Highlights
-
•
We generated transgenic pig expressing PRRSV-specific siRNA.
-
•
Stability of siRNA expression was proved in two generations.
-
•
Type I interferon was not elicited by the expression of siRNA in vivo.
-
•
We proved that transgenic pigs showed substantially decreased virus load in serum after PRRSV infection.
Keywords: Porcine reproductive and respiratory syndrome virus (PRRSV), RNA interference, Somatic cell nuclear transplantation (SCNT), Transgenic pig
Abstract
Porcine reproductive and respiratory syndrome (PRRS) is an economically devastating viral disease causing heavy losses to the swine industry worldwide. Many studies have shown that transient delivery of small interfering RNA (siRNA) or adenovirus-mediated RNA interfere (RNAi) could potentially inhibit porcine reproductive and respiratory syndrome virus (PRRSV) replication in vivo and in vitro. Here, we applied RNAi to produce transgenic (TG) pigs that constitutively expressed PRRSV-specific siRNA derived from small hairpin RNA (shRNA). First, we evaluated siRNA expression in the founding and F1 generation pigs and confirmed stable transmission. Then, we detected the expression of IFN-β and protein kinase R (PKR) and found no difference among TG, non-transgenic (NTG), and wild-type pigs. Lastly, the F1 generation pigs, including TG and NTG piglets, were challenged with 3 × 104.5 TCID50 of JXA1, a highly pathogenic porcine reproductive and respiratory syndrome virus (HP-PRRSV). Our results showed that the in vivo siRNA expression substantially reduced the serum HP-PRRSV titers and increased survival time by 3 days when TG pigs were compared with the NTG controls. These data suggested that RNAi-based genetic modification might be used to breed viral-resistant livestock with stable siRNA expression with no complications of siRNA toxicity.
1. Introduction
Porcine reproductive and respiratory syndrome (PRRS) is a highly contagious disease and one of the most devastating to the swine industry worldwide. PRRS is characterized by severe reproductive failure in gilts and respiratory illness in pigs of all ages. The etiological agent PRRS virus (PRRSV) is a member of the family Arteriviridae that contains a linear, positive-sense, single-stranded RNA genome and transcripts using a unique mechanism involving discontinuous subgenomic synthesis (Meulenberg et al., 1993). And an unprecedented “high fever” disease caused by HP-PRRSV broke out in China in 2006, which spread more than 10 provinces and brought about 4,000,000 fatal cases (Tian et al., 2007). Currently, the principal method to control and prevent PRRSV infection is vaccination. However, the vaccines are unable to provide potent and lasting disease control because of the immune evasion strategies of PRRSV and the heterogeneity of its genome (Thanawongnuwech and Suradhat, 2010).
RNA interference (RNAi) is an RNA-guided sequence-specific process in which small interfering RNA (siRNA) induces post-transcriptional gene silencing. RNA silencing was initially recognized as an anti-viral mechanism to protect host organisms from RNA viral infections (Waterhouse et al., 2001). Many studies have confirmed antiviral immunity mediated by virus-derived small interfering RNAs (viRNAs) in plants and invertebrates, although viRNAs have not been identified in mammals which have evolved the interferon (IFN) pathway instead (Ding and Voinnet, 2007). It has been demonstrated that siRNA can elicit specific intracellular antiviral resistance and may, therefore, be a valuable therapeutic tool (Gitlin et al., 2002). Hence, RNAi has great potential for the inhibition of RNA viral replication in vitro and in vivo. Since the first reported case of RNAi therapy against human pathogen respiratory syncytial virus (RSV) (Bitko and Barik, 2001), many other types of human viruses have been successfully targeted, including human immunodeficiency virus type 1 (Coburn and Cullen, 2002), hepatitis C virus (Kronke et al., 2004), hepatitis B virus (McCaffrey et al., 2003), severe acute respiratory syndrome (He et al., 2003), and influenza A virus (Ge et al., 2003). Subsequently, many viruses infecting large farm animals have also been chosen as therapy targets, such as foot and mouth disease virus (Chen et al., 2006), porcine circovirus virus type 2 (Feng et al., 2008), porcine endogenous retrovirus (Ramsoondar et al., 2009), and PRRSV (Li et al., 2009) (Guo et al., 2013).
A recent report has declared that siRNA transgenesis could confer viral infection resistance to pseudorabies virus (PRV) on mice (Daniel-Carlier et al., 2013). Although it has been shown that transient delivery of siRNA by targeting viral genes protects susceptible cell lines (He et al., 2007), stable transgenic cell line presented stable inhibition to virus (Zhou et al., 2012), and recombinant adenovirus-mediated RNAi guards animals against viral challenges (Li et al., 2009), it remains unclear whether transgenesis of siRNA in pigs can confer permanent resistance against PRRSV infection. Previously, we designed siRNAs to target the open reading frame (ORF) 1b and 6 regions of PRRSV and showed that siRNA-1B372 targeting ORF1b had the strongest virus-inhibiting effect in MARC-145 cells. Importantly, the viral yield in siRNA-1B372-transfected cells was 600-fold lower than that in control cells (Bao et al., 2012). ORF1b encodes non-structural proteins (NSP), including NSP 9–12, which plays a key role in the initiation of viral replication (Music and Gagnon, 2010). Based on our previous in vitro data, our aim in the present study was to produce transgenic animals perpetually expressing siRNA-1B372 and then challenge these animals with PRRSV. Here, we report that we generated transgenic pigs with stable siRNA expression over two generations, and the reduction of PRRSV viremia was observed in them.
2. Materials and methods
2.1. Plasmid construction
Based on our previous findings (Bao et al., 2012), we selected siRNA-1B372 as an RNAi trigger, targeting sequence 5′-GGTGTACTTCCGTTCATTA-3′ (9323–9341 nt) of PRRSV RNA genome. Double-stranded (ds) oligonucleotides encoding siRNA-1B372 were annealed and inserted into vectors and then cloned into a BamHI/HindIII-linearized pGenesil-1 plasmids (Wuhan Genesil Biotechnology, Hubei, China) containing the human U6 (hU6) promoter to generate pshRNA-1B372 (Fig. 2a). Further details of vector construction are described in our previous study (Bao et al., 2012).
Fig. 2.
