Abstract
The study of N-linked glycosylation as it relates to virus biology has become an area of intense interest in recent years due to its ability to impart various advantages to virus survival and virulence. HIV and influenza, two clear threats to human health, have been shown to rely on expression of specific oligosaccharides to evade detection by the host immune system. Additionally, other viruses such as Hendra, SARS-CoV, influenza, hepatitis and West Nile rely on N-linked glycosylation for crucial functions such as entry into host cells, proteolytic processing and protein trafficking. This review focuses on recent findings on the importance of glycosylation to viral virulence and immune evasion for several prominent human pathogens.
Viral modification by glycosylation
Glycans are perhaps one of the most important molecular components of cells and confer a diversity of structure and function that is still underappreciated. Although the field of glycobiology has a nearly one-hundred year history that began with investigations on the basic constituents of the cell, a renaissance has occurred with the development of tools to better understand and explore the role of glycosylation in health and disease. The limited expression of proteins by the mammalian genome has made clear the importance and value of glycosylation as a post-translational modification for the diversity of protein function. The past decade of research in this field has led to the elucidation of the enzymes involved and the function and role that they have in biological processes including cell adhesion, receptor activation, signal transduction, molecular trafficking and endocytosis. The current area of intensive study in this field is the description of organismal glycomes, or the carbohydrate repertoire of an organism. It has been estimated that the mammalian cell could have a library of thousands of potential carbohydrate structures. This range of diversity, although theoretically vast, is likely to be constrained by mechanisms within the cell.
One of the most common forms of protein modification is N-linked glycosylation, in which a high mannose core is attached to the amide nitrogen of asparagine in the context of the conserved motif Asn-X-Ser/Thr. This attachment occurs early in protein synthesis, followed by a complex process of trimming and remodeling of the oligosaccharide during transit through the endoplasmic reticulum (ER) and Golgi (Figure 1 ). The result is a glycoprotein with varying oligosaccharide structures. Viruses use this host cell process to modify proteins present on their surface, which ultimately impacts the role of the viral glycoproteins in stability, antigenicity and host cell invasion. Here, we review recent findings on the role of glycosylation in virus biology and host response to viral infection. These results provide a better understanding of carbohydrate in the biology of infection and host response and additionally provide potential avenues to explore better therapeutics.
Figure 1.
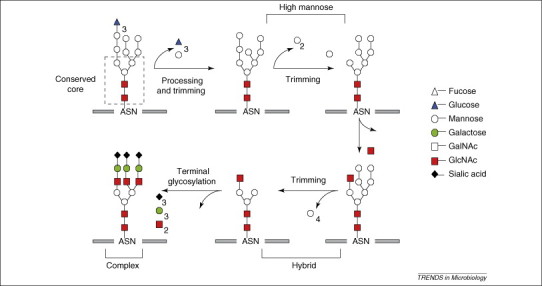
Biosynthetic pathway for the generation of N-linked glycosylation. Processing of N-linked oligosaccharide occurs in the ER and Golgi. Following glucose trimming in the ER, high mannose glycans are available for further processing and trimming by glycosidase and mannosidases to yield high-mannose, hybrid and complex glycan structures.
Viral glycoproteins
Viruses co-opt cellular biosynthetic pathways to generate their genetic and structural material, and several viruses have been shown to take advantage of the cellular glycosylation pathway to modify viral proteins. The majority of recent evidence on virus utilization of glycosylation pathways has included descriptions of attachment of N-linked oligosaccharides. This modification results in at least two changes in the viral life cycle. First, N-glycosylation of envelope or surface proteins can promote proper folding and subsequent trafficking using host cellular chaperones and folding factors. Of the viruses that have been studied thus far, all of them used either calnexin and/or calreticulin to facilitate the proper folding and handling of the viral protein. Although the cell cannot distinguish between host and viral proteins, one difference that is often seen is a general increase in the level of glycosylation in many viral glycoproteins. During viral evolution, sites are easily added and deleted and with this potential diversity of modification, the complexity of viral glycoproteins is increased. Alteration of a site or sites of glycosylation can have dramatic impacts on survival and transmissibility of the virus. Small changes can alter folding and conformation, affecting portions of the entire molecule 1, 2, 3. Second, changes in glycosylation can affect interaction with receptors and cause a virus to be more recognizable by the innate factors of host immune cells and less recognized by antibody, thus impacting viral replication and infectivity. Many viruses that impact human health use glycosylation for important functions in pathogenesis and immune evasion including influenza, HIV, hepatitis and West Nile virus, among others.
Influenza
Influenza A virus is one of the most studied viruses with respect to glycosylation. The surface proteins hemagglutinin (HA) and neuraminidase (NA) both use glycosylation for a variety of important functions including receptor binding, infectivity, virus release and neurovirulence 4, 5, 6, 7, 8, 9, 10. HA is the major surface glycoprotein on the envelope of influenza and mediates attachment and entry into the host through interactions with sialylated receptors. HA undergoes post-translational, host-cell dependent glycosylation that is crucial to the proper folding and trafficking of the molecule during infection [11]. All HA molecules are glycosylated to varying degrees, and conservation between strains and subtypes is generally not seen [12]. The number of sites has been described to range between five and 11 sites with the majority of sites residing in the globular head of the molecule. In the human H3N2 subtype, additional sites have been introduced over the past 30 years that increase the potential for elevated glycosylation [13]. In recent years, the development of reverse genetics and site-directed mutagenesis to construct recombinant and reassortant viruses [14] has allowed for more careful analysis of the role of glycosylation.
