Abstract
The truncated fragment M′ gene, encoding the exterior of the viral envelope protein of PEDV, was subcloned into prokaryotic expression vector pGEX-6p-1. The recombinant plasmid pGEX-6p-M′ was constructed and transformed into E. coli BL21(DE3)pLysS for expression. SDS-PAGE analysis showed recombinant truncated M′ protein was highly expressed by pGEX-6p-M′ and the product fusion protein GST-M′ reached 45% in the total bacteria proteins with the analysis of software AlphaImager2200. The preliminary purified recombinant protein was evaluated for its antigenicity and reactivity through Western blotting and indirect enzyme-linked immunosorbent assay (ELISA) with monoclonal antibody against M protein of PEDV and porcine polyclonal anti-PEDV antiserum as the primary antibody. The results indicated the recombinant truncated M′ protein should be candidate as a feasible recombinant diagnostic reagent.
Keywords: PEDV, Recombinant truncated M′ protein, Prokaryotic expression
1. Introduction
Porcine epidemic diarrhea (PED) is a highly contagious enteric disease of swine and results in high mortality in piglets (Ducatelle et al., 1981). Since PED was first reported in England in 1971 (Wood, 1977). It has become one of the severe viral diarrhea diseases that lead to serious economic losses in swine-raising countries (Debouck et al., 1982, Hofmann and Wyler, 1987). Although some currently established methods for serodiagnosis of PED virus infection had been reported, most of all their detecting reagents are all prepared with virus antigen from PED virus infected Vero cells or intestine and fecal samples of experimentally infected sows (Jin et al., 2004, Jin et al., 2005, Rodak et al., 2005). However, the narrow infection spectrum and the lower cell infection titre of PED virus limited the yield in laboratory, and the clinical application of PED whole virus antigen presents potential risk. Hence, it is very necessary and important to develop effective diagnostic reagents for preventing and control of this disease.
Porcine epidemic diarrhea virus (PEDV) is an enveloped and single-stranded RNA virus that belongs to the Coronaviridae family (Murphy et al., 1999, Egberink et al., 1988). The envelope contains three main viral proteins, spike (S, 180–220 kDa), membrane (M, 27–32 kDa) and small membrane(sM, 7 kDa) (Locker et al., 1992, Sang-Geon et al., 2003). The M protein is the most abundant of three, it is a triple-spanning membrane protein with a short amino-terminal domain on the outside of the virus (the exterior of the viral envelope) and a long carboxy-terminal domain on the inside (Utiger et al., 1995a). Except playing an important role in the assembly process of viral nucleocapsid and membrane, M can neutralize anti-M antibody with the presence of complement (Rottier, 1995; de Haan et al., 1998). In order to obtain and assess the recombinant M protein used for a diagnostic reagent of PED (Woods et al., 1987, Guscetti et al., 1998), in this study, the recombinant M′ protein (rMP) containing only the exterior of the viral envelope of M has been highly expressed and identified after preliminary purified.
2. Materials and methods
2.1. Virus strain, plasmids and bacterial strains
The PEDV LJB/03 was collected from the feces of piglets suffering from severe diarrhea in HeiLongJiang, China. Expression plasmid pGEX-6p-1 contains the coding region for glutathione S-transferase (GST), and the expressed product will be in the form of a fusion protein with GST, molecular mass of 27 kDa. The expression is driven by the P tac promoter, and can be induced by the addition of isopropyl β-d-thiogalactoside (IPTG). Plasmid pMD18-T-M, containing the full length M gene, was constructed and verified by Fanjinghui et al. (Jinhui and Yijing, 2005). E. coli strain BL21(DE3)pLysS was used as host strain for recombinant protein expression in this study.
2.2. Antibodies and sera
Monoclonal antibody (McAb) anti-PEDV M protein was obtained from the Institute of Veterinary Pathology, of University of Zurich. Porcine polyclonal anti-PEDV positive serum and negative serum were kindly provided by Dr. Tian ZH. J., National Key Laboratory of Veterinary Biotechnology, Harbin Veterinary Research Institute of Chinese Academy of Agricultural Sciences. Mouse polyclonal antiserum to PEDV M protein was prepared in this laboratory.
