Abstract
On the example of menthofuran, a naturally abundant compound, it has been shown for the first time that the furan ring can be readily cross-coupled with acylhaloacetylenes in the solid Al2O3 powder at room temperature to afford the corresponding 2-ethynyl derivatives in up to 88% yield. The reaction represents a ring closing/ring opening process that includes reversible formation of the intermediate cycloadducts further producing acetylene derivatives with elimination of HHal.
Keywords: Menthofuran, Haloacetylenes, Cycloadducts, Ethynylation, Al2O3
Graphical abstract
1. Introduction
After the pioneering work,1 , 2 which showed that pyrroles are cross-coupled with electrophilic acylhaloacetylenes under exceptionally mild conditions (room temperature) in the solid metal oxides and salts media to give 2-acylethynylpyrroles, this methodology has been developed into a general and efficient tool for the synthesis of diverse alkyl-, aryl-, hetaryl-, cycloalkyl-2-ethynylpyrroles, having acyl,3, 4, 5 trifluoroacyl,6 , 7 ester,8 , 9 aldehyde,10 phosphonate,11 ethynyl,12 and butadiynyl13 functions at the triple bond.
The mechanism of this ethynylation was proved to involve the addition-elimination sequence (Scheme 1 ), probably promoted by the coordinately unsaturated center of the used metal oxides and salts (electrophilic assistance) initiated by mechanoactivation (grinding up the reactants). In some cases, intermediates of this reaction, 2-(1-haloethenyl)pyrroles A, were isolated and under the same conditions transformed to 2-ethynylpyrroles.1 , 2 , 5
Scheme 1.
Reaction of menthofuran 1 with benzoylbromoacetylene 2a in the Al2O3 medium.
The development of expedient synthesis of ethynylfurans represents a challenge for heterocyclic chemistry, because such motifs are frequently met in bioactive molecules and natural products,14 , 15 for example, in inhibitors of mast cell β-triptase,16, 17, 18 SARS coronavirus main protease,19 leukocyte calcium uptake,20 lipoxygenase,21 and carlina oxide (a natural polyacetylene from Carlina acaulis) with potent antitrypanosomal and antimicrobial activities.22 They also are prospective building blocks for the synthesis of more complex biomolecules due to the rich chemistry of the triple bond and the furan ring, especially in their combination.23, 24, 25, 26, 27, 28, 29, 30, 31
A logic development of previous ethynylation of pyrroles with haloacetylenes1 , 2 , 12 , 13 , 32 might be translation of this methodology to the furan compounds. In this line, just one short note that 2-(2-furyl)pyrrole33 was capable of the ethynylating by haloacetylenes was reported. It was mentioned inter alia that the cross-coupled products with furan ethynylated moiety were isolated in small yields (4–5%).
2. Results and discussion
Here we report, on the example of natural abundant menthofuran (3,6-dimethyl-4,5,6,7-tetrahydrobenzofuran 1), first synthetically appropriate results on the transition metal-free cross-coupling of the furan ring with haloacetylenes 2a-g initiated by their grinding with most common solid oxides and salts (10-fold amount) without solvent and then allowing them to stand at room temperature for 1–72 h. The reaction course (conversion of reactants 1, 2 and the products ratio) was controlled by 1H NMR spectra of the CDCl3 extracts from the reaction mixture.
Menthofuran was chosen as a furan representative, first, due to its higher nucleophilicity (donor effect of the cyclohexane ring and two methyl groups) compared to commonly available furan compounds and, second, because menthofuran is a popular natural product, which is contained in the peppermint and exercises a great effect on the aroma of that oil.34 Also, it is the precursor of menthofurolactone and dehydromenthofurolactone, two compounds whose sweet and persistent coumarinic odor is the hallmark of premium-quality peppermint oils.35, 36, 37 This well-known fragrance is also a potent hepatotoxin and is obtained from Mentha pulegium L., a plant used in folk medicine as an abortifacient.37
A preliminary attempt to realize this reaction for furan and 2-methylfuran has shown that only the corresponding cycloadducts are formed instead of the expected ethynylated furans.38
We have started to study this reaction for benzoylbromoacetylene 2a (as acylhaloacetylene representative) using Al2O3 as a solid medium.
According to the experiments, after 1 h the reaction results in formation of ethynylfuran 3a along with the pair of diastereomeric cycloadducts of oxanorbornadiene structure 4a in 44:56 ratio (Scheme 1). The reaction is strictly regioselective: the bromine atom is neighboring the position 2 of the furan ring exclusively: NOESY interaction between protons of CH2-5 group and H-ortho protons of phenyl ring confirms C-4 location of benzoyl fragment.
After standing reaction mixture for 24 h, 48 h and 72 h the content of ethynylfuran 3a increased to 64%, 80% and 88%, correspondingly, while cycloadduct content was diminished. These results indicate that cycloadduct 4a converts to ethynylfuran 3a with elimination of hydrogen bromide, i.e. the cycloadduct 4a is kinetic intermediate of the ethynylation. As it was shown on the example of cycloadduct 4a, such derivatives of menthofuran can be isolated and handled under normal conditions.
We then turned our attention to other oxides and salts (SiO2, NaCl, K2CO3 and K3PO4) as solid media to implement the same reaction (Table 1 ).
Table 1.
