Abstract
Ethnopharmacological relevance
The genus Psoralea (Fabaceae) harbours 105 accepted species that are extensively used by local peoples and medicinal practitioners of China, India, and other countries for treatment of tooth decay, psoriasis, leucoderma, leprosy, kidney problems, tuberculosis, indigestion, constipation and impotence. Presently, pharmacological research reports are available on only few species namely Bituminaria bituminosa (Syn: P. bituminosa), P. canescens, P. corylifolia, P. esculenta, P. plicata and P. glandulosa which are valued for their chemical constituents and traditional uses.
Aim of the review
This review article provides explicit information on traditional uses, phytochemistry, and pharmacological activities of selected Psoralea species. The possible trends and perspectives for future research on these plants are also discussed.
Materials and methods
An extensive and systematic review of the extant literature was carried out, and the data under various sections were identified using a computerized bibliographic search via the PubMed, Web of Science and Google Scholar, CAB Abstracts, MEDLINE, EMBASE, INMEDPLAN, NATTS as well as several websites.
Key findings
A total of 291 bioactive compounds from 06 species of genus Psoralea have been isolated and characterized. However, P. bituminosa alone possess nearly 150 compounds. These bioactive compounds belong to different chemical classes, including flavonoids, coumarins, furanocoumarins, chalcones, quinines, terpenoids and some others due to which these species exhibit significant anti-oxidant, anti-bacterial, anti-fungal, anti-viral, anti-helmintic, anti-diabetic, diuretic, hepatoprotective, anti-cancer and anti-tumor activities. P. corylifolia L. (Babchi), a Chinese traditional medicinal plant has been used in traditional medicine for many decades for its healing properties against numerous skin diseases such as leprosy, psoriasis and leucoderma.
Conclusions
The in vitro studies and in vivo models have provided a simple bio-scientific justification for various ethnopharmacological uses of Psoralea species. From the toxicological perspective, the root, leaf, and seed extracts and their preparations have been proven to be safe when consumed in the recommended doses. But, meticulous studies on the pharmaceutical standardization, mode of action of the active constituents, and sustainable conservation of Psoralea species are needed, to meet the growing demands of the pharmaceutical industries, and to fully exploit their preventive and therapeutic potentials.
Abbreviations: ABA, Abscisic acid; BAP, 6-benzyl aminopurine; BVN, Bavistin; CH, Casein hydrolysate; 2, 4-D, 2, 4-dichlorophenoxyacetic acid; DPPH, 2,2-Diphenyl-1-picrylhydrazyl; FRAP, Ferric reducing antioxidant power; HPLC, High performance liquid chromatography; HPTLC, High performance thin layer chromatography; IAA, Indole 3-acetic acid; IBA, Indole 3-butyric acid; 2ip, N6-(2-isopentenyl) adenine; KIN, Kinetin; MS, Murashige and Skoog; NAA, α-naphthalene acetic acid; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; NO, Nitric oxide; PG, Phloroglucinol; ROS, Reactive oxygen species; PGR, Plant growth regulator; TDZ, Thidiazuron; TLC, Thin-layer chromatography; TRAP, Total reactive anti-oxidant properties; TNF-alpha, Tumor necrosis factor alpha; TRAIL, Tumor necrosis factor-related apoptosis-inducing ligand
Keywords: Psoralea corylifolia, Anti-leprosy, Anti-vitiligo, Psoralen, Isopsoralen
1. Introduction
The medicinal herbs have been a major source of biodynamic compounds of therapeutic value in Ayurveda, Unani, Homeopathy, Traditional Chinese medicine (TCM) and other traditional system of medicines. Several dreadful diseases including cancer, AIDS, kidney damage, cardiovascular diseases and many more, can be cured by the use of medicinal herbs (Ceylan-Isik et al., 2008, Duke, 2009, Kolasani et al., 2011, Turchi et al., 2009). Owing to the global trend towards improved ‘quality of life’, the demands for herbal medicines have tremendously grown. Therefore, due to overexploitation, a large number of medicinal plant species are under the threat of extinction (Sidhu, 2010).
There are several plant genera which are reputedly known for their contribution to traditional as well as modern medicine(s). The genus Psoralea of the legume family (Fabaceae) is one among them, which was first established by Linnaeus in 1742. Till 1753, only one species, Psoralea americana (native to America), was recognized and described by Linnaeus. According to The Plant List (2013), Psoralea is a cosmopolitan genus encompassing 105 accepted species, with 23 species reported in Australia and 14 species in the Northern Territory. Two widespread species occur in the Dominican Republic. The name ‘Psoralea’ is derived from the Greek term ‘Psoraleos’, which means “affected with itch or with leprosy” (Chopra et al., 2013a, Chopra et al., 2013b).
This genus is a source of several bioactive compounds which belong to the chemical class of flavonoids, coumarins, furanocoumarins, chalcones, terpenoids, and meroterpenes. Some of the medicinally important compounds obtained from Psoralea species are ‘psoralen’, ‘isopsoralen’ (angelicin), ‘bakuchiol’, ‘corylifol’, ‘psoralidin’, ‘bavachinin’, ‘corylifolinin’, ‘caryophyllene’, ‘β-farnesene’, ‘α-pinene’, ‘camphene’ and ‘germacrene D’ (Bertoli et al., 2004, Li et al., 2016, Tava et al., 2007). Although, some species are poisonous, the starchy roots of P. hypogaea Torr. & A. Gray, P. esculenta Pursh and P. macrostachya DC. are edible. At present, there are pharmacological research reports available on only few species namely P. bituminosa (accepted name: Bituminaria bituminosa), P. canescens Michx. [synonym: Pediomelum canescens (Michx.) Rydb.], P. corylifolia L. [accepted name: Cullen corylifolium (L.) Medik.], P. esculenta Pursh, P. plicata Delile [accepted name: Cullen plicatum (Delile) C.H.Stirt.] and P. glandulosa L. P. corylifolia is one among these medicinal herbs which is commonly known as ‘babachi’, ‘bakuchi’, ‘bavachi’, ‘Indian bread root’, ‘fountain-bush’ or ‘scurf pea’ (Alam et al., 2017, Shilandra et al., 2010, Zhang et al., 2016). It is a highly valued, endangered medicinal herb that is grown in tropical and subtropical areas of the world (China, Japan, Burma and India) due to its folkloric medicinal properties (Sehrawat et al., 2013). The dried ripe fruit of P. corylifolia is commonly known as ‘BuguZhi’ or ‘Poguzhi’ in traditional Chinese medicine (TCM) (Oudhiya, 2001).
The pharmacological activities of Psoralea species have been scientifically proven and well-documented (Alam et al., 2017, Li et al., 2016, Zhang et al., 2016). Psoralen and isopsoralen compounds obtained from Psoralea species are also under trials against syndromes like AIDS (Anis et al., 2005; Bhattacharjee, 1998; Duke, 2009). In a nut-shell, Psoralea species have served humanity as valuable medicinal herbs and are an unmatched remedy for treating leprosy, leucoderma, psoriasis and several other diseases (Ali et al., 2008, Khushboo et al., 2010, Newton et al., 2002, Wong et al., 2013, Zhang et al., 2016). But, it is unfortunate that several Psoralea species are vulnerable to extinction due to climate change, overexploitation and interspecific competition. Therefore, meticulous studies on the pharmacological-potential, pharmaceutical-standardization, mode of action of the active constituents, and sustainable conservation of several Psoralea species, are required (Li et al., 2016). In the past few decades, some reports have been published regarding its traditional uses but, a compendious review of its traditional and modern uses, phytochemistry, pharmacology and conservation has not been published so far. This review aims to bridge the gap and provides explicit information on the aforementioned parameters by studying six highly exploited species of this genus (Bituminaria bituminosa (L.) C.H.Stirt, Psoralea canescens Michx., Cullen corylifolium (L.) Medik., Psoralea esculenta Pursh, Cullen plicatum (Delile) C.H.Stirt., Psoralea glandulosa L.) which are important from ethnobotanical, chemical and pharmacological point of view. This shall create awareness among the environmentalists, botanists and pharmacists, for sustainable utilization and conservation of Psoralea species.
2. Methodology
An extensive and systematic review of the extant literature was carried out, and the data under various sections on distribution, traditional and modern uses, phytochemistry and biological activities of Psolaea species were identified using various databases including PubMed, Web of Science and Google Scholar, CAB Abstracts, MEDLINE, EMBASE, INMEDPLAN, NATTS, as well as websites such as www.jstor.org, www.sciencedirect.com, www.eflora.org and www.pfaf.org. The names of the species mentioned in this review have been validated taxonomically using the medicinal plants repositories available at http://mpns.kew.org/mpns-portal/ and www.theplantlist.org/. The chemical structures of the bioactive compounds were searched in ChemSpider and PubChem databases and redrawn using the ChemDraw® software (version 8.0). The literature reviewed consists of several abstracts, full-text articles, books, Ph.D. theses and blogs. The most relevant literature contained in 396 references was selected for further examination and inclusion in this review.
3. Distribution
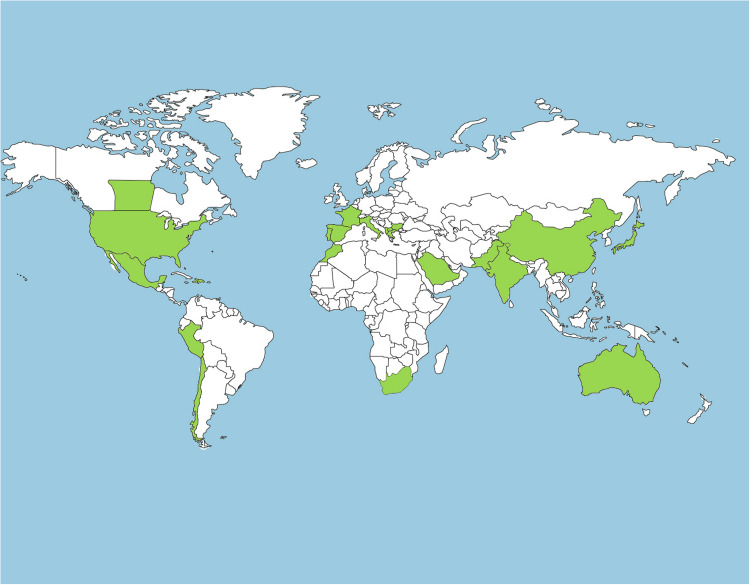
As already mentioned, 105 species have been reported so far in genus Psoralea (Fabaceae) (Supplemental information 1, Table S1). Bituminaria bituminosa (L.) C.H.Stirt. is widely distributed in Africa; Psoralea canescens Michx. [synonym: Pediomelum canescens (Michx.) Rydb.] is native of Florida and eastern parts of South America; Psoralea esculenta Pursh is found in United States; Psoralea plicata Delile is native of Pakistan and Arabian countries; Psoralea pinnata L. is a native of South Africa; Psoralea argophylla Pursh is native of the central United States, as well as the three Canadian prairie provinces, Alberta, Saskatchewan, and Manitoba, and Psoralea arborea Sims is native of South Africa. Psoralea corylifolia L., though native of Asia, is cultivated in other continents as well (Australia, North America, and Africa). It is extensively grown in the plains, in tropical and sub-tropical areas of the world (Krishnamurthi et al., 1969). In Asia, its distribution is vast ranging from China, Japan Pakistan and India ( Fig. 1). In India, it is found in Uttar Pradesh, Rajasthan, and eastern parts of Punjab. It is also reported in the Himalayan regions (up to 1000 m above sea level), Dehra Dun, Bundelkhand, West Bengal, Mumbai, Bihar, Deccan, and Karnataka. In Pakistan, it is well distributed along Baluchistan coast and Peshawar area (Saif et al., 2007, Sharma et al., 2001).
Fig. 1.
World map showing the distribution of Psoralea species (green).
4. Traditional and modern uses
Psoralea species have been used in folklore and indigenous system of medicine for a long time. Several Psoralea products are successfully commercialized and available in the markets (Supplemental information 2, Table S2). The roots of P. argophylla Pursh (Silver leaf scurf pea, Silver leaf Indian breadroot) are eaten raw or cooked (Tanaka, 1976, Yanovsky, 1936). The dried roots may be ground into a powder and used an ingredient of soups and bread (Yanovsky, 1936). A tea prepared from the leaf and stem powder possess anti-pyretic properties (Weiner, 1990). A decoction of the plant is used as a wash for wounds (Moerman, 1998). The root extract is used as a remedy for chronic constipation (Moerman, 1998). Bituminaria bituminosa (L.) C.H.Stirt. (Arabian pea or pitch trefoil) is used as a forage crop. Psoralea canescens Michx. (Buckroot) roots are eaten raw or cooked (Hedrick, 1972, Tanaka, 1976, Yanovsky, 1936). The powdered roots are used in preparation of soups or breads (Yanovsky, 1936). As the plant has analgesic properties, a poultice prepared from roots is applied on painful areas of the body (Moerman, 1998). An infusion of the roots and its steam is used for the treatment of cold, cough, headache and sore throat (Moerman, 1998). Psoralea castorea S. Watson (Beaver Indian breadroot) roots are also eaten raw or cooked and the root-powder can be used in soups or breads (Hedrick, 1972, Tanaka, 1976, Uphof, 1968, Yanovsky, 1936). Psoralea corylifolia L. (Bu Gu Zhi) is revered in Chinese traditional medicine as a tonic to improve general vitality. The name ‘Buguzhi’ (Fructus psoraleae) actually comprises of three Chinese words: ‘Bu’ means ‘to invigorate’; ‘Gu’ means ‘bone’ and the third word ‘Zhi’ means ‘fat’. The Chinese name of the herb suggests the function of the herb to provide fat for the invigorating bones. One of the most important features of P. corylifolia is that each and every part of the plant is beneficial which includes roots, stems, leaves, seeds and even blooms (Hodges, 2015). Since P. corylifolia is a leprosy destroyer it is revered to as “Kushtanashini” in Sanskrit. Moreove, it is an ancient remedy for leucoderma among the traditional system of medicines in India and China and also among the people in the West (Chopra and Chopra, 1958). In Unani system, the plant has been effective against fever, skin diseases and internal ulcers (Chopra and Chatterjee, 1927). It is also found to be an effective anti-helmintic and sedative (Nadkarni, 1976). For treating leprosy and leucoderma the leaves are consumed as powder and also apllied on skin in the form of paste (Anon, 1998; Nadkarni, 1976; Panda, 2000). However, some precaution should be taken when applying the herbal-paste externally, since it can cause a skin-allergic reaction when exposed to sunlight (Anderson and Voorhees, 1980). The leaves are also used to treat dermatitis, inflammation, mucomembranous disorders, oedematous conditions of the skin and to alleviate diarrhea (Rajpal, 2005, Sharma et al., 2001, Krishnamurthi et al., 1969). The plant possesses blood purifying properties and therefore used to treat boils, itching eruptions or red papules, ringworm-infection, extensive eczema, rough and discolored dermatosis with fissures, and scabies (Khare, 2004). The essential oil from plant is reported to have a strong effect on Streptococcal infection of the skin (Rajpal, 2005). Moreover, it is known to improve the color of skin, hair and nails.
Seeds are sweet, bitter, acrid, and astringent. The seeds are anti-pyretic and also possess alexiteric properties and therefore are given in scorpion-sting or snake bite and in bilious disorders (Agharkar, 1991, Kapoor and Boca, 2001, Nadkarni, 1976, Panda, 2000). Both the seeds and fruits contain psoralen (furocoumarin), known to regulate pigmentation (Rashid and Agarwala, 1965, Sebastian, 2006). P. corylifolia seed extracts have been reported to possess anti-hyperglycemic, anti-depressant, anti-tumor, anti-bacterial and anti-oxidant property (Steven and Russell, 2007, Wang et al., 1990). Seed extract and powder are beneficial as anti-helmintic, laxative, diuretic, and for healing wounds (Rajpal, 2005). They are used as stomachic, diaphoretic and aphrodisiac (Sharma et al., 2001). The major components psoralen (92) and isopsoralen (2) are known to possess anti-bacterial, anti-viral and anti-tumor properties (Liu et al., 2004). Therefore, the seeds are used for curing various disorders such as cough, asthma, nephritis, alopecia areata, menstruation, uterine disorders and haemorrhages (Qiao et al., 2007, Ruan et al., 2007). The crude extracts of seeds are used in the treatment of febrile diseases, impotence, spermatorrhea, premature ejaculation, lower back pains, incontinence, enuresis, pollakiuria, and cold symptoms in the waist and knees (Chopra et al., 1956, Lin et al., 2007, Zhao et al., 2005a, Zhao et al., 2005b). It also possesses coronary vasodilatory activity (Ruan et al., 2007). The seeds act as deobstruent and heal ulcers, heart troubles, cure blood disorders and elephantiasis (Anonymous, 1969, Drury, 1873). The extracts also possess cytotoxic, anti-mutagenic and anti-repellant properties (Sah et al., 2006). The seeds are also used to make perfumed oil (Gupta et al., 1979, Nadkarni, 1976). In Japan, the ethanol extract of the seeds has been used as a preservative for pickles and some processed foods (Qiao et al., 2007). The seed cake is rich in nitrogen and minerals and is used as cattle feed or manure (Krishnamurthi et al., 1969).
The fruits of the P. corylifolia also have valuable medicinal uses. The seeds or the seed with the seed pod, possess high aphrodisiac properties and are used as a tonic to strengthen the genital organs (Ven, 1987). The fruits are bitter in taste, can prevent vomiting, cure difficulty in micturition, cure piles, bronchitis and anaemia and are known to improve complexion (Joshi, 2000). Moreover, fruit extract inhibits the growth of Mycobacterium tuberculosis (Yeung, 1985). The roots and seeds of P. corylifolia are used for preventing tooth decay (Duke, 2009). They also promote bone calcification, and hence are beneficial for treating bone fractures and osteoporosis (Joshi, 2000, Krishnamurthi et al., 1969, Wong and Rabie, 2010a, Wong and Rabie, 2010b).
Psoralea esculenta Pursh. (Breadroot, Large Indian breadroot) roots are starchy (70% starch), glutinous (9% protein) with a sweetish turnip-like taste (5% sugars) and are eaten raw or cooked (Hedrick, 1972, Saunders, 2011, Tanaka, 1976, Uphof, 1968, Yanovsky, 1936). The dried roots are pulverized and used with cereals in making cakes and porridge (Facciola, 1983). A poultice prepared from the crushed roots is applied to sprains and fractures. An infusion of the dried roots is used in the treatment of sore throats, gastro-enteritis and chest problems. The roots are chewed by children as a treatment for bowel complaints (Moerman, 1998).
Psoralea glandulosa L. is cultivated in Chile for its leaves and young shoots, which are used to make a refreshing cold drink. The leaves are anti-helmintic. Psoralea hypogaea Torr. & A. Gray (Small Indian breadroot) roots are eaten raw or cooked (Elias and Dykeman, 2009; Harrington, 1974; Hedrick, 1972; Moerman, 1998; Yanovsky, 1936). The roots are rich in starch and can be ground into a powder and used in soups or with cereals for making bread (Yanovsky, 1936). The roots were used an important source of food for the native North American Indians (Diggs et al., 1999). Psoralea macrostachya DC. (Large Leather Root) roots are eaten raw or cooked and may be dried for winter use. Moreover, the plant has been used in the treatment of ulcers and sores (Moerman, 1998). A strong fibre is obtained from the inner bark of the stem (Saunders, 2011, Usher, 1974). A fibre is also obtained from the roots, which is used to make ropes and bags (Moerman, 1998, Uphof, 1968). Roots are aromatic and the perfume persists for several months (Saunders, 2011). A yellow dye is also obtained from the roots (Moerman, 1998). Psoralea orbicularis Lindl. (Roundleaf leather root) leaves are cooked and eaten (Moerman, 1998, Tanaka, 1976, Yanovsky, 1936). A decoction of the root is used to purify blood and to treat prexia (Moerman, 1998). The plant is a good soil stabilizer (Huxley, 1992). Psoralea pedunculata (Mill.) Vail (Sampson's snakeroot) is used as a bitter tonic.
