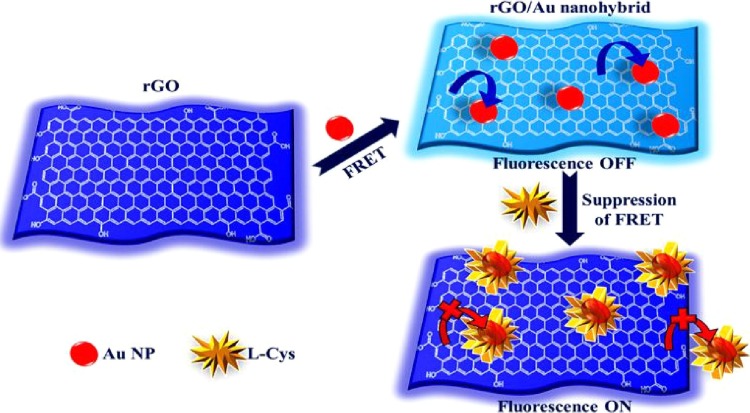
Graphical abstract
Keywords: Reduced graphene oxide, Gold nanoparticles, L-Cysteine, Bioimaging, Energy transfer, Fluorescence
Highlights
-
•
A fluorogenic detection of L-Cysteine was investigated using rGO/Au nanohybrids.
-
•
The rGO/Au nanohybrids was prepared by one-pot hydrothermal method.
-
•
A lower LOD (0.51 nm) was achieved.
-
•
Practicality of the sensor was evaluated in live cancer cells.
Abstract
A highly sensitive and selective fluorogenic sensing of L-Cysteine (L-Cys) was implemented based on gelatin stabilized gold nanoparticles decorated reduced graphene oxide (rGO/Au) nanohybrid. The rGO/Au nanohybrid was prepared by the one-pot hydrothermal method and well characterized by different physiochemical techniques. The nanohybrid exhibits a weak fluorescence of rGO due to the energy transfer from the rGO to Au NPs. The rGO/Au nanohybrid shows enhanced fluorescence activity due to the restoration of quenched fluorescence of rGO/Au nanohybrid in presence of L-Cys. The rGO/Au nanohybrid exhibits much lower detection limit of 0.51 nM for L-Cys with higher selectivity. The fluorescence sensing mechanism arose from the fluorescence recovery due to the stronger interaction between Au NPs and L-Cys, and consequently, the energy transfer was prevented between rGO and Au NPs. The practicability of rGO/Au sensor was implemented by invitro bioimaging measurements in Colo-205 (colorectal adenocarcinoma) and MKN-45 (gastric carcinoma) cancer live cells with excellent biocompatibility.
1. Introduction
The lower molecular weight of thiol-containing molecules such as L-Cysteine (L-Cys), homocysteine (Hcy) and glutathione (GSH) are frequently involved in various biological actions due to its wide distribution in living organisms. In addition, thiols are playing important role in reversible redox homeostasis and cellular functions in the biological system [[1], [2], [3], [4]]. Especially, L-Cys as a semi-essential thiol-containing amino acid, which is most important in peptide and protein synthesis, detoxification, metabolism, and neuronal tissues [[5], [6], [7]]. In addition, L-Cys can be easily cross-linked with bio-macromolecules via disulfide bonds and coupled with enlargement and hindrance of senility of the cells and tissues in the living system [[8], [9]]. However, a series of disorders could be associated with the problem of L-Cys such as slow growth, muscle and fat loss, hair depigmentation, weakness, edema, skin lesions, liver damage, lethargy, Alzheimer’s disease, Parkinson’s disease and acquired immune deficiency syndromes [[10], [11], [12]]. Hence, there is an urgent demand for accurate and low-level detection of L-Cys with simple and highly sensitive analytical techniques. Thus far, L-Cys was quantified with various classical analytical techniques such as UV–vis and fluorescence spectroscopy, Fourier-transform infrared spectroscopy (FT-IR) spectroscopy, mass spectrometry, gas chromatography and immunoassay, high-performance liquid chromatography (HPLC), fluorescence-coupled HPLC and electrochemical methods [[13], [14], [15], [16], [17], [18], [19], [20], [21], [22]]. Among these traditional methods, herein the fluorescence method was used for detection of L-Cys by taking advantage of the merits of simplicity, high sensitivity, low cost, selectivity and versatility [[23], [24]].
During the past decades, the carbon-based nanomaterials such as carbon quantum dots, graphene, and other carbon nanomaterials have been adopted widely for the fluorescence sensing of biomolecules [[25], [26], [27], [28], [29], [30]]. Particularly, reduced graphene oxide (rGO), a two-dimensional (2D) thin layer nanosheet with hexagonal honeycomb lattices of carbon sheet, has been widely used in various fields such as nanotechnology, materials science and biotechnology [31]. On the other hand, the noble-metal nanoparticles, especially gold nanoparticles (Au NPs), have attracted tremendous interest in biological applications due to its specific large surface area, superior biocompatibility, and excellent conductivity. In addition, they also act as an efficient catalyst for electron transfer process [[32], [33], [34]]. Accordingly, the Au NPs have been widely applied for ion detection, molecular identification, and chemical catalysis, while showing the prospects in bio-labeling and bio-sensing [22]. For instance, the biocompatible polymer and peptides conjugated with Au NPs could be used for biomedical applications during the decades. Specifically, Gelatin (GEL) is a helically structured biopolymer matrix, is most abundant in animal skin and bone. Moreover, GEL is a denatured product of collagen and utilized for the entrapment of biomolecules for the preparation of biosensors [[35], [36], [37]]. Zhang et al. adopted gelatin as reducing and stabilizing agent to immobilize Au NPs with carboxylic single-walled carbon nanotubes for use of cytosensing and drug uptake [38].
