Highlights
-
•
Rotavirus genotype distribution was not different in vaccinated and non-vaccinated herds.
-
•
G6, P[5] and G6P[5] genotypes predominated in both groups.
-
•
No selection of rotavirus genotypes associated with the use of vaccine was observed.
-
•
Calicivirus prevalence was similar in calves from vaccinated and non-vaccinated herds.
Keywords: Rotavirus, Vaccine, Genotype, Bovine, Diarrhoea, Calicivirus
Abstract
Group A rotaviruses are a leading cause of neonatal calf diarrhoea worldwide and prevention of this disease includes vaccination against these viruses. In order to highlight the potential selection of rotavirus genotypes due to immune pressure driven by vaccination, the aim of this study was to compare group A rotavirus genotypes circulating in French diarrhoeic calves in rotavirus vaccinated herds (G6P[5] vaccine) with those in non-vaccinated herds during one calving season in 2010. This study showed a high prevalence of rotavirus in both groups with no significant difference between the two. No significant differences regarding G, P and G/P rotavirus genotype distribution between the two groups were observed, with G6, P[5] and G6P[5] genotypes being by far the most prevalent. Moreover, sequence analyses of the VP7 and VP4 partial coding genes of the G6P[5] strains from this study did not allow us to distinguish them according to their origin. This study also showed that other pathogens responsible for calf diarrhoea, such as genogroup III noroviruses and neboviruses, were not more frequently associated with calf diarrhoea in vaccinated herds. Altogether, these results suggest that the studied vaccine did not promote the emergence of rotavirus genotypes or variants different from those of the vaccine or other viruses responsible for calf diarrhoea, such as caliciviruses.
1. Introduction
Diarrhoea is a major disease that affects calves worldwide and is a leading cause of neonatal mortality. This disease can be caused by several different infectious agents (viruses, bacteria and protozoa) and other factors including herd management, environment and nutritional and immunological condition of the host can influence disease severity [1]. Among infectious agents, group A rotaviruses (RVA), which belong to the Reoviridae family, are one of the foremost causes of calf diarrhoea worldwide [2]. Although the clinical signs are of short duration, viral shedding persists for up to three weeks after infection, often leading to a seasonal permanent outbreak of diarrhoea in young calves once the infection has been declared in a herd. Increases in morbidity, mortality and treatment costs, and reductions in growth rates caused by these viral infections lead to substantial economic losses, highlighting the need for effective prevention methods like vaccination.
Group A rotaviruses are classified into G (glycoprotein) and P (protease sensitive) genotypes according to the two immunogenic proteins VP7 and VP4 that form the outer layer of the viral capsid, respectively [3]. Several international studies have shown that G6, G10, and G8 in combination with P[1], P[5], P[11], P[15] and P[21] are the most epidemiologically relevant RVA genotypes in cattle worldwide [4]. This gives key information for the development of appropriate vaccines for cattle. The most effective rotavirus vaccination strategy in cattle consists of promoting increased and prolonged anti-rotavirus protective antibody titres in mammary secretions of vaccinated pregnant cows [5], [6], thus conferring passive protection to newborn calves. Indeed, it has been demonstrated that when calves are fed with colostrum from vaccinated dams from the first hours of life until they develop their own immunity, anti-rotavirus specific antibodies present in colostrum reduce the incidence of diarrhoea caused by rotavirus and viral shedding by calves infected with rotavirus. The effectiveness of vaccines for preventing neonatal calf diarrhoea caused by rotavirus has already been proven: depending on studies, decreased mortality and morbidity due to diarrhoea, reduced diarrhoea, decreased RVA shedding by infected calves, and/or delayed onset of diarrhoea have all been reported [7]. In France, four vaccines are commercially available. All these vaccines combine rotavirus with coronavirus, and the majority also includes enterotoxigenic Escherichia coli. Burgundy is the region of France where rotavirus vaccination of beef herds is the most widely used, with nearly 30% of beef herds vaccinated annually these last three years. In this region, vaccines against rotavirus were introduced more than ten years ago. Among the commercially available vaccines, one inactivated G6P[5] (strain UK-Compton) rotavirus vaccine is by far the most widely used in Burgundy, with nearly 80% of sales in the last three years (personal communication of a vaccine manufacturer).
Little is known about the potential of these vaccines to promote the emergence of rotavirus strains of genotypes different from those present in the vaccines. Such a phenomenon has been described since the introduction of RVA vaccines in humans [8], [9], [10]. Although the role of vaccination must be clarified and remains uncertain, these studies showed a transient changing pattern of RVA genotypes circulating in humans once the vaccines were introduced. In this context, this study aims to describe the circulating genotypes of rotaviruses in vaccinated and non-vaccinated herds and to monitor the potential emergence of rotavirus strains with genotypes or variants different from the vaccines.
In addition to rotaviruses, neboviruses and genogroup III noroviruses, two genera of the Caliciviridae family have also been reported as being responsible for neonatal calf diarrhoea, [11], [12], [13], [14] but are not yet included in vaccines. The circulation of these bovine caliciviruses in French diarrhoeic calves from the same region has already been demonstrated [15] but the impact of rotavirus vaccination on their circulation is not known. In this context, detection of these viruses was also performed in parallel with rotavirus detection in order to determine their involvement in calf diarrhoea in rotavirus vaccinated and non-vaccinated herds.
