Abstract
In Chinese Meishan/German Landrace cross-bred swine F2 generation interferon gamma (IFN-γ) production by peripheral blood mononuclear cells (PBMC) was determined directly ex vivo at different time points after survival of a virulent pseudorabies virus (PRV) infection. This reactivity was compared with the reactivity of naïve PBMC. Significant IFN-γ production was determined in ELISA and ELISPOT only after in vitro PBMC re-stimulation with PRV and not with the closely related bovine herpesvirus BHV-1.
The PRV-specific IFN-γ secretion from re-stimulated PBMC showed high levels 6 days after infection, before the presence of serum antibodies, and it persisted at a high level over a 3 months period. The response of a group of eight piglets infected intranasally with PRV varied. Only two animals showed the expected typical fever response. PRV specific IFN-γ production by PBMC clearly indicated that infection had occurred. Early significant IFN-γ production by primed PBMC turned out to be a reliable and specific ex vivo marker for cellular response against productive PRV infection in swine before antibody formation.
Keywords: Swine, Pseudorabies virus infection, Interferon gamma
1. Introduction
Swine are the principal host for pseudorabies virus (PRV) infection and develop Aujeszky's disease with high morbidity and mortality as a juveniles after infection with virulent strains or field isolates (Wittmann and Rziha, 1989, Mettenleiter, 2000, Mettenleiter, 2003). Immune response against PRV have been the subject of many studies primarily in vaccine trials (for review see Zuckermann, 2000, Mettenleiter, 2000). After acute infection swine develop specific antibody and T-cell responses against PRV (Wittmann and Rziha, 1989, Kimman et al., 1994, Mettenleiter, 1996). The latter can be characterised by the activity of antigen specific cytolytic T-lymphocytes (CTL) and by PRV-mediated proliferation of PBMC (Kimman et al., 1995, Summerfield et al., 1996, De Bruin et al., 1998, De Bruin et al., 2000, Zuckermann, 2000). Besides the induction of type I interferons, IFN-γ production of PBMC has been reported to be a sign of cell-mediated immunity in PRV and other viral infections of swine (Mateu de Antonio et al., 1998, Zuckermann et al., 1998, Zuckermann et al., 1999, Zuckermann, 2000, Suradhat et al., 2001). The interferons (IFNs) are representative cytokines in viral infections that not only play an important role as antiviral agents but are inter-connected regulators in innate and adoptive immune responses (Brinkman et al., 1993, Fearon and Locksley, 1996, Biron, 1998). Within the interferon family, IFN-γ (formerly “immune IFN”) represents a single gene encoded cytokine that has been found to be secreted by NK cells and predominantly by activated T-lymphocytes which outlines its role in adoptive immune responses (Vilcek and Sen, 1996). Moreover, the determination of IFN-γ allows the detection and quantization of antigen specific CD8+ T-lymphocytes and a efficacy detection of other cytokines such as IL-12 and IL-18 (Pittet et al., 2001, Harrington et al., 2002, Speller and Warren, 2002, Domeika et al., 2002).
We were interested in the direct ex vivo follow up of PBMC-derived IFN-γ, in context with disease resistance and survival of Meishan cross-bred swine against a virulent PRV infection (Reiner et al., 2002). To determine virus specific IFN-γ production, ELISA and ELISPOT technique were applied to monitor PBMC reactivity to in vitro PRV re-exposure at different time points after infection.
2. Materials and methods
2.1. Animals
F2 generation cross-bred Meishan/German Landrace swine were obtained from the Institute of Animal Breeding and Biotechnology, University of Hohenheim, Stuttgart, Germany (Reiner et al., 2002). All piglets were used for PRV infections at an average age of 10 (±2) weeks (between 20 and 30 kg body weight). The animals were housed and fed in groups of two or four in the isolated stables of the Federal Research Center under standardized conditions. Infection experiments were performed by intranasal PRV spray application (spray bottle, Roth, Karlsruhe, Germany) with 105 p.f.u. of the highly virulent PRV strain NIA-3 (McFerran and Dow, 1975) according to the permission No. Ak. Z. 37-9185-81-4 after the German legislation for animal experiments. Starting 3 days before until 12 day after infection (p.i.) rectal temperatures were recorded daily. None of the experimental animals had PRV-specific serum antibodies before the PRV challenge. The relative resistance of cross-bred Meishan piglets to a productive PRV infection without the development of lethal neurological symptoms but accompanied by phases of fever with temperatures >40 °C has been reported earlier (Reiner et al., 2002). The development of fever was defined according to Gillespie et al. (2000) when the rectal body temperature was measured >40 °C. Only animals (in total n = 20) that did not show any clinical signs of neurological symptoms such as trembling, in-coordination, ataxia, paralysis circling or paddling and regained physiological body temperature were selected for long-term immunological investigation. Among the experimental infection experiments one was performed with a group of eight animals showing a heterogeneous course of body temperature profiles after intranasal PRV application. Thus this group of animals was divided into three subgroups according to their body temperature course: group A with the expected rise in rectal temperature (n = 2); group B with a moderate rise in rectal temperature (n = 2); group C with no rise in rectal temperature (n = 4) until day 6 after PRV application (Fig. 1 ). To control the take of PRV infection within the subgroups B and C, the six non-febrile animals received a second PRV application but this time intramuscularly (i.m.) at day 6 following the intranasal PRV application (Table 1 ).