In this work, GEL was used as a stabilizing and reducing agent for the preparation of rGO/Au nanohybrid via the one-pot hydrothermal synthesis. The obtained rGO/Au nanohybrid shows excellent sensitivity and selectivity towards the detection of L-Cys. The fluorescence sensing of L-Cys was investigated based on the fluorescence turn-on process. When coordinated with Au NPs, the fluorescence of rGO was diminished due to the energy transfer from rGO to Au NPs. Then, the fluorescence recovery of rGO was obtained upon the addition of L-Cys owing to the strong interaction between Au NPs and L-Cys prevents energy transfer from rGO to Au NPs. For practical application, this nanohybrid based sensor was successfully demonstrated to detection of L-Cys in Colo-205 (colorectal adenocarcinoma) and MKN-45 (gastric carcinoma) cancer live cells by using confocal fluorescence microscopy.
2. Experimental section
2.1. Materials
Pristine graphite powder (size <20 mm), gelatin from bovine skin and HAuCl4·3H2O were purchased from Sigma-Aldrich. L-Cys and other chemicals were obtained from Acros and Sigma-Aldrich, and used as purchased without further purification. Phosphate buffered saline (PBS, 1X, pH 7.4) was purchased from Life technology.
2.2. Instruments
Transmission electron microscope (TEM) observations were conducted with a JEOL JEM-1200 EX-II. Atomic force microscopy (Veeco, Bio-Scope SZ) was used to determine the thickness of rGO. Raman spectra were recorded by using a Raman spectrometer (Dong Woo 500i, Korea) equipped with a 50 x objective and a charge-coupled detector. The FTIR spectra were carried out using the Thermo Scientific Nicolet iS10 instrument. The UV–vis absorption spectra were recorded with a Thermo Scientific evolution 220 UV–vis spectrophotometer. The fluorescence spectra were recorded with a Perkin-Elmer LS45 spectrometer. Confocal fluorescence images of cells were performed on a Leica SP5 confocal system under 405 nm excitation light source.
2.3. Synthesis of rGO/Au nanohybrid
The graphite oxide (GO) was synthesized via modified Hummers' method [39]. The GEL solution was prepared as per our previous report [40]. The GEL stabilized rGO/Au nanohybrid was prepared by the one-pot hydrothermal method. Briefly, the GO was redispersed in Millipore water and stirred for 1 h to obtain a fully exfoliated GO suspension. Then, the as-prepared aqueous solution of GEL (10 mL, 3%, wt%) was added to the GO suspension. After that, 1.0 mL of the HAuCl4 solution (1%, wt%) was added to the above GO/GEL suspension under magnetic stirring. Then, appropriate amount of 0.1 M NaOH solution was added to the suspension and stirred vigorously for 6 h at 80 °C. The composite was cooled at room temperature and washed with Millipore water through centrifugation to remove the unreacted Au NPs and GEL suspension. The composite was then dried for 2 h at 60 °C. The obtained GEL stabilized rGO/Au nanohybrid was further used for characterizations and fluorescence sensing studies. The plausible mechanism for fluorogenic detection of L-Cys using rGO/Au nanohybrid was illustrated in Scheme 1 .
Scheme 1.
Schematic illustration of fluorescence turn-on mechanism over rGO/Au nanohybrid.
2.4. Procedure for fluorescence sensing experiments
All diverse biomolecules solutions were buffered in pH 7.4 PBS (0.1X). The stock solution of rGO/Au nanohybrid (15 μg/mL) was prepared by the dilution of concentrated rGO/Au nanohybrid with pH 7.4 PBS. The fluorescence titration measurements were carried out by adding L-Cys (up to 20 μL, with the maximum concentration ∼0.4 μM) to the rGO/Au nanohybrid solution in a quartz cuvette. Then the solution was stirred well and the absorption and fluorescence spectra were recorded.
The selectivity experiments of rGO/Au nanohybrid were examined with diverse biomolecules. In a typical assay, a set of each rGO/Au solution (1 mL) was prepared, then 0.5 μL of 10 μM each biomolecule (concentration of biomolecule in solution is 5 nM) was added into each rGO/Au solution. Afterwards, the fluorescence spectra of each solution were monitored at the excitation of 380 nm. The matrix interference studies of rGO/Au nanohybrid with L-Cys were carried out in the presence of biomolecules. Herein, the concentration of L-Cys and other biomolecules in mixture solution was fixed at 5 nM. For example, each selected biomolecule solution (0.5 μL of 5 μM) was added individually into a mixture solution of rGO/Au (1.0 mL of stock) and L-Cys (0.5 μL of 5 μM). Then, all solutions were stirred well, and the fluorescence spectra were recorded.
