Abstract
Administration of recombinant feline interferon-ω (rFeIFN) has been proposed for the prophylaxis of canine and feline parvovirosis. In the present study, the influence of the administration of rFeIFN on blood markers of inflammation (α-globulins, α1-acid glycoprotein) and immune system activation (γ-globulins, IgG, IgM, specific anti-feline parvovirus IgG or IgM) was evaluated in a cattery developing an outbreak of feline panleukopenia due to feline parvovirus (FPV) infection few days after initial administration of rFeIFN. Kittens (n = 23) were injected with rFeIFN (1 MU/kg subcutaneoulsy, once a day for 3 days) and their blood parameters were compared with those of 17 untreated cats. Cats that survived the outbreak were vaccinated and re-sampled 1 month after the last rFeIFN administration. Time of emergence of clinical signs and survival rate were not significantly different between the two groups. Controls and treated cats surviving the infection had high levels of γ-globulins, total- and anti-FPV specific IgGs, likely due to passive transfer of maternal immunity. Compared to controls, treated kittens had lower levels of α1-globulins and higher mean values of γ-globulins and immunoglobulins. Data from samples collected after vaccination revealed a higher level of γ-globulins, total- and anti-FPV specific IgGs in treated kittens, compared with controls, suggesting that rFeIFN stimulates antibody production. Based on this results, rFeIFN should be administered to the queen, to increase passive maternal immunity, or to kittens before introduction in a potentially contaminated environment.
Keywords: Interferon-ω, Feline panleukopenia, Parvovirus, AGP, Electrophoresis, Immunoglobulin
1. Introduction
The “Interferon family” includes cytokines with non-specific antiviral, anti-proliferative, anti-inflammatory and immunomodulatory activities. Specifically, type I interferons (IFN) include IFN-α, IFN-β e IFN-ω (Wonderling et al., 2002). Type I IFNs are produced by virus-infected cells and interact with specific receptors on adjacent cells, which are induced to transcribe genes coding for antiviral proteins. As a consequence, viral replication and viral protein synthesis decrease (Ueda et al., 1993b, Uchino, 1995, Tilg, 1997, Stark et al., 1998, Wonderling et al., 2002). Type II interferons include IFN-γ (Wonderling et al., 2002), produced by T-lymphocytes and NK cells. IFN-γ has a prevalent immunomodulatory activity, characterized by the activation of macrophages, T-lymphocytes, NK cells and, to a lesser extent, of B cells (Mond et al., 1986, Bohem et al., 1997). Several virus types have been demonstrated to modulate IFN production (Uchino, 1995, Stark et al., 1998). Moreover, the administration of exogenous IFNs in a population with high prevalence of viral diseases or during the early phase of viral infection inhibits viral replication. Thus, the administration of IFNs may demonstrate a prophylactic effect or may prevent the worsening of certain viral diseases (Truyen et al., 2002). The administration of exogenous human IFN does not induce severe side effects in cats (Müller, 2002), but, as an exogenous antigen, induces the production of neutralizing antibodies with inhibition of the therapeutic effects of the active principle (Zeidner et al., 1990, Müller, 2002). The administration of a species-specific feline IFN is able to by-pass this problem (Müller, 2002, Truyen et al., 2002).
Recombinant feline IFN-ω (rFeIFN) has 65% homology with human IFNs (Ueda et al., 1993b), and has antiviral, pharmacokinetic and pharmacological properties similar to that of human IFN-ω (Ueda et al., 1993a, Ueda et al., 1993b). In vitro, rFeIFN has a proven antiviral effect against several viruses including parvovirus, herpesvirus, calicivirus, coronavirus and rotavirus (Mochizuki et al., 1994, Müller, 2002, Truyen et al., 2002). In vivo, rFeIFN has been mainly used in dogs with experimental or spontaneous parvoviral infections, with consequent clinical improvement, normalization of CBC results and reduction of the mortality rate (Ishiwata et al., 1998, Minagawa et al., 1999, Martin et al., 2002). In dogs, rFeIFN has also been successfully used, associated with vaccination, to prevent outbreaks of canine parvovirosis (Uchino, 1995). The efficacy of rFeIFN in cats with feline infectious peritonitis, or infected with feline immunodeficiency virus, feline leukaemia virus and feline calicivirus has also been reported (Uchino, 1995, Mihaljevic, 2003, De Mari et al., 2004, Ishida et al., 2004). However, no data on the effect of rFeIFN in cases of spontaneous feline panleukopenia (FP), caused by the feline parvovirus (FPV), are available. The administration of rFeIFN in kittens with FP seems not to influence the course of the disease (personal observations). Nevertheless, in the case rFeIFN would modulate inflammatory or immune response, the administration of rFeIFN may be integrated in conventional therapies or it may be considered for prophylactic plans against FP in catteries.
