Abstract
Safety-tested modified vaccinia virus Ankara (MVA) has been established as a potent vector system for the development of candidate recombinant vaccines. The versatility of the vector system was recently demonstrated by the rapid production of experimental MVA vaccines for immunization against severe acute respiratory syndrome associated coronavirus. Promising results were also obtained in the delivery of Epstein-Barr virus or human cytomegalovirus antigens and from the clinical testing of MVA vectors for vaccination against immunodeficiency virus, papilloma virus, Plasmodium falciparum or melanoma. Moreover, MVA is considered to be a prime candidate vaccine for safer protection against orthopoxvirus infections. Thus, vector development to challenge dilemmas in vaccinology or immunization against poxvirus biothreat seems possible, yet the right choice should be made for a most beneficial use.
Abbreviations: CEF, chicken embryo fibroblast; CMV, cytomegalovirus; HIV, human immunodeficiency virus; HPV, human papillomavirus; MVA, modified vaccinia virus Ankara; SARS, severe acute respiratory syndrome; SIV, simian immunodeficiency virus; VV, vaccinia virus
Introduction
Poxviruses engineered to express foreign genes are established tools for target protein synthesis and vaccine development in biomedical research. A large packaging capacity for recombinant DNA, precise virus-specific control of target gene expression, lack of persistence or genomic integration in the host, high immunogenicity as vaccine, and ease of vector and vaccine production were important features enabling this success story. Concerns about the safety of poxviruses, including vaccinia virus (VV) as the former smallpox vaccine, have been addressed by the use of viruses that are replication-defective in human cells. Among these, modified vaccinia virus Ankara (MVA) can be considered as one of the virus strains of choice for preclinical and clinical research [1].
Historically, MVA was developed through attenuation by serial passage in primary chicken embryo cells to serve as a safer vaccine against smallpox (for a review see [2]). After more than 570 passages in tissue culture MVA had lost the broad cellular host range of VV, being unable to productively grow in many cells of mammalian origin. The concurrent avirulence of MVA for laboratory animals and its entirely unproblematic use for primary smallpox vaccination in more than 100 000 humans founded a high safety profile for recombinant MVA that, depending on the nature of the inserted target gene, can be used under conditions of biosafety level 1. The capacity to produce similar levels of viral and recombinant antigen when compared with replication-competent viruses is a relevant feature of MVA vaccines. In recent years, significant progress has been made in the development of MVA vaccine technologies.
This review informs about the newest developments in the generation of recombinant MVA and illustrates the principal features that have an impact on MVA immunogenicity. We also describe advances made in the preclinical and clinical evaluation of MVA as a second-generation poxvirus vaccine or for the delivery of heterologous antigens targeting infectious diseases and cancer. Finally, we consider the compatibility of different MVA exploitations and raise the question as to what future use priority should be given.
Genetic engineering of recombinant MVA
Recombinant MVA are among the most promising live viral vector systems, because of their well-established safety and their versatility for the production of heterologous proteins. The recent engineering of recombinant MVA to synthesize all components necessary for the assembly and delivery of alphavirus replicon particles serves as an elegant example of their application [3]. For most purposes, however, the generation of MVA vectors is straightforward requiring a single genomic insertion mediated by homologous recombination between the virus genome and DNA from a plasmid that carries one or two recombinant genes being placed under control of a VV-specific promoter (Figure 1 ). The sites of naturally occurring deletions within the MVA genome or the classical gene loci encoding for the VV proteins thymidine kinase or hemagglutinin serve as sites for the insertion of recombinant gene sequences. There are several well-established techniques for clonal isolation of recombinant MVA involving the coexpression of chromogenic agents (e.g. Escherichia coli β-galactosidase and β-glucuronidase) or providing resistance against antimicrobials (e.g. E. coli xanthine-guanine-phosphoribosyl-transferase) (for an overview see 2., 4.). Recently, methods relying on growth selection of recombinant MVA have been developed. These protocols take advantage of selective propagation in cell cultures that are non-permissive for non-recombinant MVA. In one protocol, the VV host range gene K1L is transiently introduced into the MVA genome and recombinant MVA can subsequently be isolated in rabbit kidney RK-13 cells 4., 5.. After clonal isolation of the vector virus, the K1L marker gene is again removed from the viral genome bearing the advantage that the same marker can be re-used subsequently to generate MVA recombinant viruses harboring multiple gene insertions. Another recently developed host range selection technique relies on growth rescue of mutant MVA in chicken embryo fibroblast (CEF) cells [6]. MVA-ΔE3L lacks the interferon resistance gene E3L and is unable to grow in CEF. Stable re-insertion of the E3L gene together with a target gene sequence allows for quick isolation of MVA recombinant viruses on CEF.
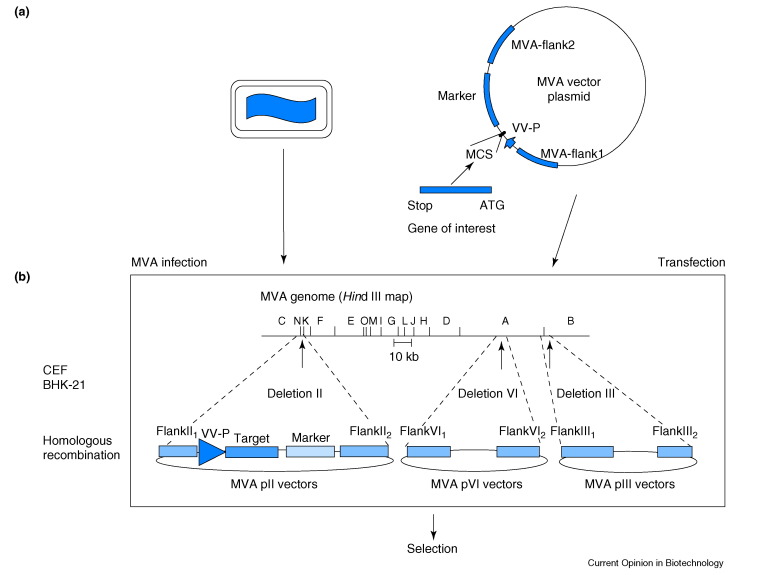
Figure 1.
