Abstract
Colostrum management is the single most important management factor in determining calf health and survival. Unfortunately, a significant proportion of North American dairy calves suffer from failure of passive transfer of antibodies from colostrum, contributing to excessively high preweaning mortality rates and other short- and long-term losses associated with animal health, welfare, and productivity. A successful colostrum management program requires producers to consistently provide calves with a sufficient volume of clean, high-quality colostrum within the first few hours of life. This article reviews the process of colostrogenesis and discusses important components of colostrum. The key components of delivering and monitoring a successful colostrum management program are discussed.
The placenta of the cow separates the maternal and fetal blood supplies, preventing in utero transmission of protective immunoglobulins (Ig) [1]. Consequently, the calf is born agammaglobulinemic and so depends almost entirely on the absorption of maternal Ig from colostrum after birth. The absorption of maternal Ig across the small intestine during the first 24 hours after birth, termed passive transfer, helps to protect the calf against common disease organisms until its own immature immune system becomes functional. Calves are defined as having failure of passive transfer (FPT) if the calf serum IgG concentration is less than 10 mg/mL when sampled between 24 and 48 hours of age [2], [3]. Achieving early and adequate intake of high-quality colostrum is widely recognized as the single most important management factor in determining health and survival of the neonatal calf ( Fig. 1) [3], [4], [5], [6]. In addition to reduced risk for preweaning morbidity and mortality, additional long-term benefits associated with successful passive transfer include reduced mortality in the postweaning period, improved rate of gain and feed efficiency, reduced age at first calving, improved first and second lactation milk production, and reduced tendency for culling during the first lactation [7], [8], [9], [10].
Fig. 1.
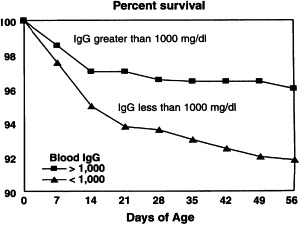
Calf survival by serum IgG concentration. (From National animal health monitoring system. National dairy heifer evaluation project. Dairy herd management practices focusing on preweaned heifers. Ft. Collins, (CO): USDA-APHIS Veterinary Services; 1993.)
Unfortunately, many producers continue to incur significant loss associated with FPT. In the United States mortality rates in preweaned dairy heifers are estimated to range between 8% and 11% [2], [4], [11]. Poor colostrum management is one of the key factors contributing to these excessive losses. In one study 41% of 2177 calves sampled between 24 and 48 hours of age had FPT (serum IgG < 10 mg/mL) [2]. It was estimated that approximately 31% of preweaning mortality events occurring in the first 3 weeks of life were attributed to FPT [9]. These studies point to the need for producers to adopt practices to improve colostrum management. This article reviews the process of colostrogenesis and discusses important components of colostrum. The key components of developing a successful colostrum management program are discussed.
Colostrogenesis and colostrum composition
Bovine colostrum consists of a mixture of lacteal secretions and constituents of blood serum, most notably Ig and other serum proteins, which accumulate in the mammary gland during the prepartum dry period [12]. This process begins several weeks before calving, under the influence of lactogenic hormones, including prolactin, and ceases abruptly at parturition. Important constituents of colostrum include Ig, maternal leukocytes, growth factors, hormones, cytokines, nonspecific antimicrobial factors, and nutrients. Concentrations of many of these components are greatest in the first secretions harvested after calving (first milking colostrum), then decline steadily over the next six milkings (transition milk) to reach the lower concentrations routinely measured in saleable whole milk ( Table 1) [12].
Table 1.
Composition of colostrum, transition milk and whole milk of Holstein cows
| Colostrum |
Transition milk (milking postpartum) |
Milk |
||
|---|---|---|---|---|
| Parameter | 1 | 2 | 3 | 6 |
| Specific gravity | 1.056 | 1.040 | 1.035 | 1.032 |
| Total solids (%) | 23.9 | 17.9 | 14.1 | 12.9 |
| Fat (%) | 6.7 | 5.4 | 3.9 | 4.0 |
| Total protein (%) | 14.0 | 8.4 | 5.1 | 3.1 |
| Casein (%) | 4.8 | 4.3 | 3.8 | 2.5 |
| Albumin (%) | 6.0 | 4.2 | 2.4 | 0.5 |
| Immunoglobulins (%) | 6.0 | 4.2 | 2.4 | 0.09 |
| IgG (g/100 mL) | 3.2 | 2.5 | 1.5 | 0.06 |
| Lactose (%) | 2.7 | 3.9 | 4.4 | 5.0 |
| IGF-I (μg/L) | 341 | 242 | 144 | 15 |
| Insulin (μg/L) | 65.9 | 34.8 | 15.8 | 1.1 |
| Ash (%) | 1.11 | 0.95 | 0.87 | 0.74 |
| Calcium (%) | 0.26 | 0.15 | 0.15 | 0.13 |
| Magnesium (%) | 0.04 | 0.01 | 0.01 | 0.01 |
| Zinc (mg/100 mL) | 1.22 | — | 0.62 | 0.3 |
| Manganese (mg/100 mL) | 0.02 | — | 0.01 | 0.004 |
| Iron (mg/100 g) | 0.20 | — | — | 0.05 |
| Cobalt (μg/100 g) | 0.5 | — | — | 0.10 |
| Vitamin A (μg/100 mL) | 295 | 190 | 113 | 34 |
| Vitamin E (μg/g fat) | 84 | 76 | 56 | 15 |
| Riboflavin (μg/mL) | 4.83 | 2.71 | 1.85 | 1.47 |
| Vitamin B12 (μg/100 mL) | 4.9 | — | 2.5 | 0.6 |
| Folic acid (μg/100 mL) | 0.8 | — | 0.2 | 0.2 |
| Choline (mg/mL) | 0.7 | 0.34 | 0.23 | 0.13 |
Data from Hammon HM, Zanker IA, Blum JW. Delayed colostrum feeding affects IGF-1 and insulin plasma concentrations in neonatal calves. J Dairy Sci 2000;83:85–92; and Foley JA, Otterby DE. Availability, storage, treatment, composition, and feeding value of surplus colostrum: a review. J Dairy Sci 1978;61:1033–60.
Immunoglobulins
IgG, IgA, and IgM account for approximately 85% to 90%, 5%, and 7%, respectively, of the total Ig in colostrum, with IgG1 accounting for 80% to 90% of the total IgG [13]. Although levels are highly variable among cows and studies, one study reported that mean colostral concentrations of IgG, IgA, and IgM were 75 mg/mL, 4.4 mg/mL, and 4.9 mg/mL, respectively [14]. IgG, and IgG1 in particular, are transferred from the bloodstream across the mammary barrier into colostrum by a specific transport mechanism: Receptors on the mammary alveolar epithelial cells capture IgG1 from the extracellular fluid, and the molecule undergoes endocytosis, transport, and finally release into the luminal secretions [13]. The alveolar epithelial cells cease expressing this receptor, most likely in response to increasing prolactin concentrations, at the onset of lactation [15]. Smaller amounts of IgA and IgM are largely derived from local synthesis by plasmacytes in the mammary gland [13]. Although not well understood, colostral transfer of IgE also occurs and may be important in providing early protection against intestinal parasites [16].
Maternal leukocytes
Normal bovine colostrum contains greater than 1 × 106 cells/mL of immunologically active maternal leukocytes, including macrophages, T and B lymphocytes, and neutrophils [13], [17]. At least a portion of colostral leukocytes are absorbed intact across the intestinal barrier [18]. Liebler-Tenorio and colleagues [19] reported that the preferential route of uptake of colostral leukocytes through the intestinal barrier is through the follicle-associated epithelium of Peyer patches in the jejunum and ileum. Reber and colleagues [20] proposed that, after entering the neonatal circulation, maternal leukocytes traffic to neonatal nonlymphoid tissues and secondary lymphoid tissues, disappearing from the neonatal circulation by 24 to 36 hours after feeding colostrum. Although their functional importance in calves is not routinely measured, early evidence suggests that colostral leukocytes enhance lymphocyte response to nonspecific mitogens, increase phagocytosis and bacterial killing ability, and stimulate humoral immune responses (IgG formation) in the calf [17], [21], [22], [23]. Presumably these cells would not be viable in pasteurized colostrum or colostrum replacer products. The role and functional significance of colostral leukocytes remains areas of active research.