1H NMR spectroscopic monitoring of reaction menthofuran with benzoylbromoacetylene in the different media.a
| Solid medium | Composition of the reaction mixture, % |
|||
|---|---|---|---|---|
| 2a | 3a | 4a | 5 | |
| Al2O3 | – | 44 | 56 | – |
| SiO2 | 60 | 26 | 13 | 1 |
| NaCl | 11 | 16 | 54 | 19 |
| K2CO3 | 24 | 12 | 63 | 1 |
| K3PO4 | – | 6 | 94 | – |
Reaction conditions: menthofuran 1 (1 mmol), benzoylbromoacetylene 2a (1 mmol), solid medium (ten-fold mass excess of the total mass of reagents), room temperature, 1 h.
In the SiO2 medium menthofuran was unstable and after 1 h the reaction mixture consisted of mainly the starting acetylene 2a (60%), content of ethynylfuran 3a and cycloadduct 4a being 26% and 13%, correspondingly. If NaCl was used as a solid medium, the main product was cycloadduct 4a (54%). The other products were ethynylfuran 3a (16%) and 3,3-bis(3,6-dimethyl-4,5,6,7-tetrahydrobenzofuran-2-yl)-1-phenylprop-2-en-1-one (5) (19%) (Scheme 2 ). Acetylene 2a was also present in the reaction mixture (11%), while menthofuran was absent.
Scheme 2.
Reaction of menthofuran 1with benzoylbromoacetylene 2a in the NaCl medium.
Contrary to the exceptions in the presence of more basic medium (K2CO3, K3PO4) ethynylation was a minor process, while the major one was cycloaddition (Table 1).
It is important to underline that, when neat reactants (1 and 2a) without a solid medium are ground, neither ethynylfuran 3a nor cycloadduct 4a are formed, while exothermic reaction take place resulting in resin-like product. Upon dropwise addition of furan 1 to cooled (10 °C) acetylene 2a the obtained reaction mixture (room temperature, 1 h) consisted of cycloadduct 4a and propenone 5 in 60:40 ratio, no ethynylfuran 3a being detected. Interestingly, under these conditions (out of a solid medium) cycloadduct 4a was not transformed to ethynylfuran 3a, whereas in CDCl3 it isomerized to 2-bromo-3-hydroxytetrahydronaphthalene 6a (Scheme 3 ).
Scheme 3.

Isomerization of cycloadduct 4a to 2-bromo-3-hydroxytetrahydronaphthalene 6a.
Thus as indicated above, Al2O3 has proven to be a medium of choice from the oxides and salts studied for the synthesis of ethynyl derivatives of menthofuran 3a. Therefore, this oxide was used to evaluate the influence of halogen atom in acylhaloacetylene on the reaction course (Table 2 ).
Table 2.
1H NMR spectroscopic monitoring of reaction of furan 1 with halobenzoylacetylenes in the Al2O3 medium.a
| Haloacetylene | Composition of the reaction mixture, % |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 h |
3 h |
72 h |
||||||||||
| 1 | 3a | 4a | 5 | 1 | 3a | 4a | 5 | 1 | 3a | 4a | 5 | |
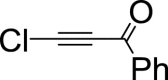 |
– | 36 | 62 | 2 | – | 38 | 61 | 1 | – | 100 | – | – |
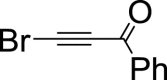 |
– | 44 | 56 | – | – | 49 | 51 | – | – | 88 | 12 | – |
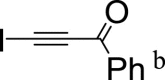 |
17 | 15 | 25 | 6 | 9 | 17 | 28 | 11 | – | 49 | – | 18 |
b Content of iodobenzoylacetylene for 1 h, 3 h and 72 h was 37%, 35% and 33% correspondingly.
Reaction conditions: 1 (1 mmol), halobenzoylacetylene (1 mmol), Al2O3 (ten-fold mass excess of the total mass of reagents), room temperature.
Chlorobenzoylacetylene reacts with furan 1 to afford after 1 h cycloadduct 4a as major product (3a: 4a ratio is the 36: 62), the reactant conversion being complete. As anticipated, after 3 h, content of ethynylfuran 3a insignificantly increased and within 72 h the only product was ethynylfuran 3a. The reaction of furan 1 with iodobenzoylacetylene proceeds slower, after 3 h composition of the reaction mixture being 1: 2a: 3a: 4a: 5 = 9: 17: 28: 11. In 72 h cycloadduct 4a disappears and the reaction mixture contains ethynylfuran 3a along with propenone 5 in the ratio of 49: 18 (Table 2).
Thus, in contrast to cross-coupling of pyrroles with acylhaloacetylenes in solid media, ethynylation of the furan moiety with acylhaloacetylenes proceeds through [4 + 2]-cycloaddition followed by ring-opening with elimination of HHal. It is also in keeping with quantitative conversion of isolated cycloadduct 4a, upon its passing through SiO2 or Al2O3 column, into ethynylfuran 3a.
As further experiments have shown, this ethynylation is also applicable for bromoacetylenes with formyl (2b), acetyl (2c), furoyl (2d), and thenoyl (2e) groups at the triple bond, which react with menthofuran 1 in the solid Al2O3 to afford the acetylenic derivatives 3b-e in 40–88% yields (Scheme 4 ).
Scheme 4.
Reaction of menthofuran 1 with haloacetylenes 2a-f in the Al2O3 medium.
For bromopropiolate 2f, the major reaction product was cycloadduct 4f, which in the CDCl3 solution was isomerized to 2-bromo-3-hydroxynaphthalene 6f. Ethynylfuran 3f in this case was minor product, its isolated yield being just ca. 10% (Scheme 4, Scheme 5 ).
Scheme 5.
Reaction of menthofuran 1 with bromopropiolate 2f in the Al2O3 medium.
In the case of bromodiacetylene 2g, the only isolated product was bicyclic propiolate 6g, the isomer of the expected cycloadduct 4g (Scheme 6 ).
