Abstract
Dental resorptive lesions (RL) are a common oral disease in cats (Felis catus) associated with pain and tooth destruction. The aetiology of RL in cats is unknown, but inflammation is often seen in conjunction with RL. Vitamin D involvement has been suggested because 1,25-dihydroxyvitamin D (1,25(OH)2D) stimulates osteoclastogenesis, through up-regulation of the nuclear vitamin D receptor (nVDR).
The aim of this study is to determine the involvement of inflammatory cytokines and the possible role of vitamin D in the pathophysiology of RL using quantitative PCR. We measured the mRNA expression of cytokines with stimulatory (IL-1β, IL-6, and TNF-α) and inhibitory effects (IL-10 and IFN-γ) on osteoclastogenesis, and the mRNA expression of the receptor activator of nuclear factor-kappaB ligand (RANKL), osteoprotegerin (OPG), and nVDR in RL samples. We found increased expression of mRNA levels for inflammatory cytokines and nVDR, but not for RANKL and OPG, in tissue from RL-affected cats compared with tissue from radiological confirmed healthy controls. The mRNA levels of nVDR were positively correlated with mRNA levels of pro-inflammatory (IL-1β, IL-6, TNF-α, and IFN-γ), anti-inflammatory (IL-10), pro-resorptive (IL-1β, IL-6, and TNF-α), and anti-resorptive (IFN-γ and IL-10) cytokines in the course of resorptive lesions. These data are consistent with our view that both inflammation and an overexpression of the nVDR are likely to be involved in RL in cats.
Keywords: Resorptive lesions, Inflammatory cytokines, Nuclear vitamin D receptor, Feline, Teeth
1. Introduction
Odontoclastic resorptive lesions (RL) are a common finding in cats. Depending on inclusion criteria and methods of diagnosis, 20–75% of cats have a least one RL (Reiter and Mendoza, 2002). The lesions often develop at the gingival margin but may be masked by overlaying gingival tissue. RL may also occur on the tooth root without affecting the crown (Gorrel and Larsson, 2002). Symptoms include swelling and bleeding of gingival tissue, pain, ptyalism, reluctance to drink/eat, and anorexia. Two types of RL have been suggested in cats; type I, characterized by inflammatory changes, and type II, characterized by replacement resorption without inflammatory changes (DuPont and DeBowes, 2002). Both can occur simultaneously and can even affect the same tooth concomitantly. There is currently no consensus on whether type I and type II are two different diseases or just different stages of the same disease. Therefore, the official classification as followed by the American Veterinary Dental College, classifies RL only to the severity of the lesion, without distinguishing between type I and type II (www.avdc.org).
In humans, dental resorptive lesions are associated with and initiated by inflammation resulting from, e.g., periodontal disease, orthodontic forces, or endodontic disease (Fuss et al., 2003). In cats, the aetiology of RL is unknown, but bacteria, viruses and the diet, and vitamin D in particular, are thought to be involved in the pathogenesis of feline RL (Okuda and Harvey, 1992, Hofmann-Lehmann et al., 1998, Reiter et al., 2005, Servet et al., 2007). While most mammals depend on exposure to sunlight for their vitamin D requirements (Holick et al., 2007), cats are dependent on their diet to meet their vitamin D requirements because they are unable to synthesize sufficient 7-dehydrocholesterol, probably due to high activity of 7-dehydrocholesterol-Δ7-reductase (How et al., 1994, Morris, 1999).
Clinically, most cats with RL show signs of inflammation. Cytokines produced during inflammation can stimulate or inhibit osteoclast activity, directly, or through the receptor activator of nuclear factor-kappaB (RANK)/receptor activator of nuclear factor-kappaB ligand (RANKL) pathway (Lindskog et al., 2007). Some pro-inflammatory cytokines, such as IL-1β, IL-6, and TNF-α, stimulate osteoclastogenesis (Takeichi et al., 1996, Baker, 2000), whereas others, such as IFN-γ, inhibit osteoclastogenesis (Gowen et al., 1986). IL-10, an anti-inflammatory cytokine, also inhibits osteoclastogenesis (Baker, 2000, Lindskog et al., 2007). 1,25-Dihydroxyvitamin D (1,25(OH)2D) stimulates osteoclastogenesis by up-regulating the expression of RANKL, mediated via the nuclear vitamin D receptor (nVDR) (Baker, 2000, Kitazawa et al., 2003, Zhang et al., 2004). RANKL is expressed in bone and dental cells (Rani and MacDougall, 2000, Fukushima et al., 2003). Dentin resorbing odontoclasts originate from the same stem cells as osteoclasts (Okuda and Harvey, 1992, Arana-Chavez and Bradaschia-Correa, 2009) and it is assumed that osteoclast properties and characteristics can be extrapolated to odontoclasts.