It is known that carbohydrate addition can have both positive and detrimental effects on the virus. For example, cleavage or ‘activation’ of the HA by host proteases is the first step in influenza entry into a cell and the susceptibility of the HA molecule to specific cellular proteases determines the tissue tropism and virulence of the virus. If carbohydrate is positioned close to the HA cleavage site, the glycan can prevent protease access and virus entry. Therefore, cleavage can only occur easily when this site is unoccupied by carbohydrate 15, 16. Alternatively, work from Klenk et al. illustrates that carbohydrates positioned near the receptor binding site are crucial to the replication and release of the virus [5] in a mechanism where glycosylation modulates binding affinity and controls receptor specificity [4]. Carbohydrate positioned around the globular head can mask antigenic sites from immune recognition. The latter example is believed to be the mechanism for antigenic drift in H3N2 viruses where successive glycosylation events have potentially created a glycan shield that prevents accessibility and recognition of antibody. Evidence for this view has come from studies where successively adding additional Asn-X-Ser/Thr sites for potential glycosylation by site-directed mutagenesis reveal the ability of the virus to evade the host response without negatively impacting survival and biological activity [9]. The addition of increased carbohydrate onto the globular head of the HA molecule of H3N2 viruses resulted in some decrease to receptor binding but had no effect on fusion activity. Importantly, however, these viruses were now more resistant to antibody recognition. The effect of carbohydrate was especially important when looking at the interaction of HA and NA by showing that a balance is needed between receptor binding activity and virus release. A virus containing HA with little carbohydrate modification can tightly bind the receptor requiring greater NA activity to promote particle release. Conversely, an HA with more extensive glycosylation interacts weakly with receptors and requires a less active NA to facilitate release. Overall, HA depends on a balance of glycosylation to mediate proper folding, interaction of virus with receptor and efficient particle release. Although additional glycosylation of H3 HA does not negatively impact function, the addition of glycosylation to the globular head of H2 HA does affect receptor binding and fusion activity 7, 8. H2N2 viruses have been seen infrequently and have been present in humans for a shorter period of time compared to the H1 and H3 influenza viruses. As a consequence, the oligosaccharide content has changed very little since H2 viruses were first encountered, suggesting that the increase in carbohydrate reduces the biological activity of the virus and therefore decreases the ability to replicate in humans.
HIV
HIV-1 is a highly mutagenic and variable virus from the Retroviridae family of viruses that contains multiple subtypes or clades that are further distinguished by wide intra-clade variation. The envelope protein (gp120) of HIV-1 is one of the most heavily glycosylated proteins in nature, with many of high mannose composition. Because glycosylation is important to the bioactivity of many proteins, the suggestion that surface carbohydrate on the mature gp120 molecule has a direct role in the interaction with gp120 and CD4 has been explored. Early studies suggested that the loss of glycan diminishes the binding of the virus to CD4 but does not abolish the interaction 17, 18. As a consequence of reduced interaction with CD4, cell infectivity and cytopathicity were also reduced without much compromise to replication or the ability of the host to become infected. More recent studies have focused on defining the role that glycosylation has in HIV-1 survival and immune evasion. A recent survey of data from global HIV gp120 sequences showed that the molecule had a range of possible N-linked glycosylation sites of between 18 and 33 with a mean of 25 [19]. Sagar et al. have shown that sequences in the V1-V2 envelope loop add glycosylation during the course of infection to alter sensitivity to neutralizing antibody [20] and Wolk et al. have shown that N-linked glycan within the V1-V2 loop of fifteen variants of the HIV-1 NL4–3 gp120 is indispensable to viral infectivity and reduced sensitivity to serum antibody [21].
Neutralizing antibodies are one of the main components of our immune response to pathogens. However, the role that neutralizing antibodies have in the biology of HIV infection is not altogether clear. The contribution of N-glycan addition to protection is a question that remains open because many glycans are highly conserved components of gp120. For example, the broadly neutralizing human monoclonal antibody 2G12 targets an epitope of the gp120 molecule that contains high mannose and/or hybrid glycans around residues 295, 332 and 392, with contributions from peripheral glycans on 386 and 448 [22]. Analysis of this region of specificity by Calarese et al. 23, 24 has highlighted the importance of the D1 and D3 arms of Man9GlcNAc2 in the interaction with 2G12 neutralizing antibody. The epitope was found to be composed primarily of carbohydrate, is mannose-dependent and is a highly conserved portion of gp120. The conservation of this epitope in gp120 suggests a functional role in infection that can be speculated to be related to the mannose-dependent attachment of HIV-1 to the mannose receptor (MMR), DC-SIGN or other lectins that could facilitate entry into host cells. By contrast, a study by Wei et al. suggests that a glycan shield formed from mutations and subsequent addition of N-linked glycan can provide protection from host neutralizing antibodies [25]. The study showed that escape virus often contained variation in the env gene that involved the addition of N-linked glycan at positions that were not known neutralization epitopes. These findings suggested that the additions provide a mechanism of antibody evasion that did not affect the ability of the virus to bind to cellular receptors. For the glycan shield to provide protection from neutralizing antibody, multiple mutations were required. This could suggest that glycosylation is a contributor but not solely responsible for neutralizing antibody resistance. A study by Frost et al. [26] suggests that the escape from neutralizing antibody is promoted by the accumulation of many amino acid substitutions and that the rate of escape is highly variable among individuals. The evolving glycan shield that is produced represents a mechanism for viral persistence despite an increasing antibody repertoire. For this reason, many research groups are attempting to exploit the glycans of gp120 in an attempt to make the virus more recognizable and susceptible to immunological neutralization. One example is illustrated by recent findings by Poon et al. [27], who examined evolutionary interactions between glycosylation sites in the HIV-1 envelope. The results of the study suggest that the configurations in the HIV-1 glycan shield are limited by functional interactions between glycan structures. Structurally, negative interactions (exclusive) were found where glycans were closely positioned and positive interactions (inclusive) were found where glycans were some distance apart. These results suggest a weakness in the HIV-1 population that might provide additional targets for neutralizing antibody.
