Abstract
Significant progress has been made in reducing the risk of pathogen transmission to transfusion recipients. Nonetheless, there remains a continuing risk of transmission of viruses, bacteria, protozoa, and prions to recipients. These include many of the viruses for which specific screening tests exist as well as pathogens for which testing is currently not being done, including various species of bacteria, babesiosis, variant Creutzfeld-Jacob disease, hepatitis A virus, human herpes virus 8, chikungunya virus, Chagas disease, and malaria. Pathogen inactivation (PI) technologies potentially provide an additional way to protect the blood supply from emerging agents and also provide additional protection against both known and as-yet-unidentified agents. However, the impact of PI on product quality and recipient safety remains to be determined. The purpose of this consensus conference was to bring together international experts in an effort to consider the following issues with respect to PI: implementation criteria; licensing requirements; blood service and clinical issues; risk management issues; cost-benefit impact; and research requirements. These proceedings are provided to make available to the transfusion medicine community the considerable amount of important information presented at this consensus conference.
ALTHOUGH SIGNIFICANT progress has been made in reducing the risk of pathogen transmission to transfusion recipients, there remains a continuing risk of the transmission of viruses, bacteria, protozoa, and prions to recipients. These include those viruses for which specific screening tests exist: hepatitis B virus (HBV), hepatitis C virus (HCV), human T-cell leukotrophic virus I/II, HIV, and West Nile virus (WNV). In addition, pathogens for which testing is not currently available and/or not being done have been shown to adversely affect the blood supply. These include various species of bacteria, babesiosis, variant Creutzfeld-Jacob disease (vCJD), hepatitis A virus (HAV), human herpes virus 8, chikungunya virus, Chagas disease, and malaria. Pathogen inactivation (PI) technologies may provide an additional way to protect the blood supply from emerging agents and also provide additional protection against both known and as-yet-unidentified agents. However, the impact of PI on product quality and safety remains to be determined.
This consensus conference entitled Pathogen Inactivation: Making Decisions About New Technologies was based on the format that had been used previously and which was initially proposed by the US National Institutes of Health (http://consensus.nih.gov/aboutcdp.htm). The participants of this consensus conference consisted of 3 distinct groups: (a) 15 international experts who summarized the available data and provided additional information relating to PI; (b) 10 individuals of diverse backgrounds who formed the consensus panel and who were responsible for writing the final consensus conference statement; and (c) approximately 220 invited delegates who participated actively in the discussion of the various presentations.
The 6 specific questions addressed to the consensus conference panel were as follows:
-
1.
Implementation criteria: Is the current risk of transfusion-transmitted diseases acceptable in relation to other risks of transfusions? (a) If so, under what new circumstances should PI be implemented? (b) Should the criteria be the same for red blood cells (RBCs), platelets, and fresh frozen plasma (FFP)? (c) Should different criteria be used for certain patient populations?
-
2.
Licensing requirements: What minimum acceptable safety and efficacy criteria should be put into place for the preapproval assessment of pathogen-inactivated products? Specifically, (a) what criteria should govern acceptable toxicology standards and how should they be assessed, and (b) what type of postmarketing surveillance should be required (if any) with the implementation of pathogen-inactivated blood components?
-
3.
Blood service and clinical issues: For PI technologies that have been approved by the regulatory authorities, what implications should be considered before their widespread adoption? Also, if pathogen-inactivated components differ in function from nonpathogen-inactivated equivalent products, how should this information be disseminated?
-
4.
Risk management issues: If PI were to be implemented for all components, in principle, what criteria would allow (a) the relaxation of any current donor deferral/exclusion policies, (b) the cessation of any currently undertaken screening tests, and (c) a decision not to implement new screening tests for agents susceptible to PI? Should multiple inventories be considered for each component, and if yes, how should allocation be decided?
-
5.
Cost-benefit impact: How should the costs/benefits of PI be assessed? Should these be aligned with other blood safety interventions and/or other health care interventions?
-
6.
Research requirements: What other information, considerations, and research-related questions would need to be answered to decide whether/when a particular PI procedure should be implemented?
The presentations by the international group of experts providing the background data for these 6 questions are summarized in this article, as is the discussion that took place after these presentations. The panel's preliminary report is summarized elsewhere.1
Microbiological Reasons for Considering PI in Transfusion Medicine
Presented by Harvey J. Alter, MD
National Institutes of Health, Bethesda, MD
The precautionary principle, which was first endorsed by the US Food and Drug Administration (FDA) after the crisis of the HIV contamination of the blood system, states that for situations of scientific uncertainty, the possibility of risk should be taken into account even in the absence of proof to the contrary. The corollary assertion of this principle is that, when potentially serious risks arise, measures must be taken to minimize that risk. It could therefore be argued that the PI of blood products represents the quintessence of the precautionary principle: almost all potential for transfusion-transmissible disease is eradicated often before the responsible agent is even recognized, let alone conclusively established as a risk to the health of the recipient.
Prevention of transfusion-transmitted infections has traditionally been accomplished by testing donors for infectious disease markers. This approach has certainly achieved some great successes in reducing the risk of blood transfusion. The risk of transfusion-acquired hepatitis, for example, has been reduced from nearly 30% to near zero through the combination of more restrictive donor selection and increasingly sensitive screening assays.2 This reduction in risk, however, occurred over a period of decades, and during the interim, countless transfusion recipients were harmed. Hepatitis B virus, for example, was first recognized as a transfusion risk in 1940, but it was only in 1970 that blood donors could be effectively screened with the first-generation assays for the historical Australia antigen, now known as the hepatitis B surface antigen. This assay was quickly refined and, in combination with the adoption of an all-volunteer donor system, reduced the overall risk of transfusion-acquired HBV to less than 1%. Nevertheless, transfusion-acquired hepatitis of all causes continued to occur at an incidence of approximately 10% throughout the 1970s, primarily because of a disease identified syndromically in 1975 as non-A/non-B hepatitis. The adoption of surrogate assays for this disease (eg, elevated donor alanine aminotransferase levels), the exclusion of donors for behavioral risk factors, and the decreased volume of blood transfused all combined to decrease the incidence of transfusion-acquired hepatitis to around 4% by 1989. It was only with the introduction of a specific serologic assay in 1990 for what was then recognized as HCV that the incidence of transfusion-associated hepatitis fell to less than 0.5%. By that point, however, much damage had already been done. If one assumes that 3 million patients are transfused per year in the United States, then it follows that some 4.8 million people acquired transfusion-acquired hepatitis between 1970 and 1990. Most of these cases were HCV, a disease that carries an 80% risk of chronicity and a 20% risk of cirrhosis and which is now the leading etiologic cause of liver transplantation in North America.
The interval between recognition of a transfusion-transmissible disease and the implementation of measures to prevent it has, over time, shortened. Thus, whereas it took 30 years to introduce screening for HBV and 15 years for HCV, it was only 3 years between the recognition of HIV and the development of a donor screening test, and for WNV, the interval was only 1 year. Yet, there continue to be exceptions to this trend: donor testing for Chagas disease was first recommended in 2002 by the Blood Products Advisory Committee of the US Food and Drug Administration but is only being implemented 5 years later. Perhaps more importantly, there is often an earlier period of delay, during which a disease is not appreciated as posing a risk to recipients of blood transfusion or, indeed, to human health in general. The harm caused by such diseases has been called a “fixed and inevitable property of transfusion practice.”3
Where do these diseases come from? Many are zoonotic in origin, their transition into significant human pathogens the result of primate viruses entering the food supply in Africa (eg, HIV) or a shifting geographic relationship between disease vectors and humans who then serve as incidental hosts (eg, WNV). Having largely safeguarded the blood supply against HIV and transfusion-transmitted hepatitis, the transfusion medicine community is confronted with an ever-growing list of new adversaries: malaria, dengue, babesiosis, ehrlichiosis, borreliosis, human herpes virus 8, and Trypanosoma cruzi (Chagas disease), to name just a few. Each of these is known to be pathogenic and to be transmissible by blood transfusion. Indeed, dengue fever may well emerge as the next true threat to the blood supply: it already causes approximately 100 million cases and 25 000 deaths around the world each year. Most cases of dengue fever are asymptomatic, with a median viremia of 5 days, and both the virus and its mosquito vector are expanding in global distribution.4 On the other hand, the blood industry may initiate screening tests for dengue only to find itself confronted with a completely unanticipated threat from a different pathogen. The dilemma we face, therefore, is whether to wait and see which pathogens emerge as serious risks to the blood supply before taking action (in which case we inevitably consign thousands and perhaps millions of patients to infectious harm during the interim) or simply start testing donors for all potentially transmissible pathogens (which would be prohibitively expensive).
In the face of such a stark choice, PI technologies are an attractive third option. Moreover, they have already proven their value. Transfusion-transmitted WNV, for example, infected 23 people in the United States, including 16 with neurologic disease, before the implementation of a donor screening assay in 2003.5 An estimated 3200 other patients experienced subclinical disease. None of these infections, however, occurred via fractionated plasma products, because of the prior implementation of a solvent/detergent (SD) method of PI by the plasma fractionation industry. It is sobering to contemplate how much harm could have been avoided had this additional safety measure been implemented in the early 1980s, when the hemophilia community was just beginning to be ravaged by HIV and HCV. Although SD treatment is not applicable to cellular blood products, such as RBCs and platelet concentrates, other processes that target nucleic acids (eg, amotosalen or riboflavin) are now available. In addition to blocking transmission of both known and unknown pathogens, including viruses, bacteria, spirochetes, and protozoa, PI may also prevent transfusion-associated graft-vs-host disease (GVHD) by disabling donor lymphocytes.
The concerns that have thus far prevented widespread adoption of PI technologies should not be exaggerated. Although PI invariably involves the addition of novel chemical substances to blood products, these substances are either benign (eg, riboflavin) or are subsequently removed, with residual levels far below established toxicity thresholds (eg, amotosalen [S-59]–treated platelets; the toxicity of residual S-303 [amustaline] in treated RBCs requires further investigation). The reduction in product yield that PI necessitates is generally modest (10%-20%) and consistent with other accepted product modifications such as cell washing or leukoreduction. The additional costs of PI are considerable, but so are the potential savings that may be subsequently realized via elimination of existing donor screening assays (eg, Chagas disease), product testing (eg, bacterial testing of platelets), and product modification (ie, irradiation). Future costs from new screening assays could also be avoided, as could the growing cost from the otherwise inexorable progression from mini-pool to single-unit nucleic acid testing (NAT). Finally, the additional layer of safety offered by PI might allow blood collection agencies to loosen donor exclusion criteria (eg, geographic exclusions for malaria) and thereby increase the size of the available blood supply.
As was the case with the implementation of NAT, close collaboration between blood bankers, governmental regulators, and the private sector will be required to make universal PI of blood products a reality. The goal should be a practicable multicomponent system that will maximize safety, maintain adequate product yield, and have acceptable toxicity. Accomplishing this in a reasonable period will require additional governmental funding, both to improve existing technologies and to develop new processes, particularly with regard to PI of RBCs. However, we must not make the perfect the enemy of the good. Until this idealized multicomponent system is a reality, existing pathogen reduction technologies should be implemented for platelets and transfusable plasma, an approach already undertaken in many parts of Europe. Taking this next step in North America will require an investment of resources and political willpower and, perhaps most importantly, a change in our collective mindset regarding the notion of acceptable risk.
Biochemical and Biologic Mechanisms of PI Methodology
Presentation by Roger Y. Dodd, PhD
American Red Cross Holland Laboratory, Transmissible Disease Department, Rockville, MD
Given by Stephen J. Wagner, PhD
American Red Cross Holland Laboratory
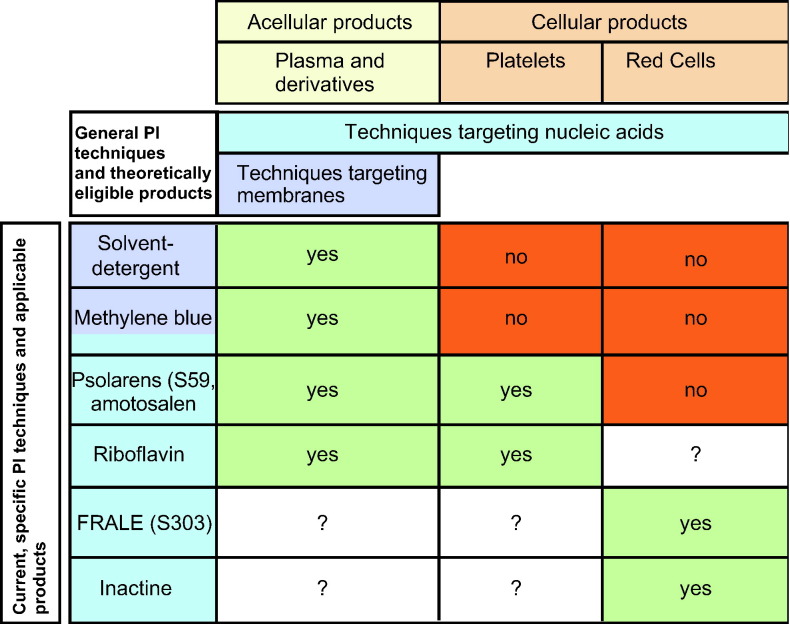
Pathogens in blood components may be intracellular or extracellular, and techniques to inactivate or remove them should not incur any significant biologic or chemical alterations upon the therapeutic product itself. Success is thus predicated on the existence of specific targets in the pathogens, which are absent in, or irrelevant to, the component's function.
Current PI techniques either target membranes or nucleic acids (Table 1 ) and are meant to go beyond the historic successes of plasma and plasma derivative PI from Cohn fractionation and heat treatment. Membrane or envelope-disrupting methods, such as organic solvents or detergents, thus remain applicable only to acellular components. When used, these methods are limited by their inability to disarm the class of nonenveloped viruses. The other methods have a more generalizable potential spectrum of use because they are designed to target nucleic acids with photoactive chemicals or alkylators, thereby shutting down the function (transcription/translation) and/or proliferation (replication) of unwanted microbial passengers, without necessarily destroying the membranes of cellular therapeutic products. As such, most pathogens (viruses, bacteria, fungi, or protozoa) and even donor leukocytes can be functionally eliminated.
Table 1.
Current PI Techniques
The assurance of a PI's nonmutagenicity with respect to the host is crucial with methods that broadly target nucleic acid integrity. Unless the PI agent is harmless to humans in all foreseeable exposure ranges of relevance, any residual quantities must either be extractable and removed or be convertible to a harmless byproduct through the exploitation of the agent's available intrinsic decay properties. Such PI processes would be appealing if they could be shown to be inexpensive and compatible with current good manufacturing practice.
The Methods
Solvent/detergent inactivation of plasma
Solvent/detergent inactivation uses the membrane-disruption solvent tri-n-butyl phosphate (1%) with a detergent such as Triton X-100 during a 4-hour, 30°C incubation of pooled plasma from thousands of donors, followed by the removal of the SD with oil and adsorption chromatography.6 Historical large-scale SD inactivation methods in the United States have been associated with an increased prothrombotic product risk, on account of the undesired incidental decrease in integral antithrombotic plasma proteins, such as protein S or α 2-antiplasmin.7
Photochemical PI in plasma and/or platelet products
Methylene blue (MB) is a phenothiazine dye that, when added to an individual unit of plasma at 1 μmol/L and photodynamically activated by UV light at 590 nm, produces singlet oxygen. Deoxyribonucleic acid is damaged by dye intercalation with free radical roles in strand cross-linking, guanosine oxidation, and indirect depurination.8 Like SD, the application of MB is restricted to plasma and is also limited in its PI abilities. However, the basis for these limitations is different. Red blood cell hemolysis can occur from the effect of reactive MB singlets on lipid and protein components of membranes. On the other hand, the capsids of nonenveloped viruses, and the membranes of bacteria, protozoa, or the infected leukocytes that persist after plasma freeze-thaw, may resist not only these perturbations but MB penetration itself. An elimination micropore filter is thus used to remove residual leukocytes alongside the dye and its photoderivatives.
The psoralens are also photoactive planar molecules, the small size of which facilitates membrane penetration. The dark reaction (base intercalation) is followed by a UV-A–dependent light reaction (320-400 nm over several minutes), in which adducts form between the psoralens and the pyrimidine bases of the nucleic acids, in turn producing irreversible cross-linkages in single- and double-stranded nucleic acids.9Significant photodegradation, and a prolonged (4-6 h) incubation with a compound absorption disk or affinity device, removes the psoralen and byproducts before storage. INTERCEPT (Cerus Europe BV, Leusden, The Netherlands), an S-59 (amotosalen) system, has been tested in plasma as well as platelets. The remarkable activity of psoralens is illustrated in their capacity to inactivate more than 5.4 logs of T lymphocytes and to disrupt disproportionately more base pairs (1:83) than γ-irradiation (1:37 000).10
Riboflavin (vitamin B2), like the other photoactive PI agents, binds to DNA by intercalation. Ultraviolet photolysis of the complex induces guanine oxidation, single-stranded breaks, and the formation of covalent adducts.11, 12 Unlike the other agents, toxicities are not a concern because this is a naturally occurring and safe compound. This agent, like the previous, has been evaluated in platelets and plasma, and its potential use in RBCs is being explored. Success with all blood components would make this a uniquely generalizable technique compared with the others.
Pathogen inactivation techniques explored in RBC products
Pathogen inactivation is challenging in RBC products because hemoglobin is capable of absorbing or scattering UV and visible light up to red wavelengths (700 nm), and the RBC membrane itself is particularly vulnerable to a variety of PI techniques.