Scheme 6.
Reaction of menthofuran 1 with bromodiacetylene 2g in the Al2O3 medium.
Thus, it appears likely that the oxanorbornadiene intermediates such as 4a, reversibly generated on the first reaction step, transform to the ethynyl derivatives of menthofuran via zwitterion B with the positive charge distributed over the whole furan moiety. The latter eliminates hydrogen bromide in the concerted process (hydrogen releases from the position 2 of the furan moiety, Scheme 7 ).
Scheme 7.
Possible reaction pathway.
An experimental evidence for the proposed mechanism is the observation that cycloadducts 4 are gradually transformed to ethynylated products 3. The formation of the intermediate zwitterion B is supported by isolation of propenone 5 resulted from substitution of bromine atom either in zwitterion B or bromovinyl intermediate 7 (Scheme 8 ).
Scheme 8.
Possible pathway of propenone 5 formation.
An alternative route to propenone 5 might be the nucleophilic addition of the second molecule of menthofuran to ethynyl derivative of menthofuran 3a (Scheme 8). However, as shown by a special experiment, ethynylfuran 3a did not add furan 1 under the reaction conditions.
The mechanistic rationalizations here considered are supported by the fact that the furan derivatives, having no donor substituents, like furan-2-carbaldehyde and its acetals as well as 6-methoxybenzofuran, happened to be reluctant in the reaction with haloacetylenes under the conditions studied (Fig. 1 ).
Fig. 1.
The furan derivatives, having no donor substituents.
Indeed, the initial [4 + 2]-cycloaddition is a typical Diels-Alder condensation with inverse electronic demand, i.e., diene (in this case furan moiety) should be electron-rich compound, correspondingly the opened form of cycloadduct, the zwitterion B, should be the more stable the stronger donor substituent are in the furan moiety.
3. Conclusion
In summary, on the example of menthofuran, it has been shown for the first time that furans bearing electron-donating substituents are capable of cross-coupling with haloacetylenes in solid media of oxides or salts under mild conditions in the absence of transition metals to afford acetylenic derivatives. Thus, it was found that a naturally abundant furan compound, menthofuran, is readily cross-coupled with haloacetylenes under transition metal-free conditions in the solid Al2O3 powder at room temperature to give 2-ethynyl derivatives in up to 88% yield. In contrast to the ethynylation of pyrroles under similar conditions, the reaction studied involves reversible [4 + 2]-cycloaddition of the furan ring to form the isolable intermediated cycloadducts, which then further transform to the acetylenic derivatives. The novel menthofuran derivatives, thus obtained, represent promising bioactive compounds and rewarding precursors for drug design.
4. Experimental
4.1. General information
IR spectra were obtained with a Bruker Vertex 70 spectrometer (400–4000 cm−1, films). 1H (400.1 MHz), 13C (100.6 MHz) spectra were recorded on a Bruker DPX-400 spectrometer at ambient temperature in CDCl3 solutions and referenced to CDCl3 (residual protons of CDCl3 in 1H NMR δ = 7.26 ppm; 13C NMR δ = 77.16 ppm). The C, H, S microanalyses were performed on a Flash EA 1112 СHNS-O/MAS analyzer. Br content was determined by combustion method. Melting point (uncorrected) was determined on a Kofler micro hot stage apparatus. 3,6-Dimethyl-4,5,6,7-tetrahydrobenzofuran is commercial product. Acylhaloacetylenes are obtained accordingly to procedures.39 , 40
4.2. General procedure for synthesis of 3-(3,6-dimethyl-4,5,6,7-tetrahydrobenzofuran-2-yl)-1-prop-2-yn-1-ones 3a-e
Equimolar amounts of 3,6-dimethyl-4,5,6,7-tetrahydrobenzofuran (0.150 g, 1 mmol) and haloacetylenes 2a-e (1 mmol) were ground at room temperature with a 10-fold amount (by weight) of Al2O3 in a porcelain mortar for 10 min and allowed to stay at room temperature for 60 min. 1H NMR analysis showed that in all cases reaction mixtures contain cycloadduct 4a-e and acetylene 3a-e in average ratio 1: 1. Slow column chromatography (SiO2, eluent n-hexane) of the reaction mixture leads to the transformation of cycloadduct 4a-e to acetylene 3a-e, which was isolated in pure state. The cycloadduct 4a was isolated according to the procedure presented below.
4.2.1. 3-(3,6-Dimethyl-4,5,6,7-tetrahydrobenzofuran-2-yl)-1-phenylprop-2-yn-1-one (3a)
Yield 150 mg (54%), yellow crystals, Rf 0.5 (n-hexane-diethyl ether, 2:1), mp 132–134°С; 1H NMR (400 MHz, CDCl3): δ 8.20–8.18 (m, 2H, Ho-Ph), 7.62–7.58 (m, 1H, Hp-Ph), 7.52–7.48 (m, 2H, Hm-Ph), 2.76–2.70 (m, 1H, CHCH3), 2.46–2.31 (m, 2H, CH2), 2.28–2.20 (m, 1H, CH2), 2.17 (s, 3H, CCH3), 2.00–1.85 (m, 2H, CH2), 1.44–1.34 (m, 1H, CH2), 1.10 (d, 3H, J = 8.0 Hz, CHCH 3); 13C NMR (100 MHz, CDCl3): δ 177.2, 156.4, 137.0, 134.7, 133.8, 130.6, 129.3, 128.6, 120.0, 97.4, 85.9, 31.8, 30.9, 29.4, 21.3, 19.9, 9.6; IR ν max 3062, 2953, 2921, 2158, 1625, 1542, 1450, 1292, 1223, 1166, 1007, 834, 791, 751, 704, 660; Anal. Calcd for C19H18O2: C, 81.99; H, 6.52%; Found: C, 82.21; H, 6.88%.
