Abstract
Vaccination has been one of the most successful breakthroughs in medical history. In recent years, epitope-based subunit vaccines have been introduced as a safer alternative to traditional vaccines. However, they suffer from limited immunogenicity. Nanotechnology has shown value in solving this issue. Different kinds of nanovaccines have been employed, among which virus-like nanoparticles (VLPs) and self-assembled peptide nanoparticles (SAPNs) seem very promising. Recently, SAPNs have attracted special interest due to their unique properties, including molecular specificity, biodegradability, and biocompatibility. They also resemble pathogens in terms of their size. Their multivalency allows an orderly repetitive display of antigens on their surface, which induces a stronger immune response than single immunogens. In vaccine design, SAPN self-adjuvanticity is regarded an outstanding advantage, since the use of toxic adjuvants is no longer required. SAPNs are usually composed of helical or β-sheet secondary structures and are tailored from natural peptides or de novo structures. Flexibility in subunit selection opens the door to a wide variety of molecules with different characteristics. SAPN engineering is an emerging area, and more novel structures are expected to be generated in the future, particularly with the rapid progress in related computational tools. The aim of this review is to provide a state-of-the-art overview of self-assembled peptide nanoparticles and their use in vaccine design in recent studies. Additionally, principles for their design and the application of computational approaches to vaccine design are summarized.
Keywords: Self-assembled peptide nanoparticles, Epitope-based vaccine, Adjuvant, Coiled-coil, β-Sheet, Computational design
1. Introduction
For hundreds of years, vaccination has been one of the most successful strategies to combat different infectious diseases and, consequently, decrease morbidity and mortality among humans (Skwarczynski and Toth, 2011, Skwarczynski and Toth, 2014). Edward Jenner was the first to vaccinate against smallpox in 1796. Jenner found that an inoculation of the pus from a cowpox lesion on a handmaid's hand to patients infected with smallpox conferred immunity (Stern and Markel, 2005). The aim of every vaccination is to present a particular antigen or set of antigens to the immune system in order to elicit immunity to the pathogen. In addition to the efficient elicitation of immune response, the ideal vaccine should be safe, show long-term stability at ambient temperatures, demonstrate high population coverage, and provide protection after the administration of a single dose. Moreover, the manufacturing, purification, and characterization processes should be effective, require a minimal number of steps, and be affordable to enable broad vaccine use.
The oldest type of vaccines or traditional vaccines consist of either live attenuated and killed microorganisms or inactivated toxins (Skwarczynski and Toth, 2011). The development of these vaccines uses the whole organisms, which is why they are more effective and elicit long-lasting innate and adaptive immune responses (Karch and Burkhard, 2016). Despite these benefits, traditional vaccines have some disadvantages, such as a risk of infection, probability of allergic and autoimmune reactions, and difficulties related to culture, production, and stability. Moreover, they cannot be developed for some diseases, such as cancer and several infectious diseases. Therefore, a new type of vaccines, named subunit vaccines, has been developed that has many advantages compared to the whole organism-based vaccines, including a lack of pathogenic organisms, safety, ease of production, stability, and low allergic and autoimmune responses. Hence, these vaccines are considered efficient (Skwarczynski and Toth, 2011, Skwarczynski and Toth, 2014). Based on the mentioned merits, many researchers, including our team, have worked extensively on the development of these types of vaccines (Farhadi et al., 2015, Hajighahramani et al., 2017, Mahmoodi et al., 2016, Nezafat et al., 2017, Nezafat et al., 2014, Nezafat et al., 2016, Shahbazi et al., 2016).
2. Epitope-based subunit vaccines
Subunit vaccines only contain the antigenic fragments of pathogens, which are most frequently protein-based antigens that are capable of stimulating appropriate adaptive immune responses. Some limitations, namely instability, production complexity, low purity, and most importantly, the induction of an autoimmune response, are observed more frequently in protein-based subunit vaccines than in epitope-based subunit vaccines; therefore, epitope-based subunit vaccines are often preferred. They are composed of multiple individual antigenic epitopes from one or several pathogens (Azmi et al., 2014, Nezafat et al., 2017, Skwarczynski and Toth, 2011). These epitopes usually consist of B-cell epitopes, helper T-cell (Th) epitopes, and cytotoxic T-cell (CTL) epitopes. After binding to B-cell receptors (BCRs), B-cell epitopes are activated in two ways: in the presence of Th cells or in a T-cell-independent manner. Generally, in the T-cell-independent pathway, activated B-cells cannot produce long-lasting B-cell responses. During the first step, both types of T-cell epitopes are processed by antigen-presenting cells (APCs), including dendritic cells (DCs), macrophages, and B-cells. Exogenous particles, such as toxins and bacterial pathogens, located on the cell surface are processed and presented to Th cells by major histocompatibility complex II (MHC-II). Afterwards, the activated Th cells directly or indirectly stimulate B-cells and CTLs and promote the secretion of cytokines and chemokines; therefore, active Th cells stimulate both adaptive immune and innate immune responses. Endogenous particles, such as viral particles and cancerous cells, which are located in the cytosol, are processed and presented to CTLs by MHC-I. The activated CTLs kill the intracellular pathogens and tumor cells by inducing cell-mediated immunity (Moyle and Toth, 2013, Skwarczynski and Toth, 2014). Thus, when designing most epitope-based subunit vaccines, researchers must identify both T-cell and B-cell epitopes to induce appropriate immune responses; however, low immunogenicity is the major drawback of these types of vaccines. Thus, due to the lack of the whole organism, the ability of these vaccines to stimulate immune responses is much weaker than traditional vaccines. Different approaches have been applied to boost the immunogenicity of subunit vaccines, including the administration of multiple doses of vaccine during the patient's life for long-lasting protection, the use of adjuvants, or the employment of nanotechnological approaches, i.e., designing nanovaccines (Moyle and Toth, 2013). Adjuvants are additional components in vaccines that stimulate APCs and target the innate immune system; in this way, they induce a robust immune response (Foged, 2011). Adjuvants are classified into three groups: 1) delivery systems composed of non-immunostimulatory components, which target and present the vaccine to the immune system, 2) immunostimulators, such as pathogen-associated molecular patterns (PAMPs), which directly stimulate the innate immune system, and 3) a combination of the two mentioned classes, which has the highest efficiency (Foged, 2011, Moyle and Toth, 2013). Many research groups have attempted to develop effective adjuvants, but only few adjuvants are being used in vaccine production due to several disadvantages, including low safety and high toxicity. Adjuvants approved for use in human are aluminum salts (alum), the first approved and a widely-used adjuvant, oil-in-water emulsions (MF59, AS03, and AF03), virosomes, and AS04 (a formulation of monophosphoryl lipid A (MPL) and alum) (Foged, 2011, Karch and Burkhard, 2016).
One promising area that is rapidly gaining attention is the use of nanotechnology in vaccine development (Yang et al., 2016, Zhao et al., 2008), which is considered one of the most effective strategies to conquer the low immunogenicity of epitope-based vaccines. Nanovaccines are new classes of vaccines that have been developed by incorporating epitopes into nanoparticles, which are microscopic particles with at least one dimension between 1 and 100 nm in size and a very high surface area to volume ratio (Horikoshi and Serpone, 2013, Srirajaskanthan and Preedy, 2011). These vaccines are highly efficacious because they mimic most features of pathogens, such as their size, shape, and PAMPs. This approach has been shown to improve antigen stability, immunogenicity, and function. Moreover, nanovaccines act as delivery systems and only target the site of the disease in the body, instead of all organs. Additionally, they have been used to prevent and treat different diseases, particularly cancers, by stimulating both humoral and cell-mediated responses (Sekhon and Saluja, 2011, Yang et al., 2016).
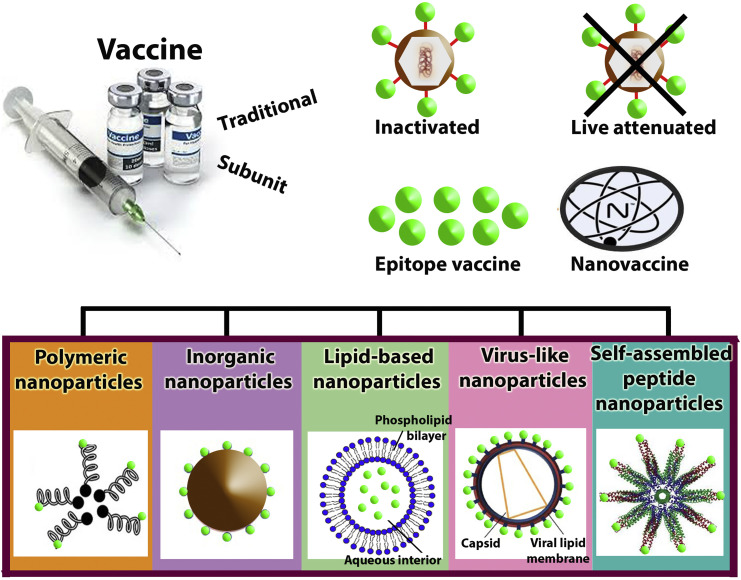
Nanovaccines are classified into several groups according to the types of nanoparticles applied in their structures, as shown in Fig. 1 . Different approved or investigational nanoparticle-based nanovaccines are summarized in Table 1, Table 2 .
Fig. 1.
Several classified nanovaccines according to the types of nanoparticles applied in their structures.
Table 1.
Different investigational nanoparticles-based nanovaccines.
| Delivery system | Type | Vaccine name | Disease | Clinical phase or licensed product name | References |
|---|---|---|---|---|---|
| Polymeric | PLGA | – | Hepatitis B | Clinical trial | (Yang et al., 2016) |
| PLGA | – | HIV | Phase I | (Bolhassani et al., 2014, Yang et al., 2016) | |
| PLGA | – | Solid tumors | Preclinical | (Bolhassani et al., 2014) | |
| PLGA | – | Cervix cancer | Phase II/III | (Bolhassani et al., 2014) | |
| PLGA | – | Measles | Preclinical | (Bolhassani et al., 2014) | |
| PLGA | – | Hepatitis C | Preclinical | (Bolhassani et al., 2014) | |
| PLGA | – | B-cell lymphoma | Preclinical | (Bolhassani et al., 2014) | |
| PGA | – | HIV | Clinical trial | (Bolhassani et al., 2014) | |
| Chitosan | – | RSV | Preclinical | (Bolhassani et al., 2014) | |
| Chitosan | – | Tuberculosis | Preclinical | (Bolhassani et al., 2014) | |
| Chitosan | – | Allergy | Preclinical | (Bolhassani et al., 2014) | |
| PEI-mannose | – | HIV | Phase I/II | (Bolhassani et al., 2014) | |
| Inorganic | Gold | – | Influenza | Clinical trial | (Vartak and Sucheck, 2016, Zhao et al., 2014) |
| Gold | – | HIV | Clinical trial | (Vartak and Sucheck, 2016, Zhao et al., 2014) | |
| Gold | – | RSV | Clinical trial | (Zhao et al., 2014) | |
| Gold | – | Foot-and-mouth disease | Clinical trial | (Vartak and Sucheck, 2016, Zhao et al., 2014) | |
| Gold | – | Malaria | Clinical trial | (Vartak and Sucheck, 2016) | |
| Gold | – | Cancer | Clinical trial | (Vartak and Sucheck, 2016) | |
| Ferric oxide | – | Malaria | Clinical trial | (Yang et al., 2016) | |
| Lipid-based | Liposome | – | Influenza | Phase I/II | (Tandrup Schmidt et al., 2016, Vartak and Sucheck, 2016) |
| Liposome | – | Hepatitis A | Phase I | (Tandrup Schmidt et al., 2016) | |
| Liposome | – | Streptococcus mutans | Phase I | (Tandrup Schmidt et al., 2016) | |
| Liposome | – | Malaria | Phase I/II | (Tandrup Schmidt et al., 2016) | |
| Liposome | – | Lung cancer | Phase II/III | (Tandrup Schmidt et al., 2016, Vartak and Sucheck, 2016) | |
| Liposome | – | Neisseria meningitides | Phase I | (Tandrup Schmidt et al., 2016) | |
| Liposome | – | Breast cancer | Phase I | (Tandrup Schmidt et al., 2016) | |
| Liposome | – | HIV | Phase I | (Tandrup Schmidt et al., 2016) | |
| Liposome | – | Mycobacterium tuberculosis | Phase I | (Tandrup Schmidt et al., 2016, Vartak and Sucheck, 2016) | |
| ISCOM | – | HPV | Clinical trial | (Zhao et al., 2014) | |
| ISCOM | – | HIV | Clinical trial | (Zhao et al., 2014) | |
| ISCOM | – | Influenza | Clinical trial | (Zhao et al., 2014) | |
| ISCOM | – | Newcastle disease | Clinical trial | (Zhao et al., 2014) | |
| Self-assembled peptides | VLPs | Heplisav | Hepatitis B | Phase III | (Shirbaghaee and Bolhassani, 2016) |
| VLPs | Nasal vaccine | Hepatitis B | Phase III | (Shirbaghaee and Bolhassani, 2016) | |
| VLPs | Edible vaccine | Hepatitis B | Phase I | (Shirbaghaee and Bolhassani, 2016) | |
| VLPs | V503 | Cervix cancer | Phase III | (Kushnir et al., 2012, Shirbaghaee and Bolhassani, 2016) | |
| VLPs | HPVB19 | Cervix cancer | Phase I/II | (Shirbaghaee and Bolhassani, 2016) | |
| VLPs | VAI-VP7051 | Parvovirus porcine infection | Phase I/II | (Kushnir et al., 2012, Shirbaghaee and Bolhassani, 2016) | |
| VLPs | – | Influenza A | Phase I/II | (Kushnir et al., 2012, López-Sagaseta et al., 2016, Shirbaghaee and Bolhassani, 2016) | |
| VLPs | NV | Norovirus infection (Norwalk virus) | Phase I | (Kushnir et al., 2012, López-Sagaseta et al., 2016, Shirbaghaee and Bolhassani, 2016) | |
| VLPs | RSV | Severe acute respiratorysyndrome relatedcoronavirus(SARS-CoV) | Phase I | (Kushnir et al., 2012, Shirbaghaee and Bolhassani, 2016) | |
| VLPs | RTS,S | Malaria | Phase I | (Kushnir et al., 2012, López-Sagaseta et al., 2016) | |
| VLPs | MalariVax | Malaria | Phase III | (Kushnir et al., 2012, López-Sagaseta et al., 2016) | |
| VLPs | PEV3 | Malaria | Phase I/II | (Kushnir et al., 2012) | |
| VLPs | CYT003-QG10 | Allergic rhinoconjunctivitis and asthma | Phase II |
(Kushnir et al., 2012) |
|
| VLPs | CAD106 | Alzheimer's disease | Phase II | (Kushnir et al., 2012, López-Sagaseta et al., 2016) | |
| VLPs | PEV7 | C. albicans | Phase I | (Kushnir et al., 2012) | |
| VLPs | CYT013-IL1bQ | Diabetes mellitus II | Phase I/IIa | (Kushnir et al., 2012, López-Sagaseta et al., 2016) | |
| VLPs | CYT006-AngQ | Hypertension | Phase II | (Kushnir et al., 2012, López-Sagaseta et al., 2016) | |
| VLPs | – | Rabies | Phase I | (Kushnir et al., 2012, López-Sagaseta et al., 2016) | |
| VLPs | NIC002 | Nicotine addiction | Phase II | (Kushnir et al., 2012, López-Sagaseta et al., 2016) | |
| VLPs | CYT004-MelQG10 | Malignant melanoma | Phase II | (Kushnir et al., 2012, López-Sagaseta et al., 2016) | |
| Ferritin | – | Epstein–Barr virus | Preclinical | (Shirbaghaee and Bolhassani, 2016) | |
| Ferritin | – | Hepatitis C | Preclinical | (Shirbaghaee and Bolhassani, 2016) | |
| Ferritin | – | HIV | Preclinical | (Shirbaghaee and Bolhassani, 2016) | |
| Ferritin | – | Influenza | Preclinical | (Shirbaghaee and Bolhassani, 2016) |
Table 2.
Different approved nanoparticles-based nanovaccines.
| Delivery system | Clinical phase or licensed product name | Disease | Vaccine name | Type | Delivery system |
|---|---|---|---|---|---|
| Lipid-based | Emulsion oil in water | Fluad | Seasonal influenza | Licensed | (Foged, 2011) |
| Focetria | Pandemic influenza | Licensed | (Foged, 2011) | ||
| Humenza | Pandemic influenza | Licensed | (Foged, 2011) | ||
| Pandemrix | Pandemic influenza | Licensed | (Foged, 2011) | ||
| Arepanrix | Pandemic influenza | Licensed | (Foged, 2011) | ||
| Prepandrix | Prepandemic influenza | Licensed | (Foged, 2011) | ||
| Aflunov | Prepandemic influenza | Licensed | (Foged, 2011) | ||
| Emulsion water in oil | CimaVax EGFTM | Non-small-cell lung cancer | Licensed | (Foged, 2011) | |
| Self-assembled peptides | VLPs | Engerix-B | Hepatitis B | Licensed | (Kushnir et al., 2012, López-Sagaseta et al., 2016, Shirbaghaee and Bolhassani, 2016) |
| VLPs | Enivac HB | Hepatitis B | Licensed | (Kushnir et al., 2012, López-Sagaseta et al., 2016, Shirbaghaee and Bolhassani, 2016) | |
| VLPs | Euvax B | Hepatitis B | Licensed | (Kushnir et al., 2012, López-Sagaseta et al., 2016, Shirbaghaee and Bolhassani, 2016) | |
| VLPs | GenHevac-B | Hepatitis B | Licensed | (Kushnir et al., 2012, López-Sagaseta et al., 2016, Shirbaghaee and Bolhassani, 2016) | |
| VLPs | Heberbiovac HB | Hepatitis B | Licensed | (Kushnir et al., 2012, López-Sagaseta et al., 2016, Shirbaghaee and Bolhassani, 2016) | |
| VLPs | Hepavax-Gene | Hepatitis B | Licensed | (Kushnir et al., 2012, López-Sagaseta et al., 2016, Shirbaghaee and Bolhassani, 2016) | |
| VLPs | Gene Vac-B | Hepatitis B | Licensed | (Kushnir et al., 2012) | |
| VLPs | Bio-Hep-B | Hepatitis B | Licensed | (Kushnir et al., 2012) | |
| VLPs | DTP-Hep B | Hepatitis B | Licensed | (Kushnir et al., 2012) | |
| VLPs | Recombivax HB | Hepatitis B | Licensed | (Shirbaghaee and Bolhassani, 2016) | |
| VLPs | Epaxal | Hepatitis A | Licensed | (Kushnir et al., 2012) | |
| VLPs | Gardasil | Cervical cancer | Licensed | (Kushnir et al., 2012, López-Sagaseta et al., 2016, Shirbaghaee and Bolhassani, 2016) | |
| VLPs | Cervarix | Cervical cancer | Licensed | (Kushnir et al., 2012, López-Sagaseta et al., 2016, Shirbaghaee and Bolhassani, 2016) | |
| VLPs | Inflexal V | Influenza A | Licensed | (Kushnir et al., 2012, Shirbaghaee and Bolhassani, 2016) |
2.1. Polymeric nanoparticles
These nanoparticles function as delivery systems and are used to formulate different drugs and vaccines, because of their stability in the body, protection of antigens from degradative agents, controlled release of antigens through a slow biodegradation rate, and biological properties, including targeting. They encapsulate antigens and carry them to the target cells, i.e., immune cells. Among this type of nanoparticles, polylactic acid (PLA), chitosan, poly lactide-co-glycolide acid (PLGA), poly glutamic acid (PGA), polypeptides, polyethylene glycol, and their copolymers are the most typical polymers. However, PLGA, which is applied to encapsulate antigens from different pathogens, such as Plasmodium vivax, hepatitis B virus (HBV), and Bacillus anthracis, and PGA, which is used to entrap hydrophobic antigens, are the two most widely used polymers due to their biocompatibility and biodegradability (Azmi et al., 2014, Skwarczynski and Toth, 2011, Zhao et al., 2014). Recently, thermo-responsive synthetic polymers (TRP) nanoparticles based on the N-isopropylacrylamide (NIPAM), and N-(2-hydroxypropyl) methacrylamide (HPMA) polymers, which are able to self-assemble into immunogenic particles at physiologic temperatures, have been developed for the co-delivery of toll like receptor 7 and 8 agonists (TLR-7/8a) as adjuvants and immunogens such as respiratory syncytial virus (RSV) fusion (F) protein trimers. TRP nanoparticles induce broad-based humoral and cellular immune responses; moreover, they are stable, soluble, filterable, colloidally dispersed, and unimolecular during purification and storage phases (Francica et al., 2016, Lynn et al., 2015). Currently, various vaccines based on polymeric nanoparticles are being tested in pre-clinical and clinical trials as treatments for different diseases, such as human immunodeficiency virus (HIV), cancer, and tuberculosis (Bolhassani et al., 2014) (Table 1).
