Abstract
Fibrous dysplasia/McCune-Albright syndrome (FD/MAS) is a rare disorder of striking complexity. It arises from somatic, gain-of-function mutations in GNAS, leading to mosaic Gα s activation and inappropriate production of intracellular cyclic adenosine monophosphate (cAMP). The clinical phenotype is largely determined by the location and extent of affected tissues, and the pathophysiological effects of Gα s activation within these tissues. In bone, Gα s activation results in impaired differentiation of skeletal stem cells, leading to discrete skeletal lesions prone to fracture, deformity, and pain. Extraskeletal manifestations include a variable combination of hyperpigmented macules and hyperfunctioning endocrinopathies. Distinctive age-related changes in disease development has key effects on histologic, radiographic, and clinical features. FD/MAS thus presents along a uniquely broad clinical spectrum, and the resulting challenges in diagnosis and management can be difficult for clinicians. This review presents FD/MAS in the context of a mosaic disorder of Gα s activation, providing an intellectual framework within which to understand, evaluate, and treat this interesting disease. It includes a comprehensive summary of current understanding of FD/MAS pathogenesis, and a detailed discussion of clinical presentation and management. Critical areas of unmet need are highlighted, including discussion of key challenges and potential solutions to advance research and clinical care in FD/MAS.
Keywords: skeletal stem cells, metabolic bone disease, somatic mosaicism, fibroblast growth factor 23, precocious puberty, growth hormone excess
Graphical Abstract
Graphical Abstract.
Essential Points.
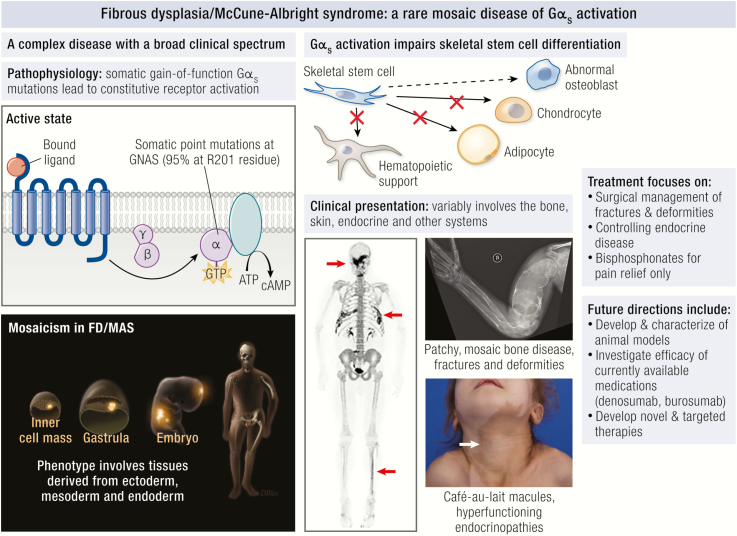
Fibrous dysplasia/McCune-Albright syndrome (FD/MAS) is a rare disorder arising from somatic activating mutations in GNAS, leading to a mosaic pattern of Gα s activation
The clinical phenotype in FD/MAS presents along a broad spectrum, involving a variable combination of hyperpigmented skin macules, hyperfunctioning endocrinopathies, and FD of bone
Gα s activation impairs differentiation of skeletal stem cells, leading to formation of expansile FD lesions prone to fracture and deformity, and presenting clinically with pain, functional impairment, and disability
Patients with FD have a distinctive age-related pattern of disease development: FD lesions become established in the first few years of life and expand during linear growth; final disease burden is established by early adulthood, after which the metabolic activity of FD lesions tends to decline
Complications of FD are more frequent and severe in patients with uncontrolled MAS endocrinopathies: Hypophosphatemia and hyperthyroidism increase the risk of deformities in weight-bearing bones, and growth hormone excess, which drives expansion of craniofacial FD
The development of effective medical therapies for FD is a critical area of unmet need; although reasonably effective treatments exist for most MAS endocrinopathies, no therapies have been shown to definitively improve bone quality or prevent the expansion of FD lesions
Fibrous dysplasia/McCune-Albright syndrome (FD/MAS) is a rare disorder of striking complexity. Somatic gain-of-function mutations lead to mosaic activation of Gα s, resulting in disease that may involve any part of the skeleton, and may be variably associated with cutaneous, endocrine, and other extraskeletal features. The combination of 2 or more classic features (FD of bone, café-au-lait skin macules, and/or associated hyperfunctioning endocrinopathies: gonadotropin-independent gonadal function, nonautoimmune hyperthyroidism, GH excess, neonatal hypercortisolism) is termed McCune-Albright syndrome (MAS) (1, 2). The resulting clinical presentation is remarkable for its uniquely broad spectrum. This complexity makes FD/MAS a compelling disorder with which to understand the role of Gα s and the interplay between systems affected by its activation. However, it also poses a unique challenge for clinicians, who seek a unified approach to a disorder that causes no 2 patients to look alike. In this review, we will provide an intellectual framework for understanding FD/MAS, in which the pathophysiology, natural history, and clinical management are determined by the tissue-specific role and distribution of Gα s signaling.
Etiology and Pathophysiology
Genetic etiology
FD/MAS results from missense mutations in the GNAS locus, located on chromosome 20q13.3 (3). This locus has a highly complex imprinted pattern of expression, with multiple alternate promoters giving rise to maternally, paternally, and biallelically expressed transcripts. Mutations associated with FD/MAS typically occur at exon 8, in which the arginine 201 is converted to either a histidine (R201H) or a cysteine (R201C). Rarely, other substitutions may occur (4), or other codons may be affected (5). GNAS mutations associated with FD/MAS are not inherited, and monozygotic twins discordant for the disease have been reported (6), consistent with a postzygotic mutational event (7). The precise timing at which the mutation occurs varies, but concomitant involvement of tissues derived from all 3 germ layers (endoderm, mesoderm, and ectoderm) in severely affected patients suggests that mutations are acquired at an early stage of development. Accordingly, the 2 most common mutations arise from aberrant methylation of the CpG dinucleotide in the R201 codon, suggesting that the mutational event may frequently take place during the active methylation phase in formation of the inner cell mass (8). This supports a model in which a disease-causing mutation acquired in a pluripotent stem cell has the potential to transmit to a broad and variable distribution of tissues. The clinical phenotype in FD/MAS therefore likely reflects the differential number, cell type, and viability of clones that arise from this mutated pluripotent cell. Epigenetic modifiers also likely contribute to phenotypic variability in FD/MAS. Random and asymmetric expression of Gα s alleles has been observed both in mutant and wild-type clonogenic osteoprogenitors (9), suggesting that mutant Gα s expression varies within and between affected tissues, potentially influencing the development and severity of clinical disease.
Molecular and cellular pathophysiology
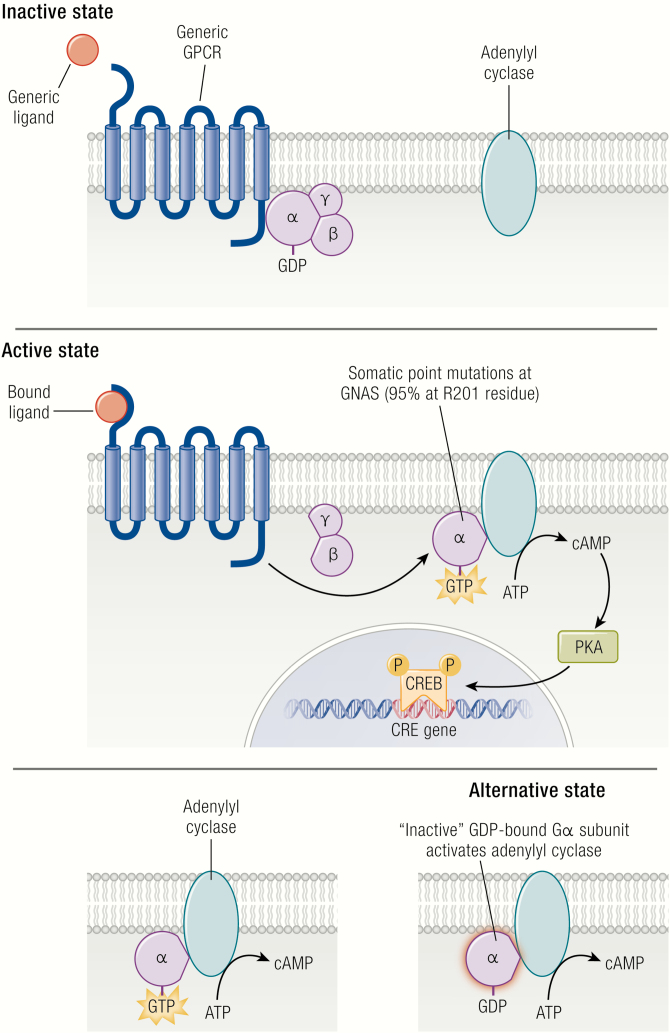
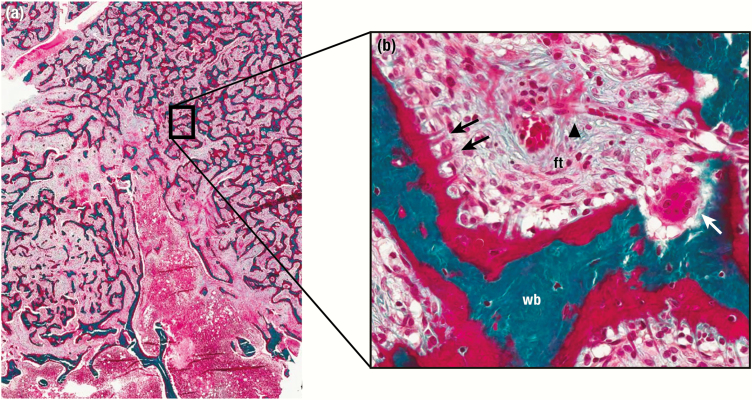
Mutations associated with FD/MAS are gain-of-function, and result from impaired intrinsic GTPase activity, leading to ligand-independent signaling and production of excess intracellular cyclic adenosine monophosphate (cAMP) (10) (Fig. 1, upper and middle panels). GDP-bound Gα s is inactive under normal physiologic conditions, however recent evidence suggests that the mutant GDP-bound Gα s interacts with adenylyl cyclase to generate cAMP, at least in the case of the R201C variant (Fig. 1, lower panel) (11). The relative contributions of activation of the mutant GDP-bound and GTP-bound receptor conformations are unknown, and further research is needed to verify this model, particularly with respect to the R201H variant. Constitutive Gα s signaling results in impaired differentiation of mutation-bearing osteoprogenitor cells, an observation supported by in vitro studies of cells isolated from FD lesions (12-14), and in normal human osteoprogenitors stably transduced with Gα sR201C (15). Proliferation of undifferentiated skeletal stem cells generates fibro-osseous tissue that expands into the marrow space, with loss of normal marrow features such as hematopoiesis and adipogenesis. Characteristic histopathologic features of FD reflect this impaired differentiation and function of osteogenic cells (16, 17) (Fig. 2). Discontinuous networks of trabeculae with woven bone result from aberrant osteoblast activity, likely through activation of Wnt/β-catenin signaling in osteoblast progenitors (13). Trabeculae are typically dense and irregularly shaped, resulting in their previous description as “Chinese characters” (Fig. 2A); however, in actuality there is little resemblance to the written Chinese language. Osteoclastogenesis is also a prominent feature and may result from local production of osteoclast-promoting factors such as interleukin-6 and receptor activator of nuclear kappa-B ligand (15, 18). Severe osteomalacic changes contribute to the structural instability of FD bone, and probably derive from an intrinsic mineralization defect as well as FD lesion production of the phosphaturic hormone fibroblast growth factor-23 (FGF23) (19).
Figure 1.
Gs G-protein-coupled signaling dysregulation in FD/MAS. In the inactive state, the αβγ-heterotrimer is bound to GDP. After ligand binding, the GTP-bound α-subunit dissociates from the βγ-complex and activates adenylyl cyclase, leading to production of intracellular cyclic AMP and activation of protein kinase A and other downstream signaling pathways. Activating GNAS mutations in FD/MAS results in loss of GTPase activity in the α-subunit, resulting in constitutive Gα s protein signaling. Alternatively, the mutant GDP-bound Gα s may also interact with adenylyl cyclase to generate cAMP. ATP indicates adenosine triphosphate; cAMP, cyclic adenosine monophosphate; CREB, cyclic adenosine monophosphate response element-binding protein; FD/MAS, fibrous dysplasia/McCune-Albright syndrome; GDP, guanosine diphosphate; GPCR, G-coupled protein receptor; GTP, guanosine triphosphate.
Figure 2.
Representative histological features of fibrous dysplasia (FD). A and B show low-power and high-power views of an FD lesion with classic dense, irregular trabeculae and marrow fibrosis (ft). Massive de novo bone formation is apparent throughout, including areas of woven bone (wb) with prominent unmineralized osteoid (o). Collagen fibers are perpendicularly oriented along forming bone surfaces, also termed Sharpey fibers (black arrows). An osteoclastic giant cell is actively resorbing an area of abnormal bone (white arrow). Note the presence of high vascularity with a red blood cell-filled vessel coursing through the lesion adjacent to an area of venous pooling (black arrowhead).
Mosaicism in FD/MAS is apparent even at the tissue level and is likely requisite for the formation of FD lesions. This has been demonstrated by genetic analysis of clones of individual cells derived from FD lesions in which mutation-positive and mutation-negative osteogenic cells are present. Further, when ectopic xenographs of patient-derived osteogenic cells are implanted in immunocompromised mice, both mutated and wild-type cells are required to generate ossicles with histologic features consistent with FD (12). The specific contributions of nonmutated cells to the establishment and progression of FD lesions, and the mechanism by which mutated skeletal stem cells co-opt the behavior of wild-type cells, remain important unanswered questions. In addition, it is possible that TRAP positive, osteoclast-like giant cells, which are a prominent feature of FD lesions, may play a contributory role in generating or promoting the expansion of FD lesions.
The effects of Gα s activation appear to be tissue specific and vary considerably in different organ systems. The downstream mechanisms that account for these differences are unknown but may relate to tissue-specific sensitivities to cAMP dysregulation. In addition, it is possible that certain cell types may not tolerate Gα s activation, leading to deselection of GNAS-mutation–bearing cells during embryogenesis.
Clinical Description
The original description of FD/MAS in 1936 included a “classic triad” of café-au-lait macules, precocious puberty, and FD (20). However, we now know that FD/MAS can involve a broad range and combination of systems, resulting in a diversity of phenotypes. Understanding the clinical presentation in FD/MAS requires an appreciation of how various disease features influence each other, and how the mosaicism underlying these features has unique and profound effects on clinical outcomes.
Fibrous dysplasia
The clinical presentation of FD varies extensively based on the location and amount of affected bone. Monostotic FD is likely far more common than polyostotic FD; however, reliable epidemiological data are lacking, in part because of the poor characterization and classification of monostotic fibro-osseous lesions (21). In cases of polyostotic FD or MAS, the diagnosis can often be made clinically based on typical radiographic features; however, isolated monostotic lesions generally require biopsy and molecular testing if diagnostic certainty is desired (22). FD lesions are most commonly found in the skull base and proximal femurs; however, any part or combination of the skeleton can be involved. The clinical sequelae of FD are region specific, with distinct manifestations involving the appendicular, craniofacial, and axial compartments of the skeleton.
Appendicular skeleton.
