Abstract
A series of pyrazinecarboxamide derivatives T-705 (favipiravir), T-1105 and T-1106 were discovered to be candidate antiviral drugs. These compounds have demonstrated good activity in treating viral infections in laboratory animals caused by various RNA viruses, including influenza virus, arenaviruses, bunyaviruses, West Nile virus (WNV), yellow fever virus (YFV), and foot-and-mouth disease virus (FMDV). Treatment has in some cases been effective when initiated up to 5–7 days after virus infection, when the animals already showed signs of illness. Studies on the mechanism of action of T-705 have shown that this compound is converted to the ribofuranosyltriphosphate derivative by host enzymes, and this metabolite selectively inhibits the influenza viral RNA-dependent RNA polymerase without cytotoxicity to mammalian cells. Interestingly, these compounds do not inhibit host DNA and RNA synthesis and inosine 5′-monophosphate dehydrogenase (IMPDH) activity. From in vivo studies using several animal models, the pyrazinecarboxamide derivatives were found to be effective in protecting animals from death, reducing viral burden, and limiting disease manifestations, even when treatment was initiated after virus inoculation. Importantly, T-705 imparts its beneficial antiviral effects without significant toxicity to the host. Prompt development of these compounds is expected to provide effective countermeasures against pandemic influenza virus and several bioweapon threats, all of which are of great global public health concern given the current paucity of highly effective broad-spectrum drugs.
Keywords: Antiviral compound, T-705, Pyrazinecarboxamide derivatives, RNA-dependent RNA polymerase, RNA viruses, Influenza virus, Arenavirus, Bunyavirus, Yellow fever virus, West Nile virus, Foot-and-mouth disease virus
1. Introduction
In order to combat infectious diseases, a number of important discoveries and inventions, such as antibiotics, vaccines and chemotherapeutic drugs, have been made. Despite significant progress, infectious diseases are still the largest cause of death in the world, and epidemics, caused by emerging and re-emerging infectious organisms, are a serious threat to human health (Heeney, 2006). Consider these recent examples: the generation and spread of severe acute respiratory syndrome (SARS) virus, West Nile virus (WNV), and H5N1 avian influenza virus. These infectious diseases can spread quickly because of increased global travel and the export and import of animals that may harbor these viruses (Tapper, 2006). The world is unprepared for the real threat of naturally occurring outbreaks or intentional virus release as a deadly bioweapon. For combating emerging viruses, as well as drug-resistant mutants of viruses for which effective treatments exist, continued and increased vigilance will be required, including the development of new broad-spectrum therapies.
Vaccines are the principal defense against viral infections (Plotkin, 2005), but their use does not negate the utility of antiviral drugs. There is a great need for the development of compounds that have therapeutic efficacy in treating diseases caused by highly pathogenic RNA viruses (Bray, 2008). For the treatment of influenza-type illnesses, M2-channel inhibitor, amantadine and neuraminidase inhibitors, oseltamivir or zanamivir exist as therapeutic drugs. However, with the exception of ribavirin, few approved drugs are available for other RNA virus infections. This is especially true for several types of viral hemorrhagic fever (Gowen and Holbrook, 2008). Application of ribavirin for the treatment of such severe infections has not become widespread because of concerns regarding adverse effects and lack of specificity and efficacy (Kilgore et al., 1997, McCormick et al., 1986, Monath, 2008).
In this article, we provide an overview of the antiviral activities of T-705 (favipiravir) and the related pyrazinecarboxamide compounds T-1105 and T-1106 against several RNA viruses and their potential as novel antiviral drugs for the treatment of some emerging pathogenic RNA virus infections. T-705, T-1106 and T-1105 were discovered and synthesized by Toyama Chemical Co., Ltd. Their structures are shown in Fig. 1 . Through the screening of a chemical library of Toyama Chemical Co., Ltd. using a plaque reduction assay for antiviral activity against influenza virus A/PR/8/34, a lead compound, which was afterwards designated as T-1105, was found to be effective. Thereafter, its derivatives were synthesized to evaluate structure–activity studies in terms of in vitro and in vivo antiviral activities as well as pharmacokinetic properties. T-705 and the related compounds were then selected for further investigation as drug candidates.
Fig. 1.
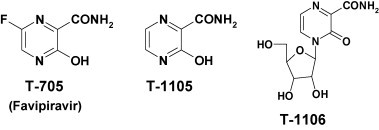
Chemical structure of T-705, T-1105 and T-1106.
2. Influenza virus
Influenza virus is a member of the Orthomyxoviridae family, and three types, A, B, and C, are known to exist. Type A influenza virus is further classified into many subtypes according to differences in surface antigens. Humans are usually infected by three of the A types, H1N1, H2N2, H3N2, and by the B type, but epidemics are repeated yearly because of frequent mutation of the virus. Some retrospective studies report that pandemic influenza outbreaks recur in cycles of about 10–40 years due to antigenic shift (Glezen, 1982, Webby and Webster, 2003).
Once an influenza virus infects the upper airway, patients usually develop fever and suffer from fatigue for a few days, followed by full recovery in approximately 1 week. However, severe illness is seen in newborns, the elderly, and patients who have chronic illness, such as cardiac disorders, pulmonary diseases, diabetes, and immunodeficiency. Furthermore, severe complications including death can occur. Recently, outbreaks of highly pathogenic avian influenza have occurred in Southeast Asia (Kaye and Pringle, 2005, Abdel-Ghafar et al., 2008), and a high mortality rate (60% or more) in humans has been reported (WHO, 2008). Authorities fear that the avian flu will acquire the ability to transmit from human to human, touching off a pandemic that could kill millions of people.
2.1. In vitro activities of T-705 against influenza virus
The anti-influenza activity of T-705 was evaluated with plaque reduction assays for influenza A (H1N1, H2N2, and H3N2), B and C viruses (Furuta et al., 2002). T-705 inhibited plaque formation of all types of laboratory-adapted and clinically isolated influenza viruses with low EC50 values (Table 1 ). The antiviral activities of T-705 against influenza A (H5N1) virus (Sidwell et al., 2007) as well as an oseltamivir-resistant virus were determined in yield reduction assays in MDCK cells (Furuta et al., 2002). The EC90 values that were effective against influenza A (H5N1) ranged from 1.3 to 7.7 μM, which is similar to effective concentrations against seasonal influenza viruses. Oseltamivir showed an anti-influenza viral activity at low concentrations except in C type and oseltamivir-resistant strains. Ribavirin also showed activity, but its potency was 14 times less effective than T-705, depending on the strain.
