Abstract
Oligodendroglial cell death is a frequent phenomenon of many neurological diseases, e.g. in demyelinating diseases such as multiple sclerosis (MS). The underlying mechanisms are largely unknown. Here, we demonstrate that in the toxic demyelination cuprizone model, oligodendroglial cell death and downregulation of myelin genes start days after initiation of the cuprizone diet and weeks before demyelination is obvious. In early – but not in later – stages, dying oligodendrocytes express activated caspase 3, suggesting a switch from classical apoptotic pathways to caspase 3-independent mechanisms during the course of the cuprizone diet. The expression level of FAS in the corpus callosum, a cell death receptor crucial for oligodendroglial cell death in experimental autoimmune encephalomyelitis (EAE), correlates with the expression of activated caspase 3 in oligodendrocytes. However, mice lacking FAS in oligodendrocytes are not protected against cuprizone-induced oligodendroglial cell death, showing that FAS is dispensable for oligodendroglial cell death in the cuprizone model.
Keywords: Myelin, Cuprizone, Apoptosis, FAS
Introduction
Oligodendroglial dysfunction and cell death are features of numerous human CNS diseases, such as demyelinating diseases (e.g. multiple sclerosis), stroke, spinal cord injury or neurodegenerative diseases (e.g. multiple system atrophy) (Barnett and Prineas, 2004, Lucchinetti et al., 2000, Wenning et al., 2008). However, although oligodendroglial cell death is a common feature in many CNS diseases, the underlying mechanisms are poorly understood. Multiple sclerosis (MS) is the most frequent human demyelinating disorder with heterogeneous clinical and pathological findings. Histopathologically, two different categories can be distinguished: One pattern is characterized by immune-mediated demyelination while the other is characterized by a primary oligodendrogliopathy. The morphology of dying oligodendrocytes in MS lesions shows some characteristics of apoptosis, e.g. condensed and fragmented nuclei; additionally, the expression of apoptosis-related proteins, such as bcl-2, p53 and FAS, has been described in the oligodendrocytes in MS lesions (Dowling et al., 1996, Kuhlmann et al., 1999, Wosik et al., 2003). The FAS signalling cascade has been shown to be involved in oligodendroglial cell death in vitro and in vivo. In vitro incubation of human oligodendroglial cell lines with FAS ligand leads to apoptotic cell death (Austin and Fehlings, 2008, Li et al., 2002). Challenging primary human oligodendrocytes with IFNγ or increased levels of p53 renders oligodendrocytes susceptible to FAS-mediated cell death (Pouly et al., 2000, Wosik et al., 2003). Additionally, FAS has been shown to be upregulated in oligodendrocytes in MS lesions as well as in experimental autoimmune encephalomyelitis, an animal model of MS (Bonetti et al., 1993, Bonetti and Raine, 1997, Dowling et al., 1996, Raine et al., 1998, Sabelko-Downes et al., 1999). Mice lacking FAS especially in oligodendrocytes show limited oligodendroglial cell death and reduced clinical symptoms in EAE (Hovelmeyer et al., 2005).
The cuprizone model is a toxic demyelination model characterized by reproducible demyelination in certain brain regions, such as the corpus callosum, while other CNS regions (e.g. spinal cord, optic nerves) are spared. Demyelination is induced by administering a cuprizone diet for up to 6 weeks, which leads to apoptotic oligodendroglial cell death via unknown pathways. While demyelination is most extensive 6 weeks after initiating the cuprizone diet, oligodendroglial cell death starts earlier. The number of oligodendrocytes decreases significantly in the second and third week and reaches its minimum 4 weeks after start of the cuprizone diet (Matsushima and Morell, 2001).
Here, we present a detailed analysis of oligodendroglial cell death in the early phase of the cuprizone model. Oligodendroglial cell death occurs within few days after initiation of the cuprizone diet and weeks before demyelination is visible. During the initial phase of the cuprizone diet (days 6 and 10), but not during later stages (day 21), activated caspase 3 can be detected in apoptotic oligodendrocytes, suggesting that caspase 3-independent pathways trigger oligodendroglial cell death in later stages. The mRNA expression levels of FAS in the corpus callosum correlate with the expression pattern of activated caspase 3 in oligodendrocytes. However, animal experiments using mice lacking FAS especially in oligodendrocytes reveal that FAS is not necessary for oligodendroglial cell death in the cuprizone model.
Materials and methods
Cuprizone treatment
Cuprizone was prepared by adding 0.3% (w/w) cuprizone (Sigma) to powdered chow. To examine oligodendroglial cell death in the cuprizone model, 7- to 8-week-old C57Bl/6 mice were fed with cuprizone for up to 21 days. The mice were sacrificed on days 6, 10 and 21. Control animals received powdered chow without cuprizone and were sacrificed at day 21.
Six- to eight-week-old male C57Bl/6 mice were purchased from Charles River (Sulzfeld, Germany). To investigate the effect of FAS on oligodendroglial cell death, we crossed mice in which exon 9 of the FAS gene was flanked by loxP sites (fasfl/fl) (Hao et al., 2004) with mice expressing Cre recombinase under the control of the endogenous MOG promoter (Hovelmeyer et al., 2005), resulting in mice lacking FAS exclusively in oligodendrocytes (MOGicrefasfl/fl). MOGicrefasfl/fl and fasfl/fl control mice were fed for up to 21 days with cuprizone. The mice were genotyped by tail clipping and PCR. Sibling female and male mice were placed on a diet of 0.2% (w/w) cuprizone mixed into powdered food, which was available ad libitum. They were sacrificed 4, 6 and 10 days after initiation of the cuprizone diet. Animal handling and experiments were conducted according to the German animal protection laws and approved by the responsible governmental authority (Bezirksregierung Braunschweig and LANUV Nordrhein-Westfalen).