Cytokines and growth factors
Other important components of colostrum include growth factors, hormones, cytokines, and nonspecific antimicrobial factors. Bioactive components of colostrum with antimicrobial activity include lactoferrin, lysozyme, and lactoperoxidase [24], [25], [26]. Oligosaccharides in colostrum may provide protection against pathogens by acting as competitive inhibitors for the binding sites on the epithelial surfaces of the intestine [27]. Growth factors in bovine colostrum include transforming growth factor beta-2 (TGF-β2), growth hormone (GH), and insulin, but their function in colostrum is not fully understood (see Table 1) [24]. Colostral insulinlike growth factor-I (IGF-I) may be a key regulator in the development of gastrointestinal tracts of bovine neonates, including stimulation of mucosal growth, brush-border enzymes, intestinal DNA synthesis, increased villus size, and glucose uptake increased [28], [29], [30]. Trypsin inhibitor, a compound found in colostrum in concentrations nearly 100 times greater than in milk, serves to protect IgG and other proteins from proteolytic degradation in the intestine of the neonatal calf.
Nutrients
Although the immunologic importance of colostrum is frequently discussed, the nutritional significance of the first colostrum meal should not be overlooked. The total solids content (%) in first milking colostrum and whole milk in Holstein cows has been reported to average 23.9% and 12.9%, respectively (see Table 1) [12]. Much of this increase in colostrum solids content is attributed to a more than fourfold increase in protein content of colostrum versus milk, this being because of significant increases in Ig and casein content [5]. The crude fat content of first milking Holstein colostrum (6.7%) is also significantly higher than for milk (3.6%) [12]. Energy from fat and lactose in colostrum is critical for thermogenesis and body temperature regulation. Certain vitamins and minerals, including calcium, magnesium, zinc, manganese, iron, cobalt, vitamin A, vitamin E, carotene, riboflavin, vitamin B12, folic acid, choline, and selenium are also found in increased concentrations in bovine colostrum versus milk (see Table 1) [12], [27].
Components of a successful colostrum management program
To achieve successful passive transfer of IgG, the calf must first consume a sufficient mass of Ig in colostrum and then be able to successfully absorb a sufficient quantity of these molecules into its circulation. Major factors affecting the mass of Ig consumed by the calf include the quality and volume of colostrum fed. The major factor affecting the absorption of Ig molecules into circulation is the quickness, after birth, with which the first colostrum feeding is provided. The remainder of this article reviews these and other important factors affecting passive transfer, management strategies for preventing bacterial contamination of colostrum, and the use of colostrum supplements and replacers, and provides recommendations for monitoring the colostrum management program.
Colostrum quality
Although it is recognized that colostrum contains a wide spectrum of important immune and nutritional components, because the relationship between Ig concentrations and calf health is best understood, and because IgG composes more than 85% of total Ig in colostrum, the concentration of IgG in colostrum has traditionally been considered the hallmark for evaluating colostrum quality. High-quality colostrum has an IgG concentration greater than 50 g/L [6]. The IgG concentration in colostrum can vary dramatically among cows. In one recent study, colostrum IgG averaged 76 g/L, but ranged from 9 to 186 g/L for individual Holstein cows [31]. Some factors affecting colostrum quality, such as breed or age of the dam, may be out of the producer's ability to manipulate. Several other important factors affecting colostrum quality, however, including preparturient vaccination, dry period length, and time to colostrum collection, can be managed by producers. This section reviews factors affecting colostrum quality and discusses cow-side testing of colostrum quality.
Breed
Comparative studies have reported that there can be a breed effect on colostrum quality [32], [33]. In one study, IgG1 concentration was greater in secretions from beef cows (113.4 g/L) than from dairy cows (42.7 g/L) [32]. In another study, Holstein cows produced colostrum with total Ig content (5.6%) that was numerically lower than for Guernsey (6.3%) and Brown Swiss (6.6%) cows, and statistically lower than for Ayrshire (8.1%) and Jersey (9.0%) cows [33]. Breed differences could be attributed to genetic differences and/or dilutional effects.
Age of dam
Most, but not all, studies report a tendency for older cows to produce higher quality colostrum, presumably because of older animals have had a greater period of exposure to farm-specific pathogens [33], [34], [35], [36]. As one example, Tyler and colleagues [36] reported that the mean colostral IgG concentration for Holstein cows in their first, second, or third and greater lactations was 66, 75, and 97 g/L, respectively. In the same study, however, there was reportedly no difference in IgG concentration for Guernsey cows in their first (119 g/L), second (113 g/L), and third and greater lactations (115 g/L). Producers should be discouraged from automatically discarding colostrum from first-calf heifers, because it may be of very good quality.
Nutrition in the preparturient period
Studies generally have shown that Ig content of colostrum is not affected by prepartum maternal nutrition [37]. In a study feeding beef cows either 100% (CO) or 57% (RS) of National Research Council (NRC) (1984) [38] protein and energy requirements, maternal nutrition did not affect either colostrum IgG concentration (43.0 versus 39.5 g/L for RS and CO, respectively) or the calves' serum IgG concentration at 24 hours (19.1 versus 20.2 mg/mL for RS and CO, respectively) [39]. Lacetera and colleagues [40] reported that cows supplemented with injections of selenium and vitamin E in late pregnancy produced a greater volume of colostrum than unsupplemented cows, when all cows were fed a prepartum diet that was deficient in Vitamin E and selenium. Treatment had no impact on colostrum IgG concentration, however. Producers should feed dry cows and heifers nonlactating rations balanced according to NRC (2001) guidelines [41].
Season of calving
Some, but not all, studies have reported that exposure to high ambient temperatures during late pregnancy is associated with poorer colostrum composition, including lower mean concentrations of colostral IgG and IgA, and lower mean percentages of total protein, casein, lactalbumin, fat, and lactose [34], [42]. These effects may be attributed to the negative effects of heat stress on dry matter intake resulting in nutritional restriction, reduced mammary blood flow resulting in impaired transfer of IgG and nutrients from the blood stream to the udder, or impaired immune reactivity of mammary gland plasmacytes that produce IgA [42]. Producers should adopt the similar heat-abatement strategies for prepartum cows and heifers as are routinely used for lactating animals.
Volume of colostrum produced
Pritchett and colleagues [35] observed that cows producing less than 8.5 kg of colostrum at first milking were more likely to produce high-quality (>50 g/L) colostrum than cows producing higher quantities of first milking colostrum (≥8.5 kg). This finding was presumed to be attributable to dilutional effects. However, more recent studies report that there is no predictable relationship between colostrum IgG concentration and weight of colostrum produced at first milking [43], [44].
Mastitis
Persistent intramammary infection (IMI) during the nonlactating period has not been associated with altered IgG1 concentration. IMI is associated with lower colostral volume produced, however [45]. Producers should not feed colostrum from cows with clinical mastitis.
Pooling
Pooling of colostrum from multiple dams is generally discouraged because larger volumes of low-quality colostrum may dilute smaller volumes of higher-quality colostrum [3]. Furthermore, pooling raw colostrum may increase the number of calves potentially exposed to colostrum-borne pathogens.
Preparturient vaccination of the dam
Although not all studies have shown positive results, a body of research has established that vaccinating the pregnant cow or heifer during the final 3- to 6-week period preceding calving results in increased concentrations of protective colostral antibodies, and increased passive antibody titers in calves of vaccinated dams, for some common pathogens including Pasteurella haemolytica, Salmonella typhimurium, Escherichia coli, rotavirus, and coronavirus [46], [47], [48], [49], [50].
Dry period length
Secretion of Ig from the dam's circulation into the mammary gland begins approximately 5 weeks before calving. In one observational study, dry period length (mean = 57.5 ± 11 days) was not associated with colostrum IgG concentration [35]. In a controlled study, Rastani and colleagues [51] also reported that colostrum quality was not different for cows with a 28- or 56-day dry period, respectively. Cows with excessively short dry periods (<21 days) or no dry period produce colostrum with significantly lower IgG concentrations [51], [52]. Furthermore, dry period length can affect the volume of colostrum produced: In a recent controlled field study cows with a short (40-day) dry period produced 2.2 kg less colostrum than did cows with a conventional (60-day) dry period [44].