The alkylators in a class derived from quinacrine mustard attempt to overcome these issues with their light-independent activity and are known as the “frangible anchor linker effector” compounds.13 An intercalating acridine ring structure is linked to an effector group that covalently attaches to the nucleic acid, and the link between these 2 structures is a hydrolyzable bond capable of “releasing the ring” under the conditions of pH change (ie, neutralizing the typical acidity of storage to a more physiologic level). The INTERCEPT system of one particular frangible anchor linker effector, S-303 (Helinx, Cerus Corp, Concord, CA), incubates with RBCs for 12 hours, during which time nucleic acid cross-linking occurs, with the release of S-300 as its degradation product. S-300 is rapidly eliminated before a final compound removal step. Some S-303 may remain bound to proteins, on or within RBCs, and thus serve as an immunogen with respect to acquired or preformed antibodies of unclear significance.
The INACTINE system represents another PI technique using PEN110, a low-molecular-weight, highly water-soluble cationic ethyleneimine derivative that instantly traverses cell membranes and damages nucleic acids without cross-linking. Over a 6-hour incubation period, PEN110's tail covalently binds to negatively charged phosphate groups, and the protonation-activated aziridine site selectively alkylates N7 on guanosine, breaking open its imidazole ring.14 The structural change is recognized as a stop signal, the base is lost, and strand breakage occurs. PEN110 is subsequently removed by washing because of its residual mutagenicity risk. As with S-303, antibody formation has also been seen, although this never appeared to be clinically significant, and antigenic RBC surface changes were never detected. Nevertheless, clinical trials with PEN110 have been suspended.
Any exponential model of PI, with a log-linear dynamic, is truly no more than a pathogen reduction technique because complete inactivation (“zero leftover pathogen”) may not be achievable with high-input inocula. Reduction is also variable, depending upon inoculum size, species, and the type and duration of PI. The applicable intensity of PI is ultimately restrained by potential collateral damage to the integrity and function of the product. Pathogen inactivation is also subject to the errors and dangers of fallible human processing, which may lead to a failed inactivation or the missed removal of toxic byproducts.
Toxicity Problems Relating to the PI of Blood Products: Impact on Recipients
Presented by John Chapman, PhD
Thermogenesis Corporation, Rancho Cordova, CA
Toxicology has been defined as “the study of adverse effects of chemical, physical, or biologic agents on living organisms and the ecosystem, including the prevention and amelioration of such adverse events.”15 For the substances used to inactivate pathogens in blood products, toxicity is required but must be selective. To be effective, these substances must have a high degree of lethality against the wide variety of living organisms that might contaminate a blood product, from viruses to simple eukaryotic parasites, while at the same time minimizing any collateral injury to the normal components of the blood product. Although SD treatment, which disrupts lipid membranes, is an option for plasma products, the only viable approach to achieving this selective toxicity in cellular blood products is to target nucleic acids. Several such technologies have been developed and have entered clinical trials. Most, such as MB, amotosalen, riboflavin, and thionine, require activation by light, whereas others may be activated by shifts in pH (eg, S-303) or are activated upon binding to the negatively charged phosphate groups present on nucleic acids (eg, PEN110). These products have demonstrated, to various degrees, a high degree of selective toxicity, in that they successfully inactivate a wide variety of transfusion-transmissible pathogens with minimal effect on the therapeutic potency of the blood product.16, 17, 18, 19, 20 Other toxicities that must be considered include the risk to personnel involved in processing the blood product, the risk to the environment from disposal of waste products, and the risk to the transfusion recipient. This last risk is the focus of the remaining discussion.
Principles of Modern Toxicology
The field of toxicology has evolved over the past 20 years into a systematic approach for characterizing hazards, exposure levels, and dose-response relationships so as to permit meaningful risk assessments to be made. A modern toxicology test battery begins with pharmacokinetic studies and is followed by assessment of single-dose toxicity, repeated-dose toxicity, reproductive toxicity, genotoxicity, and carcinogenicity. To detect all potential hazards, the dosages selected for study should range to high enough to induce generalized toxicity syndromes in test animals, such as weight loss and decreased food consumption. The combined results of these toxicity studies provides a comprehensive hazard assessment, which comprises all potential adverse effects at maximal tolerated doses and the dose-response relationship for these toxic effects. The comprehensive risk assessment is in turn weighed against an exposure assessment, which involves assumptions regarding the typical dose of the substance, the frequency with which it will be administered, and the expected duration of treatment. Ideally, the maximum safe dose determined by animal studies should have a 1000-fold safety margin: a 10-fold margin to account for extrapolation from animal to human physiology, another 10-fold margin to account for physiologic variation in humans, and another 10-fold margin again to account for variability in the detection of all toxicology end points.
Toxicology Studies of Pathogen-Inactivating Processes
For PI substances currently in phase 3 clinical trials or higher, toxicity studies have so far been encouraging. The dose threshold for acute toxicity from UV-A–activated amotosalen, for example, is 150 mg/kg in rats (mortality) and 30 mg/kg in dogs (central nervous system effects). These doses are 150 000 and 30 000 times higher, respectively, than what would be delivered in a typical dose of amotosalen-inactivated blood products. For other toxicities, however, the safety margin is below 1000. Reproductive toxicity (determined by histologic rather than functional assessment), for example, was demonstrated at a threshold of 0.35 mg/kg, or 350 times the expected clinical exposure.16 The reproductive risk of PEN110 has also been found to have an appropriate safety margin.18 It should be borne in mind that many widely used medications, such as acetaminophen, have safety margins of only 100-fold. It should also be noted that all PI substances are water soluble and rapidly excreted, and therefore, the trace amounts infused in treated blood products will not bioaccumulate. The pharmacokinetic characterization of amotosalen has been published.20
Genotoxicity and carcinogenicity are particularly important concerns with PI substances because these substances actively target nucleic acids and do not have any particular tissue specificity. Because carcinogenicity is now known to be caused by damage to nucleic acid, a battery of assays to measure chemical-induced DNA mutations have been established and are designated as genotoxicity assays.17 For example, positive indicators of genotoxicity include induction of DNA repair in cultured rat hepatocytes (as manifested by unscheduled DNA synthesis); induction of mutations in bacteria such as the Ames assay (Salmonella base pair substitutions, frame shifts); and the induction of mutations in mammalian cell lines such as the in vitro mouse lymphoma assay. A positive result in these assays means not that the chemical is carcinogenic but that it does have an increased risk of causing cancer, and exposure assessments are particularly important. A negative result is strong assurance that the chemical is not carcinogenic.
Because traditional bioassays in rats and mice are time consuming (>2 years), there has been increasing interest in recent years in assessing carcinogenicity risk directly using transgenic mice heterozygous for the wild-type p53 gene in a 6-month cancer bioassay. Because this mouse strain is innately vulnerable to carcinogenesis because of the loss of the tumor suppressor gene, only relatively brief exposure to the chemical under study is required before tumor formation is observed, and fewer animals need be tested. The p53+/− cancer model also has the advantage of providing whole-animal dosimetry data, while allowing researchers to focus on specific molecular events relevant to human malignancy.
The carcinogenicity of the PI substance PEN110 has been assessed using the mouse p53+/− model, and the results obtained were reassuring. If one begins by assuming that the residual concentration of PEN110 in a unit of treated RBCs is 0.05 μg/mL, a patient receiving 2 U of RBCs every 3 weeks for 40 years would have a lifetime exposure of 0.034 mg/kg. In a study of 28 animals receiving 3 infusions of PEN110 per week for 26 weeks, by contrast, the dose had to exceed 4.5 mg/kg before tumor induction exceeded what was observed in the control arm. In a 70-kg patient, this dose would be equivalent to receiving nearly 40 000 U of PEN110-treated RBCs per week. The carcinogenicity of a more typical human exposure can therefore only be calculated by extrapolating backwards from the dose-response curve observed in experimental studies, and unsurprisingly, the degree of added risk is very small. Even if one were to base risk assessment on the upper limit of the 95% confidence interval (CI), treating the entire US blood supply (approximately 14 million RBCs per year) with PEN110 would be expected to result in only 1 extra malignancy every 70 years.
The p53+/− cancer model has been used with both the PI compounds and the blood products treated with PI. The testing of the PI blood product is an important consideration because all PI substances participate in a variety of side reactions before their removal from the blood product, and although the resulting metabolic byproducts generally appear to be less potent than the parent compound, they may still carry their own toxicity risks. Wide safety margins for these byproducts are impossible to confirm in traditional in vitro genotoxicity studies because of the dosing limitations imposed by the volume of the blood product. In the p53+/− model, however, the ability to expose the animals on a regular basis over a 6-month period combined with the increased susceptibility of the mice to carcinogenesis allows for a more rigorous assessment of carcinogenic risk. In one experiment, 240 mL of blood was collected on a weekly basis from 300 different mice. This blood volume (equivalent to a unit of packed RBCs) was treated with PEN110, only partially washed, and then infused as a series of weekly doses for 26 weeks into 50 p53+/− mice. There was no observed toxicity or increase in tumor formation compared with control animals, demonstrating that PEN110-treated RBC products were not carcinogenic. Similar studies in a p53 +/− model have been reported for amotosalen and for S-303.
It is informative to contrast the risk assessment of PI substances with ethylene oxide (ETO), a sterilant used for years in drug products and medical devices. This substance has been demonstrated to be mutagenic, carcinogenic, and teratogenic in animal studies, and hypersensitivity reactions in patients undergoing hemodialysis have shown strong correlation with immune responses to the ETO used to sterilize dialysis device. Nevertheless, the US FDA is proposing to approve the use of ETO to sterilize the components of cord blood processing systems and storage containers, so long as the residue level does not exceed 5 mg per device. The residual quantity of amotosalen in treated platelets, in contrast, is 50 μg, whereas the residual concentration of PEN110 in treated RBCs is only 15 μg. The issues concerning ETO use as a sterilant for disposables processing stem cells has been reviewed.21
In addition to the risks described, another type of toxicologic effect is the elicitation of an immune response to the PI blood product. This occurs because of the modification of macromolecules on the cell surface so as to create a new antigen (neoantigen). The elicitation of the immune response can result in the product of antibodies, which can bind to the blood cell product and cause them to be cleared from the circulation. During the conduct of a phase 3 trial, PEN110 was determined to have elicited such an immunologic response with PEN110-treated RBCs. As a result, the PEN110 technology has been put on hold until this technical issue can be resolved. A similar immunologic problem was observed with the S-303–treated RBCs, causing a delay in that product development effort; however, new trials with a modified S-303 process for RBCs are currently underway with this technology.
Conclusion
Current toxicology testing strategies are working to identify hazards both before and during the clinical trial phase of drug development. Although no PI substance (or indeed any substance) can be considered completely nontoxic, available assays suggest that PI blood products do not impart any measurable toxicity or significant risk of long-term carcinogenicity to patients This is not to say that these products pose no risk at all: clinical trials of RBCs treated with PEN110 and S-303, for example, were stopped early because of the development of positive, albeit asymptomatic, direct antiglobulin tests in selected patient populations. This finding supports the idea that the current approach for evaluating the PI technologies (preclinical studies in animals and clinical trials in humans) is working to identify potential hazards. Whether the risks (observed or theoretical) of PI blood products are justified by the additional benefit of safety they provide is still a matter of discussion. However, it is clear that those PI blood products that have successfully completed preclinical and clinical trials have a favorable safety profile when compared with other drugs and chemicals in common use, including substances that are already being added to blood products.
Efficacy of Pathogen-Inactivated Plasma
Presented by Christopher Prowse, PhD
Scottish National Blood Transfusion Service, Edinburgh, Scotland
There has been longer clinical experience with PI of plasma products than with RBCs or platelets, in part because the cellular blood components pose particular technical challenges to the development of PI technologies. At the time of writing, 3 different plasma PI processes have been licensed. The first involves pooling individual plasma donations and treating with SD so as to disrupt the lipid membrane present on enveloped pathogenic viruses.22, 23 The second process requires the addition of a psoralen (amotosalen hydrochloride, previously known as S-59) to individual plasma donations with subsequent activation by UV light, a process that results in inhibition of DNA RNA replication.24 The third process also involves the addition of a nucleic acid sensitizer, MB, to individual plasma donations, with subsequent activation by visible light.25 There is an additional photoinactivation process involving the addition of riboflavin and subsequent activation by UV light26 that is just now entering clinical trials and that will not be discussed in detail.
Much of the published clinical experience with PI plasma products comes from Europe, where many countries have a regulatory requirement for either PI plasma or a quarantine process in which collected plasma is held until the donor is retested at a later date for transfusion-transmissible diseases. Although PI plasma products had previously been licensed in North America, none are currently being distributed to hospitals by licensed blood collection agencies.
Safety
Because the chemical agents required to inactivate pathogens may also be harmful to transfusion recipients, all of the aforementioned plasma PI processes include an additional step to remove these chemicals from the final product. In vitro studies have demonstrated that the residual concentration of these substances is far below those that animal studies would suggest are toxic doses. Moreover, clinical experience with these products suggests they are safe: at the time of writing, more than 6 million units of SD plasma and 3 million units of MB-treated plasma have been transfused without any apparent toxicity to recipients. There is less clinical experience with amotosalen-treated plasma, with only about 5000 U infused at the time of writing.
Another issue of potential concern with PI technologies is the creation of neoantigens as a result of the modification of native proteins. However, although this phenomenon has been observed for 2 PI techniques applicable to RBCs, animal studies, human clinical trials, and postmarketing surveillance have not revealed a similar problem when available PI processes have been applied to plasma.
Despite the absence of any evidence of toxicity, however, SD plasma products have been burdened by additional safety concerns. The first of these relates to the effect of the SD treatment process on the hemostatic equilibrium that exists between the procoagulant and anticoagulant proteins in native plasma. The distribution of SD plasma in the United States by the American Red Cross in the late 1990s was followed by several postmarketing reports of thrombosis, bleeding secondary to hyperfibrinolysis, and death, particularly in patients undergoing liver transplantation,27, 28 and it was partly on the basis of these reports that SD plasma was effectively withdrawn from the market in the United States in 2002. These adverse events were attributed to a loss of protein S and antiplasmin that occurred during SD treatment of plasma. Notably, the SD plasma product provided in Europe, which is made using a somewhat different process, does not appear to have been associated with the same adverse events. Although there have been cases of possible thrombotic events in patients with thrombotic thrombocytopenia purpura (TTP) receiving European-manufactured SD plasma,29 it is not clear whether SD plasma was the causative factor. Perhaps more importantly, a study focusing on patients undergoing liver transplantation, an ostensibly higher risk population, failed to document any excessive bleeding with the use of European-manufactured SD plasma.30, 31 Other studies have shown that the SD plasma products manufactured in Europe have reduced loss of protein S and antiplasmin in comparison with the US product.32, 33 It has also been suggested that the risk from US-manufactured SD plasma was itself exaggerated.34
An additional concern with SD plasma is that although it is effective against most of the known clinically significant (lipid-enveloped) transfusion-transmitted viruses, it is ineffective against non–lipid-enveloped viruses such as parvovirus B19 and HAV (other PI processes have variable impact on these pathogens). The risk of transmission is further exacerbated by the fact that SD plasma batches are made from pools of up to 2500 individual donations; in the United States, 37 lots of SD plasma were voluntarily recalled because of parvovirus B19 contamination, although none of these cases involved clinical disease. In one phase 4 study, 18 of 77 volunteers seroconverted against parvovirus B19 after infusion of SD plasma, and it was found that all implicated lots had high levels of viremia (>107 GEq/L). Individual plasma donations in SD plasma batches are now checked for parvovirus B19 and HAV using NAT , with European Union regulations requiring that all lots with B19 viremia exceeding a considerably lower threshold of 104 GEq/L be discarded (approximately 1 in 800 lots will exceed this threshold for parvovirus B19). It should be noted in passing that, although SD treatment is less effective than other PI processes against bacteria or other cellular organisms, these pathogens are already eliminated in all plasma products via the physical processing that clears most cellular components and the freeze-thaw cycle that destroys whatever remains. Reports of bacterial contamination from plasma transfusion are for the most part attributable to contamination of the water baths used for thawing.
The transmission of parvovirus B19 before the initiation of donor screening highlights the issue of donor pooling, which, among PI plasma products, is a specific concern with SD plasma. For transfusion-transmissible diseases such as vCJD, in which the pathogenic agent can neither be tested for nor demonstrably eliminated by available PI technologies, the high number of donor exposures per transfusion from any pooled product is especially worrying. On the other hand, pooling also greatly dilutes the infectious load present in any single donation, possibly to levels below the minimal infectious dose, particularly if the pool also contains a neutralizing antibody. It is notable that with more than 6 million doses of SD plasma transfused worldwide, there has not been a single report of either transfusion-transmissible vCJD or transfusion-related acute lung injury (ALI) (TRALI) with this product. In the case of TRALI, the effect of pooling has been demonstrated to reduce the concentration of donor antileukocyte antibodies, thought to contribute to TRALI, to below the limits of detection. Pooling of plasma from donors of varying ABO groups, when performed in proper proportions, also allows for the production of universally ABO-compatible plasma. Additional precautionary steps available to blood collection agencies include the importation of plasma from North America (to avoid vCJD risks) and use of plasma from male donors (to avoid donations with a higher risk of containing antileukocyte antibodies.) The United Kingdom has recently adopted imported SD plasma as its PI plasma product of choice for patients with TTP despite the aversion toward the use of pooled products in the United Kingdom due to vCJD concerns.