The generation of recombinant MVA. (a) Schematic representation of an MVA particle on the left and the MVA transfer plasmid on the right. MVA DNA sequences adjacent to the deletion site (MVA-flank1, MVA-flank2) were cloned into the plasmid and target genes are inserted between these sequences and placed under transcriptional control of vaccinia virus-specific promoters (VV-P). Recombinant MVA are generated by infection of chicken embryo fibroblast (CEF) or baby hamster kidney (BHK-21) cells with MVA and concurrent transfection with transfer vectors, resulting in recombination between homologous DNA sequences of vector and virus sequences. (b) An MVA-infected, transfected cell. Schematic map of the MVA genome and plasmids designed for the insertion of foreign DNA. Sites of the restriction endonuclease Hind III within the genome of MVA are indicated at the top. The positions of the naturally occurring deletions II, III, and VI are marked by arrows. MVA DNA sequences adjacent to the deletion sites (flank1, flank2) were cloned into plasmids to generate the pII, pIII and pVI transfer vectors.
Basics of MVA immunogenicity and smallpox vaccination
In the 1970s, highly attenuated MVA was primarily considered to provide a means for safer vaccination against smallpox. Although there was knowledge about its avirulence and immunostimulating capacity from infection experiments in multiple animal models, more systematic efforts to characterize the molecular basis of MVA vaccine immunogenicity date from the more recent past. Important achievements include the characterization of the MVA molecular life cycle upon infection of non-permissive mammalian cells, the elucidation of the MVA genome, and our understanding that MVA has lost relevant poxvirus immune evasion genes that target innate host responses based on cytokine and chemokine functions. Consequently, defects in inhibitory virus genes are likely to be responsible for the MVA-specific induction of host cytokine synthesis proposed decades ago (for a review see [2]). Interestingly, inactivation of the VV interferon resistance gene E3L in the MVA genome resulted in enhanced production of type I interferon in CEFs, suggesting that the capacity of MVA to stimulate innate responses may be further enhanced by rational mutagenesis [6]. It can be assumed that such immunostimulation contributes to the particular immunogenicity of MVA and might compensate for the advantage of live replication-competent VV in sustained antigen production. In contrast to other viruses (e.g. members of the herpesvirus family), MVA or replication-competent VV do not appear to specifically interfere with host cell antigen processing or presentation, allowing for an apparently unimpaired induction of adaptive immune responses. This assumption is in agreement with the finding that VV-specific humoral and cellular immunity can be detected decades after primary vaccination [7]. When recently compared with conventional VV vaccines, MVA vaccines were found to elicit similar patterns of VV-specific immune responses that provided protection against experimental orthopoxvirus infections but required inoculation of higher dosage 8.•, 9., 10.•, 11.••. Remarkably, Earl and coworkers [11••] demonstrated substantial protective capacity of MVA vaccines in non-human primates against challenge with monkeypox virus, thus making MVA a valuable candidate as second-generation smallpox vaccine.
Antivector immunity and impact on MVA vaccination
With more recombinant MVA used for antigen delivery in clinical research 12., 13.•, 14.•, 15., there is an increasing need to evaluate MVA-specific immune responses following immunization. Although there are established means to monitor vaccine-induced antibodies, cell-mediated immunity has scarcely been assessed 11.••, 16., 17., 18.. Several approaches allow VV-induced T-cell immunity to be quantified without knowledge of defined VV-specific target antigens 19., 20., 21.. In addition, the use of recently identified human leukocyte antigen HLA-A*0201-restricted VV-specific CD8+ T-cell epitopes 8.•, 22.• now makes it possible to compare epitope-specific responses elicited against vector or recombinant antigens (Figure 2 ; I Drexler and G Sutter, unpublished). Further knowledge as to how these responses might influence each other is highly relevant for developing optimal modes of MVA vector immunization. Although replication-competent VV vaccines and other viral vectors are more likely to be hampered by antivector immunity than MVA-based vectors 23., 24., vaccinia-specific immune responses can be assumed to affect immunogenicity of the target antigen delivered by the MVA vector vaccine. Yet, pre-existing MVA or VV immunity did not interfere with subsequent immunizations of recombinant MVA expressing a cytomegalovirus (CMV) glycoprotein gB antigen [25]. In addition, a variety of methods has been derived to possibly circumvent the influence of vector-specific responses, including the application of DNA prime MVA boost immunization [26]. One particularly promising approach to enhance target antigen specific immune responses is based on combining different viral vector vaccines (e.g. influenza [24], avipox 27., 28., Semliki Forest [29] or vesicular stomatitis virus [30]), non-viral vector vaccines (e.g. DNA- 31., 32. or Salmonella-based vaccines [33]) or protein vaccines [34] with MVA vaccines producing a common antigen. These so-called prime-boost regimens can vary in dosage, or in numbers and intervals of application [13•], and can combine different application routes with various vector systems [35]. Depending on the kind of disease, the type of immune response that needs to be induced by the test vaccine can also influence the formulation. In the case of cancer, human immunodeficiency virus (HIV) or malaria, an immune response biased towards T helper 1 (Th1) cell immunity will be promoted using a combined DNA and MVA vaccination [36].