2.5. In vitro fluorescence bioimaging experiments and MTT assay
All cell lines used in this study were obtained from the Bioresource Collection and Research Center in Taiwan. The cells were cultivated in RPMI1640 supplemented with 10% fetal bovine serum (FBS) and 250 units/mL of penicillin/streptomycin solution. All cell culture media and supplements were purchased from Gibco (Invitrogen, CA, USA). The cell viability of sensor in Colo-205 and MKN-45 were determined using a 3-(4, 5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The Colo-205 and MKN-45 cells (1 × 104 cells/well) were seeded in 96 wells for 24 h and then treated with various concentrations of rGO/Au for another 24 h. Subsequently, 20 μL of MTT solution (5 mg/mL) was added and the mixture was incubated for additional 3 h. The formazan precipitate was dissolved in 100 μL of DMSO, and the solution was vigorously mixed to dissolve the reacted dye. The absorbance of each well was read at 490 nm by a spectrophotometer. The cell lines were seeded in Ibitreat μ-slide 8-well plates (Ibidi, Munich, Germany) at a density of 8 × 104/well with media. Cells were pretreated with rGO/Au solution. After 1 h incubation, the cells were washed with DPBS solution to remove extracellular rGO/Au. Then cells were treated with L-Cys (200 nM) for 30 min. Fluorescence images of cells were performed on a Leica SP5 confocal system under a controlled environment (37 °C, 5% CO2).
3. Results and discussion
3.1. Material characterizations
The as-prepared rGO/Au nanohybrid was characterized by TEM, AFM, FT-IR, and Raman spectroscopy measurements. TEM images of rGO showed as a nanosheet (Fig. 1 a), and Au NPs were uniformly dispersed on the surface of rGO (Fig. 1b). Moreover, the size of Au NPs was observed in the wide range from ∼2 to 20 nm with the average size of ∼7 nm. AFM measurement was carried out to determine thickness of rGO/Au and the resultant image is shown in Fig. 1c. The average thickness of rGO/Au was calculated about 3.5 nm (Fig. 1d). The crystalline disorder and structural changes of GO, rGO and rGO/Au nanohybrid were characterized by Raman spectroscopy as shown in Fig. 1e. The Raman spectra of GO exhibited two predominant peaks at 1608 and 1374 cm−1 corresponding to the sp2 carbon domains of the hexagonal lattice of G band and the partially disordered nature of sp3 domains of the D band, respectively [41]. The relative intensity of G band (E2g mode of sp2 carbon atoms) and D band (symmetry A1g mode) can be changed during the reduction of GO, revealing that the change of the electronic conjugation state [[42], [43]]. The intensity ratio (ID/IG) for GO, rGO and rGO/Au were calculated to be 0.86, 1.01 and 1.36, respectively. The intensity ratio of rGO/Au dramatically increased than that of rGO and GO, suggesting that the rGO/Au nanohybrid was successfully formed using gelatin as a reducing and stabilizing agent.
Fig. 1.
TEM images of (a) rGO and (b) rGO/Au nanohybrid. AFM image of (c) rGO/Au and (d) the plot of height distributions (inset shows height profile). (e) Raman spectra of GO, rGO and rGO/Au nanohybrid, and (f) FTIR spectra of GO and rGO.
Furthermore, the structural changes and de-oxidation of GO to rGO were confirmed by FT-IR spectroscopy. The FT-IR spectra of GO and rGO are shown in Fig. 1f. It was found that the FT-IR spectra of GO and rGO depict a board peak around 3400–3600 cm−1 which is ascribed to the —OH stretching vibrations, possibly from —COOH group and H2O. The characteristic peaks of oxygen in GO were observed at ∼1726, 1248 and 1068 cm−1 corresponding to the —C O, C—OH (carboxylic) and C—O (alkoxy) stretching vibrations, respectively. This observation confirms that the GO was successfully synthesized via modified Hummers' method. To note that, the intensity of all oxygen characteristics peaks were significantly decreased which revealing the successful formation of rGO from GO and consistent with the literature [[44], [45], [46], [47]].
3.2. Optical properties
The absorption and fluorescence spectra of rGO/Au nanohybrid were acquired in pH 7.4 PBS buffered solution and the results are shown in Figure S-1. The absorption spectrum of rGO/Au exhibits the strong absorption band in the UV and visible regions (200–650 nm) with a prominent peak at ∼530 nm and a shoulder at ∼290 nm. The strong absorption peak at ∼530 nm is due to the presence of Au NPs, and the shoulder at ∼290 nm is ascribed to the rGO (n-π* transition of the C O bond) [[48], [49], [50]]. The fluorescence spectra of rGO/Au nanohybrid were recorded with different excitation wavelengths. The resultant fluorescence spectra reveal that the rGO/Au nanohybrid exhibits a weak fluorescence which is dependent on the excitation wavelength, consistent with the literature [[49], [50]]. The higher fluorescence intensity peaking at 447 nm was observed on the excitation of 380 nm.
3.3. Selectivity of the sensor
In order to reveal the selectivity of the sensor, the fluorescence characteristic of rGO/Au nanohybrid was investigated in the presence of diverse biomolecules. The selectivity experiments were carried out to determine the biomolecule influence on the fluorescence intensity of rGO/Au. Interestingly, rGO/Au nanohybrid shows a good fluorescence enhancement towards the detection of L-Cys among the biomolecules used in this work including α- and β-alanine (α- and β-Ala), L-arginine (Arg), ascorbic acid (Asc), aspartic acid (Asp), bovine serum albumin (BSA), cisplatin (CPT), dopamine (DA), glutathione (GSH), homocysteine (Hcy), l-histidine (His), human serum albumin (HSA), α-lipoic acid (LA), lysine (Lys) and uric acid (UA), and the obtained results are shown in Fig. 2 . A drastic fluorescence enhancement of rGO/Au nanohybrid was observed in the presence of L-Cys.
Fig. 2.

The fluorescence intensity of rGO/Au nanohybrid with various biomolecules. Concentration of each biomolecules: 5 nM.