Efficacy
The clinical efficacy of PI plasma has traditionally been evaluated through comparison with untreated FFP. It must be acknowledged, however, that the evidence base for the efficacy of FFP is itself not very strong. In a recent systematic review of 58 trials that evaluated the efficacy of FFP, the only clinical indication found to be adequately supported by randomized controlled trials (RCTs) was TTP. For other standard indications, the evidence of efficacy of FFP was limited to either nonrandomized trials (massive transfusion, disseminated intravascular coagulation, reversal of coumarin, correction of congenital coagulation factor deficiencies) or expert opinion (neonatal hemorrhagic disease). Most of the trials identified were either small or had inadequate protections against bias.35
Even with this caveat in mind, the evidence that PI plasma is therapeutically equivalent to FFP is not always convincing. Most of the published clinical studies on MB plasma are case reports or uncontrolled observational studies, and comparative studies on MB plasma vs FFP in healthy volunteers36 or patients undergoing cardiopulmonary bypass37 are too small and of insufficient methodological rigor to conclude therapeutic equivalence. Moreover, more recent studies comparing MB plasma and FFP in patients with TTP suggested that MB plasma may actually be less effective.38, 39, 40 It is notable that the introduction of MB plasma in the Catalonia region of Spain in 1997 was followed by a dramatic increase in the ratio of plasma to RBC transfusions and was accompanied by growing demand for fibrinogen in the form of cryoprecipitate.41 The implication of this observation is that MB plasma has reduced hemostatic quality, perhaps due to the 20% loss of fibrinogen that is observed after MB treatment of native plasma.42 Experience elsewhere shows little difference between the use of FFP and MB plasma,43 and the extensive experience with MB plasma in Europe would tend to support this observation. Although all licensed PI plasma products have approximately 20% to 30% less coagulation factor VIII than native plasma, this is unlikely to be clinically significant except in patients with congenital factor VIII deficiency.
The evidence suggesting clinical efficacy of SD plasma is also relatively weak. Although there have been several published RCTs and controlled observational studies suggesting equivalence with FFP, most of these studies were underpowered.44 Postmarketing surveillance in the Republic of Ireland, where SD plasma was introduced in 2002, has not raised any concern regarding inadequate hemostasis in several high-risk populations such as women with obstetric or gynecologic emergencies, critically ill neonates, and patients with liver disease.45
Although there is less clinical experience with amotosalen plasma than with the other 2 licensed pathogen-inactivated plasma products, the trials evaluating its clinical efficacy have been more rigorously designed. In one observational study, the transfusion of amotosalen plasma achieved incremental coagulation factor recoveries comparable with FFP, with the exception of fibrinogen, prothrombin, and factor XIII, and provided clinically effective hemostasis. A subsequent RCT of amotosalen plasma vs FFP in patients with acquired coagulopathy (predominantly patients with liver disease) showed noninferiority in the correction of the prothrombin time; although statistical noninferiority was not demonstrated in correction of the activated partial thromboplastin time, there was in fact a trend toward greater efficacy with amotosalen plasma, and no difference in clinical outcomes was observed.46 In a separate RCT comparing amotosalen plasma with FFP for the treatment of TTP, no differences in clinical outcomes were observed, although the study was underpowered to prove noninferiority.47
Cost
If one only takes account of those viruses screened for by transfusion services, the marginal cost effectiveness of SD plasma, and probably PI plasma products in general, is astronomical (>US$2 000 000 per quality-adjusted life-year [QALY]). For SD plasma, avoidance of TRALI may reduce this to acceptable levels (approximately US$100 000 per QALY).48, 49, 50 For untested or emerging pathogens, there are theoretical benefits of PI plasma use, if testing can be avoided, but these are difficult to quantify.
Summary
All licensed PI plasma products effectively reduce the pathogen load to clinically insignificant levels for most of the clinically significant (lipid-enveloped) viruses; the residual risk from HAV and parvovirus B19 in SD plasma can be addressed by enhanced donor screening. Solvent/detergent plasma requires pooling of individual donors, which may increase the risk from untested and noninactivated pathogens such as vCJD but which may decrease the risk of TRALI. Toxicity and neoantigen induction have not been concerns to date, despite extensive clinical experience with SD plasma and MB plasma and preliminary experience with amotosalen plasma. Pathogen inactivation of plasma does induce some loss of potency, for example, of factor VIII, and with one SD plasma product (no longer marketed), the loss of protein S and antiplasmin has been associated with adverse clinical events. The quality of trials evaluating the efficacy of PI plasma vs FFP has historically been poor but has improved in recent years.
Efficacy of Pathogen-Inactivated Platelets
Presented by Sherrill S. Slichter, MD
Puget Sound Blood Centre, Seattle, WA
Efficacy of PI platelets can be evaluated by both in vitro and in vivo studies. Because in vitro studies rarely reflect what happens in vivo, only the in vivo studies will be discussed. In vivo studies can be used to assess both platelet viability and function. Platelet viability is usually evaluated first by performing autologous radiolabeled platelet recovery and survival measurements in healthy volunteers. After these studies are completed, platelet transfusion studies in thrombocytopenic patients are conducted to determine platelet viability by assessing posttransfusion platelet increments, corrected count increments, and days to next transfusion. In vivo hemostatic function of transfused platelets given to thrombocytopenic patients can be evaluated by bleeding time measurements correlated with pre- and posttransfusion platelet counts, direct visual and/or diagnostic assessments of bleeding within multiple organ systems, and red cell transfusion requirements.
Studies have shown that PI platelets have inferior platelet recoveries and survivals as compared with conventional platelets. In a study by Goodrich et al,51 autologous platelets were collected from 18 healthy volunteers by apheresis. Platelets were then prepared with (n = 11) or without (n = 7) a prestorage PI procedure and stored for 5 days. The PI procedure used the same dose of riboflavin (50 μmol/L) with varying doses of UV-A exposure (7.2 J/mL in 5 healthy volunteers and 12.4 J/mL in 6 healthy volunteers). At the end of storage, platelets were labeled with 111indium and reinfused. Pathogen inactivation platelets had significantly lower percentages of recovery and shorter survivals than similarly stored conventional platelets at both UV-A doses (P < .05 for all measurements). Moreover, increasing the UV-A dose produced logarithmic decreases in both post-transfusion platelet recoveries and survivals. These results are in keeping with a single-blind, crossover 5-day platelet storage study by AuBuchon et al.52 111Indium-radiolabeled autologous platelet recoveries and survivals were statistically significantly lower for all measurements (P < .05) in volunteers who received platelets treated with riboflavin and 6.2 J/mL UV-A vs conventional platelets (platelet recoveries were 50% ± 19% vs 67% ± 13%, and survivals were 4.3 ± 1.1 vs 5.9 ± 1.1 days, respectively). Snyder53 observed similar outcomes with platelets treated with amotosalen (150 μmol/L) and 3.0 J/mL UV-A. 111Indium-radiolabeled autologous 5-day stored platelet recoveries and survivals were both statistically significantly lower (P < .01) for treated vs conventional platelets (platelet recoveries were 39 ± 10% vs 50 ± 9%, and survivals were 4.4 ± 1.4 vs 5.9 ± 1.3 days, respectively). On the other hand, neither the use of a compound absorption device to remove residual amotosalen or its degradation products nor γ-irradiation had any further negative impact on the viability of pathogen-treated platelets.
There have been no head-to-head comparisons of riboflavin/UV-A– vs amotosalen/UV-A–treated platelets. However, comparing the data from the studies by AuBuchon et al52 and Snyder et al,53 platelet recoveries were somewhat better with riboflavin/UV-A–treated platelets, whereas platelet survivals were slightly better with amotosalen/UV-A–treated platelets. For riboflavin/UV-A–treated platelets, platelet recoveries and survivals were reduced by 25% and 27%, respectively, compared with their corresponding conventional autologous radiolabeled platelet transfusion studies. Similar data for amotosalen/UV-A–treated platelets demonstrated reductions in platelet recoveries and survivals of 14% and 20%, respectively.
The assumption is that transfused platelets are able to maintain hemostasis. A correlation between platelet count and bleeding time has been demonstrated previously.54 To examine the in vivo platelet function of amotosalen/UV-A–treated platelets, bleeding times were compared in 10 thrombocytopenic patients who were transfused in random order with both amotosalen/UV-A–treated platelets and conventional platelets.55 Pretransfusion bleeding times and platelet counts were similar in both groups, and there were no statistically significant differences between bleeding times at 1 to 2 and 18 to 24 hours posttransfusion for the treated vs the conventional platelets. Moreover, this same study demonstrated a robust direct relationship between posttransfusion platelet counts and bleeding times for both the treated and conventional platelets.
Finally, PI platelets were shown to be noninferior to conventional platelets in reducing bleeding in thrombocytopenic patients. In the SPRINT trial, a large, multicenter, RCT, chronically thrombocytopenic patients were assigned to receive either pathogen-treated apheresis platelets (amotosalen/UV-A, n = 318) or conventional apheresis platelets (n = 327) for up to 28 days.56 For the primary outcome measure of the incidence of World Health Organization grade 2 bleeding, there was no statistically significant difference between patients in the treated arm (57.5%) vs patients in the conventional arm (58.5%). For the incidence of World Health Organization grade 3 or 4 bleeding, again, no difference was observed between the study arms (4.1% for patients in the treated arm vs 6.1% for patients in the conventional arm). The number of red cell transfusions given in both arms was the same (5.5 vs 5.0, respectively). On the other hand, posttransfusion platelet counts (37 000/μL vs 50 000/μL), platelet increments (21 000/μL vs 34 000/μL), and corrected count increments (11 000/μL vs 16 000/μL) at 1 and 24 hours posttransfusion were significantly lower in the treated vs the conventional arm (1-hour data are provided, P < .001 for all values at both 1 and 24 hours posttransfusion). These differences in posttransfusion platelet responses, in part, could be explained by the fact that patients in the treated arm received a slightly lower mean platelet mean dose of 3.7 × 1011 vs 4.0 × 1011 platelets per transfusion (P < .001). Patients in the treated arm also had shorter mean intervals between transfusions (1.9 vs 2.4 days, respectively; P < .001) and thus received 26% more platelet transfusions per patient (8.4 vs 6.2, respectively; P < .001).
In conclusion, PI using UV-A exposure with either amotosalen or riboflavin results in statistically significant decreases in autologous platelet recoveries of 14% to 25%, respectively, and survivals of 20% to 27%, respectively. Loss of platelet viability with PI using amotosalen/UV-A was confirmed in a large transfusion trial in thrombocytopenic patients. Patients receiving treated platelets compared with patients receiving conventional platelets had statistically significant decreases in both posttransfusion platelet counts and intervals between transfusions, resulting in the need for more platelet transfusions. This could be partially accounted for by a lower mean platelet dose/transfusion in patients who received treated platelets. However, in spite of poorer platelet responses, amotosalen/UV-A–treated platelets were comparable to conventional platelets in ability to maintain hemostasis as evidenced by assessing bleeding time measurements, clinical evidence of bleeding, and RBC transfusion requirements in thrombocytopenic patients.
Clinical Experience with Pathogen-Inactivated Platelets
Presented by Jean-Pierre Cazenave, MD
Établissement Français du Sang–Alsace, Strasbourg, France
The INTERCEPT Blood System for platelets uses amotosalen (S-59) and UV-A to pathogen-inactivate either leukoreduced apheresis platelet components (APC-LR; 46% plasma + 54% InterSol, Cerus Corp) or leukoreduced buffy coat platelet components (BCPC-LR; 35% plasma + 65% InterSol) according to International Conference on Harmonisation pharmaceutical standards. A number of clinical trials with INTERCEPT platelets have been completed in healthy subjects and thrombocytopenic patients in both Europe and the United States, with the largest number evaluated in the “Transfusion of pooled buffy coat platelet components prepared with photochemical pathogen inactivation" study (euroSPRITE) (phase 3 RCT, n = 103, 4 centers)57 and the “Therapeutic efficacy and safety of platelets treated with a photochemical process for pathogen inactivation" study (SPRINT) (phase 3 RCT, n = 645),56 respectively. The primary end point of 1-hour count increments were comparable for equivalent doses of INTERCEPT and conventional buffy coat platelets (P = .53), and all secondary end points were similarly comparable (platelet transfusion refractoriness, acute transfusion reactions, intertransfusion intervals, RBC transfusion requirements, and hemorrhages). Analogous phase 3B evaluation in apheresis platelets, with and without PI, revealed similar primary and secondary end points once again.58
The platelet concentrates obtained for the INTERCEPT Blood System, during either the apheresis platelet collections or whole blood–derived buffy coat platelets (in pools of 6), demand less than 10 minutes of hands-on time and permit same-day, overnight, or next-day processing. InterSol platelet additive solution maximizes the divertible plasma yield from donors, with the hope that reduced plasma content in platelets may reduce transfusion reactions. The platelet content of these units is 2.5 to 6.0 × 1011, in a volume of 300 to 390 mL, with less than 2 μmol/L residual amotosalen in the final products. The regulatory status of these products now includes Conformité Européenne mark registration (2002), Agence française de sécurité sanitaire des produits de santé approval (2005), and Paul-Ehrlich-Institut (PEI) marketing authorization (Germany, 2007).
The Établissement Français du Sang (EFS), the French national transfusion service, consists of 18 regional blood centers in France and 4 overseas centers (Ile de La Réunion, Martinique, Guadeloupe, and French Guyana, in 2005, transfused 243 000 platelet doses). The rapid introduction (more than weeks in May 2006) of INTERCEPT PI platelets in EFS–Ile de La Réunion was rationalized by the emergence of the chikungunya virus, for which specific testing was not available. Although EFS–Nord de France was able to export plasma and RBCs, the rapid transport of platelets (the use of which averaged 100 platelet concentrates per month) was not feasible. Implementation and training of personnel did not require additional staff, and there was no impact on platelet use, although a reduced rate of acute transfusion reactions from these products was observed.
EFS-Alsace, a large blood center, which transfuses 12 000 U of platelets annually in a buffy-coat–to–apheresis ratio of 60:40, also adopted the INTERCEPT Blood System for platelets without complications in July 2006. To date, more than 7000 INTERCEPT platelet concentrates have been transfused to 1700 patients, with a processing loss of 7.4% ± 1.2% (24 ± 4 mL or 0.3 ± 0.07 × 1011 platelets). There was no impact on platelet use, γ-irradiation was not required, and cytomegalovirus (CMV) serology was unnecessary. Six-unit pools of BCPC-LR were distributed at a median of 2.9 days after a day 2 product release, and APC-LR at 2.3 days. Clinician responses were positive, and once again, acute reactions were reduced (1.2% vs 3.0% for untreated platelet concentrates with 65% T-Sol [Baxter Healthcare Corp, Lessines, Belgium] and 6.9% for untreated platelet concentrates in 100% original plasma). There were no reported episodes of TRALI, bacterial sepsis, or death.
The EFS national strategy is to train other centers of excellence to adopt this technology, with a transition to additive solutions to increase plasma for fractionation and to reduce transfusion reactions. More than 60 000 doses of INTERCEPT platelets have now been transfused in 56 centers across 11 countries, and INTERCEPT plasma is now undergoing validation studies at 8 European blood centers. Net costs may be balanced by the shift from APC-LR to BCPC-LR and the lack of requirement for γ-irradiation, bacterial detection measures, and CMV serology testing. New tests, such as HBV NAT and protozoal testing for Chagas, malaria, or leishmania, will not need to be adopted, and donor deferral criteria may become less stringent and more inclusive, without sacrificing confidence in protection against (reemerging/emerging infections such as avian influenza H5N1, severe acute respiratory syndrome, and chikungunya virus.
In summary, the INTERCEPT system was adopted rapidly and successfully in small and large regional blood centers, with the reassurance of quality control processes and acceptable net cost, gaining the intended benefits of PI and noninferiority (or superiority) for observed acute transfusion reaction rates.
Assessment of RBC Systems
Presented by James P. AuBuchon, MD
Dartmouth-Hitchcock Medical Center, Lebanon, NH
New RBC collection, preparation, or storage systems are usually subjected to both in vitro and in vivo analyses to determine their suitability for clinical application. Most regulatory agencies attempt to document safety and efficacy of the system as part of their assessment before authorization for clinical use in their jurisdiction. The focus of this presentation is on the approach used by the FDA in the United States.
The FDA uses situation-specific approval criteria that require progressively more extensive testing when more substantial changes from established approaches are proposed. Generally speaking, with increasing risk of patient impact, more complex tests are required for a new technology. For example, minor changes to existing technology may require only in vitro testing, whereas major changes, such as introduction of PI technology, will require in vivo experiments (eg, radiolabeled autologous recovery and survival) as well as patient clinical trials to establish its safety and efficacy. Success at this juncture may lead to licensure; however, the FDA may additionally require implementation of a postmarketing surveillance program to detect rare events. This situational approach assures the agency and the manufacturer that the system is performing as expected before proceeding to each new level of testing, maximizing the likelihood of success and minimizing the risks to subjects/patients at the next testing level.
The FDA currently uses both absolute and relative criteria to assess the outcome of required tests. Absolute criteria specify that hemolysis must be less than 1% at the end of storage, the residual leukocyte content less than 5 × 106/U (for leukoreduced units), and postfiltration RBC recovery more than 85% of the original RBC mass, with 95% confidence that at least 95% of the population of units meets each of these requirements. The criteria assessed on a relative basis are judged against a control population of units from a previously licensed system with the trial having the power to detect a 20% difference. These analyses include standard hematologic parameters (concentrations of RBC, WBC, and platelets); concentrations of adenosine triphosphate (ATP), 2,3-DPG, glucose, and lactate; as well as determination of pH and morphology score. The absolute criteria are arbitrary but adequate and appropriate. The relative criteria provide interesting information but are of very limited utility. For example, only ATP concentration was shown to be predictive of RBC recovery, and this is only a dichotomous function with 40% to 50% of day 0 levels being necessary for viability.
In vivo recovery has long been regarded as the gold standard of viability. The proportion of 51chromium RBCs remaining in circulation 24 hours after autologous reinfusion can be determined through estimation of the total RBC mass by dilution/back extrapolation (single-label recovery [SLR]) or through actual determination by simultaneous infusion of 99mtechnetium-labeled freshly collected RBCs (double-label technique [DLR]). Double-label technique provides improved accuracy, particularly at lower recoveries, whereas SLR is simpler and quicker for subjects and researchers, is associated with less radiation exposure, and is less expensive. Analysis of 513 paired comparisons of SLR and DLR recoveries revealed that results obtained by these 2 methods were statistically significantly different. However, the difference was small, with SLR producing recoveries about 1 percentage point higher than DLR.59 This issue will be further explored by future studies of the Biomedical Excellence for Safer Transfusion (BEST) Collaborative. Determination of the long-term survival of RBCs is usually only required when the biochemical integrity of the RBCs may have been affected, such as through PI.