Aim of the current study was, thus, to investigate the possible influence of rFeIFN administration on the inflammatory and immune response of kittens living in a rescue shelter characterized by a high prevalence of FPV infection and treated just before the occurrence of an FP outbreak.
2. Materials and methods
2.1. Animals and experimental design
Serum samples were collected from cats living in a rescue shelter which hosts approximately 80 adult cats and more than 250 kittens per year. The shelter was chosen since it had a record of recurring, early summer severe outbreaks of FP in kittens.
All sheltered kittens with an age range of 50–70 days were included in the study. They were randomly divided in two groups: 17 “controls”, which did not receive the treatment, and 23 “treated”, which received 1 MU/kg of rFeIFN (Virbagen omega, Virbac, Carros Cedex, France) subcutaneoulsy, once a day for 3 days, as suggested for cats affected by other viral disorders (Ishida et al., 2004). Approximately 1 week prior to the expected time of the FP outbreak, the referring veterinarian started the administration of rFeIFN to the “treated group”.
After rFeIFN administration (day 0), all cats received a complete daily physical examination for 32 days. Any abnormal clinical finding or any death were recorded, especially in the case of clinical signs consistent with feline panleukopenia. In the case of clinical signs, supportive therapy (chosen on the basis of the prevalent clinical signs) was administered, to both controls and treated kittens.
Blood samples were collected from 9 controls and 12 treated cats, irrespective of the presence of clinical signs, in a period ranging from days 5 to 11.
In each of the two groups, 7 cats among those that were still alive and not symptomatic after the outbreak were randomly selected and were vaccinated against feline panleukopenia, calicivirosis and herpesvirosis (Feligen CRP, Virbac) 7 or 14 days after rFeIFN administration.
Additional blood samples were collected on day 32 from 2 vaccinated controls and 5 vaccinated treated cats.
Blood was taken from the jugular vein and put in plain tubes. Blood samples were immediately submitted to the laboratory and processed in a blind manner. Serum was obtained by centrifugation, separated and stored at −20 °C until the analysis.
A complete post-mortem examination was performed in 9 out of the 24 cats that died with clinical signs consistent with feline parvovirosis (5 from the control group and 4 from the treated group).
2.2. Serum protein electrophoresis and specific protein measurement
Total proteins were measured in 28 serum samples by a discrete analyser (EOS-BRAVO, Hospitex, Firenze, Italy) using the Biuret method (Hospitex Firenze, Italy). Serum protein electrophoresis was performed using the semimicro method, with cellulose polyacetate strips (SEAC, Firenze, Italy) in a barbitone and tris-buffer (Helena Lab. Italia Spa, Assago, MI, Italy). The strips were run for 40 min, 150 V and then stained for 15 min in Red Ponceau (0.5 g in 100 ml of 5% trichloroacetic acid), destained in 5% acetic acid and put in a diaphanising solution (Helena Lab. Italia Spa). The gels were scanned in a densitometer (BT512, Biotecnica Instruments, Roma, Italy).
Radial immunodiffusion (RID) kits were used to measure IgG, IgM (VET-RID, Bethyl Laboratories, Inc., Montgomery, USA) and AGP (Tridelta Ltd., Dublin, Ireland), according with manufacturer's instructions.