2.3. Construction of the expression plasmid
The sequence coding for the exterior of viral envelope was amplified from pMD18-T-M by 25 cycles of PCR using 10 ng of the primers chosen by the analysis of CV777 and Brl/87 sequences available in GenBank. The sense primer PM1 and antisense primer PM2 were 5′-GGCGAATTCAATATGTCTAACGGTTC-3′ and 5′-CATGTCGACACCATACAAGAACA-3′ which carried an EcoRIand a SalI restriction site, respectively. The amplified products of the truncated M gene, 0.149 kb in length from the N terminal of M gene, were gel purified and cloned into the pGEX-6p-1 expression vector, and the constructs transformed into E. coli BL21(DE3)pLysS. Transformts selected on Luria bertani (LB) agar plates (GibicoBRL) containing ampicillin (100 μg/ml) were screened by direct colony PCR, and by restriction digestion of purified plasmids. The sequence of inserts was verified. The sequence data obtained were compared with the sequence of the M gene of CV777 and Brl/87 strains. The alignment performed by DANMAN software revealed the 100% of nucleotide identity between the three sequences.
2.4. Expression and purification of rMP
The expression of rMP in the bacterial cytosol was accomplished in E. coli BL21(DE3)pLsS. A colony of cells of E. coli BL21(DE3)pLsS transformed recently was cultured in LB broth supplemented with ampicillin (50 μg/ml) with shaking at 37 °C until the optical density of the culture at 600 nm reached 0.6. IPTG was then added to a final concentration of 0.5 mM to induce the expression of rMP, and the incubation was continued for further 6 h. Control cultures containing the empty pGEX-6p-1 vector were processed in parallel. The rMP accumulated in the bacteria as inclusion bodies (IBs). The cells were harvested by centrifugation at 5000 × g for 10 min and used for the preparation of IBs. To lyse cells, the pellet was resuspended in buffer A (50 mM Tris and 1 mM EDTA [pH 8.0]) at 1/10 of the original culture volume and the lysozyme was added to a final concentration of 100 μg/L on ice. 30 min later, the cell suspension was sonicated on ice five times, each for 30 s with 30 s intervals. After sonication, the lysate was centrifuged at 5000 × g for 10 min, the supernatant was discarded and the pellet was gently resuspended in buffer B (50 mM Tris, 0.5 mM EDTA, and 1% Triton-100 [pH 8.0]) at the same volume as buffer A. Centrifugation at 5000 × g for 10 min, the pellet was saved again. To eliminate bacterial contaminants present in IBs, as much as possible, this step was repeated three times. Finally, the pellet IBs of rMP which was resuspended again in the same volume of buffer B was saved as crude purified sample for further size-exclusion chromatography purification using Sephardex G-75 (Pharmacia)column 1.6 × 60 cm at 1 ml/min velocity of flow. The pooled purified rMP, after confirmed by SDS-PAGE analysis and contents determination with Lowry's method, will be used for immunization of mice and other assay.
2.5. Preparation of mouse sera against PEDV M protein
In order to generate specific polyclonal antiserum to the rMP antigen, each female BLAB/c mouse (6–8 weeks) was immunized with 0.20 ml containing 100 μg purified rMP antigen emulsified with eaqual amount of Freund's Complete Adjuvant via abdominal cavity. Two weeks later, 0.2 ml antigen mixed with Freund's Incomplete Adjuvant was injected in every 7 days interval for two times. Antisera were collected from blood that was drawn from the mice 4 d after the last boosting. The collected antisera were diluted 100-fold and used for the immunoblotting ananlysis.
2.6. Western blotting
The immunoblotting was carried out as described previously. Briefly, purified rMP and whole virus were subjected to SDS-PAGE and transferred to a nitrocellulose (NC) membrane. The membranes were blocked overnight at 4 °C using a 0.5% blocking solution of polyvinyl alcohol (PVA, Sigama) in PBS buffer, then sliced into strips and incubated for 2 h at room temperature with monoclonal antibody (McAb) anti-PEDV M protein and mouse positive serum against rMP and porcine polyclonal anti-PEDV positive serum and negative serum, respectively. After washing in phosphate-buffered saline (PBS) containing 0.05% Tween 20 (PBS-T), the membranes were incubated with 1:3000 peroxidase labeled goat anti-mouse IgG and peroxidase-conjugated goat anti-porcine IgG (Sigma, St. Louis, MO, USA), respectively. 4-Chloro-1-naphthol (4-CN, Amresco) was used to visualize the reaction.