Delayed colostrum collection
The concentration of Ig in colostrum is highest immediately after calving, but begins to decrease over time if milking is delayed. In one study, delaying harvest of colostrum for 6 hours, 10 hours, or 14 hours after calving resulted in a 17%, 27%, and 33% decrease in colostral IgG concentration, respectively [53]. To collect the highest quality colostrum, producers should aim to milk the cow within 1 to 2 hours after calving if possible, with a maximum delay of 6 hours.
Cow-side testing of colostrum quality
Empiric recommendations suggest rejecting colostrum that is visibly watery, bloody, or is from cows that leaked before calving [54]. It is difficult to predict, based on such factors as dam parity, weight of colostrum produced at first milking, or visual consistency, which colostrum collected will be of high (>50 g/L IgG) versus low quality [43]. The colostrometer, a hydrometer instrument that estimates IgG concentration by measuring colostrum specific gravity, is one rapid and inexpensive cow-side test that may be useful to differentiate high- from low-quality colostrum (specific gravity >1.050 approximates IgG concentration >50 g/L IgG). Factors such as content of fat and other solids, plus colostrum temperature, affect the hydrometer reading, however. Pritchett and colleagues [55] reported that the sensitivity and specificity of the instrument for detecting low-quality colostrum were 0.32 and 0.97, respectively, meaning that the instrument would incorrectly classify two of every three low-quality colostrum samples as acceptable. Pritchett and colleagues [55] suggested that to avoid misclassification error, producers should alter the hydrometer cutoff points to 45, 60, or 110 g/L if feeding either 3.78, 2.84, or 1.89 L of colostrum at first feeding, respectively. Test specificity would be severely compromised by using higher cutpoints, however, resulting in an excessive portion of colostrums being misclassified as deficient [3]. Others have suggested that if a large enough volume (eg, 3.78 L) is fed at first feeding, then there may be limited value to using a hydrometer. Despite its limitations, the hydrometer may still be useful to differentiate high- from low-quality colostrum used for first versus later feedings, respectively.
An alternate tool for differentiating high- from low-quality colostrum may be a commercially available cow-side immunoassay kit (Colostrum Bovine IgG Quick Test Kit, Midland Bio-Products, Boone, Iowa). Chigerwe and colleagues [56] recently reported that the sensitivity and specificity of this test kit to identify poor-quality colostrum (IgG<50 g/L) were 0.93 and 0.76, respectively. With this relatively low specificity, the immunoassay test would incorrectly classify one in every four high-quality colostrum samples as unacceptable. One additional limitation of the immunoassay is that it yields only a positive or negative result, but does not provide an estimate of the actual IgG concentration. The immunoassay costs approximately $4 (United States dollars [USD]) per sample and takes approximately 20 minutes to run.
Volume of colostrum consumed at first feeding
To achieve successful passive transfer in an average 43-kg (90 lb) Holstein calf, experts calculate that producers should feed at least a minimum mass of 100 g of IgG in the first colostrum feeding [5]. So what volume of colostrum should producers feed to meet or exceed this minimum dose? Obviously the answer to this question depends on the IgG concentration in the colostrum being fed. For example, if colostrum was known to contain 50 g/L IgG, then the producer would only need to feed 1.89 L (2 qt) to achieve the minimum goal of ingesting more than 100 g IgG. If the colostrum contained only 25 g/L of IgG, however, then the producer would need to feed 3.78 L (4 qt) to achieve the same ingested mass of IgG. Besser and colleagues [57] noted that only 36% of colostrum samples tested would be of high enough quality to provide greater than 100 g IgG if calves were only fed 1.89 L. Some 85% of colostrum samples tested would be of high enough quality to provide greater than 100 g IgG if calves were fed 3.78 L, however. Because producers frequently do not know the concentration of IgG in the colostrum being fed, it is currently recommended that calves be fed 10% to 12% of their body weight of colostrum at first feeding (3.78 L for a 43-kg calf). In one study mean serum IgG at 24 hours was significantly higher for calves fed 4 L of high-quality colostrum at 0 hours and a further 2 L at 12 hours (31.1 mg/mL IgG) as compared with calves fed only 2 L of high-quality colostrum at 0 hours and a further 2 L at 12 hours (23.5 mg/mL) ( Fig. 2) [58]. Another study reported that Brown Swiss calves fed 3.78 L (versus 1.89 L) of colostrum at first feeding experienced significantly higher rates of average daily gain and greater levels of milk production in both the first and second lactation [10]. In national surveys, 26.1%, 35.9%, and 38.2% of producers reported feeding 4 or more quarts of colostrum within the first 24 hours in 1992, 1996, and 2002, respectively [2], [4], [11], indicating that increasing the volume of colostrum fed is still an area of opportunity for most dairy producers.
Fig. 2.
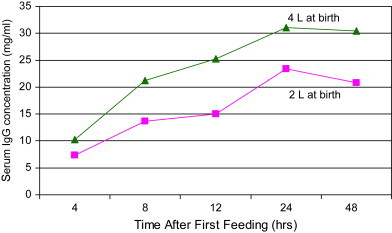
Serum IgG concentrations in calves fed either 4 L or 2 L of colostrum at birth (all calves were fed an additional 2 L of colostrum at 12 hours of age). (Data from Morin DE, McCoy GC, Hurley WL. Effects of quality, quantity, and timing of colostrum feeding and addition of a dried colostrum supplement on immunoglobulin G1 absorption in Holstein bull calves. J Dairy Sci 1997;80:747–53.)
Efficiency of absorption of immunoglobulins
The term “open gut” refers to the unique ability of the neonatal enterocyte to nonselectively absorb intact large molecules, such as Ig, by pinocytosis [59]. From there, Ig molecules are transported across the cell and released into the lymphatics by exocytosis, after which they enter the circulatory system through the thoracic duct [60]. In a process referred to as “closure,” the efficiency of colostral Ig absorption through the intestinal epithelium of the calf decreases linearly with time from birth to completely close at approximately 24 hours [3]. Feeding colostrum after the gut has closed still offers the benefit of local immunity in the gut lumen, but Ig absorption into the circulation no longer occurs. The following section discusses factors affecting the efficiency of Ig absorption, many of which are under management's control.
Time to first colostrum feeding
The major factor affecting efficiency of Ig absorption is age of the calf at feeding. The efficiency of Ig transfer across the gut epithelium is optimal in the first 4 hours postpartum, but after 6 hours there is a progressive decline in the efficiency of Ig absorption over time [61], [62]. Delaying the first colostrum feeding can only slightly postpone gut closure (36 hours) [63]. Producers should aim to feed all calves within 1 to 2 hours after birth and by 6 hours at a maximum.
Method of feeding
The method of feeding colostrum is worth considering because this can influence the time to first feeding, the volume consumed, and the efficiency of Ig absorption. High rates of FPT have been reported in calves left to suckle the dam [57], [64]. This finding may be attributable to failure of the calf to voluntarily consume a sufficient volume of colostrum and delays in suckling. Edwards and Broom [65] reported that 46% of calves born to second parity and older cows had failed to suckle within 6 hours after birth. By comparison, 11% of calves born to first-calf heifers had failed to suckle within 6 hours after birth. These delays could be caused by numerous factors, including weak or injured cow or calf, mastitis or other illness in the cow, low pendulous udders or large teats, or poor mothering ability. It is for this reason that it is currently recommended that the calf be removed from the dam within 1 to 2 hours of birth, and that the calf then be hand-fed a known volume of colostrum using either a nipple bottle or esophageal feeder [6]. In national surveys, 68.1%, 70.5%, and 76.2% of calves were reportedly fed using a nipple bottle or esophageal tube in 1992, 1996, and 2002, respectively [2], [4], [11], indicating that progressively fewer producers are relying on suckling the dam for colostrum delivery.
Producers may have a personal preference for using either a nipple bottle or esophageal feeder for the first colostrum feeding. Although the esophageal feeder method is quicker, it is known that when fluid is given with an esophageal feeder, the esophageal groove reflex is not triggered, resulting in fluid being deposited into the forestomachs. This limitation is not significant, however, because outflow of colostrum from the forestomachs to the abomasum and small intestine occurs for the most part within 3 hours [66]. Adams and colleagues [67] reported that calves fed colostrum using a bottle had only slightly higher serum IgG concentrations versus calves fed with an esophageal feeder, but that these differences were numerically small and statistically insignificant. It is generally accepted that either method of feeding achieves acceptable rates of passive transfer provided a sufficient volume of colostrum is consumed [67], [68]. Veterinarians should train interested producers on how to properly use and clean esophageal feeders.
