stems, and this distribution feature of flavones also was similar to hexadecanoic acid (4). So the leaves and flowers of Lonicera japonica also would be used in TCM to treat various diseases.
Due to the difficulty in the separation and purification of flavones, the pharmacology activities of these compounds had not been studied systematically, except hyperoside and the crude extract. Tang (2008) found that hyperoside (44) combined with β-lactams or quinolones could enhance the antibacterial effects on some common pathogenic bacteria. At the sub-MIC (<0.5 mg/ml), hyperoside (44) could enhance the antibacterial effects of hydrophilic quinolones on bacteria SA26592 (pUT-norA). It also could relieve the cell injury induced by CCl4 in hepatocyte L-02 with the decrease of ALT, AST and MDA, and increase of GSH and cell survival rate. It also showed a significant hepatoprotective effect in CCl4-attatcked rats. The biochemical indexes and liver histopathology examination of rats treated with hyperoside of 30 mg/kg were almost similar to that of normal animals.
3.4. Iridoids
In the last decades, more than 30 iridoids have been found from Lonicera japonica and HPLC with evaporative light-scattering detector or multi-spectrum detection could be used to analyze theses compounds. In 2008, 9 iridoids, loganin (63), sweroside (64), secoxyloganin (67), secologanin (72), kingiside (74), ketologanin (78), 7α-morroniside (79), 7β-morroniside (80) and secologanoside (81), with 12 pyridinium inner salt alkaloids lonijaposides A–L (82–93) were isolated from the flower buds. And in an in vitro assay, lonijaposide C (84) showed inhibitory activity against the release of glucuronidase in rat polymorphonuclear leukocytes (PMNs) induced by the platelet-activating factor (PAF) with an inhibition rate of 69.5% at 10 μM, while compounds lonijaposides A (82) and B (83) give inhibitory activities with 11.0% and 35.8% inhibition rates at the same concentration, respectively. This suggested that the ethanol-2-yl unit at N-1 and the acid form of C-11 may increase the activity (Song, 2008, Song et al., 2008). Then, four new iridoid glycosides, named l-phenylalaninosecologanin (94), 7-O-(4-β-d-glucopyranosyloxy-3-methoxy-benzoyl) secologanolic acid (95), 6′-O-(7α-hydroxyswerosyloxy) loganin (96) and (Z)-aldsecologanin (97), also were isolated, together with a known one, newly named (E)-aldosecologanin (98), from the stems and leaves (Machida et al., 2002). From the dry flower buds of Lonicera japonica, 10 known iridoids and loniceracetalides A, B (99, 100) (secoiridoid glycosides) have been identified (Kakuda et al., 2000).
Using pharmacophore-assisted docking, Ehrman et al. (2010) screened for compounds which may be active against four targets involving in inflammation. The results showed that iridoids, as the active composition, presented the marked anti-inflammation against COX, p38 and JNK. At the same time, the pharmacology researches suggested that iridoids also have good anti-tumor, anti-inflammation, antioxidant activity and hepatoprotective effects (Shang, 2010).
3.5. Saponins
Most of saponins from Lonicera japonica belong to the oleanane type and hederagenin type. In 1988, Kawai et al. (1988) firstly studied the saponins of the aerial parts of Lonicera japonica. 15 chemical compounds were found, and four compound were named, which were 3-O-α-l-arabinopyranosyl-28-O-[β-d-glucopyranosyl(1 → 6)-β-d-glucopyranosyl] oleanolic acid (101), 3-O-[α-l-rhamnopyranosyl(1 → 2)-α-l-arabinopyranosyl]-28-O-β-d-glucopyranosyl hederagenin (102), 3-O-[α-l-rahmnopyranosyl(1 → 2)-α-l-arabinopyranosyl]-28-O-[β-d-glucopyranosyl(1 → 6)-β-d-glucopyranosyl] oleanolic acid (103), 3-O-[α-l-rhamnopyranosyl(1 → 2)-α-l-arabinopyranosyl]-28-O-[6-acetyl-β-d-glucopyranosyl(1 → 6)-β-d-glucopyranosyl] hederagenin (104). At the same time, the pharmacology test proved that monodesmosides showed strong hemolytic activity, but bisdesmosides showed weak hemolytic activity. In 1996, Lou et al. (1996) has isolated three triterpenes compounds from the flower buds of Lonicera japonica. 3-O-α-l-rhamnopyranosyl-(1 → 2)-α-l-arabinopyranosy hederagenin 28-O-β-d-xylpyranosyl(1 → 6)-β-d-glucopyranosyl ester (105), 3-O-α-l-arabinopyranosy hederagenin 28-O-α-d-rhamnopyranosyl(1 → 2)[β-d-xylpyranosyl(1 → 6)-β-d-glucopyranosyl ester (106), 3-O-α-l-rhamnopyranosyl-(1 → 2)-α-l-arabinopyranosy hederagenin 28-O-α-d-rhamnopyranosyl(1 → 2)[β-d-xylpyranosyl(1 → 6)-β-d-glucopyranosyl ester (107). Then in 2000, 3-O-β-d-glucopyranosyl-(1 → 4)-β-l-glucopyranosyl(1 → 3)-α-l-rhamnopyranosyl(1 → 2)-α-l-arabinopyranosy hederagenin 28-O-β-d-glucopyranosyl(1 → 6)-β-d-glucopyranosyl ester (108), hederagenin-3-O-α-l-rhamnopyranosyl(1 → 2)-α-l-arabinopyranoside (109), 3-O-β-d-glucopyranosyl-(1 → 3)-α-l-rhamnopyranosyl(1 → 2)-α-l-arabinopyranosyl hederagenin 28-O-β-d-glucopyranosyl-(1 → 6)-β-d-glucopyranosyl ester (110), 3-O-α-l-rhamnopyranosyl-(1 → 2)-α-l-arabinopyranosy hederagenin 28-O-β-d-glucopyranosyl(1 → 6)-β-D-glucopyranosyl ester (107) have been identified (Chen et al., 2000).
Then in 2003, a new triterpenoid saponin, loniceroside C (114) was isolated from the aerial parts, which showed in vivo anti-inflammatory activity at the doses of 50, 100, 200 mg/kg (p.o.) against mouse ear edema provoked by croton oil with 15%, 31% and 28.7% inhibition rates, respectively. The positive drug aspirin (100 mg/kg, p.o.) was 15% inhibition (Kwak et al., 2003). And in 2008, two new triterpeniod saponins, loniceroside D (115) and loniceroside E (116), were isolated from the dry flowers and buds of Lonicera japonica (Lin et al., 2008).
3.6. Other compounds
From Lonicera japonica, 15 trace elements were found, such as Fe, Mn, Cu, Zn, Ti, Sr, Mo, Ba, Ni, Cr, Pb, V, Co, Li, Ca (Wu, 1988). In 2006, Kumar et al. identified six novel chemical compounds, lonijaposides A1–A4 (129–132), and lonijaposides B1–B2 (129–130) (Kumar et al., 2006). And the name of lonijaposide A1 was similar with lonijaposide A, but the chemical structures of them were different (Fig. 2). In 2008, Li isolated a new compound—shuangkangsu (141) with the marked anti-viral activity against influenza B virus (P < 0.5), influenza A3 virus (P < 0.5), and respiratory syncytial virus (RSV) (P < 0.005), respectively (Li, 2008a). (+)-N-(3-methybutyryl-β-d-glucopyranoyl)-nicotinate (142), (+)-N-(3-methybut-2-enoyl-β-d-glucopyranosyl)-nicotinate (143) and three nucleosides also have been found from flower buds of Lonicera japonica (Song, 2008).
4. Effects of crude extract
4.1. Anti-inflammatory activity
Recently, more and more experiments, in vivo and in vitro, showed different extracts of Lonicera japonica can inhibit various inflammatory reactions, and suppress various inflammatory factors.
In 1998, Lee et al. (1998) evaluated the anti-inflammatory activity of the n-butanol (BuOH, 4.2% based on the dry weight) fraction of Lonicera japonica. At oral doses of 400 mg/kg, it showed significant anti-inflammatory activities against AA ear edema, croton-oil ear edema, CGN-paw edema, rat cotton pellet granulomatic and AIA-inflammation models in mice and rats, the inhibitions were 27%, 23%, 26%, 18% and 42%, respectively. And the inhibition rate of the positive drug, aspirin (100 mg/kg) were 27%, 13%, 13%, 0% and 58%.
