Summary
The evaluation of the efficacy of an immunochemotherapy protocol to treat symptomatic dogs naturally infected with Leishmania chagasi was studied. This clinical trial had the purpose to test the combination of N-methyl meglumine antimoniate (Glucantime®) and the second generation recombinant vaccine Leish-110f® plus the adjuvant MPL-SE® to treat the canine leishmaniasis (CanL). Thirty symptomatic naturally infected mongrel dogs were divided into five groups. Animals received standard treatment with Glucantime® or treatment with Glucantime®/Leish-110f® + MPL-SE® as immunochemotherapy protocol. Additional groups received Leish-110f® + MPL-SE® only, MPL-SE® only, or placebo. Evaluation of haematological, biochemical (renal and hepatic function) and plasmatic proteins, immunological (humoral and cellular immune response) and the parasitological test revealed improvement of the clinical parameters and parasitological cure in dogs in both chemotherapy alone and immunochemotherapy cohorts. However, the immunotherapy and immunochemotherapy cohorts had reduced number of deaths, higher survival probability, and specific cellular reactivity to leishmanial antigens, in comparison with chemotherapy cohort only and control groups (adjuvant alone and placebo).
These results support the notion of using well-characterized recombinant vaccine as an adjunct to improve the current chemotherapy of CanL.
Keywords: Leishmania chagasi, Leishmaniasis, Canine visceral leishmaniasis, Immunochemotherapy
Introduction
Canine leishmaniasis (CanL) is a disease caused by Leishmania (Leishmania) chagasi, in the New World. This protozoan is an intracellular parasite found in the mononuclear system, mainly in macrophages of vertebrate host. Classical CanL appears as chronic wasting diseases with generalised or localised lymphadenopathy, muscular atrophy, weakness, weight loss, cachexia, anorexia, diarrhoea, vomiting, melena, abdominal pain, lameness, polyuria, polydipsy, oligury, haematury, cough/nose discharge, epistaxis, rhinitis, onychogryphosis, hepatomegaly and splenomegaly. In addition, cutaneous lesions such as alopecia, desquamation, dry seborrhoea, hyperpigmentation, and erythema [1], [2], [3], [4], [5], [6], [7] occur in a number of cases.
Control of the CanL in many countries is basically performed through three procedures: (a) control of the vector by using residual action insecticide; (b) culling and elimination of the seropositive dogs detected in epidemiological surveys in endemic areas, and (c) treatment of the human and canine cases [8]. Such measures can control [10] or drastically reduce transmission [11] when vigorously employed throughout the years [9]. Among the strategies for controlling zoonotic visceral leishmaniasis, the culling of seropositive dogs in endemic areas has proved ineffective because it is expensive, the impact on human disease is limited and it is not socially accepted [12]. Several studies showed that treatment of canine leishmaniasis with pentavalent antimonials or pentamidine might result in clinical recovery with remission of the symptoms and even parasitological cure to the animals, although relapse normally occurs in the following 5–12 months [1].
The use of immunotherapy for the treatment of canine leishmaniasis has represented a new approach to control the infection [13], [14]. When crude parasite extracts were used in the immunotherapy associated with conventional chemotherapy, clinical improvement [15] and partial parasitological cure were observed [16], [17]. An immunochemotherapy protocol [18] using a purified Leishmania infantum antigen LiF2 (L. infantum-derived Fraction 2, 94–67 kDa) and N-methyl meglumine antimoniate (Glucantime®) in dogs resulted in 100% parasitological cure up to 6 months after treatment, in contrast to the parasitological healing rates for chemotherapy or immunotherapy alone (37.5 and 25%, respectively). These results were corroborated by previous study using murine models [19].
Three leishmanial recombinant antigens (TSA, LmSI1 and LeIF) have been extensively tested as a subunit based vaccine and shown to induce good protection in both the murine and non-human primate models of cutaneous leishmaniasis [20]. Moreover the LeIF component was shown to have therapeutic activity in the Balb/c model of cutaneous leishmaniasis [20], [21], [22], [23], [24]. More importantly, these antigens were recently and successfully used in an immunochemotherapeutic protocol to treat human mucosal leishmaniasis [25]. The three antigens are present in both amastigotes and promastigotes forms of the parasite and are highly conserved among Leishmania sp., a requisite for ensuring cross-species protection [23], [26], [27]. A polyprotein constituted of these three antigens have been engineered to a final vaccine candidate, namely Leish-110f®, and when formulated with the adjuvant monophosphoryl lipid A (MPL) plus squalene (MPL-SE®) elicited excellent protective immunity against L. major infection in the murine model [28]. In addition, a clinical investigation in dogs immunized with these recombinant antigens (TSA, LmSTI1 and LeIF) formulated with adjuvant MPL-SE® induced almost exclusively a Th1 response (IgG2/IgG1 ≥ 40), suggesting its use in field trials against CanL [29]. However, further studies demonstrated the failure of this multi-subunit vaccine to protect dogs against CanL in experimental and natural conditions [30], [31].
Here we describe the evaluation of the immunochemotherapeutic properties of Leish-110f® plus MPL-SE® associated with N-methyl meglumine antimoniate (Glucantime®) to treat naturally infected and symptomatic dogs with CanL.
Materials and methods
Animals
Thirty mongrel dogs (males and females) aging from 3 to 5 years old, naturally infected with L. chagasi were included in the clinical trial. Animals were selected after seroepidemiological survey for CanL conducted in the city of Montes Claros, Minas Gerais State, Brazil. All dogs included in this trial had typical clinical signs for symptomatic visceral leishmaniasis [2] as they presented lymphadenopathy, slight decrease of weight and opaque eye, alopecia, eczema, and skin ulcers and never received any treatment for CanL. Demonstration of parasites was performed before the beginning of the clinical trial using direct microscopic examination of Giemsa stain smears of bone marrow aspirates and ear skin biopsies. Bone marrow aspirates were also used to isolate the parasites in NNN-LIT culture medium. Serological tests were done using immunofluorescence test (IFAT) and ELISA using cut-off dilutions of 1:40 and 1:80, respectively. Crude Leishmania antigen and rk39 were used in the ELISA tests to follow the infection [32].
Before enrolment, all animals were pre-treated with large spectrum anthelmintic drugs (Endal plus®, Schering-Plough Coopers, Brazil) and vaccinated against infections by Parvovirus, Adenovirus type I, Distemper virus, Parainfluenza virus, Corona virus and Leptospirosis (C6/Cv Recombitek vaccine®, Merial, Brazil) and rabies virus (Ravisin-i®, Merial, Brazil). Dogs were fed with commercial balanced animal food (Cherokee®, PET, Brazil) and drinking water was provided ad libitum.