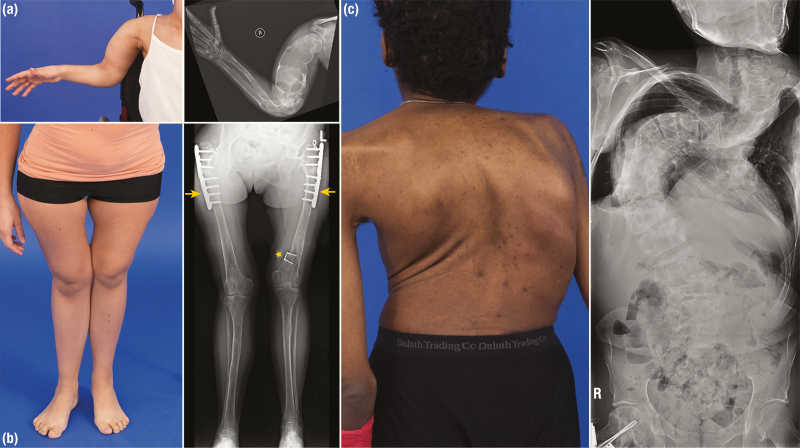
Complications of appendicular FD arise primarily due to bone fragility, resulting in fractures and deformation under weight-bearing forces (Fig. 3A and 3B). Patients typically present with pain or limp. The tension placed on the femoral neck classically results in coxa vara (“shepherd’s crook”) or, less commonly, valga deformities, which are key sources of morbidity and ambulation impairment (23). Deformities may also occur in the upper extremities, but typically result in less functional sequelae. Multiple factors may contribute to the development of deformities, including malalignment from fractures and surgeries, muscle weakness, and uncontrolled MAS-associated endocrinopathies. Secondary aneurysmal bone cysts may rarely arise in the appendicular skeleton, leading to rapid deformation with severe pain or fracture.
Figure 3.
Skeletal deformities and radiographic findings in patients with fibrous dysplasia (FD). A, Images of a patient with a bowing deformity of the proximal upper extremity. Radiographs reveal extensive FD involvement, including an expansile humeral lesion demonstrating severe cortical thinning and a characteristic “ground-glass” appearance. B, A patient with coxa vara deformity proximally (at the hip) and genu valgum deformity distally (at the knees). Note the leg length discrepancy with the resulting asymmetry of the knees apparent in the photograph. This patient’s radiographs demonstrate diffuse FD involvement of the lower extremities with cortical thinning and “ground-glass” radiolucency. Plate and nail implants have been placed in the bilateral proximal femurs to correct shepherd’s crook deformities (yellow arrows). Note the presence of an 8-plate initially implanted in the distal femoral epiphyses, which has migrated proximally during skeletal growth (yellow star). This implant was intended to treat leg length discrepancy by slowing growth in the left leg, which is unfortunately now shorter than the right. C, Posterior view of a patient with severe spinal curvature, resulting in shortening of the torso and asymmetry of the shoulders and scapulae. This patient’s radiographs demonstrate severe thoracic scoliosis with loss of lung volumes bilaterally. Reproduced with permission from Hartley I et al. (24).
Craniofacial skeleton.
Complications in the craniofacial skeleton arise because of FD’s tendency to expand, leading to asymmetry and functional impairment (Fig. 4). The typical presentation is a painless “bump” or subtle asymmetry that expands during childhood; however, asymptomatic lesions are often detected incidentally on dental radiographs, computed tomography (CT), or magnetic resonance imaging. Very rarely, the initial presentation may involve new-onset functional impairment, such as vision or hearing loss. Complications of craniofacial FD are highly region-specific. The skull base is the most commonly affected location and is typically asymptomatic; however, neurologic complications from skull-base deformities such as basilar invagination and Chiari I malformation have been reported (25). Facial asymmetry is common in patients with FD affecting the frontal, maxillary, or mandibular regions, particularly in those with unilateral involvement. Hearing loss may occur in association with temporal bone FD and is generally mild and nonprogressive (26). Vision loss is an uncommon but serious complication that may result from deformation of the optic canals, or in the setting of a secondary aneurysmal bone cyst. Vision and hearing loss both occur more frequently in patients with MAS-associated growth hormone (GH) excess (27, 28). Additional sequelae of craniofacial FD include chronic nasal congestion, hyposmia, and malocclusion (22, 29).
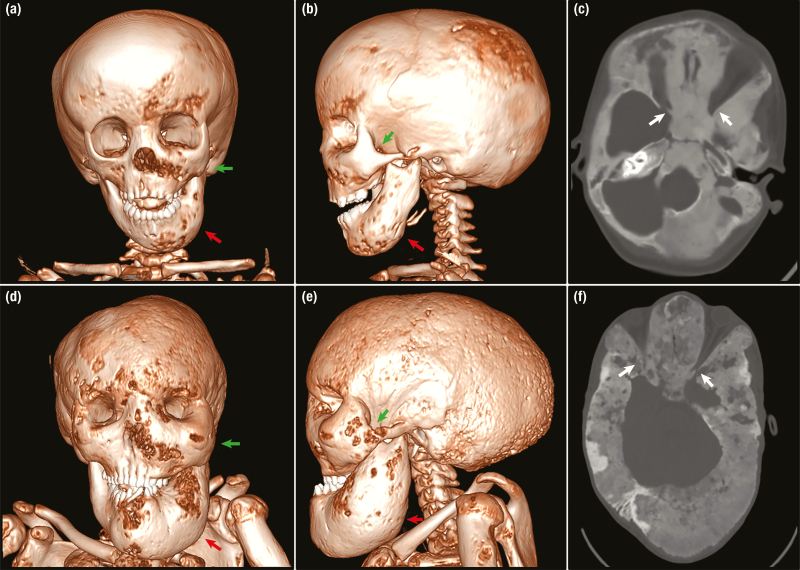
Figure 4.
Progression of craniofacial fibrous dysplasia (FD) in a patient with uncontrolled growth hormone excess. A, B, and C, Upper panels show computed tomography (CT) images at age 4 years. Note mild expansion of the left mandible (red arrows) and zygomatic bone (green arrows) visible on A and B, 3-dimensional (3D) reconstruction. C, An axial view demonstrates the characteristic homogenous. A patent optic canal surrounded by FD is visible on the right (white arrow). The left optic canal (not depicted) is also patent. D, E, and F lower panels show images from the same patient at age 17 after 13 additional years of uncontrolled growth hormone excess. D and E, On 3D reconstruction the left mandible has massively expanded (red arrows), leading to severe malocclusion and distorted dentition. The zygomatic bones are now expanded bilaterally (green arrows) with resulting orbital asymmetry. E, Note the severe macrocephaly with enlargement of the posterior cranium leading to a scaphocephalic appearance. F, An axial view demonstrates typical age-related changes with increased heterogeneity and areas of radiolucency. The left optic canal is visible and severely narrowed (white arrow), and the patient is unfortunately now blind.
Axial skeleton.
Scoliosis is a common complication of FD affecting the axial skeleton, and in severe cases may be progressive and rarely fatal (30, 31) (Fig. 3C). Risk factors for scoliosis progression include leg length discrepancy and uncontrolled endocrinopathies (30). Expansile rib lesions are prone to fractures and pain, and in rare cases may be associated with pleural effusions (32, 33).
Fibroblast growth factor-23–mediated disease.
FGF23 overproduction is an inherent feature of FD. Although most patients have elevated circulating levels of FGF23, increased cleavage of intact FGF23 to its inactive fragments often prevents the development of frank hypophosphatemia (34). The mechanism of FGF23 overproduction in FD is unknown but is presumably related to effects of Gα s activation in abnormally differentiated osteocytes (35) Because the degree of FGF23 overproduction is correlated with FD severity and tissue burden, frank hypophosphatemia occurs only in patients with substantial skeletal involvement (19, 36). Hypophosphatemia may wax and wane over time and may occur more frequently during periods of high phosphate requirement, such as phases of rapid linear growth in infancy and adolescence. Clinical sequelae of hypophosphatemia are substantial and include earlier and more frequent fractures (37), pain, poor surgical outcomes, and an increased propensity for deformities (25, 30). Rickets may be seen; however, it is important to note that even in the absence of frank metaphyseal changes, FD-related complications such as fractures and deformities may occur at an increased prevalence in patients with hypophosphatemia.
Endocrinological features
Gonadotropin-independent gonadal dysfunction.
Autonomous ovarian activation is the most common endocrinopathy in girls and women with MAS, affecting approximately 85% of patients in one large cohort (38). Ovarian Gα s activation results in recurrent estrogen-producing cysts. The typical presentation in infancy and childhood includes signs of estrogen exposure in a previously prepubertal girl, such as rapid breast development, growth acceleration, and vaginal discharge (38, 39). Serum estradiol levels are typically elevated with suppressed gonadotropins. Pelvic ultrasonography reveals unilateral or bilateral ovarian cysts, which may vary in size and be simple or complex, often accompanied by an enlarged uterus with an endometrial stripe (40) (Fig. 5C). Girls frequently present for care after cyst resolution, when declining estrogen levels result in shedding of the uterine lining and vaginal bleeding. Between episodes girls typically appear prepubertal, with appropriately low serum estrogen levels and normal pelvic ultrasonography findings, which may contribute to delayed diagnoses. The onset and frequency of cysts are variable, and cysts have been detected as early as in utero (41). Untreated peripheral precocious puberty may lead to bone age advancement with compromised adult stature and the development of secondary central precocious puberty (42). In adulthood, cysts frequently result in dysfunctional uterine bleeding, which may be severe, necessitating repeated blood transfusions and early hysterectomy (43, 44). The effects of MAS ovarian disease on fertility are unclear. Spontaneous pregnancies occur; in one series of 39 women, 43% had reduced fertility, likely in part related to frequent cysts that disrupt ovulatory cycles leading to delayed time to conception (44). Pregnancy does not appear to have a clear negative impact on skeletal disease (44).
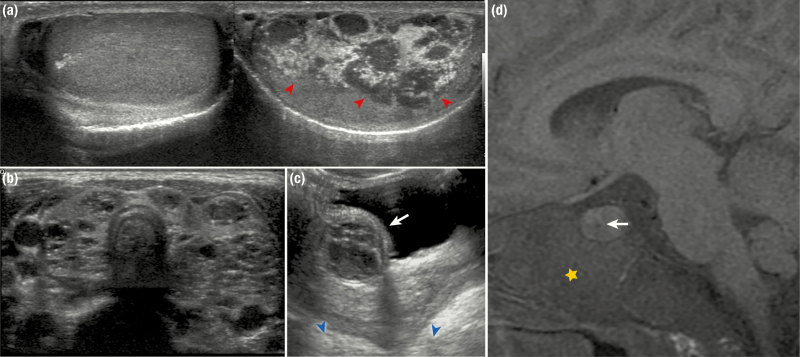
Figure 5.
Radiographic features of endocrine disease in patients with McCune-Albright syndrome. A, Testicular ultrasound images show extensive right-sided involvement with mixed radiolucent and radio-opaque lesions involving most of the testis (red arrowheads). The left side is unaffected and demonstrates a normal homogeneous echotexture. B, A thyroid ultrasound shows diffuse involvement with heterogeneous mixed radiolucent and radio-opaque lesions, resulting in a spongiform appearance. C, A pelvic ultrasound from a 2-year-old girl shows a complex ovarian cyst (white arrow). Note the enlarged, mature-appearing uterus (blue arrowheads) that has grown under the influence of hyperestrogenism. D, An MR image from a patient with growth hormone excess shows pituitary enlargement and a hypoechoic area (white arrow) consistent with an adenoma. Note the expanded skull base involved with fibrous dysplasia (yellow star).
Testicular involvement similarly affects approximately 85% of boys and men with MAS (45). Patients can present with normal testicular volume, or unilateral or bilateral macro-orchidism, and variable ultrasonographic abnormalities, including discrete lesions, diffuse heterogeneity, and microlithiasis (45) (Fig. 5A). Histopathology shows Leydig and Sertoli cell hyperplasia. Unlike MAS ovarian involvement, which appears to be necessarily associated with hyperestrogenism, autonomous testosterone production is detectable in only 15% of patients with testicular disease. In boys this presents with progressive signs of premature androgen excess, including pubic and axillary hair, body odor, penile growth, and androgen-associated behavioral changes.
Thyroid disease.
Thyroid involvement is reported in approximately two-thirds of patients with MAS, resulting in frank hyperthyroidism in around half of affected patients (38, 46). Thyrotoxicosis results from cAMP-mediated increased deiodinase activity and overproduction of triiodothyronine. Ultrasonographic features are variable and include patchy areas of heterogeneity along with discrete cystic nodules (46, 47) (Fig. 5B). The clinical presentation includes typical signs of hyperthyroidism such as tachycardia, growth acceleration, hyperactivity, and sleep disturbances. Thyroid storm has been reported, including in conjunction with anesthesia for orthopedic procedures (48, 49). Like FGF23 overproduction, hyperthyroidism in MAS has been associated with increased skeletal morbidity and progressive deformities, likely due to detrimental effects of excess thyroid hormone on bone metabolism (25, 30).
Pituitary disease.
Pituitary involvement is uncommon in MAS, affecting 10% to 15% of patients (27, 50). Approximately 85% of the patients with pituitary disease have both GH and prolactin excess, which is due and abundance of cosecreting mammosomatotroph cells (51) (Fig. 5D). GH excess may be frank, with clearly elevated GH and insulin-like growth factor-1 levels, or subtle, evident only by altered secretion dynamics on glucose tolerance testing and overnight GH sampling. GH excess fuels FD expansion, particularly in the craniofacial region, which can lead to significant morbidity (Fig. 4). Untreated GH excess has been associated with vision loss (28), hearing loss (26), macrocephaly (28, 52), facial deformity (53), and poor surgical outcomes (53). Patients are also subject to more common effects of GH excess, including diabetes, heart disease including cardiomegaly aortic root dilatation and aortic valvular insufficiency, and secondary pituitary hormone deficiencies. Prolactinemia in MAS is typically mild and asymptomatic, but in rare cases may result in hypogonadism and galactorrhea (50).
Adrenal disease.
Hypercortisolism is the rarest endocrine feature of MAS, occurring in less than 5% of patients (38, 54). Adrenocorticotropic hormone–independent adrenal Gα s activation results in a characteristic diffuse and nodular infiltration of the adrenal glands, leading to hypercortisolism that can range from mild to severe and life threatening. Disease presents exclusively within the first year of life and may develop as early as in utero, likely reflecting involvement of fetal adrenal tissue (54, 55). Infants may present with low birth weight, failure to thrive, facial plethora, or hirsutism. Cutaneous features such as violaceous striae are typically absent in infants (56). Neonatal hypercortisolism is associated with high mortality from secondary infections, which emphasizes the need for prophylaxis against opportunistic infections, and other complications, particularly in patients with delayed diagnoses.
Dermatologic features
Café-au-lait macules affect two-thirds of patients with MAS, and are often the earliest presenting feature, visible at or shortly after birth (38). An astute clinician may recognize these as an early clue to the diagnosis; however, their significance is often appreciated only in retrospect, after additional features of FD/MAS have become apparent. Café-au-lait macules arise as a result of cutaneous Gα s activation and constitutive melanocyte-stimulating hormone receptor signaling, leading to local increased melanin production in areas of affected skin. As such, skin lesions tend to follow a characteristic distribution reminiscent of embryonic cell migration patterns (Fig. 6). Macules often occur or reflect along the midline of the body (referred to as “respecting” the midline). Common locations include the chest, neck, and superior portion of the intergluteal cleft; however, any region may be affected. Borders tend to be jagged, resembling the “coast of Maine” (in contrast to the smooth-bordered “coast of California” lesions seen in neurofibromatosis). However, like FD, skin macules present along a broad spectrum, and many exceptions to these patterns exist. Mucosal pigmentation has also been reported involving the lips and intraoral and vaginal mucosa (57).
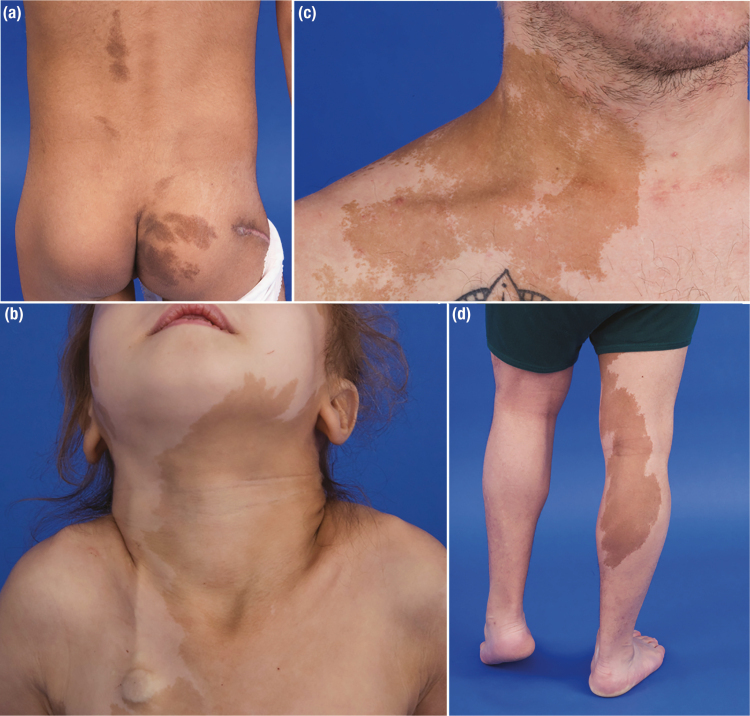
Figure 6.