4.2.2. 3-(3,6-Dimethyl-4,5,6,7-tetrahydrobenzofuran-2-yl)propiolaldehyde (3b)
Yield 93 mg (46%), yellow oil, Rf 0.5 (n-hexane-diethyl ether, 2:1); 1H NMR (400 MHz, CDCl3): δ 9.40 (s, 1H, CHO), 2.72–2.66 (m, 1H, CHCH3), 2.42–2.30 (m, 2H, CH2), 2.22–2.16 (m, 1H, CH2), 2.08 (s, 3H, CH3), 1.97–1.84 (m, 2H, CH2), 1.41–1.33 (m, 1H, CH2), 1.08 (d, 3H, J = 8.0 Hz, CHCH 3); 13C NMR (100 MHz, CDCl3): δ 175.3, 157.4, 136.0, 130.2, 120.3, 99.5, 88.2, 31.9, 30.8, 29.3, 21.3, 19.9, 9.6; IR ν max 3024, 2951, 2920, 2847, 2163, 1649, 1546, 1451, 1435, 1381, 1290, 1216, 1162, 1139, 1099, 987, 812, 719, 678; Anal. Calcd for C13H14O2: C, 77.20; H, 6.98%; Found: C, 77.38; H, 7.23%.
4.2.3. 4-(3,6-Dimethyl-4,5,6,7-tetrahydrobenzofuran-2-yl)but-3-yn-2-one (3c)
Yield 86 mg (40%), yellow crystals, Rf 0.65 (n-hexane-diethyl ether, 2:1), mp 84–86°С; 1H NMR (400 MHz, CDCl3): δ 2.71–2.65 (m, 1H, CHCH3), 2.41 (s, 3H, COCH 3), 2.37–2.28 (m, 2H, CH2), 2.22–2.15 (m, 1H, CH2), 2.06 (s, 3H, CCH3), 1.96–1.83 (m, 2H, CH2), 1.38–1.33 (m, 1H, CH2), 1.08 (d, 3H, J = 8.0 Hz, CHCH 3); 13C NMR (100 MHz, CDCl3): δ 183.6, 156.3, 134.6, 130.2, 119.9, 98.4, 83.2, 32.2, 31.8, 30.8, 29.3, 21.4, 19.8, 9.5; IR ν max 2952, 2919, 2850, 2164, 1653, 1619, 1453, 1436, 1359, 1295, 1189, 1093, 1069, 1021, 911, 733, 576; Anal. Calcd for C14H16O2: C, 77.75; H, 7.46%; Found: C, 77.96; H, 7.78%.
4.2.4. 3-(3,6-Dimethyl-4,5,6,7-tetrahydrobenzofuran-2-yl)-1-(furan-2-yl)prop-2-yn-1-one (3d)
Yield 236 mg (88%), yellow crystals, Rf 0.4 (n-hexane-diethyl ether, 2:1), mp 131–133°С; 1H NMR (400 MHz, CDCl3): δ 7.68–7.66 (m, 1H, H-5 furan), 7.38–7.37 (m, 1H, H-3, furan), 6.58–6.57 (m, 1H, H-4, furan), 2.74–2.70 (m, 1H, CHCH3), 2.44–2.31 (m, 2H, CH2), 2.25–2.18 (m, 1H, CH2), 2.14 (s, 3H, CCH3), 2.00–1.84 (m, 2H, CH2), 1.43–1.33 (m, 1H, CH2), 1.10 (d, 3H, J = 8.0 Hz, CHCH 3); 13C NMR (100 MHz, CDCl3): δ 164.4, 156.5, 153.3, 147.7, 134.9, 130.5, 120.1, 119.9, 112.6, 96.5, 84.8, 31.9, 30.9, 29.4, 21.4, 19.9, 9.6; IR ν max 3120, 2923, 2847, 2173, 1617, 1544, 1458, 1394, 1297, 1271, 1165, 1044, 1006, 789, 732; Anal. Calcd for C17H16O3: C, 76.10; H, 6.01%; Found: C, 76.32; H, 6.27%.
4.2.5. 3-(3,6-Dimethyl-4,5,6,7-tetrahydrobenzofuran-2-yl)-1-(thiophen-2-yl)prop-2-yn-1-one (3e)
Yield 190 mg (67%), yellow crystals, Rf 0.37 (n-hexane-diethyl ether, 2:1), mp 143–145°С; 1H NMR (400 MHz, CDCl3): δ 7.96–7.95 (m, 1H, H-5, thiophene), 7.69–7.68 (m, 1H, H-3, thiophene), 7.18–7.16 (m, 1H, H-4, thiophene), 2.75–2.69 (m, 1H, CHCH3), 2.43–2.31 (m, 2H, CH2), 2.25–2.19 (m, 1H, CH2), 2.15 (s, 3H, CCH3), 2.00–1.86 (m, 2H, CH2), 1.43–1.34 (m, 1H, CH2), 1.10 (d, 3H, J = 8.0 Hz, CHCH 3); 13C NMR (100 MHz, CDCl3): δ 169.2, 156.5, 145.0, 134.7, 134.6, 134.4, 130.5, 128.4, 120.0, 96.6, 84.6, 31.9, 30.9, 29.4, 21.4, 19.9, 9.6; IR ν max 3095, 2949, 2920, 2846, 2166, 1595, 1543, 1407, 1348, 1294, 1238, 1046, 970, 858, 722; Anal. Calcd for C17H16O2S: C, 71.80; H, 5.67; S, 11.28%; Found: C, 71.67; H, 5.93; S, 11.34%.