Myelin gene expression
To profile whole gene expression differences at different time points during the cuprizone diet, we used the Mice 4x44K array (Agilent Technologies, Cat No. G4122F). Microarrays were performed using the “Low RNA Input Linear Amplification Kit Plus, One Color” Protocol (Agilent Technologies, Cat No. 5188-5339) and the Agilent Spike-In Kit for One Color (Agilent Technologies, Cat No. 5188-5282). Using the mircroRNA Kit (Qiagen, Hilden, Germany), total RNA was isolated from micro-dissected corpus callosum tissue specimens taken from mice treated for 6, 10 and 21 days with cuprizone as well as from control mice (n = 3 for each time point) and was treated with DNaseI. RNA samples were quantified using the Nanodrop ND-1000 (Thermo Scientific, Wilmington) and the quality of the samples was controlled using RNA pico Chips (Agilent 2100 bioanalyzer).
Total RNA was used as a starting material to prepare cDNA. cDNA synthesis and in vitro transcription were performed according to the manufacturer's protocol. The hybridization was carried out for 17 h at 10 rpm and 65 °C in the hybridization oven (Agilent). The arrays were washed and stained according to the manufacturer's recommendation. They were then scanned using an Agilent DNA microarray scanner at 5 μm resolution. Scanned image files were visually inspected for artefacts and analyzed. Data analysis included ANOVA modelling, non-linear local regression normalization and component-wise t-tests and p-value adjustments for high dimensional microarray data to control for the family-wise error rate (FWER) or the false discovery rate (FDR).
Quantitative real-time PCR
RNA was isolated from micro-dissected corpus callosum tissue specimens using the MicroRNA kit (Qiagen, Germany) and was treated with DNaseI to eliminate genomic DNA. RNA quantity and quality was assessed by using NanoDrop ND 1000 (Thermo Scientific, Wilmington) and the Agilent 2100 bioanalyzer. The 500 ng of total RNA was used in 20 μl of reverse transcription (RT) reaction, using Omniscript (Qiagen, Hilden, Germany). Quantitative real-time PCR was performed using SYBR green PCR master mix (ABgenes) in the iQ5 Realtime-PCR detection system (Bio-Rad). The melting curve of each sample was determined to ensure the specificity of the products. The following primers were used: MBP_forward 5′-AAG-AAC-ATT-GTG-ACA-CCT-CGA-A, MBP_reverse 5′-CTC-TTC-CTC-CCA-GCT-TAA-AGA-T-3′; FAS_forward 5′-AAA-GTC-CCA-GAA-ATC-GCC-TAT-G-3′, FAS_reverse 5′-TGG-TAT-GGT-TTC-ACG-ACT-GGA-G-3′. The PCR results were normalized to human acidic ribosomal protein (hARP) message (forward primer: CGA-CCT-GGA-AGT-CCA-ACT-AC, reverse primer: 5′-ATC-TGC-TGC-ATC-TGC-TTG-3′). Cycle conditions consisted of an initial period of 15 min followed by 40 cycles; each cycle consisted of denaturation at 94 °C for 30 s, annealing at 59–62 °C for 30 s, and extension at 72 °C for 30 s.
Immunohistochemistry
The mice were sacrificed under deep anaesthesia and were perfused intracardially with ice-cold phosphate-buffered saline (PBS) followed by 4% paraformaldehyde (PFA) perfusion. Brains, spinal cords, spleen and livers were removed and post-fixed in PFA overnight. Brains were cut at the chiasma opticum level and embedded in paraffin. The 4-μm-thick sections were stained with haematoxylin and eosin (HE) and Luxol-fast blue (LFB). Immunohistochemical staining was performed with an avidin–biotin technique. After deparaffinization intrinsic peroxidase activity was blocked by incubation with 5% H2O2 in PBS for 20 min. Non-specific antibody binding was inhibited with 10% FCS in PBS for 25 min. Microwave pre-treatment for better antigen retrieval was performed for mouse anti-Nogo-A. The primary antibodies were rabbit anti-myelin basic protein (MBP) (1:1 000) (Boehringer Mannheim, Mannheim, Germany), mouse anti-CNPase (1:200) (Sternberger Monoclonals), rabbit anti-Mac3 (1:200) (PharMingen), rabbit anti-CPP32 (activated caspase 3) (1:100) (PharMingen), rabbit anti-GFAP (1:2 000) (Dako, Denmark), mouse anti-GFAP (1:50) (Dako, Denmark), rabbit anti-Olig2 (1:300) (IBL, Spring Lake Park, Minnesota) and mouse anti-Nogo-A (1:15 000) (11c7, a generous gift from M.E. Schwab, Brain Research Institute, University of Zürich and Department of Biology, Swiss Federal Institute of Technology Zürich, Switzerland). Secondary antibodies were anti-mouse, anti-rabbit or anti-rat biotinylated Ig (1:200) (Amersham Biosciences, Freiburg, Germany).
TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labelling)
Sections were deparaffinized and pretreated with Proteinase K (50 μg/ml) for 20 min followed by an incubation with a reaction mix containing terminal transferase, fluorescin-labelled nucleotides and reaction buffer for 1 h at 37 °C (Roche, Mannheim, Germany). Sections were either counterstained with DAPI or were washed in PBS, followed by incubation with alkaline phosphatase-labeled anti-fluorescin antibody (Roche, Mannheim, Germany) for 30 min at 37 °C. The color reaction was developed with nitroblue tetrazolium (NBT) and 5-bromo-4-chloro-3-indolylphosphate (BCIP) (Roche, Mannheim, Germany). TUNEL-positive oligodendrocytes were detected by immunohistochemistry with an anti-CNPase antibody as described above.