Fig. 1.
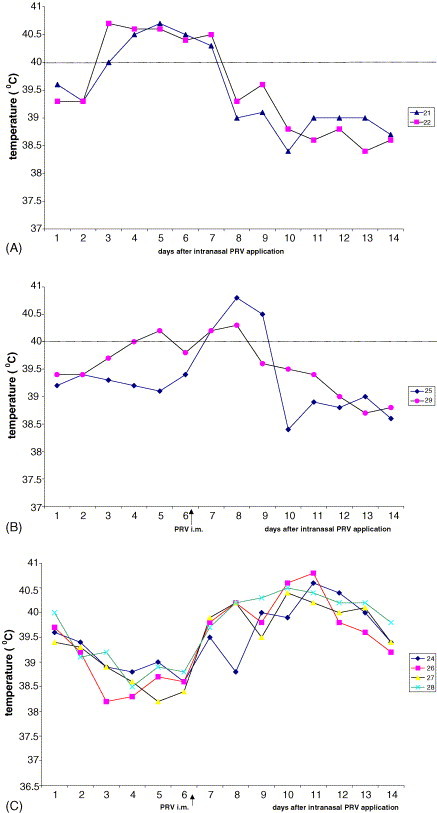
Differential course of rectal body temperature in eight piglets after intranasal infection with virulent PRV strain NIA-3. (A) The course of the temperature of two animals which developed fever (>40 °C) until day 6 post infection. (B) The course of rectal temperature of two animals from which one (No. 29) showed a moderate rise in body temperature. (C) The mean temperature course is summarized for four animals which showed rather a drop in body temperature than a rise after intranasal infection. After intramuscular (i.m.) injection of the same dose of PRV in non-febrile animals (B and C) at day 6 after intranasal application (arrows) all six piglets developed fever within 6 days.
Table 1.
Diverse temperature profiles, IFN-γ secretion by PBMC and antibody titres from piglets after take of intranasal PRV NIA-3 infection (group A) and failure of productive infection (groups B and C)
| Days after intranasal PRV application | IFN-γ secretion (pg/ml) | PRV-specific antibody titre 1 | Body temperature (°C) | Animal no.: temperature response |
|---|---|---|---|---|
| Group A | ||||
| 6 | 2450 | Not detectable | >40.5 | 21 fever |
| 12 | 23450 | 1280 | <40.0 | |
| 19 | 30200 | 2800 | <40.0 | |
| 6 | 1838 | Not detectable | >40.5 | 22 fever |
| 12 | 17500 | 640 | <40.0 | |
| 19 | 30000 | 2078 | <40.0 | |
| Group B | ||||
| 6 i.m. injection | <40 | Not detectable | <40.0 | 25 moderate temp. rise |
| 12 | 28400 | 160 | >40.0 | |
| 19 | 28400 | 2560 | <40.0 | |
| 6 i.m. injection | <40 | Not detectable | 40.0 | 29 short-term fever |
| 12 | 18900 | 320 | >40.0 | |
| 19 | 32000 | 5120 | <40.0 | |
| Group C | ||||
| 6 i.m. injection | <40 | Not detectable | <40.0 | 24 no temperature rise |
| 12 | 13892 | 5 | >40.0 | |
| 19 | 7500 | 6400 | <40.0 | |
| 6 i.m. injection | <40 | Not detectable | <40.0 | 26 no temperature rise |
| 12 | 9167 | Not detectable | >40.0 | |
| 19 | 73600 | 1600 | <40.0 | |
| 6 i.m. injection | <40 | Not detectable | <40.0 | 27 no temperature rise |
| 12 | 14800 | Not detectable | >40.0 | |
| 19 | 18000 | 1600 | <40.0 | |
| 6 i.m. injection | <40 | Not detectable | <40.0 | 28 no temperature rise |
| 12 | 12200 | Not detectable | >40.0 | |
| 19 | 35600 | 1600 | <40.0 | |
Response after second i.m. PRV application (day 6 post intranasal application) is indicated below the dotted lines and reflects a productive infection in animals from groups B and C accompanied by the development of fever (see Fig. 1). IFN-γ production and rise of body temperature in bold for day 6 post PRV application. i.m. = intramuscular.