Concentration of specific anti-parvoviral serum antibodies were evaluated using commercially available multiwell-slides coated with Crandell Feline Kidney Cells infected by the FPV strain TN/CEK-3 (VMRD Inc., Pullman, WA, USA). Briefly, 50 μl of each serum sample, diluted 1:25 in PBS were put in each well. After an incubation of 30 min at 37 °C and washes with PBS, an anti-feline IgG (Nordic Immunological Laboratories, Titburg, The Netherlands) was added to each well. After incubating the well for 30 min at 37 °C and washes with PBS, slides were coverslipped with glycerine and examined at 400× under a fluorescence microscope. Positive wells were considered those with 30–50% of fluorescent cells. Positive sera were diluted on a two-fold basis until a negative result was obtained. The antibody titre corresponded to the last dilution which provided a positive result.
All the above steps were repeated on different slides using an anti-feline IgM antibody (VMRD Inc.) as a secondary antibody instead of the anti-feline IgG. Both positive (FPL positive control, VMRD Inc.) and negative controls (PBS alone) were included in each session of tests.
2.3. Statistical analysis
Statistical analysis was performed utilizing the software Statistica (Statsoft Inc., Tulsa, OK, USA). Survival curves were designed using the Kaplan–Meyer method, and differences between groups were calculated using the Gean's Wilcoxon test. Data from controls and treated animals were compared with a Student's t-test for independent samples or, when a normality test showed that data had a non-normal distribution, with the corresponding Mann–Withney non-parametric U-test. Data from repeated samples of the same animal were compared with a Student's t-test for dependent samples or with the corresponding Wilcoxon non-parametric test.
3. Results
3.1. Clinical findings and survival rates
An outbreak of feline panleukopenia occurred in the cattery during the week following rFeIFN administration (from days 2 to 14 after the beginning of the study period). Clinical signs consistent with FP (vomiting, diarrhoea, anorexia and sudden death after hyperacute gastroenteric episodes) were recorded in 13/17 control cats (76.5%) and in 17/23 treated cats (73.9%). Among the symptomatic cats, 10/13 controls (76.9%) and 14/17 treated kittens (82.4%) died. Survival rates were 41.2% (7/17) among controls and 39.1% (9/23) among treated animals. No significant differences between the two groups were recorded in terms of time of appearance of clinical signs or of survival rates (Fig. 1 ).
Fig. 1.
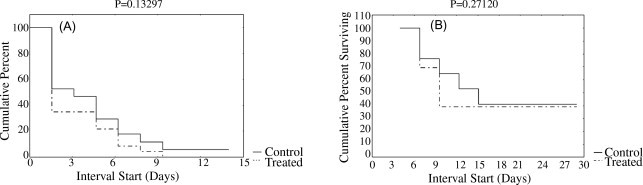
Time of appearance of signs consistent with feline panleukopenia (A), survival times (B) and results of the statistical comparison between controls and treated animals.
Necropsies confirmed the diagnosis of acute feline parvovirosis in all nine cats examined. All cats had necrotizing and hemorragic enteritis involving the small intestine (jejunum and/or ileum). Histology was characterized by necrosis of the crypt epithelium of variable severity. Crypts were often dilated, contained abundant luminal cellular debris and, in some areas, showed a flattened epithelial lining. In some crypts, epithelial cell dysplasia was detected. Additional findings were severe lymphoid depletion in lymph node cortex and splenic white pulp. Bone marrow was also moderately to severely hypocellular. The severity of lesions in lymphoid organs and bone marrow was similar in controls and treated cats.
3.2. Blood tests
Individual data obtained from control and treated cats during the outbreak (Fig. 2 ), evidenced that most cats surviving the outbreak had higher levels of γ-globulin, IgG and specific anti-FPV IgG, compared with those that died. Treated cats had significantly lower levels of α1-globulins and of AGP compared to controls (Table 1 ).
Fig. 2.
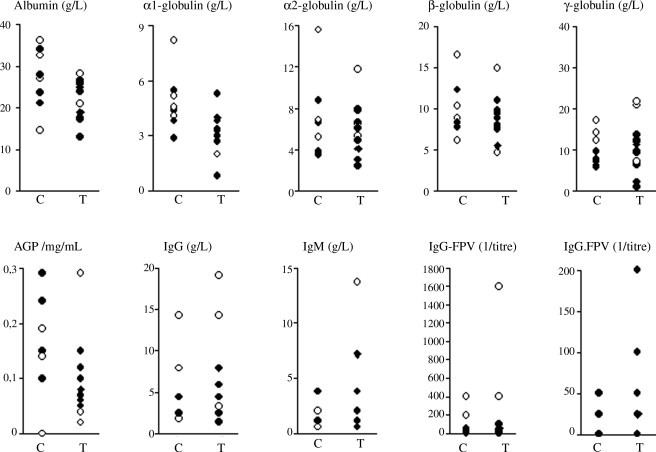
Distribution of results regarding serum protein electrophoresis, AGP, and total and FPV specific IgG and IgM recorded during the outbreak of feline panleukopenia in controls (C) and in cats treated with rFeIFN (T). White dots: cats that survived the outbreak; black dots: cats that died during the outbreak.