Table 1.
Anti-influenza viral activity of T-705 and reference drugs.
| Virus type | Number of strains | Range of inhibition concentration (μM) |
||
|---|---|---|---|---|
| T-705 | Oseltamivir carboxylate | Ribavirin | ||
| A (H1N1)a | 5 | 0.19–1.3 | 0.0070–0.034 | 6.6–49 |
| A (H2N2)a | 4 | 0.083–1.9 | 0.00060–2.4 | 20–57 |
| A (H3N2)a | 4 | 0.5–3.1 | 0.0018–0.011 | 18–82 |
| Ba | 3 | 0.25–0.57 | 0.022–0.11 | 4.9–78 |
| Ca | 3 | 0.19–0.36 | >352 | NT |
| A (H5N1)b | 4 | 1.3–7.7 | 0.007–0.92 | 18–33 |
| Oseltamivir-resistant A/PR/8/34 (H1N1)b | 1 | 0.61 | 88 | NT |
NT: not tested.
Antiviral activity (EC50) as determined by plaque reduction assay (Furuta et al., 2002).
Antiviral activity (EC90) as determined by yield reduction assay (Sidwell et al., 2007, Furuta et al., 2002).
2.2. In vivo activities of T-705 against influenza virus
The in vivo therapeutic efficacy of T-705 was evaluated using BALB/c mice infected with influenza A/Duck/MN/1525/81 (H5N1), A/Osaka/5/70(H3N2) and A/Victoria/3/75 (H3N2) viruses (Furuta et al., 2002, Sidwell et al., 2007). In one example, mice were infected intranasally with a lethal dose of the influenza A/H5N1 virus, and T-705 or oseltamivir was administered orally for 5 days, beginning from 1 h post-infection. Oral treatment with T-705 at the dose of 30 mg/kg/day or more prevented death, inhibited lung consolidation and reduced lung virus titers (Table 2 ) (Sidwell et al., 2007).
Table 2.
Efficacy of T-705 against H5N1 influenza virus infection in mice.
| Treatment | Dose (mg/kg/day) | Time of start of therapy (h) | Survivors/total | Day of death (mean ± S.D.) |
|---|---|---|---|---|
| Expt 1: twice daily for 5 days | ||||
| 0.4% CMC | – | 1 | 0/20 | 8.2 ± 1.6 |
| T-705 | 3 | 1 | 0/10 | 7.4 ± 1.1 |
| 10 | 1 | 2/10 | 8.5 ± 2.8 | |
| 30 | 1 | 8/10** | 11.0 ± 4.2 | |
| 100 | 1 | 10/10** | >21.0* | |
| 300 | 1 | 10/10** | >21.0* | |
| Expt 2: every 6 h for 5 days | ||||
| 0.4% CMC | – | 72 | 0/20 | 7.9 ± 2.7 |
| T-705 | 300 | 72 | 9/10** | 7.0 |
| 300 | 84 | 10/10** | >21.0* | |
| 300 | 96 | 8/9** | 7.0 | |
| 300 | 120 | 3/10* | 7.9 ± 2.6 | |
Expt 1: effect of drug dose on survival. Mice were treated twice daily with T-705 or with CMC vehicle for 5 days beginning 1 h after virus exposure. Expt 2: effect of delay in initiation of treatment. Mice were treated with T-705 or with CMC vehicle every 6 h for 5 days, beginning at the indicated hour post-infection. S.D.: standard deviation.
P < 0.05.
P < 0.001 compared to controls treated with CMC only (Sidwell et al., 2007).
An important and desired characteristic of an antiviral drug is to be effective even if treatment is begun after viral infection is established and clinical symptoms have appeared. The results of studies of delayed initiation of treatment using influenza strains A/NewCaledonia/20/90 (H1N1), A/NWS/33(H1N1) and B/Sichuan/379/99 showed a marked reduction in mortality even when treatment with T-705 was initiated from 60 to 96 h post-virus infection. Significantly, T-705 was highly effective against the recently emerged, highly virulent H5N1 virus (Table 2) (Sidwell et al., 2007). Mice treated with T-705 also displayed significantly reduced declines in arterial oxygen saturation (SaO2) in these infection models. In a comparative experiment with oseltamivir, using mice infected with a high challenge dose of influenza A/PR/8/34 virus, T-705 completely prevented death, and the survival rate was significantly higher than in oseltamivir-treated animals (Takahashi et al., 2003).
2.3. Mechanism of action
A time-of-addition experiment revealed that T-705 inhibited an early to middle stage of influenza viral replication, but not the adsorption or release stage. The in vitro antiviral activity of T-705 was attenuated when purines or purine nucleosides but not pyrimidines were added to the culture. This phenomenon suggests that T-705 acts as a purine or purine nucleoside in viral RNA replication (Furuta et al., 2005).
In experiments using [14C] radio-labeled T-705, T-705 ribofuranosyl-5′-triphosphate (T-705RTP) and T-705 ribofuranosyl-5′-monophosphate (T-705RMP) were detected in cultured MDCK cells. Furthermore, it was found that T-705RTP inhibited influenza virus RNA polymerase activity in a dose-dependent manner and a GTP-competitive manner (K i = 1.52 μM). The inhibitory potency was approximately 10 times higher than that of ribavirin triphosphate. On the other hand, T-705RMP did not inhibit the viral RNA-dependent RNA polymerase. The inhibition of cellular inosine 5′-monophosphate dehydrogenase (IMPDH) by this metabolite was about 150-fold weaker than by ribavirin monophosphate (Furuta et al., 2005).