Luxol-Fast-Blue, MBP and CNP quantification
To determine the extent of demyelination we used a semiquantitative score. Coronal brain sections were analyzed using an Olympus Bx41 light microscope. A score of 0 was assigned to fully myelinated sections, fully demyelinated sections were given a score of 3. Similar scores have been previously used to assess LFB and histochemical stains (Liebetanz and Merkler, 2006).
Morphometry and Statistics
The number of apoptotic cells as well as the number of Nogo-A-, CPP32-, Olig2-, GFAP- and Mac3-positive cells stained with the corresponding antibodies in the corpus callosum was determined in 10 standardized microscopic fields of 10,000 μm2 each defined by an ocular morphometric grid. In the text and figures, the mean number of cells/mm2 ± SD is given.
Bonferroni-corrected one-way or two-way ANOVA tests were performed for statistical analysis. All tests were classified as significant if the p-value was < 0.05. GraphPad PRISM™ software was used (Graph Pad Software, Inc., San Diego, CA, USA) for these analyses.
Results
Oligodendroglial cell death occurs early in cuprizone-induced demyelination
Mice were fed up to 21 days with cuprizone and sacrificed on days 6, 10 and 21 after start of the cuprizone diet. Cells with morphological characteristics typical for apoptosis such as condensed and/or fragmented nuclei were seen on day 6 (97 ± 32 cells/mm2) and were continuously present in similar numbers on day 10 (110 ± 17 cells/mm2). Control animals fed with powdered food without cuprizone were lacking apoptotic cells in the corpus callosum (Fig. 1, Fig. 2 ). On day 21, the morphology of the dying oligodendrocytes was slightly different; the nuclei were condensed, but only rarely fragmented nuclei were observed (Fig. 1b). Similar numbers of dying oligodendrocytes were found compared to days 6 and 10 (117 ± 61 cells/mm2). To confirm that these cells underwent cell death, we also performed TUNEL stainings (Fig. 1d); quantitative analysis revealed similar numbers of apoptotic and TUNEL-positive cells on day 21 (data not shown). In additional studies, apoptotic oligodendrocytes were present 2 days after the start of the cuprizone diet (data not shown). Not only in the corpus callosum but also in the cortex apoptotic cells were observed (Fig. 1c), although in somewhat reduced numbers on days 6 and 10 compared to the corpus callosum. Immunohistochemistry with CNPase confirmed that the apoptotic cells were oligodendrocytes (Fig. 1c). In contrast, we did not observed GFAP- or MAC3-positive astrocytes or microglia/macrophages with condensed or fragmented nuclei characteristic for apoptotic cell death in the cortex or corpus callosum.
Fig. 1.
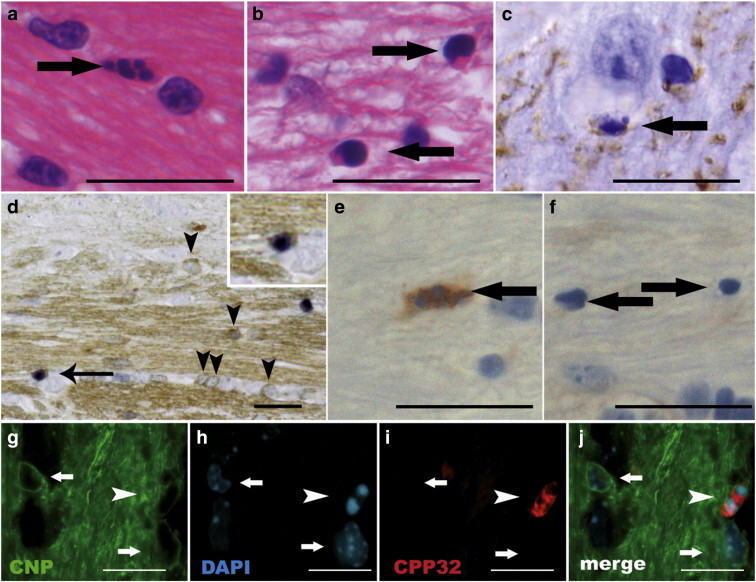
On day 6 after the start of the cuprizone diet, numerous cells with the typical morphological characteristics of apoptosis, such as round, condensed and fragmented nuclei were detected (a). On day 21, the dying cells displayed condensed nuclei, but they were rarely fragmented (b). Dying cells with apoptotic morphology (arrow) were also detected in the cortex and identified as oligodendrocytes by immunohistochemistry for CNPase (brown) (c). To detect DNA double strand breaks, TUNEL was performed. Some of the TUNEL-positive cells (black) were also positive for CNP (arrow). TUNEL-negative CNP-positive oligodendrocytes are indicated by arrow heads (d). To determine whether the cells underwent apoptosis, immunohistochemistry for activated caspase 3 was performed (e and f). On day 6 (e), almost all oligodendrocytes with condensed and fragmented nuclei expressed activated caspase 3, whereas after 21 days of cuprizone diet all dying oligodendrocytes were negative for activated caspase 3 (f). The expression of activated caspase 3 was confined to apoptotic oligodendrocytes as double immunohistochemistry for CNPase (green) and activated caspase 3 (red) demonstrated (g–j). The CNPase-positive oligodendrocyte indicated by the arrow head contains a fragmented and condensed nucleus and expresses activated caspase 3 in the cytoplasm. Arrows indicate non-apoptotic oligodendrocytes. Scale bars in a–f, 20 μm; scale bars in g–j, 10 μm.
Fig. 2.