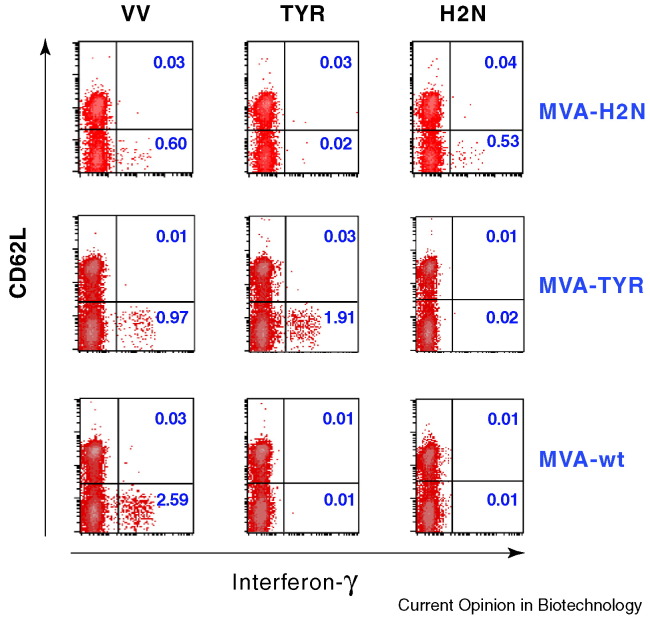
Figure 2.
Comparative monitoring of epitope-specific CD8+ T-cell responses directed against vaccinia virus and recombinant antigens. Quantitation of epitope-specific T cells was performed following one immunization of humanized HLA-A*0201-transgenic mice with 108 IU of either wild-type MVA (MVA-wt), recombinant MVA-TYR or MVA-H2N expressing the human tumor antigens tyrosinase and Her-2/neu, respectively. Ten days post vaccination, peptide-specific intracellular cytokine release of splenocytes was determined after stimulation with vaccinia epitope VP35#1 (VV), tyrosinase (TYR) or Her-2/neu peptides (H2N). Cells were analyzed by flow cytometry for the presence of peptide-specific, activated (CD62Llow) CD8+ T cells. The magnitude of the specifically induced T-cell response is depicted as the cells shifted to the lower right and indicated as a percentage (numbers in blue) of interferon-γ-secreting CD8+ T cells within the CD8+ cell population.
Recombinant MVA as a candidate vaccine against viral diseases
Much previous research has been dedicated to the development of MVA candidate recombinant vaccines against multiple virus infections of humans, including those causing AIDS, influenza, early childhood respiratory diseases, measles, Japanese encephalitis or dengue fever (for a review see [2]). As an effective vaccine against AIDS is urgently needed, recombinant MVA producing immunodeficiency virus antigens are among the first vector viruses to be evaluated as candidate vaccines in humans 14.•, 37.. Substantial data from studies in different simian immunodeficiency virus (SIV) or simian human immunodeficiency virus (SHIV, a chimera of SIV and HIV) infection models strongly support thorough clinical evaluation of MVA-based vaccines. The use of heterologous prime-boost strategies including MVA vaccines and delivering multiple SIV or HIV antigens proved successful to elicit antibody and high-level cytotoxic T-cell responses in macaques. The immune response effectively controlled a mucosal challenge with SHIV 89.6P and SIVmac251-derived viruses or significantly reduced viral loads after challenge infection with neutralization-resistant and highly replication-competent SIVmac239 30., 38., 39., 40., 41., 42.. Importantly, recent data suggest that SIV/HIV-specific mucosal immunity can be boosted by peripheral MVA immunization after oral priming with a Salmonella vector vaccine [33] and intranasal inoculation of recombinant MVA can stimulate immunodeficiency virus-specific immunity in the genital or rectal tract 35., 43.. Elicitation of potent mucosal immunity and induction of broadly virus-neutralizing antibody responses are important milestones still to be reached for the derivation of a successful HIV vaccine. The principal capacity of MVA to elicit highly effective virus-neutralizing antibody responses has been highlighted by the characterization of vector vaccines against severe acute respiratory syndrome (SARS) coronavirus and human CMV 25., 44.•. The successful engineering of MVA expressing SARS coronavirus spike protein also demonstrated the suitability of the vector system to readily respond to the potential threat of rapidly emerging infectious diseases. A recently developed recombinant MVA delivering multiple CMV antigens represents a promising candidate for the clinical testing of MVA-based T-cell vaccines 25., 45.•, 46.. This study also mirrors a general interest in the development of MVA vector vaccines against human herpesvirus infections 47., 48..
Recombinant MVA as candidate vaccines against cancer, parasitic and bacterial diseases
The potential to activate robust cellular major histocompatibility complex (MHC) class I- and II-restricted CD8+ and CD4+ T-cell responses against recombinant antigens has made MVA vaccines attractive for immunotherapeutic approaches against cancer and selected intracellular parasitic or bacterial infectious diseases. For experimental cancer therapy, virus-associated malignancies seem to be predestined targets for MVA vaccines. Taylor and co-workers [48] demonstrated the immunogenicity of an Epstein-Barr virus-associated nasopharyngeal carcinoma vaccine by reactivating Epstein-Barr virus-specific CD8+ and CD4+ memory T cells in vitro. Recently, Corona Gutierrez and colleagues [49••] showed evidence for the therapeutic efficacy of an MVA vaccine delivering human papillomavirus (HPV) E2 antigen against cervical cancer associated with HPV infection in a phase I/II clinical trial. Vaccines based on recombinant MVA expressing different tumor-associated antigens specific for a variety of cancers are currently being tested in mice 27., 50., 51. and humans [52]. Often these are combined with cytokines such as interleukin-2 [52], costimulatory molecules such as B7-1 [27], measures to circumvent immune inhibitory signalling (by CTLA-4 blockade) [51] or cellular adjuvants like dendritic cells [15] to enhance immune responses against antigens that are likely to be tolerogenic self-proteins.