The clinical trial was conducted in the kennel for the leishmaniasis experimentation of the Federal University of Minas Gerais State after certification by Ethic Committee of Animal research of the Federal University of Minas Gerais (Protocol #062/2003). The trial was performed in agreement with the Ethical Principles in Animal Experimentation, following the guidelines for animal experimentation of the National Institutes of Health (USA) in order to keep animal suffering to a minimum.
Drug, vaccine and protocol of treatments
Conventional drug N-methyl meglumine antimoniate (Glucantime®, Aventis Pharma, Brazil) was used as standard treatment. The vaccine used in the study was a formulation of lyophilized Leish-110f® (Corixa Corporation, USA) and the adjuvant monophosphoryl lipid A plus squalene (MPL-SE®, Corixa Corporation, USA), which were maintained at 4 °C until use.
Thirty dogs were randomly allocated into five groups with six animals each and were treated using the following protocols—(a) group 1: 100 mg/kg/day of N-methyl meglumine antimoniate (Glucantime®); (b) group 2: Glucantime® in the same concentration as group 1 plus 20 μg of rLeish-110f® plus 25 μg MPL-SE®; (c) group 3: Leish-110f® plus MPL-SE® in the same concentration; group 4: 25 μg MPL-SE® only; group 5: placebo constituted of 0.9% saline solution.
Animals from groups 1 and 2 were treated with Glucantime® in two cycles of 10 days with intervals of 10 days between each cycle with 100 mg/kg/day of intramuscularly injections. Immunochemotherapy was performed by administration of three subcutaneous doses of formulated vaccine at 21 days of intervals; the first dose of the vaccine was administered as the animals received the first injection of drug treatment. Treatment of animals that received Leish-110f®/MPL-SE® only, adjuvant only (MPL-SE®) or placebo consisted of three subcutaneous injections at the same intervals.
Follow-up
The dogs were followed up until 180 days after treatment. To assess their clinical response to treatment, weekly clinical examination, haematological and biochemical assays and immunological evaluation were performed on day 0 (before treatment) and at days 30, 60, 90, 120, 150 and 180. Parasitological examination and cellular immune response evaluation were performed at day 0 (before treatment) and at days 90 and 180.
Haematological and biochemical evaluations
Whole Blood Cell Counting (WBC) was performed using the T890 haematological Microcell counter (Coulter®, USA). Differential leukocyte count was made using a blood smear stained with May Grünwald-Giemsa®. References values given by Jain [33] were applied to interpret the WBC parameters.
The amount of total proteins, albumin, alpha- (α), beta- (β) and gamma- (γ) globulin fractions and the ratio albumin/globulin were evaluated in dog's sera by electrophoresis (Celm®, São Paulo, Brazil). References values given by Amusategui et al. [7] were used to calculate protein concentrations. Alanine Amino Transferase (ALT) enzyme activity to evaluate liver function was measured using a commercially available kit (Roche®, Brazil). Levels of serum creatinine and urea were evaluated using commercially available kits from Biotécnica®, Brazil. References values to interpret the parameters of the liver and kidney functions were from Kaneko et al. [34] were used.
Immune responses to Leishmania antigens
Specific anti-Leishmania antibodies were evaluated monthly in all dogs by immunofluorescence test (IFAT) and ELISA. IFAT was performed using a fluorescein conjugated anti-canine IgG antiserum (Sigma, USA) and a cut-off dilution of 1:40. ELISA using a cut-off of 1:80 with crude antigens and recombinant rk39 was performed according to Rosário et al. [32].
Cellular immune response was evaluated by lymphocyte proliferation assay. Briefly, peripheral blood mononuclear cells (PBMC) were obtained from heparinized blood samples collected from jugular vein of the dogs and then separated using ficoll-hypaque gradient (Histopaque®, Sigma). 2.5 × 105 cells per well were cultured in triplicate in 96 well flat-bottom microplates after stimulation with 10 μg/ml of the recombinant vaccine Leish-110f®, 10 μg/ml of soluble L. chagasi antigen or 2 μg/ml of Concanavalin A (ConA). Additional cultures were performed without any stimulation. Cells were cultured in a final volume of 200 μl of RPMI 1640 (Sigma®, USA), supplemented with 100 UI ml of penicillin, 100 μg/ml of streptomycin, 2 mM l-glutamine, 50 μM 2-mercaptoetanol and 10% heat-inactivated FCS (Biological Industries, Kibbutz, Beit Haemek, Israel). PBMCs were incubated for 5 days at 37 °C, 5% CO2 and pulsed during the last 18 h of culture with 1 μCi per well of [3H] thymidine (Amersham, Bucks, United Kingdom). Cells were harvested onto glass fibre filter (model 943-AH Whatman, USA), and the 3H-thymidine incorporation was counted using a liquid scintillation beta counter (Titertek Cell Harvest, Flow Laboratories, USA). Proliferative responses were expressed as stimulation index (SI), which represents the ratio between the mean of the cpm obtained for stimulated cultures and the cpm of unstimulated cultures [35], [36].
Parasitological examination
Bone marrow puncture was performed in the intercondineal fossa of the tibia. Previously to the procedure dogs were anaesthetised with Acepromazine (Acepran®, 1 mg/kg) and sodium thiopental (Thionembutal®, 10 mg/kg). Bone marrow was aspirated and cultured in duplicate tubes containing NNN-LIT medium. Tubes were then incubated at 23 °C and examined every 10 days for 30 days and then discarded if negative. Ear skin biopsies were collected from a small pinch of the ear lobe obtained with a scalpel. Smears from both, bone marrow aspirates and skin biopsies were Giemsa stained and submitted to microscopic examination.
Xenodiagnosis
To assess whether the treatment with Leish-110f® + MPL-SE®/Glucantime® (immunochemotherapeutic protocol) might block the transmission to the vector, the xenodiagnosis was performed specifically on this group. At the end of the study each dog was sedated with 1 mg/kg of Acepromazine (Acepran®, UNIVET SA, São Paulo, Brazil). Twenty females F1 from laboratory reared Lutzomyia longipalpis were placed in round plastic boxes (10 cm diameter × 5 cm height) with an open side covered by a fine-mesh nylon screen and placed over the skin of the internal ear of each dog. The roll set was covered with a piece of black fabric to achieve ideal condition to stimulate the feeding. After 30 min, the sand flies were transferred to holding cages that were kept at temperature of 25–28 °C and 90% of relative air humidity. Fifth day after blood meal, genomic DNA for PCR was extracted from the females that were alive according to Michalsky et al. [37].
Survival rate and statistical analysis
Survival rate and the death risk of the animals in each group were analyzed by the Kaplan–Maier test and the Cox model test, respectively. Statistical analysis was carried out using the t-student test [38], the non-parametric test of Kruskall–Wallis [39], the Tukey test [40], the Kaplan–Maier test [41], [42], and the Cox model [43]. All analyses were performed using the software SPSS 11.0 for PC.