2.2. Inorganic nanoparticles
These synthetic nanoparticles have also been applied as a delivery system, but they are frequently non-biodegradable. Inorganic nanoparticles are extensively used in vaccine formulations because of their appropriate physicochemical properties, including porous and rigid structure, controlled synthesis, and surface functionalization with different ligands. Gold, silver, ferric oxide, alumina nanoparticles, and carbon nanotubes are examples of inorganic nanoparticles that are easily converted into desired shapes and sizes. Gold nanoparticles are more widely used in vaccine delivery compared to the other nanoparticles. For example, gold nanoparticles have been exploited for the development of vaccines against RSV infections, influenza, HIV, and foot-and-mouth disease by conjugating or absorbing antigens on their surface (Karch and Burkhard, 2016, Skwarczynski and Toth, 2014, Zhao et al., 2014).
2.3. Lipid-based nanoparticles
These nanoparticles encapsulate antigens within their cores and then deliver them to the target cells. Lipidation leads to the formation of amphiphiles, which improve diffusion across epithelial barriers and provide stability against degradation agents (Golkar et al., 2016a, Golkar et al., 2016b, Zhao et al., 2014). These structures target vaccines to DCs and stimulate the activation of these cells through self-adjuvant properties. Lipid-based nanoparticles may have different structures, such as emulsions, including oil-in-water or water-in-oil forms, an immunostimulating complex (ISCOM), which is a cage-like lipid particle (Zhao et al., 2014), and liposomes (Golkar et al., 2016c, Tavakoli et al., 2015). Among these three types, liposomes are broadly used and are composed of biodegradable and non-toxic natural or synthetic bilayer phospholipids that surround an aqueous core (Azmi et al., 2014). In vaccine formulations, the antigenic determinants, which are most often peptide-based antigens, integrate within the lipid bilayer of liposomes and are protected from enzymatic degradation. Moreover, liposome-antigen complexes efficiently stimulate cellular immune response because of their nature. Therefore, liposome-based structures are potential efficient vaccine adjuvants (Azmi et al., 2014, Skwarczynski and Toth, 2011, Skwarczynski and Toth, 2014). Currently, several liposomal vaccines are being tested in clinical studies (Table 1).
2.4. Virus-like nanoparticles
Virus-like nanoparticles (VLPs) are one type of self-assembled proteins, but are discussed separately due to their viral origin and specific conformation. In fact, VLPs are recombinant, self-assembled capsid proteins with an ideal diameter of 20–100 nm that are non-infectious and non-replicating. Therefore, they have been applied as an effective diagnostic approach and in drug, DNA, peptide, protein, and vaccine delivery. For vaccine delivery, a variety of different antigens have been genetically or chemically fused to the VLPs and presented on their surface. These VLPs strongly stimulate the immune system without the need for adjuvant, because they mimic the structure of native viruses (Azmi et al., 2014, Yang et al., 2016). In fact, VLPs induce the innate immune response by activating TLRs and pattern recognition receptors (PRRs), as well as humoral immune response, particularly IgM. Due to the particulate nature of VLPs, they also increase the uptake of antigens by APCs. Currently, a few approved VLP-based vaccines are available on the market, including a recombinant hepatitis B virus vaccine and the human papilloma virus vaccines. Several VLP vaccines are also being tested in clinical trials for different diseases (Shirbaghaee and Bolhassani, 2016) (Table 1, Table 2).
2.5. Self-assembled peptide nanoparticles
Another method for developing effective epitope-based nanovaccines is to use self-assembled peptides nanoparticles (SAPNs). SAPNs are typically peptides with helical or β-sheet secondary structures that assemble together through non-covalent interactions to form nano-sized tubular, fibrillar, or spherical structures (Fujita and Taguchi, 2011, Jung et al., 2009).
The use of SAPNs as potential vaccine platforms is discussed in detail in the subsequent sections.
3. Introduction of self-assembled peptide nanoparticles
3.1. The concept of self-assembly
Self-assembly is defined as the ability of some molecules to spontaneously arrange themselves into well-ordered structures (Whitesides and Grzybowski, 2002). In nature, this common phenomenon results in the production of a variety of ensembles using finite building blocks (Indelicato et al., 2016, Whitesides and Grzybowski, 2002). Natural self-assembled molecules are primarily composed of DNA, polypeptides, and proteins and are found in a wide variety of living organisms, such as virus capsids, cell membranes, enzymes, and the cellular cytoskeleton. These molecules are involved in a diverse range of sophisticated cellular functions, including transport, motility, storage, and cellular morphology, based on their specific shape, small size, physicochemical, and mechanical features (Howorka, 2011).
The simple fact underlying the self-assembly of a molecule is to reach a more stable and compatible structure with a lower energy level. Self-assembly occurs when the various attractive/repulsive forces within and between molecules are balanced (Mandal et al., 2014), leading to the formation of numerous different non-covalent weak interactions, such as hydrogen bonds, electrostatic, hydrophobic, van der Waals, and π–π interactions (Webber et al., 2016). Water-mediated hydrogen bonds are also very important in biological systems, where molecules primarily contact water. All these weak, minor interactions lead to the formation of supramolecules with specific 3D conformations and shapes the interaction of these structures with other molecules (Zhao and Zhang, 2006).
3.2. History of SAPNs
The first examples of natural self-assembled particles are proteins derived from viruses, such as tobacco mosaic virus (TMV) capsid and hepatitis B virus (HBV) surface antigen (HBsAg). In fact, the first HBV vaccine to be licensed in 1981 was developed based on VLP nanoparticles that were obtained from viruses (López-Sagaseta et al., 2016). Molecular engineering of proteins as the monomeric building blocks through self-assembly was first suggested by Drexler (1981), who foresaw the development of advanced molecular machineries and the construction of devices and materials with atomic precision based on this bright new idea. Since then, self-assembled peptides have been employed to produce a broad range of materials, such as nanofibers (Yanlian et al., 2009, Zhang, 2003), nanowires (Sun et al., 2016), nanorings (Carlson et al., 2006, Shah et al., 2016), nanotubes (Hartgerink et al., 1996, Scanlon and Aggeli, 2008), and nanoparticles (Kaba et al., 2009, Raman et al., 2006), among others. They may also form some ordered nanoscale structures at mesoscopic and macroscopic levels (Whitesides and Grzybowski, 2002), including monolayers, hydrogels, and three-dimensional scaffolds (Yang et al., 2012).
3.3. Applications of SAPNs
Mimicking the self-assembly concept of some natural structures has enabled scientists to build diverse self-assembling nanomaterials from the four biomolecules classes (peptides, lipids, nucleic acids, and sugars) in different size scales with a broad range of applications in biomedicine, environmental sciences, information technologies, and materials science (Busseron et al., 2013, Zhao and Zhang, 2006). Peptidic self-assembled nanostructures have been used as peptide surfactants (Mandal et al., 2014, Zhao and Zhang, 2006), membranes for separation and purification (Busseron et al., 2013), or research models of protein folding and protein conformational disease studies, in addition to enzyme immobilization and blood cell substitution (Doll et al., 2015a, Zhao and Zhang, 2006). Some nanoscale self-assembled peptides possess a central cavity, which enables them to carry drugs or genes, and have been used for controlled drug release, gene, and drug delivery (Doll et al., 2015a, Habibi et al., 2016). SAPNs have also been harnessed in 2D and 3D cell-cultures, for reparative and regenerative medicine, tissue engineering (Yu et al., 2016), as pharmacological antagonists (Yu et al., 2016), in imaging, as biosensors, and in diagnostic device development (Busseron et al., 2013, Habibi et al., 2016, Hauser and Zhang, 2010). They have also shown antimicrobial and anticancer activities in some studies (Roy et al., 2013). One promising application of SAPNs is in vaccine development (Kaba et al., 2009, Zhang et al., 2014, Zhao et al., 2014), which will be discussed further in the next section.
3.4. Employment of SAPNs in vaccine design
SAPNs usually consist of a series of repetitive motifs, which enables the incorporation of epitopes and antigens in their subunit structures in such a way that these incorporated molecules are displayed on the surface of the assembled particles, thereby building chimeric SAPNs for use as adjuvant or adjuvant-carriers in vaccines. Two structural features of SAPNs, molecular specificity and multivalency, make them suitable vaccine adjuvants and carriers. The high antigen density and structurally ordered antigen arrangement on SAPNs resembles the recognition patterns on pathogens and thus facilitates the cross-linking of antigens with the BCRs (López-Sagaseta et al., 2016). This multivalent interaction is a key step in provoking a potent immune response and is a solution for the weak immunogenicity of subunit vaccines. In fact, SAPNs elicit high titers of high affinity neutralizing IgG in addition to CD8 + T-cell-mediated protection (Chen et al., 2013, Chesson et al., 2014). They not only trigger complement activation but also help in creating microenvironments that promote the interaction of the vaccine with APCs (Eskandari et al., 2016, Hubbell et al., 2009). Therefore, SAPNs have been used in both prophylactic and immunotherapeutic vaccines (Hubbell et al., 2009). The incorporation of antigens on self-assembled peptides and subsequent production of chimeric SAPNs is accomplished through a direct self-assembly process or covalent chemical bonding of antigens to nanoparticles (López-Sagaseta et al., 2016). SAPNs with β-sheet and coiled-coil structures have been studied by several groups as vaccine carriers and/or adjuvants (El Bissati et al., 2014, Karch and Burkhard, 2016); however, more research is needed.
The development of efficient vaccine adjuvants is an emerging area. The mechanism of action of many adjuvants is not yet clear (Shah et al., 2014) and many questions remain about the best parameters for an ideal adjuvant, such as its particle size (Oyewumi et al., 2010). These questions add more complexity to studies of SAPNs in vaccines. Despite the conflicts regarding the optimum size of nanoparticles suitable for vaccine adjuvants, particles with diameters of 10–150 nm seem suitable because they effectively interact with different cells of the immune system (Bachmann and Jennings, 2010, Oyewumi et al., 2010).
3.5. Addition of adjuvants to SAPN vaccines
Despite the self-adjuvanticity of SAPNs, the employment of vaccine adjuvants, including TLR agonists, has also been investigated. TLRs are a category of PRRs that recognize PAMPs from bacteria and viruses, thereby triggering the innate immune system and promoting antigen processing by APCs. Therefore, some TLR agonists have been recently introduced as efficient vaccine adjuvants (Reed et al., 2013).
TLR7/8 agonist was used as adjuvant during the development of an HIV vaccine on a self-assembling peptide (EAK16-II) platform. The TLR agonist was co-assembled with the peptide (Ding et al., 2016). In another study, polyI:C (polyinosinic:polycytidylic acid), a TLR3 agonist, was incorporated into a novel vaccine constructed as immune polyelectrolyte multilayer (iPEMs) capsules, which were electrostatically assembled entirely from peptide antigens and molecular adjuvants, without any polymer (Chiu et al., 2016).
The use of other adjuvants in vaccines developed on SAPN platforms has also been evaluated. For instance, the addition of Freund's adjuvant to a SAPN vaccine for avian influenza infections elicited a higher antibody titer (Babapoor et al., 2011). However, in some studies, the employment of SAPNs alone as adjuvants has been sufficient to promote immune responses. In this context, a peptide hydrogel adjuvanted antigen vaccine developed against West Nile virus induced a stronger immune response and conferred protection compared to alum adjuvant (Friedrich et al., 2016).
4. Principles for designing and generating SAPNs
The fabrication of SAPNs is of special interest due to the unique potential of these nanostructures. Advances in SAPN synthesis were delayed for several years, due to the complexities in the design of these molecular assemblies. However, in addition to the continuing development of computational tools, novel ideas have recently helped researchers to make considerable progress in this area (King and Lai, 2013).
The creation of complex structures through the highly ordered repetition of a limited number of building blocks is the fascinating feature of self-assembly. SAPNs are fabricated through a bottom-up approach (Lakshmanan et al., 2012), which begins with the selection of peptide building blocks or subunits, and needs a proper mastery of the molecular structure of the building blocks and their assembly tendencies (Zhang, 2003). The primary and secondary structures of peptides control their self-assembling properties (Yang et al., 2012). For instance, the exact structure of coiled-coils and the parallel and antiparallel orientations of dimers and tetramers are predictable based on their sequences (Ramisch et al., 2015). Furthermore, non-covalent interactions among main and side chain atoms lead to the generation of secondary structures, such as α-helices or β-sheets, and ultimately drive the formation of the 3D structures of SAPNs (Fairman and Akerfeldt, 2005, Yang et al., 2012).
Peptide building blocks are borrowed from natural molecules or synthesized from a novel computational sequence design (Brunette et al., 2015). The most common subunits or oligomerization motifs observed in natural proteins are α-helical coils (Burkhard et al., 2001, Lupas, 1996), which are convenient building blocks for designing SAPNs due to their stability and well-defined structures (Doll et al., 2015b, Yang et al., 2012). Coiled-coils and β-sheets are broadly used in vaccine development (Chen et al., 2013, Hudalla et al., 2013, Rudra et al., 2012b, Rudra et al., 2012a, Rudra et al., 2010), which will be discussed in more detail in the next sections.
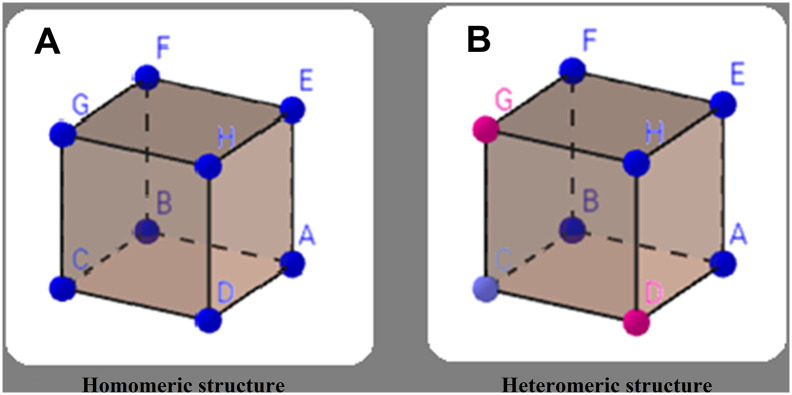
Self-assembly of similar subunits in nature almost always follows the central rule of symmetry. The rationale behind this phenomenon, as foreseen by Crick and Watson in 1956 (Crick and Watson, 1956), is that fewer definite kinds of specific interaction interfaces are needed for a symmetric assembly compared to an asymmetric assembly (Lai et al., 2012). This key rule immensely simplifies the process of designing SAPNs because it is based on the assumption that some building blocks with identical structure and interactions participate in shaping the homomeric complexes. Moreover, the number of potential structures is limited when the symmetry rule is followed (Fig. 2 ). Avidity is the second governing characteristic of self-assembly, which enables the formation of stable complexes based on the weak interactions among building blocks (Norn and André, 2016). For example, in homomeric icosahedral protein capsids, avidity induces a stability value that is six orders of magnitude higher than the affinity among the individual symmetric building blocks (Mateu, 2013, Norn and André, 2016). The avidity concept helps in designing higher-order assemblies using computational tools.
Fig. 2.
Homomeric versus heteromeric structures: In homomeric structures (A), which are composed of identical building blocks, the number of possible structures is limited based on the rule of symmetry. While in heteromeric structures (B), building blocks are not the same, leading to several different possible structures. The structure shown here is only one of the possible heteromeric cubes that can be formed with diverse units.
The ideal architecture of the designed SAPNs depends on the intended usage; for example, hollow core and shell structures, like cages, are desired for drug and gene delivery or masking and shielding molecules. In the same manner, more compact structures with repetitive units are excellent candidates for the presentation of multiple copies of antigens in vaccines or chemicals for other applications (Elsawy et al., 2016, Raman et al., 2006, Sanner et al., 2005).
Notably, self-assembly is usually a dynamic equilibrium, which can occur in both forward and reverse directions, and seems to be controlled by kinetics instead of thermodynamics (Marsh et al., 2013, Norn and André, 2016). The hydrophobic-hydrophilic balance is a key player in peptide self-assembly (Mandal et al., 2014). Regarding vaccine activities, peptides antigens are usually hydrophilic (Zhao et al., 2017, Parker et al., 1986); therefore, hydrophilic parts of SAPNs are ideal regions for epitope carriage. On the other hand, adjuvants are primarily hydrophobic (Liu et al., 2013), and hydrophobic portions may trigger the innate immune system (Seong and Matzinger, 2004). Thus, the dual nature of SAPNs favors a vaccine function.
Different methods have been employed to create the driving force needed for self-assembly, as listed in Table 3 .
Table 3.
Methods used for promotion of assembly in designing SAPNs.
| Method | Example | Advantage | Consideration | References |
|---|---|---|---|---|
| Coiled-coil and helical bundle interactions | A peptide containing a pentameric and a trimeric coiled-coil domains for antigen display (T = 1 icosahedron and T = 3 icosahedron) | Stable and well defined oligomers | Buffer conditions impact the assembly through affecting the non-covalent interactions between the oligomers. | (Yang et al., 2012) |
| Engineered disulfide bonds |
|
|
Intermolecular disulfide bonds should be avoided. | (Raman et al., 2006, Usui et al., 2009) |
| Metal-mediated interactions | 1-D nanotubes and 2-D and 3-D crystalline arrays from a monomeric protein |
|
|
(Brodin et al., 2012) |
| Chemical cross-links | A quadratic network of a C4-symmetric tetrameric aldolase | Production of a protein network with adjustable mesh | Network assembly is very sensitive to inhomogeneities of the subunits. | (Ringler and Schulz, 2003) |
| Genetic fusion of multiple protein domains or fragments that naturally self-associate | A tetrahedral SAPN consisting two oligomers linked by a helical linker | Production of a wide variety of highly symmetric structures or extended material |
|
(Padilla et al., 2001) (Sinclair et al., 2011) |
| Directing the assembly on the surface of a nonbiological material along with computational interface design | Virus-like protein assemblies on carbon nanotube surfaces | Creation of a richly textured molecular surface |
|
(Grigoryan et al., 2011) |
| Computational de novo design of possible folds using a tandem repetition of a helix–loop–helix–loop subunit | 74 helical structures with new folds not identified before from tandem repeating of a simple helix–loop–helix–loop structural motif | Design of SAPNs with folding unlike to nature | As the structures that can be obtained from a helix–loop repeat unit are limited to straight rods, a helix–loop–helix–loop subunit was adopted. |
(Brunette et al., 2015) |
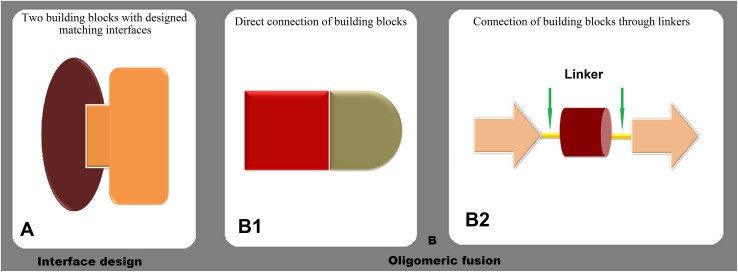
Three key aspects should be considered when designing self-assembled molecules: subunit conformation, subunit interactions, and regulation of assembly (King and Lai, 2013). Two basic strategies have been adopted to design SAPNs: oligomeric fusion and interface design (Lai et al., 2012) (Fig. 3 ). In the oligomeric fusion method, oligomers or building blocks are fused together to form different structures. The interface design approach is a newer method that has evolved along with the accumulation of the researchers' knowledge of the first approach and the improvement of computational algorithms. It enjoys the advantage of a more accurate control of the interface geometry, leading to a higher structural order. Moreover, the protein-protein interfaces control the assembly and stabilize the quaternary structure of SAPNs (Norn and André, 2016). The nature and number of these interfaces depend on the symmetry, stoichiometry, and similarities between monomers (Ardejani and Orner, 2015), whereas the 3D structures of the resulting proteins are closely related to their assembly pathway (Marsh et al., 2013). Structures with only one interface composed of monomers include cyclic, fibril, and internal symmetries. Cyclic symmetrical building blocks generate more complex symmetrical structures with two interfaces: dihedral, cubic, and 2D lattices. The design of three interfaces from monomers can shape crystals, the most complex structures (Norn and André, 2016).
Fig. 3.
Two main strategies for designing SAPNs: (A) Interface design, the connecting interfaces of the building blocks can be designed by computational tools, which brings more control on the geometry of the interfaces and the linking. (B) Oligomeric fusion, the building blocks are connected with each other either directly (B1) or through linkers (B2). Based on the desired structure, the linkers can be rigid or flexible.
4.1. Development of SAPN vaccines
SAPN synthesis is highly dependent on the selection of proper building blocks, as discussed above. For example, in most artificial and natural SAPN β-sheets, the polar and hydrophobic amino acids are located in an alternating pattern (Mandal et al., 2014). Self-assembly can occur spontaneously if the units and conditions are appropriate.