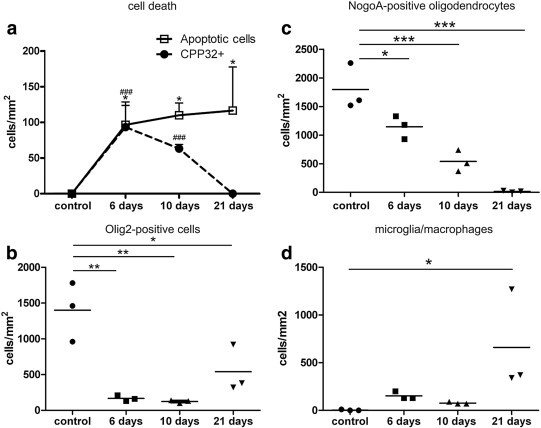
Mice (n = 3 for each time point) were fed for 6, 10 or 21 days with powdered food containing cuprizone while controls received a cuprizone-free diet. Significantly more apoptotic cells were detected in the corpus callosum on all time points compared to controls. On day 6, almost all apoptotic oligodendrocytes expressed activated caspase 3 (97 ± 5%); on day 10, the percentage is reduced to 58 ± 8% and after 21 days of cuprizone diet none of the dying oligodendrocytes expressed activated caspase 3 (a). Feeding of cuprizone over 21 days induced a significantly reduction of Nogo-A-positive oligodendrocytes on days 6, 10 and 21. (b) Olig2 is expressed in oligodendroglial progenitor cells and mature oligodendrocytes. The number of Olig2-positive cells decreased significantly during the cuprizone diet with lowest numbers observed on day 10; 21 days after the start of the cuprizone diet, a slight recovery of Olig2-positive cells was observed, suggesting a recruitment of progenitor cells. Oligodendroglial cell death was accompanied by a significant increase in the number of microglial cells on day 21 (d). ⁎p < 0.05; ⁎⁎p < 0.01; ⁎⁎⁎p < 0.001. ###p < 0.001 for activated caspase-3-positive cells compared to controls.
To further confirm that the dying oligodendrocytes undergo apoptotic cell death, we stained for activated caspase 3 combined with and without immunohistochemistry for CNP (Figs. 1e–j). On days 6 and 10 after the start of the cuprizone diet, 97 ± 5% and 58 ± 8% of the apoptotic cells expressed activated caspase 3. On day 21, none of the dying cells showed a signal for activated caspase 3 in the corpus callosum or the cortex.
In line with the notion of continuous oligodendroglial cell death, we found a significant decrease in the number of Nogo-A-positive mature oligodendrocytes in mice fed with cuprizone after 10 (540 ± 187 cells/mm2) and 21 days (17 ± 15 cells/mm2) compared to untreated controls (1797 ± 404 cells/mm2) (Fig. 2b). Olig2, which is expressed in mature oligodendrocytes as well as in oligodendroglial progenitor cells, decreased significantly on day 6 (166 ± 40 cells/mm2) and day 10 (123 ± 21 cells/mm2) compared to controls (1400 ± 413 cells/mm2); however, on day 21 a slight, but not significant increase, in the number of Olig2-positive cells was observed compared to day 10 (540 ± 331 cells/mm2) (Fig. 2c). This indicates – together with the low number of Nogo-A-expressing mature oligodendrocytes – a recruitment of oligodendroglial progenitor cells on day 21, even during ongoing cuprizone treatment as has been described in previous studies (Matsushima and Morell, 2001). In the cuprizone model, the loss of oligodendrocytes is accompanied by the expansion of microglia cells and infiltration of macrophages, whereby microglia cells substantially outnumber macrophages (Remington et al., 2007). This is in line with the observation that cuprizone does not promote a leakage of the blood–brain barrier (Matsushima and Morell, 2001, McMahon et al., 2002). We observed a significant increase in microglia/macrophages on day 21 (660 ± 528 cells/mm2) (Fig. 2d).
Myelin gene expression during early cuprizone-induced demyelination
To investigate the effects on myelin gene expression, we determined the mRNA expression levels of different myelin genes in the corpus callosum by microarray studies. All major myelin proteins (MBP, PLP, CNP, MAG, MOG, MAL, MOBP) showed a marked reduction in expression on day 6 compared to controls (between 11- and 115-fold reduction compared to controls) and the mRNA levels remained low on days 10 and 21 (Fig. 3 a). These results were confirmed by qRT-PCR for MBP (data not shown). Our results are consistent with previous studies analyzing the myelin gene expression patterns 2, 4 and 6 weeks after beginning cuprizone (Jurevics et al., 2002).
Fig. 3.
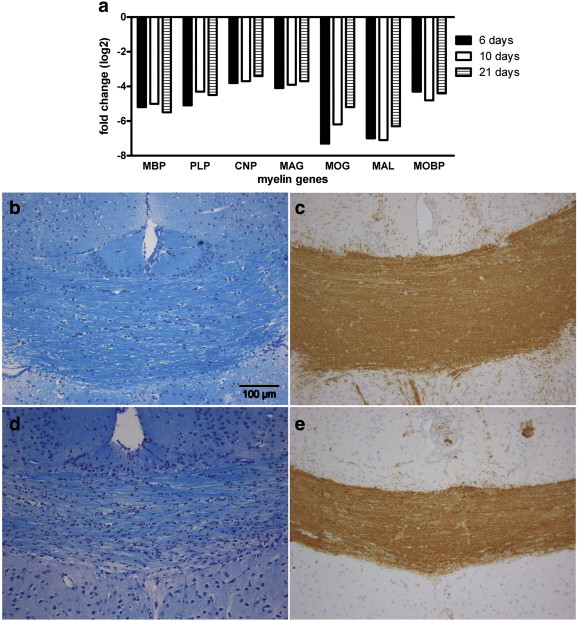
During the cuprizone diet, myelin genes were strongly downregulated at all time points compared to controls (a). On day 6, no obvious decrease in the staining intensity was observed in the LFB-PAS staining (b) nor in the CNPase immunohistochemistry (c). After 21 days of cuprizone diet, a markedly increased cellularity was associated with a slightly patchy LFB-PAS staining (d) whereas the CNPase staining revealed no signs of demyelination (e).