Major efforts are underway to develop vaccines against malaria caused by Plasmodium spp. parasites. Preclinically, heterologous prime-boost immunization regimens have elicited strong CD8+ T-cell immunity and have shown substantial protection in mouse malaria challenge models against Plasmodium berghei [28] or Plasmodium yoelii [53]. In addition, safety and immunogenicity have been established in clinical trials with human volunteers, experimentally [13•] or naturally exposed to Plasmodium falciparum [54]. Recently, the first prime-boost vaccinations against tuberculosis — combining DNA [55] or the classical bacille Calmette-Guérin vaccine [56] with recombinant MVA expressing Mycobacterium tuberculosis antigen 85A — proved to be protective in mice.
Conclusions
Recombinant MVA is a prime candidate poxvirus vector for a generation of new vaccines against infections and tumors. The portrait of the virus combines desirable elements such as high-level biological safety, the ability to activate appropriate innate immune mediators upon vaccination, and the capacity to deliver substantial amounts of heterologous antigens. The adoption of up-to-date methodology for convenient vector generation, vector quality control, and vector vaccine immune monitoring has increased the pace of development, bringing recombinant MVA vaccines into clinical trials. Initial studies testing MVA vaccines for prophylaxis or immunotherapy of AIDS, malaria, human papilloma virus-associated cancer or melanoma have already been completed (Table 1 ). First results are, overall, very encouraging and confirm clinical safety. Importantly, however, they also demonstrate clinical efficacy, despite the intrinsic difficulties associated with these target diseases. Renewed interest in the development of MVA as candidate vaccine against an orthopoxvirus-related biothreat is likely to provide the basis for feasible large-scale manufacturing of MVA vaccines. Indeed, first studies suggest that MVA would provide a suitable orthopox vaccine, if necessary. The use of recombinant MVA to simultaneously provide immunity against smallpox has been proposed. Nevertheless, one should keep in mind that hasty population-wide smallpox vaccinations might not be desirable, as high level antivector immunity could limit the future potential of poxvirus-based vector vaccines more urgently needed for prophylaxis or therapy of uncontrolled infectious diseases, cancer or emerging infections.
Table 1.
First clinical evaluation of recombinant MVA vaccines.
| Target disease | Antigen | Clinical trial | Reference |
|---|---|---|---|
| AIDS | HIV-1 Nef | Phase I/II, immunotherapy | [14•] |
| AIDS | HIVA multiantigen | Phase I, prophylaxis | [37] |
| Cervical cancer | HPV E2 | Phase I/II, immunotherapy | [49••] |
| Breast cancer | MUC1 | Phase I, immunotherapy | [52] |
| Melanoma | Tyrosinase | Phase I/II | 15., 57.• |
| Malaria | P. falciparum ME-Trap | Phase I, prophylaxis | [54] |
| Malaria | P. falciparum ME-Trap | Phase I, prophylaxis | [13•] |
Update
Recent data from a phase I clinical trial for treatment of metastatic melanoma indicated that vaccination with MVA-transduced dendritic cells can in vivo activate T-cell responses directed against the recombinant antigen tyrosinase and against the recently identified epitope within vaccinia virus envelope antigen H3 8.•, 57.•.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
The authors wish to acknowledge support by the EU (grants QLK2-CT-2002-01867 and QLK2-CT-2002-1034) and the Deutsche Forschungsgemeinschaft (SFB 455/A10 and SFB 456/B7).
References
- 1.Sutter G., Moss B. Nonreplicating vaccinia vector efficiently expresses recombinant genes. Proc Natl Acad Sci USA. 1992;89:10847–10851. doi: 10.1073/pnas.89.22.10847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sutter G., Staib C. Vaccinia vectors as candidate vaccines: the development of modified vaccinia virus Ankara for antigen delivery. Curr Drug Targets Infect Disord. 2003;3:263–271. doi: 10.2174/1568005033481123. [DOI] [PubMed] [Google Scholar]
- 3.Vasilakis N., Falvey D., Gangolli S.S., Coleman J., Kowalski J., Udem S.A., Zamb T.J., Kovacs G.R. Transfection-independent production of alphavirus replicon particles based on poxvirus expression vectors. Nat Biotechnol. 2003;21:932–935. doi: 10.1038/nbt845. [DOI] [PubMed] [Google Scholar]
- 4.Staib C., Drexler I., Sutter G. Construction and isolation of recombinant MVA. Methods Mol Biol. 2004;269:77–100. doi: 10.1385/1-59259-789-0:077. [DOI] [PubMed] [Google Scholar]
- 5.Staib C., Lowel M., Erfle V., Sutter G. Improved host range selection for recombinant modified vaccinia virus Ankara. Biotechniques. 2003;34:694–696. doi: 10.2144/03344bm02. 698, 700. [DOI] [PubMed] [Google Scholar]
- 6.Hornemann S., Harlin O., Staib C., Kisling S., Erfle V., Kaspers B., Häcker G., Sutter G. Replication of modified vaccinia virus Ankara in primary chicken embryo fibroblasts requires expression of the interferon resistance gene E3L. J Virol. 2003;77:8394–8407. doi: 10.1128/JVI.77.15.8394-8407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammarlund E., Lewis M.W., Hansen S.G., Strelow L.I., Nelson J.A., Sexton G.J., Hanifin J.M., Slifka M.K. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9:1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 8.•.Drexler I., Staib C., Kastenmüller W., Stefanovitc S., Schmidt B., Lemonnier F.A., Rammensee H.G., Busch D.H., Bernhard H., Erfle V., Sutter G. Identification of vaccinia virus epitope-specific HLA-A*0201-restricted T-cells and comparative analysis of smallpox vaccines. Proc Natl Acad Sci USA. 2003;100:217–222. doi: 10.1073/pnas.262668999. [DOI] [PMC free article] [PubMed] [Google Scholar]; The paper reports the identification of a first immunodominant HLA-A*0201-restricted VV epitope permitting comparative analysis of peptide-specific CD8+ T-cell responses in vaccination experiments. The authors demonstrate that MVA vaccination is fully protective against a lethal respiratory orthopoxvirus challenge.