4.3. (3-Bromo-1,6-dimethyl-5,6,7,8-tetrahydro-2H-2,4a-epoxynaphthalen-4-yl)(phenyl)methanone (4a)
Equimolar amounts of 3,6-dimethyl-4,5,6,7-tetrahydrobenzofuran (0.150 g, 1 mmol) and benzoylbromoacetylene (0.209 g, 1 mmol) were ground at room temperature with a 10-fold amount (by weight) of Al2O3 in a porcelain mortar for 10 min and allowed to stay at room temperature for 60 min. Reaction mixture was placed on the glass filter and washed with n-hexane, solvent removed and oily residue recrystallized from 5 to 7 ml of n-hexane to give acetylene 3a. Mother liquor was evaporated to give cycloadduct 4a as yellow oil, Rf 0.63 (n-hexane-diethyl ether, 2:1), yield 144 mg (40%); 1H NMR (400 MHz, CDCl3): δ 7.85 (m, 2H, Ho-Ph’), 7.84 (m, 2H, Ho-Ph), 7.56 (m, 2H, Hp-Ph, Hp-Ph’), 7.45 (m, 4H, Hm-Ph, Hm-Ph’), 4.99 (s, 1H, H-2′), 4.97 (s, 1H, H-2), 2.58 (m, 1H, H-8), 2.45 (m, 2H, H-8′), 2.33 (m, 1H, H-5), 2.27 (m, 1H, H-8), 2.19 (m, 1H, H-6), 2.07 (m, 1H, H-5′), 1.93 (m, 1H, H-5′), 1.85 (m, 1H, H-7), 1.83 (d, J = 2.8 Hz, 3H, CCH 3), 1.82 (dd, J = 1.2, 2.0 Hz, 3H, CCH3′), 1.77 (m, 1H, H-7′), 1.68 (m, 1H, H-6′), 1.64 (m, 1H, H-7′), 1.51 (m, 1H, H-5), 1.08 (m, 1H, H-7), 0.93 (d, J = 6.0 Hz, 3H, CHCH 3′), 0.92 (d, J = 6.60 Hz, 3H, CHCH 3); 13C NMR (100 MHz, CDCl3): δ 193.0 (C O), 192.7 (C O′), 152.6 (C-4), 152.0 (C-4′), 146.8 (C-8a’), 146.5 (C-8a), 143.5 (C-3′), 143.2 (C-3), 140.4 (C-1′), 139.5 (C-1), 136.8 (C-i), 136.6 (C-i'), 133.7 (C-p, C-p’), 129.7 (C-o, C-o’), 128.7 (C-m, C-m’), 98.2 (C-4a’), 96.7 (C-4a), 91.6 (C-2′), 91.0 (C-2), 38.1 (C-5), 35.4 (C-5′), 32.6 (C-7), 30.0 (C-7′), 29.1 (C-6), 28.1 (C-6′), 23.6 (C-8), 22.6 (CHCH3), 21.7 (CHCH3′), 19.4 (C-8′), 12.1 (CCH3′), 11.9 (CCH3); IR ν max 3060, 3032, 2922, 2871, 1673, 1591, 1446, 1409, 1334, 1282, 1223, 1089, 1007, 906, 723, 690; Anal. Calcd for C19H19BrO2: C, 63.52; H, 5.33; Br, 22.24; Found: C, 63.80; H, 5.11; Br, 22.56%.
4.4. (2-Bromo-3-hydroxy-4,7-dimethyl-5,6,7,8-tetrahydronaphthalen-1-yl)(phenyl) methanone (6a)
This product was formed (0.230 g, 64%) as yellow crystals (Rf 0.56 (n-hexane-diethyl ether, 2:1), mp 68–70 °C) from 0.359 g of cycloadducts 4a under storage in n-hexane solution and purified by flash chromatography (SiO2, n-hexane); 1H NMR (400 MHz, CDCl3): δ 7.85–7.83 (m, 2H, Ho-Ph), 7.61–7.59 (m, 1H, Hp-Ph), 7.47–7.45 (m, 2H, Hm-Ph), 5.53 (br s, 1H, OH), 2.81 (m, 1H, H-5), 2.77 (m, 1H, H-8), 2.59 (m, 1H, H-5), 2.31 (m, 1H, H-8), 2.25 (s, 3H, CCH 3), 1.90 (m, 1H, H-6), 1.70 (m, 1H, CHCH3), 1.35 (m, 1H, H-6), 0.91 (d, 3H, J = 5.8 Hz, CHCH 3); 13C NMR (100 MHz, CDCl3): δ 197.2, 147.9, 137.0, 136.1, 134.0 (two signals), 129.8, 129.0 (two signals), 124.5, 104.4, 35.5, 30.9, 28.7, 27.5, 21.7, 12.5; IR ν max 2921, 2873, 1671, 1589, 1446, 1409, 1336, 1283, 1225, 1087, 1007, 908, 832, 729, 690; Anal. Calcd for C19H19BrO2: C, 63.52; H, 5.33; Br, 22.24%; Found: C, 63.84; H, 5.51; Br, 22.51%.