Results
Overall clinical evolution
Animals that received either Glucantime® alone or Glucantime® plus Leish-110f®/MPL-SE® had improvement in clinical response. However, in the animals that received both Glucantime® plus Leish-110f®/MPL-SE® showed better clinical response to the treatment. In contrast, all animals from all other groups had worsened their symptoms during and at the end of the study. Seven dogs died during the treatment: two from group 1 (Glucantime®), one from group 2 (Glucantime® + Leish-110f®/MPL-SE®), one from group 4 (MPL-SE® only) and three dogs from group 5 (placebo). Dead animals were considered as oligosymptomatic (Glucantime® alone and Glucantime® + Leish-110f®/MPL-SE®) or symptomatic (Leish-110f®/MPL-SE®, MPL-SE® and Placebo) at the end of the study. Also, at the end of the study, the remaining dogs were reclassified as asymptomatic, olygosymptomatic or symptomatic according to Mancianti et al. [2], and the results are shown on Table 1 .
Table 1.
Clinical condition of the dogs under different therapeutic protocols at the end of the studya
| Groups | Asymptomatic | Oligosymptomatic | Symptomatic | Total of dogs alive/deadb |
|---|---|---|---|---|
| Glucantime® | 2(50%) | 2(50%) | NO | 4/2 |
| Glucantime® + Leish110f®/MPL-SE® | 3(60%) | 2(40%) | NO | 5/1 |
| Leish110f®/MPL-SE® | NO | NO | 6(100%) | 6/0 |
| MPL-SE® | NO | NO | 5(100%) | 5/1 |
| Placebo (saline) | NO | NO | 3(100%) | 3/3 |
Results were expressed in number of observed animals (percentage); NO: non-observed.
Dead animals were classified as oligosymptomatic (Glucantime® alone and Glucantime® + Leish-110f®/MPL-SE®) or symptomatic (Leish-110f®/MPL-SE®, MPL-SE® and Placebo) at the end of the study.
Haematological and biochemical parameters
Haematological analysis showed no significant differences on values of neutrophils and eosinophils among all groups during the study. A slight decrease in the number of monocytes was observed in animals treated with Glucantime® (chemo and immunochemotherapy groups) suggesting an adverse effect of the drug. However, this reduction was not significant when compared with proper reference values [33]. Increase of lymphocyte counts was also seen in all groups at the end of the study but it was not significant when compared with baseline values. From day 60 of treatment, a statistically significant increase (P < 0.05) on values of red blood cells (RBCs) was demonstrated for animals that received either chemo or immunochemotherapy. Conversely, animals from remaining groups presented a progressive anaemia during the study.
An increasing of albumin values compared to reference values (2–2.7 g/dl) was demonstrated in animals that were treated with chemo and immunochemotherapy during all times of evaluation. In contrast, significant reduction of this parameter was observed in the remaining groups. The values for α globulin (≤1.3 g/dl) and β globulin (≤2.2 g/dl) remained within normal reference values during the study for all groups. At the end of the study the levels of γ globulin reached normal values (<1.5 g/dl) in the chemo and immunochemotherapy groups while animals the others groups had hypergammaglobulinemia. The indicators of renal function such as the levels of creatinine, urea retaining, as well as the liver function evaluated by enzyme alanine amino transferase were not statistically different among all groups at all times of evaluations. The cured animals number 11 and 68 returned the values for renal and liver function normal reference parameters.
Parasitological examination
As a criterion for inclusion in the study, all animals had a positive parasitological test either by direct microscopy examination of bone marrow smears or skin biopsies stained with Giemsa, or by bone marrow cultures in NNN-LIT medium. At the end of the studies the conventional parasitological tests (smear and culture) were negative in all animals of groups 1 and 2. In contrast, all animals from groups 3, 4, and 5 remained positive. To ascertain the negative parasite burden in groups 1 and 2, xenodiagnosis detected by PCR was also performed. The results indicated that all animals of group 1 had positive PCR. In contrast two dogs of group 2 were negatives. These dogs were considered cured (Fig. 1 and Table 2 ).
Figure 1.
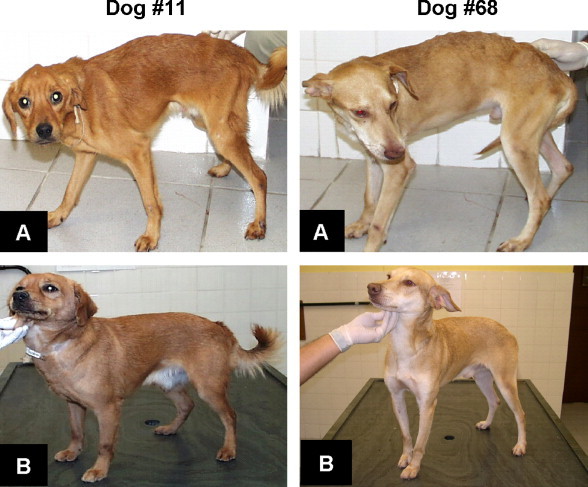
Dogs number 11 and 68 cured by immunochemotherapy using Glucantime® plus Leish-110f® + MPL-SE® before (A) day 0 and after (B) day 180 of treatment.
Table 2.
Parasitological findings in the smear of the ear skin biopsies, bone marrow smears, bone marrow cultures, and entomological PCR from infected sand fly before treatment at days 90 and 180 after therapy
| Groups | Dog number | Before treatment | Day 90 after treatment | Day 180 after treatment |
|---|---|---|---|---|
| Glucantime® | 05 | BMC | Negative | Negative |
| 108 | ES | ES | ES | |
| 110 | ES | Death | Death | |
| 112 | BMC | BMS, BMC | Negative | |
| 119 | ES | Death | Death | |
| 138 | ES | ES | Negative | |
| Glucantime® + Leish-110f®/MPL-SE® | 11 | BMC | ES, BMC, BMS | Negative |
| 26 | BMC | ES, BMC, BMS | BMC, PCR | |
| 50 | BMC | ES, BMC | ES, PCR | |
| 68 | ES, BMS | ES, BMS | Negative | |
| 77 | BMS | ES, BMS | BMS, PCR | |
| 135 | BMS, BMC | ES | Death | |
| Leish-110f®/MPL-SE® | 04 | BMS | ES, BMC, BMS | BMS, BMC |
| 83 | BMC | ES, BMS | ES, BMC, BMS | |
| 87 | ES, BMC | ES, BMC, BMS | ES, BMC | |
| 98 | ES, BMC | ES, BMC, BMS | BMC, BMS | |
| 128 | BMC | ES, BMS | BMS | |
| 146 | BMC, ES | ES, BMC, BMS | BMC, ES | |
| MPL-SE® | 106 | ES, BMC | ES | BMS, ES |
| 123 | BMS, BMC | BMS | BMS | |
| 129 | BMC | ES | ES | |
| 136 | BMS | ES, BMC, BMS | ES, BMC, BMS | |
| 141 | ES | Death | Death | |
| 143 | ES | ES, BMC | BMC | |
| Placebo | 36 | BMC | BMC | Death |
| 88 | BMC | ES, BMC, BMS | ES, BMC, BMS | |
| 91 | BMC | Death | Death | |
| 96 | BMC, ES | BMC | Death | |
| 118 | BMC | ES, BMS | ES, BMC, BMS | |
| 137 | BMC | BMS | BMC | |
ES: ear skin smear; BMC: bone marrow culture; BMS: bone marrow smears; PCR: sand fly positives; Death: death of dogs before the parasitological examination; Negative: negative dogs after complete parasitological evaluation.
