Highlights
-
•
This smartphone-based reader can be used to quantitatively reading AuNPs-LFISs of different principles and analytical targets.
-
•
Smartphone’s ambient light sensor be used to measure the transmitted light intensities of the strips.
-
•
The reader provided an on-site quantitative analysis tool and enhances the applied range of lateral flow immunoassay strips.
Keywords: Smartphone, Ambient light sensor, Lateral flow immunoassay strip, Colloidal gold, Quantitative detection
Abstract
Colloidal gold lateral flow immunoassay strips (AuNPs-LFIS) have been widely applied as qualitative diagnostic tools for point-of-care tests (POCT). If strip readers were incorporated, their use could be extended to quantitative analysis. However, their cost and non-portability render commercial strip readers unavailable for use in either home testing, community or rural hospital diagnosis. This is particularly true for on-site testing. Here, a smartphone-based reader was designed and 3D-printed for quantitatively assess AuNPs-LFIS. The basic principle of the devise was relying on a smartphone’s ambient light sensor (SPALS). This sensor was harnessed to measure the transmitted light intensities originating from the T-lines on the strips, the transmitted light intensities vary with concentration of AuNP on the T-lines. To validate this approach, our newly developed smartphone’s ambient light sensor-based reader (SPALS-reader) was used to readout AuNPs-LFIS of three analytical targets: cadmium ion (Cd2+; limit of detection (LOD) was 0.16 ng/mL), clenbuterol (CL; LOD was 0.046 ng/mL), and porcine epidemic diarrhea virus (PEDV; LOD was 0.055 μg/mL). The result showed good consistency with the results of conventional image analysis approaches, indicating that the smartphone-based device is appropriate for use in AuNPs-LFIS readouts. Compared with the traditional analysis method, the developed AuNPs-LFIS reader is easier operated, lower cost and more portable, which provided an on-site quantitative analysis tool for AuNPs-LFIS and enhances the applied range of AuNPs-LFIS.
1. Introduction
Modern sectors like health care, agriculture, environmental monitoring, forensic diagnosis, food safety, and industrial applications depend heavily on low-cost, point-of-care tests (POCT) [[1], [2], [3], [4]]. Across all areas, a variety of technologies and hybrid devices have been extensively reported, indicating the rapid improvement and spread of the point-of-care (POC) analysis. To this end, it is now possible to perform rapid, efficient, inexpensive, and easy analyses almost anywhere and at any time [[5], [6], [7], [8], [9]]. Over the past few decades, lateral flow immunoassay strips (LFIS) have become an increasingly popular diagnostic tool for use in POCT because of their use-friendliness, rapidity, inexpensive nature, and specificity [[10], [11], [12], [13], [14]]. Currently, LFIS are considered to be one of the easiest methods to commercialize for use as a POC diagnostic tool.
Given this, different signal material have been developed to meet different detection requirements, including ones containing colloidal gold [15], magnetic nanoparticles [16], quantum dots [17], fluorescence [18], biological enzyme [19], Raman enhancement probe [20] and nano-enzyme [21], etc. In addition, different types of LFISs have been developed through conjunction with different technologies to enable the detection of different analytes, such as barcodes lateral flow strip [22,23], biochemical-immunological hybrid biosensor [19], etc. Despite this variety, AuNPs-LFIS are one of the most common types of LFIS due to its inexpensive cost of production, ease of manufacture, stability, and simple readout. However, classic AuNPs-LFIS detection uses the naked eye, only providing a “yes/no‿ result. If quantitative analysis is needed, professional image analysis software and commercial instruments would be required. This limits the wide application of AuNPs-LFIS in homes or in on-site testing in resource-poor areas [24]. Therefore, a simple, inexpensive, user friendly, and pocket-sized reader would be an extremely useful tool to further advance the usability of the AuNPs-LFIS analytic technique.
Over the past decade, mobile phones have seen rapid development and gained ever-increasing levels of computational power. Globally, there are more than 5 billion mobile phone subscriptions, which is largely due to their ease of use and wide functionality. Critically, this popularity provides a development opportunity for their use in portable detection [25,26]. Due to their multifunctional capacities (e.g. permanent, built-in physical sensors, multi-core processors, digital cameras, USB ports, audio jacks, wireless transmission devices, and application software), smartphones have potential application in a wide variety of biosensor platforms [27,28]. In addition, the wireless telecommunication technologies incorporated within them allows for data upload to the internet. This could then help establish a ubiquitous platform for real-time, on-site monitoring [29]. Given this, coupling smartphones with LFIS could provide a portable, equipment-free, rapid, low-cost, and user-friendly POCT platform. To this end, Mudanyali et al. designed a cellphone-based reader platform that could work with various LFISs and get instrument similar tests to sense the presence of target analyte in samples. This platform could detect malaria, tuberculosis and HIV RDTs by installing it on both Android-based smartphones and iPhones [30]. Ling et al. reported a lateral flow-through strip for use with a smartphone camera that could quantitatively detect alkaline phosphatase activity in milk [31]. Hou et al. developed a smartphone-based, dual-modality imaging system that quantitatively detected either color or fluorescent LFIS [32]. In previous reports, smartphone-based LFIS readers were used directly with smartphone cameras to capture images of the LFIS test results. The data from these images were digitally processed using software. However, this approach has limits to its accuracy, resulting from the different pixilation and focal lengths between different smartphone’s cameras. To this end, the ambient light sensor on a given smartphone is sensitive and accurate and offers a more consistent starting point to develop a biosensor readout system. A microplate reader based on smartphone’s ambient light sensor to measures transmitted light intensities of liquid assay have been developed in our laboratory [[33], [34], [35], [36]]. The results were consistent with those obtained from a professional microplate reader and demonstrated its potential use in a variety of detection strategies for different targets.