2.2. Cells and viruses
Porcine peripheral blood mononuclear cells (PBMC) were prepared as described previously (Büttner et al., 1995, Saalmüller et al., 1989) and were maintained in RPMI 1640 medium supplemented with 300 mg/l stable l-Glutamine and 10% pestivirus-free fetal bovine serum (culture medium, Biochrom, Berlin, Germany; FBS, PAA, Linz, Austria). Viability of cells was determined using trypan blue dye exclusion in light microscopy. PBMC from naïve as well as PRV immune or primed animals were treated in vitro with PRV strain NIA-3 (McFerran and Dow, 1975), or BHV-1 strain Los Angeles (L.A.) (virus collection, Institute of Immunology) at a multiplicity of infection of 1 (MOI = 1).
For the production of PRV and bovine herpesvirus 1 (BHV-1) a pestivirus-free bovine kidney cell line, MDBK (ATCC, CCL22), was used and maintained in Dulbecco's minimal essential medium (DMEM) supplemented with 5% horse serum (Gibco-Invitrogen, Paisley, UK).
The PRV strain NIA-3 was propagated and quantified in MDBK cells, stocks of 2 ml containing 108 p.f.u./ml were aliquoted and stored at −70 °C. The control bovine herpesvirus 1 (BHV-1) strain L.A. was prepared in the same way.
2.3. Antibody detection
For the detection of PRV-specific antibodies a commercial ELISA kit (Chekit Aujeszkytest II, Hoechst, Schleißheim, Germany) was used as prescribed by the manufacturer.
2.4. IFN-γ detection
2.4.1. ELISA
The specificity of mAb reactive with porcine IFN-γ has been described (Mateu de Antonio et al., 1998). For the determination of cell-free IFN-γ a specific ELISA was used according to the recommendation of the manufacturer (Cyto Sets Swine IFN-γ, Biosource, Amarillo, CA, USA). Cytokine concentrations were calculated using the linear portion of the ELISA titration curve obtained using the recombinant standard of the kit. The titration of the standard was run on each plate and the concentrations of the test samples expressed in pg/ml.
2.4.2. ELISPOT assay
An ELISPOT assay was performed essentially as described by Zuckermann et al. (1998). Analysis of IFN-γ secretion by single cells was done using 96-well nitrocellulose-lined plates (MAIP 54510, Millipore, Eschborn, FRG) that were coated with 100 μl of anti-porcine IFN-γ mAb (Endogen, Bedford, MA, USA) (5 μg/ml) in PBS without calcium/magnesium (PBS-A) overnight at 37 °C. Wells were washed with PBS-A (wash buffer) and blocked for 1 h at room temperature (RT) with culture medium. Cell preparations were added at a concentration of 2 × 105/well and incubated for 48 h with ConA (5 μg/ml), PRV or BHV-1 (MOI ∼ 1) in a final volume of 200 μl. After the incubation period the plate was washed and 100 μl of an anti-porcine IFN-γ serum (2.5 μg/ml) (Endogen, Bedford, MA, USA) was added and incubated for 1 h at RT. The plate was washed again and incubated with anti-rabbit alkaline phosphate conjugate (100 μl/well, Promega, Mannheim, Germany) for 1 h at room temperature. After five times washing, the wells were incubated with 100 μl of the substrate solution BCIP/NBT (Sigma Taufkirchen, Germany) for 5–10 min. The plates were finally dried and the spots per well were counted with the aid of a dissecting scope or after photographing the wells.
2.5. Statistical analysis
Comparison of mean differences in interferon production was done using the Wilkoxon rank sum test. Differences were considered significant if P < 0.05.