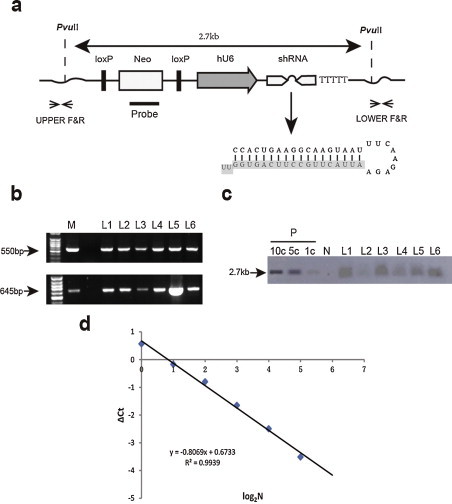
Analysis of transgene integration. (a) Schematic diagram of transgenic vector (pshRNA-1B372). The head-to-head arrows denote the primers used to identify the transgenic insert in TG pigs. Two PvuII sites, 2.7 kb apart, and the probe position used for Southern blotting are indicated. (b) PCR for detecting TG mice and assessment of integrity of the inserts. The two ends of pshRNA-1B372 transgenes were amplified (550 bp and 645 bp) separately from TG pig genomic DNA using 5′- and 3′-specific primers. Two PCR products at the length of 550 bp and 645 bp were identified as 5′ end and 3′ end of the inserted transgene. M, 100 bp DNA ladder; L1–L6, TG founders; N, genomic DNA from WT pig as a negative control; P, pshRNA-1B372 as a positive control. (c) Detection of the transgene integrated into the genome by Southern blotting analysis. The siRNA expression cassette was inserted into the genome, as indicated by the band. The positive control was the vector plasmid. After electrophoresis and Southern blot transfer, membranes were probed for integrated vector DNA. Copy number standards (the number of inserted vector DNA per diploid genomic equivalent) are indicated as 1c, 3c and 5c. (d) A standard curve for the number of transgenes and the corresponding Ct value. QRT-PCR was performed to determine the copy number of transgene. A standard set of mixtures, including 1, 2, 4, 8, 16, and 32 copies of plasmid DNA in 10 ng of WT pig genomic DNA, was used to establish a standard curve. The Y axis represents the ΔCt value (Ct of pshRNA-1b372 gene minus Ct of MSTN) and X axis represents the log2 ratio of the transgene copy number.
2.2. Generation of transgenic pigs
Fetal pig fibroblast cell lines (from Landrace pigs) were established and cultured as described previously (Zhang et al., 2006). The fibroblast cells were transfected with linearized shRNA-expressing constructs, and then cultured on a 10-cm plate. After 24 h, the cells were transferred to six 10-cm plates with selective medium that contained G418 (400 μg/ml, Promega). After selection with G418 for a week, the antibiotic-resistant colonies were selected and pooled in 24-well plates. Forty-eight hours later, half of the cells in each well were subjected to PCR using primers 1B372-F (5′-CTGTTCCACATACACTTCATTCT-3′) and 1B372-R (5′-CACAGATGCGTAAGGAGAAA-3′) to identify the transgenic positive clones. The other half of the cells was frozen for later use in somatic cell nuclear transfer (SCNT). Approximately, 400 embryos were transferred into each surrogate sows and five sows received embryo implantation. Cloned pigs were delivered by natural birth after approximate 106 days, and F1 is generated by fertilizing TG sows with semen of wild-type pigs.
TG founder pigs and offspring were identified by polymerase chain reaction (PCR) analysis of genomic DNA derived from ear biopsies. Two sets of primers were designed to cover the integrity of the 5′-region (UPPER1F: 5′-CAGCGGTAAGATCCTTGAG-3′; UPPER1R: 5′-ACTCCCCGTCGTGTAGATAAC-3′) and the 3′-region (LOWER1F: 5′-ATTCCCCCAGTGGAAAGAC-3′; LOWER1R: 5′-TGCGGCATCAGAGCAGATT-3′). TG founder pigs were confirmed by Southern blot analysis. The plasmid vector was served as positive control. Copy number standards (the number of inserted vector DNA per diploid genomic equivalent) are indicated as 1c, 3c and 5c, and the corresponding amount of plasmid loaded in each well was calculated according to the equation:
l, the length of the plasmid; c, the copy number standard; 2 × L means diploid genome. Proper amount of plasmid and ear DNA samples (10 μg) were digested with PvuII and electrophoresed through a 1% agarose gel followed by transfer to positively charged nylon membranes (Roche Applied Science, Mannheim, Germany). The membrane was hybridized with adigoxigenin (DIG)-labeled probes (761 bp) generated by PCR using the following set of primers (PROBE2F: 5′-ACTGGGCACAACAGACAATC-3′ and PROBE2R: 5′-CGATCCCCTCAGAAGAACTC-3′) with the pGenesil-1b273 plasmid as the template (Roche DIG-PCR DNA Labeling Kit). Hybridization signals were detected using the Roche DIG DNA Labeling and Detection Kit according to the manufacturer's instructions.
2.3. Determination of transgene copy number
To determine the transgene copy number, the 2−ΔΔCt method (Livak and Schmittgen, 2001) was used to compare the ΔCt value (cycle threshold (Ct); Ct of target minus Ct of control gene) of TG animal samples with unknown transgene copy numbers with the ΔCt value of a known calibrator. A wild-type (WT) pig genomic DNA sample, carrying a single copy of the myostatin (MSTN) gene, was used as a calibrator to determine the transgene copy number in TG founder pigs (MSTN110-F: 5′-TCTGAGACCCGTCAAGACTCCTA-3′, MSTN110-R: 5′-TGTCAAGTTTCAGAGATCGGATTC-3′). The transgene was amplified using 5′-CTGAAG CGGGAAGGGACTGG-3′ and 5′-TACTTTCTCGGCAGGAGCAA-3′ as specific primers.
Quantitative real-time PCR (qRT-PCR) was used to detect the copy number, and all of the reactions were performed in 96-well plates using the Roche LightCycler 480 System (LC 480, Roche, Basel, Switzerland). The amplification was performed in a 20-μL reaction volume containing 1 μl of template DNA (10 ng/μl), 0.3 μl of each primer (10 μM), 10 μl of Power SYBR Green Mix (Applied Biosystems, Foster City, CA, USA), and 8.4 μl of ddH2O. All reactions were performed using the following cycle conditions: 95 °C for 10 min; 40 cycles of 95 °C for 10 s, 60 °C for 10 s and 72 °C for 10 s; followed by 95 °C for 5 s, 65 °C for 1 min, and a final cycle at 97 °C to generate a melting curve. A standard set of mixtures, representing 1, 2, 4, 8, 16, and 32 copies of plasmid DNA in 10 ng of WT pig genomic DNA, was used to construct a standard curve to determine the correlation between the ΔCt value (Ct of the pshRNA-1B372 gene minus Ct of MSTN) and the log2 N value (N = copy number of the transgene).