Here, we have developed a LFIS reader based on the ambient light sensor of a smartphone and have extended our initial approach to now allow for the direct quantification of AuNPs-LFIS. The analytical system comprised a smartphone with ambient light sensor and an accessory (height 42 mm; width 30 mm; length 66 mm; weight 30 g) made by a 3D-printer. The principle behind this approach is as follows: (1) AuNPs accumulate at the testing zone positions, which decrease light transmittance through the NC membrane. (2) When the reader's switch is opened, the light emitted from the light-emitting diode (LED) projects over the testing zone positions and is then recorded by the ambient light sensor. (3) The light intensity signal is displayed on the smartphone’s screen using an app specifically designed to measure illuminance. (4) Light intensity relies on the degree of AuNPs coloration, AuNPs coloration is dependent on the concentration of the measured substance. Given this relationship, the light transmittance intensity can be used to directly quantify the tested sample (Fig. 1 ). We then analyzed three different analytes (cadmium ion (Cd2+), clenbuterol (CL), and porcine epidemic diarrhea virus (PEDV)) to validate our newly developed smartphone-based colloidal gold LFIS reader. Readout results were consistent with those obtained from traditional approaches, demonstrating that our smartphone based-reader could accurately quantify AuNPs-LFIS.
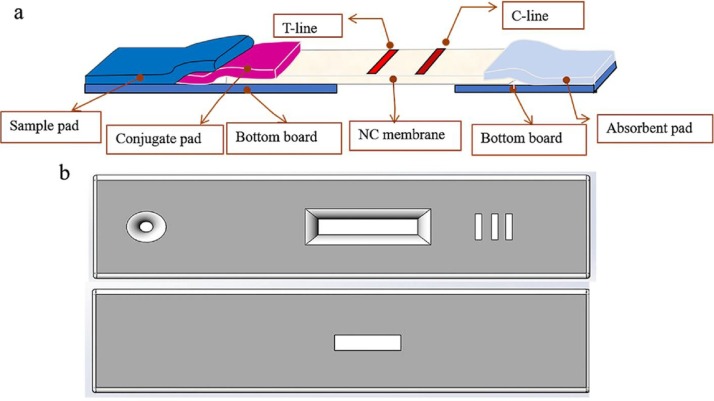
Fig. 1.
AuNPs-LFIS and plastic housing schematic representations for the SPALS-based reading system: (a) LFIS section; (b) Plastic housing.
2. Materials and methods
2.1. Material
Goat anti-mouse immunoglobulins G (IgG) were obtained from Sun gene Biotech Co., Ltd (Tianjin, China). Nitrocellulose (NC) membranes were purchased from Millipore (Shanghai, China). Plastic backing and absorbent pads, conjugation pads, and sample pads were obtained from JN Bio Co., Ltd (Shanghai, China). Chloroauric acid (HAuCl4), Bovine serum albumin (BSA), Trisodium citrate (99.8%), polyethylene glycol (PEG, MW, 2000), polyvinylpyrrolidone (PVP, MW 40,000), and S-17 (Rhoda surf ON-870) were all purchased from Sigma-Aldrich. Isothiocyanate benzyl-EDTA (iEDTA) was purchased from Dojindo Laboratories (Kumamoto, Japan). All aqueous solutions were prepared with double-distilled water (Milli-Q, Millipore). Cadmium chloride (99.99%) was purchased from Aldrich Chemical (Milwaukee, WI, USA). All other metal ions were purchased from Merck Chemical (Darmstadt, Germany). All other chemicals were of analytical grade and used without any further purification. The anti-Cd2+-EDTA monoclonal antibody(mAb)and the coating antigen BSA-Cd2+-EDTA were provided by our laboratory. The complete clenbuterol antigen (CL-BSA) and anti-CL monoclonal antibody(mAb)was purchased from Fapon Biotech Inc. (Shenzhen, China). The pierce TM BCA protein assay kit was purchased from Thermo Scientific (Rockford, USA). The PED Ag test kit was purchased from BioNote, Inc. (Gyeonggi-do, Korea). PEDV solution (106 TCID50/ml) was isolated, identified, and provided by Guangdong HAId Group Co., Ltd. LEDs (510 nm) were purchased from Shenzhen OCtai Co., Ltd. A battery (3 V) was purchased from VSAI while resistor (30,000 Ω), switch, and wires were collected from a local store.
2.2. Design of SPALS-based LFIS reader
The auxiliary device consisted of a switch, a 30,000 Ω resistor, three batteries (3 v), and a 520 nm LED. All of these electrical and optical elements were consolidated into a 3D printed, fixed structure (66 × 30 × 42 mm3) weighing approximately 30 g. The 3D printed, fixed structure was designed by SolidWorks 2016 software and fabricated using an EinStart-S 3D printer (SHINING 3D Technology Co., LTD). The printing material was thermoplastic black butadiene styrene (ABS) polymers, and was purchased from SHINING 3D Technology Co., LTD (Hangzhou, China).