Drugs directed against the carbohydrate component of the protein could potentially select for viruses that contain deletions and unmask previously hidden epitopes. Several groups have successfully used the antimalarial drugs chloroquine and hydroxychloroquine in combination therapy to reduce the viral load of HIV in patients 28, 29, 30, 31. The suggestion has also been made that this approach can be applied to other viruses that contain a glycosylated envelope [32] because the mechanism of action of chloroquine interferes with the viral life cycle at several stages. First, chloroquine accumulates in the endosome and prevents acidification, a process that is crucial to several viruses in promoting fusion and entry into the cytoplasm. Second, chloroquine can interfere with the activity of glycosyltransferases in the ER and Golgi. This interference results in improper carbohydrate addition and lack of association with calnexin and calreticulin. Without assistance from these two chaperone proteins, many viral proteins will be misfolded and non-functional.
West Nile virus
West Nile virus (WNV), a member of the Flaviviridae family of viruses, has recently become a pathogen of global concern and interest. The envelope protein of WNV has an important role in the replication and maturation process of the virus. Studies have suggested that the flavivirus envelope protein E is involved in several biological activities including receptor binding, membrane fusion and virus assembly. The glycosylation of this protein has also been proposed to be linked to neuroinvasiveness and replication efficiency in several WNV strains 33, 34, 35. Lad et al. have also shown that glycosylation is important in maintaining the native conformation and expression of the E protein in much the same way that glycosylation is important to the conformation and folding of influenza HA [36]. More recent data would suggest a further role for glycosylation in the infectivity and particle assembly of WNV. Hanna et al. have shown that there are two proteins (E and prM) in WNV that are glycosylated and affect the efficiency of virus release and infections in a manner that is both cell-type dependent and species dependent [37]. Removal of glycosylation on either the prM or E proteins results in a decrease of sub-viral particle release. Interestingly, the loss of glycosylation of the E protein resulted in virus that was modestly more infectious per genome copy when used to infect two cell types but was greatly enhanced in other cells. These results suggest a role for envelope glycosylation in cell and tissue tropism and an importance in the overall biology of WNV [37].
Other important human viral pathogens
The importance of N-glycosylation is not limited to the pathogens described earlier; many viruses are dependent on N-linked glycosylation for vital biological functions. Enveloped viruses have integral envelope proteins that participate in host–cell interactions such as receptor binding and internalization and, in most viruses, glycosylation has a role in biogenesis, stability, antigenicity and infectivity. For example, infectivity and intracellular transport is heavily influenced by glycosylation in hepatitis C virus (HCV). Investigations of the E1 protein in HCV, which contains four glycosylation sites, showed that mutagenesis of these sites impacted the translocation of the protein to the cell surface [38]. Furthermore, this study showed that glycosidase treatment of the E1 protein led to a 35% decrease in the viral titer. Goffard et al. showed in a more comprehensive study of several HCV envelope glycoproteins that the presence of glycan on some HCV envelope glycoproteins has a role in protein folding while glycosylation of other HCV proteins has a role in virus entry 39, 40.
New and emerging viruses such as Ebola, Hantaan, Newcastle, Hendra, Nipah, metapneumovirus and SARS-CoV have all been shown to have N-linked glycosylation with vital roles in infectivity, protein folding, tropism, proteolytic processing and immune evasion 41, 42, 43, 44, 45, 46, 47, 48, 49. Interestingly, in the case of Nipah virus, glycosylation has a dual role: glycosylation leads to enhanced resistance to antibody neutralization but alternatively causes a reduction in membrane fusion and viral entry [42]. Clearly, in many viruses the balance of glycosylation is important to the proper protein functions and life cycle.
Impact of viral glycosylation on immune factor interaction
It has become apparent that glycosylation in many enveloped viruses is important to the biology of infection. One of the early mechanisms for recognition of pathogens is likewise dependent on the glycosylation state of the pathogen. Over the past decade or so, an increased interest has been directed at the identification of novel carbohydrate recognition molecules that have been divided into cell-associated and soluble categories. One family of note is the calcium-dependent lectin group of molecules, examples of which are the cell-associated macrophage mannose receptor (MMR) and the dendritic cell-specific ICAM-3 grabbing non-integrin (DC-SIGN), the soluble lectins surfactant associated protein-A and -D (SP-A and SP-D), and mannose binding lectin (MBL). Each of these has an important role in the sensing and recognition of pathogens. Within the immune system, these C-type lectins have been shown to participate as pathogen recognition molecules and adhesion receptors 50, 51. Normally, these molecules would participate in the clearance of pathogens but several viruses have subverted these molecules as vehicles of binding and entry (Table 1 and Figure 2a).