Typical café-au-lait macules in patients with McCune-Albright syndrome. A, Lesions in a young child involving the lower back and buttocks approach but do not cross the midline of the body. Note the relatively even borders on the back lesions, whereas the buttock lesions show irregular borders. An osteotomy scar related to a femoral fixation procedure is visible on the right lateral buttock. B, A lesion involving the neck and shoulder of a young adult terminates sharply at the midline, with serpiginous borders involving the lateral aspects. C, An extensive lesion involving the neck and upper trunk of a young child extends past the midline. A port visible in the right lower chest was placed to facilitate intravenous access during a period of neonatal hypercortisolism. D, A lower extremity lesion in an adolescent extends downward from the upper medial thigh to the calf area.
Gastrointestinal involvement
GNAS is a known driver mutation for multiple gastrointestinal (GI) abnormalities in the general population (58-61); it is therefore not surprising that MAS is associated with a broad spectrum of GI involvement. Reported hepatobiliary abnormalities include hepatocellular adenomas (62, 63), inflammatory adenomas (62, 63), choledochal cysts (63), neonatal cholestasis (64), and hepatoblastoma (64). Recent cohort studies have identified a high prevalence of intraductal papillary mucinous neoplasms (IPMNs), a potentially precancerous pancreatic cyst, affecting up to 50% of patients with MAS (63, 65). The malignant potential of these cysts is discussed further in ‘’Malignancies.’’ Although most patients in these series were asymptomatic, some reported associated functional deficits, including acute and chronic pancreatitis and diabetes. A variety of upper GI pathology has been identified in patients with MAS, including gastric heterotopia/metaplasia (66), gastric hyperplastic polyps (66), fundic gland polyps (66), and hamartomatous polyps (66, 67).
Intramuscular myxomas
An association between FD and intramuscular myxomas was first noted in 1926 (68), and later characterized by Mazabraud and colleagues in 1967 (69). The constellation of FD and soft tissue myxomas has since been widely referred to as Mazabraud syndrome. However, given the multisystemic nature of Gα s activation, it is more pathophysiologically consistent to consider these myxomas as a feature of MAS rather than a distinct syndrome. In a retrospective multicenter review that included 1446 patients with a diagnosis of FD, myxomas were present in 32 patients, for a prevalence of 2.2% (70). Most myxomas present as a painless, palpable mass that is firm in consistency and partially movable. Uncommonly, lesions may progressively enlarge causing pain and functional impairment, particularly when located near joints or weight-bearing structures such as the buttocks. Myxomas can be visualized radiographically on ultrasound, CT, or magnetic resonance imaging, where they appear as distinct hypoechoic or low-density lesions with well-demarcated borders (71). On histopathology they present as well-encapsulated, hypovascular fibrous lesions composed of relatively hypocellular mucoid material (72). Most reported patients with myxomas have polyostotic as opposed to monostotic FD, likely reflecting a larger tissue distribution of mutated GNAS in these individuals (70). Case reports have associated the presence of myxomas with malignant transformation of FD lesions (73-76); however, this was not noted in larger series (70) and likely represents reporting bias.
Malignancies
G-protein dysregulation is a frequent contributor to tumorigenesis, and recent studies have identified that up to 4.2% of all cancers in the general population harbor gain-of-function GNAS mutations (77). These mutations are also commonly associated with sporadic, hyperfunctioning pituitary tumors (28%) and thyroid adenomas (5%) (10), which is unsurprising given their causative role in the development of MAS-related endocrinopathies.
Pancreatic tumors.
GNAS plays a key role in driving pancreatic tumorigenesis, with 66% of IPMNs harboring gain-of-function GNAS mutations (60, 61). IPMNs occur in up to 2% of the general population and are considered a precursor lesion to pancreatic adenocarcinoma (78). GNAS mutations have been identified as early driver mutations for IPMN development, particularly in the setting of concurrent KRAS mutations (79, 80). IPMNs occur in up to 50% in patients with MAS; however, pancreatic adenocarcinoma appears to be a rare development in this population, with only 1 reported case (81). It is unclear whether this reflects an inherently low malignant potential of pancreatic tumorigenesis in MAS, or whether the transformation of Gα s-associated IPMNs to invasive adenocarcinomas may be less common than previously thought (82).
Breast cancer.
Breast cancer is another malignancy that has been associated with MAS. In 1 series combining Dutch and US cohorts, women with MAS had a 3.5- to 4-fold increased risk of breast cancer compared to the general population (83). Cancers developed at the notably young age of 36 to 46 years; however, outcomes were favorable, with 100% survival and no cases of recurrence or distal metastases. Thoracic FD was identified as a risk factor, and GNAS mutations were identified in affected tissues, pointing to a potentially causative role for Gα s activation. Women with breast cancer had a high prevalence of MAS-associated precocious puberty, suggesting that, as in the general population, increased estrogen exposure in MAS may play a role in tumorigenesis. Results of this study led to the recommendations to initiate breast cancer screening in MAS at an earlier age compared to the general population, that is, at age 40 years both for the United States and the Netherlands.
Skeletal malignancies.
The transformation of FD into bone cancers has been well documented, including osteosarcoma, chondrosarcoma, fibrous histiocytoma, and others (84-87). Malignant transformation typically presents with new expansion and/or focal pain in a previously quiescent lesion, and radiographs may demonstrate expansion of the lesion through the cortex. Determining the prevalence of malignant transformation in FD is difficult because of the absence of rigorous epidemiologic studies. In the National Institutes of Health (NIH) cohort of 250 patients, 2 cases of osteosarcoma have been identified for a prevalence of less than 1%. The abandoned practice of therapeutic external beam radiation was clearly demonstrated to be a risk factor for malignant transformation as was seen in a large retrospective review of the Mayo Clinic data (85). An additional risk factor may be GH excess, both as NIH patients and several published cases report GH excess in association with malignant transformation (88-90). Pituitary irradiation for treatment of GH excess has been associated with sarcomatous transformation of skull base FD in 2 patients (88, 90), consistent with the tumorigenic effects of ionizing radiation.
Other malignancies.
Additional rarely reported tumors in MAS patients include testicular cancer (45), thyroid cancer (91, 92), ovarian virilizing sclerosing-stromal tumor (93), ovarian epithelial tumor (94), and hepatoblastoma (64). Although additional epidemiologic studies are clearly needed, the existing evidence points to a relatively low prevalence of malignancies associated with MAS, despite a potentially large and longstanding tissue burden of Gα s activation in many patients. This may reflect relatively weak prototumorigenic effects of GNAS mutations, which may require the presence of coalterations in other oncogenic pathways to produce clinical disease. It is also possible that (outside IPMN development, where GNAS is known to be an early driver mutation), Gα s mutations may occur as late events in already dedifferentiated cancers, making them less likely to be associated with malignancies in the setting of congenital mutations. More speculative is the possibility of protective mechanisms in patients with MAS, potentially related to apoptosis or senescence in mutation-bearing cells. This is discussed further in “Clinical Description,’’ which addresses age-related changes in disease activity.
Quality of life
Quality of life in FD/MAS has been studied in several large European and North American cohorts (95-97), all of which reported significant impairments in measures of physical function, bodily pain, and general health, with mental health and emotional domains within general population norms. A Dutch cohort reported impairments in vitality and social function (95); however, these domains were not different from the general population in the North American cohort (96). Impairments in quality of life indices were associated with psychosocial factors, including negative patient illness perceptions (98) and use of passive coping strategies (99). Total skeletal disease burden was negatively correlated with quality of life; however, interestingly, bone pain did not show a direct relationship with quality of life indices (95).
Age-Related Changes
FD/MAS is characterized by distinct age-related features that affect nearly every aspect of the disease, from molecular and cellular activity to long-term clinical outcomes. Defining these changes is central to understanding systemic and local effects of Gα s activation, and, importantly, provides a critical perspective in developing effective approaches to clinical evaluation, monitoring, and treatment.
Establishment of fibrous dysplasia burden
The establishment of FD follows a characteristic and predictable time course. Although GNAS mutations are acquired early in embryogenesis, skeletal development appears to occur relatively normally in utero, with no frank clinical signs of FD present at birth. FD lesions become apparent over the first several years of life and expand during childhood and adolescence. One study of patients who underwent serial scintigraphy scans showed that FD burden was established in a region-specific pattern, with 90% of craniofacial FD present by age 3.4 years, 90% of extremity FD by 13.7 years, and 90% of axial FD by 15.5 years (100). Approximately 90% of future clinically significant FD was apparent in some form by age 5 years (100). These data support a course in which FD burden is established at a young age, roughly corresponding to the period during which patients undergo skeletal growth. The appearance of new lesions or expansion of existing lesions in adulthood is atypical with the natural history of FD and should raise suspicions for secondary processes.
Age-related clinical and radiographic features of fibrous dysplasia
Clinical sequelae in FD/MAS also reflect age-related patterns. Fractures peak around age 6 to 10 years before declining steadily in adulthood (37); this is particularly true of femoral fractures, which decline precipitously after adolescence (37). When it occurs, significantly impaired ambulation is also established early. In 1 large series the median age for initiating assistive devices was approximately 6 years, with 92% of assistive devices initiated by age 17 years (100).
Age-related changes are also apparent in the radiographic appearance of FD (71). During infancy FD is typically heterogeneous with streak-like features on X-ray; however, by school-age it has transitioned to the classic, homogeneous “ground-glass” appearance. In adulthood, lesions again become heterogeneous, often with sclerotic areas along the borders (Fig. 7A, 7B, and 7C). During childhood, craniofacial FD appears as homogenous “ground-glass” lesions on CT, but by adolescence lesions begin to develop progressive heterogeneity, with focal lucent areas reminiscent of cyst-like structures (Fig. 4C and 4F).
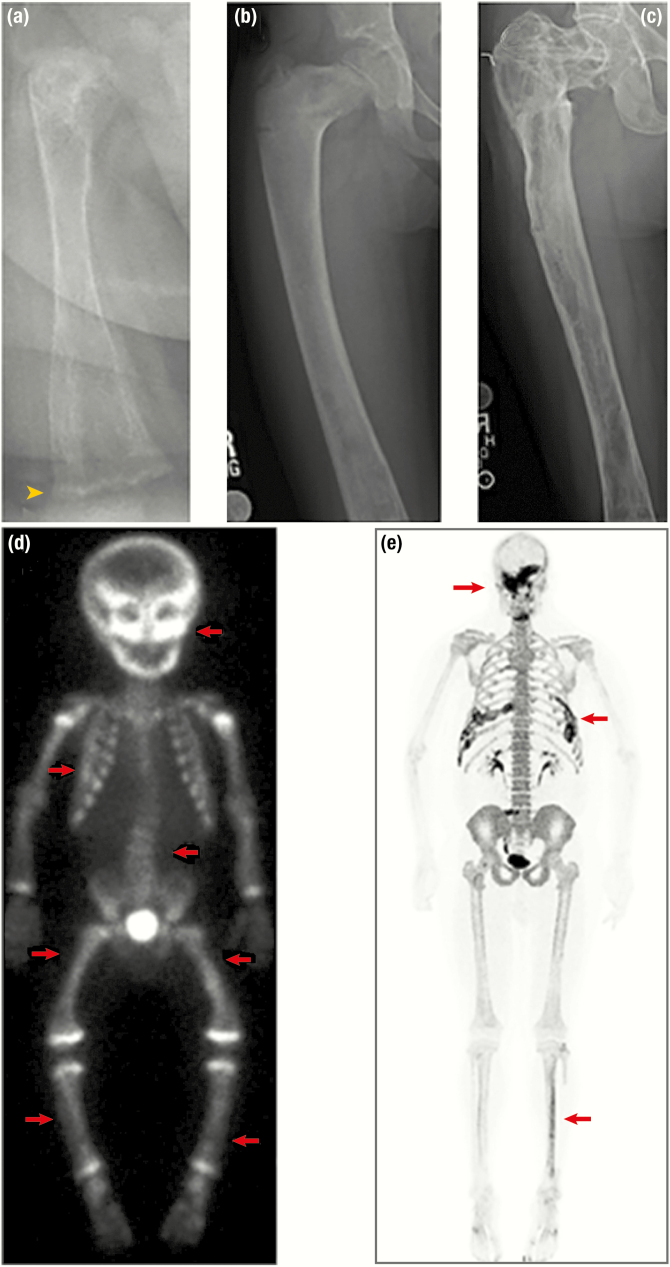
Figure 7.
Radiographic features of fibrous dysplasia (FD). A, B, and C, Upper panels demonstrate age-related radiographic changes in 3 patients with diffuse femoral involvement. A, In a 6-month-old patient, FD appears heterogeneous with streak-like features. Note the irregular metaphyses resulting from uncontrolled FGF23-mediated hypophosphatemia (yellow arrowhead). B, Radiograph from a 6-year-old demonstrates the classic “ground-glass” homogeneity. C, In a 31-year-old, FD again appears heterogeneous, with sclerotic areas interspersed with areas of radiolucency. D and E, Lower panels depict nuclear medicine scan images used to evaluate total skeletal FD burden. D, Technetium-99 scintigraphy scan in a young child with near panostotic disease shows increased tracer uptake in most of the skeleton, including the skull, spine, and long bones (red arrows). Note the symmetric increased uptake at the metaphyses in this growing child. E, 18F-NaF PET/CT scan in an adult with mild disease shows tracer uptake in areas of FD involving the skull, ribs, and left tibia (red arrows). Note the superior resolution and anatomical characterization of 18F-NaF in comparison to technetium.
Age-related biochemical and histologic features of fibrous dysplasia
Evaluation of biochemical markers can provide insight into the skeletal processes underlying age-related clinical and radiographic changes. Bone turnover markers increase in early childhood while FD lesions are established and begin a steady decline through adolescence and adulthood (101). A similar pattern holds true for receptor activator of nuclear factor kappa-Β ligand (RANKL) and FGF23 production (101). In vitro studies support this pattern of activity, with FD lesions from older individuals demonstrating the recurrence of histological features of normal bone with restoration of hematopoiesis, and decreased prevalence of mutated cells (102, 103).
Taken together, these clinical, radiographic, biochemical, and histological findings support a potential model in which FD lesions increase in childhood as a result of a greater proliferation of mutation-bearing stromal cells relative to unaffected cells. After final disease burden is established, the metabolic activity of FD lesions decreases over time, potentially because of a shortened life span of mutated stromal cells through apoptosis or senescence. This decrease in mutated cell proliferation allows the proportion of nonmutated stromal cells within FD lesions to increase over time, corresponding to improvements in histology, decreased biochemical bone turnover, changes in radiographic appearance, and clinical improvements. If this model is accurate, it has important implications for potential therapeutic strategies. Treatments designed to prevent the development or expansion of FD lesions would need to be targeted to childhood and initiated soon after FD development becomes apparent. In patients with established disease, a potential strategy may be to harness the natural decrease in FD activity by promoting proliferation and ultimately apoptosis of mutated stromal cells, with the goal of shortening the time during which lesions are metabolically active.
Bone pain and age-related changes
Bone pain in FD is common and poorly understood. In 1 large, cross-sectional study, 80% of adults and 50% of children reported the presence of bone pain, suggesting a tendency for pain to increase with age (104). Interestingly, neither the presence nor severity of pain was associated with total disease burden (104) or with serum bone turnover markers (101, 105). These findings suggest that the pathophysiology of FD pain is distinct from other skeletal complications and is not directly related to increased skeletal activity or stromal cell proliferation. The potential contribution of neuropathic or other extraskeletal elements to FD-related bone is an important unmet research need.