To our knowledge, few studies have measured, using semi-quantitative assays, the expression of cytokines in feline oral tissues (Harley et al., 1999, Harley et al., 2003, DeLaurier et al., 2002). The recent sequencing of the feline genome has opened the way for highly sensitive quantitative PCR (Q-PCR) assays for use in feline dental research (Penning et al., 2007). The aim of this study was to determine the involvement of inflammatory cytokines and the possible role of vitamin D in the pathophysiology of RL, using quantitative PCR to measure the mRNA expression of cytokines with stimulatory (IL-1β, IL-6, TNF-α, and RANKL) and inhibitory (IL-10, IFN-γ, and OPG) effects on osteoclastogenesis in samples from cats with RL and normal controls. We also measured nVDR mRNA expression as a first step to evaluating the role of vitamin D in RL.
2. Materials and methods
All procedures were approved by the local ethics committee, as required by Dutch law.
2.1. Tissue collection
Client owned cats aged 2–16 years referred to the university clinic for specific dental treatment and diagnosed with radiographically confirmed RL (Gendex AC90, Gendex Dental Systems, Milan, Italy and Vista-scan, Dürr Dental, Beuningen, The Netherlands) had affected teeth removed under general anaesthesia. Food was withheld for at least 12 h prior to anaesthesia. The cats were premedicated with medetomidine IV (30–50 μg/kg, Domitor®, Pfizer Animal Health BV, The Netherlands) and anesthesia was induced with propofol (1–2 mg/kg Propovet® ASTfarma, Oudewater, The Netherlands) at a dose adequate to allow uneventful endotracheal intubation. After intubation, anesthesia was maintained with isoflurane (IsoFlo® Schering-Plough BV, Maarssen, The Netherlands) administered in oxygen:air (1:1) with a non-rebreathing technique. During the entire procedure, isoflurane administration was adjusted to ensure a sufficient level of anaesthesia. During anaesthesia, Sterofundin Iso (Braun, Melsungen AG, Germany) was administered at 10 ml/(kg h). ECG, pulse-oximetry, inspiratory and expiratory gas analysis, and respiration rate were monitored throughout the procedure (AS3 monitor, Datex Ohmeda, Helsinki, Finland). At the end of the procedure medetomidine was reversed by atipamezole (Antisedan®, Pfizer Animal Health BV, The Netherlands, at 2.5 times the dose of medetomidine). Post-operative analgesia with buprenorphine IM/IV (20 μg/kg Schering-Plough BV, Maarssen, The Netherlands) and/or carprofen 4 mg/kg IV (Pfizer Animal Health BV, The Netherlands) was adjusted to the expected level of pain.
On the basis of clinical and radiological signs, teeth were classified as either RL with periodontitis or without. Periodontitis, defined as loss of attachment (gingival recession, periodontal probing dept, furcation involvement and bone loss on radiographs) was present in a wide range of severity in most cats with RL. The extracted tooth and adjacent tissue including gingival and inflammatory tissue was collected, immediately snap frozen in liquid nitrogen and stored in −70 °C until analysed. Control teeth were removed from cats aged 9 months without signs of oral disease and with radiographically confirmed healthy teeth within 2 h after euthanasia. The cats were euthanized for a project not related to this study. The following samples were obtained; 35 samples from 13 cats affected by RL (gingival tissue n = 13, upper premolar and molar n = 13, lower premolar n = 3, lower molar n = 6) and 43 samples from 10 cats as controls (gingival tissue n = 10, upper premolar and molar n = 6, lower premolar n = 17, lower molar n = 8, lower canine and incisors n = 2).
2.2. RNA isolation and cDNA synthesis
Total RNA was extracted from tissue samples using the RNAeasy minikit (Qiagen, Leiden, The Netherlands) according to the manufacturer's protocol. Teeth and adjacent tissue were removed from liquid nitrogen, wrapped in tinfoil, and crushed while still frozen with a hammer. Gingival tissue was minced using an IKA T20 ultra turrax. Powder and debris were put into the lysis buffer supplied by the kit and the standard procedure for the isolation of RNA from animal tissues was followed. RNA was quantified spectrophotometrically using Nanodrop ND-1000 (Isogen Life Science, IJsselstein, The Netherlands). The RNA isolates were subjected to an on-column DNase-I treatment (Qiagen). From each total RNA sample 250 ng RNA was used to synthesize cDNA using the i-script cDNA synthesis kit (Bio-Rad, Veenendaal, The Netherlands) according to standard operation protocols. After synthesis a standard sample was prepared by pooling 10 μl of each sample, which was used to make a 4-fold dilution series to calculate PCR efficiency.