The requirements for RBC in vivo recovery have evolved over the years. Mean 70% recovery 24 hours after reinfusion of radiolabeled RBCs was established as a standard without much supportive scientific evidence in the 1940s. Over the years, the mean was increased to 75% recovery and then to 75% recovery with a lower bound of 95% CI of more than 70%. The current criteria call for mean recovery of more than 75%, SD of less than 9%, and 95% CI 1-sided lower confidence limit for population proportion of successes of more than 75% in more than 70% of cases with N ≥ 20 at least in 2 sites.60 This new requirement appears to pose a significant hurdle that many currently used systems could not meet. The BEST Collaborative is currently collecting data on RBC recovery for the blood systems currently in use in the United States. The proposed requirements and their variations are being examined to determine which aspects of the tripartite concept present the greatest challenge.
What about RBC survival? The assumption is that if the RBC circulates, it will survive normally. Survival studies present a number of challenges. They require prolonged (≥4 weeks) dedication of subjects, for one. As well, radioactive decay and label elution may need to be taken into account. Currently, the FDA requires RBC survival studies only for major alterations in blood systems. Because PI technology represents a major alteration, RBC survival studies may be necessary. Pathogen inactivation agents are able to affect biologic systems, so it is not surprising that the RBC PI systems appear to have reduced viability. For example, inactine resulted in RBC recoveries not different from control but an acceleration of clearance from circulation beyond 24 hours by approximately one third.61 The original S-303 treatment process resulted in a slight decrease in recovery vs control but an unchanged survival.62 However, both of these processes resulted in generation of antibodies directed at moieties deposited on the RBC membrane through chemical interaction.63 This led to the abandonment of inactine and a revision of S-30364 process that yields RBCs nonreactive to antiacridine antibodies. This revised technique is currently undergoing trials.
If you cannot get something for nothing, what is the appropriate trade? If PI does reduce the efficacy of RBCs in circulating, how should this be balanced against the benefits of reducing infectious risks? This is a question for the panel to deliberate. The FDA has signaled that some reduced efficacy could be tolerated, but the details of and commitment to this policy are not yet known. Nevertheless, the current systems used for evaluation of RBC systems provide useful predictability of the cellular viability.
Immunogenic Issues With the Use of Pathogen-Inactivated RBCs
Presented by George Garratty, PhD, FRCPath.
American Red Cross Blood Services, Southern California Region, University of California,
Los Angeles, CA
In 2000, Lublin65 wrote an editorial in Transfusion concerning various efforts to modulate the RBC membrane to produce safer transfusion products.65 These included the following: (1) converting group A, B, and AB donor RBCs to group O by treating RBCs with specific enzymes, in vitro, to remove the immunodominant A and B sugar, yielding group O RBCs (ECO RBCs)66; (2) bonding polyethylene glycol to the RBC membrane to block all RBC antigens, creating “stealth RBCs” that would not be antigenic or immunogenic66; and (3) PI of leukoreduced RBCs. Lublin used a cartoon showing a conveyer belt making leukoreduced ECO-pegylated PI RBCs. He projected this would be happening in blood centers in 2005. So far (in 2007), only one of these processes, leukoreduction, has been achieved.
The other processes described by Lublin had all shown great promise in the laboratory and some of them in limited clinical trials in animals and humans. Pathogen inactivation procedures, in particular, have led to in vitro serologic problems when treated RBCs were crossmatched using human sera. This is not unexpected given that human sera contain an amazing variety of antibodies to chemicals,67 the presence of some of which are probably due to environmental exposure to these chemicals (eg, polyethylene glycol is present in a wide variety of foods and cosmetics); thus, the antibodies can be found in healthy donors and patients. Other antibodies are present because of inadvertent immunization with the chemical used in the PI process. This would involve the same mechanisms occurring in drug-induced immune reactions, that is, the antibody could be directed against the chemical/drug itself (eg, penicillin-induced serology) or to a combination of the chemical and the RBC membrane. Two further possibilities are that (1) the chemical/drug may change the RBC membrane, creating a new antigen (neoantigen); or (2) the changed RBC membrane may adsorb proteins nonimmunologically, leading to a positive antiglobulin test (in vitro and possibly in vivo). All of these mechanisms have been observed after PI processes, although the evidence for antibodies to neoantigens is slim. The finding of antibodies due to this phenomena (eg, naturally occurring antibodies to chemicals, drug-induced antibodies, or immune response to chemicals used in PI) does not necessarily mean that decreased RBC survival will occur. Examples of both clinically significant and insignificant antibodies in all categories have been described.
Of the available PI procedures, the best documented example of immunogenicity with RBCs is the problem encountered during the clinical trials of the INTERCEPT system. Incompatible crossmatches have been found to be due to antibodies to the chemical, S-303, that remained on the RBC surface after the process.68 S-303 is closely related to acriflavine, a dye that was added to anti-B blood typing reagents more than 20 years ago, and caused serologic anomalies because of an antibody to acriflavine.67 The antibodies that have been found in 2 patients were not likely to be clinically significant because the 2 patients were asymptomatic, and monocyte monolayer RBC phagocytic assays were negative.68 Because of these serologic issues, the clinical trials in place were stopped. As a result, a modified process to reduce S-303 binding on the RBC surface was developed by the company. Sera from patients reactive with S-303 did not react with modified-process S-303 RBCs,69 and in a hyperimmune S-303 rabbit model, RBCs survived normally.70 Clinical trials of the modified-process S-303 RBCs are currently in progress.
Serologic problems after PI are similar to problems that have been encountered in immunohematology for years. Subjecting RBCs to in vitro processes can lead to antigen-antibody reactions by providing antigens in the form of RBC-bound chemicals, neoantigens, or nonimmunologic binding of proteins. It will be difficult, if not impossible, to predict these serologic problems using animal models. In addition, the immune responses to the chemicals may only occur in a few patients. Even if companies fulfill all FDA requirements, crossmatching problems may still occur in the future, but the incidence may not be any higher than the incidence of alloantibodies to RBC antigens, and the same decision-making approaches can be used to sort out these issues. As with the RBC blood group antibodies, the most important data will be to evaluate RBC survival of the transfused RBCs. To determine immune RBC destruction, the 24-hour recovery will not suffice; RBC survival studies will also be necessary.
Pathogen Inactivation in the Context of Overall Transfusion Safety
Presented by Walter H. (“Sunny”) Dzik, MD
Massachussetts General Hospital, Boston, MA
The impact, importance, and urgency of pathogen reduction as a method to improve the overall safety of transfusion are best assessed in the context of both infectious and noninfectious hazards. Data are presented on the frequency of noninfectious risks of transfusion and the cost of technologies available to reduce those risks. Such data can be used to establish the priority for pathogen reduction technology amid other measures designed to improve transfusion safety.
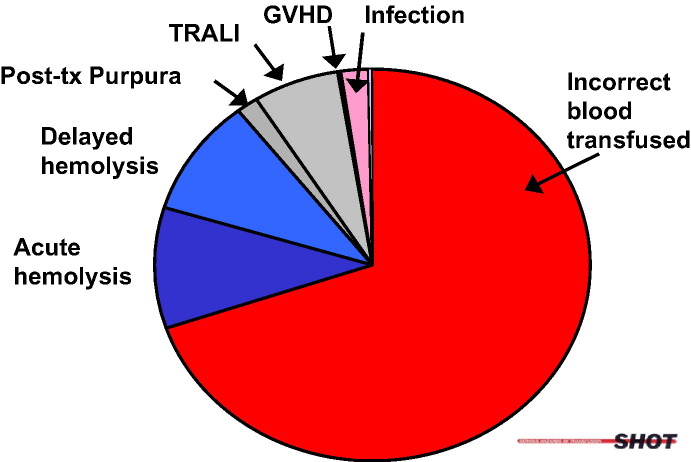
Hemovigilance programs provide us with the best estimates of transfusion risk. One such system is the Serious Hazards of Transfusion (SHOT)–United Kingdom, which has centrally gathered and classified voluntary reports of adverse transfusion events from 400 hospitals since 1996. Another hemovigilance program is under the direction of the Institut national de santé publique in Quebec, Canada. Since 2003, this program has reviewed every in-hospital adverse transfusion event reported by local transfusion safety officers using an information technology system shared by all 20 regional hospitals in the province of Quebec. Such programs have invariably found that most continuing threats to transfusion safety are noninfectious (Fig 1 ).
Fig 1.
Breakdown of the transfusion hazards, reported to SHOT, 1996 to 2004.71 TRALI, transfusion-related acute lung injury; GVHD, graft-versus-host disease.
Incorrect blood component transfused (IBCT) accounts for the largest proportion of serious transfusion hazards. The implication of human error is underscored in the higher incidence of errors in pretransfusion testing and blood administration outside core hours. Major morbidity and deaths due to transfusion errors are most often due to IBCT, hemolytic complications, and TRALI. Transfusion-transmitted infections, in contrast, comprise only 1.8% of 2630 serious hazards reported to SHOT between 1996 and 2004.71
Serious Hazards of Transfusion found that 1 (14%) in 7 IBCT events were ABO mistransfusions. Quebec hemovigilance reported that nearly 70% of ABO mistransfusions were due to failures in the proper identification of the transfused patient, with the remaining minority divided between laboratory, clerical, and technical events. Errors in patient identification occurred during specimen collection (15% of errors) as well as at the time of blood administration (85% of errors).
To address sample miscollection errors, which occur in every 1 in 2000 specimens submitted to blood banks,72, 73 some institutions have adopted policies to transfuse only group O blood until at least 2 identical ABO types have been obtained on a patient.74, 75 Addressing the more substantial problem of patient misidentification at the time of transfusion will likely require the use of machine-readable technology. Although such technology is commonplace throughout commercial and security enterprises, it is unfortunately unusual in bedside patient care. The cost to implement hospitalwide pretransfusion bedside positive patient identification using barcode technology may be extrapolated from experience at the John Radcliffe Hospital in the United Kingdom, where the equipment, training, and maintenance over the service life of technology was approximately $10 to $20 per transfused unit of blood.
Acute and delayed hemolytic transfusion reactions are commonly reported by hemovigilance programs. In the Quebec experience, 30% of hemolytic reactions were due to ABO incompatibility with the balance due to alloimmune or autoimmune hemolysis. Delayed hemolytic transfusion reactions commonly result from antibodies that were previously identified but which are no longer reactive in the pretransfusion specimen. Preventing such reactions depends upon retrieval of data from patient blood bank records. Because patients receive health care at multiple hospitals, sharing data regarding prior sensitization to blood groups between hospitals would be expected to reduce the rate of hemolytic reactions. This strategy was put to the test in Quebec, where all hospital blood banks were given access to a common database of patient records during the period from 2003 to 2005. Importantly, the incidence of both acute and delayed hemolytic transfusion reactions significantly decreased after implementation of this online interhospital database. Reactions were cut by more than 50% at a cost of approximately $6 per RBC (or $3 per component) transfused (P. Robillard, personal communication, 2007). Centralized data registries such as this could also store data obtained from high-throughput gene chip technologies applied to donor and recipient antigen typing.76 Such prospective matching may further decrease hemolytic events by reducing rates of alloimmunization to RBC antigens.
Transfusion-related ALI is a widely recognized and serious noninfectious hazard and now a leading cause of transfusion-related mortality. Strongly associated with the passive transfer of donor human leukocyte antigen (HLA) alloantibodies, options for its prevention include screening questions during the donor questionnaire for risk factors of HLA sensitization (multiparity) or laboratory screening for HLA antibodies among at-risk donors. At the Rhode Island Blood Center in Providence, previously pregnant donors were tested for HLA antibodies, and donations from those who tested positive were not transfused. The program was remarkably inexpensive. Program costs were estimated to be $25 per donor, corresponding to an incremental cost of $1.40 per component transfused, and is expected to result in an inventory of more than 500 000 low-risk components prepared from among 63 000 donors.
Compared with PI, which imposes an additional $100 to $165 per unit cost, the technologies designed to reduce the risk of misidentification error, hemolytic transfusion reactions, and TRALI are not expensive (Table 2 ). The incremental cost per unit appears to be at least 10-fold higher for PI compared with all 3 technologies for noninfectious hazards combined. When one further considers that serious infectious hazards are at least 6-fold less common than serious noninfectious hazards, the incremental cost to prevent a serious adverse transfusion event may be more than 60 times higher for PI technology compared with the other 3 technologies combined.
Table 2.
Incremental Cost (US dollars) of Technologies Designed to Improve Transfusion Safety
| Adverse event | Technology solution | Incremental cost/U |
|---|---|---|
| IBCT or ABO mistransfusion | PPI technologies (bar coding, RFID), check-type (second sample) policies | <$20 |
| Acute or delayed alloimmune hemolytic transfusion reactions | Database unification, definitive genotyping | <$10 |
| TRALI | HLA antibody screening | <$2 |
| Transfusion-transmitted infection, bacterial or viral | Pathogen reduction technology | >$100 |
Abbreviations: IBCT, incorrect blood component transfused; PPI, positive patient identification; RFID, radiofrequency identification; TRALI, transfusion-related acute lung injury; HLA, human leukocyte antigen.
Because health care funding is finite, a strategy for prioritization of available transfusion safety technologies is both important and appropriate. Decisions regarding PI technology need to be made in the context of overall transfusion safety for blood recipients.
Regulatory Issues: FDA Perspective
Presented by Jaroslav Vostal, MD, PhD
Laboratory of Cellular Hematology, Division of Hematology, Center of Biologics Evaluation and Research, Food and Drug Administration, Bethesda, MD
The FDA encourages pathogen reduction in transfusion products by all currently approved methods and by development of novel technologies. The currently approved methods include donor screening for risks of infectious diseases, skin disinfection, aseptic collection, the use of a diversion pouch and closed systems, and detection using donor testing for markers of infectious diseases (antibody; antigen and/or nucleic acids of HIV-1/2, HCV, HBV, human T-cell leukotrophic virus I/II, CMV, WNV, T cruzi, and syphilis) and bacterial detection. Novel technologies that have not yet been approved include alternate storing conditions (cold-stored platelets), rapid bacterial detection (at point of release), and PI with chemical additives.
There are concerns about novel PI methods because such processes create a novel mixture of chemicals and biologic products that could be infused intravenously into a wide range of patients of different ages and different states of health. Important considerations on implementation of such processes include the efficacy of the PI process against pathogens; the efficacy of the treated product; the safety to handlers of the PI chemicals, to the environment, and to the recipients of treated products; and, as a result of potential decreased product efficacy, the impact on the blood supply and of increased patient exposure to blood products.
From the FDA's perspective, the aim of PI validation will be to provide direct evidence that the PI process will effectively inactivate or reduce pathogens that could be transmitted by the transfusion product and indirect evidence that the pathogen reduction process has the capacity to inactivate or reduce novel or yet-unidentified agents. In vitro studies on efficacy should demonstrate log kill of pathogens in a transfusion product in the range of 6 to 10 logs; include evaluation for viral, bacterial and protozoal pathogens; and be demonstrated with the final marketed device. It is acknowledged that conducting clinical studies to determine infectivity rates in a patient population would require extremely large numbers because of the current low rates of infection in the setting of NAT and would not be a requirement for approval.
Evaluation of the transfusion product efficacy after PI treatment must be performed in clinical trials because the PI chemical may have many sites of interaction with the transfusion product, and the full extent of damage to the product may not be evident on individual in vitro tests. Initial studies should address product efficacy post–PI treatment with radiolabeling studies. For example, the FDA has introduced new standards for the evaluation of new platelet products using radiolabeled platelet studies.60 Recovery at 24 hours in healthy volunteers should be at least 66% and survival at least 58% of fresh platelets.77 In certain cases where the novel platelet product does not meet these radiolabeling acceptance criteria, limited clinical applications may still be approved if the product has clear benefits that would offset the loss in efficacy. Phase 3 clinical trials should be conducted to demonstrate the clinical efficacy of PI-treated products in a patient population requiring repeated transfusions of these products with functional end points (ie, control of hemostasis in trials assessing platelet products and oxygen delivery in trials assessing RBCs) as well as transfusion end points (ie, frequency of transfusions and transfusion increments).
Toxicity to recipients of PI-treated products may occur because of the parent compound, metabolites, and/or the treated transfusion product mixture. General toxicity and potential for mutagenic, carcinogenic and teratogenic effects will require evaluation. Guidance documents on design and interpretation of such studies can be found on the FDA Center for Drug Evaluation and Research web site.78 A caveat is that these tests model toxicity in a healthy organism but may not predict toxicity in a stressed or diseased organism. Because transfusion products will be infused into patients who have significant underlying disease, there is potential for a different toxicity profile. In addition to the mentioned toxicity studies, the PR processes should also be evaluated in models that mimic the physiologic stresses induced by various clinical situations. One example would be an animal model of platelet-mediated organ toxicity, the ALI murine model.79 In this model, platelets mediate the pathophysiology of acid- or septicemia-induced ALI by anchoring neutrophils to lung endothelial cells. Platelet P-selectin mediates neutrophil accumulation in the lung, with the hypothesis being that platelets expressing more P-selectin could support more neutrophil accumulation in lungs and exacerbate ALI symptoms. A hypothetical clinical scenario that reflects this mechanism could apply to an immunosuppressed thrombocytopenic patient who develops sepsis and mild ALI. Some PI-treated platelets have higher P-selectin expression than control platelets, and in this setting, infusion of PI-treated platelets could exacerbate the ALI symptoms. Examples of other such models that implicate platelets in pathophysiology of disease include mouse models of allergic pneumonitis80 and ischemia-reperfusion models.81 After preclinical trials, clinical trials should monitor all adverse events to identify unexpected toxicities and evaluate immune response for antibody development. It is acknowledged that low-frequency events may not be detected in preclinical or clinical studies but will require postmarketing surveillance studies.