A summary of these experimental observations and the mechanisms of action for anti-influenza drugs are illustrated in Fig. 2 . T-705 has a different mechanism of action from that of other approved anti-influenza virus drugs, and the virus selectivity of T-705 is a favorable feature distinguishing it from ribavirin.
Fig. 2.
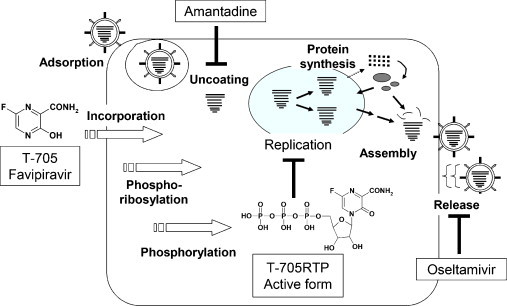
Mode of action of T-705 and other anti-influenza drugs. T-705 is converted to the ribofuranosyl triphosphate form and inhibits influenza virus RNA polymerase in the host cells. Amantadine inhibits virus M2 proteins, and oseltamivir inhibits the release of virus.
3. Arenavirus and bunyavirus
Because of the severe disease and high mortality rate associated with infections caused by agents of viral hemorrhagic fever (VHF), the viruses that cause these diseases are among the most feared. VHF agents belong to the Arenaviridae, Bunyaviridae, Filoviridae, and Flaviviridae RNA virus families. Currently, there is a paucity of safe and effective antiviral drugs for the treatment of VHF. In a limited number of studies in humans and primates, ribavirin has proven to be effective against several of the arenaviral and bunyaviral hemorrhagic fevers (Enria and Maiztegui, 1994, Ergonul, 2008, Ergonul et al., 2004, Kilgore et al., 1997, Mardani et al., 2003, McCormick et al., 1986), but because of concerns with dose-related hemolytic anemia and potential teratogenicity, it is only approved for compassionate use under investigational new drug protocols (Borio et al., 2002). The threat of intentional release of hemorrhagic fever agents as bioweapons and the increasing frequency of naturally occurring outbreaks stress the need for the development of novel therapeutics and disease management strategies.
3.1. Antiviral activity of T-705 against bunyaviruses and arenaviruses
T-705 was shown to be highly active in several bunyavirus (La Crosse, Punta Toro, Rift Valley fever, sandfly fever) and arenavirus (Junin, Pichinde, Tacaribe) cell culture systems that measured inhibition of cytopathic effect and virus yield reduction (Gowen et al., 2007). The EC50 values for T-705 ranged from 32 to 191 μM and 5.1 to 5.7 μM against the bunyaviruses and arenaviruses examined, respectively, with robust therapeutic indices (SI) (Gowen et al., 2007). EC50s for ribavirin when tested against the same panel of bunyaviruses and arenaviruses were 53–172 μM and 10–13 μM (Gowen et al., 2007). Thus, the in vitro activity of T-705 was comparable, if not superior, to ribavirin, and the SI values were higher.
The in vivo efficacy of T-705 was evaluated against Punta Toro virus (PTV) infection in mouse and hamster models of Rift Valley fever (Gowen et al., 2007). Robust protection against highly lethal PTV challenge was seen in both mice and hamsters when T-705 was administered 4 h prior to or 24 h after virus inoculation. A 10-mg/kg/day dose given twice daily for 5 days was sufficient for efficacy when treatment was initiated 4 h before challenge in mice. Treatment beginning 1 day after virus inoculation was also effective, although a higher dose of 30 mg/kg/day was needed (Gowen et al., 2007). In hamsters, a slightly higher dose of T-705 (15 mg/kg/day) was required to obtain optimally efficacy when starting treatment 4 h pre-infection (Table 3 ). Because hamsters are considerably more susceptible to PTV (Gowen and Holbrook, 2008, Gowen et al., 2006), they are generally more difficult to treat. Further animal studies examining T-705 activity against authentic highly pathogenic strains of Rift Valley fever are needed.
Table 3.
Efficacy of T-705 against Punta Toro virus infection in hamsters.
| Treatment | Dose (mg/kg/day) | Survival/total | Mean virus titer ± S.D. |
Mean ALT ± S.D. | |
|---|---|---|---|---|---|
| Liver | Serum | ||||
| 0.4% CMC | – | 1/20 | <6.6 ± 2.2 (90) | <5.4 ± 3.4 (40) | 1228 ± 1622 |
| T-705 | 15 | 5/10** | >6.9 ± 1.5 (100) | <4.1 ± 3.0 (20) | 204 ± 441 |
| 30 | 9/10*** | <3.5 ± 1.7* (40) | <4.5 ± 2.6 (40) | 25 ± 18 | |
| 60 | 9/10*** | <3.4 ± 1.3* (20) | <3.8 ± 2.2 (20) | 17 ± 3 | |
| Ribavirin | 30 | 7/10*** | <2.8** (0) | <2.8 (0) | 13 ± 13 |
Hamsters were orally treated with T-705 or ribavirin twice daily for 6 days beginning 4 h pre-virus inoculation. Viral titers were determined on day 4 of infection, and are expressed as log10 cell culture 50% infectious dose/0.1 g of liver or ml of serum. The percentage of animals presenting with detectable virus levels is indicated in parenthesis. ALT: alanine aminotransferase, in international units per liter. S.D.: standard deviation.
P < 0.05.
P < 0.01.
P < 0.001 compared to controls treated with CMC vehicle only (Gowen et al., 2007).
In the Pichinde virus (PICV) hamster infection model of arenaviral hemorrhagic fever, both prophylactic and therapeutic T-705 treatments have yielded dramatic protection (Gowen et al., 2007, Gowen et al., 2008). Initial studies in PICV-infected hamsters established an effective daily dose of 30 mg/kg/day if 7-day twice-daily treatment was initiated within 4 h after infection (Table 4 ). When treatment was begun 1–3 days after challenge, a dose of 50 mg/kg/day was effective, suggesting that higher doses were needed to effectively control the infection (Gowen et al., 2007). Recently, significant protection was achieved by employing a high-dose regimen, in which a loading dose was used to knock down the marked viral burden present when treatment was delayed until day 7 (Gowen et al., 2008). Twice-daily doses at 320 mg/kg were given on either day 6 or 7, followed by 7 days of twice-daily doses of 100 mg/kg (Fig. 3 ). Notably, PICV-infected hamsters normally begin to succumb on day 8 of infection, and peak serum and tissue viral burden is present in most animals by day 7, underscoring the significance of these findings.