3. Results and discussion
3.1. Clinical observations and antibody detection
The majority of infected and long-term monitored animals (n = 12) showed a rise in rectal body temperature above 40 °C between days 3 and 8 after intranasal PRV application (not shown) in the absence of other clinical signs. Following the febrile course of productive intranasal infection PRV-specific serum antibodies were first found 12 days p.i. by ELISA reaching a plateau (mean titer: 1/2800) around 8 weeks post challenge (not shown). Thus a correlation with the development of fever and take of infection could be confirmed by the presence of PRV-specific serum antibodies around 2 weeks after experimental infection. This is consistent with the reported data from vaccination and challenge experiments as well as from survival of natural PRV field infections (Wittmann and Rziha, 1989, Kimman et al., 1994, Mettenleiter, 1996).
In the group of eight animals showing a heterogeneous course of temperature reaction, only two (group A: 21 and 22) reacted with a fever response showing a sharp rise in rectal temperature around day 4 post virus application (Fig. 1A). In this experiment the take of infection was questionable because of the failure in rectal temperature rise among the remaining six animals from which only one in group B (No. 29) reacted with short-term fever (Fig. 1B, Table 1). The two animals showing the typical fever response (group A: 21, 22) developed high titers of PRV-specific antibodies as early as 12 days after intranasal virus application (Table 1). The course of body temperature of one piglet (29) in group B showed a short-term moderate rise pointing to a PRV sensitization (Fig. 1B). Both animals had developed low titers of PRV specific antibodies at day 12 after intranasal application (Table 1). The remaining four animals (group C) did not respond with a rise of body temperature within 6 days and also did not have detectable serum antibodies at day 12 after intranasal PRV application indicating a non PRV-sensitized status (Fig. 1C; Table 1). To control the assumption of a PRV sensitization (group B) versus the complete failure of PRV take (group C) a second virus application by the intramuscular (i.m.) route was performed to avoid insecurity in local virus application. The i.m. route of second virus application induced a fever response in all animals from groups B and C (Fig. 1B and C). However, the kinetics of antibody response revealed the difference between preceding PRV sensitization versus the failure of triggering an immune response by intranasal PRV application. Whereas both animals in group B had developed serum antibody titers already 6 days after i.m. infection, the non-sensitized animals in group C needed 12 days for the formation of PRV specific serum antibodies after i.m. infection (Table 1).
3.2. PBMC-mediated IFN-γ production after PRV re-stimulation
After ConA stimulation as a positive control IFN-γ was detectable in supernatants of PBMC from naïve as well as PRV challenged swine (Fig. 2 ). However, after in vitro exposure to live PRV naïve PBMC never secreted IFN-γ higher than 40 pg/ml independent of a short (24 h) or longer incubation period (72 h). In contrast after PRV infection, in vitro PRV re-stimulation of PBMC from the same animals resulted in the release of high amounts of IFN-γ in the culture supernatants exceeding by far those after ConA stimulation (Fig. 2). Although the mean cell viability had decreased by 12% as compared to the beginning of in vitro culture, a significant increase (P < 0.01) in PRV induced IFN-γ secretion was observed after longer in vitro exposure (72 h versus 24 h) of PBMC to PRV (Fig. 2). The significantly higher IFN-γ concentrations after longer (72 h) in vitro presence of PRV as the PBMC re-stimulating agent can be explained by a promotion through type I IFN and other cytokines (Brinkman et al., 1993, Manetti et al., 1995; Saraneva et al., 1998, Taniguchi and Takaoka, 2001) as well as by the autocrine IFN-γ mediated longevity of activated T-cells (Marrack et al., 1999, Akbar et al., 2000). A specificity of PBMC response to in vitro PRV re-stimulation was evident in PBMC supernatants tested 4 weeks after PRV infection. The mean IFN-γ concentration in the supernatants of PRV re-stimulated PBMC of 12 individuals was 5.195 pg/ml (Fig. 2) whereas the highest IFN-γ concentration after 72 h in vitro stimulation of the same cells with the closely related alpha herpesvirus BHV-1 was only 82 pg/ml (not shown). The PRV dependence of IFN-γ release was also demonstrated by the frequency of IFN-γ producing cells in IFN-γ specific ELISPOTs (Fig. 3 ). Stimulation of PBMC with the same MOI of bovine alpha herpesvirus BHV-1 (Fig. 3B) and medium cultivated cells (Fig. 3A) served as controls. As shown in Fig. 3B, BHV-1 stimulation of PBMC at 4 weeks after PRV infection survival only caused a slightly higher background compared to the cells incubated for 72 h in culture medium alone. In contrast, the same PRV re-stimulated PBMC regularly produced a high frequency of spots indicating a specificity in alpha herpesvirus-mediated induction of IFN-γ (Fig. 3C). Together with the identification of responding lymphocyte subtypes (Saalmüller et al., 1999) it remains to be determined which component(s) of PRV in contrast to related alpha herpesviruses such as BHV-1 are essential for the induction of IFN-γ synthesis in antigen primed porcine PBMC. It cannot be excluded that a glycoprotein per se or even certain epitopes might be sufficient for PBMC-mediated IFN production in re-stimulation experiments as already shown with porcine cells for corona virus type I IFN inducing capacity (Charley and Laude, 1988) and recently for herpes simplex virus glycoprotein D induced IFN-γ production with murine CD4+ peripheral T-lymphocytes (BenMohamed et al., 2003).