The anti-inflammatory properties of aqueous extracts from Lonicera japonica flower were evaluated in A549 cells. This extract directly inhibited both COX-1 and COX-2 activity, the expression of IL-1β-induced COX-2 protein and mRNA. But higher dose of the extract was required to suppress the expression of mRNA than protein. This result indicated that the extract acted translationally or post-translationally at lower doses and transcriptionally or post-transcriptionally at higher doses (Xu et al., 2007). Kang et al. examined the effect of water fraction of Lonicera japonica on trypsin-induced mast cell (HMC-1) activation. After stimulated with trypsin (100 μM), Lonicera japonica could inhibit TNF-α secretion, tryptase mRNA expression and trypsin-induced ERK phosphorylation at a dose-dependent manner. However, it did not affect the trypsin activity even with 1000 μg/ml. These all indicated that Lonicera japonica might inhibit trypsin-induced mast cell activation through the inhibition of ERK phosphorylation than the inhibition of trypsin activity (Kang et al., 2004). On the other hand, the anti-inflammatory effects of water extract in proteinase-activated receptor 2 (PAR2)-mediated mouse paw edema has been investigated. The results indicated that at doses of 50, 100 and 200 mg/kg o.p., it showed significant inhibition of both change in paw thickness and vascular permeability induced by PAR2, the inhibition rates were 41.8%, 69.1%, 70.9%, and 40.2%, 69.7%, 68.8%, respectively. The water extracts (100 mg/kg) also significantly inhibited PAR2 agonists-induced myeloperoxidase (MPO) activity and TNF-α expression in paw tissue. (Tae et al., 2003)
Su et al. (2006) used the supercritical CO2 extraction process, the temperature 35 °C, the pressure 12 MPa, the CO2 flowing 4.0 kg/h and time 1.5 h, to obtain 1.08% volatile oil from Flos lonicerae. The pharmacological studies suggested the potent anti-inflammatory effect of the volatile oil on the ear swelling model in mice.
All of these reports supported the traditional use of Lonicera japonica, and suggested it be a safe and mild anti-inflammatory agent for treating various inflammatory disorders.
4.2. Antiviral activity
Since 1980s, the antiviral activity of Lonicera japonica has been studied and proved, such as anti-RSV, anti-HIV, anti-HSV, anti-PRV and anti-NDV. At the same time, as important traditional medicines, Lonicera japonica had been used to treat some viral diseases in China. These pharmacology activities were supported to the traditional uses and drug's nature of Lonicera japonica in TCM.
Firstly, Ma et al. (2002) selected forty-four medicinal herbs, which are used for the treatment of respiratory tract infectious diseases in China, and tested the antiviral activities against respiratory syncytial virus (RSV) by means of the cytopathologic effect (CPE) assay. Lonicera japonica showed potent antiviral activities against RSV, the 50% inhibition concentration (IC50) was 50.0 μg/ml, and selectivity index (SI) was more than 20.0. Chang et al. (2003) applied a syncytia formation inhibition assay to study the anti-HIV agents of 80 MeOH extracts of Korean plants. This test based on the interaction between the HIV-1 envelope glycoprotein gp120/41 and the cellular membrane protein CD4 of T lymphocytes. Flower of Lonicera japonica showed an inhibition of 5.8 ± 1.7% at a concentration 100 μg/ml. And in 2008, Xi (2008) suggested that Lonicera japonica extraction showed an obvious therapeutic action on the influenza A virus infected Pneumonia mouse. The lung indexes of Lonicera japonica group and the ribovirin group were lower than the model group with the significance difference (P < 0.01), but no significance difference (P > 0.05) between these two groups. Lonicera japonica could reduce the histopathological changes, the viral duplication, and the contents of influenza virus nucleic acid (P < 0.01), compared to the model group). At the same time, the TNF-α, IL-1β expressions of Lonicera japonica group and the ribovirin group are lower than the model group with significant difference (P < 0.01).
Then in 2009, Chen et al. (2009) proved that Lonicera japonica extracts and chlorogenic acid had the significant anti-cytomegalovirus activity, and the 0% toxic dose, minimum effective concentration (MEC) and therapeutic index (TI) of these two composites for human cytomegalovirus were 3000 μg/ml, 3000 μg/ml, 1, and 100 μg/ml, 1 μg/ml, 100, respectively. In vitro tests, Lonicera japonica showed 104 and 72 times of TI for anti-HSV (Herpes simplex virus)-1F and anti-HSV-1HS-1 to acyclovir (ACV). But in vivo tests, the anti-viral activity of Lonicera japonica was closed to ACV (Wang, 1999). As to the caviid beta herpesvirus 1, Lonicera japonica showed significant inhibition of the duplication of guinea pig cytomegalovirus in cell level. TI and the inhibitory duplication index (100 and 2.61) (Wang et al., 2005). The anti-virus (H9N2) activity and anti-AIV (LED = 3.90 mg/ml, in vitro) of the flavones from flower buds of Lonicera japonica were also found (Li et al., 2001, Wang et al., 2006). At the same time, as the main composition, Lonicera japonica was used widely in TCM prescriptions, which were published by State Administration of TCM of China, to prevent and control SARS coronavirus in 2003 (http://www.satcm.gov.cn). And from the statistics of Beijing Youan hospital of capital medical university, the practical frequency of Lonicera japonica to treat influenza A virus subtype H1N1 was second in 113 species between May and November, 2009 (Zheng, 2010).
In Vero cells, three different extracts of Lonicera japonica (Hunan province), including volatile oil (P1), chlorogenic acids extracts (P2) and flavones extract (P3), were tested for the antiviral activity to the Pseudo rabies virus (PRV) and Newcastle disease virus (NDV). At the dose of 232.7 μg/ml, 116.35 μg/ml, 58.18 μg/ml, 29.09 μg/ml, the interdiction rates of P1 for PRV and NDV were 40.13%, 17.83%, 13.16%, 2.24%, and 75.40%, 32.01%, 12.05%, 2.34%, in CPE respectively, and the LED of P1 (Least Effective Dose) were 232.7 μg/ml and 232.7 μg/ml. The interdiction rates of P2 (3.125 mg/ml, 1.563 mg/kg, 0.781 mg/ml and 0.391 mg/ml) for PRV and NDV were 63.74%, 46.27%, 13.10%, 3.51%, and 65.23%, 36.71%, 32.61%, 28.96% in CPE respectively. The interdiction rates of P3 (1.954 mg/ml, 0.977 mg/kg, 0.489 mg/ml and 0.244 mg/ml) for PRV and NDV were 94.00%, 78.42%, 42.30%, 3.36%, and 78.07%, 27.63%, 16.37%, 6.73%, respectively. LED against PRV and NVD of P2 and P3 were 0.997 mg/ml, 3.097 mg/ml (P < 0.05), and 0.781 mg/ml, 1.563 mg/ml. These studies suggested that the extracts decrease CPE lesions and neutralize virus in dosages dependent, behave in inhibiting virus directly and promoting cell antivirus (Wang, 2008c).
As another kind of antiviral agents, several tannins of Lonicera japonica were investigated. 3,5-di-O-caffeoylquinic acid (6) and methyl 3,5-di-O-caffeoylquinic acid (30) had a strong inhibitory effect on HIV-1 RT and HDNAP-α. The ratio of IC50 of these two compounds for HIV-1 RT and HDNAP-α was 2.0 and 2.2. While 3,4-di-O-caffeoylquinic acid (8) and methyl 3,4-di-O-caffeoylquinic acid (31) exhibited higher inhibitory effects on HDNAP-α than HIV-1 RT (Chang et al., 1995). Meanwhile other 13 caffeoylquinic acids, caffeic acid (3) and caffeic acid methyl ester (32) isolated from Lonicera japonica also presented antiviral activities against respiratory viruses (Ma et al., 2005).
4.3. Antibacterial activity
Antibacterial activity, as another important property of Lonicera japonica, has been comprehensively studied. In 2009, Rahman et al. evaluated the antibacterial potential of essential oil from flowers and ethanol extracts from leaf. A remarkable antibacterial effect of the oil and extracts has been revealed against Listederia monocytogenes ATCC 19116, Bacillus subtilis ATCC 6633, Bacillus cereus SCK 111, Staphylococcus aureus (ATCC 6538 and KCTC 1916), and Salmonella enteritidis KCTC 12021, Salmanella typhimurium KCTC 2515, Enterobacter aerogenes KCTC 2190 and Escherichia coli ATCC 8739. The diameters of inhibitions zone were 20.3, 17.8, 15.2, 16.3, 14.1, 15.3, 14.0, 12.4, 12.1, and 16.2, 15.4, 14.0, 15.0, 14.1, 14.1, 14.2, 12.2, 10.3 mm, respectively. MIC values were 62.5, 62.5, 250, 125, 250, 125, 250, 500, 500, and 125, 125, 250, 125, 125, 250, 250, 500, 500 μg/ml. These findings suggested that the oil and extracts from Lonicera japonica may be a potential source of preservatives for the food or pharmaceutical industries (Rahman and Kang, 2009). And the antibacterial activities, against Bacillus cereus and Staphylococcus aureus, of the floral bud from Lonicera japonica were found by agar-well diffusion method in vitro. The diameters of inhibition zone were 6.3 and 7.2 mm, and this activity may be closely associated with the existence of phenolic constituents (Shan et al., 2007). Meanwhile Lonicera japonica also has showed the marked antibacterial activity against fourteen strains, including Staphylococcus aureus, Streptococcus hemolyticus, Escherichia coli, Bacillus dysenteriae, Bacillus comma, Bacillus typhosus, Bacillus paratyphosus, Pseudomonas aeruginosa, Klebsiella pneumoniae, Bacillus tuberculosis, Streptococcus mutans, Bacillus adhaerens, Bacteroides melanogenicus and Haemophilus actinomycetemcomitans, and so on. And the antibacterial study against different serotypes of Streptococcus mutans demonstrated that the extracts of Lonicera japonica could inhibit 87.5% strains with MIC 25 mg/ml (Sun, 2002, Song et al., 2003).
