Abstract
Purpose
This study evaluates the use of high-resolution computed tomography (HRCT) to differentiate smear-positive, active pulmonary tuberculosis (PTB) from other pulmonary infections in the emergency room (ER) setting.
Methods
One hundred and eighty-three patients diagnosed with pulmonary infections in an ER were divided into an acid fast bacillus (AFB) smear-positive, active PTB group (G1 = 84) and a non-AFB smear-positive, pulmonary infection group (G2 = 99). HRCT images from a 64-Multidetector CT were analyzed, retrospectively, for the morphology, number, and segmental distribution of pulmonary lesions.
Results
Utilizing multivariate analysis, five variables were found to be independent risk factors predictive of G1: (1) consolidation involving the apex segment of right upper lobe, posterior segment of the right upper lobe, or apico-posterior segment of the left upper lobe; (2) consolidation involving the superior segment of the right or left lower lobe; (3) presence of a cavitary lesion; (4) presence of clusters of nodules; (5) absence of centrilobular nodules. A G1 prediction score was generated based on these 5 criteria to help differentiate G1 from G2. The area under the receiver operating characteristic (ROC) curve was 0.96 ± 0.012 in our prediction model. With an ideal cut-off point score of 3, the specificity, sensitivity, positive predictive value (PPV), and negative predictive value (NPV) are 90.9%, 96.4%, 90.0% and 96.8%, respectively.
Conclusion
The use of this AFB smear-positive, active PTB prediction model based on 5 key HRCT findings may help ER physicians determine whether or not isolation is required while awaiting serial sputum smear results in high risk patients.
Key words: High-resolution CT, Patient isolation, Sputum smear, Tuberculosis
1. Introduction
Infection with Mycobacterium tuberculosis currently affects nearly one-third of the world's population [1]. Highly contagious pulmonary tuberculosis (PTB) can masquerade as community-acquired pneumonia (CAP) and escape initial diagnosis by emergency room (ER) physicians, thereby increasing the risk of nosocomial infection and further transmission to the general population [1], [2], [3], [4], [5]. Current screening methods recommended by the Centers for Disease Control and Prevention (CDC) involve respiratory isolation (RI) of suspected PTB patients until 3 sputum samples obtained over 48 h are negative [6]. This expanded RI policy for all patients suspected of PTB in the United States led to the proper isolation of over 95% of PTB upon admission; however, it also resulted in an 8-fold overuse of the isolation room, and eventually led to an insufficient number of isolation rooms [7]. Under the threatening of acute lung disease such as the severe acute respiratory syndrome (SARS) virus and the new H1N1 influenza nowadays, a 3-day isolation policy for waiting times for smear results for all patients merely suspected of active PTB, may prove too burdensome for most hospitals [8], [9]. Furthermore, an isolation policy based solely upon negative sputum samples may not adequately account for the truly positive sputums missed due to low organism loads [10]. Therefore, more effective diagnostic tests and/or criteria for differentiating those patients at highest risk of active PTB are desperately needed.
High-resolution computed tomography (HRCT) has been shown to be more effective in the differential diagnosis of acute parenchymal lung disease than chest radiographs (CXR) [11]. HRCT can indicate findings suggestive of pneumonia almost 5 days earlier than CXR [12]. More recently, Ito et al., used HRCT to differentiate bacterial CAP from non-bacterial CAP [11]. Although several prediction models for the diagnosis of active PTB have been proposed in recent years [13], [14], [15], none have specifically focused on the diagnosis of smear-positive active PTB with HRCT.
The objective of this study is to investigate a proposed triage protocol using HRCT to initiate RI for patients with AFB smear positive PTB in the ER before the sputum smear results are known, with the ultimate intention of reducing the spatial and financial burden to hospitals, while minimizing any additional risk to patients and healthcare workers.
2. Materials and methods
2.1. Patients
From July 2005 through March 2008, a retrospective study was performed on all 19990 patients evaluated in the ER of PingTung Christian Hospital, a 706-bed teaching hospital in PingTung, Taiwan, to identify those with pulmonary symptoms. Initial inclusion criteria were presence of infiltrate on CXR and symptoms suggestive of a CAP. CAP was defined as the presence of an acute illness of 21 days or less duration with (1) one or more symptoms such as fever >38.5, confusion, sweating aches, etc., (2) at least two or more of new or increasing cough, sputum production, shortness of breath, wheeze, new focal or diffuse signs on physical examinations, (3) CXR consistent with infection and which was neither pre-existing nor of other known cause and (4) treatment with antibiotics for pneumonia by the pulmonologists or infection clinicians [16]. Patients were treated by the ER physician, followed by either a pulmonologist or infectious disease specialist. There were 1719 patients with pulmonary infiltrates, and 1300 were selected for further analysis after excluding 419 patients refused to participate in the study or were lost to follow-up.
The majority of these 1300 patients were also excluded from the study for various reasons: HIV infection (n = 5); hospitalization within 7 days of ER presentation (n = 125); no HRCT study (n = 750); presence of tumors (n = 25); presence of an underlying disease (collagen vascular disease) or co-infection (n = 16); those not requiring hospitalization (n = 196) who isolated at home if symptom and sign were mild and anti-TB drugs were tolerated [17]. The remaining 183 patients suspected of CAP or PTB received an HRCT study for enrollment into this study.