Fig. 2.
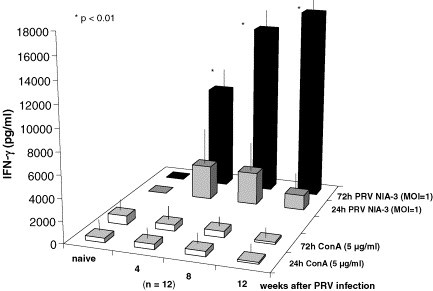
IFN-γ response after in vitro (re)stimulation. PBMC from non infected (naïve) and PRV infected animals (4, 8, 12 weeks after infection) were either stimulated with ConA or with PRV. The content of IFN-γ in the PBMC supernatants was quantified after 24 and 72 h in the IFN-γ specific ELISA.
Fig. 3.
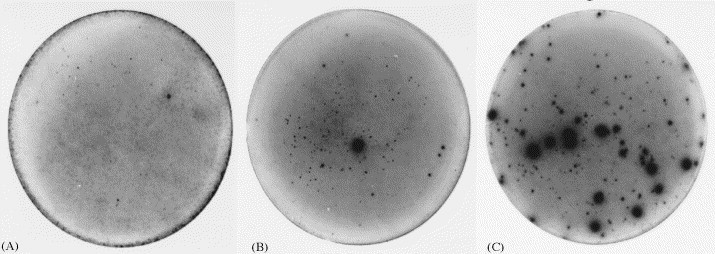
Determination of antigen-specific induction of the frequency of IFN-γ producing PBMC in ELISPOT assays (2 × 105 cells/well, 72 h) either re-stimulated with PRV (C) or stimulated with the closely related alpha herpesvirus BHV-1 (B). Only slightly elevated background spot numbers compared to culture medium controls (A) are present when BHV-1 is used as the stimulating virus in PRV primed PBMC from PRV challenge surviving piglets.
The PBMC-mediated, PRV-specific IFN-γ secretion was also tested after the fortuitous diverse course of body temperature reaction following intranasal virus application in a group of eight animals (Table 1). As early as 6 days after productive infection accompanied by a strong fever response the IFN-γ secretion of PBMC was significantly higher (2450 and 1838 pg/ml) than the pre-infection level (<40 pg/ml) in the two piglets from subgroup A (Table 1). At that early time point after intranasal PRV application PBMC from the animals in groups B and C which had not reacted with a significant rise in body temperature failed to produce IFN-γ in response to in vitro PRV re-stimulation (Table 1). However, again in correlation with the development of fever, PBMC from all animals in groups B and C produced IFN-γ 6 days after the second and secure i.m. virus application (Table 1). Thus the productive PRV infection in any case was linked to a fever response and later on to the formation of serum antibodies within 12 days. The latter were also detectable after i.m. application of PRV in the previously non-responding piglets (intranasal application group C) but with a time delay of 1 week compared to the earlier antibody detection (day 12 p.i.m. application) in sensitized animals (group B, Table 1). In addition the PBMC from obviously PRV-sensitized piglets in group B responded with a higher IFN-γ secretion early after the i.m. virus application (day 6) than the animals keeping a naïve status after the failure of intranasal virus take. Although strong individual differences in IFN-γ production exist similar to the antibody response (Hessing et al., 1995), a general rise in the IFN-γ levels in PBMC supernatants was observed later after productive PRV infection persisting at least for 12 weeks (Fig. 2). This is the first example of a direct ex vivo correlation of PBMC-mediated IFN-γ production depending on productive PRV infection of piglets accompanied by fever and followed by the presence of serum antibodies 12 days after infection. The detection of PRV-specific IFN-γ production by PBMC offers an early and reliable parameter for cellular immune response preceding the specific antibody formation (Zuckermann et al., 1998, Zuckermann et al., 1999, Mateu de Antonio et al., 1998). Following our observations with sensitized and non-responding animals the IFN-γ production of PBMC to in vitro PRV (re-)stimulation may be a sensitive reaction to indicate efficient vaccination and to assess the potency of vaccines (Fischer et al., 2000, Dufour et al., 2000, Zuckermann, 2000, Robinson and O’Garra, 2002).