2.4. Detection of siRNA by real-time PCR
We used Custom TaqMan Small RNA Assays (Applied Biosystems) to evaluate siRNA expression in TG pigs (Chen et al., 2005). Blood samples of founder were collected. After challenged with PRRSV, all animals’ heart, liver, spleen, lung, tonsil and blood were obtained for siRNA detection. Total RNA was purified using the mirVana miRNA Isolation Kit (Ambion, Austin, TX, USA). Reverse transcriptase reactions and real-time PCR were performed according to manufacturer's protocol. The U6 gene served as an internal reference control and Real-time PCR for U6 gene was performed in the same way. The siRNA expression was normalized to the expression of U6 using the 2−ΔCt method(Ct of siRNA1B372 minus Ct of U6).
2.5. Quantification of protein kinase R (PKR) and IFN-β
We applied qRT-PCR to detect PKR and IFN-β expression in TG pigs. Whole blood RNA was purified using the QIAamp RNA Blood Mini kit (Qiagen, Hilden, Germany) according to manufacturer's protocol. Genomic DNA was removed using DNase I (Qiagen). RNA (2 μg) was reverse-transcribed into first-strand cDNA with Moloney Murine Leukemia Virus transcriptase (Promega) and oligo (dT) 18 primers (TaKaRa Bio Inc., Shiga, Japan). PCR amplification was performed using 1 μL of RT product with PKR- and IFN-β-specific primers (forward PKR primer: 5′-AATTGGCTCAGGTGGATTTG-3′, reverse PKR primer: 5′-CTCTACCTTCTCGCAATCA-3′; forward IFN-β primer: 5′-CAAATCGCTCTCCTGATGTGT-3′, reverse PKR primer: 5′-TTCTGACATGCCAAATTGCTG-3′). The mRNA concentration was normalized to that of glyceraldehyde 3-phosphate dehydrogenase (GAPDH; forward GAPDH primer: 5′-ATCACCATCTTCCAGGAGCGA-3′, reverse GAPDH primer: 5′-AGCCTTCTCCATGGTCGTGAA-3′).
2.6. Viral challenge of pigs and serology analysis
All animal studies were performed according to protocols approved by the animal welfare committee of China Agricultural University. Viral challenge was performed using the PRRSV isolate JXA1 (Tian et al., 2007), which is a highly pathogenic strain. Highly pathogenic porcine reproductive and respiratory syndrome (HP-PRRS) is a PRRSV variant, characterized by high fever and high mortality (Tian et al., 2007). Three groups were established: the TG, non-transgenic (NTG), and immunized control (IC) groups (n = 5 for the TG group; n = 4 for the NTG group; n = 5 for the IC group). All pigs were 2 months old. The pigs in the IC group were vaccinated with 2 mL of the modified-live PRRSV vaccine Ingelvac PRRS MLV (Boehringer Ingelheim Vetmedica) via the intramuscular route 7 days before virus challenge. The non-attenuated parental virus (VR2332, isolated in 1991 in Minnesota, the attenuated form of this virus is commercially available under the trade-name Ingelvac® PRRS MLV) and challenge strain were 90% homologous. The TG and NTG pigs were from the F1 generation, i.e., the offspring of the founder pigs (Table 1 ). Before virus challenge, all animals in TG and NTG group were tested to be PRRSV negative by PCR and serology. Each pig received 3 mL of 3 × 104.5 TCID50 virus, which is an appropriate dosage for vaccine evaluation. The effect of the infection on rectal temperature was recorded daily, starting 2 days prior to challenge until necropsy at day 16 post-infection. At 2, 4, 6, 8, 11, and 14 days post-infection (dpi), blood samples were collected. Serum samples were tested using a commercial PRRSV enzyme-linked immunosorbent assay (ELISA; IDEXX Laboratories, Inc., Westbrook, ME, USA) to assess the presence of PRRSV antibodies.
Table 1.
Mortality and viral load data for the challenge test.
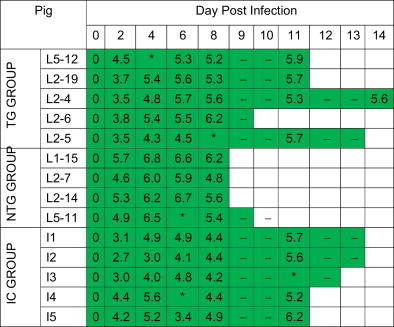
The pigs in the TG and NTG groups were from the F1 generation of the TG founders, and those in IC group were the same old pigs vaccined with Ingelvac PRRS MLV. All pigs were challenged with JXA1 at 0 dpi. Sample invalid (*), sample unavailable (−). Survival is indicated by the length of the green bar. Numbers for each day represent the estimated viral titration of the blood samples, expressed as log10 TCID50/ml.
2.7. Quantification of serum viral RNA content
Quantitative RT-PCR amplification of RNA was performed to examine PRRSV replication in pig blood, which used the QuantiTect Probe RT-PCR kit (Qiagen). The oligonucleotide primers and dual-labeled probe used for amplification were 5′-ATGATGAGGCTGGCATTCT-3′ (forward); 5′-ACACGGTCGCCCTAATTG-3′ (reverse); and 5′-HEX-TGTGGTGAATGGCACTGATTGACA-BHQ2-3′ (dual-labeled probe), which correspond to base pair positions 15,167–15,184, 15,262–15,279, and 15,216–15,239 of the JXA1 (GenBank accession number FJ548855).
We constructed a standard curve to relate the viral titer of a sample expressed as the TCID50 with Ct values obtained in qRT-PCR using serial 10-fold dilutions of virus lysates ranging from 106 to 102 TCID50 (Data not shown). Ct values were determined after amplification and were plotted against the TCID50 value.