2.2. Microbiology evaluation
Regular sampling included at least 3 sputum specimens, 2 or more blood cultures, and paired serologic specimens (at admission and within the 4th and 8th week thereafter). Additional diagnostic techniques utilized included pleural puncture, transbronchial aspirates, flexible bronchoscopy with a protected specimen brush, and urine samples for Legionella species antigen [16].
Sputum samples were gram stained. Sputum appropriate for analysis and originating from the lower respiratory tract was defined as containing at least 25 granulocytes and less than 10 epithelial cells per low-power field. Validated sputum samples, blood culture samples, pleural fluid, and protected specimen brush samples were plated on sheep blood agar. Microorganism identification was performed according to standard protocols [16].
2.3. Confirmation of PTB
The 183 patients were assigned to groups according to sputum and culture results. Those with AFB sputum smear positive, active PTB (n = 84) included patients with at least one positive sputum smear and a positive culture for Mycobacterium tuberculosis (Group 1-G1). The remaining 99 patients had negative sputum smears and culture of Mycobacterium tuberculosis (Group 2-G2).
G2 was subdivided into 3 additional groups. Group 2a included 40 patients who were AFB-smear negative with active PTB based upon at least three negative sputum smears and a positive culture [18]. Group 2b included 27 patients with inactive PTB based upon findings of residual fibrotic changes on CXR, absence of disease progression on CXR at recruitment when compared to 6 months previously or on follow-up, and absence of AFB in sputum or bronchial washings on the smears and cultures [18]. Group 2c included 32 patients without evidence of PTB and definite pathogens was identified when (1) an isolate such as Pneumococcus pneumoniae, Klebsiella pneumonia, or Hemophilus influenzae was cultured from the blood or pleural effusion, (2) atypical pneumonia or bacterial pneumonia, such as Legionella pneumophila or Mycoplasma pneumoniae caused a 4-fold rise in antibody titers in serological testing, or (3) lung biopsy or wedge section proved the presence of herpes simplex virus, Actinomycosis, or Aspergillosis [16], [19], [20].
2.4. Criteria for recommendation of HRCT
Patients with following characteristics were recommended to process HRCT: age > 65, complicated CAP, interstitial lung disease, or tumor was suspected.
2.5. HRCT morphology description
The HRCT morphology patterns of PTB are described in Fig. 1, Fig. 2 [21], [22], [23].
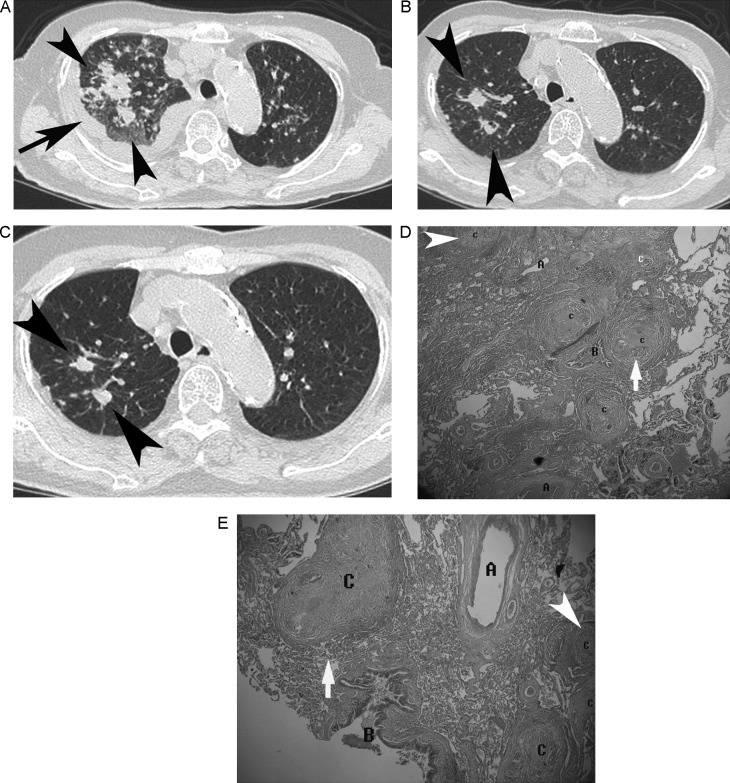
Fig. 1.
An 81-year-old woman with smear-positive, active PTB presenting with hemoptysis and pleural effusion. The score of this case is 3. (A) Axial HRCT shows clusters of nodules (black arrowhead) with spiculated margins, peribroncho-arterial distribution in the right upper lobe, and pleural effusion (black arrow). (B) Four months later, axial HRCT shows regression of clusters of nodules (black arrowhead) after tuberculosis treatment. (C) Eight months later, axial HRCT shows residual nodules (black arrowhead). (D) Histologic specimen (biopsies of the right upper lobe via video-assisted thoracoscopy, H&E stain, 40×) photomicrograph shows more concentrated granulomas at the center of the nodule clusters and granulomatous inflammation with peribroncho-arterial distribution (C, white arrow) and a small granulomata (C, white arrowhead) at the periphery of the large nodules. (E) Histologic specimen (biopsies of the right upper lobe via video-assisted thoracoscopy, H&E stain, 40×) photomicrograph shows large tuberculous nodules (C, white arrow) produced by numerous small nodules and a small granulomata (C, white arrowhead) at the periphery of the large nodules. Peripheral low attenuation spots on HRCT correspond to spaces between partially coalescent small nodules (C = clusters of nodules; B = bronchus; A = artery).