2.3. Primer design and testing
The primer sets for feline IL-1β, IL-6, IL-10, IFN-γ, and TNF-α were developed using known sequences from the public databases (www.ensembl.org or www.ncbi.nlm.nih.gov). Primer design was performed with Oligo explorer 1.1.0 software (www.genelink.com/tools/gldownloads.asp, Table 1 ). For nVDR and OPG a set of canine primers was used, after checking the homology using a BLAST against the cat genome search. Homology for the primers was 95% and 100% for the nVDR primers and 100% and 95% for the OPG primers (forward and reverse, respectively). To avoid the risk of amplifying traces of genomic DNA, the primers were positioned in different exons. The uniqueness and specificity of each primer was verified using the BLAST (www.ncbi.nlm.nih.gov/blast/) returning Genebank accession numbers. Amplicons were validated by checking product size on an agarose gel and the sequence was confirmed by sequencing on an ABI prism 3130XL genetic analyzer (Applied Biosystems, Nieuwerkerk a/d Ijssel, The Netherlands). No minus-RT amplicons were observed.
Table 1.
Primers for the feline genes of interest (GOI). For the sequences of the used reference genes, see Penning et al. (2007).
| Gene | Acc | Forward oligo (5′ → 3′) | Reverse oligo (5′ → 3′) | Tm (°C) | Fragment (bp) |
|---|---|---|---|---|---|
| IL-1β | NM_001009351 | ATGACCCACTTCATGAGGACTG | TCACCACACTCTTCTTGAGGG | 64 | 99 |
| IL-6 | NM_001009211 | GACTTCCTTCAGTTCAGCCTCAGG | AGGAATGCCCGTGAACTACAGC | 61 | 81 |
| IL-10 | NM_001009209 | ACAGCACGTGAACTCCCTGG | TCTTCACCTGCTCCACCACC | 66 | 113 |
| TNF-α | NM_001009835 | TCTTCTCCTTCCTCCTCGTCG | GGGGTTTGCTACTACATGGGC | 65 | 185 |
| IFN-γ | NM_001009873 | CAGATGTAGCAGATGGTGGGTC | CATGTCTTCCTTGATGGTGTCC | 60 | 176 |
| nVDR | XM_543714 | TCTCTGACCCTGGACCTGTC | GAAGTGAGGTCTCTGAACCCTG | 62 | 122 |
| RANKL | DQ027064 | AGCCTGATACCCAGCCT | GATACTCTGTGGCGAGGTC | 58 | 230 |
| OPG | XM_539146 | GGGTTCTTCTCGAATGAGACG | CCTGAAGAATGCCTCCTCAC | 59 | 183 |
2.4. Quantitative PCR for feline cytokines IL-1β, IL-6, IL-10, TNF-α, IFN-γ, nVDR, RANKL, and OPG
The PCR was based on the high affinity, double-stranded DNA-binding dye SYBR green, using a Bio-Rad My-IQ PCR-machine and IQ SYBR-green supermix (Bio-Rad) according to manufacturer's instructions. All primers (Eurogentech, Liège, Belgium) were used at a final concentration of 400 μM. One μl cDNA template was used per reaction.
Reactions started with 3 min at 95 °C followed by 40 cycles of 20 s at 95 °C and 30 s at T m. Annealing and extension were both performed at T m. This reaction was followed by a melting curve, with stepwise increase in temperature each 15 s by 0.5 °C, ranging from 60 °C to 95 °C. Optimal T m was determined using a temperature gradient ranging from 55 °C to 67 °C on a 16-fold dilution series with cDNA derived from pooled feline cDNA from random cat tissues. Analysis was performed with My-IQ software (Bio-Rad). Five reference genes were included; HPRT, RPL17, RPL30, RPS19, and YWHAZ. Large-scale evaluation of feline reference genes indicates that these reference genes were optimal for feline dental roots and crowns (Penning et al., 2007).
