Abstract
Community-acquired pneumonia remains a common illness with substantial morbidity and mortality. Current management challenges focus on identifying the likely etiologic pathogens based on an assessment of host risk factors, while attempting to make a specific etiologic diagnosis, which is often not possible. Therapy is necessarily empiric and focuses on pneumococcus and atypical pathogens for all patients, with consideration of other pathogens based on specific patient risk factors. It is important to understand the expected response to effective therapy, and to identify and manage clinical failure at the earliest possible time point. Prevention is focused on smoking cessation and vaccination against pneumococcus and influenza.
Keywords: Community-acquired pneumonia, Mortality, Lungs, Pneumococcus
Community-acquired pneumonia (CAP) is a common disease causing considerable morbidity and mortality in the United States. CAP is an alveolar infection that develops in the outpatient setting or within 48 hours of admission to a hospital. However, some patients developing pneumonia out of hospital have had recent contact with the health care environment, and these individuals are designated as having health care–associated pneumonia (HCAP), which may need to be managed differently from CAP. The clinical spectrum varies from a mild outpatient illness, with rapid resolution, to severe sepsis with multiorgan failure and death. The potential grave consequences are more likely with extremes of age and among patients with comorbid conditions.1
The annual incidence of CAP is between 5 and 11 per 1000 population, with the incidence being higher in elderly patients.2 Mortality from CAP continues to be unchanged even though newer antimicrobial therapy has been introduced in the last several decades.1 According to National Vital Statistics Report (2011), pneumonia, along with influenza, was the eighth leading cause of death in 2009.3 In 2006, 1.2 million people in the United States were hospitalized with pneumonia and 55,477 people died of the disease.4 Most cases of CAP occur in outpatients, in whom the mortality is less than 5%, but, when patients are admitted to the hospital, the mortality increases to more than 10%, and can exceed 30% when patients are admitted to the intensive care unit (ICU).5 The data from the German CAPNETZ Network trial showed that the mortality among patients hospitalized with CAP ranged from 5% to 20%, but was up to 50% in patients admitted to the ICU.6 Recently, investigators have shown that patients with CAP in a Medicare population have a 1-year mortality of more than 40%, suggesting that pneumonia may be a surrogate marker of severe underlying comorbidity, or that it initiates a series of adverse consequences for some patients that leads to their eventual death.7
Despite the availability of different guidelines and treatment options, the economic burden associated with CAP remains high at more than $17 billion annually in United States alone.8 Although most patients with CAP are outpatients, the greatest portion of the cost for this illness is borne by those admitted to hospital, making the decision about admission an important one for several reasons. A recent study noted that decreasing the length of stay by 1 day in a patient with CAP had a potential economic benefit of $2000.9 With new health care reforms imminent and the emphasis on better health care delivery, cost-effective treatment of pneumonia will assume greater significance.
There are several challenges with the management of CAP, from the accurate diagnosis of lung infiltrates, decisions about the site of care, and the choice of appropriate antibiotics. The Infectious Disease Society of America (IDSA)/American Thoracic Society (ATS) guideline from 2007 provides a summary of the approach to the treatment of CAP directed mainly towards primary care physicians, hospitalists, and emergency medicine physicians.1 Multiple validated severity assessment scores have been developed that stratify patients according to the risk of death and can be used as decision support tools to guide site-of-care decisions.10, 11 The emergence of drug-resistant organisms, particularly drug-resistant Streptococcus pneumoniae (DRSP), is another challenge in disease management. Biomarkers are increasingly being used to distinguish bacterial pneumonia from other causes and to help reduce the duration of antibiotic therapy.12 This article reviews the recent advances in the diagnosis, management, and potential complications associated with CAP.
Pathogenesis
In CAP, the major route of infection is microaspiration from a previously colonized oropharynx, but inhalation of suspended aerosolized microorganisms is the mechanism of infection for viruses, Legionella, and tuberculosis. Interactions between the host immune response, the virulence of the infecting organism, and the size of the inoculums determine whether a patient develops pneumonia.13 Defective cough, mucociliary clearance, and impaired local and humoral immunity predispose to severe pneumonia. Alcohol consumption and smoking are independent risk factors for the development of pneumonia. Medical comorbidities such as chronic obstructive pulmonary disease (COPD), congestive heart failure, chronic kidney disease, liver disease, and immune deficiency states have an increased predisposition for the development of CAP. Recent use of proton pump inhibitor therapy started within 30 days has been identified as a risk factor for CAP.14 Elderly patients are at increased risk for development of pneumonia and, when it occurs, they are more likely to die than younger individuals.2 Although many patients develop severe pneumonia because of immune impairment, others develop acute lung injury (acute respiratory distress syndrome [ARDS]) as a consequence of unilateral pneumonia because of an inability to localize the immune response to the initial site of infection, possibly because of the presence of a genetic variation in their immune responsiveness.15, 16
Causes
The most common organism causing CAP, in all patient populations, is S pneumoniae, or pneumococcus. Other pathogens include Hemophilus influenzae (particularly in cigarette smokers), Moraxella catarrhalis, Staphylococcus aureus (after influenza and recently in the form of methicillin-resistant S aureus [MRSA]), viruses (including influenza, respiratory syncytial virus, parainfluenza, and epidemic viruses), and atypical pathogens such as Mycoplasma pneumoniae, Chlamydophila pneumoniae, and Legionella pneumophila. In most series, atypical pathogens are common, including in those admitted to the ICU, where they can account for up to 20% of the identified pathogens. In addition, many investigators have documented that atypical pathogens may coexist with bacterial pathogens, accounting for their presence in up to 60% of patients with CAP, when serologic testing is used.17
Gram-negative bacteria (Pseudomonas aeruginosa, Klebsiella pneumoniae, Escherichia coli, Enterobacter spp, Serratia spp, Proteus spp) are the causal agents in up to 10% of patients with CAP, but may be more common in patients who develop pneumonia out of the hospital and have HCAP risk factors. Gram-negative bacteria have been associated with severe CAP, and K pneumoniae was noted to be an independent risk factor for mortality in severe CAP.18 In one study from Korea, in a multivariate analysis, the risk factors associated with gram-negative CAP were septic shock (with an odds ratio of 4.1), cardiac disease, smoking, hyponatremia, and dyspnea, emphasizing the association of these organisms with severe illness.19 Enterobacter CAP behaves more like hospital-acquired pneumonia and is associated with prolonged mechanical ventilation, delay in initiation of antibiotics, and longer ICU stay.20 Risk factors for community-acquired P aeruginosa pneumonia include bronchiectasis, immunocompromised state, use of multiple courses of antibiotics, prolonged glucocorticoids in patients with COPD, and recent hospitalization.21 Anaerobic organisms should be considered when aspiration is suspected.
Influenza is a common viral cause of CAP, with a seasonal variation in frequency. Primary influenza pneumonia tends to cause severe pneumonia, which can be either caused by the virus itself or a result of secondary bacterial infection with pneumococcus, S aureus, or H influenzae. High-risk patients include those with chronic heart or lung disease, diabetes, immunosuppression, hemoglobinopathy, renal disease, and otherwise healthy individuals more than 65 years of age. Other viruses that cause CAP include Parainfluenza virus, respiratory syncytial virus (RSV), human metapneumovirus, severe acute respiratory syndrome virus, varicella, Hantavirus, and adenovirus. Many of these patients have viral infection as part of a mixed infection, often with bacterial pathogens.22
Emergence of DRSP and community-acquired MRSA is a matter of concern that has complicated the empiric therapy choices for patients with CAP. DRSP is seen most often in patients older than 65 years of age, and in those with a history of alcoholism, antibiotic therapy within 3 months, multiple medical comorbid conditions, exposure to children in day care, or those with immune-compromised states.23 Community-associated MRSA (CA-MRSA) pneumonia occurs in patients with no prior health care exposure, usually after influenza, and may lead to a severe necrotizing pneumonia, although milder forms of illness have also been reported.24 In patients with severe illness, the organism may produce a variety of exotoxins, including the Panton-Valentine leukocidin (PVL), which may contribute to lung necrosis.25 Multidrug resistance has been reported with CA-MRSA strains but, in general, these organisms are more drug sensitive than their hospital-acquired counterparts.26
Other less common causes of CAP include Mycobacterium tuberculosis, Coxiella burnetii (Q fever), Burkholderia pseudomallei (melioidosis), Chlamydophila psittaci (psittacosis), endemic fungi (histoplasmosis, coccidioidomycosis, blastomycosis), Pasteurella multocida, Bacillus anthracis, Actinomyces israeli, Francisella tularensis (tularemia), and Nocardia spp. These organisms should be included in the differential diagnosis when evaluating a patient with CAP, depending on the presence of specific risk factors that are noted in the clinical history.
