Abstract
The Pelargonium sidoides extract EPs® 7630 is an approved drug for the treatment of acute bronchitis in Germany. The postulated mechanisms underlying beneficial effects of EPs® 7630 in bronchitis patients include immunomodulatory and cytoprotective effects, inhibition of interaction between bacteria and host cells, and increase of cilliary beat frequency on respiratory cells. Here, we investigated the influence of EPs® 7630 on replication of a panel of respiratory viruses. Determination of virus-induced cytopathogenic effects and virus titres revealed that EPs® 7630 at concentrations up to 100 μg/ml interfered with replication of seasonal influenza A virus strains (H1N1, H3N2), respiratory syncytial virus, human coronavirus, parainfluenza virus, and coxsackie virus but did not affect replication of highly pathogenic avian influenza A virus (H5N1), adenovirus, or rhinovirus. Therefore, antiviral effects may contribute to the beneficial effects exerted by EPs® 7630 in acute bronchitis patients.
Keywords: Pelargonium sidoides, Respiratory viruses, Acute bronchitis
Introduction
In southern Africa, the roots of Pelargonium sidoides have been used for centuries for the treatment of different aliments including infections of the airways (Conrad et al., 2007, Brendler and van Wyk, 2008, Kolodziej, 2008). In Germany, a standardised extract of Pelargonium sidoides (EPs® 7630) was registered in 2005 by the Federal Institute for Drugs and Medical Devices (BfArM) for the indication “acute bronchitis” (Conrad et al. 2007). A couple of randomised, double-blind, placebo-controlled clinical trials support the efficacy and safety of EPs® 7630 for the treatment of acute bronchitis in adults and children (Conrad et al., 2007, Agbabiaka et al., 2008, Matthys and Funk, 2008, Timmer et al., 2008, Kamin et al., 2010a, Kamin et al., 2010b, Matthys et al., 2010). The postulated mechanisms underlying beneficial effects of EPs® 7630 in bronchitis patients include immunomodulatory and cytoprotective effects, inhibition of interaction between bacteria and host cells, and increase of cilliary beat frequency on respiratory cells (Conrad et al., 2007, Kolodziej, 2008).
Notably, bacteria are estimated to cause <10% of cases of uncomplicated acute bronchitis while >90% of cases are estimated to be caused by respiratory viruses (Gonzales and Sande 2000). Therefore, effects of EPs® 7630 on replication of respiratory viruses are of interest in the context acute bronchitis. An aqueous extract of Pelargonium sidoides had been already tested for antiviral effects against herpes simplex virus type 1 (HSV-1) and 2 (HSV-2) (Schnitzler et al. 2008) common viruses that cause recurrent orofacial or genital lesions (Cernik et al. 2008). The extract inhibited replication of both viruses through interference with virus surface resulting in virus inactivation (Schnitzler et al. 2008).
Here, we investigated the effects of EPs® 7630 on virus replication of a broad panel of respiratory viruses including Influenza A virus (H1N1, H3N2, H5N1), respiratory syncytial virus (RSV), human coronavirus, adenovirus, parainfluenza virus, coxsackie virus, and rhinovirus.
Materials and methods
Drugs
EPs® 7630 was provided by the Phytochemistry Section of the Department of Preclinical Research, Dr. Willmar Schwabe Pharmaceuticals (Karlsruhe, Germany).
Cells and viruses
The following viruses were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA): respiratory syncytial virus (RSV) strain A2 (ATCC-No. VR-1540), adenovirus 3 strain GB (adeno 3, ATCC-No. VR-3), adenovirus 7 strain Gomen (adeno 7, ATCC-No. VR-7), parainfluenza virus type 3 strain C243 (parainfluenza 3, ATCC-No. VR-93), human rhinovirus 16 strain 11757 (rhino 16, ATCC-No. VR-283).
The influenza virus strains A/New Caledonia/20/99 (H1N1) and A/California/7/2004 (H3N2) were received from the World Health Organization (WHO) Influenza Centre (National Institute for Medical Research, London, UK). The H5N1 influenza strain A/Thailand/1(Kan-1)/04 was obtained from Prof. Pilaipan Puthavathana (Mahidol University, Bangkok, Thailand). The human coronavirus strain 229E (HCo-229E) was obtained from John Ziebuhr (University of Würzburg, Germany). Coxsackie virus A9 (coxsackie A9) was isolated from one of our patients.