2.5. Analysis/statistics
For each experimental sample, the amount of gene product of interest (threshold cycle) and of the five independent reference genes were determined from the standard curve in an autonomous experiment. Normalization was based on the average amount of the five reference genes. These normalized values were divided by the normalized values of the calibrator (healthy teeth) group to generate relative expression levels. To assess differences in expression, the log of the ratios of the RL-affected tissues was compared to the log of the ratios of the controls. On the basis of radiographs and clinical findings, we classified the cats initially as either affected by RL with signs of periodontitis (i.e., type I) or as affected by RL without signs of periodontitis (i.e., type II) but with radiographically visible replacement resorption and/or ankylosis. For statistical analysis, mixed models with random cat effects and affected and control groups as fixed effects followed by Bonferroni's post-test were used. Q–Q plots were made to examine normal distribution. For correlations between nVDR and the inflammatory cytokines, the cytokines were grouped in pro-inflammatory and anti-inflammatory, and pro-resorptive and anti-resorptive cytokines. Differences in expression, Q–Q plots and correlations were evaluated using SPSS 15.0 for Windows (SPSS Inc., Chicago, IL). Graphs showing linear regression analysis in the course of resorptive lesions were created using SigmaPlot 10.0 for windows (Systat Software Inc., San Jose, CA). For all tests, values of p < 0.05 were considered as statistical significant.
3. Results
Statistical analyses showed no significant differences (p > 0.05) in gene expression of IL-1β, IL-6, IL-10, TNF-α, IFN-γ, nVDR, RANKL, and OPG between the initial subgroups (type I vs type II lesions). Both subgroups were also compared to the control group. They both revealed similar significant differences. Therefore data from the initial groups were combined in the further analyses.
Q–Q plots (data not shown) showed no deviation from normality for any cytokines or nVDR. The expression of IL-1β, IL-6, IL-10, TNF-α, IFN-γ, and nVDR was all significantly higher in the RL group than in the control group (p ≤ 0.001), with the expression of the pro-inflammatory and pro-resorptive cytokine IL-6 being increased nearly 100-fold in the RL group relative to the control. The expression of IL-1β and TNF-α (both pro-resorptive cytokines) and the expression of IL-10 and IFN-γ (anti-resorptive cytokines) were increased 10-fold in the RL group compared with the control group. Levels of nVDR mRNA showed a strongly significant 3-fold increase in the RL group (p = 0.001), whereas levels of RANKL and OPG mRNA were not significantly different between the RL affected group and control group (Fig. 1 ).
Fig. 1.
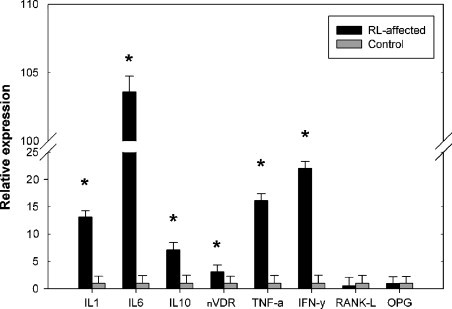
Relative expression of genes of interest corrected for 5 reference genes. Bars represent relative expression (+SD n-fold), controls were set at 1. *p ≤ 0.001.
Nuclear VDR was weakly, but positively correlated with pro-inflammatory (IL-1β, IL-6, TNF-α, and IFN-γ), anti-inflammatory (IL-10), pro-resorptive (IL-1β, IL-6, and TNF-α), and anti-resorptive (IFN-γ and IL-10) cytokines (all correlations p < 0.001) in the course of resorptive lesions (Fig. 2 , Table 2 ).
Fig. 2.
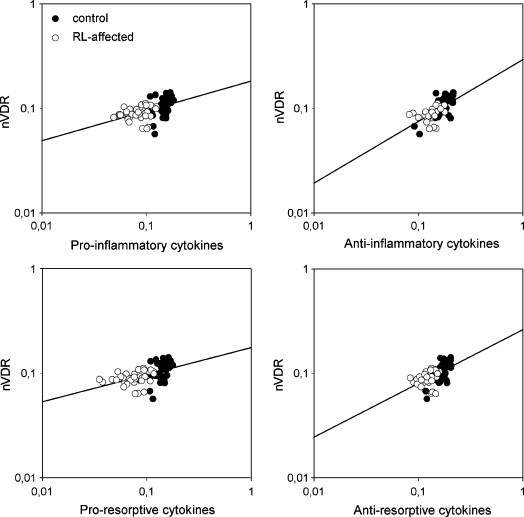
Linear regression of the quantitative gene expression LOG values between the nuclear VDR and the different groups of cytokines. All correlations were positive and statistically significant in the course of resorptive lesions (Table 2).
Table 2.
Pearson correlations between the gene expression levels of nuclear VDR (nVDR) and the individual and different groups of cytokines in cats without RL, with RL, and all cats.
| nVDR | IL-1β | IL-6 | TNF-α | IFN-γ | IL-10 (anti-inflammatory) | Pro-inflammatory | Pro-resorptive | Anti-resorptive |
|---|---|---|---|---|---|---|---|---|
| Control | 0.622*** | 0.374* | 0.396* | 0.510*** | 0.605*** | 0.443** | 0.616*** | |
| RL affected | 0.418* | 0.451** | ||||||
| Control + RL affected | 0.615*** | 0.506*** | 0.541*** | 0.528*** | 0.669*** | 0.569*** | 0.581*** | 0.639*** |
Statistical significant r values are depicted.
p < 0.05.
p < 0.01.
p < 0.001.
