Summary
The Japanese encephalitis virus (JEV) serocomplex-group consists of mosquito-borne flaviviruses, which include West Nile virus (WNV) and JEV, and both may cause severe encephalitis in humans. WNV has spread rapidly across the United States since its introduction in 1999 and its geographical distribution within the western hemisphere is expected to further expand, whereas, JEV is the most common cause of viral encephalitis in Southeast Asia, China and India. Currently, there is no registered human vaccine or specific therapy to prevent or treat WNV infection. Here we describe the efficacy of recombinant domain III (DIII) of WNV glycoprotein E in a mouse model. It induces high neutralizing antibody titers, as well as, protection against lethal WNV infection in C57BL/6 mice. This vaccine preparation also afforded partial protection against lethal JEV infection.
Keywords: West Nile virus, Domain III, Vaccine, C57BL/6 mice, Cross-protection, Japanese encephalitis virus
The flaviviruses West Nile virus (WNV) and Japanese encephalitis virus (JEV) are responsible for a large proportion of viral encephalitis in humans [1], [2]. WNV infects a wide range of avian and mammalian species, including humans. WNV has also been shown to be transmitted through blood transfusion, organ transplantation, and breast-feeding [3], [4], [5], [6], [7], [8]. JEV is the single-most important cause of viral encephalitis in Asia, with case fatality rates averaging 30% [9], [10], [11]. JEV is a major problem in South-East Asia, India, and China, where the virus is endemic. In recent years, JEV has spread to other geographic areas such as Australia and Pakistan, and has thus become an important emerging virus infection in these areas. Vaccination against JEV using a mouse brain-derived, inactivated vaccine has been shown to be very effective and has led to a decreased disease burden [12], [13], [14]. However, there are concerns about the immunogenicity and the safety of this vaccine [13], [15]. A live-attenuated (SA14-14-2 strain) and a cell culture-based JEV vaccine that are produced on primary hamster kidney (PHK) cells have been licensed for use in China and have been shown to be safe and effective [13]. However, since PHK is not an approved cell line for production of human vaccine, many countries will not use this JEV vaccine. Therefore, a recombinant protein-based vaccine represents an attractive alternative. The E protein of flaviviruses is the most immunogenic and suitable for the purpose of vaccine development. The protein E consists of three structural domains (DI–DIII) [16], [17], of which DIII contains predominantly sub-complex- and type-specific epitopes, many of which induce neutralizing antibodies [17], [18], [19], [20], [21], [22], [23]. Several vaccines based on DIII have been shown to be immunogenic and effective under certain conditions [24], [25], [26].
The 315 nucleotides of WNV-NY99 E protein (accession no. AF196835, passage 5, obtained from the Health Protection Agency, Porton Down, UK) that constitute the ectodomain of DIII were BamH1 (caggatccaGGAACAACCTATGGCGTCT) and Sal1 (atgtcgacTTTGCCAATGCTGCTTCCA) cloned into the pTRHis2A bacterial vector (Invitrogen), upstream of the histidine repeat string. Recombinant DIII (rDIII) protein expression was induced with isopropyl-β-d-thiogalactopyranoside. Purification of rDIII was performed with nickel-affinity chromatography as previously described [27]. Purification of the solubilised rDIII resulted in 80–90% pure fractions of rDIII (not shown), which were used in subsequent vaccination experiments. The authenticity of the purified recombinant WNV E DIII protein was confirmed by western blot analyses using a WNV monoclonal antibody (7H2; Bioreliance Corp., Rockville, USA). The rDIII protein had a molecular weight of 13 kDa, which corresponds to what has been described previously [24]. To produce an inactivated whole virus vaccine, WNV was propagated in Vero E6 cells, concentrated and purified by sucrose gradient centrifugation and subsequently inactivated with β-propiolactone (BPL) in a biosafety-level 3 laboratory, using the same method as previously described for inactivation of the SARS coronavirus [28]. All vaccine preparations used for primary immunization were adjuvanted with synthetic CpG oligonucleotide (ODN: TCCATGACGTTCCTGACGTT, Sigma, Haverhill, UK), which was mouse optimized [29]. For booster immunizations, vaccine candidates were adjuvanted with oil (Stimune®; Cedi-Diagnostics, Lelystad, The Netherlands) as recommended by the manufacturer.