Acknowledgements
We thank Angelika Braun for excellent technical assistance. The work was supported by grants from the German Research Community DFG PF 257/3-1 and from the European Community EU Project QLK2-1999-00459.
References
- Akbar A.N., Lord J.M., Salmon M. IFN-α and IFN-β: a link between immune memory and chronic inflammation. Immunol. Today. 2000;21:337–342. doi: 10.1016/s0167-5699(00)01652-2. [DOI] [PubMed] [Google Scholar]
- BenMohamed L., Bertrand G., McNamara C.D., Gras-Masse H., Hammer J., Wechsler S.L., Nesburn A.B. Identification of novel immunodominant CD4+ Th1-type T-cell peptide epitopes from herpes simplex virus glycoprotein D that confer protective immunity. J. Virol. 2003;77:9463–9473. doi: 10.1128/JVI.77.17.9463-9473.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biron C.A. Role of early cytokines, including α and β interferons (IFN-α/β), in innate and adaptive immune responses to viral infections. Semin. Immunol. 1998;10:383–390. doi: 10.1006/smim.1998.0138. [DOI] [PubMed] [Google Scholar]
- Brinkman V., Geiger T., Alkan S., Heusser C.H. Interferon α increases the frequency of interferon γ-producing human CD4+ T cells. J. Exp. Med. 1993;178:1655–1663. doi: 10.1084/jem.178.5.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büttner, Czerny C.-P., Wertz K., Lehner K.H., Kaaden O.-R. Interferon induction in peripheral blood mononuclear leukocytes (PBML) of man and farm animals by poxvirus vector candidates and some poxvirus constructs. Vet. Immunol. Immunopathol. 1995;146:179–184. doi: 10.1016/0165-2427(94)05357-x. [DOI] [PubMed] [Google Scholar]
- Charley B., Laude H. Induction of alpha interferon by transmissible gastroenteritis coronavirus: role of transmembrane glycoprotein E1. J. Virol. 1988;62:8–11. doi: 10.1128/jvi.62.1.8-11.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bruin M.G.M., De Visser Y.E., Kimman T.G., Bianchi A.T.J. Time course of the porcine cellular and humoral immune responses in vivo against pseudorabies virus after inoculation and challenge: significance of in vitro antigenic restimulation. Vet. Immunol. Immunopathol. 1998;65:75–87. doi: 10.1016/s0165-2427(98)00175-5. [DOI] [PubMed] [Google Scholar]
- De Bruin T.G.M., Van Rooij E.M.A., De Visser Y.E., Voermans J.J.M., Samsom J.N., Kimman T.G., Bianchi A.T.J. Discrimination of different subsets of cytolytic cells in pseudorabies virus-immune and naïve pigs. J. Gen. Virol. 2000;81:1529–1537. doi: 10.1099/0022-1317-81-6-1529. [DOI] [PubMed] [Google Scholar]
- Domeika K., Berg M., Eloranta M.-L., Alm G.V. Porcine interleukin-12 fusion protein and interleukin-18 in combination induce interferon-γ production in porcine natural killer cells and T cells. Vet. Immunol. Immunopathol. 2002;86:11–21. doi: 10.1016/s0165-2427(01)00431-7. [DOI] [PubMed] [Google Scholar]
- Dufour V., Chevallier S., Cariolet R., Somasundaram S., Lefevre F., Jestin A., Albina E. Induction of porcine cytokine mRNA expression after DNA immunization and pseudorabies virus infection. J. Interferon Cytok. Res. 2000;20:889–895. doi: 10.1089/10799900050163262. [DOI] [PubMed] [Google Scholar]
- Fearon D.T., Locksley R.M. The instructive role of innate immunity in the acquired immune response. Science. 1996;272:50–53. doi: 10.1126/science.272.5258.50. [DOI] [PubMed] [Google Scholar]