2.7. Indirect ELISA
Briefly, ELISA plates were coated at 4 °C overnight with rMP (dilutions from 1:50 to 1:1 2800, from 100 ng/original well to 0.015 ng/well) in carbonate–bicarbonate buffer (15 mM Na2CO3, 35 mM NaHCO3 [pH 9.6]), protein negative control wells coated with GST were processed in parallel. Then each well of the plate was incubated with 0.5% PVA blocking solution for 2 h at 37 °C. The wells were washed four times with PBS-T. One hundred microliters of the primary antibody McAb diluted 1:200 in PVA blocking solution was added in duplicate and the plates were incubated for 1 h at 37 °C, negative primary antibody control used by culture supernatant of SP2/0 myeloma cell were processed in parallel. After four washes with PBS-T, plates were then incubated with 1:5000 goat anti-mouse IgG antibody labeled with peroxidase for 1 h at 37 °C. OPD substrate (100 μl/well) was added after four washes with PBS-T and the wells were incubated for 15 min at 37 °C. Fifty microliters of stop buffer (2 M H2SO4) was added to each well and the optical density (OD) was read at 492 nm.
3. Results
The coding sequence for the M protein's exterior of PEDV was cloned in the pGEX-6p-1 expression vector and the rMP with a 26 kDa fusion protein GST binding at the N-terminus was expressed as IBs in the bacterial cytosol. After SDS-PAGE analysis, a protein band corresponding to the expected molecular mass of approximate 31 kDa was revealed in IPTG-induced culture (Fig. 1 , lane 3) which was not present in the culture before the induction (data not shown) or in the control culture after the induction (Fig. 1, lane 2). With the analysis of software AlphaImager2200, the product of expression reached approximately 45% in the total bacteria protein of each 1 ml culture, and the 4.4 kDa recombinant truncated M′ protein reached approxamitely 7%. It was crudely purified by the extraction of IBs and further purified by size-exclusion chromatography. The purity was confirmed by single band in SDS-PAGE (Fig. 2 , lane 2). In order to identify the specific reactivity of rMP, Western blotting was carried out using the McAb anti-PEDV M protein and a band of 31 kDa (Fig. 3 , lane 2) showed the strong and specific reaction between them whereas the same size band was not demonstrated in negative control (data not shown). Contrarily, the reactivity of mouse polyclonal antiserum of rMP with the purified PED virion was revealed by the band of approximate 29 kDa (Fig. 3, lane 2) as well as by the positive control (Fig. 3, lane 3). As shown in Fig. 4 , the rMP antigen strongly reacted with the positive porcine polyclonal anti-PEDV serum with a band of 31 kDa in lane 2. In contrast, no reactivity was observed in negative control in lane 1.
Fig. 1.

SDS-PAGE analysis of expression of rMP in BL21(DE3)pLysS. The solid arrowhead indicates the position of the rMP. 1: a low molecular weight standard (LMW). 2: BL21(DE3)pLysS/pGEX-6p-1, induced cell pellet(control). 3: BL21(DE3)pLysS/pGEX-6p-M′, 0.5 mM IPTG induction at OD600 equates 1.60.
Fig. 2.

SDS-PAGE analysis of extraction inclusion body and purification of rMP. 1: the extract products of inclusion bodies formed in BL21(DE3)pLysS/pGEX-6p-M′, which were washed once with buffer A (50 mM Tris, 0.5 mM EDTA, 100 mM NaCl, 1% Triton X-100, 2 mM Urea, pH 8.0), after induced and cultured at 37 °C for 5 h. 2: purified by size-exclusion chromatography. 3: a low molecular weight standard (LMW).
Fig. 3.