Then, the antimicrobial activity of water and alcohol extract from Lonicera japonica was investigated. MIC and MBC of water extract on Staphyloccocu saureus were 19.25% and 38.50%; MIC and MBC of alcohol extract on Salmonella and Staphyloccocu aureus were 9.80%, 19.60%, and 19.60%, 39.20%, respectively (Kang et al., 2010). Meanwhile the water decoctum from Lonicera japonica showed the reasonable eliminating effect on R plasmid from Pseudomonas aeruginosa in vivo. The elimination rate was 8% (Wang et al., 2000).
Finally, Tang et al. indicated that flavonoids from Lonicera japonica also have a strong antibacterial action, especially for Methicillin Resistant Staphylococcus aureus (MIC ≤ 5 mg/ml) (Tang, 2008).
4.4. Antioxidant activity
Chio et al. has evaluated the antioxidant effects of Lonicera japonica flowers in 2007. The EtOAc fraction exhibited marked scavenging/inhibitory activities, as follows: IC50 values of 4.37, 27.58 ± 0.71, 0.47 ± 0.05, and 12.13 ± 0.79 μg/ml in the 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical, total reactive oxygen species (ROS), hydroxyl radical (−OH), and peroxynitrite (ONOO−) assays, respectively. And as the main compounds of the EtOAc fraction, luteolin, caffeic acid, protocatechuic acid, isorhamnetin-3-O-β-d-glucopyranoside, quercetin 3-O-β-d-glucopyranoside, and luteolin 7-O-β-d-glucopyranoside, also evidenced marked scavenging activities, with IC50 values of 2.08–11.76 μM for DPPH radicals, and 1.47–6.98 μM for ONOO− (Choi et al., 2007). And the results of other antioxidant tests indicated that the Trolox equivalent antioxidant capacity (TEAC) values and total phenolic content for methanolic extracts of the flora bud from Lonicera japonica were 589.1 μmol Trolox equivalent/100 g dry weight (DW), and 3.63 gallic acid equivalent/100 g DW. These studies suggested that Lonicera japonica might be potential natural antioxidants and beneficial chemopreventive agent (Cai et al., 2004).
Then, antioxidant activity of polysaccharides with different molecule weights separated from Flos lonicerae by ultra-filtration was also studied. The reducing power of the polysaccharides has a direct correlation between antioxidant activity and concentration of certain plant extracts, and ultra-filtration fraction has a significant inhibit effect on superoxide radicals generated in a PMS/NADA/NBT system. Administered to rats, crude polysaccharides extracts (50–400 mg/kg) could reduce lipid peroxidate malony dialdehyde (MDA) content, improve glutathione peroxidase (GSH-Px) and catalase (CAT) activity, and enhance significantly superoxide dismutase (SOD) activity in serum and tissue (Li, 2008b).
Lan et al. (2007) used the biochemical method to investigate the activity and scavenging capability on free radicals of the membrane-protecting enzyme, which was extracted from flower bud, leaf and wattle of Lonicera japonica in different harvest time. The activity of the enzyme was increased since April and reached the highest level in June. The activity of the enzyme extracted from leaf was higher than those from flower bud and wattle, and the scavenging capacity of the extract from the flower bud on OH and H2O2 free radicals was stronger than those from leaf and wattle. Meanwhile the scavenging capacities on free radicals of five medicinal plants of Lonicerae from several provinces of China have been investigated. All the samples showed the scavenging capacities on three kinds of free radicals (O2 −, −OH, H2O2) at some extent, and the samples from Henan and Shandong showed stronger scavenging capacities than those from Jiangsu province. The descending order of scavenging capacities from different species is Lonicera macranthoides, Lonicera japonica, Lonicera similis, Lonicera fulvotomentosa and Lonicera hypoglauca (Li et al., 2002b).
4.5. Hepatoprotective
In the dimethylnitrosamine (35 mg/kg o.p. 7 days) induced liver fibrosis rats, 75% ethanol extract of Lonicera japonica showed significantly hepatoprotective effect by pathological analysis. 19 compounds, such as 8-phenyl-8-azbicyclo[4,3,0]non-3-ene-7,9-dione, 3-[1,3]dioxolan-2-yl-4-methoxy-6-nitro-benzo[d]isoxazole, (E)-2-(5,5,5-Yip richloro-3-penten-1-yl)-1,3-dioxolane, 3,3-diphenyl-cyclopropene, 2-(methoxy-imino)-hexanedioic acid, gamma-lactone-2-methoximinegluconic acid, 2-(6-heptynyl)-1,3-dioxolane, and bis(o-methyloxime)-4-ketoglucose, have been isolated and identified by GC–MS method from 75% ethanol extract (Sun et al., 2010). But, Hu et al. suggested that the total flavones of Lonicera japonica have significantly protective effect on immunological liver injury in mice. Administered ig*10d, the total flavones (100, 200, 400 mg/kg) could significantly decrease the raised liver and spleen indexes, and improve the aggravated liver histopathologic changes. In addition, this extract could reduce the high levels of NO and iNOS in liver homogenate and inhibit the expression of TNF-α in liver tissue. This mechanism possibly was due to diminishing the inflammation mediators (Hu et al., 2008).
4.6. Anti-tumor activity
The mechanisms of apoptosis induced by photodynamic therapy (PDT) in lung CH27 carcinoma cells, cultured with alcohol extract from Lonicera japonica as photosensitizer, have been explored. This extract exhibited significant photocytotoxicity in CH27 cells at a concentration range of 50–150 μg/ml, with 0.4–1.2 J/cm2 light dose. The apoptosis induced by PDT combined with Lonicera japonica extract was accompanied by DNA condensation, externalization of phosphatidylserine and formation of apoptotic bodies, it showed to be caspase-3-independent apoptosis via activation of AIF. P38-associated pathway may be involved in apoptosis induced by PDT with Lonicera japonica in CH27 cells. These demonstrated that they induced CH27 cells apoptosis was probably related to its ability to change the protein expression and distribution of heat shock protein 27 (Leung et al., 2008). When the aqueous extract of Lonicera japonica (100 μg/ml) was applied to the HepG2 cells, the lysates of the treated cells were associated with increased stimulatory phosphorylation of JNK and p38 as compared to the basal value, similarly to the MAPK activation profile of PCA. Further experiments showed that this aqueous extract also decreased the viability of HepG2 cells to 50%. So Yip thought that the aqueous extract of Lonicera japonica could trigger HepG2 cell death in a JNK-dependent manner (Yip et al., 2006).
4.7. Insecticidal and acaricidal activities
95% methanol extracts from leaves and twigs of Lonicera japonica were tested at 10,000 ppm for evaluating their insecticidal and acaricidal activities against Tetranychus urticae Koch, Aphis gossypii Glover, Myzus persicae Sulzer, Trialeurodes vaporariorum (Westwood), and Panonychus citri (McGregor). 24 and 48 h after application, the mortality (%) of adult Tetranychus urticae were 31.6 and 37.0, and the control group was 3.3 and 3.3. After 24, 48 and 72 h application, the number of laid eggs against the reproduction and repellent indexes were 2.2, 2.7, 4.0 and 91.1, 80.0, 55.6 gh, and the control group was 6.1, 6.4, and 11.7. At the same time, survival rates of Panonychus citri after 5 and 10 days application of the extracts were 41.7 and 31.3 (Kim et al., 2005).
Then, the repellent activities against Aedes albopitus of methanol extracts from different parts of Lonicera japonica have been studied on mouse skin. The results showed that the mortalities of n-hexane, ethyl acetate, n-butanol and water fractions of methanol extracts were 97%, 80%, 97%, 97% (stem), 97%, 80%, 0%, 0% (leaf) and 77%, 90%, 50%, 80% (flower) (Yoon and Kyung, 2002). These results suggested that Lonicera japonica had the marked insecticidal and acaricidal activities.
4.8. Anti-pregnant activity
The good anti-pregnant effect of Lonicera japonica extract has been found in mice, dogs and monkeys. The extract has marked inhibitory effect on the deciduoma of pseudopregnant in mice, with a significant decrease on the level of plasma progesterone in pregnant rats after administration for 24 h. These results implied that the interruption effect of extract was likely concerned with the decrease on the level of plasma progesterone and/or prostaglandin-like action (Cao et al., 1986a, Cao et al., 1986b).