Immunization experiments were performed as follows: six groups of 10 mice each were immunized subcutaneously with 25 μg of the purified rDIII (groups A and B), 15 μg BPL-inactivated WNV (WNV-BPL; groups C and D), and a control vaccine (15 μg BPL-inactivated rabies; groups E and F). Animals were immunized first with 25 μg of ODN adjuvanted vaccine on day 0 and boosted with the same oil-adjuvanted vaccine on day 14. To determine the virus-specific IgG response upon vaccination, an enzyme-linked immunosorbent assay (ELISA) was developed using WNV-NY99 or JEV-Beijing-1 strains as antigens. To this end, purified virus was solubilised (end-concentration 250 μg/ml) in PBS containing 4% (w/v) Mega-10 (Sigma, Zwiijndrecht, The Netherlands) and microtitre plates (Costar, Cambridge, MA, USA) were coated with 2 μg total protein (diluted in PBS) per well. Twofold dilutions of sera (1:25–1:3200) were prepared in PBS containing 0.2% (w/v) bovine serum albumin, 0.1% (w/v), dry milk powder and 3% (w/v) sodium chloride (Sigma, Zwijndrecht, The Netherlands). Virus specific IgG was measured using a HRPO labeled goat-anti-mouse (1:1000; Dako, Glostrup, Denmark). Titers were expressed as the reciprocal of the highest serum dilution with OD450 nm values higher than three times the background OD450 nm value. Titers <50 were considered negative based on cutoff values established with sera from mice naïve towards WNV or JEV. After primary vaccination, some mice in groups A–B developed low IgG antibody titers (50) against WNV, and no titers against JEV, whereas, some animals in groups C–D developed IgG antibody titers (range: <50–200) against WNV, but no antibody titers to JEV. It is worth realizing that 2 weeks post-primary vaccination, mainly low affinity antibodies are produced, which could explain why no cross-reactive antibodies were measured against JEV. After booster vaccination, IgG titers to WNV increased to 880 ± 130 in groups A–B and up to 8800 ± 1416 in groups C–D. Titers to JEV increased to 540 ± 90 in groups A–B and up to 1520 ± 221 in groups C–D. None of the mock-vaccinated animals developed specific antibody titers.
To determine virus neutralizing (VN) antibody titers, serial twofold dilutions of heat-inactivated mouse sera were incubated with 100 TCID50 of WNV or JEV and VN titers were expressed as the reciprocal of the highest serum dilution still giving 100% suppression of cytopathic effect. After primary immunization, low VN titers were measured against WNV in some of the vaccinated mice (Fig. 1A ). No VN titers were measured against JEV (Fig. 1B). After booster, all animals developed high VN titers against WNV, but cross-neutralizing antibody titers measured against JEV (Fig. 1B) were lower than against homologous virus (Fig. 1A). In the method that we have used to solubilize rDIII inclusion bodies prior to purification, renaturation was a critical step, since correct folding of DIII determines the formation of neutralizing epitopes [18], [30], [31]. The observation that mice vaccinated with rDIII developed neutralizing antibodies against homologous WNV and heterologous JEV, indicates that using this procedure at least a portion of the solubilised rDIII folded correctly. However, targeted experiments are needed to prove that renaturation was successful and to determine the percentage of solubilised proteins that folded correctly.
Figure 1.
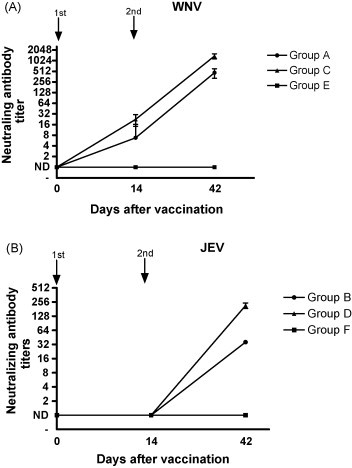
Neutralizing and cross-neutralizing antibody responses to WNV or JEV following vaccination with ODN-adjuvanted (first) and oil-adjuvanted (second) vaccines. Antibody titers in sera collected from individual animals at days 0, 14, and 42. Logarithms of the mean titers and 95% confidence intervals are indicated. ND: not detected; groups: A + B (●); C + D (▴); E + F (■).