An analysis of the risks vs benefits of using PI transfusion products will also be required. Current benefits of PI would include the reduction of transfusion-transmitted diseases, with bacteria representing the highest current risk of 1 septic reaction per 75 000 products.82 This benefit would need to be weighed against an acceptable risk of adverse events, which would be anticipated to be much smaller than 1 in 75 000. A study to detect an adverse event with a frequency of 1 in 75 000 would be unlikely because it would require 225 000 participants.83 Nevertheless, justification for requesting an in-depth safety evaluation of PI products in clinical studies revolves around the following arguments. The benefit derived from the PI process is relatively small because of a safe blood supply, and any adverse event associated with the treated transfusion product would need to be of very low frequency to be acceptable. Available data indicate that PI platelets are activated with increased P-selectin expression and damaged with decreased recovery and survival. Currently available animal models present a plausible mechanism that involves activated platelets in the mediation of respiratory adverse events similar to those seen in a large clinical trial with PI-treated platelets.84
Until further elucidated, the current safety profile of PI-treated RBC and platelet products argues against justifying a general clinical use of the products at this time. However, there may be more specific clinical indications where the benefit of PI products would outweigh a potentially high adverse event rate; for example, an emerging transfusion-transmitted pathogen epidemic with a high mortality rate or a specific patient population that may be more susceptible to a transfusion-transmitted infection.
In summary, the FDA's current perspective on regulation of PI processes requires defining pathogen reduction capacity, the loss of transfusion product efficacy post–PI treatment, the toxicity and adverse event rate in both preclinical and clinical trials, and the current risks of transfusion transmitted diseases, so that a risk-benefit balance of PI processes can be determined. If the risk-benefit balance is favorable, then PI processes would be approved with provisions to limit their use based on transfusion product efficacy, with a request for phase 4 postmarketing studies for adverse event monitoring. If the risk-benefit is deemed unfavorable, approval could be considered in situations where the transfusion-transmitted disease risk would be increased, as in a case of an emerging pathogen epidemic.
Regulatory Issues: European Community Perspective
Presented by Margarethe Heiden, MD
Paul-Erlich-Institut, Langen, Germany
All blood establishments in Europe must abide by minimal requirements set out in the directive 2002/98/EC of the European Parliament and of the Council, a key legislative document with respect to blood and blood components, amended by technical directives setting standards for blood collection, testing, processing, storage, and distribution of blood components. In addition, legally binding directives 93/42/EC and 98/79/EC define standards for medical devices such as blood collection bags and in vitro diagnostics such as screening tests. Moreover, each nation may have its own, sometimes more stringent, regulations of the transfusion system. For example, in Germany, collection, testing, and use of blood components is governed by the German Transfusion Act. Production and distribution of blood components, on the other hand, are regulated by the German Drug Law. Blood banks are required to apply to the regional authorities for a manufacturing license. Blood components are viewed as proprietary medicinal products and as such require marketing authorization from the PEI, a German federal agency.
To obtain marketing authorization in Germany, all new technologies pertaining to blood component preparation must be assessed and approved by the PEI. In case of PI technology, the PEI requires preclinical pharmacologic and toxicological studies as well as clinical studies conducted by the applicant. To be approved, pathogen-inactivated components had to exhibit a state-of-the-art quality (confirmed by experimental data); safety (based on experimental preclinical data, experimental data on validation of virus reduction, and clinical data); and efficacy (based on clinical data showing noninferiority in a prespecified clinical outcome). In general, depending on the results of such an assessment, a marketing authorization could be granted under the following conditions: introduction of additional in-process controls, inclusion of specific safety information in the package leaflet, or requirement for postmarketing surveillance. Conditional approval is reviewed after 5 years, and at that point, full approval may be granted. In January 2007, MB/UV light–treated FFP and amotosalen/UV light–treated platelets were conditionally approved by the PEI. Both products are required to have postmarketing surveillance of adverse events and additional safety information indicated in the package insert. For example, the package insert for MB/UV light–treated FFP states that treated FFP may be less effective than untreated FFP especially when used in massive transfusion or plasma exchange. The package insert for amotosalen/UV light–treated platelets cautions against use in newborns with hyperbilirubinemia. Also, it reminds the user that the risk of transmission of nonenveloped viruses, certain bacterial species, and bacterial spores is not eliminated. For both PI products, the user is reminded that PI does not obviate testing for HIV-1/2, HCV, HBV, and syphilis.
After careful consideration, the PEI recommended against universal implementation of PI blood components. The agency felt that the blood supply in Germany was already safe. With introduction of minipool NAT, residual risks of HIV-1 and HCV were 1 in 5.5 million and 1 in 4.4 million, respectively.85 Moreover, there have been no reports of transfusion-transmitted HIV or HCV for the past 2 years. In contrast, bacterial contamination continued to account for a significant portion of severe transfusion reactions. In 1995 to 2005, 125 cases including 17 deaths were reported.86 Unfortunately, screening for bacterial contamination did not eliminate this problem.87 Pathogen inactivation also would not be able to prevent all cases because some bacteria (applicant's data) and bacterial spores 88 appear to be resistant to amotosalen/UV treatment. The institute felt that the introduction of current PI technology does not eliminate the need for bacterial detection or testing for transfusion-transmitted viruses.
Despite an extremely low risk of transfusion-transmitted infectious diseases, the PEI felt that it was important to review and give conditional approval to the PI technology. This technology was expected to enhance the safety of the blood supply in case of errors or test failures. Moreover, it could potentially protect the blood supply against emerging pathogens for which tests did not yet exist. Finally, it was felt that PI technology could become paramount if testing for transmissible diseases was interrupted because of a pandemic or another public health emergency.
Pathogen Inactivation Technologies: Challenges and Opportunities
Presented by Peter Ganz, PhD
Director, Centre for Biologics Evaluation, Health Canada, Ottawa, Ontario, Canada
Given the blood system's history, blood safety is considered to be of paramount importance to the health of Canadians. Health Canada (Ottawa, Ontario, Canada), as the regulator for the Canadian blood supply system, provides regulatory oversight that is appropriate, science based, proactive, risk based, and flexible. Consultation with interested and affected stakeholders is essential to Health Canada's regulatory process.
Background
Pathogen inactivation technologies are technologies that are aimed at inactivating known and/or unknown blood-borne pathogens in blood products, whole blood, blood components, or plasma to improve the safety of these products. These technologies are being developed to reduce the risk of transmission of infectious agents that are not detected by current screening or testing. These technologies are designed to take advantage of the fact that the labile components of blood do not contain DNA or RNA. Therefore, treatments that target the DNA and/or RNA of pathogens should not have a negative impact on the function of RBCs, platelets, and plasma.
The various PI technologies available have been discussed elsewhere. In general, the process involves the addition of a chemical compound to the transfusion product. This compound interacts with the nucleic acids of the pathogen(s), which are then inactivated spontaneously or by exposure to light energy. Usually, there is also a nucleic acid cross-linking event that prevents transcription and/or replication of the RNA and/or DNA. Existing technologies include chemical nucleic acid inactivators such as psoralens, riboflavin, binary ethyleneimines, and alkalating agents; SD treatment; pasteurization; ozone; and radiation.
Regulatory Process in Canada
The regulatory review process for PI technologies in Canada will follow the requirements defined by Canadian regulations. For example, for technologies that function with a device component, the process will be defined by the Medical Device Regulations, and devices intended for the PI of blood are classified as Class IV medical devices according to rule 6 (2), Schedule I, part 1 of the Medical Device Regulations. For technologies that function with a drug component, the regulatory review process will be defined by the Food and Drugs Act and Regulations.
In general, there are 2 issues that are important to consider when reviewing new PI technologies: (1) safety and quality and (2) efficacy. Safety and quality refer to both the absence of toxicity to the recipient as well as the absence of toxicity to the component itself. Efficacy refers to the efficacy of the component as well as the efficacy of the PI process.
Assessment of safety and quality
The kinds of data required to support a lack of toxicity to recipients fall into 4 major categories: (1) general toxicity of residual inactivating substance(s) (animal studies); (2) genotoxicity (in vitro and in vivo) and carcinogenicity (long term in animals); (3) reproductive toxicity and effects on the fetus; and (4) immune toxicity, because the alkylation of proteins and binding to lipids can raise antibodies against neoantigens. Even after extensive preclinical and clinical work, postmarketing studies will likely be needed to evaluate long-term safety.
Assessment of the potential toxicity to treated components should include evaluation of potential toxicity due to cell remnants resulting from damage due to process or direct effect of inactivating substances; the potential effect on treated cellular components and functional condition of treated component; and the potential effect on other blood or plasma proteins (eg, decreased levels of protein C and protein S due to SD treatment).
Each technique/substance has its own characteristics, and the analyses of risk and benefit must be undertaken for each. Other considerations for evaluation will include component-specific benefits; risk vs benefit in vulnerable patients, such as neonates, premature infants, and pregnant patients; geographic considerations (eg, malaria or Chagas disease); and expected patient survival.
Assessment of effectiveness
The efficacy of the inactivating process must be evaluated to determine its effectiveness against different pathogens. Appropriate representative pathogens (blood-borne enveloped and nonenveloped viruses, bacteria, and parasites) must be chosen and used to demonstrate effectiveness (qualitative and quantitative in log reduction with expectation of 6 to 10 logs' reduction). In addition, evaluation of loss of cellular components during the process and potential effect on treated components as well as functional condition of treated components must be performed. For evaluation of toxicity to cellular components and simultaneous evaluation of efficacy of the treated components, studies should include recovery and survival after reinfusion of labeled cells and evaluation of clinical end points such as the effect on bleeding for platelets and on oxygen delivery for RBCs.
Other Considerations/Regulatory Challenges
Current limitations of processes
The ideal PI process should have a high effectiveness against a wide range of pathogens; maintain the biologic activity of components; produce no or minimal levels of reactive oxygen species; and be nontoxic, nonmutagenic and noncarcinogenic. However, there is not currently a universal inactivating agent that is able to eliminate all pathogens. Therefore, the benefit of PI is the elimination or reduction of as many blood-borne pathogens as possible. In addition, it should be noted that among current PI technologies, each has clear limitations. For example, they have no effect on spores or accumulated endotoxins, and none has been shown to inactivate prions.
General risk perspectives
From a risk-benefit perspective, blood and blood components produced and distributed in Canada (and in other developed countries) are extraordinarily safe. Therefore, it is reasonable to consider whether the introduction of PI would add another necessary layer of safety and at what cost or risk? Similarly, given the current low risk of blood products, one must consider what a tolerable risk-benefit profile for PI might be.
Screening test phaseout or replacement
Introduction of PI will not immediately replace the need for screening tests until such time as results are obtained from extensive field trials of the PI technologies and one can be confident that there were no serious effects secondary to the introduction of these technologies. The importance of determining long-term toxicity and the absence of low-frequency serious effects must be stressed.
Need for public consultation
Unlike traditional individual informed consent for trials of new drugs, widespread introduction of PI should require the broader informed consent of the public, also termed societal informed consent, before the types of extensive postmarketing or field trials that would be needed for the introduction of these types of technologies. It is currently unclear what the best mechanism to determine societal acceptance of the risks/benefits of PI technologies is.
Current Status
To date, Health Canada has issued notices of compliance, or approvals, and issued drug identification numbers for SD-treated plasma for use in Canada. However, there have not been any submissions for PI technology as part of the establishment licensing requirements for blood establishments, which is essentially the equivalent to a Biologics License Application (BLA) in the United States. However, in anticipation of future requests, the Canadian federal government has initiated several actions: (1) the formation of internal working group to consider unique scientific, medical, ethical, regulatory issues of PI; (2) consultation with Health Canada's Expert Advisory Committee on Blood Regulation (April 2007); and (3) the sharing of information concerning PI with stakeholders (this meeting).
Public Health Aspects of Residual Risks Relating to Transfusions
Presented by Matthew Kuehnert, MD
Centers for Disease Control and Prevention, Atlanta, GA
There has been a marked decrease in the risk of transmission of viral agents through transfusion over the past 25 years as a result of improved donor selection, advances in laboratory screening, improved use of allogeneic blood components, and advances in blood component manufacturing. However, until recently, the risk of transmission of bacteria and parasites through transfusion had remained essentially unchanged. Recent concerns in blood safety also have included prion transmission and noninfectious risks.
Residual risk of transfusion to a given threat is a relatively straightforward concept but can be extremely difficult to measure. Pre- and postintervention risk, surveillance sensitivity, and intervention efficacy are critical factors to accurately measure residual risk. One can simply measure the risk after a given intervention, but to determine the benefit of the intervention, one must know the preintervention risk, the postintervention risk, and the sensitivity of each measurement. When assessing the risk of a pathogen, one must consider the plausibility of transmission through transfusion, the likelihood of exposure, the availability of effective screening tests, and the potential for clinically significant disease in a transfusion recipient.
In 2003, the American Association of Blood Banks (AABB) introduced a new standard mandating that procedures to limit and detect bacterial contamination of all platelet components be in place in AABB-accredited facilities by March 1, 2004. The performance of active surveillance is crucial to assess the success of such interventions because passive surveillance methods may understate risk by up to 10 to 15 times as compared with active surveillance methods. In the case of efforts to mitigate bacterial risk, the lack of active surveillance data made it exceedingly difficult to assess the success of any intervention. The situation was further compounded by the different processes in use for platelet collection and processing and by the multiple potential sources of bacterial contamination of a blood product. Wide variations in estimates of preintervention risk existed, most likely as a result of differences in surveillance intensity. The same caveats applied to postintervention risk estimates, although countries with active hemovigilance systems were better able to make this assessment because of their enhanced surveillance mechanisms and more robust adverse event definitions. Estimates of false-negative testing varied widely, although based on American Red Cross data, it is possible to determine an approximate 50% reduction in reported septic reactions and sepsis-related fatalities after apheresis platelet transfusions after implementation of the 2003 AABB standard.
Parasites currently constitute a significant and largely underrecognized infectious disease risk associated with transfusion, although recent steps are being taken to intervene. In particular, agents such as Babesia species and T cruzi (agent of Chagas disease) are emerging as significant threats because of immigration patterns in the United States and elsewhere. Preintervention risks are unknown for these agents because of lack of adequate surveillance.
Taken in perspective, however, the infectious risks of transfusion are uncommon, and perhaps noninfectious risks should be the focus for future interventions relating to transfusion safety. It should also be appreciated that even safety interventions can result in adverse events as illustrated by the “red eye syndrome” and back pain syndromes associated with leukoreduced blood components in 1999 and 2000, respectively. White particulate matter observed in blood products in 2003, although not associated with an increased incidence of reported adverse reactions in patients, provided a reminder that when phenomena of unknown significance occur, there is a need for surveillance to determine the impact on recipient health. Active surveillance is essential to provide objective data on the impact of new technologies for patients and can also help guide the actions of regulatory bodies.
Therefore, there is a need for good surveillance methods to determine the impact of any new technology for the health of recipients. One must weigh the effectiveness of the intervention and the potential for toxicity to mitigate the risk. Even where the risk from implementing an intervention is thought to be zero, the greatest postimplementation risk may relate to uncertainty inherent in designing new technologies without an effective system for evaluating efficacy because in that situation, the benefit is unknown. A focus on outcomes may be the optimal approach to evaluating the overall benefit of new technologies for transfusion, but this broader approach is costly.
In summary, both infectious and noninfectious risks to the blood supply will remain despite the implementation of PI technologies in the future. Regardless of what measures are put in place to improve blood safety, there will be a continuing need to assess residual risks; to evaluate the efficacy of an intervention, as well as any expected or unanticipated toxicity; and to monitor donor and recipient health outcomes. It is likely that a layered approach to interventions will be most effective in addressing future residual risks of transfusion.
Economics Issues: Costs and Benefits of PI in Relation to Other Aspects of Transfusion Medicine
Presented by Brian Custer, MPH, PhD
Blood Systems Research Institute, Pharmaceutical Outcomes Research and Policy, University of Washington, Seattle, WA
The economics of PI technologies pose complex questions for the health care system. Evidence indicating the potential for PI to substantially decrease the risk of both infectious and noninfectious transfusion threats is mounting,89, 90 but incorporating multiple lines of evidence into models that can be used to assess costs and potential benefits has only recently occurred.
Although PI may have broad-ranging capability to reduce transfusion threats, from an economic perspective, the technology is expensive. Two review articles have sought to assess the economic evidence of various transfusion medicine interventions, both highlighting how blood safety interventions do not conform to standard notions of cost effectiveness.91, 92 With the reduction of infectious transfusion risks through the adoption of additional testing such as NAT for HIV and HCV and bacterial culture of platelets in many settings, it is unlikely any PI technologies can be classified as cost effective when judged by traditional thresholds such as $50 000 to 100 000 per QALY saved. However, given the context of blood safety expectations and robust capabilities of PI, traditional cost-effectiveness thresholds may not be relevant. These thresholds typically apply to specific interventions as opposed to interventions with broad-spectrum capability. Furthermore the precedent set in blood safety is that interventions that cost well over $1 million per QALY are candidates for adoption.