Table 1.
Viral protein glycan structure, function and host glycoprotein interaction
| Virus protein | Glycan structure | Role | Host glycoprotein | Refs |
|---|---|---|---|---|
| Influenza HA | High mannose | Attachment | MBL | [64] |
| GlcNAc | Release | SP-D | 72, 73 | |
| Glycan shield? | MMR | [52] | ||
| Gp340 | 74, 75 | |||
| HIV-1 gp120 | High mannose | Attachment | MBL | 76, 77 |
| Hybrid | Glycan shield | SP-D | [67] | |
| Sialic acid | DC-SIGN | 46, 78 | ||
| DC-SIGNR | [46] | |||
| MMR | [53] | |||
| Hepatitis C virus E1, E2 | High mannose | Infectivity | DC-SIGN | [79] |
| Entry | L-SIGN | [55] | ||
| West Nile virus E | High mannose | Replication | DC-SIGN | 57, 80 |
| Fucose | Neuroinvasion | DC-SIGNR | ||
| SARS-CoV S, M | High mannose | Assembly | LSECtin | 59, 62 |
| Hybrid | Attachment | DC-SIGN | ||
| GalNAc | ||||
| Ebola GP | High mannose | Infectivity | DC-SIGN | [58] |
| DC-SIGNR |
Figure 2.

Glycan interactions result in virus neutralization, internalization or degradation. Interactions between virus envelope glycoprotein and host molecules comprise several general interactions. (a) Virus glycan can interact with cell-surface-bound lectin molecules such as MMR and DC-SIGN to obtain entry into the target cell. (b) Lectin binding to virus can lead to internalization of complexed virus into a degradative pathway for processing and presentation to the immune system. (c) Soluble lectin such as SP-D or MMR can interact with virus glycans to neutralize virus infectivity and block associations with host cell receptors.
Influenza A virus and HIV have been reported to use MMR as a potential uptake receptor 52, 53, 54 (Figure 2a). These findings might be especially important in the biology of influenza because the virus requires delivery to an acidic compartment for proper fusion. The MMR participates in both endocytic and phagocytic uptake of proteins and particles, and might provide a direct infection route for influenza in addition to providing a mechanism of evasion from macrophage recognition. The HCV E2 protein has been found to associate with DC-SIGN and the related liver lectin (L-SIGN) through high-mannose N-glycans. These results suggest a possible mechanism for liver tropism and dendritic cell tropism by illustrating two novel HCV binding receptors [55]. The presence of glycosylation on the prM or E proteins of WNV is sufficient to allow for interaction and uptake by DC-SIGN and DC-SIGNR. Davis et al. have further shown that glycosylated WNV proteins display a preference for DC-SIGNR, although the virus can attach to both 56, 57. In these studies, the location of the glycosylation sites on the virion determined the types of glycan that were incorporated and that then controlled the specificity for either DC-SIGN or DC-SIGNR. DC-SIGNR recognized viruses with broad specificity and DC-SIGN recognized viruses with predominantly mannose-rich surfaces. Lin et al. have shown that Ebola virus glycoproteins with high-mannose content interact with DC-SIGNR, whereas those glycoproteins with complex glycan do not [46]. Marzi et al. have shown that the signal peptide of Ebola and Marburg virus glycoproteins has a role in the interactions with both DC-SIGN and DC-SIGNR 58, 59. Finally, MMR and DC-SIGN have been shown to bind and transmit HIV to T-cells through interaction with the envelope glycoprotein 53, 54, 60, 61.
Gramberg et al. have also recently shown that interaction between the SARS-CoV S protein and filovirus glycoproteins with the lectin LSECtin can enhance infection [62]. LSECtin is a cell-associated lectin that is expressed from the same chromosomal locus as DC-SIGN and is co-expressed in the liver and lymph nodes. Interestingly, this same study showed that LSECtin does not interact with either HIV or HCV envelope proteins, perhaps because LSECtin does not recognize high-mannose glycan. The use of MMR, DC-SIGN and LSECtin as attachment and potential entry receptors could confer an additional advantage to viruses. Cellular attachment to these receptors might augment viral infection and promote viral dissemination between cell and hosts. Because these receptors are resident on cells of the innate immune system, an additional advantage of beginning their life cycle in cells tasked with clearance of infectious agents might make these agents invisible to immune recognition for a short time.
As a counterpoint to the binding of various viruses to DC-SIGN, soluble molecules such as MBL can bind to Ebola and Marburg envelope glycoproteins and block the association of both viruses with DC-SIGN. Similarly, SP-A and SP-D can both interact with influenza to prevent the association of the HA with its receptor. Binding of MMR, MBL or SP-D to influenza A virus involves the carbohydrate recognition domain of the molecule and high-mannose oligosaccharides of the viral proteins hemagglutinin and neuraminidase 63, 64, 65, 66 and can potentially lead to internalization of the lectin–virus complex and delivery of complexed virus to degradative compartments for processing and presentation by the immune system (Figure 2b). MMR and SP-D can additionally bind to HIV and prevent entry and replication [67]. As soluble recognition molecules of innate immunity, these molecules are able to bind directly to various viruses, including retroviruses and influenza viruses, and can interfere with the association of virus with its cognate receptor [64] (Figure 2c).