At day 42 animals from groups A, C, and E were challenged intra-peritoneally with a lethal dose of WNV-NY99 (1 × 106 TCID50) and animals in group B, D, and F were challenged intra-peritoneally with a lethal dose of JEV-Beijing-1 (1 × 104 TCID50). All mock-vaccinated mice (E and F) showed more than 15% body weight loss (Fig. 2 ) and died within 11 days p.i., irrespective of the virus that was used for challenge infection (Fig. 3 ). Mice vaccinated with rDIII and challenged with WNV (A) or JEV (B) were protected against weight loss at a degree comparable to WNV-BPL vaccinated animals (Fig. 2), indicating that rDIII provided significant protection of C57BL/6 mice against developing weight loss. The survival rates of five animals in each group were also monitored after lethal challenge with WNV or JEV (Fig. 3). Survival rates of 80 (4/5) and 60% (3/5) were observed in groups A and B, respectively, whereas rates of 100 (5/5) and 80% (4/5) were seen in groups C and D. The differences in survival curves between groups were analyzed with the logrank test incorporated in the GraphPad Prism Version 4 software (Graphpad Software, San Diego, USA). The test uses the complete survival-curve for comparing groups. Values of P ≤ 0.05 were considered to be statistically significant. The differences between groups A and E and between groups C and E were statistically significant (P = 0.009 and P = 0.002, respectively). Similarly, significant differences were measured between groups B and F, and between groups D and F (P = 0.043, 0.008, respectively). When groups A and C were compared, no significant difference in survival rate was observed (P = 0.51), indicating that the efficacy of rDIII-based vaccine was similar to the WNV-BPL vaccine in protecting mice against lethal WNV challenge.
Figure 2.
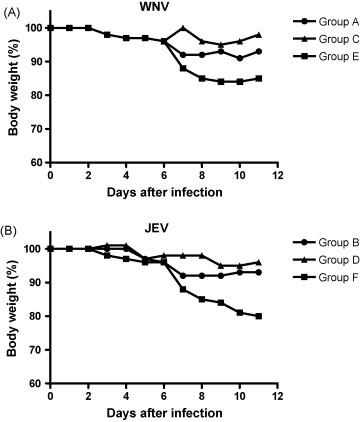
Loss of body weight of vaccinated mice after challenge with WNV or JEV. Vaccinated mice were challenged intraperitoneally with 106 TCID50 WNV (A) or 104 TCID50 JEV (B). After challenge mice were weighed daily. Percent body weight per group was calculated compared to the body weight at the time of challenge. Groups: A + B (●); C + D (▴); E + F (■).
Figure 3.
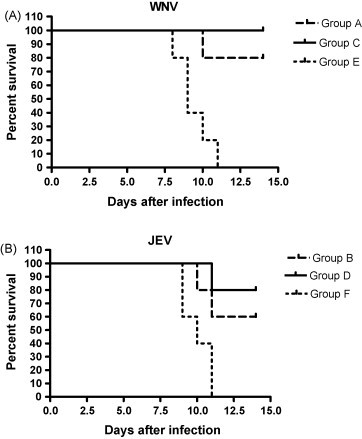
Kaplan–Meier survival curves of vaccinated mice after challenge with WNV or JEV. (A) Groups of five mice were challenged with 106 TCID50 of WNV-NY99. The number of mice surviving was recorded daily. (B) Groups of five mice were challenged with 104 TCID50 of JEV-Beijing-1. The number of mice surviving was recorded daily.
Five mice in each group were sacrificed on day 8 p.i., which is the timepoint at which the first signs of paralysis appeared in the mock-vaccinated animals, and brains were collected for virus titration. High WNV (average: 105.2 TCID50/g brain) and JEV (average: 105.7 TCID50/g brain) titers were detected in the brains of groups E and F, respectively (Fig. 4 ). Only one out of five mice in group A had detectable WNV (101 TCID50/g brain), while two out of five animals in group B had detectable JEV (101 and 102 TCID50/g brain, respectively).
Figure 4.
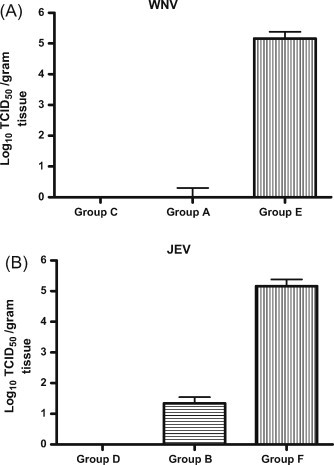
Virus titers in brain of mice after challenge with WNV and JEV. Vaccinated mice were challenged intraperitoneally with (A) 106 TCID50 WNV-NY99 and (B) 104 TCID50 JEV-Beijing-1. On day 8 p.i., five mice were sacrificed, brain tissues were collected and virus titers were determined in Vero E6 cells. The mean virus titer per group was calculated. Error bars indicate the standard deviation.