General principles should be followed for vaccine development based on SAPN platforms, and epitopes would also be incorporated into the SAPN structures either through linkers or direct attachment. One approach is to synthesize the SAPN systems first, followed by their functionalization with epitopes under proper conditions to achieve a vaccine construct (Friedrich et al., 2016, Rudra et al., 2012b). Using this method, epitopes are covalently attached to both termini and presented on the surface of the peptide nanofibers, while preserving their immunogenicity and eliciting high antibody titers and cellular responses in mice, without the need for any adjuvants (Branco et al., 2011). Alternatively, peptide monomers that include the self-assembling domain and epitopes may be produced by expressing recombinant proteins in Escherichia coli hosts, which will be self-assembled after production (El Bissati et al., 2014, Kaba et al., 2012, Pimentel et al., 2009). The self-assembling peptide may be synthesize alone or in tandem with the epitope via the solid phase peptide synthesis method with high purity (Chen et al., 2013, Coin et al., 2007, Rudra et al., 2010). The amino acid pairing (AAP) strategy has also been used for SAPN synthesis. This method is based on three major interactions (hydrogen bonding, electrostatic attractions, and hydrophobic interactions) that are created between the amino acid side chains of a self-assembling peptide with an adjacent peptide molecule (Eskandari et al., 2016, Fung et al., 2011). Although this approach was used to fabricate β-sheet fibrils for drug delivery (Sadatmousavi, 2009), it may also be applied to vaccine design.
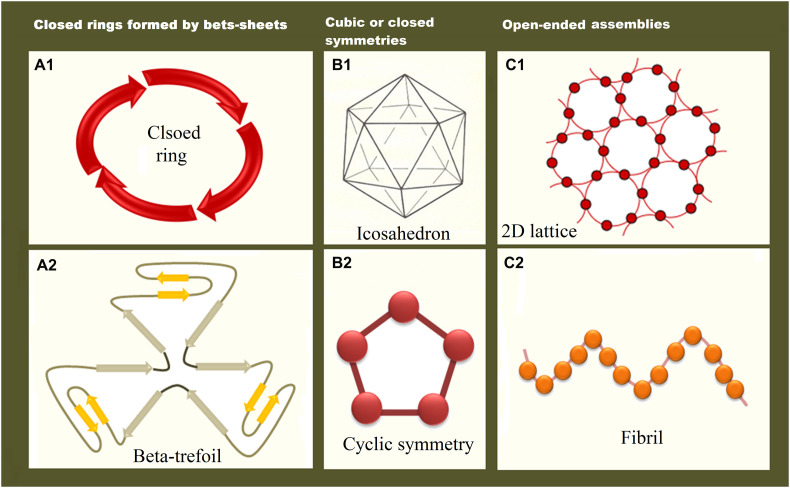
4.2. Symmetries used in SAPNs
Three major types of symmetries used in SAPNs (Fig. 4 ) are discussed in the next section.
Fig. 4.
Major types of symmetries used in SAPNs: (A) Repetition of covalently bound oligomers with internal symmetry- Repetition of beta sheets formed closed rings (A1). Beta-trefoil (A2) is mainly composed of beta sheets, but may also contain alpha helices in more complex types. (B) Cubic or closed symmetries- Icosahedron, which are similar to virus capsids, and are very suitable for vaccine design (B1). An example of simple cyclic symmetries, which are the structural components of closed symmetries (B2). (C) Open-ended assemblies- 2D lattice, made from cyclic symmetries (C1). Fibril, constituted from monomers as a front-to-end assembly (C2).
4.2.1. Repetition of covalently bound oligomers with internal symmetry
In this category, the subunits are more stabilized, because they are fused into repeat proteins through covalent bonds. These repeats can further assemble to form open linear structures with a helical curvature or closed ring structures with rotational symmetries (Huang et al., 2016). The generated SAPNs in this class may also possess β-sheet structures, such as β-trefoil (Broom et al., 2012, Longo et al., 2014), β-propeller (Voet et al., 2014), and TIM barrels (Huang et al., 2016). Many of these structures are either natural or synthetic scaffolds. These repeat proteins may be generated through gene duplication and fusion of a single repeat. The geometric constraints of linear repeat proteins are lower than cyclic proteins. Moreover, they can adopt various supramolecular forms, some of which may not exist in nature (Norn and André, 2016). The supramolecular shapes should be controlled either through computational de novo design (Brunette et al., 2015, Doyle et al., 2015, Park et al., 2015) or by using slightly different oligomeric repeats to produce structures with predefined geometries for these molecular architectures (Ramisch et al., 2014). Additionally, different conformational repeats may be used to generate structures with a non-uniform curvature (Park et al., 2015).
4.2.2. Cubic or closed symmetries
Large protein complexes with closed symmetries are generated from cyclic symmetrical building blocks. These polyhedral structures are usually cage-like with a hollow center. The icosahedral arrangement, which is ubiquitous among spherical virus capsids (Zandi et al., 2004), has been imitated as a stable model in many studies (Doll et al., 2015a, Doll et al., 2015b, Indelicato et al., 2016, Raman et al., 2006). The highly repetitive geometry of icosahedrons and their similarity with virus capsids, make them ideal for repetitive antigen display and, consequently, the triggering of high antibody titers (Sanner et al., 2005).
Cubic symmetries are fabricated using two previously mentioned approaches: oligomeric fusion and interface design. In the fusion approach, a linker sequence, which is usually an α-helical polypeptide, is needed to join the oligomerization domains together in a relatively rigid and fixed way to obtain a regular self-assembled structure (Padilla et al., 2001) (Fig. 3B2). Otherwise, the oligomeric units may adopt different orientations, leading to irregular assemblies. Generally, in epitope vaccines, linkers, which are short sequences that are sensitive to proteasomal degradation (Nezafat et al., 2014), are commonly used for two reasons: separating the antigenic domains (Arai et al., 2001) to prevent the generation of junctional epitopes (neoepitopes) and promoting the processing and presentation of HLA-II binding epitopes (Nezafat et al., 2016). In SAPNs, different types of linkers are being employed for architectural purposes according to the desired structure (Lai et al., 2012).
In the interface design approach, the most commonly designed cages are homomeric (Norn and André, 2016). However, protein cages with tetrahedral and octahedral symmetries were computationally designed using trimeric oligomers and the introduction of a single new interface with high accuracy (King et al., 2012). More recently, tetrahedral cages were designed from two distinct oligomers, again with a single interface (King et al., 2014). The diverse combinations that can be built with two building blocks open possibilities of developing a wide variety of SAPNs with different characteristics and applications.
4.2.3. Open-ended assemblies
In open-ended assemblies, including fibrils and 2D lattices, nanoscale protein-protein interactions are linked to the mesoscale properties of the material (Norn and André, 2016).
Fibrils are fabricated from monomers with a single self-compatible interface that can generate a front-to-end assembly (Norn and André, 2016), as reported for the production of antifreeze amyloid fibers (Peralta et al., 2015). However, many natural protein fibers present more complex structures. For the engineering of such fibrils, one interface is usually associated with another motif or structure to limit the number of available interfaces (Grigoryan et al., 2011, Kaltofen et al., 2015).
The design of 2D lattices can be similar to cubic symmetries, because the common oligomers used in both cases are cyclic symmetries. Both the fusion approach or interface design have been employed. Symmetric 3D crystals are extended from 2D lattices or engineered from monomeric oligomers, which requires at least three interfaces (Norn and André, 2016).
4.3. General considerations in SAPN generation
Some general important factors should be considered during SAPN synthesis, regardless of the classifications described above.
Basically, peptide building blocks are synthesized through two main approaches: recombinant technology and solid phase chemical methods (Habibi et al., 2016). Chemical methods may be used to synthesize short peptides, whereas the recombinant technology is the rational choice for the production of more complex peptide chains. However, fermentation costs and control requirements, in addition to difficulties in detecting impurities and purification are some of the challenges that should be considered, particularly if large-scale production is required.
In chemical methods, the building blocks sequences and buffer conditions may affect the molecular weight of the produced SAPN and its polymorphism (Indelicato et al., 2016). The nanoscale assembly is based on a fundamental parameter: the peptide solution concentration. Another factor influencing the self-assembly of the SAPNs is pH, which determines the overall charges of the different oligomers, thereby affecting SAPN formation (Doll et al., 2015a).
Some common issues in fabrication of SAPNs and methods used in studies as successful solutions are briefly described in Table 4 .
Table 4.
Some main issues in SAPN fabrication and applied solutions.
| Objective | Issue | Method | Reference |
|---|---|---|---|
| Control and limitation of assembly | Aggregation and undesired extension of the nanoparticles, resulting in polydisperse structures | Charge repulsion at the N-termini of the helical subunits | (Fletcher et al., 2013) |
| Capped oligomers, lacking some binding elements necessary for assembly at the ends of the subunits | (Usui et al., 2009) | ||
| Production of recombinant proteins in a soluble form during bacterial protein expression | Large aggregates and inclusion bodies formed because of subunits linkage inside the host cell | Separate production of components or subunits in different bacterial strains and mixing the purified soluble components in situ afterwards | (Ringler and Schulz, 2003, Sinclair et al., 2011, Usui et al., 2009) |
| Addition of metals or ligands that trigger the assembly to the mixed solution of discrete subunits | (Brodin et al., 2012, Carlson et al., 2006, Lai et al., 2012) | ||
| Reaching oligomerization specificity | Biophysical challenges, such as existence of competing oligomerization states with slight differences in energy gaps or isoenergetic energy levels | Avoiding kinetic traps and aggregation during the assembly process, for example by combination of de novo structure modeling with stability calculations to simultaneously predict structure and oligomeric states of homomeric coiled-coils. | (Marsh et al., 2013, Norn and André, 2016) (Ramisch et al., 2015) |
4.4. Employing computational tools in SAPN design
Currently, vaccinology integrates immunology, systems biology, immunoinformatics, structural biology, and nanotechnology sciences, which, like pieces of a puzzle, should be put together to develop efficient vaccines. Bioinformatics provides great help for researchers aiming to generate predictions before performing lab experiments to design SAPNs, similar to some other fields (Negahdaripour et al., 2016, Negahdaripour et al., 2017, Rahmatabadi et al., 2016, Zamani et al., 2015), which helps save time and energy (Negahdaripour et al., 2017, Rahmatabadi et al., 2016). On the other hand, the use of computational tools in vaccine design or SAPN development seems unavoidable, considering the huge volume of data that must be compiled. SAPN design usually begins with an initial computer design that calculates the expected molecular mass, diameter, and symmetry in advance. In the past few years, other factors that impact design complexity, such as the rotational and translational degree of freedoms (Marsh et al., 2013) and different competing assembly states with similar stabilities (Fleishman and Baker, 2012, Ramisch et al., 2015), have been also calculated computationally. However, some deviations from the predicted models have been reported (Brunette et al., 2015). By taking advantage of molecular modeling and simulation programs, various α-helices and oligomerization domains have been combined to design novel self-assembling nanoparticles (Sanner et al., 2005). Moreover, point mutations within the oligomerization domains and adjacent to the linker, or within the linker, and their impacts on the assembly can be investigated with these programs (Ardejani and Orner, 2015). Additionally, protein stability has been optimized with computer programs by modifying surface polarity, core hydrophobicity, and backbone regularity (Netzer and Fleishman, 2016).
In interface design through computation, the most challenging task is achieving geometrically precise interfaces (Lai et al., 2012); however, great headway has recently been made in this area (Netzer and Fleishman, 2016). A general computational method used for the design of SAPNs through interface design was explained by (King et al. (2012) and includes two steps: 1) the symmetrical docking of protein building blocks in a target symmetric architecture and 2) the design of low-energy protein-protein interfaces between the building blocks to promote self-assembly.
Protein assembly can be predicted with several different computational tools, which are usually based on two main approaches: 1) template-based modeling (including homology modeling and threading), and 2) free modeling (Wang et al., 2015). For example, several tools, including Rosetta (Huang et al., 2011, Leaver-Fay et al., 2011), CABS-FOLD (Blaszczyk et al., 2013), and FALCON (Wang et al., 2015), have been used for de novo protein prediction. The servers and tools are being improved constantly. The Protein Structure Prediction Center (http://predictioncenter.org/) biennially performs an objective test of the ability of the servers to identify a protein structure from sequence through a process of blind prediction to support improvements to these methods. The most recently published results of the critical assessment of protein structure prediction (CASP) experiments in 2014, or CASP 11, were published in Proteins Journal, 2015, Oct. and Dec. issue (Lensink et al., 2016, Monastyrskyy et al., 2015, Ovchinnikov et al., 2015), and the CASP12 outcomes are awaited.
5. Classification of SAPNs
SAPNs have been categorized from different perspectives, based on their indication, shape (cage-like, 2D lattices, nanofibers, etc.), building block structures (cyclic, alpha-helix (α-helix), and β-sheets (β-sheets)) (Ardejani and Orner, 2013, Kim et al., 2012), building block source (natural or ab initio designed), design approaches, and many other features.
This review focuses on the most common subunits used to build SAPNs, including β-sheets and coiled-coils, and their usage in vaccines.
5.1. β-Sheet SAPNs
5.1.1. Brief history of the discovery of the β-sheet structure
In 1951, Linus Pauling and Robert Corey of the California Institute of Technology and Herman Branson presented the β-sheet structure, along with the α-helix, in a series of eight papers submitted to PNAS. Their suggested models were deduced from the characteristics of smaller molecules (Eisenberg, 2003). The β-sheet was explained in their second paper as “a hydrogen-bonded layer structure of polypeptide chains, with alternate chains oppositely oriented”. They also introduced pleated sheets, which were formed from a hydrogen-bonded layered structure of polypeptide chains, in which all chains were similarly oriented (Pauling and Corey, 1951). They called this structure the β-sheet, because it was the second structure identified (i.e., the α-helix was identified first).
5.1.2. Basic characteristics of β-sheet SAPNs
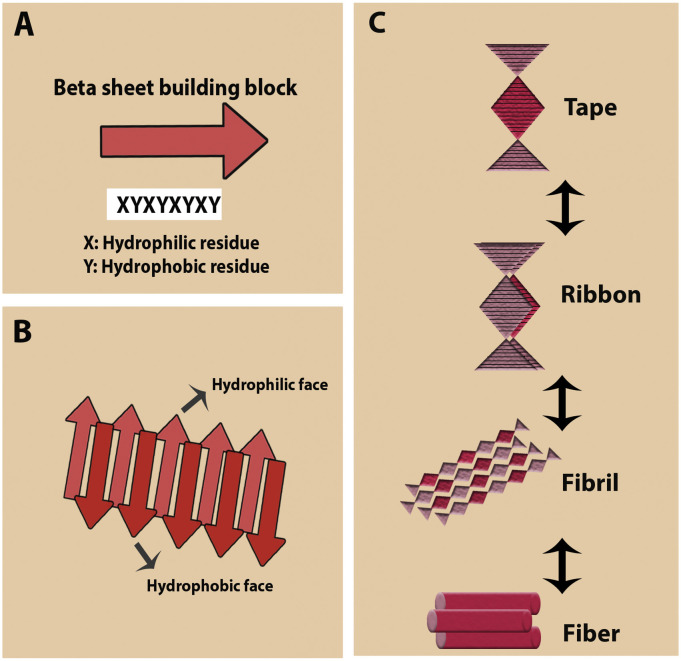
β-Sheets are one of the most common natural motifs that can be utilized to create self-assembled peptides (Habibi et al., 2016). Peptides composed of alternate hydrophilic (charged, polar) and hydrophobic (uncharged, nonpolar) amino acid repeats, which provide a backbone with amphiphilic properties, possess a tendency to form a β-sheet structure with one hydrophilic face and one hydrophobic face (Doll et al., 2013, Eskandari et al., 2016, Habibi et al., 2016, Zhang et al., 1993). These amino acid arrangements promote hydrogen bonds among residues (hydrogen donors and acceptors) in various polypeptide chains or in different parts of a folded polypeptide, resulting in parallel/anti-parallel sheet-like structures (Eskandari et al., 2016). Two sheets interact such that the surrounding aqueous media contacts the hydrophilic faces of the sheets, whereas the aqueous media is excluded from the hydrophobic faces (Doll et al., 2013). The β-sheet motif has been used to fabricate supramolecular structures. Various hierarchical structural arrays have been developed based on the number of packed sheets, such as tapes, ribbons, fibrils, and fibers (Fig. 5 ) (Eskandari et al., 2016, Kumar et al., 2011, Loo et al., 2012). However, under physiological conditions, these short peptides primarily self-assemble into β-sheet-rich nanofibers (Doll et al., 2013, Rudra et al., 2010).
Fig. 5.
Schematic representation of β-sheet peptides and their possible self-assembled structures: (A) a sequence of peptide including alternating hydrophilic (X) and hydrophobic (Y) residues, (B) β-sheet peptides assembly, and (C) self-assembly of the peptide into various filamentous particles such as tape, ribbon, fibril, and fiber (depended on the packing density).
The initiation of self-assembly is regulated by the introduction of stimuli, such as phosphate-buffered saline (PBS) and a pH change (Bowerman and Nilsson, 2012, Chen, 2005, Sun et al., 2016). β-Sheet peptides primarily self-assemble when they are dissolved in an aqueous solution and are allowed to fibrillize overnight at 4 °C (at low concentrations of < 1% W/V). The mechanical characteristics of the self-assembled β-sheets are controlled by adjusting the molar ratio of enantiomeric peptides, where different ratios of L-peptides (composed of L-amino acids) and D-peptides (composed of D-amino acids) are applied to design self-assembled β-sheet structures. The use of chirality in peptide sequences may greatly enhance the stability of the prepared peptide scaffolds and protect them from enzymatic degradation (Eskandari et al., 2016, Nagy et al., 2011). Furthermore, polypeptides rich in alanine (A) and glycine (G) formed larger beta strands (Zhang et al., 2011). Various self-assembled β-sheet peptides and their physicochemical characteristics are presented in Table 5 . The physicochemical properties of peptides were calculated using the ProtParam tool at (http://web.expasy.org/protparam/) server (Gasteiger et al., 2005).
Table 5.
Various self-assembled β-sheet peptides and their physicochemical characteristics.
| Name | Amino acid composition* | Number of amino acid | Charge | Stability (instability index (II)) | Size of nanofibers | PI | Molecular weight | Half-life | Aliphatic index | Grand average of hydropathicity (GRAVY) | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Q11 | QQKFQFQFEQQ | 11 | Neutral | Unstable (47.91) | 10–20 nm | 6.00 | 1485.6 | 0.8 h (mammalian reticulocytes, in vitro), 10 min (yeast, in vivo), 10 h (E. coli, in vivo) | 00.00 | − 1.818 | (Chen et al., 2013, Jung et al., 2009, Rudra et al., 2010) |
| KFE8 | FKFEFKFE | 8 | Neutral | Stable (− 28.82) | 6.14 | 1121.3 | 1.1 h (mammalian reticulocytes, in vitro), 3 min (yeast, in vivo), 2 min (E. coli, in vivo) | 00.00 | − 0.450 | (Rudra et al., 2016) | |
| QAAAAGGG | 8 | Negative | Stable (39.60) | 5–8 nm | 5.52 | 601.6 | 0.8 h (mammalian reticulocytes, in vitro), 10 min (yeast, in vivo), 10 h (E. coli, in vivo) | 50.00 | 0.312 | (Silva et al., 2004) | |
| EAK16-I | AEAEAKAK | 8 | Neutral | Stable (8.75) | 10–20 nm | 6.19 | 816.9 | 4.4 h (mammalian reticulocytes, in vitro), > 20 h (yeast, in vivo), > 10 h (E. coli, in vivo). | 50.00 | − 0.950 | (Bowerman and Nilsson, 2012, Hong et al., 2004, Loo et al., 2012) |
| EAK16-II | AEAEAKAKAEAEAKAK | 16 | Neutral | Stable (9.38) | 9.2 nm | 6.33 | 1615.8 | 4.4 h (mammalian reticulocytes, in vitro), > 20 h (yeast, in vivo), > 10 h (E. coli, in vivo). | 50 | − 0.950 | (Bowerman and Nilsson, 2012, Ding et al., 2016, Hong et al., 2003, Loo et al., 2012, Zhang et al., 1993, Zhang et al., 1995, Zhang et al., 1994) |
| EAK16-IV | (AEAE) 2(AKKE)2 | 16 | Negative (− 2) | Stable (9.38) | 4.77 | 1731.8 | 4.4 h (mammalian reticulocytes, in vitro). > 20 h (yeast, in vivo). > 10 h (E. coli, in vivo). | 37.50 | − 1.613 | (Bowerman and Nilsson, 2012, Hong et al., 2003, Zhang and Rich, 1997) | |
| EMK16-II | MEMEMKMK | 8 | Neutral | Unstable (49.50) | 5.90 | 1057.3 | 30 h (mammalian reticulocytes, in vitro), > 20 h (yeast, in vivo), > 10 h (E. coli, in vivo). | 0.00 | − 0.900 | (Bowerman and Nilsson, 2012, Zou et al., 2010) | |
| RADA16-I | RADARADARADARADA | 16 | Neutral | Stable (− 11.85) | 6 nm | 6.10 | 1671.7 | 1 h (mammalian reticulocytes, in vitro), 2 min (yeast, in vivo), 2 min (E. coli, in vivo). | 50.00 | − 1.100 | (Bowerman and Nilsson, 2012, Loo et al., 2012, Yokoi et al., 2005) |
| RAD16-II | (RARARDRD)2 | 16 | Positive (+ 4) | Stable (− 4.76) | 11.78 | 2012.1 | 1 h (mammalian reticulocytes, in vitro), 2 min (yeast, in vivo), 2 min (E. coli, in vivo). | 25.00 | − 2.675 | (Bowerman and Nilsson, 2012, Loo et al., 2012, Zhang et al., 1995, Zhang et al., 1994) | |
| KLD16 | (KLDL)4 | 16 | Neutral | Stable (− 27.77) | 6.10 | 1896.3 | 1.3 h (mammalian reticulocytes, in vitro), 3 min (yeast, in vivo), 3 min (E. coli, in vivo). | 195.00 | 0.050 | (Bowerman and Nilsson, 2012, Sieminski et al., 2008) | |
| FKFE2 | (FKFE)2 | 8 | Neutral | Stable (− 28.82) | 6.14 | 1121.3 | 1.1 h (mammalian reticulocytes, in vitro), 3 min (yeast, in vivo), 2 min (E. coli, in vivo). | 0.00 | − 0.450 | (Bowerman and Nilsson, 2012, Caplan et al., 2002, Hwang et al., 2003, Marini et al., 2002) | |
| EFK12 | (FKFE)3 | 12 | Neutral | Stable (− 28.41) | 6.23 | 1672.9 | 1.1 h (mammalian reticulocytes, in vitro), 3 min (yeast, in vivo). 2 min (E. coli, in vivo). |
0.00 | − 0.450 | (Bowerman and Nilsson, 2012, Caplan et al., 2002) | |
| EFK16 | (FEFEFKFK)2 | 16 | Neutral | Stable (− 28.20) | 6.29 | 2224.5 | 1.1 h (mammalian reticulocytes, in vitro), 3 min (yeast, in vivo), 2 min (E. coli, in vivo). | 0.00 | − 0.450 | (Bowerman and Nilsson, 2012, Caplan et al., 2002) | |
| F9 | FEFKFEFKK | 9 | Positive (+ 1) | Stable (− 24) | 8.50 | 1249.4 | 1.1 h (mammalian reticulocytes, in vitro), 3 min (yeast, in vivo), 2 min (E. coli, in vivo). | 0.00 | − 0.833 | (Elsawy et al., 2016) | |
| P11 | QQRFEWEFEQQ | 11 | Negative (− 2) | Unstable (47.95) | 4.25 | 1554.6 | 0.8 h (mammalian reticulocytes, in vitro), 10 min (yeast, in vivo), 10 h (E. coli, in vivo). | 0.00 | − 2.209 | (Aggeli et al., 2001, Bowerman and Nilsson, 2012, Loo et al., 2012) | |
| FEFQFNFK | 8 | Neutral | Stable (− 38.25) | 6.00 | 1106.2 | 1.1 h (mammalian reticulocytes, in vitro), 3 min (yeast, in vivo), 2 min (E. coli, in vivo). | 0.00 | − 0.400 | (Eskandari et al., 2016, Sadatmousavi, 2009) |
* A:alanine, C:cysteine, D:aspartic acid, E:glutamic acid, F:phenylalaninr, G:glycine, H:histidine, I:isoleucine, K:lysine, L:leucine, M:methionine, N:aspargine, P:proline, Q:glutamine, R:arginine, S:serine, T:threonine, V:valine, W:tryptophan, Y:tyrosine
β-Sheet filamentous particles are able to increase drug bioavailability by increasing the circulation times and the maximum tolerable dose, promoting tumor cell apoptosis, delaying the clearance of the drug (by the liver and spleen), and participating in fewer interactions with serum proteins due to the neutral charge of their surfaces (Eskandari et al., 2016, Lee et al., 2010).