3.4. Detection of L-Cys
The absorption and fluorescence spectra of rGO/Au nanohybrid with different concentrations of L-Cys were recorded successively by fluorescence titration method to scrutiny the sensitivity level of L-Cys. There was not much change observed in the absorption spectra of rGO/Au with different concentrations of L-Cys (Figure S-2). This result excludes that the ground state interaction between the rGO/Au and L-Cys. On the contrary, the fluorescence intensity of rGO/Au nanohybrid was gradually increased with increasing the concentrations of L-Cys and also observed slight red-shift of emission wavelength (∼4 nm). Fig. 3 a and b displays the fluorescence spectra of rGO/Au nanohybrid in the presence of different concentrations of L-Cys and the calibration plot for fluorescence intensity change (ΔF) vs. different concentrations of L-Cys, respectively. The detection limit (LOD) was determined from the values of fluorescence intensity as the function of L-Cys by using the relation 3(σ/slope), where σ is the standard deviation of the response.
Fig. 3.
(a) The fluorescence spectra of rGO/Au nanohybrid with different concentration of L-Cys. (b) The plot of fluorescence intensity as a function of concentration of L-Cys; inset shows linearity graph for initial concentration of L-Cys.
The fluorescence intensity change (ΔF = F − F0, where F0 and F are the fluorescence intensity in the absence and presence of L-Cys) at 447 nm was plotted as the function of L-Cys concentration. The resultant plot shows a linearity over the initial concentrations of L-Cys (Fig. 3b). The LOD of L-Cys was calculated to be 0.51 nM, revealing that this nanohybrid based sensor system showed a higher sensitivity towards the detection of L-Cys. The obtained parameters are compared with a previously reported L-Cys sensors as summarized in Table 1 . This result suggests that our proposed sensor is better than that of the previous reports [[9], [18], [19], [25], [28], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63]].
Table 1.
Comparison of various chemosensors for L-Cys detection using fluorescence technique.
| Sensors | LOD (nM) | Ref. |
|---|---|---|
| Pyrene based probe | 0.01 | 9 |
| N-doped graphene quantum dots | 1.3 | 18 |
| Cyanine-based probe | 160 | 19 |
| Graphene quantum dot/gold NPs | 0.32 | 25 |
| Coumarin-based probe | 657 | 28 |
| Carbon dots | 0.34 | 51 |
| N-doped carbon dots | 0.79 | 52 |
| DAPI dye | 2.4 | 53 |
| P-acid-aldehyde probe | 3.0 | 54 |
| Iminocoumarin based probe | 6.6 | 55 |
| Mitochondria-targeted near-IR probe | 14.5 | 56 |
| 1,8-naphthalimide-based probe | 45.0 | 57 |
| BODIPY −based glyoxal hydrazone | 54.6 | 58 |
| Squaraine based near-IR probe | 59.0 | 59 |
| Coumarin and N-(4-aminobenzoyl)-b-alanine | 150 | 60 |
| Carbon dots | 290 | 61 |
| ESIPT-based probe | 640 | 62 |
| Gold nanoclusters & nanoparticles | 3600 | 63 |
| Gelatin-stabilized rGO/Au | 0.51 | This work |
3.5. Matrix interference of the sensor
The matrix interference studies were also investigated to scrutinize the effect of other biomolecules on the interaction between rGO/Au nanohybrid and L-Cys. The fluorescence spectra of rGO/Au with a certain concentration of L-Cys were recorded in presence of each diverse biomolecules. The obtained changes in the fluorescence intensity are shown in Figure S-3. It was found that the fluorescence intensity of rGO/Au with L-Cys was not significantly changed upon addition of other biomolecules. This result minimizes the matrix effect by other biomolecules.
3.6. Mechanism for fluorescence detection of L-Cys
Based on acquired fluorescence sensing results, a plausible mechanism was proposed for the fluorescence enhancement of rGO/Au nanohybrid on the addition of L-Cys. When the Au NPs are attached with rGO, the energy transfer takes place from rGO to Au NPs on the excitation. Consequently, the fluorescence intensity of rGO is diminished via the effect of energy transfer. This process was confirmed by the comparison of fluorescence intensity of rGO in the absence and presence of Au NPs. Indeed, the rGO exhibited strong fluorescence as compared to that of rGO/Au (Figure S-4), verifying the energy transfer occurrence from rGO to Au NPs. Similar FRET phenomena between Au nanocluster and GO were reported previously by Nguyen et al. and Xiaofei et al. [[64], [65]]. Then, upon the addition of L-Cys, the energy transfer process could be probably suppressed. The L-Cys may strongly interact with Au NPs because the presence of thiol group in L-Cys induces strong binding with Au NPs [[25], [63]]. Accordingly, the composite like L-Cys surrounded Au NPs may be formed via Au-S binding formation and thus affected the interaction between rGO and Au NPs to prevent the energy transfer between them. That is why the fluorescence of rGO was enhanced upon the addition of L-Cys. To demonstrate the fluorescence enhancement of rGO is caused by the block of energy transfer, and the fluorescence spectra of rGO (without Au NPs) were recorded with various concentrations of L-Cys (Figure S-5). The fluorescence intensity of rGO does not alter even in the presence of higher concentration of L-Cys (0.1 μM).