DIII proteins are highly conserved between several WNV strains. The WNV strain used in this study (WNV-NY99) shared overall amino acid identity and similarity values with the JEV-Beijing-1 strain of 81 and 94%, respectively, which explains the level of protection against lethal WNV and JEV infection seen in mice. DIII functions as a receptor-binding domain [32], [33], forming a continuous polypeptide segment that can fold independently. Certain mutations within DIII have been shown to affect virulence and tropism of flaviviruses [27], [34]. rDIII is a stable protein, and therefore is an attractive candidate to be used as a subunit vaccine. The lack of glycosylation of the protein during expression in prokaryotic cells most likely did not affect its antigenicity since native DIII is not glycosylated as well. Recombinant DIII of JEV and dengue virus has been shown to be immunogenic and protective in mice challenged with the respective virulent viruses [25], [26], [35], [36], underlining the suitability of DIII-based vaccine formulations against flaviviruses. Our survival results are consistent with a recent study that showed the efficacy of DIII in protecting BALB/c mice against intracerebral challenge with WNV and JEV [24]. In this study, however, mice were vaccinated with 100 μg rDIII adjuvanted only with CpG. The relatively high concentrations of rDIII needed for induction of neutralizing antibody responses may indicate that rDIII is poorly immunogenic. More experiments are needed to determine the percentage of solubilised proteins that folded correctly and how the purification procedure used in our study may affect immunogenicity of rDIII.
Collectively the results obtained in the present study indicate that DIII is a promising well-defined vaccine candidate that in combination with a good adjuvant can be used for the induction of protective immunity against WNV and JEV. Although the WNV-BPL vaccine was more immunogenic and resulted in better protection than the DIII-based vaccine, it did not result in statistically better results, indicating similar efficacy of the two vaccine formulations against WNV. Therefore further evaluation of this DIII-based vaccine in other mammalian species, including humans seems warranted.
Acknowledgements
The authors would like to thank Pieter van der Pol for technical assistance and Dr. B. Haagmans for comments on the manuscript.
References
- 1.Solomon T. Exotic and emerging viral encephalitides. Curr Opin Neurol. 2003;16(3):411–418. doi: 10.1097/01.wco.0000073944.19076.56. [DOI] [PubMed] [Google Scholar]
- 2.Solomon T. Recent advances in Japanese encephalitis. J Neurovirol. 2003;9(2):274–283. doi: 10.1080/13550280390194037. [DOI] [PubMed] [Google Scholar]
- 3.Possible West Nile virus transmission to an infant through breast-feeding—Michigan, 2002. MMWR MorbMortalWklyRep. 2002;51(39):877–878. [PubMed] [Google Scholar]
- 4.From the Centers for Disease Control and Prevention. Possible West Nile virus transmission to an infant through breast-feeding—Michigan, 2002. JAMA 2002;288(16):1976–7. [PubMed]
- 5.Armstrong W.S., Bashour C.A., Smedira N.G., Heupler F.A., Hoeltge G.A., Mawhorter S.D. A case of fatal West Nile virus meningoencephalitis associated with receipt of blood transfusions after open heart surgery. Ann Thorac Surg. 2003;76(2):605–607. doi: 10.1016/s0003-4975(03)00271-6. [DOI] [PubMed] [Google Scholar]
- 6.Harrington T., Kuehnert M.J., Kamel H., Lanciotti R.S., Hand S., Currier M. West Nile virus infection transmitted by blood transfusion. Transfusion. 2003;43(8):1018–1022. doi: 10.1046/j.1537-2995.2003.00481.x. [DOI] [PubMed] [Google Scholar]
- 7.Pealer L.N., Marfin A.A., Petersen L.R., Lanciotti R.S., Page P.L., Stramer S.L. Transmission of West Nile virus through blood transfusion in the United States in 2002. N Engl J Med. 2003;349(13):1236–1245. doi: 10.1056/NEJMoa030969. [DOI] [PubMed] [Google Scholar]