4.5. 3,3-Bis(3,6-dimethyl-4,5,6,7-tetrahydrobenzofuran-2-yl)-1-phenylprop-2-en-1-one (5)
This product was isolated from the reaction of 3,6-dimethyl-4,5,6,7-tetrahydrobenzofuran (0.150 g, 1 mmol) and benzoylbromoacetylene (0.209 g, 1 mmol) when NaCl was used instead of Al2O3 (See 4.2. general procedure); Yield 0.043 g (10%), yellow oil, Rf 0.4 (n-hexane-diethyl ether, 2:1); 1H NMR (400 MHz, CDCl3): δ 7.76–7.74 (m, 2H, Ho-Ph), 7.39–7.36 (m, 1H, Hp-Ph), 7.31–7.29 (m, 2H, Hm-Ph), 6.84 (s, 1H, =CH), 2.76–2.71 (m, 1H, CHCH3), 2.57–2.52 (m, 1H, CHCH3), 2.36–1.84 (m, 12H, 6CH2), 1.68 (s, 3H, CCH3), 1.59 (s, 3H, CCH3), 1.10 (d, 3H, J = 6.6 Hz, CHCH 3), 1.01 (d, 3H, J = 6.6 Hz, CHCH 3); 13C NMR (100 MHz, CDCl3): δ 193.2, 153.2, 151.5, 145.8, 142.8, 139.9, 132.9, 131.3, 128.2, 127.8, 125.6, 123.8, 121.4, 119.5, 119.2, 31.7, 31.5, 31.2, 31.1, 29.6, 29.5, 21.5, 21.4, 20.1, 19.9, 9.5, 9.2; IR ν max 3060, 2921, 2853, 1634, 1545, 1446, 1378, 1271, 1218, 1108, 1071, 1021, 913, 858, 732, 702, 647; Anal. Calcd for C29H32O3: C, 81.27; H, 7.53%; Found: C, 81.42; H, 7.85%.
4.6. Reaction of furan 1 with ethyl 3-bromopropiolate 1f
Equimolar amounts of 3,6-dimethyl-4,5,6,7-tetrahydrobenzofuran (0.150 g, 1 mmol) and ethyl 3-bromopropiolate (0.177 g, 1 mmol) were ground at room temperature with a 10-fold amount (by weight) of Al2O3 in a porcelain mortar for 10 min and allowed to stay at room temperature for 60 min. 1H NMR analysis of crude reaction mixture shows that it contains both isomeric cycloadducts 4f and ethynylfuran 3f in 2:1 ratio. Fractionating (SiO2, eluent n-hexane + 0.5% of diethyl ether) of the reaction mixture gives pure acetylene as white crystals (3f) and cycloadducts mixture (4f) as colorless oil.
4.6.1. Ethyl-3-(3,6-dimethyl-4,5,6,7-tetrahydrobenzofuran-2-yl)propiolate (3f)
Yield 25 mg (10%), white crystals, Rf 0.62 (n-hexane-diethyl ether, 2:1), mp 46–48°С; 1H NMR (400 MHz, CDCl3): δ 4.27 (q, 2H, J = 16 Hz, CH 2CH3), 2.69–2.64 (m, 1H, CHCH3), 2.41–2.30 (m, 2H, CH2), 2.20–2.14 (m, 1H, CH2), 2.06 (s, 3H, CCH3), 1.94–1.82 (m, 2H, CH2), 1.40–1.35 (m, 1H, CH2), 1.33 (t, 3H, J = 8.0 Hz, CH2 CH 3), 1.07 (d, 3H, J = 8.0 Hz, CHCH 3); 13C NMR (100 MHz, CDCl3): δ 155.6, 154.5, 133.5, 130.3, 119.5, 89.7, 78.5, 61.9, 31.8, 31.0, 29.4, 21.4, 19.9, 14.3, 9.4; IR ν max 2951, 2924, 2873, 2855, 2192, 1706, 1549, 1455, 1371, 1293, 1210, 1025, 862, 742; Anal. Calcd for C15H18O3: C, 73.15; H, 7.37%; Found: C, 73.36; H, 7.62%.
4.6.2. Ethyl-3-bromo-1,6-dimethyl-5,6,7,8-tetrahydro-2H-2,4a-epoxynaphthalene-4-carboxylate (4f) (the pair of diastereoisomers)
Yield 141 mg (43%), colorless oil, Rf 0.55 (n-hexane-diethyl ether, 2:1); 1H NMR (400 MHz, CDCl3): δ 4.90 (s, 1H, CH′), 4.88 (s, 1H, CH), 4.26–4.24 (m, 2H, CH 2CH3), 2.63–2.61 (m, 1H, CH2), 2.60–2.58 (m, 1H, CH2), 2.20–2.18 (m, 1H, CHCH3), 1.92–1.88 (m, 1H, CH2), 1.85–1.83 (m, 3H, CCH 3), 1.84–1.80 (m, 1H, CH2), 1.51–1.47 (m, 1H, CH2), 1.35–1.31 (m, 3H, CH2 CH 3), 1.12–1.10 (m, 1H, CH2), 1.05–1.01 (m, 3H, CHCH3). 13C NMR (100 MHz, CDCl3): δ 163.7 (C O′), 163.1 (C O), 149.8 (C-3), 148.2 (C-3′), 146.9 (C-8a, C-8a’), 144.9 (C-4′), 144.3 (C-4), 141.1 (C-1′), 139.7 (C-1), 96.1 (C-4a’), 94.7 (C-4a), 91.3 (C-2′), 90.9 (C-2), 60.6 (CH2CH3, CH2CH3′), 38.1 (C-5), 35.5 (C-5′), 32.5 (C-7), 30.0 (C-7′), 29.2 (C-6), 28.0 (C-6′), 24.3 (C-8), 22.4 (CHCH3), 21.7 (CHCH3′), 19.8 (C-8′), 14.2 (CH2 CH3, CH2 CH3′), 12.1 (C-Me’), 11.8 (C-Me); IR ν max 2950, 2926, 2873, 1725, 1588, 1446, 1416, 1374, 1293, 1213, 1080, 1023, 1014, 913, 860, 733, 650; Anal. Calcd for C15H19BrO3: C, 55.06; H, 5.85; Br, 24.42%; Found: C, 55.28; H, 6.03; Br, 24.74%.