4.9. Antihyperlipidemic and antithrombotic activities
In 1998, Pan et al. has found that Lonicera japonica could inhibit the increase of blood sugar and the content of high density lipoprotein cholesterin (HDL-C) in blood, reduce the level of serum cholesterol and accumulation index atherosclerosis in mice-induced alloxan (ALX) (Pan et al., 1998). Meanwhile Fan et al. observed the antithrombotic effects of the organic acid compounds in Lonicera japonica on the oxidative injury of HUVEC (Human Umbilical Vein Endothelial Cells). The IC50 of isomeric compounds of chlorogenic acids (2), caffeic acid, and isochlorogenic acid (3) were 0.0286 mg/ml, 1.707 mg/ml, 2.411 mg/ml, 0.026 mg/ml, 0.328 mg/ml, and 0.539 mg/ml respectively. The protect effect on the oxidative injury of HUVEC by H2O2 indicated a dosage depended manner. Caffeic acid and isochlorogenic acid have evident resistance to the oxidative injury, and chlorogenic acid has the preventive protect effect (Fan et al., 2007).
4.10. Anti-lipase activity
By screening for 75 medicinal plants on new pancreatic lipase (triacylglycerol lipase, EC 3.1.1.3), Sharma et al. suggested that 80% methanolic extract of whole plant from Lonicera japonica (0.2 mg/ml) present the marked anti-lipase activity, and the inhibition was 40.9% (Sharma et al., 2005).
5. Application on veterinary and agriculture
5.1. Application on veterinary
As a new medical feed additive, the effect of two Chinese medicinal herbs (Astragalus membranaceus and Lonicera japonica) and boron on non-specific immune response of Nile tilapia (Oreochromis niloticus) was investigated. After four weeks of feeding (contained 0.1% Astragalus, 0.1% Lonicera and a mixture of the two herbs with 0.05% boron), fish were infected with Aeromonas hydrophila and mortalities were recorded. Feeding tilapia with two herbs alone or in combination significantly enhanced phagocytic and respiratory burst activity of blood phagocytic cells, reduced the mortality following Aeromonas hydrophila infection. The lowest mortality was observed in the group fed with the combination of both herbs and boron. So both extracts and boron supplementation added to fish feed can act as immunostimulants and enhance the immune response and disease resistance of cultured fish (Ardó et al., 2008).
The fish (mean body weight of ca. 110 g) were fed diets with a 0.1% supplement of Astragalus radix, Lonicera japonica or a mixture of these herbs for 8 weeks. Statistically significant intergroup differences were noted in the value of the hepatosomatic index, hepatocyte size, nucleus and nucleus/cytoplasm diameter ratio, and the appearance of the hepatic parenchyma and the protein content of the whole fish body. The analysis of the proximal composition of the fish viscera indicated significant differences in the fat content (P < 0.05). Among the analyzed group of fatty acids (saturated – SFA, monoenoic – MUFA, polyenoic – PUFA) contained in the whole fish, the fillets and the viscera, significant intergroup differences were noted with regard to SFA (viscera) and MUFA (whole fish) (P < 0.05). The total PUFA content was stable, although significant intergroup differences were noted with regard to a few of the acids that belong to this group (P < 0.05) (Zdzisław et al., 2008).
At the same time, the volatile oil extract and flavones extract promoted proliferation of chicken splenic lymphocytes significantly by MTT method (P < 0.05), which were induced by ConA or LPS, and the effects were related to concentration of the extracts (Wang, 2008c).
5.2. Cadmium-hyperaccumulator
Phytoremediation using hyperaccumulators is a promising technique of removing soil pollutants. The growth responses, cadmium (Cd) accumulation capability and physiological mechanisms of Lonicera japonica under Cd stress were investigated. Exposed to 5 and 10 mg/L Cd, the plants did not show any visual symptoms. Furthermore, the height, dry biomass of leaves, roots and total and the chlorophyll content were obtained different grade increase. When the concentration of Cd was up to 50 mg/L, the height, dry biomass of leaves and roots had not significant differences compared with the control. The indexes of tolerance were all above 0.8. The maintenance of high superoxide dismutase and catalase activities was observed along with the increased Cd concentration, suggesting strong internal detoxification mechanisms inside plant cells. After 21 days exposure to 25 mg/L Cd, stem and shoot Cd concentrations reached 344.49 ± 0.71 μg/g and 286.12 ± 9.38 μg/g DW, respectively and the plant had higher bioaccumulation coefficient and translocation factor. According to these results, Liu et al. suggested that Lonicera japonica has strong tolerance and accumulation capability to Cd, and it is a potential Cd-hyperaccumulator (Liu et al., 2009).
6. Preparations, the qualitative and quantitative analysis
In 2010, more than 12 preparations, in which Lonicera japonica was the main and active compositions, were listed in Chinese Pharmacopoeia and used to clear away the heat-evil and expel superficial evils, and cure fever, cough and pharyngalgia and the swell of throat, constipation, conjunctival congestion, etc., such as Shuang Huang Lian Ke Li, Xiao Yin Pian, Ying Huang Kou Fu Ye (Table 1).
In 2005, luteolin was added in Chinese Pharmacopoeia with chlorogenic acid to control the quality of medical materials by TLC and HPLC methods. The content of luteolin and chlorogenic acid in the Flos Lonicera should be more than 0.1% and 1.5%. An efficient microwave-assisted extraction (MAE) technique has been developed to extract chlorogenic acid from flower buds of Lonicera japonica. The yield of chlorogenic acid rapidly reached 6.14% within 5 min under the optimal MAE conditions, i.e. 50% ethanol as extraction solvent, 1:10 (w/v) of the solid/liquid ratio and 60 °C of extraction temperature (Zhang et al., 2008). Today, with the development of modern separation and identify techniques, it is widely accepted that the quality cannot be measured by mono-content. As the main and effective components of Lonicera japonica, essential oils and flavones were paid attention to qualitative and quantitative analysis Lonicera japonica. In 2008, Wang et al. (2008d) used normal HPLC–MS to separate and analyze the essential oils. 192 compounds were identified, and this technology provided the better method to analyze the chemical compounds of Lonicera japonica and should be beneficial for the study of the contents and active compounds.
Otherwise, a fast liquid chromatography method with diode-array detection (DAD) and time-of-flight mass spectrometry (TOF-MS) has been developed for analysis of constituents in Flos Lonicera. By accurate mass measurements within 4 ppm error for each molecular ion and subsequent fragment ions, as well as the ‘full mass spectral’ information of TOF-MS, a total of 41 compounds including 13 iridoid glycosides, 11 phenolic acids, 7 saponins, and 10 flavonoids were identified in a methanolic extract (Qi et al., 2009). In order to differentiate the sources and comprehensively control the quality of this medicinal plant, Chen et al. (2007) thought coupled with principal component analysis would be a well acceptable strategy. At the same time, capillary electrophoresis with electrochemical detection (CE-ED) also was employed to analyze active ingredients of Lonicera japonica. Operating in a wall-jet configuration, a 300 mm diameter carbon-disk electrode was used as the working electrode, which exhibits a good response at +0.90 V (vs. saturated calomel electrode) for four analytes. Under the optimum conditions, the analytes were baseline separated within 20 min in a 50 mmol/L borax buffer (pH 8.7). Notably, excellent linearity was obtained over two orders of magnitude with detection limits (S/N = 3) ranging from 0.1 to 0.5 mg/L for all the analytes (Peng et al., 2005).
Chemical fingerprint analysis has been introduced and accepted by WHO (1991), SFDAC (2000) and other authorities as a strategy for quality assessment of herbal medicines. It has been recognized as a rapid and reliable means for the identification and qualification of herbal medicines. Li et al. (2006) has employed the binary chromatographic profiling in fingerprint analysis of Lonicera japonica, Correlation analysis showed that six chromatographic peaks in ethanol extract were positively correlated with in vitro bacteriostasis activity. Two standard fingerprints were developed with 10 genuine samples of Lonicera japonica. Similarity analysis with a limited number of samples showed a fair consistence in the chromatographic profiling of Lonicera japonica from various sources and two harvests, and significant differences from other species. Combination use of the two fingerprints demonstrated confirmative identification and quality assessment of Lonicera japonica.
7. Acute and subacute toxicity
Thanabhorn et al. (2006) has evaluated the acute and subacute toxicity of the ethanol extract from the leaves of Lonicera japonica. The single oral dose of the ethanol extract at 5000 mg/kg did not produce mortality or significant changes in the general behavior and gross appearance of the internal organs of rats. In subacute toxicity study, the ethanol extract was administered orally at a dose of 1000 mg/kg/day for a period of 14 days. The satellite group was treated with the ethanol extract at the same dose and the same period and kept for another 14 days after treatment. There were no significant differences in the body and organ weights between the control and the treated group of both sexes. Hematological analysis and clinical blood chemistry revealed no toxicity effects of the extract. Pathologically, neither gross abnormalities nor histopathological changes were observed. Then, Chang et al. (2003) assessed the toxicological assessment on food safety of the flower bud of Lonicera japonica. Acute toxicity test showed LD50 > 15 g/kg Bw. on mice orally. And micronucleus test of bone marrow cell and Salmanella typhimurium/mammals microsomal enzyme test showed it was safe without mutagenesis. Meanwhile no toxicity test on mice and in antifertility test on SD female mouse. In summary, the extract was found to be fairly nontoxic when oral acute and subacute toxicities in rats were performed. Chronic toxicity study is needed for further support the safe use of this plant.