4. Discussion
We found that expression of IL-1β, IL-6, IL-10, TNF-α, IFN-γ, and nVDR was higher in tissue from cats with RL than in tissue from control cats. Although the cats in the two groups were not age matched, all cats had a permanent dentition. Because the yield of RNA from individual dental tissues was limited, we used similar RL affected teeth for histological investigations, which revealed odontoclasts, fibroblasts and inflammatory cells in the vicinity of the resorption lacunae (data not shown).
The factors involved in bone resorption, i.e., osteoclast formation and activity, form a complex cascade. Pathological alveolar bone destruction is seen in periodontitis due to elevated osteoclastic activity (Takayanagi, 2005). Bone loss was observed in cats that are affected by periodontitis, which was also seen in cats with RL in this study. The anti-inflammatory cytokine IL-10 inhibits osteoclastogenesis by down-regulating the expression of the pro-inflammatory and osteoclast-stimulating cytokines, IL-1β, IL-6, and TNF-α (Baker, 2000), and RANKL (Liu et al., 2006). The inflammatory cytokine IFN-γ also inhibits osteoclastogenesis by down-regulation of RANKL and the inflammatory cytokines IL-1β and TNF-α (Gowen et al., 1986, Takayanagi et al., 2000). Therefore both IL-10 and IFN-γ might be involved in the inhibition of alveolar bone resorption (Takayanagi et al., 2000, Liu et al., 2006). Our findings are consistent with those of other studies reporting elevated mRNA levels for the pro-inflammatory cytokines IL-1β and IL-6 in teeth affected by RL (DeLaurier et al., 2002) and elevated levels of RNA for the pro-inflammatory cytokines IL-6 and IFN-γ and anti-inflammatory cytokine IL-10 in cats with chronic stomatitis (Harley et al., 1999). In humans with periodontitis, the pro-inflammatory cytokines IL-1β, IL-6 and TNF-α are up-regulated and promote the resorptive process (Baker, 2000). It is beyond the scope of this study to discuss osteoimmunology further, but it may be obvious that the cytokines we studied do have complex actions on the differentiation and activity of osteoclasts and therefore most likely also on the odontoclasts that resorb dental tissues.
The ligand 1,25(OH)2D stimulates osteoclastogenesis by up-regulating the expression of RANKL, through up-regulation of the nVDR (Krishnan and Feldman, 1997, Baker, 2000, Kitazawa et al., 2003, Zhang et al., 2004). Studies on the possible role of vitamin D in the aetiology of RL have concentrated on the plasma levels of vitamin D metabolites, and not on the nuclear receptor levels (Reiter and Mendoza, 2002, Reiter et al., 2005). The finding that nVDR is increased in teeth affected by RL suggests that 1,25(OH)2D might play a role in the pathological tooth resorption in cats. The active metabolite 1,25(OH)2D is produced in the kidney through hydroxylation of 25(OH)D by 1α-hydroxylase (CYP27B1) or locally by cells that express CYP27B1 (Peterlik and Cross, 2005). On a para-/auto-crine level 1,25(OH)2D production by macrophages and endothelial cells is increased by inflammatory cytokines (Zehnder et al., 2002, Overbergh et al., 2000). We propose that, in periodontitis, inflammatory cytokines induce either homologous and/or heterologous up-regulation of the expression of nVDR. Nuclear VDR mRNA levels were measured as a first step to determine the possible role of 1,25(OH)2D in RL. The possible causative relationship between vitamin D and RL needs further investigation with the aid of in vivo and in vitro mechanistical studies.
Differences in the expression of the gene products of RANKL and OPG in dental material were not significant, whereas increased expression of RANKL would be expected in active bone and tooth resorption. It is possible that by the time the cats were referred, active resorption had already occurred (Okuda and Harvey, 1992). This is consistent with earlier findings, where differences in levels for RANKL and OPG were not significant in feline teeth with or without RL (DeLaurier et al., 2002). We would expect RANKL to be up-regulated in a very early resorptive lesion, but it is virtually impossible to obtain representative samples from clinical patients to test this because symptoms from these early lesions (if present) may not be noticed by owners, are not clinically visible, and are difficult to detect on dental radiographs. It is possible that RANKL and OPG activity was in an equilibrium because IFN-γ down-regulates RANKL (Takayanagi et al., 2000), whereas 1,25(OH)2D up-regulates RANKL (Baker, 2000, Kitazawa et al., 2003, Zhang et al., 2004) and down-regulates OPG (Kondo et al., 2004).