Table 1.
Mean ± S.D. (median) and reference interval at 95th percentiles recorded during the outbreak (CI: controls; TI: cats treated with recombinant feline interferon-ω) and 1 month later (CII: controls; TII: cats treated with recombinant feline interferon-ω)
| CI | TI | CII | TII | |
|---|---|---|---|---|
| Alb | 2.72 ± 0.72 (2.75) | 2.25 ± 0.49 (2.45) | 3.48 ± 1.92 (3.48) | 3.31 ± 0.86 (2.96) |
| (g/dl) | 1.57–3.58 | 1.41–2.78 | 2.19–4.76 | 2.50–4.43 |
| α1-glob | 0.48 ± 0.16 (0.45) | 0.32 ± 0.12* (0.34) | 0.18 ± 0.09 (0.18) | 0.24 ± 0.15 (0.18) |
| (g/dl) | 0.31–0.77 | 0.11–0.49 | 0.11–0.24 | 0.15–0.48 |
| α2-glob | 0.72 ± 0.38 (0.67) | 0.57 ± 0.27 (0.57) | 0.40 ± 0.21 (0.40) | 0.60 ± 0.12 (0.55) |
| (g/dl) | 0.36–1.44 | 0.24–1.07 | 0.26–0.54 | 0.49–0.75 |
| β-glob | 0.98 ± 0.33 (0.86) | 0.87 ± 0.26 (0.85) | 0.70 ± 0.39 (0.70) | 0.72 ± 0.10 (0.76) |
| (g/dl) | 0.64–1.58 | 0.49–1.38 | 0.43–0.96 | 0.57–0.81 |
| γ-glob | 1.00 ± 0.42 (0.86) | 1.06 ± 0.63 (1.05) | 0.81 ± 0.29 (0.81) | 2.44 ± 0.71† (2.66) |
| (g/dl) | 0.58–1.67 | 0.13–2.15 | 0.61–1.00 | 1.65–3.23 |
| AGP | 0.17 ± 0.08 (0.17) | 0.10 ± 0.07* (0.09) | 0.06 ± 0.04 (0.06) | 0.05 ± 0.04 (0.03) |
| (mg/ml) | 0.04–0.28 | 0.03–0.25 | 0.03–0.09 | 0.02–0.11 |
| IgG | 0.55 ± 0.43 (0.35) | 0.67 ± 0.53 (0.59) | 0.66 ± 0.31 (0.66) | 1.64 ± 0.35† (1.85) |
| (g/dl) | 0.19–1.32 | 0.14–1.78 | 0.45–0.87 | 1.15–1.91 |
| IgM | 0.24 ± 0.13 (0.20) | 0.56 ± 0.46 (0.55) | 0.22 ± 0.03 (0.22) | 0.39 ± 0.21 (0.38) |
| (g/dl) | 0.07–0.38 | 0.07–1.37 | 0.20–0.24 | 0.20–0.69 |
| IgG–FPV | 1:144 ± 1:169 (1:50) | 1:194 ± 1:457 (1:25) | 1:300 ± 1:141 (1:300) | 1:1160 ± 1:1268† (1:400) |
| 1:4–1:400 | 0–1:1270 | 1:205–1:395 | 1:220–1:3040 | |
| IgM–FPV | 1:16 ± 1:19 (1:12) | 1:50 ± 1:58 (1:25) | 1:37 ± 1:18 (1:37) | 1:75 ± 1:79 (1:50) |
| 0–1:46 | 0–1:172 | 1:25–1:50 | 1:2–1:190 | |
P < 0.05 vs. CI.
P < 0.05 vs. CII and vs. TI.