5. Phytochemistry
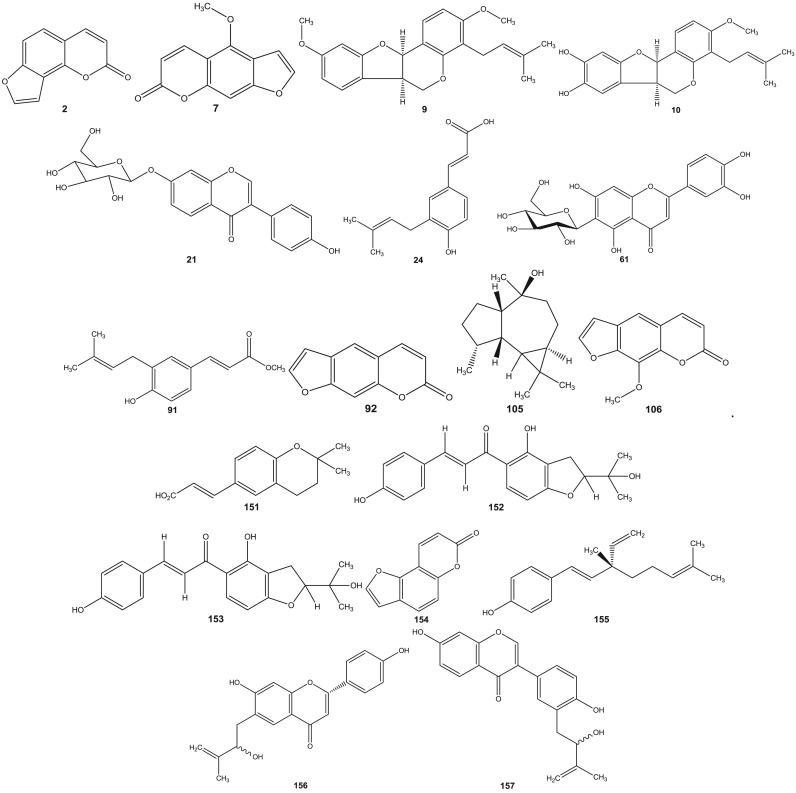
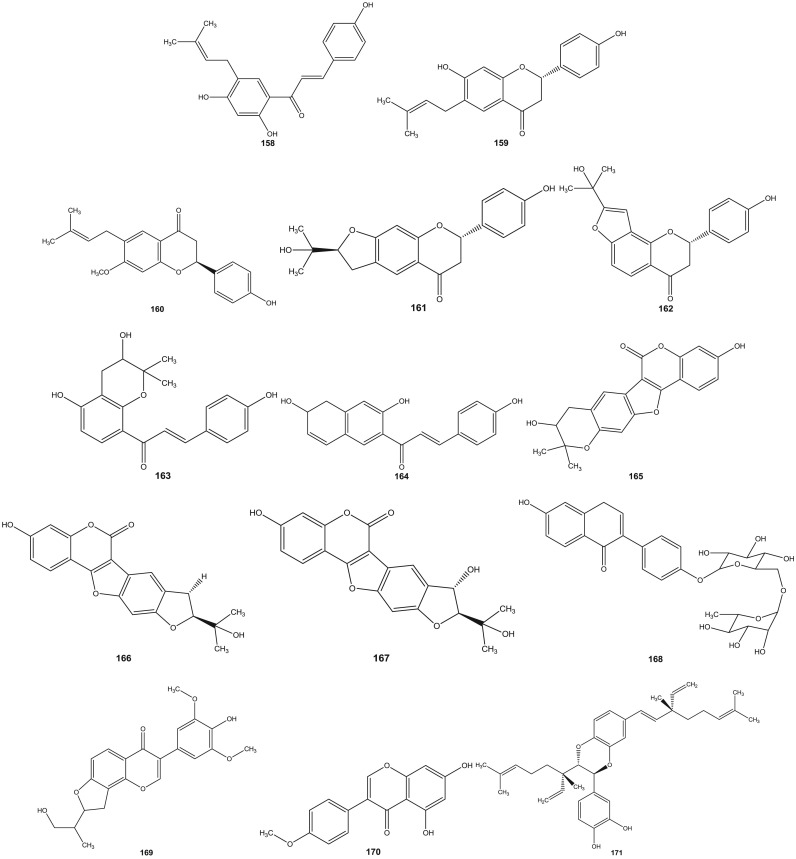
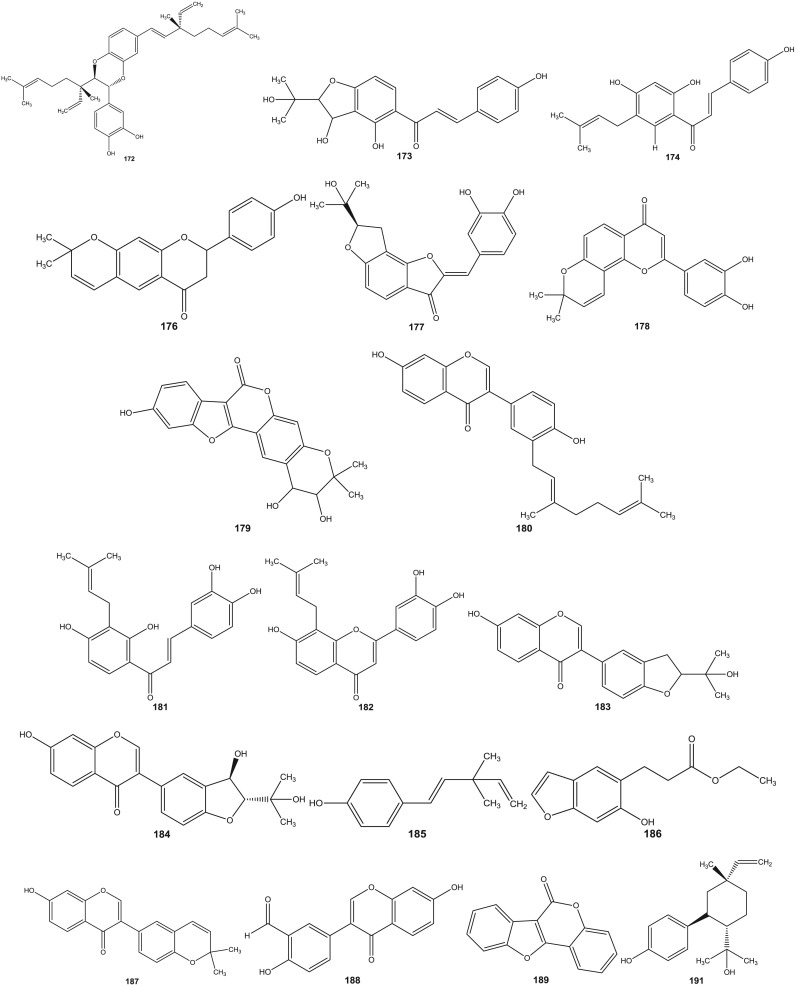
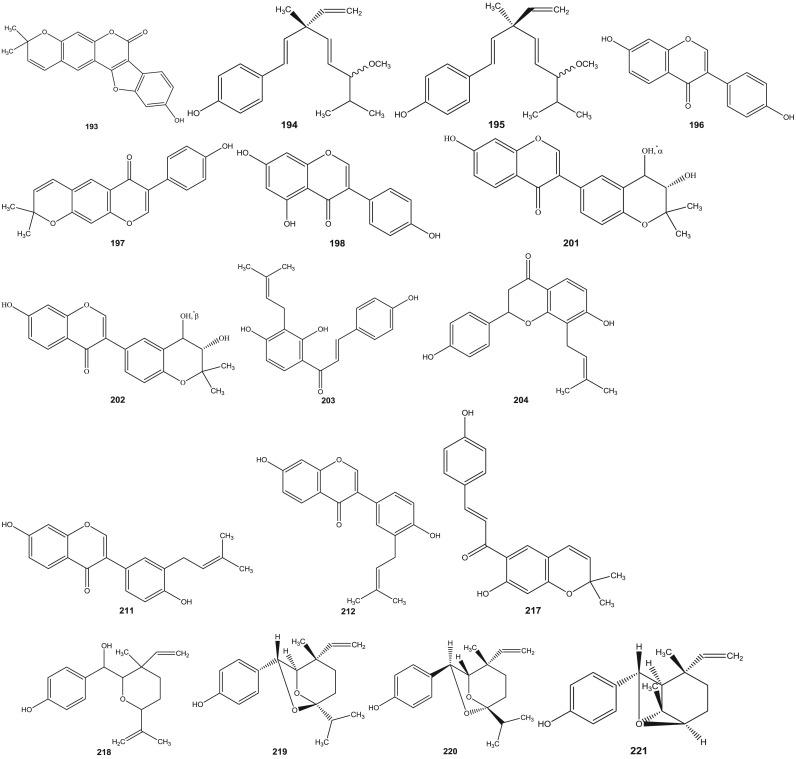
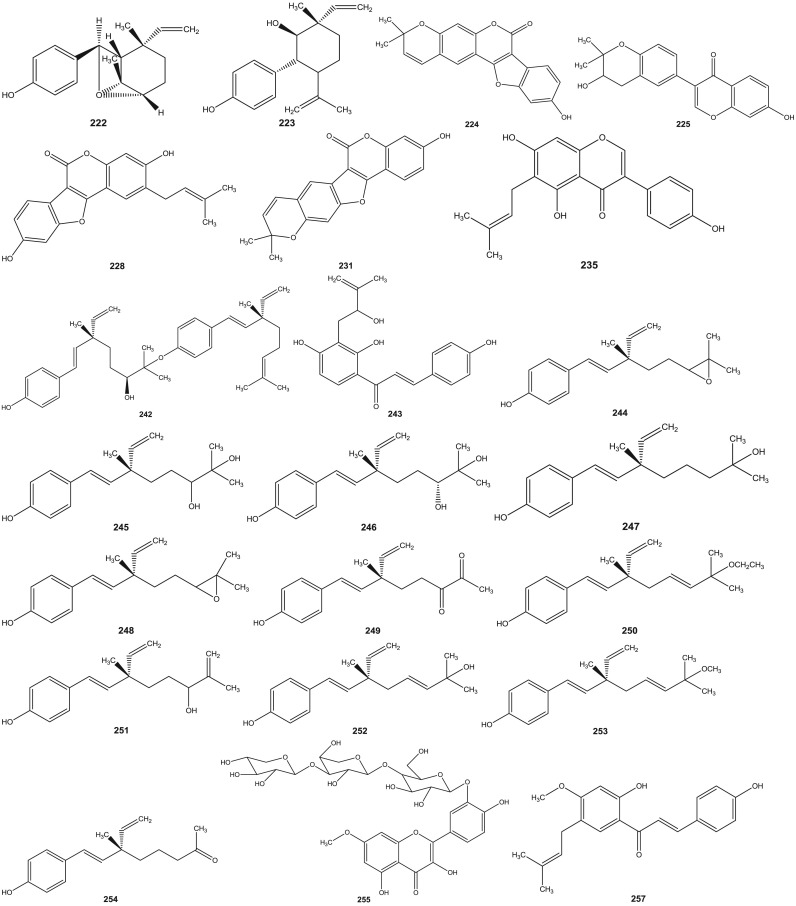
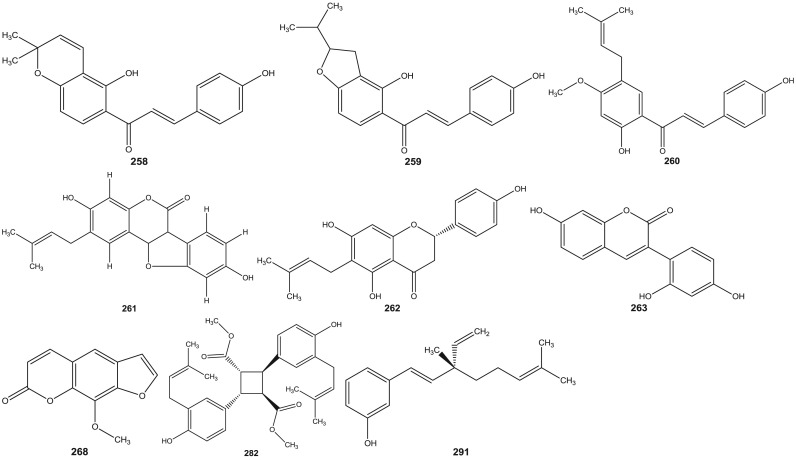
Psoralea species have been investigated since 1890s (Dymock et al., 1893). Leaf, rhizome, root, seeds, fruit and resinous extracts of Psoralea species have been subjected to HPLC and HPTLC followed by pharmacological analyses (Pandey et al., 2012, Shailajan et al., 2012, Uikey et al., 2010, Won et al., 2015, Yin et al., 2015, Zhao et al., 2005a). Detailed and extensive chemical investigation of six Psoralea species viz: P. bituminosa L. (Syn: Bituminaria bituminosa), P. canescens Michx., P. corylifolia L., P. esculenta Pursh, P. plicata Delile and P. glandulosa L. led to the characterization of a large number of bioactive constituents. Review of literature reveals the presence of altogether 291 chemical constituents including volatile compounds in the aforementioned species ( Table 1). These chemical compounds have been categorized into coumarins, furanocoumarins, flavonoids (polyphenols), isoflavones, meroterpenes, chalcones, phenols, phenolic cinnamates, phenylpropene, sterols, terpenes, tocopherols, benzofurans, sesquiterpenes, acids, fatty acids, alkyl aldehydes, alcohols and esters (Agarwal et al., 2006, Bertoli et al., 2004, Hsu et al., 2001, Qiao et al., 2006, Ruan et al., 2007, Tava et al., 2007, Yin et al., 2006, Yin et al., 2007, Yu et al., 2005). The chemical structures of 1–291 compounds are shown in the supplemental information (Supplemental information 3, Fig. S1). Their chemical names, chemical class and the corresponding plant sources are compiled in Table 1. The structures of those bioactive compounds which are representative of the genus and with reported pharmacological activities are presented in the main text ( Fig. 2).
Table 1.
Pharmacologically active compounds isolated from Psoralea species.
| Plant(s) | Part used | Chemical constituents (structure number)[chemicalclass/sub-class] | Reference(s) |
|---|---|---|---|
| Psoralea bituminosaL. | Whole plant | Allo-Aromadendrene (1)u | (Azzouzi et al., 2014a, Azzouzi et al., 2014b, Bertoli et al., 2004, Innocenti et al., 1998, Khatune et al., 2004, Pazos-Navarro et al., 2011, Pistelli et al., 2003, Tava et al., 2007) |
| [accepted name: Bituminariabituminosa (L.) C.H.Stirt] | Angelicin (Isopsoralen) (2)n | ||
 |
Apiol (3)s | ||
| Benzaldehyde (4)d | |||
| Benzoicacid (5)a | |||
| Benzyl alcohol (6)b | |||
| Bergapten (7)n | |||
| Bicyclogermacrene (8)u | |||
| Bitucarpin A (9)o | |||
| Bitucarpin B (10)o | |||
| Camphene (11)u | |||
| Caryophyllen-5-ol (12)u | |||
| Caryophyllene oxide (13)u | |||
| Chavicol (14)s | |||
| (Z,Z)-Nepetalactone (15)u | |||
| (Z,E)-Nepetalactone (16)u | |||
| (Z)-γ-Cadinene (17)c | |||
| Citronellylacetate (18)u | |||
| Cubebol (19)u | |||
| Cyclododecane (20)c | |||
| Diadzin (21)o | |||
| Docosanol (22)b | |||
| Dodecanol (23)b | |||
| Drupanin (24)h | |||
| (E)-2-Hexenal (25)d | |||
| (E)-2-Hexenol (26)b | |||
| (E)-Anethole (27)s | |||
| (E)-Phytol (28)b | |||
| (E)-Werneriachromene (29)n | |||
| (E)-β-Farnesene (30)t | |||
| (E)-β-Ocimene (31)u | |||
| (E,E)-Farnesol (32)u | |||
| (E,E)-α-Farnesene (33)u | |||
| (E,Z)-Farnesol (34)u | |||
| Ethylbenzoate (35)k | |||
| Ethyl butanoate (36)k | |||
| Ethyl hexanoate (37)k | |||
| Ethyl linoleate (38)k | |||
| Ethyl linolenate (39)k | |||
| Ethyl palmitate (40)k | |||
| Ethyl salicilate (41)k | |||
| Eudesma-4 (15),7-dien-1-β-ol (42)u | |||
| Eugenol (43)s | |||
| Ferulic acid ethyl ester (44)r | |||
| Furanmethanol (45)b | |||
| Furfural (46)d | |||
| Furfuryl methyl sulphide (47)x | |||
| Geraniol (48)u | |||
| Germacrene D (49)u | |||
| Germacrene D-4-ol (50)u | |||
| Guaiacol (51)r | |||
| Heptadecane (52)c | |||
| Heptanal (53)d | |||
| Heptane (54)c | |||
| Hexadecanol (55)b | |||
| Hexanal (56)d | |||
| Hexane (57)c | |||
| Hexanol (58)b | |||
| Humulene oxide II (59)u | |||
| Indole (60)x | |||
| Isoorientin (61)m | |||
| Isopentyl alcohol (62)b | |||
| Limonane (63)u | |||
| Limonene (64)u | |||
| Linalool (65)u | |||
| Linoleic acid (66)l | |||
| Linolenic acid (67)l | |||
| Maltol (68)x | |||
| Methyl benzoate (69)k | |||
| Methyl chavicol (70)s | |||
| Methyl hexadecanoate (71)k | |||
| Methyl linolenate (72)k | |||
| Methyl octadecanoate (73)k | |||
| Methyl tetradecanoate (74)k | |||
| Mint sulphide (75)x | |||
| n-Docosane (76)c | |||
| n-Eicosane (77)c | |||
| n-Heneicosane (78)c | |||
| n-Hexadecane (79)c | |||
| Nonanal (80)d | |||
| Nonane (81)c | |||
| Oct-1-en-3-ol (82)b | |||
| Octadecanoicacid (83)a | |||
| Octadecanol (84)b | |||
| Octane (85)c | |||
| Palmitic acid (86)l | |||
| Pentanol (87)b | |||
| Phenol (88)r | |||
| Phenylacetaldehyde (89)d | |||
| Phenylethyl alcohol (90)b | |||
| Plicatin B (91)q | |||
| Psoralen (92)n | |||
| p-Vinyl guaiacol (93)r | |||
| Sabinene (94)u | |||
| Spathulenol (95)u | |||
| Squalene (96)c | |||
| Terpinen-4-ol (97)u | |||
| Terpinolene (98)u | |||
| Tetradecanoicacid (99)1 | |||
| Tetradecanol (100)b | |||
| Thymol (101)p | |||
| (E)-γ-Cadinene (102)c | |||
| Tricyclene (103)u | |||
| Vanillicacidethylester (104)r | |||
| Viridiflorol (105)u | |||
| Xanthotoxin (106)n | |||
| (Z)-Phytol (107)b | |||
| (Z)-2-Pentenol (108)b | |||
| (Z)-3-Hexenol (109)b | |||
| (Z)-3-Hexenyl acetate (110)k | |||
| (Z,Z)-9,12-Octadecadien-1-ol (111)b | |||
| (Z,Z,Z)-9,12,15-Octadecadien-1-ol (112)b | |||
| α-Bisabolol (113)u | |||
| α-Cadinol (114)u | |||
| α-Cedrene (115)u | |||
| α-Copaene (116)u | |||
| α-Cubebene (117)u | |||
| α-Humulene (118)u | |||
| α-Longipinene (119)u] | |||
| α-Muurolene (120)u | |||
| α-Muurolol (121)u | |||
| α-Pinene (122)u | |||
| α-Terpineol (123)b | |||
| α-Ylangene (124)u | |||
| β-Bourbonene (125)u | |||
| β-Caryophyllene (126)u | |||
| β-Cedrene (127)t | |||
| β-Copaene (128)u | |||
| β-Cubebene (129)u | |||
| β-(E)-Damascenone (130)v | |||
| β-(E)-Ocimene (131)p | |||
| β-Elemene (132)u | |||
| β-Farnesene (133)u | |||
| β-Gurjunene (134)u | |||
| β-Myrcene (135)u | |||
| β-Phellandrene (136)u | |||
| β-Pinene (137)u | |||
| β-Ionone (138)v | |||
| γ-Muurolene (139)u | |||
| γ-Terpinene (140)u | |||
| δ-Cadinene (141)u | |||
| 2,3-Dihydrobenzofuran (142)e | |||
| 2-Methyl hexane (143)c | |||
| 2-Methyl pentane (144)c | |||
| 3-(E)-Hexen-1-ol (145)b | |||
| 3-(Methylthio) propanal (146)x | |||
| 3-Methyl-2-buten-1-ol (147)b | |||
| 4-Allylanisole (148)s | |||
| 4-Methoxy acetophenone (149)x | |||
| 4-Methyl pentanol (150)b | |||
| Psoralea canescensMichx. | Rhizome | Angelicin (Isopsoralen) (2)n | (Innocenti et al., 1997) |
| [synonym: Pediomelum canescens (Michx.) Rydb.] | Drupacin (151)a | ||
 |
Plicatin-B (91)q | ||
| Psoralen (92)n | |||
| Psoralea corylifoliaL. | Whole plant | Angelicin (Isopsoralen) (2)n | (Agarwal et al., 2006; |
| [accepted name: Cullen corylifolium (L.) Medik.] | Astragalin (152)m | Alam et al., 2017) | |
 |
Bakuchalcone (153)g | Anwar et al., 2011; Bajwa et al., 1972; Behloul and Wu, 2013; Bhalla et al., 1968; Bourgaud et al., 2008; Chen et al., 2010; Chen et al., 2010; Chen et al., 2011; Chen et al., 2013; Chen et al., 2014; Choi et al., 2008a, Choi et al., 2008b; Chopra and Chopra, 1958; Damodaran and Dev, 1973; Farahani et al., 2015; Gupta et al., 1977; Gupta et al., 1980; Gupta et al., 2005; Gupta et al., 2013a, Gupta et al., 2013b; Hao et al., 2014; Hsu et al., 2001; Huang et al., 2014; Iwamura et al., 1989; Jeong et al., 2013; Ji and Xu, 1995; Kapoor and Raton, 2001; Katsura et al., 2001; Kawl, 1976; Khatune et al., 2002; Khatune et al., 2004; Kim et al., 2016; Kondo et al., 1990; Krishnamurthi et al., 1969; Lau et al., 2010; Lau et al., 2014; Lee et al., 2015; Li et al., 2014; Li et al., 2015; Li et al., 2016; Lim et al., 2011; Limper et al., 2013; Lin and Kuo, 1992; Lin et al., 2007; Liu et al., 2014; Manohar and UdayaSankar, 2012; Mar et al., 2001; Matsuda et al., 2007; Newton et al., 2002; Peng et al., 1995; Prakasarao et al., 1973; Prasad et al., 2004; Qiao et al., 2006; Rajpal, 2005; Rajput et al., 2008; Rastogi and Mehrotra, 1998; Rastogi and Mehrotra, 1999; Nordin et al., 2015; Rastogi and Mehrotra, 2001; Rastogi and Mehrotra, 2004; Ruan et al., 2007; Sah et al., 2006; Sardari et al., 1999; Seo et al., 2013; Shah et al., 1997; Shan et al., 2014; Sharma et al., 2001; Sheng et al. 2004; Shirwaikar et al., 2010; Siddapa et al., 1956; Siddapa et al., 1957; Siva et al., 2015; Somani et al., 2015; Song et al., 2013; Song et al., 2015; Srinivasan and Sarada, 2012; Sun et al., 2016; Teschke et al., 2014; Tewari and Bhakuni, 2010; Wang et al., 2014; Won et al., 2015; Wu et al., 2007a, Wu et al., 2007b; Wu et al., 2008; Xiao et al., 2012; Xu et al., 2012; Yadava and Verma, 2003; Yadava and Verma, 2005; Yang et al., 2006; Yang et al., 2009; Yin et al., 2004; Yu et al., 2005; Zhang et al., 2016; Zhao et al., 2005a) | |
| Bakuchicin (154)n | |||
| Bakuchiol (155)p | |||
| Bakuflavonone (156)m | |||
| Bakuisoflavone (157)o | |||
| Bavachalcone (158)g | |||
| Bavachin/Corlifolin (159)m | |||
| Bavachinin (160)m | |||
| Bavachinone A (161)m | |||
| Bavachinone B (162)m | |||
| Bavachromanol (163)g | |||
| Bavachromene (164)g | |||
| Bavacoumestan A (165)h | |||
| Bavacoumestan B (166)h | |||
| Bavacoumestan C (167)h | |||
| Bavadin (168)m | |||
| Bavarigenin (169)m | |||
| Biochanin A (170)m | |||
| Bisbakuchiols A (171)p | |||
| Bisbakuchiols B (172)p | |||
| Brosimacutin G (173)m | |||
| Broussochalcone B (174)g | |||
| Chalcone (175)g | |||
| Chromenoflavanone (176)m | |||
| Coryaurone A (177)j | |||
| Coryfolia D (178)m | |||
| Corylidin (179)i | |||
| Corylifol A (Corylinin) (180)m | |||
| Corylifol B (181)g | |||
| Corylifol C (182)m | |||
| Corylifol D (183)o | |||
| Corylifol E (184)o | |||
| Corylifolin (185)m | |||
| Corylifonol (186)e | |||
| Corylin (187)m | |||
| Corylinal (188)o | |||
| Coumestan (189)o | |||
| Coumesterol (190)i | |||
| Cyclobakuchiol C (191)p | |||
| Daucosterol (192)t | |||
| Dehydroisopsoralidin (193)h | |||
| Delta(10)-12,13-dihydro-12-(R)- | |||
| methoxyisobakuchiol (194)p | |||
| Delta(10)-12,13-dihydro-12-(S)- | |||
| methoxyisobakuchiol (195)p | |||
| Diadzein (196)o | |||
| Diadzin (21)o | |||
| Erythrinin A (197)m | |||
| Genistein (198)o | |||
| Geranylacetate (199)p | |||
| Hydroquinone (200)r | |||
| Hydroxypsoralenol A (201)o | |||
| Hydroxypsoralenol B (202)o | |||
| Isobavachalcone (corylifolinin) (203)g | |||
| Isobavachin (204)m | |||
| Isocorylifonol (205)e | |||
| Isoneobavachalcone (206)g | |||
| Isoneobavaisoflavone (207)m | |||
| Isopsoralenoside (208)e | |||
| Isopsoralidin (209)n | |||
| Isowighteone (210)m | |||
| Neobavachalcone (211)g | |||
| Neobavaisoflavone (212)o | |||
| Neocorylin (213)m | |||
| Neopsoralen (214)h | |||
| p-Hydroxybenzoicacid (215)a | |||
| Protocatechualdehyde (216)d | |||
| Psorachromene (217)g | |||
| Psoracorylifol A (218)p | |||
| Psoracorylifol B (219)p | |||
| Psoracorylifol C (220)p | |||
| Psoracorylifol D (221)p | |||
| Psoracorylifol E (222)p | |||
| Psoracorylifol F (223)p | |||
| Psoracoumestan (224)h | |||
| Psoralen (92)n | |||
| Psoralenol (225)o | |||
| Psoralenoside (226)e | |||
| Psoralester (227)k | |||
| Psoralidin (228)n | |||
| Psoralidin-2',3'-oxide (229)i | |||
| Pyranocoumarin (230)h | |||
| Sophoracoumestan A (231)o | |||
| Stigmasterol (232)t | |||
| Terpinen-4-ol (97)u | |||
| Triacontane (233)c | |||
| Trilaurin (234)l | |||
| Wighteone (235)m | |||
| Xanthoangelol (236)m | |||
| α-Elemene (237)u | |||
| β-Caryophyllene (126)u | |||
| β-Caryophyllenoxide (238)u | |||
| β-D-Glucosyl-cis-O-hydroxycinnamicacid (239)a | |||
| β-Sitosterol-D-glucoside (240)t | |||
| γ-elemene (241)u | |||
| (12'S)-Bisbakuchiol C (242)p | |||
| 1-[2,4-Dihydroxy-3-(2-hydroxy-3-methyl-3-butenyl) phenyl]-3-(4-hydroxyphenyl-2-propen-1-one (Psorachalcone A) (243)m | |||