2.8. Preparation of pulmonary alveolar macrophages (PAMs)
Pulmonary alveolar macrophages (PAMs) were prepared using lung lavage technique (Wensvoort et al., 1991) from 6-week-old TG pigs (n = 3) and NTG pigs (n = 3). We confirmed that the pigs were PRRSV-free by qRT-PCR and ELISA. In brief, the lungs from piglets were washed 3–6 times with HBSS (Hanks's Balanced Salt Solution) supplemented with 2% fetal bovine serum. Each eliquot of washing fluid was centrifuged at 1000 rpm at 4 °C for 10 min. Then the cell pellets were resuspended with 20 mL RPMI-1640 medium (GIBCO, Invitrogen Corporation, CA) containing 10% fetal bovine serum, 100 units/mL of penicillin, and 100 μg/ml of streptomycin. The cell concentration of PAMs was adjusted to 1 × 106/mL with RPMI-1640 medium containing 10% fetal bovine serum, then added to 10 cm2 petri dish (Thermo) and incubated for 12 h at 37 °C in a humidified compartment for adhering. This procedure yielded approximately 95% pure macrophages as determined by light microscopy.
2.9. Virus Inoculation and qRT-PCR
PAMs were incubated for 12 h at 37 °C in 5% CO2 in RPMI-1640 medium, and the non-adherent cells were removed by gentle washing with RPMI-1640 medium before inoculation. Then, the cells were inoculated with 0.05 MOI of JXA1, and were collected at 6, 12, 24, and 48 h post inoculation (pi) respectively. The uninfected cells served as mock-infected cells. Total RNA in infected cells was isolated and subject to qPCR analysis. GAPDH served as internal control. The ΔΔCt method for relative quantitation of gene expression was used to determine viral RNA levels. The ΔCt was calculated by subtracting the Ct of GAPDH from the Ct of viral RNA. The ΔΔCt was calculated by subtracting the ΔCt of the reference sample (RNA of TG PAMs at pi 12 h) from the ΔCt of each sample. Fold change was generated using the equation 2−ΔΔCt.
2.10. Immunofluorescence assay (IFA)
For detection of PRRSV in PAMs, an immunofluorescence assay (IFA) was performed on PAMs infected with PRRSV JXA1 at an MOI of 0.05 for 24 h. The cells were fixed with cold methanol–acetone (1:1) for 10 min at 4 °C, and washed with phosphate-buffered saline (PBS) three times. After blocked with 10% goat serum–PBS for 30 min at room temperature, cells were then incubated with monoclonal antibodies (MAbs) (SDOW17; Rural Technologies) against PRRSV nucleocapsid protein at 80 rpm for 1 h. After being washed with PBS three times, cells were incubated with Alexa Fluor 488-labeled goat antimouse IgG antibody (Beyotime Institute of Biotechnology) at 80 rpm for 1 h. After washed with PBS, cells were examined under fluorescence microscopy.
2.11. Statistical analysis
Student's t-test was used to determine significant differences among groups. A P-value <0.05 was considered statistically significant. n-Values represent the number of independent experiments.
3. Results
3.1. Generation of transgenic pigs expressing siRNA
Given the outstanding performance of siRNA-1B372 (Bao et al., 2012), we produced the transgenic pigs harboring the sequence using the SCNT technique (Fig. 1 ). Six female TG founder pigs (L1–L6) derived from the same cell clone were obtained from seven newborn piglets with preliminary PCR evidence of siRNA-1B372 expression cassette (Fig. 2b). Subsequently, we performed Southern blotting to confirm our results (Fig. 2c). The transgene copy number was measured by real-time PCR. As shown in Fig. 2(d), the ΔCt value (Ct of siRNA-1B372 gene minus Ct of MSTN) of each sample was plotted against the respective log2 N value (N = transgene copy number). Accordingly, the copy number of the inserted vector was calculated to be 3, which was roughly consistent with the Southern blotting results. All the 6 positive TG pigs were inseminated artificially with wild-type semen that brought about 62 offspring (32 females and 30 males), but only 11 piglets grew healthily to one month old, 6 (54.5%) of which were TG. It was probably due to the insufficient nursing ability of the cloned sows, because most death occurred in the first 10 days and the survivals grew as well as wild-type pigs.
Fig. 1.
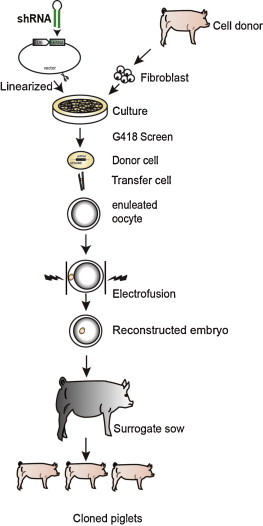
Generation of transgenic pigs expressing siRNA. The plasmids were linearized and transfected into pig fibroblast cells. Cell clones containing the siRNA-expressing cassette were isolated by G418 screening and served as donors for SCNT. Finally, pigs that endogenously expressed the siRNA were generated via SCNT.
Custom TaqMan Small RNA Assays were used to further confirm the siRNA levels in the transgenic pigs. siRNA production was normalized to U6 expression, which is reportedly abundant and stable in all tissues and proven to be a suitable internal control (Jurcevic et al., 2013, Kiss, 2002). Initially, we only examined siRNA expression in blood of the founder pigs because we needed to keep them alive for the following propagation. As shown in Fig. 3(a), the siRNA levels in the blood of the transgenic pigs were similar and around 4 × 10−5 when normalized to U6, which confirmed that they were derived from the same clone mass. After necropsy of the six F1 TG pigs, the siRNA levels in lung, tonsil, liver, blood, spleen and heart tissues were examined, and the ratio of siRNA/U6 RNA was approximately 10−5 (Fig. 3b). The results from the founder pigs showed that siRNA levels were close among the individuals, although they were diverse among different tissues. In addition, siRNA levels in blood were similar between the founder and F1 generation pigs. Thus, siRNA expression was stably transmitted over at least one generation.
Fig. 3.
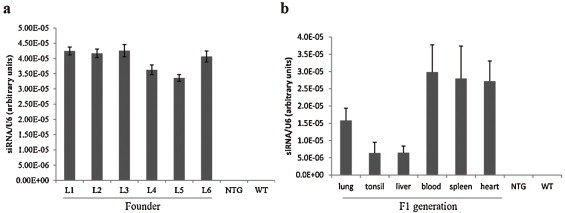
Analysis of siRNA expression in transgenic pigs. (a) RNA was isolated from TG founder blood and siRNA-1B372-specific small RNA TaqMan was performed. The expression of siRNA was expressed as a relative ratio to U6 used as a reference gene. Data are presented as means ± SD from six repeats for each individual. (b) After necropsy of the F1 TG pigs, RNAs in several tissues (lung, tonsil, liver, blood, spleen, and heart) were isolated and the corresponding siRNAs were quantified. Data are presented as mean ± SD from six TG pigs.