Survival rate and death risk evaluation
Table 1 illustrates the rates of all groups of dogs the end of the clinical observations. As expected, the survival rate was the lowest in the control saline group (0%). In both groups treated with Glucantime® (groups 1 and 2) the survival rate was 66.6 and 83.3%, respectively. Survival in the adjuvant group (MPL-SE® only) was 83.3%. Of note was the observation that no animal died (100% survival) in the group treated with the immunotherapeutic protocol only (Leish-110f® plus MPL-SE®). The survival probability over time of therapeutic intervention was estimated using Kaplan–Maier's analysis. By day 30 this probability was 100% for all groups (there was not death at this period of time). At the end of the study, the survival probability decreased to 66.67% for animals treated with Glucantime® alone; 75% for animals treated with Glucantime® plus Leish-110f protocol and 41.67% for animals treated with placebo. Animals treated with adjuvant alone had 83.33% survival probability at the end of study. Interestingly the survival probability for animals of the Leish-110f® plus MPL-SE® (immunotherapy only) was 100% (Fig. 2 ).
Figure 2.
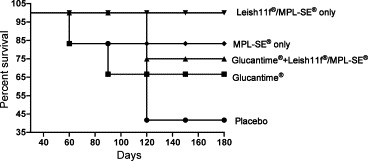
Survival probability for dogs under different protocols of treatment during and at the end of the study. The survival rate was estimated by statistical analysis using the Kaplan–Maier test.
Immune responses to Leishmania antigens
Antibody response
Animals treated with Glucantime® alone or Glucantime® + Leish-110f®/MPL-SE® presented a significant reduction (P < 0.05) of specific antibody titters at the end of the study, as determined by IFAT, ELISA with crude antigen and ELISA with rK39 antigen (Table 3 ). In contrast dogs treated with Leish-110f® vaccine alone, MPL-SE® alone, or placebo showed increase of specific antibody titters. However, despite the reduction of antibody titers in the former two groups, the antibody titers remained above the specified positive cut-off. Only two animals from group 2 showed negative results for all serological tests at days 150 and 180.
Table 3.
Humoral immune responses determined by IFAT, ELISA with crude antigen and ELISA rK39
| Glucantime® |
Glucantime® + Leish-110f®/MPL-SE® |
Leish-110f®/MPL-SE® |
MPL-SE® |
Placebo |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n = 6 | n = 4 | n = 6 | n = 5 | n = 6 | n = 6 | n = 6 | n = 5 | n = 6 | n = 3 day | |
| Day 0 | Day 180 | Day 0 | Day 180 | Day 0 | Day 180 | Day 0 | Day 180 | Day 0 | Day 180 | |
| IFAT (antibody titer) | 3733.3 ± 3538.4 | 1520 ± 1208 | 7466.7 ± 7076.8 | 480.0 ± 226.3 | 17493 ± 31579 | 17520 ± 5120 | 5573 ± 4058 | 6672 ± 7997 | 5720 ± 8186 | 1706 ± 739 |
| ELISA crude antigen (antibody titer) | 1173.3 ± 1083.6 | 300.0 ± 247.6 | 1013.3 ± 820.0 | 256.0 ± 87.6 | 4533 ± 4765 | 2468 ± 1568 | 1400 ± 1894 | 3200 ± 1280 | 2226 ± 3962 | 426.6 ± 184.7 |
| ELISA rK39 (antibody titer) | 3733.0 ± 3870.0 | 1800.0 ± 2260.0 | 16426 ± 19330 | 5180.0 ± 5842.0 | 25013 ± 31881 | 14080 ± 14971 | 4293 ± 3459 | 28688 ± 33478 | 4186 ± 8035 | 14506 ± 22909 |
Values are m ± σ. Values are expressed as mean of antibody titers by groups at day 0 and 180 after therapy. σ: standard deviation, n: numbers of dogs evaluated.
T cell response
Proliferation of PBMC stimulated with leishmanial antigens (crude L. chagasi extract and Leish-110f® antigen) and with the mitogen Concanavalin A (Con A) was used to investigate the T cell responses of all dogs enrolled in the study. The proliferation assay was evaluated at day 0, 90 and 180 after enrolment. At the end of study (day 180) the PBMC from animals of the two groups that were treated with Leish-110f® vaccine responded better to in vitro stimulation to this antigen than the other three groups (Fig. 3 ). As expected, animals that received the vaccine presented higher cell proliferative response to Leish-110f® than that observed in the groups treated with adjuvant or placebo only (P < 0.05). Similar tendency was also observed for the response to L. chagasi crude antigen but no statistically significance was achieved. Response to the mitogenic Con A was high in all but the placebo group, which is indication of the known immunosuppression of T cell response in untreated visceral leishmaniasis.
Figure 3.
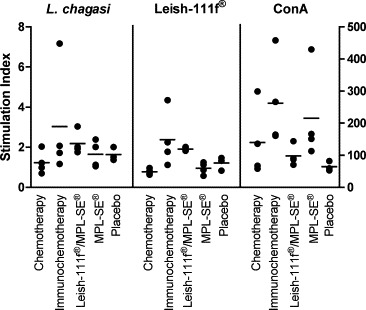
Lymphoproliferative responses of dogs under different protocols of treatment at the end of study (day 180). Specific Leishmania-cell proliferative response was evaluated by using crude L. chagasi antigen extract or Leish-110f® (left Y-axis). Mitogenic response was assessed by stimulation with Concanavalin A (ConA, on right Y-axis). Each dot represents individual values for each animal. Bars represent the median of the values for the each group. Statistical significant differences were observed between animals that received Leish-110f® and all remaining groups (P < 0.05), and also between animals treated with vaccine in comparison with control (adjuvant and placebo) groups (P < 0.05).