Concluding remarks and future perspectives
Glycosylation clearly has importance to both the pathogen and the host. Many of the interactions might be a result of a lack of adaptation by the virus. The viruses that have been discussed in this article are recent pathogens of humans and might not have been in circulation for a sufficient time to have adapted. In the case of influenza, which is the pathogen that has circulated in humans for the longest period of time, the level of glycosylation has gradually increased with a concomitant attenuation of disease. As yet, all of these pathogens are of concern to global public health and an understanding of the role of cellular processes such as glycosylation in the biology of viral infection is one step towards developing successful treatment strategies. Much has been learned about the importance of glycosylation to proper viral protein function and the interaction between pathogen-associated carbohydrate and portions of the innate and adaptive immune systems. This knowledge can now be applied to the development of novel therapies to strike at this vital element in virus biology. Recently, investigators have discovered plant- and bacterial-derived lectins that have high specificity for the carbohydrate found on the virus surface. These compounds, which include the cyanobacterial cyanovirin-N (CV-N), plant lectins Urtica dioica agglutinin, Galanthus nivalia and concanavalin A, can interfere with attachment and also directly neutralize HIV-1 and influenza virus in vitro 68, 69, 70, 71. The future of research in this area provides the ability to reveal avenues for new antiviral therapeutics and prophylaxis against a wide range of enveloped viruses that contain oligosaccharide-rich membrane glycoproteins.
References
- 1.Land A., Braakman I. Folding of the human immunodeficiency virus type 1 envelope glycoprotein in the endoplasmic reticulum. Biochimie. 2001;83:783–790. doi: 10.1016/s0300-9084(01)01314-1. [DOI] [PubMed] [Google Scholar]
- 2.Slater-Handshy T. HCV E2 glycoprotein: mutagenesis of N-linked glycosylation sites and its effects on E2 expression and processing. Virology. 2004;319:36–48. doi: 10.1016/j.virol.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 3.Meunier J.C. Analysis of the glycosylation sites of hepatitis C virus (HCV) glycoprotein E1 and the influence of E1 glycans on the formation of the HCV glycoprotein complex. J. Gen. Virol. 1999;80:887–896. doi: 10.1099/0022-1317-80-4-887. [DOI] [PubMed] [Google Scholar]
- 4.Wagner R. Interdependence of hemagglutinin glycosylation and neuraminidase as regulators of influenza virus growth: a study by reverse genetics. J. Virol. 2000;74:6316–6323. doi: 10.1128/jvi.74.14.6316-6323.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klenk H.D. Importance of hemagglutinin glycosylation for the biological functions of influenza virus. Virus Res. 2002;82:73–75. doi: 10.1016/s0168-1702(01)00389-6. [DOI] [PubMed] [Google Scholar]
- 6.Baigent S.J., McCauley J.W. Glycosylation of haemagglutinin and stalk-length of neuraminidase combine to regulate the growth of avian influenza viruses in tissue culture. Virus Res. 2001;79:177–185. doi: 10.1016/s0168-1702(01)00272-6. [DOI] [PubMed] [Google Scholar]
- 7.Tsuchiya E. Role of overlapping glycosylation sequons in antigenic properties, intracellular transport and biological activities of influenza A/H2N2 virus haemagglutinin. J. Gen. Virol. 2002;83:3067–3074. doi: 10.1099/0022-1317-83-12-3067. [DOI] [PubMed] [Google Scholar]
- 8.Tsuchiya E. Effect of addition of new oligosaccharide chains to the globular head of influenza A/H2N2 virus haemagglutinin on the intracellular transport and biological activities of the molecule. J. Gen. Virol. 2002;83:1137–1146. doi: 10.1099/0022-1317-83-5-1137. [DOI] [PubMed] [Google Scholar]
- 9.Abe Y. Effect of the addition of oligosaccharides on the biological activities and antigenicity of influenza A/H3N2 virus hemagglutinin. J. Virol. 2004;78:9605–9611. doi: 10.1128/JVI.78.18.9605-9611.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaverin N.V. Structure of antigenic sites on the haemagglutinin molecule of H5 avian influenza virus and phenotypic variation of escape mutants. J. Gen. Virol. 2002;83:2497–2505. doi: 10.1099/0022-1317-83-10-2497. [DOI] [PubMed] [Google Scholar]
- 11.Daniels R. N-linked glycans direct the cotranslational folding pathway of influenza hemagglutinin. Mol. Cell. 2003;11:79–90. doi: 10.1016/s1097-2765(02)00821-3. [DOI] [PubMed] [Google Scholar]
- 12.Keil W. Carbohydrates of influenza virus. V. Oligosaccharides attached to individual glycosylation sites of the hemagglutinin of fowl plague virus. Virology. 1984;133:77–91. doi: 10.1016/0042-6822(84)90427-6. [DOI] [PubMed] [Google Scholar]
- 13.Skehel J.J., Wiley D.C. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu. Rev. Biochem. 2000;69:531–569. doi: 10.1146/annurev.biochem.69.1.531. [DOI] [PubMed] [Google Scholar]