| 12,13-Dihydro-12,13-epoxybakuchiol) (244)p | |||
| 12,13-Diolbakuchiol (245)p | |||
| 12,13-Dihydro-12,13-dihydroxybakuchiol C (246)p | |||
| 12,13-Dihydro-13-hydroxybakuchiol (247)p | |||
| 12,13-Epoxybakuchiol (248)p | |||
| 12-Oxoisobakuchiol (249)p | |||
| 13-Ethoxyisobakuchiol (250)p | |||
| 12-Hydroxyisobakuchiol / 3-Hydroxy-∆1-bakuchiol (251)p | |||
| 13-Hydroxyisobakuchiol / 2-Hydroxy-∆3-bakuchiol (252)p | |||
| 13-Methoxyisobakuchiol (253)p | |||
| 15-Demetyl-12,13-dihydro-13-ketobakuchiol (254)p | |||
| 3,5,3',4'-tetrahydroxy-7-methoxyflavone-3'-O-α-L-xylopyranosyl(1→3)-O-α-L-arabinopyranosyl(1→4)-O-β-D-galactopyranoside (255)m | |||
| 3-Hydroxybenzaldehyde (256)d | |||
| 4,2'-Dihydroxy-4'-methoxy-5'-(3''',3'''-dimethyl allyl)-chalkone (257)m | |||
| 4-Hydroxylonchocarpin (Isobavachromene) (258)m | |||
| 4,2'-dihydroxy-2''-(1'''-methylethyl)-2''-3''-dihydro-(4'',5'',3',4') furanochalkone (259)m | |||
| 4-O-Methylbavachalcone (260)g | |||
| 6-(-3-Methylbut-2-enyl)-6'-7-dihydroxycoumestan (261)m | |||
| 6-Prenylnaringenin (262)m | |||
| 7,2',4'-Trihydroxy-3-arylcoumarin (263)h | |||
| 7-Methoxybavachin (264)m | |||
| 7-O-Isoprenylcorylifol A (265)m | |||
| 7-O-Methylbavachin(266)m | |||
| 7-O-Methylcorylifol A (267)m | |||
| 8-Methoxypsoralen (268)n | |||
| 8-Oxo-8H-furo [2, 3-f][l]benzopyran (269)f | |||
| 8-Prenyldaidzin (270)m | |||
| Psoralea esculentaPursh | Root | Diadzin (21)o | (Christensen et al., 2008; Kaye and Moodie, 1978; Perara, Reese, 2002; Stahnke et al., 2008) |
| [synonym: Pediomelum esculentum (Pursh) Rydb.] | Genistein (198)o | ||
 | |||
| Psoralea plicataDelile | Leaf, Flower, Seed | Angelicin (Isopsoralen) (2)n | (Arfaoui et al., 2013, Cheikh et al., 2015; El-Abgy et al., 2012; Hamed et al., 1997, Hamed et al., 1999; Khatune et al., 2004; Menon et al., 1999; Rasool et al., 1990, Rasool et al., 1991; Youssef et al., 2013) |
| [accepted name: Cullen plicatum (Delile) C.H.Stirt.] | Bakuchicin (154)n | ||
 |
Corylifonol (186)e | ||
| Coumestan (189)o | |||
| Diadzin (21)o | |||
| (E)-Werneriachromene (29)n | |||
| Isocorylifonol (205)e | |||
| Isopsoralicacid (271)h | |||
| Isopsoralicacid-O-glucopyranosyl (272)w | |||
| Isovitexin (273)m | |||
| Lupeol (274)u | |||
| p-Dimethyl coumaric acid (275)a | |||
| Plicadin (276)i | |||
| Plicatin A (277)q | |||
| Plicatin B (91)q | |||
| Psoracinol (278)p | |||
| Psoralen (92)n | |||
| Psoralic acid (279)h | |||
| Roseoside A (280)w | |||
| Stigmasterol (232)t | |||
| (Z)-Werneriachromene (281)n | |||
| α-Diplicatin-B (282)q | |||
| α-Tocopherol (283)t | |||
| β-Caryophyllene (126)u | |||
| β-Caryophyllenoxide (238)u | |||
| β-Duplicatin-B (284)q | |||
| 3-(-3-Methyl-2,3-epoxybutyl)-p-coumaric acid methyl ester (285)q | |||
| Psoralea glandulosaL. | Resinous exudate, young shoots, leaves | Angelicin (2)n | (Backhouse et al., 2001, Labbe et al., 1996, Li et al., 2016, Madrid et al., 2012, Madrid et al., 2013, Madrid Villegas et al., 2015a, Madrid et al., 2015b) |
 | |||
| Bakuchiol (155)p | |||
| Bakuchiol acetate (286)p | |||
| Bakuchiol methyl ether (287)p | |||
| Cyclobakuchiol A (288)p | |||
| Cyclobakuchiol B (289)p | |||
| Drupanin(24)h | |||
| Drupanin methyl ester (290)h | |||
| Psoralen (92)n | |||
| 3-Hydroxybakuchiol (291)p | |||
| 12,13-Dihydro-12,13-epoxybakuchiol (244)p | |||
| 12-Hydroxyisobakuchiol (251)p | |||
| 13-Hydroxyisobakuchiol (252)p |
Acids
Alcohols
Aliphatichydrocarbons
Alkylaldehyde
Benzofuran
Benzopyran
Chalcone
Coumarins
Coumesterol
Dihydrofuran
Esters
Fattyacids
Flavonoids
Furanocoumarin
Isoflavone
Meroterpene
Phenoliccinnamate
Phenols
Phenylpropene
Sterols
Terpenes
Rose-ketones
Glycoside
Miscellaneous.
Fig. 2.
Chemical structures of selected phytochemicals isolated and characterized from Psorelea species.
Recently, an isoflavone synthase (IFS) gene has been isolated and functionally characterized from P. corylifolia (Misra et al., 2010). The active principle found in P. corylifolia is bakuchiol (155) and psoralen (92) (Dev, 1999, Wang et al., 2009). Psoralen is linear in structure and may be called as a derivative of umbelliferone (Jois et al., 1933, Rangari and Agrawal, 1992). The production of psoralen enhances when the plant is exposed low dose of gamma radiation (Jan et al., 2011). Isopsoralen (2) is a structural isomer of psoralen (92), and it was found to be identical to angelicin (2) (Jois et al., 1933, Jois and Manjunath, 1934; Seshadri and Venkata Rao, 1937). Angelicin (isopsoralen) (2) is a photosensitizing agent and used for determination of DNA and RNA structures in cells and microorganisms (Kittler et al., 1980). Angelicin (2) and its derivatives occur in a number of plants belonging to the family Umbelliferae. The active compound, bakuchiol (155) is a monoterpene phenol, has been obtained in a pure state and named after Sanskrit name of the plant (Mehta et al., 1973) and possess the potent anti-bacterial property (Satyavati et al., 1987).
The seed contains volatile oils, monoterpenes, flavones, coumarins, stigmasteroids, resins, lipid compounds and phenols. The volatile oils include limonene (64), linalool (65), β-caryophyllene (126), geranyl acetate (199) and terpinen-4-ol (97), coumarin derivatives include psoralen (92), isopsoralen (2), isopsoralidin (209), corylidin (179), psoralidin (228), bavacoumestan A (165), bavacoumestan B (166), 8-methoxypsoralen (268) and sophoracoumestan A (231). Flavones include corylifolinin (203), bavachin/corlifolin (159), neobavaisoflavone (212), bavachromene (164), isobavachin (204), bavachalcone (158), corylin (187), and neobavachalcone (211). Lipids include triglycerides, diglycerides and monoglycerides. Monoterpene phenol includes bakuchiol (155). Others include free fatty acids, stigmasterol (232), triacontane (233), daucosterol (192), glucose and saponin. The fatty acids obtained from oil were found to be primarily palmitic acid (86) and linoleic acid (66) together with small fraction of linolenic acid (67). The pharmacologically active oil is identical with the unsaponifiable oil isolated by earlier workers (Gaind et al., 1965; Gupta et al., 1962). More that 188 chemical constituents (belonging to furanocoumarins, coumestrol group, chalcones and flavones) have been reported from the seeds (Bhalla et al., 1968, Chakrovarti et al., 1948, Chen et al., 2005; Satyavati et al., 1987; Tsai et al., 1996).
6. Pharmacological activities
Psoralea species have received tremendous attention because of their bioactive principles possessing remarkable pharmaceutical properties ( Fig. 3; Supplemental information 4, Table S3).
Fig. 3.
Medicinal properties of Psoralea specie(s).
6.1. Anti-oxidant activity
Several protocols have been followed to analyse the anti-oxidant property of seeds and leaf extracts of P. bituminosa, P. glandulosa, P. corylifolia, P. plicata, P. esculenta and P. glandulosa. The phenolic compounds obtained from different extracts were found to protect the biological membranes from oxidative stresses (Haraguchi et al., 2002, Guo et al., 2005, Borchardt et al., 2008, Kim et al., 2013, Madrid et al., 2013, Huang et al., 2014). The antioxidant activity of fruit extracts of Psoralea plicata was evaluated by DPPH assay. The study indicated that the methanol extract shows an anti-oxidant activity (288.32 micromol Trolox equivalent/100 g dry material) which is slightly higher as compare to aqueous extract (258,65μmol Trolox equivalent/100 g dry material) (Cheikh et al., 2015). In a study on P. corylifolia, several bioactive compounds such as bakuchiol (155), psoralen (92), isopsoralen (2), corylin (187), corylifolin (185) and psoralidin (228) were screened for their anti-oxidant potential. Their antioxidant activities were investigated individually and compared with butylated hydroxytoluene (BHT) and α-tocopherol by the oxidative stability instrument (OSI) at 100ºC Among these compounds psoralidin exhibited highest anti-oxidant activity (5.23) than that of standard compounds (Psoralidin>BHT>α-tocopherol>bakuchiol>corylifolin>corylin>isopsoralen~psoralen) (Jiangning et al., 2005). However, antioxidant activity alone cannot be of any pharmacological significance. It is accompanied by other specific pharmacological activities to claim any therapeutic benefit.
6.2. Anti-bacterial activity
There are several reports on the anti-bacterial activitiy of P. bituminosa and P. corylifolia. The ethanol and methanol extracts obtained from the aerial parts of P. bituminosa contain flavones and isoflavones, while the seed extracts of P. corylifolia contain psoralidin (228), bakuchicin (154), psoralen (92) and angelicin (2), which have shown significant anti-bacterial activities. Techniques such as disc-diffusion method, UV (ultraviolet), H NMR (proton nuclear magnetic resonance), C NMR (carbon nuclear magnetic resonance), anti-bacterial assay, broth-dilution method, column chromatography, HPLC (high performance liquid chromatography) and TLC (thin layer chromatography) were used to quantitate and analyse the anti-bacterial property of these natural compounds (Azzouzi et al., 2014a, Azzouzi et al., 2014b; Bhawna et al., 2013; Cui et al., 2015). Bakuchiol obtained from seed extract of P. corylifolia has been reported to inhibit the growth of Staphylococcus mutans and Actinomycess viscosus and hence possess strong anti-bacterial activity and MIC value of bakuchiol was found to be 9.76–19.5 µg/mL (Khushboo et al., 2010; Rao et al., 2011). In a study, the compounds psoralidin (228), bakuchicin (154), psoralen (92) and angelicin (2) obtained from different extracts of the plant were found to show anti-bacterial activity against different gram-positive and gram-negative bacteria. Among them, psoralidin (228) showed highest anti-bacterial activity against Shigella sonnei and S. flexneri as depicted by disc diffusion assay (Khatune et al., 2004).
6.3. Anti-fungal activity
P. corylifolia and P. glandulosa are known to possess significant anti-fungal activity. Aqueous and methanol extract of seeds and petroleum ether extract of the aerial parts P. corylifolia have been tested against various seed borne fungi such as (Alternaria, Cladosporium, Dreschslera and Rhizophus spp.) of maize. In aqueous extract, maximum inhibition (95.4% inhibition at 50% concentration) was observed against A. alternata followed by C. lunata (86.0%), Rhizopus sp. (82.3%), D. halodes (68.0%) and C. cladosporioides (57.7%). Among the solvents used for the preparation of extracts, maximum inhibition was observed with petroleum ether extract and moderate activity was observed with methanol extract (Kiran et al., 2011). In an anti-fungal assay, the essential oil extracted from P. corylifolia, was studied against three dermatophytic fungi Microsporum canis, Trichophyton rubrum and Trichophyton mentagrophytes. The zone of inhibition (by disc-diffusion assay) for M. canis, T. rubrum and T. mentagrophytes was found to be 20, 35 and 37 mm respectively, while the minimum inhibitory concentration was reported to be 1.4, 0.4 and 0.5 μl/mL, respectively (Sharma and Tiwari, 2012). In another study, methanol extract of P. corylifolia seeds was found to be most effective against tomato late blight (Phytophthora infestans) and wheat leaf rust (Puccinia recondite) (Shim et al., 2009). The crude extract of P. corylifolia exhibited significant anti-fungal activity against Candida albicans (Anwar et al., 2011). In a study by Borate et al. (2014), methanol extract of P. corylifolia seeds was reported to exhibit a maximum zone of inhibition against C. albicans (16.25 mm) followed by Malassezia furfur (14.25 mm) and A. niger (12.22 mm). Two active compounds bakuchiol (155) and 3-hydroxy-bakuchiol (291) obtained from resinous exudate of aerial plant parts of P. glandulosa were reported to show MIC 80 ranging from 4 to 416 and 0.125–16 mg/mL, respectively against various strains of Candida (Madrid et al., 2012).