Besides, the other thiol-containing biomolecules such as GSH and Hcy showed a less selectivity compared with that of L-Cys. The reason for the lower selectivity of GSH and Hcy might be mainly due to the steric hindrance effect of these thiol-containing biomolecules. For instance, GSH contains more functional groups such as amino, carbonyl and carboxylic groups around −SH group causing strong steric hindrance effect. This effect weakens the interaction of −SH group in GSH with rGO/Au. Consequently, rGO/Au nanohybrid exhibits lower selectivity towards GSH compared to that of Hcy and L-Cys. When compared to Hcy, the L-Cys exhibits lower steric hindrance due to its smaller structural size, which can feasibly allow for the stronger interaction between the rGO/Au and L-Cys.
3.7. In vitro bioimaging and cytotoxicity
The cell viability of rGO/Au was investigated in Colo-205 and MKN-45 using a MTT assay. The live cells were treated with various concentrations of rGO/Au (3, 6, 12, 25, 50 and 100 μg/mL) for 24 h. Then, the cell viability was determined from the observed absorbance value at each concentration. The resultant cell viability in both cells is shown in Fig. 4 a and b. This histogram plots clearly indicate that the rGO/Au sensor was maintained their viability of about 80% in both cells even at the higher concentration of rGO/Au. This results confirm that the rGO/Au sensor exhibits biocompatibility with non-toxicity in cells and it could be used to probe the L-Cys in live cells.
Fig. 4.
Cell viability of (a) Colo-205 and (b) MKN-45 cells incubated with different concentrations of rGO/Au for 24 h. Percentage of cell viability was calculated with respect to 100% control.
Finally, in vitro bioimaging experiments were demonstrated for practical application towards the detection of L-Cys in Colo-205 and MKN-45 live cells by confocal fluorescence microscopy measurement. The live cells were incubated with a certain concentration of rGO/Au nanohybrid in the absence and presence of L-Cys. Then, the fluorescence images of incubated cells were recorded at the excitation of 405 nm, as shown in Fig. 5, Fig. 6 . For rGO/Au nanohybrid in the absence of L-Cys, the confocal fluorescence images of cells showed a very weak fluorescence signal (Figs. 5a–c and 6a–c). Then, the fluorescence signal became stronger with the presence of L-Cys as similarly observed in the steady-state fluorescence measurement. Figs. 5d–f and 6d–f show the confocal fluorescence images of rGO/Au in the presence of L-Cys (200 nM). This result reveals that the L-Cys was successfully detected in the live cells based on the fluorescence recovery of rGO/Au nanohybrid. At the same time, this rGO/Au nanohybrid sensor system showed good biocompatibility in both live cancer cells. Based on these confocal image results, we can conclude that the rGO/Au nanohybrid could be used as a superior sensor system towards the detection of L-Cys within live cells.
Fig. 5.
Fluorescence images of Colo-205 cells with rGO/Au nanohybrid in the (a–c) absence and (d–f) presence of L-Cys (200 nM).
Fig. 6.
Fluorescence images of MKN-45 cells with rGO/Au nanohybrid in the (a–c) absence and (d–f) presence of L-Cys (200 nM).
4. Conclusions
The GEL stabilized Au NPs enriched rGO nanohybrid was prepared by the one-pot hydrothermal method to investigate the detection of L-Cys. The fluorescence sensing of L-Cys was demonstrated based on fluorescence turn-on mechanism. Interestingly, the rGO/Au nanohybrid shows a weak fluorescence signal due to the energy transfer from rGO to Au NPs. The fluorescence intensity of rGO/Au nanohybrid was significantly restored by the addition of L-Cys. The fact was ascribed to the interaction between L-Cys and Au NPs, and subsequently, the energy transfer from rGO to Au NPs is blocked. This rGO/Au nanohybrid sensor exhibits excellent selectivity and sensitivity towards the detection of L-Cys with the detection limit of 0.51 nM. The confocal fluorescence microscopy was used to demonstrate the sensing capability of rGO/Au nanohybrid towards the detection of L-Cys in MKN-45 and Colo-205 cancer live cells.
Acknowledgments
This work was supported by Ministry of Science and Technology of Taiwan, Republic of China, under contract number MOST 106-2113-M-027-003 (Shen-Ming Chen) and NSC 102-2113-M-002-009-MY3(King-Chuen Lin). Authors thank to C.-Y. Chien of Ministry of Science and Technology (National Taiwan University) for her assistance in the TEM experiments. The authors Balamurugan Thirumalraj and Namasivayam Dhenadhayalan are contibuted equally.
Biographies
Dr. Balamurugan Thirumalraj is working as a Post-Doctoral Fellow (Advisor: Prof. Bing-Joe Hwang) in Department of Chemical Engineering, National Taiwan University of Science and Technology, Taiwan. He received PhD degree (2017) in National Taipei University of Technology (Advisor: Prof. Shen-Ming Chen), Taiwan. He received B.Sc. (2008) and M.Sc (2010) degrees from Madurai Kamaraj University, India. He specializes in electrochemical sensors and biosensors using carbon based nanomaterials. Currently, His research focus on the development of Li-ion battery and electrocatalyst for HER and ORR applications.
Dr. Namasivayam Dhenadhayalan is working as a Post-Doctoral Fellow in Institute of Atomic and Molecular Sciences, Academia Sinica, Taipei, Taiwan. He received his Ph.D. (2013) degree in chemistry from University of Madras, India. He specializes in ultrafast spectroscopy, femtosecond dynamics and materials designed for sensors application. His current research interests are two dimensional nanomaterials based chemo- and bio-sensors.