Presence of the dam
It has been reported that efficiency of Ig absorption was improved when calves were housed with the dam [69]. Considering that acceptable levels of serum IgG can be achieved without housing the calf with the dam, however, and given that the latter practice may increase the calf's risk for exposure to pathogens from the dam or her environment, it is currently recommended that the calf be removed from the dam within 1 to 2 hours of birth and then hand-fed a known volume of colostrum [6].
Metabolic disturbances
Decreased colostral Ig absorption in the first 12 hours has been reported in calves with postnatal respiratory acidosis, associated with prolonged parturition [70]. Although hypoxic calves may have delayed IgG absorption initially, studies have reported that there is no difference in overall absorptive capacity between hypoxic and normoxic calves and that there is no difference in serum IgG concentrations by the time of gut closure [71], [72]. Weaver and colleagues [3] suggested that an increased rate of FPT seen in calves with metabolic or respiratory acidosis may be caused by a delay in the animal getting up to nurse, not by reduced absorptive capacity.
Cold stress
Absorption of Ig may be impaired when newborn calves are exposed to extreme cold, possibly because of direct effects on intestinal absorption and transport and indirect effects on the calf's ability to stand and nurse [73].
Bacterial contamination of colostrum
Bacteria in colostrum may bind free Ig in the gut lumen or directly block uptake and transport of Ig molecules across intestinal epithelial cells, thus interfering with passive absorption of colostral Ig [74], [75], [76]. This effect was demonstrated in a recent controlled study wherein newborn calves were fed either 3.8 L of pasteurized (60°C × 60 min) colostrum or 3.8 L of raw colostrum, with the geometric mean total bacteria counts in the two colostrum treatment groups being 813 cfu/mL or 40,738 cfu/mL, respectively [77]. Although the volume, timing, and quality of colostrum fed to the two feeding groups was not different, calves fed pasteurized colostrum had significantly higher mean serum IgG levels at 24 hours of age (22.3 mg/mL) versus calves fed raw colostrum (18.1 mg/mL). This improvement was attributed to reduced bacterial interference with IgG absorption across the gut, resulting in higher efficiency of IgG absorption in calves fed pasteurized colostrum (35%) versus calves fed raw colostrum (27%) [77]. Strategies for preventing or minimizing bacterial contamination of colostrum are discussed in the next section.
Strategies for preventing bacterial contamination of colostrum
Although colostrum is an important source of nutrients and immune factors, it can also represent one of the earliest potential exposures of dairy calves to infectious agents including Mycoplasma spp, Mycobacterium avium subsp paratuberculosis, fecal coliforms, and Salmonella spp [78], [79], [80]. This exposure is a concern because pathogenic bacteria in colostrum could cause diseases such as diarrhea or septicemia. It is also a concern because bacteria in colostrum may interfere with absorption of Ig [74], [75], [76]. Experts recommend that fresh colostrum fed to calves contain fewer than 100,000 cfu/mL total bacteria count (TPC) and fewer than 10,000 cfu/mL total coliform count [6]. Unfortunately, average bacteria counts in colostrum fed on commercial dairies frequently far exceeds this cutpoint [31], [76]. In one study of Wisconsin dairy herds, 82% of samples tested exceeded the upper limit of 100,000 cfu/mL TPC [76]. The following section describes management techniques for minimizing bacterial contamination of colostrum.
Preventing contamination during colostrum harvest, storage, and feeding procedures
Methods for reducing the risk for pathogen exposure to calves include avoiding feeding colostrum from known infected cows and avoiding pooling of raw colostrum. Additionally, all producers should take steps to avoid contamination during colostrum harvest, storage, or feeding processes. In a study of colostrum harvesting and feeding practices on one dairy, total bacteria counts (TPC cfu/mL) were very low or nil in colostrum stripped directly from the gland (geometric meanudder TPC = 27.5 cfu/mL). Significant bacterial contamination occurred, however, during the process of milking the colostrum into the bucket (geometric meanbucket TPC = 97,724 cfu/mL) [81]. These results emphasize the importance of minimizing colostrum contamination by properly prepping udders before harvesting colostrum, milking into a clean, sanitized bucket, and handling colostrum using clean, sanitized storage or feeding equipment.
Minimizing bacterial growth in stored colostrum
Bacteria can multiply rapidly if colostrum or milk is stored at warm ambient temperatures [81]. Unless colostrum is to be fed right away, it should be frozen or refrigerated within 1 hour after collection. It is generally accepted that colostrum may be frozen for up to 1 year, provided multiple freeze–thaw cycles do not occur. When thawing frozen colostrum, producers should avoid overheating colostrum (avoid temperatures >60°C or 140°F) or some denaturation of colostral Ig can occur [82]. Options for producers who wish to store fresh colostrum include refrigeration with or without the use of preservatives such as potassium sorbate [81]. IgG in raw refrigerated colostrum is stable for at least 1 week. Average bacteria counts in raw refrigerated colostrum may reach unacceptably high concentrations (>100,000 cfu/mL) after 2 days of refrigeration, however. By comparison, average colostrum bacteria counts remained less than 100,000 cfu/mL for 6 days of refrigeration when colostrum was preserved with potassium sorbate in a 0.5% final solution [81]. Information on potassium sorbate sources and mixing directions can be found at http://www.atticacows.com/orgMain.asp?orgid=19&storyTypeID=&sid=&.
Pasteurizing colostrum
An additional tool that may be useful to reduce bacterial contamination of colostrum is pasteurization. Early studies tried to pasteurize colostrum using the same conventional methods and high temperatures as are typically used to pasteurize milk (63°C [145°F] for 30 minutes or 72°C [161°F] for 15 seconds). This process yielded unacceptable results, however, including thickening or congealing of colostrum and denaturation of approximately one third of colostral IgG [83]. Despite these early setbacks, more recent research has determined that using a lower-temperature, longer-time approach (60°C [140°F] for 60 minutes) to batch-pasteurize colostrum is sufficient to maintain IgG activity and colostrum fluid characteristics, while eliminating or significantly reducing important pathogens including E. coli, Salmonella enteritidis, Mycoplasma bovis and Mycobacterium avium subsp paratuberculosis [82], [84]. In one recent on-farm controlled study, calves fed pasteurized colostrum (60°C × 60 minutes) experienced a significant reduction in colostrum bacterial exposure and significantly higher serum IgG levels at 24 hours of age versus calves fed 3.8 L of raw colostrum [77]. If stored in a clean covered container, the shelf life of pasteurized refrigerated colostrum is at least 8 to 10 days [85]. The potential short- and long-term health and economic benefits of feeding pasteurized colostrum have not yet been described.
Use of colostrum supplements or replacement products
Farms can occasionally experience periods in which an adequate supply of clean, high-quality, fresh or stored colostrum is not available to feed to all newborn calves. Contributing to this problem, some producers may discard colostrum from cows that test positive for M avium subsp paratuberculosis, bovine leukosis virus, or M bovis mastitis. Under such circumstances, using colostrum supplements (CS) or colostrum replacement (CR) products may offer producers a convenient way to improve levels of passive immunity in calves while reducing the risk for pathogen exposure through colostrum. Powdered commercial CS or CR products contain bovine Ig that is typically either lacteal- or plasma-derived. It is recommended that CS or CR products be mixed in water (according to label directions) and fed as a separate meal after any natural colostrum has been fed [6]. There are important differences between the less expensive CS products ($5–$7 per dose) and more expensive CR products ($25–$30 per dose). Colostrum supplement products typically contain less than 50 g IgG per dose, contain no nutrient pack, and are only intended to supplement (not replace) existing colostrum. If given alone, feeding CS products results in significantly lower serum Ig and greater risk for FPT in calves as compared with feeding fresh colostrum [86]. There is no added benefit of feeding CS products if already feeding 3 to 4 L of high-quality bovine colostrum [87], [88]. By comparison, CR products contain a minimum of 100 g IgG per dose, provide a nutritional source of protein, energy, vitamins, and minerals, and are designed to completely replace (or feed in the absence of) maternal colostrum [89].