3.2. Type I IFN response in transgenic pigs is similar to that of control pigs
IFN system could be activated by siRNA, and PKR was a necessary inducer of type I interferon (Sledz et al., 2003), Activation of the IFN pathway is a major contributor to the off-target effects of siRNA in mammalian cells (Kenworthy et al., 2009). In this regard, we measured IFN-β and PKR by real-time PCR from blood extracts in TG, non-TG, and WT pigs. The TG and non-TG pigs were from the F1 generation, i.e., the offspring of the founders. As shown in Fig. 4 , there is no significant difference both in the levels of IFN-β and PKR among the three groups (P > 0.5), indicating that endogenous siRNA expression did not induce a type I IFN response.
Fig. 4.
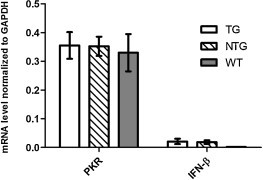
IFN-β and PKR mRNA levels in the blood of TG, NTG and wild-type pigs. IFN-β and PKR mRNA levels were determined by qRT-PCR from blood extracts as described in Section 2. There were no significant differences in the IFN-β or PKR mRNA levels among the TG, NTG, and wild-type pigs (P > 0.5). Data are presented as means ± SD from three pigs from each group.
3.3. Transgenic pigs were resistant to PRRSV infection
To evaluate the susceptibility of the TG pigs to PRRSV infection, viral challenges were administered by intramuscular injections with a HP-PRRSV strain (Tian et al., 2007) that is homologous to JXwn06 and designated as JXA1. The challenged animals included a TG group (five pigs), a NTG group (four pigs), and an IC group (five pigs) (Table 1). As shown in Fig. 5(d), prior to the day of infection, no animals in the TG and NTG groups tested positive for PRRSV. On post-infection day 1, all animals exhibited a high fever. The trend in body temperature was similar in the NTG group and the IC group, which remained elevated until post-infection day 9, when it then decreased. The body temperature of the TG pigs increased slowly during the first 4 dpi and then climbed drastically (Fig. 5a), which might suggest that siRNA worked in the early stage of PRRSV infection. The average survival time after infection was calculated for each group and the results showed that the TG pigs exhibited significantly longer survival times (11 days) than the NTG pigs (8 days), while TG and IC groups performed similarly (Fig. 5b).
Fig. 5.
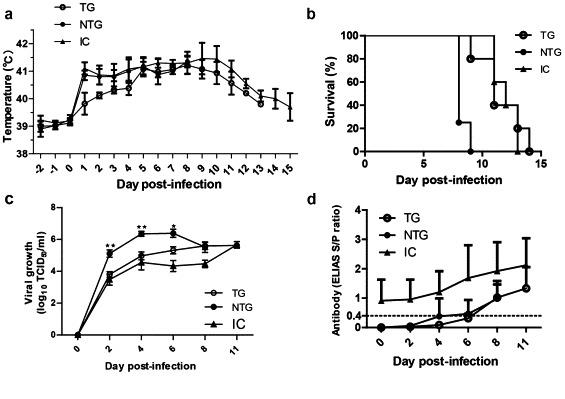
Transgenic pigs exhibits antiviral response after PRRSV infection. (a) Rectal temperature curve of the infected pigs. The rectal temperatures of three groups (TG group, n = 5; NTG group, n = 4; IC group, n = 5) had been measured at 24-h intervals since 2 days before JXA1 infection until the end of the experiment. (b) Survival curves of pigs after PRRSV JXA1 infection. The pigs in the TGP group survived significantly longer than NTG group, 11 and 8 days, respectively (P < 0.05), and IC group was 12 days. (c) Viral growth curves in pigs of three groups. Serum samples were collected at the indicated times post-transfection. The Ct value of each sample was obtained by qRT-PCR and the corresponding TCID50 values were determined according to the standard curve. The viral loads of the TG pigs were significantly higher than those of NTG pigs on dpi 2, 4, and 6. *P < 0.05; **P < 0.01. (d) Antibody curves of the three groups. Specific antibodies of the PRRSV M protein was measured by ELISA (HerdCheck, Idexx Laboratories) at the indicated times. Results are expressed as S/P ratio averages. S/P ratios <0.4 were considered negative. Differences between the two S/P ratios (TG < IC) was significant at all indicated times (P < 0.01). Data in panels are presented as means ± SE. Statistical significance was analyzed using Student's-test.
We subsequently quantified the viral genome RNA and PRRSV-specific antibodies in the serum of the infected animals. Blood was collected from all animals for serology analysis at 0, 2, 4, 6, 8, 11, and 16 dpi. The overall trends in the dynamics of the viremia after infection were similar for the animals between the TG and IC groups. For all challenged pigs, the viral content increased dramatically and immediately after infection until approximately 11 dpi, when it peaked. Meanwhile, the serum viral load of the NTG group underwent an abrupt decrease before the animals began to die (from 6 to 8 dpi). Consistent with the survival time data, the lowest viral load was detected in the IC group and the viral load in the TG group was lower than that in the NTG group (Fig. 5c). The average viral RNA load in serum was 20-fold lower in the TG group than that in the NTG group at 2 and 4 dpi (P < 0.005).
Furthermore, PRRSV-specific antibody was examined using HerdChek PRRS 2XR ELISA (IDEXX Inc.). The humoral immune response to PRRSV infection showed that all of the pigs were infected after the viral challenge. As shown in Fig. 5(d), the magnitude of the antibody response in the IC group was higher than that in the unvaccinated pigs and there was no significant difference between the TG and NTG groups (P > 0.5), suggesting that these pigs presented similar immune responses.