5.1.3. Vaccine development studies using β-sheets
β-Sheet peptide self-assemblies have been studied less extensively than coiled-coil peptide self-assemblies. However, several self-assembled β-sheet peptides have been investigated in vaccine-related research. A natural protein that self-assembled in water and utilized β-sheet peptide motifs as a peptide scaffold was accidentally discovered in the initial attempts in the early 1990s. The first member of this class is AEAK16-II, which was discovered in a yeast protein, Zuotin (Zhang et al., 1993).
A self-assembled peptide named EAK16-II was utilized for the co-delivery of a CD8 + T-cell epitope and TLR7/8 agonists (R848 or R837). A conjugate of an HIV-1 cytotoxic T lymphocyte (CTL) epitope, SL9, with the EAK16-II peptide (SL9-EAK16-II) self-assembled with R848 or R837 into nanofibers in an aqueous solution. The ex vivo produced dendritic cells of HIV-1 + patients, who had been treated with the nanofibers of SL9-EAK16-II/R848, stimulated more SL9-specific CTLs than the cells that had been treated with either SL9 alone or the mixture of both SL9 and the TLR agonist. Furthermore, the developed nanofibers induced a stronger CTL response in vaccinated mice (Ding et al., 2016).
One of the most well-studied nanofibers, Q11, consists of 11 amino acids that have the ability to self-assemble into unbranched antiparallel β-sheets (Collier and Messersmith, 2003, Jung et al., 2009, Karch and Burkhard, 2016). Schematic representation of nanofibers that self-assemble through a β-sheet fibrilizing domain, Q11, conjugated to B and T cell epitopes peptide are shown in Fig. 6 . In a study performed by Rudra and colleagues, the fibrillized peptides Q11 (Ac-QQKFQFQFEQQ-Am) and KFE8 (FKFEFKFE) were used to develop a self-adjuvanting vaccine delivery system. A peptide antigen of OVA323–339 was covalently conjugated to the N-terminus of nonimmunogenic amphipathic fibrillizing KFE8 peptide as a model to produce β-sheet nanoparticles. In the absence of any adjuvant, this system resulted in strong and long-lasting antibody responses. In addition, the cocaine-KFE8 conjugate assembles into β-sheet rich nanofibers in aqueous buffers, leading to higher titers of anticocaine antibodies in vaccinated mice, without the need for other adjuvants (Rudra et al., 2016).
Fig. 6.
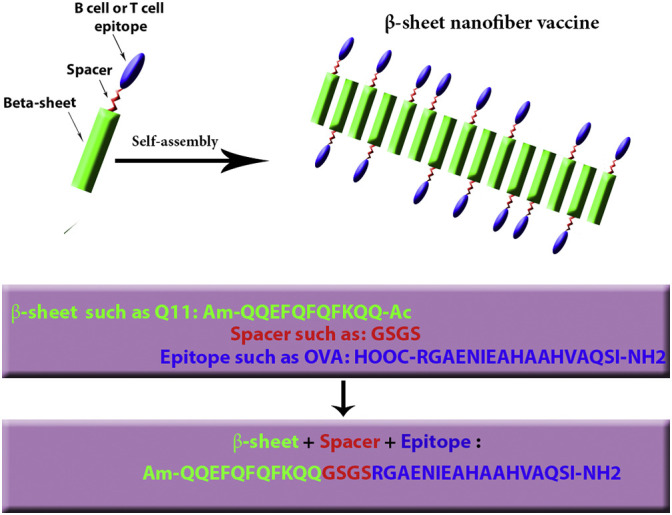
Schematic representation of epitope-bearing self-assembling peptides (β-sheet nanofiber vaccine); The β-sheet Q11 domain (green) assembles into fibrillar aggregates, presenting T or B cell epitopes peptide (blue) at the end of a flexible spacer (red). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
TLR ligand responses, which are often associated with inflammatory signals, can be omitted when self-adjuvanting vaccines are used. This approach seems very promising. In one investigation, the Q11 peptide domain (QQKFQFQFEQQ) was conjugated to a model peptide antigen from ovalbumin, OVA323-339. The resulting conjugate vaccine (Q11-OVA323-339) that self-assembled into β-sheet-rich nanofibers in water or physiological buffers was shown to be noncytotoxic in vitro and lacked several important features in vivo, such as quantifiable inflammation, swelling at the site of injection, accumulation of either inflammatory cytokines or cells, and the generation of an allergic IgE response. Furthermore, the prepared nanofibers showed a strong antibody response in mice that was CD4 T-cell-dependent (Chen et al., 2013).
Vaccines that are able to produce strong CD8 + T-cell responses are effective against infectious diseases and cancers. In another study by Chesson and colleagues, Q11 was conjugated to the OVA CD8 + T-cell ovalbumin epitope. The resulting peptide conjugates that self-assembled into nanofibers induced a strong CD8 + T-cell response. Moreover, this self-assembled vaccine was able to protect against repeated influenza virus infection in a vaccination regimen. Remarkably, these nanofibers were not accumulated at the site of injection as an inflammatory antigen deposit (Chesson et al., 2014).
In the design of an anti-malaria vaccine, an epitope of Plasmodium falciparum circumsporozoite protein, (NANP)3, was attached to the self-assembling peptide, Q11, to investigate the immunogenicity mechanism and the quality of the antibody responses. (NANP)3-Q11 conjugates self-assembled into nanofibers. A notable increase in the antibody titers and a significant inhibition of sporozoite infection were produced by the prepared self-assembled nanofibers. The antibody responses were both T-cell- and MyD88-dependent. Furthermore, two different epitopes self-assembled together without impacting the strength and duration of the antibody responses. These developed nanofibers were proposed to be a favorable platform for self-adjuvanting multi-antigenic immunotherapies (Karch and Burkhard, 2016, Rudra et al., 2012a).
Another kind of self-adjuvanting peptide nanofibers was produced through the co-assembly of a common high-affinity CD4 + T-cell epitope named PADRE and a B-cell epitope of Staphylococcus aureus, which were then conjugated to the Q11 peptide. Increasing the PADRE concentration resulted in bell-shaped dose responses that were distinct to various populations of T-cells. Interestingly, the epitope ratios that maximized the T follicular helper and antibody responses were different by an order of magnitude from the ratios that maximized Th1 or Th2 responses (Karch and Burkhard, 2016, Pompano et al., 2014).
In a study performed by Friedrich et al. (2016), the adjuvanting potential of peptide hydrogels was examined using the model peptide KFE8 in combination with the immunoprotective envelope protein domain III (EIII) of West Nile virus (WNV) as a protective subunit antigen in a mouse model. Vaccination with WNV EIII emulsified with KFE8 peptide hydrogel (EIII + KFE8) generated strong antibody responses and significantly protected the mouse model against lethal infection compared to WNV EIII adjuvanted with alum (EIII + alum), implying that peptide hydrogel adjuvants might be a viable alternative to alum for producing protective immunity.
The β-sheet peptides that have been used for other applications in addition to vaccines also possess various features. These β-sheet peptides may also have a potential for vaccine development in the future. Some examples are briefly presented below.
The effect of functionalization of β-sheet peptides on the hydrophobic face on the self-assembly of band gelation was evaluated in the investigation performed by Elsawy and co-workers. A self-assembling peptide FEFKFEFKK (F9) was utilized in their study and formed stable β-sheet-rich fibers and hydrogels (Elsawy et al., 2016).
Aggeli et al. (2001) developed a class of self-assembling β-sheet peptides named K24 (KLEALVYVLGFFTLGIMLSYIR), which formed nanotapes that self-assembled into regular nanofibers.
In another report, the peptide GNNDESNISFKEK with a β-sheet structure formed nanofibers with a morphology comprising twisted tape structures at concentrations greater than the critical aggregation concentration (0.5 wt%) (Chen, 2005).
A study by Sadatmousavi (2009) investigated the ability of the self-assembling all complementary peptide AC8 (β-sheet structures) to stabilize an anticancer drug, ellipticine, and the in vitro therapeutic efficacy of the system was then determined.
5.2. Coiled-coil SAPNs
5.2.1. A brief history of the discovery of the coiled-coil structure
Like β-sheets, the coiled-coil structure was discovered by William Astbury in the early 1930s (Astbury, 1933). Astbury applied the first X-ray diffraction measurement of natural protein fibers (the keratins from wool, horsehair, and collagen) and discovered three main structures in native wool (a-form), in the denatured form (b-form), and in collagen (Astbury, 1933). Then, he observed meridional arcs of 5.15 Å. He proposed that the α-helix may be deformed into a coiled-coil to clarify the 5.15 Å reflection on the meridian and indicated that the energy is involved in this deformation is likely to be small (Crick, 1953b). In the 1950s, L. Pauling at Caltech in the USA and L. Bragg at Cambridge in the UK attempted to interpret the meridional arcs observed at 5.15 Å (Pauling and Corey, 1950, Pauling et al., 1951). In the same year, L. Pauling, R. Corey, and H. Branson at Caltech in the USA elaborated mathematical models of 3.7 residues per turn and 5.1 residues per turn in an α-helical structure (Pauling et al., 1951). In 1952–1953, F. H. C. Crick (Cambridge, UK), L. Pauling, and R. Corey (Caltech, USA) separately submitted reports to Nature showing that the 5.15 Å diffraction pattern was attributed to the packing of two or more strands of α-helices (Crick, 1953b, Pauling and Corey, 1953). Subsequently, in 1953, Crick described the Fourier transform of a continuous coiled-coil domain, and, in a follow-up paper, explained that the knobs-in-holes configuration leads to the packing of α-helices in the form of coiled-coils (Apostolovic et al., 2010, Crick, 1952, Crick, 1953a, Crick, 1953b, Pauling and Corey, 1953).
5.2.2. Basic characteristics of coiled-coils
The coiled-coil structure is a common protein-folding motif that has garnered particular attention due to the relationship between the three-dimensional structure of the peptide and the amino acid sequence. A primary analysis of different protein sequences predicts that 2.5–10% of all protein residues possess α-helical coiled-coil motifs (Burkhard et al., 2001, Pechar and Pola, 2013, Robson Marsden and Kros, 2010). An α-helical coiled-coil consists of two or more α-helical peptides that wrap around each other in a superhelical fashion to produce a stable complex in aqueous solutions. The majority of peptides that form coiled-coil structures are based on the heptad repeats (a repetition of seven amino acid residues). Heptad repeat patterns have the form (a-b-c-d-e-f-g) n, in which n is the number of heptad repeats. Hydrophobic amino acids are often located at the ‘a’ and ‘d’ positions, whereas hydrophilic amino acids are placed at the ‘e’ and ‘g’ positions (Parry et al., 2008, Pechar and Pola, 2013, Robson Marsden and Kros, 2010).
The number of the helices in the superhelix is determined by the selection of hydrophobic amino acid residues at positions ‘a’ and ‘d’. The hydrophobic core of the coiled-coil is described by the tight packing of the hydrophobic amino acid side chains in a ‘knobs-in-holes’ fashion. Ionic interactions between residues in positions ‘e’ and ‘g’ of the two helices significantly contribute to the stability of the coiled-coil motif (Pechar and Pola, 2013).
The seven main factors that influence the stability of coiled-coils include: 1) the hydrophobicity of the core residues in positions ‘a’ and ‘d’, 2) the number of heptad repeats (a-b-c-d-e-f-g) n, 3) the helix melting temperature, as a high thermal stability and high binding constants result in reduced immunogenicity and a lower synthesis cost, 4) pH, as changes in pH induce unfolding of the coiled-coils by (de)protonation of the side chain functional groups of amino acids at residues ‘e’ and ‘g’, followed by disruption of the interchain salt bridges and destabilization of the coiled-coil, 5) specific interstrand interactions, such as disulfide bridges, 6) metal ions, which are important in direct protein folding, stabilization of the tertiary structure, or electron transfer and mainly influence the orientation of the helices in a coiled-coil, and 7) the helical propensity of amino acid residues in the remaining positions (Apostolovic et al., 2010). The amino acid sequence determines many aspects of coiled-coil binding, such as stability, the direction of binding (parallel or antiparallel), the oligomerization state (two or more peptides), size (2 nm–200 nm long), rigidity, and homomeric or heteromeric binding (Robson Marsden and Kros, 2010). The coiled-coil domains of cartilage oligomerization matrix protein (COMP) (Malashkevich et al., 1996), tetrabrachion (Stetefeld et al., 2000), fibritin (Tao et al., 1997), and the GCN4 leucine zipper (O'Shea et al., 1991) are some of the well-known examples of α-helical coiled-coils and represent pentameric, tetrameric, trimeric, and dimeric coiled-coils, respectively (Yang et al., 2012).
The coiled-coil motif is a common natural protein folding motif (Table 6 ), in contrast to the de novo coiled-coils (Table 7 ), which are used to produce peptide/protein hybrid materials. The de novo coiled-coils have novel and well-defined structures and properties, because they are controlled via the engineering of the protein/peptide primary structure (Apostolovic et al., 2010, Pechar and Pola, 2013).
Table 6.
Overview of natural coiled-coils.
| Natural coiled-coils | Orientation and oligomerization number | Organism | References |
|---|---|---|---|
| Non-structural RNA binding protein | Parallel dimer | Simian Rotavirus sa11 | (Groft and Burley, 2002) |
| Transcription of DNA, CREB | Parallel dimer | Mouse | (Schumacher et al., 2000) |
| Contractile protein, tropomyosin | Parallel dimer | Pig | (Whitby and Phillips, 2000) |
| Transcription activator, c-Myc/Max | Parallel dimer | Human & mouse | (Lavigne et al., 1998) |
| Transcription of DNA, ATF4/EBP | Parallel dimer | Human & mouse | (Podust et al., 2001) |
| Gene regulator, Fos/Jun | Parallel dimer | Human | (Glover and Harrison, 1995) |
| Contractile protein, troponin Ca2 +-saturated from cardiac muscle | Parallel dimer | Human | (Takeda et al., 2003) |
| Vimentin | Parallel dimer | Human | (Strelkov et al., 2002) |
| Transcription, CueR | Antiparallel dimer | Escherichia coli | (Changela et al., 2003) |
| Transcription, ZntR | Antiparallel dimer | Escherichia coli | (Changela et al., 2003) |
| Transcription, SlyA | Antiparallel dimer | Enterococcus faecalis | (Wu et al., 2003) |
| Glycoprotein, pilin | Antiparallel dimer | Neisseria gonorrhoeae | (Parge et al., 1995) |
| Lectin, fungal immunomodulatory protein Fve | Antiparallel dimer | Flammulina velutipes | (Paaventhan et al., 2003) |
| Transcription of DNA (MADS-box), Myocyte enhancer factor 2 (MEF2) | Antiparallel dimer | Human | (Santelli and Richmond, 2000) |
| Viral protein, glycoprotein41 | Trimer | Human immunodeficiency virus | (Chan et al., 1997) |
| Viral protein, glycoprotein F | Trimer | Simian virus | (Baker et al., 1999) |
| Coat protein | Trimer | Moloney murine leukemia virus | (Strelkov et al., 2002) |
| Viral protein E2 glycoprotein | Trimer | SARS coronovirus | (Deng et al., 2006) |
| Viral protein, hemagglutinin glycoprotein | Trimer | Influenza virus H1N1 from 1918 | (Gamblin et al., 2004) |
| Membrane protein, Murein-lipoprotein LPP | Trimer | Escherichia coli | (Shu et al., 2000) |
| Lectin, mannose binding protein A | Trimer | Norway rat | (Ng et al., 1998) |
| Apotosis, TNF receptor associated factor 3 (TRAF3) | Trimer | Human | (Ni et al., 2000) |
| Sugar binding protein, pulmonary surfactant-associated protein D | Trimer | Human | (Shrive et al., 2003) |
| Blood clotting protein fibrinogen beta | Trimer | Human | (Pechik et al., 2006) |
| Lectin, Mannose binding protein MBP | Trimer | Human | (Sheriff et al., 1994) |
| Lectin, tetranectin | Trimer | Human | (Håkansson et al., 1999) |
| Transcription, regulatory protein Rop | Tetramer | Escherichia coli | (Banner et al., 1987) |
| Complex GrpE/DnaK | Tetramer | Escherichia coli | (Harrison et al., 1997) |
| Transcription regulation, Lac repressor | Tetramer | Escherichia coli | (Lewis et al., 1996) |
| Protein binding, tetrabrachion | Tetramer | Staphylothermus marinus | (Stetefeld et al., 2000) |
| Hydrolase, tyrosine hydrolase | Tetramer | Norway rat | (Goodwill et al., 1997) |
| Signaling protein, heterogeneous nuclear ribonucleoprotein C (hnRNP-C) | Tetramer | Human | (Whitson et al., 2005) |
| SNARE, synaptosomal associated protein 23 (SNAP23) | Tetramer | Human | (Freedman et al., 2003) |
| Mechanosensitive ion channel protein | Pentamer | Mycobacterium tuberculosis | (Steinbacher et al., 2007) |
| Membrane protein, CorA | Pentamer | Thermatoga maritime | (Eshaghi et al., 2006) |
| Metal transport protein | Pentamer | Thermatoga maritime | (Hattori et al., 2007) |
| Protein binding, cartilage oligomeric matrix protein | Pentamer | Mouse | (Özbek et al., 2002) |
| Membrane protein, cardiac phospholamban | Pentamer | Human | (Oxenoid and Chou, 2005) |
| Transferase, cobalamin adenosyltransferase | Hexamer | Lactobacillus reuteri | (Maurice et al., 2007) |
Table 7.