Western blotting analysis of reactivity and antigenisity of rMP. M: a low molecular weight standard (LMW). 1: rMP was applied and incubated with McAb anti-PEDV M protein. 2 and 3 were both applied with purified PED viral original from piglet gut, and incubated with polyclonal antiserum rMP and McAb anti-PEDV M protein, respectively.
Fig. 4.

Western blotting analysis of reactivity and antigenisity of rMP. M: a low molecular weight standard (LMW). 1 and 2 rMP was applied and incubated with negative and positive porcine serum against PEDV, respectively.
The optical density (OD) value of plot a1 (Fig. 5A) decreased progressively with serial dilutions of rMP antigen from 1:50 to 1:800, which showed the sensitive reactivity of rMP antigen combining McAb anti-PEDV M protein, whereas, the OD value of control plot a2 and negative GST antigens control plot b1 and b2 (Fig. 5B) were very low between in 0.10 and 0.25. This rMP-ELISA results correlated with the results of the immunoblotting assay well, and the specific reactivity of rMP was identified.
Fig. 5.

(A). Detection of rMP antigens in ELISA using McAb anti-PEDV M protein. The average (plot a1  for McAb + rMP and plot a2
for McAb + rMP and plot a2 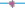 for culture supernatant of SP2/0 meyloma cell + rMP) optical density (OD) readings were plotted for each dilution. The assay was carried out in duplicate for each antigen dilution. (B). GST as negative antigen control was processed, in parallel with (A) in ELISA. The same McAb and supernatant in Fig. 4A were used. The average (plot b1
for culture supernatant of SP2/0 meyloma cell + rMP) optical density (OD) readings were plotted for each dilution. The assay was carried out in duplicate for each antigen dilution. (B). GST as negative antigen control was processed, in parallel with (A) in ELISA. The same McAb and supernatant in Fig. 4A were used. The average (plot b1  for McAb + GST and plot b2
for McAb + GST and plot b2  for culture supernatant of SP2/0 meyloma cell + GST) optical density (OD) readings were plotted for each dilution. The assay was carried out in duplicate for each antigen.
for culture supernatant of SP2/0 meyloma cell + GST) optical density (OD) readings were plotted for each dilution. The assay was carried out in duplicate for each antigen.
4. Discussion
Expression of Coronavirus M protein is very difficult due to many unknown complex factors, one of the most likely possibilities was deduced to be the sprout function of it which might as well as cause damage to cell wall while it was expressed and accumulated to a certain amount in cells, hence, the growth inhibition phenomenon appeared in some experiments (Kopecky et al., 2001, Kopecky and Lyles, 2003, Wang et al., 1997). In 1995, Eukaryotic expression system (e.g., baculovirus) had been evaluated to express PEDV M protein, but the expression level is very low (Utiger et al., 1995b). Although the full length M protein may be considered an ideal antigen to use in diagnosis, the poor-level of expression of it limited the potential production and application in future. In order to improve the expression level and avoid the possible sprout function of it, we take two strategies, firstly we truncated the M gene by primers design, to obtain the N terminal gene fragment encoding the approximate 4.4 kDa envelope exterior of the M protein, and secondly, we made use of the prokaryotic expression vector pGEX-6P-1, which can combine 26 kDa fusion protein GST as the vector of truncated M′ protein antigenic determinant. After induction conditions optimized, the expected high-level expression result was obtained (Fig. 1, lane 3) and as we expected, the expression product was accumulated in the form of IBs in E. coli cytosol, therefore, the extraction process of IBs was carried out expediently. After the preliminary size-exclusion chromatography purification, the pooled purified rMP was confirmed by SDS-PAGE analysis and identified using McAb against PEDV M protein by western blotting and indirect ELISA. Especially, the practical use of rMP antigen was evaluated by authentic positive porcine polyclonal anti-PEDV serum and negative serum control (Fig. 4). The assays results showed that antigenicity of rMP was not only very close to its native viral proteins’ but also determined by linear antigenic determinant of truncated M′ protein, hence, the suspicion that the antigenic integrity of the small target M′ protein fragment may partly affected by a big fusion protein GST among a single recombinant protein was eliminated.