2. Materials and methods
2.1. Samples
A total of 81 samples were collected in Burgundy, France, during one calving season from February through May 2010, from calves (Bos taurus) showing diarrhoea for less than four days. All of the calves were Charolais and were born on the farms included in the study.
Two groups were compared: the first group (38 samples) included calves born from vaccinated cows in farms using a rotavirus vaccine (strain UK-Compton G6P[5]) for at least three years, whereas the second group (43 samples) included calves from farms without a rotavirus vaccination schedule. In the first group, one sample each was collected in 36 herds, and two were collected in one herd. In the second group, one and two samples were collected in 37 and 3 herds, respectively.
There was no significant difference between the two groups concerning the age of the sampled calves (P = 0.116) which ranged from 2 to 18 days (mean: 8.3 days; median: 8 days) and from 1 to 15 days (mean: 7.2 days; median: 7 days) in the vaccinated and non-vaccinated herds, respectively.
Samples were stored at −20 °C prior to analysis.
2.2. RNA extraction
Viral RNA was extracted from 20% faecal suspensions in phosphate-buffered saline (PBS) using the NucliSENS® EasyMAG™ platform (bioMérieux, Marcy l’Etoile, France) according to the manufacturer's instructions.
2.3. Virus detection and genotyping
Group A rotavirus and bovine calicivirus were detected using the Qiagen One Step RT-PCR kit (Qiagen, Hilden, Germany) in separate reactions and according to the manufacturer's instructions. For RVA, VP6-F/R primers [16] targeting the VP6 coding gene were used, while CBECU-F/R and NBU-F/R primers [17] were used to amplify the 3′end polymerase region of genogroup III noroviruses (NoVsGIII) and neboviruses, respectively.
RVA strains were then G and P genotyped by hemi-nested multiplex RT-PCRs using the EuroRotaNet protocol (http://www.eurorota.net/) modified with primers previously described [18], [19] in order to allow the specific amplification of genotypes G5, G6, G8, G10 and G11 and genotypes P[1], P[5]-P[7] and P[11]. All the RVA genotyping results were confirmed by sequencing the VP4 and VP7 coding gene amplicons with the same primers as for amplification. NoVsGIII and neboviruses strains were genotyped by direct sequencing of the PCR products. All the sequencing reactions were performed using the ABI PRISM® Big Dye® Terminator Cycle Sequencing Kit on a 3130XL DNA Genetic Analyzer (Applied Biosystems, Foster City, CA, USA).
2.4. Sequence analyses
A selection of reference strains available in the National Center for Biotechnology Information GenBank database (http://www.ncbi.nlm.nih.gov/genbank/) was used for all sequence analyses. Molecular Evolutionary Genetics Analysis (MEGA) software [20] was used to calculate nucleotide and amino-acid sequence identities between strains using the p-distance substitution model. Phylogenetic analyses were performed online in the Phylogeny.fr platform [21] according to the following workflow: first, nucleotide and deduced amino-acid sequences were aligned using the MUSCLE programme [22], and then phylogenetic trees were inferred using the PhyML programme [23] and were finally edited with the Seaview programme [24]. Of note, MUSCLE and PhyML programmes were used rather than ClustalW and Neighbour-Joining programmes because it has been demonstrated that the first two programmes were more accurate than the last ones [22], [25].
A selection of representative VP7 and VP4 partial sequences of French RVA G6P[5] strains from both vaccinated and non-vaccinated herds were submitted to the European Nucleotide Archive database (http://www.ebi.ac.uk/ena/) under the following accession numbers: HE646639 to HE646657 for VP7 and HE646658 to HE646676 for VP4.
2.5. Statistical analyses
To compare the mean age in both groups (vaccination vs. no vaccination), the underlying assumptions of analysis of variance (normally distributed populations with equal variances or homoscedasticity) were checked using the Jarque-Bera (S–K) test and Bartlett test. Transformations of the raw data based on skewness and kurtosis were also performed. A nonparametric test (i.e. Kruskal Wallis test) was performed if necessary.
The Chi square or Fischer exact test were used to compare virus prevalence and genotypes between the two groups.
All statistical analyses were performed with Stata® software (StataCorp release 10, 2007; College Station, TX, USA). For all analyses, a P value of less than 0.05 was considered the threshold of significance.