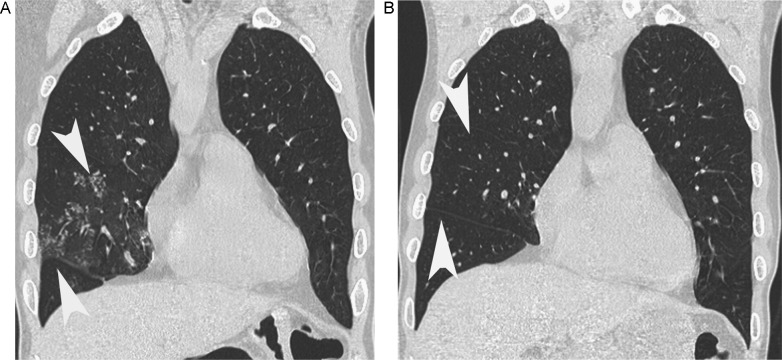
Fig. 2.
A 66-year-old-male with mycoplasma pneumonia. (A) Coronal HRCT shows centrilobular nodules (white arrowhead) in right middle lobe. (B) Two months later, after antibiotic treatment, coronal HRCT shows the centrilobular nodules disappear (white arrowhead). The score of this case is −1.
2.6. HRCT imaging evaluation
Chest CT scans without contrast media (0.625 mm collimation, 100–120 kV, 250 mA s), table speed of 57.5 mm/s and a rotation time of 0.75 s, pitch of 1.07) using a 64-MDCT scanner (Brilliance, Philips Medical Systems, Cleveland, OH, USA) were performed on 183 patients. Images were acquired during a single breath-hold 5–8 s long, making respiratory motion artifacts very uncommon. The images were reconstructed with a 1-mm slice thickness in the axial plane (no gap) and in the coronal plane (5-mm apart) using a high spatial-frequency algorithm, and then sent to PACS for review. All thin-section MDCT images were displayed on a monitor at the pulmonary window level setting (level, −600 HU; width, 1200 HU).
2.7. Reading criteria
The HRCT scans were evaluated by two radiologists and a chest physician. Each specialist had more than 15 years of experience reading thoracic radiology studies and was unaware of the sputum smear and clinical examination results. Findings were reached by consensus. The HRCT scans were assessed for the presence and distribution of parenchyma abnormalities. Location of lung involvement was reported as one or more of 18 designated segments in the lungs.
2.8. Statistical analyses
Statistical analyses were performed using SPSS 15.0 statistics software (SPSS Inc, Chicago, IL, USA). Data is presented as mean ± standard deviation (SD) for continuous data, such as age, lag time of TB smear, and lag time of TB culture. Number with percentage (%) is used for other categorical data by group. Two-sample t-test is used to compare the difference between groups for continuous data. Pearson's chi-square test or Fisher's exact test was used to compare the difference in distribution of the categorical data between groups. Furthermore, a multiple logistic regression model was performed to indicate the predictors of the subjects with smear-positive, active PTB. The estimated beta (β) with standard error (Std. Err.) and odds ratio (OR) with 95% confidence interval (CI) are presented from the multivariate logistic regression model in Table 4. In addition, a relative score is given by using the estimated β as a base according to the study of Kanaya et al. [14]. The relative score is given as 1, 2, or 3 if the relative ratio is <1.5, >1.5, and >2.5. The area under the receiver operating characteristic (ROC) curve is shown to indicate the best cut-off point (Fig. 3 ). All statistical analyses are considered significant at p < 0.05.
Table 4.
Summary of the results of multivariate logistic regression for patients with smear-positive, active PTB (N = 183).
| Term | Estimated β (Std. Err.) | OR [95% CI] | p-Value | Weighting score |
|---|---|---|---|---|
| Consolidation s1, s2, s1 + s2 | 1.83 (0.71) | 6.21 [1.54–24.99] | 0.010* | 1 |
| Consolidation s6 | 3.44 (0.98) | 31.31 [4.58–213.95] | <.001* | 2 |
| Cavitation | 1.72 (0.81) | 5.6 [1.15–27.29] | 0.033* | 1 |
| Clusters of nodules/mass | 5.21 (0.96) | 183.83 [28.25–1196.16] | <.001* | 3 |
| Centrilobular nodules | −1.77 (0.69 | 0.17 [0.04–0.67] | 0.011* | −1 |
Abbreviations: s1 = apical segment; s2 = posterior segment right upper lobe; s1 + s2 = apico-posterior segment left upper lobe; s6 = superior segment of right or left lower lobe. Weighting score is observed according the ratio of each estimated β using the estimated β 1.72 of cavitation as for the base. The relative score is given 1 as the ratio <1.5, 2 as ratio > 1.5, and 3 as ratio > 2.5. Since the effect of centrilobular nodules is negative with a ratio < 1.5, then the relative score is set as for −1.
p-Value < 0.05.