At the same time, Zhang (2007) used MTT method to study the toxicity of Lonicera japonica against THP-1, MT-2 cell in vitro. The result showed that compare to control group, 50 mg/ml could marked induce the cell death (P < 0.05).
8. Conclusion
As the above said, Lonicera japonica was used and planted in China and East Asia. In 2003, Lonicera japonica was used as the most popular TCM to treat SARS coronavirus in China. But in Argentina, Australia, Brazil, Mexico, New Zealand and much of the United States, Lonicera japonica was thought as a major nuisance, and restricted in parts of North America and New Zealand (Starr et al., 2003). So, there are more potential utilization and development of Lonicera japonica out of Asia, especially out of China.
Obviously, the chemical components and pharmacology activities of Lonicera japonica have been studied, and many active compounds were isolated and identified. Of these, due to the good antibacterial, anti-inflammatory and anti-tumor activities, chlorogenic acid and luteolin were used as the indicator compound to characterize the quality of Lonicera japonica and the preparations in Chinese Pharmacopoeia. But recently, chlorogenic acid and luteolin were found in other medicinal plants, and even the contents were higher than Lonicera japonica (Committee for the Pharmacopoeia of PR China, 2010). So employing chlorogenic acid and luteolin to control the quality of Lonicera japonica lacked specificity. At the same time, Wu suggested that the antibacterial of the flavones of fresh flower buds were 4 times of chlorogenic acid, and iridoids were also presented the marked biology activities (Wu et al., 2001). The different habitat, harvest's time, medicinal parts, extraction methods, fresh flowers and dry flowers would result in the different chemical compositions and the quality of Lonicera japonica. So, how to exclusively and accurately control the quality of Lonicera japonica in TCM and preparations should be studied further.
In Chinese Pharmacopoeia (Committee for the Pharmacopoeia of PR China, 2010), only the flowers and flower buds have been officially used as the active parts to treat diseases. But the results of phytochemical researches indicated that some active compositions and compounds also existed in the leaves, vines and stems, such as essential oils, flavones and iridoids. So the intrinsically active compositions and the mechanisms of action of Lonicera japonica were also ambiguous.
In Chinese clinical practice, the traditional medicines with clearing away the heat-evil and expelling superficial evils effects are usually used to treat various infectious diseases. And the pharmacological studies proved that these medicines also have the anti-inflammatory, antiviral, antibacterial commonly. From the development of Lonicera japonica, the relationship between TCM and modern pharmacy also has been embodied.
In a word, phytochemical and pharmacological studies of Lonicera japonica have received much interest, more and more extracts and active compounds have been isolated and proved which has the anti-inflammatory, antiviral, antibacterial, antioxidant and enhance the immune response effects, etc. But until now, poor quality control, and fail development of Lonicera japonica always existed in. Further research especially on in vitro/in vivo antibacterial and antiviral effects should have priority.
Acknowledgements
This work was financed by The Special Fund of Chinese Central Government for Basic Scientific Research Operations in Commonweal Research Institutes (no. 1610322011009). The authors would also like to express their gratitude to Lanzhou University PhD English writing foreign teacher Allan Grey who thoroughly corrected the English in the paper.
Contributor Information
Xiaofei Shang, Email: shangxf928@163.com.
Maoxing Li, Email: lm730530xing@yahoo.com.cn.
References
- Ardó L., Yin G.J., Xu P., Váradi L., Szigeti G., Jeney Z., Jeney G. Chinese herbs (Astragalus membranaceus and Lonicera japonica) and boron enhance the non-specific immune response of Nile tilapia (Oreochromis niloticus) and resistance against Aeromonas hydrophila. Aquaculture. 2008;275:26–33. [Google Scholar]
- Bassoli B.K., Cassolla P., Borba-Murad G.R., Constantin J., Salgueiro-Pagadigorria C.L., Bazotte R.B., da Silva R.S.d.S.F., de Souza H.M. Chlorogenic acid reduces the plasma glucose peak in the oral glucose tolerance test: effects on hepatic glucose release and glycaemia. Cell Biochemistry and Function. 2008;26:320–328. doi: 10.1002/cbf.1444. [DOI] [PubMed] [Google Scholar]
- Cai Y.Z., Luo Q., Sun M., Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sciences. 2004;74:2157–2184. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao C.P., Huang Z.N., Qian P.L., Pan X.X. Studies on the anti-pregnant effect of the Lonicera japonica thunb extract. Pharmaceutical Industry. 1986;17:115–117. (in Chinese) [Google Scholar]
- Cao C.P., Huang Z.N., Qian P.L., Yan D.P., Shen Y.P., Pan X.X. Pharmacological study of pregnancy termination effect of Lonicera japonica thunb. Pharmaceutical Industry. 1986;17:319–321. (in Chinese) [Google Scholar]
- Chang C.W., Lin M.T., Lee S.S., Karin C.S., Liu C., Hsu F.L., Lin J.Y. Differential inhibition of reverse transcriptase and cellular DNA polymerase-a activities by lignans isolated from Chinese herbs, Phyllanthus myrtifolius Moon, and tannins from Lonicera japonica Thunb and Castanopsis hystrix. Antiviral Research. 1995;27:367–374. doi: 10.1016/0166-3542(95)00020-m. [DOI] [PubMed] [Google Scholar]
- Chang J., Sheng P.P., Zhang X.M. The toxicological assessment of Lonicera japonica on food safety. The Chinese Academic Medical Magazine of Organisms. 2003;2:63–64. (in Chinese) [Google Scholar]
- Choi C.W., Jung H.A., Kang S.S., Choi J.S. Antioxidant constitutes and a new triterpenoid glycoside from Flos lonicerae. Archives of Pharmacal Research. 2007;30:1–7. doi: 10.1007/BF02977770. [DOI] [PubMed] [Google Scholar]
- Chen C.X., Wang W.W., Ni W., Chen N.Y., Zhou Jun. Triterpenoid glycosides from the Lonicera japonica. Acta Botanica Yunnanic. 2000;22:201–208. (in Chinese) [Google Scholar]
- Chen C.Y., Qi L.W., Li H.J., Li P., Yi L., Ma H.L., Tang D. Simultaneous determination of iridoids, phenolic acids, flavonoids, and saponins in Flos lonicerae and Flos lonicerae japonicae by HPLC-DAD-ELSD coupled with principal component analysis. Journal of Separation Science. 2007;30:318–3192. doi: 10.1002/jssc.200700204. [DOI] [PubMed] [Google Scholar]
- Chen J.J., Fang J.G., Wan J., Feng L., Zhang Y.W., Zhao J.H., Zhao J.J., Wang N., Chen S.H. An in vitro study of the anti-cytomegalovirus effect of chlorogenic acid. Herald of Medicine. 2009;28:1138–1140. [Google Scholar]
- Chen X.C. Utilization of Lonicera japonca. Forest By-Product and Speciality in China. 2008;92:37–39. (in Chinese) [Google Scholar]
- Committee for the pharmacopoeia of PR China . Chemical Industrial Press; Guangdong Science and Technology Publishing House: 1995. Pharmacopoeia of PR China, Part I. (in Chinese) [Google Scholar]
- Committee for the Pharmacopoeia of PR China . China Medical Science and Technology Press; PR China: 2010. Pharmacopoeia of PR China, Part I. (in Chinese) [Google Scholar]
- Du H.F., Zhang Y., Wen D.Q., Yang D.J. Identify the contents of fresh flower of L. japonica using GC–MS with the different extraction methods. TCM Research of Chongqing. 2009;60:13–15. (in Chinese) [Google Scholar]
- Ehrman T.M., Barlow D.J., Hylands P.J. In silico search for multi-target anti-inflammatories in Chinese herbs and formulas. Bioorganic & Medicinal Chemistry. 2010;18:2204–2218. doi: 10.1016/j.bmc.2010.01.070. [DOI] [PubMed] [Google Scholar]
- Fan H.W., Li Y.B., Sun M., Zhu Q. Antithrombus effect of honeysuckle and its organic acid compounds in vitro. Pharmacology and Clinics of Chinese Materia Medica. 2007;23:34–36. (in Chinese) [Google Scholar]
- Feng Y. Study on the quality of Lonicerae japonica flos from different habitats. Chinese Medicine Journal Research Practice. 2010;24:34–35. (in Chinese) [Google Scholar]
- Gao Y.M., Mu H.J. Studies on the chemical constitutes of Japanese Honeysuckle (Lonicera Japonica) Chinese Traditional and Herbal Drugs. 1995;26:568–656. (in Chinese) [Google Scholar]
- Gu G.G. Harrbing Publishing House; Harrbing: 2007. Sheng Nong Ben Cao Jing. (in Chinese) [Google Scholar]
- He S.Q., Hu Q.F., Yang G.Y. Research of honeysuckle. Yunnan Chemical Technology. 2010;37:72–75. (in Chinese) [Google Scholar]
- http://www.satcm.gov.cn. State Administration of Traditional Chinese Medicine of the People's Republic of China (in Chinese).
- http://www.zysj.com.cn (in Chinese).