Prof. Shen-Ming Chen is currently working as a Distinguished Professor in Department of Chemical Engineering and Biotechnology, National Taipei University of Technology, Taiwan. His current research interests in sensors, biosensors, electroanalytical chemistry, bioelectrochemistry, fabrication of energy storage devices, synthesis of carbon nanomaterial and hybrid nanomaterials for various electrochemical applications.
Dr. Yan-Jin Liu is working as a post-doctoral fellow in Institute of Biological Chemistry (IBC), Academia Sinica, Taipei, Taiwan. She received her Ph.D. (2015) degree in Program for Pharmacy China Medical University, Taiwan. Her research specially focus on developing anti-cancer drug discovery, mechanism of resistance to apoptosis in cancer and bioactive natural products. Her current research interest is investigation the mechanism of small molecular compounds in cancer metastasis.
Mr. Tse-Wei Chen is studying Master degree in department of Chemical Engineering and Biotechnology at National Taipei University of Technology. He received B.Sc. (2016) degrees in Chemistry from Fu-Jen Catholic University, Taiwan. He specialist in nanomaterial synthesis and application for electrochemical sensors and biosensors. His research interest also includes the studies on nanomaterials for different applications.
Prof. Po-Huang Liang is working as Research Fellow & Acting Associate Director in Institute of Biological Chemistry (IBC), Academia Sinica, Taipei, Taiwan. His research interests are focusing on (i) mechanistic and structural studies of prenyltransferases catalyzing chain elongation, such as undecaprenyl diphosphate synthase, octaprenyl diphosphate synthase, and geranylgeranyl diphosphate synthase; (ii) antivirus and anti-cancer drug discovery targeting 3C protease from Enterovirus 71, 3C-like proteases from SARS-CoV and MERS-CoV, neuraminidase from avian flu virus, and protein–protein interactions; and (iii) multi-functional cellulose/hemicellulose degrading enzymes for biofuel production.
Prof. King-Chuen Lin is working as distinguished Professor in Department of Chemistry at National Taiwan University and a Distinguished Research Fellow of National Science Council, Taiwan. His research interests are photo dissociation and reaction dynamics in gas and condensed phases, atmospheric chemistry, materials designed for sensors and catalysts, and single molecule spectroscopy. He received Academic Award of Ministry of Education, Taiwan, in 2014, and serves as an Associate Editor for J. Chin. Chem. Soc. (Taipei) and a member of Editorial Board for Scientific Reports (Nature publisher).
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.snb.2017.12.028.
Electronic Supplementary Material: Supplementary material (The absorption and fluorescence spectra of rGO/Au nanohybrid; the absorption spectra of rGO/Au with different concentrations of L-Cys; comparison of various chemosensors for L-Cys detection; the fluorescence intensity of rGO/Au with L-Cys in the presence of various biomolecules; the fluorescence spectra of rGO and rGO/Au; the fluorescence spectra of rGO with different concentrations of L-Cys).
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Chen X., Zhou Y., Peng X., Yoon J. Chem. Soc. Rev. 2010;39:2120–2135. doi: 10.1039/b925092a. [DOI] [PubMed] [Google Scholar]
- 2.Kim D.H., Han M.S. Bioorg. Med. Chem. Lett. 2003;13:2543–2546. doi: 10.1016/s0960-894x(03)00468-2. [DOI] [PubMed] [Google Scholar]
- 3.Shahrokhian S. Anal. Chem. 2001;73:5972–5978. doi: 10.1021/ac010541m. [DOI] [PubMed] [Google Scholar]
- 4.Spataru N., Sarada B.V., Papa E., Tryk D.A., Fujishima A. Anal. Chem. 2001;73:514–519. doi: 10.1021/ac000220v. [DOI] [PubMed] [Google Scholar]
- 5.Weerapana E., Wang C., Simon G.M., Richter F., Khare S., Dillon M.B., Bachovchin D.A., Mowen K., Baker D., Cravatt B.F. Nature. 2010;468:790–795. doi: 10.1038/nature09472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gazit V., Ben-Abraham R., Coleman R., Weizman A., Katz Y. Amino Acids. 2004;26:163–168. doi: 10.1007/s00726-003-0045-5. [DOI] [PubMed] [Google Scholar]
- 7.Zhu F., Li X.Y., Li Y.C., Yan M., Liu S.Q. Anal. Chem. 2015;87:357–361. doi: 10.1021/ac504017f. [DOI] [PubMed] [Google Scholar]