In the blood samples collected at day 32, treated cats had a significantly higher level of γ-globulins, IgG and anti-FPV specific IgG (Table 1). The same parameters were significantly higher in treated cats sampled at 32 days after rFeIFN administration compared to treated cats sampled during the outbreak (Table 1).
4. Discussion
The aim of this study was to evaluate the possible influence of the administration of recombinant feline IFN-ω on serum markers of inflammation and immunity in kittens living in a rescue shelter characterized by a high prevalence of feline parvovirus infection. This is, to our knowledge, the first “in field” study evaluating the influence of rFeIFN. As in any other “in field” study in feline rescue shelters, this type of approach has the advantage to correspond to the routine daily practice. However, the major pitfall is the consequent lack of standardization. As a matter of fact, in “field studies” it is not possible to define the time intercurring between initial infection and IFN-administration. Additionally, some cats were not completely worked up since they died during the night or in the weekends impeding the collection of “fresh” blood or tissues. Also, in some instances, samples were hemolytic and had to be excluded from the study.
Although the day of the FP outbreak could not be calculated with precision, the rFeIFN administration was initiated based on the mean outbreak starting dates recorded in the past years. As expected, the outbreak occurred during the week after the beginning of the administration. Specifically, the first clinical signs and/or deaths were recorded on day 2, when kittens from the “treated” group were still receiving rFeIFN.
The dose of rFeIFN was the same administered to cats with other viral diseases (Ishida et al., 2004). Our protocol closely resembled the one applied to the canine species that is characterized by rFeIFN administration followed by vaccination either during the introduction of animals in a contaminated environment or immediately after the appearance of clinical signs consistent with the disease (Uchino, 1995, Martin et al., 2002, Truyen et al., 2002) Unfortunately, it was not possible to perform necropsies in all dead cats due to the poor conditions of the carcasses or to investigate the presence of feline panleukopenia viruses in the faeces of survivors or to perform leukocyte counts in all the alive symptomatic animals. The incomplete collection of material as a consequence of the high temperatures, the long lapse time from death and collection of samples, did not allow the demonstration of FPV in all cats included. Nevertheless, the clinical signs recorded were consistent with acute forms of FP, and necropsies and histology confirmed this diagnosis in the nine cats that could be examined. These findings, coupled with our knowledge regarding FPV ecology, epidemiology and clinical presentation, together with the finding of specific anti-FPV IgM, which are produced during acute phase of infection, allowed us to conclude that FP was present in the cattery, and likely affected all cats.
Contrarily to what reported in dogs, in this study the administration of rFeIFN did not influence the development of clinical signs or the survival rate during the outbreak (Minagawa et al., 1999, Martin et al., 2002, De Mari et al., 2003). Nevertheless, the analysis of serum levels of indirect markers of inflammation/immunity demonstrated that rFeIFN influenced the development of inflammation and the immune response to the FPV.
Although the number of animals included in the study was comparable with, or greater than, previous laboratory experiments (Martin et al., 2002, De Mari et al., 2003), the total number of cats per group was too low to detect statistically significant differences between cats with or without clinical signs. Moreover, a type I statistical error (e.g. overestimation of statistical significance) might have affected data analysis, due to the multiple different analyses of biochemical data. Nevertheless, individual data collected during the outbreak evidenced high levels of total- and anti-FPV specific IgG in cats of both groups that were non-symptomatic at sampling, especially if they survived the infection. These data support the hypothesis of Pollock and Carmichael (1982) that maternal passive IgG transfer protects against parvovirus and allows the kittens to mount a moderate systemic inflammatory response that seems to protect them from worsening or from death. On the contrary, symptomatic cats, including one kitten that was vaccinated 2 days before sampling, did not have major electrophoretic changes (data not shown), likely due to the hyperacute course of the disease.