Table 4.
Efficacy of T-705 against Pichinde virus infection in hamsters.
| Treatment | Dose (mg/kg/day) | Survivors/total | MDD ± S.D. | Mean virus titer ± S.D. |
Mean ALT ± S.D. | |
|---|---|---|---|---|---|---|
| Liver | Serum | |||||
| 0.4% CMC | – | 0/20 | 8.6 ± 0.8 | >9.1 ± 0.3 (100) | 5.5 ± 0.1 (100) | 2619 ± 778 |
| T-705 | 15 | 2/10 | 11.1 ± 4.2 | 7.7 ± 0.9* (100) | <5.3 ± 1.8 (80) | 1220 ± 926 |
| 30 | 6/10*** | 12.5 ± 5.7* | 6.4 ± 1.0** (100) | <5.2 ± 1.9 (80) | 378 ± 370** | |
| 60 | 9/10*** | 22.0 | <2.8** (0) | <2.8** (0) | 23 ± 12** | |
| Ribavirin | 40 | 9/10*** | 11.0 | <3.2 ± 0.9** (20) | <3.0 ± 0.3** (40) | 30 ± 47** |
Hamsters were orally treated with T-705 or ribavirin twice daily for 7 days beginning 4 h pre-virus inoculation. Viral titers and serum alanine aminotransferase (ALT) levels were determined on day 7 post-infection for five animals per group. MDD: mean day of death. Viral titers: log10 cell culture 50% infectious dose/0.1 g of liver or ml of serum. Percentage of animals presenting with detectable virus levels is indicated in parenthesis. ALT: in international units per liter. S.D.: standard deviation.
P < 0.05.
P < 0.01.
P < 0.001 compared to controls treated with CMC vehicle only (Gowen et al., 2007).
Fig. 3.
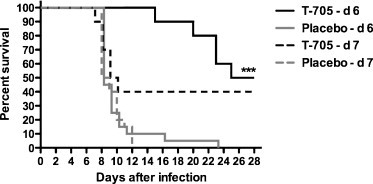
Survival of Pichinde virus-infected hamsters treated with T-705 or placebo beginning on day 6 or 7 post-infection. T-705 was given twice daily at 320 mg/kg/day on the first day and 100 mg/kg/day thereafter. ***P < 0.001 compared to respective placebo-treated hamsters by log-rank test (Gowen et al., 2007).
As regards toxicity, the LD50 of T-705 in hamsters was six times or more higher than that of ribavirin, suggesting that T-705 is superior to ribavirin from the perspective of safety. These results support an assumption that T-705 may be a viable alternative for the treatment of life-threatening bunyaviral and arenaviral infections. Experiments investigating the activity of T-705 against Lassa fever virus and South American hemorrhagic fever viruses are underway.
4. Yellow fever virus
YFV, a member of the Flaviviridae family of arboviruses, has been responsible for many outbreaks of hemorrhagic disease in many countries in Africa and South America, causing an estimated 30,000 deaths each year, despite the availability of an effective vaccine (Tomori, 2004). Adverse vaccination events and imported YFV by unvaccinated travelers have been documented outside of areas of endemnicity. There are no approved antivirals for the treatment of YFV (Monath, 2008). Therefore, the development of therapeutic agents for the treatment of YFV is important, especially those that are effective after the onset of clinical symptoms.
4.1. Antiviral activity of T-1106 against YFV
In vitro cytopathic effect reduction assays in Vero cells with T-705 and T-1106 showed only weak activity against YFV, with EC50 values of 335 μM and >369 μM, respectively (Julander et al., 2007, Julander et al., 2009). However, despite these in vitro results, T-1106 showed surprising therapeutic efficacy in a hamster model of YF (Julander et al., 2007). Intraperitoneal treatment with a dose of 32 mg/kg/day of T-1106, beginning 4 h before virus inoculation and continuing twice daily for 7 days, resulted in lower ALT and AST levels in the serum, less weight loss and significantly improved survival. T-1106 was also effective when administered orally with a minimal effective dose of 32 mg/kg/day, which was similar to intraperitoneal treatment. Survival rates were significantly improved even when hamsters were treated with 100 mg/kg/day of T-1106 twice daily beginning as late as 4 days post-infection (Fig. 4 ). For comparison, ribavirin at a dose of 50 mg/kg/day, significantly improved survival rates and ALT levels in infected hamsters when given 4 h prior to virus challenge.
Fig. 4.
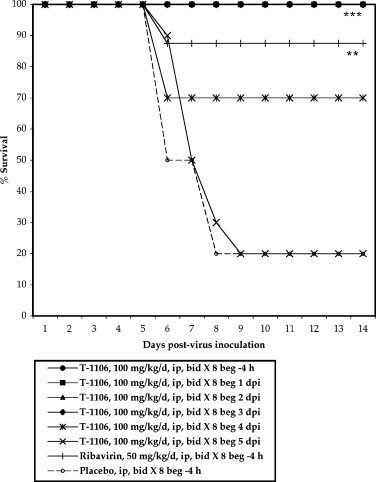
Survival of YFV-infected hamsters treated i.p. with 100 mg/kg/day of T-1106 starting at various times after virus challenge. beg, beginning of treatment. ***P < 0.001, **P < 0.01 compared to placebo-treated animals (Julander et al., 2007).
On the other hand, a dose of 400 mg/kg/day of T-705 was required to achieve results similar to those obtained after treatment with T-1106 (Table 5 ) (Julander et al., 2009). Protection from mortality was also observed after hamsters were treated with 200 mg/kg/day. There are insufficient data to clarify the reason why the in vivo antiviral effect of T-1106 did not correspond to its in vitro activity and why there were such differences in antiviral activities between T-1106 and T-705. A possible explanation is that the conversion rate of T-1106 to an active form in the hamster liver was higher than in cultured cells (Furuta et al., 2004). In any case, it is expected that T-1106 will be a useful drug for YF in humans.