- 8.Voet D., Voet J.E. 2nd ed. John Wiley & Sons; NewYork: 1995. Biochemistry. [Google Scholar]
- 9.Kirthika Rani B., Abraham John S. Biosens. Bioelectron. 2016;83:237–242. doi: 10.1016/j.bios.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 10.van Meurs J.B.J., Dhonukshe-Rutten R.A.M., Pluijm S.M.F., van der Klift M., de Jonge J., de Groot A., Witt man J.C.M., van J.P.T.M., Breteler P., Pols H.A.P., Uitterlinden A.G. N. Engl. J. Med. 2004;350:2033–2041. doi: 10.1056/NEJMoa032546. [DOI] [PubMed] [Google Scholar]
- 11.Goodman M.T., McDuffie K., Hernandez B., Wilkens L.R., Selhub J. Cancer. 2000;89:376–382. doi: 10.1002/1097-0142(20000715)89:2<376::aid-cncr24>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 12.Su H., Qiao F., Duan R., Chen L., Ai S. Biosens. Bioelectron. 2013;43:268–273. doi: 10.1016/j.bios.2012.12.032. [DOI] [PubMed] [Google Scholar]
- 13.Lee J.S., Ulmann P.A., Han M.S., Mirkin C.A. Nano Lett. 2008;8:529–533. doi: 10.1021/nl0727563. [DOI] [PubMed] [Google Scholar]
- 14.Sato Y., Iwata T., Tokutomi S., Kandori H. J. Am. Chem. Soc. 2005;127:1088–1089. doi: 10.1021/ja0436897. [DOI] [PubMed] [Google Scholar]
- 15.Küster A., Tea I., Sweeten S., Rozé J., Robins R.J., Darmaun D. Anal. Bioanal. Chem. 2008;390:1403. doi: 10.1007/s00216-007-1799-5. [DOI] [PubMed] [Google Scholar]
- 16.Refsum H., Smith A.D., Ueland P.M., Nexo E., Clarke R., McPartlin J., Johnston C., Engbaek F., Schneede J., McPartlin C., Scott J.M. Clin. Chem. 2004;50:3–32. doi: 10.1373/clinchem.2003.021634. [DOI] [PubMed] [Google Scholar]
- 17.Kwon N.Y., Kim D., Jang G., Lee J.H., So J.H., Kim C.H., Kim T.H., Lee T.S. ACS Appl. Mater. Interfaces. 2012;4:1429–1433. doi: 10.1021/am201677r. [DOI] [PubMed] [Google Scholar]
- 18.Liu Z., Gong Y., Fan Z. J. Lumin. 2016;175:129–134. [Google Scholar]
- 19.Zhang J., Wang J., Liu J., Ning L., Zhu X., Yu B., Liu X., Yao X., Zhang H. Anal. Chem. 2015;87:4856–4863. doi: 10.1021/acs.analchem.5b00377. [DOI] [PubMed] [Google Scholar]
- 20.Ogasawara Y., Mukaib Y., Togawa T., Suzuki T., Tanabe S., Ishii K. J. Chromatogr. B. 2007;845:157–163. doi: 10.1016/j.jchromb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 21.Tcherkas Y., Denisenko A. J. Chromatogr. A. 2001;913:309–313. doi: 10.1016/s0021-9673(00)01201-2. [DOI] [PubMed] [Google Scholar]
- 22.Hosseini H., Ahmar H., Dehghani A., Bagheri A., Tadjarodi A., Fakhari A.R. Biosens. Bioelectron. 2013;42:426–429. doi: 10.1016/j.bios.2012.09.062. [DOI] [PubMed] [Google Scholar]
- 23.Zhang M., Yu M., Li F., Zhu M., Li M., Gao Y., Li L., Liu Z., Zhang J., Zhang D., Yi T., Huang C. J. Am. Chem. Soc. 2007;129:10322–10323. doi: 10.1021/ja073140i. [DOI] [PubMed] [Google Scholar]
- 24.Kim T.K., Lee D.N., Kim H.J. Tetrahedron Lett. 2008;49:4879–4881. [Google Scholar]
- 25.Huang S., Wang Y., Huang C., Hu B., Su W., Xiao Q. Microchim. Acta. 2016;183:1855–1864. [Google Scholar]
- 26.Ge J., Huang Z.M., Xi Q., Yu R.Q., Jiang J.H., Chu X. Chem. Commun. 2014;50:11879–11882. doi: 10.1039/c4cc05309e. [DOI] [PubMed] [Google Scholar]
- 27.Chen X., Zhou H., Zhai N., Liu P., Chen Q., Jin L., Zheng Q. Anal. Lett. 2015;48:1892–1906. [Google Scholar]
- 28.Dai X., Wu Q.H., Wang P.C., Tian J., Xu Y., Wang S.Q., Miao J.Y., Zhao B.X. Biosens. Bioelectron. 2014;59:35–39. doi: 10.1016/j.bios.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 29.Xiao C., Chen J., Liu B., Chu X., Wu L., Yao S. Phys. Chem. Chem. Phys. 2011;13:1568–1574. doi: 10.1039/c0cp00980f. [DOI] [PubMed] [Google Scholar]
- 30.Tam T.V., Hong S.H., Choi W.M. RSC Adv. 2015;5:97598–97603. [Google Scholar]
- 31.Gu C.J., Kong F.Y., Chen Z.D., Fan D.H., Fang H.L., Wang W. Biosens. Bioelectron. 2016;78:300–307. doi: 10.1016/j.bios.2015.11.035. [DOI] [PubMed] [Google Scholar]
- 32.Tao A., Sinsermsuksakul P., Yang P.D. Nat. Nanotechnol. 2007;2:435–440. doi: 10.1038/nnano.2007.189. [DOI] [PubMed] [Google Scholar]
- 33.Kong B., Zhu A., Luo Y., Tian Y., Yu Y., Shi G. Angew. Chem. 2011;123:1877–1880. doi: 10.1002/anie.201007071. [DOI] [PubMed] [Google Scholar]
- 34.Schrinner M. Science. 2009;323:617–620. doi: 10.1126/science.1166703. [DOI] [PubMed] [Google Scholar]
- 35.Li N., Xu J.Z., Yao H., Zhu J.J., Chen H.Y.J. J. Phys. Chem. B. 2006;110:11561–11565. doi: 10.1021/jp060653n. [DOI] [PubMed] [Google Scholar]