- 9.Belyakov I.M., Earl P., Dzutsev A., Kuznetsov V.A., Lemon M., Wyatt L.S., Snyder J.T., Ahlers J.D., Franchini G., Moss B., Berzofsky J.A. Shared modes of protection against poxvirus infection by attenuated and conventional smallpox vaccine viruses. Proc Natl Acad Sci USA. 2003;100:9458–9463. doi: 10.1073/pnas.1233578100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.•.Wyatt L.S., Earl P.L., Eller L.A., Moss B. Highly attenuated smallpox vaccine protects mice with and without immune deficiencies against pathogenic vaccinia virus challenge. Proc Natl Acad Sci USA. 2004;101:4590–4595. doi: 10.1073/pnas.0401165101. [DOI] [PMC free article] [PubMed] [Google Scholar]; Comparative immunogenicity and protection study using MVA and licensed Dryvax vaccines in mice. Confirms the safety of MVA vaccination in SCID (severe combined immunodeficiency disease) mice and shows that induced humoral and cellular immune responses can complement each other to protect normal and partially immunodeficient animals.
- 11.••.Earl P.L., Americo J.L., Wyatt L.S., Eller L.A., Whitbeck J.C., Cohen G.H., Eisenberg R.J., Hartmann C.J., Jackson D.L., Kulesh D.A. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature. 2004;428:182–185. doi: 10.1038/nature02331. [DOI] [PubMed] [Google Scholar]; This study compares MVA and standard Dryvax vaccine in a monkeypox virus/macaque challenge model demonstrating similar immunogenicity and substantial protective capacity of MVA vaccination. The work represents an important step in developing MVA as second-generation smallpox vaccine.
- 12.Drexler I., Antunes E., Schmitz M., Wölfel T., Huber C., Erfle V., Rieber P., Theobald M., Sutter G. Modified vaccinia virus Ankara for delivery of human tyrosinase as melanoma-associated antigen: induction of tyrosinase- and melanoma-specific human leukocyte antigen A*0201-restricted cytotoxic T cells in vitro and in vivo. Cancer Res. 1999;59:4955–4963. [PubMed] [Google Scholar]
- 13.•.McConkey S.J., Reece W.H., Moorthy V.S., Webster D., Dunachie S., Butcher G., Vuola J.M., Blanchard T.J., Gothard P., Watkins K. Enhanced T-cell immunogenicity of plasmid DNA vaccines boosted by recombinant modified vaccinia virus Ankara in humans. Nat Med. 2003;9:729–735. doi: 10.1038/nm881. [DOI] [PubMed] [Google Scholar]; This is the first study showing that heterologous prime/boost immunization regimens using a DNA vaccine and recombinant MVA encoding liver-stage antigens elicit partial, but not complete, protection against P. falciparum challenge in human volunteers.
- 14.•.Cosma A., Nagaraj R., Buhler S., Hinkula J., Busch D.H., Sutter G., Goebel F.D., Erfle V. Therapeutic vaccination with MVA-HIV-1 nef elicits Nef-specific T-helper cell responses in chronically HIV-1 infected individuals. Vaccine. 2003;22:21–29. doi: 10.1016/s0264-410x(03)00538-3. [DOI] [PubMed] [Google Scholar]; This report describes the first clinical evaluation of a recombinant MVA vaccine in an immunotherapeutic approach against HIV infection demonstrating clinical safety and evidence for Nef-specific immunostimulation in patients under highly active antiretroviral treatment.
- 15.Di Nicola M., Carlo-Stella C., Anichini A., Mortarini R., Guidetti A., Tragni G., Gallino F., Del Vecchio M., Ravagnani F., Morelli D. Immunization of patients with malignant melanoma with autologous CD34+ cell-derived dendritic cells transduced ex vivo with a recombinant replication-deficient vaccinia vector encoding the human tyrosinase gene: a phase I trial. Hum Gene Ther. 2003;14:1347–1360. doi: 10.1089/104303403322319426. [DOI] [PubMed] [Google Scholar]
- 16.Hooper J.W., Thompson E., Wilhelmsen C., Zimmerman M., Ait Ichou M., Steffen S.E., Schmaljohn C.S., Schmaljohn A.L., Jahrling P.B. Smallpox DNA vaccine protects nonhuman primates against lethal monkeypox. J Virol. 2004;78:4433–4443. doi: 10.1128/JVI.78.9.4433-4443.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cosma A., Buhler S., Nagaraj R., Staib C., Hammarin A.L., Wahren B., Goebel F.D., Erfle V., Sutter G. Neutralization assay using a modified vaccinia virus Ankara vector expressing the green fluorescent protein is a high-throughput method to monitor the humoral immune response against vaccinia virus. Clin Diagn Lab Immunol. 2004;11:406–410. doi: 10.1128/CDLI.11.2.406-410.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrop R., Ryan M.G., Golding H., Redchenko I., Carroll M.W. Monitoring of human immunological responses to vaccinia virus. Methods Mol Biol. 2004;269:243–266. doi: 10.1385/1-59259-789-0:243. [DOI] [PubMed] [Google Scholar]
- 19.Speller S.A., Warren A.P. Ex vivo detection and enumeration of human antigen-specific CD8+ T lymphocytes using antigen delivery by a recombinant vaccinia expression vector and intracellular cytokine staining. J Immunol Methods. 2002;262:167–180. doi: 10.1016/s0022-1759(02)00025-x. [DOI] [PubMed] [Google Scholar]
- 20.Harrington L.E., van der Most R., Whitton J.L., Ahmed R. Recombinant vaccinia virus-induced T-cell immunity: quantitation of the response to the virus vector and the foreign epitope. J Virol. 2002;76:3329–3337. doi: 10.1128/JVI.76.7.3329-3337.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ober B.T., Bruhl P., Schmidt M., Wieser V., Gritschenberger W., Coulibaly S., Savidis-Dacho H., Gerencer M., Falkner F.G. Immunogenicity and safety of defective vaccinia virus lister: comparison with modified vaccinia virus Ankara. J Virol. 2002;76:7713–7723. doi: 10.1128/JVI.76.15.7713-7723.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.•.Terajima M., Cruz J., Raines G., Kilpatrick E.D., Kennedy J.S., Rothman A.L., Ennis F.A. Quantitation of CD8+ T cell responses to newly identified HLA-A*0201-restricted T cell epitopes conserved among vaccinia and variola (smallpox) viruses. J Exp Med. 2003;197:927–932. doi: 10.1084/jem.20022222. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this study, the authors analysed vaccinia virus-specific immune responses of the acute and memory phase in recently smallpox-immunized individuals using two new peptide epitopes that they identified.