Discussion
The use of chemotherapy based on pentavalent antimonial (Glucantime®) was introduced over 50 years ago for visceral leishmaniasis and still constitute the first drug of choice to treat the diseases caused by Leishmania. However, recent development of drug resistance associated with variation in the sensitivity of Leishmania species to this drug strongly reveals the need to develop (or re-develop) alternative treatment, which might replace or complement existing or newer therapeutic drugs [44], [45], [46]. Indeed, drug combinations for treating VL have been tested with some promising results to treat leishmaniasis caused by drug resistant [45]. Similarly, combination of drugs and vaccines or even vaccine alone has been successfully used to treat American cutaneous leishmaniasis over the past years [47], with low number of recurrence cases. Combined anti-Leishmania treatment using vaccine and drug would enhance drug efficacy and stimulate host immune responses, resulting in a therapeutic harness of cellular immune protection. The therapeutic use of recombinant antigens with standard drugs for treatment of leishmaniasis would be an advantage due their well-characterized nature required to meet both scientific and regulatory standards. Indeed, the leishmanial recombinant antigens TSA, LmSTI1 and LeIF in combination with the cytokine Granulocyte and Monocyte Colony Stimulator Factor (GM-CSF) used as adjuvant were recently and successfully used to treat human mucosal leishmaniasis [27].
The most common drugs used for the treatment of canine leishmaniasis are the pentavalent antimonies, which destroy the parasites through the inhibition of two leishmanial essential enzymes: the phosphofructokinase and dehydrogenase pyruvate, both needed for glycolytic and fatty acid oxidation simultaneously [48], [49]. In dogs, chemotherapy using pentavalent antimonies has been mostly unsuccessful and in many occasions has reportedly caused exacerbation of disease [50], [51]. Although major clinical signs of disease disappear after treatment and treated animals may present a good general health status, this might not indicate the complete absence of parasites in spleen, bone marrow or skin [52]. Indeed, many treated animals remain infective to sand flies several months post-treatment [50], [52].
In our clinical trial, L. chagasi naturally infected symptomatic dogs were submitted to the immunochemotherapy with a standard pentavalent antimony drug (Glucantime®) associated with a recombinant vaccine (Leish-110f®) formulated with an adjuvant (MPL-SE®). Treatment with Glucantime® in identical dose to that used by Mancianti et al. [2], associated with Leish-110f® + MPL-SE® resulted in the clinical improvement of the infected animals, associated with normalization of biochemical and haematological parameters and reduction of anti-Leishmania antibodies. These results resemble the initial immunochemotherapy of CanL using Glucantime® plus leishmanial crude extract as antigen [16], [17]. At the end of our studies at day 180, two dogs from group 2 were clinically cured by the conversion of parasitological and immunological parameters and also according to the clinical classification of Mancianti et al. [2]. Moreover, the xenodiagnosis technique performed to identification of infected phlebotomine sand flies and potential transmission of the parasite [37], [53] from animals treated with Glucantime® and Leish-110f® + MPL-SE® indicated that both clinically cured dogs were also PCR negatives. On the other hand, the transmission blocking effect conferred by the immunochemotherapeutic protocol still remains uncertain since animals treated under the same protocol also presented a positive xenodiagnosis. Moreover, the lack of data from animals treated with remaining protocols makes difficult to assess whether the proposed treatment is able to effectively avoid or reduce transmission to invertebrate host.
Visceral leishmaniasis treatment is typically considered successful when clinical signs have disappeared, results of haematological and serum biochemical's analyses are within the reference ranges [54]. While the clinical recovery observed in those groups (Glucantime® + Leish-110f®/MPL-SE and Glucantime® alone) could be related to the use of the drug itself, the immunochemotherapy cohort had a reduced number of deaths, higher survival probability and higher specific cellular reactivity to leishmanial antigens, in comparison with the treatment with Glucantime® alone. Resistance to leishmaniasis is well known to be mediated by cell-mediated protective immune response to specific Leishmania antigens [55]. Although each clinical manifestation of Leishmania infection has a different immunological picture, patients with acute visceral leishmaniasis lack cell reactivity particularly when specific antibody titers are high. This T cell deficiency is manifested in vitro by failure of the T lymphocytes to proliferate after stimulation to parasite antigens [56]. Usually, these patients become responsive after resolution of their symptoms [57]. Therefore, T cell reactivity shown either in vitro (e.g. lymphocyte proliferative responses to Leishmania antigens) or in vivo (e.g. delayed hypersensitivity to leishmanial antigens) is an important surrogate of protection against leishmaniasis [52], [58]. Consequently, a treatment that provides clinical recovery and restore, at least partially, the cell reactivity against leishmanial components is highly desirable. Hence, CanL vaccines that promote at least partial protection and trigger a Th1 type of immune response, which eventually might lead to protection against disease, should be also considered in future protocols of immunochemotherapy of infected dogs. In this way, it was recently reported that a heterologous prime-boost vaccination with a non-replicative vaccinia recombinant vector expressing LACK confers protection against canine visceral leishmaniasis, which was correlated with absence of visceral leishmaniasis symptoms, lower parasite-specific antibodies, higher degree of T cell activation in parasitized organs and higher synthesis of Th1 cytokines [59]. In addition, the long lasting protective fucose mannose ligand (FML)-vaccine (Leishmune) [60] was demonstrated to be also effective in the immunotherapy against visceral leishmaniasis of asymptomatic [61] and symptomatic [14] infected dogs. The treatment of symptomatic dogs with Leishmune vaccine reduced the clinical symptoms and evidence of parasite, modulating the outcome of the infection and blocking transmission of the parasites to phlebotomines [14].
The three leishmanial antigens (TSA, LmSTIl and LeIF) selected for the development of a subunit vaccine (Leish-110f®) were considered promising candidates to vaccination or therapy protocols based on their demonstrated abilities to induce protection in the Balb/c mouse model of L. major in either prophylactic (TSA and LmSTIl) or therapeutic (LeIF) applications [20], [23]. On the other hand, we chose to use monophosphoryl lipid A (MPL) as adjuvant because of its strong immunostimulatory effects of the innate immune system by the direct activating of antigen presenting cells to produce IL-12, TNF-α, GM-CSF and IFN-γ, which results in enhanced phagocytosis and microbicidal activities [62]. In addition, a formulation of these antigens plus with (MPL-SE®) has been previously used in dogs and shown to be highly efficient in inducing a powerful Th1 response to the recombinant antigens [29], although it failure to induce protection after immunization in dogs [30], [31].