3. Results and discussion
A high prevalence of RVA associated with calf diarrhoea was observed in both groups: they were similarly (P = 0.495) detected in 21/38 (55.3%) and 28/43 (65.1%) diarrhoeic faecal samples from vaccinated and non-vaccinated herds, respectively. To the best of our knowledge, this is the first study to compare the prevalence of rotaviruses in diarrhoeic calves from vaccinated and non-vaccinated herds. A large number of international studies have reported the prevalence of rotaviruses in diarrhoeic calves, but the use of vaccines against rotavirus is rarely documented, and we can assume that the majority of these studies certainly included calves from both vaccinated and non-vaccinated herds. Nevertheless, some of these studies aimed to determine the prevalence of rotaviruses in diarrhoeic calves from vaccinated or non-vaccinated herds. For example, studies from Italy [26] and Japan [27] reported different prevalences in non-vaccinated herds of 9.6% and 37%, respectively, which is lower than the prevalence of 65.1% reported here. Similarly, a slightly lower prevalence of rotavirus (45.1% vs. 55.3%) compared with the present study was reported in a previous study conducted during the late 90 s in the same region of France [28] in which more than 80% of the herds included were vaccinated. The high prevalence of RVA observed in the vaccinated herds was hardly surprising. Indeed, rotavirus vaccination of pregnant cows was not developed to completely prevent infection by these viruses but ideally to supply calves with anti-rotavirus specific antibodies to allow them to withstand the infectious pressure without developing clinical signs. Thus RVA could infect calves even in vaccinated herds and we can thus assume that diarrhoea in these calves involves other factors or pathogens.
To identify these other pathogens, samples were screened for the presence of genogroup III noroviruses and neboviruses, which are rarely investigated routinely in neonatal calf diarrhoea. With detection rates of 36.8% (14/38) and 34.9% (15/43), the overall prevalence of these bovine caliciviruses was not significantly different (P = 1.000) in diarrhoeic calves from vaccinated and non-vaccinated herds, respectively. NoVsGIII were the most frequently detected bovine caliciviruses in this study, and were detected at similar rates (P = 1.000) of 31.6% (12/38) and 30.2% (13/43) in faecal samples from vaccinated and non-vaccinated herds, respectively. Moreover, sequence analyses revealed that genotype 2 NoVsGIII were more frequently detected than genotype 1 NoVsGIII in both groups (data not shown). Of note, the predominance of the genotype 2 NoVsGIII among bovine caliciviruses in French diarrhoeic calves has already been reported [15] but the vaccine status of the herds studied was unknown. Similar to NoVsGIII, nebovirus detection rates were similar (P = 1.000) in samples from vaccinated and non-vaccinated herds, with 5/38 (13.2%) and 5/43 (11.6%) positive samples, respectively. Sequence analyses showed that all the nebovirus strains from this study were closely related to the Nebraska/80/US reference strain (data not shown). Finally, the present study indicated that neboviruses and NoVsGIII, which are among the numerous pathogens responsible for calf diarrhoea, were not promoted in diarrhoeic calves from herds where the rotavirus vaccine was used. Nevertheless, other pathogens, notably bacteria and protozoa, should be investigated in order to better understand the aetiology of diarrhoea in calves from vaccinated dams.
Genotyping of all the RVA positive samples showed that the G6 genotype clearly predominated in both groups with a prevalence of 90.5% in vaccinated herds and 89.3% in non-vaccinated herds (Table 1 ). The G10 genotype co-circulated at low frequencies of 9.5% and 7.1% in vaccinated and non-vaccinated herds, respectively. Of note, the G genotype of one sample from a non-vaccinated herd could not be determined. Regarding VP4 protein, the most frequently detected genotype in the vaccinated and non-vaccinated herds was P[5] (76.2% and 89.3%, respectively), followed by P[11] (4.8% and 7.1%, respectively). The P genotype could not be assigned for four samples from vaccinated herds and one sample from non-vaccinated herds. Rotavirus strains G6P[5] noticeably predominated in the two groups of diarrhoeic calves studied, reaching a prevalence of 76.2% in vaccinated herds and 85.7% in non-vaccinated herds. Strains of genotypes G10P[11] were also detected but at low rates (4.8% and 7.1% in vaccinated and non-vaccinated herds, respectively). Only partial genotyping was achieved for four (19%) strains from vaccinated herds (3 G6 strains and 1 G10 strain) and for two (7.1%) strains from non-vaccinated herds (1 G6 strain and 1 P[5] strain). Of note, no rotavirus co-infection was observed in any group. These rotavirus genotyping results are in accordance with previous studies conducted in diarrhoeic calves in the same region during 2005–2007 [29] and thereafter (unpublished data). In these previous studies, although the rotavirus vaccine status of herds where samples were collected was not known, G6P[5] followed by G10P[11] strains also predominated, indicating the relative stability of rotavirus genotypes over time. Of note, the predominance of G6P[5] strains was also observed during the last decade in several parts of the world, notably in Australia [30], Ireland [31] and Argentina [32], highlighting the fact that G6P[5] are one of the most common rotavirus strains in cattle. In the present study, statistical analyses showed that there were no significant differences regarding G (P = 0.778), P (P = 0.275) or G/P (P = 0.457) genotype distribution between the two groups. These results suggested that in spite of the immune pressure due to long-term vaccination on farms using the selected vaccine, there was no selection of RVA genotypes. To the best of our knowledge, only one previous study compared RVA strains in diarrhoeic calves born to vaccinated and non-vaccinated dams [33]. In this study, conducted in Brazil in 2000–2001, the authors reported a different RVA strain distribution in calves from vaccinated and non-vaccinated herds. Notably, the P[1] genotype was not observed in calves from herds vaccinated with a commercially available G6P[1] (strain NCDV) vaccine whereas it was the most frequent genotype detected in non-vaccinated herds. These discrepant results may be partly explained by the different rotavirus vaccines used (strain UK-Compton G6P[5] in our study and strain NCDV G6P[1] in Brazil) and suggest that the impact on rotavirus genotypes circulating under immune pressure may be vaccine-dependent.