Age-related features of endocrine disease
Unlike FD, which follows a waxing and waning pattern of activity, MAS endocrinopathies appear persistent across the lifespan and do not improve over time. However, endocrinopathies manifest at variable times and may have profound effects on growth and development; thus, they have inherent age-related clinical implications. The timing of disease establishment has been less well elucidated for endocrinopathies in comparison to FD; however, most endocrinopathies appear to present in childhood before age 10 years (personal observations, A.M.B. and M.T.C.). GH excess and hyperthyroidism may infrequently become apparent in adulthood; however, in most cases, these patients likely had longstanding subclinical disease that could have been detected in childhood through careful evaluation. Linear growth changes are a hallmark feature of MAS endocrinopathies in children, and untreated disease may result in bone age advancement and disabling short stature. However, interpretation of linear growth in MAS is complex and affected by multiple variables. Growth acceleration is common in early precocious puberty, hyperthyroidism, and GH excess, whereas growth may be impaired by advanced puberty, hypophosphatemia, and skeletal deformities. Behavior changes are also common manifestations of endocrinopathies in children; these may also be affected by bone pain.
Model Systems
The ideal model system in FD/MAS would reflect all critical disease-specific and clinically relevant aspects, such as mosaicism, multisystem involvement (bone and endocrine), and age-related changes. Realistically it may be impractical for any single model to encompass all these features, and multiple models may be necessary to investigate various aspects of pathophysiology, and to develop and test therapeutics. The following sections will discuss the benefits and limitations of currently available models.
In vitro models
Primary cultures from patient tissue.
Attempts to isolate mutation-bearing stromal cells from patient tissue highlight the essential mosaic nature of FD lesions. In one study, single cell suspensions isolated from FD tissue and plated at low density led to colony-forming units that were a mixture of mutated and wild-type stromal cells (12). Further, in an established in vivo ossicle model, when clones of mutant, wild-type, or mixed mutant + wild-type cells implanted in immunocompromised mice, FD-like ossicles developed only in mice that received a combination of mutant + wild-type clones (12). This suggests that mutant GNAS-bearing stromal cells are unable to propagate clonally in the absence of wild-type cells, supporting a critical, thus far unelucidated contribution of wild-type stromal cells to FD lesion formation. Mixed cultures can also be useful for evaluating FD cell expression profiles and behavior, evidenced by studies of increased RANKL production in FD cells and osteoclastogenic induction in cocultured monocytes, consistent with the high levels of osteoclastogenesis present in FD tissue (106). Evaluation of specific transcriptional targets in these mixed cultures is also possible, as shown in studies demonstrating upregulated Wnt/β-catenin signaling in patient-derived tissue and cell cultures (13). However, the degree of heterogeneity seen in FD lesions, which are an admixture mutant and wild-type cells, must be considered and presents a challenge in interpreting data and developing informative models (102). One approach to overcoming this limitation has been to generate mixtures of homogeneous, clonally derived strains both of wild-type and GNAS-mutated stromal cells from individual FD lesions. This approach demonstrated increased basal and induced interleukin-6 (IL-6) production in clonally derived mutation-bearing stromal cells compared to cognate wild-type cells (18, 107), suggesting that Gα s activation specific to mutant cells (as opposed to wild-type) is a source of osteoclast-promoting factors in FD. Recent evidence that demonstrated osteoclasts have paracrine effects on osteogenic cells via osteoclast-produced vesicular RANK raise the question of whether osteoclasts, which are so prevalent in active FD lesions, may play a role in FD lesion formation and/or expansion (108).
Transfected cells.
Targeting activating GNAS mutations to normal human stromal cells is a strategy that limits the variability found in patient-derived cells and more easily facilitates investigation of molecular pathways downstream from Gα s activation. One study used lentiviral vectors to engineer stably transfect normal human skeletal progenitor cells with R201C mutations. Transfected cells demonstrated enhanced cAMP production consistent with constitutive Gα s activation (15). These cells displayed altered osteogenic differentiation in vitro and, like findings in patient-derived cells, showed dramatic upregulation of the osteoclast promotor RANKL. This model also identified upregulation of several phosphodiesterase isoforms in transfected cells that could potentially inform development of therapeutic targets (15).
Animal models
Transplanted models.
Cellular models have been used to generate FD-like tissue (ossicles) ex vivo through transplantation of patient-derived stromal cells into immunocompromised mice (12). Subcutical transplants of clonally derived mutant and wild-type cells resulted in formation of ossicles that recapitulated key histologic aspects of FD, including abnormal bone tissue devoid of adipocytes and hematopoietic marrow. Transplantation of stromal cells stably transfected with R201C mutations produced similar findings (15). As mentioned above, it is important to note that in experiments of patient-derived cells, a mixture both of mutant and wild-type cells was required for FD-like ossicle formation; wild-type cells alone generated ossicles resembling normal bone, whereas transplantation of only mutant cells resulted in transplant loss and no ossicle. This model provides experimental evidence for the hypothesis that GNAS mutation-bearing cells are less viable than wild-type cells and necessitate mosaicism for survival. Additional advantages of this model include the potential to investigate Gα s activation effects on wild-type cells. As such, this model may prove useful in studying FD lesion expansion and in testing therapies to prevent lesion growth. However, this model has important limitations inherent to use of patient-derived tissue, including availability and adequacy of samples, the growth restriction typical of most GNAS-mutated colonies, and variability in mutational loads between samples. Additional limitations include the inability to account for developmental features of FD and nonskeletal features associated with MAS.
Germline models.
Germline animal models offer practical advantages that bypass some of the limitations posed by cell culture-based methods. Saggio et al used lentiviral constructs to generate transgenic mice that constitutively express the R201C mutation found in human disease in all tissues, resulting in inherited, panostotic skeletal disease that histopathologically replicates FD (109). Like human disease, skeletal lesions in these mice developed postnatally, with normal development and cell differentiation during the embryonic and neonatal periods. Lesions developed through a distinct sequence of histopathological stages, including a primary phase of increased bone formation and a secondary remodeling phase with marked narrowing of the marrow cavity. The tertiary phase, which most resembled human FD, was not established until mice were well into adulthood at age 1 year or older; the equivalent of approximately 50 years in a human. Although this model demonstrates a developmental context to disease expression in FD, the late onset of disease is markedly different from the human phenotype, in which lesions develop in early childhood. It is possible that differences in the relative number of cell divisions in humans and mice may confound the ability of murine models to replicate this age-related phenotype. Interestingly, transgenic germline expression of R201C targeted to mature osteoblasts by a 2.3 kb Col1a1 promoter resulted in mice with progressive high bone mass without features of FD, suggesting that Gα s activation involving less-differentiated skeletal progenitors is required for FD lesion formation (110). By modeling an age-related pattern of development, transgenic germline models may prove useful for investigating the pathogenesis of FD lesion formation and expansion, and to test preventive therapies. Further work is needed to determine whether these models reproduce the multisystem involvement characteristic of MAS. The lack of embryonic lethality in these models indicates that key pathogenic aspects of human disease, including those related to obligate mosaicism, may not be captured in these systems and require other methods of investigation.
Inducible models.
Inducible animal models offer the ability to modify the timing of Gα s activation, providing another approach to replicating developmental patterns of disease expression. The first inducible model of FD was developed through use of a G-coupled protein receptor (Rs1) engineered to constitutively activate Gα s signaling in osteoblasts (111, 112). Constitutive Rs1 expression led to dramatically increased trabecular bone mass with histopathologic features reminiscent of FD; however, the presentation varied widely depending on the timing of induction, with delayed expression leading to a progressively attenuated phenotype, and no skeletal abnormalities with induction after age 4 weeks (111, 112). Withdrawal of tetracycline-induced Rs1 expression in adult mice led to a marked reversion of skeletal disease (112). This important finding supports 3 important hypotheses: 1) There is a critical postnatal window during which osteoblasts respond to Gα s activation, consistent with the age-related phenotype observed in human disease; 2) ongoing Gα s activation may be required to maintain a disease state in FD, and 3) if a therapy could “turn off” mutant Gαs activation, lesion regression could take place.
Two recently developed models show promise in recapitulating FD both pathogenically and phenotypically. Zhao et al created a conditional doxycycline-inducible model expressing the human R201C mutation in skeletal stem cells under a Prrx1-Cre promoter, resulting in rapid development of skeletal lesions both in embryonic and postnatal mice (113). Like in human disease, conditional mutant Gα s expression resulted in increased cAMP production and proliferation of skeletal progenitor cells without differentiation into mature osteoblasts. The resulting skeletal lesions demonstrated multiple histopathologic features of FD, including abnormal woven bone, increased RANKL expression, and prominent osteoclastogenesis. Consistent with the tissue-specific distribution of Cre-recombinase expression, lesions developed exclusively in the long bones and calvarium, locations frequently associated with morbidity in human disease. The presence and degree of mosaicism within skeletal lesions in this model is unclear; however, the variable Cre-recombinase (and therefore mutant Gα s) expression in the skull offers a potential opportunity to investigate the interface between mutant and wild-type cells. Like the Rs1 FD mouse, doxycycline withdrawal resulted in resolution of skeletal lesions, adding support for Gα s inhibition as a potential treatment strategy.
Khan and colleagues concurrently published a model in which the human R201H mutation was conditionally knocked in to the endogenous mouse GNAS locus (114). Pre-embryonic induction targeted to mouse oocytes resulted in embryonic lethality; however, expression targeted to osteochondral progenitor cells, early osteoblasts, and bone marrow stromal cells under a Prrx1-Cre promoter resulted in rapid formation of severe skeletal lesions, which like FD were characterized by increased trabecular volume and loss of marrow space. Wnt/β-catenin signaling was increased in skeletal tissue, consistent with findings in patient-derived tissues and cells (13), and the pathogenic role of Wnt/β-catenin was further confirmed by rescue of the FD phenotype in mice missing 1 copy of LRP6, a Wnt coreceptor gene. An inducible mosaic state was created by crossing R201H knock-in mice with a Sox9-CreER line, leading to selective mutant Gα s expression in bone marrow stromal cells and the development of a similar but attenuated skeletal phenotype. The spatial and temporal control of Gα s activation offered by this model is particularly useful in investigating Gα s’s role in skeletal formation and differentiation. For example, further work in this system demonstrated Gα s regulation of intramembranous ossification through Hedgehog and Wnt1/β-catenin, and reduced cartilage dissolution associated with Gα s activation in cranial bones (115). This knock-in approach could also potentially be targeted to extraskeletal tissues, providing an opportunity to investigate multisystemic involvement in MAS.
Treatment
Clinical management of fibrous dysplasia
Surgical management.
Surgery is the mainstay approach to treatment for FD. Techniques must be highly individualized, tailored both to the area of the affected skeleton and the specific patient characteristics. The timing of surgical intervention is a critical aspect of decision making that cannot be overemphasized. Skeletal complications characteristically become apparent in childhood; however, FD tends to be more metabolically active during this period, and surgical techniques appropriate for use in a growing skeleton are limited. Together these factors conspire to produce poorer surgical outcomes and contribute to long-term disability.
Appendicular skeleton.
Surgical management of appendicular FD focuses on mechanical stabilization with correction and prevention of fractures and deformities. FD involving the upper extremities and tibias can generally be approached conservatively, with intervention needed only in cases of acute or recurrent fractures, severe deformity, or ongoing pain with weight-bearing. The proximal femur is a particularly vulnerable location and poses one of the most challenging clinical management dilemmas in FD/MAS. Deformity in this area is common, and surgery should be considered at an early stage to avoid progression to a clinically significant degree. Femoral involvement is most common in the proximal region, but it may extend to any portion of the shaft. The most common finding is the classical coxa vara (“shepherd’s crook”) deformity, but coxa valga is also possible. There is no consensus on how to manage FD in the proximal femur. Plate and screw devices may be effective when anchored to healthy cortical bone; however, in cases in which FD extends to the femoral shaft this typically leads to inadequate fixation (116). Intramedullary rods are often effective at providing stability to the femoral shaft; however, they do not provide adequate support for the neck shaft angle. In addition, there is extremely limited availability of small surgical devices appropriate for pediatric use (116). Bone grafting procedures have been proposed as an alternative technique; however, a large retrospective analysis of femoral grafts showed only 50% graft survival over a 15-year period, with poorer outcomes in patients younger than 18 years (117). An analysis of allogenic cortical strut grafts showed similar results, with satisfactory outcomes in patients with limited disease, but decreased graft survival in patients with previous fractures and more extensive FD involvement (118). In general, reasonable proximal femoral stabilization can often be achieved in mature patients who have completed or nearly completed linear growth by tailoring these existing methods to the specific lesion of interest (119). However, surgical outcomes are considerably poorer in young children, which may contribute significantly to long-term morbidity and functional impairment. Treatment should be individualized to fit the clinical setting (119), which in complicated cases can involve a multistep approach (120).
Craniofacial skeleton.
The best-defined indication for craniofacial surgery in FD is optic nerve encasement. In the setting of acute, clinically significant vision loss, optic nerve decompression surgery can preserve or restore vision (22). Although FD is commonly located in the optic canal, it only rarely results in vision loss (121). Large cohort studies have demonstrated that prophylactic optic nerve decompression increases the risk of vision loss (121) and that expectant management with close monitoring leads to superior outcomes (122). In contrast, there is little evidence to inform other craniofacial surgical indications. The tendency of FD to regrow after subtotal resections is an important limitation that frequently leads to suboptimal outcomes. In 1 large retrospective series, postoperative regrowth occurred after 68% of craniofacial resections, and more frequently after more conservative recontouring procedures, and in patients with GH excess (53). At present, surgical intervention in the craniofacial area is clearly indicated to address functional impairment, such as compressive neuropathies, otic canal obstruction, severe malocclusion, and symptomatic cranial base deformities (25, 53). The decision to intervene for cosmetic purposes must be individualized and made in conjunction with a multidisciplinary team, with a clear understanding of the potential likelihood for recurrent deformity.
Axial skeleton.
Progressive scoliosis is a potentially serious complication of axial FD that may require surgical intervention in severe cases. Unlike the more common adolescent idiopathic scoliosis, which typically stabilizes after completion of linear growth, FD-associated scoliosis may continue to progress into adulthood (30). Despite the often-poor durability of orthopedic procedures in appendicular FD, outcomes from spinal fusion are generally favorable. In 1 retrospective series, 9 of 10 patients who underwent spinal fusion using a variety of standard approaches showed hardware stability during up to 15 years of follow-up (30). Use of scoliosis braces is often limited by FD in the spine and pelvis, which may develop worsening deformity when subjected to indirect forces from bracing (116).
Functional management.
Physiatry is a cornerstone of management that is often overlooked to the detriment of patient care. Fractures and surgeries result in progressive loss of muscle strength, particularly in the hip extensors, leading to decreased ambulation ability (123). Because FD is mechanically unsound, optimizing gait and positioning to align weight-bearing forces is important to prevent deformities, manage pain, and prevent late complications such as arthritis. Malalignment in FD is complex and multifactorial. Leg length discrepancies arise through a combination of FD overgrowth, which increases limb length, and fractures and deformities, which decrease limb length. Discrepancies as small as 1/8 inch (3.18 mm) have been associated with decreased hip strength and range of motion (123), decreased gait efficiency (123), and progressive scoliosis (30). Conservative management with lifts and other orthotic devices is typically effective and should be reassessed regularly. Clinicians are often tempted to treat growing children with epiphysiodesis or other growth-altering procedures; however, because of the multiplicity of factors contributing to malignment in FD, these are frequently ineffective and should be undertaken with caution.
Medical management.
There are currently no medical therapies shown to increase bone quality or prevent skeletal complications in FD. Studies of commercially available bone-altering therapies such as bisphosphonates have shown mixed results and are discussed in “Commercially available medications.” Hyperfunctioning endocrinopathies have direct and deleterious effects on bone metabolism and have been linked to poor skeletal outcomes; therefore, screening and treatment of extraskeletal features is essential medical therapy in FD. FGF23-mediated hypophosphatemia can lead to rickets and compromised mechanical integrity, contributing to fractures and deformities (25, 30, 37). Precocious puberty increases growth velocity and may compromise adult height, which has important implications for progression and correction of skeletal deformities (25, 116). Hyperthyroidism increases bone turnover and is associated with skeletal deformities (25, 30), whereas GH excess is an important driver of craniofacial expansion and is associated with functional deficits (26-28). Specific management of these features is discussed in “Clinical management of extraskeletal features.”