6.4. Anti-viral activity
The crude ethanol extract of the seeds of P. corylifolia exhibited significant anti-viral activity against the severe acute respiratory syndrome corona virus (SARS‐CoV) papain‐like protease (PLpro). The IC50 value for the same was 15 μg/mL. SARS‐CoV‐PLpro is the enzyme that is crucial for replication of SARS virus (Kim et al., 2014). In a recent study, it was reported that bakuchiol (155) present in seeds of P. corylifolia inhibited the influenza A viral infection and growth and activated the nuclear factor erythroid 2-related factor (Nrf2) pathway. This pathway is responsible for cellular defense against electrophilic or oxidative stress (Shoji et al., 2015).
6.5. Anti-inflammatory activity
Bakuchiol (155) extracted (petroleum ether extract, dichloromethane extract, and methanol extract) from the aerial parts of P. glandulosa inhibits degranulation in human neutrophils and decreases the cell migration, eicosanoid levels and myeloperoxidase activity in mice thus, confirming its anti-inflammatory potential (Ferrándiz et al., 1996, Backhouse et al., 2001). In another study, bakuchiol (155) from P. corylifolia was reported to inhibit the expression of inducible nitric oxide synthase (NOS) gene through the inactivation of nuclear transcription factor-B in RAW 264.7 macrophages (Pae et al., 2001). The bioactive compounds obtained from leaves, fruits and seeds of P. corylifolia inhibit the functioning of tumor necrosis factor-alpha (TNF-α) and exhibt anti-inflammatory activity (Mueller et al., 2010). The production of inflammatory mediators such as, reactive oxygen species (ROS), reactive nitrogen species (RNS), and cytokines such as IL-1β, IL-6 and TNF-α (tumor necrosis factor) in PMA (phorbol 12-myristate 13-acetate)/LPS (lipopolysaccharide), in IFN-stimulated murine peritoneal macrophage cell line (RAW 264.7) was inhibited by neobavaisoflavone (212) (Szliszka et al., 2011a). Cytotoxicity of neobavaisoflavone (212) was tested by using LDH assay (lactate dehydrogenase assay). Psoralidin (228) suppresses the activity of pro-inflammatory cytokines which regulate pulmonary inflammation in human lung-fibroblasts and in mice, by ionizing radiation, has been reported (Yang et al., 2011). In a recent study the activity of IL-6-induced STAT3 (Signal transducer and activator of transcription 3) was found to be inhibited by bavachin (159), bakuchiol, bavachinin (160), corylin (187), corylifol A (180), neobavaisoflavone (212), and isobavachalcone (203). Hence, these bioactive compounds hold promise to cure inflammatory diseases (Lee et al., 2012).
6.6. Anti-leprotic activity
In a study by Newton et al. (2002), forty-three plant species were screened for their anti-mycobacterial activities. Among them, bakuchiol (155) extracted from P. corylifolia hexane seed-extract exhibited significant anti-bacterial activity (MIC = 31.25 g/mL) against Mycobacterium aurum and M. smegmatis thus, confirming its potential for treatment of leprosy. However, these studies further require in vitro and in vivo analyses for their large-scale utilization (Newton et al., 2002).
6.7. Anti-psoriatic activity
In a study by Dwarampudi et al. (2012), the ethanolic seed extract of P. corylifolia showed an IC50 value of 255 μg/mL and a considerable anti-psoriatic activity (75.87%), using the mouse tail model. The seed extract converted parakeratosis stage (keratinization) to orthokeratosis (formation of anuclear keratin layer) stage of the cell, thus confirming its anti-psoriatic potential (Dwarampudi et al., 2012). In a recent report, different micro-emulsions containing single and both Commiphora mukul powder and babchi-oil from P. corylifolia were used to assess the anti-psoriatic efficacy on diseased rat paw. The synergistic effect of both the natural products gave better results, hence this herbal combination could be a cheap and effective source of anti-psoriatic agent (Marwaha, 2013). In a study involving human subjects, P. corylifolia hexane-seeds extract was prepared into a cream using stearic acid followed by an open clinical trial on thirty patients suffering from eczema, for a period of one month. The placebo preparation, for this experiment contained all the ingredients except the seed extract. The parameters studied were length of the lesion, exudation rate and rate of itching. The symptoms score reduced after two weeks of cream application. Finally, the length of the lesion reduced from 6.367 ± 1.098–0.333 ± .279, exudation rate reduced from 1.333 ± .994–0.165 ± .087 and the rate of itching reduced from 2.567 ± .504–0.165 ± .132. This study concluded that P. corylifolia seed extracts could be used effectively used for the treatment of eczema (Gidwani et al., 2010a).
6.8. Anti-vitiligo/anti-leucoderma activity
The compounds obtained from the P. corylifolia extract have played an influential role for the treatment of vitiligo. The furocoumarins, psoralen (92) and isopsoralen (2) present in Psoralea species assists the skin to produce new pigment. They initiate the transformation of dihydroxyphenylalanine (DOPA) to melanin when exposed to sunlight and are therefore being used for treating vitiligo, psoriasis and leprosy (Fitzpatrick and Pathak, 1959, Hussain et al., 2016, Song et al., 2004, Yin et al., 2004). Psoralen (92) alone can effectively treat psoriasis and alopecia areata (Ji and Xu, 1995, Wang and Wang, 2007). In a report by Abu Tahir et al. (2010), human melanocytes were used to test the anti-vitiligo activity of the oleo-resinous extracts of the seeds. The seed extract acted as an effective anti-vitiligo agent by restoring the melanocytes of the affected area (Abu Tahir et al., 2010).
6.9. Anti-diabetic activity
Ethanol seed extract of P. corylifolia when administrated orally to streptozotocin-nicotinamide (STZ) induced diabetic rats lead to an increase in glycogen content of liver and insulin level in plasma with a decrease in plasma cholesterol and blood glucose level (Kamboj et al., 2011). In a report by Bera et al. (2013), the aqueous seed extract of P. corylifolia was found to regulate the functioning of insulin sensitive enzyme activities in Wistar strain male albino rats. The seed extract tends to increase the activity of liver hexokinase, glucose-6-phosphate and decrease the level of glucose-6-phosphatase (Bera et al., 2013).
6.10. Anti-depressant activity
The furanocoumarins psoralen (92) and isopsoralen (2) obtained from the seed extract of P. corylifolia have showed anti-depressant activity in mice by hindering the MAO (monoamine oxidase) activity, hypothalamic-pituitary-adrenal axis action and oxidative stress (Chen et al., 2005, Chen et al., 2007, Chen et al., 2008). Another compound, psoralidin (228) inhibits the transcription of CRF (corticotrophin releasing factor) gene, which is responsible for stress response (Chen et al., 2007). The anti-depressant activity of psoralidin (228) was evaluated by applying the forced swimming test on rats (Yi et al., 2008). Psoralen was reported to successfully change the level of 5-hydroxyindoleacetic acid (5-HIAA) and serotonin (5-HT) in Hippocampus and frontal cortex of mice (Xu et al., 2008). Bakuchiol (155) decreases the immobilization time in behavioral despair mouse and plasma epinephrine and norepinephrine (neurotransmitter) levels in bovine adrenal medullary cells hence, exhibits anti-depressant activity (Hao et al., 2014). These results depict the anti-depressant activity of psoralen and bakuchiol.
6.11. Anti-cancer activity
Psoralea species possess unique bioactive compounds with anti-cancer properties. P. corylifolia leaves contain remarkably high concentrations (more than 2 g per Kg dry weight) of genistein (198), the anti-cancer metabolite. Bioactivity of two furocoumarins psoralen (92) and isopsoralen (2) was determined for their cytotoxicity on carcinoma lines KB, KBv200 (vincristine resistance subline of KB), human erythroleukemia cell K562 and K562/ADM (doxorubicin resistance subline of K562). Both the compounds induced apoptosis in these cells thus, confirming their anti-cancer potential. The IC50 values of psoralen were 88.1, 86.6, 24.4 and 62.6, which of isopsoralen were 61.9, 49.4, 49.6 and 72.0, respectively (Wang et al., 2011). Psolaren (92) when subjected to human hepatocarcinoma cells, showed its inhibitory activity by inducing the mechanism of apoptosis. Psoralen (92) was able to inhibit the growth of SMMC-7721 cells in a dose- and time-dependent manner and had a strong proapoptotic effect on these cells (Jiang and Xiong, 2014, Khan et al., 2015). The bioactive compounds 7,2',4'-trihydroxy-3-arylcoumarin (263), psoracoumestan (224) and corylifol C (182) from P. corylifolia showed strong anti-cancer potential by inhibiting the enzyme MAPK/ERK kinase phosphorylation and inducing aptotic cell death (Limper et al., 2013). In a study, psoralidin (228) showed its activity in MCF‐7 cancer cells (isolated from human breast) by induction of pS2 gene activity, with an EC50 value of ERE‐reporter gene transcription activity of 1.85 μM (Liu et al., 2014). The seed extract of P. corylifolia induced apoptosis in the human breast cancer (MCF-7) cells followed by mitochondrial cell death (Rajan et al., 2014). In another study, psoralidin (228) was reported to generate reactive oxygen species and also inhibited A549 cell proliferation. The method adopted was MTT assay and the IC50 values obtained after 24‐, 48‐ and 72‐h treatment were 19.2, 15.4, and 11.8 μM, respectively (Hao et al., 2014). Psoralidin (228) and neobavaisoflavone (212) in combination with TRAIL (tumor necrosis factor-related apoptosis-inducing ligand) have showed their anti-cancer property by inducing apoptosis in LNCaP (human adinocarcinoma prostate cancer cells) (Szliszka et al., 2011a). In another report, psoralidin (228) in combination with TRAIL influenced apoptosis in HeLa cells by increasing the expression of death receptor (TRAIL-R2) (Bronikowska et al., 2012). In a similar report, psoralidin (228) compound obtained from different extracts of P. corylifolia exhibited the anti-cancer activitiy against human lung cancer (A549) cells. Psoralidin dramatically decreased the cell viabilities in dose and time-dependent manner (Hao et al., 2014). Psoralea fructus suppressed the proliferation of human colorectal cancer cell lines, such as LoVo (IC50: 23.3 ± 1.9 μg/mL), SW480 (IC50: 37.9 ± 1.6 μg/mL), HT‐29 (IC50 value: 40.7 ± 1.5 μg/mL), and HCT116 (IC50: 45.3 ± 1.2 μg/mL) by decreasing the protein expression of cyclin D1 and CDK4. Succeeding experiments with many kinase inhibitors revealed that P. fructus‐mediated degradation of cyclin D1 and CDK4 is dependent on GSK3β and/or ERK1/2 (Park et al., 2016). Psoralen (92), isoporalen (2) and bakuchiol (155) identified in P. corylifolia extracts (chloroform and ethanol) have shown their cytotoxic property against HEp-2 cell line (Whelan and Ryan, 2003), BGC-823 cancer cell (Guo et al., 2003) and cultured human cancer cells (SK-OV-3, A549, XF498 and SK-MEL-2HCT15) (Ryu et al., 1992). Bakuchiol (155) is one of the active ingredients of the dried ripe fruit of P. corylifolia. In a study by Miao et al., 2013, bakuchiol (155) suppressed the testosterone induced cell proliferation and gene expression in androgen‐dependent prostate cancer (PCa) cell line (LNCaP). The IC50 of bakuchiol (155) to androgen receptor was 8.87 × 104, which was similar to the standard flutamide (10.00 × 104). Bakuchiol (155) has also showed a strong anti-cancer action against human lung adenocarcinoma cell line A549 and showed better results than its analogue resveratrol. IC50 of bakuchiol (155) at 72 h was 9.58 ± 1.12 μmol/L, much lower than that of resveratrol (33.02 ± 2.35 μmol/L). Compared to resveratrol, bakuchiol (155) triggered the process of apoptosis to a higher level. It was also observed that oxygen species mediated apoptosis contributes to the cytotoxic properties of bakuchiol (155) and can therefore be used against non‐small‐cell lung cancer (Chen et al., 2010). Invitro experiments with A2058 melanoma cells using P. glandulosa resinous exudates revealed that it can inhibit the growth of cancer cells after 48 h of treatment. These experiments confirmed the anti-cancer potential of P. glandulosa which can be attributed to the presence of bakuchiol, 3-hydroxy-bakuchiol and 12-hydroxy-isobakuchiol (Madrid et al., 2015b).
Ethanol, methanol, chloroform and aqueous seed extracts of P. corylifolia were tested against the tumor cells of mice and were found to stimulate the antibody complement-mediated cytotoxicity during tumor development (Latha et al., 2000). Anti-tumor property of bakuchiol (155) was also compared with resveratrol and it was observed that bakuchiol was more efficient in inhibiting the growth of tumor cells, when tested against human lung adeno-carcinoma A549 cell line (Chen et al., 2010). In a similar study on tumor cells of murine origin, radioiodinated bakuchiol showed greater cytotoxic effect than bakuchiol (Bapat et al., 2005). Bakuchiol (155) also induced ERβ expression and suppressed the ERα expression in MCF‐7 cells (breast cancer cells). It also caused the arrest of S-phase in MCF‐7 and MDA‐MB 231 cells and showed a stronger anti-proliferative effect than resveratrol. Additionally, bakuchiol (155) caused the apoptotic cell induction and interrupted membrane potential in mitochondria of MCF‐7 cells via an intrinsic apoptotic pathway. The same compound when tested for in vivo anti‐breast cancer effect in Zebrafish xenografts showed a significant reduction in MCF‐7 cell mass (Li et al., 2017). Similarly, two more compounds from the same species identified as isobavachalcone (203) and bavachinin (160) attenuate Aβ42‐induced cell toxicity. The investigation was carried out on yeast two‐hybrid system. During this study, eight compounds were tested, and among them IBC (3 μM) and BCN (30 μM) proved to be active at non‐toxic concentrations (Chen et al., 2013).
6.12. Osteogenic activity
This is a significant property which is associated with the P. corylifolia fruit extract, the osteoblastic proliferation in cultured UMR106 (osteosarcoma) cell line and enhancing the formation of bones (Wang et al., 2001). In several studies, P. corylifolia extracts showed significant inhibitory effect on osteoclasts (Yang et al., 2007, Zhang et al., 1995). Corylin (187) and bavachin/coryfilolin (159) were reported to promote the proliferation of osteoblasts and inhibit bone resorption (Wang et al., 2001). Bakuchiol (155) compound in the extracts showed inhibitory effect on osteoporosis, mediated through estrogen deficiency (Lim et al., 2009, Tsai et al., 2007). In a report by Tsai et al. (2007), it was observed that P. corylifolia extract lead to a decrease in calcium and osteocalcin through urinary excretion in ovariectomized (OVX) rat model and thus, maintained the bone density (Tsai et al., 2007). In a similar report, ethanolic bakuchiol present in seed extract of P. corylifolia reduced the bone loss in OVX rat model thus, showed promising anti-osteoporosis activity (Lim et al., 2009).
6.13. Estrogenic activity
The ethanolic fruit extract of P. corylifolia showed estrogenic activity, which was demonstrated by transcription of lacZ in recombinant yeast system (Zhang et al., 2005). In another report by Zhao et al. (2007), proliferation rates of MCF-7 cells increased significantly when treated with the P. corylifolia extract (Zhao et al., 2007). In a study by Lim et al. (2011) Bakuchiol (155) showed a higher estrogenic activity and estrogen receptor (ER) binding affinity than genistein, both in vitro and in vivo (Lim et al., 2009, Lim et al., 2011). Among the seven bioactive components of P. corylifolia extract exhibited isobavachalcone (203), bavachin (159), corylifol A (180), neobavaisoflavone (212), bakuchiol (155), and two coumarins psoralen (92) and isopsoralen (2) compounds selectively activated ER-α while the other compounds activated both ER-α and ER-β (Xin et al., 2010). Park et al. (2012), reported that bavachin (159) could bind and activate the estrogen receptor (Park et al., 2012). In addition to it, psoralidin (228) was reported as aganist for both ER-α and ER-β (Liu et al., 2014). In a similar study by Xin et al. (2010), seed extract of P. corylifolia containing psoralen (92) and isopsoralen (2) enhanced the MCF7 cell prolification by enhancing the activity of ERα (estrogen receptors), hence showed significant estrogenic activity.
6.14. Hepatoprotective activity
P. corylifolia is revered for its hepatoprotective potential (Dai et al., 2009, Ye et al., 1999). Three compounds psoralen (92), bakuchicin (153) and bakuchiol (155) (EC50 values of 1.0, 47.0, and 50.0 μg/mL, respectively) have shown hepatoprotective activity towards the liver cells (Hep G2 cells) in which the cytotoxicity was induced by tacrine (cholinesterase inhibitor) (Cho et al., 2001). In a similar study, bakuchiol (155) reduced the toxic activity of carbon tetrachloride (CCl4), D-galactosamine (D-GaIN), and tert-butylhydroperoxide (tBH) in primary rat hepatocytes (Park et al., 2005). The activity of hepatic stellate cells (involved in liver fibrosis) was reduced by bakuchiol in liver injured rats thus, confirming its hepatoprotective activity (Park et al., 2007). In a recent report, bakuchiol (155) obtained from the seed extract of the P. corylifolia exhibited hepatoprotective activity by inhibiting the production of reactive oxygen species (ROS) and malfunctioning of the mitochondria in human diploid fibroblast (HDF) (Seo et al., 2013).
6.15. Neuroprotective activity
In a study, the cultured rat pheochromocytoma (PC12) cells pretreated with P. corylifolia L. seed extract significantly attenuated 3‐NP induced cell death, reduced ATP levels, and lowered the mitochondrial membrane potential. Thus, P. corylifolia seed extract has the potential to treat neurodegenerative diseases (Im et al., 2014). In another study, it was revealed that isobavachalcone, a flavonoid from P. corylifolia, has the ability to ameliorate the neuronal injury in brain diseases related to inflammation, and this was accomplished through inhibition of the expression of lipopolysaccharide induced intercellular adhesion molecule‐1 and leukocyte adhesion to brain endothelial cell, by blocking toll‐like receptor 4 signaling (Lee et al., 2015).
6.16. Immunomodulatory activity
P. corylifolia extract effectively increased the proliferation rate of diploid fibroblasts (in mice) and increased the ability of non-specific immunity (Wang et al., 1990). In the study, a mixture of fruit extracts of P. corylifolia and Brucea javanica improved the immunological regulation in rats that were infected with Pneumocystis carinii pneumonia. As a result of the treatment, gain in body weight, decline in the number of cysts produced in the lungs and the level of T cells (CD4 + and CD8 + ) and TNF-alpha in the serum also increased considerably in the immunosuppressed rats (Qin et al., 2006). In another study, the ethanol seed-extracts of P. corylifolia stimulated the immune system in mice by increasing both cell-mediated and humoral immune responses in mice (Latha et al., 2000).
6.17. Anti-asthma activity
P. corylifolia has long been used for its anti-asthmatic properties. Experiments have shown that coumarins isolated from P. corylifolia exhibits anti-asthmatic activity by markedly increasing the level of serum cAMP (Deng et al., 2001, Yu et al., 2006). In another study, a Chinese herbal decoction, which contained six herbs, along with P. corylifolia seeds, could prompt treatment for asthma in the convalescent stage, to prevent emphysema (Fu, 1989). In a study by Hongfen (2007), the preparations obtained from the P. corylifolia showed high anti-asthma activity by stabilization of mast cells and inhibition of histamine release. Thus, the natural compounds obtained from P. corylifolia can act as promising molecules to cure asthma.