Finally, an important point that deserves further investigation was the observation that no death occurred in the group of animals submitted to the immunotherapeutic protocol only. Although the number of animals in each group was not large enough to warrant a statistical analysis this was an intriguing result. Unfortunately, this protocol alone was not sufficient to lead to total parasite elimination as it has occurred in two animals of the group treated with both Glucantime® and Leish-110f®. However, these results together suggest that the immunotherapy with Leish-110f® can be an important adjunct to anti-CanL therapeutic protocol that uses lower doses of Glucantime®. In other words, the immunotherapy can be an important factor that may favour the administration of much lower concentration of Glucantime® that is administered in the current protocols (also used in the current studies). It is well known that Glucantime® has serious side effects including death. In the present studies using Cox's statistical analysis based on serum levels of albumin, gamma globulin, and creatinine it was defined that the animals treated with Glucantime® had an instantaneous death risk of 3 times higher than animals under other treatments. Therefore, the institution of an immunochemotherapeutic protocol constituted of Leish-110f® together with Glucantime® may help to define new schemes with lower doses of the latter, consequently minimizing the risk of death. This is an interesting possibility that deserves further investigation.
In conclusion, the present study shows that combination of standard drugs to treat leishmaniasis with Leish-110f® + MPL-SE® might improve the current therapy of CanL. Several former studies have described immunochemotherapy protocols using a whole parasite vaccine as immunogenic component [47]. However, the use of multiple recombinant antigens delivered as a single recombinant polyprotein would result in a better approach to the immunochemotherapy as they represent higher standardized products, which simplify the manufacturing process and are attractive to distribution in developing countries due the associated reduced cost. This approach also represents a valid alternative treatment for those cases where conventional chemotherapy is not effective.
Acknowledgements
This research is part of the Master Degree's Dissertation in Parasitology (M.Sc.) of Jorge Miret with fellowship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil) in the Graduate Academic Program in Parasitology of the Instituto de Ciências Biológicas of the Universidade Federal de Minas Gerais, Brazil. We thank the members of Control Center of Zoonosis of Montes Claros, MG and Empresa Júnior de Estatística ICEX/UFMG for help with statistical analysis. This work was supported by Infectious Disease Research Institute, Seattle, WA, USA.
References
- 1.Slappendel R.J. Canine leishmaniasis. A review based on 95 cases in the Netherlands. Vet Quarterly. 1988;10:1–16. doi: 10.1080/01652176.1988.9694140. [DOI] [PubMed] [Google Scholar]
- 2.Mancianti F., Gramiccia M., Gradoni L., Pieri S. Studies on canine leishmaniasis control. 1. Evolution of infection of different clinical forms of canine leishmaniasis following antimonial treatment. Trans R Trop Med Hyg. 1988;82:566–567. doi: 10.1016/0035-9203(88)90510-x. [DOI] [PubMed] [Google Scholar]
- 3.Denerolle P. Leishmaniose canine: difficulté du diagnostic et du traitement (125 cas) Prat Méd Chir Animal Comp. 1996;31:137–145. [Google Scholar]
- 4.Ciaramella P., Oliva G., De Luna R., Gradoni L., Ambrosio R., Cortese L. A retrospective clinical study of canine leishmaniasis in 150 dogs naturally infected by Leishmania infantum. Vet Rec. 1997;141:539–543. doi: 10.1136/vr.141.21.539. [DOI] [PubMed] [Google Scholar]
- 5.Koutinas A.F., Saridomichelakis M.N., Mylonakis M., Leontides L., Polizoulou Z., Billinis C. A randomized, blinded, placebo-controlled clinical trial with allopurinol in canine leishmaniasis. Vet Parasitol. 2001;98:247–261. doi: 10.1016/s0304-4017(01)00399-5. [DOI] [PubMed] [Google Scholar]
- 6.Blavier A., Keroack S., Denerolle P., Goy-Thollot I., Chabanne L., Cadoré J.L. Atypical forms of canine leishmaniasis. Vet J. 2001;162:108–120. doi: 10.1053/tvjl.2000.0556. [DOI] [PubMed] [Google Scholar]
- 7.Amusategui I., Sainz A., Rodríguez F., Tesouro M.A. Distribution and relationship between clinical and histopathological parameters in canine leishmaniasis. Eur J Epidemiol. 2003;18:147–156. doi: 10.1023/a:1023090929302. [DOI] [PubMed] [Google Scholar]
- 8.Manual of surveillance and control of visceral leishmaniasis. Brasilia: Ministry of Healthy/Brazil; 2003. http://portal.saude.gov.br/portal/arquivos/pdf/manual_leish_visceral2006.pdf.
- 9.Magalhães P., Mayrink W., Costa C., Melo M.N., Dias M., Batista S.M. Calazar na zona do Rio Doce. Minas Gerais. Resultados de medidas profiláticas. Rev Inst Med Trop São Paulo. 1980;22:197–202. [PubMed] [Google Scholar]
- 10.Palatnik-de-Sousa C.B., dos Santos W.R., França-Silva J.C., da Costa R.T., Reis A.B., Palatnik M. Impact of canine control on the epidemiology of canine and human visceral leishmaniasis in Brazil. Am J Trop Med Hyg. 2001;65(5):510–517. doi: 10.4269/ajtmh.2001.65.510. [DOI] [PubMed] [Google Scholar]
- 11.Jerónimo S.M., Teixeira M.J., Sousa A.D., Thielking P., Pearson R.D., Evans T.G. Natural history of Leishmania (Leishmania) chagasi infection in northeastern Brazil: long-term follow-up. Clin Infect Dis. 2000;30:608–609. doi: 10.1086/313697. [DOI] [PubMed] [Google Scholar]
- 12.Tesh R. Control of zoonotic visceral leishmaniasis: its time to change strategies? Am J Trop Med Hyg. 1995;52:287–292. doi: 10.4269/ajtmh.1995.52.287. [DOI] [PubMed] [Google Scholar]
- 13.Dedet J.P. Traitement des leishmanioses: realités et perspectives. Méd et Armées. 1994;22:73–78. [Google Scholar]