- 36.Yao H., Li N., Xu J.Z., Zhu J.J. Talanta. 2007;71:550–554. doi: 10.1016/j.talanta.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 37.Piao Y., Chen B. RSC Adv. 2016;6:6171–6181. [Google Scholar]
- 38.Zhang J.J., Gu M.M., Zheng T.T., Zhu J.J. Anal. Chem. 2009;81:6641–6648. doi: 10.1021/ac900628y. [DOI] [PubMed] [Google Scholar]
- 39.Thirumalraj B., Rajkumar C., Chen S.M., Barathi P. J. Mater. Chem. B. 2016;4:6335–6343. doi: 10.1039/c6tb01576j. [DOI] [PubMed] [Google Scholar]
- 40.Rajkumar C., Thirumalraj B., Chen S.M., Chen H.A. J. Colloid Interface Sci. 2017;487:149–155. doi: 10.1016/j.jcis.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 41.Li W.-W., Kong F.-Y., Wang J.-Y., Chen Z.-D., Fang H.-L., Wang W. Electrochim. Acta. 2015;157:183–190. [Google Scholar]
- 42.Tuinstra F., Koenig J.L. J. Chem. Phys. 1970;53:1126–1130. [Google Scholar]
- 43.Zhang J., Yang H., Shen G., Cheng P., Zhang J., Guo S. Chem. Commun. 2010;46:1112–1114. doi: 10.1039/b917705a. [DOI] [PubMed] [Google Scholar]
- 44.Das A.K., Srivastav M., Layek R.K., Md. Uddin E., Jung D., Kim N.H., Lee J.H. J. Mater. Chem. A. 2014;2:1332–1340. [Google Scholar]
- 45.Kellici S., Acord J., Ball J., Reehal H.S., Morgan D., Saha B. RSC Adv. 2014;4:14858–14861. [Google Scholar]
- 46.Rajaura R.S., Srivastava S., Sharma V., Sharma P.K., Lal C., Singh M., Palsania H.S., Vijay Y.K. Int. J. Hydrogen Energy. 2016;41:9454–9461. [Google Scholar]
- 47.Thirumalraj B., Rajkumar C., Chen S.-M., Palanisamy S. Sci. Rep. 2017;7:41213. doi: 10.1038/srep41213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu Y., Loh W.Q., Ananthanarayanan A., Yang C., Chen P., Xu C. RSC Adv. 2014;4:35673–35677. [Google Scholar]
- 49.Cushing S.K., Li M., Huang F., Wu N. ACS Nano. 2014;8:1002–1013. doi: 10.1021/nn405843d. [DOI] [PubMed] [Google Scholar]
- 50.Shang J., Ma L., Li J., Ai W., Yu T., Gurzadyan G.G. Sci. Rep. 2012;2:792. doi: 10.1038/srep00792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zong J., Yang X., Trinchi A., Hardin S., Cole I., Zhu Y., Li C., Muster T., Wei G. Biosens. Bioelectron. 2014;51:330–335. doi: 10.1016/j.bios.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 52.Zhang Y., Cui P., Zhang F., Feng X., Wang Y., Yang Y., Liu X. Talanta. 2016;152:288–300. doi: 10.1016/j.talanta.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 53.Chen X., Liu H., Wang C., Hu H., Wang Y., Zhou X., Hu J. Talanta. 2015;138:144–148. doi: 10.1016/j.talanta.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 54.Yang C., Wang X., Shen L., Deng W., Liu H., Ge S., Yan M., Song X. Biosens. Bioelectron. 2016;80:17–23. doi: 10.1016/j.bios.2016.01.044. [DOI] [PubMed] [Google Scholar]
- 55.Liu X., Yang D., Chen W., Yang L., Qi F., Song X. Sens. Actuators B. 2016;234:27–33. [Google Scholar]
- 56.Han C., Yang H., Chen M., Su Q., Feng W., Li F. ACS Appl. Mater. Interfaces. 2015;7:27968–27975. doi: 10.1021/acsami.5b10607. [DOI] [PubMed] [Google Scholar]
- 57.Liu T., Huo F., Li J., Chao J., Zhang Y., Yin C. Sens. Actuators B. 2016;232:619–624. [Google Scholar]
- 58.Gong D., Tian Y., Yang C., Iqbal A., Wang Z., Liu W., Qin W., Zhu X., Guo H. Biosens. Bioelectron. 2016;85:178–183. doi: 10.1016/j.bios.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 59.Liu T., Huo F., Yin C., Li J., Niu L. R.S.C Adv. 2015;5:28713–28716. [Google Scholar]
- 60.Yan L., Kong Z., Shen W., Du W., Zhou Y., Qi Z. Anal. Biochem. 2016;500:1–5. doi: 10.1016/j.ab.2016.01.027. [DOI] [PubMed] [Google Scholar]
- 61.Yan F., Shi D., Zheng T., Yun K., Zhou X., Chen L. Sens. Actuators B. 2016;224:926–935. [Google Scholar]
- 62.Lv H.-M., Yuan D.-H., Liu W., Chen Y., Au C.-T., Yin S.-F. Sens. Actuators B. 2016;233:173–179. [Google Scholar]
- 63.Xu X., Qiao J., Li N., Qi L., Zhang S. Anal. Chim. Acta. 2015;879:97–103. doi: 10.1016/j.aca.2015.03.036. [DOI] [PubMed] [Google Scholar]
- 64.Nguyen P.-D., Cong V.T., Baek C., Min J. Biosens. Bioelectron. 2017;89:666–672. doi: 10.1016/j.bios.2015.12.031. [DOI] [PubMed] [Google Scholar]
- 65.Xiaofei W., Ruiyi L., Zaijun L., Junkang L., Guangli W., Zhiguo G. R.S.C Adv. 2014;4:24978–24985. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.