The following cell lines were used for the cultivation of viruses: Madin-Darby canine kidney cell line MDCK (ATCC-No. CCL-34; H3N2, H1N1, influenza B), Vero (ATCC-No. CCL-81; RSV, H5N1), Caco-2 (ATCC-No. HTB-37, adeno 3, adeno 7, HCo-229E), LLC-MK2 (ATCC-No. CCL-7; parainfluenza 3), Mel-Ho (obtained from DSMZ, Braunschweig, Germany, DSMZ-No. ACC 62, rhino 16), human foreskin fibroblasts (HFFs, established as described previously (Cinatl et al. 1994); coxsackie A9). All cells were cultured in MEM supplemented with 10% foetal calf serum (FSC).
Virus stocks were prepared by infecting cells with the respective viruses. Aliquots were stored at −80 °C. Virus titres were determined as 50% tissue culture infectious dose (TCID50/ml) in 96-well microtiter plates. The infectious titre was determined by the method of Spearman and Kärber (Spearman, 1908, Kärber, 1931). The maintenance medium during infection was for influenza viruses serum-free MEM supplemented with 2 μg/ml trypsine and for all other viruses MEM supplemented with 2% FCS.
Cytopathogenic effect (CPE) reduction assay
The cytopathogenic effect (CPE) reduction assay was performed as described before (Michaelis et al. 2010). Confluent cell monolayers grown in 96-well microtitre plates were infected with respective viruses in the presence or absence of EPs® 7630. The following multiplicities of infection (MOIs) were used: H1N1, 0.005; H3N2, 0.005; influenza B, 0.01; coxsackie A9, 0.005; RSV, 0.01; adeno 3, 0.01; adeno 7, 0.02, HCo-229E, 0.01; parainfluenza 3; 0.01; rhino 16, 0.005; H5N1, 0.001.
The virus-induced CPE were recorded using an inverted light microscope at 24 h (H1N1, H3N2, H5N1, influenza B, coxsackie A9, coxsackie B5), 48 h (coxsackie B4, rhino 16), 72 h (RSV, HCo-229E, parainfluenza 3), or 96 h (adeno 3, adeno 7, parainfluenza 2) post infection (p.i.). EPs® 7630 was continuously present starting with a 1 h pre-incubation period.
Cell viability assay
The effect of EPs® 7630 on cell viability was investigated in non-infected cells that were treated in the same way with EPs® 7630 like the virus-infected cells in the CPE reduction assay. Cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) dye reduction assay as described before (Michaelis et al. 2004).
Virus yield reduction assay
Infectious virus titres were determined as described before at “Cells and viruses”. Cells were infected with the respective viruses in the absence or presence of EPs® 7630 at the following MOIs: H1N1, 0.005 (titres were determined 24 h post infection); H3N2, 0.005 (24 h); coxsackie B5, 0.005 (24 h); coxsackie A9, MOI 0.005 (24 h); parainfluenza 3, 0.01 (72 h); RSV, 0.01 (72 h); HCo-229E, 0.01 (72 h).
Results
Influence of EPs® 7630 on virus-induced cytopathogenic effect (CPE) formation
EPs® 7630 did not decrease viability of all investigated cell types in concentrations up to 100 μg/ml. Moreover, EPs® 7630 did not affect CPE formation induced by H5N1, adeno 3, adeno 7, or rhino 16 in the examined concentrations up to 100 μg/ml. However, EPs® 7630 interfered with CPE formation caused by H1N1, H3N2, RSV, HCo-229E, parainfluenza 3, or coxsackie A9 (Table 1 ).
Table 1.
Influence of EPs® 7630 on virus-induced formation of cytopathogenic effects (CPE) and on cell viability.
| Virus | Cell | IC50a (μg/ml) | CC50b (μg/ml) | TIc |
|---|---|---|---|---|
| H1N1 | MDCK | 9.45 ± 2.94 | >100 μg/ml (114 ± 15%)d | >10.6 |
| H3N2 | MDCK | 8.66 ± 1.06 | >100 μg/ml (114 ± 15%) | >11.5 |
| H5N1 | Vero | >100 | >100 μg/ml (85 ± 10%) | n.d.e |
| RSV | Vero | 19.65 ± 1.77 | >100 μg/ml (85 ± 10%) | >5.1 |
| HCo-229E | Caco-2 | 44.50 ± 15.84 | >100 μg/ml (86 ± 12%) | >2.3 |
| Adeno 3 | Caco-2 | >100 | >100 μg/ml (86 ± 12%) | n.d. |
| Adeno 7 | Caco-2 | >100 | >100 μg/ml (86 ± 12%) | n.d. |
| Parainfluenza 3 | LLC-MK2 | 74.35 ± 17.89 | >100 μg/ml (81 ± 14%) | >1.3 |
| Coxsackie A9 | HFF | 14.80 ± 3.39 | >100 μg/ml (85 ± 9%) | >6.8 |
| Rhino 16 | Mel-Ho | >100 | >100 μg/ml (87 ± 13%) | n.d. |
Concentration that inhibits CPE formation by 50%.