Although our quantitative PCR results do not necessarily reflect the actual amount of active protein, they supplement earlier semi-quantitative data on cytokine expression in feline RL (Harley et al., 1999, Harley et al., 2003, DeLaurier et al., 2002). They also highlight the possible involvement of nVDR and thus vitamin D in tooth resorption. Previous quantitative PCR studies have shown the role of cytokine expression in the pathophysiology of feline coronavirus (Kipar et al., 2006) and allergic skin disease (Taglinger et al., 2008).
Periodontal pathogens are probably at least partly responsible for feline dental RL. Inflammation of gingival tissues is initiated by bacteria in dental plaque possibly in conjunction with other components, which could aggravate the inflammatory condition. Besides inflammatory cytokines, lipopolysaccharide (LPS), a cell component of gram-negative bacteria (Lindemann et al., 1988), is also present in dental plaque. Both inflammatory cytokines and LPS are involved in osteoclast differentiation and function. In a previous study we showed dental plaque accumulation, together with gram-negative bacteria, in cats with periodontitis (Vrieling et al., 2005).
In conclusion, we hypothesize the following sequence of events. Cats with inflammatory changes of gingival tissues produce more stimulatory regulators of osteoclastogenesis and up-regulate at least on a para/auto-crine level the nVDR levels. The activated vitamin D pathway, as reflected by the increased expression of nVDR mRNA levels, stimulates odontoclast activity. Inflammatory changes and the nuclear VDR overexpression might therefore both play an important role in the pathophysiology of RL in cats. Long-term clinical studies are needed to investigate their causative role pathogenesis of dental RL in cats.
Acknowledgments
We thank Dr J.J. van den Broek, Center for Biostatistics for his assistance with the statistical analysis, the pathology department from the veterinary faculty for providing control teeth and Dr J.E.C. Sykes for revision for English language, grammar and style.
Contributor Information
Henriëtte E. Booij-Vrieling, Email: h.e.booij-vrieling@uu.nl.
Marianna A. Tryfonidou, Email: M.A.Tryfonidou@uu.nl.
References
- Arana-Chavez V.E., Bradaschia-Correa V. Clastic cells: mineralized tissue resorption in health and disease. Int. J. Biochem. Cell Biol. 2009;41(3):446–450. doi: 10.1016/j.biocel.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Baker P.J. The role of immune responses in bone loss during periodontal disease. Microbes Infect. 2000;2(10):1181–1192. doi: 10.1016/s1286-4579(00)01272-7. [DOI] [PubMed] [Google Scholar]
- DeLaurier A., Allen S., deFlandre C., Horton M.A., Price J.S. Cytokine expression in feline osteoclastic resorptive lesions. J. Comp. Pathol. 2002;127(2–3):169–177. doi: 10.1053/jcpa.2002.0577. [DOI] [PubMed] [Google Scholar]
- DuPont G.A., DeBowes L.J. Comparison of periodontitis and root replacement in cat teeth with resorptive lesions. J. Vet. Dent. 2002;19(2):71–75. doi: 10.1177/089875640201900202. [DOI] [PubMed] [Google Scholar]
- Fukushima H., Kajiya H., Takada K., Okamoto F., Okabe K. Expression and role of RANKL in periodontal ligament cells during physiological root-resorption in human deciduous teeth. Eur. J. Oral Sci. 2003;111(4):346–352. doi: 10.1034/j.1600-0722.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- Fuss Z., Tsesis I., Lin S. Root resorption—diagnosis, classification and treatment choices based on stimulation factors. Dent. Traumatol. 2003;19(4):175–182. doi: 10.1034/j.1600-9657.2003.00192.x. [DOI] [PubMed] [Google Scholar]
- Gorrel C., Larsson A. Feline odontoclastic resorptive lesions: unveiling the early lesion. J. Small Anim. Pract. 2002;43(11):482–488. doi: 10.1111/j.1748-5827.2002.tb00018.x. [DOI] [PubMed] [Google Scholar]