Clinical evaluation
Presentation
Patients with CAP usually present with an acute illness of 1 to 2 days duration. In those with intact immune response, systemic and respiratory symptoms such as cough, dyspnea, fever, and pleuritic chest pain predominate. Fever and chills have a sensitivity of 50% to 85%, and dyspnea a sensitivity of 70% for the diagnosis of CAP, whereas purulent sputum has a sensitivity of only 50%.23 Hemoptysis suggests necrotizing infection, such as lung abscess, tuberculosis, or gram-negative pneumonia, but is also a common finding, even in patients with bronchitis. In patients with disease and age-associated impairments in the immune response, the clinical presentation may be subtle, and involve primarily nonrespiratory findings. In the elderly, chest pain and cough may be absent in the early course of the disease, and fever and confusion may be the only symptoms.23 Other complaints such as lethargy, falling, poor oral intake, and decompensation of a chronic illness could also occur in patients with comorbid conditions and among the elderly.
History and Physical Examination
A good history and physical examination are essential for determining the possible causal agent and assessing the severity of illness, which in turn helps with management. Risk factors for HCAP, such as hospitalization or antibiotic therapy in the past 90 days, residence in a long-term care facility, chronic dialysis, outpatient wound care, or home infusion therapy, needs to be identified, because these patients are at risk for drug-resistant gram-negative organisms and S aureus. The history should identify risk factors for DRSP and gram-negative organisms, as discussed earlier. It is also important to elicit recent travel history and exposure to birds, bats, farm animals, and rabbits (Table 1 ).
Table 1.
Pathogens by risk factors and underlying conditions
| Underlying Conditions | Suspected Pathogens |
|---|---|
| Chronic obstructive lung disease | H influenza, P aeruginosa, Legionella species, S pneumonia, M pneumonia, C pneumoniae |
| Alcoholism | S pneumonia, oral anaerobes, K pneumonia, Acinetobacter species, M tuberculosis |
| HIV infection | S pneumoniae, H influenzae, Salmonella, Cytomegalovirus, Cryptococcus, P jiroveci, anaerobes, M tuberculosis |
| Aspiration | Anaerobes, enteric gram-negative bacilli, chemical pneumonitis |
| Exposure to bats | Histoplasma capsulatum |
| Exposure to birds | C psittaci, Cryptococcus neoformans, H capsulatum |
| Contact with farm animals | Coxiella burnetii (Q fever) |
| Exposure to rabbits | Francisella tularensis |
| Travel to southwest United States | Coccidioides immitis |
| Nursing home resident | S pneumoniae, gram-negative bacilli, anaerobes, H influenzae, MRSA, C pneumoniae, M tuberculosis |
| Recent influenza infection | S pneumoniae, S aureus (including MRSA), H influenzae |
| Structural disease of lung (bronchiectasis, cystic fibrosis) | P aeruginosa, P cepacia, S aureus |
| Bioterrorism | Bacillus anthracis, Yersinia pestis, Francisella tularensis |
| Cruise ship, sauna, hot tub, or hotel stay within 2 wk | Legionella species |
Abbreviation: HIV, human immunodeficiency virus.
On physical examination, patients may have tachypnea, tachycardia, crackles, bronchial breath sounds, and findings of pleural effusion. Clinicians should pay attention to other clues, such as relative bradycardia in relation to fever, which can be seen in infections caused by agents like Legionella, Chlamydophila, and Mycoplasma.27 Mycoplasma can also cause cervical lymphadenopathy, arthralgia, and bullous myringitis. Poor outcomes are noted in patients with a respiratory rate greater than 30 breaths/min, diastolic blood pressure less than 60 mm Hg, systolic blood pressure less than 90 mm Hg, heart rate greater than 125 beats/min, and temperature less than 35°C or greater than 40°C.23 These clinical findings can be used to determine the risk of death, by incorporating them into prognostic scoring, using the Pneumonia Severity Index (PSI), the CURB-65 criteria (a modification of the British Thoracic Society scoring system), or other tools (discussed later).
Other than raising clinical suspicion, no combination of symptoms and signs can accurately diagnose pneumonia in the clinical setting, and the definitive diagnosis requires a chest radiograph.28 The clinical diagnosis has an overall sensitivity ranging from 70% to 90% and specificity between 40% and 70%. Therefore, whenever there is suspicion of CAP, a chest radiograph should be obtained for corroboration of the physical findings.29 Certain chest radiographic findings can also suggest more severe illness, including the presence of multilobar infiltrates, rapid progression of infiltrates, pleural effusion, and findings of necrotizing pneumonia.
Diagnostic Approach
In the outpatient setting, extensive diagnostic testing is not routinely performed, because results are nonspecific, and antibiotic treatment should be initiated empirically.1 Even for inpatients, the value of diagnostic testing is limited and, when outcomes were compared using pathogen-directed therapy, compared with empiric therapy, there was limited benefit of testing.30 In one prospective study of 262 patients from the Netherlands, a pathogen was identified in 60% of cases. Adequate sputum samples were obtained from only 44 patients, Gram stain was diagnostic and confirmed by a positive sputum in 82%, urine pneumococcal antigen was positive in 54% of cases, blood cultures were positive in 16%, and bronchoscopic samples added benefit to diagnostic yield when sputum could not be expectorated.31 In most studies, a specific causal diagnosis is obtained in less than 50% of patients with CAP, even with extensive diagnostic testing, and the major focus of laboratory testing should be to assess severity of illness and allow early identification of the presence of pneumonic complications.
White blood cell count may be normal on admission, and leukopenia is seen in patients with overwhelming pneumococcal pneumonia with sepsis and pneumonia caused by gram-negative organisms.27 Thrombocytosis and thrombocytopenia are associated with worse 30-day mortality in patients admitted with CAP.32 Hyponatremia (<130 mEq/L) is also associated with a poor outcome, if present on admission, in patients with CAP.33 The IDSA/ATS guidelines recommended testing for patients with pneumonia (Table 2 ).
Table 2.
Recommended tests per IDSA/ATS 2007 guidelines
| Tests | Indications |
|---|---|
| Blood culture | ICU admission. Consider if multiple of: cavitary infiltrate, leucopenia, active alcohol abuse, chronic liver disease, asplenia, pneumococcal UAT positive, pleural effusion |
| Sputum culture | ICU admission, failure of outpatient antibiotics, cavitary infiltrate, severe COPD/structural disease, active alcohol abuse, legionella or pneumococcal UAT positive, pleural effusion |
| Legionella UAT | ICU admission, failure of outpatient antibiotics, active alcohol abuse, recent travel within 2 wk, pleural effusion |
| Pneumococcal UAT | ICU admission, failure of outpatient antibiotics, leukopenia, asplenia, active alcohol abuse, chronic liver disease, pleural effusion |
| Pleural fluid culture/thoracentesis | Significant pleural effusion |
| Endotracheal aspirate/bronchoscopic washings | ICU admission |
| Fungal culture and TB testing | Cavitary infiltrate |
| Special media for legionella | Positive UAT for legionella |
Abbreviations: TB, tuberculosis; UAT, urinary antigen testing.