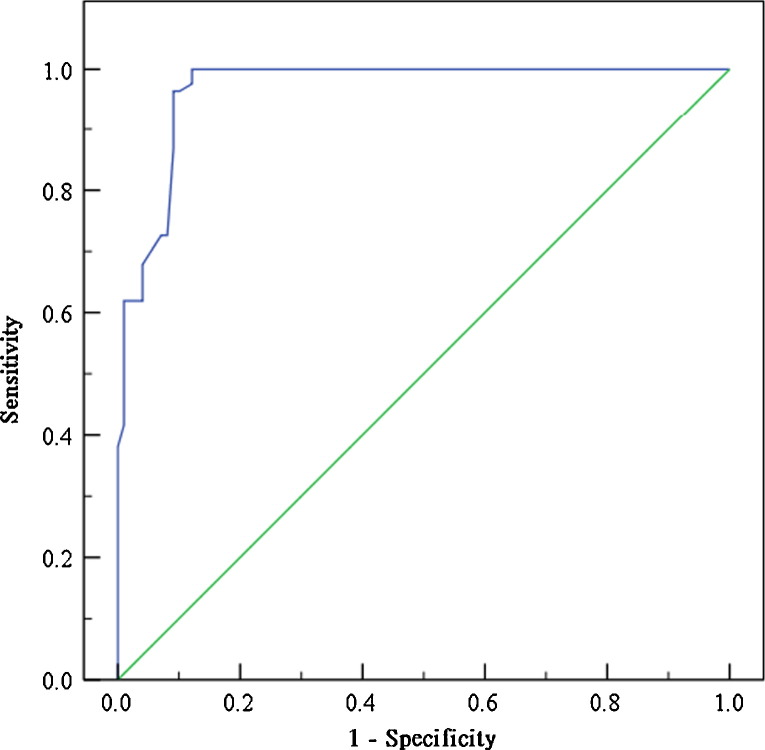
Fig. 3.
A receiver operating characteristic (ROC) curve plots the false positive rate against the true positive rate for each possible cutoff for a diagnostic test. The area under this curve generated from the multiple logistic regression model with a 95% CI is 0.968 [0.945–0.990]. The best cut-off probability is 0.68 and the cutoff point of relevance is 3. The specificity, sensitivity, positive predictive value (PPV), and negative predictive value (NPV) are 90.9%, 96.4%, 90.0%, and 96.8%, respectively.
2.9. Ethics
All participants in this study gave written consent after being fully informed. This study was approved by the Ethics Review Board of our institution.
3. Results
A total of 183 subjects receiving HRCT analysis are included for evaluation. The subjects are classified as Group 1 AFB sputum smear positive, active PTB (n = 84) and Group 2 sputum smear negative pulmonary infection patients (n = 99). Table 1 presents the demographic data, and methods of confirmation of smear-positive PTB and non-AFB smear-positive pulmonary infection for all the patients by group and subgroup. There was no significant age difference between the groups. Males comprised the majority in each study group. Co-morbid conditions such as anemia, diabetes, alcoholism, chronic steroid use, uremia, and hypoalbuminemia were noted in the 183 studied patients; however, only the presence of anemia proved to be a statistically significant finding among those with AFB sputum positive, active PTB (Group 1). A history of previous PTB was a significant finding among those with negative sputum smears. Group 1 generated spontaneous sputum for examination (p < 0.001), while a greater percentage of Group 2 patients required bronchoscopy for sputum examination (p < 0.001).
Table 1.
Summary of clinical profiles and methods of confirmation by group (N = 183).
| Variablesa | Group 1 | Group 2 |
|||
|---|---|---|---|---|---|
| (n = 84) | 2a (n = 40) | 2b (n = 27) | 2c (n = 32) | p-Valueb | |
| Age | 72.8 ± 5.6 | 72.1 ± 7.7 | 72.4 ± 5.7 | 72.4 ± 5.0 | 0.556 |
| Gender, males (%) | 60 (71.4) | 18 (46.2) | 18 (66.7) | 20 (62.5) | 0.046 |
| Previous PTB | 2 (2.4) | 5 (12.5) | 26 (96.3) | 3 (9.4) | <.001* |
| Cancer history | 8 (9.5) | 0 (0) | 2 (7.4) | 3 (9.4) | 0.240 |
| Anemia | 41 (48.8) | 6 (15.0) | 3 (11.1) | 4 (12.5) | <.001* |
| DM | 32 (38.1) | 12 (30.0) | 9(33.3) | 4 (12.5) | 0.062 |
| Alcoholism | 11 (13.1) | 6 (15.0) | 4 (14.8) | 4 (12.5) | 0.837 |
| Steroid use | 27 (32.1%) | 14 (35.0) | 9 (33.3) | 5 (15.6) | 0.570 |
| Uremia | 12 (14.3) | 7 (17.5) | 4 (14.8) | 6 (18.8) | 0.594 |
| Albumin <3 | 17 (20.2) | 9 (22.5) | 3 (11.1) | 4 (12.5) | 0.475 |
| Sputums– | |||||
| Spontaneous | 70 (83.3) | 21 (52.5) | 0 (0) | 0 (0) | <.001* |
| Bronchoscopy (TBLB/washing/brushing) | 19 (22.6) | 19 (47.5) | 27 (100) | 32 (100) | <.001* |
| Lag time of TB smear (days) | 7.49 ± 2.66 | 6.63 ± 3.33 | 9.26 ± 2.88 | 9.34 ± 2.73 | 0.101 |
| Lag time of TB culture (days) | 37.80 ± 7.49 | 45.60 ± 5.47 | 44.48 ± 2.01 | 39.44 ± 5.85 | <.001* |
| Surgical intervention | 1 (1.2) | 0 (0) | 0 (0) | 5 (15.6) | 0.221 |