Pathogen inactivation is a term for several different technologies and processes that are most effective for different blood components, and for 2 technologies, published studies have provided insight into cost effectiveness. The peer-reviewed, published evidence for SD FFP highlights the challenges in conducting meaningful economic analyses of PI. A 1994 publication estimated a cost effectiveness of US $289 000 per QALY, but a 1999 update of this same analysis using more recent estimates of the risk transmission of HIV, HCV, and HBV coupled with a 5-times-higher estimate of the cost of the technology generated a result of $9.7 million per QALY for the US setting.48, 93 A 1999 study from Spain evaluating a similar SD technology had a cost effectiveness of US $2.2 million per QALY.49 These studies focused on the risk of transmitting viral infections with and without SD treatment. However, a recent study from the United Kingdom included the noninfectious threat of TRALI. Under the assumption that the SD treatment and component manufacturing processes can reduce the risk of TRALI, the cost effectiveness of SD FFP improved to £23 000 to £99 000 per QALY (US $45 000-$194 000 per QALY).94
Psoralen light treatment (INTERCEPT) for single-donor apheresis (AP) and random-donor (RD) platelets has the largest body of available economic evidence. An analysis conducted for the US setting predated current bacterial culture. Results were reported for 4 different patient groups (pediatric acute lymphocytic leukemia, hip arthroplasty, coronary artery bypass grafts, and adult non-Hodgkin lymphoma) and with and without bacterial culture. Depending on the patient population, baseline results ranged from US $4.8 million to $23.0 million per QALY with bacterial culture and US $1.3 million to $4.5 million per QALY without bacterial culture for AP platelets.95 For RD platelets, the profile improved to US $1.0 million to $6.0 million per QALY with bacterial culture and US $460 000 to $1.8 million per QALY without bacterial culture. This difference was mainly attributed to higher risk of bacterial contamination in RD vs AP platelets. In a similar analysis from Japan with the same patient populations, results ranged from ¥99 million to ¥433 million per QALY (US $818 000-$3.6 million per QALY) without bacterial culture for AP platelets.96 Three studies from Europe have generated results with the same order of magnitude. Patient populations were different, reflecting those of health care institutions within each country. One study from the Netherlands found the cost effectiveness to be €2.8 million per QALY (US $3.6 million per QALY) with bacterial culture and €382 000 per QALY (US $497 000 per QALY) without bacterial culture in RD platelets for the average recipient.97 Another study from the Netherlands reported results of €261 000 to €679 000 per life year gained (US $339 000-$889 000 per life year gained) for 3 different patient populations.98 A study from Belgium reported results ranging between €195 000 and €3.5 million per QALY (US $255 000-$4.6 million per QALY) for RD platelet transfusion to 9 patient populations.99 In all of these studies, the best cost-effectiveness profile was evident for the pediatric population. Results were most sensitive to the risk of sepsis, death attributable to sepsis, and the age and underlying medical conditions of the transfused population. Results were not sensitive to viral infections, except in scenario analyses that included significant risks of clinically important emerging unknown viruses. Published cost utility evidence for riboflavin (Mirasol, Gambro BCT, Inc, Lakewood, CO) and MB light treatment are currently not available. Likewise, studies that seek to determine the cost effectiveness of PI for RBCs have not been published.
There are limitations to the evidence generated by cost-effectiveness studies, and future analyses will need to take these factors into consideration. Studies should compare each technology with all interventions in place in a given setting. Studies should be based on infectious threats reduced or inactivated by each technology including analyses on known emerging agents such as chikungunya virus in Reunion Island as opposed to unknown emerging agents. Noninfectious threats may hold potential to be incorporated into economic analyses, but clear evidence of the effectiveness of PI in reducing these threats will first need to be established. The measure of increased product use resulting from the loss of component therapeutic activity from PI will also need to be considered.
The greatest potential for improving the cost effectiveness of PI comes from the possibility that several current interventions such as bacterial culture, testing for WNV, and γ-irradiation could potentially be discontinued, or that implementation of individual donation testing by NAT for HIV, HCV, HBV, and WNV be avoided along with avoidance of the implementation of additional assays for emerging viruses (eg, dengue, chikungunya) or parasites (eg, T cruzi, malaria, Leishmania). Perhaps even donor selection criteria, which can be complex and costly, could be modified. The potential broad-spectrum coverage of multiple infectious and noninfectious threats using PI will require careful accounting of what events can and cannot be averted by the use of each technology and what health care costs can be prevented. Given the broad range of potential benefits, future studies that seek to estimate the cost effectiveness of specific PI technologies will require the use of the most advanced methods to account for uncertainty such as probabilistic sensitivity analysis. Should newer PI technologies be adopted, the cost of blood components will increase. The question these technologies pose for the health care sector of each country is whether the increase in blood costs will come with improved transfusion safety and with potentially reduced health care expenditures resulting from averted adverse transfusion events, modified donor selection criteria, and laboratory screening interventions.
Overview of newer PI technologies
Presented by Stephen J. Wagner, PhD
American Red Cross Holland Laboratories
This presentation provides an overview of selected developing PI technologies that are not addressed in other presentations and discussion of future directions in PI technologies.
Ongoing Research Activities
CryoFacets technology
Cryofacets, Inc, (Durham, NC) has been developing different techniques for the PI of plasma, platelets, and RBCs. In plasma, infectious agents are inactivated using a 2-step process consisting of germicidal UV-B followed by ozonation. To prevent undesirable photo-oxidation of plasma proteins by UV-B light sensitization, the plasma is first deoxygenated using ultrasound technology. After treatment, sterilized units can be fractionated according to molecular weight (above albumin, below albumin, and an albumin fraction) and concentrated by freezing using an ultrasonic assist. This technology is currently in development, and data about maintenance of coagulation factor activity levels are not yet available.
The CryoFacet RBC and platelet technology relies on using a planned new design for counterflow elutriation to remove infectious agents in plasma, followed by subsequent ozonation of the resulting cell suspensions. Platelets can be stored in a pathogen-reduced plasma fraction (lower molecular weight). Before infusion, platelets can be washed, suspended in degassed saline, and further treated with germicidal UV. Studies looking at in vitro and in vivo platelet and RBC activities have not been reported and will be of interest given the high degree of manipulation of product involved.
Sanquin technology
Sanquin International Limited (Amsterdam, The Netherlands) has investigated the use of light for plasma inactivation and later for platelet inactivation. Early work focused on the use of high-intensity, broad-spectrum light pulses with platelets suspended in 30% plasma. A 3-log inactivation of virus was demonstrated with maintenance of in vitro platelet properties during storage. The company did not further develop the technology. Subsequent studies treated platelets suspended in 10% plasma and 90% additive solution with UV-C light. Although a number of pathogens were inactivated by more than 5 logs, the treatment activated the platelet fibrinogen receptor, glycoprotein IIb/IIIa, which caused platelet aggregation and resulted in a decrease in platelet counts. It was also noted that HIV was relatively insensitive to the activity of UV-C treatment. Currently, Sanquin is involved in a clinical trial comparing S59-treated platelets with untreated platelets stored in platelet additive solution III.
Sanquin International Limited has studied inactivation of pathogens in RBCs using positively charged porphryin compounds. One compound, mono-phenyl-tri-(N-methyl-4-pyridyl)-porphyrin chloride, yielded the least hemolysis and was able to inactivate the model single-stranded RNA virus, vesicular stomatitis virus (VSV), by 5 logs.100 However, other agents, including intracellular HIV, required prolonged illumination for inactivation which resulted in unacceptable hemolysis.101
Other Future Directions
Hemolysis and treatment of RBC suspensions
The problem of hemolysis after virucidal photoinactivation of RBC suspensions using these agents has been a common theme reported in several laboratories. This theoretically may be minimized through the use of dyes such as MB and dimethylene blue, which bind strongly to nucleic acids. This provides a basis for specificity because pathogens, unlike RBCs, contain genomic nucleic acid. Hemolysis upon illumination with these compounds may occur by several mechanisms: (1) freely floating dye near RBCs produces singlet oxygen when illuminated, which results in RBC damage; (2) many nucleic acid binding dyes also bind to the RBC membrane, resulting in photo-induced hemolysis. Another problem with photosensitizing dyes is the fact that not all dyes are able to cross cell membranes, which is necessary to kill intracellular viruses. Examples of such dyes include MB and positively changed porphyrins.
Development of flexible photosensitizers
In general, photosensitizing dyes are rigid, planar aromatic compounds that are activated by light whether free in solution or bound to a substrate. However, the structure of some sensitizers permits separated aromatic ring systems to rotate about adjoining covalent linkages of partial single-bond character when they are not rigidly bound to substrate. This rotation permits the energy from absorbed light to be dissipated as heat through bond rotation. However, once these dyes are intercalated into nucleic acids, they become rigid photosensitizers. The discovery of flexible dyes has allowed the development of RBC cell inactivation systems that are more specific for pathogens and that do not cause excessive RBC cell damage. The first example of a flexible photosensitizer used for RBC cell decontamination was a nucleic acid staining dye, thiopyrylium (TP). Because TP bound to RBCs cells in addition to nucleic acids, a second substance, dipyridamole, a commonly used vasodilator drug, and also an antioxidant, may be added to inhibit binding and photodamage to RBC membranes. Thiopyrylium phototreatment of RBC suspensions containing 200 μmol/L dipyridamole with 1.1 J/cm2 of red light resulted in robust inactivation of a number of intracellular and extracellular viruses as well as bacteria and parasites.64, 102 After storage for 42 days, the RBCs exhibited acceptable levels of hemolysis, morphology scores, extracellular pH, ATP, glucose use rates, and lactate production. However, treated samples exhibited substantially increased potassium efflux compared with controls, and day 42 ATP levels were significantly different from controls.64 Autologous infusion of fresh TP-phototreated canine RBCs cells resulted in comparable 24-hour recovery and survival as untreated fresh canine RBCs.103
One disadvantage of the TP system is the requirement for the presence of a second drug, dipyridamole, to limit RBC photodamage because any commercial development of the system would require toxicological investigation of TP, dipyridamole, as well as the combination of the 2 drugs. This would likely make it very difficult to get such a system approved by a regulatory agency. The identification of a flexible dye that binds poorly to RBCs may make the addition of a competitive inhibitor unnecessary. One such a dye, thiazole orange, a nucleic acid–intercalating dye that has been used as a stain to identify reticulocytes and malaria-infected RBCs, has showed promise as a photosensitizer for RBC decontamination without the need for a second drug because thiazole orange binds to RBCs less than TP. Treatment of RBC suspensions with 80 μmol/L thiazole orange and up to 7.9 J/cm2 cool white light also resulted in robust inactivation of intracellular and extracellular viruses (6 to 8 logs).104 Bacteria were photoinactivated with thiazole orange to differing extents depending on the species, but at least 2.3 logs, and for some species, more than 7 logs of bacteria were inactivated.104 Preliminary experiments indicate that micromolar concentrations of thiazole orange, without illumination, completely inactivated the parasite Leishmania donovani infantum as well as the parasite T cruzi. At day 42, hemolysis of RBCs were approximately 0.4%, and ATP levels were essentially unchanged.104
Open Discussion
Led by Gilles Delage, MD
Héma-Québec, Montreal, Québec, Canada
Rationale for PI and Relevance of This Technology
The discussion opened with a question about the utility of this emerging technology (PI) for the prevention of transfusion-related infections in the developing world. It was acknowledged that PI technology in its present form is expensive and could not be implemented in many less developed regions of the world at this time. There was optimism, however, that with refinement, PI technologies could eventually have a major impact on the prevention of transfusion-transmissible infections in less financially capable nations. This was seen as a significant impetus to the continued need for the development of PI technology. An additional point made was that it was likely not feasible to continue to introduce additional tests for every emerging pathogen. The finite resources available to ensure transfusion safety precluded such an approach because the costs of introducing additional tests for emerging pathogens would be considerable. It was suggested that a cost-effectiveness model would be invaluable for assessing the impact of new technologies in the context of the resources available, particularly in disadvantaged countries.
Risk-Benefit Assessment of PI Technology
The main benefits of PI technology were identified as (a) the further reduction, but not total elimination, of the risk of transfusion-transmitted bacterial sepsis; (b) minimization of an already extremely low risk of viral transmission; (c) additional protection against cell-associated viruses, such as CMV; (d) probable elimination of transfusion-associated GVHD; and (e) possible attenuation of the immunomodulatory effects of transfusion. It was noted that the risk of transmission of HIV, HCV, and HBV is already extremely low because of the sensitive viral screening assays currently in place. In Canada, the residual risk of acquiring HIV infection through transfusion is approximately 1 per 3 million blood donations or 1 infection per 3 years.105 The figure is similar for HCV. The residual risk of HBV, which remains the most common viral hepatitis transmitted through transfusion, is 1 per 150 000 or approximately 20 cases per year in Canada.
It was pointed out that the current approaches to screening the blood supply for the prevention of viral hepatitis and HIV have reduced these risks to extremely low levels; however, the risk associated with unknown pathogens remains. It was suggested that pathogens appear to be emerging with increased frequency. This statement was disputed because emerging pathogens have always been present, although not necessarily found to be relevant to blood transfusion recipients.
It noted that, as with most biologic fluids and medications given by intravenous administration, blood products should be sterile and endotoxin-free. Ideally the safety of all blood components should match that of the fractionation protein products that currently have a very low risk of disease transmission due to the use of robust viral inactivation steps built into their manufacturing process.
One participant felt that it was essential that countries introducing PI technology have an active surveillance system in place before PI implementation. It was noted that the benefit and margin of safety of PI products may differ for the different patient groups; for example, the risk associated with transfusion of PI products may be higher in the neonatal population than for adults because of their higher life expectancy. Such considerations may therefore necessitate the maintenance of multiple inventories of the various blood components.
It was pointed out that despite the use of PI products, it may not be possible to eliminate the requirement for bacterial screening of blood products, particularly platelets. Although PI appears to be more effective at reducing the risk of transfusion-transmitted sepsis than bacterial screening techniques, how much more effective is as yet uncertain. Current estimates suggest that 50% to 75% of bacterially contaminated products are detected by the available screening methods, whereas PI may reduce that risk to close to 0%. For example, it must be recognized that PI fails to eliminate bacterial spores.
It was reported that Norway has used SD-treated plasma routinely since 1993 and that this intervention has eliminated the occurrence of TRALI.90 This likely relates to the dilution of HLA antibodies in the SD plasma pool. The use of SD plasma also appears to be associated with an 80% decrease in the incidence of allergic reactions.90
It was also pointed out that there are currently no tests available to screen for prion-associated diseases, and PI appears to be ineffective against these agents. Therefore the implementation of PI technologies would not mitigate the risks associated with such agents. The need for continued efforts to screen the blood supply for, or to remove, this pathogen would thus remain.
Role of Public Health Agencies in Protecting the Blood Supply From Emerging Threats
The issue is as to whether the emergence of HBV in the 1960s, followed by HIV in the 1980s and HCV in the 1990s, could serve as a basis for public health agencies to model the risks of other emerging pathogens. The health care economist on the consensus panel advised that in modeling such situations, it is best to work with a concrete or a clearly definable emerging agent. He suggested that dengue virus could serve as a useful prototype in this regard. It was pointed out that health policy agencies have a responsibility to develop valid models for assessing the cost effectiveness of various interventions addressing emerging threats to public safety. The importance of the hemovigilance systems now in place in many countries was emphasized, not only for detecting the emergence of new pathogens but also for monitoring the potential toxicity of new therapeutic interventions.
It was noted that many emerging microbiological-related illnesses are caused by zoonotic agents, and as such, it may be difficult to justify expenditures on measures to prevent the spread of an agent through transfusion for diseases that are endemic in a particular community. Both the effectiveness of transmission through other means and the burden of illness in that community must be considered in allocating resources.
Ethical Concerns Relating to the Implementation of PI Technologies
The widespread implementation of PI technologies would considerably change the nature of blood products as we know them today. It was thus suggested that PI should, therefore, not be pursued without adequate public consultation as well as the introduction of active surveillance. The latter must involve the follow-up of large cohorts of transfusion recipients. Many surveillance systems currently in place are voluntary and passive in approach. Surveillance of potential complications arising from the widespread introduction of PI technology must be active and must also have regulatory oversight. In addition, such surveillance must be long term, following recipients for many years. There was considerable discussion around the level of informed consent necessary for postmarketing surveillance. It was pointed out that precedents exist in some countries, such as Canada, where active surveillance for severe adverse reactions to vaccinations is carried out without the requirement for specific informed consent.
Another ethical consideration raised concerned the approach to be taken in the event that universal implementation of PI technologies is not feasible. Ideally, a universal approach to implementation of PI should be pursued; however, with the limitations of currently proposed PI technology, a universal approach to PI of all blood components would probably not be possible at this time. For example, obstacles to the PI of RBCs remain substantial. Under these circumstances, implementation of PI technology would likely result in 2 levels of safety for the different blood components, and the attendant needs to continue to perform microbiological screening of RBC components. If universal implementation of PI for all blood components were not possible, the ethical considerations of selective implementation of PI technology would have to be addressed, including issues relating to varying the levels of the safety of blood products and the need for explicit informed consent by the patient receiving the PI products. For example, would it be appropriate to offer PI-treated products to the pediatric population exclusively, similar to the approach taken in the United Kingdom with the transfusion of MB-treated plasma to persons younger than 16 years? Similar concerns could arise during the crossover from the existing inventory to a PI inventory. Moreover, if significant differences were known to exist between PI and non-PI products, this could become an important element of informed consent for transfusion. It was also pointed out that policy makers must retain flexibility in making decisions with respect to the appropriate allocation of limited resources if a universal approach to PI is not attainable.
Clinical Trials and Surveillance to Assess the Benefit and Risk of PI
The characteristics of PI products can vary considerably depending on the process by which they are manufactured. Solvent/detergent plasma from one manufacturer could be very different biochemically from another manufacturer's SD plasma product. Such differences relate to processing parameters such as the hold time before extraction and the different chemicals and/or enzymes used in the extraction process. One cannot therefore assume that all SD-processed plasma products are equivalent. Hence, there is a need for clinical trials and active surveillance over time to assess the properties and potential complications of each of these products. It was therefore suggested that manufacturers should be encouraged to work together in an effort to standardize their processes so the available products are comparable.
A representative from the US FDA indicated that the FDA would be in a position to ensure effective postmarketing surveillance if they perceived that a safety issue may exist. Health Canada concurred, as did the European regulators. It was acknowledged, however, that such oversight might be logistically difficult because of the many different PI technologies currently under development and/or likely to be developed in the foreseeable future.
It was noted that to adequately power a randomized clinical trial to assess viral removal would be financially prohibitive because many of the measures currently in place have reduced the risk of viral transmission through transfusion to such low levels. However, clinical trials are essential to assess the clinical effectiveness of the various PI-treated products.