Results of CR studies have been mixed, with many products failing to routinely provide the necessary 10 mg/mL IgG in serum of calves fed CR [31], [89], [90], [91]. In a controlled study of 12 dairy herds in Minnesota and Wisconsin, Swan and colleagues [31] reported that 239 commercial dairy calves fed a commercially available CR product (Acquire, American Protein Corporation, Inc., Ames, Iowa) had significantly lower serum IgG concentrations (5.8 mg/mL IgG) than 218 calves fed maternal colostrum (14.8 mg/mL IgG). Although a trend was present, the preweaning morbidity and mortality rates were not different for calves fed CR (morbidity = 59.6%; mortality = 12.4%) versus calves fed maternal colostrum (morbidity = 51.9%; mortality = 10%). Other studies have reported better rates of successful passive transfer (mean serum IgG >10.0 mg/mL), particularly when calves were fed two doses of CR product [89], [92]. In one such study, the average 24-hour serum IgG level for calves fed either one dose (100 g IgG) or two doses (200 g IgG) of a lacteal-derived CR, or 3.78 L of maternal colostrum, were 11.6, 16.9, and 27.2 mg/mL IgG, respectively (Land O' Lakes Colostrum Replacement, Land O' Lakes Inc., St. Paul, Minnesota) [93]. Feeding higher doses of CR products may increase the rate of successful passive transfer, but the cost–benefit of this practice has yet to be described. Similarly, the effectiveness and cost–benefit of routinely using CR products in Johne's or other infectious disease control programs has yet to be described. Because of the highly variable performance among different products, veterinarians should review results of peer-reviewed controlled trials when selecting a CR product.
Monitoring the colostrum management program
Veterinarians can help producers develop programs to routinely monitor colostrum management. Possible laboratory-based test methods for directly measuring or estimating serum IgG concentrations in calves include radial immunodiffusion (RID), turbidimetric immunoassay (TIA), enzyme-linked immunosorbent assay (ELISA), sodium sulfite turbidity test, zinc sulfate turbidity test, serum gamma glutamyltransferase (GGT) activity, and whole-blood glutaraldehyde coagulation test [94], [95], [96]. In a recent review of these tests, Weaver and colleagues [3] raised concerns about unacceptably high levels of inaccurate results for the sodium sulfite turbidity test when using the 14% and 16% sodium sulfite test solutions, the zinc sulfate turbidity test if samples are exposed to CO2 or are hemolyzed, GGT test results, and whole-blood glutaraldehyde coagulation test results. Although RID, TIA, or ELISA would be acceptable tests for use in periodic outbreak investigations, the expense and inconvenience of routinely submitting serum samples to a veterinary diagnostic laboratory would generally discourage their adoption for ongoing monitoring programs.
A lateral-flow immunoassay is one tool that could be used for on-farm testing (Midland Quick Test Kit – Calf IgG, Midland BioProducts Corp., Boone, Iowa). The manufacturer has reported the sensitivity, specificity, and overall accuracy of this assay to identify calves with serum IgG less than 10.0 mg/mL as being 0.99, 0.89, and 0.94, respectively [97]. Independent validation of this test is still required. One limitation of the immunoassay is that it yields only a positive or negative result, but does not provide an estimate of the actual serum IgG concentration. The assay requires approximately 20 minutes to complete and costs approximately $4.50 (USD) per sample.
Measurement of serum total solids (STS) by hand-held refractometer offers a convenient, simple, rapid, and inexpensive on-farm tool by which producers can monitor the colostrum feeding program. The refractometer instrument costs approximately $250 (USD). In an early study of 185 calves, STS had a good correlation with serum IgG concentration as measured using RID (R 2 = 0.72) [98]. Calloway and colleagues [99], reported that STS concentration test endpoints of 5.0 and 5.2 g/dL yielded the most accurate results in estimating the adequacy of passive transfer as defined by serum IgG 10.0 mg/mL or greater (sensitivity >0.80; specificity >0.80; proportion classified correctly >0.85). In that study lower or higher test endpoints misclassified larger numbers of calves. Because STS results do result in periodic misclassification of individual calves, the use of STS results as an individual animal diagnostic tool is discouraged. When results are interpreted at the group or herd level, however, STS results accurately reflect the proportion of calves that have FPT, thereby making it a useful on-farm tool for monitoring whether the colostrum management program is succeeding. It is recommended that serum samples be collected from a minimum of 12 clinically normal (not scouring) calves between 24 hours and 7 days of age [6]. Wallace and colleagues [100] reported that the results of STS refractometry from centrifuge- and noncentrifuge-harvested sources of serum were highly correlated (R 2 = 0.95), so producers can conduct this test on-farm without need of a centrifuge. McGuirk and Collins [6] suggest that a goal is for 80% or more of calves tested to meet or exceed a STS cutpoint of 5.5 g/dL. Tyler suggests that 90% or more of calves tested should meet or exceed the more accurate STS cutpoint of 5.0 g/dL (J Tyler, personal communication, 2002). If it is determined that a disproportionate number of calves have FPT, then the veterinarian and producer must investigate to identify and then correct the root causes of FPT within the colostrum management program. In addition to periodically sampling groups of calves to assess FPT, producers can also periodically submit frozen colostrum samples to a microbiology laboratory for culture. A goal is for a majority of samples submitted to have at total bacteria count of less than 100,000 cfu/mL and a total coliform count less than 10,000 cfu/mL [6].
Summary
Colostrum management is the single most important management factor in determining calf health and survival. Unfortunately, a significant proportion of North American dairy calves suffer from failure of passive transfer, contributing to excessively high preweaning mortality. There is considerable opportunity for most dairy producers to improve their colostrum management practices, resulting in improved short- and long-term health and performance of the animal. A successful colostrum management program requires producers to consistently provide calves with a sufficient volume of clean, high-quality colostrum within the first few hours of life. Colostrum replacers are useful tools if a sufficient quantity of clean, high-quality maternal colostrum is not available. Ongoing monitoring helps producers to more quickly identify and correct problems within the colostrum management program.
References
- 1.Arthur G.H. The development of the conceptus. In: Arthur G.H., Nokes D.E., Pearson H., editors. Pregnancy and parturition in veterinary reproduction and obstetrics. 7th edition. W.B. Saunders; Philadelphia: 1996. pp. 51–109. [Google Scholar]
- 2.National Animal Health Monitoring System . USDA-APHIS Veterinary Services; Ft. Collins (CO): 1996. Dairy 1996: National dairy health evaluation project. Dairy heifer morbidity, mortality, and health management focusing on preweaned heifers. [Google Scholar]