- 23.Ramirez J.C., Gherardi M.M., Rodriguez D., Esteban M. Attenuated modified vaccinia virus Ankara can be used as an immunizing agent under conditions of preexisting immunity to the vector. J Virol. 2000;74:7651–7655. doi: 10.1128/jvi.74.16.7651-7655.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gherardi M.M., Najera J.L., Perez-Jimenez E., Guerra S., Garcia-Sastre A., Esteban M. Prime-boost immunization schedules based on influenza virus and vaccinia virus vectors potentiate cellular immune responses against human immunodeficiency virus Env protein systemically and in the genitorectal draining lymph nodes. J Virol. 2003;77:7048–7057. doi: 10.1128/JVI.77.12.7048-7057.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Z., La Rosa C., Maas R., Ly H., Brewer J., Mekhoubad S., Daftarian P., Longmate J., Britt W.J., Diamond D.J. Recombinant modified vaccinia virus Ankara expressing a soluble form of glycoprotein B causes durable immunity and neutralizing antibodies against multiple strains of human cytomegalovirus. J Virol. 2004;78:3965–3976. doi: 10.1128/JVI.78.8.3965-3976.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Z.Y., Wyatt L.S., Kong W.P., Moodie Z., Moss B., Nabel G.J. Overcoming immunity to a viral vaccine by DNA priming before vector boosting. J Virol. 2003;77:799–803. doi: 10.1128/JVI.77.1.799-803.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hodge J.W., Poole D.J., Aarts W.M., Gomez Yafal A., Gritz L., Schlom J. Modified vaccinia virus Ankara recombinants are as potent as vaccinia recombinants in diversified prime and boost vaccine regimens to elicit therapeutic antitumor responses. Cancer Res. 2003;63:7942–7949. [PubMed] [Google Scholar]
- 28.Anderson R.J., Hannan C.M., Gilbert S.C., Laidlaw S.M., Sheu E.G., Korten S., Sinden R., Butcher G.A., Skinner M.A., Hill A.V. Enhanced CD8+ T cell immune responses and protection elicited against Plasmodium berghei malaria by prime boost immunization regimens using a novel attenuated fowlpox virus. J Immunol. 2004;172:3094–3100. doi: 10.4049/jimmunol.172.5.3094. [DOI] [PubMed] [Google Scholar]
- 29.Hanke T., Barnfield C., Wee E.G., Agren L., Samuel R.V., Larke N., Liljestrom P. Construction and immunogenicity in a prime-boost regimen of a Semliki Forest virus-vectored experimental HIV clade A vaccine. J Gen Virol. 2003;84:361–368. doi: 10.1099/vir.0.18738-0. [DOI] [PubMed] [Google Scholar]
- 30.Ramsburg E., Rose N.F., Marx P.A., Mefford M., Nixon D.F., Moretto W.J., Montefiori D., Earl P., Moss B., Rose J.K. Highly effective control of an AIDS virus challenge in macaques by using vesicular stomatitis virus and modified vaccinia virus Ankara vaccine vectors in a single-boost protocol. J Virol. 2004;78:3930–3940. doi: 10.1128/JVI.78.8.3930-3940.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amara R.R., Villinger F., Staprans S.I., Altman J.D., Montefiori D.C., Kozyr N.L., Xu Y., Wyatt L.S., Earl P.L., Herndon J.G. Different patterns of immune responses but similar control of a simian-human immunodeficiency virus 89.6P mucosal challenge by modified vaccinia virus Ankara (MVA) and DNA/MVA vaccines. J Virol. 2002;76:7625–7631. doi: 10.1128/JVI.76.15.7625-7631.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vogel T.U., Horton H., Fuller D.H., Carter D.K., Vielhuber K., O’Connor D.H., Shipley T., Fuller J., Sutter G., Erfle V. Differences between T cell epitopes recognized after immunization and after infection. J Immunol. 2002;169:4511–4521. doi: 10.4049/jimmunol.169.8.4511. [DOI] [PubMed] [Google Scholar]
- 33.Evans D.T., Chen L.M., Gillis J., Lin K.C., Harty B., Mazzara G.P., Donis R.O., Mansfield K.G., Lifson J.D., Desrosiers R.C. Mucosal priming of simian immunodeficiency virus-specific cytotoxic T-lymphocyte responses in rhesus macaques by the Salmonella type III secretion antigen delivery system. J Virol. 2003;77:2400–2409. doi: 10.1128/JVI.77.4.2400-2409.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Earl P.L., Wyatt L.S., Montefiori D.C., Bilska M., Woodward R., Markham P.D., Malley J.D., Vogel T.U., Allen T.M., Watkins D.I., Miller N., Moss B. Comparison of vaccine strategies using recombinant env-gag-pol MVA with or without an oligomeric Env protein boost in the SHIV rhesus macaque model. Virology. 2002;294:270–281. doi: 10.1006/viro.2001.1345. [DOI] [PubMed] [Google Scholar]