Radiographic Evaluation
Radiographic evidence of lung infiltration provides a sensitive, but not specific, confirmation of community-acquired pneumonia. Chest radiograph may show areas of consolidation, pleural effusion, lung abscess, necrotizing pneumonia, or multilobar illness. It may help in pattern recognition of the disease process: H influenzae has a peribronchial distribution of bronchopneumonia; S pneumoniae infection can have either lobar consolidation or bronchopneumonia; atypical pathogens may have an alveolar and interstitial pattern; aspiration most commonly involves the superior segment of the right lower lobe or the posterior segment of the right upper lobe; hematogenous dissemination follows the distribution of blood flow and may lead to bilateral nodular infiltrates.27 Cavitation or necrotizing pneumonia suggests infection with anaerobes, gram-negative bacteria, or S aureus, including MRSA. Loculated effusion can be ruled out by decubitus film or computed tomography (CT). Chest ultrasound is increasingly being used to assess the size, and to identify a safe site for sampling of pleural fluid.
The usefulness of chest radiography is suboptimal in patients with very early infection, dehydration, severe granulocytopenia, structural changes such as with bullous emphysema, and in obese patients. It is reasonable to repeat a follow-up radiograph in 24 to 48 hours in patients who have had a negative initial finding, but have clinical signs of pneumonia.1 There may be interobserver variability in chest radiographic interpretation of pneumonia. In a study that compared the readings of at least 2 radiologists, positive agreement (59%) was less frequent than negative agreement (94%).34 CT has better sensitivity in diagnosing an infiltrate than chest radiography, but it is not routinely used, because there is a lack of evidence that use of CT scan improves outcomes.35
Sputum Examination
Sputum should be sent for Gram stain and culture before starting therapy, but primarily in patients suspected of infection with drug-resistant or unusual pathogens. A good specimen contains no more than 10 squamous epithelial cells and more than 25 polymorphonuclear cells per low per field. The Gram stain pattern on sputum can help with tailoring of antibiotics, particularly if it shows a pathogen that would not be treated routinely (such as clumps of gram-positive cocci, suggesting S aureus). The sensitivity of identifying S pneumonia is only 50% to 60% and specificity is greater than 80%.27 It is less likely to have S aureus or gram-negative pneumonia in the absence of these organisms on Gram stain of a good sputum sample, but this test is more valuable if positive than if negative. Routine culture of expectorated sputum is not useful in the absence of an informative Gram stain. The usefulness of real-time polymerase chain reaction testing of sputum samples has not been shown. Culture can be obtained from intubated patients by collecting an endotracheal aspirate.
Blood Culture
A positive blood or pleural culture is seen in less than 20% of patients with pneumonia but, if present, helps with establishing the diagnosis. Most positive cultures are of S pneumoniae. The IDSA/ATS guidelines recommend blood culture testing in patients admitted to ICU, and in those with multiple other risk factors, including active alcohol abuse, liver disease, cavitatory lung disease, asplenia, leukopenia, and pleural effusion. These recommendations are based, in part, on the data from 13,043 Medicare patients who showed that a true-positive blood culture was associated with no previous antibiotics, underlying liver disease, systolic blood pressure less than 90 mm Hg, fever less than 35°C or greater than 40°C, pulse greater than 125 beats/min, blood urea nitrogen greater than 10.71 mmol/L (30 mg/dL), serum sodium less than 130 mmol, and leukocyte count less than 5000 or greater than 20,000 cells/mL. The diagnostic yield of blood cultures increased in patients with 1 or more risk factor and in those who had not received antibiotics before blood was collected.36
Urinary Antigen Testing
Urinary antigen testing (UAT) is commercially available for detection of capsular polysaccharide of S pneumoniae and L pneumophilia serogroup 1. Pneumococcal urinary antigen tests have a sensitivity of 50% to 80% and specificity of more than 90%.37 The degree of positivity is correlated with the PSI for S pneumoniae.38 False-positive tests occur in patients who have had CAP from pneumococcus within the previous 3 months. UAT for Legionella has a sensitivity of 70% to 90% and a specificity of up to 99% for detection of infection with serogroup 1, by far the commonest species to infect humans.27 However, it does not detect other types of Legionella, so a negative finding cannot rule out this infection. In one study, the use of UAT for Legionella had increased with time, leading to more diagnoses of serogroup 1 infection, but a decreased mortality from Legionella, suggesting that urinary antigen testing was finding milder illness than had been recognized previously.39 Although one prospective study of 474 episodes of CAP from Spain found that S pneumoniae was diagnosed by urinary antigen test in 43.8% and helped physicians optimize antibiotic choice,40 in general, it remains uncertain whether a positive result of any urinary antigen test changes CAP management, or whether it is primarily of epidemiologic interest.
Serology Testing
Serologic tests are of questionable importance in the initial setting, but are useful for the epidemiologic diagnosis of agents that are not readily cultured, although results are generally not available for weeks, and require the collection of both acute and convalescent serum samples. The diagnosis of most pathogens is based on acute and convalescent blood serologies showing a fourfold increase in immunoglobulin (Ig) G obtained 2 to 6 weeks apart, which applies to C pneumoniae, C psittaci, Q fever, and M pneumoniae. Ig M antibodies start to increase in the acute phase and are useful in the early course of the disease. Cold agglutinins are sometimes present in patients with M pneumoniae.
Polymerase Chain Reaction
Nucleic acid amplification tests provide rapid test results in CAP for atypical agents such as viruses, Mycoplasma, Chlamydophila, and Legionella. Polymerase chain reaction (PCR) assays were widely used for detecting influenza virus in the recent H1N1 epidemic. Direct immunofluorescence or enzyme immunoassay are available for detection of viral antigens like influenza, RSV, adenovirus and parainfluenza viruses. The usefulness of PCR assays in managing CAP has not been proven, and the concern with this method is that it is so sensitive that, if a respiratory sample is positive, it cannot distinguish colonization from infection unless the presence of a specific pathogen is itself diagnostic of infection (such as M tuberculosis). However, the test may be valuable if negative, because the absence of a suspected pathogen by PCR may permit a more focused antibiotic therapy approach.
Biomarkers
Several newer biomarkers have been developed (midregional proadrenomedullin, midregional proatrial natriuretic peptide, proarginin-vasopressin, proendothelin-1, procalcitonin [PCT], C-reactive protein [CRP]) to identify patients with bacterial infection and to define the prognosis of CAP. In one recent study, cardiac biomarkers, such as midregional proadrenomedullin, were better predictors of 28-day and 180-day mortality than inflammatory biomarkers such as PCT. In that study, biomarkers correlated with disease severity and mortality, but did not help with causal diagnosis.41 In another prospective study evaluating the relationship between biomarkers and ICU admission, inflammatory biomarkers helped identify patients needing intensive care monitoring, including those requiring delayed ICU admission.42
The inflammatory biomarkers that have been studied most extensively are CRP and PCT, both of which are acute-phase reactants primarily produced by the liver in the presence of bacterial infection, but not viral illness. CRP may identify which patients with acute respiratory symptoms have infectious pneumonia; levels are higher in patients who require hospitalization and in those with pneumococcal and Legionella infection.43 PCT is a hormokine, produced in response to microbial toxins and certain host responses associated with bacterial infection, but inhibited by viral-related cytokines. Serum levels tend to be high in patients with CAP, who benefit from antibiotic therapy, and in those with an increased risk of death from CAP. Serial measurements of serum levels have also been used to define when antibiotics can be safely stopped in the presence of CAP.12, 44, 45 In one study of 302 patients with radiographic infiltrates and suspected CAP, initiation of antibiotics and duration of therapy were determined by randomizing patients to management by an algorithm dictated by serial PCT measurements versus management by clinical assessment. The PCT-guided group had significantly fewer antibiotic prescriptions on admission and less antibiotic usage, and the duration of therapy was reduced from 12 to 5 days with similar clinical success.46
Severity assessment
One of the most important decisions in the management of pneumonia is to assess the severity of the disease, which can be used to predict mortality risk and may be a surrogate measure to define the site of care (outpatient, hospital ward, or ICU). Proper site-of-care decisions can have an impact on mortality, with several studies showing that delayed admission to the ICU leads to a poor outcome.47, 48 The most widely used prognostic scoring systems are the PSI and the CURB-65 score. In clinical practice, the PSI is not widely used because it is complex and difficult to calculate a score.49 In addition to these general scoring tools, some evaluations are designed to identify the need for ICU admission, including the IDSA/ATS criteria for severe CAP, and an Australian method called the SMART-COP, which is designed to predict the need for intensive respiratory or vasopressor support. Other prediction rules are available and their clinical application varies widely.