- 8.Shepherd J.C., Subramanian A., Montgomery R.A., Samaniego M.D., Gong G., Bergmann A. West Nile virus encephalitis in a kidney transplant recipient. Am J Transplant. 2004;4(5):830–833. doi: 10.1111/j.1600-6143.2004.00410.x. [DOI] [PubMed] [Google Scholar]
- 9.Kumar R., Tripathi P., Singh S., Bannerji G. Clinical features in children hospitalized during the 2005 epidemic of Japanese encephalitis in Uttar Pradesh, India. Clin Infect Dis. 2006;43(2):123–131. doi: 10.1086/505121. [DOI] [PubMed] [Google Scholar]
- 10.Misra U.K., Kalita J. Movement disorders in Japanese encephalitis. J Neurol. 1997;244(5):299–303. doi: 10.1007/s004150050090. [DOI] [PubMed] [Google Scholar]
- 11.Murgod U.A., Muthane U.B., Ravi V., Radhesh S., Desai A. Persistent movement disorders following Japanese encephalitis. Neurology. 2001;57(12):2313–2315. doi: 10.1212/wnl.57.12.2313. [DOI] [PubMed] [Google Scholar]
- 12.Schioler K., Samuel M., Wai K. Vaccines for preventing Japanese encephalitis. Cochrane Database Syst Rev. 2007;3:CD004263. doi: 10.1002/14651858.CD004263.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bharati K., Vrati S. Japanese encephalitis: development of new candidate vaccines. Expert Rev Anti Infect Ther. 2006;4(2):313–324. doi: 10.1586/14787210.4.2.313. [DOI] [PubMed] [Google Scholar]
- 14.Hoke C.H., Nisalak A., Sangawhipa N., Jatanasen S., Laorakapongse T., Innis B.L. Protection against Japanese encephalitis by inactivated vaccines. N Engl J Med. 1988;319(10):608–614. doi: 10.1056/NEJM198809083191004. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi H., Pool V., Tsai T.F., Chen R.T. Adverse events after Japanese encephalitis vaccination: review of post-marketing surveillance data from Japan and the United States. The VAERS Working Group. Vaccine. 2000;18(26):2963–2969. doi: 10.1016/s0264-410x(00)00111-0. [DOI] [PubMed] [Google Scholar]
- 16.Heinz F.X., Mandl C.W., Holzmann H., Kunz C., Harris B.A., Rey F. The flavivirus envelope protein E: isolation of a soluble form from tick-borne encephalitis virus and its crystallization. J Virol. 1991;65(10):5579–5583. doi: 10.1128/jvi.65.10.5579-5583.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nybakken G.E., Nelson C.A., Chen B.R., Diamond M.S., Fremont D.H. Crystal structure of the West Nile virus envelope glycoprotein. J Virol. 2006;80(23):11467–11474. doi: 10.1128/JVI.01125-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin C.W., Wu S.C. A functional epitope determinant on domain III of the Japanese encephalitis virus envelope protein interacted with neutralizing-antibody combining sites. J Virol. 2003;77(4):2600–2606. doi: 10.1128/JVI.77.4.2600-2606.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mason P.W., Dalrymple J.M., Gentry M.K., McCown J.M., Hoke C.H., Burke D.S. Molecular characterization of a neutralizing domain of the Japanese encephalitis virus structural glycoprotein. J Gen Virol. 1989;70(Pt 8):2037–2049. doi: 10.1099/0022-1317-70-8-2037. [DOI] [PubMed] [Google Scholar]
- 20.Nybakken G.E., Oliphant T., Johnson S., Burke S., Diamond M.S., Fremont D.H. Structural basis of West Nile virus neutralization by a therapeutic antibody. Nature. 2005;437(7059):764–769. doi: 10.1038/nature03956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu K.P., Wu C.W., Tsao Y.P., Kuo T.W., Lou Y.C., Lin C.W. Structural basis of a flavivirus recognized by its neutralizing antibody: solution structure of the domain III of the Japanese encephalitis virus envelope protein. J Biol Chem. 2003;278(46):46007–46013. doi: 10.1074/jbc.M307776200. [DOI] [PubMed] [Google Scholar]
- 22.Holzmann H., Stiasny K., York H., Dorner F., Kunz C., Heinz F.X. Tick-borne encephalitis virus envelope protein E-specific monoclonal antibodies for the study of low pH-induced conformational changes and immature virions. Arch Virol. 1995;140(2):213–221. doi: 10.1007/BF01309857. [DOI] [PubMed] [Google Scholar]