4.7. Ethyl-2-bromo-3-hydroxy-4,7-dimethyl-5,6,7,8-tetrahydronaphthalene-1-carboxylate (6f)
This product was formed (196 mg, 60%) as yellow crystals (Rf 0.7 (n-hexane-diethyl ether, 2:1), mp 105–107 °C) from 0.327 g of cycloadducts 4f under storage and purified by flash chromatography (SiO2, n-hexane);
1H NMR (400 MHz, CDCl3): δ 5.51 (br s, 1H, OH), 4.41 (q, 2H, J = 16 Hz, CH 2CH3), 2.76–2.72 (m, 1H, H-5), 2.68–2.64 (m, 1H, H-8), 2.54–2.52 (m, 1H, H-5), 2.32–2.26 (m, 1H, H-8), 2.18 (s, 3H, CCH 3), 1.93–1.90 (m, 1H, H-6), 1.75–1.73 (m, 1H, CHCH3), 1.40 (t, 3H, J = 8.0 Hz, CH2 CH 3), 1.36–1.32 (m, 1H, H-6), 1.03 (d, 3H, J = 8.0 Hz, CHCH 3); 13C NMR (100 MHz, CDCl3): δ 168.1, 147.9, 137.0, 133.0, 127.0, 125.0, 104.7, 61.8, 35.7, 31.0, 28.6, 27.5, 21.8, 14.3, 12.4; IR ν max 2923, 2873, 1726, 1583, 1442, 1413, 1373, 1333, 1289, 1212, 1074, 1036, 861, 816, 765, 710; Anal. Calcd for C15H19BrO3: C, 55.06; H, 5.85; Br, 24.42%; Found: C, 55.29; H, 6.08; Br, 24.80%.
4.8. Methyl-3-(2-bromo-3-hydroxy-4,7-dimethyl-decahydronaphthalen-1-yl)propiolate (6g)
Equimolar amounts of 3,6-dimethyl-4,5,6,7-tetrahydrobenzofuran (0.150 g, 1 mmol) and methyl-5-bromopenta-2,4-diynoate (2g) (0.177 g, 1 mmol) were ground at room temperature with a 10-fold amount (by weight) of Al2O3 in a porcelain mortar for 10 min and allowed to stay at room temperature for 60 min. 1H NMR analysis of crude reaction mixture shows that it contains only both isomeric cycloadducts 4g. TLC (SiO2, eluent - n-hexane: diethyl ether 1: 1) of the reaction mixture give bicyclic phenol 6g as light yellow oil, Rf 0.7 (n-hexane-diethyl ether, 1:1); Yield 34 mg (10%); 1H NMR (400 MHz, CDCl3): δ 5.56 (br, 1H, OH), 3.86 (s, 3H, OCH 3), 3.08–3.02 (m, 1H, CHCH3), 2.74–2.70 (m, 1H, CH2), 2.57–2.48 (m, 2H, CH2), 2.40–2.33 (m, 1H, CH2), 2.21 (s, 3H, CCH 3), 1.95–1.74 (m, 2H, CH2), 1.08 (d, 3H, J = 4.0 Hz, CHCH 3); 13C NMR (100 MHz, CDCl3): δ 154.7, 148.3, 136.8, 135.4, 127.5, 118.1, 111.8, 88.0, 84.0, 53.0, 37.3, 30.9, 28.6, 27.3, 21.7, 12.8; IR ν max 3012, 2949, 2924, 2857, 2216, 1712, 1648, 1440, 1237, 1106, 1074, 1022, 765; Anal. Calcd for C16H17BrO3: C, 56.99; H, 5.08; Br, 23.70%; Found: C, 57.20; H, 5.31; Br, 24.02%.
Conflicts of interest
There are no conflicts of interest to declare.