- 3.Weaver D.M., Tyler J.W., VanMetre D.C. Passive transfer of colostral immunoglobulins in calves. J Vet Intern Med. 2000;14:569–577. doi: 10.1892/0891-6640(2000)014<0569:ptocii>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 4.National Animal Health Monitoring System . USDA-APHIS Veterinary Services; Ft. Collins (CO): 1993. National dairy heifer evaluation project. Dairy herd management practices focusing on preweaned heifers. [Google Scholar]
- 5.Davis C.L., Drackley J.K. The development, nutrition, and management of the young calf. 1st edition. © 1998. Iowa State University Press; Ames (IA): 1998. pp. 179–206. [Google Scholar]
- 6.McGuirk S.M., Collins M. Managing the production, storage and delivery of colostrum. Vet Clin North Am Food Anim Pract. 2004;20(3):593–603. doi: 10.1016/j.cvfa.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Robison J.D., Stott G.H., DeNise S.K. Effects of passive immunity on growth and survival in the dairy heifer. J Dairy Sci. 1988;71:1283–1287. doi: 10.3168/jds.S0022-0302(88)79684-8. [DOI] [PubMed] [Google Scholar]
- 8.DeNise S.K., Robison J.D., Stott G.H. Effects of passive immunity on subsequent production in dairy heifers. J Dairy Sci. 1989;72:552–554. doi: 10.3168/jds.S0022-0302(89)79140-2. [DOI] [PubMed] [Google Scholar]
- 9.Wells S.J., Dargatz D.A., Ott S.L. Factors associated with mortality to 21 days of life in dairy heifers in the United States. Prev Vet Med. 1996;29:9–19. [Google Scholar]
- 10.Faber S.N., Faber N.E., McCauley T.C. Effects of colostrum ingestion on lactational performance. The Professional Animal Scientist. 2005;21:420–425. [Google Scholar]
- 11.National Animal Health Monitoring System . USDA-APHIS Veterinary Services; Ft. Collins (CO): 2002. Dairy 2002. Part 1: reference of dairy health and management in the United States. [Google Scholar]
- 12.Foley J.A., Otterby D.E. Availability, storage, treatment, composition, and feeding value of surplus colostrum: a review. J Dairy Sci. 1978;61:1033–1060. [Google Scholar]
- 13.Larson B.L., Heary H.L., Jr., Devery J.E. Immunoglobulin production and transport by the mammary gland. J Dairy Sci. 1980;63:665–671. doi: 10.3168/jds.S0022-0302(80)82988-2. [DOI] [PubMed] [Google Scholar]
- 14.Newby T.J., Stokes C.R., Bourne F.J. Immunological activities of milk. Vet Immunol Immunopathol. 1982;3:67–94. doi: 10.1016/0165-2427(82)90032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barrington G.M., Besser T.E., Gay C.C. Effect of prolactin on in vitro expression of the bovine mammary immunoglobulin G1 receptor. J Dairy Sci. 1997;80:94–100. doi: 10.3168/jds.S0022-0302(97)75916-2. [DOI] [PubMed] [Google Scholar]
- 16.Thatcher E.F., Gershwin L.J. Colostral transfer of bovine immunoglobulin E and dynamics of serum IgE in calves. Vet Immunol Immunopathol. 1989;20:325–334. doi: 10.1016/0165-2427(89)90078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Jan C. Cellular components of mammary secretions and neonatal immunity: a review. Vet Res. 1996;27:403–417. [PubMed] [Google Scholar]
- 18.Schnorr K.L., Pearson L.F. Intestinal absorption of maternal leucocytes by newborn lambs. J Reprod Immunol. 1984;6:329–337. doi: 10.1016/0165-0378(84)90031-7. [DOI] [PubMed] [Google Scholar]
- 19.Liebler-Tenorio E.M., Riedel-Caspari G., Pohlenz J.F. Uptake of colostral leukocytes in the intestinal tract of newborn calves. Vet Immunol Immunopathol. 2002;85:33–40. doi: 10.1016/s0165-2427(01)00404-4. [DOI] [PubMed] [Google Scholar]
- 20.Reber A.J., Lockwood A., Hippen A.R. Colostrum induced phenotypic and trafficking changes in maternal mononuclear cells in a peripheral blood leukocyte model for study of leukocyte transfer to the neonatal calf. Vet Immunol Immunopathol. 2006;109:139–150. doi: 10.1016/j.vetimm.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 21.Reidel-Caspari G. The influence of colostral leukocytes on the course of an experimental Escherichia coli infection and serum antibodies in neonatal calves. Vet Immunol Immunopathol. 1993;35:275–288. doi: 10.1016/0165-2427(93)90039-7. [DOI] [PubMed] [Google Scholar]
- 22.Reber A.J., Hippen A.R., Hurley D.J. Effects of the ingestion of whole colostrum or cell-free colostrum on the capacity of leukocytes in newborn calves to stimulate or respond in one-way mixed leukocyte cultures. Am J Vet Res. 2005;66:1854–1860. doi: 10.2460/ajvr.2005.66.1854. [DOI] [PubMed] [Google Scholar]
- 23.Donovan D., Reber A., Gabbard J. Effect of maternal cells transferred with colostrum on cellular response to pathogen antigens in neonatal calves. Am J Vet Res. 2007;68:778–782. doi: 10.2460/ajvr.68.7.778. [DOI] [PubMed] [Google Scholar]
- 24.Pakkanen R., Aalto J. Growth factors and antimicrobial factors of bovine colostrum. Int Dairy J. 1997;7:285–297. [Google Scholar]
- 25.Shah N.P. Effects of milk-derived bioactives: an overview. Br J Nutr. 2000;84(Suppl 1):S3–S10. doi: 10.1017/s000711450000218x. [DOI] [PubMed] [Google Scholar]
- 26.Elfstrand L., Lindmark-Månsson H., Paulsson M. Immunoglobulins, growth factors and growth hormone in bovine colostrum and the effects of processing. Int Dairy J. 2002;12:879–887. [Google Scholar]
- 27.Przybylska J., Albera E., Kankofer M. Antioxidants in bovine colostrum. Reprod Domest Anim. 2007;42:402–409. doi: 10.1111/j.1439-0531.2006.00799.x. [DOI] [PubMed] [Google Scholar]
- 28.Baumrucker C.R., Hadsell D.L., Blum J.W. Effects of dietary insulin-like growth factor I on growth and insulin-like growth factor receptors in neonatal calf intestine. J Anim Sci. 1994;72:428–433. doi: 10.2527/1994.722428x. [DOI] [PubMed] [Google Scholar]
- 29.Bird A.R., Croom W.J., Fan Y.K. Peptide regulation of intestinal glucose absorption. J Anim Sci. 1996;74:2523–2540. doi: 10.2527/1996.74102523x. [DOI] [PubMed] [Google Scholar]
- 30.Bühler C., Hammon H., Rossi G.L. Small intestinal morphology in eight-day-old calves fed colostrum for different durations or only milk replacer and treated with long-R3-insulin-like growth factor I and growth hormone. J Anim Sci. 1998;76:758–765. doi: 10.2527/1998.763758x. [DOI] [PubMed] [Google Scholar]
- 31.Swan H., Godden S., Bey R. Passive transfer of immunoglobulin g and preweaning health in Holstein calves fed a commercial colostrum replacer. J Dairy Sci. 2007;90:3857–3866. doi: 10.3168/jds.2007-0152. [DOI] [PubMed] [Google Scholar]
- 32.Guy M.A., McFadden T.B., Cockrell D.C. Regulation of colostrum formation in beef and dairy cows. J Dairy Sci. 1994;77:3002–3007. doi: 10.3168/jds.S0022-0302(94)77241-6. [DOI] [PubMed] [Google Scholar]
- 33.Muller L.D., Ellinger D.K. Colostral immunoglobulin concentrations among breeds of dairy cattle. J Dairy Sci. 1981;64:1727–1730. doi: 10.3168/jds.S0022-0302(81)82754-3. [DOI] [PubMed] [Google Scholar]
- 34.Morin D.E., Constable P.D., Maunsell F.P. Factors associated with colostral specific gravity in dairy cows. J Dairy Sci. 2001;84:937–943. doi: 10.3168/jds.S0022-0302(01)74551-1. [DOI] [PubMed] [Google Scholar]
- 35.Pritchett L.C., Gay C.C., Besser T.E. Management and production factors influencing Immunoglobulin G1 concentration in colostrum from Holstein cows. J Dairy Sci. 1991;74:2336–2341. doi: 10.3168/jds.S0022-0302(91)78406-3. [DOI] [PubMed] [Google Scholar]
- 36.Tyler J.W., Steevens B.J., Hostetler D.E. Colostral immunoglobulin concentrations in Holstein and Guernsey cows. Am J Vet Res. 1999;60:1136–1139. [PubMed] [Google Scholar]
- 37.Blecha G.K., Bulls R.C., Olson D.P. Effects of prepartum protein restriction in the beef cow on immunoglobulin content in blood and colostral whey and subsequent immunoglobulin absorption by the neonatal calf. J Anim Sci. 1981;53:1174–1180. doi: 10.2527/jas1981.5351174x. [DOI] [PubMed] [Google Scholar]