2.3. AuNPs-LFISs preparation for use with SPALS-based reader
The AuNPs-LFIS structure was slightly altered when compared with conventional LFIS for use specifically with the SPALS-based reading system. Briefly, the bottom of the board was hollowed under the T/C-lines (Fig. 1a). The plastic housing was specially modified and equipped with a light transparent hole in the bottom (Fig. 1b). These modifications were necessary to allow for light transmission. Additional information regarding the preparation and fabrication process for the model AuNPs-LFIS, Cd2+-LFIS, CL-LFIS, and PEDV-LFIS are provided in Supplementary Information.
2.4. Quantitative reading of LFIS using both the SPALS-based reader and traditional image analysis method
Briefly, the procedure for using the SPALS-based reader to assess LFIS results was as follows: First, samples were added to sample orifice. After the immunoreaction was completed, LFIS were placed into the SPALS-based reader and the resulting, transmitted light intensities were measured using the Light Meter App. Simultaneous to light intensity measurements, a picture of the LFIS was obtained using the smartphone’s camera. Images were then analyzed using the free image analysis software Image J (NIH). Additional details regarding these experiments are provided in Supplementary information.
3. Results and discussion
3.1. Work principle behind the SPALS-based LFIS reader
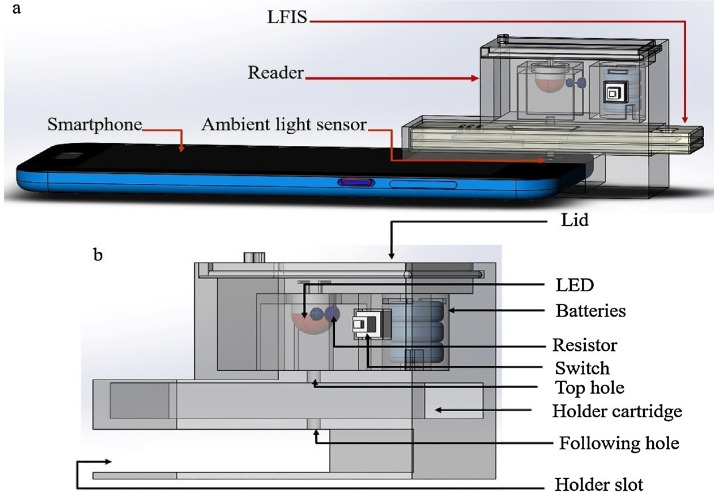
In this study, we developed a battery powered, 3D-printed attachment that could be connected to a smartphone’s ambient light sensor (SPALS). This could then be used to quantitatively read the results of colloidal gold lateral flow immunoassay strips (Fig. 2 a). Here, we chose an Android-based phone as the baseline smartphone for fabricating the reader. However, the SPSLS-based LFIS reader is easily adaptable to different mobile phones with minor modifications to the accessory (e.g. XIAOMI Note or HUAWEI P10). The attachment module included the LED, batteries, resistors, switch, wires, and a 3D-printed structure (Fig. 2b). LED wavelengths were specifically matched with the maximum absorption spectra of the AuNPs. The resistor (30,000 Ω) was used to regulate the intensity of the excitation light. Three batteries (3 V) was used to power the LED. The switch allowed the reader to turn on and off while the wires were used to connect all of the electronic components. The 3D-printed accessory held two light transparent holes of 2 mm diameter: The top hole was connected to the LFIS’s light transparent hole, the following hole was connected to the ambient light sensor. The 3D-printed accessory was equipped with a holder cartridge (64 × 21 × 7 mm3) as well as a holder slot (49 × 30 × 8.4 mm3), which were used to place the LFIS and fix the accessory in the cell phone, respectively. The holder slot is easily modifiable to fit any smartphone. The smartphone application for light intensity signal acquisition and data display was a free android app, Light Meter App. Collectively, the SPALS-based reader accessory weighed approximately 30 g, was manufactured in 2 h, and only cost $1.30 (Table S1).
Fig. 2.
Schematic illustration of the smartphone based AuNPs-LFIS reading system: (a) Three-dimensional structure of the AuNPs-LFIS reader installed on an Android-based smartphone and placed LFIS; (b) Design of the smartphone-based AuNPs-LFIS reader.
3.2. Work principle behind the SPALS-based LFIS reader
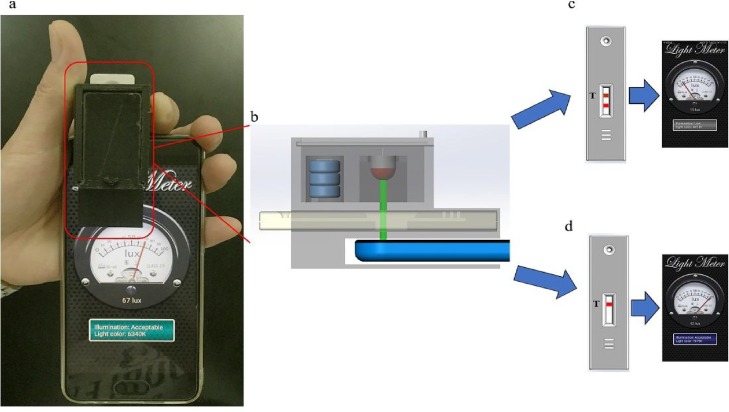
The working principle behind the SPALS-based LFIS readers is diagrammed and displayed in Fig. 3 . The immunoreaction on the NC membrane was completed by the sample, AuNPs-immunoreagents, and T-line’s immunoreagents. AuNPs-colored bands were formed since the label accumulated at specific positions on the NC membrane. The degree of the analyte concentration was then displayed as its corresponding color intensity. AuNPs have specific optical properties; as such, AuNPs color can decrease light transmittance through the NC membrane. When the LFIS assay was completed, the LFIS was put into the SPALS-based LFIS reader. The reader’s switch was opened and the light emitted by the LED was projected over the NC membrane. This was then measured by the ambient light sensor of the smartphone. The light intensity signals were displayed on the smartphone’s screen using the Light Meter app. When AuNPs concentration on the NC membrane was low, measured transmitted light intensity was high. Conversely, when AuNPs concentration on the NC membrane was high, measured transmitted light intensity was low. Given this relationship, it is clear that the transmitted light intensity depends on AuNPs concentration.