Acknowledgements
Authors acknowledge Baikal Analytical Center for collective use SB RAS for the equipment.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.tet.2018.02.024.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Trofimov B.A., Stepanova Z.V., Sobenina L.N., Mikhaleva A.I., Ushakov I.A. Tetrahedron Lett. 2004;45:6513–6516. [Google Scholar]
- 2.Trofimov B.A., Sobenina L.N. In: Attanasi O.A., Spinelli D., editors. vol. 13. Società Chimica Italiana; Roma: 2009. pp. 92–119. (Targets in Heterocyclic Systems). [Google Scholar]
- 3.Trofimov B.A., Sobenina L.N., Stepanova Z.V., et al. Synthesis. 2007;2007:447–451. [Google Scholar]
- 4.Trofimov B.A., Sobenina L.N., Stepanova Z.V., et al. Tetrahedron. 2008;64:5541–5544. [Google Scholar]
- 5.Sobenina L.N., Tomilin D.N., Petrova O.V., et al. Russ J Org Chem. 2010;46:1373–1377. [Google Scholar]
- 6.Tomilin D.N., Gotsko M.D., Sobenina L.N., et al. J Fluorine Chem. 2016;186:1–6. [Google Scholar]
- 7.Tomilin D.N., Soshnikov D.Y., Trofimov A.B., et al. Mendeleev Commun. 2016;26:480–482. [Google Scholar]
- 8.Trofimov B.A., Sobenina L.N., Demenev A.P., et al. Tetrahedron Lett. 2007;48:4661–4664. [Google Scholar]
- 9.Trofimov B.A., Sobenina L.N., Stepanova Z.V., Petrova O.V., Ushakov I.A., Mikhaleva A.I. Tetrahedron Lett. 2008;49:3946–3949. [Google Scholar]
- 10.Gotsko M.D., Sobenina L.N., Tomilin D.N., et al. Tetrahedron Lett. 2016;57:4961–4964. [Google Scholar]
- 11.Gotsko M.D., Sobenina L.N., Tomilin D.N., Ushakov I.A., Dogadina A.V., Trofimov B.A. Tetrahedron Lett. 2015;56:4657–4660. [Google Scholar]
- 12.Tomilin D.N., Pigulski B., Gulia N., et al. RSC Adv. 2015;5:73241–73248. [Google Scholar]
- 13.Pigulski B., Arendt A., Tomilin D.N., Sobenina L.N., Trofimov B.A., Szafert S. J Org Chem. 2016;81:9188–9198. doi: 10.1021/acs.joc.6b01732. [DOI] [PubMed] [Google Scholar]
- 14.Li Y., Brand J.P., Waser J. Angew Chem Int Ed. 2013;52:6743–6747. doi: 10.1002/anie.201302210. [DOI] [PubMed] [Google Scholar]
- 15.Romashov L.V., Ananikov V.P. Chem Asian J. 2017;12:2652–2655. doi: 10.1002/asia.201700940. [DOI] [PubMed] [Google Scholar]
- 16.Pauls HW, Aldous SA, Merriman GH, Farr RA, Sledeski AW. US2004192734 (A1), 2004.
- 17.Costanzo M.J., Yabut S.C., Zhang H.-C., et al. Bioorg Med Chem Lett. 2008;18:2114–2121. doi: 10.1016/j.bmcl.2008.01.093. [DOI] [PubMed] [Google Scholar]
- 18.Costanzo MJ, Yabut SC, Tounge B, Maryanoff BE, Zhang H-C. US2012165327 (A1), 2012.
- 19.Wu S-Y, Hsieh H-P, Hsu T-A, Lu I-L. US2006019967 (A1), 2006.
- 20.Dinerstein RJ, Sabol JS, Diekema KA. CA2051504 (A1), 1992.
- 21.Brooks DW, Stewart AO, Kerkman DJ, Bhatia PA, Basha A. WO9201682 (A1), 1992.
- 22.Herrmann F., Hamoud R., Sporer F., Tahrani A., Wink M. Planta Med. 2011;77:1905–1911. doi: 10.1055/s-0031-1279984. [DOI] [PubMed] [Google Scholar]
- 23.Schmittel M., Steffen J.-P., Bohn I. Heterocycl Commun. 1997;3:443–448. [Google Scholar]
- 24.Dudnik A.S., Schwier T., Gevorgyan V. Tetrahedron. 2009;65:1859–1870. [Google Scholar]
- 25.Du D., Hu Z., Jin J., et al. Org Lett. 2012;14:1274–1277. doi: 10.1021/ol300148f. [DOI] [PubMed] [Google Scholar]
- 26.Chatzopoulou E., Davies P.W. Chem Commun. 2013;49:8617–8619. doi: 10.1039/c3cc45410j. [DOI] [PubMed] [Google Scholar]
- 27.Lu Y., Tang W., Zhang Y., Du D., Lu T. Adv Synth Catal. 2013;355:321–326. [Google Scholar]
- 28.Hussain M.K., Ansari M.I., Kant R., Hajela K. Org Lett. 2014;16:560–563. doi: 10.1021/ol403420z. [DOI] [PubMed] [Google Scholar]
- 29.Al-Amin M., Johnson J.S., Blum S.A. Organometallics. 2014;33:5448–5456. doi: 10.1021/om500747m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siyang H.X., Ji X.Y., Wu X.R., Wu X.Y., Liu P.N. Org Lett. 2015;17:5220–5223. doi: 10.1021/acs.orglett.5b02556. [DOI] [PubMed] [Google Scholar]
- 31.Hou Z.-W., Mao Z.-Y., Zhao H.-B., et al. Angew Chem Int Ed. 2016;55:9168–9172. doi: 10.1002/anie.201602616. [DOI] [PubMed] [Google Scholar]
- 32.Tomilin D.N., Petrushenko K.B., Sobenina L.N., et al. Asian J Org Chem. 2016;5:1288–1294. [Google Scholar]
- 33.Sobenina L.N., Petrova O.V., Tomilin D.N., et al. Tetrahedron. 2014;70:9506–9511. [Google Scholar]
- 34.Din ZU, Ho TL, Traynor SG. US4240969 (A), 1980.
- 35.Näf R., Velluz A. Flavour Fragrance J. 1998;13:203–208. [Google Scholar]
- 36.Frérot E., Bagnoud A., Vuilleumier C. Flavour Fragrance J. 2002;17:218–226. [Google Scholar]
- 37.De Lucia M., Mainieri F., Verotta L., et al. J Org Chem. 2007;72:10123–10129. doi: 10.1021/jo701992r. [DOI] [PubMed] [Google Scholar]
- 38.Tomilin D.N., Sobenina L.N., Gotsko M.D., Ushakov I.A., Trofimov B.A. Mendeleev Commun. 2018;28:20–21. [Google Scholar]
- 39.Leroy J. Synth Commun. 1992;22:567–572. [Google Scholar]
- 40.Tomilin D.N., Petrova O.V., Sobenina L.N., Mikhaleva A.I., Trofimov B.A. Chem Heterocycl Comp. 2013;49:341–344. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.