6.18. Anti‐obesity activity
Several studies on animal models showed that a trihydroxyflavone, genistein (198) has the potential to decrease body weight by decreasing the food intake. One such study was conducted on ovariectomised mice wherein, it reduced the fat pad weight and enhanced the apoptosis of adipose tissues. Genistein (198) isolated from P. corylifolia, exhibited a potential anti‐obesity and ant-diabetic activity through multiple mechanisms and cell signaling pathways by the action on adipocyte life cycle, obesity‐related low‐grade inflammation, and oxidative stress (Behloul and Wu, 2013).
6.19. Anti-filarial activity
In a report, the aqueous and alcohol extracts of the leaves and seeds of P. corylifolia showed significant anti-filarial activity against Setaria cervi. Alcohol extracts of both leaves and seeds caused death of microfilariae in vitro. The LC50 and LC90 for alcohol extract of leaves and seed was 15, 25 ng/mL and 12, 18 ng/mL respectively (Qamaruddin et al., 2002). The extracts also caused the inhibition of spontaneous movements of the whole worm and the nerve muscle preparation of S. cervi (Qamaruddin et al., 2002).
6.20. Anti-platelet activity
The methanolic P. corylifolia seed extracts was observed to inhibit the aggregation of rabbit platelets induced by arachidonic acid, collagen, and platelet activating factors. The anti-platelet aggregation activity of isobavachalcone was found to be most effective against aggregation induced by arachidonic acid, with a 50% inhibitory concentration (IC50) of about 0.5 µM (Tsai et al., 1996).
6.21. Anti-pyretic activity
Using rabbit as an animal model, and Escherichia coli endotoxin (13 ng/kg) as a pyrogen, the petroleum ether, dichloromethane, and methanol extracts of the aerial parts of P. glandulosa have been reported to possess anti-pyretic activity. The anti-pyretic effect (21.7%) of the petroleum ether extract was reported because of bakuchiol compound present in the extract. Maximum anti-pyretic effect (68%) was observed at the dose of 17 mg/kg (Backhouse et al., 2001).
6.22. Anti‐Alzheimer's activity
Isobavachalcone (203) and bavachinin (160) from Psoralea modulate amyloid β (Aβ) peptides, especially the peptides with 40 (Aβ40) or 42 (Aβ42) residues, which are believed to be responsible for the development of amyloid plaques in Alzheimer's disease. Isobavachalcone significantly inhibits both oligomerization and fibrillarization of Aβ42, whereas, bavachinin converts Aβ42 into large unstructured aggregates in neuroblastoma cells (Chen et al., 2013). Psoralen (92) isolated from P. corylifolia fruits was investigated as an inhibitor of AChE enzyme in an attempt to explore its potential for the management of Alzheimer's disease. The concentration of psoralen used was 25–400 μg/mL. It inhibited the AchE in a dose‐dependent manner in animal models. Adult male Wistar rats, weighing 180–250 g, were used in the study. While molecular docking study was also carried out, which showed that psoralen (92) binds well within the binding site of the enzyme showing interactions such as hydrogen bonding and π‐π stacking (Somani et al., 2015). These findings may provide valuable information for the synthesis of new drugs or for the treatment of Alzheimer’s disease.
7. Side-effects and toxicity
Although having excellent bioactivities, excess intake or use of Psoralea is not free from side-effects. P. fascicularis (synonym: Psoralea tenuifolia Thunb.) has been reported to be toxic to horses, cattle and therefore is not recommended as a fodder. There have been reports on skin allergic reactions after the oral and injected preparations of Psoralea (Bensky et al., 2004). In over-dose, Psoralea has been associated with dizziness, general weakness, blurred vision, rapid breathing, and vomiting. Severe cases of overdose have been associated with vomiting of blood, loss of consciousness, and coma (Bensky et al., 2004, Chen and Chen, 2004).
Psoralen plus UVA (PUVA) therapy has been used to treat skin diseases, such as vitiligo and psoriasis. The reported side-effects include dermatitis, blistering, edema, renal complications, loose-motions, biliousness, malaise, sleeplessness, annoyance and mental depression. Prolonged therapy has reported to affect liver, eyes, and the immune system (Rajpal, 2005). The psoralen (92) treatment along with UVA can cumulatively cause extensive chromosome damage to mammalian cells and could lead to malignancy. A mixture of psoralen (92), isopsoralen (2), and imperatorin caused hypertrophy of liver, kidney, and spleen in rats at a daily dose of 2.5 mg/75 g for 60 days (Sharma, 2001). In a similar study, different concentrations of P. corylifolia extracts were administered to the rats for 90 days. The treatment decreased the body weight and gonad weight (testes and ovaries) thus indicating psoralen-induced reproductive toxicity (Takizawa et al., 2002). There are several reports on hepatotoxicity symptoms in mice and rats after long-term usage of psoralen or isopsoralen (Khushboo et al., 2010, Totonchy and Chiu, 2014, Wang et al., 2012). In an investigation, P. corylifolia and its natural compounds (bavachin, corylifol A, neobavaisoflavone, IBC, and BCN) were evaluated for their potential toxicity, and the results showed it had a potent inhibitory effect against human UDP‐glucuronosyltransferase 1A1 (UGT1A1) which is considered a stimulant for P. corylifolia related toxicity, including hepatic injury and raised bilirubin levels (Wang et al., 2015). In another study, P. corylifolia extract and fractionated compounds such as psoralen (92) and isopsoralen (2) were incubated with the recombinant CYP3A4 enzyme or differentiated HuH‐7 and HepaRG cells. P. corylifolia extract, psoralen, and isopsoralen caused the inhibition of concentration CYP3A4 activity in a dose dependent manner with different potency in vitro. It was also noted that none of the sample tested showed any toxicity (Liu and Flynn, 2015). Three cases of liver injury have been reported in Chinese population associated with consumption of P. corylifolia dried seeds (Fructus psoraleae) (Cheung et al., 2009). Pregnant women are discouraged from consuming P. corylifolia (Bensky et al., 2004, Chen et al., 2007). In a case study, a 44-year-old female ingested P. corylifolia seeds every 1 h for seven weeks for treating osteoporosis but, developed acute cholestatic hepatitis (Nam et al., 2005). In another case study, a 64-year-old female developed severe hepatotoxicity after administration for nine months of three kinds of herbal tablets and herbal tea for treating vitiligo. Tablets made from P. corylifolia leaves were identified as the most probable cause of the hepatotoxicity (Teschke and Bahre, 2009, Teschke et al., 2014). However, the underlying hepatotoxic mechanisms of P. corylifolia and its major components are not well elucidated. A text on traditional Chinese medicine reports that no adverse effects of Psoralea occur when a normal dose range (decoction of 4.5–9.0 g) is consumed (Bensky et al., 2004). In all the case-studies/experiments on psoralea induced toxicity one thing was found common and that is purposeful/deliberate and continuous/prolonged intake/application of a high dose. Going through the available literature we can conclude that there are mixed opinions about the Psoralea induced toxicity and therefore there is still a research gap with regard to its mode of action and its pharmaceutical standardization.
8. Conclusions
As already mentioned the genus Psoralea has immense potential to cure various diseases and the most important among these are psoriasis, leprosy and vitiligo. Although pre-clinical studies have shown promising results, further studies are required to explore the in-depth molecular mechanisms responsible for the afore-mentioned pharmacological activities and to test the efficacy of isolated compounds, in properly designed experiments. In addition, long term toxicity studies and data on interaction with other drugs are also required to establish the safety profile of extracts before the commencement of clinical trials.
The efficiency and efficacy of the bioactive compounds present in the Psoralea species has been evaluated from time to time. Now, since there is awareness complemented with scientific proof regarding the medicinal benefits, their mass cultivation/propagation and extraction of bioactive compounds should also be the objective of further research. The commercialization of pharmaceutical drugs containing Psoralea as a sole or a part of the ingredients shall bring relief to millions of people suffering from psoriasis, leprosy and vitiligo, in a natural way. Looking into the available literature on the genus Psoralea, including the discovery of the number of bioactive compounds from each species (as mentioned in Table 1) it is very clear that P. corylifolia is an important member of the genus from ethnobotanical, ethnopharmacological, biological, and phytochemical point of view.
Several bioactive compounds isolated from Psoralea are commercially available. The compounds such as Daidzin (CAS 552‐66‐9) is a potent inhibitor of human mitochondrial aldehyde dehydrogenase that indicates it chemopreventive property (Keung and Vallee, 1993); Angelicin (CAS 523‐50‐2) is an antifungal compound (Sardari et al., 2000); 8-Methoxypsoralen (CAS 298‐81‐7) has been known as a potent suicide inhibitor of cytochrome P‐450 (Tinel et al., 1987); Corylifol C and xanthoangelol are potent protein kinase inhibitors and induce apoptotic cell death therefore, possess anti-cancer property (Limper et al., 2013); Bakuchiol (CAS 10309‐37‐2) is a PTP1B and DNA polymerase inhibitor (Choi et al., 2015); Genistein (CAS 446‐72‐0) is a highly specific inhibitor of protein tyrosine kinase (Zarmouh et al., 2016); Psoralen has been used as photochemical probe in DNA mutation and repair studies (Gasparro, 1988); and Bavachin (CAS 19879‐32‐4) stimulates bone formation (anti-osteoporotic activity) (Wang et al., 2001, Xin et al., 2010). These examples indicate the utility of bioactive compounds isolated from Psoralea species.
Psoralea species are difficult to propagate because of poor seed-germination and high seedling-mortality (Mitter et al., 1993, Saxena et al., 1997, Siva et al., 2014). To ensure sustained production and benefits of Psoralea products we must aim at its mass cultivation through conventional approaches as well as micropropagation (Koul and Chase, 2015). Psoralea guenzii Harv. has become extinct while species like P. asarina (P.J.Bergius) T.M.Salter, P. fascicularis DC. and P. glaucina Harv. are vulnerable to extinction. Their bioactive principles and mode of action has not been explored yet. Their conservation would also ensure an alternative source of continuous and sustainable supply of elite bioactive compounds such as bakuchiol, psoralen, angelicin and psoralidin, for the pharmaceutical industry (Supplemental information 5, Table S4). Although, reproducible or rapid regeneration protocols for all the Psoralea species are not yet developed, but the same can ensure the enhancement of bioactive compound(s) production in near future, through suspension culture or transgenic technology (Arya and Gothalwal, 2015). These techniques may also streamline the path for further research on the bioactive principles and for the discovery of new compounds with novel properties in future. This is the first report wherein a detailed botanical description, traditional uses, phytochemistry and conservation of Psoralea species has been discussed. To conclude, Psoralea species have immense potential to act as panacea to several health-related maladies and so its conservation before excessive exploitation should be a prerequisite.
Acknowledgements
The authors are thankful to Lovely Professional University (LPU), Punjab, India for the infrastructural support. The authors are also thankful to the Center for Chromatography and Mass Spectrometry, Industrial University of Santander, Columbia for awarding the postdoctoral fellowship (Apoyo a estancias postdoctorales-UIS) to Arvind Kumar and to CSIR, New Delhi for awarding the research fellowship to Anil Kumar.
Acknowledgments
Ethical statement
There are no ethical issues related to this manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jep.2018.11.036
Appendix A. Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
References
- Abu Tahir M., Pramod K., Ansari S.H., Ali J. Current remedies for vitiligo. Autoimmun. Rev. 2010;9(7):516–520. doi: 10.1016/j.autrev.2010.02.013. [DOI] [PubMed] [Google Scholar]
- Agarwal D., Garg S.P., Sah P. Isolation of chalcones from the seeds of Psoralea corylifolia Linn. Indian J. Chem. 2006;45B:2574–2579. [Google Scholar]
- Agharkar S.P. Medicinal plants of Bombay presidency. India Sci. Publ. 1991:176–177. [Google Scholar]
- Alam F., Khan G.N., Hassan Bin Asad H.M. Psoralea corylifolia L: Ethnobotanical, biological, and chemical aspects: a review. Phytother. Res. 2017;2017:1–19. doi: 10.1002/ptr.6006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali J., Akhtar N., Sultana Y., Baboota S., Ahuja A. Antipsoriatic microemulsion gel formulations for topical drug delivery of babchi oil (Psoralea corylifolia) Methods Find. Exp. Clin. Pharmacol. 2008;30:277–285. doi: 10.1358/mf.2008.30.4.1185802. [DOI] [PubMed] [Google Scholar]
- Anderson T.F., Voorhees J.J. Psoralen photochemotherapy of cutaneous disorders. Annu. Rev. Pharmacol. Toxicol. 1980;22:235–257. doi: 10.1146/annurev.pa.20.040180.001315. [DOI] [PubMed] [Google Scholar]
- Anonymous . CSIR; New Delhi, India: 1969. The Wealth of India, A Dictionary of Indian Raw Materials and Industrial Products. [Google Scholar]
- Anwar M., Ahmad M., Mehjabeen, Jahan N., Mohiuddin O.A., Qureshi M. Phytopharmacological assessment of medicinal properties of Psoralea corylifolia. Afr. J. Pharm. Pharmacol. 2011;5:2627–2638. [Google Scholar]
- Arfaoui Y., Al-Ayed A.S., Said R.B., Hamed A.I. Experimental and density functional theory study of a new dimer with tetra-substituted cyclobutane ring system isolated from Psoralea plicata seeds. Int. J. Chem. 2013;5(4):73–79. [Google Scholar]
- Azzouzi S., Zaabat N., Medjroubi K., Akkal S., Benlabed K., Smati F., Franca M.G.D. Phytochemical and biological activities of Bituminaria bituminosa L (Fabaceae) Asian Pac. J. Trop. Med. 2014;1:481–484. doi: 10.1016/S1995-7645(14)60278-9. [DOI] [PubMed] [Google Scholar]
- Azzouzi S., Zaabat N., Medjroubi K., Akkal S., Benlabed K., Smati F., Franca M.G.D. Anti-oxidant potential of n-BuOH extract was evaluated through two methods, DPPH and cupric ion reducing anti-oxidant capacity assay. Asian Pac. J. Trop. Med. 2014;7:481–484. [Google Scholar]
- Backhouse C.N., Delporte C.L., Negrete R.E., Erazo S., Zuniga A., Pinto A., Cassels B.K. Active constituents isolated from Psoralea glandulosa L. with anti-inflammatory and anti-pyretic activities. J. Ethnopharmacol. 2001;78:27–31. doi: 10.1016/s0378-8741(01)00309-9. [DOI] [PubMed] [Google Scholar]
- Bajwa B.S., Pyare L.K., Seshadri T.R. A new chromenochalcone bavachromene from the seeds of Psoralea corylifolia. Curr. Sci. 1972;41(22):814–815. [Google Scholar]
- Bapat K., Chintalwar G.J., Pandey U., Thakur V.S., Sarma H.D., Samuel G., Pillai M.R.A., Chattopadhyay S., Venkatesh M. Preparation and in vitro evaluation of radio iodinated bakuchiol as an anti-tumor agent. Appl. Radiat. Isot. 2005;62:389–393. doi: 10.1016/j.apradiso.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Behloul N., Wu G. Genistein: a promising therapeutic agent for obesity and diabetes treatment. Eur. J. Pharmacol. 2013;698(1):31–38. doi: 10.1016/j.ejphar.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Bensky D., Clavey S., Stoger E. Chinese Herbal Medicine: Materia Medica. 3rd ed. Eastland Press; Seattle: 2004. [Google Scholar]
- Bera T.K., Ali K.M., Jana K., Ghosh A., Ghosh D. Protective effect of aqueous extract of seed of Psoralea corylifolia (Somraji) and seed of Trigonella foenum-graecum L (Methi) in streptozotocin-induced diabetic rat, comparative evaluation. Pharm. Res. 2013;5:277–285. doi: 10.4103/0974-8490.118840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoli A., Menichini F., Noccioli C., Morelli I., Pistelli L. Volatile constituents of different organs of Psoralea bituminosa L. Flav. Frag. J. 2004;19:166–171. [Google Scholar]
- Bhalla V.K., Nayak U.R., Dev S. Some new flavonoids from Psoralea corylifolia. Tetrahedron Lett. 1968;9:2401–2406. [Google Scholar]
- Bhattacharjee S.K. Pointer Publishers; Jaipur: 1998. Handbook of Medicinal Plants. [Google Scholar]
- Chopra B., Dhingra A.K., Dhar K.L. Antimicrobial activity of Psoralea corylifolia Linn. (Baguchi) seeds extracts by organic solvents and supercritical fluids. Int. J. Pharm. Clin. Res. 2013;5(1):13–16. [Google Scholar]
- Borate A., Udgire M., Khambhapati A. Anti-fungal activity associated with Psoralea corylifolia Linn (Bakuchi) seed and chemical profile crude methanol seed extract. Mintage J. Pharm. Med. Res. 2014;3(3):4–6. [Google Scholar]
- Borchardt J.R., Wyse D.L., Sheaffer C.C., Kauppi K.L., Fulcher R.G., Ehlke N.J., Bey R.F. Antioxidant and antimicrobial activity of seed from plants of the Mississippi river basin. J. Med. Plants Res. 2008;2(4):081–093. [Google Scholar]
- Bourgaud F., Poutaraud A., Guckert A. Extraction of coumarins from plant material. Phytochem. Anal. 2008;5:127–132. [Google Scholar]
- Bronikowska J., Szliszka E., Jaworska D., Czuba Z.P., Krol W. The coumarin psoralidin enhances anti-cancer effect of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) Molecules. 2012;17:6449–6464. doi: 10.3390/molecules17066449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceylan-Isik A.F., Fliethman R.M., Wold L.E., Ren J. Herbal and traditional Chinese medicine for the treatment of cardiovascular complications in diabetes mellitus. Curr. Diabetes Rev. 2008;4:320–328. doi: 10.2174/157339908786241142. [DOI] [PubMed] [Google Scholar]
- Chakrovarti K.K., Bose A.K., Siddiqui S. Chemical examination of the seeds of Psoralea corylifolia Linn (Babchi) J. Sci. Ind. Res. 1948;7B(2):24–26. [Google Scholar]
- Cheikh A., Samba M., Hadou A., Boumediana A.I., Kaihil A., Deida M.V., Essassi E.L.M., Dieng S., Minnih M.S. Ethnobotanic study phytochemical screening and anti-oxidant activity of Psoralea plicata (Cullen plicatum) Delile. J. Chem. Pharm. Res. 2015;7:1743–1747. [Google Scholar]
- Chen H., Luo J., Wang Q., Zhang D., Jiang R. Psoralen and isopsoralen’s cloud-point extraction from Psoralea corylifolia L. Sep. Sci. Technol. 2014;49:2169–2174. [Google Scholar]
- Chen J., Chen C., Lai R., Chen H., Kuo W., Liao T. New isoflavones and bioactive constituents from the fruits of Psoralea corylifolia. Planta Med. 2011;77:1339–1340. [Google Scholar]
- Chen J.K., Chen T.T. CA: Art of Medicine Press; 2004. Chinese Medical Herbology and Pharmacology. City of Industry. [Google Scholar]
- Chen X., Yang Y., Zhang Y. Isobavachalcone and bavachinin from Psoraleae Fructus modulate Aβ42 aggregation process through different mechanisms in vitro. FEBS Lett. 2013;587(18):2930–2935. doi: 10.1016/j.febslet.2013.07.037. [DOI] [PubMed] [Google Scholar]
- Chen Y., Kong L.D., Xia X., Kung H.F., Zhang L. Behavioral and biochemical studies of total furocoumarins from seeds of Psoralea corylifolia in the forced swimming test in mice. J. Ethnopharmacol. 2005;96:451–459. doi: 10.1016/j.jep.2004.09.033. [DOI] [PubMed] [Google Scholar]
- Chen Y., Wang H.D., Xia X., Kung H.F., Pan Y., Kong L.D. Behavioral and biochemical studies of total furocoumarins from seeds of Psoralea corylifolia in the chronic mild stress model of depression in mice. Phytomedicine. 2007;14(7–8):523–529. doi: 10.1016/j.phymed.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Chen Y.C., Cheung Y.T., Kong L.D., Ng T.B., Qiao C., Mo S.F., Xu H.X., Kung H.F. Transcriptional regulation of corticotrophin releasing factor gene by furocoumarins isolated from seeds of Psoralea corylifolia. Life Sci. 2008;82(21–22):1117–1121. doi: 10.1016/j.lfs.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Chen Z., Jin K., Gao L. Anti-tumor effects of bakuchiol an analogue of resveratrol on human lung adenocarcinoma A549 cell line. Eur. J. Pharmacol. 2010;643(2–3):170–179. doi: 10.1016/j.ejphar.2010.06.025. [DOI] [PubMed] [Google Scholar]
- Cheung W.I., Tse M.L., Ngan T., Lin J., Lee W.K., Poon W.T., Mak T.W., Leung V.K., Chau T.N. Liver injury associated with the use of Fructus psoraleae (Bol-gol-zhee or Bu-gu-zhi) and its related proprietary medicine. Clin. Toxicol. 2009;47:683–685. doi: 10.1080/15563650903059136. [DOI] [PubMed] [Google Scholar]
- Cho H., Jun J.Y., Song E.K., Kang K.H., Baek H.Y., Kd Y.S., Kim Y.C. Bakuchiol, A hepatoprotective compound of Psoralea corylifolia on tacrine-induced cytotoxicity in Hep G2 cells. Planta Med. 2001;67:84–749. doi: 10.1055/s-2001-18347. [DOI] [PubMed] [Google Scholar]
- Choi J.H., Rho M.C., Lee S.W., Choi J.N., Kim K., Song G.Y., Kim Y.K. Bavachin and isobavachalcone acyl-coenzyme A, cholesterol acyltransferase inhibitors from Psoralea corylifolia. Arch. Pharm. Res. 2008;31:1419–1423. doi: 10.1007/s12272-001-2126-x. [DOI] [PubMed] [Google Scholar]
- Choi Y.H., Yon G.H., Hong K.S., Yoo D.S., Choi C.W., Park W.K., Kong J.Y., Kim Y.S., Ryu S.Y. In vitro BACE-1 inhibitory phenolic components from the seeds of Psoralea corylifolia. Planta Med. 2008;74:1405–1408. doi: 10.1055/s-2008-1081301. [DOI] [PubMed] [Google Scholar]
- Chopra B., Dhingra A.K., Dhar K.L. Psoralea corylifolia L (Buguchi)-Folklore to modern evidence, Review. Fitoterapia. 2013;90:44–56. doi: 10.1016/j.fitote.2013.06.016. [DOI] [PubMed] [Google Scholar]
- Chopra R.N., Chatterjee N.R. Psoralea corylifolia (Babchi), its constituents their pharmacological action and therapeutic properties. Ind. J. Med. Res. 1927;15:49–55. [Google Scholar]
- Chopra R.N., Chopra I.C. Indigenous Drugs of India. second ed. Academic Publishers; Kolkata: 1958. [Google Scholar]
- Chopra R.N., Nayar S.L., Chopra I.C. Council of Scientific and Industrial Research; New Delhi: 1956. Glossary of Indian Medicinal Plants. [Google Scholar]
- Christensen S., Kavan A., Weber P.L. Identification and quanti-tation of genistein daidzein and related compounds in Psoralea esculenta (prairie Turnip) Anal. Chem. J. 2008;107:2–4. [Google Scholar]
- Cui Y., Taniguchi S., Kuroda T., Hatano T. Constituents of Psoralea corylifolia fruits and their effects on methicillin-resistant Staphylococcus aureus. Molecules. 2015;20(7):12500–12511. doi: 10.3390/molecules200712500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Z., Zhao C., Zhou K., Qu C.Q., Hu L.M. Effect of fructus Psoraleae on rats’ bile secretion and contractile activity guinea-pigs’ gall bladder smooth muscle. Strait Pharm. J. 2009;22(4):37–38. [Google Scholar]
- Damodaran N.P., Dev S. Meroterpenoids. III. Psoralea corylifolia Linn. 3. Synthesis of (±) – bakuchiol methyl ether. Tetrahedron. 1973;29:1209–1213. [Google Scholar]
- Deng S.G., Li A.Q., Ou R.M., Qu Y.Q. The anti-asthma effect of total-coumarines of fructus Psoraleae. Chin. J. Mod. Appl. Pharm. 2001;18(6):439–440. [Google Scholar]
- Dev S. Ancient-modern concordance in Ayurvedic plants, some examples. Environ. Health Perspect. 1999;107(10):783–789. doi: 10.1289/ehp.99107783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diggs, G.M., Lipscomb, B.L., O’Kennon, R.J., 1999. Shinners and Mahler’s illustrated flora of North Central Texas. Sida, Bot. Misc. No. 16. Botanical Research Institute of Texas, Fort Worth.