Table 1.
Genotype distribution of rotavirus strains circulating in diarrhoeic calves from vaccinated and non-vaccinated herds. Specific amplification of genotypes G5, G6, G8, G10 and G11 and genotypes P[1], P[5]-P[7] and P[11] was performed. Of note, genotypes G5, G8, G11, P[1], P[6] and P[7] were not detected.
| G6 | G10 | G-UDa | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Vaccinated herds | P[5] | 16 | (76.2%) | 16 | (76.2%) | ||||
| P[11] | 1 | (4.8%) | 1 | (4.8%) | |||||
| P-UDa | 3 | (14.3%) | 1 | (4.8%) | 4 | (19.0%) | |||
| Total | 19 | (90.5%) | 2 | (9.5%) | 21 | ||||
| Non-vaccinated herds | P[5] | 24 | (85.7%) | 1 | (3.6%) | 25 | (89.3%) | ||
| P[11] | 2 | (7.1%) | 2 | (7.1%) | |||||
| P-UDa | 1 | (3.6%) | 1 | (3.6%) | |||||
| Total | 25 | (89.3%) | 2 | (7.1%) | 1 | (3.6%) | 28 | ||
| Total | 44 | (89.8%) | 4 | (8.2%) | 1 | (2.0%) | 49 | ||
UD: undetermined.
Sequence and phylogenetic analyses of the partial VP7 and VP4 gene segments revealed that G6P[5] strains from this study did not segregate on separated branches according to their origin (i.e. from vaccinated/non-vaccinated herds). Considering that nucleotide and amino-acid phylogenetic analyses were in agreement and that the amino-acid analysis is more informative than the nucleotide analysis according to the purpose of this study (by indicating the existence of potential antigenic variants due to the use of vaccine), only the amino-acid analyses were presented (Fig. 1, Fig. 2 ). Analysis of the partial VP7 gene segment indicated that all these G6P[5] strains were closely related to each other (Table 2 ), with nucleotide and amino-acid identities between the two groups ranging from 94.3% to 100% and 95.8% to 100%, respectively. Furthermore, they were all closely related to the selected G6 lineage IV reference strain RVA/Cow-tc/GBR/UK/1973/G6P[5], with high identities ranging from 93.5% to 95.3% (nucleotides) and 96.7% to 97.5% (amino-acids) for strains from vaccinated herds and from 93.6% to 95.4% (nucleotides) and 95.8% to 97.5% (amino-acids) for strains from non-vaccinated herds. Partial VP4 gene segment analysis confirmed the close relationship between G6P[5] strains from vaccinated and non-vaccinated herds (nucleotide identity: 91.8% to 100%, amino-acid identity: 95.4% to 100%), and also confirmed that they were closely related to the selected rotavirus reference strain RVA/Cow-tc/GBR/UK/1973/G6P[5], with identities ranging from 88.6% to 90.5% (nucleotides) and 92.0% to 93.7% (amino-acids) for strains from vaccinated herds and from 88.4% to 90.7% (nucleotides) and 92.0% to 94.3% (amino-acids) for strains from non-vaccinated herds. Alignment and comparison of the deduced amino-acid sequences of the partial VP7 and VP4 coding genes from G6P[5] strains did not reveal any major differences between strains from vaccinated and non-vaccinated herds (Fig. 3, Fig. 4 ). Actually, G6P[5] strains from this study showed several mutations mainly in the variable and major antigenic regions when compared with the RVA/Cow-tc/GBR/UK/1973/G6P[5] reference strain, but the large majority of these mutations was shared by strains from both vaccinated and non-vaccinated herds. Only three mutations involving three strains (one mutation per strain) from vaccinated herds and four mutations involving three strains (two strains with one mutation and one strain with three mutations) from non-vaccinated herds were not shared by the two groups based on the partial VP7 deduced amino-acid sequences (Fig. 3). Regarding the partial VP4 deduced amino-acid sequences, only two mutations were exclusively observed in four strains (three strains with one identical mutation and one strain with another mutation) from vaccinated herds and four mutations were exclusively observed in two strains (two mutations per strain) from non-vaccinated herds (Fig. 4). Finally, these further analyses performed on the G6P[5] strains from our study did not allow us to distinguish them according to their origin (i.e. from vaccinated/non-vaccinated herds). This suggested that in addition to not promoting selection of RVA genotypes, the studied G6P[5] vaccine did not promote the emergence of G6P[5] variants on the farms where vaccination was used. Nevertheless, the complete genome analysis of these strains should be undertaken in order to better assess the impact of the vaccine on rotavirus circulating strains.