| Blood culture | 0 (0) | 0 (0) | 0 (0) | 21 (65.6) | <.001* |
| Urine culture | 0 (0) | 0 (0) | 0 (0) | 3 (9.4) | 0.251 |
| Pleural effusion culture | 0 (0) | 0 (0) | 0 (0) | 4 (12.5) | 0.126 |
| Antigen of atypical pneumonia and bacteria | 0 (0) | 0 (0) | 0 (0) | 8 (8.1) | 0.008* |
| Non-TB pathogen | |||||
| Bacteria | 0 (0) | 0 (0) | 0 (0) | 24 (22.2) | <.001* |
| Fungus | 0 (0) | 0 (0) | 0 (0) | 1 (1.0) | 1.000 |
| Virus | 0 (0) | 0 (0) | 0 (0) | 1 (1.0) | 1.000 |
| Mycoplasma | 0 (0) | 0 (0) | 0 (0) | 6 (6.1) | 0.032* |
HRCT was performed on all 183 patients within 24 h of presentation in the ER, and the results are described in Table 2 . HRCT patterns like ground glass opacity, consolidation, bronchial wall thickening, clusters of nodules, paratracheal adenopathy, interlobular septal thickening, and cavitations were more frequent in G1 (p < 0.05). Meanwhile, HRCT patterns of calcification and fibrosis were more frequent in G2 (all p < 0.05). There were no statistically significant differences in centrilobular nodules and tree-in-bud between G1 and G2 (p = 0.156 and p = 0.781, respectively).
Table 2.
Summary of HRCT morphology between groups (N = 183).
| CT morphologya | Group 1 | Group 2 |
p-Valueb | ||
|---|---|---|---|---|---|
| (n = 84) | 2a (n = 40) | 2b (n = 27) | 2c (n = 32) | ||
| Consolidation | 72 (85.7) | 14 (35.0) | 3 (11.1) | 28 (87.5) | <.001* |
| Cavitation | 51 (60.7) | 7 (17.5) | 2 (7.4) | 3 (9.4) | <.001* |
| Clusters of small nodules/mass | 69 (82.1) | 10 (25.0) | 0 (0) | 0 (0) | <.001* |
| Septal thickening | 55 (65.5) | 18 (45.0) | 2 (7.4) | 12 (37.5) | <.001* |
| Bronchial wall thickening | 69 (82.1) | 20 (50.0) | 7 (25.9) | 10 (31.3) | <.001* |
| Ground glass opacity | 74 (88.1) | 27 (67.5) | 11 (40.7) | 22 (68.8) | <.001* |
| Centrilobular nodules | 43 (51.2) | 35 (87.5) | 5 (18.5) | 21 (65.6) | 0.156 |
| Tree-in-bud | 45 (53.6) | 35 (87.5) | 2 (7.4) | 14 (43.8) | 0.781 |
| Paratracheal adenopathy | 56 (66.7) | 8 (20.0) | 4 (14.8) | 4 (12.5) | <.001* |
| Fibrosis | 2 (2.4) | 11 (27.5) | 17 (63.0) | 0 (0) | <.001* |
| Calcification | 6 (7.1) | 6 (15.0) | 19 (70.4) | 0 (0) | 0.001* |
Data are presented as numbers with percentage (%).
p-Value derived from the difference between group 1 and group 2 with Pearson's chi-square test or Fisher's exact test.
p-Value < 0.05.
The anatomic distribution and numbers of lung segments/sub-segments with consolidation, cavitations, and clusters of nodules are described in Table 3 . Consolidation involving an apex segment (s1), posterior segment of the right upper lobe (s2), or apico-posterior segment of the left upper lobe (s1 + s2); consolidation involving the superior segment of the right or left lower lobe (s6); cavitation in the apex segment (s1), posterior segment of the right upper lobe (s2), or apico-posterior segment of the left upper lobe (s1 + s2); number of cavitation >1, number of consolidations >2; number of clusters of nodules >3 were more frequent in G1 (all p < 0.05).
Table 3.
Anatomic distribution of involved segments/sub-segments and the number of segments with consolidation, cavitation, and clusters of nodules (N = 183).