3.4. PRRSV inhibition on TG PAMs
Since viral suppression was observed in TG pigs, we next analyzed the PRRSV replication in infected PAMs, which is the target cell of PRRSV (Teifke et al., 2001), SiRNA expression in TG PAMs was examined firstly, and was 9 × 10−5 as referred to U6 RNA (Fig. 6a). Then cells were infected by JXA1, and intracellular viral replication was detected. Viral growth was suppressed by about 13-folds at pi 12 h and 38-folds at pi 24 h as compared with NTG PAMs. At pi 48 h, no viral RNA could be detected due to the cell lysis while that of TG PAMs was around 20-folds of the reference sample (Fig. 6b). Importantly, TG PAMs exhibited apparently relieved PRRSV-induced cytopathogenic effect (CPE) in comparison to NTG PAMs (Fig. 6c). To further investigate the effect of RNA interference on PRRSV infection, we applied an immunofluorescence assay to detect PRRSV in PAMs. Results showed that PRRSV infection was suppressed in TG PAMs as compared with NTG PAMs at early stage of infection (pi 12 h). However, the difference between the two subjects became smaller at pi 24 h (Fig. 6d). Taken together, transgenic siRNA inhibited the PRRSV replication in PAMs of TG pigs.
Fig. 6.
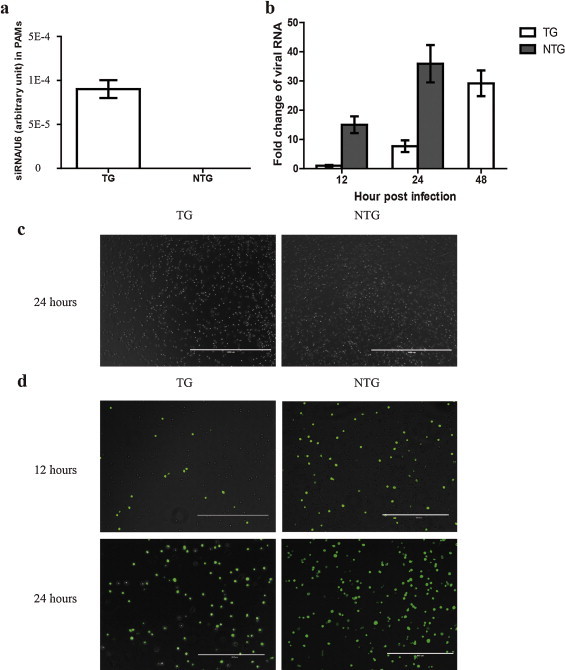
Transgenic siRNA in PAMs inhibited PRRSV replication. (a) qRT-PCR analysis of siRNA expression in PAMs isolated from TG pigs. The expression of siRNA was expressed as a relative ratio to U6. Data are presented as mean ± SD from three TG pigs. (b) qRT-PCR analysis of viral ORF7 RNA from TG and NTG PAMs both inoculated with PRRSV JXA1 for 12, 24 and 48 h. Fold change was generated by 2−ΔΔCt. ΔCt = Ct(viral RNA) − Ct(GAPDH); ΔΔCt = ΔCt − Ct (reference, viral RNA at pi 12 h). The default fold of viral RNA at pi 12 h was 1. Data are representative of the results of three individuals (means ± SD). (c) PAMs of TG and NTG pigs were observed for development of CPE by bright-field microscopy at 24 h pi. (d) Immunofluorescence assay analysis of PRRSV N protein in TG and NTG PAMs infected with PRRSV JXA1 for 12 and 24 h.
4. Discussion
Li et al. (2009) reported that adenovirus-mediated RNAi can inhibit PRRSV replication in swine, and a large proportion of reports on RNAi-based therapies thus far have evaluated viral vectors (see review in Davidson and McCray, 2011). However, the safety and efficacy of RNAi are challenged by significant problems stemming from host cellular and humoral immune responses (Huang and Yang, 2009). Here, we report for the first time transgenic pigs harboring RNA polymerase III promoter U6 driving a siRNA targeting a conserved region of the PRRSV genome. Moreover, according to the similar siRNA expression level in blood of two generations, we presumed that the siRNA expression in founder and F1 pigs was similar, which may indicate reliable and stable transgenic expression. More passages are needed to demonstrate the sustainable heredity of siRNA expression.
As porcine alveolar macrohphages (PAMs) are the primary target cells when PRRSV invades (Teifke et al., 2001), RNAi effect occurs in them could play major role in virus inhibition. Results of qRT-PCR and IFA in PRRSV-infected PAMs confirmed that integrated siRNA expression could also have the inhibitory effect on virus in vitro, which in turn prove that it is the transgenic siRNA reducing viral load of the blood in vivo.
A characteristic feature of RNA viruses, such as arteriviruses, is their high mutation rate, which allows them to adapt to and escape from host immune responses. Since RNAi relies on a perfect match between the siRNA and the target sequence in the viral RNA genome, even single mutations would suffice to abolish the antiviral effect of the siRNA (Du et al., 2005). To avoid viral escape from RNAi, given our former work in cell line development, we selected the siRNA-1B372 which targeted the most conserved region of PRRSV genome among 20 siRNAs targeting ORFs 1b, 5, 6 and 7 (Bao et al., 2012) to generate the transgenic pigs. The highly pathogenic PRRSV strain, JXwn06 (GenBank accession number: EF641008) was used as a reference sequence for this study, and the target sequence of siRNA-1B372 was located in 9324–9335nt in the PRRSV genome, which sites in NSP9 coding region. This sequence is highly conserved in all American PRRSV strains, as confirmed by comparisons using the Basic Local Alignment Search Tool algorithm on the National Center for Biotechnology Information website (www.ncbi.nlm.nih.gov/). Additionally, ORF1b encodes NSP 9–12, of which NSP9 is a putative RNA-dependent RNA polymerase that plays a central role in viral replication (Wang et al., 2012).
Although many studies have reported that the RNA polymerase III promoter can induce strong expression of siRNA, and others have found that RNAi vectors in mammalian cells may induce a distinct IFN response (Bridge et al., 2003), our results showed that the siRNA expression in the transgenic pigs was low and no significant IFN response was detected. There could be the possibility that low siRNA expression account for the absence of IFN response. The positional effect (an effect of the local chromosomal environment on the levels or patterns of transgene expression) probably accounted for the low production of siRNA as the small inserted DNA fragments were likely subject to this effect. Therefore, developing new transgenic technology to improve the PRRSV specific RNAi could be helpful to enhance the antiviral capacity in pigs, such as artificial microRNA (Xia et al., 2013, Boudreau et al., 2009). Nonetheless, stably expressed siRNA can still block PRRSV replication to some extent without causing any visible toxicity.