Pain management.
Bone pain is one of the most challenging aspects of clinical management in FD. There are multiple factors that may contribute to pain, and clinicians should carefully assess for treatable underlying causes. Pain that is limited to a focal area or is elicited with weight bearing may portend a current or impending fracture that requires orthopedic intervention. Clinicians should also consider functional causes such as muscle weakness and malalignment that may respond to physiatric interventions, such as orthotics or targeted strengthening exercises. Diffuse pain may result from metabolic disturbances, particularly hypophosphatemia. In the absence of any metabolic issues, pain that occurs diffusely in multiple affected areas is likely due to intrinsic FD pain. Knowledge gaps in the pathophysiology of this pain (which appears to be distinct from other skeletal complications) has prevented the development of targeted treatment strategies. Bisphosphonate treatment has shown mixed results (discussed in a subsequent section); however, uncontrolled studies suggest intravenous formulations may be effective for pain relief in some patients (124, 125). Osteonecrosis of the jaw (ONJ) is a rare but potentially serious sequela of bisphosphonate use, particularly in patients receiving long-term, high-dose intravenous formulations. In 1 large cohort of patients with FD, ONJ was reported in 5.4% of those treated with bisphosphonates (126), a prevalence similar to that seen in oncology patients (127) and indicating that patients with FD are likely at increased risk for this complication. In this series, affected patients (age 23-57 years) had features known to be associated with ONJ in the general population, including treatment with long-term, high-dose intravenous formulations (treatment ranging 2-13 years), dental infections, and dentoalveolar surgical procedures. Therefore, the risk may be mitigated by using the lowest dose and longest interval between doses needed to control symptoms and by maintaining regular dental care and excellent dental hygiene.
Clinical management of extraskeletal features
Endocrine
Gonadal disease.
Treatment for precocious puberty involves blocking sex steroid activity, with the goal of preventing disabling short stature and mitigating psychosocial effects of early pubertal development. Treatment in girls with MAS has primarily used medications developed for women with breast cancer. A prospective trial of the selective estrogen receptor modulator tamoxifen was shown to have beneficial effects on growth velocity, skeletal maturation, and bleeding episodes, however treatment led to increased uterine volumes, consistent with its agonist effect on endometrial stroma and raising concerns about the potential for increasing the risk of malignancy (128). Fulvestrant is a pure estrogen receptor antagonist that was shown to benefit skeletal maturation in girls with MAS; however, there was no benefit on growth velocity or predicted adult height (129). First- and second-generation aromatase inhibitors have shown disappointing results (130-132); however, studies using letrozole, a more potent third-generation formulation, showed beneficial effects on bleeding episodes, skeletal maturation, and final adult height (42). At present letrozole is considered first-line treatment in girls with MAS, with tamoxifen and fulvestrant available as adjuvant therapies if needed.
Treatment in boys requires combination therapy with the goals of 1) blocking testosterone activity to prevent premature physical and psychological masculinization, and 2) blocking estrogen activity to prevent skeletal maturation and compromised final height. Because precocious puberty is much less common in boys with MAS than girls, there have been comparatively fewer studies. One retrospective cohort study reported improved predicted adult height using a combination of androgen receptor blockers, including spironolactone and testolactone, and the aromatase inhibitor letrozole (45). Additional case reports have shown efficacy using a combination of bicalutamide and anastrozole (133), and ketoconazole and cyproterone acetate (134).
Secondary central puberty is a potential complication of peripheral puberty, which arises most frequently in girls with MAS on reaching a bone age of approximately 10 to 13 years (42). The most common presentation is “breakthrough” pubertal signs in a child who had previously been well controlled with medical therapy (ie, growth acceleration, bone age advancement, breast development, and/or vaginal bleeding in girls, increased penile size and secondary sexual hair in boys). Children typically respond well to gonadotropin-releasing hormone agonists, which can be used in combination with sex steroid blocking therapies.
When to discontinue pubertal blockade in children with MAS-related precocious puberty is determined by a combination of the age and psychosocial status of the child, and clinical judgment. Generally, these factors are similar to those in idiopathic precocious puberty, with the important caveat that in MAS once the medication(s) controlling pubertal advancement are discontinued patients tend to advance in puberty quite rapidly. This point should be made to the child and parents and should be taken into consideration in predicting final height, which may be reduced in comparison to children with idiopathic precocious puberty.
Thyroid disease.
Because of the known deleterious effects on skeletal outcomes, clinicians should maintain a low threshold for treating hyperthyroidism in patients with MAS, even if there are no clear symptoms. Hyperthyroidism generally responds to typical antithyroidal medications, which are first-line treatment in children (46, 47). Because hyperthyroidism in MAS is persistent, most patients ultimately elect to undergo definitive treatment. Thyroidectomy performed in a high-volume surgical center is generally preferred. Radioablation is also an effective strategy, particularly if the patient has surgical risks or if a high-volume center is unavailable. However, clinicians should be aware that most patients have mosaic thyroid involvement, and there is a theoretical risk of subtherapeutic radiation exposure to areas of normal tissue. The presence of the growth-promoting GNAS mutation in remnant tissue following radioablation or thyroidectomy leaves patients with risk recurrence of hyperthyroidism. This typically presents with decreasing levothyroxine requirements, new-onset goiter, or the development of typical symptoms.
Growth hormone excess.
Expansion of craniofacial FD can occur even under the influence of mild, otherwise asymptomatic GH excess. It is therefore prudent for clinicians to have a low threshold to start treatment, and to aim for aggressive control, targeting insulin-like growth factor-1 Z scores to the mid- to lower end of the normal range. Medication is first-line management, and patients typically respond well to somatostatin analogues (octreotide, lanreotide) or GH receptor antagonists (pegvisomant), used alone or in combination. Patients who do not respond to medical therapy are particularly challenging. Surgery is often technically difficult because of FD involvement of the skull base. In addition, mutation-bearing somatomammotrophs are typically spread diffusely throughout the pituitary, and total hypophysectomy is required for surgical cure (51). This is true even if pituitary imaging suggests a focal adenoma (28, 51). Radiation therapy can be effective but has been associated with malignant transformation of skull base FD, making this an undesirable option that should be used as a final resort in severely affected patients (88, 90).
Adrenal disease.
Treatment of neonatal hypercortisolism is often dictated by the severity of illness. Adrenalectomy provides definitive treatment and is the preferred approach in patients with severe disease who are sufficiently hemodynamically stable to tolerate surgery. Spontaneous resolution of hypercortisolism has been reported in approximately one-third of cases (54); therefore, if patients are mildly affected and well controlled with medications, observation may be warranted. Metyrapone is typically used first-line for medical management because of its low risk of hepatotoxicity; however, ketoconazole and mitotane may also be considered (135). Partial adrenal insufficiency has been reported in patients who have undergone spontaneous resolution of hypercortisolism, and these patients should be monitored with periodic adrenocorticotropic hormone stimulation testing, particularly before surgical procedures (54). The development of testicular adrenal rest tissue has also been reported as a late complication of bilateral adrenalectomy (45). Neurodevelopmental sequelae of neonatal hypercortisolism have not been well defined; however one series reported an increased incidence of speech disorders and developmental delays (54). Clinicians should therefore initiate developmental intervention services as early as possible.
Fibroblast growth factor-23–mediated hypophosphatemia.
Intervention should also be instituted early in patients with hypophosphatemia, even if asymptomatic. Like other disorders of FGF23 excess, hypophosphatemia in FD responds well to oral calcitriol and phosphorus supplementation (136). Hypophosphatemia may wax and wane, and patients may have varying requirements for supplementation throughout their lifespan. It is therefore important to monitor phosphorus regularly in all patients with polyostotic disease, and to consider hypophosphatemia as a potential contributor in patients who develop pain or worsening skeletal complications. Of note, supplements should be withheld prior to surgical procedures to decrease the risk of immobilization hypercalcemia, particularly in adolescents.
Skin
Café-au-lait macules in MAS are asymptomatic and not typically a source of morbidity, although lesions that are especially large or occur in highly visible locations such as the face may lead to psychosocial stress. Patients should be advised that lesions may become darker when exposed to sunlight. There are no well-established treatments known to decrease skin pigmentation, although a single case report describes improvement after laser therapy (137).
Intramuscular myxomas
Intramuscular myxomas that are asymptomatic can typically be managed by observation alone; however, myxomas that are particularly large or associated with pain or functional impairment may require resection. Postoperative recurrences have been reported in up to 30% of patients (70, 138).
Psychosocial management
Management of psychosocial stress is a critical component of care in FD/MAS. Stressors vary between individuals; however, common concerns in the FD/MAS population include challenges related to physical disability, pain, craniofacial differences, health care access, and others typical of chronic illness. These stressors may also extend to parents, caregivers, and other family members. Patients and/or families should undergo structured assessment for quality of life to identify individual stressors and develop therapeutic plans to address them. In a Dutch cohort, patients who used avoidant or passive coping strategies, such as ignoring problems, feeling overwhelmed by them, or worrying over past problems, had reduced quality of life (99). Negative perceptions of their disease were also associated with impaired quality of life (98). These findings suggest that patients with ineffective coping strategies may benefit from psychotherapy or other interventions focused on developing positive strategies to improve quality of life.
Unmet Needs and Future Directions
Novel treatment approaches
Medical therapies.
The development of effective medical therapies for FD is a critical area of unmet need. Although reasonably effective treatments exist for most MAS endocrinopathies, no therapies have been shown to definitively improve bone quality or prevent the expansion of FD lesions. The development of novel therapies targeting specific pathogenic mechanisms in FD is thus a high priority for research. In addition, the adaptation of commercially available therapies developed for other skeletal disorders provides an opportunity for more immediate clinical application.
Commercially available medications.
Bisphosphonates.
Antiresorptive medications such as bisphosphonates are an intuitive potential treatment because of the increased bone turnover and osteoclastogenesis in FD tissue. Bisphosphonates have been studied in FD for several decades, and although much has been learned about their limitations, their specific role in clinical management has yet to be clearly defined. Early studies generated excitement with encouraging reports, including a series of 9 patients treated with pamidronate who reported improvements in pain, bone turnover markers, and decreased radiolucency on radiographs with thickening of the femoral cortices (125). Longer-term studies using this regimen showed similar findings (124, 139); however, although reports from other investigators described similar benefits on pain and bone turnover markers, they were unable to replicate the radiographic benefits (140-143). Plotkin et al found no difference in histomorphometric analyses on FD tissue from 9 pamidronate-treated and 7 untreated children, calling into question the ability of bisphosphonates to affect bone turnover within dysplastic lesions (142). Similarly, a retrospective comparison of FD burden in 20 treated and 15 untreated children found no association between bisphosphonate treatment and FD lesion expansion (101). This series also demonstrated that bisphosphonate treatment was associated with lower bone turnover markers; however, it did not alter the expected age-dependent pattern of decline (101). Additional retrospective studies reported no associations between bisphosphonate treatment and progression of spinal deformities (30) or cranial base deformities (25).
There has been one controlled trial of bisphosphonate treatment in FD, which included 40 subjects treated for 2 years with either the oral formulation alendronate or placebo. Alendronate treatment was associated with a significant decrease in bone resorption markers, but no improvements in pain or radiographic appearance of FD lesions (144). Concerningly, 1 retrospective series reported ONJ in 4 of 76 patients treated with bisphosphonates, suggesting that patients with FD/MAS may be at increased risk for this complication.
Taken together, these findings do not support a role for bisphosphonates in preventing FD lesion expansion or in improving the mechanical properties of FD lesions. The oral bisphosphonate alendronate does not improve FD-related pain; however, open-label studies suggest that intravenous formulations, including pamidronate and zoledronate, may have a role in treatment of FD-related bone pain. In the absence of rigorous controlled data, clinicians should limit bisphosphonate use to the lowest dose and interval needed to control pain and make efforts to limit dental risks. Future studies should include controlled trials to determine the analgesic effects of intravenous bisphosphonates.
Denosumab.
Denosumab is a fully human, monoclonal antibody to RANKL that has emerged as a promising therapy for osteoporosis and complications of skeletal tumors. Interest in a potential role for denosumab in FD was piqued after reports emerged of its efficacy in treatment of giant cell tumors. These benign but locally aggressive tumors typically affect adults in their second to fourth decades and share key histologic features with FD, including abundant RANKL expression (145). Phase 2 trials of denosumab treatment reported decreased giant cell tumor progression and prevention or reduction in surgical morbidity (146, 147), leading to its approval for this indication by the US Food and Drug Administration in 2013 and the European Medicines Agency in 2014 (148). Based on these findings, denosumab treatment was initiated as part of a compassionate use trial in a 9-year-old boy with a highly morbid, rapidly expanding femoral FD lesion (149, 150). Treatment was associated with a rapid improvement in pain and bone turnover markers, along with evidence of a decreased rate of FD expansion. However, after denosumab discontinuation there was a rapid rebound in bone resorption markers, which was associated with severe and life-threatening hypercalcemia that fortunately responded to bisphosphonate treatment. Similar postdiscontinuation rebound has been reported in pediatric and adult patients treated for a variety of indications, variably associated with hypercalcemia and vertebral compression fractures (151, 152). Subsequent cases and small series of denosumab treatment in FD patients report consistent decreases in bone turnover, but variable effects on pain and no clear descriptions of lesional or postdiscontinuation effects (153-155). Current evidence suggests a potential role for denosumab treatment in FD; however, safety concerns about postdiscontinuation effects should limit use to compassionate treatment of highly morbid patients by clinicians experienced in managing complex cases of altered mineral homeostasis, or in the setting of clinical trials. A phase 2 trial that includes intensive postdiscontinuation monitoring is ongoing (NCT03571191).
Tocilizumab.
Based on the demonstration of high levels of IL-6 produced by FD-derived bone marrow stromal cells (18), anti–IL-6 pathway treatment with drugs such as the anti–IL-6 receptor drug, tocilizumab, has been explored. A case report demonstrated tocilizumab was effective in relieving FD-related bone pain in a patient who did not respond to bisphosphonates (156). A placebo-controlled study of tocilizumab on bone turnover and bone pain in FD (NCT01791842) was recently completed, the results of which are pending.
Burosumab.
Burosumab is a fully human, monoclonal antibody to FGF23 that has been studied in patients with X-linked hypophosphatemia, another disorder of FGF23 excess. A phase 2 study in children reported improvements in serum phosphorus, rickets severity, and functional parameters (157, 158), and a phase 3 study in adults similarly demonstrated improvements in serum phosphorus and fracture healing (159). Based on these findings, burosumab was approved for treatment of X-linked hypophosphatemia by the US Food and Drug Administration and the European Medicines Agency in 2018 (160). Investigation is needed to determine the potential utility of burosumab to treat FGF23-mediated hypophosphatemia in patients with FD. Hypophosphatemia in FD can generally be managed with conventional calcitriol and phosphate supplementation; however, this regimen can be associated with hypercalciuria and potential adverse renal effects (such as nephrocalcinosis and nephrolithiasis), complications that may be mitigated by burosumab. An unanswered question is whether systemic burosumab would be effective in treating the tissue-level osteomalacia observed in FD lesions, an issue that may be separate from the systemic complications of systemic hypophosphatemia.
Novel drug development.
Molecular targeting.
Because disease-causing mutations in FD/MAS occur almost exclusively at the R201 position, targeting the mutated Gα s protein is a strategy that could potentially treat disease affecting multiple systems. Pharmacologic efforts to target G-proteins has proved difficult, but efforts are underway to identify small molecules that can specifically target the gain-of-function activity that is brought about by the R201 mutations (161). Recent success in the development of high-potency inhibitors targeting Gα q/11 oncoproteins provides support for this potential therapeutic strategy (162, 163).
Gene therapy.
Theoretically, the genetic variants in GNAS that give rise to FD/MAS should be amenable to gene therapy, including gene replacement, correction, and silencing oligonucleotide therapy. The tissue most in need of better therapies is the bone but targeting bone cells adds another level of complexity to the already unsurmounted problems that accompany gene therapy in general. However, gene therapy is an active area of research that is making great strides, and progress made in other conditions will ultimately prove applicable to FD/MAS.