- Hu C.M., Jiang H., Liu H.F., Li R., Li J. Effects of LJTF on immunological liver injury in mice. Anhui Medical and Pharmaceutical Journal. 2008;12:295–296. (in Chinese) [Google Scholar]
- Hu K.J., Sun K.X., Wang J.L. Inhibited effect of chlorogenic acid on virus in vitro. Journal of Harrbing Medical University. 2001;35:430–432. (in Chinese) [Google Scholar]
- Huang L.Y., Lv Z.Z., Li J.B. Studies on the chemical constitutes of Japanese Honeysuckle. Chinese Traditional and Herbal Drugs. 1996;27:645–647. (in Chinese) [Google Scholar]
- Huo X.F., Tang Y.P., Zhang Q.L., Luo S.H. Effect of chlorogenic acid on the macrophages induced by lipoplysaccharide. Acta Academiae Medicine Zunyi. 2003;26:507–508. (in Chinese) [Google Scholar]
- Ikeda N., Ishihara M., Tsuneya T., Kawakita M., Yoshihara M. Volatile components of honeysuckle (Lonicera japonica Thunb.) flowers. Flavour and Fragrance Journal. 1994;9:325–331. [Google Scholar]
- Iwanhashi H., Negoroy I. Inhibation by chlorogenic acid of haematin-catalysed retinoic acid 5,6-epoxidation. Journal of Biochemistry. 1986;239:641–646. doi: 10.1042/bj2390641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong J.J., Ha Y.M., Jin Y.C., Lee E.J., Kim J.S., Kim H.J., Seo H.G., Lee J.H., Kang S.S., Kim Y.S., Chang K.C. Rutin from Lonicera japonica inhibits myocardial ischemia/reperfusion-induced apoptosis in vivo and protects H9c2 cells against hydrogen peroxide-mediated injury via ERK1/2 and PI3K/Akt signals in vitro. Food and Chemical Toxicology. 2009;47:1569–1576. doi: 10.1016/j.fct.2009.03.044. [DOI] [PubMed] [Google Scholar]
- Ji L., Pan J.G., Xu Z.L. The GC–MS analysis of volatile oil from Lonicera japonica thunb. Chinese Journal of Chinese Materia Medica. 1990;l5:680. (in Chinese) [PubMed] [Google Scholar]
- Jiao S.G. Research and comprehensive utilization of honeysuckle. Qilu Pharmaceutical Affairs. 2009;28:487–489. (in Chinese) [Google Scholar]
- Kakuda R., Imai M., Yaoita Y., Machida K., Kikuchi M. Secoiridoid glycosides from the flower buds of Lonicera japonica. Phytochemistry. 2000;55:879–881. doi: 10.1016/s0031-9422(00)00279-x. [DOI] [PubMed] [Google Scholar]
- Kang O.H., Choi Y.A., Park H.J., Lee J.Y., Kim D.K., Choi S.C., Kim T.H., Nah Y.H., Yun K.J., Choi S.J., Kim Y.H., Bae K.H., Lee Y.M. Inhibition of trypsin-induced mast cell activation by water fraction of Lonicera japonica. Archives of Pharmacal Research. 2004;27:1141–1146. doi: 10.1007/BF02975120. [DOI] [PubMed] [Google Scholar]
- Kang X., Li D.S., Deng C., Yuan J.L. Study on antimicrobial activity of extracts from Lonicera japonica Thunb. Journal of Anhui Agricultural Science. 2010;38:14935–14936. (in Chinese) [Google Scholar]
- Kawai H., Kuroyanngi M., Umehara K., Ueno A., Satake M. Studies on the saponins of Lonicera japonica Thunb. Chemical & Pharmaceutical Bulletin. 1988;36:4769–4775. doi: 10.1248/cpb.36.4769. [DOI] [PubMed] [Google Scholar]
- Kim D.I., Park J.D., Kim S.G., Kuk H., Jang M.S., Kim S.S. Screening of some crude plant extracts for their acaricidal and insecticidal efficacies. Journal of Asia-Pacific Entomology. 2005;8:93–100. [Google Scholar]
- Kotanidou A., Xagorari A., Bagli E., Kitsanta P., Fotsis T., Papapetropoulos A., Roussos C. Luteolin reduces lipopolysaccharide-induced lethal toxicity and expression of proinflammatory molecules in mice. American Journal of Respiratory and Critical Care Medicine. 2002;165:818–823. doi: 10.1164/ajrccm.165.6.2101049. [DOI] [PubMed] [Google Scholar]
- Kumar N., Singh B., Bhandari P., Gupta A.P., Uniyal S.K., Kaul V.K. Biflavonoids from Lonicera japonica. Phytochemistry. 2005;66:2740–2744. doi: 10.1016/j.phytochem.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Kumar N., Singh B., Gupta A.P., Kaul V.K. Lonijaposides, novel cerebrosides from Lonicera japonica. Tetrahedron. 2006;62:4317–4322. [Google Scholar]
- Kwak W.J., Han C.K., Chang H.W. Loniceroside C, an antiinflammatory saponin from Lonicera japonica. Chemical & Pharmaceutical Bulletin. 2003;51:333–433. doi: 10.1248/cpb.51.333. [DOI] [PubMed] [Google Scholar]
- Lan H., Giao D., Shen H., Chen Z.D. Studies on the activity of antioxidant enzyme and capability of scavenge free radicals extracted from Lonicera Japonica Thunb. Journal of Chinese Institute of Food Science and Technology. 2007;7:27–29. (in Chinese) [Google Scholar]
- Lee E.J., Kim J.S., Kim H.P., Lee J.H. Sam Sik Kang phenolic constituents from the flower buds of Lonicera japonica and their 5-lipoxygenase inhibitory activities. Food Chemistry. 2010;120:134–139. [Google Scholar]
- Lee S.J., Son K.H., Chang H.W., Kang S.S., Kim H.P. Antiinflammatory activity of Lonicera japonica. Phytotherapy Research. 1998;12:445–447. [Google Scholar]
- Lee S.J., Shin E.J., Son K.H., Chang H.W., Kang S.S., Kim H.P. Anti-inflammatory activity of the major constituents of Lonicera japonica. Archives of Pharmacal Research. 1995;18:133–135. [Google Scholar]
- Lee S.J., Choi J.H., Son K.H., Chang H.W., Kang S.S., Kim H.P. Suppression of mouse lymphocyte proliferation in vitro by naturally-occurring biflavovoids. Life Sciences. 1995;57:558. doi: 10.1016/0024-3205(95)00305-p. [DOI] [PubMed] [Google Scholar]
- Leung H.W.C., Hour M.J., Chang W.T., Wud Y.C., Lai M.Y., Wang M.Y., Lee H.Z. P38-associated pathway involvement in apoptosis induced by photodynamic therapy with Lonicera japonica in human lung squamous carcinoma CH27 cells. Food and Chemical Toxicology. 2008;46:3389–3400. doi: 10.1016/j.fct.2008.08.022. [DOI] [PubMed] [Google Scholar]
- Li, D.Z., 2008a. Study on the synthesis and bioactivities of analogs of shuangkangsu – an anti-virus principle of Jinyinhua. Chinese Academy of Medical Science & Peking Union Medical College. Doctoral thesis (in Chinese).
- Li, E.C., 2008b. Studies on the isolation, purification and bioactivity of polysaccharides from Flos lonicerae. Shaanxi Normal University. Master thesis (in Chinese).
- Li F.M., Yuan B., Xiong Z.L., Lu X.M., Qin F., Chen H.S., Liu Z.G. Fingerprint analysis of Flos Lonicerae japonicae using binary HPLC profiling. Biomedical Chromatography. 2006;20:634–641. doi: 10.1002/bmc.678. [DOI] [PubMed] [Google Scholar]
- Li H.J., Zhang C.Y., Li P. Comparative study on volatile oils in flower and stem of Lonicera japonica. Journal of Chinese Medicinal Materials. 2002;25:476–477. (in Chinese) [PubMed] [Google Scholar]
- Li H.J., Li P., Zhang C.Y., Zhao M.Q. Scavenging capacities on radicals of Flos Lonicerae by chemiluminescence. Journal of China Pharmaceutical University. 2002;33:496–549. (in Chinese) [Google Scholar]
- Li, H.J., Li, P., Wang, M.C., Ye, W.C., 2003. A new secoiridoid glucoside from Lonicera japonica. Chinese Journal of Natural Medicines 9, 132–133 (in Chinese).