Overview of de novo coiled-coils.
| De novo coiled-coil peptides name | Oligomerization number | Orientation | References |
|---|---|---|---|
| Val19a | Homodimer | Parallel | (Mant et al., 2003) |
| Coil-VL | Homotetramer | Antiparallel | (Betz and DeGrado, 1996) |
| Coil-LL | Homotetramer | Antiparallel | (Betz and DeGrado, 1996) |
| WSPLB | Homopentamer | Antiparallel | (Slovic et al., 2005) |
| EE1234L | Heterodimer | Parallel | (Moll et al., 2001) |
| CCSL | Intramolecular dimer | Antiparallel | (Myszka and Chaiken, 1994) |
| Ac-AB4C-OH | Homodimer | Parallel | (Hodges et al., 1981) |
| a1B-Pro-a1B | Homotrimer | Antiparallel | (Ho and DeGrado, 1987) |
| VSAL E4 | Heterodimer | Parallel | (Litowski and Hodges, 2001) |
| E/E35, K/K35 | Heterodimer | Parallel | (Graddis et al., 1993) |
| IZ-2dE,IZ-2aE | Homotrimer | Parallel | (Suzuki et al., 1999) |
| TZ1H | Homotrimer | Parallel | (Zimenkov et al., 2006) |
| RLP-1, RLP-2, RLP-3 | Heterodimer of helixturn-helix peptides into 4-helix bundle | Antiparallel | (Betz et al., 1997) |
| CSP-6 | Homodimer | Parallel | (Pandya et al., 2004) |
| IZ-3adH | Homotrimer | Parallel | (Suzuki et al., 1998) |
| IZ-3aH | Homotrimer | Parallel | (Kiyokawa et al., 2004) |
| IZ-AC | Homotrimer | Parallel | (Li et al., 2000) |
| H3a4, H6a4 | Homotetramer | Antiparallel | (Handel et al., 1993) |
| Asn16Ala | Homodimer to homotrimer switch | Parallel | (Gonzalez et al., 1996) |
| aFFP | Homopentamer | Parallel | (Potekhin et al., 2001) |
| Variant 2, Variant 3 | Homodimer | Parallel | (Vagt et al., 2006) |
| SAF-p1, SAF-p2, SAF-p2a | Heterodimer | Parallel | (Papapostolou et al., 2007) |
| C2A16, C33A16 | Homodimer ((C2A16)2 and (C33A16)2); heterodimer (C2A16/C33A16) | Parallel homodimer; antiparallel heterodimer | (Monera et al., 1993, Zhou et al., 1992) |
Coiled-coil proteins, particularly heterodimeric coiled-coils, have different applications in de novo coiled-coil-based biomaterials/hybrids, including (hybrid) hydrogels (Fig. 7 ) (Yang et al., 2006), and drug-delivery (Fig. 8 ) (Hodges, 1996).
Fig. 7.
Schematic representation of hydrogel self-assembly through dimeric coiled-coils association.
Fig. 8.
A two-step targeting and drug delivery system applying coiled-coil heterodimerization technology.
5.2.3. Vaccine development studies using coiled-coils
Hodges and colleagues used the E/K coiled-coil, which is composed of E and K peptides, to immobilize proteins on Surface Plasmon Resonance (SPR) sensor chips (Apostolovic et al., 2010, Chao et al., 1998); the K peptide is composed of five heptad (g′–a′–b′–c′–d′–e′–f′) repeats of the sequence K–V–S–A–L–K–E and the E peptide consists of the same number of heptad repeats with the sequence E–V–S–A–L–E–K (g–a–b–c–d–e–f). The hydrophobic residues at positions a, a′, d and d′ interact and are mainly responsible for the formation and stability of the coiled-coil. In one study, a range of epitopes obtained from the synthetic peptide of an anti-Pseudomonas aeruginosa (PA) vaccine were immobilized through the E/K coiled-coils on an SPR sensor chip and were then characterized according to their affinity and cross-reactivity to a variety of antibodies (Cachia et al., 2004, Chao et al., 1998).
The Burkhard group constructed self-assembling peptides that were exploited as a multiple antigen-display platform based on a pentameric coiled-coil oligomerization domain derived from cartilage oligomeric matrix protein (COMP) and a de novo minimal trimeric coiled-coil oligomerization domain. This group has used this platform to present a B-cell immunodominant repeated epitope, (DPPPPNPN)2D, derived from the circumsporozoite protein of the malaria parasite Plasmodium berghei on the surface of SAPNs constructed from a COMP structure, known as P4c-Mal. The administration of the P4c-Mal vaccine formulation in complete Freund's adjuvant (CFA) successfully generated high antibody titers, which protected most of the mice against the malaria challenge for up to six months. This conjugated malaria vaccine is now under pre-clinical evaluation in the USA. As shown in the study by Burkhard and co-workers, the delivery of P4c-Mal in phosphate-buffered saline (PBS) induced a long-lived protective immune response in mice without the need for a heterologous adjuvant. Schematic display of peptide nanoparticle that self-assemble through pentameric and trimeric coiled-coil domain conjugated to B and T cell epitopes peptide are represented in Fig. 9 .
Fig. 9.
Schematic representation of peptide nanoparticle that self-assemble through pentameric and trimeric coiled-coil domain conjugated to B and T cell epitopes peptide. Green: T-cell epitope; Red: trimeric coiled-coil domain; Blue: pentameric coiled coil domain; Orange: B-cell epitope. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Moreover, immunization with P4c-Mal generated CD4+ T-cell-dependent antibodies that promoted protection (Kaba et al., 2009).
In another study, Kaba et al. (2012) constructed a vaccine (PfCSP-SAPN vaccine) based on SAPNs, which displayed B-cell and T-cell epitopes derived from the circumsporozoite protein (CSP) of Plasmodium falciparum (PfCSP), the human malaria parasite. This vaccine induced the production of both epitope-specific antibodies and long-lived poly-functional interferon-γ (IFNγ)-producing central memory T-cells. It protected mice against a lethal dose of a transgenic P. berghei that expressed the CSP from P. falciparum. Furthermore, the PfCSP-SAPN vaccine considerably protected cultured human hepatocytes from the invasion and growth of native P. falciparum.
Wahome et al. (2012) developed an HIV vaccine using a nanoparticle composed of two covalently linked coiled-coil domains joined to the membrane proximal external region (MPER) of HIV-1 gp41. However, this vaccine was not able to generate HIV-1-neutralizing antibodies. Although these nanoparticles induced high MPER-specific titers, none of the sera exhibited a noticeable neutralizing activity against HIV-1.
Pimentel et al. (2009) designed and produced a prototypic SARS (severe acute respiratory syndrome) vaccine to induce neutralizing antibodies against the SARS virus epitope in vitro. This SARS vaccine was constructed by inserting a trimeric coiled-coil epitope from HRC (C-terminal heptad repeat of the SARS-CoV virus spike protein) into SAPNs. The SARS nanoparticles evoked specific neutralizing antibodies for the trimeric coiled-coil epitopes from HRC in mice.
Babapoor et al. (2011) devised two novel vaccines to immunize chickens against avian influenza (AI) that are based on the insertion of monomeric (Mono- M2e) and tetrameric (Tetra-M2e) forms of the immunogenic epitopes from the external domain of the avian influenza virus matrix protein 2 (M2e) into SAPNs. Both vaccines raised M2e-specific IgG responses in the vaccinated chickens (specific pathogen-free chickens) compared to a non-vaccinated group. The use of an adjuvant with the vaccine construct, Tetra-M2e, induced a considerably higher antibody response.
Raman et al. (2006) prepared 16 nm diameter regular polyhedral peptidic nanoparticles by covalently bonding coiled-coil peptides with C3- and C5-symmetries to elicit an immune response. Each peptide chain of this nanoparticle consisted of two coiled-coil oligomerization domains, which formed peptide nanoparticles with a diameter of approximately 16 nm in an aqueous solution. Polyhedral peptidic nanoparticles were suitable for drug targeting, drug delivery systems, or repetitive antigen display applications.
El Bissati et al. (2014) produced a novel self-assembling nanoparticle platform to deliver peptide epitopes to evoke CD8 + and CD4 + T-cells against a Toxoplasma gondii infection. These SAPN platforms were compose of a dense granule 7 (GRA720–28 LPQFATAAT) peptide, linear peptide (LP) monomers (containing two coiled-coil oligomerization domains), and a universal CD4 + T-cell epitope (derived from PADRE). GRA720–28 and PADRE SAPNs induced protective immunity against T. gondii in HLA-B*0702 transgenic mice.
6. Advantages and disadvantages of using SAPNs in vaccines
The nanoscale sizes of SAPNs make them similar to pathogens (Cordeiro et al., 2015). Moreover, their morphological shape, repetitive structure, and multivalency (Howorka, 2011) allows condensation of antigens and are similar to PAMPs on microbes, leading to recognition by PRPs and activation of the immune system (Rudra et al., 2012b). The antigen attachment position is well controlled. Additionally, SAPNs are biocompatible and biodegradable because they are derived from proteins, which are the main building blocks of living organisms (Sanner et al., 2005), and the different units are held together by weak interactions instead of chemical covalent bonds.
One of the significant challenges in vaccine design is creating and maintaining the balance between immunogenicity and inflammation, mainly for vaccines that incorporate pro-inflammatory adjuvants, such as aluminum hydroxide (Al(OH)3) or aluminum phosphate (AlPO4) microparticles, or even some TLR agonists. The self-adjuvanticity of SAPNs allows vaccines to be developed that do not incorporate adjuvants, which is a key benefit considering the possible inflammation and toxicity caused by adjuvants (Chen et al., 2013).
Recently, interest in the development of non-parenteral vaccines has emerged due to their different advantages, including usage convenience, compliance of a greater number of patients, lack of a need for professionals to administer the injection, and economical advantages. SAPNs are a candidate vaccine carrier for these formulations. In this context, SAPNs containing squalenyl derivatives, which are known to possess excellent oral bioavailability, was studied for the development of an oral dosage form of fondaparinux (an anticoagulant with a low oral bioavailability) (Ralay-Ranaivo et al., 2014). Similar approaches can be employed for oral vaccine delivery.
Other general beneficial characteristics of SAPNs that are important in all applications, including vaccine design, have been reported. SAPNs are chemically well defined (Skwarczynski and Toth, 2014) and manufacturable (Sun et al., 2016). In addition, the speed of protein expression, purification, and self-assembly into nanoparticles leads to acceptable cost and time needed for the large-scale production of SAPNs (Babapoor et al., 2011, Kaba et al., 2009). Their production process is environmentally friendly, as it does not involve the use of large amounts of chemicals (Zhao et al., 2017). Overall, SAPN fabrication is affordable and lacks complexity through its bottom up approach (Zhao and Zhang, 2006). Other advantages of SAPNs include their stability at high temperatures, resistance to enzymatic degradation, organic solvents and extreme pH values (Roy et al., 2013), as well as their symmetrical nature, modularity, responsiveness to stimuli (Yu et al., 2016), and bioactivity (Yang et al., 2012). The chemical diversity and possibility of structure design and engineering to match the specific needs (Fairman and Akerfeldt, 2005), which leads to their versatility (Mandal et al., 2014), are other merits. In other words, SAPNs can be synthesized from a variety of natural and non-natural amino acid building blocks with diverse characteristics (Webber et al., 2016).
Several special features of SAPNs make them superior to other nano systems used in vaccine development. As an example, the mechanical and chemical stabilities of SAPNs are considered important advantages over liposomes (Raman et al., 2006). Biodegradability is the primary advantage of SAPNs over synthetic nanoparticle carriers and polymers. Some polymerization reactions may lead to the generation of several products with various lengths and molecular weights. Moreover, the reaction between polymer and epitopes sometimes results in structures with different degrees of substitution (Zhao et al., 2017). The toxicity and immunogenicity of polymers and carriers used in micelles have not yet been thoroughly studied, which is a concern in vaccine development (Yokoyama, 2014). Generally, SAPNs are preferred over structures containing lipids, due to the potential toxicity of lipopeptides and the risk of membrane disruption. Additionally, some lipid-based nanostructures are not strong inducers of cellular immune responses (Zhao et al., 2017).
On the other hand, some concerns regarding SAPNs have persisted, such as their stability in liquid environments and accurate size control during fabrication. Given the diversity of SAPN immunogenicity, this feature should be studied for each type of SAPN. Although SAPNs have not shown toxicity in cells, their long-term effects should be further investigated (Habibi et al., 2016).
7. Conclusions and future perspectives
Subunit epitope-based vaccines have been developed to overcome the safety concerns regarding the use of traditional vaccines. However, increasing the safety of new vaccines has adversely affected their immunogenicity, leading to a requirement for adjuvant usage. Susceptibility to enzymatic degradation is another issue with these vaccines. Recently, nanotechnology has been employed in vaccine development to augment vaccine immunogenicity and stability. SAPNs have shown some benefits over other nano-structured systems used for vaccine development, and their usage in vaccine design is emerging due to their advantages of multivalency, biodegradability, biocompatibility, and molecular specificity. The orderly repetitive array of antigens on SAPNs structures and their small sizes resemble PAMPs, leading to strong immune reactions. SAPN vaccines are mainly constructed from coiled-coil or β-sheet biostructural motifs to which the immunodominant B- and T-cell epitopes for various antigens that protect against bacteria or viruses are fused. Under controlled conditions, coiled-coils and β-sheets assemble into single nanoparticles in a variety of forms. These structures act as carriers and immune adjuvants to enhance both humoral and cellular immunity. This self-adjuvanting property is considered a key benefit of SAPNs and allows the use of vaccines without the unwanted inflammatory side effects and toxicities of adjuvants.
SAPNs are promising candidates as adjuvants/carriers for vaccines, including non-parenteral formulations. Despite the outstanding recent advances in SAPN design, a deeper understanding of the self-assembly phenomenon, factors that influence the process, and rational design principles is required to produce peptide nanoparticles with favorable characteristics.
Some challenges in the design, functionalization, and fabrication of SAPNs persist. In this context, achieving a controlled, specific size and monodispersity in production is very important, due to its key role in the uptake of antigens by APCs and, consequently, the induction of immune responses. Solid-phase methods usually allow the size of the synthesize peptides to be controlled. The increased stability of SAPNs at a wider pH range is another concern. Moreover, more detailed studies on the relations between the physicochemical and morphological features of SAPNs and their biological and immunological activities are needed.
In addition to the coiled-coils and β-sheets that are currently used in vaccine development research, more novel architectures can be introduced, such as combinations of α and β structures, coiled-coil hybrids that are not found in nature, and helix-loop-helix-loop repetitions. Each of these structures has its own special features and potential, and different desired characteristics may be achieved by selecting appropriate building subunits. Because the identified repeat protein motifs are a minor part of all probable structures, many possibilities can be explored using synthetic biology. In this regard, in silico modeling will assist in the discovery of novel self-assembled peptides with more appropriate structures and effectiveness from thousands of available natural peptides. The ab initio design of building blocks will substantially increase the opportunities to develop novel SAPNs. However, the immunogenicity of novel synthetic oligomers should be carefully tested. The architecture and the assembly pathway both should be investigated and controlled to achieve higher success rates in accurate SAPN design. Additionally, the specificity of oligomerization should be improved. The use of immunoinformatics to select immunodominant epitopes in addition to structural biology and computational modeling to achieve the desired biomolecules may also help. Continuous progress in improving computational algorithms would be an undeniable support in this process. Overall, SAPN usage in vaccine design seems very promising. The combination of the mentioned knowledge may lead to the exploitation of the auspicious potential of SAPNs in vaccine manufacturing and their successful transition to clinical use in the future.
Declarations of interest statement
The authors declare that there is no conflict of interest.
Acknowledgements
The authors wish to thank Shiraz University of Medical Sciences (13435) for supporting the conduct of this research.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.biotechadv.2017.05.002.