The PSI was developed to identify patients with a low risk of dying who could be safely discharged home and receive outpatient treatment. The PSI stratifies patients into 5 categories based on 30-day mortality, by using a scoring system based on 20 factors. It includes demographic characteristics, coexisting illnesses, physical examination findings, laboratory measurements, and radiographic finding.50 Patients in classes IV (30-day mortality risk of 4%–10%) and V (27% risk of death at 30 days) are usually admitted to the hospital and often to the ICU. Those in low-risk classes I and II are often treated as outpatients, whereas it is a clinical judgment whether those in class III should be hospitalized. The PSI score includes age as an important determinant of point scoring and hence can overestimate the severity of illness in the elderly and in those with comorbidity. In one study of patients in PSI class V, only approximately 20% needed ICU admission, and these tended to be individuals who scored points based on acute illness features, and not on age and comorbid illness factors.51 In contrast, the PSI may underestimate severity of illness in young patients without comorbid illness, especially if their vital sign abnormalities are slightly less than the cutoffs used in the scoring system.52 This was a particular problem during recent influenza epidemics that have involved primarily younger populations, in which PSI scoring was not valuable for defining the need for ICU admission.
The CURB-65 score from the British Thoracic Society is an easy scoring system to use, with the score (0–5) being defined (1 point each) by the presence of confusion, blood urea nitrogen greater than 7.0 mol/L (19.6 mg/dL), respiratory rate of 30 breaths/min or greater, systolic blood pressure less than 90 mm Hg or diastolic blood pressure no greater than 60 mm Hg, and age 65 years or older. Patients with 2 of these criteria have a high enough risk of death that they should probably be admitted to the hospital, while those with 3 or more points should be considered for ICU admission. Modifications of this tool, without the laboratory measurement of blood urea nitrogen (CRB-65) have also been found to be similarly accurate.53 The limitation of this approach is its focus on assessment of only clinical parameters, such as vital signs, but without measurement of oxygenation or serial measurement of severity of illness after the initial hospital admission, and that it does not evaluate the presence of comorbid illness and its decompensation from baseline.54
Serum biomarkers can be used to supplement data obtained by prognostic scoring.55 Data from the German Competence Network for the Study of Community Acquired Pneumonia (CAPNETZ) Study Group, showed that all new biomarkers were good predictors of short-term and long-term all-cause mortality and correlated with CRB-65 score.41 In other studies, low levels of PCT were able to define patients at low risk of death regardless of findings using severity scoring. Huang and colleagues55 as well as Kruger and colleagues56 found, that even in patients identified as high risk using CURB-65 or PSI, a low PCT value predicted a low chance of dying.55, 56
Severe CAP
Scoring systems can also be used to help define which patients need ICU care, identifying those with severe illness. The IDSA/ATS guidelines and the PIRO (predisposition, insult, response, and organ dysfunction) scoring system were developed to help define mortality risk in patients with severe pneumonia. According to the 2007 IDSA/ATS guidelines, severe CAP is present if a patient needs invasive mechanical ventilation or requires vasopressors or has any 3 of 9 from the minor criteria listed later. Liapakou and colleagues57 found that patients meeting the major criteria needed ICU admission, but those patients who had only minor criteria present had no increased mortality risk, regardless of how many criteria were met. More recently, Brown and colleagues58 found that both the positive and negative predictive value of minor criteria exceeded 80% if 4 criteria were used to define the need for ICU admission rather than just 3 criteria.
IDSA/ATS 2007 criteria for severe CAP.
- Major criteria
-
1.Invasive mechanical ventilation
-
2.Septic shock with the need for vasopressors
-
1.
- Minor criteria
-
1.Respiratory rate 30 breaths/min
-
2.Alveolar oxygen partial pressure (Pao 2)/forced inspiratory oxygen (Fio 2) ratio 250
-
3.Multilobar infiltrates
-
4.Confusion/disorientation
-
5.Uremia (blood urea nitrogen level 1.1 mmol/L)
-
6.Leukopenia (white blood cells <4000 cells/mm3)
-
7.Thrombocytopenia (platelet count <100,000 cells/mm3)
-
8.Hypothermia (core temperature <36°C)
-
9.Hypotension requiring aggressive fluid resuscitation
-
1.
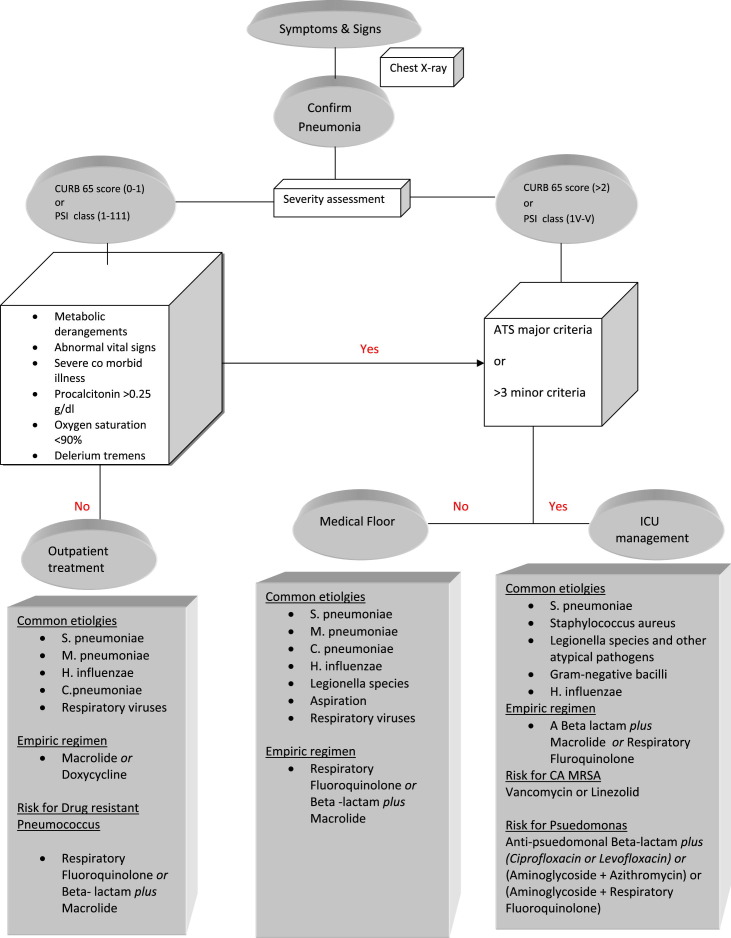
The PIRO score is calculated within 24 hours of ICU admission, with 1 point given for each variable: comorbidities (COPD, immunocompromise), age greater than 70 years, multilobar opacities on chest radiograph, shock, severe hypoxemia, acute renal failure, bacteremia, and acute respiratory distress syndrome. The maximum score that can be achieved is 8. Patients are stratified into 4 levels of risk: (a) low, 0 to 2 points; (b) mild, 3 points; (c) high, 4 points; and (d) very high, 5 to 8 points. The PIRO score performed well as a 28-day mortality prediction tool in patients with CAP requiring ICU admission, with a better performance than APACHE II and IDSA/ATS criteria.59 The SMART–COP tool was developed to identify the need for intensive respiratory or vasopressor support (IRVS), rather than a specific site-of-care decision. This tool uses a complex scoring system with the following values: low systolic blood pressure (<90 mm Hg) (2 points), multilobar pneumonia (1 point), low albumin level (<3.5 g/dL) (1 point), high respiratory rate (≥25–30 breaths/min) (1 point), tachycardia (>125 beats/min) (1 point), confusion (1 point), poor oxygenation (2 points), and low arterial pH (<7.35) (2 points). When this method was used, the finding of a patient with a score of more than 3 points identified 92% of those needing IRVS, with a specificity of 62.3%, whereas the PSI and CURB-65 did not perform as well for this endpoint.60 An algorithm for decision on site of care based on scoring system and treatment strategy is provided later (Fig. 1 ).