- 38.NRC. National Research Council . 6th revised edition. National Academy Press; Washington, DC: 1984. Nutrient requirements of beef cattle. 1984. [Google Scholar]
- 39.Hough R.L., McCarthy F.D., Kent H.D. Influence of nutritional restriction during late gestation on production measures and passive immunity in beef cattle. J Anim Sci. 1990;68:2622–2627. doi: 10.2527/1990.6892622x. [DOI] [PubMed] [Google Scholar]
- 40.Lacetera N., Bernabucci U., Ronchi B. Effects of selenium and vitamin E administration during a late stage of pregnancy on colostrum and milk production in dairy cows, and on passive immunity and growth of their offspring. Am J Vet Res. 1996;57:1776–1780. [PubMed] [Google Scholar]
- 41.NRC. National Research Council 2001 . 7th revised edition. National Academy Press; Washington, DC: 2001. Nutrient requirements of dairy cattle. [Google Scholar]
- 42.Nardone A., Lacetera N., Bernabucci U. Composition of colostrum from dairy heifers exposed to high air temperatures during late pregnancy and the early postpartum period. J Dairy Sci. 1997;80:838–844. doi: 10.3168/jds.S0022-0302(97)76005-3. [DOI] [PubMed] [Google Scholar]
- 43.Maunsell F.P., Morin D.E., Constable P.D. Use of mammary gland and colostral characteristics for prediction of colostral IgG1 concentration and intramammary infection in Holstein cows. J Am Vet Med Assoc. 1999;214:1817–1823. [PubMed] [Google Scholar]
- 44.Grusenmeyer D.J., Ryan C.M., Galton D.M. Shortening the dry period from 60 to 40 days does not affect colostrum quality but decreases colostrum yield by Holstein cows. J Dairy Sci. 2006;89(Suppl 1):336. [Google Scholar]
- 45.Maunsell F.P., Morin D.E., Constable P.D. Effects of mastitis on the volume and composition of colostrum produced by Holstein cows. J Dairy Sci. 1998;81:1291–1299. doi: 10.3168/jds.S0022-0302(98)75691-7. [DOI] [PubMed] [Google Scholar]
- 46.Jones P.W., Collins P., Aitkin M.M. Passive protection of calves against experimental infection with Salmonella typhimurium. Vet Rec. 1988;123:536–541. doi: 10.1136/vr.123.21.536. [DOI] [PubMed] [Google Scholar]
- 47.Myers L.L., Snodgrass D.R. Colostral and milk antibody titers in cows vaccinated with a modified live rotavirus-coronavirus vaccine. J Am Vet Med Assoc. 1982;181:486–488. [PubMed] [Google Scholar]
- 48.Waltner-Toews D., Martin S.W., Meek A.H. A field trial to evaluate the efficacy of a combined rotavirus-coronavirus Escherichia coli vaccine in dairy cattle. Can J Comp Med. 1985;49:1–9. [PMC free article] [PubMed] [Google Scholar]
- 49.Archambault D., Morin G., Elazhary Y. Immune response of pregnant heifers and cows to bovine rotavirus inoculation and passive protection to rotavirus infection in newborn calves fed colostral antibodies or colostral lymphocytes. Am J Vet Res. 1988;49:1084–1091. [PubMed] [Google Scholar]
- 50.Hodgins D.C., Shewen P.E. Preparturient vaccination to enhance passive immunity to the capsular polysaccharide of Pasteurella haemolytica A1. Vet Immunol Immunopathol. 1996;50:67–77. doi: 10.1016/0165-2427(95)05493-6. [DOI] [PubMed] [Google Scholar]
- 51.Rastani R.R., Grummer R.R., Bertics S.J. Reducing dry period length to simplify feeding transition cows: Milk production, energy balance and metabolic profiles. J Dairy Sci. 2005;88:1004–1014. doi: 10.3168/jds.S0022-0302(05)72768-5. [DOI] [PubMed] [Google Scholar]
- 52.Dixon F.J., Weigle W.O., Vasquez J.J. Metabolism and mammary secretion of serum protein in the cow. Lab Invest. 1961;10:216–237. [PubMed] [Google Scholar]
- 53.Moore M., Tyler J.W., Chigerwe M. Effect of delayed colostrum collection on colostral IgG concentration in dairy cows. J Am Vet Med Assoc. 2005;226(8):1375–1377. doi: 10.2460/javma.2005.226.1375. [DOI] [PubMed] [Google Scholar]
- 54.BAMN. Bovine Alliance on Management and Nutrition . American Feed Industry Association; Arlington (VA): 1995. A guide to colostrum and colostrum management for dairy calves. [Google Scholar]
- 55.Pritchett L.C., Gay C.C., Hancock D.D. Evaluation of the hydrometer for testing immunoglobulin G1 concentrations in Holstein colostrum. J Dairy Sci. 1994;77:1761–1767. doi: 10.3168/jds.S0022-0302(94)77117-4. [DOI] [PubMed] [Google Scholar]
- 56.Chigerwe M., Dawes M.E., Tyler J.W. Evaluation of a cow-side immunoassay kit for assessing IgG concentration in colostrum. J Am Vet Med Assoc. 2005;227:129–131. doi: 10.2460/javma.2005.227.129. [DOI] [PubMed] [Google Scholar]
- 57.Besser T.E., Gay C.C., Pritchett L. Comparison of three methods of feeding colostrum to dairy calves. J Am Vet Med Assoc. 1991;198:419–422. [PubMed] [Google Scholar]
- 58.Morin D.E., McCoy G.C., Hurley W.L. Effects of quality, quantity, and timing of colostrum feeding and addition of a dried colostrum supplement on immunoglobulin G1 absorption in Holstein bull calves. J Dairy Sci. 1997;80:747–753. doi: 10.3168/jds.S0022-0302(97)75994-0. [DOI] [PubMed] [Google Scholar]
- 59.Broughton C.W., Lecce J.G. Electron microsopic studies of the jejunal epithelium from neonatal pigs fed different diets. J Nutr. 1970;100:445–449. doi: 10.1093/jn/100.4.445. [DOI] [PubMed] [Google Scholar]
- 60.Staley T.E., Corles C.D., Bush L.J. The ultrastructure of neonatal calf intestine and absorption of heterologous proteins. Anat Rec. 1972;172:559–579. doi: 10.1002/ar.1091720310. [DOI] [PubMed] [Google Scholar]
- 61.Besser T.E., Garmedia A.E., McGuire T.C. Effect of colostral immunoglobulin G1 and immunoglobulin M concentrations on immunoglobulin absorption in calves. J Dairy Sci. 1985;68:2033–2037. doi: 10.3168/jds.S0022-0302(85)81065-1. [DOI] [PubMed] [Google Scholar]
- 62.Michanek P., Ventorp M., Weström B. Intestinal transmission of macromolecules in newborn dairy calves of different ages at first feeding. Res Vet Sci. 1989;46:375–379. [PubMed] [Google Scholar]
- 63.Stott G.H., Marx D.B., Menefee B.E. Colostral immunoglobulin transfer in calves I. Period of absorption. J Dairy Sci. 1979;62:1632–1638. doi: 10.3168/jds.S0022-0302(79)83472-4. [DOI] [PubMed] [Google Scholar]
- 64.Brignole T.J., Stott G.H. Effect of suckling followed by bottle feeding colostrum on immunoglobulin absorption and calf survival. J Dairy Sci. 1980;63:451–456. doi: 10.3168/jds.S0022-0302(80)82952-3. [DOI] [PubMed] [Google Scholar]
- 65.Edwards S.A., Broom D.M. The period between birth and first suckling in dairy calves. Res Vet Sci. 1979;26:255–256. [PubMed] [Google Scholar]
- 66.Lateur-Rowet H.J.M., Breukink H.J. The failure of the oesophageal groove reflex, when fluids are given with an oesophageal feeder to newborn and young calves. Vet Q. 1983;5:68–74. doi: 10.1080/01652176.1983.9693874. [DOI] [PubMed] [Google Scholar]
- 67.Adams G.D., Bush L.J., Horner J.L. Two methods for administering colostrum to newborn calves. J Dairy Sci. 1985;68:773–775. doi: 10.3168/jds.S0022-0302(85)80887-0. [DOI] [PubMed] [Google Scholar]
- 68.Kaske M., Werner A., Schuberth H.J. Colostrum management in calves: effects of drenching vs. bottle feeding. J Anim Physiol Anim Nutr (Berl) 2005;89:151–157. doi: 10.1111/j.1439-0396.2005.00535.x. [DOI] [PubMed] [Google Scholar]
- 69.Selman I.E., McEwan A.D., Fisher E.W. Studies on dairy calves allowed to suckle their dams at fixed times postpartum. Res Vet Sci. 1971;12:1–6. [PubMed] [Google Scholar]
- 70.Besser T.E., Szenci O., Gay C.C. Decreased colostral immunoglobulin absorption in calves with postnatal respiratory acidosis. J Am Vet Med Assoc. 1990;196:1239–1443. [PubMed] [Google Scholar]