- Li S.Z. People's Medical Publishing House; Beijing: 1979. Ben Cao Gang Mu. (in Chinese) [Google Scholar]
- Li Y.M., Li L., Bo C., Li D., Wang T.Z. Study on the anti-adenovir effects of L. japonica. West China Journal of Pharmaceutical Sciences. 2001;16:327–329. [Google Scholar]
- Lin K. Analysis of essential oil from lonicera japonicain Fujian. Acta Agriculturae Jiangxi. 2009;21:102–104. (in Chinese) [Google Scholar]
- Lin L.M., Zhang X.G., Zhu J.J., Gao H.M., Wang Z.M., Wang W.H. Two new triterpenoid saponins from the flowers and buds of Lonicera japonica. Journal of Asian Natural Products Research. 2008;10:925–929. doi: 10.1080/10286020802217366. [DOI] [PubMed] [Google Scholar]
- Liu Z.L., He X.Y., Chen W., Yuan F.H., Yan K., Tao D.L. Accumulation and tolerance characteristics of cadmium in a potential hyperaccumulator – Lonicera japonica Thunb. Journal of Hazardous Materials. 2009;169:170–175. doi: 10.1016/j.jhazmat.2009.03.090. [DOI] [PubMed] [Google Scholar]
- Lou H.X., Lang W.J., Lu M.J. Water soluble constituents from Lonicera japonica. Chinese Traditional and Herbal Drugs. 1996;27:195. (in Chinese) [Google Scholar]
- Ma S.C., Du J., But P.P.-H., Deng X.L., Zhang Y.W., Ooi V.E.-C., Xu H.X., Lee S.H-S., Lee S.F. Antiviral Chinese medicinal herbs against respiratory syncytial virus. Journal of Ethnopharmacology. 2002;79:205–211. doi: 10.1016/s0378-8741(01)00389-0. [DOI] [PubMed] [Google Scholar]
- Ma, S.C., Paul But, P.H., Vincent Ooi, E.C., Spencer Lee, H.S., Lee, S.F., 2005. Determination of the antiviral caffeoyl quinic acids isolated from L. japonica thunb. Chinese Journal Pharmaceutical Analysis 25, 751–753 (in Chinese).
- Machida K., Asano J., Kikuchi M. Caeruleosides A and B, bis-iridoid glucosides from Lonicera caerulea. Phytochemistry. 1995;39:111. [Google Scholar]
- Machida K., Sasaki H., Iijima T., Kikuchi M. Studies on the constituents of Lonicera species XVII. New iridoid glycosides of the stems and leaves of Lonicera japonica Thunb. Chemical & Pharmaceutical Bulletin. 2002;50:1041–1044. doi: 10.1248/cpb.50.1041. [DOI] [PubMed] [Google Scholar]
- Myung W.B., Cheorun J., Tae W.J., Cheul H.H. Effects of gamma irradiation on color characteristics and biological activities of extracts of Lonicera japonica (Japanese honeysuckle) with methanol andacetone. Lebensmittel-Wissenschaft & Technologie. 2004;37:29–33. [Google Scholar]
- Pan J.K., Liu H.C., Liu G.G., Chen L.X., Qiu Z.Q. L. japonica could reduce the level of blood sugar and blood fat of mice. Guangzhou Medicine. 1998;29:59–61. (in Chinese) [Google Scholar]
- Park E., Kum S., Wang C., Park S.Y., Kim B.S., Schuller-Levis G. Antiinflammatory activity of herbal medicines: inhibition of nitric oxide production and tumor necrosis factor-alpha secretion in an activated macrophage-like cell line. The American Journal of Chinese Medicine. 2005;33:415–424. doi: 10.1142/S0192415X05003028. [DOI] [PubMed] [Google Scholar]
- Peng L.Y., Mei S.X., Jiang B., Zhou H., Sun H.D. Constituents from Lonicera japonica. Fitoterapia. 2000;71:713–715. doi: 10.1016/s0367-326x(00)00212-4. [DOI] [PubMed] [Google Scholar]
- Peng Y.Y., Liu F.H., Ye J.N. Determination of phenolic acids and flavones in Lonicera japonica Thumb. By capillary electrophoresis with electrochemical detection. Electroanalysis. 2005;17:356–359. [Google Scholar]
- Qi L.W., Chen C.Y., Li P. Structural characterization and identification of iridoid glycosides, saponins, phenolic acids and flavonoids in Flos Lonicerae Japonicae by a fast liquid chromatography method with diode-array detection and time-of-flight mass spectrometry. Rapid Communications in Mass Spectrometry. 2009;23:3227–3242. doi: 10.1002/rcm.4245. [DOI] [PubMed] [Google Scholar]
- Rahman A., Kang S.C. In vitro control of food-borne and food spoilage bacteria by essential oil and ethanol extracts of Lonicera japonica Thunb. Food Chemistry. 2009;116:670–675. [Google Scholar]
- Ren M.T., Song J.C.Y., Sheng L.S., Li P., Qi L.W. Identification and quantification of 32 bioactive compounds in Lonicera species by high performance liquid chromatography coupled with time-of-flight mass spectrometry. Journal of Pharmaceutical and Biomedical Analysis. 2008;48:1351–1360. doi: 10.1016/j.jpba.2008.09.037. [DOI] [PubMed] [Google Scholar]
- Robinson W.E., Cordeiro J.M., Abdel-Malek S., Jia Q., Chow S.A., Reinecke M.G., Mitchell W.M. Dicaffeoylquinic acid inhibitors of human immunodeficiency virus integrase: inhibition of the core catalytic domain of human immunodeficiency virus integrase. Molecular Pharmacology. 1996;50:846–855. [PubMed] [Google Scholar]
- Shan B., Cai Y.Z., Brooks J.D., Corke H. The in vitro antibacterial activity of dietary spice and medicinal herb extracts. International Journal of Food Microbiology. 2007;117:112–119. doi: 10.1016/j.ijfoodmicro.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Shang, X.F., 2010. Antinociceptive and anti-inflammatory activities of iridoid glycosides extract of Lamiophlomis rotata (Benth.) Kudo. Lanzhou University. Master thesis (in Chinese). [DOI] [PubMed]
- Shanghai Institute of Pharmaceutical Industry Study on the active compositions of Lonicera japonica Thunb. Chinese Journal of Pharmaceuticals. 1975;11:24. (in Chinese) [Google Scholar]
- Sharma N., Sharma V.K., Seo S.Y. Screening of some medicinal plants for anti-lipase activity. Journal of Ethnopharmacology. 2005;97:453–456. doi: 10.1016/j.jep.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Shu Y.B., Zhang J.R., Ma X.W., Zhang J.S. Supercritical extraction of volatile oil in honeysuckle and its application in cigarettes flavoring. Anhui Agricultural Science Bulletin. 2008;14:76–78. (in Chinese) [Google Scholar]
- Son K.H., Park J.O., Chung K.C., Chang H.W., Kim H.P., Kim J.S., Kang S.S. Flavonoids from the aerial parts of Lonicera japonica. Archives of Pharmacal Research. 1992;15:365–370. [Google Scholar]
- Son K.H., Jung K.Y., Chang H.W., Kim H.P., Kang S.S. Triterpenoid saponins from the aerial parts of Lonicera japonica. 1994;35:1005–1008. doi: 10.1016/s0031-9422(00)90656-3. [DOI] [PubMed] [Google Scholar]
- Son M.J., Moon T.C., Lee E.K., Son K.H., Kim H.P., Kang S.S., Son J.K., Leet S.H., Chang H.W. Naturally occurring biflavonoid, ochanflavone, inhibits cyclooxygenases-2 and 5-lipoxygenase in mouse bone marrowderived mast cells. Archives of Pharmacal Research. 2006;29:282–286. doi: 10.1007/BF02968571. [DOI] [PubMed] [Google Scholar]
- Song H.Y., Qiu S.C., Wang Z.Q., Zhang Q., Yang X. Research on the in vitro growth inhibition effect of Lonicera japonica Thunb. (LJT) on bacteri. Lishizhen Medicine and Materia Research. 2003;14:269. (in Chinese) [Google Scholar]
- Song W.X. Chinese Academy of Medical Science & Peking Union Medical College; 2008. Study on the Water-soluble Chemical Constituents of the Flower Buds of Lonicera japonica. (in Chinese) [Google Scholar]
- Song W.X., Li S., Wang S.J.W.Y., Zi J.C., Gan M.L., Zhang Y.L., Liu M.T., Lin S., Yang Y.C., Shi J.G. Pyridinium alkaloid-coupled secoiridoids from the flower buds of Lonicera japonica. Journal of Natural Product. 2008;71:922–925. doi: 10.1021/np800040k. [DOI] [PubMed] [Google Scholar]
- Song Y., Li S.L., Wu M.H., Li H.J., Li P. Qualitative and quantitative analysis of iridoid glycosides in the flower buds of Lonicera species by capillary high performance liquid chromatography coupled with mass spectrometric detector. Analytica Chimica Acta. 2006;564:211–218. [Google Scholar]
- State Food Drug Administration of China . SFDA; Beijing: 2000. Technical Requirements for the Development of Fingerprints of TCM Injections. (in Chinese) [Google Scholar]
- Starr F., Starr K., Loope L. United States Geological Survey – Biological Resources Division; 2003. Lonicera japonica. Caprifoliaceae. [Google Scholar]
- Su X.P., Song B.W., Chen Z.H., Niu Y.P. Study on the volatile oil of Flos lonicerae by supercritical CO2 extraction and its anti-inflammatory activity. Natural Product Research and Development. 2006;18:663–666. (in Chinese) [Google Scholar]
- Sun C.H., Teng Y., Li G.Z., Yoshioka S., Yokota J., Miyamura M., Fang H.Z., Zhang Y. Metabonomics study of the protective effects of Lonicera japonica extract on acute liver injury in dimethylnitrosamine treated rats. Journal of Pharmaceutical and Biomedical Analysis. 2010;53:98–102. doi: 10.1016/j.jpba.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Sun D.M. Study on activity of antioxidation and effect of restraining bacterium of extract components in Lonicera japonica Thunb's leaf. Henan Science. 2002;20:511–513. (in Chinese) [Google Scholar]
- Tae J., Han S.W., Yoo J.Y., Kim J.A., Kang O.H., Baek O.S., Lim J.P., Kim D.K., Kim Y.H., Bae K.H., Lee Y.M. Anti-inflammatory effect of Lonicera japonica in proteinase-activated receptor 2-mediated paw edema. Clinica Chimica Acta. 2003;330:165–171. doi: 10.1016/s0009-8981(03)00017-2. [DOI] [PubMed] [Google Scholar]
- Tang, M., 2008. Study of isolation and biological effects of active flavonoid components extracted from Lonicera Japonica. The Third Military Medical University (China). Doctoral thesis (in Chinese).