- Drury C.H. The Useful Plants of India. second ed. Willium H. Allen and Co.; London: 1873. [Google Scholar]
- Duke, J.A., 2009. Dr Duke’s phytochemical and ethnobotanical databases http//wwwars–gringov/duke.
- Dwarampudi L.P., Dhanabal S.P., Shanmugam Muruganantham N. Antipsoriatic activity and cytotoxicity of ethanolic extract of Psoralia corylifolia seeds. Hygeia J. D. Med. 2012;4(2):41–48. [Google Scholar]
- Dymock W., Warden C.J.H., Hooper D. Paul, K., Trench, Trubner & Co., Ltd.; London: 1893. Pharmacographia Indica, A History of the Principal Drugs of the Vegetable Origin. [Google Scholar]
- Farahani M.S., Bahramsoltani R., Farzaei M.H., Abdollahi M., Rahimi R. Plant‐derived natural medicines for the management of depression: an overview of mechanisms of action. Rev. Neurosci. 2015;26(3):305–321. doi: 10.1515/revneuro-2014-0058. [DOI] [PubMed] [Google Scholar]
- Ferrándiz M.L., Gil B., Sanz M.J., Úbeda A., Erazo S., González E., Negrete R., Pacheco S., Payá M., Alcaraz M.J. Effect of bakuchiol on leukocyte functions and some inflammatory responses in mic. J. Pharm. Pharmacol. 1996;48:975–980. doi: 10.1111/j.2042-7158.1996.tb06016.x. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick T.B., Pathak M.A. Basic considerations of the Psoralens: historical impact of methoxsalen and other furocoumarins. J. Investig. Dermatol. 1959;32:223–229. [PubMed] [Google Scholar]
- Fu J.X. Measurement of MEFV in 66 cases of asthma in the convalescent stage and after treatment with Chinese herbs. Zhong Xi Yi JieHe Za Zhi. 1989;9(11):658–659. [PubMed] [Google Scholar]
- Gaind K.N., Dar R.N., Chopra B.M., Kaul R.N. Anti-helmintic properties of the seeds of Psoralea corylifolia Linn. Ind. J. Pharmacol. 1965;27:198. [Google Scholar]
- Gasparro F.P. CRC Press, Taylor and Francis; 1988. Psoralen DNA Photobiology. [Google Scholar]
- Guo J., Weng X., Wu H., Li Q. Anti-oxidants from a Chinese medicinal herb-Psoralea corylifolia L. Food Chem. 2005;91:287–292. [Google Scholar]
- Guo J., Wu H., Weng X., Yan J., Bi K. Studies on extraction and isolation of active constituents from Psoralea corylifolia and the anti-tumor effect of the constituents in vitro. J. Chin. Med. Mat. 2003;26(3):185–187. [PubMed] [Google Scholar]
- Gupta A.K., Neeraj T., Madhu S. Vol. 3. ICMR; New Delhi: 2005. (Quality Standards of Indian Medicinal Plants). [Google Scholar]
- Gupta B.K., Dhar K.L., Atal C.K. Cyclodehydrogenation of psoralidin with dichlorodicyanobenzoquinone. Indian J. Chem. 1977;15B:657–658. [Google Scholar]
- Gupta B.K., Gupta G.K., Dhar K.L., Atal C.K. The essential oil from the seeds of P. corylifolia. Indian Perfum. 1979;23:174. [Google Scholar]
- Gupta B.K., Gupta G.K., Dhar K.L., Atal C.K. A C-formylated chalcone from P. corylifolia. Phytochemistry. 1980;19(9):2034–2035. [Google Scholar]
- Gupta M., Gupta A., Gupta S. Insecticidal activity of essential oils obtained from Piper nigrum and Psoralea corylifolia seeds against agricultural pests. Asian J. Res. Chem. 2013;6(4):360–363. [Google Scholar]
- Gupta M., Gupta A., Gupta S. Phytochemical analysis of methanol extracts of Psoralea corylifolia. Int. J. Indigenous Med. Plants. 2013;46:2051–4263. [Google Scholar]
- Hamed A.I., Springuel I., El-Emary N.A., Mitome H., Yamada Y. A phenolic cinnamate dimer from Psoralea plicata. Phytochemistry. 1997;45:1257–1261. [Google Scholar]
- Hamed A.I., Springuel I.V., El-Emary N.A. Benzofuran glycosides from Psoralea plicata seeds. Phytochemistry. 1999;50:887–890. [Google Scholar]
- Hao W., Zhang X., Zhao W., Chen X. Psoralidin induces autophagy through ROS generation which inhibits the proliferation of human lung cancer A549 cells. Peer J. 2014;2:e555. doi: 10.7717/peerj.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraguchi H., Inoue J., Tamura Y., Mizutani K. Anti-oxidative components of Psoralea corylifolia (Leguminosae) Phytother. Res. 2002;16:539–544. doi: 10.1002/ptr.972. [DOI] [PubMed] [Google Scholar]
- Harrington H.D. University of New Mexico Press; 1974. Edible Native Plants of the Rocky Mountains. [Google Scholar]
- Hedrick U.P. Dover Publications; 1972. Sturtevant's Edible Plants of the World. [Google Scholar]
- Hongfen, Li, 2007. Composition for treatment of asthma. US Patent number, 7255884 B2.
- Hsu Y.T., Wu C.J., Chen J.M., Yang Y.C., Wang S.Y. The presence of three isoflavonoid compounds in Psoralea corylifolia. J. Chromatogr. Sci. 2001;39(10):441–444. doi: 10.1093/chromsci/39.10.441. [DOI] [PubMed] [Google Scholar]
- Huang M.Y., Chen L., Li L., Jia X., Hong R. Synthesis of (±)-bakuchiol via a pot–economy approach. Chin. J. Chem. 2014;32(8):715–720. [Google Scholar]
- Hussain I., Hussain N., Manan A., Rashid A., Khan B., Bakhsh S. Fabrication of anti-vitiligo ointment containing Psoralea corylifolia: in vitro and in vivo characterization. Drug Des. Dev. Ther. 2016;10:3805–3816. doi: 10.2147/DDDT.S114328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley A. Grove's and Macmillan; 1992. The New RHS Dictionary of Gardening. [Google Scholar]
- Im A.R., Chae S.W., Zhang G.J., Lee M.Y. Neuroprotective effects of Psoralea corylifolia Linn seed extracts on mitochondrial dysfunction induced by 3-nitropropionic acid. BMC Comp. Alt. Med. 2014;14(1):370–378. doi: 10.1186/1472-6882-14-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innocenti G., Bourgaud F., Piovan A., Favretto D. Furocoumarins and other secondary metabolites from Psoralea canescens. Int. J. Pharm. 1997;35:232–236. [Google Scholar]
- Innocenti G., Piovan A., Filippini R., Caniato R., Cappelletti E.M. Quantitative recovery of furanocoumarins from Psoralea bituminosa. Phytochem. Anal. 1998;8:84–86. [Google Scholar]
- Iwamura J., Dohi T., Tanaka H., Odani T., Kubo M. cytotoxicity of corylifoliae fructus. II. cytotoxicity of bakuchiol and the analogues. Yakugaku zasshi. 1989;109(12):962–965. doi: 10.1248/yakushi1947.109.12_962. [DOI] [PubMed] [Google Scholar]
- Jan S., Talat P., Siddiqui T.O., Uzzafar M. Gamma radiation effects on growth and yield attributes of Psoralea corylifolia L. with reference to enhanced production of psoralen. J. Plant Growth Regul. 2011;64:163–171. [Google Scholar]
- Ji L., Xu Z. Review of constituents in fruits of Psoralea corylifolia L. Zhongguo Zhong Yao Za Zhi. 1995;20(2):120–128. [PubMed] [Google Scholar]
- Jiangning G., Xinchu W., Hou W., Qinghua L., Kaishun B. Anti-oxidants from a Chinese medicinal herb–Psoralia corylifolia L. Food Chem. 2005;91:287–292. [Google Scholar]
- Jiang Z., Xiong J. Induction of apoptosis in human hepatocarcinoma SMMC-7721 cells in vitro by psoralen from Psoralea corylifolia. Cell Biochem. Biophys. 2014;70(2):1075–1081. doi: 10.1007/s12013-014-0025-2. [DOI] [PubMed] [Google Scholar]
- Jois, H.S., Manjunath, B.L., 1934. Isopsoralen from the seeds of Psoralea corylifolia L. In: Proceedings of the Indian Science Congress, 21(111), p. 243.
- Jois H.S., Manjunath B.L., Venkatarao S. Chemical examination of the seeds of Psoralea corylifolia. J. Indian Chem. Soc. 1933;10:41–46. [Google Scholar]
- Joshi, S.G., 2000. New Delhi, Oxford and IBH Publishing Co Pvt Ltd; Medicinal Plants, pp. 206–207.
- Kamboj J., Sharma S., Kumar S. In vivo anti-diabetic and anti-oxidant potential of Psoralea corylifolia seeds in Streptozotocin induced type-2 diabetic rats. J. Health Sci. 2011;57:225–235. [Google Scholar]
- Kapoor L.D., Boca R. CRC Press; Florida: 2001. Handbook of Ayurvedic Medicinal Plants; pp. 274–275. [Google Scholar]
- Katsura H., Tsukiyama R.‐I., Suzuki A., Kobayashi M. In vitro antimicrobial activities of bakuchiol against oral microorganisms. Antimicrob. Agents Chemother. 2001;45(11):3009–3013. doi: 10.1128/AAC.45.11.3009-3013.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawl R. Kinetics of the anti-staphylococcal activity of bakuchiol in vitro. GMBH. 1976;45:2401–2403. [PubMed] [Google Scholar]
- Kaye B., Moodie D.W. The Psoralea food resource of northern plains. Plains Anthropol. 1978;23:329–336. [Google Scholar]
- Keung W.M., Vallee B.L. Daidzin: a potent, selective inhibitor of human mitochondrial aldehyde dehydrogenase. PNAS. 1993;90(4):1247–1251. doi: 10.1073/pnas.90.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S., Iqbal T., Ahmed N., Jamil A. Anti-oxidant, hemolytic and mutagenic potential of Psoralea corylifolia. J. Anim. Plant Sci. 2015;25(5):1451–1456. [Google Scholar]
- Khare C.P. Springer-Verlag; New York: 2004. Encyclopedia of Indian Medicinal Plants. [Google Scholar]
- Khatune N.A., Islam M.E., Haque M.E., Khondkar P., Rahman M.M. Anti-bacterial compounds from the seeds of Psoralea corylifolia. Fitoterapia. 2004;75:228–230. doi: 10.1016/j.fitote.2003.12.018. [DOI] [PubMed] [Google Scholar]
- Khatune N.A., Islam M.E., Rahman M.A.A., Baki M.A., Gadik G., Haque M.E. Pesticidal activity of a novel coumestan derivative isolated from Psoralea corylifolia L. against Tribolium casteneum Herbst adults and larvae (Coleoptera, Tenebrionidae) Pak. J. Agron. 2002;(4):112–115. [Google Scholar]
- Khushboo P.S., Jadhav V.M., Kadam V.J., Sathe N.S. Psoralea corylfolia Linn-Khushtanashini. Pharm. Rev. 2010;4(7):69–76. doi: 10.4103/0973-7847.65331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K.A., Shim S.H., Ahn H.R., Jung S.H. Protective effects of the compounds isolated from the seed of Psoralea corylifolia on oxidative stress-induced retinal damage. Toxicol. Appl. Pharmacol. 2013;269(2):109–120. doi: 10.1016/j.taap.2013.03.017. [DOI] [PubMed] [Google Scholar]
- Kim D.W., Seo K.H., Curtis‐Long M.J., Oh K.Y., Oh J.‐W., Cho J.K., Park K.H. Phenolic phytochemical displaying SARS‐CoV papain‐like protease inhibition from the seeds of Psoralea corylifolia. J. Enzym. Inhib. Med. Chem. 2014;29(1):59–63. doi: 10.3109/14756366.2012.753591. [DOI] [PubMed] [Google Scholar]
- Kim S.J., Oh H.C., Kim Y.C., Jeong G.C., Lee S. Selective inhibition of bakuchicin isolated from Psoralea corylifolia on CYP1A in human liver microsomes. Evid. Based Complement. Altern. Med. 2016 doi: 10.1155/2016/5198743. (Article ID 5198743) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiran B., Lalitha V., Raveesha K.A. Anti-fungal activity of aqueous and solvent extracts of seeds of Psoralea corylifolia L. against seed borne fungi of maize. Int. J. Pharm. Life Sci. 2011;2(10):1133–1136. [Google Scholar]
- Kittler L., Hradecna Z., Suhnel J. Cross-link formation of phage lambda DNA in situ photochemically induced by the furocoumarin derivative angelicin. BBA. 1980;607:215–220. doi: 10.1016/0005-2787(80)90074-x. [DOI] [PubMed] [Google Scholar]
- Kolasani A., Xu H., Millikan M. Determination and comparison of mineral elements in traditional chienese herbal formulae at different decoction times used to improve kidney function -chemometric approach. Afr. J. Tradit. Complement. Altern. Med. 2011;8(S):191–197. doi: 10.4314/ajtcam.v8i5S.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo Y., Kato A., Kubota Y., Nozoe S. Bakuchicin a new simple furanocoumarin from Psoralea corylifolia. Heterocycles. 1990;31:187–190. [Google Scholar]
- Koul B., Chase N. Moringa oleifera Lam, panacea to several maladies. J. Chem. Pharm. Res. 2015;7(6):687–707. [Google Scholar]
- Krishnamurthi, A.K., Manjunath, B.L., Sastri, B.N., Deshaprabhu, S.B., Chadha, Y.R., New Delhi, CSIR; 1969. The Wealth of India, Raw Materials, vol. 7, pp. 295–298.