Fig. 1.
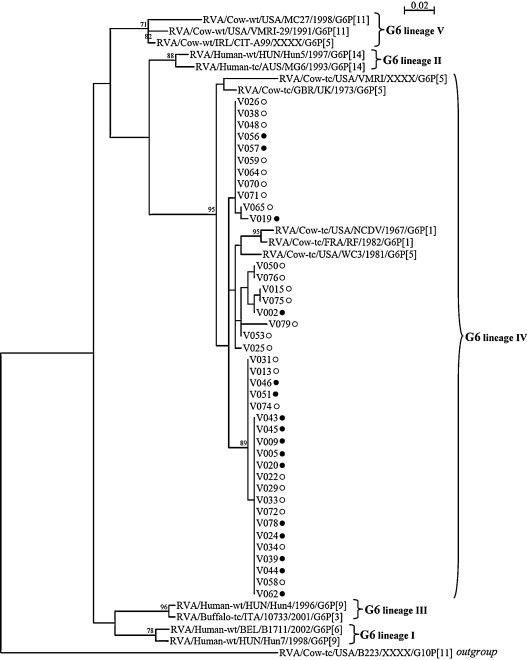
Phylogenetic tree based on the partial amino-acid sequences of the rotavirus G6P[5] VP7 coding gene. Phylogeny was reconstructed using the maximum likelihood method implemented in the PhyML programme with the Jones Taylor Thornton substitution model. The number of substitutions per site is indicated by the scale bar. Bootstrap values were calculated for 500 replicates and are indicated at each node when ≥70%. Strains from this study which were collected in a vaccinated herd are indicated with a full circle (●), while those collected in a non-vaccinated herd are indicated with an empty circle (○). GenBank accession numbers of the reference strains used for this analysis are: RVA/Huma-wt/BEL/B1711/2002/G6P[6]: AF532202; RVA/Human-wt/HUN/Hun7/1998/G6P[9]: AJ488134; RVA/Human-wt/HUN/Hun4/1996/G6P[9]: AJ487833; RVA/Buffalo-tc/ITA/10733/2001/G6P[3]: AY281360; RVA/Human-wt/HUN/Hun5/1997/G6P[14]: EF554109; RVA/Human-tc/AUS/MG6/1993/G6P[14]: U22011; RVA/Cow-wt/USA/VMRI-29/1991/G6P[11]: U50332; RVA/Cow-wt/IRL/CIT-A99/XXXX/G6P[5]: GQ377868; RVA/Cow-wt/USA/MC27/1998/G6P[11]: AF162435; RVA/Cow-tc/USA/NCDV/1967/G6P[1]: M12394; RVA/Cow-tc/FRA/RF/1982/G6P[1]: X65940; RVA/Cow-tc/USA/WC3/1981/G6P[5]: AY050272; RVA/Cow-tc/GBR/UK/1973/G6P[5]: X00896; RVA/Cow-tc/USA/VMRI/XXXX/G6P[5]: U53924; RVA/Cow-tc/USA/B223/XXXX/G10P[11]: X57852.
Fig. 2.
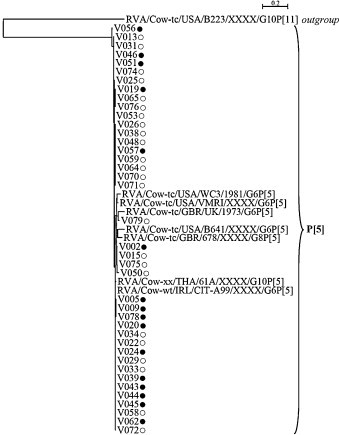
Phylogenetic tree based on the partial amino-acid sequences of the rotavirus G6P[5] VP4 coding gene. Phylogeny was reconstructed using the maximum likelihood method implemented in the PhyML programme with the Jones Taylor Thornton substitution model. The number of substitutions per site is indicated by the scale bar. Bootstrap values were calculated for 500 replicates and are indicated at each node when ≥70%. Strains from this study which were collected in a vaccinated herd are indicated with a full circle (●), while those collected in a non-vaccinated herd are indicated with an empty circle (○). GenBank accession numbers of the reference strains used for this analysis are: RVA/Cow-wt/IRL/CIT-A99/XXXX/G6P[5]: GQ414745; RVA/Cow-xx/THA/61A/XXXX/G10P[5]: D13396; RVA/Cow-tc/USA/VMRI/XXXX/G6P[5]: U53923; RVA/Cow-tc/USA/WC3/1981/G6P[5]: AY050271; RVA/Cow-tc/GBR/678/XXXX/G8P[5]: D32054; RVA/Cow-tc/GBR/UK/1973/G6P[5]: M22306; RVA/Cow-tc/USA/B641/XXXX/G6P[5]: M63267; RVA/Cow-tc/USA/B223/XXXX/G10P[11]: M92986.
Table 2.