Concentration that decreases cell viability by 50%.
Therapeutic index = CC50/IC50.
Cell viability in the presence of EPs® 7630 100 μg/ml.
Not determinable.
Influence of EPs® 7630 on infectious virus titres
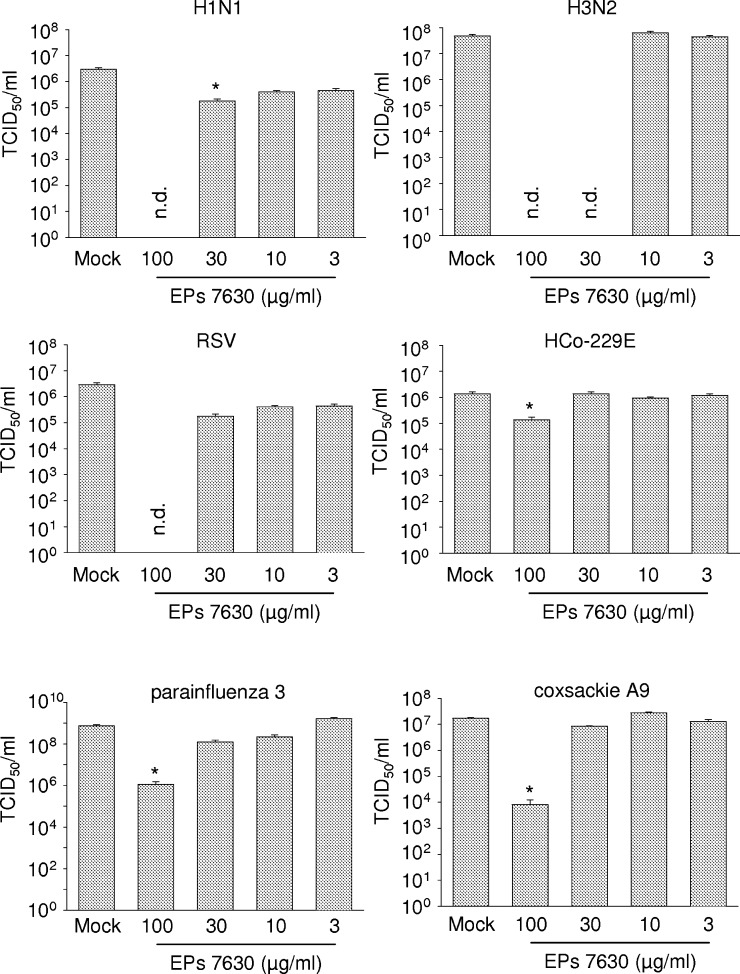
Inhibition of virus-induced CPE formation may be a consequence of inhibition of virus replication, of cytoprotective effects without influence on virus replication, or of a combination of both. Therefore, the influence of EPs® 7630 was investigated on H1N1, H3N2, RSV, HCo-229E, parainfluenza 3, or coxsackie A9 virus titres. EPs® 7630 decreased titres of all susceptible viruses in a dose-dependent manner (Fig. 1 ).
Fig. 1.
Influence of EPs® 7630 on infectious virus titres indicated as 50% tissue culture infectious dose (TCID50/ml). EPs® 7630 was continuously present in cell culture media beginning with a 1 h pre-incubation period. Cells were infected with the respective viruses in the absence (Mock) or presence of EPs® 7630 at the following MOIs: influenza A virus H1N1, 0.005 (titres were determined 24 h post infection, cell line MDCK); influenza A virus H3N2, 0.005 (24 h, MDCK); coxsackie virus A9 (coxsackie A9), MOI 0.005 (24 h, human foreskin fibroblasts); parainfluenza virus type 3 (parainfluenza 3), 0.01 (72 h, LLC-MK2); respiratory syncytial virus (RSV), 0.01 (72 h, Vero); human coronavirus strain 229E (HCo-229E), 0.01 (72 h, Caco-2). *P < 0.05 relative to Mock (as determined by ANOVA with subsequent Student-Newmam–Keuls test); n.d. = not detectable.