Fig. 1.
A proposed algorithm for site of care and treatment of CAP and common organisms per the IDSA/ATS guidelines. CA, community acquired.
Treatment and prognosis
Early diagnosis and timely administration of antibiotics are associated with improved outcomes in patients with CAP.61, 62 Although administration of therapy within 4 to 6 hours of arrival at the hospital can reduce mortality, it is important to only use antibiotics when the diagnosis is certain, because indiscriminate use of antibiotics in the absence of radiographic pneumonia has limited benefit and a real risk of antibiotic-associated adverse events, including drug-induced infectious diarrhea. According to IDSA/ATS guidelines, the first dose of antibiotic should be given in the emergency department, preferably within 4 to 6 hours of arrival, but no time period is specified. Because no diagnostic testing can rapidly identify the causal pathogens in a patient with CAP, initial therapy is empiric, based on an epidemiologic assessment of patient risk factors for specific pathogens. This assessment requires a careful history of patient comorbidity, recent antibiotic therapy history (within the past 3 months), and identification of pathogen-specific risk factors (see Table 1; Box 1 ).
Box 1. Treatment regimen per ATS/IDSA 2007 guidelines in different settings.
Outpatient treatment
- Previously healthy individual
-
•A macrolide or doxycycline.
-
•
- Presence of comorbid disease or risk factors for DRSP
-
•A respiratory fluoroquinolone (gemfloxacin, levofloxacin, moxifloxacin) or a β-lactam (high-dose amoxicillin or amoxicillin-clavulanate) plus a macrolide (azithromycin, clarithromycin)
-
•
Inpatient non-ICU
-
•
A respiratory fluoroquinolone (levofloxacin 750 mg or moxifloxacin) or
-
•
β-lactam (cefotaxime, ceftriaxone, or ertapenem in selected patients) plus a macrolide (intravenous azithromycin)
Patients in ICU
- No pseudomonal risk factors present
-
•A β-lactam (cefotaxime, ceftriaxone, or ampicillin-sulbactam) plus a macrolide (azithromycin) or respiratory fluoroquinolone (levofloxacin 750 mg or moxifloxacin).
-
•In patients allergic to penicillin – respiratory fluoroquinolone plus aztreonam.
-
•
- If community-acquired MRSA is suspected
-
•Vancomycin (and possibly clindamycin) or linezolid alone added to above regimen.
-
•
- If Pseudomonas is suspected
-
•A β-lactam with activity against P aeruginosa (piperacillin-tazobactam, cefepime, imipenem, or meropenem) plus either ciprofloxacin or levofloxacin, or
-
•A β-lactam with activity against Pseudomonas plus an aminoglycoside and azithromycin or a nonpseudomonal respiratory fluoroquinolone (moxifloxacin)
-
•
The IDSA/ATS guidelines recommend outpatient treatment with a macrolide or doxycycline for previously healthy adult patients with no risk factors for DRSP. In patients with risk factors for DRSP, a respiratory fluoroquinolone or a β-lactam antibiotic plus a macrolide or doxycycline is recommended. In choosing between these options, it is important to take a history about antibiotic usage in the past 3 months and to use an agent that is different from what has recently been used, because recent therapy may predispose to pneumococcal resistance to the agent used, rendering that therapy less effective.
For patients admitted to the hospital, but not to the ICU, an intravenous respiratory fluoroquinolone or a β-lactam plus a macrolide should be used. As mentioned earlier, the choice should be influenced by a history of which antibiotics have been used in the past 3 months, using agents from a different class, if possible. Doxycycline is an alternative to a macrolide. Ertapenem is an alternative to β-lactam agents such as cefotaxime, ceftriaxone, or ampicillin-sulbactam, and should be considered for patients with risk factors for infection with gram-negative pathogens other than P aeruginosa. All patients with CAP should have routine therapy directed at pneumococcus and atypical pathogens, plus other organisms, as dictated by specific risk factors. The routine coverage for atypical pathogens is based on outcome studies that show that the addition of a macrolide to a β-lactam, or the use of a quinolone alone, leads to better outcome than β-lactam monotherapy.1 In addition, some studies have shown a high frequency of atypical pathogen coinfection in patients with bacterial CAP.
Current CAP guidelines do not recommend monotherapy with any agent, including a quinolone, for patients with severe CAP who are admitted to the ICU. In patients with bacteremia (pneumococcal and other), atypical pathogen coverage with a macrolide (monotherapy or combination) improves mortality compared with treatment regimens with a quinolone, particularly quinolone monotherapy.63, 64 Combination therapy with a β-lactam and a macrolide has a survival advantage compared with quinolones alone in patients in the ICU, and in the 1 prospective study that compared quinolone monotherapy with a β-lactam/quinolone combination therapy the monotherapy arm was not as effective.65 In addition, in patients with pneumococcal bacteremia, especially in those with severe illness, the use of dual therapy (usually by adding a macrolide to a β-lactam) is associated with better outcome than with monotherapy, implying benefit from atypical pathogen coverage or from the antiinflammatory effect of the macrolide.64 In a prospective study by Rodriguez and colleagues66 on 279 patients with CAP and shock requiring vasopressors, combination therapy with either a β-lactam and a macrolide or a β-lactam and a quinolone had a 28-day survival advantage compared with monotherapy with a β-lactam or a quinolone alone.
Based on these data, in patients in the ICU, an intravenous β-lactam plus either a macrolide or respiratory fluoroquinolone is recommended for patients without pseudomonal risk factors. In patients with risk factors for pseudomonal infection, an antipseudomonal β-lactam should be combined with either levofloxacin or ciprofloxacin, or the antipseudomonal β-lactam can be combined with both an aminoglycoside and either azithromycin or a respiratory quinolone. In patients allergic to penicillin, a respiratory fluoroquinolone should be used with aztreonam as an alternative regimen. When CA-MRSA is suspected, vancomycin or linezolid should be added to the other recommended agents. However, it may be necessary to add an anti–toxin producing agent, because part of the illness caused by CA-MRSA is mediated by bacterial exotoxin production. To stop toxin production, it may be necessary to add clindamycin to vancomycin, or to use linezolid alone.
Duration of Therapy
Outpatients with mild-to-moderate CAP are treated for 7 days or fewer with oral antibiotics, and therapy is stopped if they are afebrile and clinical features of pneumonia are resolving (cough, dyspnea, and sputum production). For inpatients, antibiotics are switched from intravenous to oral once the patient is afebrile for at least 2 occasions 8 hours apart, is able to take food by mouth, and there are clinical signs of improvement (in parameters such as cough, dyspnea, sputum production, oxygenation, and vital sign abnormalities), and this usually happens by the second or third hospital day. The switch to oral antibiotics can also be done for bacteremic patients, although it may take longer for these patients to reach clinical stability compared with nonbacteremic patients.67 Use of PCT as a guide to decide on the duration of antibiotic use is supported by clinical trial data.46 The duration of therapy should be a minimum of 5 days, providing that the patient is afebrile for 48 to 72 hours, there is no sign of extrapulmonary infection, the correct therapy was used initially, and the organism identified is not S aureus or P aeruginosa.