Economic Implications of the Implementation of PI Technologies
It was suggested that with PI in place, cost savings could be realized because there should probably be no reason to continue to perform bacterial screening. In addition, the donor questionnaire could be modified, WNV and anti–hepatitis B core testing would become redundant, and screening for syphilis would no longer be required. There was considerable skepticism as to whether cost savings would actually be realized, because regulatory constraints would likely not permit the discontinuation of existing donor screening practices. It was considered unlikely that the discontinuation of HIV, HCV, or HBV testing would be acceptable based on political considerations and other societal pressures. It would thus take many years for PI technology to be validated to allow discontinuation of various microbiological screening testing. Similar to the situation with CMV serologic testing after implementation of universal leukoreduction, it was considered unlikely that physicians would accept the discontinuation of established screening practices, including the irradiation of blood products for transplant patients, despite the apparent likely effectiveness of PI in the prevention of GVHD. It was pointed out that for some countries, such as Canada, which provides a universally leukoreduced product and in addition performs CMV serologic testing for specific indications, there would likely be no benefit to PI in reduction of CMV testing. It was acknowledged that cost-savings measures after implementation of PI may vary from country to country depending on existing practices as well as the regulatory environment.
The opportunity costs relating to PI were discussed. It was stated that the problem is not one of limitation of resources but rather our concept of resource availability. For example, the cost increment associated with the use of positive identification systems to prevent one of the most common cause of transfusion-related mortality is modest compared with many other costs associated with the collection, processing, and administration of microbiologically safe blood products.
It was pointed out that several countries in Europe that have implemented PI routinely for platelet production have observed reduced product loss during processing. Platelet outdates in Alsace, France, for example, approach 1%, in contrast to platelet outdate levels, which are much higher in most other centers. This was attributed to stabilization of the supply of this short-lived product.
It was noted that the high blood donor loss inherent in the current approach to transmissible disease testing of each donation should be included in any economic analysis. The ability to retain some of these donors would be significant in maintaining the adequacy of the blood supply and would impact donor recruitment strategies. For example, Héma-Québec, Montreal, Quebec, collects roughly 250 000 donations per year, and since 1990, approximately 35 000 donors have been excluded from their donor registry on the basis of transmissible disease testing. Most tests currently in use for screening blood donations have a false-positive rate in the order of 1 per 1000 donations. Donor reinstatement protocols are not widely available, and even with reinstatement protocols, experience has shown that only a minority of donors can be returned to the donor pool.
For economic analysis to inform decisions relating to the implementation of new technologies, cost-consequence analysis would be optimal in that such an analysis takes into consideration the context of the decision. Cost-effectiveness analysis, on the other hand, determines benefit in number of QALYs saved without regard to context. An intervention with the same benefit in number of QALYs saved may have an entirely different value in different countries depending on the context of the decision. Therefore, it was suggested that cost-consequence analysis would be the preferable approach for assessing the potential value of a new technology.
Role of the Regulatory Bodies in Approving and Facilitating the Implementation of Advances in Transfusion Medicine
Considerable concern was expressed over the potential inability of blood regulatory agencies to allow the PI process to move forward. For example, it was asked what amount of data was required to allow regulators to make a decision. There was a strong perception that current regulations inhibit the ability to implement change. History shows that regulators find it difficult to discontinue certain transmissible disease testing, even where the risk had been shown to no longer exist. For example, the Blood Products Advisory Committee in the United States recommended discontinuation of syphilis testing of the blood supply many years ago, yet this test continues to be performed on all blood donations in the United States. It was further suggested that the requirements for the testing of a new technology have become so detailed as to be counterproductive to the rational assessment of new approaches to transfusion safety. It was recommended that the regulators clearly define what data might be required for licensure of a new technology. In the past, manufacturers were required to develop the concept and move it through the regulatory process to bring new technology to the market. This was felt to be a time-consuming and laborious approach, which has become cost prohibitive based on the sophistication of newer technologies under development. It was felt that the role of blood operators in facilitating this process must also be clarified and that there should be a cooperative effort between the innovator of new technology, the regulator, and the blood operator before the implementation of new technologies.
Finally, it was suggested that the regulators need to communicate more effectively among themselves in an effort to achieve international harmonization of approaches used in the assessment and acceptance of new technology. Universally applicable regulations are needed together with a decision-making process that must be capable of quickly addressing new pathogens or other threats that may emerge in the future.
Pathogen Inactivation Represents a New Paradigm Shift for Transfusion Medicine
It was observed that there have been major changes in thinking about transfusion safety since the emergence of the HIV pandemic in the early 1980s. It was suggested that PI represents a new paradigm shift, which moves the focus away from the reactive development of new testing methods to eliminate microbiological threats to a preemptive thinking about blood safety. Dr M.A. Blajchman (Hamilton, Ontario) referred to the work of Thomas Kuhn, a philosopher/scientist at the Massachusetts Institute of Technology (Cambridge, MA), who noted that “all major changes in science represent paradigm shifts.”106 Kuhn noted that major conflicts often occur when the supporters of the old paradigm are confronted with a new paradigm (a new way of thinking). This is where the international transfusion community finds itself today. The old paradigm tends to be reactive, that is, “to test” for new microbiological threats as they appear. It was felt that the transfusion medicine community needs to move forward in its thinking to the new paradigm of preemptively implementing new approaches toward improving microbiological safety, using global approaches that have a high likelihood of dealing with most emerging pathogens.
Thus, the decision with respect to introduction of PI technology must be based on an assessment of both the benefits and the risks. Separation of the safety/quality of care from the economic aspects of the decision-making process may be essential. Efforts should therefore be made by the transfusion medicine community to support manufacturers in evaluating the risks and benefits of these new technologies by targeted research initiatives and to assist regulators in the assessment and approval of new approaches to blood safety. The transfusion medicine community needs to take some responsibility for this process to ensure the realization of the potentially pre-emptive new paradigm for dealing with microbiological blood safety.
Acknowledgments
The authors of these proceedings would like to thank and acknowledge the significant contributions of the panel of experts, who presented data to the PI consensus conference, particularly for their cooperation in reviewing the summaries of their presentation for accuracy. We would also like to thank the members of the consensus conference steering committee: Morris Blajchman (Chair), Giles Delage, Dana Devine, Sunny (Walter) Dzik, Heather Hume, Harvey Klein, Jaroslav Vostal, Stephen Wagner, Kathryn Webert, and Lorna Williamson, as well as the members of the consensus conference panel, chaired by Dr Harvey Klein, for their participation and leadership in this meeting. We are grateful to Canadian Blood Services, Héma-Québec, and the BEST Collaborative for their sponsorship of this consensus conference. Finally, we would like to thank Malachite Management (Vancouver, British Columbia) and, in particular, Ms Lisa Markus, for helping with the many organizational details of this conference.
Footnotes
This Consensus Conference took place at the Park Hyatt Hotel in Toronto, Ontario, Canada, on March 29 to 30, 2007, and was chaired by Dr Morris A. Blajchman.
References
- 1.Klein H.G., Anderson D., Bernardi M.J. Pathogen inactivation: making decisions about new technologies—Preliminary report of a consensus conference. Vox Sang. 2007;93:179–182. doi: 10.1111/j.1423-0410.2007.00937.x. [DOI] [PubMed] [Google Scholar]
- 2.Busch M.P. Transfusion-transmitted viral infections: Building bridges to transfusion medicine to reduce risks and understand epidemiology and pathogenesis. Transfusion. 2006;46:1624–1640. doi: 10.1111/j.1537-2995.2006.00957.x. [DOI] [PubMed] [Google Scholar]
- 3.Murphy W. Managing threats rather than risks in blood transfusion: Robust design for a complex system. Transfusion. 2006;46:2011–2013. doi: 10.1111/j.1537-2995.2006.01010.x. [DOI] [PubMed] [Google Scholar]
- 4.Gubler D.J. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998;11:480–496. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stramer S.L., Fang C.T., Foster G.A. West Nile virus among blood donors in the United States, 2003 and 2004. N Engl J Med. 2005;353:451–459. doi: 10.1056/NEJMoa044333. [DOI] [PubMed] [Google Scholar]
- 6.Klein H.G., Dodd R.Y., Dzik W.H. Current status of solvent/detergent-treated frozen plasma. Transfusion. 1998;38:102–107. doi: 10.1046/j.1537-2995.1998.38198141508.x. [DOI] [PubMed] [Google Scholar]
- 7.Bryant B.J., Klein H.G. Pathogen inactivation: The definitive safeguard for the blood supply. Arch Pathol Lab Med. 2007;131:719–733. doi: 10.5858/2007-131-719-PITDSF. [DOI] [PubMed] [Google Scholar]
- 8.Wagner S.J. Virus inactivation in blood components by photoactive phenothiazine dyes. Transfus Med Rev. 2002;16:61–66. doi: 10.1053/tmrv.2002.29405. [DOI] [PubMed] [Google Scholar]
- 9.Hanson C.V. Photochemical inactivation of viruses with psoralens: an overview. Blood Cells. 1992;18:7–25. [PubMed] [Google Scholar]
- 10.Grass J.A., Hei D.J., Metchette K. Inactivation of leukocytes in platelet concentrates by photochemical treatment with psoralen plus UVA. Blood. 1998;91:2180–2188. [PubMed] [Google Scholar]
- 11.Ennever J.F., Speck W.T. Short communication. Photochemical reactions of riboflavin: Covalent binding to DNA and to poly (dA). poly (dT) Pediatr Res. 1983;17:234–236. doi: 10.1203/00006450-198303000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Ito K., Inoue S., Yamamoto K. 8-Hydroxydeoxyguanosine formation at the 5′ site of 5′-GG-3′ sequences in double-stranded DNA by UV radiation with riboflavin. J Biol Chem. 1993;268:13221–13227. [PubMed] [Google Scholar]
- 13.Corash L. Helinx technology for inactivation of infectious pathogens and leukocytes in labile blood components: from theory to clinical application. Transfus Apheresis Sci. 2001;25:179–181. doi: 10.1016/s1473-0502(01)00116-1. [DOI] [PubMed] [Google Scholar]
- 14.Purmal A., Valeri C.R., Dzik W. Process for the preparation of pathogen-inactivated RBC concentrates by using PEN110 chemistry: Preclinical studies. Transfusion. 2002;42:139–145. doi: 10.1046/j.1537-2995.2002.00020.x. [DOI] [PubMed] [Google Scholar]
- 15.McQueen C.A., Devito M., Chapin R. Definition of toxicology. Society of Toxicology web site. http://www.toxicology.org/ai/pub/si05/SI05_Define.asp [accessed August 27, 2007]
- 16.Ciaravino V. Preclinical safety of a nucleic acid-targeted Helinx compound: A clinical perspective. Semin Hematol. 2001;38:12–19. doi: 10.1016/s0037-1963(01)90119-2. [DOI] [PubMed] [Google Scholar]
- 17.Ciaravino V., McCullough T., Cimino G. The role of toxicology assessment in transfusion medicine. Transfusion. 2003;43:1481–1492. doi: 10.1046/j.1537-2995.2003.00544.x. [DOI] [PubMed] [Google Scholar]
- 18.Chapman J.R., Moore K., Butterworth B.E. Pathogen inactivation of RBCs: PEN110 reproductive toxicology studies. Transfusion. 2003;43:1386–1393. doi: 10.1046/j.1537-2995.2003.00497.x. [DOI] [PubMed] [Google Scholar]
- 19.Ciaravino V., McCullough T., Cimino G. Preclinical safety profile of plasma prepared using the INTERCEPT Blood System. Vox Sang. 2003;85:171–182. doi: 10.1046/j.1423-0410.2003.00351.x. [DOI] [PubMed] [Google Scholar]
- 20.Ciaravino V., McCullough T., Dayan A.D. Pharmacokinetic and toxicology assessment of INTERCEPT (S-59 and UVA treated) platelets. Hum Exp Toxicol. 2001;20:533–550. doi: 10.1191/096032701718120319. [DOI] [PubMed] [Google Scholar]
- 21.Butterworth B.E., Chapman J. Exposure of hematopoietic stem cells to ethylene oxide during processing represents a potential carcinogenic risk for transplant recipients. Regul Toxicol Pharmacol. 2007 doi: 10.1016/j.yrtph.2007.07.004. [E-publication 01 Aug 2007] [DOI] [PubMed] [Google Scholar]
- 22.Horowitz B., Bonomo R., Prince A.M. Solvent/detergent-treated plasma: A virus-inactivated substitute for fresh frozen plasma. Blood. 1992;79:826–831. [PubMed] [Google Scholar]
- 23.Biesert L., Suhartono H. Solvent/detergent treatment of human plasma—A very robust method for virus inactivation. Validated virus safety of OCTAPLAS. Vox Sang. 1998;74:207–212. doi: 10.1111/j.1423-0410.1998.tb05474.x. [Suppl 1] [DOI] [PubMed] [Google Scholar]
- 24.Lin L., Cook D.N., Wiesehahn G.P. Photochemical inactivation of viruses and bacteria in platelet concentrates by use of a novel psoralen and long-wavelength ultraviolet light. Transfusion. 1997;37:423–435. doi: 10.1046/j.1537-2995.1997.37497265344.x. [DOI] [PubMed] [Google Scholar]
- 25.Lambrecht B., Mohr H., Knuver-Hopf J. Photoinactivation of viruses in human fresh plasma by phenothiazine dyes in combination with visible light. Vox Sang. 1991;60:207–213. doi: 10.1111/j.1423-0410.1991.tb00907.x. [DOI] [PubMed] [Google Scholar]
- 26.Goodrich R.P. The use of riboflavin for the inactivation of pathogens in blood products. Vox Sang. 2000;78:211–215. [Suppl 2] [PubMed] [Google Scholar]
- 27.Flamholz R., Jeon H.R., Baron J.M. Study of three patients with thrombotic thrombocytopenic purpura exchanged with solvent/detergent-treated plasma: Is its decreased protein S activity clinically related to their development of deep venous thromboses? J Clin Apheresis. 2000;15:169–172. doi: 10.1002/1098-1101(2000)15:3<169::aid-jca2>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 28.Coignard B., Nguyen G.T., Tokars J. A cluster of intra-operative deaths in a liver transplant center associated with the use of solvent/detergent plasma. Proceedings of the 11th Annual Meetings of the Society for Healthcare Epidemiology of America; 2001. [abstract] [Google Scholar]
- 29.Yarranton H., Cohen H., Pavord S.R. Venous thromboembolism associated with the management of acute thrombotic thrombocytopenic purpura. Br J Haematol. 2003;121:778–785. doi: 10.1046/j.1365-2141.2003.04360.x. [DOI] [PubMed] [Google Scholar]
- 30.Williamson L.M., Llewelyn C.A., Fisher N.C. A randomized trial of solvent/detergent-treated and standard fresh-frozen plasma in the coagulopathy of liver disease and liver transplantation. Transfusion. 1999;39:1227–1234. doi: 10.1046/j.1537-2995.1999.39111227.x. [DOI] [PubMed] [Google Scholar]
- 31.Williamson L.M., Llewelyn C.A. Efficacy of SD-treated plasma during liver transplantation (reply) Transfusion. 2000;40:887–888. doi: 10.1046/j.1537-2995.2000.40070886.x. [letter] [DOI] [PubMed] [Google Scholar]
- 32.Salge-Bartels U., Breitner-Ruddock S., Hunfeld A. Are quality differences responsible for different adverse reactions reported for SD-plasma from USA and Europe? Transfus Med. 2006;16:266–275. doi: 10.1111/j.1365-3148.2006.00672.x. [DOI] [PubMed] [Google Scholar]