Appendix A. Supplementary data
Supplementary material
References
- Aggeli A., Fytas G., Vlassopoulos D., McLeish T.C., Mawer P.J., Boden N. Structure and dynamics of self-assembling β-sheet peptide tapes by dynamic light scattering. Biomacromolecules. 2001;2(2):378–388. doi: 10.1021/bm000080z. [DOI] [PubMed] [Google Scholar]
- Apostolovic B., Danial M., Klok H.-A. Coiled coils: attractive protein folding motifs for the fabrication of self-assembled, responsive and bioactive materials. Chem. Soc. Rev. 2010;39(9):3541–3575. doi: 10.1039/b914339b. [DOI] [PubMed] [Google Scholar]
- Arai R., Ueda H., Kitayama A., Kamiya N., Nagamune T. Design of the linkers which effectively separate domains of a bifunctional fusion protein. Protein Eng. 2001;14(8):529–532. doi: 10.1093/protein/14.8.529. [DOI] [PubMed] [Google Scholar]
- Ardejani M.S., Orner B.P. Obey the peptide assembly rules. Science. 2013;340(6132):561–562. doi: 10.1126/science.1237708. [DOI] [PubMed] [Google Scholar]
- Ardejani M.S., Orner B.P. Computationally assisted engineering of protein cages. In: Orner P.B., editor. Protein Cages: Methods and Protocols. Springer New York; New York, NY: 2015. pp. 51–59. [DOI] [PubMed] [Google Scholar]
- Astbury W. Some problems in the X-ray analysis of the structure of animal hairs and other protein fibres. Trans. Faraday Soc. 1933;29(140):193–205. [Google Scholar]
- Azmi F., Ahmad Fuaad A.A.H., Skwarczynski M., Toth I. Recent progress in adjuvant discovery for peptide-based subunit vaccines. Hum. Vaccin Immunother. 2014;10(3):778–796. doi: 10.4161/hv.27332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babapoor S., Neef T., Mittelholzer C., Girshick T., Garmendia A., Shang H., Khan M.I., Burkhard P. A novel vaccine using nanoparticle platform to present immunogenic M2e against avian influenza infection. Influenza Res. Treat. 2011;2011:126794. doi: 10.1155/2011/126794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann M.F., Jennings G.T. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 2010;10(11):787–796. doi: 10.1038/nri2868. [DOI] [PubMed] [Google Scholar]
- Baker K.A., Dutch R.E., Lamb R.A., Jardetzky T.S. Structural basis for paramyxovirus-mediated membrane fusion. Mol. Cell. 1999;3(3):309–319. doi: 10.1016/s1097-2765(00)80458-x. [DOI] [PubMed] [Google Scholar]
- Banner D.W., Kokkinidis M., Tsernoglou D. Structure of the ColE1 rop protein at 1.7 Å resolution. J. Mol. Biol. 1987;196(3):657–675. doi: 10.1016/0022-2836(87)90039-8. [DOI] [PubMed] [Google Scholar]
- Betz S.F., DeGrado W.F. Controlling topology and native-like behavior of de novo-designed peptides: design and characterization of antiparallel four-stranded coiled coils. Biochemistry. 1996;35(21):6955–6962. doi: 10.1021/bi960095a. [DOI] [PubMed] [Google Scholar]
- Betz S.F., Liebman P.A., DeGrado W.F. De novo design of native proteins: characterization of proteins intended to fold into antiparallel, rop-like, four-helix bundles. Biochemist. 1997;36(9):2450–2458. doi: 10.1021/bi961704h. [DOI] [PubMed] [Google Scholar]
- Blaszczyk M., Jamroz M., Kmiecik S., Kolinski A. CABS-fold: server for the de novo and consensus-based prediction of protein structure. Nucleic Acids Res. 2013;41(Web Server issue):W406–W411. doi: 10.1093/nar/gkt462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolhassani A., Javanzad S., Saleh T., Hashemi M., Aghasadeghi M.R., Sadat S.M. Polymeric nanoparticles: potent vectors for vaccine delivery targeting cancer and infectious diseases. Hum. Vaccin Immunother. 2014;10(2):321–332. doi: 10.4161/hv.26796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowerman C.J., Nilsson B.L. Review self-assembly of amphipathic β-sheet peptides: insights and applications. Biopolymers. 2012;98(3):169–184. doi: 10.1002/bip.22058. [DOI] [PubMed] [Google Scholar]
- Branco M.C., Sigano D.M., Schneider J.P. Materials from peptide assembly: towards the treatment of cancer and transmittable disease. Curr. Opin. Chem. Biol. 2011;15(3):427–434. doi: 10.1016/j.cbpa.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodin J.D., Ambroggio X.I., Tang C., Parent K.N., Baker T.S., Tezcan F.A. Metal-directed, chemically tunable assembly of one-, two- and three-dimensional crystalline protein arrays. Nat. Chem. 2012;4(5):375–382. doi: 10.1038/nchem.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broom A., Doxey A.C., Lobsanov Y.D., Berthin L.G., Rose D.R., Howell P.L., McConkey B.J., Meiering E.M. Modular evolution and the origins of symmetry: reconstruction of a three-fold symmetric globular protein. Structure. 2012;20(1):161–171. doi: 10.1016/j.str.2011.10.021. [DOI] [PubMed] [Google Scholar]
- Brunette T.J., Parmeggiani F., Huang P.-S., Bhabha G., Ekiert D.C., Tsutakawa S.E., Hura G.L., Tainer J.A., Baker D. Exploring the repeat protein universe through computational protein design. Nature. 2015;528(7583):580–584. doi: 10.1038/nature16162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhard P., Stetefeld J., Strelkov S.V. Coiled coils: a highly versatile protein folding motif. Trends Cell Biol. 2001;11(2):82–88. doi: 10.1016/s0962-8924(00)01898-5. [DOI] [PubMed] [Google Scholar]
- Busseron E., Ruff Y., Moulin E., Giuseppone N. Supramolecular self-assemblies as functional nanomaterials. Nano. 2013;5(16):7098–7140. doi: 10.1039/c3nr02176a. [DOI] [PubMed] [Google Scholar]
- Cachia P.J., Kao D.J., Hodges R.S. Synthetic peptide vaccine development: measurement of polyclonal antibody affinity and cross-reactivity using a new peptide capture and release system for surface plasmon resonance spectroscopy. J. Mol. Recognit. 2004;17(6):540–557. doi: 10.1002/jmr.682. [DOI] [PubMed] [Google Scholar]
- Caplan M.R., Schwartzfarb E.M., Zhang S., Kamm R.D., Lauffenburger D.A. Control of self-assembling oligopeptide matrix formation through systematic variation of amino acid sequence. Biomaterials. 2002;23(1):219–227. doi: 10.1016/s0142-9612(01)00099-0. [DOI] [PubMed] [Google Scholar]
- Carlson J.C.T., Jena S.S., Flenniken M., Chou, T.-f., Siegel, R.A., Wagner, C.R. Chemically controlled self-assembly of protein nanorings. J. Am. Chem. Soc. 2006;128(23):7630–7638. doi: 10.1021/ja060631e. [DOI] [PubMed] [Google Scholar]
- Chan D.C., Fass D., Berger J.M., Kim P.S. Core structure of gp41 from the HIV envelope glycoprotein. Cell. 1997;89(2):263–273. doi: 10.1016/s0092-8674(00)80205-6. [DOI] [PubMed] [Google Scholar]
- Changela A., Chen K., Xue Y., Holschen J., Outten C.E., O'Halloran T.V., Mondragón A. Molecular basis of metal-ion selectivity and zeptomolar sensitivity by CueR. Science. 2003;301(5638):1383–1387. doi: 10.1126/science.1085950. [DOI] [PubMed] [Google Scholar]
- Chao H., Bautista D.L., Litowski J., Irvin R.T., Hodges R.S. Use of a heterodimeric coiled-coil system for biosensor application and affinity purification. J. Chromatogr. B Biomed. Sci. Appl. 1998;715(1):307–329. doi: 10.1016/s0378-4347(98)00172-8. [DOI] [PubMed] [Google Scholar]
- Chen P. Self-assembly of ionic-complementary peptides: a physicochemical viewpoint. Colloids Surf. A Physicochem. Eng. Asp. 2005;261(1):3–24. [Google Scholar]
- Chen J., Pompano R.R., Santiago F.W., Maillat L., Sciammas R., Sun T., Han H., Topham D.J., Chong A.S., Collier J.H. The use of self-adjuvanting nanofiber vaccines to elicit high-affinity B cell responses to peptide antigens without inflammation. Biomaterials. 2013;34(34):8776–8785. doi: 10.1016/j.biomaterials.2013.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesson C.B., Huelsmann E.J., Lacek A.T., Kohlhapp F.J., Webb M.F., Nabatiyan A., Zloza A., Rudra J.S. Antigenic peptide nanofibers elicit adjuvant-free CD8(+) T cell responses. Vaccine. 2014;32(10):1174–1180. doi: 10.1016/j.vaccine.2013.11.047. [DOI] [PubMed] [Google Scholar]
- Chiu Y.C., Gammon J.M., Andorko J.I., Tostanoski L.H., Jewell C.M. Assembly and immunological processing of polyelectrolyte multilayers composed of antigens and adjuvants. ACS Appl. Mater. Interfaces. 2016;8(29):18722–18731. doi: 10.1021/acsami.6b06275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coin I., Beyermann M., Bienert M. Solid-phase peptide synthesis: from standard procedures to the synthesis of difficult sequences. Nat. Protoc. 2007;2(12):3247–3256. doi: 10.1038/nprot.2007.454. [DOI] [PubMed] [Google Scholar]
- Collier J.H., Messersmith P.B. Enzymatic modification of self-assembled peptide structures with tissue transglutaminase. Bioconjug. Chem. 2003;14(4):748–755. doi: 10.1021/bc034017t. [DOI] [PubMed] [Google Scholar]
- Cordeiro A.S., Alonso M.J., de la Fuente M. Nanoengineering of vaccines using natural polysaccharides. Biotechnol. Adv. 2015;33(6 Pt 3):1279–1293. doi: 10.1016/j.biotechadv.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick F.H. Is α-keratin a coiled coil? Nature. 1952;170(4334):882–883. doi: 10.1038/170882b0. [DOI] [PubMed] [Google Scholar]
- Crick F.H. The Fourier transform of a coiled-coil. Acta Cryst. 1953;6(8–9):685–689. [Google Scholar]
- Crick F.H. The packing of α-helices: simple coiled-coils. Acta Cryst. 1953;6(8–9):689–697. [Google Scholar]
- Crick F.H.C., Watson J.D. Structure of small viruses. Nature. 1956;177(4506):473–475. doi: 10.1038/177473a0. [DOI] [PubMed] [Google Scholar]
- Deng Y., Liu J., Zheng Q., Yong W., Lu M. Structures and polymorphic interactions of two heptad-repeat regions of the SARS virus S2 protein. Structure. 2006;14(5):889–899. doi: 10.1016/j.str.2006.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y., Liu J., Lu S., Igwize J., Xu W., Kuang D., Zealey C., Liu D., Gregor A., Bozorgzad A. Self-assembling peptide for co-delivery of HIV-1 CD8 + T cell epitope and toll-like receptor 7/8 agonists R848 to induce maturation of monocyte derived dendritic cell and augment polyfunctional cytotoxic T lymphocyte sCTL response. J. Control. Release. 2016;236:22–30. doi: 10.1016/j.jconrel.2016.06.019. [DOI] [PubMed] [Google Scholar]
- Doll T.A., Raman S., Dey R., Burkhard P. Nanoscale assemblies and their biomedical applications. J. R. Soc. Interface. 2013;10(80):20120740. doi: 10.1098/rsif.2012.0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll T.A., Dey R., Burkhard P. Design and optimization of peptide nanoparticles. J. Nanobiotechnology. 2015;13:73. doi: 10.1186/s12951-015-0119-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll T.A., Neef T., Duong N., Lanar D.E., Ringler P., Muller S.A., Burkhard P. Optimizing the design of protein nanoparticles as carriers for vaccine applications. Nanomedicine. 2015;11(7):1705–1713. doi: 10.1016/j.nano.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle L., Hallinan J., Bolduc J., Parmeggiani F., Baker D., Stoddard B.L., Bradley P. Rational design of alpha-helical tandem repeat proteins with closed architectures. Nature. 2015;528(7583):585–588. doi: 10.1038/nature16191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drexler K.E. Molecular engineering: an approach to the development of general capabilities for molecular manipulation. Proc. Natl. Acad. Sci. U. S. A. 1981;78(9):5275–5278. doi: 10.1073/pnas.78.9.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg D. The discovery of the α-helix and β-sheet, the principal structural features of proteins. Proc. Natl. Acad. Sci. U. S. A. 2003;100(20):11207–11210. doi: 10.1073/pnas.2034522100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Bissati K., Zhou Y., Dasgupta D., Cobb D., Dubey J.P., Burkhard P., Lanar D.E., McLeod R. Effectiveness of a novel immunogenic nanoparticle platform for toxoplasma peptide vaccine in HLA transgenic mice. Vaccine. 2014;32(26):3243–3248. doi: 10.1016/j.vaccine.2014.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsawy M.A., Smith A.M., Hodson N., Squires A., Miller A.F., Saiani A. Modification of β-sheet forming peptide hydrophobic face: effect on self-assembly and gelation. Langmuir. 2016;32(19):4917–4923. doi: 10.1021/acs.langmuir.5b03841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshaghi S., Niegowski D., Kohl A., Molina D.M., Lesley S.A., Nordlund P. Crystal structure of a divalent metal ion transporter CorA at 2.9 angstrom resolution. Science. 2006;313(5785):354–357. doi: 10.1126/science.1127121. [DOI] [PubMed] [Google Scholar]
- Eskandari S., Guerin T., Toth I., Stephenson R.J. Recent advances in self-assembled peptides: implications for targeted drug delivery and vaccine engineering. Adv. Drug. Deliv. Rev. 2016 doi: 10.1016/j.addr.2016.06.013. [DOI] [PubMed] [Google Scholar]
- Fairman R., Akerfeldt K.S. Peptides as novel smart materials. Curr. Opin. Struct. Biol. 2005;15(4):453–463. doi: 10.1016/j.sbi.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Farhadi T., Nezafat N., Ghasemi Y., Karimi Z., Hemmati S., Erfani N. Designing of complex multi-epitope peptide vaccine based on omps of Klebsiella pneumoniae: an in silico approach. Int. J. Pept. Res. Ther. 2015;21(3):325–341. [Google Scholar]
- Fleishman Sarel J., Baker D. Role of the biomolecular energy gap in protein design, structure, and evolution. Cell. 2012;149(2):262–273. doi: 10.1016/j.cell.2012.03.016. [DOI] [PubMed] [Google Scholar]
- Fletcher J.M., Harniman R.L., Barnes F.R., Boyle A.L., Collins A., Mantell J., Sharp T.H., Antognozzi M., Booth P.J., Linden N., Miles M.J., Sessions R.B., Verkade P., Woolfson D.N. Self-assembling cages from coiled-coil peptide modules. Science. 2013;340(6132):595–599. doi: 10.1126/science.1233936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foged C. Subunit vaccines of the future: the need for safe, customized and optimized particulate delivery systems. Ther. Deliv. 2011;2(8):1057–1077. doi: 10.4155/tde.11.68. [DOI] [PubMed] [Google Scholar]
- Francica J.R., Lynn G.M., Laga R., Joyce M.G., Ruckwardt T.J., Morabito K.M., Chen M., Chaudhuri R., Zhang B., Sastry M., Druz A., Ko K., Choe M., Pechar M., Georgiev I.S., Kueltzo L.A., Seymour L.W., Mascola J.R., Kwong P.D., Graham B.S., Seder R.A. Thermoresponsive polymer nanoparticles co-deliver RSV F trimers with a TLR-7/8 adjuvant. Bioconjug. Chem. 2016;27(10):2372–2385. doi: 10.1021/acs.bioconjchem.6b00370. [DOI] [PubMed] [Google Scholar]
- Freedman S.J., Song H.K., Xu Y., Sun Z.-Y.J., Eck M.J. Homotetrameric structure of the SNAP-23 N-terminal coiled-coil domain. J. Biol. Chem. 2003;278(15):13462–13467. doi: 10.1074/jbc.M210483200. [DOI] [PubMed] [Google Scholar]
- Friedrich B.M., Beasley D.W.C., Rudra J.S. Supramolecular peptide hydrogel adjuvanted subunit vaccine elicits protective antibody responses against West Nile virus. Vaccine. 2016;34(46):5479–5482. doi: 10.1016/j.vaccine.2016.09.044. [DOI] [PubMed] [Google Scholar]
- Fujita Y., Taguchi H. Current status of multiple antigen-presenting peptide vaccine systems: application of organic and inorganic nanoparticles. Chem. Cent. J. 2011;5(1):1. doi: 10.1186/1752-153X-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung S.Y., Yang H., Sadatmousavi P., Sheng Y., Mamo T., Nazarian R., Chen P. Amino acid pairing for de novo design of self-assembling peptides and their drug delivery potential. Adv. Funct. Mater. 2011;21(13):2456–2464. [Google Scholar]
- Gamblin S., Haire L., Russell R., Stevens D., Xiao B., Ha Y., Vasisht N., Steinhauer D., Daniels R., Elliot A. The structure and receptor binding properties of the 1918 influenza hemagglutinin. Science. 2004;303(5665):1838–1842. doi: 10.1126/science.1093155. [DOI] [PubMed] [Google Scholar]
- Gasteiger E., Hoogland C., Gattiker A., Duvaud S.e., Wilkins M.R., Appel R.D., Bairoch A. Springer; 2005. Protein Identification and Analysis Tools on the ExPASy Server. [DOI] [PubMed] [Google Scholar]
- Glover J.M., Harrison S.C. Crystal structure of the heterodimeric bZIP transcription factor c-Fos–c-Jun bound to DNA. Nature. 1995;373(6511):257–261. doi: 10.1038/373257a0. [DOI] [PubMed] [Google Scholar]
- Golkar N., Samani S.M., Tamaddon A.M. Cholesterol-conjugated supramolecular assemblies of low generations polyamidoamine dendrimers for enhanced EGFP plasmid DNA transfection. J. Nanopart. Res. 2016;18(5):1–20. [Google Scholar]
- Golkar N., Samani S.M., Tamaddon A.M. Modulated cellular delivery of anti-VEGF siRNA (bevasiranib) by incorporating supramolecular assemblies of hydrophobically modified polyamidoamine dendrimer in stealth liposomes. Int. J. Pharm. 2016;510(1):30–41. doi: 10.1016/j.ijpharm.2016.06.026. [DOI] [PubMed] [Google Scholar]
- Golkar N., Tamaddon A.M., Samani S.M. Effect of lipid composition on incorporation of trastuzumab-PEG-lipid into nanoliposomes by post-insertion method: physicochemical and cellular characterization. J Liposome Res. 2016;26(2):113–125. doi: 10.3109/08982104.2015.1048692. [DOI] [PubMed] [Google Scholar]
- Gonzalez L., Plecs J.J., Alber T. An engineered allosteric switch in leucine-zipper oligomerization. Nat. Struct. Mol. Biol. 1996;3(6):510–515. doi: 10.1038/nsb0696-510. [DOI] [PubMed] [Google Scholar]
- Goodwill K.E., Sabatier C., Marks C., Raag R., Fitzpatrick P.F., Stevens R.C. Crystal structure of tyrosine hydroxylase at 2.3 Å and its implications for inherited neurodegenerative diseases. Nat. Struct. Mol. Biol. 1997;4(7):578–585. doi: 10.1038/nsb0797-578. [DOI] [PubMed] [Google Scholar]
- Graddis T.J., Myszka D.G., Chaiken I.M. Controlled formation of model homo-and heterodimer coiled coil polypeptides. Biochemistry. 1993;32(47):12664–12671. doi: 10.1021/bi00210a015. [DOI] [PubMed] [Google Scholar]
- Grigoryan G., Kim Y.H., Acharya R., Axelrod K., Jain R.M., Willis L., Drndic M., Kikkawa J.M., DeGrado W.F. Computational design of virus-like protein assemblies on carbon nanotube surfaces. Science. 2011;332(6033):1071–1076. doi: 10.1126/science.1198841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groft C.M., Burley S.K. Recognition of eIF4G by rotavirus NSP3 reveals a basis for mRNA circularization. Mol. Cell. 2002;9(6):1273–1283. doi: 10.1016/s1097-2765(02)00555-5. [DOI] [PubMed] [Google Scholar]
- Habibi N., Kamaly N., Memic A., Shafiee H. Self-assembled peptide-based nanostructures: smart nanomaterials toward targeted drug delivery. Nano Today. 2016;11(1):41–60. doi: 10.1016/j.nantod.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajighahramani N., Nezafat N., Eslami M., Negahdaripour M., Rahmatabadi S.S., Ghasemi Y. Immunoinformatics analysis and in silico designing of a novel multi-epitope peptide vaccine against Staphylococcus aureus. Infect. Genet. Evol. 2017;48:83–94. doi: 10.1016/j.meegid.2016.12.010. [DOI] [PubMed] [Google Scholar]
- Håkansson K., Lim N.K., Hoppe H.-J., Reid K.B.M. Crystal structure of the trimeric α-helical coiled-coil and the three lectin domains of human lung surfactant protein D. Structure. 1999;7(3):255–264. doi: 10.1016/s0969-2126(99)80036-7. [DOI] [PubMed] [Google Scholar]
- Handel T.M., Williams S.A., DeGrado W.F. Metal ion-dependent modulation of the dynamics of a designed protein. Science. 1993;261:879. doi: 10.1126/science.8346440. [DOI] [PubMed] [Google Scholar]
- Harrison C.J., Hayer-Hartl M., Di Liberto M., Hartl F.-U., Kuriyan J. Crystal structure of the nucleotide exchange factor GrpE bound to the ATPase domain of the molecular chaperone DnaK. Science. 1997;276(5311):431–435. doi: 10.1126/science.276.5311.431. [DOI] [PubMed] [Google Scholar]
- Hartgerink J.D., Granja J.R., Milligan R.A., Ghadiri M.R. Self-assembling peptide nanotubes. J. Am. Chem. Soc. 1996;118(1):43–50. [Google Scholar]
- Hattori M., Tanaka Y., Fukai S., Ishitani R., Nureki O. Crystal structure of the MgtE Mg2 + transporter. Nature. 2007;448(7157):1072–1075. doi: 10.1038/nature06093. [DOI] [PubMed] [Google Scholar]
- Hauser C.A.E., Zhang S. Designer self-assembling peptide nanofiber biological materials. Chem. Soc. Rev. 2010;39(8):2780–2790. doi: 10.1039/b921448h. [DOI] [PubMed] [Google Scholar]
- Ho S.P., DeGrado W.F. Design of a 4-helix bundle protein: synthesis of peptides which self-associate into a helical protein. J. Am. Chem. Soc. 1987;109(22):6751–6758. [Google Scholar]
- Hodges R.S. De novo design of α-helical proteins: basic research to medical applications. Biochem. Cell Biol. 1996;74:133–154. doi: 10.1139/o96-015. [DOI] [PubMed] [Google Scholar]
- Hodges R.S., Saund A.K., Chong P., St-Pierre S., Reid R.E. Synthetic model for two-stranded alpha-helical coiled-coils. Design, synthesis, and characterization of an 86-residue analog of tropomyosin. J. Biol. Chem. 1981;256(3):1214–1224. [PubMed] [Google Scholar]
- Hong Y., Legge R.L., Zhang S., Chen P. Effect of amino acid sequence and pH on nanofiber formation of self-assembling peptides EAK16-II and EAK16-IV. Biomacromolecules. 2003;4(5):1433–1442. doi: 10.1021/bm0341374. [DOI] [PubMed] [Google Scholar]
- Hong Y., Lau L.S., Legge R.L., Chen P. Critical self-assembly concentration of an ionic-complementary peptide EAK16-I. J. Adhes. 2004;80(10 − 11):913–931. [Google Scholar]
- Horikoshi S., Serpone N. 2013. Introduction to Nanoparticles. Microwaves in Nanoparticle Synthesis: Fundamentals and Applications; pp. 1–24. [Google Scholar]
- Howorka S. Rationally engineering natural protein assemblies in nanobiotechnology. Curr. Opin. Biotechnol. 2011;22(4):485–491. doi: 10.1016/j.copbio.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Huang P.S., Ban Y.E.A., Richter F., Andre I., Vernon R., Schief W.R., Baker D. RosettaRemodel: a generalized framework for flexible backbone protein design. PLoS One. 2011;6(8) doi: 10.1371/journal.pone.0024109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P.S., Feldmeier K., Parmeggiani F., Fernandez Velasco D.A. De novo design of a four-fold symmetric TIM-barrel protein with atomic-level accuracy. Nat. Chem. Biol. 2016;12(1):29–34. doi: 10.1038/nchembio.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbell J.A., Thomas S.N., Swartz M.A. Materials engineering for immunomodulation. Nature. 2009;462(7272):449–460. doi: 10.1038/nature08604. [DOI] [PubMed] [Google Scholar]
- Hudalla G.A., Modica J.A., Tian Y.F., Rudra J.S., Chong A.S., Sun T., Mrksich M., Collier J.H. A self-adjuvanting supramolecular vaccine carrying a folded protein antigen. Adv. Healthc. Mater. 2013;2(8):1114–1119. doi: 10.1002/adhm.201200435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang W., Marini D.M., Kamm R.D., Zhang S. Supramolecular structure of helical ribbons self-assembled from a β-sheet peptide. J. Chem. Phys. 2003;118(1):389–397. [Google Scholar]