As reviewed by (Clark and Whitelaw, 2003), the advent of new methods, such as RNAi, can advance transgenic livestock research, thus the development of transgenic animals that constitutively express siRNAs targeting the knockdown of a pathogenic virus or its transcription products is expected in the near future. In the present study, the transgenic pigs were able to reduce viral load in blood. The quantitative reduction of the amount of serum viral load may have impaired the forward transmission of PRRS, as previously described (Lyall et al., 2011). Even though we are aware that the low production of siRNA can only induce moderate inhibition of PRRSV, RNAi combined with transgenic technology offers the possibility to genetically engineer livestock to promote resistance to viral infections.
Acknowledgment
The study was supported by the earmarked fund for National Transgenic Breeding Project of China (2011ZX08010-00).
References
- Bao Y., Guo Y., Zhang L., Zhao Z., Li N. Inhibition of porcine reproductive and respiratory syndrome virus replication by RNA interference in MARC-145 cells. Mol. Biol. Rep. 2012;39:2515–2522. doi: 10.1007/s11033-011-1003-z. [DOI] [PubMed] [Google Scholar]
- Bitko V., Barik S. Phenotypic silencing of cytoplasmic genes using sequence-specific double-stranded short interfering RNA and its application in the reverse genetics of wild type negative-strand RNA viruses. BMC Microbiol. 2001;1:34. doi: 10.1186/1471-2180-1-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau R.L., Martins I., Davidson B.L. Artificial microRNAs as siRNA shuttles: improved safety as compared to shRNAs in vitro and in vivo. Mol. Ther. 2009;17:169–175. doi: 10.1038/mt.2008.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge A.J., Pebernard S., Ducraux A., Nicoulaz A.L., Iggo R. Induction of an interferon response by RNAi vectors in mammalian cells. Nat. Genet. 2003;34:263–264. doi: 10.1038/ng1173. [DOI] [PubMed] [Google Scholar]
- Chen C., Ridzon D.A., Broomer A.J., Zhou Z., Lee D.H., Nguyen J.T., Barbisin M., Xu N.L., Mahuvakar V.R., Andersen M.R., Lao K.Q., Livak K.J., Guegler K.J. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005;33:e179. doi: 10.1093/nar/gni178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Liu M., Jiao Y., Yan W., Wei X., Chen J., Fei L., Liu Y., Zuo X., Yang F., Lu Y., Zheng Z. Adenovirus-mediated RNA interference against foot-and-mouth disease virus infection both in vitro and in vivo. J. Virol. 2006;80:3559–3566. doi: 10.1128/JVI.80.7.3559-3566.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J., Whitelaw B. A future for transgenic livestock. Nat. Rev. Genet. 2003;4:825–833. doi: 10.1038/nrg1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn G.A., Cullen B.R. Potent and specific inhibition of human immunodeficiency virus type 1 replication by RNA interference. J. Virol. 2002;76:9225–9231. doi: 10.1128/JVI.76.18.9225-9231.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel-Carlier N., Sawafta A., Passet B., Thepot D., Leroux-Coyau M., Lefevre F., Houdebine L.M., Jolivet G. Viral infection resistance conferred on mice by siRNA transgenesis. Transgenic Res. 2013;22:489–500. doi: 10.1007/s11248-012-9649-4. [DOI] [PubMed] [Google Scholar]
- Davidson B.L., McCray P.B., Jr. Current prospects for RNA interference-based therapies. Nat. Rev. Genet. 2011;12:329–340. doi: 10.1038/nrg2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S.W., Voinnet O. Antiviral immunity directed by small RNAs. Cell. 2007;130:413–426. doi: 10.1016/j.cell.2007.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Q., Thonberg H., Wang J., Wahlestedt C., Liang Z. A systematic analysis of the silencing effects of an active siRNA at all single-nucleotide mismatched target sites. Nucleic Acids Res. 2005;33:1671–1677. doi: 10.1093/nar/gki312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z., Jiang P., Wang X., Li Y., Jiang W. Adenovirus-mediated shRNA interference against porcine circovirus type 2 replication both in vitro and in vivo. Antiviral Res. 2008;77:186–194. doi: 10.1016/j.antiviral.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Ge Q., McManus M.T., Nguyen T., Shen C.H., Sharp P.A., Eisen H.N., Chen J. RNA interference of influenza virus production by directly targeting mRNA for degradation and indirectly inhibiting all viral RNA transcription. Proc. Natl. Acad. Sci. U.S.A. 2003;100:2718–2723. doi: 10.1073/pnas.0437841100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin L., Karelsky S., Andino R. Short interfering RNA confers intracellular antiviral immunity in human cells. Nature. 2002;418:430–434. doi: 10.1038/nature00873. [DOI] [PubMed] [Google Scholar]
- Guo X.K., Zhang Q., Gao L., Li N., Chen X.X., Feng W.H. Increasing expression of microRNA 181 inhibits porcine reproductive and respiratory syndrome virus replication and has implications for controlling virus infection. J. Virol. 2013;87:1159–1171. doi: 10.1128/JVI.02386-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M.L., Zheng B., Peng Y., Peiris J.S., Poon L.L., Yuen K.Y., Lin M.C., Kung H.F., Guan Y. Inhibition of SARS-associated coronavirus infection and replication by RNA interference. JAMA. 2003;290:2665–2666. doi: 10.1001/jama.290.20.2665. [DOI] [PubMed] [Google Scholar]