- 14.Hoffmann E. Eight-plasmid system for rapid generation of influenza virus vaccines. Vaccine. 2002;20:3165–3170. doi: 10.1016/s0264-410x(02)00268-2. [DOI] [PubMed] [Google Scholar]
- 15.Deshpande K.L. Glycosylation affects cleavage of an H5N2 influenza virus hemagglutinin and regulates virulence. Proc. Natl. Acad. Sci. U. S. A. 1987;84:36–40. doi: 10.1073/pnas.84.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zambon M.C. Epidemiology and pathogenesis of influenza. J. Antimicrob. Chemother. 1999;44(Suppl. B):3–9. doi: 10.1093/jac/44.suppl_2.3. [DOI] [PubMed] [Google Scholar]
- 17.Fenouillet E. Role of N-linked glycans of envelope glycoproteins in infectivity of human immunodeficiency virus type 1. J. Virol. 1990;64:2841–2848. doi: 10.1128/jvi.64.6.2841-2848.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montefiori D.C. Role of protein N-glycosylation in pathogenesis of human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. U. S. A. 1988;85:9248–9252. doi: 10.1073/pnas.85.23.9248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korber B. Evolutionary and immunological implications of contemporary HIV-1 variation. Br. Med. Bull. 2001;58:19–42. doi: 10.1093/bmb/58.1.19. [DOI] [PubMed] [Google Scholar]
- 20.Sagar M. Human immunodeficiency virus type 1 V1-V2 envelope loop sequences expand and add glycosylation sites over the course of infection, and these modifications affect antibody neutralization sensitivity. J. Virol. 2006;80:9586–9598. doi: 10.1128/JVI.00141-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolk T., Schreiber M. N-Glycans in the gp120 V1/V2 domain of the HIV-1 strain NL4-3 are indispensable for viral infectivity and resistance against antibody neutralization. Med. Microbiol. Immunol. (Berl.) 2006;195:165–172. doi: 10.1007/s00430-006-0016-z. [DOI] [PubMed] [Google Scholar]
- 22.Sanders R.W. The mannose-dependent epitope for neutralizing antibody 2G12 on human immunodeficiency virus type 1 glycoprotein gp120. J. Virol. 2002;76:7293–7305. doi: 10.1128/JVI.76.14.7293-7305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calarese D.A. Dissection of the carbohydrate specificity of the broadly neutralizing anti-HIV-1 antibody 2G12. Proc. Natl. Acad. Sci. U. S. A. 2005;102:13372–13377. doi: 10.1073/pnas.0505763102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calarese D.A. Antibody domain exchange is an immunological solution to carbohydrate cluster recognition. Science. 2003;300:2065–2071. doi: 10.1126/science.1083182. [DOI] [PubMed] [Google Scholar]
- 25.Wei X. Antibody neutralization and escape by HIV-1. Nature. 2003;422:307–312. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- 26.Frost S.D. Neutralizing antibody responses drive the evolution of human immunodeficiency virus type 1 envelope during recent HIV infection. Proc. Natl. Acad. Sci. U. S. A. 2005;102:18514–18519. doi: 10.1073/pnas.0504658102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poon A.F. Evolutionary interactions between N-linked glycosylation sites in the HIV-1 envelope. PLoS Comput. Biol. 2007;3:e11. doi: 10.1371/journal.pcbi.0030011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romanelli F. Chloroquine and hydroxychloroquine as inhibitors of human immunodeficiency virus (HIV-1) activity. Curr. Pharm. Des. 2004;10:2643–2648. doi: 10.2174/1381612043383791. [DOI] [PubMed] [Google Scholar]
- 29.Boelaert J.R. Chloroquine exerts an additive in vitro anti-HIV type 1 effect when associated with didanosine and hydroxyurea. AIDS Res. Hum. Retroviruses. 1999;15:1241–1247. doi: 10.1089/088922299310133. [DOI] [PubMed] [Google Scholar]
- 30.Sperber K. Hydroxychloroquine treatment of patients with human immunodeficiency virus type 1. Clin. Ther. 1995;17:622–636. doi: 10.1016/0149-2918(95)80039-5. [DOI] [PubMed] [Google Scholar]
- 31.Paton N.I., Aboulhab J. Hydroxychloroquine, hydroxyurea and didanosine as initial therapy for HIV-infected patients with low viral load: safety, efficacy and resistance profile after 144 weeks. HIV Med. 2005;6:13–20. doi: 10.1111/j.1468-1293.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- 32.Savarino A. New insights into the antiviral effects of chloroquine. Lancet Infect. Dis. 2006;6:67–69. doi: 10.1016/S1473-3099(06)70361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shirato K. Viral envelope protein glycosylation is a molecular determinant of the neuroinvasiveness of the New York strain of West Nile virus. J. Gen. Virol. 2004;85:3637–3645. doi: 10.1099/vir.0.80247-0. [DOI] [PubMed] [Google Scholar]
- 34.Scherret J.H. Biological significance of glycosylation of the envelope protein of Kunjin virus. Ann. N. Y. Acad. Sci. 2001;951:361–363. doi: 10.1111/j.1749-6632.2001.tb02719.x. [DOI] [PubMed] [Google Scholar]
- 35.Beasley D.W. Envelope protein glycosylation status influences mouse neuroinvasion phenotype of genetic lineage 1 West Nile virus strains. J. Virol. 2005;79:8339–8347. doi: 10.1128/JVI.79.13.8339-8347.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lad V.J. Effect of tunicamycin on expression of epitopes on Japanese encephalitis virus glycoprotein E in porcine kidney cells. Acta Virol. 2000;44:359–364. [PubMed] [Google Scholar]