- 23.Wu S.C., Lin C.W. Neutralizing peptide ligands selected from phage-displayed libraries mimic the conformational epitope on domain III of the Japanese encephalitis virus envelope protein. Virus Res. 2001;76(1):59–69. doi: 10.1016/s0168-1702(01)00246-5. [DOI] [PubMed] [Google Scholar]
- 24.Chu J.H., Chiang C.C., Ng M.L. Immunization of flavivirus West Nile recombinant envelope domain III protein induced specific immune response and protection against West Nile virus infection. J Immunol. 2007;178(5):2699–2705. doi: 10.4049/jimmunol.178.5.2699. [DOI] [PubMed] [Google Scholar]
- 25.Mota J., Acosta M., Argotte R., Figueroa R., Mendez A., Ramos C. Induction of protective antibodies against dengue virus by tetravalent DNA immunization of mice with domain III of the envelope protein. Vaccine. 2005;23(26):3469–3476. doi: 10.1016/j.vaccine.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 26.Wu S.C., Yu C.H., Lin C.W., Chu I.M. The domain III fragment of Japanese encephalitis virus envelope protein: mouse immunogenicity and liposome adjuvanticity. Vaccine. 2003;21(19/20):2516–2522. doi: 10.1016/s0264-410x(03)00042-2. [DOI] [PubMed] [Google Scholar]
- 27.Chu J.J., Rajamanonmani R., Li J., Bhuvanakantham R., Lescar J., Ng M.L. Inhibition of West Nile virus entry by using a recombinant domain III from the envelope glycoprotein. J Gen Virol. 2005;86(Pt 2):405–412. doi: 10.1099/vir.0.80411-0. [DOI] [PubMed] [Google Scholar]
- 28.Zakhartchouk A.N., Liu Q., Petric M., Babiuk L.A. Augmentation of immune responses to SARS coronavirus by a combination of DNA and whole killed virus vaccines. Vaccine. 2005;23(35):4385–4391. doi: 10.1016/j.vaccine.2005.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chu R.S., Targoni O.S., Krieg A.M., Lehmann P.V., Harding C.V. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J Exp Med. 1997;186(10):1623–1631. doi: 10.1084/jem.186.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beasley D.W., Barrett A.D. Identification of neutralizing epitopes within structural domain III of the West Nile virus envelope protein. J Virol. 2002;76(24):13097–13100. doi: 10.1128/JVI.76.24.13097-13100.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guirakhoo F., Heinz F.X., Kunz C. Epitope model of tick-borne encephalitis virus envelope glycoprotein E: analysis of structural properties, role of carbohydrate side chain, and conformational changes occurring at acidic pH. Virology. 1989;169(1):90–99. doi: 10.1016/0042-6822(89)90044-5. [DOI] [PubMed] [Google Scholar]
- 32.Modis Y., Ogata S., Clements D., Harrison S.C. A ligand-binding pocket in the dengue virus envelope glycoprotein. Proc Natl Acad Sci USA. 2003;100(12):6986–6991. doi: 10.1073/pnas.0832193100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roehrig J.T. Antigenic structure of flavivirus proteins. Adv Virus Res. 2003;59:141–175. doi: 10.1016/s0065-3527(03)59005-4. [DOI] [PubMed] [Google Scholar]
- 34.Rey F.A., Heinz F.X., Mandl C., Kunz C., Harrison S.C. The envelope glycoprotein from tick-borne encephalitis virus at 2 A resolution. Nature. 1995;375(6529):291–298. doi: 10.1038/375291a0. [DOI] [PubMed] [Google Scholar]
- 35.Alka, Bharati K., Malik Y.P., Vrati S. Immunogenicity and protective efficacy of the E. coli-expressed domain III of Japanese encephalitis virus envelope protein in mice. Med Microbiol Immunol. 2007;196(4):227–231. doi: 10.1007/s00430-007-0043-4. [DOI] [PubMed] [Google Scholar]
- 36.Zulueta A., Martin J., Hermida L., Alvarez M., Valdes I., Prado I. Amino acid changes in the recombinant Dengue 3 Envelope domain III determine its antigenicity and immunogenicity in mice. Virus Res. 2006;121(1):65–73. doi: 10.1016/j.virusres.2006.04.003. [DOI] [PubMed] [Google Scholar]