Nucleotide and amino-acid identities of the partial VP7 and VP4 coding gene of G6P[5] rotavirus strains from vaccinated and non-vaccinated herds. Identities were determined using MEGA software. The GenBank accession numbers of the reference strain RVA/Cow-tc/GBR/UK/1973/G6P[5] used were: X00896 (VP7) and M22306 (VP4).
| G6P[5] strain identities (%) nucleotide (amino-acids) | VP7 coding gene |
VP4 coding gene |
||
|---|---|---|---|---|
| Strains from vaccinated herds (n = 16) | Strains from non-vaccinated herds (n = 24) | Strains from vaccinated herds (n = 16) | Strains from non-vaccinated herds (n = 24) | |
| Strains from vaccinated herds (n = 16) | 95.1–100 | 94.3–100 | 92.4–100 | 91.8–100 |
| (96.7–100) | (95.8–100) | (97.1–100) | (95.4–100) | |
| Strains from non-vaccinated herds (n = 24) | 94.3–100 | 94.4–100 | 91.8–100 | 92.2–100 |
| (95.8–100) | (95.8–100) | (95.4–100) | (95.4–100) | |
| Reference strain RVA/Cow-tc/GBR/UK/1973/G6P[5] | 93.5–95.3 | 93.6–95.4 | 88.6–90.5 | 88.4–90.7 |
| (96.7–97.5) | (95.8–97.5) | (92.0–93.7) | (92.0–94.3) | |
Fig. 3.
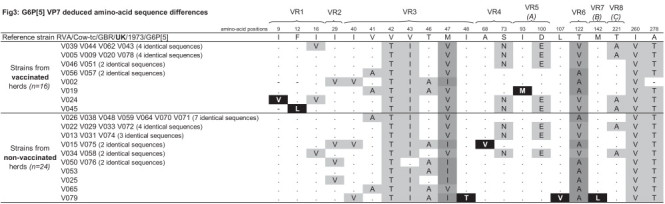
Schematic representation of the G6P[5] partial VP7 deduced amino-acid sequence differences. Comparison was made from amino-acid number 9 through to amino-acid number 288 (total number of positions: 280), and only the diverging positions of G6P[5] strains from this study with the reference strain RVA/Cow-tc/GBR/UK/1973/G6P[5] (GenBank accession number: X00896) are shown. Black shade (■) indicates the amino-acid mutations only present in strains from one of the two groups of herds, dark grey shades ( and
and  ) allow to highlight different amino-acid mutations at the same position shared by strains from both groups of herds and light grey shade (
) allow to highlight different amino-acid mutations at the same position shared by strains from both groups of herds and light grey shade ( ) indicates single amino-acid mutations shared by strains from both groups of herds. VR1 to VR8: Variable Region 1 to 8; (A–C): major antigenic region A–C.
) indicates single amino-acid mutations shared by strains from both groups of herds. VR1 to VR8: Variable Region 1 to 8; (A–C): major antigenic region A–C.
Fig. 4.
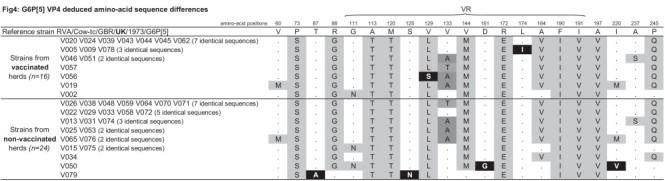
Schematic representation of the G6P[5] partial VP4 deduced amino-acid sequence differences. Comparison was made from amino-acid number 51 through to amino-acid number 259 (total number of positions: 209), and only the diverging positions of G6P[5] strains from this study with the reference strain RVA/Cow-tc/GBR/UK/1973/G6P[5] (GenBank accession number: M22306) are shown. Black shade (■) indicates the amino-acid mutations only present in strains from one of the two groups of herds, dark grey shades ( and
and  ) allow to highlight different amino-acid mutations at the same position shared by strains from both groups of herds and light grey shade (
) allow to highlight different amino-acid mutations at the same position shared by strains from both groups of herds and light grey shade ( ) indicates single amino-acid mutations shared by strains from both groups of herds. VR: Variable Region.
) indicates single amino-acid mutations shared by strains from both groups of herds. VR: Variable Region.
Interestingly, coinfections by caliciviruses and rotaviruses occurred and were similarly observed in both groups (P = 0.788), with an occurrence of 21.1% (8/38) in samples from vaccinated herds and 18.6% (8/43) in samples from non-vaccinated herds. This fairly high prevalence of coinfections by these viruses observed in our study illustrated the fact that calf diarrhoea is a disease involving several infectious agents in co-circulation. To the best of our knowledge, this is the first study to describe both RVA and bovine calicivirus prevalence in faecal samples from diarrhoeic calves. Although the prevalence of bovine caliciviruses was lower than that of RVA, it was significant, raising the hypothesis that these viruses could be included in vaccines in order to prevent calf diarrhoea as efficiently as possible.
4. Conclusion
Our study shows that the rotavirus G6P[5] vaccine studied did not seem to promote the emergence of rotavirus strains with genotypes or variants different from those of the vaccine. Moreover, this vaccine did not seem to promote the emergence of other viruses, such as caliciviruses, which also cause calf diarrhoea.