- 37.Hanna S.L. N-linked glycosylation of West Nile virus envelope proteins influences particle assembly and infectivity. J. Virol. 2005;79:13262–13274. doi: 10.1128/JVI.79.21.13262-13274.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beyene A. Influence of N-linked glycans on intracellular transport of hepatitis C virus E1 chimeric glycoprotein and its role in pseudotype virus infectivity. Virology. 2004;324:273–285. doi: 10.1016/j.virol.2004.03.039. [DOI] [PubMed] [Google Scholar]
- 39.Goffard A. Role of N-linked glycans in the functions of hepatitis C virus envelope glycoproteins. J. Virol. 2005;79:8400–8409. doi: 10.1128/JVI.79.13.8400-8409.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goffard A., Dubuisson J. Glycosylation of hepatitis C virus envelope proteins. Biochimie. 2003;85:295–301. doi: 10.1016/s0300-9084(03)00004-x. [DOI] [PubMed] [Google Scholar]
- 41.Eichler R. The role of single N-glycans in proteolytic processing and cell surface transport of the Lassa virus glycoprotein GP-C. Virol. J. 2006;3:41. doi: 10.1186/1743-422X-3-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aguilar H.C. N-glycans on Nipah virus fusion protein protect against neutralization but reduce membrane fusion and viral entry. J. Virol. 2006;80:4878–4889. doi: 10.1128/JVI.80.10.4878-4889.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Melanson V.R., Iorio R.M. Addition of N-glycans in the stalk of the Newcastle disease virus HN protein blocks its interaction with the F protein and prevents fusion. J. Virol. 2006;80:623–633. doi: 10.1128/JVI.80.2.623-633.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shi X. Role of N-linked glycans on bunyamwera virus glycoproteins in intracellular trafficking, protein folding, and virus infectivity. J. Virol. 2005;79:13725–13734. doi: 10.1128/JVI.79.21.13725-13734.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moll M. Influence of N-glycans on processing and biological activity of the nipah virus fusion protein. J. Virol. 2004;78:7274–7278. doi: 10.1128/JVI.78.13.7274-7278.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin G. Differential N-linked glycosylation of human immunodeficiency virus and Ebola virus envelope glycoproteins modulates interactions with DC-SIGN and DC-SIGNR. J. Virol. 2003;77:1337–1346. doi: 10.1128/JVI.77.2.1337-1346.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bossart K.N. Receptor binding, fusion inhibition, and induction of cross-reactive neutralizing antibodies by a soluble G glycoprotein of Hendra virus. J. Virol. 2005;79:6690–6702. doi: 10.1128/JVI.79.11.6690-6702.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oostra M. Glycosylation of the severe acute respiratory syndrome coronavirus triple-spanning membrane proteins 3a and M. J. Virol. 2006;80:2326–2336. doi: 10.1128/JVI.80.5.2326-2336.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schowalter R.M. Characterization of human metapneumovirus F protein-promoted membrane fusion: critical roles for proteolytic processing and low pH. J. Virol. 2006;80:10931–10941. doi: 10.1128/JVI.01287-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cambi A. Microdomains of the C-type lectin DC-SIGN are portals for virus entry into dendritic cells. J. Cell Biol. 2004;164:145–155. doi: 10.1083/jcb.200306112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cambi A., Figdor C.G. Dual function of C-type lectin-like receptors in the immune system. Curr. Opin. Cell Biol. 2003;15:539–546. doi: 10.1016/j.ceb.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 52.Reading P.C. Involvement of the mannose receptor in infection of macrophages by influenza virus. J. Virol. 2000;74:5190–5197. doi: 10.1128/jvi.74.11.5190-5197.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nguyen D.G., Hildreth J.E. Involvement of macrophage mannose receptor in the binding and transmission of HIV by macrophages. Eur. J. Immunol. 2003;33:483–493. doi: 10.1002/immu.200310024. [DOI] [PubMed] [Google Scholar]
- 54.Vigerust D.J. HIV-1 Nef mediates post-translational down-regulation and redistribution of the mannose receptor. J. Leukoc. Biol. 2005;77:522–534. doi: 10.1189/jlb.0804454. [DOI] [PubMed] [Google Scholar]
- 55.Lozach P.Y. DC-SIGN and L-SIGN are high affinity binding receptors for hepatitis C virus glycoprotein E2. J. Biol. Chem. 2003;278:20358–20366. doi: 10.1074/jbc.M301284200. [DOI] [PubMed] [Google Scholar]
- 56.Davis C.W. The location of asparagine-linked glycans on West Nile virions controls their interactions with CD209 (DC-SIGN) J. Biol. Chem. 2006;281:37183–37194. doi: 10.1074/jbc.M605429200. [DOI] [PubMed] [Google Scholar]