- 14.Santos F.N., Borja-Cabrera G.P., Miyashiro L.M., Grechi J., Reis A.B., Moreira M.A. Immunotherapy against experimental canine visceral leishmaniasis with the saponin enriched-Leishmune vaccine. Vaccine. 2007;25:6176–6190. doi: 10.1016/j.vaccine.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guarga J.L., Moreno J., Lucientes J., Gracia M.J., Peribañez M.A., Castillo J.A. Evaluation of a specific immunochemotherapy for the treatment of canine visceral leishmaniasis. Vet Immunol Immunopathol. 2002;88:13–20. doi: 10.1016/s0165-2427(02)00128-9. [DOI] [PubMed] [Google Scholar]
- 16.Mayrink W., Genaro O., Costa C.A., França-Silva J.C., Hermeto M.V., Oliveira-Lima A. Combined immunotherapy and chemotherapy in canine visceral Leishmaniasis preliminary findings. Mem Inst Oswaldo Cruz. 1992;87:201. [Google Scholar]
- 17.Melo M.A., França-Silva J.C., Azevedo E.O., Tabosa I.M., Da Costa R., Da Costa C.A. Clinical trial on the efficacy of the N-methyl glucamine associated to immunotherapy in dogs, experimentally infected with Leishmania (Leishmania) chagasi. Revue Méd Vét. 2002;153:75–84. [Google Scholar]
- 18.Neogy A.B. Exploitation of parasite-derived antigen in therapeutic success against canine visceral leishmaniasis. Vet Parasitol. 1994;54:367–373. doi: 10.1016/0304-4017(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 19.Monjour L., Vouldoukis I., Ogunkolade B.W., Hetzel C., Ichen M., Frommel D. Vaccination and treatment trials against murine leishmaniasis with semi-purified Leishmania antigens. Trans R Soc Trop Med Hyg. 1988;82:412–415. doi: 10.1016/0035-9203(88)90140-x. [DOI] [PubMed] [Google Scholar]
- 20.Campos-Neto A., Porrozi R., Greeson K., Coler R.N., Weeb J.R., Skeiky Y.A.M. Protection against cutaneous leishmaniasis induced by recombinant antigens in murine and non-human primate models of human disease. Infect Immun. 2001;9:4103–4108. doi: 10.1128/IAI.69.6.4103-4108.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skeiky Y.A.W., Guderian J.A., Benson D.R., Bacelar O., Carvalho E.M., Kubin M. A recombinant Leishmania antigen that stimulates human peripheral blood mononuclear cells to express a Th1 cytokine profile and to produce interleukin12. J Exp Med. 1995;181:1527–1537. doi: 10.1084/jem.181.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Probst P., Skeiky Y., Steeves M., Gervassi A., Grabstein K., Reed S. A Leishmania protein that modulates interleukin IL-12, IL-10 and tumor necrosis factor production and expression of B7-1 in human monocyte-derived antigen presenting cells. Eur J Immunol. 1997;27:2634. doi: 10.1002/eji.1830271024. [DOI] [PubMed] [Google Scholar]
- 23.Skeiky Y.A.W., Kennedy M., Kaufman D., Borges M.M., Guderian J.A., Scholler J.K. LeIF: a recombinant Leishmania protein that induces an IL-12 mediated Th1 cytokine profile. J Immunol. 1998;161:6171–6179. [PubMed] [Google Scholar]
- 24.Borges M.M., Campos-Neto A., Sleath P., Grabstein K.H., Morrisey P.J., Skeiky Y.W.A. Potent stimulation of the innate immune system by a Leishmania brasiliensis recombinant protein. Infect Immun. 2001;69:5270–5277. doi: 10.1128/IAI.69.9.5270-5277.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Badaró R., Lobo I., Munos A., Netto E.M., Modabber F., Campos-Neto A. Immunotherapy for drug-refractory mucosal leishmaniasis. J Infect Dis. 2006;194:1151–1159. doi: 10.1086/507708. [DOI] [PubMed] [Google Scholar]
- 26.Weeb J.R., Kaufman D., Campos-Neto A., Reed S.G. Molecular cloning of a novel protein antigen of that elicits a potent immune protein antigen of Leishmania major that elicits a potent response in experimentally murine leishmaniasis. J Immun. 1996;157:5034–5041. [PubMed] [Google Scholar]
- 27.Weeb J.R., Campos-Neto A., Ovendale P., Martin T.I., Stromberg E.J., Badaro J. Human and murine immune responses to a novel Leishmania major recombinant protein encoded by members of a multicopy gene family. Infect Immun. 1998;66:3279–3289. doi: 10.1128/iai.66.7.3279-3289.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skeiky Y.A.W., Coler R.N., Brannon M., Stromberg E., Greeson K., Thomas-Crane R. Protective efficacy of an tandemly linked, multi-subunit recombinant leishmanial vaccine (Leish-110f®) formulated in MPL® adjuvant. Vaccine. 2002;20:3293–3303. doi: 10.1016/s0264-410x(02)00302-x. [DOI] [PubMed] [Google Scholar]
- 29.Fujiwara R.T., Vale A.M., França-Silva J.C., Da Costa R.T., Da Silva J.Q., Reis A.B. Immunogenicity in dogs of three recombinant antigens (TSA, LeIF and LmSTI1) potential candidates for canine visceral leishmaniasis. Vet Res. 2005;36:1–13. doi: 10.1051/vetres:2005033. [DOI] [PubMed] [Google Scholar]
- 30.Gradoni L., Foglia Manzillo V., Pagano A., Piantedosi D., De Luna R., Gramiccia M. Failure of a multi-subunit recombinant leishmanial vaccine (MML) to protect dogs from Leishmania infantum infection and to prevent disease progression in infected animals. Vaccine. 2005;23:5245–5251. doi: 10.1016/j.vaccine.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Moreno J., Nieto J., Masina S., Cañavate C., Cruz I., Chicharro C. Immunization with H1, HASPB1 and MML Leishmania proteins in a vaccine trial against experimental canine leishmaniasis. Vaccine. 2007;25:5290–5300. doi: 10.1016/j.vaccine.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosário E.Y., Genaro O., França-Silva J.C., Da Costa R.T., Mayrink W., Barbosa-Reis A. Evaluation of enzyme-linked immunosorbent assay using crude Leishmania and recombinant antigens as a diagnostic marker for canine visceral leishmaniasis. Mem Inst Oswaldo Cruz. 2005;100:197–203. doi: 10.1590/s0074-02762005000200015. [DOI] [PubMed] [Google Scholar]
- 33.Jain N.C. Lea & Febirger; Philadelphia: 1993. Essentials of veterinary haematology. [Google Scholar]
- 34.Kaneko J.J., Harvey J.W., Bruss M.L. Academic Press; San Diego: 1997. Clinical biochemistry of domestic animals. p. 932. [Google Scholar]
- 35.Pinelli E., Kellick-Kendrick R., Wagenaar J., Bernardina W., Del Real G., Ruitenberg J. Cellular and humoral immune response in dogs experimentally infected with Leishmania infantum. Infect Immun. 1994;62:229–235. doi: 10.1128/iai.62.1.229-235.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fujiwara R.T., Loukas A., Mendez S., Williamson A.L., Bueno L.L., Wang Y. Vaccination with irradiated Ancylostoma caninum third stage larvae induces a Th2 protective response in dogs. Vaccine. 2006;24:501–509. doi: 10.1016/j.vaccine.2005.07.091. [DOI] [PubMed] [Google Scholar]