Fig. 3.
SPALS-based AuNPs-LFIS reading system schematic: (a) Image of the actual optical reader installed on the smartphone HUAWEI P10; (b) Work principle behind the smartphone-based AuNPs-LFIS reading system. Light emitted by the LED was projected over the T-line of the strip, which was then record by the smartphone’s ambient light sensor; (c) When AuNPs concentration on the NC membrane was high, transmitted light intensity as measured by the SPALS-based AuNPs-LFIS reader was low; (d) When AuNPs concentration on the NC membrane was low, intensities of measured light transmittance by the SPALS-based AuNPs-LFIS reader was high.
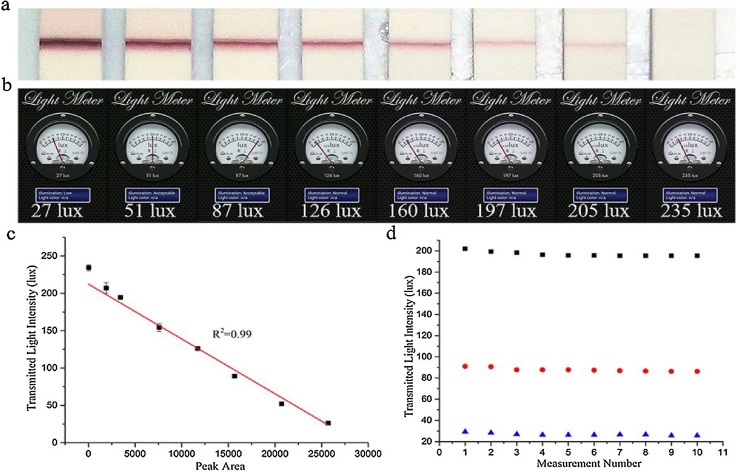
In order to study the accuracy of the smart phone based LFIS reader, we prepared a simple AuNPs-LFIS model. When the goat anti-mouse IgG-AuNPs and mouse IgG immunoreactions are performed on the T-line, the AuNPs colored bands will result. The transmitted light intensities of the LFIS were then record by the SPALS-based reader. Simultaneous to this, color images were obtained by the smartphone’s camera and were analyzed using the image analysis software Image J. As shown in Fig. 4 a and b, increasing AuNPs concentrations resulted in decreasing transmitted light intensities. To quantitatively determine the agreement between our SPALS-based reader and more conventional image analysis, we then performed a linear correlation. Results between the SPALS-based reader and image analysis showed strong agreement (R2 = 0.992, Fig. 4c). Transmitted light intensities were obtained from 10 runs of the quantitative AuNPs LFISs assays and each had nearly identical results (Fig. 4d). Taken together, these findings demonstrate the smartphone-based LFIS reader’s feasible and stability.
Fig. 4.
Performance of SPALS-based AuNPs-LFIS reader: a. Photograph of the quantitative AuNPs-LFISs for detecting different AuNPs concentration; b. Smartphone images of assays for detecting different AuNPs concentration; c. Agreement between detected results obtained by the SPALS-based reader and image analysis; d. Stability of the SPALS-based reader. Each value represents the mean of three independent experiments (n = 3).
3.3. Performance of the SPALS-based LFIS reader for quantitative detection of Cd2+ and CL
Small analytes are one of the most commonly used indicators to test for environmental pollution, pesticide residue, food safety, and drug inspection [37]. Due to their smaller molecular mass and single antigenic determinant, competitive format lateral flow assay has been widely employed for testing for the presence of small analytes. Cadmium (Cd2+) and clenbuterol (CL) have been important testing targets for both environmental pollutants and food safety, respectively. As established by the World Health Organization (WHO), the maximum contaminant level (MCL) for Cd2+ in drinking water is 3 ng/mL [38,39]. Detecting Cd2+ in either drinking water or agricultural irrigation water has been shown to be a very effective way to reduce cadmium pollution. Clenbuterol (CL) is a synthesized β2-agonist/antagonist and is used to promote animal growth and decrease fat synthesis. Poisoning symptoms can occur in individuals who consume food containing CL residues. Although many countries have banned the use CL in livestock, CL poisoning has been frequently reported [14,40,41].