Table 5.
Effect of treatment with T-705 after virus exposure in hamsters challenged with YFV.
| Treatment | Time of start of therapy (h) | Survivors/total | MDD ± S.D. | Serum ALT ± S.D. |
|---|---|---|---|---|
| Saline | −4 | 3/10 | 7.4 ± 1.8 | 188 ± 94 |
| T-705 | −4 | 10/10*** | >21.0 | 75 ± 23** |
| +24 | 10/10*** | >21.0 | 80 ± 32** | |
| +48 | 10/10*** | >21.0 | 84 ± 47** | |
| +72 | 8/10* | 7.0 ± 1.4 | 139 ± 71 | |
| +96 | 6/10 | 6.5 ± 0.6 | 115 ± 82* | |
| +120 | 5/10 | 6.8 ± 0.8 | 157 ± 74 | |
| Ribavirin | −4 | 10/10*** | >21.0 | 110 ± 61* |
T-705 was orally administered twice daily for 8 days at a dose of 400 mg/kg/day and ribavilrin was administered at a dose of 50 mg/kg/day. MDD: mean day of death. S.D.: standard deviation. ALT: serum alanine aminotransferase, in international units per liter.
P < 0.05.
P < 0.01
P < 0.001 as compared with saline-treated controls (Julander et al., 2009).
5. West Nile virus
Like YFV, dengue virus, and Japanese encephalitis virus, WNV is a mosquito-borne member of the Flaviviridae family. WNV was first isolated in 1937 from a patient in the West Nile district of Uganda. Since then, the virus has been isolated in various regions of the world and has spread to North America (Hayes, 2001). Because it has been shown that such a virus could spread to the whole world and an effective vaccine has not been developed, countermeasures are required.
5.1. Antiviral activity of T-705 against WNV
The in vitro antiviral activity of T-705 was evaluated in Vero cells against the New York 1999 isolate of WNV, in which its EC50 value was 318 μM (Morrey et al., 2008). For in vivo efficacy studies, C57BL/6 mice and golden Syrian hamsters were used (Morrey et al., 2008). Mice and hamsters were infected subcutaneously with the WNV New York 1999 strain, and T-705 was administered orally twice daily beginning 4 h after infection for 14 days. Orally administered T-705 at 200 mg/kg/day protected mice against WNV-induced mortality, and reduced viral RNA levels in brains and spleens without reduction of body weight. Treatment could be delayed out to 2 days after challenge and still achieve efficacy in mice (Fig. 5 A). The minimal effective oral dose administered twice a day beginning from 1 day after viral challenge was between 20 and 65 mg/kg/day in mice. T-705 at 200 mg/kg/day under the same dose regimen also significantly improved the survival in hamsters. Notably, immunohistochemically detected WNV envelope protein was not observed in the brains of T-705 treated hamsters, although it was seen in the placebo-treated animals (Fig. 5B). Further investigation of T-705 should be considered for the treatment of WNV, because it is one of only a few compounds known to reduce WNV-induced mortality in rodents.
Fig. 5.
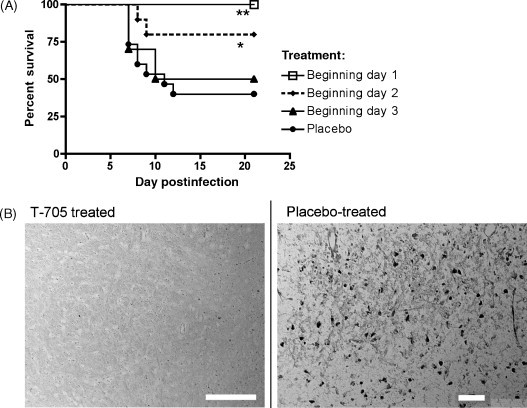
(A) Effect of twice-daily oral treatment with 200 mg/kg of T-705 beginning on day 1, 2 or 3 post-infection on the survival of WNV-infected C57Bl/6 mice. *P ≤ 0.05, **P ≤ 0.01 compared to placebo controls. (B) Immunohistochemical detection of WNV envelope glycoprotein on day 7 of infection in the brains of Syrian golden hamsters treated twice daily with 200 mg/kg of T-705 or with a placebo beginning on the day of infection. Scale bar: 50 μm (Morrey et al., 2008).
6. Foot-and-mouth disease virus
Foot-and-mouth disease (FMD) is a highly contagious and economically devastating disease of cloven-hoofed animals, including swine and bovines. The causative agent FMDV is an aphthovirus of the Picornaviridae family. It causes a rapidly spreading, acute infection characterized by fever, lameness, and vesicular lesions on the feet, tongue, snout, and teats (Grubman and Baxt, 2004). In regions in which FMD is enzootic, disease control is achieved by the two policies of “test and slaughter” and/or “vaccination.” When FMD outbreaks occur, countries use either or both approaches, depending on the epidemiological situation. Recent outbreaks occurred in 1997, in Taiwan (Dunn and Donaldson, 1997) and in 2001, in the UK (Gibbens et al., 2001), causing serious economic damage.
The rate of FMD transmission depends on several factors, among which are the infected species and breed, the virulence of the FMDV strain, the route of infection, the environment and differences in the immune status of the individual animal, and the quality of the FMD vaccines. It generally takes about 7 days for vaccination to elicit protective antibodies (Doel, 1996). To compensate for this disadvantage of FMD vaccines, new control methods that exhibit prompt effectiveness must be developed. Therefore, an antiviral agent can be one of several powerful tools to reduce the expansion of FMD outbreaks, thus providing additional means of controlling the spread of the disease.