Cell-based therapy.
Because FD originates from dysfunctional skeletal stem cells, stem cell therapy is another intuitive potential treatment strategy. These multipotential, self-renewing cells have been shown to differentiate into a miniature bone organ in vivo, demonstrating their potential role in promoting skeletal regeneration (164, 165). Preclinical studies have shown that in vivo transplantation of mesenchymal stem cells with appropriate carriers may promote regeneration of skeletal tissue; however, this is highly dependent on the qualities of the transplanted cells (166-168). Intravenous mesenchymal stem cell transfusion has been performed in numerous skeletal, inflammatory, and immune-mediated disorders in attempts to channel the potential paracrine and immunomodulatory effects of these cells, but results have been variable (169, 170). At present, the primary barriers to determining the clinical application of mesenchymal stem cell transplantation are critical knowledge gaps in understanding the nature and function of these cells, which prevents development of standardized methods for their retrieval, handling, and storage (171). In addition, techniques to deliver cells to affected tissues in such a way that will allow them to establish biomechanically organized and functional fashion represents an additional level of challenge.
Challenges in clinical research and trials
Research in rare diseases is fraught with challenges; however, clinical investigation in FD/MAS is characterized by a unique combination of obstacles and strengths. Robust natural history studies have broadly outlined the disease course, allowing investigators to focus interventions on the most clinically relevant long-term complications. However, issues such as patient heterogeneity and evolving disease activity over time necessitate taking a considered and creative approach.
Clinical end points and surrogate disease markers.
In comparison to similarly rare disorders, the natural history of FD/MAS has been relatively well established, in part from the activities of a longstanding NIH protocol that has extensively phenotyped a cohort of more than 200 individuals followed prospectively for up to 20 years (NCT00001727). This study has identified and broadly outlined many clinically relevant skeletal outcomes, including fractures (37), deformities (23, 30, 100, 172), pain (96, 104), and functional impairment (26, 28, 96, 100, 121-123). Although these are meaningful problems to target therapeutically, they present important practical barriers as clinical trial end points, and identification of clinically relevant surrogate markers is thus a critical unmet need. A method of quantitating total FD burden using technetium-99 scintigraphy (Fig. 7D) has been widely employed in retrospective studies and has been shown to correlate with mobility impairment (173). However, its inability to quantitate activity within individual FD lesions represents a critical limitation. Other studies have used serum bone turnover markers (143, 144); however, FD-specific markers have not been identified, making these an impractical end point for therapies that affect both FD and non-FD bone, such as antiresorptive medication. Recently (18), 18F-NaF–positron emission tomography (PET)/CT imaging has shown great promise in quantifying disease-specific activity in patients with FD, correlating with clinical outcome measures such as fractures, craniofacial and orthopedic surgeries, spinal deformity, and mobility (174) (Fig. 7E).
Other potential challenges in trial design relate to changes in disease activity over time (100, 101). Understanding these age-related changes is critical to choosing surrogate end points that target specific therapeutic goals. For example, therapies aimed at preventing FD lesion formation should be targeted to children in whom FD lesions are in a growth phase and may be most accurately measured using radiographic techniques such as 18F-NaF–PET/CT. Therapies for pain are likely better targeted to adults and may potentially be evaluated using more standardized assessments, such as validated pain questionnaires.
Registry development and role of patient advocacy organizations.
Patient registries are increasingly recognized as an important strategy to address hurdles in rare disease research. Although the purposes of registries can vary widely, in rare diseases such as FD/MAS they have frequently been used to collect patient-reported natural history data and create repositories that engage stakeholders to stimulate future research. In FD/MAS and a growing number of other disorders, registry creation has been largely patient centered, driven, and supported by the activities of patient advocacy organizations. Active registries in the United States and United Kingdom have been used to support successful funding applications and generate original research (175, 176). Patient advocacy organizations also play a central role in building rare-disease communities, which among other activities can improve care by disseminating accurate information to patients and clinicians. The FD/MAS field has benefited from the activities of longstanding advocacy organizations such as the Fibrous Dysplasia Foundation (United States), the Fibrous Dysplasia Support Society (United Kingdom), and L’Associazione EAMAS (Italy), along with a growing number of organizations representing France, the Netherlands, Brazil, and other countries.
FD/MAS International Consortium.
Collaboration between investigators, clinicians, patients, and advocates is an effective approach to building innovative research programs and advancing clinical care. Particularly in rare and complex disorders such as FD/MAS, input from multiple stakeholders is needed to develop strategies for diverse needs such as basic and translational science, clinical trials, patient recruitment and engagement, and regulatory issues. The strong patient advocacy presence in FD/MAS has been bolstered by collaborations with international, multidisciplinary clinicians, and investigators, culminating in the formation of the FD/MAS International Consortium in 2016 (177). With growing membership from the United States, Europe, and South America, the Consortium has held 3 international conferences and launched productive collaborations combining international datasets (83, 177). The Consortium has recently developed comprehensive clinical care recommendations for FD/MAS (2). Future activities include the dissemination of these recommendations and plans for international clinical trials. Multidisciplinary, multistakeholder organizations such as the FD/MAS International Consortium will facilitate progress in FD/MAS research that will advance our understanding of the disease, accelerate the development of improved therapies, and lead to better quality of life for patients with FD/MAS.
Acknowledgments
Financial Support: This work was supported by the Intramural Research Program of the National Institute of Dental and Craniofacial Research, National Institutes of Health. (ZIA DE000649)
Glossary
Abbreviations
- ATP
adenosine triphosphate
- cAMP
cyclic AMP
- CREB
cAMP response element-binding protein
- FD/MAS
fibrous dysplasia/McCune-Albright syndrome
- GDP
guanosine diphosphate
- GPCR
G-coupled protein receptor
- GTP
guanosine triphosphate
Additional Information
Disclosure Summary: NIDCR receives research funding from Amgen, Inc, for an investigator-sponsored study of denosumab treatment in FD. The authors have submitted the International Committee of Medical Journal Editors Form for disclosure of potential conflicts of interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Boyce AM, Florenzano P, de Castro LF, Collins MT. Fibrous dysplasia/McCune-Albright syndrome. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A, eds. GeneReviews®. Seattle, WA: University of Washington, Seattle; 1993-2020. [PubMed] [Google Scholar]
- 2. Javaid MK, Boyce A, Appelman-Dijkstra N, et al. Best practice management guidelines for fibrous dysplasia/McCune-Albright syndrome: a consensus statement from the FD/MAS International Consortium. Orphanet J Rare Dis. 2019;14(1):139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, Spiegel AM. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N Engl J Med. 1991;325(24):1688–1695. [DOI] [PubMed] [Google Scholar]
- 4. Riminucci M, Fisher LW, Majolagbe A, et al. A novel GNAS1 mutation, R201G, in McCune-Albright syndrome. J Bone Miner Res. 1999;14(11):1987–1989. [DOI] [PubMed] [Google Scholar]
- 5. Idowu BD, Al-Adnani M, O’Donnell P, et al. A sensitive mutation-specific screening technique for GNAS1 mutations in cases of fibrous dysplasia: the first report of a codon 227 mutation in bone. Histopathology. 2007;50(6):691–704. [DOI] [PubMed] [Google Scholar]
- 6. Endo M, Yamada Y, Matsuura N, Niikawa N. Monozygotic twins discordant for the major signs of McCune-Albright syndrome. Am J Med Genet. 1991;41(2):216–220. [DOI] [PubMed] [Google Scholar]
- 7. Happle R. The McCune-Albright syndrome: a lethal gene surviving by mosaicism. Clin Genet. 1986;29(4):321–324. [DOI] [PubMed] [Google Scholar]
- 8. Riminucci M, Saggio I, Robey PG, Bianco P. Fibrous dysplasia as a stem cell disease. J Bone Miner Res. 2006;21(Suppl 2):P125–P131. [DOI] [PubMed] [Google Scholar]
- 9. Michienzi S, Cherman N, Holmbeck K, et al. GNAS transcripts in skeletal progenitors: evidence for random asymmetric allelic expression of Gs alpha. Hum Mol Genet. 2007;16(16):1921–1930. [DOI] [PubMed] [Google Scholar]
- 10. Landis CA, Masters SB, Spada A, Pace AM, Bourne HR, Vallar L. GTPase inhibiting mutations activate the alpha chain of Gs and stimulate adenylyl cyclase in human pituitary tumours. Nature. 1989;340(6236):692–696. [DOI] [PubMed] [Google Scholar]
- 11. Hu Q, Shokat KM. Disease-causing mutations in the G protein Gαs subvert the roles of GDP and GTP. Cell. 2018;173(5):1254–1264.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bianco P, Kuznetsov SA, Riminucci M, Fisher LW, Spiegel AM, Robey PG. Reproduction of human fibrous dysplasia of bone in immunocompromised mice by transplanted mosaics of normal and Gsalpha-mutated skeletal progenitor cells. J Clin Invest. 1998;101(8):1737–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Regard JB, Cherman N, Palmer D, et al. Wnt/β-catenin signaling is differentially regulated by Gα proteins and contributes to fibrous dysplasia. Proc Natl Acad Sci U S A. 2011;108(50):20101–20106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Riminucci M, Fisher LW, Shenker A, Spiegel AM, Bianco P, Gehron Robey P. Fibrous dysplasia of bone in the McCune-Albright syndrome: abnormalities in bone formation. Am J Pathol. 1997;151(6):1587–1600. [PMC free article] [PubMed] [Google Scholar]
- 15. Piersanti S, Remoli C, Saggio I, et al. Transfer, analysis, and reversion of the fibrous dysplasia cellular phenotype in human skeletal progenitors. J Bone Miner Res. 2010;25(5):1103–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Riminucci M, Liu B, Corsi A, et al. The histopathology of fibrous dysplasia of bone in patients with activating mutations of the Gs alpha gene: site-specific patterns and recurrent histological hallmarks. J Pathol. 1999;187(2):249–258. [DOI] [PubMed] [Google Scholar]
- 17. Robinson C, Collins MT, Boyce AM. Fibrous dysplasia/McCune-Albright syndrome: clinical and translational perspectives. Curr Osteoporos Rep. 2016;14(5):178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Riminucci M, Kuznetsov SA, Cherman N, Corsi A, Bianco P, Gehron Robey P. Osteoclastogenesis in fibrous dysplasia of bone: in situ and in vitro analysis of IL-6 expression. Bone. 2003;33(3):434–442. [DOI] [PubMed] [Google Scholar]
- 19. Riminucci M, Collins MT, Fedarko NS, et al. FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J Clin Invest. 2003;112(5):683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McCune D. Osteitis fibrosa cystica; the case of a nine year old girl who also exhibits precocious puberty, multiple pigmentation of the skin and hyperthyroidism. Am J Dis Child (1960). 1936;52:743–744. [Google Scholar]
- 21. El-Mofty SK. Fibro-osseous lesions of the craniofacial skeleton: an update. Head Neck Pathol. 2014;8(4):432–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee JS, FitzGibbon EJ, Chen YR, et al. Clinical guidelines for the management of craniofacial fibrous dysplasia. Orphanet J Rare Dis. 2012;24(Suppl 1):S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ippolito E, Farsetti P, Boyce AM, Corsi A, De Maio F, Collins MT. Radiographic classification of coronal plane femoral deformities in polyostotic fibrous dysplasia. Clin Orthop Relat Res. 2014;472(5):1558–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hartley I, Zhadina M, Collins MT, Boyce AM. Fibrous dysplasia of bone and mccune-albright syndrome: a bench to bedside review. Calcif Tissue Int.2019;104(5):517–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pan KS, Heiss JD, Brown SM, Collins MT, Boyce AM. Chiari I malformation and basilar invagination in fibrous dysplasia: prevalence, mechanisms, and clinical implications. J Bone Miner Res. 2018;33(11):1990–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Boyce AM, Brewer C, DeKlotz TR, et al. Association of hearing loss and otologic outcomes with fibrous dysplasia. JAMA Otolaryngol Head Neck Surg. 2018;144(2):102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Akintoye SO, Chebli C, Booher S, et al. Characterization of gsp-mediated growth hormone excess in the context of McCune-Albright syndrome. J Clin Endocrinol Metab. 2002;87(11):5104–5112. [DOI] [PubMed] [Google Scholar]
- 28. Boyce AM, Glover M, Kelly MH, et al. Optic neuropathy in McCune-Albright syndrome: effects of early diagnosis and treatment of growth hormone excess. J Clin Endocrinol Metab. 2013;98(1):E126–E134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. DeKlotz TR, Kim HJ, Kelly M, Collins MT. Sinonasal disease in polyostotic fibrous dysplasia and McCune-Albright syndrome. Laryngoscope. 2013;123(4):823–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Berglund JA, Tella SH, Tuthill KF, et al. Scoliosis in fibrous dysplasia/McCune-Albright syndrome: factors associated with curve progression and effects of bisphosphonates. J Bone Miner Res. 2018;33(9):1641–1648. [DOI] [PubMed] [Google Scholar]
- 31. Mancini F, Corsi A, De Maio F, Riminucci M, Ippolito E. Scoliosis and spine involvement in fibrous dysplasia of bone. Eur Spine J. 2009;18(2):196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hamadani M, Awab A, Rashid A, Ali T, Brown B. Fibrous dysplasia protuberans in a patient with McCune-Albright syndrome. J Coll Physicians Surg Pak. 2006;16(5):376–377. [PubMed] [Google Scholar]
- 33. Valentino V, Onoscuri E, Gallo E, Carrata Thomes A. Albright’s syndrome. Report of a case, complicated by pleural effusion and diabetes [article in Italian]. Minerva Med. 1982;73(17):1077–1086. [PubMed] [Google Scholar]
- 34. Bhattacharyya N, Wiench M, Dumitrescu C, et al. Mechanism of FGF23 processing in fibrous dysplasia. J Bone Miner Res. 2012;27(5):1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Boyce AM, Bhattacharyya N, Collins MT. Fibrous dysplasia and fibroblast growth factor-23 regulation. Curr Osteoporos Rep. 2013;11(2):65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Collins MT, Chebli C, Jones J, et al. Renal phosphate wasting in fibrous dysplasia of bone is part of a generalized renal tubular dysfunction similar to that seen in tumor-induced osteomalacia. J Bone Miner Res. 2001;16(5):806–813. [DOI] [PubMed] [Google Scholar]