- 35.Gherardi M.M., Perez-Jimenez E., Najera J.L., Esteban M. Induction of HIV immunity in the genital tract after intranasal delivery of a MVA vector: enhanced immunogenicity after DNA prime-modified vaccinia virus Ankara boost immunization schedule. J Immunol. 2004;172:6209–6220. doi: 10.4049/jimmunol.172.10.6209. [DOI] [PubMed] [Google Scholar]
- 36.Woodberry T., Gardner J., Elliott S.L., Leyrer S., Purdie D.M., Chaplin P., Suhrbier A. Prime boost vaccination strategies: CD8 T cell numbers, protection, and Th1 bias. J Immunol. 2003;170:2599–2604. doi: 10.4049/jimmunol.170.5.2599. [DOI] [PubMed] [Google Scholar]
- 37.Mwau M., Cebere I., Sutton J., Chikoti P., Winstone N., Wee E.G., Beattie T., Chen Y.H., Dorrell L., McShane H. A human immunodeficiency virus 1 (HIV-1) clade A vaccine in clinical trials: stimulation of HIV-specific T-cell responses by DNA and recombinant modified vaccinia virus Ankara (MVA) vaccines in humans. J Gen Virol. 2004;85:911–919. doi: 10.1099/vir.0.19701-0. [DOI] [PubMed] [Google Scholar]
- 38.Amara R.R., Villinger F., Altman J.D., Lydy S.L., O’Neil S.P., Staprans S.I., Montefiori D.C., Xu Y., Herndon J.G., Wyatt L.S. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science. 2001;292:69–74. doi: 10.1126/science.1058915. [DOI] [PubMed] [Google Scholar]
- 39.Allen T.M., Mortara L., Mothe B.R., Liebl M., Jing P., Calore B., Piekarczyk M., Ruddersdorf R., O’Connor D.H., Wang X. Tat-vaccinated macaques do not control simian immunodeficiency virus SIVmac239 replication. J Virol. 2002;76:4108–4112. doi: 10.1128/JVI.76.8.4108-4112.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horton H., Vogel T.U., Carter D.K., Vielhuber K., Fuller D.H., Shipley T., Fuller J.T., Kunstman K.J., Sutter G., Montefiori D.C. Immunization of rhesus macaques with a DNA prime/modified vaccinia virus Ankara boost regimen induces broad simian immunodeficiency virus (SIV)-specific T-cell responses and reduces initial viral replication but does not prevent disease progression following challenge with pathogenic SIVmac239. J Virol. 2002;76:7187–7202. doi: 10.1128/JVI.76.14.7187-7202.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vogel T.U., Reynolds M.R., Fuller D.H., Vielhuber K., Shipley T., Fuller J.T., Kunstman K.J., Sutter G., Marthas M.L., Erfle V. Multispecific vaccine-induced mucosal cytotoxic T lymphocytes reduce acute-phase viral replication but fail in long-term control of simian immunodeficiency virus SIVmac239. J Virol. 2003;77:13348–13360. doi: 10.1128/JVI.77.24.13348-13360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Negri D.R., Baroncelli S., Catone S., Comini A., Michelini Z., Maggiorella M.T., Sernicola L., Crostarosa F., Belli R., Mancini M.G. Protective efficacy of a multicomponent vector vaccine in cynomolgus monkeys after intrarectal simian immunodeficiency virus challenge. J Gen Virol. 2004;85:1191–1201. doi: 10.1099/vir.0.79794-0. [DOI] [PubMed] [Google Scholar]
- 43.Bertley F.M., Kozlowski P.A., Wang S.W., Chappelle J., Patel J., Sonuyi O., Mazzara G., Montefiori D., Carville A., Mansfield K.G., Aldovini A. Control of simian/human immunodeficiency virus viremia and disease progression after IL-2-augmented DNA-modified vaccinia virus Ankara nasal vaccination in nonhuman primates. J Immunol. 2004;172:3745–3757. doi: 10.4049/jimmunol.172.6.3745. [DOI] [PubMed] [Google Scholar]
- 44.•.Bisht H., Roberts A., Vogel L., Bukreyev A., Collins P.L., Murphy B.R., Subbarao K., Moss B. Severe acute respiratory syndrome coronavirus spike protein expressed by attenuated vaccinia virus protectively immunizes mice. Proc Natl Acad Sci USA. 2004;101:6641–6646. doi: 10.1073/pnas.0401939101. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper describes the characterization of a first MVA candidate vaccine against SARS accomplished within the first year after the emergence of this infectious disease.
- 45.•.Wang Z., La Rosa C., Mekhoubad S., Lacey S.F., Villacres M.C., Markel S., Longmate J., Ellenhorn J.D., Siliciano R.F., Buck C. Attenuated poxviruses generate clinically relevant frequencies of CMV-specific T cells. Blood. 2004;104:847–856. doi: 10.1182/blood-2003-10-3469. [DOI] [PubMed] [Google Scholar]; This study demonstrates the clinical feasibility to efficiently amplify multiple human CMV-specific cytotoxic T-lymphocyte populations and to induce robust T-cell responses in HLA A2.1 transgenic mice. The data are important with regard to possible usefulness of these recombinant MVA for clinical evaluation in the context of CMV-specific immunotherapy for hematopoietic stem cell transplantation recipients or donor vaccination.