- Tao H.J. People's Medical Publishing House; Beijing: 1986. Ming Yi Bie Lu. (in Chinese) [Google Scholar]
- Thanabhorn S., Jaijoy K., Thamaree S., Ingkaninan K., Panthong A. Acute and subacute toxicity study of the ethanol extract from Lonicera japonica Thunb. Journal of Ethnopharmacology. 2006;107:370–373. doi: 10.1016/j.jep.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Wagner W.L., Herbst D.R., Sohmer S.H. Manual of the Flowering Plants of Hawaii. University of Hawaii Press; Honolulu, Hawaii: 1999. Colocasia. pp. 1356–1357. [Google Scholar]
- Wang L.J. The study progress of Lonicera japonica. Medical Information. 2010;8:2293–2296. (in Chinese) [Google Scholar]
- Wang J.M., Ji Z.Q., Kang W.Y. The volatile oil from different parts of Lonicera japonica Thunb. Fine Chemicals. 2008;25:1075–1077. (in Chinese) [Google Scholar]
- Wang L.M., Li M.T., Yan Y.Y., Ao M.Z., Wu G., Yu L.J. Influence of flowering stage of Lonicera japonica Thunb. on variation in volatiles and chlorogenic acid. Journal of the Science of Food and Agriculture. 2009;89:953–957. [Google Scholar]
- Wang, L.Q., 2008c. Studies on antiviral effect and immunopotentiating activity of Lonicera japonica Thunb. and flos lonicerae in vitro. Agricultural University of Henan. Mater thesis (in Chinese).
- Wang X.R., Chen S.H., Qiao F.Y. Experimental study of honeysuckle flower against guinea pig cytomegalovirus in vitro. Journal on Maternal and Children Health Care of China. 2005;17:2241–2243. (in Chinese) [Google Scholar]
- Wang X.Y., Jia W., Zhao A.H., Wang X.R. Anti-influenza agents from plants and traditional Chinese medicine. Phytotherapy Research. 2006;20:335–341. doi: 10.1002/ptr.1892. [DOI] [PubMed] [Google Scholar]
- Wang Y., Yu J., Xiao Y., Cheng L., Guan X.Z. Studies on the elimination of resistance plasmid from P. aeruginosa in vitro and vivo with Lonicera japonica. Journal of Norman Bethune University of Medical Science. 2000;26:139–141. (in Chinese) [Google Scholar]
- Wang Y.P., Xue X.Y., Zhang F.F., Xu Q. Separation and analysis of volatile oil from Lonicera japonica thunb. Modernization of Traditional Chinese Medicine and Materia Medica. 2008;10:45–55. (in Chinese) [Google Scholar]
- Wang Y.P., Xue X.Y., Zhang F.F., Liang X.M. Separation and analysis of volatile oil from Lonicera japonica thunb using normal phase liquid chromatography–gas chromatography hyphenated with mass spectrometry. World Science and Technology/Modernization of Traditional Chinese Medicine and Materia Medica. 2008;10:45–48. (in Chinese) [Google Scholar]
- Wang Z.J. Study on the anti-HSV-1F and anti-HSV-1HS-1 activities of L. japonica. Journal of Shandong University of TCM. 1999;23:377–379. (in Chinese) [Google Scholar]
- World Health Organization . WHO; Munich, Geneva: 1991. Guidelines for the Assessment of Herbal Medicines. [Google Scholar]
- Wu E.X. Analysis on the trace element between Lonicera japonica Thunb and Lonicera macranthoides Hand.-Mazz. Chinese Traditional and Herbal Drugs. 1988;19:45–47. (in Chinese) [Google Scholar]
- Wu C.X., Wang J.M., Kang W.Y. Component analysis on the volatile oil in different medicinal part of Lonicera japonica Thunb from Henan Province. China Pharmacy. 2009;20:1412–1414. (in Chinese) [Google Scholar]
- Wu, L., 2005. Extraction and antioxidiant ability of chlorogenic acid from flos lonicera. Tianjin University of Science and Technology. Master thesis (in Chinese).
- Wu X.F., Jiao X.Q., Li G.R. Extracting of medicinal components Lonicera japonica Thunb's leaf and studying of their restraining bacterium test. Natural Product Research and Development. 2001;23:448. (in Chinese) [Google Scholar]
- Xagorari A., Papapetropoulos A., Mauromatis A., Economou M., Fotsis T., Roussos C. Luteolin inhibits an endotoxin-stimulated phosphorylation cascade and proinflammatory cytokine production in macrophages. The Journal of Pharmacology and Experimental Therapeutics. 2001;296:181–187. [PubMed] [Google Scholar]
- Xi, Q., 2008. Experimental study on the intervention of active component of Lonicerae Powder on immune cytokines of influenza viral pneumonia in mice. TCM University of Liaoning. Master thesis (in Chinese).
- Xing J.B., Li H.J., Li P. Studies on the quality control of Flos Lonicerae – Determination of total flavonoids. Chinese Journal of Modern Applied Pharmacy. 2002;19:169–170. (in Chinese) [Google Scholar]
- Xu Y.B., Oliverson B.G., Simmons D.L. Trifunctional inhibition of COX-2 by extracts of Lonicera japonica: direct inhibition, transcriptional and post-transcriptional down regulation. Journal of Ethnopharmacology. 2007;111:667–670. doi: 10.1016/j.jep.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Xu, Y.C., 2008. Study on extraction and isolation of the chlorogenic acid in Honeysuckle flowers and its bacteticidal activity. Chongqing University. Master thesis (in Chinese).
- Yang M.L., Zhao Y.G. A comparative study of composition of volatile oil of Flos Lonicerae of Yuanzhou in Ningxia in different months. Journal of Ningxia University (Natural Science Edition) 2007;28:140–142. (in Chinese) [Google Scholar]
- Yip E.C.H., Chan A.S.L., Pang H., Tam Y.K., Wong Y.H. Protocatechuic acid induces cell death in HepG2 hepatocellular carcinoma cells through a c-Jun N-terminal kinase-dependent mechanism. Cell Biology and Toxicology. 2006;22:293–302. doi: 10.1007/s10565-006-0082-4. [DOI] [PubMed] [Google Scholar]
- Yoon Y.H., Kyung S.H. Repellent activity of n-hexane, ethyl acetate, n-butanol and water extracts of Native Plants against Aedes albopitus. Korean Journal of Entomology. 2002;32:61–64. [Google Scholar]
- Zdzisław Z., Kowalska A., Demska-Zak̨esŁ K., Jeney G., Jeney Z. Effect of two medicinal herbs (Astragalus radix and Lonicera japonica) on the growth performance and body composition of juvenile pikeperch [Sander lucioperca (L.)] Aquaculture Research. 2008;39:1149–1160. [Google Scholar]
- Zhang B., Yang R.Y., Liu C.Z. Microwave-assisted extraction of chlorogenic acid from flower buds of Lonicera japonica Thunb. Separation and Purification Technology. 2008;62:480–483. [Google Scholar]
- Zhang H.F., Bai Y.J., Lan Y.S. Study on the anti-bacterium effect in vitro of Lonicera japonica. Journal of East China Normal University (Natural Science) 2000;1:107–110. (in Chinese) [Google Scholar]
- Zhang, N., 2008. Extraction of the antiseptic of cosmetic from honeysuckle's leaves. Jiangnan University. Master thesis (in Chinese).
- Zhang T.D., Pan R.E., Bi Z.M., Li H.J., Li P. Flavonoides from aerial parts of Lonicera cheysantha. Chinese Pharmaceutical Journal. 2006;41:741–743. (in Chinese) [Google Scholar]
- Zhang, X.C., 2007. Inhibition effects of allicin and flos lonicerae on the cells apoptosis induced by VSV and the possible molecular mechanism involved. Zhejiang University. Master thesis (in Chinese).
- Zheng Y.H. Investigation of prescription to treat influenza A virus subtype H1N1 in Beijing YouAn Hospital. Shanxi Medical Journal. 2010;39:897–898. (in Chinese) [Google Scholar]