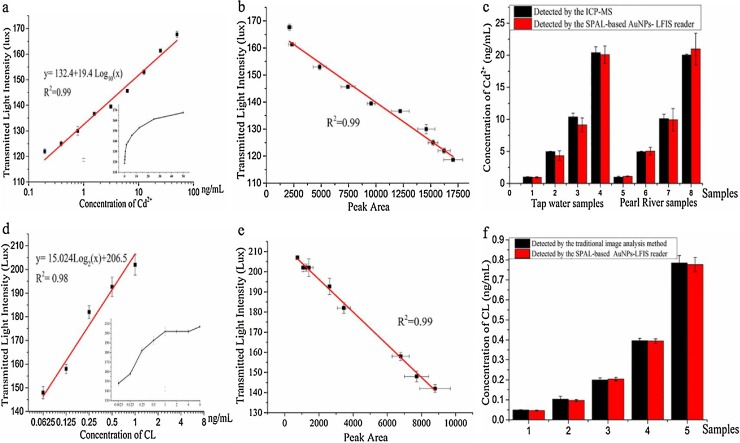
As shown in Fig. 5 a and d, we conducted an analysis for Cd2+ and CL levels using our SPALS-based reader. Results indicated that increasing Cd2+ analyte concentration corresponded to increasing transmitted light intensities, with a strong linear correlation between the two (R2 = 0.989). Cd2+ concentrations were found to be within the range of 0.1953–50 ng/mL and the limit of detection (LOD) was 0.16 ng/mL. To detect CL, results indicated a good linear correlation (R2 = 0.978) between the transmitted light intensities and CL concentration. The range obtained was 0.0625–1 ng/mL and the LOD for the CL assay was 0.046 ng/mL. As shown in Fig. 5b and e, the two reading methods for Cd2+-LIFS and CL-LIFS showed excellent correlations. We next validated the feasibility of using the SPALS-based reader for the analysis of spiked Cd2+ tap water samples and separate samples from the Pearl River (Guangzhou, China, see Fig. 5d). CL in swine urine samples was measured using LFIS and result were read using the SPALS-based reader (Fig. 5f). These results indicated that the SPALS reader had a high reliability and could successfully determine LFIS in water and urine samples.
Fig. 5.
Evaluation of SPALS-based AuNPs-LFIS reader for quantitative detection of Cd2+ and CL: (a) AuNPs-LIFS transmitted light intensities for quantitative detection of different Cd2+ concentrations; (b) Agreement between Cd2+ results obtained by both the SPALS-based reader and image analysis; (c) Cd2+ results from spiked samples obtained using the SPALS-based AuNPs-LFIS reader; (d) AuNPs-LIFS transmitted light intensities for quantitative detection of different CL concentrations; (e) Agreement between CL results obtained by both the SPALS-based reader and image analysis; (f) CL results in spiked samples obtained by the SPALS-based AuNPs-LFIS reader. Each value represents the mean of three independent experiments (n = 3).
3.4. Performance of SPSLS-based LFIS reader for quantitative detection of PEDV
Large analytes are one of the most common testing markers used in health care, including enzymes, protein markers, bacteria, and viruses. Due to their large molecular mass and multiple antigenic determinants, double antibody sandwich LFIS has been widely employed in testing for the presence of large analytes [37]. Porcine epidemic diarrhoea virus (PEDV) is a member of the coronavirus family that destroys that epithelial cells of the small intestine. This results in the development of an acute intestinal infectious disease in piglets and fattening pigs known as porcine epidemic diarrhoea (PED). A diagnosis of PED is conclusively made by detecting PEDV in pig waste [42,43]. Currently, the colloidal gold LFIS is one of the most common, qualitative detection methods used in veterinary diagnostics for on-site PEDV detection.
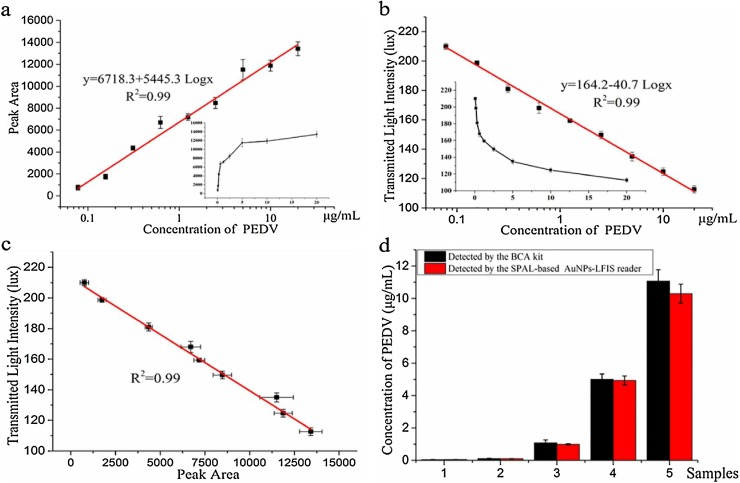
Here, quantitative analyte of PEDV-LFIS was obtained using both the conventional image analysis approach and our newly developed SPALS-based reader. The results from these two methods are shown in Fi. 6a and 6b. The image analysis system enabled discrimination of PEDV over a linear range of 0.078–20 μg/mL with a LOD of 0.062 μg/mL. The linear detection range for CL-LIFS using the SPALS-based reader was 0.078–20 μg/mL with a LOD of 0.055 μg/mL. As shown in Fig. 6 c, the results from these two methods had good agreement. The swine faecal samples were then spiked with different concentrations of PEDV and detected using the PEDV-LFIS. These results were then measured using the SPALS-based reader (Fig. 6d). Taken together, these results demonstrate the feasibility of using our smartphone reader in clinical samples.
Fig. 6.