| Locationa | Group 1 | Group 2 |
p-Valueb | ||
|---|---|---|---|---|---|
| (n = 84) | 2a (n = 40) | 2b (n = 27) | 2c (n = 32) | ||
| Consolidation | 72 (85.7) | 14 (35.0) | 3 (11.1) | 28 (87.5) | |
| s1, s2, s1 + s2 | 69 (82.1) | 10 (25.0) | 3 (11.1) | 6 (18.8) | <.001* |
| s3, s4, s5 | 15 (17.9) | 2 (5.0) | 1 (3.7) | 5 (15.6) | 0.047* |
| s6 | 41 (48.8) | 6 (15.0) | 1 (3.7) | 3 (9.4) | <.001* |
| s7, s8, s7 + s8, s9, s10 | 13 (15.5) | 3 (7.5) | 0 (0) | 28 (87.5) | 0.012* |
| Number >2 | 36 (42.9) | 1 (2.5) | 0 (0) | 17 (53.1) | <.001* |
| Cavitation | 51 (60.7) | 7 (17.5) | 2 (7.4) | 3 (9.4) | |
| s1, s2, s1 + s2 | 51 (60.7) | 5 (12.5) | 1 (3.7) | 1 (3.1) | <.001* |
| s3, s4, s5 | 5 (6.0) | 0 (0) | 0 (0) | 1 (3.1) | 0.095 |
| s6 | 21 (25.0) | 2 (5.0) | 1 (3.7) | 0 (0) | <.001* |
| s7, s8, s7 + s8, s9, s10 | 3 (3.6) | 0 (0) | 0 (0) | 3 (9.4) | 1.000 |
| Number >1 | 32 (38.1) | 0 (0) | 0 (0) | 2 (6.3) | <.001* |
| Clusters of nodules/mass | 69 (82.1) | 10 (25.0) | 0 (0) | 0 (0) | |
| s1, s2, s1 + s2 | 66 (78.6) | 8 (20.0) | 0 (0) | 0 (0) | <.001* |
| s3, s4, s5 | 8 (9.5) | 2 (5.0) | 0 (0) | 0 (0) | 0.046* |
| s6 | 37 (44.0) | 3 (7.5) | 0 (0) | 0 (0) | <.001* |
| s7, s8, s7 + s8, s9, s10 | 11 (13.1) | 3 (7.5) | 0 (0) | 0 (0) | 0.011* |
| Number >3 | 46 (54.8) | 0 (0) | 0 (0) | 0 (0) | <.001* |
Abbreviations: s1 = apical segment; s2 = posterior segment right upper lobe; s1 + s2 = apico-posterior segment left upper lobe; s3 = anterior segment; s4 = lateral segment of right middle lobe or super segment of left lingual lobe; s5 = medial segment of right middle lobe or inferior segment of left lingual lobe; s6 = superior segment of right or left lower lobe; s7 = medical segment of right lower lobe; s8 = anterior segment of left lower lobe; s7 + s8 = medial-anterior segment of left lower lobe; s9 = lateral segment of right or left basal lower lobe; s10 = posterior segment of right or left basal lower lobe.
Data are presented as numbers with percentage by group (%).
p-Value derived from the comparison between group 1 and group 2 with Pearson's chi-square test or Fisher's exact test.
p-Value < 0.05 statistically significant.
In the multivariate analysis, consolidations in the apex segment (s1), posterior segment of the right upper lobe (s2), or apico-posterior segment of the left upper lobe (s1 + s2) (p = 0.010); consolidation of the superior segment of either lower lobe (s6) (p < 0.001); cavitations (p = 0.033); clusters of nodules (p < 0.001) not related to the number and location were independently significant factors that were predictive for G1. Only the centrilobular nodules had an independently negative predictive value for G1 (p = 0.011) (Table 4 ).
Using the five independent variables that were associated with G1, a prediction score was generated to help differentiate between G1 and G2. The ROC curve analysis is shown in Fig. 3. The area under the curve is 0.96 ± 0.014 for our prediction model. With an ideal cut-off point score of 3, the specificity, sensitivity, positive predictive value (PPV), and negative predictive value (NPV) are 90.9%, 96.4%, 90.0%, and 96.8%, respectively.
4. Discussion
The clusters of nodules represent the peri-bronchovascular nodules, which were significant findings in G1. Poey et al. and Heo et al. found similar results among patients with active PTB [24], [25]. In our study, no clusters of nodules were identified in the G2c patients, which is comparable to the Tanaka et al. study which revealed no nodules on HRCT examination of CAP patients (no active PTB cases) [26].
Physicians in the ER play a crucial role in the identification of potential PTB patients. Hsieh et al. revealed that a high proportion of hospitalized, active PTB cases are often admitted through the ERwith an initial diagnosis of CAP, which further delays RI and increases the risk of nosocomial infection [4], [27]. Furthermore, 34% of active PTB cases had atypical CXR presentations, and only 37.7% with sputum from spontaneous cough [4]. Steen reported 31 of 212 (15%) smear-positive PTB patients also had one or more negative initial sputum smears [28]. The diverse clinical presentation of ER patients with PTB, as well as the limited number of isolation rooms available (especially during influenza outbreaks), not only complicates triage, but also makes development and implementation of effective triage screening criteria crucial to the success of PTB management [29], [30].
While reducing the number of isolated patients who have a low likelihood of active PTB may reduce hospital costs, it is important not to misdiagnose patients who have smear-positive PTB. The subsequent costs associated with late treatment and contact investigation could actually offset the original savings [31]. During flu season, influenza is one of the leading causes of ER visits, and the availability of isolation rooms is essential [30]. With this in mind, we created a G1 prediction score based on 5 key HRCT findings: (1) consolidation involving the apex segment of either lung (s1), posterior segment of the right upper lobe (s2), or apico-posterior segment of the left upper lobe (s1 + s2); (2) consolidation involving the superior segment of the right or left lower lobe (s6); (3) presence of a cavitary lesion; (4) presence of clusters of nodules; (5) absence of centrilobular nodules. The ROC curve (Fig. 3) identified a score of 3 points as the cut-off. At this point, the sensitivity hits 96.4%, and the NPV approaches 96.8%. An ideal test would have both 100% sensitivity to avoid missing any patients with active disease and 100% NPV to assure negative results truly represented disease-free status. This is extremely important in public health situations that deal with highly infectious diseases like AFB sputum positive, active PTB.