The AGP levels from symptomatic and non-symptomatic control cats were within the range reported in controls included in previous studies (Kajikawa et al., 1999). The lack of inflammatory changes in symptomatic controls may be the consequence of the hyperacute course of the disease although, in experimentally induced inflammation AGP increases within 24 h (Kajikawa et al., 1999). Most cats included in the present study died within few hours from the appearance of clinical signs or, in some cases, were found in pre-agonic or agonic state, thus, probably before the possible increase of circulating AGP. The concentration of AGP and of α-globulins (the electrophoretic fraction on which AGP and other acute phase proteins migrate) in treated cats was lower than in controls, suggesting that rFeIFN treatment depressed the so called “acute phase reaction”, most likely trough its possible influence on the “cytokine network” responsible for the systemic response to inflammation. On the contrary, rFeIFN administration did not induce significant changes in γ-globulins or in total and FPV specific antibody classes. However, this should not be considered surprising, since the interval between the last treatment and the blood samplings was too low (3–8 days) to evidence changes in these parameters. Nevertheless, the analysis of individual data indicated that most cats from the treated group had higher levels of IgM and higher anti-FPV IgM titres compared with untreated cats, thus, suggesting that, in spite of the short delay mentioned above, cats from the treated group were mounting an early IgM-mediated immune-response against FPV. The mechanism by which rFeIFN exerts this effect cannot be inferred from this study. However, the hypothesis that rFeIFN stimulates an immune system response was confirmed by the results obtained on day 32, when treated cats had a more intense response to the vaccinal trial, with higher levels of γ-globulins, total and FPV specific IgG compared to control cats.
5. Conclusion
The results of the present study suggest that, in spontaneous feline panleukopenia, rFeIFN does not have an antiviral effect typical of type I interferons contrary to what reported in vitro and in dogs (Mochizuki et al., 1994, Truyen et al., 2002, Ishiwata et al., 1998, Minagawa et al., 1999, Martin et al., 2002, De Mari et al., 2003). In treated and in controls kittens, the viral spread caused similar morbidity and mortality rates. However, due to the limits of “in field” studies it is not possible to conclude that rFeIFN does not have an immunotherapeutic role in FPV. On the contrary, treated animals seem to have a more reactive immune system (as demonstrated by the vaccinal trial) and a reduced acute inflammatory responsiveness. This powerful immunomodulatory activity suggests that rFeIFN treatment of kittens 2 weeks prior to their introduction into a high risk environment, followed by vaccination, or preceded by the treatment of the queen to improve the transfer of passive maternal immunity, may provide kittens with a good level of IgG when exposed to the virus. This immunomodulatory effect should be taken into consideration when designing prophylactic schemes for the prevention of feline parvoviral outbreaks.
Acknowledgements
This work was supported by the University grant FIRST 2003. The authors are grateful to Drs. Alessia Giordano and Valentina Spagnolo for their assistance.
References
- Bohem U., Klamp T., Groot M., Howard J.C. Cellular responses to interferon-gamma. Ann. Rev. Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- De Mari K., Maynard L., Eun H.M., Lebreux B. Treatment of canine parvoviral enteritis with interferon-omega in a placebo-controlled field trial. Vet. Rec. 2003;152:105–108. doi: 10.1136/vr.152.4.105. [DOI] [PubMed] [Google Scholar]
- De Mari K., Maynard L., Sanquer A., Lebreux B., Eun H.M. Therapeutic effect of recombinant feline interferon-omega on FeLV-infected and FeLV/FIV-coinfected symptomatic cats. J. Vet. Int. Med. 2004;18:477–482. doi: 10.1892/0891-6640(2004)18<477:teorfi>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Ishida T., Shibanai A., Tanaka S., Uchida K., Mochizuki M. Use of recombinant feline interferon and glucocorticoid in the treatment of feline infectious peritonitis. J. Fel. Med. Surg. 2004;6:107–109. doi: 10.1016/j.jfms.2003.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiwata K., Minagawa T., KAjimoto T. Clinical effects of the recombinant feline interferon-ω on experimental parvovirus infection in Beagle dogs. J. Vet. Med. Sci. 1998;60:911–917. doi: 10.1292/jvms.60.911. [DOI] [PubMed] [Google Scholar]