- Indelicato G., Wahome N., Ringler P., Muller S.A., Nieh M.P., Burkhard P., Twarock R. Principles governing the self-assembly of coiled-coil protein nanoparticles. Biophys. J. 2016;110(3):646–660. doi: 10.1016/j.bpj.2015.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J.P., Nagaraj A.K., Fox E.K., Rudra J.S., Devgun J.M., Collier J.H. Co-assembling peptides as defined matrices for endothelial cells. Biomaterials. 2009;30(12):2400–2410. doi: 10.1016/j.biomaterials.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaba S.A., Brando C., Guo Q., Mittelholzer C., Raman S., Tropel D., Aebi U., Burkhard P., Lanar D.E. A nonadjuvanted polypeptide nanoparticle vaccine confers long-lasting protection against rodent malaria. J. Immunol. (Baltimore, Md.: 1950) 2009;183(11):7268–7277. doi: 10.4049/jimmunol.0901957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaba S.A., McCoy M.E., Doll T.A., Brando C., Guo Q., Dasgupta D., Yang Y., Mittelholzer C., Spaccapelo R., Crisanti A. Protective antibody and CD8 + T-cell responses to the Plasmodium falciparum circumsporozoite protein induced by a nanoparticle vaccine. PLoS One. 2012;7(10) doi: 10.1371/journal.pone.0048304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltofen S., Li C., Huang P.S., Serpell L.C., Barth A., Andre I. Computational de novo design of a self-assembling peptide with predefined structure. J. Mol. Biol. 2015;427(2):550–562. doi: 10.1016/j.jmb.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Karch C.P., Burkhard P. Vaccine technologies: from whole organisms to rationally designed protein assemblies. Biochem. Pharmacol. 2016;120:1–14. doi: 10.1016/j.bcp.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Kwon S., Kim S.H., Lee C.K., Lee J.H., Cho S.J., Lee H.S., Ihee H. Microtubes with rectangular cross-section by self-assembly of a short beta-peptide foldamer. J. Am. Chem. Soc. 2012;134(51):20573–20576. doi: 10.1021/ja3088482. [DOI] [PubMed] [Google Scholar]
- King N.P., Lai Y.-T. Practical approaches to designing novel protein assemblies. Curr. Opin. Struct. Biol. 2013;23(4):632–638. doi: 10.1016/j.sbi.2013.06.002. [DOI] [PubMed] [Google Scholar]
- King N.P., Sheffler W., Sawaya M.R., Vollmar B.S., Sumida J.P., André I., Gonen T., Yeates T.O., Baker D. Computational design of self-assembling protein nanomaterials with atomic level accuracy. Science. 2012;336(6085):1171–1174. doi: 10.1126/science.1219364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King N.P., Bale J.B., Sheffler W., McNamara D.E., Gonen S., Gonen T., Yeates T.O., Baker D. Accurate design of co-assembling multi-component protein nanomaterials. Nature. 2014;510(7503):103–108. doi: 10.1038/nature13404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyokawa T., Kanaori K., Tajima K., Koike M., Mizuno T., Oku J.I., Tanaka T. Binding of Cu (II) or Zn (II) in a de novo designed triple-stranded α-helical coiled-coil toward a prototype for a metalloenzyme. J. Pept. Res. 2004;63(4):347–353. doi: 10.1111/j.1399-3011.2004.00109.x. [DOI] [PubMed] [Google Scholar]
- Kumar P., Pillay V., Modi G., Choonara Y.E., du Toit L.C., Naidoo D. Self-assembling peptides: implications for patenting in drug delivery and tissue engineering. Recent Pat. Drug. Deliv. Formul. 2011;5(1):24–51. doi: 10.2174/187221111794109510. [DOI] [PubMed] [Google Scholar]
- Kushnir N., Streatfield S.J., Yusibov V. Virus-like particles as a highly efficient vaccine platform: diversity of targets and production systems and advances in clinical development. Vaccine. 2012;31(1):58–83. doi: 10.1016/j.vaccine.2012.10.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y.T., King N.P., Yeates T.O. Principles for designing ordered protein assemblies. Trends Cell Biol. 2012;22(12):653–661. doi: 10.1016/j.tcb.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Lakshmanan A., Zhang S., Hauser C.A.E. Short self-assembling peptides as building blocks for modern nanodevices. Trends Biotechnol. 2012;30(3):155–165. doi: 10.1016/j.tibtech.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Lavigne P., Crump M.P., Gagné S.M., Hodges R.S., Kay C.M., Sykes B.D. Insights into the mechanism of heterodimerization from the 1H-NMR solution structure of the c-Myc-Max heterodimeric leucine zipper. J. Mol. Biol. 1998;281(1):165–181. doi: 10.1006/jmbi.1998.1914. [DOI] [PubMed] [Google Scholar]
- Leaver-Fay A., Tyka M., Lewis S.M., Lange O.F., Thompson J., Jacak R., Kaufman K., Renfrew P.D., Smith C.A., Sheffler W., Davis I.W., Cooper S., Treuille A., Mandell D.J., Richter F., Ban Y.E., Fleishman S.J., Corn J.E., Kim D.E., Lyskov S., Berrondo M., Mentzer S., Popovic Z., Havranek J.J., Karanicolas J., Das R., Meiler J., Kortemme T., Gray J.J., Kuhlman B., Baker D., Bradley P. ROSETTA3: an object-oriented software suite for the simulation and design of macromolecules. Methods Enzymol. 2011;487:545–574. doi: 10.1016/B978-0-12-381270-4.00019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.h., Choi Y.J., Lim Y.b. Self-assembled filamentous nanostructures for drug/gene delivery applications. Expert. Opin. Drug Deliv. 2010;7(3):341–351. doi: 10.1517/17425240903559841. [DOI] [PubMed] [Google Scholar]
- Lensink M.F., Velankar S., Kryshtafovych A., Huang S.Y., Schneidman-Duhovny D., Sali A., Segura J., Fernandez-Fuentes N., Viswanath S., Elber R., Grudinin S., Popov P., Neveu E., Lee H., Baek M., Park S., Heo L., Rie Lee G., Seok C., Qin S., Zhou H.X., Ritchie D.W., Maigret B., Devignes M.D., Ghoorah A., Torchala M., Chaleil R.A., Bates P.A., Ben-Zeev E., Eisenstein M., Negi S.S., Weng Z., Vreven T., Pierce B.G., Borrman T.M., Yu J., Ochsenbein F., Guerois R., Vangone A., Rodrigues J.P., van Zundert G., Nellen M., Xue L., Karaca E., Melquiond A.S., Visscher K., Kastritis P.L., Bonvin A.M., Xu X., Qiu L., Yan C., Li J., Ma Z., Cheng J., Zou X., Shen Y., Peterson L.X., Kim H.R., Roy A., Han X., Esquivel-Rodriguez J., Kihara D., Yu X., Bruce N.J., Fuller J.C., Wade R.C., Anishchenko I., Kundrotas P.J., Vakser I.A., Imai K., Yamada K., Oda T., Nakamura T., Tomii K., Pallara C., Romero-Durana M., Jimenez-Garcia B., Moal I.H., Fernandez-Recio J., Joung J.Y., Kim J.Y., Joo K., Lee J., Kozakov D., Vajda S., Mottarella S., Hall D.R., Beglov D., Mamonov A., Xia B., Bohnuud T., Del Carpio C.A., Ichiishi E., Marze N., Kuroda D., Roy Burman S.S., Gray J.J., Chermak E., Cavallo L., Oliva R., Tovchigrechko A., Wodak S.J. Prediction of homo- and hetero-protein complexes by protein docking and template-based modeling: a CASP-CAPRI experiment. Proteins. 2016;(Suppl. 1):323–348. doi: 10.1002/prot.25007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M., Chang G., Horton N.C., Kercher M.A. Crystal structure of the lactose operon repressor and its complexes with DNA and inducer. Science. 1996;271(5253):1247. doi: 10.1126/science.271.5253.1247. [DOI] [PubMed] [Google Scholar]
- Li X., Suzuki K., Kanaori K., Tajima K., Kashiwada A., Hiroaki H., Kohda D., Tanaka T. Soft metal ions, cd (II) and Hg (II), induce triple-stranded [alpha]-helical assembly and folding of a de novo designed peptide in their trigonal geometries. Protein Sci. 2000;9(07):1327–1333. doi: 10.1110/ps.9.7.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litowski J., Hodges R. Designing heterodimeric two-stranded α-helical coiled-coils: the effect of chain length on protein folding, stability and specificity. J. Pept. Res. 2001;58(6):477–492. doi: 10.1034/j.1399-3011.2001.10972.x. [DOI] [PubMed] [Google Scholar]
- Liu Y., Yin Y., Wang L., Zhang W., Chen X., Yang X., Xu J., Ma G. Surface hydrophobicity of microparticles modulates adjuvanticity. J. Mater. Chem. B Mater. Biol. Med. 2013;1:3888–3896. doi: 10.1039/c3tb20383b. [DOI] [PubMed] [Google Scholar]
- Longo L.M., Kumru O.S., Middaugh C.R., Blaber M. Evolution and design of protein structure by folding nucleus symmetric expansion. Structure. 2014;22(10):1377–1384. doi: 10.1016/j.str.2014.08.008. [DOI] [PubMed] [Google Scholar]
- Loo Y., Zhang S., Hauser C.A. From short peptides to nanofibers to macromolecular assemblies in biomedicine. Biotechnol. Adv. 2012;30(3):593–603. doi: 10.1016/j.biotechadv.2011.10.004. [DOI] [PubMed] [Google Scholar]
- López-Sagaseta J., Malito E., Rappuoli R., Bottomley M.J. Self-assembling protein nanoparticles in the design of vaccines. Comput. Struct. Biotechnol. J. 2016;14:58–68. doi: 10.1016/j.csbj.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupas A. Coiled coils: new structures and new functions. Trends Biochem. Sci. 1996;21(10):375–382. [PubMed] [Google Scholar]
- Lynn G.M., Laga R., Darrah P.A., Ishizuka A.S. In vivo characterization of the physicochemical properties of polymer-linked TLR agonists that enhance vaccine immunogenicity. Nat. Biotechnol. 2015;33(11):1201–1210. doi: 10.1038/nbt.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoodi S., Nezafat N., Barzegar A., Negahdaripour M., Nikanfar A.R., Zarghami N., Ghasemi Y. Harnessing bioinformatics for designing a novel multiepitope peptide vaccine against breast cancer. Curr. Pharm. Biotechnol. 2016;17(12):1100–1114. doi: 10.2174/1389201017666160914191106. [DOI] [PubMed] [Google Scholar]
- Malashkevich V.N., Kammerer R.A., Efimov V.P., Schulthess T., Engle J. The crystal structure of a five-stranded coiled coil in COMP: a prototype ion channel? Science. 1996;274(5288):761. doi: 10.1126/science.274.5288.761. [DOI] [PubMed] [Google Scholar]
- Mandal D., Nasrolahi Shirazi A., Parang K. Self-assembly of peptides to nanostructures. Org. Biomol. Chem. 2014;12(22):3544–3561. doi: 10.1039/c4ob00447g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mant C.T., Tripet B., Hodges R.S. Temperature profiling of polypeptides in reversed-phase liquid chromatography: II. Monitoring of folding and stability of two-stranded α-helical coiled-coils. J. Chromatogr. A. 2003;1009(1):45–59. doi: 10.1016/s0021-9673(03)00919-1. [DOI] [PubMed] [Google Scholar]
- Marini D.M., Hwang W., Lauffenburger D.A., Zhang S., Kamm R.D. Left-handed helical ribbon intermediates in the self-assembly of a β-sheet peptide. Nano Lett. 2002;2(4):295–299. [Google Scholar]
- Marsh J.A., Hernandez H., Hall Z., Ahnert S.E., Perica T., Robinson C.V., Teichmann S.A. Protein complexes are under evolutionary selection to assemble via ordered pathways. Cell. 2013;153(2):461–470. doi: 10.1016/j.cell.2013.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateu M.G. Assembly, stability and dynamics of virus capsids. Arch. Biochem. Biophys. 2013;531(1–2):65–79. doi: 10.1016/j.abb.2012.10.015. [DOI] [PubMed] [Google Scholar]
- Maurice M.S., Mera P.E., Taranto M.P., Sesma F., Escalante-Semerena J.C., Rayment I. Structural characterization of the active site of the PduO-type ATP: Co (I)rrinoid adenosyltransferase from Lactobacillus reuteri. J. Biol. Chem. 2007;282(4):2596–2605. doi: 10.1074/jbc.M609557200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J.R., Ruvinov S.B., Pastan I., Vinson C. Designed heterodimerizing leucine zippers with a ranger of pIs and stabilities up to 10–15 M. Protein Sci. 2001;10(3):649–655. doi: 10.1110/ps.39401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monastyrskyy B., D'Andrea D., Fidelis K., Tramontano A., Kryshtafovych A. New encouraging developments in contact prediction: assessment of the CASP11 results. Proteins Suppl. 2015;1:131–144. doi: 10.1002/prot.24943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monera O.D., Zhou N.E., Kay C.M., Hodges R.S. Comparison of antiparallel and parallel two-stranded alpha-helical coiled-coils. Design, synthesis, and characterization. J. Biol. Chem. 1993;268(26):19218–19227. [PubMed] [Google Scholar]
- Moyle P.M., Toth I. Modern subunit vaccines: development, components, and research opportunities. ChemMedChem. 2013;8(3):360–376. doi: 10.1002/cmdc.201200487. [DOI] [PubMed] [Google Scholar]
- Myszka D.G., Chaiken I.M. Design and characterization of an intramolecular antiparallel coiled coil peptide. Biochemistry. 1994;33(9):2363–2372. doi: 10.1021/bi00175a003. [DOI] [PubMed] [Google Scholar]
- Nagy K.J., Giano M.C., Jin A., Pochan D.J., Schneider J.P. Enhanced mechanical rigidity of hydrogels formed from enantiomeric peptide assemblies. J. Am. Chem. Soc. 2011;133(38):14975–14977. doi: 10.1021/ja206742m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negahdaripour M., Nezafat N., Ghasemi Y. A panoramic review and in silico analysis of IL-11 structure and function. Cytokine Growth Factor Rev. 2016;32:41–61. doi: 10.1016/j.cytogfr.2016.06.002. [DOI] [PubMed] [Google Scholar]
- Negahdaripour M., Nezafat N., Hajighahramani N., Rahmatabadi S.S., Morowvat M.H., Ghasemi Y. In silico study of different signal peptides for secretory production of interleukin-11 in Escherichia coli. Curr. Proteomics. 2017;14:1–10. [Google Scholar]
- Netzer R., Fleishman S.J. Programming protein shapes and specificities by modular design. Science. 2016;352(6286):657–658. doi: 10.1126/science.aaf7599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezafat N., Ghasemi Y., Javadi G., Khoshnoud M.J., Omidinia E. A novel multi-epitope peptide vaccine against cancer: an in silico approach. J. Theor. Biol. 2014;349:121–134. doi: 10.1016/j.jtbi.2014.01.018. [DOI] [PubMed] [Google Scholar]
- Nezafat N., Karimi Z., Eslami M., Mohkam M., Zandian S., Ghasemi Y. Designing an efficient multi-epitope peptide vaccine against Vibrio cholerae via combined immunoinformatics and protein interaction based approaches. Comput. Biol. Chem. 2016;62:82–95. doi: 10.1016/j.compbiolchem.2016.04.006. [DOI] [PubMed] [Google Scholar]
- Nezafat N., Eslami M., Negahdaripour M., Rahbar M.R., Ghasemi Y. Designing an efficient multi-epitope oral vaccine against Helicobacter pylori using immunoinformatics and structural vaccinology approaches. Mol. BioSyst. 2017;13:699–713. doi: 10.1039/c6mb00772d. [DOI] [PubMed] [Google Scholar]
- Ng K.K.S., Park-Snyder S., Weis W.I. Ca2 +-dependent structural changes in C-type mannose-binding proteins. Biochemistry. 1998;37(51):17965–17976. doi: 10.1021/bi981972a. [DOI] [PubMed] [Google Scholar]
- Ni C.Z., Welsh K., Leo E., Chiou, C.-k., Wu, H., Reed, J.C., Ely, K.R. Molecular basis for CD40 signaling mediated by TRAF3. Proc. Natl. Acad. Sci. U. S. A. 2000;97(19):10395–10399. doi: 10.1073/pnas.97.19.10395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norn C.H., André I. Computational design of protein self-assembly. Curr. Opin. Struct. Biol. 2016;39:39–45. doi: 10.1016/j.sbi.2016.04.002. [DOI] [PubMed] [Google Scholar]
- O'Shea E.K., Klemm J.D., Kim P.S., Alber T. Two-stranded, parallel coiled coil. Science. 1991;254(5031):539–544. doi: 10.1126/science.1948029. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov S., Kim D.E., Wang R.Y., Liu Y., DiMaio F., Baker D. Improved de novo structure prediction in CASP11 by incorporating co-evolution information into rosetta. Proteins Suppl. 2015;1:67–75. doi: 10.1002/prot.24974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxenoid K., Chou J.J. The structure of phospholamban pentamer reveals a channel-like architecture in membranes. Proc. Natl. Acad. Sci. U. S. A. 2005;102(31):10870–10875. doi: 10.1073/pnas.0504920102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyewumi M.O., Kumar A., Cui Z. Nano-microparticles as immune adjuvants: correlating particle sizes and the resultant immune responses. Expert. Rev. Vaccines. 2010;9(9):1095–1107. doi: 10.1586/erv.10.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özbek S., Engel J., Stetefeld J. Storage function of cartilage oligomeric matrix protein: the crystal structure of the coiled-coil domain in complex with vitamin D3. EMBO J. 2002;21(22):5960–5968. doi: 10.1093/emboj/cdf628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paaventhan P., Joseph J.S., Seow S.V., Vaday S., Robinson H., Chua K.Y., Kolatkar P.R. A 1.7 Å structure of Fve, a member of the new fungal immunomodulatory protein family. J. Mol. Biol. 2003;332(2):461–470. doi: 10.1016/s0022-2836(03)00923-9. [DOI] [PubMed] [Google Scholar]
- Padilla J.E., Colovos C., Yeates T.O. Nanohedra: using symmetry to design self assembling protein cages, layers, crystals, and filaments. Proc. Natl. Acad. Sci. U. S. A. 2001;98(5):2217–2221. doi: 10.1073/pnas.041614998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya M.J., Cerasoli E., Joseph A., Stoneman R.G., Waite E., Woolfson D.N. Sequence and structural duality: designing peptides to adopt two stable conformations. J. Am. Chem. Soc. 2004;126(51):17016–17024. doi: 10.1021/ja045568c. [DOI] [PubMed] [Google Scholar]
- Papapostolou D., Smith A.M., Atkins E.D., Oliver S.J., Ryadnov M.G., Serpell L.C., Woolfson D.N. Engineering nanoscale order into a designed protein fiber. Proc. Natl. Acad. Sci. U. S. A. 2007;104(26):10853–10858. doi: 10.1073/pnas.0700801104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parge H.E., Forest K.T., Hickey M.J., Christensen D.A., Getzoff E.D., Tainer J.A. Structure of the fibre-forming protein pilin at 2.6 A resolution. Nature. 1995;378(6552):32–38. doi: 10.1038/378032a0. [DOI] [PubMed] [Google Scholar]
- Park K., Shen B.W., Parmeggiani F., Huang P.S., Stoddard B.L., Baker D. Control of repeat-protein curvature by computational protein design. Nat. Struct. Mol. Biol. 2015;22(2):167–174. doi: 10.1038/nsmb.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker J.M.R., Guo D., Hodges R.S. New hydrophilicity scale derived from high-performance liquid chromatography peptide retention data: correlation of predicted surface residues with antigenicity and X-ray-derived accessible sites. Biochemistry. 1986;25(19):5425–5432. doi: 10.1021/bi00367a013. [DOI] [PubMed] [Google Scholar]
- Parry D.A., Fraser R.B., Squire J.M. Fifty years of coiled-coils and α-helical bundles: a close relationship between sequence and structure. J. Struct. Biol. 2008;163(3):258–269. doi: 10.1016/j.jsb.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Pauling L., Corey R.B. Two hydrogen-bonded spiral configurations of the polypeptide chain. J. Am. Chem. Soc. 1950;72(11):5349. [Google Scholar]
- Pauling L., Corey R.B. The pleated sheet, a new layer configuration of polypeptide chains. Proc. Natl. Acad. Sci. U. S. A. 1951;37:251–256. doi: 10.1073/pnas.37.5.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauling L., Corey R.B. Compound helical configurations of polypeptide chains: structure of proteins of the α-keratin type. Nature. 1953;171(4341):59–61. doi: 10.1038/171059a0. [DOI] [PubMed] [Google Scholar]
- Pauling L., Corey R.B., Branson H.R. The structure of proteins: two hydrogen-bonded helical configurations of the polypeptide chain. Proc. Natl. Acad. Sci. U. S. A. 1951;37(4):205–211. doi: 10.1073/pnas.37.4.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechar M., Pola R. The coiled coil motif in polymer drug delivery systems. Biotechnol. Adv. 2013;31(1):90–96. doi: 10.1016/j.biotechadv.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Pechik I., Yakovlev S., Mosesson M.W., Gilliland G.L., Medved L. Structural basis for sequential cleavage of fibrinopeptides upon fibrin assembly. Biochemistry. 2006;45(11):3588–3597. doi: 10.1021/bi0525369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peralta M.D.R., Karsai A., Ngo A., Sierra C., Fong K.T., Hayre N.R., Mirzaee N., Ravikumar K.M., Kluber A.J., Chen X., Liu, G.-y., Toney, M.D., Singh, R.R., Cox, D.L. Engineering amyloid fibrils from β-solenoid proteins for biomaterials applications. ACS Nano. 2015;9(1):449–463. doi: 10.1021/nn5056089. [DOI] [PubMed] [Google Scholar]
- Pimentel T.A., Yan Z., Jeffers S.A., Holmes K.V., Hodges R.S., Burkhard P. Peptide nanoparticles as novel immunogens: design and analysis of a prototypic severe acute respiratory syndrome vaccine. Chem. Biol. Drug Des. 2009;73(1):53–61. doi: 10.1111/j.1747-0285.2008.00746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podust L.M., Krezel A.M., Kim Y. Crystal structure of the CCAAT box/enhancer-binding protein β activating transcription factor-4 basic leucine zipper heterodimer in the absence of DNA. J. Biol. Chem. 2001;276(1):505–513. doi: 10.1074/jbc.M005594200. [DOI] [PubMed] [Google Scholar]
- Pompano R.R., Chen J., Verbus E.A., Han H., Fridman A., McNeely T., Collier J.H., Chong A.S. Titrating T-cell epitopes within self-assembled vaccines optimizes CD4 + helper T cell and antibody outputs. Adv. Healthc. Mater. 2014;3(11):1898–1908. doi: 10.1002/adhm.201400137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potekhin S.a., Melnik T., Popov V., Lanina N., Vazina A.a., Rigler P., Verdini A., Corradin G., Kajava A. De novo design of fibrils made of short α-helical coiled coil peptides. Chem. Biol. 2001;8(11):1025–1032. doi: 10.1016/s1074-5521(01)00073-4. [DOI] [PubMed] [Google Scholar]
- Rahmatabadi S.S., Nezafat N., Negahdaripour M., Hajighahramani N., Morowvat M.H., Ghasemi Y. Studying the features of 57 confirmed CRISPR loci in 29 strains of Escherichia coli. J. Basic Microbiol. 2016;56(6):645–653. doi: 10.1002/jobm.201500707. [DOI] [PubMed] [Google Scholar]
- Ralay-Ranaivo B., Desmaele D., Bianchini E.P., Lepeltier E., Bourgaux C., Borgel D., Pouget T., Tranchant J.F., Couvreur P., Gref R. Novel self assembling nanoparticles for the oral administration of fondaparinux: synthesis, characterization and in vivo evaluation. J. Control. Release. 2014;194:323–331. doi: 10.1016/j.jconrel.2014.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman S., Machaidze G., Lustig A., Aebi U., Burkhard P. Structure-based design of peptides that self-assemble into regular polyhedral nanoparticles. Nanomedicine. 2006;2(2):95–102. doi: 10.1016/j.nano.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Ramisch S., Weininger U., Martinsson J., Akke M., André I. Computational design of a leucine-rich repeat protein with a predefined geometry. Proc. Natl. Acad. Sci. U. S. A. 2014;111(50):17875–17880. doi: 10.1073/pnas.1413638111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramisch S., Lizatovic R., Andre I. Exploring alternate states and oligomerization preferences of coiled-coils by de novo structure modeling. Proteins. 2015;83(2):235–247. doi: 10.1002/prot.24729. [DOI] [PubMed] [Google Scholar]
- Reed S.G., Orr M.T., Fox C.B. Key roles of adjuvants in modern vaccines. Nat. Med. 2013;19(12):1597–1608. doi: 10.1038/nm.3409. [DOI] [PubMed] [Google Scholar]
- Ringler P., Schulz G.E. Self-assembly of proteins into designed networks. Science. 2003;302(5642):106–109. doi: 10.1126/science.1088074. [DOI] [PubMed] [Google Scholar]
- Robson Marsden H., Kros A. Self-assembly of coiled coils in synthetic biology: inspiration and progress. Angew. Chem. Int. Ed. Eng. 2010;49(17):2988–3005. doi: 10.1002/anie.200904943. [DOI] [PubMed] [Google Scholar]
- Roy A., Franco O.L., Mandal S.M. Biomedical exploitation of self assembled peptide based nanostructures. Curr. Protein Pept. Sci. 2013;14(7):580–587. doi: 10.2174/1389203711209070687. [DOI] [PubMed] [Google Scholar]
- Rudra J.S., Tian Y.F., Jung J.P., Collier J.H. A self-assembling peptide acting as an immune adjuvant. Proc. Natl. Acad. Sci. U. S. A. 2010;107(2):622–627. doi: 10.1073/pnas.0912124107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudra J.S., Mishra S., Chong A.S., Mitchell R.A., Nardin E.H., Nussenzweig V., Collier J.H. Self-assembled peptide nanofibers raising durable antibody responses against a malaria epitope. Biomaterials. 2012;33(27):6476–6484. doi: 10.1016/j.biomaterials.2012.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudra J.S., Sun T., Bird K.C., Daniels M.D., Gasiorowski J.Z., Chong A.S., Collier J.H. Modulating adaptive immune responses to peptide self-assemblies. ACS Nano. 2012;6(2):1557–1564. doi: 10.1021/nn204530r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudra J.S., Ding Y., Neelakantan H., Ding C., Appavu R., Stutz S., Snook J.D., Chen H., Cunningham K.A., Zhou J. Suppression of cocaine-evoked hyperactivity by self-adjuvanting and multivalent peptide nanofiber vaccines. ACS Chem. Neurosci. 2016;7(5):546–552. doi: 10.1021/acschemneuro.5b00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadatmousavi P. Peptide-mediated Anticancer Drug Delivery. 2009. http://hdl.handle.net/10012/4577 UWSpace.