Campos et al. evaluated the efficacy of a single first-sputum nucleic acid amplification (NAA) test to rapidly and accurately identify the subset of patients with suspected TB who require isolation [10]. Compared to serial sputum smear analysis currently endorsed by the CDC, first sputum NAA demonstrated an 87% sensitivity and 99% NPV in this study. The future of diagnostic testing may soon include the use of first sputum NAA, but current guidelines are still in effect. Furthermore, if sputum was not available from spontaneous cough or specimens of bronchoscope, the NAA test was not feasible. Our study demonstrates the effective use of HRCT to predict smear-positive, active PTB cases in advance of the serial sputum results or patients without spontaneous sputum, thereby decreasing the transmission rate and opening up isolation rooms needed for other communicable diseases, such as influenza and SARS.
4.1. Limitations
We limited our scope to the reliability and reproducibility of the five variables predictive of PTB. The demographic and clinical characteristics of patients with PTB may differ in other geographic areas, so the model needs to be validated in other settings before it can be implemented in clinical practice. Although HRCT is widely available today, some countries may not have access to HRCT technology. Reader experience is another limitation also.
5. Conclusion
The smear-positive, active PTB prediction model based upon five characteristic HRCT findings may ease the burden of RI in areas where availability and financial resources are limited.
Conflict of interest statement
The author reports that there is no conflict of interest.
Acknowledgement
None.
References
- 1.Moran G.J., Barrett T.W., Mower W.R. Decision instrument for the isolation of pneumonia patients with suspected pulmonary tuberculosis admitted through US emergency departments. Ann Emerg Med. 2009;53(5):625–632. doi: 10.1016/j.annemergmed.2008.07.027. [DOI] [PubMed] [Google Scholar]
- 2.Liam C.K., Pang Y.K., Poosparajah S. Pulmonary tuberculosis presenting as community-acquired pneumonia. Respirology. 2006;11(6):786–792. doi: 10.1111/j.1440-1843.2006.00947.x. [DOI] [PubMed] [Google Scholar]
- 3.Craig S.E., Bettinson H., Sabin C.A., Gillespie S.H., Lipman M.C. Think TB! Is the diagnosis of pulmonary tuberculosis delayed by the use of antibiotics? Int J Tuberc Lung Dis. 2009;13(2):208–213. [PubMed] [Google Scholar]
- 4.Hsieh M.J., Liang H.W., Chiang P.C. Delayed suspicion, treatment and isolation of tuberculosis patients in pulmonology/infectious diseases and non-pulmonology/infectious diseases wards. J Formos Med Assoc. 2009;108(3):202–209. doi: 10.1016/S0929-6646(09)60053-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haley C.E., McDonald R.C., Rossi L., Jones W.D., Jr., Haley R.W., Luby J.P. Tuberculosis epidemic among hospital personnel. Infect Control Hosp Epidemiol. 1989;10(5):204–210. doi: 10.1086/646003. [DOI] [PubMed] [Google Scholar]
- 6.Jensen P.A., Lambert L.A., Iademarco M.F., Ridzon R. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep. 2005;54(RR-17):1–141. [PubMed] [Google Scholar]
- 7.Bock N.N., McGowan J.E., Jr., Ahn J., Tapia J., Blumberg H.M. Clinical predictors of tuberculosis as a guide for a respiratory isolation policy. Am J Respir Crit Care Med. 1996;154(5):1468–1472. doi: 10.1164/ajrccm.154.5.8912766. [DOI] [PubMed] [Google Scholar]
- 8.Siddiqui A.H., Perl T.M., Conlon M., Donegan N., Roghmann M.C. Preventing nosocomial transmission of pulmonary tuberculosis: when may isolation be discontinued for patients with suspected tuberculosis? Infect Control Hosp Epidemiol. 2002;23(3):141–144. doi: 10.1086/502024. [DOI] [PubMed] [Google Scholar]
- 9.American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: controlling tuberculosis in the United States. Am J Respir Crit Care Med. 2005;172(9):1169–1227. doi: 10.1164/rccm.2508001. [DOI] [PubMed] [Google Scholar]
- 10.Campos M., Quartin A., Mendes E. Feasibility of shortening respiratory isolation with a single sputum nucleic acid amplification test. Am J Respir Crit Care Med. 2008;178(3):300–305. doi: 10.1164/rccm.200803-381OC. [DOI] [PubMed] [Google Scholar]
- 11.Ito I., Ishida T., Togashi K. Differentiation of bacterial and non-bacterial community-acquired pneumonia by thin-section computed tomography. Eur J Radiol. 2008 doi: 10.1016/j.ejrad.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heussel C.P., Kauczor H.U., Heussel G., Fischer B., Mildenberger P., Thelen M. Early detection of pneumonia in febrile neutropenic patients: use of thin-section CT. AJR Am J Roentgenol. 1997;169(5):1347–1353. doi: 10.2214/ajr.169.5.9353456. [DOI] [PubMed] [Google Scholar]