- Labbe C., Faini F., Coll J., Connolly J.D. Bakuchiol derivatives from the leaves of Psoralea glandulosa. Phytochemistry. 1996;42(5):1299–1303. [Google Scholar]
- Lau K.M., Fu L.H., Cheng L., Wong C.W., Wong Y.L., Lau C.P., Han S.Q., Chan P.K., Fung K.P., Lau C.B., Hui M., Leung P.C., Leung P.C. Two antifungal components isolated from Fructus psoraleae and Folium eucalypti globuli by bioassay‐guided purification. Am. J. Chin. Med. 2010;38(05):1005–1014. doi: 10.1142/S0192415X10008421. [DOI] [PubMed] [Google Scholar]
- Lau K.M., Wong J.H., Wu Y.O., Cheng L., Wong C.W., To M.H., Lau C.P., Yew D.T., Leung P.C., Fung K.P., Hui M., Ng T.B., Lau C.B. Anti‐dermatophytic activity of bakuchiol: In vitro mechanistic studies and in vivo Tinea pedis‐inhibiting activity in a guinea pig model. Phytomedicine. 2014;21(7):942–945. doi: 10.1016/j.phymed.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Latha P.G., Evans D.A., Panikkar K.R., Jayavardhanan K.K. Immunomodulatory and anti-tumour properties of Psoralea corylifolia seeds. Fitoterapia. 2000;71(3):223–231. doi: 10.1016/s0367-326x(99)00151-3. [DOI] [PubMed] [Google Scholar]
- Lee G.J., Cho I.A., Kang K.R., Kim D.K., Sohn H.M., You J.W., Oh J.S., Seo Y.S., Yu S.J., You J.S., Kim C.S., Kim S.G., Im H.J., Kim J.S. Biological effects of the herbal plant-derived phytoestrogen bavachin in primary rat chondrocytes. Biol. Pharm. Bull. 2015;38(8):1199–1207. doi: 10.1248/bpb.b15-00198. [DOI] [PubMed] [Google Scholar]
- Lee S.W., Yun B.R., Kim M.H., Park C.S., Lee W.S., Oh H.M., Rho M.C. Phenolic compounds isolated from Psoralea corylifolia inhibits IL-6-induced STAT3 activation. Planta Med. 2012;78(9):903–906. doi: 10.1055/s-0031-1298482. [DOI] [PubMed] [Google Scholar]
- Li W.D., Yan C.P., Wu Y., Weng Z.B., Yin F.Z., Yang G.M., Cai B.C., Chen Z.P. Osteoblasts proliferation and differentiation stimulating activities of the main components of Fructus Psoraleae corylifoliae. Phytomedicine. 2014;21(4):400–405. doi: 10.1016/j.phymed.2013.09.015. [DOI] [PubMed] [Google Scholar]
- Li Y.G., Hou J., Li S., Lv X., Ning J., Wang P., Liu Z.M., Guang-Bo G., Jia-Yan R., Ling Y. Fructus Psoraleae contains natural compounds with potent inhibitory effects towards human carboxylesterase 2. Fitoterapia. 2015;101:99–106. doi: 10.1016/j.fitote.2015.01.004. [DOI] [PubMed] [Google Scholar]
- Li L., Liu C.C., Chen X., Xu S., Hernandez Cortes-Manno S., Cheng S.H. Mechanistic study of bakuchiol-induced anti-breast cancer stem cell and in vivo anti-metastasis effects. Front. Pharmacol. 2017;8:1–10. doi: 10.3389/fphar.2017.00746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S.H., Ha T.Y., Kim S.R., Ahn J., Park H.J., Kim S. Ethanol extract of Psoralea corylifolia L. and its main constituent bakuchiol reduce bone loss in ovariectomised Sprague-Dawley rats. Br. J. Nutr. 2009;101(7):1031–1039. doi: 10.1017/S0007114508066750. [DOI] [PubMed] [Google Scholar]
- Lim S.H., Ha T.Y., Ahn J., Kim S. Estrogenic activities of Psoralea corylifolia L. seed extracts and main constituents. Phytomedicine. 2011;18:425–430. doi: 10.1016/j.phymed.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Limper C., Wang Y., Ruhl S., Wang Z., Lou Y., Totzke F., Kubbutat M.H.G., Chovolou Y., Proksch P., Wätjen W. Compounds isolated from Psoralea corylifolia seeds inhibit protein kinase activity and induce apoptotic cell death in mammalian cells. J. Pharm. Pharmacol. 2013;65(9):1393–13408. doi: 10.1111/jphp.12107. [DOI] [PubMed] [Google Scholar]
- Lin C.F., Huang Y.L., Chiem M.Y., Sheu S.J., Chen C.C. Analysis of bakuchiol psoralen and angelicin in crude drugs and commercial concentrated products of Fructus Psoraleae. J. Food Drug Anal. 2007;15:433–437. [Google Scholar]
- Lin Y.L., Kuo Y.H. Two new benzofuran derivatives corylifonol and isocorylifonol from the seeds of Psoralea corylifolia. Heterocycles. 1992;34:1555–1564. [Google Scholar]
- Liu R., Aifeng L.I., Sun A., Kong L. Preparative isolation and purification from Psoralea corylifolia by high speed counter current chromatography. J. Chromatogr. 2004;1057:225–228. doi: 10.1016/j.chroma.2004.09.049. [DOI] [PubMed] [Google Scholar]
- Liu X., Nam J.W., Song Y.S., Viswanath A.N.I., Pae A.N., Kil Y.S., Kim H.D., Park J.H., Seo E.K., Chang M. Psoralidin, a coumestan analogue, as a novel potent estrogen receptor signaling molecule isolated from Psoralea corylifolia. Bioorg. Med. Chem. Lett. 2014;24(5):1403–1406. doi: 10.1016/j.bmcl.2014.01.029. [DOI] [PubMed] [Google Scholar]
- Liu Y., Flynn T.J. CYP3A4 inhibition by Psoralea corylifolia and its major components in human recombinant enzyme differentiated human hepatoma HuH‐7 and HepaRG cells. Toxicol. Rep. 2015;2:530–534. doi: 10.1016/j.toxrep.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Chen X., Liu C.C., Lee L.S., Man C., Cheng S.H. Phytoestrogen bakuchiol exhibits in vitro and in vivo anti‐breast cancer effects by inducing S phase arrest and apoptosis. Front. Pharmacol. 2016:7. doi: 10.3389/fphar.2016.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrid A., Espinoza L., Gonzalez C., Mellado M., Joan V., Santander R., Silva V., Montenegro I. Anti-fungal study of the resinous exudates and of meroterpenoids isolated from Psoralea glandulosa (Fabaceae) J. Ethnopharmacol. 2012;144(3):809–811. doi: 10.1016/j.jep.2012.10.027. [DOI] [PubMed] [Google Scholar]
- Madrid A., Jara C., Espinoza L., Santander R., Mellado M., González C., Villena J., Montenegro I. Study of the chemical composition of the resinous exudate isolated from Psoralea glandulosa and evaluation of the antioxidant properties of the terpenoids and the resin. B. Latinoam. Caribe Pl. 2013;12(4):338–345. [Google Scholar]
- Madrid Villegas A., Díaz Peralta K., González Tapia C., Catalán Marín K., Espinoza Catalán L. Antiphytopathogenic activity of Psoralea glandulosa (Fabaceae) against Botrytis cinerea and Phytophthora cinnamomi. Nat. Product. Res. 2015;29(6):586–588. doi: 10.1080/14786419.2014.955486. [DOI] [PubMed] [Google Scholar]
- Madrid A., Cardile V., González C., Montenegro I., Villena J., Caggia S., Graziano A., Russo A. Psoralea glandulosa as a potential source of anticancer agents for melanoma treatment. Int. J. Mol. Sci. 2015;16:7944–7959. doi: 10.3390/ijms16047944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manohar B., Udaya Sankar K. Extraction modelling and characterization of bioactive components from Psoralea corylifolia L. obtained by supercritical carbon dioxide. J. Food Process Technol. 2012;3(2):1–8. [Google Scholar]
- Mar W., Je K.H., Seo E.K. Cytotoxic constituents of Psoralea corylifolia. Arch. Pharm. Res. 2001;24:211–213. doi: 10.1007/BF02978259. [DOI] [PubMed] [Google Scholar]
- Marwaha T.K. Formulation design and evaluation of herbal anti-psoriatic emulgel. J. Pharm. Sci. Innov. 2013;2(3):30–42. [Google Scholar]
- Matsuda H., Sugimoto S., Morikawa T., Matsuhira K., Mizuguchi E., Nakamura S., Yoshikawa M. Bioactive constituents from Chinese natural medicines XX Inhibitors of antigen-induced degranulation in RBL–2H3 cells from the seeds of Psoralea corylifolia. Chem. Pharm. Bull. 2007;55(1):106–110. doi: 10.1248/cpb.55.106. [DOI] [PubMed] [Google Scholar]
- Mehta G., Naik U.R., Dev S. Meroterpenoids-1 Psoralea corylifolia Linn-1 Bakuchiol a novel monoterpene phenol. Tetrahedron. 1973;29:1119–1125. [Google Scholar]
- Menon S.R., Patel V.K., Mitscher L.A., Shih P., Pillai S.P., Shankel D.M. Structure-anti-mutagenic activity relationship study of plicatin B. J. Nat. Prod. 1999;62(1):102–106. doi: 10.1021/np980304n. [DOI] [PubMed] [Google Scholar]
- Misra P., Pandey A., Tewari S.K., Nath P., Trivedi P.K. Characterization of isoflavone synthase gene from Psoralea corylifolia, a medicinal plant. Plant Cell Rep. 2010;29:747–755. doi: 10.1007/s00299-010-0861-5. [DOI] [PubMed] [Google Scholar]
- Mitter V., Srinivasan K., Singh B.M. Overcoming hard seededness on Psoralea corylifolia. Seed Res. 1993;21(1):31–34. [Google Scholar]
- Moerman D. Timber Press; Oregon: 1998. Native American Ethnobotany. [Google Scholar]
- Nadkarni, K.M., 1976. Mumbai, Popular Prakashan Pvt. Ltd. Indian Materia Medica, pp. 1019–1022.
- Nam S.W., Baek J.T., Lee D.S., Kang S.B., Ahn B.M., Chung K.W. A case of acute cholestatic hepatitis associated with the seeds of Psoralea corylifolia (Boh-Gol-Zhee) Clin. Toxicol. 2005;43:589–591. doi: 10.1081/clt-200068863. [DOI] [PubMed] [Google Scholar]
- Newton S.M., Lau C., Gurcha S.S., Besra G.S., Wright C.W. The evaluation of forty-three plant species for in vitro antimycobacterial activities; isolation of active constituents from Psoralea corylifolia and Sanguinaria canadensis. J. Ethnopharmacol. 2002;79(1):57–67. doi: 10.1016/s0378-8741(01)00350-6. [DOI] [PubMed] [Google Scholar]
- Nordin M.F., Razak F.A., Himratul-Aznita W.H. Assessment of antifungal activity of bakuchiol on oral-associated Candida spp. Evid. Based Complement Altern. Med. 2015:1–7. doi: 10.1155/2015/918624. (Article ID 918624) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudhiya P. Traditional medicinal knowledge about herb Bemchi (Psoralea corylifolia) in Chhatisgarh. India Pharm. 2001;61(7):312–332. [Google Scholar]
- Panda H. National Institute of Industrial Research; New Delhi: 2000. Herbs Cultivation and Medicinal Uses; pp. 479–481. [Google Scholar]
- Pandey A., Niranjan A., Misra P., Lehri A., Tewari S.K., Trivedi P.K. Simultaneous separation and quanti-fication of targeted group of compounds in Psoralea corylifolia L. using HPLC-PDA-MS-MS. J. Liq. Chromatogr. 2012;35:2567–2583. [Google Scholar]
- Park J., Kim D.H., Ahn H.N., Song Y.S., Lee Y.J., Ryu J.H. Activation of estrogen receptor by bavachin from Psoralea corylifolia. Biomol. Ther. 2012;20(2):183–188. doi: 10.4062/biomolther.2012.20.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park G.H., Sung J.H., Song H.M., Jeong J.B. Anti‐cancer activity of Psoralea fructus through the downregulation of cyclin D1 and CDK4 in human colorectal cancer cells. BMC Complement. Altern. Med. 2016;16(1):373. doi: 10.1186/s12906-016-1364-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pae H.O., Cho H., Oh G.S., Kim N.Y., Song E.K., Kim Y.C., Yun Y.G., Kang C.L., Kim J.D., Kim J.M., Chung H.T. Bakuchiol from Psoralea corylifolia inhibits the expression of inducible nitric oxide synthase gene via the inactivation of nuclear transcription factor- κB in RAW264.7 macrophages. Int. Immunopharmacol. 2001;1:1849–1855. doi: 10.1016/s1567-5769(01)00110-2. [DOI] [PubMed] [Google Scholar]
- Pazos-Navarro M., Del Río J.A., Ortuño A., Romero-Espinar P., Correal E., Dabauza M. Micropropagation from apical and nodal segments of Bituminaria bituminosa and the furanocoumarin content of propagated plants. J. Hort. Sci. Biotechnol. 2011;87:29–35. [Google Scholar]
- Peng G., Wu P., Li H., Chen D. Neo-psoralen isolated from Psoralea corylifolia Linn. Nat. Prod. Res. Dev. 1995;8:31–34. [Google Scholar]
- Perara, G., Reese, R.N., 2002. Nutritional value of Psoralea esculenta. Proceedings South Dakota Acad. Sci., 81, p. 271.
- Pistelli L., Noccioli C., Appendino G., Bianchi F., Sterner O., Ballero M. Pterocarpans from Bituminaria morisiana and Bituminaria bituminosa. Phytochemistry. 2003;64:595–598. doi: 10.1016/s0031-9422(03)00190-0. [DOI] [PubMed] [Google Scholar]
- Prakasarao A.S.C., Bhalla V.K., Nayak U.R., Dev S. Meroterpenoids II Psoralea corylifolia Linn 2 Absolute configuration of + (–) bakuchiol. Tetrahedron. 1973;29:1127–1130. [Google Scholar]
- Prasad N.R., Anandi C., Balasubramanian S., Pugalend K.V. Anti-dermatophytic activity of extracts from Psoralea corylifolia (Fabaceae) correlated with the presence of a flavonoid compound. J. Ethnopharmacol. 2004;91:21–24. doi: 10.1016/j.jep.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Qamaruddin A., Parveen N., Khan N.U., Singhal K.C. Potential anti-filarial activity of the leaves and seed extracts of Psoralea corylifolia on cattle filarial parasite Setaria cervi. J. Ethnopharmacol. 2002;82:23–28. doi: 10.1016/s0378-8741(02)00141-1. [DOI] [PubMed] [Google Scholar]
- Qiao C.F., Han Q.B., Mo S.F., Song J.Z., Xu L.J., Chen S.L., Yang D.J., Kong L.D., Kung H.F., Xu H.X. Psoralenoside and isopsoralenoside two new benzofuran glycosides from Psoralea corylifolia. Chem. Pharm. Bull. 2006;54:714–716. doi: 10.1248/cpb.54.714. [DOI] [PubMed] [Google Scholar]
- Qiao C.F., Han Q.B., Song J.Z., Mo S.F., Kong L.D., Kung H.F. Chemical fingerprint and quanti-tative analysis of Fructus psoraleae by high-performance liquid chromatography. J. Sep. Sci. 2007;30:813–818. doi: 10.1002/jssc.200600339. [DOI] [PubMed] [Google Scholar]
- Qin Y.H., Cui Y., Ren Y.X., Zhang X.L., Wang Y., Zheng X.Y., Chen X.W., Sun M. Immunological regulation and treatment of Brucea javanica and Fructus psoraleae on rats with Pneumocystis carinii pneumonia. Chin. J. Parasitol. Parasit. Dis. 2006;24(1):59–62. [PubMed] [Google Scholar]
- Rajpal V. Vol. 2. Eastern Publishers; New Delhi: 2005. pp. 284–295. (Standardization of Botanicals). [Google Scholar]
- Rajput S.J., Vijaya Z., Pallavi R. Studies on extraction isolation and estimation of psoralen from the fruits of Psoralea corylifolia. Pharmacol. Mag. 2008;4(13):52–56. [Google Scholar]
- Rangari V.D., Agrawal S.R. Chemistry and pharmacology of Psoralea corylifolia. Indian Drugs. 1992;29:662–670. [Google Scholar]
- Rashid A., Agarwala S.C. Mode of action of psoralen in pigment production III photooxidation of dihydroxydiphenylalanine in the presence of psoralen. Ind. J. Biochem. 1965;2:271. [PubMed] [Google Scholar]
- Rasool N., Khan A., Malik A. Plicatin A and B two phenolic cinnamates from Psoralea plicata. Phytochemistry. 1990;29:3979–3981. [Google Scholar]
- Rasool N., Khan A.Q., Ahmad V.U., Malik A. A benzoquinone and a coumestan from Psoralea plicata. Phytochemistry. 1991;30(8):2800–2803. [Google Scholar]
- Rastogi R.P., Mehrotra B.N. Vol. 5. CDRI; Lucknow and NISCIR, New Delhi: 1998. pp. 703–704. (Compendium of Indian Medicinal Plants). [Google Scholar]
- Rastogi R.P., Mehrotra B.N. Vol. 2. CDRI; Lucknow and NISCIR, New Delhi: 1999. pp. 567–568. (Compendium of Indian Medicinal Plants). [Google Scholar]
- Rastogi R.P., Mehrotra B.N. Vol. 3. CDRI; Lucknow and NISCIR, New Delhi: 2001. pp. 535–536. (Compendium of Indian Medicinal Plants). [Google Scholar]
- Rastogi R.P., Mehrotra B.N. Vol. 1. CDRI; Lucknow and NISCIR, New Delhi: 2004. pp. 332–333. (Compendium of Indian Medicinal Plants). [Google Scholar]
- Ruan B., Kong L.Y., Takaya Y., Niwa M.J. Studies on the chemical constituents of Psoralea corylifolia L. J. Asian Nat. Prod. Res. 2007;9(1):41–44. doi: 10.1080/10286020500289618. [DOI] [PubMed] [Google Scholar]
- Ryu S.Y., Choi S.U., Lee C.O., Zee O.P. Anti-tumor activity of Psoralea corylifolia. Arch. Pharm. Res. 1992;15(4):356–359. [Google Scholar]
- Sah P., Agrawal D., Garg S.P. Isolation and identification of furocoumarins from the seeds of Psoralea corylifolia L. Indian J. Pharma. Sci. 2006;68 (768–671) [Google Scholar]
- Saif A., Arshad J., Inayat S.F., Tanweer A., Mather A.K., Kamaluddin, Malik Z.A. Estimation of psoralen content by HPLC method in different organs of Psoralea corylifolia L. as influenced by sulphur and nitrogen nutrition. J. Med. Arom. Pl. Sci. 2007;29:197–203. [Google Scholar]
- Sardari S., Mori Y., Horita K., Micetich R.J., Nishibe S., Daneshtalab M. Synthesis and anti-fungal activity of coumarins and angular furanocoumarins. Bioorg. Med. Chem. 1999;7:1933–1940. doi: 10.1016/s0968-0896(99)00138-8. [DOI] [PubMed] [Google Scholar]
- Sardari S., Amin G., Sekhon A., Micetich R., Daneshtalab M. Antifungal activity of Diplotaenia damavandica. Pharm. Pharmacol. Commun. 2000;6(10):455–458. [Google Scholar]
- Satyavati G.V., Gupta A.K., Tandon N. Vol. 2. Indian Council of Medical Research; New Delhi: 1987. (Medicinal Plants of India). [Google Scholar]
- Saunders C.F. Dover Publications; USA: 2011. Edible and Useful Wild Plants of the United States and Canada. [Google Scholar]
- Saxena C., Palai S.K., Samantaray S., Rout G.R., Das P. Plant regeneration from callus cultures of Psoralea corylifolia Linn. J. Plant Growth Regul. 1997;22:13–17. [Google Scholar]
- Sebastian P. Elsevier Health Sciences; New York: 2006. Ayurvedic Medicine: The Principles of Traditional Practice, Part 2. [Google Scholar]
- Sehrawat N., Yadav M., Jaiwal P.K. Development of an efficient in vitro regeneration protocol for rapid multiplication and genetic improvement of an important endangered medicinal plant Psoralea corylifolia. Asian J. Plant Sci. Res. 2013;3:88–94. [Google Scholar]
- Seo E., Oh Y.S., Kim D., Lee M.Y., Chae S., Jun H.S. Protective role of Psoralea corylifolia L. seed extract against hepatic mitochondrial dysfunction induced by oxidative stress or aging. Evid. Based Complement. Altern. Med. 2013;2013:1–9. doi: 10.1155/2013/678028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshadri, T.R., Venkata, R.C., 1937. A new separation of the components of P. corylifolia L. In: Proceedings Indi. Acad. Sci. Sec A. Section A 5, p. 351.