6.1. Antiviral activity of T-1105 against FMDV
In vitro anti-FMDV activities of T-705, T-1106 and T-1105 were assessed by a plaque reduction assay with the O/JPN/2000 strain. T-1105 showed the most potent antiviral activity with an EC50 value of 12 μM. T-1105 was administered by feed to four pigs at a dose of 200 mg/kg twice daily for 6 days beginning 1 h prior to FMDV inoculation (Sakamoto et al., 2006). The animals in the control group showed typical signs of FMD, such as fever, vesicles on their feet and lameness, but no clinical signs of FMD were observed in T-1105-treated pigs throughout the experiment. Viremia was effectively reduced to below the levels of detection and the increase of anti-FMD antibody titers was suppressed after T-1105 treatment, suggesting that the virus replication was effectively inhibited by this compound in the infected pigs.
It should also be noted that the virus was not detected in nasal swabs from T-1105-treated animals. The inhibition of virus secretion is a very important factor in the treatment of pigs infected with FMDV, given that pigs are known to be important amplifying hosts and excrete approximately 1000 times more virus than other hosts such as cattle. Since this compound can be administered through food, large numbers of animals can be treated through relatively little effort. Thus, T-1105 could be a powerful tool to control FMD in pigs.
7. Other viruses
Alphaviruses, such as TC-83 Venezuelan equine encephalitis virus (VEEV) and Western equine encephalitis virus (WEEV), are also inhibited in cell culture by T-705, with SIs comparable to those seen against flaviviruses. The effect in vivo, however, is less than that seen with the flaviviruses. Treatment with a dose of 400 mg/kg/day resulted in a modest but significant improvement in survival in a mouse model of WEEV (Julander, 2009). T-1106 showed no activity against either VEEV or WEEV.
Other RNA viruses, including poliovirus, rhinovirus, and respiratory syncytial virus (RSV), have also been shown to be susceptible to T-705, with EC50s of 4.8 (31 μM), 23 (150 μM) and 41 (260 μM) μg/mL, respectively (Furuta et al., 2002). However, with EC50 values >100 μg/mL (640 μM), T-705 showed no effect on the plaque formation of herpes simplex virus type 1 (HSV-1), human cytomegalovirus (HCMV), and adenovirus. On the other hand, ribavirin has been shown to inhibit the growth of these DNA viruses (Allen et al., 1978, Markland et al., 2000).
In addition to activity seen against YFV, T-1106 is also active against bovine viral diarrhea virus (BVDV), another flavivirus, as well as hepatitis C virus (HCV) (Furuta et al., 2004). T-1106 reduced BVDV replication in MDBK cells with an EC90 value of 38 μM by the yield reduction assay. Similar activity was observed in the inhibition of BVDV in cell culture with T-1105, which had an EC90 of 34 μM, while T-705 showed less activity with an EC90 of 330 μM. In addition, the phosphorylation of T-1106 was observed in several mammalian cells and in the livers of animals treated with this compound. T-1106 triphosphate was also found to inhibit the viral RNA polymerase of HCV in an enzyme assay (Furuta et al., 2004). Further investigation of therapeutic efficacy of T-1106 against HCV is warranted in an appropriate animal model.
8. Discussion
In our studies, pyrazinecarboxamide derivatives including T-705 have been found to possess favorable antiviral activity against RNA viruses and to show a therapeutic effect in a number of animal models. Regarding the mechanism of action, the experimental findings suggest that T-705 is converted to the ribofuranosyltriphosphate derivative by host cellular enzymes, and the metabolite inhibits the viral RNA-dependent RNA polymerase. It is noteworthy that T-705 inhibits viral replication without influencing synthesis of cellular DNA and RNA (Furuta et al., 2005). The high error rate of RNA polymerase is considered to be one of the strategies executed by RNA viruses to escape the host immune response, and false recognition of nucleotides by the viral polymerase might occur more frequently, as compared to the host RNA polymerase (Day et al., 2005, Leyssen et al., 2006).
Although there is considerable evidence supporting the inhibition of the influenza polymerase by T-705, it is not fully understood how the active form inhibits RNA-dependent RNA polymerase. For example, the following questions remain to be clarified. Does T-705 suppress enzyme function by binding to enzyme molecules? Does it incorporate into nascent viral RNA during the replication process? Does it suppress the translation of viral proteins? Is it a viral mutagen like ribavirin? In addition, we tried to obtain a T-705-resistant strain through serial passage of influenza A/PR/8/34 (H1N1), but resistant viruses could not be isolated after 30 passages (unpublished data). Although the inability to develop drug resistance is clearly advantageous, it makes analyzing the mechanism of action at the molecular level difficult.
On the other hand, we observed that the antiviral activities of pyrazinecarboxamide derivatives varied largely depending on the kind of host cells used. For example, the antiviral activity of T-705 against influenza A/WSN/33 was higher than that of T-1106 in MDCK cells, but their antiviral potencies were reversed in MDBK cells (unpublished data). Since it is presumed that these compounds are converted to their active ribofuranosyltriphosphate forms by cellular enzymes, antiviral activity would be greatly affected by the uptake of the parent compounds into host cells and/or the conversion rates to active forms. These are important points to consider when we estimate the therapeutic efficacy of these compounds in various viral diseases. Variation in the distribution and conversion of compounds in targeted organs and cells could be the cause of differences in their antiviral spectrum.
T-705 is now in clinical phase I/II trials for influenza in the USA and Japan, and non-clinical and clinical data on toxicology and pharmacokinetics are accumulating. For example, non-clinical pharmacokinetic studies revealed that the plasma levels of T-705 after oral administration rose in a dose-dependent manner with high linearity, and the oral bioavailability in mice was almost 100%, as compared to intravenous administration. In addition, no serious adverse events have been reported in the clinical studies, suggesting that T-705 is well tolerated in humans. These data will help us to consider the possibility of T-705 as a novel antiviral drug against various RNA viral infections. The first indication for T-705 is influenza, but in our development plan hereafter it is necessary also to consider other diseases caused by medically important viruses such as Lassa, Junin, WNV and YFV for T-705 and T-1106. Regarding the development of T-1105, to elucidate its effectiveness as a powerful tool to control FMD in pigs, more large-scale experiments are expected using an infection model of FMDV. In conclusion, the pyrazinecarboxamide derivatives appear to be promising tools to satisfy unmet needs for treating highly pathogenic RNA viral infections.