- Kajikawa T., Furuta A., Onishi T., Tajima T., Sugii S. Changes in oncentrations of serum amyloid A protein, α1-acid glycoprotein, haptoglobin, and C-reactive protein in feline sera due to induced inflammation and surgery. Vet. Immunol. Immunopathol. 1999;68:91–98. doi: 10.1016/s0165-2427(99)00012-4. [DOI] [PubMed] [Google Scholar]
- Martin V., Najbar W., Gueguen S., Grousson D., Eun H.M., Lebreux B., Aubert A. Treatment of canine parvoviral enteritis with interferon-omega in a placebo-controlled challenge trial. Vet. Microbiol. 2002;89:115–127. doi: 10.1016/s0378-1135(02)00173-6. [DOI] [PubMed] [Google Scholar]
- Mihaljevic S.Y. First clinical experiences with omega-interferon in the treatment of chronic gingivitis-stomatitis-oropharyngitis of cats. Der Prakt. Tierarzt. 2003;84:350–361. [Google Scholar]
- Minagawa T., Ishiwata K., Kajimoto T. Feline interferon-ω treatment on canine parvovirus infection. Vet. Microbiol. 1999;69:51–53. doi: 10.1016/s0378-1135(99)00087-5. [DOI] [PubMed] [Google Scholar]
- Mochizuki M., Nakatani H., Yoshida M. Inibitory effects of recombinant feline interferon on the replication of feline enteropathogenic viruses in vitro. Vet. Microbiol. 1994;39:145–152. doi: 10.1016/0378-1135(94)90095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mond J.J., Carman J., Sarma C., Ohara J., Finkelman F.D. Interferon-gamma suppresses B cell stimulation factor (BSF-1) induction of class II MHC determinants on B cells. J. Immunol. 1986;137:3534–3537. [PubMed] [Google Scholar]
- Müller D. Interferon therapy in dogs and cats. Kleintiermedizin. 2002;8:334–337. [Google Scholar]
- Pollock R.V., Carmichael L.E. Maternally derived immunity to canine parvovirus infection: transfer, decline and interference with vaccination. J. Am. Vet. Med. Assoc. 1982;180:37–42. [PubMed] [Google Scholar]
- Stark G.R., Kerr I.M., Williams B.R.G., Silverman R.H., Schreiber R.D. How cells respond to interferons. Ann. Rev. Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- Tilg H. New insights into the mechanisms of interferon alfa: an immunoregulatory and anti-inflammatory cytokine. Gastroenterology. 1997;112:1017–1021. doi: 10.1053/gast.1997.v112.pm9041265. [DOI] [PubMed] [Google Scholar]
- Truyen U., Blewaska S., Schultheiss U. A study of the antiviral activity of interferon-omega (IFN-ω) against selected canine and feline viruses. J. Mod. Vet. Med. 2002;10:862–864. [Google Scholar]
- Uchino T. Future prospect of feline interferon in prevention of viral disorders. Preventive medicine for dogs and cats. J. Vet. Med. 1995;48:663–666. [Google Scholar]
- Ueda Y., Sakurai T., Yanai A. Homogeneous production of feline interferon in silkworm by replacing single amino acid code in signal peptide region in recombinant baculovirus and characterization of the product. J. Vet. Med. Sci. 1993;55:251–258. doi: 10.1292/jvms.55.251. [DOI] [PubMed] [Google Scholar]
- Ueda Y., Sakurai T., Kasama K., Satoh Y., Atsumi K., Hanawa S., Uchino T., Yanai A. Pharmacokinetic properties of recombinant feline interferon and its stimulatory effect on 2′, 5′-oligoadenylate synthetase activity in the cat. J. Vet. Med. Sci. 1993;55:1–6. doi: 10.1292/jvms.55.1. [DOI] [PubMed] [Google Scholar]
- Wonderling R., Powell T., Baldwin S., Morales T., Snyder S., Keiser K., Hunter S., Best E., McDermott M.J., Milhausen M. Cloning, expression, purification and biological activity of five feline type I interferons. Vet. Immunol. Immunopathol. 2002;89:13–27. doi: 10.1016/s0165-2427(02)00188-5. [DOI] [PubMed] [Google Scholar]
- Zeidner N.S., Myles M.H., Mathiason-Dubard C.K., Dreitz M.J., Mullins J.I., Hoover E.A. Alpha interferon (2b) in combinantion with zidovudine for the treatment of presymptomatic feline leukaemia virus-induced immunodeficiency syndrome. Antimicrob. Agents Chemother. 1990;34:1749–1756. doi: 10.1128/aac.34.9.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]