- 37.Michalsky E.M., Rocha M.F., Rocha Lima A.C.V., França-Silva J.C., Pires M.Q., Oliveira F.S. Infectivity of seropositive dogs, showing different clinical forms of leishmaniasis, to Lutzomyia longipalpis phlebomine sand flies. Vet Parasitol. 2007;147:67–76. doi: 10.1016/j.vetpar.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 38.Fisher R.A. Applications of Student's distribution. Metron. 1925;5:90–104. [Google Scholar]
- 39.Kruskall W.H., Wallis W.A. Use of ranks in one-criterion variance analysis. J Am Stat Assoc. 1952;47:583–621. [Google Scholar]
- 40.Tukey J.W. University of Princeton; Princeton, NY: 1953. The problem of multiple comparisons. [Google Scholar]
- 41.Collet D. Chapman & Hall; London: 1994. Modelling survival data in medical research. [Google Scholar]
- 42.Klein J.P., Moeschberger J. Springer; New York: 1997. Survival analysis techniques for censored and truncated data. [Google Scholar]
- 43.Cox D.R. Chapman & Hall; London: 1984. Analysis of survival data. [Google Scholar]
- 44.Croft S.L., Coombs G.H. Leishmaniasis—current chemotherapy and recent advances in the search for novel drugs. Trends Parasitol. 2003;19:502–508. doi: 10.1016/j.pt.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 45.Hailu A., Musa A.M., Royce C., Wasunna M. Visceral leishmaniasis: new health tools are needed. PLoS Med. 2005;2:590–594. doi: 10.1371/journal.pmed.0020211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Croft S.L., Sundar S., Fairlamb A.H. Drug resistance in leishmaniasis. Clin Microbiol Rev. 2006;19:111–126. doi: 10.1128/CMR.19.1.111-126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mayrink W., Botelho A.C., Magalhães P.A., Batista S.M., Lima A.O., Genaro O. Immunotherapy, immunochemotherapy and chemotherapy for American cutaneous leishmaniasis treatment. Rev Soc Bras Med Trop. 2006;39:14–21. doi: 10.1590/s0037-86822006000100003. [DOI] [PubMed] [Google Scholar]
- 48.Herwaldt B.L., Berman J.D. Recommendations for treating leishmaniosis with sodium stibogluconate (Pentostan) and review of pertinent clinical studies. Am J Trop Med Hyg. 1992;46:296–306. doi: 10.4269/ajtmh.1992.46.296. [DOI] [PubMed] [Google Scholar]
- 49.Amusategui I., Rodríguez F., Tesouro M.A. Tratamiento de la leishmaniosis canina, Parte I. Med Vet. 1995;12:289–298. [Google Scholar]
- 50.Alvar J., Molina R., San Andrés M., Tesouro M., Nieto J., Vitutia M. Canine leishmaniasis: clinical, parasitological and entomological follow-up after chemotherapy. Ann Trop Med Parasitol. 1994;88:371–378. doi: 10.1080/00034983.1994.11812879. [DOI] [PubMed] [Google Scholar]
- 51.Oliva G., Gradoni L., Cortese L., Orsini S., Ciaramella P., Scalone A. Comparative efficacy of meglumine antimoniate and aminosidine sulphate, alone, or in combination in canine leishmaniasis. Ann Trop Med Parasitol. 1998;92:165–171. doi: 10.1080/00034989860003. [DOI] [PubMed] [Google Scholar]
- 52.Rhalem A., Sahibi H., Lasri S., Jaffe C.L. Analysis of immune responses in dogs with canine visceral leishmaniasis before, and after, drug treatment. Vet Immunol Immunopathol. 1999;71:69–76. doi: 10.1016/s0165-2427(99)00088-4. [DOI] [PubMed] [Google Scholar]
- 53.Gradoni L., Maroli M., Gramiccia M., Mancianti F. Leishmania infantum infection rates in Phlebotomus perniciosus fed in naturally infected dogs under antimonial treatment. Med Vet Entomol. 1987;1:339–342. doi: 10.1111/j.1365-2915.1987.tb00364.x. [DOI] [PubMed] [Google Scholar]
- 54.Pasa S., Ozensoy-Toz S., Vodyvoda H., Ozbel Y. Clinical and serological follow-up in dogs with visceral leishmaniosis treated with allopurinol and sodium stibogluconate. Vet Parasitol. 2005;128:243–249. doi: 10.1016/j.vetpar.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 55.Reed S.G., Scott P. T-cell and cytokine responses in leishmaniasis. Curr Opin Immunol. 1993;5:524–531. doi: 10.1016/0952-7915(93)90033-o. [DOI] [PubMed] [Google Scholar]
- 56.Carvalho E.M., Badaró R., Reed S.G., Jones T.C., Johnson W.D. Absence of gamma interferon and interleukin 2 during active visceral leishmaniasis. J Clin Invest. 1985;76:2006–2009. doi: 10.1172/JCI112209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Badaró R., Carvalho E.M., Orge M.G. Imunidade humoral e celular em indivíduos curados de leishmaniose visceral. Rev Soc Bras Med Trop. 1985;18:77–83. [Google Scholar]
- 58.Moreno J., Nieto J., Chamizo C., González F., Blanco F., Barrer D.C. The immune response and PBMC subsets in canine visceral leishmaniasis before, and after chemotherapy. Vet Immunol Immunopathol. 1999;71:181–195. doi: 10.1016/s0165-2427(99)00096-3. [DOI] [PubMed] [Google Scholar]
- 59.Ramos I., Alonso A., Marcen J.M., Peris A., Castillo J.A., Colmenares M. Heterologous prime-boost vaccination with a non-replicative vaccinia recombinant vector expressing LACK confers protection against canine visceral leishmaniasis with a predominant Th1-specific immune response. Vaccine. 2007 doi: 10.1016/j.vaccine.2007.11.021. [November 29, Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 60.Borja-Cabrera G.P., Correia Pontes N.N., da Silva V.O., Paraguai de Souza E., Santos W.R., Gomes E.M. Long lasting protection against canine kala-azar using the FML-QuilA saponin vaccine in an endemic area of Brazil (São Gonçalo do Amarante RN) Vaccine. 2002;20:3288–3384. doi: 10.1016/s0264-410x(02)00294-3. [DOI] [PubMed] [Google Scholar]
- 61.Borja-Cabrera G.P., Cruz Mendes A., Paraguai de Souza E., Hashimoto Okada L.Y., de A Trivellato F.A., Kawasaki J.K. Effective immunotherapy against canine visceral leishmaniasis with the FML-vaccine. Vaccine. 2004;22:2234–2243. doi: 10.1016/j.vaccine.2003.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ulrich J.T., Myers K.R. Monophosphoryl A as an adjuvant. In: Powel M.F., Newman M.J., editors. Vaccine design: the subunit and adjuvant approach. Plenum Press; New York: 1995. p. 495. [Google Scholar]