Acknowledgements
We thank Drs. Heather Greenstone, Chris Tseng and Catherine Laughlin for their extensive advice. We also acknowledge Dr. Keiichi Tanaka and Ms. Kyo Kozaki for their beneficial discussion and assistance. This work was supported in part by contract grants NO1-AI-15435 and NO1-AI-30048 from the Virology Branch, NIAID, NIH.
References
- Abdel-Ghafar A.N., Chotpitayasunondh T., Gao Z., Hayden F.G., Nguyen D.H., de Jong M.D., Naghdaliyev A., Peiris J.S., Shindo N., Soeroso S., Uyeki T.M. Update on avian influenza A (H5N1) virus infection in humans. N. Engl. J. Med. 2008;358:261–273. doi: 10.1056/NEJMra0707279. [DOI] [PubMed] [Google Scholar]
- Allen L.B., Boswell K.H., Khwaja T.A., Meyer R.B., Jr., Sidwell R.W., Witkowski J.T., Christensen L.F., Robins R.K. Synthesis and antiviral activity of some phosphates of the broad-spectrum antiviral nucleoside, 1-beta-d-ribofuranosyl-1,2,4-triazole-3-carboxamide (ribavirin) J. Med. Chem. 1978;21:742–746. doi: 10.1021/jm00206a005. [DOI] [PubMed] [Google Scholar]
- Borio L., Inglesby T., Peters C.J., Schmaljohn A.L., Hughes J.M., Jahrling P.B., Ksiazek T., Johnson K.M., Meyerhoff A., O’Toole T., Ascher M.S., Bartlett J., Breman J.G., Eitzen E.M., Jr., Hamburg M., Hauer J., Henderson D.A., Johnson R.T., Kwik G., Layton M., Lillibridge S., Nabel G.J., Osterholm M.T., Perl T.M., Russell P., Tonat K. Hemorrhagic fever viruses as biological weapons: medical and public health management. JAMA. 2002;287:2391–2405. doi: 10.1001/jama.287.18.2391. [DOI] [PubMed] [Google Scholar]
- Bray M. Highly pathogenic RNA viral infections: challenges for antiviral research. Antiviral Res. 2008;78:1–8. doi: 10.1016/j.antiviral.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Day C.W., Smee D.F., Julander J.G., Yamshchikov V.F., Sidwell R.W., Morrey J.D. Error-prone replication of West Nile virus caused by ribavirin. Antiviral Res. 2005;67:38–45. doi: 10.1016/j.antiviral.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Doel T.R. Natural and vaccine-induced immunity to foot-and-mouth disease: the prospects for improved vaccine. O. I. E. Bull. 1996;15:883–911. doi: 10.20506/rst.15.3.955. [DOI] [PubMed] [Google Scholar]
- Dunn C.S., Donaldson A.I. Natural adoption to pigs of a Taiwanese isolate of foot-and-mouth disease virus. Vet. Rec. 1997;141:174–175. doi: 10.1136/vr.141.7.174. [DOI] [PubMed] [Google Scholar]
- Enria D.A., Maiztegui J.I. Antiviral treatment of Argentine hemorrhagic fever. Antiviral Res. 1994;23:23–31. doi: 10.1016/0166-3542(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Ergonul O. Treatment of Crimean–Congo hemorrhagic fever. Antiviral Res. 2008;78:125–131. doi: 10.1016/j.antiviral.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Ergonul O., Celikbas A., Dokuzoguz B., Eren S., Baykam N., Esener H. Characteristics of patients with Crimean–Congo hemorrhagic fever in a recent outbreak in Turkey and impact of oral ribavirin therapy. Clin. Infect. Dis. 2004;39:284–287. doi: 10.1086/422000. [DOI] [PubMed] [Google Scholar]
- Furuta Y., Takahashi K., Fukuda Y., Kuno M., Kamiyama T., Kozaki K., Nomura N., Egawa H., Minami S., Watanabe Y., Narita H., Shiraki K. In vitro and in vivo activities of anti-influenza virus compound T-705. Antimicrob. Agents Chemother. 2002;46:977–981. doi: 10.1128/AAC.46.4.977-981.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y., Takahashi K., Maekawa M., Maegawa H., Egawa H., Terashima N. Abstr. 44th Intersci. Conf. Antimicrob. Agents Chemother. 2004. T-1106, a novel pyrazine nucleoside, hepatitis C virus polymerase inhibitor; pp. 199–200. (Abstr. F-487) [Google Scholar]
- Furuta Y., Takahashi K., Kuno-Maekawa M., Sangawa H., Uehara S., Kozaki K., Nomura N., Egawa H., Shiraki K. Mechanism of action of T-705 against influenza virus. Antimicrob. Agents Chemother. 2005;49:981–986. doi: 10.1128/AAC.49.3.981-986.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbens J.C., Sharpe C.E., Wilesmith J.W., Mansley L.M., Michalopoulou E., Ryan J.B., Hudson M. Descriptive epidemiology of the 2001 foot-and-mouth disease epidemic in Great Britain: the first five months. Vet. Rec. 2001;149:729–743. [PubMed] [Google Scholar]
- Glezen W.P. Serious morbidity and mortality associated with influenza epidemics. Epidemiol. Rev. 1982;4:25–44. doi: 10.1093/oxfordjournals.epirev.a036250. [DOI] [PubMed] [Google Scholar]
- Gowen B.B., Holbrook M.R. Animal models of highly pathogenic RNA viral infections: hemorrhagic fever viruses. Antiviral Res. 2008;78:79–90. doi: 10.1016/j.antiviral.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Gowen B.B., Smee D.F., Wong M.H., Hall J.O., Jung K.H., Bailey K.W., Stevens J.R., Furuta Y., Morrey J.D. Treatment of late stage disease in a model of arenaviral hemorrhagic fever: T-705 efficacy and reduced toxicity suggests an alternative to ribavirin. PLoS ONE. 2008;3:e3725. doi: 10.1371/journal.pone.0003725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowen B.B., Smee D.F., Wong M.H., Judge J.W., Jung K.H., Bailey K.W., Pace A.M., Rosenberg B., Sidwell R.W. Recombinant Eimeria protozoan protein elicits resistance to acute phlebovirus infection in mice but not hamsters. Antimicrob. Agents Chemother. 2006;50:2023–2029. doi: 10.1128/AAC.01473-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowen B.B., Wong M.H., Jung K.H., Sanders A.B., Mendenhall M., Bailey K.W., Furuta Y., Sidwell R.W. In vitro and in vivo activities of T-705 against Arenavirus and Bunyavirus infections. Antimicrob. Agents Chemother. 2007;51:3168–3176. doi: 10.1128/AAC.00356-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubman M.J., Baxt B. Foot-and-mouth disease. Clin. Microbiol. Rev. 2004;17:465–493. doi: 10.1128/CMR.17.2.465-493.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes C.G. West Nile virus: Uganda, 1937 to New York City, 1999. Ann. N. Y. Acad. Sci. 2001;951:25–37. doi: 10.1111/j.1749-6632.2001.tb02682.x. [DOI] [PubMed] [Google Scholar]