S, N and M proteins are three main structure proteins of PEDV particles, there's been no related report on making use of any one of them as diagnostic reagent in serology diagnosis at present, we select M protein as the main study object in this paper based on the following considerations. Briefly, compared with S, N proteins of PEDV in theory, we found M protein to be superior as a diagnostic antigen. The M protein accounts for a considerable proportion in envelope components of PEDV particles and the exterior of envelope of M is more stable than that of S, especially, in the process of whole virus antigen extraction treatment. Hence, based on whole viral particles as diagnostic reagent, it would be more reliable making use of M protein as diagnostic antigen in serology diagnosis; N protein located inside of envelope of virus particles, to expose it outside is the first necessary step before making use of it as a diagnostic antigen, by comparison, the envelope exterior of M protein originally exposes outside. Obviously, that would improve diagnosis efficiency of PED when directly using M protein in actual diagnosis process.
Based on the good antigenicity and practical use of rMP evaluated as described above, we concluded that the high level expression rMP obtained in the E. coli systerm will provide base for the establishment and standardization of the rMP-based ELISA (Hofmann and Wyler, 1990, Knuchel et al., 1992, Gabriella et al., 2003) for clinical diagnosis of PEDV infection.
Acknowledgements
This research was supported by a grant from the 10th 5-year Plan of Science and Technology Research Program (GC01B510) funded by Heilongjiang Province PR China. Specially, we thank Ackermann M. and Andreas Pospischil of Institute of Veterinary Pathology and Virology, University of Zurich, for kindly providing the monoclonal antibody against PEDV M protein; and Dr. Tian ZH. J., National Key Laboratory of Veterinary Biotechnology, Harbin Veterinary Research Institute of Chinese Academy of Agricultural Sciences, for kindly providing positive and negative porcine polyclonal anti-PEDV serum.
References
- de Haan C.A.M., Kuo L., Masters P.S., Vennema H., Rottier P.J.M. Coronavirus particle assembly primary structure requirements of the membrane protein. J. Virol. 1998;72:6838–6850. doi: 10.1128/jvi.72.8.6838-6850.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducatelle R., Coussement W., Pensaert M., Debouck P., Hoorens J. In vivo morphogenesis of a new porcine enteric coronavirus, CV777. Arch. Virol. 1981;68:35–44. doi: 10.1007/BF01315165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debouck P., Callebaut P., Pensaert M. Prevalence of the porcine epidemic diarrhea (PED) virus in the pig population of different countries. Proc. Int. Pig. Vet. Soc. Congr. 1982;7:53. [Google Scholar]
- Egberink H.F., Ederveen J., Callebant P., Horzinek M.C. Characterization of the structural proteins of porcine epizootic diarrhea virus, strain CV777. Am. J. Vet. Res. 1988;49:1320–1324. [PubMed] [Google Scholar]
- Guscetti F., Bernasconi C., Tobler K., Van Reeth K., Pospischil A., Ackermann M. Immunohistochemical detection of porcine epidemic diarrhea virus compared to other methods. Clin. Diagn. Lab. Immunol. 1998;5:412–414. doi: 10.1128/cdli.5.3.412-414.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriella E., Giuseppe F., Annamaria P., Vito M., Michele C., Francesco C., Canio B. Recombinant M protein-based ELISA test for detection of antibodies to canine coronavirus. J. Virol. Methods. 2003;109:139–142. doi: 10.1016/S0166-0934(03)00064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann M., Wyler R. Study of the occurrence of epizootic viral diarrhea in swine in Switzerland. Schweiz Arch. Tierheilkd. 1987;129:437–442. [PubMed] [Google Scholar]