To further analyze the effects of the oligodendroglial cell death on the myelin proteins, we stained for LFB-PAS, CNP and MBP. On days 6 and 10, the intensity of the LFB-PAS and CNP staining was comparable to controls (Figs. 3b, c and e). A slightly patchy LFB-PAS and MBP staining associated with an increased cellularity was found in the middle of the corpus callosum in two out of three mice on day 21, whereas the CNP staining revealed no obvious differences compared to earlier time points (Figs. 3c and d). Since demyelination of the cortex has been observed as well in the cuprizone model, we also analyzed the expression of the myelin proteins in the cortex by immunohistochemistry (Gudi et al., 2009, Skripuletz et al., 2008). Neither in the LFB-PAS nor in the MBP staining did we observe a significant demyelination of the cortex on day 6 or 10. However, on day 21 some cortical regions displayed a less well-defined and patchy MBP staining (data not shown).
Role of FAS in toxin-induced demyelination
The majority of dying oligodendrocytes expressed high levels of activated caspase 3 on days 6 and 10, indicating an activation of the apoptosis cascade in oligodendrocytes early during cuprizone treatment. FAS, an activator of the extrinsic apoptotic cell cascade, has been described as a key molecule for oligodendroglial cell death in experimental autoimmune encephalomyelitis (Hovelmeyer et al., 2005). We found an upregulation of FAS in the corpus callosum following a time course similar to activated caspase 3 in oligodendrocytes (Fig. 4 ).
Fig. 4.
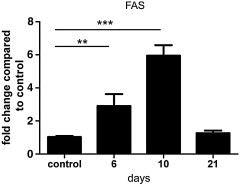
Corpus callosum from mice fed with cuprizone for 6, 10 and 21 days as well as from mice without cuprizone diet (n = 3 each) was micro-dissected and RNA was extracted. The FAS mRNA expression levels were determined in the corpus callosum by qRT-PCR. The mRNA expression was significantly increased on days 6 and 10 compared to controls. ⁎⁎p < 0.01; ⁎⁎⁎p < 0.001.
To further elucidate the role of FAS in oligodendroglial cell death in the cuprizone model, we crossed mice in which exon 9 of the FAS gene was flanked by loxP sites (fasfl/fl) (Hao et al., 2004) with mice expressing Cre recombinase under the control of the endogenous MOG promoter (Hovelmeyer et al., 2005). This resulted in mice lacking FAS exclusively in oligodendrocytes (MOGicre+/-fasfl/fl). One MOGicre allele is sufficient to ensure the FAS-KO type. Since the cre recombinase open reading frame followed by a polyadenylation signal replaced the first MOG exon and its start codon, mice homozygous for the MOGicre allele fail to express MOG while other myelin genes (e.g. MBP) are expressed (data not shown).
MOGicre+/-fasfl/fl mice (subsequently named FAS-KO) and MOGicre-/-fasfl/fl mice (subsequently called FAS-wild-type (FAS-wt) were fed for up to 10 days with cuprizone. We focussed on the first 10 days since the majority of dying oligodendroglial cells express activated caspase 3 during this time period, indicating elimination of oligodendrocytes via classical apoptotic cell death pathways. The number of apoptotic cells, Nogo-A-positive oligodendrocytes, activated caspase 3-expressing oligodendrocytes, macrophages and astrocytes, as well as the extent of demyelination, was determined on days 4, 6 and 10 and in mice fed with a cuprizone-free diet (controls). Similar numbers of apoptotic cells were found on days 4, 6 and 10 (between 97 ± 46 and 143 ± 12 cells/mm2; Fig. 5 a). As in the previous experiments, a high percentage of the dying oligodendrocytes expressed activated caspase 3 on day 4 (FAS-KO 98 ± 3%; FAS-wt 100 ± 0%), and day 6 (FAS-KO 100 ± 0%; FAS-wt 83 ± 17%). On day 10 after the start of the cuprizone diet, 47 ± 18% or 62 ± 23%, respectively, expressed activated caspase 3 (Fig. 5b). However, no significant differences in the number of apoptotic cells or in the percentage of activated caspase 3-positive cells were found between FAS-KO and FAS-wt animals indicating that FAS is not necessary for oligodendroglial cell death in toxic demyelination (Figs. 5a and b). In neither FAS-wt nor in FAS-KO mice did the LFB-PAS staining reveal significant demyelination (data not shown). As expected, the number of Nogo-A-positive oligodendrocytes decreased significantly during the cuprizone diet, whereas the numbers of microglia/macrophages increased significantly. Similar numbers of oligodendrocytes, microglia/macrophages and astrocytes were found in FAS-KO compared to FAS-wt mice on the vast majority of time points analyzed (Figs. 5c–e).
Fig. 5.