- 37. Leet AI, Chebli C, Kushner H, et al. Fracture incidence in polyostotic fibrous dysplasia and the McCune-Albright syndrome. J Bone Miner Res. 2004;19(4):571–577. [DOI] [PubMed] [Google Scholar]
- 38. Collins MT, Singer FR, Eugster E. McCune-Albright syndrome and the extraskeletal manifestations of fibrous dysplasia. Orphanet J Rare Dis. 2012;7(Suppl 1):S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Boyce AM, Collins MT. Fibrous dysplasia/McCune-Albright syndrome. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, eds. GeneReviews®. Seattle, WA: University of Washington, Seattle; 1993-2020. [PubMed] [Google Scholar]
- 40. Pienkowski C, Cartault A, Carfagna L, et al. Ovarian cysts in prepubertal girls. Endocr Dev. 2012;22:101–111. [DOI] [PubMed] [Google Scholar]
- 41. Gaspari L, Paris F, Nicolino M, et al. Fetal ovarian cysts: an early manifestation of McCune-Albright syndrome? Prenat Diagn. 2012;32(9):859–863. [DOI] [PubMed] [Google Scholar]
- 42. Estrada A, Boyce AM, Brillante BA, Guthrie LC, Gafni RI, Collins MT. Long-term outcomes of letrozole treatment for precocious puberty in girls with McCune-Albright syndrome. Eur J Endocrinol. 2016;175(5):477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Escobar ME, Gryngarten M, Domené H, Ropelato G, López MR, Bergadá C. Persistence of autonomous ovarian activity after discontinuation of therapy for precocious puberty in McCune-Albright syndrome. J Pediatr Adolesc Gynecol. 1997;10(3):147–151. [DOI] [PubMed] [Google Scholar]
- 44. Boyce AM, Casey RK, Ovejero Crespo D, et al. Gynecologic and reproductive outcomes in fibrous dysplasia/McCune-Albright syndrome. Orphanet J Rare Dis. 2019;14(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Boyce AM, Chong WH, Shawker TH, et al. Characterization and management of testicular pathology in McCune-Albright syndrome. J Clin Endocrinol Metab. 2012;97(9):E1782–E1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Celi FS, Coppotelli G, Chidakel A, et al. The role of type 1 and type 2 5’-deiodinase in the pathophysiology of the 3,5,3’-triiodothyronine toxicosis of McCune-Albright syndrome. J Clin Endocrinol Metab. 2008;93(6):2383–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tessaris D, Corrias A, Matarazzo P, et al. Thyroid abnormalities in children and adolescents with McCune-Albright syndrome. Horm Res Paediatr. 2012;78(3):151–157. [DOI] [PubMed] [Google Scholar]
- 48. Isotani H, Sanda K, Kameoka K, Takamatsu J. McCune-Albright syndrome associated with non-autoimmune type of hyperthyroidism with development of thyrotoxic crisis. Horm Res. 2000;53(5):256–259. [DOI] [PubMed] [Google Scholar]
- 49. Lawless ST, Reeves G, Bowen JR. The development of thyroid storm in a child with McCune-Albright syndrome after orthopedic surgery. Am J Dis Child. 1992;146(9):1099–1102. [DOI] [PubMed] [Google Scholar]
- 50. Salenave S, Boyce AM, Collins MT, Chanson P. Acromegaly and McCune-Albright syndrome. J Clin Endocrinol Metab. 2014;99(6):1955–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vortmeyer AO, Gläsker S, Mehta GU, et al. Somatic GNAS mutation causes widespread and diffuse pituitary disease in acromegalic patients with McCune-Albright syndrome. J Clin Endocrinol Metab. 2012;97(7):2404–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Roszko KL, Collins MT, Boyce AM. Mosaic effects of growth hormone on fibrous dysplasia of bone. N Engl J Med. 2018;379(20):1964–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Boyce AM, Burke A, Cutler Peck C, DuFresne CR, Lee JS, Collins MT. Surgical management of polyostotic craniofacial fibrous dysplasia: long-term outcomes and predictors for postoperative regrowth. Plast Reconstr Surg. 2016;137(6):1833–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Brown RJ, Kelly MH, Collins MT. Cushing syndrome in the McCune-Albright syndrome. J Clin Endocrinol Metab. 2010;95(4):1508–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Carney JA, Young WF, Stratakis CA. Primary bimorphic adrenocortical disease: cause of hypercortisolism in McCune-Albright syndrome. Am J Surg Pathol. 2011;35(9):1311–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tatsi C, Stratakis CA. Neonatal Cushing syndrome: a rare but potentially devastating disease. Clin Perinatol. 2018;45(1):103–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pichard DC, Boyce AM, Collins MT, Cowen EW. Oral pigmentation in McCune-Albright syndrome. JAMA Dermatol. 2014;150(7):760–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Matsubara A, Sekine S, Kushima R, et al. Frequent GNAS and KRAS mutations in pyloric gland adenoma of the stomach and duodenum. J Pathol. 2013;229(4):579–587. [DOI] [PubMed] [Google Scholar]
- 59. Liu C, McKeone DM, Walker NI, Bettington ML, Leggett BA, Whitehall VLJ. GNAS mutations are present in colorectal traditional serrated adenomas, serrated tubulovillous adenomas and serrated adenocarcinomas with adverse prognostic features. Histopathology. 2017;70(7):1079–1088. [DOI] [PubMed] [Google Scholar]
- 60. Wu J, Matthaei H, Maitra A, et al. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med. 2011;3(92):92ra66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Furukawa T, Kuboki Y, Tanji E, et al. Whole-exome sequencing uncovers frequent GNAS mutations in intraductal papillary mucinous neoplasms of the pancreas. Sci Rep. 2011;1:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nault JC, Fabre M, Couchy G, et al. GNAS-activating mutations define a rare subgroup of inflammatory liver tumors characterized by STAT3 activation. J Hepatol. 2012;56(1):184–191. [DOI] [PubMed] [Google Scholar]
- 63. Gaujoux S, Salenave S, Ronot M, et al. Hepatobiliary and pancreatic neoplasms in patients with McCune-Albright syndrome. J Clin Endocrinol Metab. 2014;99(1):E97–E101. [DOI] [PubMed] [Google Scholar]
- 64. Johansen L, Haller W, Thyagarajan M, Kelly D, McKiernan P. Hepatic lesions associated with McCune Albright syndrome. J Pediatr Gastroenterol Nutr. 2019;68(4):e54–e57. [DOI] [PubMed] [Google Scholar]
- 65. Robinson C, Estrada A, Zaheer A, et al. Clinical and radiographic gastrointestinal abnormalities in McCune-Albright syndrome. J Clin Endocrinol Metab. 2018;103(11):4293–4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wood LD, Noë M, Hackeng W, et al. Patients with McCune-Albright syndrome have a broad spectrum of abnormalities in the gastrointestinal tract and pancreas. Virchows Arch. 2017;470(4):391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zacharin M, Bajpai A, Chow CW, et al. Gastrointestinal polyps in McCune Albright syndrome. J Med Genet. 2011;48(7):458–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Henschen F. Fall von Ostitis Fibrosa mit multipelen Tumoren in den umgebenden Muskulatur. Verh Dtsch Ges Pathol. 1926;21:93–97. [Google Scholar]
- 69. Mazabraud A, Semat P, Roze R. Apropos of the association of fibromyxomas of the soft tissues with fibrous dysplasia of the bones [article in French]. Presse Med. 1967;75(44):2223–2228. [PubMed] [Google Scholar]
- 70. Majoor BCJ, van de Sande MAJ, Appelman-Dijkstra NM, et al. Prevalence and clinical features of Mazabraud syndrome: a multicenter European study. J Bone Joint Surg Am. 2019;101(2):160–168. [DOI] [PubMed] [Google Scholar]
- 71. Kushchayeva YS, Kushchayev SV, Glushko TY, et al. Fibrous dysplasia for radiologists: beyond ground glass bone matrix. Insights Imaging. 2018;9(6):1035–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Nielsen GP, O’Connell JX, Rosenberg AE. Intramuscular myxoma: a clinicopathologic study of 51 cases with emphasis on hypercellular and hypervascular variants. Am J Surg Pathol. 1998;22(10):1222–1227. [DOI] [PubMed] [Google Scholar]
- 73. Crawford EA, Brooks JS, Ogilvie CM. Osteosarcoma of the proximal part of the radius in Mazabraud syndrome. A case report. J Bone Joint Surg Am. 2009;91(4):955–960. [DOI] [PubMed] [Google Scholar]
- 74. Jhala DN, Eltoum I, Carroll AJ, et al. Osteosarcoma in a patient with McCune-Albright syndrome and Mazabraud’s syndrome: a case report emphasizing the cytological and cytogenetic findings. Hum Pathol. 2003;34(12):1354–1357. [DOI] [PubMed] [Google Scholar]
- 75. Lopez-Ben R, Pitt MJ, Jaffe KA, Siegal GP. Osteosarcoma in a patient with McCune-Albright syndrome and Mazabraud’s syndrome. Skeletal Radiol. 1999;28(9):522–526. [DOI] [PubMed] [Google Scholar]
- 76. Szymanski C, Bourgault C, Penel N, Maynou C. Chondrosarcoma of the femur in Mazabraud’s syndrome: a first case study. Orthop Traumatol Surg Res. 2015;101(7):875–878. [DOI] [PubMed] [Google Scholar]
- 77. O’Hayre M, Vázquez-Prado J, Kufareva I, et al. The emerging mutational landscape of G proteins and G-protein-coupled receptors in cancer. Nat Rev Cancer. 2013;13(6):412–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Khan S, Sclabas G, Reid-Lombardo KM. Population-based epidemiology, risk factors and screening of intraductal papillary mucinous neoplasm patients. World J Gastrointest Surg. 2010;2(10):314–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Omori Y, Ono Y, Tanino M, et al. Pathways of progression from intraductal papillary mucinous neoplasm to pancreatic ductal adenocarcinoma based on molecular features. Gastroenterology. 2019;156(3):647–661.e2. [DOI] [PubMed] [Google Scholar]
- 80. Hollstein PE, Shaw RJ. GNAS shifts metabolism in pancreatic cancer. Nat Cell Biol. 2018;20(7):740–741. [DOI] [PubMed] [Google Scholar]
- 81. Parvanescu A, Cros J, Ronot M, et al. Lessons from McCune-Albright syndrome-associated intraductal papillary mucinous neoplasms: GNAS-activating mutations in pancreatic carcinogenesis. JAMA Surg. 2014;149(8):858–862. [DOI] [PubMed] [Google Scholar]
- 82. Rossi RE, Massironi S. Intraductal papillary mucinous neoplasms of the pancreas: a clinical challenge. Expert Rev Gastroenterol Hepatol. 2018;12(11):1123–1133. [DOI] [PubMed] [Google Scholar]
- 83. Majoor BC, Boyce AM, Bovée JV, et al. Increased risk of breast cancer at a young age in women with fibrous dysplasia. J Bone Miner Res. 2018;33(1):84–90. [DOI] [PubMed] [Google Scholar]
- 84. Beuerlein ME, Schuller DE, DeYoung BR. Maxillary malignant mesenchymoma and massive fibrous dysplasia. Arch Otolaryngol Head Neck Surg. 1997;123(1):106–109. [DOI] [PubMed] [Google Scholar]
- 85. Ruggieri P, Sim FH, Bond JR, Unni KK. Malignancies in fibrous dysplasia. Cancer. 1994;73(5):1411–1424. [DOI] [PubMed] [Google Scholar]
- 86. Yalniz E, Er T, Ozyilmaz F. Fibrous dysplasia of the spine with sarcomatous transformation: a case report and review of the literature. Eur Spine J. 1995;4(6):372–374. [DOI] [PubMed] [Google Scholar]
- 87. Kaushik S, Smoker WR, Frable WJ. Malignant transformation of fibrous dysplasia into chondroblastic osteosarcoma. Skeletal Radiol. 2002;31(2):103–106. [DOI] [PubMed] [Google Scholar]
- 88. Liu F, Li W, Yao Y, et al. A case of McCune-Albright syndrome associated with pituitary GH adenoma: therapeutic process and autopsy. J Pediatr Endocrinol Metab. 2011;24(5-6):283–287. [DOI] [PubMed] [Google Scholar]
- 89. Hall MB, Sclar AG, Gardner DF. Albright’s syndrome with reactivation of fibrous dysplasia secondary to pituitary adenoma and further complicated by osteogenic sarcoma. Report of a case. Oral Surg Oral Med Oral Pathol. 1984;57(6):616–619. [DOI] [PubMed] [Google Scholar]
- 90. Hansen MR, Moffat JC. Osteosarcoma of the skull base after radiation therapy in a patient with McCune-Albright syndrome: case report. Skull Base. 2003;13(2):79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Collins MT, Sarlis NJ, Merino MJ, et al. Thyroid carcinoma in the McCune-Albright syndrome: contributory role of activating Gs alpha mutations. J Clin Endocrinol Metab. 2003;88(9):4413–4417. [DOI] [PubMed] [Google Scholar]
- 92. Yang GC, Yao JL, Feiner HD, Roses DF, Kumar A, Mulder JE. Lipid-rich follicular carcinoma of the thyroid in a patient with McCune-Albright syndrome. Mod Pathol. 1999;12(10):969–973. [PubMed] [Google Scholar]
- 93. Boussaïd K, Meduri G, Maiza JC, et al. Virilizing sclerosing-stromal tumor of the ovary in a young woman with McCune Albright syndrome: clinical, pathological, and immunohistochemical studies. J Clin Endocrinol Metab. 2013;98(2):E314–E320. [DOI] [PubMed] [Google Scholar]
- 94. Chevalier N, Paris F, Fontana S, et al. Postpubertal persistent hyperestrogenemia in McCune-Albright syndrome: unilateral oophorectomy improved fertility but detected an unexpected borderline epithelial ovarian tumor. J Pediatr Adolesc Gynecol. 2015;28(6):e169–e172. [DOI] [PubMed] [Google Scholar]
- 95. Majoor BCJ, Andela CD, Bruggemann J, et al. Determinants of impaired quality of life in patients with fibrous dysplasia. Orphanet J Rare Dis. 2017;12(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kelly MH, Brillante B, Kushner H, Gehron Robey P, Collins MT. Physical function is impaired but quality of life preserved in patients with fibrous dysplasia of bone. Bone. 2005;37(3):388–394. [DOI] [PubMed] [Google Scholar]
- 97. Forestier-Zhang L, Watts L, Turner A, et al. Health-related quality of life and a cost-utility simulation of adults in the UK with osteogenesis imperfecta, X-linked hypophosphatemia and fibrous dysplasia. Orphanet J Rare Dis. 2016;11(1):160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Majoor BCJ, Andela CD, Quispel CR, et al. Illness perceptions are associated with quality of life in patients with fibrous dysplasia. Calcif Tissue Int. 2018;102(1):23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Rotman M, Andela CD, Majoor BCJ, et al. Passive coping strategies are associated with more impairment in quality of life in patients with fibrous dysplasia. Calcif Tissue Int. 2018;103(5):469–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hart ES, Kelly MH, Brillante B, et al. Onset, progression, and plateau of skeletal lesions in fibrous dysplasia and the relationship to functional outcome. J Bone Miner Res. 2007;22(9):1468–1474. [DOI] [PubMed] [Google Scholar]
- 101. Florenzano P, Pan KS, Brown SM, et al. Age-related changes and effects of bisphosphonates on bone turnover and disease progression in fibrous dysplasia of bone. J Bone Miner Res. 2019;34(4):653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kuznetsov SA, Cherman N, Riminucci M, Collins MT, Robey PG, Bianco P. Age-dependent demise of GNAS-mutated skeletal stem cells and “normalization” of fibrous dysplasia of bone. J Bone Miner Res. 2008;23(11):1731–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Isobe Y, Takahashi K, Kiso H, et al. Direct evidence for the age-dependent demise of GNAS-mutated cells in oral fibrous dysplasia. Arch Oral Biol. 2018;93:133–140. [DOI] [PubMed] [Google Scholar]
- 104. Kelly MH, Brillante B, Collins MT. Pain in fibrous dysplasia of bone: age-related changes and the anatomical distribution of skeletal lesions. Osteoporos Int. 2008;19(1):57–63. [DOI] [PubMed] [Google Scholar]
- 105. Guerin Lemaire H, Merle B, Borel O, Gensburger D, Chapurlat R. Serum periostin levels and severity of fibrous dysplasia of bone. Bone. 2019;121:68–71. [DOI] [PubMed] [Google Scholar]
- 106. de Castro LF, Burke AB, Wang HD, et al. Activation of RANK/RANKL/OPG pathway is involved in the pathophysiology of fibrous dysplasia and associated with disease burden. J Bone Miner Res. 2019;34(2):290–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Stanton RP, Hobson GM, Montgomery BE, Moses PA, Smith-Kirwin SM, Funanage VL. Glucocorticoids decrease interleukin-6 levels and induce mineralization of cultured osteogenic cells from children with fibrous dysplasia. J Bone Miner Res. 1999;14(7):1104–1114. [DOI] [PubMed] [Google Scholar]