- 13.Tattevin P., Casalino E., Fleury L., Egmann G., Ruel M., Bouvet E. The validity of medical history, classic symptoms, and chest radiographs in predicting pulmonary tuberculosis: derivation of a pulmonary tuberculosis prediction model. Chest. 1999;115(5):1248–1253. doi: 10.1378/chest.115.5.1248. [DOI] [PubMed] [Google Scholar]
- 14.Kanaya A.M., Glidden D.V., Chambers H.F. Identifying pulmonary tuberculosis in patients with negative sputum smear results. Chest. 2001;120(2):349–355. doi: 10.1378/chest.120.2.349. [DOI] [PubMed] [Google Scholar]
- 15.El-Solh A.A., Hsiao C.B., Goodnough S., Serghani J., Grant B.J. Predicting active pulmonary tuberculosis using an artificial neural network. Chest. 1999;116(4):968–973. doi: 10.1378/chest.116.4.968. [DOI] [PubMed] [Google Scholar]
- 16.Lim W.S., Macfarlane J.T., Boswell T.C. Study of community acquired pneumonia aetiology (SCAPA) in adults admitted to hospital: implications for management guidelines. Thorax. 2001;56(4):296–301. doi: 10.1136/thorax.56.4.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levy M.L., Le Jeune I., Woodhead M.A., Macfarlaned J.T., Lim W.S. Primary care summary of the British Thoracic Society Guidelines for the management of community acquired pneumonia in adults: 2009 update. Endorsed by the Royal College of General Practitioners and the Primary Care Respiratory Society UK. Prim Care Respir J. 2010;19(1):21–27. doi: 10.4104/pcrj.2010.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO, editor. WHO tuberculosis programme—framework for effective tuberculosis control. Switzerland; Geneva: 1994. [Google Scholar]
- 19.Community-acquired pneumonia in adults in British hospitals in 1982–1983: a survey of aetiology, mortality, prognostic factors and outcome. The British Thoracic Society and the Public Health Laboratory ServiceQ J Med. 1987;62(239):195–220. [PubMed] [Google Scholar]
- 20.Venkatesan P., Gladman J., Macfarlane J.T. A hospital study of community acquired pneumonia in the elderly. Thorax. 1990;45(4):254–258. doi: 10.1136/thx.45.4.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Webb W.R. Sarcoidosis. In: Webb W.R., Higgins C.B., editors. Thoracic imaging. 1st ed. LWW; Philadelphia: 2005. pp. 439–449. [Google Scholar]
- 22.Webb W.R., Muller N.L., Naidich D.P. LWW; Philadelphia: 2009. Illustrated glossary of high-resolutuion computed tomography term. High-resolution CT of the lung. pp. 585–602. [Google Scholar]
- 23.Webb W.R., Nestor L.M., Naidich D.P. Sarcoidosis. In: Webb W.R., Nestor L.M., Naidich D.P., editors. High resolution CT of the lung. 4th ed. LWW; Philadelphia: 2009. pp. 273–300. [Google Scholar]
- 24.Poey C., Verhaegen F., Giron J., Lavayssiere J., Fajadet P., Duparc B. High resolution chest CT in tuberculosis: evolutive patterns and signs of activity. J Comput Assist Tomogr. 1997;21(4):601–607. doi: 10.1097/00004728-199707000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Heo J.N., Choi Y.W., Jeon S.C., Park C.K. Pulmonary tuberculosis: another disease showing clusters of small nodules. AJR Am J Roentgenol. 2005;184(2):639–642. doi: 10.2214/ajr.184.2.01840639. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka N., Matsumoto T., Kuramitsu T. High resolution CT findings in community-acquired pneumonia. J Comput Assist Tomogr. 1996;20(4):600–608. doi: 10.1097/00004728-199607000-00019. [DOI] [PubMed] [Google Scholar]
- 27.Weber A.M., Areerat P., Fischer J.E., Thamthitiwat S., Olsen S.J., Varma J.K. Factors associated with diagnostic evaluation for tuberculosis among adults hospitalized for clinical pneumonia in Thailand. Infect Control Hosp Epidemiol. 2008;29(7):648–657. doi: 10.1086/588684. [DOI] [PubMed] [Google Scholar]
- 28.Steen T.W., Mazonde G.N. Pulmonary tuberculosis in Kweneng District, Botswana: delays in diagnosis in 212 smear-positive patients. Int J Tuberc Lung Dis. 1998;2(8):627–634. [PubMed] [Google Scholar]
- 29.Critical Care Services and 2009 H1N1 Influenza in Australia and New Zealand. N Engl J Med. 2009 doi: 10.1056/NEJMoa0908481. [DOI] [PubMed] [Google Scholar]
- 30.Schull M.J., Mamdani M.M., Fang J. Community influenza outbreaks and emergency department ambulance diversion. Ann Emerg Med. 2004;44(1):61–67. doi: 10.1016/j.annemergmed.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 31.Kellerman S., Tokars J.I., Jarvis W.R. The cost of selected tuberculosis control measures at hospitals with a history of Mycobacterium tuberculosis outbreaks. Infect Control Hosp Epidemiol. 1997;18(8):542–547. doi: 10.1086/647669. [DOI] [PubMed] [Google Scholar]