- 71.Tyler H., Ramsey H. Hypoxia in neonatal calves: Effect on intestinal transport of immunoglobulins. J Dairy Sci. 1991;74:1953–1956. doi: 10.3168/jds.S0022-0302(91)78361-6. [DOI] [PubMed] [Google Scholar]
- 72.Drewry J.J., Quigley J.D., Geiser D.R. Effect of high arterial carbon dioxide tension on efficiency of immunoglobulin G absorption in calves. Am J Vet Res. 1999;60:609–614. [PubMed] [Google Scholar]
- 73.Olson D.P., Bull R.C., Woodward L.F. Effects of maternal nutritional restriction and cold stress on young calves: absorption of colostral immunoglobulins. Am J Vet Res. 1981;42:876–880. [PubMed] [Google Scholar]
- 74.James R.E., Polan C.E. Effect of orally administered duodenal fluid on serum proteins in neonatal calves. J Dairy Sci. 1978;61:1444–1449. doi: 10.3168/jds.S0022-0302(78)83747-3. [DOI] [PubMed] [Google Scholar]
- 75.James R.E., Polan C.E., Cummins K.A. Influence of administered indigenous microorganisms on uptake of [iodine-125] gamma-globulin in vivo by intestinal segments of neonatal calves. J Dairy Sci. 1981;64(1):52–61. doi: 10.3168/jds.S0022-0302(81)82528-3. [DOI] [PubMed] [Google Scholar]
- 76.Poulsen K.P., Hartmann F.A., McGuirk S.M. Mira Digital Publishing; St. Luois (MO): 2002. Bacteria in colostrum: impact on calf health [abstract 52] in Proc. 20th American College of Internal Veterinary Medicine. p. 773. [Google Scholar]
- 77.Johnson J., Godden S., Molitor T. The effect of feeding heat-treated colostrum on passive transfer of cellular and humoral immune parameters in neonatal dairy calves. J Dairy Sci. 2007;90:5189–5198. doi: 10.3168/jds.2007-0219. [DOI] [PubMed] [Google Scholar]
- 78.Steele M.L., McNab W.B., Poppe C. Survey of Ontario bulk tank raw milk for food-borne pathogens. J Food Prot. 1997;60(11):1341–1346. doi: 10.4315/0362-028X-60.11.1341. [DOI] [PubMed] [Google Scholar]
- 79.Streeter R.N., Hoffsis G.F., Bech-Nielsen S. Isolation of Mycobacterium paratuberculosis from colostrum and milk of subclinically infected cows. Am J Vet Res. 1995;56(10):1322–1324. [PubMed] [Google Scholar]
- 80.Walz P.H., Mullaney T.P., Render J.A. Otitis media in preweaned Holstein dairy calves in Michigan due to Mycoplasma bovis. J Vet Diagn Invest. 1997;9:250–254. doi: 10.1177/104063879700900305. [DOI] [PubMed] [Google Scholar]
- 81.Stewart S., Godden S., Bey R. Preventing bacterial contamination and proliferation during the harvest, storage and feeding of fresh bovine colostrum. J Dairy Sci. 2005;88:2571–2578. doi: 10.3168/jds.S0022-0302(05)72933-7. [DOI] [PubMed] [Google Scholar]
- 82.McMartin S., Godden S., Metzger L. Heat-treatment of bovine colostrum I: Effects of temperature on viscosity and immunoglobulin G. J Dairy Sci. 2006;89:2110–2118. doi: 10.3168/jds.S0022-0302(06)72281-0. [DOI] [PubMed] [Google Scholar]
- 83.Godden S.M., Smith S., Feirtag J.M. Effect of on-farm commercial batch pasteurization of colostrum on colostrum and serum immunoglobulin concentrations in commercial dairy calves. J Dairy Sci. 2003;86:1503–1512. doi: 10.3168/jds.S0022-0302(03)73736-9. [DOI] [PubMed] [Google Scholar]
- 84.Godden S., McMartin S., Feirtag J. Heat-treatment of bovine colostrum II: Effects of heating duration on pathogen viability and immunoglobulin G. J Dairy Sci. 2006;89:3476–3483. doi: 10.3168/jds.S0022-0302(06)72386-4. [DOI] [PubMed] [Google Scholar]
- 85.Bey R, Godden S, Lillegaard H, et al. Improving cleanliness and shelf-life of refrigerated colostrum using heat-treatment and chemical preservatives. Proc. Annu. Meet. Minnesota Dairy Health Management Conference. St. Paul, Minnesota, May 15–17, 2007.
- 86.Quigley J.D., Fike D.L., Egerton M.N. Effects of a colostrum replacement product derived from serum on immunoglobulin G absorption by calves. J Dairy Sci. 1998;81:1936–1939. doi: 10.3168/jds.S0022-0302(98)75766-2. [DOI] [PubMed] [Google Scholar]
- 87.Francisco S.F.A., Quigley J.D. Serum immunoglobulin concentrations after feeding maternal colostrum or maternal colostrum plus colostral supplement to dairy calves. Am J Vet Res. 1993;54:1051–1054. [PubMed] [Google Scholar]
- 88.Zaremba W., Guterbock W.M., Holmberg C.A. Efficacy of a dried colostrum powder in the prevention of disease in neonatal Holstein calves. J Dairy Sci. 1993;76:831–836. doi: 10.3168/jds.S0022-0302(93)77408-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Quigley J.D., Strohbehn R.E., Kost C.J. Formulation of colostrum supplements, colostrums replacers and acquisition of passive immunity in neonatal calves. J Dairy Sci. 2001;84:2059–2065. doi: 10.3168/jds.S0022-0302(01)74650-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mee J.F., O'Farrell K.J., Reitsma P. Effect of a why protein concentrate used as a colostrum substitute or supplement on calf immunity, weight gain, and health. J Dairy Sci. 1996;79:886–889. doi: 10.3168/jds.S0022-0302(96)76437-8. [DOI] [PubMed] [Google Scholar]
- 91.Smith G.W., Foster D.M. Short Communication: absorption of protein and immunoglobulin G in calves fed a colostrum replacer. J Dairy Sci. 2007;90:2905–2908. doi: 10.3168/jds.2006-682. [DOI] [PubMed] [Google Scholar]
- 92.Jones C.M., James R.E., Quigley J.D. Influence of pooled colostrum or colostrum replacement on IgG and evaluation of animal plasma in milk replacer. J Dairy Sci. 2004;87:1806–1814. doi: 10.3168/jds.S0022-0302(04)73337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Foster D.M., Smith G.W., Sanner T.R. Serum IgG and total protein concentrations in dairy calves fed two colostrum replacement products. J Am Vet Med Assoc. 2006;229:1282–1285. doi: 10.2460/javma.229.8.1282. [DOI] [PubMed] [Google Scholar]
- 94.Etzel L.R., Strohbehn R.E., McVicker J.K. Development of an automated turbidimetric immunoassay for quantification of bovine serum immunoglobulin G. Am J Vet Res. 1997;58:1201–1205. [PubMed] [Google Scholar]
- 95.Tyler J.W., Hancock D.D., Parish S.M. Evaluation of 3 assays for failure of passive transfer in calves. J Vet Intern Med. 1996;10:304–307. doi: 10.1111/j.1939-1676.1996.tb02067.x. [DOI] [PubMed] [Google Scholar]
- 96.Pfeiffer N.E., McGuire T.C., Bendel R.B. Quantitation of bovine immunoglobulins: comparison of single radial immunodiffusion, zinc sulphate turbidity, serum electrophoresis, and refractometer methods. Am J Vet Res. 1977;38:693–698. [PubMed] [Google Scholar]
- 97.McVicker J.K., Rouse G.C., Fowler M.A. Evaluation of a lateral-flow immunoassay for use in monitoring passive transfer of immunoglobulins in calves. Am J Vet Res. 2002;63:247–250. doi: 10.2460/ajvr.2002.63.247. [DOI] [PubMed] [Google Scholar]
- 98.McBeath D.G., Penhale W.J., Logan E.F. An examination of the influence of husbandry on the plasma immunoglobulin level of the newborn calf, using a rapid refractometer test for assessing immunoglobulin content. Vet Rec. 1971;88:266–270. doi: 10.1136/vr.88.11.266. [DOI] [PubMed] [Google Scholar]
- 99.Calloway C.D., Tyler J.W., Tessman R.K. Comparison of refractometers and test endpoints in the measurement of serum protein concentration to assess passive transfer status in calves. J Am Vet Med Assoc. 2002;221:1605–1608. doi: 10.2460/javma.2002.221.1605. [DOI] [PubMed] [Google Scholar]
- 100.Wallace M.M., Jarvie B.D., Perkins N.R. A comparison of serum harvesting methods and type of refractometer for determining total solids to estimate failure of passive transfer in calves. Can Vet J. 2006;47:573–575. [PMC free article] [PubMed] [Google Scholar]