Performance of SPALS-based AuNPs-LFIS reader for the quantitative detecting of PEDV: (a) AuNPs-LIFS PEDV results obtained by image analysis; (b) AuNPs-LIFS PEDV results obtained using the SPALS-based reader; (c) Agreement between the detected results obtained by the SPALS-based reader and image analysis; (d) Detected results of PEDV in spiked samples obtained using the SPALS-based AuNPs-LFIS reader. Each value represents the mean of three independent experiments (n = 3).
4. Conclusions
In summary, we have development a novel SPALS-based detection device that can quantitatively read colloidal gold lateral flow immunoassay strips (AuNPs-LFIS). We illustrated the performance of the SPALS-based AuNPs-LFIS reader by quantifying the detected AuNPs concentrations on the NC membrane. First, the linearity results for the AuNP quantification showed good correlation (R2 = 0.992) between transmitted light intensity analysis and the image analysis, indicating the device was validated with good sensitivity and stability. In addition, our technological evaluation analyzed three types AuNPs-LFIS and showed good agreement between the SPALS-based AuNPs-LFIS reader and the traditional image analysis method (Table S5). Additional evaluation the device with spiked samples revealed excellent recovery rates (Table S2,3 and 4). Compared with the conventional analysis system, our reading system has following advantages: 1) our reader operated more easily, the result could be directly obtained did not required additional data analysis; 2) our approach can be implemented on different smartphones and would only require an adjustment to the size of the holder slot for better fixation of the accessory; 3) our device is favored since ambient sensors do not require a focal adjustment; 4) the ambient light sensors do not require a large dark field, thus minimizing the size of accessory (height 42 mm; width 30 mm; length 66 mm; weight 30 g), even it can be put it in pocket; 5) our device doesn’t require expensive optical component to improve the performance of the sensor, thus the production cost of the instrument are cheaper (about 1.3 $, Table S1). Taken together, our new smartphone-based device provides a quantitative analysis tool for AuNPs-LFIS and enhances the applied range of AuNPs-LFIS. This approach will have wide-ranging applications prospect s, including in environmental testing, public health monitoring, and disease prevention. In future studies, to reduce fabrication difficulty of the LFIS, more advanced materials and manufactures is essential, such as transparent bottom board. On the side, a higher resolution smartphone application should be used to improve the accuracy and stability of SPALS-based detection device.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2016YFD0500600) and the Technology Research Program of Guangzhou City (201508020100).
Biographies
Wei Xiao presently is a PhD student at Jinan University. His research interests are in the area of biosensors and analytical chemistry.
Caihong Huang is currently enrolled in the Department of Bioengineering at Jinan University. She is studying for his Master's degree. His current research interest focuses on biosensors.
Fei Xu is currently studying for her Master's degree in the Department of Bioengineering, Jinan University. Her current research interest focuses on lateral-flow immunochromatographic strip.
Junjie Yan is currently studying for his Master's degree in the Department of Bioengineering, Jinan University. His current research interest focuses on monoclonal antibody preparation.
Hongfen Bian is currently studying for her Master's degree in the Department of Bioengineering, Jinan University. Her current research interest focuses on antigen purification and monoclonal antibody preparation.
Qiangqiang Fu Has gotten his Doctor's degree in the Department of Bioengineering, Jinan University. His current research interest focuses on biosensors and analytical chemistry.
Kaixin Xie is currently enrolled in the Department of Bioengineering at Jinan University. He is studying for his Master's degree. His current research interest focuses on biosensors.
Lei Wang currently is a master's student at Jinan university. His current research interest focuses on preparation and purification of antibodies.
Yong Tang is a professor of the Department of Bioengineering, Jinan University, PR China. He spends most of his time in preparation of monoclonal antibodies and biosensor analytical tools.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.snb.2018.03.110.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Kaushik A., Vasudev A., Arya S.K., Pasha S.K., Bhansali S. Recent advances in cortisol sensing technologies for point-of-care application. Biosens. Bioelectron. 2014;53:499. doi: 10.1016/j.bios.2013.09.060. [DOI] [PubMed] [Google Scholar]