- Gowen M., Nedwin G.E., Mundy G.R. Preferential inhibition of cytokine-stimulated bone resorption by recombinant interferon gamma. J. Bone Miner. Res. 1986;1(5):469–474. doi: 10.1002/jbmr.5650010511. [DOI] [PubMed] [Google Scholar]
- Harley R., Helps C.R., Harbour D.A., Gruffydd-Jones T.J., Day M.J. Cytokine mRNA expression in lesions in cats with chronic gingivostomatitis. Clin. Diagn. Lab. Immunol. 1999;6(4):471–478. doi: 10.1128/cdli.6.4.471-478.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley R., Gruffydd-Jones T.J., Day M.J. Characterization of immune cell populations in oral mucosal tissue of healthy adult cats. J. Comp. Pathol. 2003;128(2–3):146–155. doi: 10.1053/jcpa.2002.0619. [DOI] [PubMed] [Google Scholar]
- Hofmann-Lehmann R., Berger M., Sigrist B., Schawalder P., Lutz H. Feline immunodeficiency virus (FIV) infection leads to increased incidence of feline odontoclastic resorptive lesions (FORL) Vet. Immunol. Immunopathol. 1998;23(2–4):299–308. doi: 10.1016/S0165-2427(98)00163-9. 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick M.F., Chen T.C., Lu Z., Sauter E. Vitamin D and skin physiology: a D-lightful story. J. Bone Miner. Res. 2007;22(Suppl. 2):V28–33. doi: 10.1359/jbmr.07s211. [DOI] [PubMed] [Google Scholar]
- How K.L., Hazewinkel H.A., Mol J.A. Dietary vitamin D dependence of cat and dog due to inadequate cutaneous synthesis of vitamin D. Gen. Comp. Endocrinol. 1994;96(1):12–18. doi: 10.1006/gcen.1994.1154. [DOI] [PubMed] [Google Scholar]
- Kipar A., Meli M.L., Failing K., Euler T., Gomes-Keller M.A., Schwartz D., Lutz H., Reinacher M. Natural feline coronavirus infection: differences in cytokine patterns in association with the outcome of infection. Vet. Immunol. Immunopathol. 2006;15(3–4):141–155. doi: 10.1016/j.vetimm.2006.02.004. 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa S., Kajimoto K., Kondo T., Kitazawa R. Vitamin D3 supports osteoclastogenesis via functional vitamin D response element of human RANKL gene promoter. J. Cell Biochem. 2003;89(4):771–777. doi: 10.1002/jcb.10567. [DOI] [PubMed] [Google Scholar]
- Kondo T., Kitazawa R., Maeda S., Kitazawa S. 1 alpha, 25 dihydroxyvitamin D3 rapidly regulates the mouse osteoprotegerin gene through dual pathways. J. Bone Miner. Res. 2004;19(9):1411–1419. doi: 10.1359/JBMR.040604. [DOI] [PubMed] [Google Scholar]
- Krishnan A.V., Feldman D. Regulation of vitamin D receptor abundance. In: Feldman D., Glorieux F.H., Pike J.W., editors. Vitamin D. Academic Press; San Diego: 1997. pp. 179–200. [Google Scholar]
- Lindemann R.A., Economou J.S., Rothermel H. Production of interleukin-1 and tumor necrosis factor by human peripheral monocytes activated by periodontal bacteria and extracted lipopolysaccharides. J. Dent. Res. 1988;67(8):1131–1135. doi: 10.1177/00220345880670081401. [DOI] [PubMed] [Google Scholar]
- Lindskog S.F., Dreyer C.W., Pierce A.M., Torabinejad M., Shabahang S. Osteoclastic activity. In: Andreassen J.O., Andreassen F.M., Andersson L., editors. Traumatic Injuries to the Teeth. Blackwell Munksgaard; Copenhagen: 2007. pp. 137–171. [Google Scholar]
- Liu D., Yao S., Wise G.E. Effect of interleukin-10 on gene expression of osteoclastogenic regulatory molecules in the rat dental follicle. Eur. J. Oral Sci. 2006;114(1):42–49. doi: 10.1111/j.1600-0722.2006.00283.x. [DOI] [PubMed] [Google Scholar]
- Morris J.G. Ineffective vitamin D synthesis in cats is reversed by an inhibitor of 7-dehydrocholestrol-delta7-reductase. J. Nutr. 1999;129(4):903–908. doi: 10.1093/jn/129.4.903. [DOI] [PubMed] [Google Scholar]
- Okuda A., Harvey C.E. Etiopathogeneses of feline dental resorptive lesions. Vet. Clin. North Am. Small Anim. Pract. 1992;22:1385–1404. doi: 10.1016/s0195-5616(92)50133-4. [DOI] [PubMed] [Google Scholar]