- He Y.X., Hua R.H., Zhou Y.J., Qiu H.J., Tong G.Z. Interference of porcine reproductive and respiratory syndrome virus replication on MARC-145 cells using DNA-based short interfering RNAs. Antiviral Res. 2007;74:83–91. doi: 10.1016/j.antiviral.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Huang X., Yang Y. Innate immune recognition of viruses and viral vectors. Hum. Gene Ther. 2009;20:293–301. doi: 10.1089/hum.2008.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurcevic S., Olsson B., Klinga-Levan K. Validation of suitable endogenous control genes for quantitative PCR analysis of microRNA gene expression in a rat model of endometrial cancer. Cancer Cell Int. 2013;13:45. doi: 10.1186/1475-2867-13-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenworthy R., Lambert D., Yang F., Wang N., Chen Z., Zhu H., Zhu F., Liu C., Li K., Tang H. Short-hairpin RNAs delivered by lentiviral vector transduction trigger RIG-I-mediated IFN activation. Nucleic Acids Res. 2009;37:6587–6599. doi: 10.1093/nar/gkp714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss T. Small nucleolar RNAs: an abundant group of noncoding RNAs with diverse cellular functions. Cell. 2002;109:145–148. doi: 10.1016/s0092-8674(02)00718-3. [DOI] [PubMed] [Google Scholar]
- Kronke J., Kittler R., Buchholz F., Windisch M.P., Pietschmann T., Bartenschlager R., Frese M. Alternative approaches for efficient inhibition of hepatitis C virus RNA replication by small interfering RNAs. J. Virol. 2004;78:3436–3446. doi: 10.1128/JVI.78.7.3436-3446.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Jiang P., Li Y., Wang X., Huang J., Bai J., Cao J., Wu B., Chen N., Zeshan B. Inhibition of porcine reproductive and respiratory syndrome virus replication by adenovirus-mediated RNA interference both in porcine alveolar macrophages and swine. Antiviral Res. 2009;82:157–165. doi: 10.1016/j.antiviral.2009.02.202. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lyall J., Irvine R.M., Sherman A., McKinley T.J., Nunez A., Purdie A., Outtrim L., Brown I.H., Rolleston-Smith G., Sang H., Tiley L. Suppression of avian influenza transmission in genetically modified chickens. Science. 2011;331:223–226. doi: 10.1126/science.1198020. [DOI] [PubMed] [Google Scholar]
- McCaffrey A.P., Nakai H., Pandey K., Huang Z., Salazar F.H., Xu H., Wieland S.F., Marion P.L., Kay M.A. Inhibition of hepatitis B virus in mice by RNA interference. Nat. Biotechnol. 2003;21:639–644. doi: 10.1038/nbt824. [DOI] [PubMed] [Google Scholar]
- Meulenberg J.J., de Meijer E.J., Moormann R.J. Subgenomic RNAs of Lelystad virus contain a conserved leader–body junction sequence. J. Gen. Virol. 1993;74(Pt 8):1697–1701. doi: 10.1099/0022-1317-74-8-1697. [DOI] [PubMed] [Google Scholar]
- Music N., Gagnon C.A. The role of porcine reproductive and respiratory syndrome (PRRS) virus structural and non-structural proteins in virus pathogenesis. Anim. Health Res. Rev. 2010;11:135–163. doi: 10.1017/S1466252310000034. [DOI] [PubMed] [Google Scholar]
- Ramsoondar J., Vaught T., Ball S., Mendicino M., Monahan J., Jobst P., Vance A., Duncan J., Wells K., Ayares D. Production of transgenic pigs that express porcine endogenous retrovirus small interfering RNAs. Xenotransplantation. 2009;16:164–180. doi: 10.1111/j.1399-3089.2009.00525.x. [DOI] [PubMed] [Google Scholar]
- Sledz C.A., Holko M., de Veer M.J., Silverman R.H., Williams B.R. Activation of the interferon system by short-interfering RNAs. Nat. Cell Biol. 2003;5:834–839. doi: 10.1038/ncb1038. [DOI] [PubMed] [Google Scholar]
- Teifke J.P., Dauber M., Fichtner D., Lenk M., Polster U., Weiland E., Beyer J. Detection of European porcine reproductive and respiratory syndrome virus in porcine alveolar macrophages by two-colour immunofluorescence and in-situ hybridization-immunohistochemistry double labelling. J. Comp. Pathol. 2001;124:238–245. doi: 10.1053/jcpa.2000.0458. [DOI] [PubMed] [Google Scholar]
- Thanawongnuwech R., Suradhat S. Taming PRRSV: revisiting the control strategies and vaccine design. Virus Res. 2010;154:133–140. doi: 10.1016/j.virusres.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Tian K., Yu X., Zhao T., Feng Y., Cao Z., Wang C., Hu Y., Chen X., Hu D., Tian X., Liu D., Zhang S., Deng X., Ding Y., Yang L., Zhang Y., Xiao H., Qiao M., Wang B., Hou L., Wang X., Yang X., Kang L., Sun M., Jin P., Wang S., Kitamura Y., Yan J., Gao G.F. Emergence of fatal PRRSV variants: unparalleled outbreaks of atypical PRRS in China and molecular dissection of the unique hallmark. PLoS ONE. 2007;2:e526. doi: 10.1371/journal.pone.0000526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F.X., Wen Y.J., Yang B.C., Liu Z., Shi X.C., Leng X., Song N., Wu H., Chen L.Z., Cheng S.P. Role of non-structural protein 2 in the regulation of the replication of the porcine reproductive and respiratory syndrome virus in MARC-145 cells: effect of gene silencing and over expression. Vet. Microbiol. 2012;161:58–65. doi: 10.1016/j.vetmic.2012.07.011. [DOI] [PubMed] [Google Scholar]
- Waterhouse P.M., Wang M.B., Lough T. Gene silencing as an adaptive defence against viruses. Nature. 2001;411:834–842. doi: 10.1038/35081168. [DOI] [PubMed] [Google Scholar]
- Wensvoort G., Terpstra C., Pol J.M., ter Laak E.A., Bloemraad M., de Kluyver E.P., Kragten C., van Buiten L., den Besten A., Wagenaar F. Mystery swine disease in The Netherlands: the isolation of Lelystad virus. Vet. Q. 1991;13:121–130. doi: 10.1080/01652176.1991.9694296. [DOI] [PubMed] [Google Scholar]
- Xia B., Song H., Chen Y., Zhang X., Xia X., Sun H. Efficient inhibition of porcine reproductive and respiratory syndrome virus replication by artificial microRNAs targeting the untranslated regions. Arch. Virol. 2013;158:55–61. doi: 10.1007/s00705-012-1455-z. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Pan D., Sun X., Sun G., Wang X., Liu X., Li Y., Dai Y., Li N. Production of porcine cloned transgenic embryos expressing green fluorescent protein by somatic cell nuclear transfer. Sci. China C Life Sci. 2006;49:164–171. doi: 10.1007/s11427-006-0164-9. [DOI] [PubMed] [Google Scholar]
- Zhou F., Liang S., Chen A.H., Singh C.O., Bhaskar R., Niu Y.S., Miao Y.G. A transgenic Marc-145 cell line of piggyBac transposon-derived targeting shRNA interference against porcine reproductive and respiratory syndrome virus. Vet. Res. Commun. 2012;36:99–105. doi: 10.1007/s11259-012-9519-9. [DOI] [PubMed] [Google Scholar]