Acknowledgements
This study was supported by the University Hospital of Dijon and the National Reference Centre for Enteric Viruses (Dijon, France) and sample collection was financially supported by Merial (Merial SAS, Lyon, France).
The authors are very grateful to the cattle breeders that took part in the study and to the veterinary practitioners that collected faecal samples: H. Clement (58-Corbigny), E. Grosbois (71-Epinac), P. Collomb and A. Fichot (71-Marcigny), N. Dehaynin (21-Semur en Auxois), C. Dumas (58-Luzy), B. Gerard (71-Genelard), L. Bellis (71-Charolles) and F. Louis (71-Tournus). We also thank C. Fontana (National Reference Centre for Enteric Viruses, University Hospital of Dijon) for technical assistance and P. Bastable (University Hospital of Dijon) for editorial assistance.
Conflict of interest: The authors declare that they have no conflict of interest. Contributors: Jérôme Kaplon designed the study, detected and characterised the viral strains, analysed and interpreted the data, drafted the manuscript and revised it critically for important intellectual content. Céline Fremy detected and characterised the viral strains. Sandrine Bernard and Liliane Rehby coordinated the veterinary practitioners, managed the sample and data collection. Serge Aho carried out all the statistical analyses. Pierre Pothier designed the study and revised the manuscript critically for important intellectual content. Katia Ambert-Balay designed the study, interpreted the data, drafted the manuscript and revised it critically for important intellectual content. All authors have approved the final article.
References
- 1.Bendali F., Sanaa M., Bichet H., Schelcher F. Risk factors associated with diarrhoea in newborn calves. Vet Res. 1999;30(5):509–522. [PubMed] [Google Scholar]
- 2.Dhama K., Chauhan R.S., Mahendran M., Malik S.V. Rotavirus diarrhea in bovines and other domestic animals. Vet Res Commun. 2009;33(1):1–23. doi: 10.1007/s11259-008-9070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Estes M., Kapikian A. Rotaviruses. In: Knipe D., Howley P., Griffin D., Lamb R., Martin M., Roizman B., editors. Fields virology. 5th ed. Lippincott Williams & Wilkins; Philadelphia: 2007. pp. 1917–1974. [Google Scholar]
- 4.Martella V., Banyai K., Matthijnssens J., Buonavoglia C., Ciarlet M. Zoonotic aspects of rotaviruses. Vet Microbiol. 2010;140(3/4):246–255. doi: 10.1016/j.vetmic.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 5.Snodgrass D.R., Fahey K.J., Wells P.W., Campbell I., Whitelaw A. Passive immunity in calf rotavirus infections: maternal vaccination increases and prolongs immunoglobulin G1 antibody secretion in milk. Infect Immun. 1980;28(2):344–349. doi: 10.1128/iai.28.2.344-349.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crouch C.F., Oliver S., Francis M.J. Serological, colostral and milk responses of cows vaccinated with a single dose of a combined vaccine against rotavirus, coronavirus and Escherichia coli F5 (K99) Vet Rec. 2001;149(4):105–108. doi: 10.1136/vr.149.4.105. [DOI] [PubMed] [Google Scholar]
- 7.Saif L.J., Fernandez F.M. Group A rotavirus veterinary vaccines. J Infect Dis. 1996;174(Suppl. 1):S98–S106. doi: 10.1093/infdis/174.Supplement_1.S98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeller M., Rahman M., Heylen E., De Coster S., De Vos S., Arijs I. Rotavirus incidence and genotype distribution before and after national rotavirus vaccine introduction in Belgium. Vaccine. 2010;28(47):7507–7513. doi: 10.1016/j.vaccine.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Patel M.M., Steele D., Gentsch J.R., Wecker J., Glass R.I., Parashar U.D. Real-world impact of rotavirus vaccination. Pediatr Infect Dis J. 2011;30(Suppl. 1):S1–S5. doi: 10.1097/INF.0b013e3181fefa1f. [DOI] [PubMed] [Google Scholar]
- 10.Usonis V., Ivaskeviciene I., Desselberger U., Rodrigo C. The unpredictable diversity of co-circulating rotavirus types in Europe and the possible impact of universal mass vaccination programmes on rotavirus genotype incidence. Vaccine. 2012;30(31):4596–4605. doi: 10.1016/j.vaccine.2012.04.097. [DOI] [PubMed] [Google Scholar]
- 11.Woode G.N., Bridger J.C. Isolation of small viruses resembling astroviruses and caliciviruses from acute enteritis of calves. J Med Microbiol. 1978;11(4):441–452. doi: 10.1099/00222615-11-4-441. [DOI] [PubMed] [Google Scholar]