- 2.Drain P.K., Hyle E.P., Noubary F., Freedberg K.A., Wilson D., Bishai W.R. Diagnostic point-of-care tests in resource-limited settings. Lancet Infect. Dis. 2014;14:239–249. doi: 10.1016/S1473-3099(13)70250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barcellona D., Fenu L., Marongiu F. Point-of-care testing INR: an overview. Clin. Chem. Lab. Med. 2016;55:800–805. doi: 10.1515/cclm-2016-0381. [DOI] [PubMed] [Google Scholar]
- 4.Majors C.E., Smith C.A., Natoli M.E., Kundrod K.A., Richardskortum R. Point-of-care diagnostics to improve maternal and neonatal health in low-resource settings. Lab Chip. 2017;17(20):3351. doi: 10.1039/c7lc00374a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tian B., Bejhed R.S., Svedlindh P., Strömberg M. Blu-ray optomagnetic measurement based competitive immunoassay for Salmonella detection. Biosens. Bioelectron. 2016;77:32–39. doi: 10.1016/j.bios.2015.08.070. [DOI] [PubMed] [Google Scholar]
- 6.Liang J., Yao C., Li X., Wu Z., Huang C., Fu Q. Silver nanoprism etching-based plasmonic ELISA for the high sensitive detection of prostate-specific antigen. Biosens. Bioelectron. 2015;69:128. doi: 10.1016/j.bios.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 7.Hu J., Cui X., Gong Y., Xu X., Gao B., Wen T. Portable microfluidic and smartphone-based devices for monitoring of cardiovascular diseases at the point of care. Biotechnol. Adv. 2016;34:305–320. doi: 10.1016/j.biotechadv.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Cheng Z., Choi N., Wang R., Lee S., Moon K.C., Yoon S.Y. Simultaneous detection of dual prostate specific antigens using surface-Enhanced raman scattering-Based immunoassay for accurate diagnosis of prostate cancer. ACS Nano. 2017;11 doi: 10.1021/acsnano.7b01536. [DOI] [PubMed] [Google Scholar]
- 9.Wu Z., Fu Q., Yu S., Sheng L., Xu M., Yao C. Pt@AuNPs integrated quantitative capillary-based biosensors for point-of-care testing application. Biosens. Bioelectron. 2016;85:657. doi: 10.1016/j.bios.2016.05.074. [DOI] [PubMed] [Google Scholar]
- 10.Wang K., Qin W., Hou Y., Xiao K., Yan W. The application of lateral flow immunoassay in point of care testing: a review. Nano Biomed. Eng. 2016;8 [Google Scholar]
- 11.Kim S.W., Cho I.H., Lim G.S., Park G.N., Paek S.H. Biochemical-immunological hybrid biosensor based on two-dimensional chromatography for on-site sepsis diagnosis. Biosens. Bioelectron. 2017;98:7. doi: 10.1016/j.bios.2017.06.032. [DOI] [PubMed] [Google Scholar]
- 12.Dzantiev B.B., Byzova N.A., Urusov A.E., Zherdev A.V. Immunochromatographic methods in food analysis. TrAC Trends Anal. Chem. 2014;55:81–93. [Google Scholar]
- 13.Fu Q., Tang Y., Shi C., Zhang X., Xiang J., Liu X. A novel fluorescence-quenching immunochromatographic sensor for detection of the heavy metal chromium. Biosens. Bioelectron. 2013;49:399–402. doi: 10.1016/j.bios.2013.04.048. [DOI] [PubMed] [Google Scholar]
- 14.Fu Q., Liang J., Lan C., Zhou K., Shi C., Tang Y. Development of a novel dual-functional lateral-flow sensor for on-site detection of small molecule analytes. Sens Actuators B: Chem. 2014;203:683–689. [Google Scholar]
- 15.Liu X., Xiang J.J., Tang Y., Zhang X.L., Fu Q.Q., Zou J.H. Colloidal gold nanoparticle probe-based immunochromatographic assay for the rapid detection of chromium ions in water and serum samples. Anal. Chim. Acta. 2012;745:99. doi: 10.1016/j.aca.2012.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Z., Wen J., Ju H., Fang Z. Magnetic nano-Fe3O4 particles targeted gathering and bio-effects on nude mice loading human hepatoma Bel-7402 cell lines model under external magnetic field exposure in vivo. Electromagn. Biol. Med. 2015;34:309. doi: 10.3109/15368378.2014.919589. [DOI] [PubMed] [Google Scholar]
- 17.Yang Q., Gong X., Song T., Yang J., Zhu S., Li Y. Quantum dot-based immunochromatography test strip for rapid, quantitative and sensitive detection of alpha fetoprotein. Biosens. Bioelectron. 2011;30:145–150. doi: 10.1016/j.bios.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Wen L., Zhu P., Liu Y., Pan Q., Qu Y., Xu X. Development of a fluorescence immunochromatographic assay for the detection of zeta globin in the blood of (--(SEA)) α-thalassemia carriers. Blood Cells Mol. Dis. 2012;49:128–132. doi: 10.1016/j.bcmd.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 19.Kim S.W., Cho I.H., Lim G.S., Park G.N., Paek S.H. Biochemical-immunological hybrid biosensor based on two-dimensional chromatography for on-site sepsis diagnosis. Biosens. Bioelectron. 2017;98:7–14. doi: 10.1016/j.bios.2017.06.032. [DOI] [PubMed] [Google Scholar]
- 20.Gao X., Peng Z., Kasani S., Wu S., Feng Y., Lewis S. Paper-based surface-enhanced Raman scattering lateral flow strip for detection of neuron-specific enolase in blood plasma. Anal. Chem. 2017;89:10104. doi: 10.1021/acs.analchem.7b03015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim M.S., Kweon S.H., Cho S., An S., Kim M.I., Doh J. Pt-decorated magnetic nanozymes for facile and sensitive point-of-care bioassay. ACS Appl. Mater. Interfaces. 2017;9(40):35133–35140. doi: 10.1021/acsami.7b12326. [DOI] [PubMed] [Google Scholar]
- 22.Yang M., Zhang W., Zheng W., Cao F., Jiang X. Inkjet-printed barcodes for a rapid and multiplexed paper-based assay compatible with mobile devices. Lab Chip. 2017;17(22):3874–3882. doi: 10.1039/c7lc00780a. [DOI] [PubMed] [Google Scholar]