Complications
With appropriate antibiotic treatment, most cases of CAP resolve without complications. However, the treating physician should be alert to potential complications that, if not detected early, can lead to adverse outcomes. If the patient is responding well to therapy, no immediate follow-up radiograph is needed, and imaging is only done 4 to 6 weeks after discharge to define a new radiographic baseline. In most patients, the chest radiograph usually clears within 4 weeks, especially in patients younger than 50 years without underlying pulmonary disease or bacteremia. However, resolution may be delayed for 12 weeks or longer in older individuals and those with underlying lung disease and bacteremia.
Treatment Failure
In about 15% of patients, there is a lack of response or clinical deterioration despite antibiotic therapy. The IDSA/ATS guidelines define early failure as progressive pneumonia or clinical deterioration, occurring in the first 72 hours of therapy, usually with respiratory failure or septic shock, and is a consequence of inappropriate antibiotic therapy or an incorrect initial diagnosis. Later failure or nonresponse is often caused by a nosocomial infection, a disease-related or therapy complication, or a noninfectious process (eg, pulmonary embolism, inflammatory lung disease).
If the patient has persistent fever, worsening dyspnea, unresolving pneumonia symptoms, and continued debility, a repeat radiograph should be done focusing on a broad differential diagnosis, including therapy for an unusual or drug-resistant pathogen (tuberculosis, endemic fungus, or a zoonosis), a pneumonic complication (empyema), an antibiotic complication (drug-induced colitis) or a nonpneumonic diagnosis (inflammatory lung disease, malignancy). Diagnostic testing can include a chest CT scan, bronchoscopy, and, in some cases, open lung biopsy. Organizing pneumonia is a complication of viral lung infection and other processes, and is characterized by fibroblast proliferation and diagnosed by a combination of radiographic findings, bronchoscopic lung biopsy, and the absence of ongoing infection. It is often managed with a therapeutic trial of steroids. The definitive investigation is an open lung biopsy.
Parapneumonic effusion and empyema are complications that can lead to apparent treatment failure. The chest radiograph shows an effusion, which should be sampled, and, if a low pleural fluid pH is present (<7.2 if previously healthy, but <7.3 if chronically ill) or if organisms are present, chest tube drainage and prolonged antibiotic therapy is required. A connection between the pleural space and the lung can develop and result in a bronchopleural fistula, which can be caused by erosion of the lung infection to the pleural surface. Bronchopleural fistula is initially treated conservatively with antibiotics and a chest tube, but sometimes requires surgical repair. Localized bronchiectasis can be a long-term sequela of CAP, as a result of injury and dilation of the bronchus, and can be seen on CT scan of the chest. Patients present with chronic productive sputum and recurrent infection on the same area. Treatment is with postural drainage, antibiotics for exacerbation, and bronchodilators for coexisting airflow obstruction.
Recurrent pneumonia can occur after clinical and radiographic resolution of pneumonia. If it is present, whether it is in the same or a different area as the original infection should be determined. If it is in the same area, an anatomic problem (obstruction by tumor or foreign body) needs to be considered, whereas, if it is at another site, it may be the consequence of general immune impairment. The risk of this problem is higher in the elderly, those with a history of alcoholism, and in smokers. An underlying systemic immune deficiency should be ruled out by measuring quantitative Ig levels.
Prevention
A detailed discussion of prevention is beyond the scope of this article. In the IDSA/ATS guidelines, the mainstay of prevention is pneumococcal and influenza vaccination for at-risk individuals, and provision of smoking cessation information to those smoking cigarettes at the time of pneumonia onset.1 Influenza vaccine is recommended during the appropriate season, for all persons aged 50 years or older, and for those with specific risk factors, including pregnant women and those with chronic heart, lung, metabolic, hematologic, or immune-compromising illnesses. Pneumococcal polysaccharide vaccine should be given to all patients aged 65 years or older, and to younger patients with chronic heart or lung disease, asplenia, diabetes mellitus, and to residents of long-term care facilities. One revaccination after 5 years should be given to those with either a poor immune response or after age 65 years for those first immunized before the age of 65 years. In guidelines, and also in performance measures for hospitalized patients, vaccination should be given before discharge for all patients admitted with CAP.
Footnotes
Conflict of interest: Dr Nair has no potential conflicts of interest to declare. Dr Niederman has served as a consultant to Pfizer, Johnson and Johnson, and Merck.
References
- 1.Mandell L.A., Wunderink R.G., Anzueto A. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44:S2–S27. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brar N.K., Niederman M.S. Management of community-acquired pneumonia: a review and update. Ther Adv Respir Dis. 2011;5:61–78. doi: 10.1177/1753465810381518. [DOI] [PubMed] [Google Scholar]
- 3.Kochanek KD, Xu J, Murphy SL, et al. Deaths: Preliminary data for 2009. National Vital Statistics Reports 59, No. 4.CDC 2011. Available at: http://www.cdc.gov/nchs/data/nvsr/nvsr59/nvsr59_04.pdf. Accessed 30, June 2011. [PubMed]
- 4.Heron MP, Hoyert DL, Murphy SL, et al. Deaths: Final data for 2006. National Vital Statistics Reports 57. National Center for Health Statistics2009. Available at: http://www.cdc.gov/nchs/fastats/pneumonia.htm. Accessed 10, July 2011.
- 5.Fine M.J., Smith M.A., Carson C.A. Prognosis and outcomes of patients with community-acquired pneumonia. A meta-analysis. JAMA. 1996;275:134–141. [PubMed] [Google Scholar]
- 6.Welte T., Köhnlein T. Global and local epidemiology of community-acquired pneumonia: the experience of the CAPNETZ network. Semin Respir Crit Care Med. 2009;30:127–135. doi: 10.1055/s-0029-1202941. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan V., Clermont G., Griffin M.F. Pneumonia: still the old man’s friend? Arch Intern Med. 2003;163:317–323. doi: 10.1001/archinte.163.3.317. [DOI] [PubMed] [Google Scholar]
- 8.File T.M., Jr., Marrie T.J. Burden of community-acquired pneumonia in North American adults. Postgrad Med. 2010;122:130–141. doi: 10.3810/pgm.2010.03.2130. [DOI] [PubMed] [Google Scholar]
- 9.Kozma C.M., Dickson M., Raut M.K. Economic benefit of a 1-day reduction in hospital stay for community-acquired pneumonia (CAP) J Med Econ. 2010;13:719–727. doi: 10.3111/13696998.2010.536350. [DOI] [PubMed] [Google Scholar]
- 10.Capelastegui A., Espana P.P., Quintana J.M. Validation of a predictive rule for the management of community-acquired pneumonia. Eur Respir J. 2006;27:151. doi: 10.1183/09031936.06.00062505. [DOI] [PubMed] [Google Scholar]