- Fischer T., Büttner M., Rziha R.-J. T helper 1-type cytokine transcription in peripheral blood mononuclear cells of pseudorabies virus (Suid herpesvirus 1)-primed swine indicates efficient immunization. Immunology. 2000;101:378–387. doi: 10.1046/j.1365-2567.2000.00124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie R.R., Hill M.A., Kanitz C.L., Knox K.E., Clark L.K., Robinson J.P. Infection of pigs by Aujesky‘s disease virus via the breath of intranasally inoculated pigs. Res. Vet. Sci. 2000;68:217–222. doi: 10.1053/rvsc.1999.0364. [DOI] [PubMed] [Google Scholar]
- Harrington L.E., van der Most R., Whitton J.L., Ahmed R. Recombinant vaccinia virus-induced T-cell immunity: quantitation of the response to the virus vector and the foreign epitope. J. Virol. 2002;76:3329–3337. doi: 10.1128/JVI.76.7.3329-3337.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessing M.J., Coenen G.J., Vaiman M., Renard C. Individual differences in cell-mediated and humoral immunity in pigs. Vet. Immunol. Immunopathol. 1995;45:97–113. doi: 10.1016/0165-2427(94)05338-s. [DOI] [PubMed] [Google Scholar]
- Kimman T.G., De Wind N., Oei-Lie N., Pol J.M.A., Berns A.J.M., Gielkens A.L.J. Inactivation of glycoprotein gE and thymidine kinase or the US3-encoded protein kinase synergistically decreases in vivo replication of pseudorabies virus and the induction of protective immunity. Virology. 1994;205:511–518. doi: 10.1006/viro.1994.1672. [DOI] [PubMed] [Google Scholar]
- Kimman T.G., De Bruin T.M.G., Voermans J.J.M., Peeters B.P.H., Bianchi A.T.J. Development and antigen specifity of the lymphoproliferation response of pigs to pseudorabies virus: dichotomy between secondary B- and T-cell response. Immunology. 1995;86:372–378. [PMC free article] [PubMed] [Google Scholar]
- Manetti R., Annunziato F., Tomasevic L., Gianno V., Parrochi P., Romagnani S., Maggi E. Polyinosinic acid: polycytidylic acid promotes T helper type 1-specific immune responses by stimulating macrophage production of interferon-α and interleukin-12. Eur. J. Immunol. 1995;25:2656–2660. doi: 10.1002/eji.1830250938. [DOI] [PubMed] [Google Scholar]
- Marrack P., Kappler J., Mitchell T. Type I interferons keep activated T cells alive. J. Exp. Med. 1999;189:521–529. doi: 10.1084/jem.189.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateu de Antonio E., Husmann R.J., Hansen R., Lunney J.K., Strom D., Martin S., Zuckermann F.A. Quantitative detection of porcine interferon-gamma in response to mitogen, superantigen and recall viral antigen. Vet. Immunol. Immunopathol. 1998;61:265–277. doi: 10.1016/s0165-2427(97)00141-4. [DOI] [PubMed] [Google Scholar]
- McFerran J.B., Dow C. Studies on immunization of pigs with the Bartha strain of Aujeszky's disease virus. Res. Vet. Sci. 1975;19:17–22. [PubMed] [Google Scholar]
- Mettenleiter T.C. Immunobiology of pseudorabies (Aujeszky's Disease) Vet. Immunol. Immunopathol. 1996;54:221–229. doi: 10.1016/s0165-2427(96)05695-4. [DOI] [PubMed] [Google Scholar]
- Mettenleiter T.C. Aujeszky's disease (pseudorabies) virus: the virus and molecular pathogenesis-state of the art, June 1999. Vet. Res. 2000;31:99–115. doi: 10.1051/vetres:2000110. [DOI] [PubMed] [Google Scholar]
- Mettenleiter T.C. Pathogenesis of neurotropic herpesviruses: role of viral glycoproteins in neuroinvasion and transneuronal spread. Virus Res. 2003;92:197–206. doi: 10.1016/s0168-1702(02)00352-0. [DOI] [PubMed] [Google Scholar]
- Pittet M.J., Zippelius A., Speiser D.E., Assenmacher M., Guillaume P., Valmori D., Lienard D., Lejeune F., Cerotti J.-C., Romero P. Ex vivo IFN-γ secretion by circulating CD8 T lymphocytes: implications of a novel approach for T cell monitoring in infections and malignant diseases. J. Immunol. 2001;166:7634–7640. doi: 10.4049/jimmunol.166.12.7634. [DOI] [PubMed] [Google Scholar]