- 57.Davis C.W. West Nile virus discriminates between DC-SIGN and DC-SIGNR for cellular attachment and infection. J. Virol. 2006;80:1290–1301. doi: 10.1128/JVI.80.3.1290-1301.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marzi A. The signal peptide of the ebolavirus glycoprotein influences interaction with the cellular lectins DC-SIGN and DC-SIGNR. J. Virol. 2006;80:6305–6317. doi: 10.1128/JVI.02545-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marzi A. DC-SIGN and DC-SIGNR interact with the glycoprotein of Marburg virus and the S protein of severe acute respiratory syndrome coronavirus. J. Virol. 2004;78:12090–12095. doi: 10.1128/JVI.78.21.12090-12095.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Turville S.G. HIV gp120 receptors on human dendritic cells. Blood. 2001;98:2482–2488. doi: 10.1182/blood.v98.8.2482. [DOI] [PubMed] [Google Scholar]
- 61.Liu Y. CD4-independent infection of astrocytes by human immunodeficiency virus type 1: requirement for the human mannose receptor. J. Virol. 2004;78:4120–4133. doi: 10.1128/JVI.78.8.4120-4133.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gramberg T. LSECtin interacts with filovirus glycoproteins and the spike protein of SARS coronavirus. Virology. 2005;340:224–236. doi: 10.1016/j.virol.2005.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reading P.C. Collectin-mediated antiviral host defense of the lung: evidence from influenza virus infection of mice. J. Virol. 1997;71:8204–8212. doi: 10.1128/jvi.71.11.8204-8212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thielens N.M. Interaction of C1q and mannan-binding lectin with viruses. Immunobiology. 2002;205:563–574. doi: 10.1078/0171-2985-00155. [DOI] [PubMed] [Google Scholar]
- 65.White M.R. Enhanced antiviral and opsonic activity of a human mannose-binding lectin and surfactant protein D chimera. J. Immunol. 2000;165:2108–2115. doi: 10.4049/jimmunol.165.4.2108. [DOI] [PubMed] [Google Scholar]
- 66.Hartshorn K.L. Evidence for a protective role of pulmonary surfactant protein D (SP-D) against influenza A viruses. J. Clin. Invest. 1994;94:311–319. doi: 10.1172/JCI117323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Meschi J. Surfactant protein D binds to human immunodeficiency virus (HIV) envelope protein gp120 and inhibits HIV replication. J. Gen. Virol. 2005;86:3097–3107. doi: 10.1099/vir.0.80764-0. [DOI] [PubMed] [Google Scholar]
- 68.Witvrouw M. Resistance of human immunodeficiency virus type 1 to the high-mannose binding agents cyanovirin N and concanavalin A. J. Virol. 2005;79:7777–7784. doi: 10.1128/JVI.79.12.7777-7784.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Williams D.C., Jr. Crystal structures of the HIV-1 inhibitory cyanobacterial protein MVL free and bound to Man3GlcNAc2: structural basis for specificity and high-affinity binding to the core pentasaccharide from n-linked oligomannoside. J. Biol. Chem. 2005;280:29269–29276. doi: 10.1074/jbc.M504642200. [DOI] [PubMed] [Google Scholar]
- 70.Balzarini J. Carbohydrate-binding agents efficiently prevent dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN)-directed HIV-1 transmission to T lymphocytes. Mol. Pharmacol. 2007;71:3–11. doi: 10.1124/mol.106.030155. [DOI] [PubMed] [Google Scholar]
- 71.O’Keefe B.R. Potent anti-influenza activity of cyanovirin-N and interactions with viral hemagglutinin. Antimicrob. Agents Chemother. 2003;47:2518–2525. doi: 10.1128/AAC.47.8.2518-2525.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hawgood S. Pulmonary collectins modulate strain-specific influenza a virus infection and host responses. J. Virol. 2004;78:8565–8572. doi: 10.1128/JVI.78.16.8565-8572.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.LeVine A.M. Surfactant protein D enhances clearance of influenza A virus from the lung in vivo. J. Immunol. 2001;167:5868–5873. doi: 10.4049/jimmunol.167.10.5868. [DOI] [PubMed] [Google Scholar]
- 74.Hartshorn K.L. Salivary agglutinin and lung scavenger receptor cysteine-rich glycoprotein 340 have broad anti-influenza activities and interactions with surfactant protein D that vary according to donor source and sialylation. Biochem. J. 2006;393:545–553. doi: 10.1042/BJ20050695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.White M.R. Cooperative anti-influenza activities of respiratory innate immune proteins and neuraminidase inhibitor. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005;288:L831–L840. doi: 10.1152/ajplung.00365.2004. [DOI] [PubMed] [Google Scholar]
- 76.Ji X. Mannose binding lectin (MBL) and HIV. Mol. Immunol. 2005;42:145–152. doi: 10.1016/j.molimm.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 77.Hart M.L. High mannose glycans and sialic acid on gp120 regulate binding of mannose-binding lectin (MBL) to HIV type 1. AIDS Res. Hum. Retroviruses. 2002;18:1311–1317. doi: 10.1089/088922202320886352. [DOI] [PubMed] [Google Scholar]
- 78.Geijtenbeek T.B. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 79.Wang Q.C. DC-SIGN: binding receptors for hepatitis C virus. Chin. Med. J. (Engl.) 2004;117:1395–1400. [PubMed] [Google Scholar]
- 80.Li J. The glycosylation site in the envelope protein of West Nile virus (Sarafend) plays an important role in replication and maturation processes. J. Gen. Virol. 2006;87:613–622. doi: 10.1099/vir.0.81320-0. [DOI] [PubMed] [Google Scholar]