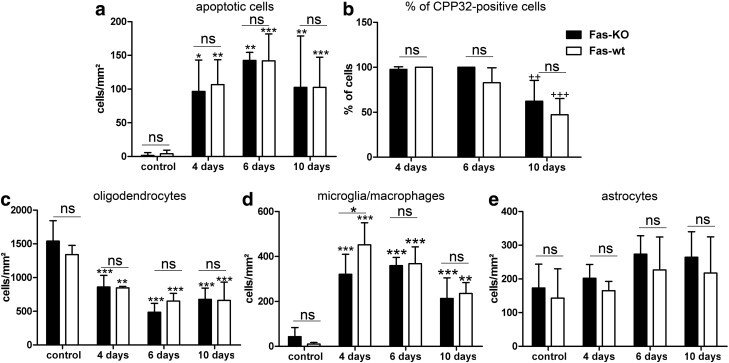
FAS-KO and FAS-wt mice were fed for up to 10 days with 0.2% cuprizone, controls received chow without cuprizone. Between 5 and 13 mice were analyzed per time point and genotype (except for FAS-wt fed for 4 days with cuprizone diet (n = 3)). High numbers of apoptotic oligodendrocytes were observed on days 4, 6 and 10, whereas in control mice only single dying cells were found independent of the genotype (a). The number of CPP32-positive cells was almost 100% in FAS-KO and FAS-wt on days 4 and 6. On day 10, 47 ± 18% of the apoptotic cells expressed activated caspase 3 in FAS-wt; in FAS-KO mice, 62 ± 23% of the mice were positive for CPP32. No significant differences were observed between FAS-KO and FAS-wt mice (b). The numbers of Nogo-A-positive oligodendrocytes were significantly decreased on days 4, 6 and 10 in FAS-wt and FAS-KO mice compared to controls; no differences were observed between FAS-KO and FAS-wt mice except for day 6 (c). The numbers of microglia/macrophages were significantly increased on days 4, 6 and 10 in both mouse lines compared to controls. On day 4, significantly more microglia/macrophages were found in FAS-wt mice than in FAS-KO mice (d). The number of astrocytes did not differ significantly between FAS-KO mice and FAS-wt (e). ⁎p < 0.05, ⁎⁎p < 0.01, ⁎⁎⁎p < 0.001 compared to controls. ++p < 0.001, +++p < 0.001 compared to day 4.
Discussion
The mechanisms responsible for oligodendroglial cell death and demyelination in the cuprizone model are not well understood. Here, we demonstrate that in this toxic demyelination model oligodendroglial cell death occurs very early and precedes demyelination by weeks. In the early disease phase oligodendroglial cell death is associated with the expression of activated caspase 3 in oligodendrocytes, whereas in later disease stages oligodendroglial cell death is apparently independent of caspase 3. FAS is upregulated in the corpus callosum in the early disease stage in the cuprizone model; however, it is not necessary for oligodendroglial cell death in cuprizone-induced demyelination.
To analyze oligodendroglial cell death in the cuprizone model, we focussed on the first 3 weeks after the start of the cuprizone diet since previous studies show that the number of oligodendrocytes is maximally reduced after 21 days. Similarly, the myelin gene expression was maximally reduced 3 weeks after initiation of the cuprizone diet while at 4 weeks a slight increase was observed (Jurevics et al., 2002, Matsushima and Morell, 2001). This is in line with our findings presented here. We observed a continuous decrease of Nogo-A-positive oligodendrocytes during the cuprizone diet and maximal numbers of apoptotic oligodendrocytes were found 10 and 21 days after start of the cuprizone diet. This was accompanied by a dramatic decrease of myelin gene expression already obvious on day 6. Together, these data confirm that the vast majority of mature oligodendrocytes undergo cell death during the first 3 weeks of the cuprizone diet.
In the initial phases of the cuprizone model, almost all dying oligodendrocytes express activated caspase 3, suggesting that this is the dominant mechanism by which oligodendrocytes are eliminated. However, in later disease stages the percentage of activated caspase 3-positive oligodendrocytes decreases gradually to the complete absence of caspase 3 on day 21. These findings are suggestive for a change from a caspase 3-dependent to a caspase 3-independent cell death mechanism in the cuprizone model. The dying oligodendrocytes still display some morphological characteristics of apoptosis, this makes it tempting to speculate that apoptotic-like cell death mechanisms might contribute to oligodendroglial cell death at later stages (for a review, see Boujrad et al., 2007). This type of cell death is controlled by mitochondria, and one of the major effectors might be the apoptosis-inducing factor (AIF). Increased Ca2+ levels and generation of oxygen species are triggers for release of AIF from the mitochondrial intermembrane space. It is conceivable that cuprizone, which has been described as damaging mitochondria, also directly or indirectly mediates the mitochondrial release of AIF (Maloff et al., 1978). Downstream signalling cascades may involve poly(ADP-ribose) polymerase, calpains or EndoG (Moubarak et al., 2007, Niikura et al., 2007). However, so far no direct evidence exists that indicates a contribution of this pathway to cuprizone-induced demyelination; further studies are required to reveal the exact mechanisms underlying caspase-independent cell death in later stages of the cuprizone diet.