- 11.Aujesky D., Auble T.E., Yealy D.M. Prospective comparison of three validated prediction rules for prognosis in community-acquired pneumonia. Am J Med. 2005;118:384–392. doi: 10.1016/j.amjmed.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Niederman M.S. Biological markers to determine eligibility in trials for community-acquired pneumonia: a focus on procalcitonin. Clin Infect Dis. 2008;47:S127–S132. doi: 10.1086/591393. [DOI] [PubMed] [Google Scholar]
- 13.Wunderink R.G., Waterer G.W. Community-acquired pneumonia: pathophysiology and host factors with focus on possible new approaches to management of lower respiratory tract infections. Infect Dis Clin North Am. 2004;18:743–759. doi: 10.1016/j.idc.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Sarkar M., Hennessy S., Yang Y.X. Proton-pump inhibitor use and the risk for community-acquired pneumonia. Ann Intern Med. 2008;149:391–398. doi: 10.7326/0003-4819-149-6-200809160-00005. [DOI] [PubMed] [Google Scholar]
- 15.Waterer G.W., Bruns A.H. Genetic risk of acute pulmonary infections and sepsis. Expert Rev Respir Med. 2010;4:229–238. doi: 10.1586/ers.10.13. [DOI] [PubMed] [Google Scholar]
- 16.Niederman M.S., Ahmed Q.A. Inflammation in severe pneumonia: act locally, not globally. Crit Care Med. 1999;27:2030–2032. doi: 10.1097/00003246-199909000-00056. [DOI] [PubMed] [Google Scholar]
- 17.Lieberman D. Atypical pathogens in community-acquired pneumonia. Clin Chest Med. 1999;20:489–497. doi: 10.1016/s0272-5231(05)70230-6. [DOI] [PubMed] [Google Scholar]
- 18.Paganin F., Lilienthal F., Bourdin A. Severe community-acquired pneumonia: assessment of microbial aetiology as mortality factor. Eur Respir J. 2004;24:779–785. doi: 10.1183/09031936.04.00119503. [DOI] [PubMed] [Google Scholar]
- 19.Kang C.I., Song J.H., Oh W.S. Asian Network for Surveillance of Resistant Pathogens (ANSORP) Study Group. Clinical outcomes and risk factors of community-acquired pneumonia caused by gram-negative bacilli. Eur J Clin Microbiol Infect Dis. 2008;27:657–661. doi: 10.1007/s10096-008-0485-7. [DOI] [PubMed] [Google Scholar]
- 20.Boyer A., Amadeo B., Vargas F. Severe community-acquired Enterobacter pneumonia: a plea for greater awareness of the concept of health-care-associated pneumonia. BMC Infect Dis. 2011;11:120. doi: 10.1186/1471-2334-11-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arancibia F., Bauer T.T., Ewig S. Community-acquired pneumonia due to gram-negative bacteria and Pseudomonas aeruginosa: incidence, risk, and prognosis. Arch Intern Med. 2002;162:1849–1858. doi: 10.1001/archinte.162.16.1849. [DOI] [PubMed] [Google Scholar]
- 22.de Roux A., Marcos M.A., Garcia E. Viral community-acquired pneumonia in non immunocompromised adults. Chest. 2004;125:1343–1351. doi: 10.1378/chest.125.4.1343. [DOI] [PubMed] [Google Scholar]
- 23.Niederman M.S. In the clinic - community-acquired pneumonia. Ann Intern Med. 2009;151 doi: 10.7326/0003-4819-151-7-200910060-01004. ITC1–14. [DOI] [PubMed] [Google Scholar]
- 24.Lobo L.J., Reed K.D., Wunderink R.G. Expanded clinical presentation of community-acquired methicillin-resistant Staphylococcus aureus pneumonia. Chest. 2010;138:130–136. doi: 10.1378/chest.09-1562. [DOI] [PubMed] [Google Scholar]
- 25.Francis J.S., Doherty M.C., Lopatin U. Severe community-onset pneumonia in healthy adults caused by methicillin-resistant Staphylococcus aureus carrying the Panton-Valentine leukocidin genes. Clin Infect Dis. 2005;40:100–107. doi: 10.1086/427148. [DOI] [PubMed] [Google Scholar]
- 26.Diep B.A., Chambers H.F., Graber C.J. Emergence of multidrug-resistant, community associated, methicillin-resistant Staphylococcus aureus clone USA300 in men who have sex with men. Ann Intern Med. 2008;148:249–257. doi: 10.7326/0003-4819-148-4-200802190-00204. [DOI] [PubMed] [Google Scholar]
- 27.Torres A., Menendez R., Wunderink R. Infectious disease of lung. In: Mason R.J., Their S.O., editors. Murray and Nadel’s Textbook of Respiratory Medicine. [Chapter: Pyogenic Bacterial Pneumonia and Lung Abscess] vol. 1. 5th edition. Saunders; Philadelphia: 2010. pp. 699–740. [Google Scholar]
- 28.Metlay J.P., Kapoor W.N., Fine M.J. Does this patient have community-acquired pneumonia? Diagnosing pneumonia by history and physical examination. JAMA. 1997;278:1440–1445. [PubMed] [Google Scholar]
- 29.Mandell L.A., Marrie T.J., Grossman R.F. Canadian guidelines for the initial management of community-acquired pneumonia: an evidence-based update by the Canadian Infectious Diseases Society and the Canadian Thoracic Society. Clin Infect Dis. 2000;31:383–421. doi: 10.1086/313959. [DOI] [PubMed] [Google Scholar]
- 30.van der Eerden M.M., Vlaspolder F., de Graaff C.S. Comparison between pathogen directed antibiotic treatment and empirical broad spectrum antibiotic treatment in patients with community acquired pneumonia: a prospective randomised study. Thorax. 2005;60:672–678. doi: 10.1136/thx.2004.030411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Eerden M.M., Vlaspolder F., de Graaff C.S. Value of intensive diagnostic microbiological investigation in low- and high-risk patients with community-acquired pneumonia. Eur J Clin Microbiol Infect Dis. 2005;24:241–249. doi: 10.1007/s10096-005-1316-8. [DOI] [PubMed] [Google Scholar]
- 32.Mirsaeidi M., Peyrani P., Aliberti S. Thrombocytopenia and thrombocytosis at time of hospitalization predict mortality in patients with community-acquired pneumonia. Chest. 2010;137:416–420. doi: 10.1378/chest.09-0998. [DOI] [PubMed] [Google Scholar]
- 33.Nair V., Niederman M.S., Masani N. Hyponatremia in community-acquired pneumonia. Am J Nephrol. 2007;27:184–190. doi: 10.1159/000100866. [DOI] [PubMed] [Google Scholar]
- 34.Hopstaken R.M., Witbraad T., van Engelshoven J.M. Inter-observer variation in the interpretation of chest radiographs for pneumonia in community-acquired lower respiratory tract infections. Clin Radiol. 2004;59:743–752. doi: 10.1016/j.crad.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 35.Syrjälä H., Broas M., Suramo I. High-resolution computed tomography for the diagnosis of community-acquired pneumonia. Clin Infect Dis. 1998;27:358–363. doi: 10.1086/514675. [DOI] [PubMed] [Google Scholar]
- 36.Metersky M.L., Ma A., Bratzler D.W. Predicting bacteremia in patients with community-acquired pneumonia. Am J Respir Crit Care Med. 2004;169:342–347. doi: 10.1164/rccm.200309-1248OC. [DOI] [PubMed] [Google Scholar]
- 37.Smith M.D., Derrington P., Evans R. Rapid diagnosis of bacteremic pneumococcal infections in adults by using the Binax NOW Streptococcus pneumoniae urinary antigen test: a prospective, controlled clinical evaluation. J Clin Microbiol. 2003;41:2810–2813. doi: 10.1128/JCM.41.7.2810-2813.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ortega L., Sierra M., Domínguez J. Utility of a pneumonia severity index in the optimization of the diagnostic and therapeutic effort for community-acquired pneumonia. Scand J Infect Dis. 2005;37:657–663. doi: 10.1080/00365540510027174. [DOI] [PubMed] [Google Scholar]
- 39.Benin A.L., Benson R.F., Besser R.E. Trends in legionnaire’s disease, 1980-1998: declining mortality and new patterns of diagnosis. Clin Infect Dis. 2002;35:1039–1046. doi: 10.1086/342903. [DOI] [PubMed] [Google Scholar]