- 108. Ikebuchi Y, Aoki S, Honma M, et al. Coupling of bone resorption and formation by RANKL reverse signalling. Nature. 2018;561(7722):195–200. [DOI] [PubMed] [Google Scholar]
- 109. Saggio I, Remoli C, Spica E, et al. Constitutive expression of Gsα(R201C) in mice produces a heritable, direct replica of human fibrous dysplasia bone pathology and demonstrates its natural history. J Bone Miner Res. 2014;29(11):2357–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Remoli C, Michienzi S, Sacchetti B, et al. Osteoblast-specific expression of the fibrous dysplasia (FD)-causing mutation Gsα(R201C) produces a high bone mass phenotype but does not reproduce FD in the mouse. J Bone Miner Res. 2015;30(6):1030–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Hsiao EC, Boudignon BM, Chang WC, et al. Osteoblast expression of an engineered Gs-coupled receptor dramatically increases bone mass. Proc Natl Acad Sci U S A. 2008;105(4):1209–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Hsiao EC, Boudignon BM, Halloran BP, Nissenson RA, Conklin BR. Gs G protein-coupled receptor signaling in osteoblasts elicits age-dependent effects on bone formation. J Bone Miner Res. 2010;25(3):584–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Zhao X, Deng P, Iglesias-Bartolome R, et al. Expression of an active Gαs mutant in skeletal stem cells is sufficient and necessary for fibrous dysplasia initiation and maintenance. Proc Natl Acad Sci U S A. 2018;115(3):E428–E437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Khan SK, Yadav PS, Elliott G, Hu DZ, Xu R, Yang Y. Induced Gnas R201H expression from the endogenous Gnas locus causes fibrous dysplasia by up-regulating Wnt/β-catenin signaling. Proc Natl Acad Sci U S A. 2018;115(3):E418–E427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Xu R, Khan SK, Zhou T, et al. Gαs signaling controls intramembranous ossification during cranial bone development by regulating both Hedgehog and Wnt/β-catenin signaling. Bone Res. 2018;6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Stanton RP, Ippolito E, Springfield D, Lindaman L, Wientroub S, Leet A. The surgical management of fibrous dysplasia of bone. Orphanet J Rare Dis. 2012;24(7 Suppl 1):S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Leet AI, Boyce AM, Ibrahim KA, Wientroub S, Kushner H, Collins MT. Bone-grafting in polyostotic fibrous dysplasia. J Bone Joint Surg Am. 2016;98(3):211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Majoor BC, Peeters-Boef MJ, van de Sande MA, Appelman-Dijkstra NM, Hamdy NA, Dijkstra PD. What is the role of allogeneic cortical strut grafts in the treatment of fibrous dysplasia of the proximal femur? Clin Orthop Relat Res. 2017;475(3):786–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Majoor BCJ, Leithner A, van de Sande MAJ, Appelman-Dijkstra NM, Hamdy NAT, Dijkstra PDS. Individualized approach to the surgical management of fibrous dysplasia of the proximal femur. Orphanet J Rare Dis. 2018;13(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Ippolito E, Farsetti P, Benedetti Valentini M, Fichera A. Two-stage surgical treatment of complex femoral deformities with severe coxa vara in polyostotic fibrous dysplasia. JBJS Essent Surg Tech. 2016;6(2):e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Lee JS, FitzGibbon E, Butman JA, et al. Normal vision despite narrowing of the optic canal in fibrous dysplasia. N Engl J Med. 2002;347(21):1670–1676. [DOI] [PubMed] [Google Scholar]
- 122. Amit M, Collins MT, FitzGibbon EJ, Butman JA, Fliss DM, Gil Z. Surgery versus watchful waiting in patients with craniofacial fibrous dysplasia—a meta-analysis. PloS One. 2011;6(9):e25179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Paul SM, Gabor LR, Rudzinski S, et al. Disease severity and functional factors associated with walking performance in polyostotic fibrous dysplasia. Bone. 2014;60:41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Chapurlat RD, Hugueny P, Delmas PD, Meunier PJ. Treatment of fibrous dysplasia of bone with intravenous pamidronate: long-term effectiveness and evaluation of predictors of response to treatment. Bone. 2004;35(1):235–242. [DOI] [PubMed] [Google Scholar]
- 125. Liens D, Delmas PD, Meunier PJ. Long-term effects of intravenous pamidronate in fibrous dysplasia of bone. Lancet. 1994;343(8903):953–954. [DOI] [PubMed] [Google Scholar]
- 126. Metwally T, Burke A, Tsai JY, Collins MT, Boyce AM. Fibrous dysplasia and medication-related osteonecrosis of the jaw. J Oral Maxillofac Surg. 2016;74(10):1983–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Khan AA, Morrison A, Hanley DA, et al. ; International Task Force on Osteonecrosis of the Jaw . Diagnosis and management of osteonecrosis of the jaw: a systematic review and international consensus. J Bone Miner Res. 2015;30(1):3–23. [DOI] [PubMed] [Google Scholar]
- 128. Eugster EA, Rubin SD, Reiter EO, Plourde P, Jou HC, Pescovitz OH; McCune-Albright Study Group . Tamoxifen treatment for precocious puberty in McCune-Albright syndrome: a multicenter trial. J Pediatr. 2003;143(1):60–66. [DOI] [PubMed] [Google Scholar]
- 129. Sims EK, Garnett S, Guzman F, Paris F, Sultan C, Eugster EA; Fulvestrant McCune-Albright study group . Fulvestrant treatment of precocious puberty in girls with McCune-Albright syndrome. Int J Pediatr Endocrinol. 2012;2012(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Feuillan PP, Jones J, Cutler GB Jr. Long-term testolactone therapy for precocious puberty in girls with the McCune-Albright syndrome. J Clin Endocrinol Metab. 1993;77(3):647–651. [DOI] [PubMed] [Google Scholar]
- 131. Nunez SB, Calis K, Cutler GB Jr, Jones J, Feuillan PP. Lack of efficacy of fadrozole in treating precocious puberty in girls with the McCune-Albright syndrome. J Clin Endocrinol Metab. 2003;88(12):5730–5733. [DOI] [PubMed] [Google Scholar]
- 132. Mieszczak J, Lowe ES, Plourde P, Eugster EA. The aromatase inhibitor anastrozole is ineffective in the treatment of precocious puberty in girls with McCune-Albright syndrome. J Clin Endocrinol Metab. 2008;93(7):2751–2754. [DOI] [PubMed] [Google Scholar]
- 133. Tessaris D, Matarazzo P, Mussa A, et al. Combined treatment with bicalutamide and anastrozole in a young boy with peripheral precocious puberty due to McCune-Albright syndrome. Endocr J. 2012;59(2):111–117. [DOI] [PubMed] [Google Scholar]
- 134. Messina MF, Aversa T, de Sanctis L, et al. Adult height following a combined treatment of ketoconazole-cyproterone acetate-leuprolide depot in a boy with atypical McCune-Albright syndrome. Hormones (Athens). 2015;14(2):286–292. [DOI] [PubMed] [Google Scholar]
- 135. Feelders RA, Hofland LJ. Medical treatment of Cushing’s disease. J Clin Endocrinol Metab. 2013;98(2):425–438. [DOI] [PubMed] [Google Scholar]
- 136. Carpenter TO, Imel EA, Holm IA, Jan de Beur SM, Insogna KL. A clinician’s guide to X-linked hypophosphatemia. J Bone Miner Res. 2011;26(7):1381–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Ozawa T, Tateishi C, Shirakawa M, Murakami E, Ishii M, Harada T. Long-term follow-up of a case of cheek hyperpigmentation associated with McCune-Albright syndrome treated with Q-switched ruby laser. Dermatol Surg. 2011;37(2):263–266. [DOI] [PubMed] [Google Scholar]
- 138. Baltu Y, Arikan ŞM, Dölen UC, Uzun H, Alkan Bİ, Aydın O. Intramuscular myxoma: clinical and surgical observation notes on eleven cases. Int Orthop. 2017;41(4):837–843. [DOI] [PubMed] [Google Scholar]
- 139. Chapurlat RD, Delmas PD, Liens D, Meunier PJ. Long-term effects of intravenous pamidronate in fibrous dysplasia of bone. J Bone Miner Res. 1997;12(10):1746–1752. [DOI] [PubMed] [Google Scholar]
- 140. Parisi MS, Oliveri B, Mautalen CA. Effect of intravenous pamidronate on bone markers and local bone mineral density in fibrous dysplasia. Bone. 2003;33(4):582–588. [DOI] [PubMed] [Google Scholar]
- 141. Lala R, Matarazzo P, Bertelloni S, Buzi F, Rigon F, de Sanctis C. Pamidronate treatment of bone fibrous dysplasia in nine children with McCune-Albright syndrome. Acta Paediatr. 2000;89(2):188–193. [DOI] [PubMed] [Google Scholar]
- 142. Plotkin H, Rauch F, Zeitlin L, Munns C, Travers R, Glorieux FH. Effect of pamidronate treatment in children with polyostotic fibrous dysplasia of bone. J Clin Endocrinol Metab. 2003;88(10):4569–4575. [DOI] [PubMed] [Google Scholar]
- 143. Majoor BC, Appelman-Dijkstra NM, Fiocco M, van de Sande MA, Dijkstra PS, Hamdy NA. Outcome of long-term bisphosphonate therapy in McCune-Albright syndrome and polyostotic fibrous dysplasia. J Bone Miner Res. 2017;32(2):264–276. [DOI] [PubMed] [Google Scholar]
- 144. Boyce AM, Kelly MH, Brillante BA, et al. A randomized, double blind, placebo-controlled trial of alendronate treatment for fibrous dysplasia of bone. J Clin Endocrinol Metab. 2014;99(11):4133–4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Al-Ibraheemi A, Inwards CY, Zreik RT, et al. Histologic spectrum of giant cell tumor (GCT) of bone in patients 18 years of age and below: a study of 63 patients. Am J Surg Pathol. 2016;40(12):1702–1712. [DOI] [PubMed] [Google Scholar]
- 146. Chawla S, Henshaw R, Seeger L, et al. Safety and efficacy of denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: interim analysis of an open-label, parallel-group, phase 2 study. Lancet Oncol. 2013;14(9):901–908. [DOI] [PubMed] [Google Scholar]
- 147. Martin-Broto J, Cleeland CS, Glare PA, et al. Effects of denosumab on pain and analgesic use in giant cell tumor of bone: interim results from a phase II study. Acta Oncol. 2014;53(9):1173–1179. [DOI] [PubMed] [Google Scholar]
- 148. Lipplaa A, Dijkstra S, Gelderblom H. Challenges of denosumab in giant cell tumor of bone, and other giant cell-rich tumors of bone. Curr Opin Oncol. 2019;31(4):329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Boyce AM, Chong WH, Yao J, et al. Denosumab treatment for fibrous dysplasia. J Bone Miner Res. 2012;27(7):1462–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Wang HD, Boyce AM, Tsai JY, et al. Effects of denosumab treatment and discontinuation on human growth plates. J Clin Endocrinol Metab. 2014;99(3):891–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Boyce AM. Denosumab: an emerging therapy in pediatric bone disorders. Curr Osteoporos Rep. 2017;15(4):283–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Lamy O, Gonzalez-Rodriguez E, Stoll D, Hans D, Aubry-Rozier B. Severe rebound-associated vertebral fractures after denosumab discontinuation: 9 clinical cases report. J Clin Endocrinol Metab. 2017;102(2):354–358. [DOI] [PubMed] [Google Scholar]
- 153. Eller-Vainicher C, Rossi DS, Guglielmi G, et al. Prompt clinical and biochemical response to denosumab in a young adult patient with craniofacial fibrous dysplasia. Clin Cases Miner Bone Metab. 2016;13(3):253–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Ganda K, Seibel MJ. Rapid biochemical response to denosumab in fibrous dysplasia of bone: report of two cases. Osteoporos Int. 2014;25(2):777–782. [DOI] [PubMed] [Google Scholar]
- 155. Benhamou J, Gensburger D, Chapurlat R. Transient improvement of severe pain from fibrous dysplasia of bone with denosumab treatment. Joint Bone Spine. 2014;81(6):549–550. [DOI] [PubMed] [Google Scholar]
- 156. de Boysson H, Johnson A, Hablani N, Hajlaoui W, Auzary C, Geffray L. Tocilizumab in the treatment of a polyostotic variant of fibrous dysplasia of bone. Rheumatology (Oxford). 2015;54(9):1747–1749. [DOI] [PubMed] [Google Scholar]
- 157. Carpenter TO, Whyte MP, Imel EA, et al. Burosumab therapy in children with X-linked hypophosphatemia. N Engl J Med. 2018;378(21):1987–1998. [DOI] [PubMed] [Google Scholar]
- 158. Whyte MP, Carpenter TO, Gottesman GS, et al. Efficacy and safety of burosumab in children aged 1-4 years with X-linked hypophosphataemia: a multicentre, open-label, phase 2 trial. Lancet Diabetes Endocrinol. 2019;7(3):189–199. [DOI] [PubMed] [Google Scholar]
- 159. Insogna KL, Briot K, Imel EA, et al. ; AXLES 1 Investigators . A randomized, double-blind, placebo-controlled, phase 3 trial evaluating the efficacy of burosumab, an anti-FGF23 antibody, in adults with X-linked hypophosphatemia: week 24 primary analysis. J Bone Miner Res. 2018;33(8):1383–1393. [DOI] [PubMed] [Google Scholar]
- 160. Lamb YN. Burosumab: first global approval. Drugs. 2018;78(6):707–714. [DOI] [PubMed] [Google Scholar]
- 161. Bhattacharyya N, Hu X, Chen CZ, et al. A high throughput screening assay system for the identification of small molecule inhibitors of gsp. PloS One. 2014;9(3):e90766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Annala S, Feng X, Shridhar N, et al. Direct targeting of Galphaq and Galpha11 oncoproteins in cancer cells. Sci Signal. 2019;12(573):eaau5948. [DOI] [PubMed] [Google Scholar]
- 163. Lapadula D, Farias E, Randolph CE, et al. Effects of oncogenic Gαq and Gα11 inhibition by FR900359 in uveal melanoma. Mol Cancer Res. 2019;17(4):963–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Méndez-Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Bianco P, Cao X, Frenette PS, et al. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med. 2013;19(1):35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Robey PG, Kuznetsov SA, Ren J, Klein HG, Sabatino M, Stroncek DF. Generation of clinical grade human bone marrow stromal cells for use in bone regeneration. Bone. 2015;70:87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Miller RP, Hanley PJ. Isolation and manufacture of clinical-grade bone marrow-derived human mesenchymal stromal cells. Methods Mol Biol. 2016;1416:301–312. [DOI] [PubMed] [Google Scholar]
- 168. Luby AO, Ranganathan K, Lynn JV, Nelson NS, Donneys A, Buchman SR. Stem cells for bone regeneration: current state and future directions. J Craniofac Surg. 2019;30(3):730–735. [DOI] [PubMed] [Google Scholar]
- 169. Wang S, Zhu R, Li H, Li J, Han Q, Zhao RC. Mesenchymal stem cells and immune disorders: from basic science to clinical transition. Front Med. 2018;13(2):138–151. [DOI] [PubMed] [Google Scholar]
- 170. Kim N, Cho SG. New strategies for overcoming limitations of mesenchymal stem cell-based immune modulation. Int J Stem Cells. 2015;8(1):54–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Bieback K, Kuçi S, Schäfer R. Production and quality testing of multipotent mesenchymal stromal cell therapeutics for clinical use. Transfusion. 2019;59(6):2164–2173. [DOI] [PubMed] [Google Scholar]
- 172. Leet AI, Magur E, Lee JS, Wientroub S, Robey PG, Collins MT. Fibrous dysplasia in the spine: prevalence of lesions and association with scoliosis. J Bone Joint Surg Am. 2004;86(3):531–537. [PubMed] [Google Scholar]
- 173. Collins MT, Kushner H, Reynolds JC, et al. An instrument to measure skeletal burden and predict functional outcome in fibrous dysplasia of bone. J Bone Miner Res. 2005;20(2):219–226. [DOI] [PubMed] [Google Scholar]
- 174. Papadakis G, Manikis G, Karantanas A, et al. 18F-NaF PET/CT imaging in fibrous dysplasia of bone. J Bone Miner Res. 2019;34(9):1619–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Javaid MK, Forestier-Zhang L, Watts L, et al. The RUDY study platform—a novel approach to patient driven research in rare musculoskeletal diseases. Orphanet J Rare Dis. 2016;11(1):150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Teare HJA, Hogg J, Kaye J, et al. The RUDY study: using digital technologies to enable a research partnership. Eur J Hum Genet. 2017;25(7):816–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Boyce AM, Turner A, Watts L, et al. Improving patient outcomes in fibrous dysplasia/McCune-Albright syndrome: an international multidisciplinary workshop to inform an international partnership. Arch Osteoporos. 2017;12(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]