The cuprizone model is a relatively artificial model and cannot be regarded as a prototypical paradigm mimicking oligodendroglial cell death in human demyelinating diseases. However, it provides insights in general cell death mechanisms that can be activated in oligodendrocytes. Furthermore, since the cuprizone model is frequently used to study remyelination, the mechanisms leading to demyelination and oligodendroglial cell death deserve deeper understanding. Little is known about potential pathways leading to the cleavage and activation of caspase 3 in the cuprizone model. Mice on the cuprizone diet lacking p53 or treated with the p53 inhibitor pifithrin-α show reduced apoptosis and improved preservation of oligodendrocytes as well myelin, suggesting that p53 is involved in oligodendroglial cell death in this toxic demyelination model (Li et al., 2008). Increased expression of p53 has been observed in MS lesions and leads in vitro to an increased susceptibility to FAS- or TRAIL-mediated oligodendroglial cell death (Wosik et al., 2003). FAS has been shown to be upregulated in oligodendrocytes in experimental autoimmune encephalomyelitis, in coronavirus-induced oligodendroglial cell death, in animal models of spinal cord injury and hypoxia-induced demyelination as well as in MS lesions and multiple systems atrophy (Casha et al., 2001, Gerstner et al., 2007, Hovelmeyer et al., 2005, Liu and Zhang, 2007). In animal models of spinal cord trauma, the lack of FAS or neutralization of FAS ligand is associated with increased oligodendroglial survival (Casha et al., 2005, Demjen et al., 2004). Mice lacking FAS, especially in oligodendrocytes, show improved clinical symptoms, reduced demyelination and oligodendroglial cell death compared to control animals in experimental autoimmune encephalomyelitis (Hovelmeyer et al., 2005). In the cuprizone model, apoptotic oligodendrocytes express activated caspase 3, and this is associated with a significant increase of FAS mRNA in the corpus callosum. However, no differences in the amount of demyelination, oligodendroglial cell death, number of apoptotic cells or inflammatory response was observed, indicating that FAS is not necessary for oligodendroglial cell death in the cuprizone model. These data strongly suggest that toxin-induced cell death is mediated via FAS-independent mechanisms, e.g. mitochondrial cell death mechanisms. These results are in contrast to the observed role of FAS for oligodendroglial cell death in EAE (Hovelmeyer et al., 2005). An obvious difference between EAE and the cuprizone model is the role of the immune system. Whereas EAE is an autoimmune-mediated disease in which T cells play a pivotal role, the immune response in the cuprizone model is limited to the activation of macrophages/microglial cells and very low numbers of T cells. In contrast to EAE, the blood–brain barrier remains intact in the cuprizone model (Remington et al., 2007). In EAE, therefore, the immune response may trigger oligodendroglial cell death via the FAS receptor, whereas in the cuprizone model cell death might be mediated via the mitochondrial cascade. In summary, we show that oligodendroglial cell death starts early after initiation of the cuprizone model and weeks before the loss of myelin is obvious by immunohistochemistry. Expression of activated caspase 3 is a prominent feature of dying oligodendrocytes during the early disease phase which is lost in later stages. Additionally, although FAS expression correlates with the expression of activated caspase 3, it is not necessary for oligodendroglial cell death. Further studies are required to identify the mechanisms leading to oligodendroglial cell death in early and late stages of the cuprizone model.
Acknowledgments
This study was supported by the Heidenreich von Siebold Program at the University Medical Center, Georg-August-University Göttingen, and by the Hertie Foundation.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.nbd.2009.10.016.
Appendix A.
References
- Austin J.W., Fehlings M.G. Molecular mechanisms of Fas-mediated cell death in oligodendrocytes. J. Neurotrauma. 2008;25:411–426. doi: 10.1089/neu.2007.0436. [DOI] [PubMed] [Google Scholar]
- Barnett M.H., Prineas J.W. Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion. Ann. Neurol. 2004;55:458–468. doi: 10.1002/ana.20016. [DOI] [PubMed] [Google Scholar]
- Bonetti B., Monaco S., Giannini C., Ferrari S., Zanusso GL., Rizzuto N. Human peripheral nerve macrophages in normal and pathological conditions. J. Neurol. Sci. 1993;118:158–168. doi: 10.1016/0022-510x(93)90105-8. [DOI] [PubMed] [Google Scholar]
- Bonetti B., Raine C.S. Multiple sclerosis: oligodendrocytes display cell death-related molecules in situ but do not undergo apoptosis. Ann. Neurol. 1997;42:74–84. doi: 10.1002/ana.410420113. [DOI] [PubMed] [Google Scholar]
- Boujrad H., Gubkina O., Robert N., Krantic S., Susin S.A. AIF-mediated programmed necrosis: a highly regulated way to die. Cell Cycle. 2007;6:2612–2619. doi: 10.4161/cc.6.21.4842. [DOI] [PubMed] [Google Scholar]
- Casha S., Yu W.R., Fehlings M.G. Oligodendroglial apoptosis occurs along degenerating axons and is associated with FAS and p75 expression following spinal cord injury in the rat. Neuroscience. 2001;103:203–218. doi: 10.1016/s0306-4522(00)00538-8. [DOI] [PubMed] [Google Scholar]
- Casha S., Yu W.R., Fehlings M.G. FAS deficiency reduces apoptosis, spares axons and improves function after spinal cord injury. Exp. Neurol. 2005;196:390–400. doi: 10.1016/j.expneurol.2005.08.020. [DOI] [PubMed] [Google Scholar]
- Demjen D., Klussmann S., Kleber S., Zuliani C., Stieltjes B., Metzger C., Hirt U.A., Walczak H., Falk W., Essig M., Edler L., Krammer P.H., Martin-Villalba A. Neutralization of CD95 ligand promotes regeneration and functional recovery after spinal cord injury. Nat. Med. 2004;10:389–395. doi: 10.1038/nm1007. [DOI] [PubMed] [Google Scholar]