- Shah C.C., Bhalla V.K., Dev S. Meroterpenoids-V Psoralea corylifolia Linn. 4 2 3- Epoxybakuchiol A1 3-hydroxybakuchiol and A3 2-hydroxybakuchiol. J. Indian Chem. Soc. 1997;74:970–973. [Google Scholar]
- Shan L., Yang S., Zhang G., Zhou D., Qiu Z., Tian L., Yuan H., Feng Y., Shi X. Comparison of the inhibitory potential of bavachalcone and corylin against UDP‐Glucuronosyltransferases. Evid. Based Complement. Altern. Med. 2014:6. doi: 10.1155/2014/958937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shailajan S., Menon S., Singh A., Mhatre M., Sayed N., Joshi H., Tiwari B. Estimation of psoralen from herbal formulations containing Psoralea corylifolia using the RP-HPLC-DAD method and its application to a pharmacokinetic study. Int. J. Green. Pharm. 2012;6(3):217–223. [Google Scholar]
- Sharma M., Tiwari M. A study of dermatophytes in dogs and in vitro response of Psoralea corylifolia essential oil. J. Essent. Oil Bear Plants. 2012;15:445–453. [Google Scholar]
- Sharma P.C., Yelne M.B., Dennis T.J. Vol. 2. Central Council for Research in Ayurveda and Siddha; New Delhi: 2001. pp. 89–93. (Database on Medicinal Plants Used in Ayurveda). [Google Scholar]
- Sheng Y., Fan C.Q., Wang Y., Dong L., Yue J.M. Anti-bacterial prenylflavone derivatives from Psoralea corylifolia and their structure activity relationship study. Bioorg. Med. Chem. 2004;12:4387–4392. doi: 10.1016/j.bmc.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Shilandra K.U., Yadav A.S., Sharma A.K., Rai A.K., Raghuwanshi D.K., Badkhane Y. The botany chemistry pharmacological and therapeutic application of Psoralea corylifolia Linn. A review. Int. J. Phytomed. 2010;2(2):100–107. [Google Scholar]
- Shim S.H., Kim J.C., Jang K.S., Choi G.J. Anti-oomycete activity of furanocoumarins from seeds of Psoralea corylifolia against Phytophthora infestans. Plant Pathol. J. 2009;25(1):103–107. [Google Scholar]
- Shirwaikar A., Khan S., Kamariya Y.H., Patel B.D., Gajera F.P. Medicinal plants for the management of post-menopausal osteoporosis: A review. Open Bone J. 2010;2:1–13. [Google Scholar]
- Shoji M., Arakaki Y., Esumi T., Kohnomi S., Yamamoto C., Suzuki Y., Takahashi E., Konishi S., Kido H., Kuzuhara T. Bakuchiol is a phenolic isoprenoid with novel enantiomer-selective anti-influenza A virus activity involving Nrf2 activation. J. Biol. Chem. 2015;290(46):28001–28017. doi: 10.1074/jbc.M115.669465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu Y. In vitro micropropagation of medicinal plants by tissue culture. Plymouth Stud. Sci. 2010;4(1):432–449. [Google Scholar]
- Siva G., Sivakumar S., Premkumar G., Baskaran P., Senthilkumar T., Jayabalan N. Enhanced seed germination of Psoralea corylifolia L. by heat treatment. World J. Agric. Res. 2014;2(4):151–154. [Google Scholar]
- Siva G., Sivakumar S., Kumar G.P., Vigneswaran M., Vinoth S., Selvan A.M., Parveez Ahamed A., Manivannan K., Rajesh Kumar R., Thajuddin N., Senthil Kumar T., Jayabalana N. Optimization of elicitation condition with jasmonic acid, characterization and antimicrobial activity of psoralen from direct regenerated plants of Psoralea corylifolia L. Biocatal. Agric. Biotechnol. 2015;4(4):624–631. [Google Scholar]
- Somani G., Kulkarni C., Shinde P., Shelke R., Laddha K., Sathaye S. In vitro acetylcholinesterase inhibition by psoralen using molecular docking and enzymatic studies. J. Pharm. Bioallied Sci. 2015;7(1):32. doi: 10.4103/0975-7406.148775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song A., Zhang J., Lebrilla C.B., Lam K.S. Solid-phase synthesis and spectral properties of 2-alkylthio-6H-pyrano[2,3-f]benzimidazole-6-ones: a combinatorial approach for 2-alkylthioimidazocoumarins. J. Comb. Chem. 2004;6(4):604–610. doi: 10.1021/cc049955u. [DOI] [PubMed] [Google Scholar]
- Song P., Yang X.Z., Yuan J.Q. Cytotoxic constituents from Psoralea corylifolia. J. Asian Nat. Prod. Res. 2013;15:624–630. doi: 10.1080/10286020.2013.793181. [DOI] [PubMed] [Google Scholar]
- Song K., Ling F., Huang A., Dong W., Liu G., Jiang C., Zhang Q., Wang G. In vitro and in vivo assessment of the effect of antiprotozoal compounds isolated from Psoralea corylifolia against Ichthyophthirius multifiliis in fish. Int. J. Parasitol. Drugs Drug Resist. 2015;5(2):58–64. doi: 10.1016/j.ijpddr.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S., Sarada D.V. Antifungal activity of phenyl derivative of pyranocoumarin from Psoralea corylifolia L. seeds by inhibition of acetylation activity of trichothecene 3-O-acetyltransferase. J. Biomed. Biotechnol. 2012 doi: 10.1155/2012/310850. (Article ID 310850) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahnke A., Hayes M., Meyer K., Witt K., Weideman J., Fernando A.P., Burrows R., Reese R.N. Prairie turnip Pediomelum esculentum (Pursh) Rydb, historical and modern use propagation and management of a new crop. Nat. Plant. J. 2008;9:46–58. [Google Scholar]
- Steven M.C., Russell J.M. Bioactive Natural Products, Detection Isolation and Structural Determination. Second ed. CRC Press; USA: 2007. [Google Scholar]
- Sun D.X., Ge G.B., Dong P.P., Cao Y.F., Fu Z.W., Ran R.X., Wu X., Zhang Y.Y., Hua H.M., Zhao Z., Fang Z.Z., Zhang Z.Z. Inhibition behavior of fructus psoraleae's ingredients towards human carboxylesterase 1 (hCES1) Xenobiotica. 2016;46(6):503–510. doi: 10.3109/00498254.2015.1091521. [DOI] [PubMed] [Google Scholar]
- Szliszka E., Czuba Z.P., Sędek L., Paradysz A., Król W. Enhanced TRAIL-mediated apoptosis in prostate cancer cells by the bioactive compounds neobavaisoflavone and psoralidin isolated from Psoralea corylifolia. Pharmacol. Rep. 2011;63:139–148. doi: 10.1016/s1734-1140(11)70408-x. [DOI] [PubMed] [Google Scholar]
- Tinel M., Belghiti J., Descatoire V., Amouyal G., Letteron P., Geneve J., Larrey D., Pessayre D. Inactivation of human liver cytochrome P-450 by the drug methoxsalen and other psoralen derivatives. Biochem. Pharmacol. 1987;36(6):951–955. doi: 10.1016/0006-2952(87)90190-0. [DOI] [PubMed] [Google Scholar]
- Tanaka T. Tanaka's Cyclopaedia of Edible Plants of the World. First ed. Keigaku Pub Co.; Tokyo, Japan: 1976. [Google Scholar]
- Tava A., Pecetti L., Ricci M., Pagnotta M.A., Russi L. Volatile compounds from leaves and flowers of Bituminaria bituminosa (L) Stirt (Fabaceae) from Italy. Flav. Frag. J. 2007;22:363–370. [Google Scholar]
- Teschke R., Bahre R. Severe hepatotoxicity by Indian ayurvedic herbal products: a structured causality assessment. Ann. Hepatol. 2009;8:258–266. [PubMed] [Google Scholar]
- Teschke R., Wolff A., Frenzel C., Schulze J. Review article: herbal hepatotoxicity–an update on traditional Chinese medicine preparations. Aliment Pharmacol. Ther. 2014;40(1):32–50. doi: 10.1111/apt.12798. [DOI] [PubMed] [Google Scholar]
- Tewari A., Bhakuni R.S. New constituents from Psoralea corlifolia. Ind. J. Chem. 2010;49B:256–259. [Google Scholar]
- Totonchy M.B., Chiu M.W. UV-based therapy. Dermatol. Clin. 2014;32:399–413. doi: 10.1016/j.det.2014.03.003. [DOI] [PubMed] [Google Scholar]
- The Plant List, 2013. Version 1.1. Published on the Internet; 〈http://www.theplantlist.org/〉 (Accessed 1 January).
- Tsai M.H., Huang G.S., Hung Y.C., Bin L., Liao L.T., Lin L.W. Psoralea corylifolia extract ameliorates experimental osteoporosis in ovariectomized rats. Am. J. Chin. Med. 2007;35:669–680. doi: 10.1142/S0192415X07005168. [DOI] [PubMed] [Google Scholar]
- Tsai W.J., Hsin W.C., Chen C.C. Anti-platelet flavonoids from seeds of Psoralea colylifolia. J. Nat. Prod. 1996;59:671–672. doi: 10.1021/np960157y. [DOI] [PubMed] [Google Scholar]
- Turchi G., Alagona G., Lubrano V. Protective activity of plicatin B against human LDL oxidation induced in metal ion-dependent and -independent processes. Experimental and theoretical studies. Phytomedicine. 2009;16:1014–1026. doi: 10.1016/j.phymed.2009.03.018. [DOI] [PubMed] [Google Scholar]
- Uikey S., Yadav A.S., Sharma A.K., Badkhane Y. The botany, chemistry pharmacological and therapeutic application of Psoralea corylifolia L - a review. Int. J. Phytomed. 2010;2:100–107. [Google Scholar]
- Uphof, J.C.Th., 1968. Dictionary of economic plants. Second ed. Cramer Wiirzburg.
- Usher G. Macmillan Pub. Co.; UK: 1974. A Dictionary of Plants Used by Man. [Google Scholar]
- Wang D., Li F., Jiang Z. Osteoblastic proliferation stimulating activity of Psoralea corylifolia extracts and two of its flavonoids. Planta Med. 2001;67(8):748–749. doi: 10.1055/s-2001-18343. [DOI] [PubMed] [Google Scholar]
- Wang S.L., Wu S.D., Li S.L., Xiao H., Dong C.T., Cui L. Experimental study on the effect of fructus lyc II and 7 other Chinese drugs on cells cultured in vitro and macrophages of the abdominal cavity in mice. J. Norman Bethune Univ. Med. Sci. 1990:4. [Google Scholar]
- Wang X., Lou Y.J., Wang M.X., Shi Y.W., Xu H.X., Kong L.D. Furocoumarins affect hepatic cytochrome P450 and renal organic ion transporters in mice. Toxicol. Lett. 2012;209:67–77. doi: 10.1016/j.toxlet.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Wang X.X., Lv X., Li S.Y., Hou J., Ning J., Wang J.Y., Cao Y.F., Ge G.B., Guo B., Yang L. Identification and characterization of naturally occurring inhibitors against UDP‐glucuronosyltransferase 1A1 in Fructus psoraleae (Bugu‐zhi) Toxicol. Appl. Pharmacol. 2015;289(1):70–78. doi: 10.1016/j.taap.2015.09.003. [DOI] [PubMed] [Google Scholar]
- Wang X.Y., Wang J.X. Synergistic effect of psoralen cooperated with substrates on tyrosinase activation. Nat. Product. Res. Dev. 2007;19(1):77–80. [Google Scholar]
- Wang Y., Hong C., Zhou C., Xu D., Qu H.B. Screening anti-tumor compounds psoralen and isopsoralen from Psoralea corylifolia L. seeds. Evid. Based Complement. Altern. Med. 2011;2011:1–7. doi: 10.1093/ecam/nen087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.F., Liu Y.N., Xiong W., Yan D.M., Zhu Y., Gao X.M., Xu Y.T., Qi A.D. A Uplc-ms/ms method for in vivo and in vitro pharmacokinetic studies of psoralenoside isopsoralenoside psoralen and isopsoralen from Psoralea corylifolia extract. J. Ethnopharmacol. 2014;151:609–617. doi: 10.1016/j.jep.2013.11.013. [DOI] [PubMed] [Google Scholar]
- Wang Y.F., Wu B., Yang J., Hu L.M., Su Y.F., Gao X.M. A rapid method for the analysis of ten compounds in Psoralea corylifolia L. by UPLC. Chromatographia. 2009;70:199–204. [Google Scholar]
- Weiner, M.A., 1990. Earth medicine, Earth Food. Ballantine Books, Revised expanded edition.
- Whelan L.C., Ryan M.P. Ethanolic extracts of Euphorbia and other ethnobotanical species as inhibitors of tumor cell growth. Phytomedicine. 2003;10:53–58. doi: 10.1078/094471103321648665. [DOI] [PubMed] [Google Scholar]
- Won T.H., Song I.H., Kim K.H., Yang W.Y., Lee S.K., Oh D.C., Oh W.K., Oh K.B., Shin J. Bioactive metabolites from the fruits of Psoralea corylifolia. J. Nat. Prod. 2015;78(4):666–673. doi: 10.1021/np500834d. [DOI] [PubMed] [Google Scholar]
- Wong R.W., Rabie A.B. Effect of Buguzhi (Psoralea corylifolia fruit) extract on bone formation. Phytother. Res. 2010;24:S155–S160. doi: 10.1002/ptr.3049. [DOI] [PubMed] [Google Scholar]
- Wong R.W., Rabie A.B. Systemic effect of Fructus psoraleae extract on bone in mice. Phytother. Res. 2010;24(10):1578–1580. doi: 10.1002/ptr.3184. [DOI] [PubMed] [Google Scholar]
- Wong B.S.T., Hsu B.A.L., Liao M.D.W. Phototherapy in psoriasis. A review of mechanisms of action. J. Cutan. Med. Surg. 2013;17(1):6–12. doi: 10.2310/7750.2012.11124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C.R., Chang C.L., Hsieh P.Y., Lin L.W., Ching H. Psoralen and isopsoralen two coumarins of Psoraleae fructus can alleviate scopolamine-induced amnesia in rats. Planta Med. 2007;73:275–278. doi: 10.1055/s-2007-967127. [DOI] [PubMed] [Google Scholar]
- Wu C.Z., Cai X.F., Dat N.T., Hong S.S., Han A.R., Seo E.K., Hwang B.Y., Nan J.X., Lee D., Lee J.J. Bisbakuchiols A and B novel dimeric meroterpenoids from Psoralea corylifolia. Tetrahedron. 2007;48:8861–8864. [Google Scholar]
- Wu C.Z., Hong S.S., Cai X.F., Dat N.T., Nan J.X., Hwang B.Y., Lee J.J., Lee D. Hypoxiainducible factor-1 and nuclear factor-κB inhibitory meroterpene analogues of bakuchiol a constituent of the seeds of Psoralea corylifolia. Bioorg. Med. Chem. Lett. 2008;18:2619–2623. doi: 10.1016/j.bmcl.2008.03.028. [DOI] [PubMed] [Google Scholar]
- Xiao G., Li X., Wu T., Cheng Z., Tang Q., Zhang T. Isolation of a new meroterpene and inhibitors of nitric oxide production from Psoralea corylifolia fruits guided by TLC bioautography. Fitoterapia. 2012;83:1553–1557. doi: 10.1016/j.fitote.2012.08.024. [DOI] [PubMed] [Google Scholar]
- Xin D., Wang H., Yang J., Su Y.F., Fan G.W., Wang Y.F., Zhu Y., Gao X.M. Phytoestrogens from Psoralea corylifolia reveal estrogen receptor subtype selectivity. Phytomedicine. 2010;17:126–131. doi: 10.1016/j.phymed.2009.05.015. [DOI] [PubMed] [Google Scholar]
- Xu M.J., Wu B., Ding T., Chu J.H., Li C.Y., Zhang J., Wu T., Wu J., Liu S.J., Liu S.L., Ju W.Z., Li P. Simultaneous characterization of prenylated flavonoids and isoflavonoids in Psoralea corylifolia L. by liquid chromatography with diode–array detection and quadrupole time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2012;26(19):2343–2358. doi: 10.1002/rcm.6361. [DOI] [PubMed] [Google Scholar]
- Xu Q., Pan Y., Yi L.T., Li Y.C., Mo S.F., Jiang F.X., Qiao C.F., Xu H.X., Lu X.B., Kong L.D., Kung H.F. Anti-depressant-like effects of psoralen isolated from the seeds of Psoralea corylifolia in the mouse forced swimming test. Biol. Pharm. Bull. 2008;31(6):1109–1114. doi: 10.1248/bpb.31.1109. [DOI] [PubMed] [Google Scholar]
- Yadava R.N., Verma V. A new biologically active flavonol glycoside from Psoralea corylifolia (Linn) J. Asian Nat. Prod. Res. 2003;7:671–675. doi: 10.1080/10286020310001608921. [DOI] [PubMed] [Google Scholar]
- Yang R.P., Shou Q.Y., Wang B.H. Effect of Psoralea corylifolia L. on the proliferation and differentiation of osteoblast isolated from neonatal rat calvarium in vitro. China Pract. Med. 2007;2(1):32–34. [Google Scholar]
- Yang T.T., Li J., Qin M.J., Zhao M., Ouyang Z., Fu Z. Two new compounds from Psoralea corylifolia L. Acta Pharm. Sin. 2009;44:1387–1390. [PubMed] [Google Scholar]
- Yang X., Li J., Wang X., Fang W., Bidochka M.J., She R., Xia Y., Pei Y. Psc-AFP an anti-fungal protein with trypsin inhibitor activity from Psoralea corylifolia seeds. Peptides. 2006;27:1726–1731. doi: 10.1016/j.peptides.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Yanovsky E. United States Department of Agriculture; 1936. Food Plants of the North American Indians. (Pub no 237) [Google Scholar]
- Ye S.M., Ou W.P., Hong X. Pharmacokinetics of fructus Psoraleae in bile elimination. traditional Chinese. Drug Res. Clin. Pharmacol. 1999;10(3):162–164. [Google Scholar]
- Yeung H.C. Handbook of Chinese Herbal Formulas. Second ed. Redwing Book Co.; United States: 1985. [Google Scholar]
- Yi L.T., Li Y.C., Pan Y., Li J.M., Xu Q., Mo S.F., Qiao C.F., Jiang F.X., Xu H.X., Lu X.B., Kong L.D., Kung H.F. Anti-depressant-like effects of psoralidin isolated from the seeds of Psoralea corylifolia in the forced swimming test in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32(2):510–519. doi: 10.1016/j.pnpbp.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Yin F.Z., Li L., Lu T.L., Li W.D., Cai B.C., Yin W. Quality assessment of Psoralea fructus by HPLC fingerprint coupled with multi-components analysis. Indian J. Pharm. Sci. 2015;77(6):715–722. doi: 10.4103/0250-474x.174996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin S., Fan C.Q., Dong L., Yue J.M. Psoracorylifols A-E, five novel compounds with activity against Helicobacter pylori from seeds of Psoralea corylifolia. State Key Lab. Drug Res. 2006;62:2569–2575. [Google Scholar]
- Yin S., Fan C.Q., Wang Y., Dong L., Yue J.M. Anti-bacterial prenylflavone derivatives from Psoralea corylifolia, and their structure-activity relationship study. Bioorg. Med. Chem. 2004;12(16):4387–4392. doi: 10.1016/j.bmc.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Yin S., Fan C.Q., Yue J.M. Cyclobakuchiol C, a new bakuchiol derivative from Psoralea coryllfolia. J. Asian Nat. Prod. Res. 2007;9(1):29–33. doi: 10.1080/10286020500289568. [DOI] [PubMed] [Google Scholar]
- Youssef A., Sulaiman A.A.A., Ben S.R., Arafa I.H. Experimental and density functional theory study of a new dimer with tetrasubstituted cyclobutane ring system isolated from Psoralea plicata seeds. Int. J. Chem. 2013;5:73–79. [Google Scholar]
- Yu L.L., Chen Y.G., Liu J.C., Lu Y.P., Gui S. Chalcones from the seeds of Psoralea corylifolia. Pol. J. Chem. 2005;79:1173–1177. [Google Scholar]
- Yu W.X., Li W.Y., Li H.Y., Han L., Chen W.L., Deng S.G. Effects of total coumarin on cGMP/cGMP of asthmatic rats. Res. Pract. Chin. Med. 2006;20(5):27–29. [Google Scholar]
- Zarmouh N.O., Messeha S.S., Elshami F.M., Soliman K.F. Evaluation of the isoflavone genistein as reversible human monoamine oxidase‐a and‐b inhibitor. Evid. Based Complement. Altern. Med. 2016 doi: 10.1155/2016/1423052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C.Z., Wang S.X., Zhang Y., Chen J.P., Liang X.M. In vitro estrogenic activities of Chinese medicinal plants traditionally used for the management of menopausal symptoms. J. Ethnopharmacol. 2005;98:295–300. doi: 10.1016/j.jep.2005.01.033. [DOI] [PubMed] [Google Scholar]
- Zhang R.Q., Shi F.Q., Pang S.Z., Yu S.F. The effect of Psoralea corylifolia on isolated osteoclasts cells. J. Mod. Stomatol. 1995;3:136–138. [Google Scholar]
- Zhang X., Zhao W., Wang Y., Lu J., Chen X. The chemical constituents and bioactivities of Psoralea corylifolia Linn. A review. Am. J. Chin. Med. 2016;44(1):35–60. doi: 10.1142/S0192415X16500038. [DOI] [PubMed] [Google Scholar]
- Zhao L., Haung C., Shan Z., Xiang B., Mei L. Fingerprint analysis of Psoralea corylifolia L.by HPLC and LC-MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2005;821(1):67–74. doi: 10.1016/j.jchromb.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Zhao L.H., Wu M.H., Xiang B.R. Analysis of Psoralea corylifolia L. fruits in different regions. Chem. Pharm. Bull. 2005;53(8):1054–1057. doi: 10.1248/cpb.53.1054. [DOI] [PubMed] [Google Scholar]
- Zhao P.W., Wang D.W., Niu J.Z., Wang J.F., Wang L.Q. Evaluation on phytoestrogen effects of ten kinds of Chinese medicine including flos carthami. Zhongguo Zhong Yao Za Zhi. 2007;32(5):436–439. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material