- Sanner M.F., Stolz M., Burkhard P., Kong X.-P., Min G., Sun T.-T., Driamov S., Aebi U., Stoffler D. Visualizing nature at work from the nano to the macro scale. NanoBiotechnol. 2005;1(1):7–21. [Google Scholar]
- Santelli E., Richmond T.J. Crystal structure of MEF2A core bound to DNA at 1.5 Å resolution. J. Mol. Biol. 2000;297(2):437–449. doi: 10.1006/jmbi.2000.3568. [DOI] [PubMed] [Google Scholar]
- Scanlon S., Aggeli A. Self-assembling peptide nanotubes. Nano Today. 2008;3(3–4):22–30. [Google Scholar]
- Schumacher M.A., Goodman R.H., Brennan R.G. The structure of a CREB bZIP· somatostatin CRE complex reveals the basis for selective dimerization and divalent cation-enhanced DNA binding. J. Biol. Chem. 2000;275(45):35242–35247. doi: 10.1074/jbc.M007293200. [DOI] [PubMed] [Google Scholar]
- Sekhon B.S., Saluja V. Nanovaccines-an overview. Int. J. Pharm. Front Res. 2011;1:101–109. [Google Scholar]
- Seong S.Y., Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat. Rev. Immunol. 2004;4:469–478. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]
- Shah R.R., O'Hagan D.T., Amiji M.M., Brito L.A. The impact of size on particulate vaccine adjuvants. Nanomedicine (London, England) 2014;9(17):2671–2681. doi: 10.2217/nnm.14.193. [DOI] [PubMed] [Google Scholar]
- Shah R., Petersburg J., Gangar A.C., Fegan A., Wagner C.R., Kumarapperuma S.C. In vivo evaluation of site-specifically PEGylated chemically self-assembled protein nanostructures. Mol. Pharm. 2016;13(7):2193–2203. doi: 10.1021/acs.molpharmaceut.6b00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbazi M., Haghkhah M., Rahbar M.R., Nezafat N., Ghasemi Y. In silico sub-unit hexavalent peptide vaccine against an Staphylococcus aureus biofilm-related infection. Int. J. Pept. Res. Ther. 2016;22(1):101–117. [Google Scholar]
- Sheriff S., Chang C.Y., Ezekowitz R.A.B. Human mannose-binding protein carbohydrate recognition domain trimerizes through a triple α-helical coiled-coil. Nat. Struct. Mol. Biol. 1994;1(11):789–794. doi: 10.1038/nsb1194-789. [DOI] [PubMed] [Google Scholar]
- Shirbaghaee Z., Bolhassani A. Different applications of virus-like particles in biology and medicine: vaccination and delivery systems. Biopolymers. 2016;105(3):113–132. doi: 10.1002/bip.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrive A.K., Tharia H.A., Strong P., Kishore U., Burns I., Rizkallah P.J., Reid K.B., Greenhough T.J. High-resolution structural insights into ligand binding and immune cell recognition by human lung surfactant protein D. J. Mol. Biol. 2003;331(2):509–523. doi: 10.1016/s0022-2836(03)00761-7. [DOI] [PubMed] [Google Scholar]
- Shu W., Liu J., Ji H., Lu M. Core structure of the outer membrane lipoprotein from Escherichia coli at 1.9 Å resolution. J. Mol. Biol. 2000;299(4):1101–1112. doi: 10.1006/jmbi.2000.3776. [DOI] [PubMed] [Google Scholar]
- Sieminski A., Semino C., Gong H., Kamm R. Primary sequence of ionic self-assembling peptide gels affects endothelial cell adhesion and capillary morphogenesis. J. Biomed. Mater. Res. A. 2008;87(2):494–504. doi: 10.1002/jbm.a.31785. [DOI] [PubMed] [Google Scholar]
- Silva G.A., Czeisler C., Niece K.L., Beniash E., Harrington D.A., Kessler J.A., Stupp S.I. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science. 2004;303(5662):1352–1355. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]
- Sinclair J.C., Davies K.M., Venien-Bryan C., Noble M.E. Generation of protein lattices by fusing proteins with matching rotational symmetry. Nat. Nanotechnol. 2011;6(9):558–562. doi: 10.1038/nnano.2011.122. [DOI] [PubMed] [Google Scholar]
- Skwarczynski M., Toth I. Peptide-based subunit nanovaccines. Curr. Drug. Deliv. 2011;8(3):282–289. doi: 10.2174/156720111795256192. [DOI] [PubMed] [Google Scholar]
- Skwarczynski M., Toth I. Recent advances in peptide-based subunit nanovaccines. Nanomedicine. 2014;9(17):2657–2669. doi: 10.2217/nnm.14.187. [DOI] [PubMed] [Google Scholar]
- Slovic A., Lear J., DeGrado W. De novo design of a pentameric coiled-coil: decoding the motif for tetramer versus pentamer formation in water-soluble phospholamban. J. Pept. Res. 2005;65(3):312–321. doi: 10.1111/j.1399-3011.2005.00244.x. [DOI] [PubMed] [Google Scholar]
- Srirajaskanthan R., Preedy V.R. Taylor & Francis; 2011. Nanomedicine and Cancer. [Google Scholar]
- Steinbacher S., Bass R., Strop P., Rees D.C. Structures of the prokaryotic mechanosensitive channels MscL and MscS. Curr. Top. Membr. 2007;58:1–24. [Google Scholar]
- Stern A.M., Markel H. The history of vaccines and immunization: familiar patterns, new challenges. Health Aff. 2005;24(3):611–621. doi: 10.1377/hlthaff.24.3.611. [DOI] [PubMed] [Google Scholar]
- Stetefeld J., Jenny M., Schulthess T., Landwehr R., Engel J., Kammerer R.A. Crystal structure of a naturally occurring parallel right-handed coiled coil tetramer. Nat. Struct. Mol. Biol. 2000;7(9):772–776. doi: 10.1038/79006. [DOI] [PubMed] [Google Scholar]
- Strelkov S.V., Herrmann H., Geisler N., Wedig T., Zimbelmann R., Aebi U., Burkhard P. Conserved segments 1A and 2B of the intermediate filament dimer: their atomic structures and role in filament assembly. EMBO J. 2002;21(6):1255–1266. doi: 10.1093/emboj/21.6.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H., Zhang X., Miao L., Zhao L., Luo Q., Xu J., Liu J. Micelle-induced self-assembling protein nanowires: versatile supramolecular scaffolds for designing the light-harvesting system. ACS Nano. 2016;10(1):421–428. doi: 10.1021/acsnano.5b05213. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Hiroaki H., Kohda D., Nakamura H., Tanaka T. Metal ion induced self-assembly of a designed peptide into a triple-stranded α-helical bundle: a novel metal binding site in the hydrophobic core. J. Am. Chem. Soc. 1998;120(50):13008–13015. [Google Scholar]
- Suzuki K., Yamada T., Tanaka T. Role of the buried glutamate in the α-helical coiled coil domain of the macrophage scavenger receptor. Biochemistry. 1999;38(6):1751–1756. doi: 10.1021/bi9821014. [DOI] [PubMed] [Google Scholar]
- Takeda S., Yamashita A., Maeda K., Maéda Y. Structure of the core domain of human cardiac troponin in the Ca2 + − saturated form. Nature. 2003;424(6944):35–41. doi: 10.1038/nature01780. [DOI] [PubMed] [Google Scholar]
- Tandrup Schmidt S., Foged C., Smith Korsholm K., Rades T., Christensen D. Liposome-based adjuvants for subunit vaccines: formulation strategies for subunit antigens and immunostimulators. Pharmaceutics. 2016;8(1):7. doi: 10.3390/pharmaceutics8010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y., Strelkov S.V., Mesyanzhinov V.V., Rossmann M.G. Structure of bacteriophage T4 fibritin: a segmented coiled coil and the role of the C-terminal domain. Structure. 1997;5(6):789–798. doi: 10.1016/s0969-2126(97)00233-5. [DOI] [PubMed] [Google Scholar]
- Tavakoli S., Tamaddon A.M., Golkar N., Samani S.M. Microencapsulation of (deoxythymidine)20–DOTAP complexes in stealth liposomes optimized by Taguchi design. J. Liposome Res. 2015;25(1):67–77. doi: 10.3109/08982104.2014.928889. [DOI] [PubMed] [Google Scholar]
- Usui K., Maki T., Ito F., Suenaga A., Kidoaki S., Itoh M., Taiji M., Matsuda T., Hayashizaki Y., Suzuki H. Nanoscale elongating control of the self-assembled protein filament with the cysteine-introduced building blocks. Protein Sci. 2009;18(5):960–969. doi: 10.1002/pro.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagt T., Zschörnig O., Huster D., Koksch B. Membrane binding and structure of de novo designed α-helical cationic coiled-coil-forming peptides. ChemPhysChem. 2006;7(6):1361–1371. doi: 10.1002/cphc.200600010. [DOI] [PubMed] [Google Scholar]
- Vartak A., Sucheck S.J. Recent advances in subunit vaccine carriers. Vaccine. 2016;4(2):12. doi: 10.3390/vaccines4020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voet A.R.D., Noguchi H., Addy C., Simoncini D., Terada D., Unzai S., Park S.-Y., Zhang K.Y.J., Tame J.R.H. Computational design of a self-assembling symmetrical β-propeller protein. Proc. Natl. Acad. Sci. U. S. A. 2014;111(42):15102–15107. doi: 10.1073/pnas.1412768111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahome N., Pfeiffer T., Ambiel I., Yang Y., Keppler O.T., Bosch V., Burkhard P. Conformation-specific display of 4E10 and 2F5 epitopes on self-assembling protein nanoparticles as a potential HIV vaccine. Chem. Biol. Drug Des. 2012;80(3):349–357. doi: 10.1111/j.1747-0285.2012.01423.x. [DOI] [PubMed] [Google Scholar]
- Wang C., Zhang H., Zheng W.M., Xu D., Zhu J., Wang B., Ning K., Sun S., Li S.C., Bu D. FALCON@home: a high-throughput protein structure prediction server based on remote homologue recognition. Bioinformatics. 2015;32(3):462–464. doi: 10.1093/bioinformatics/btv581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber M.J., Appel E.A., Meijer E.W., Langer R. Supramolecular biomaterials. Nat. Mater. 2016;15(1):13–26. doi: 10.1038/nmat4474. [DOI] [PubMed] [Google Scholar]
- Whitby F.G., Phillips G.N. Crystal structure of tropomyosin at 7 Ångstroms resolution. Proteins Struct. Funct. Bioinforma. 2000;38(1):49–59. [PubMed] [Google Scholar]
- Whitesides G.M., Grzybowski B. Self-assembly at all scales. Science. 2002;295(5564):2418–2421. doi: 10.1126/science.1070821. [DOI] [PubMed] [Google Scholar]
- Whitson S.R., LeStourgeon W.M., Krezel A.M. Solution structure of the symmetric coiled coil tetramer formed by the oligomerization domain of hnRNP C: implications for biological function. J. Mol. Biol. 2005;350(2):319–337. doi: 10.1016/j.jmb.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Wu R.y., Zhang R.g., Zagnitko O., Dementieva I., Maltzev N., Watson J.D., Laskowski R., Gornicki P., Joachimiak A. Crystal structure of Enterococcus faecalis SlyA-like transcriptional factor. J. Biol. Chem. 2003;278(22):20240–20244. doi: 10.1074/jbc.M300292200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Xu C., Wang C., Kopecek J. Refolding hydrogels self-assembled from N-(2-hydroxypropyl) methacrylamide graft copolymers by antiparallel coiled-coil formation. Biomacromolecules. 2006;7:1187–1195. doi: 10.1021/bm051002k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Ringler P., Müller S.A., Burkhard P. Optimizing the refolding conditions of self-assembling polypeptide nanoparticles that serve as repetitive antigen display systems. J. Struct. Biol. 2012;177(1):168–176. doi: 10.1016/j.jsb.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Li W., Kirberger M., Liao W., Ren J. Design of nanomaterial based systems for novel vaccine development. Biomater Sci. 2016;4(5):785–802. doi: 10.1039/c5bm00507h. [DOI] [PubMed] [Google Scholar]
- Yanlian Y., Ulung K., Xiumei W., Horii A., Yokoi H., Shuguang Z. Designer self-assembling peptide nanomaterials. Nano Today. 2009;4(2):193–210. [Google Scholar]
- Yokoi H., Kinoshita T., Zhang S. Dynamic reassembly of peptide RADA16 nanofiber scaffold. Proc. Natl. Acad. Sci. U. S. A. 2005;102(24):8414–8419. doi: 10.1073/pnas.0407843102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama M. Polymeric micelles as drug carriers: their lights and shadows. J. Drug Target. 2014;22(7):576–583. doi: 10.3109/1061186X.2014.934688. [DOI] [PubMed] [Google Scholar]
- Yu Z., Cai Z., Chen Q., Liu M., Ye L., Ren J., Liao W., Liu S. Engineering [small beta]-sheet peptide assemblies for biomedical applications. Biomater. Sci. 2016;4(3):365–374. doi: 10.1039/c5bm00472a. [DOI] [PubMed] [Google Scholar]
- Zamani M., Nezafat N., Negahdaripour M., Dabbagh F., Ghasemi Y. In silico evaluation of different signal peptides for the secretory production of human growth hormone in E. coli. Int. J. Pept. Res. Ther. 2015;21(3):261–268. [Google Scholar]
- Zandi R., Reguera D., Bruinsma R.F., Gelbart W.M., Rudnick J. Origin of icosahedral symmetry in viruses. Proc. Natl. Acad. Sci. U. S. A. 2004;101(44):15556–15560. doi: 10.1073/pnas.0405844101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S. Fabrication of novel biomaterials through molecular self-assembly. Nat. Biotechnol. 2003;21(10):1171–1178. doi: 10.1038/nbt874. [DOI] [PubMed] [Google Scholar]
- Zhang S., Rich A. Direct conversion of an oligopeptide from a β-sheet to an α-helix: a model for amyloid formation. Proc. Natl. Acad. Sci. U. S. A. 1997;94(1):23–28. doi: 10.1073/pnas.94.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Holmes T., Lockshin C., Rich A. Spontaneous assembly of a self-complementary oligopeptide to form a stable macroscopic membrane. Proc. Natl. Acad. Sci. U. S. A. 1993;90(8):3334–3338. doi: 10.1073/pnas.90.8.3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Lockshin C., Cook R., Rich A. Unusually stable β-sheet formation in an ionic self-complementary oligopeptide. Biopolymers. 1994;34(5):663–672. doi: 10.1002/bip.360340508. [DOI] [PubMed] [Google Scholar]
- Zhang S., Holmes T.C., DiPersio C.M., Hynes R.O., Su X., Rich A. Self-complementary oligopeptide matrices support mammalian cell attachment. Biomaterials. 1995;16(18):1385–1393. doi: 10.1016/0142-9612(95)96874-y. [DOI] [PubMed] [Google Scholar]
- Zhang J., Hao R., Huang L., Yao J., Chen X., Shao Z. Self-assembly of a peptide amphiphile based on hydrolysed Bombyx mori silk fibroin. Chem. Commun. 2011;47(37):10296–10298. doi: 10.1039/c1cc12633d. [DOI] [PubMed] [Google Scholar]
- Zhang Y.M., Liu Y.H., Zhang J., Liu Q., Huang Z., Yu X.-Q. Cationic gemini lipids with cyclen headgroups: interaction with DNA and gene delivery abilities. RSC Adv. 2014;4(83):44261–44268. [Google Scholar]
- Zhao X., Zhang S. Molecular designer self-assembling peptides. Chem. Soc. Rev. 2006;35(11):1105–1110. doi: 10.1039/b511336a. [DOI] [PubMed] [Google Scholar]
- Zhao X., Pan F., Lu J.R. Recent development of peptide self-assembly. Prog. Nat. Sci. 2008;18(6):653–660. [Google Scholar]
- Zhao L., Seth A., Wibowo N., Zhao C.-X., Mitter N., Yu C., Middelberg A.P. Nanoparticle vaccines. Vaccine. 2014;32(3):327–337. doi: 10.1016/j.vaccine.2013.11.069. [DOI] [PubMed] [Google Scholar]
- Zhao G., Chandrudu S., Skwarczynski M., Toth I. The application of self-assembled nanostructures in peptide-based subunit vaccine development. Eur. Polym. J. 2017 doi: 10.1016/j.eurpolymj.2017.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N., Kay C., Hodges R. Synthetic model proteins. Positional effects of interchain hydrophobic interactions on stability of two-stranded alpha-helical coiled-coils. J. Biol. Chem. 1992;267(4):2664–2670. [PubMed] [Google Scholar]
- Zimenkov Y., Dublin S.N., Ni R., Tu R.S., Breedveld V., Apkarian R.P., Conticello V.P. Rational design of a reversible pH-responsive switch for peptide self-assembly. J. Am. Chem. Soc. 2006;128(21):6770–6771. doi: 10.1021/ja0605974. [DOI] [PubMed] [Google Scholar]
- Zou D., Tie Z., Lu C., Qin M., Lu X., Wang M., Wang W., Chen P. Effects of hydrophobicity and anions on self-assembly of the peptide EMK16-II. Biopolymers. 2010;93(4):318–329. doi: 10.1002/bip.21340. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material