- 33.Heger A., Romisch J., Svae T.E. A biochemical comparison of a pharmaceutically licensed coagulation active plasma (Octaplas) with a universally applicable development product (Uniplas) and single-donor FFPs subjected to methylene-blue dye and white-light treatment. Transfus Apheresis Sci. 2006;35:223–233. doi: 10.1016/j.transci.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 34.Hellstern P. Solvent/detergent-treated plasma: Composition, efficacy, and safety. Curr Opin Hematol. 2004;11:346–350. doi: 10.1097/01.moh.0000137915.88478.23. [DOI] [PubMed] [Google Scholar]
- 35.Stanworth S.J., Brunskill S.J., Hyde C.J. Is fresh frozen plasma clinically effective? A systematic review of randomized controlled trials. Br J Haematol. 2004;126:139–152. doi: 10.1111/j.1365-2141.2004.04973.x. [DOI] [PubMed] [Google Scholar]
- 36.Simonsen A.C., Sorensen H. Clinical tolerance of methylene blue virus–inactivated plasma. A randomized crossover trial in 12 healthy human volunteers. Vox Sang. 1999;77:210–217. doi: 10.1159/000031129. [DOI] [PubMed] [Google Scholar]
- 37.Wieding J.U. Prospective randomized and controlled trial on solvent/detergent plasma versus methylene blue light inactivated plasma. Transfusion. 2007;39:23S. [abstract] [Suppl] [Google Scholar]
- 38.Varez-Larran A., Del R.J., Ramirez C. Methylene blue-photoinactivated plasma vs. fresh-frozen plasma as replacement fluid for plasma exchange in thrombotic thrombocytopenic purpura. Vox Sang. 2004;86:246–251. doi: 10.1111/j.0042-9007.2004.00506.x. [DOI] [PubMed] [Google Scholar]
- 39.de la R.J., Arriaga F., Linares D. Role of methylene blue-treated or fresh-frozen plasma in the response to plasma exchange in patients with thrombotic thrombocytopenic purpura. Br J Haematol. 2001;114:721–723. doi: 10.1046/j.1365-2141.2001.02991.x. [DOI] [PubMed] [Google Scholar]
- 40.del Rio-Garma J., Alvarex-Larran A., Martinez C. Methylene blue photoinactivated plasma (MBPIP) vs fresh frozen plasma (FFP) in the treatment of idiopathic thrombotic thrombocytopenic purpura (TTP): a multicentre prospective-cohort study. Vox Sang. 2007;93:41. [abstract] [suppl 1] [Google Scholar]
- 41.Atance R., Pereira A., Ramirez B. Transfusing methylene blue-photoinactivated plasma instead of FFP is associated with an increased demand for plasma and cryoprecipitate. Transfusion. 2001;41:1548–1552. doi: 10.1046/j.1537-2995.2001.41121548.x. [DOI] [PubMed] [Google Scholar]
- 42.Osselaer J.C., Debry C., Goffaux M. Coagulation function in fresh frozen plasma prepared with two photochemical treatment methods. Vox Sang. 2006;91:183. doi: 10.1111/j.1537-2995.2007.01488.x. [abstract] [Suppl 3] [DOI] [PubMed] [Google Scholar]
- 43.Politis C., Kavallierou L., Hantziara S. Quality and safety of fresh-frozen plasma inactivated and leucoreduced with the Theraflex methylene blue system including the Blueflex filter: 5 years' experience. Vox Sang. 2007;92:319–326. doi: 10.1111/j.1423-0410.2007.00898.x. [DOI] [PubMed] [Google Scholar]
- 44.Pehta J.C. Clinical studies with solvent detergent–treated products. Transfus Med Rev. 1996;10:303–311. doi: 10.1016/s0887-7963(96)80006-x. [DOI] [PubMed] [Google Scholar]
- 45.Chekrizova V., Murphy W.G. Solvent-detergent plasma: Use in neonatal patients, in adult and paediatric patients with liver disease and in obstetric and gynaecological emergencies. Transfus Med. 2006;16:85–91. doi: 10.1111/j.1365-3148.2006.00626.x. [DOI] [PubMed] [Google Scholar]
- 46.Mintz P.D., Bass N.M., Petz L.D. Photochemically treated fresh frozen plasma for transfusion of patients with acquired coagulopathy of liver disease. Blood. 2006;107:3753–3760. doi: 10.1182/blood-2004-03-0930. [DOI] [PubMed] [Google Scholar]
- 47.Mintz P.D., Neff A., MacKenzie M. A randomized, controlled Phase III trial of therapeutic plasma exchange with fresh-frozen plasma (FFP) prepared with amotosalen and ultraviolet A light compared to untreated FFP in thrombotic thrombocytopenic purpura. Transfusion. 2006;46:1693–1704. doi: 10.1111/j.1537-2995.2006.00959.x. [DOI] [PubMed] [Google Scholar]
- 48.Jackson B.R., AuBuchon J.P., Birkmeyer J.D. Update of cost-effectiveness analysis for solvent-detergent–treated plasma. JAMA. 1999;282:329. doi: 10.1001/jama.282.4.329. [letter] [DOI] [PubMed] [Google Scholar]
- 49.Pereira A. Cost-effectiveness of transfusing virus-inactivated plasma instead of standard plasma. Transfusion. 1999;39:479–487. doi: 10.1046/j.1537-2995.1999.39050479.x. [DOI] [PubMed] [Google Scholar]
- 50.Riedler G.F., Haycox A.R., Duggan A.K. Cost-effectiveness of solvent/detergent-treated fresh-frozen plasma. Vox Sang. 2003;85:88–95. doi: 10.1046/j.1423-0410.2003.00329.x. [DOI] [PubMed] [Google Scholar]
- 51.Goodrich R.P., Li J., Pieters H. Correlation of in vitro platelet quality measurements with in vivo platelet viability in human subjects. Vox Sang. 2006;90:279–285. doi: 10.1111/j.1423-0410.2006.00761.x. [DOI] [PubMed] [Google Scholar]
- 52.AuBuchon J.P., Herschel L., Roger J. Efficacy of apheresis platelets treated with riboflavin and ultraviolet light for pathogen reduction. Transfusion. 2005;45:1335–1341. doi: 10.1111/j.1537-2995.2005.00202.x. [DOI] [PubMed] [Google Scholar]
- 53.Snyder E., Raife T., Lin L. Recovery and life span of 111indium-radiolabeled platelets treated with pathogen inactivation with amotosalen HCl (S-59) and ultraviolet A light. Transfusion. 2004;44:1732–1740. doi: 10.1111/j.0041-1132.2004.04145.x. [DOI] [PubMed] [Google Scholar]
- 54.Harker L.A., Slichter S.J. The bleeding time as a screening test for evaluation of platelet function. N Engl J Med. 1972;287:155–159. doi: 10.1056/NEJM197207272870401. [DOI] [PubMed] [Google Scholar]
- 55.Slichter S.J., Raife T.J., Davis K. Platelets photochemically treated with amotosalen HCl and ultraviolet A light correct prolonged bleeding times in patients with thrombocytopenia. Transfusion. 2006;46:731–740. doi: 10.1111/j.1537-2995.2006.00791.x. [DOI] [PubMed] [Google Scholar]
- 56.McCullough J., Vesole D.H., Benjamin R.J. Therapeutic efficacy and safety of platelets treated with a photochemical process for pathogen inactivation: The SPRINT Trial. Blood. 2004;104:1534–1541. doi: 10.1182/blood-2003-12-4443. [DOI] [PubMed] [Google Scholar]
- 57.van Rhenen R.D., Gulliksson H., Cazenave J.P. Transfusion of pooled buffy coat platelet components prepared with photochemical pathogen inactivation treatment: The euroSPRITE trial. Blood. 2003;101:2426–2433. doi: 10.1182/blood-2002-03-0932. [DOI] [PubMed] [Google Scholar]
- 58.Janetzko K., Cazenave J.P., Kluter H. Therapeutic efficacy and safety of photochemically treated apheresis platelets processed with an optimized integrated set. Transfusion. 2005;45:1443–1452. doi: 10.1111/j.1537-2995.2005.00550.x. [DOI] [PubMed] [Google Scholar]
- 59.Pickard C., AuBuchon J.P., Tosteson A.N.A. Influence of gender and collection order on radiolabeled red blood cell recovery. Transfusion. 1995;35:6S. [abstract] [Suppl] [Google Scholar]
- 60.US Food and Drug Administration Blood Products Advisory Committee transcript. Blood Products Advisory Committee. 2004. http://www.fda.gov/OHRMS/DOCKETS/ac/04/transcripts/2004-4057t1.htm [accessed August 27, 2007]
- 61.AuBuchon J.P., Pickard C.A., Herschel L.H. Production of pathogen-inactivated RBC concentrates using PEN110 chemistry: A phase I clinical study. Transfusion. 2002;42:146–152. doi: 10.1046/j.1537-2995.2002.00021.x. [DOI] [PubMed] [Google Scholar]
- 62.Rios J.A., Hambleton J., Viele M. Viability of red cells prepared with S-303 pathogen inactivation treatment. Transfusion. 2006;46:1778–1786. doi: 10.1111/j.1537-2995.2006.00973.x. [DOI] [PubMed] [Google Scholar]
- 63.Benjamin R.J., McCullough J., Mintz P.D. Therapeutic efficacy and safety of red blood cells treated with a chemical process (S-303) for pathogen inactivation: A phase III clinical trial in cardiac surgery patients. Transfusion. 2005;45:1739–1749. doi: 10.1111/j.1537-2995.2005.00583.x. [DOI] [PubMed] [Google Scholar]
- 64.Wagner S.J. Pathogen inactivation. Presented at AABB Annual Meeting, Seattle, WA; 2005. [Google Scholar]
- 65.Lublin D.M. Universal RBCs. Transfusion. 2000;40:1285–1289. doi: 10.1046/j.1537-2995.2000.40111285.x. [DOI] [PubMed] [Google Scholar]
- 66.Garratty G. Progress in modulating the RBC membrane to produce transfusable universal/stealth donor RBCs. Transfus Med Rev. 2004;18:245–256. doi: 10.1016/j.tmrv.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 67.Garratty G. In vitro reactions with red blood cells that are not due to blood group antibodies: A review. Immunohematol. 1998;14:1–11. [PubMed] [Google Scholar]
- 68.Conlan M.G., Stassinopoulos A., Garratty G. Antibody formation to S-303–treated RBCs in setting of chronic RBC transfusion. Blood. 2004;104:112a. [abstract] [Suppl] [Google Scholar]
- 69.North A., Garratty G., Schott M. A modified process for preparation of S-303 RBCs for pathogen inactivation substantially reduces potential for reactivity. Transfusion. 2006;46:116A. [abstract] [Suppl] [Google Scholar]
- 70.Stassinopoulos A., Schott M.A., Castro G.M. Modification of the S-303 RBC pathogen inactivation process results in normal S-303 RBC viability in rabbits hyper-immunized to S-303 [abstract] Vox Sang. 2005;89:138–139. [Supp] [Google Scholar]
- 71.Stainsby D., Jones H., Asher D. Serious hazards of transfusion: a decade of hemovigilance in the UK. Transfus Med Rev. 2006;20:273–282. doi: 10.1016/j.tmrv.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 72.Dzik W.H., Murphy M.F., Andreu G. An international study of the performance of sample collection from patients. Vox Sang. 2003;85:40–47. doi: 10.1046/j.1423-0410.2003.00313.x. [DOI] [PubMed] [Google Scholar]
- 73.Murphy M.F., Stearn B.E., Dzik W.H. Current performance of patient sample collection in the UK. Transfus Med. 2004;14:113–121. doi: 10.1111/j.0958-7578.2004.0488.x. [DOI] [PubMed] [Google Scholar]
- 74.Figueroa P.I., Ziman A., Wheeler C. Nearly two decades using the check-type to prevent ABO incompatible transfusions: One institution's experience. Am J Clin Pathol. 2006;126:422–426. doi: 10.1309/C6U7VP87GC030WMG. [DOI] [PubMed] [Google Scholar]
- 75.Cserti C.M., Ward M., Uhl L. Impact of a policy to use only group O red cell transfusions for recipients with fewer-than-twice established ABO types: A feasible means to reduce potential ABO incompatible transfusion errors. Blood. 2006;108:289a. [abstract] [Suppl] [Google Scholar]
- 76.Denomme G.A., Van O.M. High-throughput multiplex single-nucleotide polymorphism analysis for red cell and platelet antigen genotypes. Transfusion. 2005;45:660–666. doi: 10.1111/j.1537-2995.2005.04365.x. [DOI] [PubMed] [Google Scholar]
- 77.Vostal J. Update on bacterial contamination. Presentation at 2005 AABB Annual Meeting, slide 18; 2005. [Google Scholar]
- 78.FDA Center for Drug Evaluation and Research Guidance documents. http://fda.gov/cder/guidance/index.htm#pharmacology/toxicology [Accessed August 27, 2007]
- 79.Zarbock A., Singbartl K., Ley K. Complete reversal of acid-induced acute lung injury by blocking of platelet-neutrophil aggregation. J Clin Invest. 2006;116:3211–3219. doi: 10.1172/JCI29499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pitchford S.C., Momi S., Giannini S. Platelet P-selectin is required for pulmonary eosinophil and lymphocyte recruitment in a murine model of allergic inflammation. Blood. 2005;105:2074–2081. doi: 10.1182/blood-2004-06-2282. [DOI] [PubMed] [Google Scholar]
- 81.Kupatt C., Wichels R., Horstkotte J. Molecular mechanisms of platelet-mediated leukocyte recruitment during myocardial reperfusion. J Leukoc Biol. 2002;72:455–461. [PubMed] [Google Scholar]
- 82.Benjamin R.J. Presentation at AABB Annual Meeting; 2006. [Google Scholar]
- 83.Hanley J.A., Lippman-Hand A. If nothing goes wrong, is everything all right? Interpreting zero numerators. JAMA. 1983;249:1743–1745. [PubMed] [Google Scholar]
- 84.Snyder E., McCullough J., Slichter S.J. Clinical safety of platelets photochemically treated with amotosalen HCl and ultraviolet A light for pathogen inactivation: The SPRINT trial. Transfusion. 2005;45:1864–1875. doi: 10.1111/j.1537-2995.2005.00639.x. [DOI] [PubMed] [Google Scholar]
- 85.Offergeld R., Faensen D., Ritter S. Human immunodeficiency virus, hepatitis C and hepatitis B infections among blood donors in Germany 2000-2002: Risk of virus transmission and the impact of nucleic acid amplification testing. Euro Surveill. 2005;10:8–11. [PubMed] [Google Scholar]
- 86.German haemovigilance, Paul-Ehrlich-Institute. 2007.
- 87.Schrezenmeier H., Walther-Wenke G., Muller T.H. Bacterial contamination of platelet concentrates: Results of a prospective multicenter study comparing pooled whole blood–derived platelets and apheresis platelets. Transfusion. 2007;47:644–652. doi: 10.1111/j.1537-2995.2007.01166.x. [DOI] [PubMed] [Google Scholar]
- 88.Knutson F., Alfonso R., Dupuis K. Photochemical inactivation of bacteria and HIV in buffy-coat–derived platelet concentrates under conditions that preserve in vitro platelet function. Vox Sang. 2000;78:209–216. doi: 10.1159/000031183. [DOI] [PubMed] [Google Scholar]
- 89.Sachs U.J., Kauschat D., Bein G. White blood cell–reactive antibodies are undetectable in solvent/detergent plasma. Transfusion. 2005;45:1628–1631. doi: 10.1111/j.1537-2995.2005.00587.x. [DOI] [PubMed] [Google Scholar]
- 90.Solheim B.G., Seghatchian J. Update on pathogen reduction technology for therapeutic plasma: An overview. Transfus Apheresis Sci. 2006;35:83–90. doi: 10.1016/j.transci.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 91.Custer B. Economic analyses of blood safety and transfusion medicine interventions: A systematic review. Transfus Med Rev. 2004;18:127–143. doi: 10.1016/j.tmrv.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 92.van Hulst M., De Wolf J.T., Staginnus U. Pharmaco-economics of blood transfusion safety: Review of the available evidence. Vox Sang. 2002;83:146–155. doi: 10.1046/j.1423-0410.2002.00198.x. [DOI] [PubMed] [Google Scholar]
- 93.AuBuchon J.P., Birkmeyer J.D. Safety and cost-effectiveness of solvent-detergent–treated plasma. In search of a zero-risk blood supply. JAMA. 1994;272:1210–1214. [PubMed] [Google Scholar]
- 94.Riedler G.F., Haycox A.R., Duggan A.K. Solvent-detergent–treated plasma may be cost-effective. Vox Sang. 2003;84:334–335. doi: 10.1046/j.1423-0410.2003.00307_1.x. [DOI] [PubMed] [Google Scholar]
- 95.Bell C.E., Botteman M.F., Gao X. Cost-effectiveness of transfusion of platelet components prepared with pathogen inactivation treatment in the United States. Clin Ther. 2003;25:2464–2486. doi: 10.1016/S0149-2918(03)80288-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Staginnus U., Corash L. Economics of pathogen inactivation technology for platelet concentrates in Japan. Int J Hematol. 2004;80:317–324. doi: 10.1532/ijh97.04131. [DOI] [PubMed] [Google Scholar]
- 97.Janssen M.P., van der Poel C.L., Buskens E. Costs and benefits of bacterial culturing and pathogen reduction in the Netherlands. Transfusion. 2006;46:956–965. doi: 10.1111/j.1537-2995.2006.00828.x. [DOI] [PubMed] [Google Scholar]
- 98.Postma M.J., van Hulst M., De Wolf J.T. Cost-effectiveness of pathogen inactivation for platelet transfusions in the Netherlands. Transfus Med. 2005;15:379–387. doi: 10.1111/j.1365-3148.2005.00609.x. [DOI] [PubMed] [Google Scholar]
- 99.Moeremans K., Warie H., Annemans L. Assessment of the economic value of the INTERCEPT blood system in Belgium. Transfus Med. 2006;16:17–30. doi: 10.1111/j.1365-3148.2006.00644.x. [DOI] [PubMed] [Google Scholar]
- 100.Trannoy L.L., Lagerberg J.W., Dubbelman T.M. Positively charged porphyrins: A new series of photosensitizers for sterilization of RBCs. Transfusion. 2004;44:1186–1196. doi: 10.1111/j.1537-2995.2004.03275.x. [DOI] [PubMed] [Google Scholar]
- 101.Trannoy L.L., Terpstra F.G., de K.D. Differential sensitivities of pathogens in red cell concentrates to Tri-P(4)–photoinactivation. Vox Sang. 2006;91:111–118. doi: 10.1111/j.1423-0410.2006.00791.x. [DOI] [PubMed] [Google Scholar]
- 102.Wagner S.J., Skripchenko A., Salata J. Photoinactivation of Leishmania donovani infantum in red cell suspensions by a flexible thiopyrylium sensitizer. Vox Sang. 2006;91:178–180. doi: 10.1111/j.1423-0410.2006.00810.x. [DOI] [PubMed] [Google Scholar]
- 103.Skripchenko A., Balch A., Mackin A. In vivo recovery and survival of red cells after photodynamic treatment with thiopyrylium and red light using a canine model. Vox Sang. 2007;92:157–159. doi: 10.1111/j.1423-0410.2006.00864.x. [DOI] [PubMed] [Google Scholar]
- 104.Skripchenko A., Wagner S.J., Thompson-Montgomery D. Thiazole orange, a DNA-binding photosensitizer with flexible structure, can inactivate pathogens in red blood cell suspensions while maintaining red cell storage properties. Transfusion. 2006;46:213–219. doi: 10.1111/j.1537-2995.2006.00703.x. [DOI] [PubMed] [Google Scholar]
- 105.O'Brien S.F., Yi Q.L., Fan W. Current incidence and estimated residual risk of transfusion-transmitted infections in donations made to Canadian Blood Services. Transfusion. 2007;47:316–325. doi: 10.1111/j.1537-2995.2007.01108.x. [DOI] [PubMed] [Google Scholar]
- 106.Kuhn T.S. 2nd enlarged ed. The University of Chicago Press; Chicago: 1970. The structure of scientific revolutions. [Google Scholar]