- 12.Bridger J.C., Hall G.A., Brown J.F. Characterization of a calici-like virus (Newbury agent) found in association with astrovirus in bovine diarrhea. Infect Immun. 1984;43(1):133–138. doi: 10.1128/iai.43.1.133-138.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smiley J.R., Chang K.O., Hayes J., Vinje J., Saif L.J. Characterization of an enteropathogenic bovine calicivirus representing a potentially new calicivirus genus. J Virol. 2002;76(20):10089–10098. doi: 10.1128/JVI.76.20.10089-10098.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Otto P.H., Clarke I.N., Lambden P.R., Salim O., Reetz J., Liebler-Tenorio E.M. Infection of calves with bovine norovirus GIII.1 strain Jena virus: an experimental model to study the pathogenesis of norovirus infection. J Virol. 2011;85(22):12013–12021. doi: 10.1128/JVI.05342-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaplon J., Guenau E., Asdrubal P., Pothier P., Ambert-Balay K. Possible novel nebovirus genotype in cattle, France. Emerg Infect Dis. 2011;17(6):1120–1123. doi: 10.3201/eid1706.100038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iturriza Gomara M., Wong C., Blome S., Desselberger U., Gray J. Molecular characterization of VP6 genes of human rotavirus isolates: correlation of genogroups with subgroups and evidence of independent segregation. J Virol. 2002;76(13):6596–6601. doi: 10.1128/JVI.76.13.6596-6601.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smiley J.R., Hoet A.E., Traven M., Tsunemitsu H., Saif L.J. Reverse transcription-PCR assays for detection of bovine enteric caliciviruses (BEC) and analysis of the genetic relationships among BEC and human caliciviruses. J Clin Microbiol. 2003;41(7):3089–3099. doi: 10.1128/JCM.41.7.3089-3099.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gouvea V., Santos N., Timenetsky Mdo C. Identification of bovine and porcine rotavirus G types by PCR. J Clin Microbiol. 1994;32(5):1338–1340. doi: 10.1128/jcm.32.5.1338-1340.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gouvea V., Santos N., Timenetsky Mdo C. VP4 typing of bovine and porcine group A rotaviruses by PCR. J Clin Microbiol. 1994;32(5):1333–1337. doi: 10.1128/jcm.32.5.1333-1337.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tamura K., Dudley J., Nei M., Kumar S. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24(8):1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 21.Dereeper A., Guignon V., Blanc G., Audic S., Buffet S., Chevenet F. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008;36(Web Server issue):W465–W469. doi: 10.1093/nar/gkn180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edgar R.C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32(5):1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guindon S., Dufayard J.F., Lefort V., Anisimova M., Hordijk W., Gascuel O. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol. 2010;59(3):307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- 24.Gouy M., Guindon S., Gascuel O. SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol Biol Evol. 2010;27(2):221–224. doi: 10.1093/molbev/msp259. [DOI] [PubMed] [Google Scholar]
- 25.Guindon S., Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol. 2003;52(5):696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- 26.Falcone E., Tarantino M., Di Trani L., Cordioli P., Lavazza A., Tollis M. Determination of bovine rotavirus G and P serotypes in italy by PCR. J Clin Microbiol. 1999;37(12):3879–3882. doi: 10.1128/jcm.37.12.3879-3882.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okada N., Matsumoto Y. Bovine rotavirus G and P types and sequence analysis of the VP7 gene of two G8 bovine rotaviruses from Japan. Vet Microbiol. 2002;84(4):297–305. doi: 10.1016/s0378-1135(01)00445-x. [DOI] [PubMed] [Google Scholar]
- 28.Vende P., Karoum R., Manet G., Rizet C., Schelcher F., Cohen J. Molecular epidemiology or bovine rotaviruses from the Charolais area. Vet Res. 1999;30(5):451–456. [PubMed] [Google Scholar]
- 29.Midgley S.E., Banyai K., Buesa J., Halaihel N., Hjulsager C.K., Jakab F. Diversity and zoonotic potential of rotaviruses in swine and cattle across Europe. Vet Microbiol. 2011;156(3/4):238–245. doi: 10.1016/j.vetmic.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 30.Swiatek D.L., Palombo E.A., Lee A., Coventry M.J., Britz M.L., Kirkwood C.D. Detection and analysis of bovine rotavirus strains circulating in Australian calves during 2004 and 2005. Vet Microbiol. 2010;140(1/2):56–62. doi: 10.1016/j.vetmic.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 31.Cashman O., Lennon G., Sleator R.D., Power E., Fanning S., O'Shea H. Changing profile of the bovine rotavirus G6 population in the south of Ireland from 2002 to 2009. Vet Microbiol. 2010;146(3/4):238–244. doi: 10.1016/j.vetmic.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 32.Badaracco A., Garaicoechea L., Rodriguez D., Uriarte E.L., Odeon A., Bilbao G. Bovine rotavirus strains circulating in beef and dairy herds in Argentina from 2004 to 2010. Vet Microbiol. 2012;158(3/4):394–399. doi: 10.1016/j.vetmic.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 33.Barreiros M.A., Alfieri A.F., Medici K.C., Leite J.P., Alfieri A.A. G and P genotypes of group A rotavirus from diarrhoeic calves born to cows vaccinated against the NCDV (P[1], G6) rotavirus strain. J Vet Med B Infect Dis Vet Public Health. 2004;51(3):104–109. doi: 10.1111/j.1439-0450.2004.00737.x. [DOI] [PubMed] [Google Scholar]