- 23.Yang M., Zhang W., Yang J., Hu B., Cao F., Zheng W. Skiving stacked sheets of paper into test paper for rapid and multiplexed assay. Sci. Adv. 2017;3 doi: 10.1126/sciadv.aao4862. eaao4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mak W.C., Beni V., Turner A.P.F. Lateral-flow technology: from visual to instrumental. TrAC Trends Anal. Chem. 2016;79:297–305. [Google Scholar]
- 25.Preechaburana Pakorn, Suska Anke, Filippini Daniel. Biosensing with cell phones. Trends Biotechnol. 2014;32:351. doi: 10.1016/j.tibtech.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 26.Zhang D., Liu Q. Biosensors and bioelectronics on smartphone for portable biochemical detection. Biosens. Bioelectron. 2016;75:273–284. doi: 10.1016/j.bios.2015.08.037. [DOI] [PubMed] [Google Scholar]
- 27.Sun A., Wambach T., Venkatesh A.G., Hall D.A. A low-cost smartphone-based electrochemical biosensor for point-of-care diagnostics. Biomedical Circuits & Systems Conference. 2014 doi: 10.1109/BioCAS.2014.6981725. (p. 312) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laksanasopin T., Guo T.W., Nayak S., Sridhara A.A., Xie S., Olowookere O.O. A smartphone dongle for diagnosis of infectious diseases at the point of care. Sci. Transl. Med. 2015;7:273re1. doi: 10.1126/scitranslmed.aaa0056. [DOI] [PubMed] [Google Scholar]
- 29.Kim J., Jeerapan I., Imani S., Cho T.N., Bandodkar A., Cinti S. 2016. Noninvasive Alcohol Monitoring Using a Wearable Tattoo-Based Iontophoretic-Biosensing System. [Google Scholar]
- 30.Mudanyali O., Dimitrov S., Sikora U., Padmanabhan S., Navruz I., Ozcan A. Integrated rapid-diagnostic-test reader platform on a cellphone. Lab Chip. 2012;12:2678. doi: 10.1039/c2lc40235a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu L., Shi Z., Fang C., Zhang Y., Liu Y., Li C. Disposable lateral flow-through strip for smartphone-camera to quantitatively detect alkaline phosphatase activity in milk. Biosens. Bioelectron. 2015;69:307–315. doi: 10.1016/j.bios.2015.02.035. [DOI] [PubMed] [Google Scholar]
- 32.Hou Y., Wang K., Xiao K., Qin W., Lu W., Tao W. Smartphone-based dual-modality imaging system for quantitative detection of color or fluorescent lateral flow immunochromatographic strips. Nanoscale Res. Lett. 2017;12:291. doi: 10.1186/s11671-017-2078-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fu Q., Wu Z., Li X., Yao C., Yu S., Wei X. Novel versatile smart phone based Microplate readers for on-site diagnoses. Biosens. Bioelectron. 2016;81:524–531. doi: 10.1016/j.bios.2016.03.049. [DOI] [PubMed] [Google Scholar]
- 34.Fu Q., Wu Z., Xu F., Li X., Yao C., Xu M. A portable smart phone-based plasmonic nanosensor readout platform that measures transmitted light intensities of nanosubstrates using an ambient light sensor. Lab Chip. 2016;16:1927. doi: 10.1039/c6lc00083e. [DOI] [PubMed] [Google Scholar]
- 35.Yu S., Xiao W., Fu Q., Wu Z., Yao C., Shen H. A portable chromium ion detection system based on a smartphone readout device. Anal. Methods. 2016;8 [Google Scholar]
- 36.Xiao W., Xiao M., Fu Q., Yu S., Shen H., Bian H. A portable smart-phone readout device for the detection of mercury contamination based on an aptamer-assay nanosensor. Sensors. 2016;16:1871. doi: 10.3390/s16111871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bahadır E.B., Sezgintürk M.K. Lateral flow assays: principles, designs and labels. TrAC Trends Anal. Chem. 2016;82:286–306. [Google Scholar]
- 38.Chantarawong W., Takeda K., Sangartit W., Yoshizawa M., Pradermwong K., Shibahara S. Microphthalmia-associated transcription factor as the molecular target of cadmium toxicity in human melanocytes. Biochem. Biophys. Res. Commun. 2014;454:594–599. doi: 10.1016/j.bbrc.2014.10.141. [DOI] [PubMed] [Google Scholar]
- 39.Vergauwen L., An H., Blust R., Knapen D. Temperature dependence of long-term cadmium toxicity in the zebrafish is not explained by liver oxidative stress: evidence from transcript expression to physiology. Aquat. Toxicol. 2013;126:52–62. doi: 10.1016/j.aquatox.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 40.Shi Z.Z., Tian Y.L., Wu X.S., Li C.M., Yu L. A one-piece lateral flow impedimetric test strip for label-free clenbuterol detection. Anal. Methods. 2015;7:4957–4964. [Google Scholar]
- 41.Zhang G.P., Wang X.N., Yang J.F., Yang Y.Y., Xing G.X., Li Q.M. Development of an immunochromatographic lateral flow test strip for detection of β-adrenergic agonist Clenbuterol residues. J. Immunol. Methods. 2006;312:27. doi: 10.1016/j.jim.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 42.Chen J., Wang C., Shi H., Qiu H., Liu S., Chen X. Molecular epidemiology of porcine epidemic diarrhea virus in China. Arch. Virol. 2010;155:1471. doi: 10.1007/s00705-010-0720-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Utiger A., Tobler K., Bridgen A., Ackermann M. Identification of the membrane protein of porcine epidemic diarrhea virus. Virus Genes. 1995;10:137. doi: 10.1007/BF01702594. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.