- Overbergh L., Decallonne B., Valckx D., Verstuyf A., Depovere J., Laureys J., Rutgeerts O., Saint-Arnaud R., Bouillon R., Mathieu C. Identification and immune regulation of 25-hydroxyvitamin D-1-alpha-hydroxylase in murine macrophages. Clin. Exp. Immunol. 2000;120(1):139–146. doi: 10.1046/j.1365-2249.2000.01204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penning L.C., Vrieling H.E., Brinkhof B., Riemers F.M., Rothuizen J., Rutteman G.R., Hazewinkel H.A. A validation of 10 feline reference genes for gene expression measurements in snap-frozen tissues. Vet. Immunol. Immunopathol. 2007;15(3–4):212–222. doi: 10.1016/j.vetimm.2007.08.006. 120. [DOI] [PubMed] [Google Scholar]
- Peterlik M., Cross H.S. Vitamin D and calcium deficits predispose for multiple chronic diseases. Eur. J. Clin. Invest. 2005;35(5):290–304. doi: 10.1111/j.1365-2362.2005.01487.x. [DOI] [PubMed] [Google Scholar]
- Rani C.S., MacDougall M. Dental cells express factors that regulate bone resorption. Mol. Cell Biol. Res. Commun. 2000;3(3):145–152. doi: 10.1006/mcbr.2000.0205. [DOI] [PubMed] [Google Scholar]
- Reiter A.M., Mendoza K.A. Feline odontoclastic resorptive lesions, an unsolved enigma in veterinary dentistry. Vet. Clin. North Am. Small Anim. Pract. 2002;32:791–837. doi: 10.1016/s0195-5616(02)00027-x. [DOI] [PubMed] [Google Scholar]
- Reiter A.M., Lyon K.F., Nachreiner R.F., Shofer F.S. Evaluation of calciotropic hormones in cats with odontoclastic resorptive lesions. Am. J. Vet. Res. 2005;66(8):1446–1452. doi: 10.2460/ajvr.2005.66.1446. [DOI] [PubMed] [Google Scholar]
- Servet E., Girard N., Biourge V. Proceedings of the 11th ESVCN Congress. 2007. Vitamin D3 status and FORL prevalence in cats fed premium dry foods; p. 43. [Google Scholar]
- Taglinger K., Van Nguyen N., Helps C.R., Day M.J., Foster A.P. Quantitative real-time RT-PCR measurement of cytokine mRNA expression in the skin of normal cats and cats with allergic skin disease. Vet. Immunol. Immunopathol. 2008;15(3–4):216–230. doi: 10.1016/j.vetimm.2007.11.014. 122. [DOI] [PubMed] [Google Scholar]
- Takayanagi H. Inflammatory bone destruction and osteoimmunology. J. Periodontal Res. 2005;40(4):287–293. doi: 10.1111/j.1600-0765.2005.00814.x. [DOI] [PubMed] [Google Scholar]
- Takayanagi H., Ogasawara K., Hida S., Chiba T., Murata S., Sato K., Takaoka A., Yokochi T., Oda H., Tanaka K., Nakamura K., Taniguchi T. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-gamma. Nature. 2000;30(6812):600–605. doi: 10.1038/35046102. 408. [DOI] [PubMed] [Google Scholar]
- Takeichi O., Saito I., Tsurumachi T., Moro I., Saito T. Expression of inflammatory cytokine genes in vivo by human alveolar bone-derived polymorphonuclear leukocytes isolated from chronically inflamed sites of bone resorption. Calcif. Tissue Int. 1996;58(4):244–248. doi: 10.1007/BF02508643. [DOI] [PubMed] [Google Scholar]
- Vrieling H.E., Theyse L.F., van Winkelhoff A.J., Dijkshoorn N.A., Logan E.I., Picavet P. Effectiveness of feeding large kibbles with mechanical cleaning properties in cats with gingivitis. Tijdschr Diergeneeskd. 2005;1(5):136–140. 130. [PubMed] [Google Scholar]
- Zehnder D., Bland R., Chana R.S., Wheeler D.C., Howie A.J., Williams M.C., Stewart P.M., Hewison M. Synthesis of 1,25-dihydroxyvitamin D(3) by human endothelial cells is regulated by inflammatory cytokines: a novel autocrine determinant of vascular cell adhesion. J. Am. Soc. Nephrol. 2002;13(3):621–629. doi: 10.1681/ASN.V133621. [DOI] [PubMed] [Google Scholar]
- Zhang D., Yang Y.Q., Li X.T., Fu M.K. The expression of osteoprotegerin and the receptor activator of nuclear factor kappa B ligand in human periodontal ligament cells cultured with and without 1alpha,25-dihydroxyvitamin D3. Arch. Oral Biol. 2004;49(1):71–76. doi: 10.1016/s0003-9969(03)00201-2. [DOI] [PubMed] [Google Scholar]