- Reiner G., Melchinger E., Kramarova M., Pfaff E., Büttner M., Saalmüller A., Geldermann H. Detection of quatitative trait loci for resistance/susceptibility to pseudorabies virus in swine. J. Gen. Virol. 2002;83:167–172. doi: 10.1099/0022-1317-83-1-167. [DOI] [PubMed] [Google Scholar]
- Robinson D.S., O’Garra A. Further checkpoints in Th1 development. Immunity. 2002;16:755–758. doi: 10.1016/s1074-7613(02)00331-x. [DOI] [PubMed] [Google Scholar]
- Saalmüller A., Hirt W., Reddehase M.J. Phenotypic discrimination between thymic and extrathymic CD4−CD8− and CD4+CD8+ porcine T lymphocytes. Eur. J. Immunol. 1989;19:2011–2016. doi: 10.1002/eji.1830191107. [DOI] [PubMed] [Google Scholar]
- Saalmüller A., Pauly T., Höhlich B.-J., Pfaff E. Characterization of porcine T lymphocytes and their immune response against viral antigens. J. Biotechnol. 1999;73:223–233. doi: 10.1016/s0168-1656(99)00140-6. [DOI] [PubMed] [Google Scholar]
- Saraneva T., Matikainen S., Kurimoto M., Julkunen I. Influenza A virus-induced IFN-α/β and IL-12 synergistically enhance IFN-γ gene expression in human T cells. J. Immunol. 1998;160:6032–6038. [PubMed] [Google Scholar]
- Speller S., Warren A.P. Ex vivo detection and enumeration of human antigen-specific CD8+ T lymphocytes using antigen delivery by a recombinant vaccinia expression vector and intracellular cytokine staining. J. Immunol. Meth. 2002;262:167–180. doi: 10.1016/s0022-1759(02)00025-x. [DOI] [PubMed] [Google Scholar]
- Summerfield A., Rziha H.-J., Saalmüller A. Functional characterisation of porcine CD4+CD8+ extrathymic T lymphocytes. Cell. Immunol. 1996;168:291–296. doi: 10.1006/cimm.1996.0078. [DOI] [PubMed] [Google Scholar]
- Suradhat S., Intrakamhaeng M., Damrongwatanapokin S. The correlation of virus-specific interferon-gamma production and protection against classical swine fever virus infection. Vet. Immunol. Immunopathol. 2001;83:177–189. doi: 10.1016/s0165-2427(01)00389-0. [DOI] [PubMed] [Google Scholar]
- Taniguchi T., Takaoka A. A weak signal for strong responses: interferon-α/β revisited. Nat. Rev. Mol. Cell. Biol. 2001;2:378–386. doi: 10.1038/35073080. [DOI] [PubMed] [Google Scholar]
- Vilcek J., Sen G.C. Interferons and other cytokines. In: Fields B.N., Knipe D.M., Howley P.M., editors. Virology. 3rd ed. Lippincott-Raven Press; Philadelphia: 1996. pp. 375–399. [Google Scholar]
- Wittmann G., Rziha H.-J. Aujeszky's disease (pseudorabies) In: Wagner E., editor. Herpesvirus Diseases of Cattle, Horses, and Pigs. Kluwer Academic Publishers; Boston, Dordrecht: 1989. pp. 230–327. [Google Scholar]
- Zuckermann F.A., Husmann R.J., Schwartz R., Brandt J., Mateu de Antonio E., Martin S. Interleukin-12 enhances the virus-specific interferon gamma response of pigs to an inactivated pseudorabies virus vaccine. Vet. Immunol. Immunopathol. 1998;63:57–67. doi: 10.1016/s0165-2427(98)00082-8. [DOI] [PubMed] [Google Scholar]
- Zuckermann F.A., Martin S., Husmann R.J., Brandt J. Use of Interleukin 12 to enhance the cellular immune response of swine to an inactivated herpesvirus vaccine. Adv. Vet. Med. 1999;41:447–461. doi: 10.1016/s0065-3519(99)80034-2. [DOI] [PubMed] [Google Scholar]
- Zuckermann F.A. Aujeszky's disease virus: opportunities and challenges. Vet. Res. 2000;31:121–131. doi: 10.1051/vetres:2000111. [DOI] [PubMed] [Google Scholar]