- 46.Gibson L., Piccinini G., Lilleri D., Revello M.G., Wang Z., Markel S., Diamond D.J., Luzuriaga K. Human cytomegalovirus proteins pp65 and immediate early protein 1 are common targets for CD8+ T cell responses in children with congenital or postnatal human cytomegalovirus infection. J Immunol. 2004;172:2256–2264. doi: 10.4049/jimmunol.172.4.2256. [DOI] [PubMed] [Google Scholar]
- 47.Meseda C.A., Elkins K.L., Merchlinsky M.J., Weir J.P. Prime-boost immunization with DNA and modified vaccinia virus ankara vectors expressing herpes simplex virus-2 glycoprotein D elicits greater specific antibody and cytokine responses than DNA vaccine alone. J Infect Dis. 2002;186:1065–1073. doi: 10.1086/344234. [DOI] [PubMed] [Google Scholar]
- 48.Taylor G.S., Haigh T.A., Gudgeon N.H., Phelps R.J., Lee S.P., Steven N.M., Rickinson A.B. Dual stimulation of Epstein-Barr Virus (EBV)-specific CD4+- and CD8+-T-cell responses by a chimeric antigen construct: potential therapeutic vaccine for EBV-positive nasopharyngeal carcinoma. J Virol. 2004;78:768–778. doi: 10.1128/JVI.78.2.768-778.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.••.Corona Gutierrez C.M., Tinoco A., Lopez C.M., Navarro T., Calzado P., Vargas L., Reyes L., Posternak R., Rosales R. Clinical protocol. A phase II study: efficacy of the gene therapy of the MVA E2 recombinant virus in the treatment of precancerous lesions (NIC I and NIC II) associated with infection of oncogenic human papillomavirus. Hum Gene Ther. 2002;13:1127–1140. doi: 10.1089/104303402753812520. [DOI] [PubMed] [Google Scholar]; This is the first study reporting substantial therapeutic efficacy of a candidate MVA vaccine against human cancer. Elicited antibody and T-cell responses after local therapeutic vaccination correlated with the elimination of precancerous cervical lesions and with a remarkable reduction of HPV viral load.
- 50.Mulryan K., Ryan M.G., Myers K.A., Shaw D., Wang W., Kingsman S.M., Stern P.L., Carroll M.W. Attenuated recombinant vaccinia virus expressing oncofetal antigen (tumor-associated antigen) 5T4 induces active therapy of established tumors. Mol Cancer Ther. 2002;1:1129–1137. [PubMed] [Google Scholar]
- 51.Espenschied J., Lamont J., Longmate J., Pendas S., Wang Z., Diamond D.J., Ellenhorn J.D. CTLA-4 blockade enhances the therapeutic effect of an attenuated poxvirus vaccine targeting p53 in an established murine tumor model. J Immunol. 2003;170:3401–3407. doi: 10.4049/jimmunol.170.6.3401. [DOI] [PubMed] [Google Scholar]
- 52.Rochlitz C., Figlin R., Squiban P., Salzberg M., Pless M., Herrmann R., Tartour E., Zhao Y., Bizouarne N., Baudin M., Acres B. Phase I immunotherapy with a modified vaccinia virus (MVA) expressing human MUC1 as antigen-specific immunotherapy in patients with MUC1-positive advanced cancer. J Gene Med. 2003;5:690–699. doi: 10.1002/jgm.397. [DOI] [PubMed] [Google Scholar]
- 53.Gonzalez-Aseguinolaza G., Nakaya Y., Molano A., Dy E., Esteban M., Rodriguez D., Rodriguez J.R., Palese P., Garcia-Sastre A., Nussenzweig R.S. Induction of protective immunity against malaria by priming-boosting immunization with recombinant cold-adapted influenza and modified vaccinia Ankara viruses expressing a CD8+-T-cell epitope derived from the circumsporozoite protein of Plasmodium yoelii. J Virol. 2003;77:11859–11866. doi: 10.1128/JVI.77.21.11859-11866.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moorthy V.S., Pinder M., Reece W.H., Watkins K., Atabani S., Hannan C., Bojang K., McAdam K.P., Schneider J., Gilbert S., Hill A.V. Safety and immunogenicity of DNA/modified vaccinia virus Ankara malaria vaccination in African adults. J Infect Dis. 2003;188:1239–1244. doi: 10.1086/378515. [DOI] [PubMed] [Google Scholar]
- 55.McShane H., Behboudi S., Goonetilleke N., Brookes R., Hill A.V. Protective immunity against Mycobacterium tuberculosis induced by dendritic cells pulsed with both CD8+- and CD4+-T-cell epitopes from antigen 85A. Infect Immun. 2002;70:1623–1626. doi: 10.1128/IAI.70.3.1623-1626.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goonetilleke N.P., McShane H., Hannan C.M., Anderson R.J., Brookes R.H., Hill A.V. Enhanced immunogenicity and protective efficacy against Mycobacterium tuberculosis of bacille Calmette-Guerin vaccine using mucosal administration and boosting with a recombinant modified vaccinia virus Ankara. J Immunol. 2003;171:1602–1609. doi: 10.4049/jimmunol.171.3.1602. [DOI] [PubMed] [Google Scholar]
- 57.•.Di Nicola M., Carlo-Stella C., Mortari R., Baldassari P., Guidetti A., Gallino G.F., Del Vecchio M., Ravagnani F., Magni M., Chaplin P. Boosting T cell-mediated immunity to tyrosinase by vaccinia virus-transduced, CD34+-derived dendritic cell vaccination: a phase I trial in metastatic melanoma. Clin Cancer Res. 2004;10:5381–5390. doi: 10.1158/1078-0432.CCR-04-0602. [DOI] [PubMed] [Google Scholar]; This is the first study reporting relevant safety and efficacy data for a candidate MVA vector vaccine delivering an endogenous human tumor-associated antigen.