- Dowling P., Shang G., Raval S., Menonna J., Cook S., Husar W. Involvement of the CD95 (APO-1/Fas) receptor/ligand system in multiple sclerosis brain. J. Exp. Med. 1996;184:1513–1518. doi: 10.1084/jem.184.4.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstner B., Sifringer M., Dzietko M., Schuller A., Lee J., Simons S., Obladen M., Volpe J.J., Rosenberg P.A., Felderhoff-Mueser U. Estradiol attenuates hyperoxia-induced cell death in the developing white matter. Ann. Neurol. 2007;61:562–573. doi: 10.1002/ana.21118. [DOI] [PubMed] [Google Scholar]
- Gudi V., Moharregh-Khiabani D., Skripuletz T., Koutsoudaki P.N., Kotsiari A., Skuljec J., Trebst C., Stangel M. Regional differences between grey and white matter in cuprizone induced demyelination. Brain Res. 2009;1283:127–138. doi: 10.1016/j.brainres.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Hao Z., Hampel B., Yagita H., Rajewsky K. T cell-specific ablation of Fas leads to Fas ligand-mediated lymphocyte depletion and inflammatory pulmonary fibrosis. J. Exp. Med. 2004;199:1355–1365. doi: 10.1084/jem.20032196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovelmeyer N., Hao Z., Kranidioti K., Kassiotis G., Buch T., Frommer F., von Hoch L., Kramer D., Minichello L., Kollias G., Lassmann H., Waisman A. Apoptosis of oligodendrocytes via Fas and TNF-R1 is a key event in the induction of experimental autoimmune encephalomyelitis. J. Immunol. 2005;175:5875–5884. doi: 10.4049/jimmunol.175.9.5875. [DOI] [PubMed] [Google Scholar]
- Jurevics H., Largent C., Hostettler J., Sammond D.W., Matsushima G.K., Kleindienst A., Toews A.D., Morell P. Alterations in metabolism and gene expression in brain regions during cuprizone-induced demyelination and remyelination. J. Neurochem. 2002;82:126–136. doi: 10.1046/j.1471-4159.2002.00954.x. [DOI] [PubMed] [Google Scholar]
- Kuhlmann T., Lucchinetti C., Zettl U.K., Bitsch A., Lassmann H., Brück W. Bcl-2-expressing oligodendrocytes in multiple sclerosis lesions. Glia. 1999;28:34–39. [PubMed] [Google Scholar]
- Li W., Maeda Y., Ming X., Cook S., Chapin J., Husar W., Dowling P. Apoptotic death following Fas activation in human oligodendrocyte hybrid cultures. J. Neurosci. Res. 2002;69:189–196. doi: 10.1002/jnr.10285. [DOI] [PubMed] [Google Scholar]
- Li J., Ghiani C.A., Kim J.Y., Liu A., Sandoval J., deVellis J., Casaccia-Bonnefil P. Inhibition of p53 transcriptional activity: a potential target for future development of therapeutic strategies for primary demyelination. J. Neurosci. 2008;28:6118–6127. doi: 10.1523/JNEUROSCI.0184-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebetanz D., Merkler D. Effects of commissural de- and remyelination on motor skill behaviour in the cuprizone mouse model of multiple sclerosis. Exp. Neurol. 2006;202:217–224. doi: 10.1016/j.expneurol.2006.05.032. [DOI] [PubMed] [Google Scholar]
- Liu Y., Zhang X. Murine coronavirus-induced oligodendrocyte apoptosis is mediated through the activation of the Fas signaling pathway. Virology. 2007;360:364–375. doi: 10.1016/j.virol.2006.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchinetti C., Brück W., Parisi J., Scheithauer B., Rodriguez M., Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann. Neurol. 2000;47:707–717. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Maloff B.L., Scordilis S.P., Tedeschi H. Assays of the metabolic viability of single giant mitochondria. Experiments with intact and impaled mitochondria. J. Cell Biol. 1978;78:214–226. doi: 10.1083/jcb.78.1.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima G.K., Morell P. The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system. Brain Pathol. 2001;11:107–116. doi: 10.1111/j.1750-3639.2001.tb00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon E., Suzuki K., Matsushima G. Peripheral macrophage recruitment in cuprizone-induced CNS demyelination despite an intact blood–brain barrier. J. Neuroimmunol. 2002;130:32–45. doi: 10.1016/s0165-5728(02)00205-9. [DOI] [PubMed] [Google Scholar]
- Moubarak R.S., Yuste V.J., Artus C., Bouharrour A., Greer P.A., Menissier-de M.J., Susin S.A. Sequential activation of poly(ADP-ribose) polymerase 1, calpains, and Bax is essential in apoptosis-inducing factor-mediated programmed necrosis. Mol. Cell Biol. 2007;27:4844–4862. doi: 10.1128/MCB.02141-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niikura Y., Dixit A., Scott R., Perkins G., Kitagawa K. BUB1 mediation of caspase-independent mitotic death determines cell fate. J. Cell Biol. 2007;178:283–296. doi: 10.1083/jcb.200702134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouly S., Becher B., Blain M., Antel J.P. Interferon-γ modulates human oligodendrocyte susceptibility to Fas-mediated apoptosis. J. Neuropathol. Exp. Neurol. 2000;59:280–286. doi: 10.1093/jnen/59.4.280. [DOI] [PubMed] [Google Scholar]
- Raine C.S., Bonetti B., Cannella B. Multiple sclerosis: expression of molecules of the tumor necrosis factor ligand and receptor families in relationship to the demyelinated plaque. Rev. Neurol. 1998;154:577–585. [PubMed] [Google Scholar]
- Remington L.T., Babcock A.A., Zehntner S.P., Owens T. Microglial recruitment, activation, and proliferation in response to primary demyelination. Am. J. Pathol. 2007;170:1713–1724. doi: 10.2353/ajpath.2007.060783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabelko-Downes K.A., Cross A.H., Russel J.H. Dual role for Fas ligand in the initiation of and recovery from experimental allergic encephalomyelitis. J. Exp. Med. 1999;189:1195–1205. doi: 10.1084/jem.189.8.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skripuletz T., Lindner M., Kotsiari A., Garde N., Fokuhl J., Linsmeier F., Trebst C., Stangel M. Cortical demyelination is prominent in the murine cuprizone model and is strain-dependent. Am. J. Pathol. 2008;172:1053–1061. doi: 10.2353/ajpath.2008.070850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenning G.K., Stefanova N., Jellinger K.A., Poewe W., Schlossmacher M.G. Multiple system atrophy: a primary oligodendrogliopathy. Ann. Neurol. 2008;64:239–246. doi: 10.1002/ana.21465. [DOI] [PubMed] [Google Scholar]
- Wosik K., Antel J., Kuhlmann T., Bruck W., Massie B., Nalbantoglu J. Oligodendrocyte injury in multiple sclerosis: a role for p53. J. Neurochem. 2003;85:635–644. doi: 10.1046/j.1471-4159.2003.01674.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