Discussion
Here, we investigated the effect of EPs® 7630 on replication of a panel of respiratory viruses. EPs® 7630 inhibited the replication of the respiratory viruses H1N1, H3N2, RSV, HCo-229E, parainfluenza 3, and coxsackie A9.
The magnitude of EPs® 7630-induced antiviral effects differed between the sensitive viruses. EPs® 7630 at a concentration of 100 μg/ml completely suppressed replication of the seasonal influenza A virus strains (H1N1, H3N2) and of RSV. Virus titres of coxsackie A9 were reduced by about 10,000-fold, of parainfluenza 3 by about 150-fold, and of HCo-229E by about 10-fold. A 10-fold decrease of virus titres may be sufficient to prevent viral disease, especially in a pre-emptive setting.
All viruses that were inhibited by EPs® 7630 are enveloped viruses (H1N1, H3N2, HCo-229E, RSV, parainfluenza 3) except coxsackie A9 while all non-sensitive viruses were non-enveloped viruses (adeno 3, adeno 7, rhino 16) with the exemption of the highly pathogenic avian influenza A virus H5N1 (Knipe and Howley 2007). Therefore, EPs® 7630 appears to primary target enveloped viruses. In accordance, an aqueous extract of Pelargonium sidoides had been found to inhibit replication of the enveloped viruses HSV-1 and HSV-2 through interference with the virus surface resulting in virus inactivation (Schnitzler et al. 2008). However, since EPs® 7630 inhibited replication of the non-enveloped coxsackie A9 other mechanisms may also play a role.
The varying sensitivities to EPs® 7630 of the seasonal influenza A strains H1N1 and H3N2 in comparison to H5N1 cannot be explained by our data. H5N1 viruses are generally known to be less sensitive to antiviral treatment than seasonal strains (Michaelis et al. 2009). Moreover, the influenza A virus haemagglutinin proteins that bind to the sialic acids on the target cell's surface as a first step in the viral infection process significantly vary between the seasonal strains and H5N1 (Michaelis et al. 2009) and may therefore be differently affected by agents that interact with the virus surface.
In conclusion, we show that the Pelargonium sidoides extract EPs® 7630 that is approved for the treatment of acute bronchitis interferes with replication of different respiratory viruses including seasonal influenza A virus strains, RSV, human coronavirus, parainfluenza virus, and coxsackie virus. Since respiratory viruses are estimated to cause >90% of cases of acute bronchitis (Gonzales and Sande 2000) antiviral effects may contribute to the beneficial effects of EPs® 7630 described in human acute bronchitis patients (Conrad et al., 2007, Agbabiaka et al., 2008, Matthys and Funk, 2008, Timmer et al., 2008, Kamin et al., 2010a, Kamin et al., 2010b, Matthys et al., 2010).
Conflict of interest
The work was financially supported by Dr. Willmar Schwabe Pharmaceuticals (Karlsruhe, Germany).
Acknowledgements
The authors thank Gesa Meincke for technical support. The work was supported by the friendly society Hilfe für krebskranke Kinder Frankfurt e.V. and its foundation Frankfurter Stiftung für krebskranke Kinder.