- Hofmann M., Wyler R. Enzyme-linked immunosorbent assay for the detection of porcine epidemic diarrhoea coronavirus antibodies in swine sera. Vet. Microbiol. 1990;21:263–273. doi: 10.1016/0378-1135(90)90037-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinhui F., Yijing L. Cloning and sequence analysis of the m gene of porcine epidemic diarrhea virus LJB/03. Virus Genes. 2005;30:69–73. doi: 10.1007/s11262-004-4583-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S.Oh., Dae S.S., Yang J.-S., Ju Y.S., Yoo H.-S., Jang Y.-S., Bong K.P. Effect of soluble porcine aminopeptidase N on antibody production against porcine epidemic diarrhea virus. J. Vet. Sci. 2004;5:353–357. [PubMed] [Google Scholar]
- Jin S.Oh., Dae S.S., Jeong S.Y., Ju Y.S., Hyoung J.M., Tae Y.K., Bong K.P. Comparison of an enzyme-linked immunosorbent assay with serum neutralization test for serodiagnosis of porcine epidemic diarrhea virus infection. J. Vet. Sci. 2005;6:349–352. [PubMed] [Google Scholar]
- Knuchel M., Ackermann M., Muller H.K., Kihm U. An ELISA for detection of antibodies against porcine epidemic diarrhoea virus (PEDV) based on the specific solubility of the viral surface glycoprotein. Vet. Microbiol. 1992;32:117–134. doi: 10.1016/0378-1135(92)90100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecky S.A., Lyles D.S. Constrasting effects of matrix protein on apoptosis in HeLa and BHK cells infected with vesicular stomatitis virus are due to inhibition of host gene expression. J. Virol. 2003;77:4658–4669. doi: 10.1128/JVI.77.8.4658-4669.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecky S.A., Willingham M.C., Lyles D.S. Matrix protein and another viral component contribute to induction of apoptosis in cells infected with vesicular stomatitis virus. J. Virol. 2001;75:12169–12181. doi: 10.1128/JVI.75.24.12169-12181.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locker J.K., Rose J.K., Horzinek M.C., Rottier P.J.M. Membrane assembly of the triple-spanning coronavirus M protein. Individual transmembrane domains show preferred orientation. J. Biol. Chem. 1992;267:21911–21918. doi: 10.1016/S0021-9258(19)36699-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy F.A., Gibbs E.P.J., HorZinek M.C., Studdert M.J. Cademic Press; San Diego: 1999. Veterinary Virology. pp. 496–501. [Google Scholar]
- Rodak L., Smid B., Nevorankova Z., Valicek L., Smitalova R. An ELISA optimized for porcine epidemic diarrhoea virus detection in faeces. J. Vet. Microbiol. 2005;105:9–17. doi: 10.1016/j.vetmic.2004.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottier P.J.M. The coronavirus membrane protein. In: Siddel S.G., editor. The Coronaviridae. Plenum Press; New York: 1995. pp. 115–139. [Google Scholar]
- Sang-Geon Y., Mercedes H., Peter J.K., Eva N. Cloning and sequence analysis of the spike gene of porcine epidemic diarrhea virus Chinju99. Virus Genes. 2003;26:239–246. doi: 10.1023/A:1024443112717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utiger A., Tobler K., Bridgen A., Suter M., Singh M., Ackermann M. Identification of proteins specified by porcine epidemic diarrhea virus. Adv. Exp. Med. Biol. 1995;380:87–90. doi: 10.1007/978-1-4615-1899-0_46. [DOI] [PubMed] [Google Scholar]
- Utiger A., Tobler K., Bridgen A., Ackermann M. Identification of the membrane protein of porcine epidemic diarrhea virus. Virus Genes. 1995;10:137–148. doi: 10.1007/BF01702594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.F., Gould A.R., Selleck P.W. Expression of equine morbillivirus (EMV) matrix and fusion proteins and their evaluation as diagnostic reagents. Arch. Virol. 1997;142:2269–2279. doi: 10.1007/s007050050241. [DOI] [PubMed] [Google Scholar]
- Wood E.N. An apparently new syndrome of porcine epidemic diarrhea. Vet. Rec. 1977;100:243–244. doi: 10.1136/vr.100.12.243. [DOI] [PubMed] [Google Scholar]
- Woods R.D., Wesley R., Kapke P.A. Complement-dependent neutralization of transmissible gastroenteritis virus by monoclonal antibodies. Adv. Exp. Med. Biol. 1987;218:493–500. doi: 10.1007/978-1-4684-1280-2_64. [DOI] [PubMed] [Google Scholar]