- Heeney J.L. Zoonotic viral diseases and the frontier of early diagnosis, control and prevention. J. Intern. Med. 2006;260:399–408. doi: 10.1111/j.1365-2796.2006.01711.x. [DOI] [PubMed] [Google Scholar]
- Julander J.G., Furuta Y., Shafer K., Sidwell R.W. Activity of T-1106 in a hamster model of yellow fever virus infection. Antimicrob Agents Chemother. 2007;51:1962–1966. doi: 10.1128/AAC.01494-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julander J.G., Shafer K., Smee D.F., Morrey J.D., Furuta Y. Activity of T-705 in a hamster model of yellow fever virus infection in comparison with a chemically related compound T-1106. Antimicrob. Agents Chemother. 2009;53:202–209. doi: 10.1128/AAC.01074-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye D., Pringle C.R. Avian influenza viruses and their implication for human health. Clin. Infect. Dis. 2005;40:108–112. doi: 10.1086/427236. [DOI] [PubMed] [Google Scholar]
- Kilgore P.E., Ksiazek T.G., Rollin P.E., Mills J.N., Villagra M.R., Montenegro M.J., Costales M.A., Paredes L.C., Peters C.J. Treatment of Bolivian hemorrhagic fever with intravenous ribavirin. Clin. Infect. Dis. 1997;24:718–722. doi: 10.1093/clind/24.4.718. [DOI] [PubMed] [Google Scholar]
- Leyssen P., De Clercq E., Neyts J. The anti-yellow fever virus activity of ribavirin is independent of error-prone replication. Mol. Pharmacol. 2006;69:1461–1467. doi: 10.1124/mol.105.020057. [DOI] [PubMed] [Google Scholar]
- Mardani M., Jahromi M.K., Naieni K.H., Zeinali M. The efficacy of oral ribavirin in the treatment of Crimean-Congo hemorrhagic fever in Iran. Clin. Infect. Dis. 2003;36:1613–1618. doi: 10.1086/375058. [DOI] [PubMed] [Google Scholar]
- Markland W., McQuaid T.J., Jain J., Kwong A.D. Broad-spectrum antiviral activity of the IMP dehydrogenase inhibitor VX-497: a comparison with ribavirin and demonstration of antiviral additivity with alpha interferon. Antimicrob. Agents Chemother. 2000;44:859–866. doi: 10.1128/aac.44.4.859-866.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick J.B., King I.J., Webb P.A., Scribner C.L., Craven R.B., Johnson K.M., Elliott L.H., Belmont-Williams R. Lassa fever. Effective therapy with ribavirin. N. Engl. J. Med. 1986;314:20–26. doi: 10.1056/NEJM198601023140104. [DOI] [PubMed] [Google Scholar]
- Monath T.P. Treatment of yellow fever. Antiviral Res. 2008;78:116–124. doi: 10.1016/j.antiviral.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Morrey J.D., Taro B.S., Siddharthan V., Wang H., Smee D.F., Christensen A.J., Furuta Y. Efficacy of orally administered T-705 pyrazine analog on lethal West Nile virus infection in rodents. Antiviral Res. 2008;80:377–379. doi: 10.1016/j.antiviral.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin S.A. Vaccines: past, present and future. Nat. Med. 2005;11:S5–S11. doi: 10.1038/nm1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K., Ohashi S., Yamazoe R., Takahashi K., Furuta Y. The inhibition of FMD virus excretion from the infected pigs by an antiviral agent, T-1105. FAO Report of the Research Group of the Standing Technical Committee of European Commission for the control of Foot-and-Mouth Disease, Paphos, Cyprus. FAO Appendix. 2006;64:418–424. [Google Scholar]
- Sidwell R.W., Barnard D.L., Day C.W., Smee D.F., Bailey K.W., Wong M.H., Morrey J.D., Furuta Y. Efficacy of orally administered T-705 on lethal avian influenza A (H5N1) virus infections in mice. Antimicrob. Agents Chemother. 2007;51:845–851. doi: 10.1128/AAC.01051-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Furuta Y., Fukuda Y., Kuno M., Kamiyama T., Kozaki K., Nomura N., Egawa H., Minami S., Shiraki K. In vitro and in vivo activities of T-705 and oseltamivir against influenza virus. Antivir. Chem. Chemother. 2003;14:235–241. doi: 10.1177/095632020301400502. [DOI] [PubMed] [Google Scholar]
- Tapper M.L. Emerging viral diseases and infectious disease risks. Haemophilia. 2006;12(Suppl. 1):3–7. doi: 10.1111/j.1365-2516.2006.01194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomori O. Yellow fever: the recurring plague. Crit. Rev. Clin. Lab. Sci. 2004;41:391–427. doi: 10.1080/10408360490497474. [DOI] [PubMed] [Google Scholar]
- Webby R.J., Webster R.G. Are we ready for pandemic influenza? Science. 2003;302:1519–1522. doi: 10.1126/science.1090350. [DOI] [PubMed] [Google Scholar]
- WHO . 2008. Epidemic and Pandemic Alert and Response (EPR)http://www.who.int/csr/disease/avian influenza/country/en/ [Google Scholar]