References
- Agbabiaka T.B., Guo R., Ernst E. Pelargonium sidoides for acute bronchitis: a systematic review and meta-analysis. Phytomedicine. 2008;15:378–385. doi: 10.1016/j.phymed.2007.11.023. [DOI] [PubMed] [Google Scholar]
- Brendler T., van Wyk B.E. A historical, scientific and commercial perspective on the medicinal use of Pelargonium sidoides (Geraniaceae) J. Ethnopharmacol. 2008;119:420–433. doi: 10.1016/j.jep.2008.07.037. [DOI] [PubMed] [Google Scholar]
- Cernik C., Gallina K., Brodell R.T. The treatment of herpes simplex infections: an evidence-based review. Arch. Intern. Med. 2008;168:1137–1144. doi: 10.1001/archinte.168.11.1137. [DOI] [PubMed] [Google Scholar]
- Cinatl J., Jr., Cinatl J., Rabenau H., Gümbel H.O., Kornhuber B., Doerr H.W. In vitro inhibition of human cytomegalovirus replication by desferrioxamine. Antiviral Res. 1994;25:73–77. doi: 10.1016/0166-3542(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Conrad A., Kolodziej H., Schulz V. Pelargonium sidoides-Extrakt (Eps® 7630): Zulassung bestätigt Wirksamkeit und Verträglichkeit. Wien. Med. Wochenschr. 2007;157:331–336. doi: 10.1007/s10354-007-0434-6. [DOI] [PubMed] [Google Scholar]
- Gonzales R., Sande M.A. Uncomplicated acute bronchitis. Ann. Intern. Med. 2000;133:981–991. doi: 10.7326/0003-4819-133-12-200012190-00014. [DOI] [PubMed] [Google Scholar]
- Kamin W., Maydannik V.G., Malek F.A., Kieser M. Efficacy and tolerability of EPs® 7630 in patients (aged 6–18 years old) with acute bronchitis. Acta Paediatr. 2010;99:537–543. doi: 10.1111/j.1651-2227.2009.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamin W., Maydannik V., Malek F.A., Kieser M. Efficacy and tolerability of EPs® 7630 in children and adolescents with acute bronchitis – a randomized, double-blind, placebo-controlled multicenter trial with a herbal drug preparation from Pelargonium sidoides roots. Int. J. Clin. Pharmacol. Ther. 2010;48:184–191. doi: 10.5414/cpp48184. [DOI] [PubMed] [Google Scholar]
- Kärber G. Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche [A contribution to the collective treatment of a pharmacological experimental series] Archiv für experimentelle Pathologie und Pharmakologie. 1931;162:480–483. [Google Scholar]
- Knipe D.M., Howley P.M., editors. Fields Virology. 5th edition. Philadelphia, Pennsylvania; USA: 2007. [Google Scholar]
- Kolodziej H. Aqueous ethanolic extract of the roots of Pelargonium sidoides – new scientific evidence for an old anti-infective phytopharmaceutical. Planta Med. 2008;74:661–666. doi: 10.1055/s-2007-993778. [DOI] [PubMed] [Google Scholar]
- Matthys H., Funk P. EPs® 7630 improves acute bronchitic symptoms and shortens time to remission. Results of a randomised, double-blind, placebo-controlled, multicentre trial. Planta Med. 2008;74:686–692. doi: 10.1055/s-2008-1074519. [DOI] [PubMed] [Google Scholar]
- Matthys H., Lizogub V.G., Malek F.A., Kieser M. Efficacy and tolerability of EPs® 7630 tablets in patients with acute bronchitis: a randomised, double-blind, placebo-controlled dose-finding study with a herbal drug preparation from Pelargonium sidoides. Curr. Med. Res. Opin. 2010;26:1413–1422. doi: 10.1185/03007991003798463. [DOI] [PubMed] [Google Scholar]
- Michaelis M., Köhler N., Reinisch A., Eikel D., Gravemann U., Doerr H.W., Nau H., Cinatl J., Jr. Increased human cytomegalovirus replication in fibroblasts after treatment with therapeutical plasma concentrations of valproic acid. Biochem. Pharmacol. 2004;68:531–538. doi: 10.1016/j.bcp.2004.04.013. [DOI] [PubMed] [Google Scholar]
- Michaelis M., Doerr H.W., Cinatl J., Jr. Of chickens and men: avian influenza in humans. Curr. Mol. Med. 2009;9:131–151. doi: 10.2174/156652409787581565. [DOI] [PubMed] [Google Scholar]
- Michaelis M., Geiler J., Naczk P., Sithisarn P., Ogbomo H., Altenbrandt B., Leutz A., Doerr H.W., Cinatl J., Jr. Glycyrrhizin inhibits highly pathogenic H5N1 influenza A virus-induced pro-inflammatory cytokine and chemokine expression in human macrophages. Med. Microbiol. Immunol. 2010 doi: 10.1007/s00430-010-0155-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzler P., Schneider S., Stintzing F.C., Carle R., Reichling J. Efficacy of an aqueous Pelargonium sidoides extract against herpesvirus. Phytomedicine. 2008;15:1108–1116. doi: 10.1016/j.phymed.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Spearman C. The method of “right and wrong cases” (“constantstimuli”) without Gauss's formulae. Br. J. Psychol. 1908;2:227–242. [Google Scholar]
- Timmer A., Günther J., Rücker G., Motschall E., Antes G., Kern W.V. Pelargonium sidoides extract for acute respiratory tract infections. Cochrane Database Syst. Rev. 2008;16 doi: 10.1002/14651858.CD006323.pub2. CD006323. [DOI] [PubMed] [Google Scholar]