- 40.Sordé R., Falcó V., Lowak M. Current and potential usefulness of pneumococcal urinary antigen detection in hospitalized patients with community-acquired pneumonia to guide antimicrobial therapy. Arch Intern Med. 2011;171:166–172. doi: 10.1001/archinternmed.2010.347. [DOI] [PubMed] [Google Scholar]
- 41.Krüger S., Ewig S., Giersdorf S. Cardiovascular and inflammatory biomarkers to predict short- and long-term survival in community-acquired pneumonia: results from the German Competence Network, CAPNETZ. Am J Respir Crit Care Med. 2010;182:1426–1434. doi: 10.1164/rccm.201003-0415OC. [DOI] [PubMed] [Google Scholar]
- 42.Ramírez P., Ferrer M., Martí V. Inflammatory biomarkers and prediction for intensive care unit admission in severe community-acquired pneumonia. Crit Care Med. 2011 doi: 10.1097/CCM.0b013e3182257445. [Epub ahead of print]. PMID:21705887. [DOI] [PubMed] [Google Scholar]
- 43.Almirall J., Bolíbar I., Toran P. Contribution of C-reactive protein to the diagnosis and assessment of severity of community-acquired pneumonia. Chest. 2004;125:1335–1342. doi: 10.1378/chest.125.4.1335. [DOI] [PubMed] [Google Scholar]
- 44.Schuetz P., Suter-Widmer I., Chaudri A. Procalcitonin-Guided Antibiotic Therapy and Hospitalisation in Patients with Lower Respiratory Tract Infections (ProHOSP) Study Group. Prognostic value of procalcitonin in community-acquired pneumonia. Eur Respir J. 2011;37:384–392. doi: 10.1183/09031936.00035610. [DOI] [PubMed] [Google Scholar]
- 45.Müller F., Christ-Crain M., Bregenzer T. Procalcitonin levels predict bacteremia in patients with community-acquired pneumonia: a prospective cohort trial. Chest. 2010;138:121–129. doi: 10.1378/chest.09-2920. [DOI] [PubMed] [Google Scholar]
- 46.Christ-Crain M., Stolz D., Bingisser R. Procalcitonin guidance of antibiotic therapy in community- acquired pneumonia: a randomized trial. Am J Respir Crit Care Med. 2006;174:84–93. doi: 10.1164/rccm.200512-1922OC. [DOI] [PubMed] [Google Scholar]
- 47.Renaud B., Santin A., Coma E. Association between timing of intensive care unit admission and outcomes for emergency department patients with community acquired pneumonia. Crit Care Med. 2009;37:2867–2874. doi: 10.1097/CCM.0b013e3181b02dbb. [DOI] [PubMed] [Google Scholar]
- 48.Restrepo M.I., Mortensen E.M., Rello J. Late admission to the ICU in patients with community-acquired pneumonia is associated with higher mortality. Chest. 2010;137:552–557. doi: 10.1378/chest.09-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aujesky D., McCausland J.B., Whittle J. Reasons why emergency department providers do not rely on the Pneumonia Severity Index to determine the initial site of treatment for patients with pneumonia. Clin Infect Dis. 2009;49:100–108. doi: 10.1086/644741. [DOI] [PubMed] [Google Scholar]
- 50.Fine M.J., Auble T.E., Yealy D.M. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336:243–250. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 51.Valencia M., Badia J.R., Cavalcanti M. Pneumonia severity index class V patients with community-acquired pneumonia: characteristics, outcomes, and value of severity. Chest. 2007;132:515–522. doi: 10.1378/chest.07-0306. [DOI] [PubMed] [Google Scholar]
- 52.Brito V., Niederman M.S. Predicting mortality in the elderly with community-acquired pneumonia: should we design a new car or set a new ’speed limit’? Thorax. 2010;65:944–945. doi: 10.1136/thx.2010.138131. [DOI] [PubMed] [Google Scholar]
- 53.Bauer T.T., Ewig S., Marre R. CRB-65 predicts death from community-acquired pneumonia. J Intern Med. 2006;260:93–101. doi: 10.1111/j.1365-2796.2006.01657.x. [DOI] [PubMed] [Google Scholar]
- 54.Blot S.I., Rodriguez A., Solé-Violán J. Effects of delayed oxygenation assessment on time to antibiotic delivery and mortality in patients with severe community-acquired pneumonia. Crit Care Med. 2007;35:2509–2514. doi: 10.1097/01.CCM.0000287587.43801.9C. [DOI] [PubMed] [Google Scholar]
- 55.Huang D.T., Weissfeld L.A., Kellum J.A. Risk prediction with procalcitonin and clinical rules in community-acquired pneumonia. Ann Emerg Med. 2008;52:48–58. doi: 10.1016/j.annemergmed.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kruger S., Ewig S., Marre R. Procalcitonin predicts patients at low risk of death from community acquired pneumonia across all CRB-65 classes. Eur Respir J. 2008;31:349–355. doi: 10.1183/09031936.00054507. [DOI] [PubMed] [Google Scholar]
- 57.Liapikou A., Ferrer M., Polverino E. Severe community-acquired pneumonia: validation of the Infectious Diseases Society of America/American Thoracic Society guidelines to predict an intensive care unit admission. Clin Infect Dis. 2009;48:377–385. doi: 10.1086/596307. [DOI] [PubMed] [Google Scholar]
- 58.Brown S.M., Jones B.E., Jephson A.R. Validation of the Infectious Disease Society of America/American Thoracic Society 2007 guidelines for severe community-acquired pneumonia. Crit Care Med. 2009;37:3010–3016. doi: 10.1097/CCM.0b013e3181b030d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rello J., Rodriguez A., Lisboa T. PIRO score for community-acquired pneumonia: a new prediction rule for assessment of severity in intensive care unit patients with community-acquired pneumonia. Crit Care Med. 2009;37:456–462. doi: 10.1097/CCM.0b013e318194b021. [DOI] [PubMed] [Google Scholar]
- 60.Charles P.G., Wolfe R., Whitby M. SMART-COP: a tool for predicting the need for intensive respiratory or vasopressor support in community-acquired pneumonia. Clin Infect Dis. 2008;47:375–384. doi: 10.1086/589754. [DOI] [PubMed] [Google Scholar]
- 61.Houck P.M., Bratzler D.W., Nsa W. Timing of antibiotic administration and outcomes for Medicare patients hospitalized with community-acquired pneumonia. Arch Intern Med. 2004;164:637–644. doi: 10.1001/archinte.164.6.637. [DOI] [PubMed] [Google Scholar]
- 62.Meehan T.P., Fine M.J., Krumholz H.M. Quality of care, process, and outcomes in elderly patients with pneumonia. JAMA. 1997;278:2080–2084. [PubMed] [Google Scholar]
- 63.Metersky M.L., Ma A., Houck P.M. Antibiotics for bacteremic pneumonia: improved outcomes with macrolides but not fluoroquinolones. Chest. 2007;131:466–473. doi: 10.1378/chest.06-1426. [DOI] [PubMed] [Google Scholar]
- 64.Baddour L.M., Yu V.L., Klugman K.P. Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia. Am J Respir Crit Care Med. 2004;170:440–444. doi: 10.1164/rccm.200311-1578OC. [DOI] [PubMed] [Google Scholar]
- 65.Leroy O., Saux P., Bédos J.P. Comparison of levofloxacin and cefotaxime combined with ofloxacin for ICU patients with community-acquired pneumonia who do not require vasopressors. Chest. 2005;128:172–183. doi: 10.1378/chest.128.1.172. [DOI] [PubMed] [Google Scholar]
- 66.Rodríguez A., Mendia A., Sirvent J.M. Combination antibiotic therapy improves survival in patients with community-acquired pneumonia and shock. Crit Care Med. 2007;35:1493–1498. doi: 10.1097/01.CCM.0000266755.75844.05. [DOI] [PubMed] [Google Scholar]
- 67.Ramirez J.A., Bordon J. Early switch from intravenous to oral antibiotics in hospitalized patients with bacteremic community-acquired Streptococcus pneumoniae pneumonia. Arch Intern Med. 2001;161:848–850. doi: 10.1001/archinte.161.6.848. [DOI] [PubMed] [Google Scholar]